
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci., 17 September 2020
Sec. Cellular Neurophysiology
Volume 14 - 2020 | https://doi.org/10.3389/fncel.2020.566418
This article is part of the Research TopicSensory Abnormalities and Primary Sensory NeuronsView all 14 articles
Sensory abnormalities generated by nerve injury, peripheral neuropathy or disease are often expressed as neuropathic pain. This type of pain is frequently resistant to therapeutic intervention and may be intractable. Numerous studies have revealed the importance of enduring increases in primary afferent excitability and persistent spontaneous activity in the onset and maintenance of peripherally induced neuropathic pain. Some of this activity results from modulation, increased activity and /or expression of voltage-gated Na+ channels and hyperpolarization-activated cyclic nucleotide–gated (HCN) channels. K+ channels expressed in dorsal root ganglia (DRG) include delayed rectifiers (Kv1.1, 1.2), A-channels (Kv1.4, 3.3, 3.4, 4.1, 4.2, and 4.3), KCNQ or M-channels (Kv7.2, 7.3, 7.4, and 7.5), ATP-sensitive channels (KIR6.2), Ca2+-activated K+ channels (KCa1.1, 2.1, 2.2, 2.3, and 3.1), Na+-activated K+ channels (KCa4.1 and 4.2) and two pore domain leak channels (K2p; TWIK related channels). Function of all K+ channel types is reduced via a multiplicity of processes leading to altered expression and/or post-translational modification. This also increases excitability of DRG cell bodies and nociceptive free nerve endings, alters axonal conduction and increases neurotransmitter release from primary afferent terminals in the spinal dorsal horn. Correlation of these cellular changes with behavioral studies provides almost indisputable evidence for K+ channel dysfunction in the onset and maintenance of neuropathic pain. This idea is underlined by the observation that selective impairment of just one subtype of DRG K+ channel can produce signs of pain in vivo. Whilst it is established that various mediators, including cytokines and growth factors bring about injury-induced changes in DRG function and excitability, evidence presently available points to a seminal role for interleukin 1β (IL-1β) in control of K+ channel function. Despite the current state of knowledge, attempts to target K+ channels for therapeutic pain management have met with limited success. This situation may change with the advent of personalized medicine. Identification of specific sensory abnormalities and genetic profiling of individual patients may predict therapeutic benefit of K+ channel activators.
Neuropathic pain is caused by disease or lesion of the somatosensory system (Treede et al., 2008). It can arise from a broad range of insults, including peripheral nerve trauma, post herpetic neuralgia, spinal cord injury, stroke or from neuropathies associated with chemotherapy, diabetes or HIV infection (von Hehn et al., 2012; Alles and Smith, 2018). Pain associated with fibromyalgia, osteoarthritis, complex regional pain syndromes, multiple sclerosis, rheumatoid arthritis and autoimmune disease is also dominated by neuropathic components (Olechowski et al., 2009; Lu et al., 2012; Sumpton and Moulin, 2014; Mifflin and Kerr, 2017; Rifbjerg-Madsen et al., 2017). Neuropathic pain is usually chronic and often intractable. Unlike nociceptive pain, which serves to protect an individual from potential or actual injury, neuropathic pain typically persists long after tissue healing and recovery has taken place. This and the fact that some symptoms of neuropathic pain persist in the absence of any type of sensory stimulus (Scholz and Woolf, 2007) means that this type of pain is maladaptive and serves no obvious biological purpose (Iadarola and Caudle, 1997; Costigan et al., 2009).
I will focus on neuropathic pain associated with peripheral nerve trauma and/or peripheral neuropathies as this aligns with the focus of the present collection of papers on “sensory abnormalities and primary sensory neurons.” Consideration of the K+ channels that play important roles in Schwann cells, satellite and other types of glial cells (Chiu and Wilson, 1989; Konishi, 1989; Olsen et al., 2015; Stebbing et al., 2015; Murata et al., 2016; Lu et al., 2017) is beyond the scope of the present review. Some of the evidence relating to the role of glial K+ channels in pathological pain processes can be found in the review by Tsantoulas and McMahon (2014).
In approved animal models, investigations into the etiology of neuropathic pain often involve surgical or chemical lesions to peripheral nerves followed by in vivo or ex vivo investigations of the properties of primary afferent neurons. These are correlated with behavioral studies which seek to assess pain intensity by indices such as mechanical or thermal withdrawal threshold or presence of hyperalgesia and touch-induced pain (allodynia) (Kim et al., 1997; Decosterd and Woolf, 2000; Mogil, 2009; Stemkowski and Smith, 2013). Unfortunately, these “classical” models may better reflect nociception rather than “pain” per se which is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage”. It may be argued, for example, that withdrawal of a foot or limb in response to a noxious stimulus may simply reflect activation of a spinal reflex (Mogil, 2009). Thus, current models for assessment of chronic pain involve determination of indices such as facial grimace score as well as observation of social interaction and nest-building (Sotocinal et al., 2011; Turner et al., 2019). A complementary approach is to use operant models such as conditioned place preference protocols. In one version of this, rodents are required to make a conscious choice between being in a pain-inducing environment and an otherwise undesirable environment such as a brightly illuminated space (Mauderli et al., 2000; Negus et al., 2006; Alles and Smith, 2016; Harte et al., 2016). The time spent in the undesirable environment gives an index of the pain the animal is experiencing.
In addition to the difficulty in assessing bona fide pain in animal models, several other considerations limit the translation between laboratory studies and beneficial human treatments. Such issues explain the slow progress in development of urgently needed new therapeutic approaches (Clark, 2016).
It is recognized, for example, that the processes initiating neuropathic pain (within 1–2 weeks of peripheral nerve injury) differ from those that maintain it over periods of months or years (Ji et al., 2006, 2019; Zhuang et al., 2006). Although the latter phenomena are more relevant to the clinical presentation of neuropathic pain, much of the work done in the laboratory has been associated with investigation of mechanisms underlying pain onset (Gold and Gebhart, 2010; Clark, 2016; Noh et al., 2020).
Two people can have virtually identical lesions and whilst one may recover, the other may develop chronic and intractable pain (Peiro et al., 2016). Genetic components must therefore play a role in human pain vulnerability (Costigan et al., 2009; Zorina-Lichtenwalter et al., 2016). This is reflected in animal studies as Fisher F-344 rats completely recover from nerve injury induced allodynia within 28 days whereas inbred Lewis and outbred Sprague-Dawley rats do not (Herradon et al., 2007). There is even considerable variation in injury-induced pain responses of different individuals of the same strain (Liu et al., 2001). Variation in pain response to injury may not only relate to hereditary genetics but also epigenetics reflecting prior exposure to noxious stimuli (Ciotu et al., 2019; Brewer and Baccei, 2020; Brewer et al., 2020; Topham et al., 2020).
Ongoing molecular genetics studies seek to identify genes that predispose certain human populations and/or individuals to pain development (Dib-Hajj and Waxman, 2014; Peiro et al., 2016). Variations in the propensity of certain individuals to develop chronic pain may even reflect differences in their gastrointestinal microbiome (Guo et al., 2019).
A further complication is that mechanisms underlying neuropathic pain in females differ from those in males (Wagner et al., 1995; Mogil, 2012; Sorge et al., 2015; Dodds et al., 2016; Mifflin et al., 2018) and women are more prone than men to develop neuropathic pain (Bannister et al., 2020). To the best of my knowledge, only one study appears to address possible differences in the effect of injury on K+ channel function in females versus males (Ritter et al., 2015b). This parallels the issue of species differences. It has been shown, for example that while human and rat peripheral neurons express the inwardly rectifying K+ channel GIRK2 (Kir3.2) these channels are not expressed in this location in mice (Nockemann et al., 2013).
Finally, the variety of peripheral nerve injury models that have been used provoke different changes in the physiology and behavioral manifestations of pain and nociception (Kim et al., 1997; Lee et al., 1998; Decosterd and Woolf, 2000; Chen et al., 2009b; Stemkowski and Smith, 2013; Noh et al., 2020). This complicates the evaluation, comparison, integration and summation of data from different research groups. It may also explain help to contradictory findings. For example, spinal nerve ligation reduces expression of mRNA for delayed rectifier Kv1.2 channels (Fan et al., 2014) but this not seen in a model of painful diabetic neuropathy (Cao et al., 2010, see section “Effects of Nerve Injury on Delayed Rectifier K+ Currents and Channels in DRG Neurons”).
Chronic neuropathic pain and models thereof are distinct from chronic inflammatory pain which is modeled by injection of irritants such as complete Freund’s adjuvant (CFA) into the tail, paw, muscle or joint (Gregory et al., 2013). Whereas neuropathic pain models provoke axonal degeneration (Ramer et al., 1997; Ma et al., 2003), tissue inflammation associated with CFA injection may not cause damage to nerves per se. Although there are doubtless molectular parallels between chronic inflammatory and neuropathic pain, important diffferences have been identified (Djouhri, 2016). Space constraints limit discussion of altered K+ channel function in inflammation and only nerve injury effects will be considered.
The use of sciatic nerve section as a model for neuropathic pain was put forward many years ago (Wall and Gutnick, 1974) but the complete loss of sensory nerve fibers prevents testing for mechanical or thermal hyperalgesia. Whole nerve transection does however promote the phenomenon of autotomy (Wall et al., 1979). This involves attacking and gnawing the foot of the nerve transected limb. Since it has been argued that the appearance of autotomy parallels the onset of neuropathic pain in humans (Coderre et al., 1986), it may be an index of pain per se rather than a manifestation of altered nociception. Despite this, sciatic nerve section is only an appropriate model for post-amputation or phantom limb pain, it has largely been superseded by manipulations that better mimic more frequently observed cases of human nerve trauma.
The first of these is the chronic constriction injury model (CCI). There are several variations. The Chung model and the Bennett and Xie model which involve the positioning of ligatures around the sciatic nerve (Bennett and Xie, 1988; Kim and Chung, 1992), the Mosconi Kruger model which involves encapsulation of the sciatic nerve in a polyethylene cuff (Mosconi and Kruger, 1996) and the Seltzer model which involves ligation of half of the sciatic nerve high within the thigh (Seltzer et al., 1990).
The spared nerve injury (SNI) model was developed over 20 years ago (Decosterd and Woolf, 2000). This involves ligation and distal transection of the tibial and common peroneal nerves whilst leaving the sural nerve intact.
Lastly most studies on the effect of nerve injury on K+ channel function have favored the spinal nerve ligation and transection models (SNL and SNT; Table 1). These involve ligation or severing the L5 dorsal root proximal to the DRG whilst leaving the L4 root intact. Studies on the properties of the L4 DRG allow examination of the properties of uninjured neurons (Ma et al., 2003) whereas those on L5 DRG allow examination of injured neurons with severed axons.
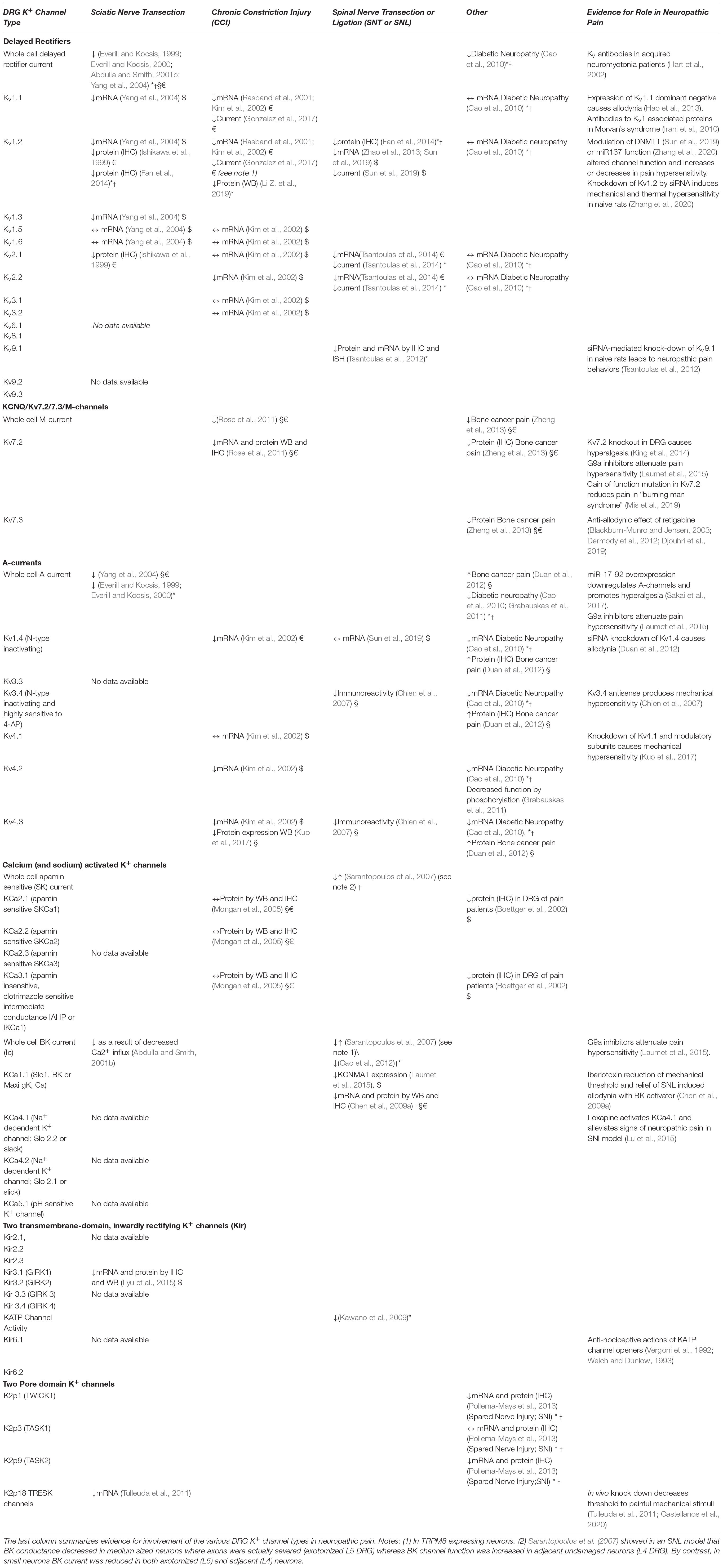
Table 1. Known changes in K+ channel function in DRG following various types of peripheral nerve injury (↑, increase; ↓, decrease; ↔, unchanged; *, in large cells only; †, in medium sized cells only; §, in small IB4 positive neurons; €, in small IB4 negative neurons; $, neuron type unspecified.
In the CCI, SNL, SNT, and SNI models some axons are subject to Wallerian degeneration whereas others remained relatively unscathed (Ramer et al., 1997; Ma et al., 2003). The release of inflammatory mediators and growth factors from the site of injury is thought to initiate changes in axons and cell bodies of both injured and uninjured axons (Wagner and Myers, 1996a,b; Sorkin et al., 1997; Sommer et al., 1999; Cui et al., 2000; George et al., 2004). Because a fraction of both sensory and motor axons survive after SNL, SNT, CCI and SNI, animals can be assessed for mechanical and/or thermal hyperalgesia. The main difference between CCI and SNI is that the behavioral responses to CCI subside within a month or so whereas those produced by SNI persist for much longer periods of time (Decosterd and Woolf, 2000; Noh et al., 2020). SNL, SNT, and SNI are thus emerging as preferred animal protocols by which to model human neuropathic pain.
Since the concept was first put forward (Govrin-Lippmann and Devor, 1978; Wall and Devor, 1983), an overwhelming body of evidence now supports the indispensable role of peripheral nerve sensitization and ongoing, aberrant spontaneous activity in the onset and persistence of pain following neuropathy or peripheral nerve injury (Costigan et al., 2009; Bedi et al., 2010; Gold and Gebhart, 2010; Sexton et al., 2017; Koplovitch and Devor, 2018; Waxman, 2019; Yatziv and Devor, 2019; Yousuf et al., 2019; Waxman, 2019). Much of this peripheral sensitization results from the dorsal root ganglia (DRG) per se as well as from nerve axons or terminals and/or the site of injury (Kajander et al., 1992; Yatziv and Devor, 2019). Aberrant spontaneous activity in injured peripheral nerves is thought to drive the sensitization of spinal circuits; an established harbinger of chronic pain (Woolf, 1983; Sandkuhler, 2009; Gold and Gebhart, 2010; Latremoliere and Woolf, 2009; Tsantoulas and McMahon, 2014; Alles and Smith, 2018).
Intracellular recordings from DRG neurons both ex vivo and in vitro confirmed that peripheral nerve injury increases their excitability and may provoke spontaneous discharge of action potentials (Study and Kral, 1996; Djouhri et al., 2018). In a study of the effect of complete nerve section, we found and that the most profound increases were seen in animals that displayed autotomy (Abdulla and Smith, 2001a). If it is accepted that the appearance of autotomy parallels the onset of neuropathic pain (Coderre et al., 1986), our findings support a role for increased DRG excitability in the etiology of pain per se as opposed to a simple consequence of altered nociception. In a complementary study, it was found that individual rats with a high propensity to exhibit autotomy following peripheral nerve injury exhibited more spontaneous primary afferent activity than those with a weaker autotomy phenotype (Liu et al., 2001).
We also found that peripheral nerve chronic constriction injury (CCI) increases the excitatory synaptic drive to putative excitatory substantia gelatinosa neurons (Balasubramanyan et al., 2006; Lu et al., 2009). Although intrinsic properties of substantia gelatinosa neurons such as excitability, rheobase and input resistance were unchanged, the frequency and amplitude of spontaneous excitatory synaptic events was increased. As will be discussed below, this may be a consequence of altered expression of K+ channels in primary afferent nerve terminals (Barkai et al., 2017; Muqeem et al., 2018). This is again consistent with the notion that injury induced-sensitization of spinal circuits is driven by, and attributed to changes in primary afferent activity (Devor, 2006). This idea is supported further by the observation that peripheral nerve injury increases the spontaneous discharge rate of wide dynamic range (WDR) neurons in the rat dorsal horn and that this effect is attenuated by peripheral application of lidocaine (Pitcher and Henry, 2008).
More recent work continues to underline the role of aberrant and persistent peripheral nerve activity in the long-term sensitization of spinal nociceptive circuits following nerve injury. Thus, application of low dose lidocaine to DRG within the intervertebral foramen transiently suppresses allodynia in a rat SNL model. Normal nerve conduction was unaffected and sustained infusion of low dose lidocaine for 2 weeks using osmotic minipumps suppressed allodynia for the duration of the infusion. Since application of low dose lidocaine to the peripheral site of injury to the sciatic nerve was ineffective, these findings not only support a role for spontaneous peripheral nerve activity in pain generation but suggest that such activity originates in the DRG (Yatziv and Devor, 2019). This study was based on the previous electrophysiological observation that low concentrations of lidocaine suppress ectopic neuropathic discharge in dorsal root ganglia without blocking propagation of action potentials along the axon (Koplovitch and Devor, 2018).
Another study used in vivo optogenetic methodology to silence nociceptive primary afferents which express the voltage gated Na+ channel Nav1.8. This was achieved by selectively expressing inhibitory archaerhodopsin-3 (Arch) proton pumps in Nav1.8(+) neurons. Activation of Arch by yellow light leads to membrane hyperpolarization. Acute transdermal illumination of the hindpaw of Nav1.8(+)-Arch(+) mice in vivo reduced mechanical allodynia under inflammatory conditions, while basal mechanical sensitivity was unaffected. Furthermore, in mice made neuropathic by SNI, prolonged optical silencing of Nav1.8(+) peripheral afferents led to lasting analgesia with a decrease in mechanical and thermal hypersensitivity (Daou et al., 2016).
Confirmation of the role of persistent increased excitability of peripheral neurons in a clinical setting was obtained from examination of amputees who suffered phantom limb pain (Vaso et al., 2014). Application of lidocaine intrathecally or to the DRG surface during intraforaminal epidural block reversibly extinguished phantom limb pain. Similar results in amputees have been reported using peripheral nerve block (Buch et al., 2019). Indeed, lidocaine patches still retain a role, albeit a minor one, in the management of neuropathic pain in adults (Finnerup et al., 2015).
Classically, Aβ fibers convey tactile and non-noxious sensation, Aδ fibers convey high threshold mechanical and thermal sensation (first pain) and polymodal C-fibers convey thermal mechanical and inflammatory pain (second pain). More recent studies have illustrated that the situation is much more complex (Snider and McMahon, 1998; Lawson, 2002; Gold and Gebhart, 2010; Chiu et al., 2014; Peirs and Seal, 2016; Arcourt et al., 2017; Zheng et al., 2019; Giacobassi et al., 2020). C-fibers are known to fall into at least two categories; nociceptors that contain neuropeptides such as substance P and calcitonin gene related peptide (CGRP) and neurons which bind the plant lectin IB4 and often express Ret, the receptor for the glial cell line-derived family of neurotrophins as well as the purinergic P2X3 receptor (Stucky and Lewin, 1999; Fang et al., 2006; Gold and Gebhart, 2010). It is suggested that IB4-positive nociceptors play a role in mediating mechanical inflammatory hypersensitivity rather than normal mechanical nociception (Pinto et al., 2019).
Although DRG neurons are heterogeneous and highly specialized (Zheng et al., 2019; Giacobassi et al., 2020) the simple answer to the question of which neuronal types are affected by nerve injury is “probably all of them depending on the nature of the injury” (see Table 2).
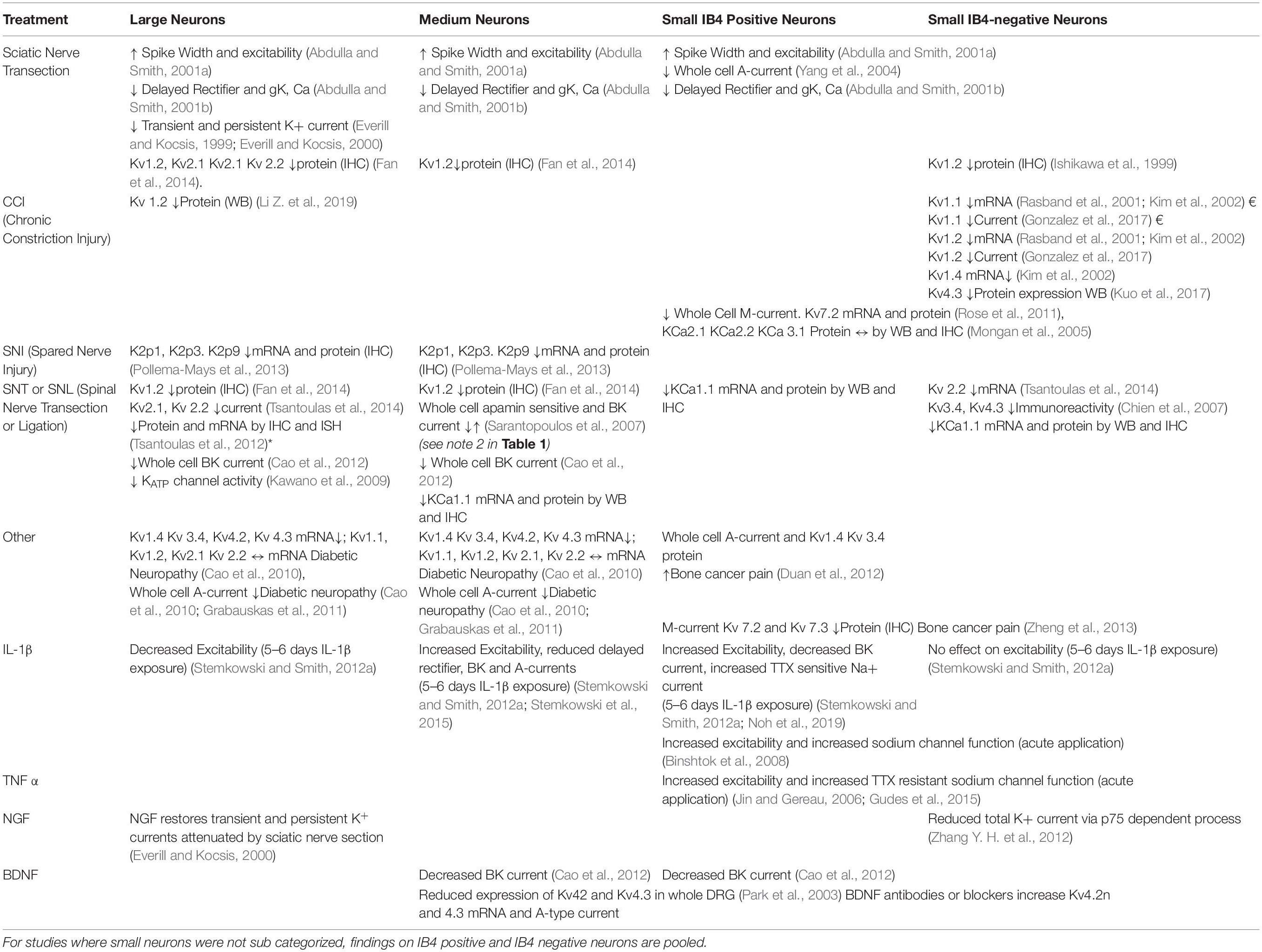
Table 2. Effects of injury and inflammatory mediators on K+ channel function in Large, Medium, Small IB4 positive and Small IB4 negative neurons.
By contrast with nociceptive pain which is primarily transmitted by Aδ and C-fibers, there is good evidence to support a role of Aβ afferents in neuropathic pain in both animal models (Devor, 2009; Sandkuhler, 2009; Tashima et al., 2018) and in clinical situations (Campbell et al., 1988). One aspect of central sensitization is loss of inhibition (Coull et al., 2003) which enables transfer of aberrant excitation between deeper spinal cord laminae and more superficial laminae (Baba et al., 2003; Schoffnegger et al., 2008; Peirs et al., 2015; Peirs and Seal, 2016; Boyle et al., 2019). Because the deeper laminae are primarily involved in the processing of tactile information and superficial laminae process nociceptive information, it is easy to see how activation of tactile Aβ fibers can lead to allodynia as tactile information is misdirected to the superficial dorsal horn. In view of this, the following discussion of effects of injury on K+ channels will include those in all DRG cell types as perturbation of any of them could be potentially involved in neuropathic pain. In addition to this, there is now evidence that some Aβ fibers play a role in “normal” pain perception (Arcourt et al., 2017) and that they may undergo a “phenotypic switch” following injury wherein they acquire the properties and sensitivities of afferent C-fibers (Abdulla et al., 2001; Nitzan-Luques et al., 2011).
There is now evidence that pain following injury to the spinal cord per se (as opposed to CCI, sciatic nerve transection or SNI of peripheral nerves as discussed above) also depends upon continuing hyperactivity of peripheral sensory neurons (Bedi et al., 2010; Yang et al., 2014). Thus, spinal contusion injury promotes persistent upregulation of protein for the voltage-gated Na+ channel, Nav1.8. Since this channel is expressed almost exclusively in primary afferent neurons, Yang et al. (2014) were able to reverse hypersensitivity of hindlimb withdrawal reflexes, and reduced ongoing pain by knocking down Nav1.8 after spinal injury. Pain (as opposed to nociception) assessed was by a conditioned place preference test.
With regard to the present focus on K+ channels, cervical unilateral spinal cord injury has also shown to reduce surface expression of Kv3.4 channels (A-type) in DRG neurons (Ritter et al., 2015a). It is likely therefore that inflammatory mediators produced as a result of spinal cord injury diffuse and affect channel expression and function in the DRG [see section “Distribution of High Threshold, N-type Inactivating, 4-AP and TEA Sensitive A-currents (Kv3.4) and Effects of Nerve Injury” below].
As summarized in Table 1, the majority of studies show that the function of all types of K+ channels is reduced following peripheral nerve injury (Everill and Kocsis, 1999; Everill and Kocsis, 2000; Abdulla and Smith, 2001b; Rasband et al., 2001; Kim et al., 2002; Park et al., 2003; Ocana et al., 2004; Kawano et al., 2009; Cao et al., 2010; Takeda et al., 2011; Tsantoulas and McMahon, 2014; Zemel et al., 2018). Many of the early studies describe attenuation of voltage-gated K+ conductances in the DRG cell body per se. In most cases, this does not lead to depolarization but may rather affect the accommodation of action potential discharge in response to persistent depolarization (Abdulla and Smith, 2001a). Spike width (action potential duration) is also increased (Gurtu and Smith, 1988; Stebbing et al., 1999; Abdulla and Smith, 2001a) and this may involve attenuation of voltage-gated K+ conductances. If this situation also applies to channels in primary afferent terminals, this may lead to increased glutamate release in the dorsal horn of the spinal cord (Muqeem et al., 2018).
It should be pointed out, however, that extrapolation of findings in DRG cell bodies to K+ channel function and distribution throughout the axons, nerve terminals and free nerve endings of the whole neuron is very much an oversimplification of processes underlying changes in excitability. The review by Tsantoulas and McMahon (2014) presents detailed information regarding K+ channel location, particularly in the nodal, paranodal and juxtaparanodal regions of axons. The idea that specific types of K+ channels are trafficked to specific subcellular regions is borne out by the observation that injury-induced early loss of Kv1.1 and 1.2 (delayed rectifier) channels at the juxtaparanodal regions of sensory axons may be later followed by appearance of Kv1.4 (A-type) and Kv1.6 channels (Calvo et al., 2016). On the other hand, it should be reiterated that the DRG themselves play an important role in generating ectopic activity after nerve injury (Kajander et al., 1992; Yatziv and Devor, 2019).
Experiments using the skin-nerve preparation that allows single fiber recording from sensory afferents of excised rat skin (Reeh, 1988; Schutze et al., 2016) have shown that sensory nerve endings are excited by the K+ channel blockers, TEA and 4AP (Kirchhoff et al., 1992). Selective blockade of nerve ending K+ channels increased spontaneous activity in Aβ, Aδ and C-fibers. Other studies have identified TREK-1 (K2p2) in sensory C-fibers of both IB4-positive and IB4-negative neurons where it appears as an important ion channel for regulation of polymodal pain (Alloui et al., 2006). TRAAK (K2p4) channels have also been detected in C-fibers (Noel et al., 2009) and Kv7.2 and 7.3 channels are expressed in the endings of D-hair Aδ fiber mechanoceptors (Schutze et al., 2016). While is it not yet known whether injury alters K+ channel expression in nerve endings in a similar fashion to cell bodies, a role for these channels in neuropathic pain is implied by three observations; (1) in some, but not all cases, nerve injury downregulates both mRNA (Rasband et al., 2001; Kim et al., 2002; Yang et al., 2004) and/or channel protein (Ishikawa et al., 1999; Fan et al., 2014). In these cases, K+ channel function would be altered at all loci within the neuron (including free nerve endings); (2) K+ channel function is impaired by various inflammatory mediators (Binshtok et al., 2008; Takeda et al., 2008; Stemkowski and Smith, 2012a; Stemkowski et al., 2015) most of which are released from damaged axons and Schwann cells in the vicinity of nerve endings (Sommer and Kress, 2004; Scholz and Woolf, 2007); (3) many types of K2p channels are mechanosensitive (Goldstein et al., 2005) and may therefore play a role in the development of nerve injury-induced allodynia.
Whereas studies on voltage-gated Na+ channels have paved the way for the development of new therapeutic targets (Dib-Hajj et al., 2010; Waxman and Zamponi, 2014; Dib-Hajj and Waxman, 2019), studies on K+ channels have been less encouraging. Voltage-gated Na+ channels are monomeric proteins resulting from the nine gene products Nav1.1 to Nav1.9 (Goldin et al., 2000). Since Nav1.7, 1.8, and 1.9 are highly expressed in DRG and have been implicated in neuropathic pain, they present attractive targets for drug development. Because these channels are much less abundant in other excitable tissues, this limits the side effect profile of drugs which target them (Dib-Hajj et al., 2013; Zakrzewska et al., 2017). Nav1.3 represents another attractive target as it only appears in adult DRG neurons after nerve injury (Samad et al., 2013; Waxman and Zamponi, 2014).
Hyperpolarization activated cyclic nucleotide gated cation (HCN) channels also display increased activity following nerve injury (Chaplan et al., 2003; Smith et al., 2015; Bernal and Roza, 2018; Djouhri et al., 2018) and may be involved in pain associated with diabetic neuropathy (Tsantoulas et al., 2017) are under consideration as therapeutic targets. For example, clinically approved drugs such as the antianginal agent ivabradine are effective in attenuating allodynia in animal models (Emery et al., 2011, 2012; Noh et al., 2014; Young et al., 2014; Tsantoulas et al., 2016). HCN2 channels are a particularly attractive target as this isoform is found predominantly in neurons (Tsantoulas et al., 2016).
By contrast, many of the K+ channel types found in primary afferent neurons are found in many tissues and cell types throughout the body in both excitable and non-excitable cells. This may limit the therapeutic potential of K+ channel activators.
The 78 K+ channel genes so far identified in the human genome give rise to four broad families of channels (Ocana et al., 2004; Goldstein et al., 2005; Gutman et al., 2005; Kubo et al., 2005; Wei et al., 2005).
(1) the six transmembrane-domain voltage gated (Kv) channels which assemble as homo- or hetero-tetramers from 40 or so human genes. These give rise to A-currents and delayed rectifier conductances;
(2) Ca2+ activated K+ (KCa) channels which often contribute to action potential duration and afterhyperpolarization. This category includes Na+ sensitive K+ channels such as “slick” (sequence like an intermediate calcium channel) and “slack” (Wei et al., 2005);
(3) two transmembrane-domain inwardly rectifying K+ channels (KIR) which also assemble as tetramers and include instantaneous inward rectifiers as well as ATP sensitive and G-protein activated channels (Kubo et al., 2005);
(4) four transmembrane-domain tandem pore domain (K2p) channels which assemble as dimers and account for K+ leak conductance (Goldstein et al., 2005; Enyedi and Czirjak, 2010).
Representatives of all four K+ channel families are found in DRG neurons (McFarlane and Cooper, 1991; Gold et al., 1996a; Abdulla and Smith, 2001b; Talley et al., 2001; Kang and Kim, 2006; Kawano et al., 2009; Zemel et al., 2018; Gada and Plant, 2019) and the distribution of genetically defined channel phenotypes in various DRG neuron types is an active area of investigation (Chien et al., 2007; Bocksteins et al., 2009, 2012; Tsantoulas et al., 2014; Ritter et al., 2015b; Zemel et al., 2018). Some of these studies also address how the distribution and function of these channels is altered by nerve injury. Other work examines the molecular mechanism of altered K+ channel function and how alteration of function relates to pain (Chien et al., 2007; Takeda et al., 2008; Cao et al., 2010; Rose et al., 2011; Stemkowski et al., 2015; Calvo et al., 2016; Zemel et al., 2017, 2018; Djouhri et al., 2019; Noh et al., 2019). The hope is that a single K+ channel type or subtype will emerge as a therapeutic target in neuropathic pain in a comparable fashion to Nav1.7. To explore this possibility, the following sections outline the current state of knowledge regarding the location and subunit composition of various types of K+ channels in sensory neurons and how they affected by nerve injury. Although a considerable amount of information is available, it should be remembered that different authors have used different criteria for definition of neuronal types or have gathered information from unidentified DRG neurons in culture.
Membrane conductances mediated by delayed rectifier channels activate in response to depolarization and show little or no inactivation. They comprise hetero- or homo-tetramers from the gene products Kv1.1, 1.2, 1.3, 1.5, 1.6, 1.7, 1.8, 2.1, 2.2, 3.1, 3.2, 7.1, 7.2, 7.3, 7.4, 7.5, 10.1, 10.2, 11.2, 11.3, 12.1, 12.2, 12.3 from the KCNA, KCNB, KCNC, KCNQ, and KCNH gene families (Gutman et al., 2005).
K+ channel conductances that display delayed outward rectification have been described in all types of DRG neuron (Akins and McCleskey, 1993; Gold et al., 1996a; Everill et al., 1998; Abdulla and Smith, 2001b). mRNA for Kv1.1, Kv1.2, Kv1.3, Kv1.5, and Kv1.6 has been detected in rat DRG (Yang et al., 2004) and Kv1.2, Kv2.1, and Kv2.2 mRNA appears to particularly abundant in medium and/or large sized DRG neurons (Fan et al., 2014; Tsantoulas et al., 2014). Other work has demonstrated the presence of Kv3.1 protein and Kv3.1 currents in small DRG neurons but these only contribute to about 19% of the delayed rectifier current (Bocksteins et al., 2012). This is consistent with the suggestion that Kv3 channels are mainly found in the CNS (Weiser et al., 1994). The silent subunit Kv9.1 (KCNS1) is present in large DRG neurons with myelinated axons but is absent from small neurons (Tsantoulas et al., 2012, 2018).
The known effects of peripheral nerve injury on all types of K+ channels in DRG neurons are summarized in Tables 1, 2. Delayed rectifier currents in cell bodies of small, medium and large sized DRG neurons are reduced by about 60% following sciatic nerve section (Abdulla and Smith, 2001b). mRNA’s for Kv1.1, 1.2, and 2.2 but not Kv1.5 and 1.6 are downregulated (Rasband et al., 2001; Kim et al., 2002; Yang et al., 2004) and immunohistochemical (IHC) studies confirm the loss of Kv1.2 protein (Ishikawa et al., 1999; Fan et al., 2014). More recent studies have confirmed that spinal nerve transection (SNT) downregulates Kv2.2 as well as Kv2.1 mRNA and this may have accounted for a shortening of action potential afterhyperpolarization and increased excitability of myelinated medium to large cells (Tsantoulas et al., 2014).
Knockdown of Kv1.2 by siRNA induces significant mechanical and thermal hypersensitivity in naive rats (Zhang et al., 2020) and considerable progress has been made in understanding the underlying epigenetic mechanisms. Three of these involve alterations in DNA methylation.
Firstly, SNL increases expression of the canonical maintenance methyltransferase DNMT1 via a CREB (cAMP response element binding protein) – dependent process. This leads to downregulation of the KCNA2 gene, reduced KCNA2 expression and a reduction of total Kv current that was attributed to loss of Kv1.2 channel function. Blockade of DNMT1 upregulation attenuated hyperexcitability in the injured DRG neurons and alleviated nerve injury-induced pain hypersensitivity (Sun et al., 2019).
A second a parallel pathway involving the MBD1 protein (Methyl-CpG-binding domain protein 1) has been described. MBD1 binds to methylated sequences of DNA and attracts the DNA methylation protein DNMT3a leading to downregulation of KCNA2 and reduced Kv1.2 expression. Overexpression of MBD1 leads to spontaneous pain and evoked pain hypersensitivities in wild type mice (Zhao et al., 2017; Mo et al., 2018).
Thirdly, SNL downregulates the expression of Kv1.2 in DRG by decreasing expression of ten-eleven translocation methylcytosine dioxygenase 1 (TET1). This promotes DNA demethylation and its overexpression in the DRG of nerve injured animals alleviated pain hypersensitivities without altering acute pain. Mechanistically, TET1 rescued the expression of Kv1.2 by reducing the level of 5-methylcytosine and increasing the level of 5-hydroxymethylcytosine in the promoter region of the KCNA2 gene (Wu et al., 2019).
A separate mechanism involving histone deacetylase2 (HDAC2) is also thought to control Kv1.2 expression in large DRG neurons in response to CCI (Li Z. et al., 2019).
There is also evidence that Kv1.2 function is controlled by the non-coding miniature RNA miR-137. Because it impairs Kv1.2 function, experimental impairment of miR-137 function, rescues channel expression and function attenuates allodynia in rats subject to CCI (Zhang et al., 2020).
Lastly, a long non-coding RNA contributes to neuropathic pain by silencing KCNA2 and thereby reducing expression of Kv1.2 in primary afferents (Zhao et al., 2013). This is of particular interest because Kv1.1–Kv1.2 heteromers function as mechanosensitive K+ currents that act as mechanical brake in the senses of touch and pain (Hao et al., 2013).
Interfering with Kv1.1 channel expression results in mechanical allodynia without a change in thermal sensitivity. A role of Kv1.1 channels in suppressing mechanical allodynia fits with their high expression in high threshold C-mechanoreceptors. These findings do not exactly correspond with the effect of CCI on TRPM8 expressing DRG neurons where there is loss of function of the “excitability brake current”. This current, which is thought to involve Kv1.1 and 1.2 channels, normally restricts the depolarizing influence of TRPM8 channel activation by cold. It has been proposed that the loss of Kv1.1/1.2 may thus promote cold allodynia (Gonzalez et al., 2017).
It is also possible that injury-induced changes in delayed rectifier channels reflect post-translational processes such as phosphorylation, endocytosis and/or trafficking (Nesti et al., 2004; Yang et al., 2007) that may be independent of any change in expression of K+ channel genes. This possibility is underlined by the observation that delayed rectifier currents were markedly reduced in medium- and large-, but not in small diameter DRG neurons in a rodent model of painful diabetic neuropathy but the mRNA levels for Kv1.1, Kv1.2, Kv2.1, and Kv2.2 were unchanged (Cao et al., 2010). mRNA for Kv1.2 was, however, downregulated in the lumbar (L5) spinal nerve ligation (SNL)model (Fan et al., 2014). This illustrates the point made above that responses of the sensory system to injury are contingent on the nature of the injury (see Tables 1, 2).
Studies of the silent subunit Kv9.1, have brought forth yet another mechanism whereby injury can influence delayed rectifier channels (Tsantoulas et al., 2012, 2018). Kv9.1 forms heterotetramers with Kv2.1 and Kv2.2 (Kerschensteiner et al., 2005) and this interaction leads to alteration of the functional channel kinetics. SNI has been shown to downregulate Kv9.1 in large DRG neurons and this may alter behavior of Kv9.1 ∼ Kv2.1. ∼ Kv2.2 heterotetramers (Tsantoulas et al., 2012). Genetic modulation of Kv9.1 expression in vivo produces changes in pain behavior consistent with its role in onset of neuropathic pain (Tsantoulas et al., 2012, 2018).
Since loss of delayed rectifier function augments nociceptive processes, restoration of function may lead to novel therapeutic approaches. Although preclinical studies have identified some substances that activate members of the delayed rectifier K+ channel family, for example RE1 and EX15 or AUT1 and AUT2 for Kv3.1 (Taskin et al., 2015; Brown et al., 2016), glycine derivatives for Kv1.1 (Manville and Abbott, 2020) and hanatoxin (from tarantula spiders) for Kv2.1 (Milescu et al., 2013), any therapeutic potential for pain management remains to be established. On the one hand, some of these substances seem highly selective for the cogent channel subtypes but on the other hand, the wide distribution of delayed rectifier channels in both excitable and non-excitable tissues may limit their therapeutic effectiveness.
These channels fall into the broad category of delayed rectifiers but are frequently discussed separately as they display limited structural similarity to other Kv channel types (Gutman et al., 2005). Unlike some other Kv channels, M-channels are modulated by G-protein coupled agonists (Selyanko et al., 1990; Suh et al., 2004), inflammatory mediators (Linley et al., 2008), including bradykinin (Cruzblanca et al., 1998) and ATP (Ford et al., 2003), src-tyrosine kinases (Gamper et al., 2003), Ca2+ acting via calmodulin (Gamper and Shapiro, 2003; Li et al., 2005) and by the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) (Ford et al., 2003; Li et al., 2005; Du et al., 2018). Gq coupled agonists suppress M-channel conductance via phospholipase C mediated depletion of PIP2 (Ford et al., 2003; Suh et al., 2004). M-channels are opened by the anti-epileptic drug, retigabine (Brown and Passmore, 2009).
Kv7.1 is derived from the KCNQ1 gene and is confined to non-neuronal tissue. It plays an important role in the repolarization of cardiac action potentials. By contrast Kv7.2, 7.3, 7.4, and 7.5 which derive from the KCNQ2, 3, 4, and 5 genes are predominantly neuronal (Gutman et al., 2005; Brown and Passmore, 2009). Classical M-channels are hetero-tetramers comprised of Kv7.2 and 7.3 (Shapiro et al., 2000; Hadley et al., 2003) which show maximal activation at −30 mV. Because of this, they exert profound attenuation of repetitive action potential discharge in several neuron types (Adams et al., 1982; Cooper et al., 2001; Brown and Passmore, 2009) including nociceptors (Barkai et al., 2017; Du et al., 2018).
Pharmacologically identified M-currents and the relevant mRNA’s have been detected in cultured rat DRG neurons (Passmore et al., 2003; Rose et al., 2011). Kv7.2 is more abundant in small and medium sized neurons than in large neurons (Rose et al., 2011; Du et al., 2018). In addition to their role in controlling excitability of DRG cell bodies, modeling studies have identified the importance of M-channels in the regulation of action potential propagation from the periphery, past the DRG to the CNS (Sundt et al., 2015; Du et al., 2018) as well as their role in controlling the excitability of primary afferent terminals in the dorsal horn (Barkai et al., 2017).
Decreases in expression of KCNQ2 and/or KCNQ3 proteins and a reduction of M-current density in the cell bodies of small-sized DRG neurons have been reported in CCI (Rose et al., 2011) and bone cancer pain (Zheng et al., 2013). Moreover, impairment of expression of Kv7.2 induces thermal and mechanical hyperalgesia in naïve animals (King et al., 2014). In line with this, the Kv7 activator, retigabine has been shown to alleviate neuropathic pain behavior in a rodent model of diabetic neuropathy (Djouhri et al., 2019). The effectiveness of retigabine paralleled that of gabapentin and its effect was reversed by the Kv7/M-channel blocker, XE991. Although some of the anti-nociceptive effectiveness of the COX2 inhibitor, celecoxib has been attributed to an ability to activate Kv7.2/7.3 channels expressed in HEK293 cells and M-type current in DRG neurons (Mi et al., 2013), this may have little or no therapeutic implication as NSAIDs generally display little or no effectiveness in the management of neuropathic pain (Yekkirala et al., 2017).
Considerable progress has been made in understanding the molecular machinery associated with down-regulation of the expression of KCNQ2 and 3 in DRG in response to peripheral nerve injury. KCNQ genes have functional repressor element 1 (NRSE) binding sites (Mucha et al., 2010) and the effects of partial sciatic nerve ligation depend on repressor element 1-silencing transcription factor (REST, also known as NRSF) (Mucha et al., 2010; Rose et al., 2011). Viral overexpression of REST in DRG neurons strongly suppresses M-current density and increases excitability (Mucha et al., 2010). REST inhibits transcription by recruiting the co-repressor complexes SIN3A/B and REST corepressor 1; these complexes, in turn, modify target gene regions through the action of HDAC1/2, the histone demethylase LSD1 and the histone methylase G9a (Ooi and Wood, 2007; Willis et al., 2016).
Inhibition or genetic deletion of G9a in DRG abolished injury-induced down-regulation of Kv7.2 and reduced neuropathic hyperalgesia. G9a may have an important role in K+ channel regulation as it has also been implicated in injury induced suppression of Kv1.4, Kv4.2 and BK channels (KCa1.1) (Laumet et al., 2015).
The effectiveness of retigabine may not only reflect reduced excitability of DRG cell bodies (Passmore et al., 2003), but may also involve reduced excitability of C-fiber axons (Lang et al., 2008). As mentioned above, this may be especially important for propagation of action potentials in unmyelinated fibers adjacent to DRG cell bodies, where the safety factor for transmission is low. Transmission is attenuated in a model where a KCNQ component is included the soma, stem axon, and proximal (to the T-junction), peripheral and central axons (Sundt et al., 2015). Another modeling study underlined the possible role of Kv7.2/7.3 channels in controlling the activity of primary afferent terminals in the dorsal horn (Barkai et al., 2017). Additional sites where Kv7.2/7.3 channels influence sensory nerve activity include the neuroma generated by injury to peripheral nerves (Cisneros et al., 2015; Bernal et al., 2016) as well as intact Aδ mechanoreceptor terminals in the skin (Schutze et al., 2016).
Taken together, these findings underline the importance of Kv7.2/7.3 channels in controlling sensory neuron excitability and outline their potential as a therapeutic target in the management of neuropathic pain (Rivera-Arconada et al., 2009; Barrese et al., 2018). Unfortunately, a clinical study of retigabine in human neuropathic pain (post herpetic neuralgia) failed to meet its efficacy endpoint (Yekkirala et al., 2017). Similarly flupirtine which is chemically related to retigabine (Abd-Elsayed et al., 2019; Osuma et al., 2019; Surur et al., 2019; Shi et al., 2020) and which was used as a non-opioid analgesic in Europe for many years, showed promise as an anti-allodynic agent. Unfortunately, this drug has now been withdrawn for all indications owing to its propensity to cause hepatotoxicity. Despite these setbacks at least 200 Kv7.2 activators are currently under development (Du and Gamper, 2013; Yekkirala et al., 2017). It is interesting to note in this regard that the frequently prescribed anti-allodynic agent gabapentin, which is thought to only interact with Ca2+ channels (Field et al., 2006), has been recently been reported to activate Kv7.3 and 7.5 channels when they are expressed in Xenopus oocytes (Manville and Abbott, 2018).
Tetrameric, rapidly activating, inactivating, voltage-gated K+ currents assemble from Kv1.4, 3.3, 3.4, 4.1, 4.2, and 4.3 subunits that are products of the KCNA4, KCNC3, KCCN4, KCND1, KCND2, and KCND3 genes (Gutman et al., 2005). Such channels are widely distributed in the cell bodies of DRG neurons (McFarlane and Cooper, 1991; Gold et al., 1996a; Safronov et al., 1996; Everill et al., 1998; Abdulla and Smith, 2001b; Matsuyoshi et al., 2012; Yunoki et al., 2014; Stemkowski et al., 2015) and display a considerable range of biophysical and pharmacological properties. For example, τ for inactivation can very between 4 and 190 ms at +50 or +60 mV and τ for recovery from inactivation can range between 2000 and 43 ms at −100 mV. Although this reflects classical N-type “ball and chain” inactivation in Kv1.4 and Kv3.4 channels (Kanda et al., 2011), the mechanism of inactivation of Kv4.1, 4.2 and 4.3 is not fully understood. Whereas Kv3.4 channels are sensitive to sub-millimolar concentrations of 4-aminopyridine (4-AP) somewhat higher concentrations are required to block Kv4.1, 4.2, and 4.3 (Zemel et al., 2018).
Kv1.4 channels are especially abundant in small diameter IB4-positive DRG neurons (Vydyanathan et al., 2005) where their function and surface expression is modulated by phosphorylation, growth factors, inflammatory mediators and auxiliary subunits (Takeda et al., 2008; Zhu et al., 2012a,b; Zemel et al., 2018). Most manipulations capable of producing painful neuropathy attenuate Kv1.4 expression and/or function in DRG neurons (Kim et al., 2002).
In a bone cancer model, however, there are time-dependent increases in A-type Kv channels that are expressed in IB4-positive, small DRG neurons. Currents are increased on post-tumor day 14 but then diminish yet remain at a higher level than control for an additional 7 days. The expression of Kv1.4 protein quantified by immunohistochemistry (IHC) and Western immunoblots (WB), as well as that for Kv3.4, and Kv4.3, show corresponding time-dependent increases (Duan et al., 2012). There is also evidence that injury-induced down regulation of delayed rectifier Kv1.1. and Kv1.2 at the neuroma that forms after nerve injury is balanced by increased expression of Kv1.4 and 1.6 (Calvo et al., 2016).
This time-dependent replacement of one channel type by another may relate to the partial recovery of mechanical hypersensitivity seen in some nerve injury models such as CCI but not after SNI (Decosterd and Woolf, 2000; Noh et al., 2020).
Although the axon, soma and spinal terminals of all sensory neurons express Kv3.4, expression is especially strong in small diameter neurons (Ritter et al., 2012, 2015a,b; Muqeem et al., 2018; Zemel et al., 2018). Kv3.4 likely corresponds to high threshold A-currents first described by Gold et al. (1996a) and since these currents were found in capsaicin sensitive neurons, Kv3.4 channels may be strongly associated with nociceptors (Zemel et al., 2018); a prediction confirmed by immunohistochemical (IHC) studies (Chien et al., 2007). Kv3.4 in nociceptors contributes 40-70% of the total repolarizing charge during the AP (Ritter et al., 2015b). These channels are especially sensitive to 4-aminopyridine (4-AP) and tetraethylammonium (TEA) block and are regulated by oxidation, phosphorylation and ancillary proteins (Zemel et al., 2018).
Dysfunction in Kv3.4 channels has been described in several chronic pain models and additional data underline their role in pain etiology. Thus, sciatic nerve ligation has been shown to reduce Kv3.4 immunoreactivity in the cell bodies, peripheral axons and central terminals of primary afferent nociceptors (Chien et al., 2007) and suppression of Kv3.4 expression following intrathecal injection of antisense oligodeoxynucleotides into uninjured animals produces mechanical hypersensitivity (Chien et al., 2007). Similar results were obtained in a model of diabetic neuropathy, the densities of total A-currents were markedly reduced in medium- and large-, but not in small-diameter DRG neurons and this was matched by changes in mRNA levels for Kv1.4, Kv3.4, Kv4.2, and Kv4.3 (Cao et al., 2010).
Muqeem et al. (2018) took advantage of the high sensitivity of Kv3.4 channels to 4-AP and TEA to demonstrate that channel blockade increased monosynaptic excitatory postsynaptic currents (EPSCs) in dorsal horn laminae I and II neurons through a presynaptic mechanism. Kv3.4 function is impaired in primary afferents after nerve injury (Chien et al., 2007; Cao et al., 2010; Duan et al., 2012) and excitatory spontaneous synaptic activity is increased in excitatory lamina II neurons (Balasubramanyan et al., 2006; Chen et al., 2009b). These findings taken together underline the importance of Kv3.4 in primary afferent terminals in central sensitization and pain.
Lastly, and already alluded to above (section “Spinal Cord Injury and Changes in Peripheral Neuron Excitability”) Kv3.4 current amplitude, inactivation, and channel membrane expression are reduced in the DRG following unilateral spinal cord contusion (Ritter et al., 2015a; Zemel et al., 2017). Since total Kv3.4 protein and mRNA in the DRG did not change, these results suggest a post-translational effect (Ritter et al., 2015b; Zemel et al., 2018) possibly involving dysregulation of calcineurin (Zemel et al., 2017).
Immunoreactivity and/or mRNA for Kv4 type channels is found in the cell bodies of small and large DRG neurons (Kim et al., 2002). Kv4.3 appears selectively in the cell bodies of a subset of non-peptidergic DRG neurons (Chien et al., 2007; Yunoki et al., 2014; Kuo et al., 2017) whereas Kv.4.1 is expressed in all sizes of DRG neurons (Matsuyoshi et al., 2012; Yunoki et al., 2014). Electrophysiological and molecular studies and the use of a Kv4-specific dominant negative probe implicate Kv4.1 and 4.3 as the molecular correlate of subthreshold A-currents in DRG neurons (Phuket and Covarrubias, 2009).
The function and expression of Kv4 channels in the DRG is controlled by signaling pathways such as MAPK (Grabauskas et al., 2011), accessory subunits such as Kv4 channel interacting proteins (KChIPs) and dipeptidyl-peptidase-like proteins (DPPLs) (Amarillo et al., 2008; Jerng et al., 2009; Pongs and Schwarz, 2010) as well as transcription factors such as the neuron restrictor silencer factor (REST), which suppresses transcription of the Kv4.3 gene (KCND3) after nerve injury (Uchida et al., 2010b).
The downregulation of mRNA for Kv4.2/4.3 channels and/or their surface expression and/or IA occurs in DRG neurons in a variety of nerve injury models, thereby implicating Kv4 dysfunction in chronic neuropathic pain (Chien et al., 2007; Cao et al., 2010; Grabauskas et al., 2011; Kuo et al., 2017; Zemel et al., 2018).
The modulatory subunits KChIP1, KChIP2, and DPP10 form a Kv4.3/KChIP1/KChIP2/DPP10 complex in DRG neurons. Knockdown of the expression of any component of this complex promotes mechanical hypersensitivity and increased excitability of non-peptidergic nociceptors. Spinal nerve ligation downregulates the expression of all Kv4 complex components but this is rescued using cDNA constructs encoding Kv4.3, KChIP1, and DPP10. This is accompanied by attenuation of SNL-induced mechanical hypersensitivity and partial recovery of Kv4.3, KChIP1, and DPP10 surface levels in the injured DRGs. This demonstrates that the K+ channel modulatory subunits KChIP1, KChIP2, and DPP10 participate in Kv4.3-mediated mechanical pain control (Kuo et al., 2017).
Although a few compounds have been identified which directly open delayed rectifier K+ channels by altering their biophysical properties (Milescu et al., 2013; Taskin et al., 2015; Brown et al., 2016; Manville and Abbott, 2020) less progress has been made in identifying direct activators of A-channels. The compound KW-7158 suppresses afferent nerve activity as a result of its ability to potentiate IA in DRG neurons (Sculptoreanu et al., 2004). Although KW-7158 was proposed as a treatment for overactive urinary bladder (Maeda et al., 2012), any efficacy of this compound in pain models remains to be established.
Drugs which target accessory subunits of A-channels may provide an alternative strategy (Zemel et al., 2018). DPPLs and KChIPs not only govern the biophysical properties of Kv channels. They also impact channel assembly, channel trafficking to and from the cellular surface, and targeting of channels to different cellular compartments (Pongs and Schwarz, 2010). The observation that knockdown of any component of the Kv4.3/KChIP1/KChIP2/DPP10 complex promotes mechanical hyperalgesia has already been alluded to (Kuo et al., 2017).
Lastly, targeting signaling pathways and epigenetic mechanisms that control expression, trafficking and properties of A-type channels may provide the theoretical basis for novel approaches to chronic pain management. Two types of mechanism may be involved,
(1) Nerve injury upregulates a micro-RNA cluster (mir-17-92) in rat DRG neurons and its overexpression reduces the expression of A-type K+ channels and some of their accessory subunits. Overexpression of certain members of this cluster elicited mechanical allodynia and specific blockade of micro-RNA function with antisense oligomers alleviated pain in nerve injury models (Sakai et al., 2017). The therapeutic potential of antisense targeting of mir- 17-92 or other micro RNAs remains to be exploited.
(2) Nerve injury increases activity of the histone-lysine N-methyltransferase 2 (G9a). This enzyme regulates euchromatic gene expression via histone modification. This in turn affects the promoters for the A-channel genes KCNA4 (Kv1.4) and KCND2 (Kv3.2), as well as that for (Kv7.2) KCNQ2 and KCNMA1 [for the BK channel KCa1.1, see sections “Distribution of KCNQ/Kv7.2/7.3/M-channels in Primary Afferent Neurons and Effects of Nerve Injury” and “Distribution of BK or Maxi K+ Channels (KCa1.1) in Primary Afferent Neurons and Effects of Nerve Injury”]. G9a inhibition or ablation restored K+ channel expression in the DRG and attenuated pain hypersensitivity. Interestingly inhibition of G9a but also normalized the expression of many other genes altered by nerve injury (Laumet et al., 2015). These findings implicate G9a in the transcriptional silencing associated with neuroplasticity in neuropathic pain. While there are some reports of the efficacy of small molecule G9a inhibitors in rodent models of neuropathic pain (Wang et al., 2017; Liang et al., 2019) any therapeutic potential of these substances is yet to be explored.
Ca2+ activated K+ channels and their corresponding conductances (gK,Ca) are divided into two broad categories on the basis of low or high single channel conductance (Wei et al., 2005; Berkefeld et al., 2010). The first group are voltage-insensitive and activated by low concentrations of intracellular Ca2+ (<1.0 μM). Ca2+ acts via binding to calmodulin which is tightly complexed to the C-terminal of the channel protein (Berkefeld et al., 2010). This group includes the small conductance KCa2.1, 2.2, and 2.3 channels (SKCa1, SKCa2, and SKCa3) which are products of the KCNN1, 2 and 3 genes and which are blocked by 100 pM–10 nM apamin (Wei et al., 2005). Single channel conductance for this group of channels is <10ps. The grouping also includes the apamin-insensitive, clotrimazole-sensitive intermediate conductance channel (KCa 3.1 encoded by KCNN4, single channel conductance 11pS; also known as the IAHP or IKCa1channel).
The second group includes the KCa1.1 (Slo or Slo1), KCa4.1 (Slack or Slo 2.2), KCa4.2 (Slick or Slo 2.1) and KCa5.1 (Slo3) channels which are products of the KCNMA1, KCNT1, KCNT2 and KCNU1 genes respectively. KCa1.1 is regulated by four different β-subunits encoded by the KCNMB1-4 genes. It is also known as the “maxi K+ channel,” the BK channel or the BK(Ca) channel by virtue of its high conductance (260pS). Its activation requires both depolarization and the direct interaction of Ca2+ with the channel. KCa1.1 is blocked by low concentrations of TEA (0.14 mM), and by charybdotoxin (2.9 nM) or iberiotoxin (1.7 nM). Channels of this type are rapidly activating and intimately associated with voltage gated Ca2+ channels (Robitaille et al., 1993; Jassar et al., 1994; Berkefeld et al., 2010; Zhang et al., 2018). They thus serve as a powerful braking mechanism for voltage-gated Ca2+ influx. This has obvious implications for the control of neurotransmitter release (Furukawa et al., 2008; Hoppa et al., 2014). The tight association of KCa1.1 with Ca2+ channels means that their activity is readily attenuated by divalent cations such as Cd2+ which block closely associated voltage-gated Ca2+ channels (Abdulla and Smith, 2001b).
The inclusion of KCa4.1 (Slack or Slo 2.2) and KCa4.2 (Slick or Slo 2.1) in this grouping is based on their structural similarity to KCa1.1 (Wei et al., 2005) but this is somewhat misleading in practical terms as they display little sensitivity to Ca2+ and are more readily activated by internal Na+ and Cl– (Yuan et al., 2003). They are known as Na+ activated K+ channels and require both depolarization and internal Na+ to activate. The category also includes the pH sensitive high conductance K+ channel KCa5.1 which is encoded by the KCNMC1 gene (Wei et al., 2005).
In rodents, KCa2.1, 2.2, and 2.3 have been localized to IB4-positive and to peptidergic nociceptors (Mongan et al., 2005) and these express an apamin-sensitive gK,Ca which is reduced by SNL (Sarantopoulos et al., 2007). This correlates with the clinical postmortum immunohistochemical (IHC) studies suggest that KCa2.1 is decreased in DRG avulsed from neuropathic pain patients (Boettger et al., 2002).
Positive modulators of KCa2 and KCa3 channels, 4,5-dichloro-1,3-diethyl-1,3-dihydro-benzoimidazol-2-one (NS4591) and 4-(2-methoxyphenylcarbamoyloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester (GW542573X) have been identified (Hougaard et al., 2009a,b) and their possible effectiveness in neuropathic pain is yet to be explored.
Although the work of Boettger et al. (2002) and Sarantopoulos et al. (2007) also reported decreased function of KCa3.1 in DRG following nerve injury, the relevance of these findings to pain etiology is questioned by the observation that KCa3.1-/1 mice show normal behavioral responses in models of neuropathic pain (Lu et al., 2017).
Iberiotoxin or charybdotoxin sensitive BK channels (KCa1.1) are present in all DRG neurons (Abdulla and Smith, 1999; Li et al., 2007; Zhang et al., 2010; Cao et al., 2012). They are especially enriched in the IB4-positive population of small neurons (Zhang et al., 2010).
Sciatic nerve section reduces overall Cd2+-sensitive Ca2+ sensitive K+ conductance in small, medium and large sized DRG neurons (Abdulla and Smith, 2001b). Although these changes were attributed to suppression of the associated voltage gated Ca2+ channels, more recent work has underlined the direct attenuation of KCa channel function (Sarantopoulos et al., 2007). This study used the SNL model and while BK conductance decreased in those medium sized neurons where axons were actually severed (axotomized L5 DRG), BK channel function was increased in adjacent undamaged neurons (L4 DRG). By contrast, in small neurons BK current was reduced in both axotomized (L5) and adjacent, undamaged (L4) neurons. SNL induced decrease in BK function in small and medium DRG neurons has been confirmed by others (Cao et al., 2012) but these authors did not address the possibility that axotomized and undamaged neurons respond differently in response to SNL.
SNL downregulates KCNMA1 in whole DRG as a result of G9a activation (Laumet et al., 2015) and the protein levels of BK channels are substantially reduced in small- and medium-sized DRG neurons (Chen et al., 2009a). These authors showed that blocking the BK channel with iberiotoxin reduced the mechanical withdrawal threshold in control and nerve-injured rats. Intrathecal injection of the BK channel opener [1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzi midazol-2-one] reversed allodynia and hyperalgesia in nerve-injured rats but it had no significant effect on nociception in control rats.
SNI is known to increase the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) in neurons in the rat superficial dorsal horn (Balasubramanyan et al., 2006; Chen et al., 2009b). In mice, iberiotoxin increased the frequency of sEPSCs in control mice to the same level as that in the nerve injured mice but did not increase sEPSC frequency in nerve injured mice. These findings support the possibility that BK channels located in presynaptic terminals control synaptic transmission in the superficial dorsal horn, and that functional downregulation of BK channels contributes to the central sensitization that characterizes neuropathic pain (Furukawa et al., 2008). In addition to this, it has been reported that activation of BK in DRG cell bodies can impede passage of impulses through the T-junction on the primary afferent fiber, effectively acting as a low pass filter for propagation of action potentials towards the CNS (Gemes et al., 2013). When BK channels are impaired, after spinal nerve ligation (SNL), a higher frequency of action potentials can pass. This too may contribute to increased frequency of sEPSC’s recorded in the spinal cord.
Given the established role of BK in determination of spike width in DRG (Scholz et al., 1998; Zhang X. L. et al., 2012; Noh et al., 2019) and action potential propagation (Gemes et al., 2013), the implications for increased neurotransmitter release in the spinal dorsal horn (Furukawa et al., 2008), a recent report that overexpression of BK increased mechanical threshold in a rodent neuropathic pain model (Zhang et al., 2018), and the availability of a BK activator (Chen et al., 2009a), these channels would seem at least as viable a target as Kv7 and A-channels for therapeutic intervention.
The work by Zhang et al. (2018) also brought forth an alternative mechanism whereby altered BK expression could relate to neuropathic pain mechanisms. It is well established that the cell surface expression and trafficking of Cav2.2 (N-type Ca2+ channels) is controlled by the α2δ1 accessory subunit (Dolphin, 2016) and these are upregulated in DRG after peripheral nerve injury (Hoppa et al., 2012). Injury would thus lead to increased abundance of Cav2.2 channels in primary afferent terminals and increased transmitter release. Because BK channels were shown to also bind to α2δ1, it has been suggested they compete with Cav2.2, thus normally restricting surface expression of Cav2.2 (Zhang et al., 2018). Following downregulation of BK by injury (Cao et al., 2012), α2δ1 would be released to interact with Cav2.2 and increase their surface expression and to increase transmitter from primary afferent terminals.
It has also been suggested that endoplasmic reticulum stress associated with nerve injury may contribute to pain by alteration of BK function (Yousuf et al., 2020).
Several positive modulators of BK function have been identified (Chen et al., 2009a; Hoshi and Heinemann, 2016), including the highly effective GoSlo-SR family of anthraquinone analogs (Roy et al., 2012) but these have not yet been evaluated as possible anti-allodynic agents. On the other hand, there is considerable discussion in the literature relating to the efficacy of cannabinoids in neuropathic pain (Cristino et al., 2020) and it has been suggested that augmentation of BK function may contribute to their therapeutic effect (Li Y. et al., 2019).
An Na+ sensitive K+ conductance was first described in small DRG neurons and myelinated sensory axons over 20 years ago (Poulter et al., 1995; Bischoff et al., 1998). Single channel activity of KCa4.1 in IB4-positive nociceptors is controlled by the anoctamin protein TMEM16C. TMEM16C knockout rats or those in which slack (KCa4.1) is knocked down using siRNA exhibit increased thermal and mechanical sensitivity (Huang et al., 2013). Similarly, global or selective ablation of slack (KCa4.1) in DRG led to increased hypersensitivity in neuropathic pain models whereas inflammatory and acute nociceptive pain were unaffected (Lu et al., 2015). These effects may be mediated via slack channels (KCa4.1) regulation of neurotransmitter release from primary afferent terminals (Evely et al., 2017). The atypical antipsychotic drug, loxapine which has been reported to activate KCa4.1/slack (Biton et al., 2012), ameliorated persisting neuropathic pain behaviors in the rat SNI model (Lu et al., 2015). Unfortunately a pilot study of loxapine effectiveness in chronic pain patients had to be terminated due to adverse events seen in all patients involved in the study (Schmiedl et al., 2019).
Inwardly rectifying channels are opened by membrane hyperpolarization and favor inward flux over outward flux of K+ ions. There are seven subfamilies of KIR channels, denoted as KIR1 – KIR7 (Kubo et al., 2005). Each functional channel contains four subunits each with two transmembrane domains.
Instantaneous inward rectifiers, which among other processes, are involved in repolarization of the cardiac Purkinje fiber action potential include KIR1.1, 2.1, 2.2, 2.3, and 2.4. These are the respective products of the KCNJ1, KCNJ2, KCNJ12, KCNJ4, and KCNJ14 genes.
KIR3.1, 3.2, 3.3, and 3.4 are also known as G-protein coupled inwardly rectifying K+ channels GIRK 1-4. They are activated by acetylcholine in the heart and by a variety of Gi coupled agonists throughout the nervous system. They are products of the KCNJ3, KCNJ6, KCNJ9, and KCNJ5 genes.
KIR6.1 and 6.2 channels which are products of KCNJ8 and KCNJ11 genes are controlled by the intracellular ATP to ADP ratio. KIR6.2 [also known as IK(ATP)] is involved in insulin secretion from pancreatic islet cells. It is also found in neurons and other excitable cells.
KIR4.1 channels are found primarily in glial cells and have important roles in brain K+ buffering. KIR4.2 and Kir 5.1channels are largely non-neuronal in distribution and KIR7.1 is of particular importance pigmented epithelial cells of the retina (Kubo et al., 2005; Kumar and Pattnaik, 2014).
An instantaneous, Ba2+–-sensitive inwardly rectifying K+ current has been described in medium sized DRG neurons (Scroggs et al., 1994). In agreement with this, confocal imaging revealed KIR2.1, KIR2.2, and KIR2.3 immunoreactivity in most DRG neurons as well as in nerve terminals in spinal lamina II (substantia gelatinosa). KIR2.3 was also seen in satellite glial cells and all three KIR channels were found in spinal astrocytes (Murata et al., 2016). Any effects of nerve injury on these currents remains to be explored.
Application of Gi coupled agonists such as μ-opioids or α2 adrenergic suppress N-type Ca2+ currents and open GIRK channels in the cell bodies of DRG neurons (Abdulla and Smith, 1997, 1998; Nockemann et al., 2013; Stotzner et al., 2018). Complementary studies reported the presence of GIRK1/KIR3.1 immunoreactivity in 70% of DRG neurons and although GIRK2/KIR3.2 only appears in 10% of neurons, these appear to be small peptidergic nociceptors. These findings are not in complete agreement with those of Nockemann et al. (2013) who described the presence of both GIRK1/Kir3.1 and GIRK2/Kir3.2 in IB4 positive non-peptidergic neurons. GIRK channels may be transported to sensory nerve endings in the skin (Nockemann et al., 2013) and to presynaptic terminals in the spinal cord, particularly lamina II (Lyu et al., 2015). Opioids and other Gi coupled agonists are well known to attenuate neurotransmitter release from primary afferents (Kohno et al., 1999) and this is thought to involve an action on Ca2+ channels (Heinke et al., 2011). Since opioid actions are not blocked by the GIRK channel blockers Ba2+ and Cs+, it is unlikely that they are involved in this process (Heinke et al., 2011). Despite this, there is good evidence to suggest a role for GIRK2/KIR 3.2 channels in sensory nerve endings in the actions of peripherally acting opioids (Nockemann et al., 2013). Although only 18% of DRG cell bodies display immunoreactivity for KIR3.3, channel protein is present in nerve endings in glabrous skin and may be present in primary afferent terminals in the spinal cord (Lyu et al., 2020).
Nerve injury down-regulates GIRK1/KIR3.1 in DRG neuron cell bodies at the mRNA and protein levels and reduces its expression in the spinal dorsal horn (Lyu et al., 2015). Despite the reported presence of functional KIR channels in DRG cell bodies (Stotzner et al., 2018) altered expression may only affect DRG excitability in the presence of appropriate Gi coupled ligands. It is well known, however, that opioid effectiveness is limited in neuropathic pain states (Abdulla and Smith, 1998; Sun et al., 2017) and this may involve a decrease in their effectiveness in limiting transmitter release from primary afferents terminals (Kohno et al., 2005). It remains to be determined whether this effect is contingent upon down regulation of KIR as reported by Lyu et al. (2015).
By contrast with the limited information and possible limited significance of effects of nerve injury on instantaneous inward rectifiers and GIRK channels in primary afferents, somewhat more information is available regarding KIR6.1 and 6.2; the KATP channels (Ocana et al., 2004). These channel proteins co-assemble with sulphonylurea receptors (SUR or ATP binding cassettes) (Campbell et al., 2003). SUR are the molecular targets of the sulphonylurea drugs such as glibenclamide and tolbutamide which are used in the treatment of diabetes. Channel activation can be achieved by pinacidil and by the anti-hypertensive agent, diazoxide. The association of four KIR6 and four SUR subunits form a functional KATP channel. These channel complexes are activated by ADP and inhibited by ATP (Kubo et al., 2005).
Immunoreactivity for SUR1, SUR2, and Kir6.2 has been detected in DRG (Kawano et al., 2009). This observation was confirmed by Western blots for Kir6.2/SUR1 and Kir6.2/SUR2 KATP channels (Zoga et al., 2010). Kawano et al. (2009) also recorded spontaneous activity of single KATP channels in cell-attached patches made from all subpopulations of DRG neuron. Higher open probabilities and longer open times were seen in large compared to small neurons. KATP activity was suppressed only in large neurons from hyperalgesic rats, but not from animals that did not develop hyperalgesia in response to spinal nerve ligation (SNL). Injury did not affect ATP sensitivity, inward rectification, unitary conductance or the pharmacological properties of KATP channels. Although the anti-nociceptive actions of KATP channel openers has been recognized for many years (Vergoni et al., 1992; Welch and Dunlow, 1993), much of the preclinical work has dealt with the ability of drugs such as pinacidil to potentiate opioid action (Vergoni et al., 1992; Fisher et al., 2019). The relevance of this to therapeutic management of neuropathic pain is yet to be explored.
Four transmembrane-domain tandem pore domain (K2p) channels account for K+ leak conductance and set the resting membrane potential of most excitable cells (Goldstein et al., 2005; Enyedi and Czirjak, 2010; Gada and Plant, 2019). They are different from other K+ channels as they assemble as dimers rather than tetramers and contain 2 pore domains.
At least 15 different types of K2p channels have been identified that are products of corresponding KCNK genes. Channels are subcategorized as TWIK (Tandem of pore domains in a weak inwardly rectifying K+ channel), TREK (TWIK related K+ channel), TASK (TWIK related acid-sensing K+ channel), TRAAK (TWIK-related arachidonic acid activated K channel, TALK (TWIK related alkaline pH activated K+ channel), THIK (Tandem pore domain halothane inhibited K+ channel) and TRESK (TWIK related spinal cord K+ channel). This nomenclature has now been standardized so that 15 available KCNK genes correspond more exactly to the identity of the channels (i.e., K2p1-7, K2p9-10, K2p12-13, K2p15-18, Gada and Plant, 2019). In symmetrical K+ solutions, conductance of K2p is instantaneous and ohmic whereas in physiological K+ solutions channels exhibit outward rectification as predicted by the Goldman–Hodgkin–Katz equation (Enyedi and Czirjak, 2010). With hindsight since the discovery of these channels (Ketchum et al., 1995), this means that most instantaneous current responses evoked by voltage commands involve K2p channels.
mRNA coding for TASK-1 (KCNK3), TASK-2 (KCNK5), TASK-3 (KCNK9), TREK-1 (KCNK2), TREK-2 (KCNK10), TRAAK (KCNK4), TWIK-1 (KCNK1), and TRESK (KCNK18) has been found in rat and mouse DRG (Talley et al., 2001; Kang and Kim, 2006; Tulleuda et al., 2011; Marsh et al., 2012; Gada and Plant, 2019). These channels preferentially localize to small neurons (Gada and Plant, 2019). The superficial layers of spinal cord and small-diameter and/or or medium sized neurons of dorsal root ganglia also showed TASK-1, 2 or 3 (K2p3, 9 and 10) immunoreactivity (Gabriel et al., 2002; Rau et al., 2006) and presumptive and functional TASK-2 (k2p10) channels have also been recorded in cell attached patches on DRG neurons (La et al., 2006).
K2p channels are highly regulated by a broad variety of intracellular and extracellular signals such as oxygen tension, pH, lipids, neurotransmitters, G-proteins, volatile and local anesthetics, temperature, kinases, cyclic AMP, small ubiquitin-related modifier proteins and scaffolding protein and their role in chronic inflammatory pain is well documented (Marsh et al., 2012). The mechanical and thermal sensitivity of K2p channels is of special relevance as it has been suggested that TRAAK, TREK-1, and TREK2 are involved in polymodal pain perception (Kang et al., 2005; Alloui et al., 2006; Noel et al., 2009).
TRESK (k2p18) channels play an important role in the leak conductance of DRG neurons and their functional knockout produced a notable decrease in rheobase (Dobler et al., 2007). The relevance of this observation to neuropathic pain is supported by the observation that sciatic nerve transection reduces TRESK/(k2p18)/KCNK18 mRNA in DRG to a greater extent than other K2p channels and in vivo knock down decreases threshold to painful mechanical stimuli (Tulleuda et al., 2011; Castellanos et al., 2020).
TRESK is not the only K2p channel involved as TASK3 (K2p9) and TWIK1 (K2p1) are also down-regulated in DRG by SNI but K2p3 (TASK1) is not. Expression of K2p9 returns to normal within weeks, whereas K2p1 channels remain depleted for months (Pollema-Mays et al., 2013).
Although volatile anesthetics are known to activate K2p2, 3, 4, 9, and 18 (Gada and Plant, 2019) and this may relate to their clinical effectiveness, it is obviously impractical to use these drugs for long term control of chronic pain. There is, however, a recent report a novel TREK2/ K2p10.1 activator presently known as GI-530159. Although this substance decreases DRG excitability (Loucif et al., 2018), its possible effectiveness in pain models has not yet been reported.
It is clear from the forgoing sections that K+ channel function is regulated by a variety of mechanisms, including trafficking, alternate splicing of gene products, protein–protein interactions, channel subunit interactions, as phosphorylation and interaction with membrane phospholipids. Channel expression can be altered by the action of transcription factors, microRNA and DNA methylation. All such processes are involved in alteration of K+ channel function in sensory neurons following injury (Uchida et al., 2010a,b; Kanda et al., 2011; Rose et al., 2011; Zhang X. L. et al., 2012; Sakai et al., 2013, 2017; Kuo et al., 2017; Zemel et al., 2017). These diverse cellular mechanisms are engaged by extracellular signals such as inflammatory mediators and growth factors released from the site of injury (Wagner and Myers, 1996a,b; Sorkin et al., 1997; Sommer et al., 1999; Cui et al., 2000; Scholz and Woolf, 2007; Takeda et al., 2008; Stemkowski and Smith, 2012a,b; Shinoda et al., 2019). Several lines of evidence collaborate the involvement of soluble mediators in injury-induced changes in DRG excitability and K+ channel function. It is for example, well established that peripheral nerve injury alters the properties of both severed and undamaged axons (Ma et al., 2003; Yang et al., 2018) and, as already mentioned (section “Spinal Cord Injury and Changes in Peripheral Neuron Excitability”), injury to the spinal cord per se can induce changes in DRG excitability and channel expression (Yang et al., 2014; Ritter et al., 2015a). Lastly the phenomenon of “mirror image pain” where unilateral injury provokes changes in contralateral nerves is well characterized. This phenomenon also involves the action of soluble inflammatory mediators and growth factors (Milligan et al., 2003; Cheng et al., 2014, 2015; Xie et al., 2016; Yuan et al., 2020).
It is widely accepted that a transient injury-induced inflammatory event is the primary trigger to the onset of neuropathic pain (Watkins and Maier, 2002; Scholz and Woolf, 2007; Kawasaki et al., 2008a,b; Leung and Cahill, 2010; Gaudet et al., 2011; Stemkowski and Smith, 2012b; von Hehn et al., 2012; Noh et al., 2020). The crucial interplay between the nervous and immune systems (Ren and Torres, 2009) has led some authors to describe neuropathic pain as a neuroimmune disorder (Scholz and Woolf, 2007; Grace et al., 2014). It should also be noted that cytokine production is not wholly maladaptive as both interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) have been implicated in functional recovery after peripheral nerve injury (Nadeau et al., 2011). Although a multiplicity of inflammatory and anti-inflammatory cytokines, chemokines, eicosanoids, neuropeptides and growth factors have been implicated, my primary focus will be on the most extensively studied agents, i.e., IL-1β, TNF-α, NGF, and BDNF.
Nerve injury orchestrates the release of IL-1β from mast cells, mononuclear cells, neutrophils, Schwann cells, fibroblasts, endothelial cells, satellite glial cells and neurons themselves (Sommer and Kress, 2004; Scholz and Woolf, 2007). A 10-fold increase in IL-1β protein expression in sciatic nerve is seen between 1 and 7 days after nerve injury (Nadeau et al., 2011) and impairment of interleukin-1 signaling following nerve injury by deletion of IL-1 receptor type 1 or transgenic over-expression of the physiological IL-1 receptor antagonist attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity (Wolf et al., 2006).
Acute or chronic exposure to IL-1β increases the excitability of DRG neurons (Binshtok et al., 2008; Stemkowski and Smith, 2012a; Stemkowski et al., 2015). The long-term actions of this particular cytokine are of special relevance as they are dominated by changes in K+ channel function. Thus, 5–6 days exposure of medium sized DRG neurons to 100pM IL-1β reduced the amplitudes of A-current, delayed rectifier and BK currents by 68, 64, and 36%, respectively. This likely reflected changes in Kv1.2, 2.1, 2.2, 3.4, 4.2 and KCa1.1. But only modest changes in HCN, Na+ and Ca2+ currents were seen. This action is neuron type-specific as small IB4-positive and large DRG neurons were little affected. Effects on small IB4-negative neurons were dominated by decrease in BK channel function (Noh et al., 2019). In our experiments, neurons were maintained in defined medium culture and exposed to IL-1β for 5–6 days to mimic the time course of cytokine increase seen after nerve injury (Stemkowski and Smith, 2012a; Stemkowski et al., 2015; Noh et al., 2019). One interesting finding that emerged from this series of experiments is that while effects of IL-1β on medium cell excitability returned to control levels following removal of cytokine for 3–4 days (Stemkowski et al., 2015), effects on small IB4-negative neurons were more persistent (Noh et al., 2019). This implies that IL-1β produces both acute effects on ion channel function as well as more enduring changes in neuronal phenotypic properties, perhaps mediated at the transcriptional level. The idea that decreased K+ channel function in nerve terminals can affect neurotransmitter release from primary afferent terminals has already been alluded to Furukawa et al. (2008) and Muqeem et al. (2018). This may relate to the observation that 6–8 days exposure to IL-1β increases sEPSC amplitude in substantia gelatinosa neurons (Gustafson-Vickers et al., 2008), perhaps reflecting an action of the cytokine on K+ channels in primary afferent terminals.
The evidence implicating TNF-α in neuropathic pain parallels that described above for IL-1β (Leung and Cahill, 2010). Thus it is detected at the site of injury following CCI (George et al., 1999), intra-sciatic injection produces pain (Wagner and Myers, 1996a) and injury induced hyperalgesia is abrogated following inhibition of TNF-α synthesis with thalidomide (George et al., 2000). The TNF-α antagonist etanercept, similarly blocks tactile allodynia in diabetic mice (Dogrul et al., 2011). Unlike IL-1β, most actions of TNF-α involve modifications of Na+ channel function (Jin and Gereau, 2006) rather than any effect on K+ channels (He et al., 2010).
Nerve injury increases levels of BDNF and NGF in peripheral nerves and attenuation of their actions leads to attenuation of allodynia and other signs of neuropathic pain (Herzberg et al., 1997; Theodosiou et al., 1999; Zhou et al., 2000; Pezet and McMahon, 2006). Neurotrophins alter expression, posttranslational modification and trafficking of TRPV1 and voltage gated sodium channels (Dib-Hajj et al., 1998, 2010; Mantyh et al., 2011).
Ligation or crush of the sciatic nerve reduces voltage-gated, transient and persistent K+ currents in large cutaneous afferent DRG neurons but these are restored by in vivo administration of NGF (Everill and Kocsis, 2000). NGF has also been reported to increase immunoreactivity for small but not intermediate Ca2+ gated K+ channels in injured human sensory neurons (Boettger et al., 2002). Other work has, however, suggested that NGF working through the p75 neurotrophin receptor reduces K+ current in small-diameter capsaicin-sensitive sensory neurons (Zhang Y. H. et al., 2012). Although NGF inhibits Kv7 channels in sympathetic neurons (Jia et al., 2008), it remains to be determined whether these channels are similarly affected in DRG neurons. These divergent effects of NGF on K+ channels may parallel its actions on Na+ channels, which involve increased activity of some channel types in some neuron types and decreased activity in others (Black et al., 1997; Dib-Hajj et al., 1998; Fjell et al., 1999). Despite this, antagonism of the actions of NGF is generally effective in alleviating pain (Ro et al., 1999; Djouhri, 2016) and a loss of function mutation in TrkA leads to congenital insensitivity to pain (Indo, 2002). It is likely therefore that NGF’s actions in generating allodynia involve a decrease K+ currents and an increase certain voltage-gated Na+ currents.
The role of microglia derived BDNF in central sensitization is well established (Coull et al., 2005; Lu et al., 2007, 2009; Smith, 2014; Boakye et al., 2019). Since injury-induced release of NGF increases expression of BDNF in nociceptors (Pezet and McMahon, 2006), it has also been implicated in peripheral pain mechanisms. BDNF levels are increased in DRG following nerve injury and this may lead to a decrease in BK currents (Cao et al., 2012). It also reduces expression of Kv4.2 and Kv4.3 genes in whole DRG (Park et al., 2003) whereas BDNF antibodies or a TrkB blocker increases Kv4.2 and Kv4.3 mRNA and A-type K+current (Cao et al., 2010). Interestingly BDNF also increases spontaneous excitatory synaptic activity in the spinal dorsal horn (Lu et al., 2007, 2009) it is therefore tempting to speculate that this may reflect downregulation of Kv4.2/4.3 in primary afferent terminals.
Several other soluble mediators have been implicated in signaling between the inflammatory response and the initiation of neuropathic pain. These include interferon γ (Vikman et al., 2003), interleukin 6 (IL-6) (Xu et al., 1997; Arruda et al., 1998), glial cell line derived neurotrophic factor (GDNF) (Cummins et al., 2000; Leffler et al., 2002), fibroblast growth factor (bFGF), transforming growth factor-beta1 (TGF-beta) (DeLeo et al., 1997; Zhu et al., 2012a) and prostaglandins (Syriatowicz et al., 1999; Kanda et al., 2017). Despite the identification of a considerable number of extracellular mediators, it is interesting to note the similarity in the transduction processes they activate; rasMAP kinase and p38 kinases are frequently involved. Since K+ channels function is clearly affected by IL-1β and BDNF, it is likely that interferon γ, IL-6, GDNF, bFGF, TGF-beta and prostaglandins work through similar transduction processes to thereby regulate K+ channel function in the context of neuropathic pain. Available evidence to support this contention comes from the observation that GDNF increases spike width of small IB4-negative DRG neurons (Stemkowski et al., 2015) and prostaglandin E2 attenuates the slow action potential afterhyperpolarization in DRG neurons in culture (Gold et al., 1996b).
The relationship between K+ channel dysfunction and neuropathic pain in animal models is borne out in the clinic where certain neurological disorders that involve peripheral hyperexcitability and pain also involve altered K+ channel function. For example, Kv antibodies have been detected in acquired neuromyotonia patients (Hart et al., 2002) and treatment of murine DRG neurons with these antibodies increased their excitability (Shillito et al., 1995). Other patients with acquired neuromyotonia or Morvan’s syndrome express antibodies to Kv1 channel complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 (Irani et al., 2010).
Gain-of-function mutations of the Nav1.7 sodium channel produce a painful inherited condition known as erythromelalgia (Dib-Hajj et al., 2005). Although individuals afflicted with this chronic “burning man syndrome” suffer debilitating chronic pain, a subset of individuals who have this mutation in Nav1.7 are relatively pain resistant. This has been ascribed to an additional gain of function mutation in KCNQ which encodes Kv7.2 (Mis et al., 2019). This study illustrates how differences in cation channel expression in sensory neurons can contribute to inter-individual differences in pain (Waxman, 2019).
It should be mentioned that K+ channels mutations are very rare in the human population and are often manifest as epilepsy or ataxia (Kullmann, 2010). There is, however, an emerging literature on K+ channelopathies and migraine (Cregg et al., 2010; Lafreniere and Rouleau, 2012; Albury et al., 2017), perhaps improved understanding of peripheral neuropathy will contribute to better understanding of migraine and vice versa.
Despite issues associated with bona fide pain measurement (section “How Is Pain Measured?”), the use of different injury models (see section “Lost in Translation”), differences in neuron classifications used by different groups, and influences of genetics and epigenetics, there can be little doubt that dysfunction of K+ channels in primary afferents is involved in both the onset and persistence of neuropathic pain. It is also clear that perturbation of K+ channel function contributes to the development and persistence of neuropathic pain as a result of increased excitability of primary afferent neurons. This leads to spontaneous activity, increased axonal conduction and increased neurotransmitter release from primary afferent terminals (Furukawa et al., 2008; Barkai et al., 2017; Evely et al., 2017; Muqeem et al., 2018; Zhang et al., 2018).
It has long been recognized that different types of DRG neuron express different palates of K+ channels (Gold et al., 1996a) and in general, nerve injury promotes decreases in their functionality. Different changes are seen in different DRG neuron types; some channels are affected whereas others are not. Table 1 summarizes how various types of nerve injury affect various channel types and Table 2 summarizes changes seen in different DRG neuron types in response to injury or the actions of inflammatory mediators.
The observed effects of nerve injury involve changes involve multiple processes affecting K+ channel expression, epigenetic modulation and trafficking. This is illustrated by the multiplicity of processes regulating Kv 1.2 delayed rectifier channels (see section “Effects of Nerve Injury on Delayed Rectifier K+ Currents and Channels in DRG Neurons”). These processes are initiated by the release of inflammatory mediators and growth factors following Wallarian degeneration at the site of injury.
One interesting point that emerges from the literature reviewed is that selective perturbation of more or less any single DRG K+ channel type can lead to chronic pain. As well as underlining the role K+ channels in neuropathic pain, this observation implies that function of all K+ channels has to be unperturbed to prevent pain onset. In other words “everything has to be right with K+ channels or else pain will occur.”
The findings of cellular and ex vivo studies need to be correlated with behavioral studies in order to verify K+ channel dysfunction with signs of pain. In vivo studies in which channel function is augmented or impaired implicate delayed rectifiers, A-currents, M-currents, BK currents, KATP channels and leak channels in the etiology of neuropathic pain. It should be noted, however, that most of the in vivo studies involve global perturbation of channels throughout the nervous system, including those in glial cells and in central neurons. It is uncertain therefore whether behavioral responses are only attributable to changes in K+ channels in peripheral neurons. These issues will be resolved as improved methods for selectively altering channel expression within different DRG neuron types are applied. This has already been done with selective optogenetic attenuation of function of Nav1.8 expressing nociceptors (Daou et al., 2016).
Despite these difficulties, recognition of the importance of K+ channel dysfunction has led to considerable interest in the potential role of K+ channel activators in pain management (Ocana et al., 2004; Busserolles et al., 2016; Barrese et al., 2018; Zemel et al., 2018; Abd-Elsayed et al., 2019; Gada and Plant, 2019). Unfortunately, this promise is yet to be fulfilled either because prospective agents do not produce statistically significant effects in sufficiently large populations (Yekkirala et al., 2017) or because unexpected toxicities limit long term drug use. It should be noted, however, that some of the actions of gabapentinoids and cannabinoids that display efficacy in neuropathic pain may be mediated by augmentation of K+ channel function (Manville and Abbott, 2018; Li Y. et al., 2019). Given the improved understanding of cellular processes controlling channel function, it may be useful to target upstream mediators such as the histone methylase G9a which controls the function of several K+ channel types (Laumet et al., 2015).
On a more positive note, however, K+ channel activators may play a role with the advent of personalized medicine approaches to pain management (Bannister et al., 2020). Determination of the sensory phenotypes and genetics of individual patients may dictate drug effectiveness and define therapeutic approaches. K+ channel activators should not be overlooked in this regard.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abd-Elsayed, A., Jackson, M., Gu, S. L., Fiala, K., and Gu, J. (2019). Neuropathic pain and Kv7 voltage-gated potassium channels: the potential role of Kv7 activators in the treatment of neuropathic pain. Mol. Pain 15:1744806919864256.
Abdulla, F. A., and Smith, P. A. (1997). Ectopic α2-adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons. J. Neurosci. 17, 1633–1641. doi: 10.1523/jneurosci.17-05-01633.1997
Abdulla, F. A., and Smith, P. A. (1998). Axotomy reduces the effect of analgesic opioids yet increases the effect of nociceptin on dorsal root ganglion neurons. J. Neurosci. 18, 9685–9694. doi: 10.1523/jneurosci.18-23-09685.1998
Abdulla, F. A., and Smith, P. A. (1999). Nerve injury increases an excitatory action of neuropeptide Y and Y2- agonists on dorsal root ganglion neurons. Neuroscience 89, 43–60. doi: 10.1016/s0306-4522(98)00443-6
Abdulla, F. A., and Smith, P. A. (2001a). Axotomy and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J. Neurophysiol. 85, 630–643. doi: 10.1152/jn.2001.85.2.630
Abdulla, F. A., and Smith, P. A. (2001b). Axotomy- and autotomy-induced changes in Ca2+and K+ channel currents of rat dorsal root ganglion neurons. J. Neurophysiol. 85, 644–658. doi: 10.1152/jn.2001.85.2.644
Abdulla, F. A., Stebbing, M. J., and Smith, P. A. (2001). Effects of substance P on excitability and ionic currents of normal and axotomized rat dorsal root ganglion neurons. Eur. J. Neurosci. 13, 545–552. doi: 10.1046/j.0953-816x.2000.01429.x
Adams, P. R., Brown, D. A., and Constanti, A. (1982). Pharmacological inhibition of the M-current. J. Physiol. 332, 223–262.
Akins, P. T., and McCleskey, E. W. (1993). Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience 56, 759–769. doi: 10.1016/0306-4522(93)90372-m
Albury, C. L., Stuart, S., Haupt, L. M., and Griffiths, L. R. (2017). Ion channelopathies and migraine pathogenesis. Mol. Genet. Genomics 292, 729–739. doi: 10.1007/s00438-017-1317-1
Alles, S. R. A., and Smith, P. A. (2016). The anti-allodynic gabapentinoids: myths, paradoxes, and acute effects. Neuroscientist 23, 40–55. doi: 10.1177/1073858416628793
Alles, S. R. A., and Smith, P. A. (2018). The etiology and pharmacology of neuropathic pain. Pharmacol. Rev. 70, 315–347. doi: 10.1124/pr.117.014399
Alloui, A., Zimmermann, K., Mamet, J., Duprat, F., Noel, J., Chemin, J., et al. (2006). TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 25, 2368–2376. doi: 10.1038/sj.emboj.7601116
Amarillo, Y., De Santiago-Castillo, J. A., Dougherty, K., Maffie, J., Kwon, E., Covarrubias, M., et al. (2008). Ternary Kv4.2 channels recapitulate voltage-dependent inactivation kinetics of A-type K+ channels in cerebellar granule neurons. J. Physiol. 586, 2093–2106. doi: 10.1113/jphysiol.2007.150540
Arcourt, A., Gorham, L., Dhandapani, R., Prato, V., Taberner, F. J., Wende, H., et al. (2017). Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron 93, 179–193. doi: 10.1016/j.neuron.2016.11.027
Arruda, J. L., Colburn, R. W., Rickman, A. J., Rutkowski, M. D., and DeLeo, J. A. (1998). Increase of interleukin-6 mRNA in the spinal cord following peripheral nerve injury in the rat: potential role of IL-6 in neuropathic pain. Brain Res. Mol. Brain Res. 62, 228–235. doi: 10.1016/s0169-328x(98)00257-5
Baba, H., Ji, R. R., Kohno, T., Moore, K. A., Ataka, T., Wakai, A., et al. (2003). Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol. Cell. Neurosci. 24, 818–830. doi: 10.1016/s1044-7431(03)00236-7
Balasubramanyan, S., Stemkowski, P. L., Stebbing, M. J., and Smith, P. A. (2006). Sciatic chronic constriction injury produces cell-type specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J. Neurophysiol. 96, 579–590. doi: 10.1152/jn.00087.2006
Bannister, K., Sachau, J., Baron, R., and Dickenson, A. H. (2020). Neuropathic pain: mechanism-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 60, 257–274. doi: 10.1146/annurev-pharmtox-010818-021524
Barkai, O., Goldstein, R. H., Caspi, Y., Katz, B., Lev, S., and Binshtok, A. M. (2017). The role of Kv7/M potassium channels in controlling ectopic firing in nociceptors. Front. Mol. Neurosci. 10:181. doi: 10.3389/fnmol.2017.00181
Barrese, V., Stott, J. B., and Greenwood, I. A. (2018). KCNQ-encoded potassium channels as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 58, 625–648. doi: 10.1146/annurev-pharmtox-010617-052912
Bedi, S. S., Yang, Q., Crook, R. J., Du, J., Wu, Z., Fishman, H. M., et al. (2010). Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J. Neurosci. 30, 14870–14882. doi: 10.1523/jneurosci.2428-10.2010
Bennett, G. J., and Xie, Y.-K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107. doi: 10.1016/0304-3959(88)90209-6
Berkefeld, H., Fakler, B., and Schulte, U. (2010). Ca2+-Activated K+ channels: from protein complexes to function. Physiol. Rev. 90, 1437–1459. doi: 10.1152/physrev.00049.2009
Bernal, L., Lopez-Garcia, J. A., and Roza, C. (2016). Spontaneous activity in C-fibres after partial damage to the saphenous nerve in mice: effects of retigabine. Eur. J. Pain 20, 1335–1345. doi: 10.1002/ejp.858
Bernal, L., and Roza, C. (2018). Hyperpolarization-activated channels shape temporal patterns of ectopic spontaneous discharge in C-nociceptors after peripheral nerve injury. Eur. J. Pain 22, 1377–1387. doi: 10.1002/ejp.1226
Binshtok, A. M., Wang, H., Zimmermann, K., Amaya, F., Vardeh, D., Shi, L., et al. (2008). Nociceptors Are Interleukin-1{beta} Sensors. J. Neurosci. 28, 14062–14073.
Bischoff, U., Vogel, W., and Safronov, B. V. (1998). Na+-activated K+ channels in small dorsal root ganglion neurones of rat. J. Physiol. 510(Pt 3), 743–754. doi: 10.1111/j.1469-7793.1998.743bj.x
Biton, B., Sethuramanujam, S., Picchione, K. E., Bhattacharjee, A., Khessibi, N., Chesney, F., et al. (2012). The antipsychotic drug loxapine is an opener of the sodium-activated potassium channel slack (Slo2.2). J. Pharmacol. Exp. Ther. 340, 706–715. doi: 10.1124/jpet.111.184622
Black, J. A., Langworthy, K., Hinson, A. W., Dib-Hajj, S. D., and Waxman, S. G. (1997). NGF has opposing effects on Na+ channel III and SNS gene expression in spinal sensory neurons. Neuroreport 8, 2331–2335. doi: 10.1097/00001756-199707070-00046
Blackburn-Munro, G., and Jensen, B. S. (2003). The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur. J. Pharmacol. 460, 109–116. doi: 10.1016/s0014-2999(02)02924-2
Boakye, P. A., Rancic, V., Whitlock, K. H., Simmons, D. A., Longo, F. M., Ballanyi, K., et al. (2019). Receptor-dependence of BDNF actions in superficial dorsal horn; relation to central sensitization and actions of macrophage colony stimulating factor 1 (CSF-1). J. Neurophysiol. 121, 2308–2322. doi: 10.1152/jn.00839.2018
Bocksteins, E., Raes, A. L., Van de Vijver, G., Bruyns, T., Van Bogaert, P. P., and Snyders, D. J. (2009). Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am. J. Physiol. Cell Physiol. 296, C1271–C1278.
Bocksteins, E., Van, D. V., Van Bogaert, P. P., and Snyders, D. J. (2012). Kv3 channels contribute to the delayed rectifier current in small cultured mouse dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 303, C406–C415.
Boettger, M. K., Till, S., Chen, M. X., Anand, U., Otto, W. R., Plumpton, C., et al. (2002). Calcium-activated potassium channel SK1- and IK1-like immunoreactivity in injured human sensory neurones and its regulation by neurotrophic factors. Brain 125, 252–263. doi: 10.1093/brain/awf026
Boyle, K. A., Gradwell, M. A., Yasaka, T., Dickie, A. C., Polgar, E., Ganley, R. P., et al. (2019). Defining a spinal microcircuit that gates myelinated afferent input: implications for tactile allodynia. Cell Rep. 28, 526–540. doi: 10.1016/j.celrep.2019.06.040
Brewer, C. L., and Baccei, M. L. (2020). The development of pain circuits and unique effects of neonatal injury. J. Neural Transm. 127, 467–479. doi: 10.1007/s00702-019-02059-z
Brewer, C. L., Li, J., O’Conor, K., Serafin, E. K., and Baccei, M. L. (2020). Neonatal injury evokes persistent deficits in dynorphin inhibitory circuits within the adult mouse superficial dorsal horn. J. Neurosci. 40:JN-RM-0029-20.
Brown, D. A., and Passmore, G. M. (2009). Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 156, 1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x
Brown, M. R., El-Hassar, L., Zhang, Y., Alvaro, G., Large, C. H., and Kaczmarek, L. K. (2016). Physiological modulators of Kv3.1 channels adjust firing patterns of auditory brain stem neurons. J. Neurophysiol. 116, 106–121. doi: 10.1152/jn.00174.2016
Buch, N. S., Ahlburg, P., Haroutounian, S., Andersen, N. T., Finnerup, N. B., and Nikolajsen, L. (2019). The role of afferent input in postamputation pain: a randomized, double-blind, placebo-controlled crossover study. Pain 160, 1622–1633. doi: 10.1097/j.pain.0000000000001536
Busserolles, J., Tsantoulas, C., Eschalier, A., and Lopez Garcia, J. A. (2016). Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. Pain 157(Suppl. 1), S7–S14.
Calvo, M., Richards, N., Schmid, A. B., Barroso, A., Zhu, L., Ivulic, D., et al. (2016). Altered potassium channel distribution and composition in myelinated axons suppresses hyperexcitability following injury. eLife 5:e12661.
Campbell, J. D., Sansom, M. S., and Ashcroft, F. M. (2003). Potassium channel regulation. EMBO Rep. 4, 1038–1042. doi: 10.1038/sj.embor.embor7400003
Campbell, J. N., Raja, S. N., Meyer, R. A., and Mackinnon, S. E. (1988). Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain 32, 89–94. doi: 10.1016/0304-3959(88)90027-9
Cao, X. H., Byun, H. S., Chen, S. R., Cai, Y. Q., and Pan, H. L. (2010). Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J. Neurochem. 114, 1460–1475.
Cao, X. H., Chen, S. R., Li, L., and Pan, H. L. (2012). Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. J. Neurochem. 121, 944–953. doi: 10.1111/j.1471-4159.2012.07736.x
Castellanos, A., Pujol-Coma, A., Andres-Bilbe, A., Negm, A., Callejo, G., Soto, D., et al. (2020). TRESK background K+ channel deletion selectively uncovers enhanced mechanical and cold sensitivity. J. Physiol. 598, 1017–1038. doi: 10.1113/jp279203
Chaplan, S. R., Guo, H. Q., Lee, D. H., Luo, L., Liu, C., Kuei, C., et al. (2003). Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J. Neurosci. 23, 1169–1178. doi: 10.1523/jneurosci.23-04-01169.2003
Chen, S. R., Cai, Y. Q., and Pan, H. L. (2009a). Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. J. Neurochem. 110, 352–362. doi: 10.1111/j.1471-4159.2009.06138.x
Chen, Y., Balasubramanyan, S., Lai, A. Y., Todd, K. G., and Smith, P. A. (2009b). Effects of sciatic nerve axotomy on excitatory synaptic transmission in rat substantia gelatinosa. J. Neurophysiol. 102, 3203–3215. doi: 10.1152/jn.00296.2009
Cheng, C. F., Cheng, J. K., Chen, C. Y., Lien, C. C., Chu, D., Wang, S. Y., et al. (2014). Mirror-image pain is mediated by nerve growth factor produced from tumor necrosis factor alpha-activated satellite glia after peripheral nerve injury. Pain 155, 906–920. doi: 10.1016/j.pain.2014.01.010
Cheng, C. F., Cheng, J. K., Chen, C. Y., Rau, R. H., Chang, Y. C., and Tsaur, M. L. (2015). Nerve growth factor-induced synapse-like structures in contralateral sensory ganglia contribute to chronic mirror-image pain. Pain 156, 2295–2309. doi: 10.1097/j.pain.0000000000000280
Chien, L. Y., Cheng, J. K., Chu, D., Cheng, C. F., and Tsaur, M. L. (2007). Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J. Neurosci. 27, 9855–9865. doi: 10.1523/jneurosci.0604-07.2007
Chiu, I. M., Barrett, L. B., Williams, E. K., Strochlic, D. E., Lee, S., Weyer, A. D., et al. (2014). Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife 3:e04660.
Chiu, S. Y., and Wilson, G. F. (1989). The role of potassium channels in Schwann cell proliferation in Wallerian degeneration of explant rabbit sciatic nerves. J. Physiol. 408, 199–222. doi: 10.1113/jphysiol.1989.sp017455
Ciotu, C. I., Tsantoulas, C., Meents, J., Lampert, A., McMahon, S. B., Ludwig, A., et al. (2019). Noncanonical ion channel behaviour in pain. Int. J. Mol. Sci. 20:4572. doi: 10.3390/ijms20184572
Cisneros, E., Roza, C., Jackson, N., and Lopez-Garcia, J. A. (2015). A new regulatory mechanism for Kv7.2 protein during neuropathy: enhanced transport from the soma to axonal terminals of injured sensory neurons. Front. Cell. Neurosci. 9:470. doi: 10.3389/fncel.2015.00470
Clark, J. D. (2016). Preclinical pain research: Can we do better? Anesthesiology 125, 846–849. doi: 10.1097/aln.0000000000001340
Coderre, T. J., Grimes, R. W., and Melzak, R. (1986). Deafferentation and chronic pain in animals: an evaluation of evidence suggesting autotomy is related to pain. Pain 26, 61–84. doi: 10.1016/0304-3959(86)90174-0
Cooper, E. C., Harrington, E., Jan, Y. N., and Jan, L. Y. (2001). M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J. Neurosci. 21, 9529–9540. doi: 10.1523/jneurosci.21-24-09529.2001
Costigan, M., Scholz, J., and Woolf, C. J. (2009). Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 32, 1–32. doi: 10.1146/annurev.neuro.051508.135531
Coull, J. A., Beggs, S., Boudreau, D., Boivin, D., Tsuda, M., Inoue, K., et al. (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021. doi: 10.1038/nature04223
Coull, J. A., Boudreau, D., Bachand, K., Prescott, S. A., Nault, F., Sik, A., et al. (2003). Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942. doi: 10.1038/nature01868
Cregg, R., Momin, A., Rugiero, F., Wood, J. N., and Zhao, J. (2010). Pain channelopathies. J. Physiol. 588, 1897–1904. doi: 10.1113/jphysiol.2010.187807
Cristino, L., Bisogno, T., and Di Marzo, V. (2020). Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 16, 9–29. doi: 10.1038/s41582-019-0284-z
Cruzblanca, H., Koh, D. S., and Hille, B. (1998). Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc. Natl. Acad. Sci. U.S.A. 95, 7151–7156. doi: 10.1073/pnas.95.12.7151
Cui, J. G., Holmin, S., Mathiesen, T., Meyerson, B. A., and Linderoth, B. (2000). Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain 88, 239–248. doi: 10.1016/s0304-3959(00)00331-6
Cummins, T. R., Black, J. A., Dib-Hajj, S. D., and Waxman, S. G. (2000). Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J. Neurosci. 20, 8754–8761. doi: 10.1523/jneurosci.20-23-08754.2000
Daou, I., Beaudry, H., Ase, A. R., Wieskopf, J. S., Ribeiro-da-Silva, A., Mogil, J. S., et al. (2016). Optogenetic Silencing of Nav1.8-Positive afferents alleviates inflammatory and neuropathic pain. eNeuro 3:ENEURO.0140-15.2016.
Decosterd, I., and Woolf, C. J. (2000). Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158. doi: 10.1016/s0304-3959(00)00276-1
DeLeo, J. A., Colburn, R. W., and Rickman, A. J. (1997). Cytokine and growth factor immunohistochemical spinal profiles in two animal models of mononeuropathy. Brain Res. 759, 50–57. doi: 10.1016/s0006-8993(97)00209-6
Dermody, A., Kamishima, T., Quayle, J., and Djouhri, L. (2012). “The KV7 Channel activator, retigabine attenuates mechanical allodynia but not heat hyperalgesia associated with painful diabetic neuropathy in rats,” in Proceedings of the 14th World Congress of Pain (IASP), Milan, 103.
Devor, M. (2006). Centralization, central sensitization and neuropathic pain. Focus on “sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons”. J. Neurophysiol. 96, 522–523. doi: 10.1152/jn.00365.2006
Devor, M. (2009). Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp. Brain Res. 196, 115–128. doi: 10.1007/s00221-009-1724-6
Dib-Hajj, S. D., Black, J. A., Cummins, T. R., Kenney, A. M., Kocsis, J. D., and Waxman, S. G. (1998). Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo. J. Neurophysiol. 79, 2668–2676. doi: 10.1152/jn.1998.79.5.2668
Dib-Hajj, S. D., Cummins, T. R., Black, J. A., and Waxman, S. G. (2010). Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 33, 325–347. doi: 10.1146/annurev-neuro-060909-153234
Dib-Hajj, S. D., Rush, A. M., Cummins, T. R., Hisama, F. M., Novella, S., Tyrrell, L., et al. (2005). Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain 128, 1847–1854. doi: 10.1093/brain/awh514
Dib-Hajj, S. D., and Waxman, S. G. (2014). Translational pain research: lessons from genetics and genomics. Sci. Transl. Med. 6:249sr4. doi: 10.1126/scitranslmed.3007017
Dib-Hajj, S. D., and Waxman, S. G. (2019). Sodium channels in human pain disorders: genetics and pharmacogenomics. Annu. Rev. Neurosci. 42, 87–106. doi: 10.1146/annurev-neuro-070918-050144
Dib-Hajj, S. D., Yang, Y., Black, J. A., and Waxman, S. G. (2013). The Na(V)1.7 sodium channel: from molecule to man. Nat. Rev. Neurosci. 14, 49–62. doi: 10.1038/nrn3404
Djouhri, L. (2016). PG110, A Humanized Anti-NGF antibody, reverses established pain hypersensitivity in persistent inflammatory pain, but not peripheral neuropathic pain, rat models. Pain Med. 17, 2082–2094. doi: 10.1093/pm/pnw007
Djouhri, L., Malki, M. I., Zeidan, A., Nagi, K., and Smith, T. (2019). Activation of Kv7 channels with the anticonvulsant retigabine alleviates neuropathic pain behaviour in the streptozotocin rat model of diabetic neuropathy. J. Drug Target. 27, 1118–1126. doi: 10.1080/1061186x.2019.1608552
Djouhri, L., Smith, T., Ahmeda, A., Alotaibi, M., and Weng, X. (2018). Hyperpolarization-activated cyclic nucleotide-gated channels contribute to spontaneous activity in L4 C-fiber nociceptors, but not Abeta-non-nociceptors, after axotomy of L5-spinal nerve in the rat in vivo. Pain 159, 1392–1402. doi: 10.1097/j.pain.0000000000001224
Dobler, T., Springauf, A., Tovornik, S., Weber, M., Schmitt, A., Sedlmeier, R., et al. (2007). TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J. Physiol. 585, 867–879. doi: 10.1113/jphysiol.2007.145649
Dodds, K. N., Beckett, E. A., Evans, S. F., Grace, P. M., Watkins, L. R., and Hutchinson, M. R. (2016). Glial contributions to visceral pain: implications for disease etiology and the female predominance of persistent pain. Transl. Psychiatry 6:e888. doi: 10.1038/tp.2016.168
Dogrul, A., Gul, H., Yesilyurt, O., Ulas, U. H., and Yildiz, O. (2011). Systemic and spinal administration of etanercept, a tumor necrosis factor alpha inhibitor, blocks tactile allodynia in diabetic mice. Acta Diabetol. 48, 135–142. doi: 10.1007/s00592-010-0237-x
Dolphin, A. C. (2016). Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J. Physiol. 594, 5369–5390. doi: 10.1113/jp272262
Du, X., and Gamper, N. (2013). Potassium channels in peripheral pain pathways: expression, function and therapeutic potential. Curr. Neuropharmacol. 11, 621–640.
Du, X., Gao, H., Jaffe, D., Zhang, H., and Gamper, N. (2018). M-type K(+) channels in peripheral nociceptive pathways. Br. J. Pharmacol. 175, 2158–2172. doi: 10.1111/bph.13978
Duan, K. Z., Xu, Q., Zhang, X. M., Zhao, Z. Q., Mei, Y. A., and Zhang, Y. Q. (2012). Targeting A-type K(+) channels in primary sensory neurons for bone cancer pain in a rat model. Pain 153, 562–574. doi: 10.1016/j.pain.2011.11.020
Emery, E. C., Young, G. T., Berrocoso, E. M., Chen, L., and McNaughton, P. A. (2011). HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 333, 1462–1466. doi: 10.1126/science.1206243
Emery, E. C., Young, G. T., and McNaughton, P. A. (2012). HCN2 ion channels: an emerging role as the pacemakers of pain. Trends Pharmacol. Sci. 33, 456–463. doi: 10.1016/j.tips.2012.04.004
Enyedi, P., and Czirjak, G. (2010). Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 90, 559–605. doi: 10.1152/physrev.00029.2009
Evely, K. M., Pryce, K. D., Bausch, A. E., Lukowski, R., Ruth, P., Haj-Dahmane, S., et al. (2017). Slack KNa channels influence dorsal horn synapses and nociceptive behavior. Mol. Pain 13:1744806917714342.
Everill, B., and Kocsis, J. D. (1999). Reduction of potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J. Neurophysiol. 82, 700–708. doi: 10.1152/jn.1999.82.2.700
Everill, B., and Kocsis, J. D. (2000). Nerve growth factor maintains potassium conductance after nerve injury in adult cutaneous afferent dorsal root ganglion neurons [In Process Citation]. Neuroscience 100, 417–422. doi: 10.1016/s0306-4522(00)00263-3
Everill, B., Rizzo, M. A., and Kocsis, J. D. (1998). Morphologically identified cutaneous afferent DRG neurons express three different potassium currents in varying proportions. J. Neurophysiol. 79, 1814–1824. doi: 10.1152/jn.1998.79.4.1814
Fan, L., Guan, X., Wang, W., Zhao, J. Y., Zhang, H., Tiwari, V., et al. (2014). Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons. Mol. Pain 10:8.
Fang, X., Djouhri, L., McMullan, S., Berry, C., Waxman, S. G., Okuse, K., et al. (2006). Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J. Neurosci. 26, 7281–7292. doi: 10.1523/jneurosci.1072-06.2006
Field, M. J., Cox, P. J., Stott, E., Melrose, H., Offord, J., Su, T. Z., et al. (2006). Identification of the {alpha}2-{delta}-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc. Natl. Acad. Sci. U.S.A. 103, 17537–17542. doi: 10.1073/pnas.0409066103
Finnerup, N. B., Attal, N., Haroutounian, S., McNicol, E., Baron, R., Dworkin, R. H., et al. (2015). Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 14, 162–173.
Fisher, C., Johnson, K., Okerman, T., Jurgenson, T., Nickell, A., Salo, E., et al. (2019). Morphine efficacy, tolerance, and hypersensitivity are altered after modulation of SUR1 subtype KATP channel activity in mice. Front. Neurosci. 13:1122. doi: 10.3389/fnins.2019.01122
Fjell, J., Cummins, T. R., Dib-Hajj, S. D., Fried, K., Black, J. A., and Waxman, S. G. (1999). Differential role of GDNF and NGF in the maintenance of two TTX- resistant sodium channels in adult DRG neurons. Brain Res. Mol. Brain Res. 67, 267–282. doi: 10.1016/s0169-328x(99)00070-4
Ford, C. P., Stemkowski, P. L., Light, P. E., and Smith, P. A. (2003). Experiments to test the role of phosphatidylinositol 4,5-bisphosphate in neurotransmitter-induced M-channel closure in bullfrog sympathetic neurons. J. Neurosci. 23, 4931–4941. doi: 10.1523/jneurosci.23-12-04931.2003
Furukawa, N., Takasusuki, T., Fukushima, T., and Hori, Y. (2008). Presynaptic large-conductance calcium-activated potassium channels control synaptic transmission in the superficial dorsal horn of the mouse. Neurosci. Lett. 444, 79–82. doi: 10.1016/j.neulet.2008.08.022
Gabriel, A., Abdallah, M., Yost, C. S., Winegar, B. D., and Kindler, C. H. (2002). Localization of the tandem pore domain K+ channel KCNK5 (TASK-2) in the rat central nervous system. Brain Res. Mol. Brain Res. 98, 153–163. doi: 10.1016/s0169-328x(01)00330-8
Gada, K., and Plant, L. D. (2019). Two-pore domain potassium channels: emerging targets for novel analgesic drugs: IUPHAR Review 26. Br. J. Pharmacol. 176, 256–266. doi: 10.1111/bph.14518
Gamper, N., and Shapiro, M. S. (2003). Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. J. Gen. Physiol. 122, 17–31. doi: 10.1085/jgp.200208783
Gamper, N., Stockand, J. D., and Shapiro, M. S. (2003). Subunit-specific modulation of KCNQ potassium channels by src tyrosine kinase. J. Neurosci. 23, 84–95. doi: 10.1523/jneurosci.23-01-00084.2003
Gaudet, A. D., Popovich, P. G., and Ramer, M. S. (2011). Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 8:110. doi: 10.1186/1742-2094-8-110
Gemes, G., Koopmeiners, A., Rigaud, M., Lirk, P., Sapunar, D., Bangaru, M. L., et al. (2013). Failure of action potential propagation in sensory neurons: mechanisms and loss of afferent filtering in C-type units after painful nerve injury. J. Physiol. 591, 1111–1131. doi: 10.1113/jphysiol.2012.242750
George, A., Buehl, A., and Sommer, C. (2004). Wallerian degeneration after crush injury of rat sciatic nerve increases endo- and epineurial tumor necrosis factor-alpha protein. Neurosci. Lett. 372, 215–219. doi: 10.1016/j.neulet.2004.09.075
George, A., Marziniak, M., Schafers, M., Toyka, K. V., and Sommer, C. (2000). Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-alpha, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain 88, 267–275. doi: 10.1016/s0304-3959(00)00333-x
George, A., Schmidt, C., Weishaupt, A., Toyka, K. V., and Sommer, C. (1999). Serial determination of tumor necrosis factor-alpha content in rat sciatic nerve after chronic constriction injury. Exp. Neurol. 160, 124–132. doi: 10.1006/exnr.1999.7193
Giacobassi, M. J., Leavitt, L. S., Raghuraman, S., Alluri, R., Chase, K., Finol-Urdaneta, R. K., et al. (2020). An integrative approach to the facile functional classification of dorsal root ganglion neuronal subclasses. Proc. Natl. Acad. Sci. U.S.A. 117, 5494–5501. doi: 10.1073/pnas.1911382117
Gold, M. S., and Gebhart, G. F. (2010). Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257. doi: 10.1038/nm.2235
Gold, M. S., Shuster, M. J., and Levine, J. D. (1996a). Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J. Neurophysiol. 75, 2629–2646. doi: 10.1152/jn.1996.75.6.2629
Gold, M. S., Shuster, M. J., and Levine, J. D. (1996b). Role of a Ca(2+)-dependent slow afterhyperpolarization in prostaglandin E2-induced sensitization of cultured rat sensory neurons. Neurosci. Lett. 205, 161–164. doi: 10.1016/0304-3940(96)12401-0
Goldin, A. L., Barchi, R. L., Caldwell, J. H., Hofmann, F., Howe, J. R., Hunter, J. C., et al. (2000). Nomenclature of voltage-gated sodium channels. Neuron 28, 365–368.
Goldstein, S. A., Bayliss, D. A., Kim, D., Lesage, F., Plant, L. D., and Rajan, S. (2005). International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol. Rev. 57, 527–540. doi: 10.1124/pr.57.4.12
Gonzalez, A., Ugarte, G., Restrepo, C., Herrera, G., José Antonio Gómez-Sánchez, J. A., Pertusa, M., et al. (2017). Role of the excitability brake potassium current IKD in cold allodynia induced by chronic peripheral nerve injury. J. Neurosci. 37, 3109–3126. doi: 10.1523/jneurosci.3553-16.2017
Govrin-Lippmann, R., and Devor, M. (1978). Ongoing activity in severed nerves: source and variation with time. Brain Res. 159, 406–410. doi: 10.1016/0006-8993(78)90548-6
Grabauskas, G., Heldsinger, A., Wu, X., Xu, D., Zhou, S., and Owyang, C. (2011). Diabetic visceral hypersensitivity is associated with activation of mitogen-activated kinase in rat dorsal root ganglia. Diabetes Metab. Res. Rev. 60, 1743–1751. doi: 10.2337/db10-1507
Grace, P. M., Hutchinson, M. R., Maier, S. F., and Watkins, L. R. (2014). Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 14, 217–231. doi: 10.1038/nri3621
Gregory, N. S., Harris, A. L., Robinson, C. R., Dougherty, P. M., Fuchs, P. N., and Sluka, K. A. (2013). An overview of animal models of pain: disease models and outcome measures. J. Pain 14, 1255–1269. doi: 10.1016/j.jpain.2013.06.008
Gudes, S., Barkai, O., Caspi, Y., Katz, B., Lev, S., and Binshtok, A. M. (2015). The role of slow and persistent TTX-resistant sodium currents in acute tumor necrosis factor-alpha-mediated increase in nociceptors excitability. J. Neurophysiol. 113, 601–619. doi: 10.1152/jn.00652.2014
Guo, R., Chen, L. H., Xing, C., and Liu, T. (2019). Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br. J. Anaesth. 123, 637–654. doi: 10.1016/j.bja.2019.07.026
Gurtu, S., and Smith, P. A. (1988). Electrophysiological characteristics of hamster dorsal root ganglion cells and their response to axotomy. J. Neurophysiol. 59, 408–423. doi: 10.1152/jn.1988.59.2.408
Gustafson-Vickers, S. L., Lu, V. B., Lai, A. Y., Todd, K. G., Ballanyi, K., and Smith, P. A. (2008). Long-term actions of interleukin-1beta on delay and tonic firing neurons in rat superficial dorsal horn and their relevance to central sensitization. Mol. Pain 4:63.
Gutman, G. A., Chandy, K. G., Grissmer, S., Lazdunski, M., McKinnon, D., Pardo, L. A., et al. (2005). International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 57, 473–508. doi: 10.1124/pr.57.4.10
Hadley, J. K., Passmore, G. M., Tatulian, L., Al-Qatari, M., Ye, F., Wickenden, A. D., et al. (2003). Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J. Neurosci. 23, 5012–5019. doi: 10.1523/jneurosci.23-12-05012.2003
Hao, J., Padilla, F., Dandonneau, M., Lavebratt, C., Lesage, F., Noel, J., et al. (2013). Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron 77, 899–914. doi: 10.1016/j.neuron.2012.12.035
Hart, I. K., Maddison, P., Newsom-Davis, J., Vincent, A., and Mills, K. R. (2002). Phenotypic variants of autoimmune peripheral nerve hyperexcitability. Brain 125, 1887–1895. doi: 10.1093/brain/awf178
Harte, S. E., Meyers, J. B., Donahue, R. R., Taylor, B. K., and Morrow, T. J. (2016). Mechanical conflict system: a novel operant method for the assessment of nociceptive behavior. PLoS One 11:e0150164. doi: 10.1371/journal.pone.0150164
He, X. H., Zang, Y., Chen, X., Pang, R. P., Xu, J. T., Zhou, X., et al. (2010). TNF-alpha contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain 151, 266–279. doi: 10.1016/j.pain.2010.06.005
Heinke, B., Gingl, E., and Sandkuhler, J. (2011). Multiple targets of mu-opioid receptor-mediated presynaptic inhibition at primary afferent Adelta- and C-fibers. J. Neurosci. 31, 1313–1322. doi: 10.1523/jneurosci.4060-10.2011
Herradon, G., Ezquerra, L., Nguyen, T., Wang, C., Siso, A., Franklin, B., et al. (2007). Changes in BDNF gene expression correlate with rat strain differences in neuropathic pain. Neurosci. Lett. 420, 273–276. doi: 10.1016/j.neulet.2007.05.014
Herzberg, U., Eliav, E., Dorsey, J. M., Gracely, R. H., and Kopin, I. J. (1997). NGF involvement in pain induced by chronic constriction injury of the rat sciatic nerve. Neuroreport 8, 1613–1618. doi: 10.1097/00001756-199705060-00012
Hoppa, M. B., Gouzer, G., Armbruster, M., and Ryan, T. A. (2014). Control and plasticity of the presynaptic action potential waveform at small CNS nerve terminals. Neuron 84, 778–789. doi: 10.1016/j.neuron.2014.09.038
Hoppa, M. B., Lana, B., Margas, W., Dolphin, A. C., and Ryan, T. A. (2012). alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature 486, 122–125. doi: 10.1038/nature11033
Hoshi, T., and Heinemann, S. H. (2016). Modulation of BK channels by small endogenous molecules and pharmaceutical channel openers. Int. Rev. Neurobiol. 128, 193–237. doi: 10.1016/bs.irn.2016.03.020
Hougaard, C., Fraser, M. O., Chien, C., Bookout, A., Katofiasc, M., Jensen, B. S., et al. (2009a). A positive modulator of K Ca 2 and K Ca 3 channels, 4,5-dichloro-1,3-diethyl-1,3-dihydro-benzoimidazol-2-one (NS4591), inhibits bladder afferent firing in vitro and bladder overactivity in vivo. J. Pharmacol. Exp. Ther. 328, 28–39. doi: 10.1124/jpet.108.143123
Hougaard, C., Jensen, M. L., Dale, T. J., Miller, D. D., Davies, D. J., Eriksen, B. L., et al. (2009b). Selective activation of the SK1 subtype of human small-conductance Ca2+-activated K+ channels by 4-(2-methoxyphenylcarbamoyloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester (GW542573X) is dependent on serine 293 in the S5 segment. Mol. Pharmacol. 76, 569–578. doi: 10.1124/mol.109.056663
Huang, F., Wang, X., Ostertag, E. M., Nuwal, T., Huang, B., Jan, Y. N., et al. (2013). TMEM16C facilitates Na(+)-activated K+ currents in rat sensory neurons and regulates pain processing. Nat. Neurosci. 16, 1284–1290. doi: 10.1038/nn.3468
Iadarola, M. J., and Caudle, R. M. (1997). Good pain, bad pain. Science 278, 239–240. doi: 10.1126/science.278.5336.239
Indo, Y. (2002). Genetics of congenital insensitivity to pain with anhidrosis (CIPA) or hereditary sensory and autonomic neuropathy type IV. Clinical, biological and molecular aspects of mutations in TRKA(NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Clin. Auton. Res. 12(Suppl. 1), I20–I32.
Irani, S. R., Alexander, S., Waters, P., Kleopa, K. A., Pettingill, P., Zuliani, L., et al. (2010). Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 133, 2734–2748. doi: 10.1093/brain/awq213
Ishikawa, K., Tanaka, M., Black, J. A., and Waxman, S. G. (1999). Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve 22, 502–507. doi: 10.1002/(sici)1097-4598(199904)22:4<502::aid-mus12>3.0.co;2-k
Jassar, B. S., Pennefather, P. S., and Smith, P. A. (1994). Changes in potassium channel activity following axotomy of B-cells in bullfrog sympathetic ganglion. J. Physiol. 479(Pt 3), 353–370. doi: 10.1113/jphysiol.1994.sp020301
Jerng, H. H., Dougherty, K., Covarrubias, M., and Pfaffinger, P. J. (2009). A novel N-terminal motif of dipeptidyl peptidase-like proteins produces rapid inactivation of KV4.2 channels by a pore-blocking mechanism. Channels 3, 448–461. doi: 10.4161/chan.3.6.10216
Ji, R. R., Donnelly, C. R., and Nedergaard, M. (2019). Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 20, 667–685. doi: 10.1038/s41583-019-0218-1
Ji, R. R., Kawasaki, Y., Zhuang, Z. Y., Wen, Y. R., and Decosterd, I. (2006). Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2, 259–269. doi: 10.1017/s1740925x07000403
Jia, Z., Bei, J., Rodat-Despoix, L., Liu, B., Jia, Q., Delmas, P., et al. (2008). NGF inhibits M/KCNQ currents and selectively alters neuronal excitability in subsets of sympathetic neurons depending on their M/KCNQ current background. J. Gen. Physiol. 131, 575–587. doi: 10.1085/jgp.200709924
Jin, X., and Gereau, R. W. IV (2006). Acute p38-Mediated Modulation of Tetrodotoxin-Resistant Sodium Channels in Mouse Sensory Neurons by Tumor Necrosis Factor-{alpha}. J. Neurosci. 26, 246–255. doi: 10.1523/jneurosci.3858-05.2006
Kajander, K. C., Wakisaka, S., and Bennett, G. J. (1992). Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci. Lett. 138, 225–228. doi: 10.1016/0304-3940(92)90920-3
Kanda, H., Kobayashi, K., Yamanaka, H., Okubo, M., and Noguchi, K. (2017). Microglial TNFalpha Induces COX2 and PGI2 Synthase Expression in Spinal Endothelial Cells during Neuropathic Pain. eNeuro 4:ENEURO.0064-17.2017.
Kanda, V. A., Lewis, A., Xu, X., and Abbott, G. W. (2011). KCNE1 and KCNE2 inhibit forward trafficking of homomeric N-type voltage-gated potassium channels. Biophys. J. 101, 1354–1363. doi: 10.1016/j.bpj.2011.08.015
Kang, D., Choe, C., and Kim, D. (2005). Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J. Physiol. 564, 103–116. doi: 10.1113/jphysiol.2004.081059
Kang, D., and Kim, D. (2006). TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 291, C138–C146.
Kawano, T., Zoga, V., McCallum, J. B., Wu, H. E., Gemes, G., Liang, M. Y., et al. (2009). ATP-sensitive potassium currents in rat primary afferent neurons: biophysical, pharmacological properties, and alterations by painful nerve injury. Neuroscience 162, 431–443. doi: 10.1016/j.neuroscience.2009.04.076
Kawasaki, Y., Xu, Z. Z., Wang, X., Park, J. Y., Zhuang, Z. Y., Tan, P. H., et al. (2008a). Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 14, 331–336. doi: 10.1038/nm1723
Kawasaki, Y., Zhang, L., Cheng, J. K., and Ji, R. R. (2008b). Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 28, 5189–5194. doi: 10.1523/jneurosci.3338-07.2008
Kerschensteiner, D., Soto, F., and Stocker, M. (2005). Fluorescence measurements reveal stoichiometry of K+ channels formed by modulatory and delayed rectifier alpha-subunits. Proc. Natl. Acad. Sci. U.S.A. 102, 6160–6165. doi: 10.1073/pnas.0500468102
Ketchum, K. A., Joiner, W. J., Sellers, A. J., Kaczmarek, L. K., and Goldstein, S. A. (1995). A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature 376, 690–695. doi: 10.1038/376690a0
Kim, D. S., Choi, J. O., Rim, H. D., and Cho, H. J. (2002). Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res. Mol. Brain Res. 105, 146–152. doi: 10.1016/s0169-328x(02)00388-1
Kim, K. J., Yoon, Y. W., and Chung, J. M. (1997). Comparison of three rodent models of neuropathic pain. Exp. Brain Res. 113, 200–206. doi: 10.1007/bf02450318
Kim, S. J., and Chung, J. M. (1992). An experimental model for peripheral neuropathy produced by segmental nerve ligation in the rat. Pain 50, 355–363. doi: 10.1016/0304-3959(92)90041-9
King, C. H., Lancaster, E., Salomon, D., Peles, E., and Scherer, S. S. (2014). Kv7.2 regulates the function of peripheral sensory neurons. J. Comp. Neurol. 522, 3262–3280. doi: 10.1002/cne.23595
Kirchhoff, C., Leah, J. D., Jung, S., and Reeh, P. W. (1992). Excitation of cutaneous sensory nerve endings in the rat by 4-aminopyridine and tetraethylammonium. J. Neurophysiol. 67, 125–131. doi: 10.1152/jn.1992.67.1.125
Kohno, T., Ji, R. R., Ito, N., Allchorne, A. J., Befort, K., Karchewski, L. A., et al. (2005). Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain 117, 77–87. doi: 10.1016/j.pain.2005.05.035
Kohno, T., Kumamoto, E., Higashi, H., Shimoji, K., and Yoshimura, M. (1999). Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J. Physiol. 518, 803–813. doi: 10.1111/j.1469-7793.1999.0803p.x
Konishi, T. (1989). Voltage-dependent potassium channels in mouse Schwann cells. J. Physiol. 411, 115–130. doi: 10.1113/jphysiol.1989.sp017564
Koplovitch, P., and Devor, M. (2018). Dilute lidocaine suppresses ectopic neuropathic discharge in dorsal root ganglia without blocking axonal propagation: a new approach to selective pain control. Pain 159, 1244–1256. doi: 10.1097/j.pain.0000000000001205
Kubo, Y., Adelman, J. P., Clapham, D. E., Jan, L. Y., Karschin, A., Kurachi, Y., et al. (2005). International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol. Rev. 57, 509–526. doi: 10.1124/pr.57.4.11
Kumar, M., and Pattnaik, B. R. (2014). Focus on Kir7.1: physiology and channelopathy. Channels 8, 488–495. doi: 10.4161/19336950.2014.959809
Kuo, Y. L., Cheng, J. K., Hou, W. H., Chang, Y. C., Du, P. H., Jian, J. J., et al. (2017). K(+) Channel Modulatory Subunits KChIP and DPP Participate in Kv4-Mediated Mechanical Pain Control. J. Neurosci. 37, 4391–4404. doi: 10.1523/jneurosci.1619-16.2017
La, J. H., Kang, D., Park, J. Y., Hong, S. G., and Han, J. (2006). A novel acid-sensitive K+ channel in rat dorsal root ganglia neurons. Neurosci. Lett. 406, 244–249. doi: 10.1016/j.neulet.2006.07.039
Lafreniere, R. G., and Rouleau, G. A. (2012). Identification of novel genes involved in migraine. Headache 52(Suppl. 2), 107–110. doi: 10.1111/j.1526-4610.2012.02237.x
Lang, P. M., Fleckenstein, J., Passmore, G. M., Brown, D. A., and Grafe, P. (2008). Retigabine reduces the excitability of unmyelinated peripheral human axons. Neuropharmacology 54, 1271–1278. doi: 10.1016/j.neuropharm.2008.04.006
Latremoliere, A., and Woolf, C. J. (2009). Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain 10, 895–926. doi: 10.1016/j.jpain.2009.06.012
Laumet, G., Garriga, J., Chen, S. R., Zhang, Y., Li, D. P., Smith, T. M., et al. (2015). G9a is essential for epigenetic silencing of K+ channel genes in acute-to-chronic pain transition. Nat. Neurosci. 18, 1746–1755. doi: 10.1038/nn.4165
Lawson, S. N. (2002). Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp. Physiol. 87, 239–244. doi: 10.1113/eph8702350
Lee, B. H., Yoon, Y. W., Chung, K., and Chung, J. M. (1998). Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Exp. Brain Res. 120, 432–438. doi: 10.1007/s002210050416
Leffler, A., Cummins, T. R., Dib-Hajj, S. D., Hormuzdiar, W. N., Black, J. A., and Waxman, S. G. (2002). GDNF and NGF reverse changes in repriming of TTX-sensitive Na(+) currents following axotomy of dorsal root ganglion neurons. J. Neurophysiol. 88, 650–658. doi: 10.1152/jn.2002.88.2.650
Leung, L., and Cahill, C. M. (2010). TNF-alpha and neuropathic pain–a review. J. Neuroinflammation 7:27. doi: 10.1186/1742-2094-7-27
Li, W., Gao, S. B., Lv, C. X., Wu, Y., Guo, Z. H., Ding, J. P., et al. (2007). Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons. J. Cell. Physiol. 212, 348–357. doi: 10.1002/jcp.21007
Li, Y., Gamper, N., Hilgemann, D. W., and Shapiro, M. S. (2005). Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25, 9825–9835. doi: 10.1523/jneurosci.2597-05.2005
Li, Y., Zhang, L., Wu, Y., Zheng, Q., Chen, M., Qian, Z., et al. (2019). Cannabinoids-induced peripheral analgesia depends on activation of BK channels. Brain Res. 1711, 23–28. doi: 10.1016/j.brainres.2019.01.007
Li, Z., Guo, Y., Ren, X., Rong, L., Huang, M., Cao, J., et al. (2019). HDAC2, but not HDAC1, regulates Kv1.2 expression to mediate neuropathic pain in CCI rats. Neuroscience 408, 339–348. doi: 10.1016/j.neuroscience.2019.03.033
Liang, L., Zhao, J. Y., Kathryn, T., Bekker, A., and Tao, Y. X. (2019). BIX01294, a G9a inhibitor, alleviates nerve injury-induced pain hypersensitivities during both development and maintenance periods. Transl. Perioper. Pain Med. 6, 106–114.
Linley, J. E., Rose, K., Patil, M., Robertson, B., Akopian, A. N., and Gamper, N. (2008). Inhibition of M current in sensory neurons by exogenous proteases: a signaling pathway mediating inflammatory nociception. J. Neurosci. 28, 11240–11249. doi: 10.1523/jneurosci.2297-08.2008
Liu, C. N., Raber, P., Ziv-Sefer, S., and Devor, M. (2001). Hyperexcitability in sensory neurons of rats selected for high versus low neuropathic pain phenotype. Neuroscience 105, 265–275. doi: 10.1016/s0306-4522(01)00161-0
Loucif, A. J. C., Saintot, P. P., Liu, J., Antonio, B. M., Zellmer, S. G., Yoger, K., et al. (2018). GI-530159, a novel, selective, mechanosensitive two-pore-domain potassium (K2P) channel opener, reduces rat dorsal root ganglion neuron excitability. Br. J. Pharmacol. 175, 2272–2283. doi: 10.1111/bph.14098
Lu, R., Bausch, A. E., Kallenborn-Gerhardt, W., Stoetzer, C., Debruin, N., Ruth, P., et al. (2015). Slack channels expressed in sensory neurons control neuropathic pain in mice. J. Neurosci. 35, 1125–1135.
Lu, R., Flauaus, C., Kennel, L., Petersen, J., Drees, O., Kallenborn-Gerhardt, W., et al. (2017). KCa3.1 channels modulate the processing of noxious chemical stimuli in mice. Neuropharmacology 125, 386–395. doi: 10.1016/j.neuropharm.2017.08.021
Lu, V. B., Ballanyi, K., Colmers, W. F., and Smith, P. A. (2007). Neuron type-specific effects of brain-derived neurotrophic factor in rat superficial dorsal horn and their relevance to ‘central sensitization’. J. Physiol. 584, 543–563. doi: 10.1113/jphysiol.2007.141267
Lu, V. B., Biggs, J. E., Stebbing, M. J., Balasubramanyan, S., Todd, K. G., Lai, A. Y., et al. (2009). BDNF drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J. Physiol. 587, 1013–1032. doi: 10.1113/jphysiol.2008.166306
Lu, V. B., Smith, P. A., and Rashiq, S. (2012). The excitability of dorsal horn neurons is affected by cerebrospinal fluid from humans with osteoarthritis. Can. J. Physiol. Pharmacol. 90, 783–790. doi: 10.1139/y2012-014
Lyu, C., Lyu, G. W., Mulder, J., Martinez, A., and Shi, T. S. (2020). G protein-gated inwardly rectifying potassium channel subunit 3 is upregulated in rat DRGs and spinal cord after peripheral nerve injury. J. Pain Res. 13, 419–429. doi: 10.2147/jpr.s233744
Lyu, C., Mulder, J., Barde, S., Sahlholm, K., Zeberg, H., Nilsson, J., et al. (2015). G protein-gated inwardly rectifying potassium channel subunits 1 and 2 are down-regulated in rat dorsal root ganglion neurons and spinal cord after peripheral axotomy. Mol. Pain 11:44.
Ma, C., Shu, Y., Zheng, Z., Chen, Y., Yao, H., Greenquist, K. W., et al. (2003). Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J. Neurophysiol. 89, 1588–1602. doi: 10.1152/jn.00855.2002
Maeda, H., Fujita, K., Kobayashi, H., Ushiki, J., Nakanishi, T., and Tamai, I. (2012). Novel LC-MS/MS method for simultaneous quantification of KW-7158, a new drug candidate for urinary incontinence and bladder hyperactivity, and its metabolites in rat plasma: a pharmacokinetic study in male and female rats. Arzneimittelforschung 62, 213–221. doi: 10.1055/s-0032-1301883
Mantyh, P. W., Koltzenburg, M., Mendell, L. M., Tive, L., and Shelton, D. L. (2011). Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 115, 189–204. doi: 10.1097/aln.0b013e31821b1ac5
Manville, R. W., and Abbott, G. W. (2018). Gabapentin is a potent activator of KCNQ3 and KCNQ5 potassium channels. Mol. Pharmacol. 94, 1155–1163. doi: 10.1124/mol.118.112953
Manville, R. W., and Abbott, G. W. (2020). Isoform-selective KCNA1 potassium channel openers built from glycine. J. Pharmacol. Exp. Ther. 373, 391–401. doi: 10.1124/jpet.119.264507
Marsh, B., Acosta, C., Djouhri, L., and Lawson, S. N. (2012). Leak K(+) channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol. Cell. Neurosci. 49, 375–386. doi: 10.1016/j.mcn.2012.01.002
Matsuyoshi, H., Takimoto, K., Yunoki, T., Erickson, V. L., Tyagi, P., Hirao, Y., et al. (2012). Distinct cellular distributions of Kv4 pore-forming and auxiliary subunits in rat dorsal root ganglion neurons. Life Sci. 91, 258–263. doi: 10.1016/j.lfs.2012.07.007
Mauderli, A. P., Acosta-Rua, A., and Vierck, C. J. (2000). An operant assay of thermal pain in conscious, unrestrained rats. J. Neurosci. Methods 97, 19–29. doi: 10.1016/s0165-0270(00)00160-6
McFarlane, S., and Cooper, E. (1991). Kinetics and voltage dependence of A-type currents on neonatal rat sensory neurons. J. Neurophysiol. 66, 1380–1391. doi: 10.1152/jn.1991.66.4.1380
Mi, Y., Zhang, X., Zhang, F., Qi, J., Gao, H., Huang, D., et al. (2013). The role of potassium channel activation in celecoxib-induced analgesic action. PLoS One 8:e54797. doi: 10.1371/journal.pone.0054797
Mifflin, K. A., and Kerr, B. J. (2017). Pain in autoimmune disorders. J. Neurosci. Res. 95, 1282–1294.
Mifflin, K. A., Yousuf, M. S., Thorburn, K. C., Huang, J., Perez-Munoz, M. E., Tenorio, G., et al. (2018). Voluntary wheel running reveals sex specific nociceptive factors in murine experimental autoimmune encephalomyelitis. Pain 160, 870–881. doi: 10.1097/j.pain.0000000000001465
Milescu, M., Lee, H. C., Bae, C. H., Kim, J. I., and Swartz, K. J. (2013). Opening the shaker K+ channel with hanatoxin. J. Gen. Physiol. 141, 203–216. doi: 10.1085/jgp.201210914
Milligan, E. D., Twining, C., Chacur, M., Biedenkapp, J., O’Connor, K., Poole, S., et al. (2003). Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J. Neurosci. 23, 1026–1040. doi: 10.1523/jneurosci.23-03-01026.2003
Mis, M. A., Yang, Y., Tanaka, B. S., Gomis-Perez, C., Liu, S., Dib-Hajj, F., et al. (2019). Resilience to pain: a peripheral component identified using induced pluripotent stem cells and dynamic clamp. J. Neurosci. 39, 382–392. doi: 10.1523/jneurosci.2433-18.2018
Mo, K., Wu, S., Gu, X., Xiong, M., Cai, W., Atianjoh, F. E., et al. (2018). MBD1 Contributes to the Genesis of Acute Pain and Neuropathic Pain by Epigenetic Silencing of Oprm1 and Kcna2 Genes in Primary Sensory Neurons. J. Neurosci. 38, 9883–9899. doi: 10.1523/jneurosci.0880-18.2018
Mogil, J. S. (2009). Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 10, 283–294. doi: 10.1038/nrn2606
Mogil, J. S. (2012). Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 13, 859–866. doi: 10.1038/nrn3360
Mongan, L. C., Hill, M. J., Chen, M. X., Tate, S. N., Collins, S. D., Buckby, L., et al. (2005). The distribution of small and intermediate conductance calcium-activated potassium channels in the rat sensory nervous system. Neuroscience 131, 161–175. doi: 10.1016/j.neuroscience.2004.09.062
Mosconi, T., and Kruger, L. (1996). Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructural morphometric analysis of axonal alterations. Pain 64, 37–57. doi: 10.1016/0304-3959(95)00077-1
Mucha, M., Ooi, L., Linley, J. E., Mordaka, P., Dalle, C., Robertson, B., et al. (2010). Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J. Neurosci. 30, 13235–13245. doi: 10.1523/jneurosci.1981-10.2010
Muqeem, T., Ghosh, B., Pinto, V., Lepore, A. C., and Covarrubias, M. (2018). Regulation of Nociceptive Glutamatergic Signaling by Presynaptic Kv3.4 Channels in the Rat Spinal Dorsal Horn. J. Neurosci. 38, 3729–3740. doi: 10.1523/jneurosci.3212-17.2018
Murata, Y., Yasaka, T., Takano, M., and Ishihara, K. (2016). Neuronal and glial expression of inward rectifier potassium channel subunits Kir2.x in rat dorsal root ganglion and spinal cord. Neurosci. Lett. 617, 59–65. doi: 10.1016/j.neulet.2016.02.007
Nadeau, S., Filali, M., Zhang, J., Kerr, B. J., Rivest, S., Soulet, D., et al. (2011). Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J. Neurosci. 31, 12533–12542. doi: 10.1523/jneurosci.2840-11.2011
Negus, S. S., Vanderah, T. W., Brandt, M. R., Bilsky, E. J., Becerra, L., and Borsook, D. (2006). Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J. Pharmacol. Exp. Ther. 319, 507–514. doi: 10.1124/jpet.106.106377
Nesti, E., Everill, B., and Morielli, A. D. (2004). Endocytosis as a mechanism for tyrosine kinase-dependent suppression of a voltage-gated potassium channel. Mol. Biol. Cell 15, 4073–4088. doi: 10.1091/mbc.e03-11-0788
Nitzan-Luques, A., Devor, M., and Tal, M. (2011). Genotype-selective phenotypic switch in primary afferent neurons contributes to neuropathic pain. Pain 152, 2413–2426. doi: 10.1016/j.pain.2011.07.012
Nockemann, D., Rouault, M., Labuz, D., Hublitz, P., McKnelly, K., Reis, F. C., et al. (2013). The K(+) channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol. Med. 5, 1263–1277. doi: 10.1002/emmm.201201980
Noel, J., Zimmermann, K., Busserolles, J., Deval, E., Alloui, A., Diochot, S., et al. (2009). The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 28, 1308–1318. doi: 10.1038/emboj.2009.57
Noh, M. C., Mikler, B., Joy, T., and Smith, P. A. (2020). Time course of inflammation in dorsal root ganglia correlates with differential reversibility of mechanical allodynia. Neuroscience 428, 199–216. doi: 10.1016/j.neuroscience.2019.12.040
Noh, M. C., Stemkowski, P. L., and Smith, P. A. (2019). Long-term actions of interleukin-1beta on K(+), Na(+) and Ca(2+) channel currents in small, IB4-positive dorsal root ganglion neurons; possible relevance to the etiology of neuropathic pain. J. Neuroimmunol. 332, 198–211. doi: 10.1016/j.jneuroim.2019.05.002
Noh, S., Kumar, N., Bukhanova, N., Chen, Y., Stemkowsi, P. L., and Smith, P. A. (2014). The heart-rate-reducing agent, ivabradine, reduces mechanical allodynia in a rodent model of neuropathic pain. Eur. J. Pain 18, 1139–1147. doi: 10.1002/j.1532-2149.2014.00460.x
Ocana, M., Cendan, C. M., Cobos, E. J., Entrena, J. M., and Baeyens, J. M. (2004). Potassium channels and pain: present realities and future opportunities. Eur. J. Pharmacol. 500, 203–219. doi: 10.1016/j.ejphar.2004.07.026
Olechowski, C. J., Truong, J. J., and Kerr, B. J. (2009). Neuropathic pain behaviours in a chronic-relapsing model of experimental autoimmune encephalomyelitis (EAE). Pain 141, 156–164. doi: 10.1016/j.pain.2008.11.002
Olsen, M. L., Khakh, B. S., Skatchkov, S. N., Zhou, M., Lee, C. J., and Rouach, N. (2015). New insights on astrocyte ion channels: critical for homeostasis and neuron-glia signaling. J. Neurosci. 35, 13827–13835. doi: 10.1523/jneurosci.2603-15.2015
Ooi, L., and Wood, I. C. (2007). Chromatin crosstalk in development and disease: lessons from REST. Nat. Rev. Genet. 8, 544–554. doi: 10.1038/nrg2100
Osuma, A. T., Xu, X., Wang, Z., Van Camp, J. A., and Freiberg, G. M. (2019). Design and evaluation of pyrazolopyrimidines as KCNQ channel modulators. Bioorg. Med. Chem. Lett. 29:126603. doi: 10.1016/j.bmcl.2019.08.007
Park, S. Y., Choi, J. Y., Kim, R. U., Lee, Y. S., Cho, H. J., and Kim, D. S. (2003). Downregulation of voltage-gated potassium channel alpha gene expression by axotomy and neurotrophins in rat dorsal root ganglia. Mol. Cells 16, 256–259.
Passmore, G. M., Selyanko, A. A., Mistry, M., Al-Qatari, M., Marsh, S. J., Matthews, E. A., et al. (2003). KCNQ/M currents in sensory neurons: significance for pain therapy. J. Neurosci. 23, 7227–7236. doi: 10.1523/jneurosci.23-18-07227.2003
Peiro, A. M., Planelles, B., Juhasz, G., Bagdy, G., Libert, F., Eschalier, A., et al. (2016). Pharmacogenomics in pain treatment. Drug Metab. Pers. Ther. 31, 131–142.
Peirs, C., and Seal, R. P. (2016). Neural circuits for pain: recent advances and current views. Science 354, 578–584. doi: 10.1126/science.aaf8933
Peirs, C., Williams, S. P., Zhao, X., Walsh, C. E., Gedeon, J. Y., Cagle, N. E., et al. (2015). Dorsal horn circuits for persistent mechanical pain. Neuron 87, 797–812. doi: 10.1016/j.neuron.2015.07.029
Pezet, S., and McMahon, S. B. (2006). Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 29, 507–538. doi: 10.1146/annurev.neuro.29.051605.112929
Phuket, T. R., and Covarrubias, M. (2009). Kv4 channels underlie the subthreshold-operating A-type K-current in nociceptive dorsal root ganglion neurons. Front. Mol. Neurosci. 2:3. doi: 10.3389/neuro.02.003.2009
Pinto, L. G., Souza, G. R., Kusuda, R., Lopes, A. H., Sant’Anna, M. B., Cunha, F. Q., et al. (2019). Non-peptidergic nociceptive neurons are essential for mechanical inflammatory hypersensitivity in mice. Mol. Neurobiol. 56, 5715–5728. doi: 10.1007/s12035-019-1494-5
Pitcher, G. M., and Henry, J. L. (2008). Governing role of primary afferent drive in increased excitation of spinal nociceptive neurons in a model of sciatic neuropathy. Exp. Neurol. 214, 219–228. doi: 10.1016/j.expneurol.2008.08.003
Pollema-Mays, S. L., Centeno, M. V., Ashford, C. J., Apkarian, A. V., and Martina, M. (2013). Expression of background potassium channels in rat DRG is cell-specific and down-regulated in a neuropathic pain model. Mol. Cell. Neurosci. 57, 1–9. doi: 10.1016/j.mcn.2013.08.002
Pongs, O., and Schwarz, J. R. (2010). Ancillary subunits associated with voltage-dependent K+ channels. Physiol. Rev. 90, 755–796. doi: 10.1152/physrev.00020.2009
Poulter, M. O., Hashiguchi, T., and Padjen, A. L. (1995). Evidence for a sodium-dependent potassium conductance in frog myelinated axon. Neuroscience 68, 487–495. doi: 10.1016/0306-4522(95)00138-9
Ramer, M. S., French, G. D., and Bisby, M. A. (1997). Wallerian degeneration is required for both neuropathic pain and sympathetic sprouting into the DRG. Pain 72, 71–78. doi: 10.1016/s0304-3959(97)00019-5
Rasband, M. N., Park, E. W., Vanderah, T. W., Lai, J., Porreca, F., and Trimmer, J. S. (2001). Distinct potassium channels on pain-sensing neurons. Proc. Natl. Acad. Sci. U.S.A. 98, 13373–13378. doi: 10.1073/pnas.231376298
Rau, K. K., Cooper, B. Y., and Johnson, R. D. (2006). Expression of TWIK-related acid sensitive K+ channels in capsaicin sensitive and insensitive cells of rat dorsal root ganglia. Neuroscience 141, 955–963. doi: 10.1016/j.neuroscience.2006.04.019
Reeh, P. W. (1988). Sensory receptors in a mammalian skin-nerve in vitro preparation. Prog. Brain Res. 74, 271–276. doi: 10.1016/s0079-6123(08)63024-1
Ren, K., and Torres, R. (2009). Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 60, 57–64. doi: 10.1016/j.brainresrev.2008.12.020
Rifbjerg-Madsen, S., Christensen, A. W., Christensen, R., Hetland, M. L., Bliddal, H., Kristensen, L. E., et al. (2017). Pain and pain mechanisms in patients with inflammatory arthritis: a Danish nationwide cross-sectional DANBIO registry survey. PLoS One 12:e0180014. doi: 10.1371/journal.pone.0180014
Ritter, D. M., Ho, C., O’Leary, M. E., and Covarrubias, M. (2012). Modulation of Kv3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion neurons. J. Physiol. 590, 145–161. doi: 10.1113/jphysiol.2011.218560
Ritter, D. M., Zemel, B. M., Hala, T. J., O’Leary, M. E., Lepore, A. C., and Covarrubias, M. (2015a). Dysregulation of Kv3.4 channels in dorsal root ganglia following spinal cord injury. J. Neurosci. 35, 1260–1273. doi: 10.1523/jneurosci.1594-14.2015
Ritter, D. M., Zemel, B. M., Lepore, A. C., and Covarrubias, M. (2015b). Kv3.4 channel function and dysfunction in nociceptors. Channels 9, 209–217. doi: 10.1080/19336950.2015.1056949
Rivera-Arconada, I., Roza, C., and Lopez-Garcia, J. A. (2009). Enhancing m currents: A way out for neuropathic pain? Front. Mol. Neurosci. 2:10. doi: 10.3389/neuro.02.010.2009
Ro, L. S., Chen, S. T., Tang, L. M., and Jacobs, J. M. (1999). Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain 79, 265–274. doi: 10.1016/s0304-3959(98)00164-x
Robitaille, R., Garcia, M. L., Kaczorowski, G. J., and Charlton, M. P. (1993). Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron 11, 645–655. doi: 10.1016/0896-6273(93)90076-4
Rose, K., Ooi, L., Dalle, C., Robertson, B., Wood, I. C., and Gamper, N. (2011). Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain 152, 742–754. doi: 10.1016/j.pain.2010.12.028
Roy, S., Morayo, A. A., Large, R. J., Webb, T. I., Camarasu, C., Sergeant, G. P., et al. (2012). Structure-activity relationships of a novel group of large-conductance Ca(2+)-activated K(+) (BK) channel modulators: the GoSlo-SR family. ChemMedChem 7, 1763–1769. doi: 10.1002/cmdc.201200321
Safronov, B. V., Bischoff, U., and Vogel, W. (1996). Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurones of rat. J. Physiol. 493(Pt 2), 393–408. doi: 10.1113/jphysiol.1996.sp021391
Sakai, A., Saitow, F., Maruyama, M., Miyake, N., Miyake, K., Shimada, T., et al. (2017). MicroRNA cluster miR-17-92 regulates multiple functionally related voltage-gated potassium channels in chronic neuropathic pain. Nat. Commun. 8:16079.
Sakai, A., Saitow, F., Miyake, N., Miyake, K., Shimada, T., and Suzuki, H. (2013). miR-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability. Brain 136, 2738–2750. doi: 10.1093/brain/awt191
Samad, O. A., Tan, A. M., Cheng, X., Foster, E., Dib-Hajj, S. D., and Waxman, S. G. (2013). Virus-mediated shRNA knockdown of Na(v)1.3 in rat dorsal root ganglion attenuates nerve injury-induced neuropathic pain. Mol. Ther. 21, 49–56. doi: 10.1038/mt.2012.169
Sandkuhler, J. (2009). Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 89, 707–758. doi: 10.1152/physrev.00025.2008
Sarantopoulos, C. D., McCallum, J. B., Rigaud, M., Fuchs, A., Kwok, W. M., and Hogan, Q. H. (2007). Opposing effects of spinal nerve ligation on calcium-activated potassium currents in axotomized and adjacent mammalian primary afferent neurons. Brain Res. 1132, 84–99. doi: 10.1016/j.brainres.2006.11.055
Schmiedl, S., Peters, D., Schmalz, O., Mielke, A., Rossmanith, T., Diop, S., et al. (2019). Loxapine for treatment of patients with refractory, chemotherapy-induced neuropathic pain: a prematurely terminated pilot study showing efficacy but limited tolerability. Front. Pharmacol. 10:838. doi: 10.3389/fphar.2019.00838
Schoffnegger, D., Ruscheweyh, R., and Sandkuhler, J. (2008). Spread of excitation across modality borders in spinal dorsal horn of neuropathic rats. Pain 135, 300–310. doi: 10.1016/j.pain.2007.12.016
Scholz, A., Gruss, M., and Vogel, W. (1998). Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J. Physiol. 513(Pt 1), 55–69. doi: 10.1111/j.1469-7793.1998.055by.x
Scholz, J., and Woolf, C. J. (2007). The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 10, 1361–1368. doi: 10.1038/nn1992
Schutze, S., Orozco, I. J., and Jentsch, T. J. (2016). KCNQ potassium channels modulate sensitivity of skin down-hair (D-hair) Mechanoreceptors. J. Biol. Chem. 291, 5566–5575. doi: 10.1074/jbc.m115.681098
Scroggs, R. S., Todorovic, S. M., Anderson, E. G., and Fox, A. P. (1994). Variation in IH, IIR, and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. J. Neurophysiol. 71, 271–279. doi: 10.1152/jn.1994.71.1.271
Sculptoreanu, A., Yoshimura, N., and de Groat, W. C. (2004). KW-7158 [(2S)-(+)-3,3,3-trifluoro-2-hydroxy-2-methyl-N-(5,5,10-trioxo-4,10-dihydrothieno[3,2-c][1]benzothiepin-9-yl)propanamide] enhances A-type K+ currents in neurons of the dorsal root ganglion of the adult rat. J. Pharmacol. Exp. Ther. 310, 159–168. doi: 10.1124/jpet.104.065409
Seltzer, Z., Dubner, R., and Shir, Y. (1990). A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43, 205–218. doi: 10.1016/0304-3959(90)91074-s
Selyanko, A. A., Smith, P. A., and Zidichouski, J. A. (1990). Effects of muscarine and adrenaline on neurones from Rana pipiens sympathetic ganglia. J. Physiol. 425, 471–500. doi: 10.1113/jphysiol.1990.sp018114
Sexton, J. E., Cox, J. J., Zhao, J., and Wood, J. N. (2017). The genetics of pain: implications for therapeutics. Annu. Rev. Pharmacol. Toxicol. 58, 123–142. doi: 10.1146/annurev-pharmtox-010617-052554
Shapiro, M. S., Roche, J. P., Kaftan, E. J., Cruzblanca, H., Mackie, K., and Hille, B. (2000). Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K(+) channels that underlie the neuronal M current. J. Neurosci. 20, 1710–1721. doi: 10.1523/jneurosci.20-05-01710.2000
Shi, S., Li, J., Sun, F., Chen, Y., Pang, C., Geng, Y., et al. (2020). Molecular mechanisms and structural basis of retigabine analogues in regulating KCNQ2 channel. J. Membr. Biol. 253, 167–181. doi: 10.1007/s00232-020-00113-6
Shillito, P., Molenaar, P. C., Vincent, A., Leys, K., Zheng, W., van den Berg, R. J., et al. (1995). Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann. Neurol. 38, 714–722. doi: 10.1002/ana.410380505
Shinoda, M., Fukuoka, T., Takeda, M., Iwata, K., and Noguchi, K. (2019). Spinal glial cell line-derived neurotrophic factor infusion reverses reduction of Kv4.1-mediated A-type potassium currents of injured myelinated primary afferent neurons in a neuropathic pain model. Mol. Pain 15:1744806919841196.
Smith, P. A. (2014). BDNF: No gain without pain? Neuroscience 283, 107–123. doi: 10.1016/j.neuroscience.2014.05.044
Smith, T., Al, O. M., Sathish, J., and Djouhri, L. (2015). Increased expression of HCN2 channel protein in L4 dorsal root ganglion neurons following axotomy of L5- and inflammation of L4-spinal nerves in rats. Neuroscience 295, 90–102. doi: 10.1016/j.neuroscience.2015.03.041
Snider, W. D., and McMahon, S. B. (1998). Tackling pain at the source: new ideas about nociceptors. Neuron 20, 629–632. doi: 10.1016/s0896-6273(00)81003-x
Sommer, C., and Kress, M. (2004). Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 361, 184–187. doi: 10.1016/j.neulet.2003.12.007
Sommer, C., Petrausch, S., Lindenlaub, T., and Toyka, K. V. (1999). Neutralizing antibodies to interleukin 1-receptor reduce pain associated behavior in mice with experimental neuropathy. Neurosci. Lett. 270, 25–28. doi: 10.1016/s0304-3940(99)00450-4
Sorge, R. E., Mapplebeck, J. C., Rosen, S., Beggs, S., Taves, S., Alexander, J. K., et al. (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18, 1081–1083. doi: 10.1038/nn.4053
Sorkin, L. S., Xiao, W. H., Wagner, R., and Myers, R. R. (1997). Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 81, 255–262. doi: 10.1016/s0306-4522(97)00147-4
Sotocinal, S. G., Sorge, R. E., Zaloum, A., Tuttle, A. H., Martin, L. J., Wieskopf, J. S., et al. (2011). The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 7:55.
Stebbing, M. J., Cottee, J. M., and Rana, I. (2015). The Role of Ion Channels in Microglial Activation and Proliferation - A Complex Interplay between Ligand-Gated Ion Channels, K(+) Channels, and Intracellular Ca(2+.). Front. Immunol. 6:497. doi: 10.3389/fimmu.2015.00497
Stebbing, M. J., Eschenfelder, S., Habler, H. J., Acosta, M. C., Janig, W., and McLachlan, E. M. (1999). Changes in the action potential in sensory neurones after peripheral axotomy in vivo. Neuroreport 10, 201–206. doi: 10.1097/00001756-199902050-00001
Stemkowski, P. L., Noh, M. C., Chen, Y., and Smith, P. A. (2015). Increased excitability of medium-sized dorsal root ganglion neurons by prolonged interleukin-1beta exposure is K(+) channel dependent and reversible. J. Physiol. 593, 3739–3755. doi: 10.1113/jp270905
Stemkowski, P. L., and Smith, P. A. (2012a). Long-term IL-1beta exposure causes subpopulation-dependent alterations in rat dorsal root ganglion neuron excitability. J. Neurophysiol. 107, 1586–1597. doi: 10.1152/jn.00587.2011
Stemkowski, P. L., and Smith, P. A. (2012b). Sensory neurons, ion channels, inflammation and the onset of neuropathic pain. Can. J. Neurol. Sci. 39, 416–435. doi: 10.1017/s0317167100013937
Stemkowski, P. L., and Smith, P. A. (2013). “An overview of animal models of neuropathic pain,” in Neuropathic Pain, Causes, Management and Understanding, eds C. Toth and D. E. Moulin (Cambridge: Cambridge University Press), 33–50. doi: 10.1017/cbo9781139152211.005
Stotzner, P., Spahn, V., Celik, M. O., Labuz, D., and Machelska, H. (2018). Mu-opioid receptor agonist induces Kir3 currents in mouse peripheral sensory neurons - effects of nerve injury. Front. Pharmacol. 9:1478. doi: 10.3389/fphar.2018.01478
Stucky, C. L., and Lewin, G. R. (1999). Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J. Neurosci. 19, 6497–6505. doi: 10.1523/jneurosci.19-15-06497.1999
Study, R. E., and Kral, M. G. (1996). Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain 65, 235–242. doi: 10.1016/0304-3959(95)00216-2
Suh, B. C., Horowitz, L. F., Hirdes, W., Mackie, K., and Hille, B. (2004). Regulation of KCNQ2/KCNQ3 Current by G Protein cycling: the kinetics of receptor-mediated signaling by Gq. J. Gen. Physiol. 123, 663–683. doi: 10.1085/jgp.200409029
Sun, L., Gu, X., Pan, Z., Guo, X., Liu, J., Atianjoh, F. E., et al. (2019). Contribution of DNMT1 to neuropathic pain genesis partially through epigenetically repressing Kcna2 in primary afferent neurons. J. Neurosci. 39, 6595–6607. doi: 10.1523/jneurosci.0695-19.2019
Sun, L., Zhao, J. Y., Gu, X., Liang, L., Wu, S., Mo, K., et al. (2017). Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons. Pain 158, 1153–1165. doi: 10.1097/j.pain.0000000000000894
Sundt, D., Gamper, N., and Jaffe, D. B. (2015). Spike propagation through the dorsal root ganglia in an unmyelinated sensory neuron: a modeling study. J. Neurophysiol. 114, 3140–3153. doi: 10.1152/jn.00226.2015
Surur, A. S., Beirow, K., Bock, C., Schulig, L., Kindermann, M. K., Bodtke, A., et al. (2019). Flupirtine analogues: explorative synthesis and influence of chemical structure on KV7.2/KV7.3 channel opening activity. ChemistryOpen 8, 41–44. doi: 10.1002/open.201800244
Syriatowicz, J. P., Hu, D., Walker, J. S., and Tracey, D. J. (1999). Hyperalgesia due to nerve injury: role of prostaglandins. Neuroscience 94, 587–594. doi: 10.1016/s0306-4522(99)00365-6
Takeda, M., Kitagawa, J., Takahashi, M., and Matsumoto, S. (2008). Activation of interleukin-1beta receptor suppresses the voltage-gated potassium currents in the small-diameter trigeminal ganglion neurons following peripheral inflammation. Pain 139, 594–602. doi: 10.1016/j.pain.2008.06.015
Takeda, M., Tsuboi, Y., Kitagawa, J., Nakagawa, K., Iwata, K., and Matsumoto, S. (2011). Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol. Pain 7:5.
Talley, E. M., Solorzano, G., Lei, Q., Kim, D., and Bayliss, D. A. (2001). Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J. Neurosci. 21, 7491–7505. doi: 10.1523/jneurosci.21-19-07491.2001
Tashima, R., Koga, K., Sekine, M., Kanehisa, K., Kohro, Y., Tominaga, K., et al. (2018). Optogenetic activation of non-nociceptive abeta fibers induces neuropathic pain-like sensory and emotional behaviors after nerve injury in rats. eNeuro 5:ENEURO.0450-17.2018.
Taskin, B., von Schoubye, N. L., Sheykhzade, M., Bastlund, J. F., Grunnet, M., and Jespersen, T. (2015). Biophysical characterization of KV3.1 potassium channel activating compounds. Eur. J. Pharmacol. 758, 164–170. doi: 10.1016/j.ejphar.2015.03.061
Theodosiou, M., Rush, R. A., Zhou, X. F., Hu, D., Walker, J. S., and Tracey, D. J. (1999). Hyperalgesia due to nerve damage: role of nerve growth factor. Pain 81, 245–255. doi: 10.1016/s0304-3959(99)00018-4
Topham, L., Gregoire, S., Kang, H., Salmon-Divon, M., Lax, E., Millecamps, M., et al. (2020). The transition from acute to chronic pain: dynamic epigenetic reprogramming of the mouse prefrontal cortex up to one year following nerve injury. Pain. doi: 10.1097/j.pain.0000000000001917 [Epub ahead of print].
Treede, R.-D., Jensen, T. S., Campbell, J. N., Cruccu, G., Dostrovsky, J. O., Griffin, J. W., et al. (2008). Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70, 1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59
Tsantoulas, C., Denk, F., Signore, M., Nassar, M. A., Futai, K., and McMahon, S. B. (2018). Mice lacking Kcns1 in peripheral neurons show increased basal and neuropathic pain sensitivity. Pain 159, 1641–1651. doi: 10.1097/j.pain.0000000000001255
Tsantoulas, C., Lainez, S., Wong, S., Mehta, I., Vilar, B., and McNaughton, P. A. (2017). Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy. Sci. Transl. Med. 9:eaam6072. doi: 10.1126/scitranslmed.aam6072
Tsantoulas, C., and McMahon, S. B. (2014). Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci. 37, 146–158. doi: 10.1016/j.tins.2013.12.002
Tsantoulas, C., Mooney, E. R., and McNaughton, P. A. (2016). HCN2 ion channels: basic science opens up possibilities for therapeutic intervention in neuropathic pain. Biochem. J. 473, 2717–2736. doi: 10.1042/bcj20160287
Tsantoulas, C., Zhu, L., Shaifta, Y., Grist, J., Ward, J. P. T., Raouf, R., et al. (2012). Sensory neuron downregulation of the Kv9.1 potassium channel subunit mediates neuropathic pain following nerve injury. J. Neurosci. 32, 17502–17513. doi: 10.1523/jneurosci.3561-12.2012
Tsantoulas, C., Zhu, L., Yip, P., Grist, J., Michael, G. J., and McMahon, S. B. (2014). Kv2 dysfunction after peripheral axotomy enhances sensory neuron responsiveness to sustained input. Exp. Neurol. 251, 115–126. doi: 10.1016/j.expneurol.2013.11.011
Tulleuda, A., Cokic, B., Callejo, G., Saiani, B., Serra, J., and Gasull, X. (2011). TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol. Pain 7:30.
Turner, P. V., Pang, D. S., and Lofgren, J. L. (2019). A review of pain assessment methods in laboratory rodents. Comp. Med. 69, 451–467. doi: 10.30802/aalas-cm-19-000042
Uchida, H., Ma, L., and Ueda, H. (2010a). Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J. Neurosci. 30, 4806–4814. doi: 10.1523/jneurosci.5541-09.2010
Uchida, H., Sasaki, K., Ma, L., and Ueda, H. (2010b). Neuron-restrictive silencer factor causes epigenetic silencing of Kv4.3 gene after peripheral nerve injury. Neuroscience 166, 1–4. doi: 10.1016/j.neuroscience.2009.12.021
Vaso, A., Adahan, H. M., Gjika, A., Zahaj, S., Zhurda, T., Vyshka, G., et al. (2014). Peripheral nervous system origin of phantom limb pain. Pain 155, 1384–1391. doi: 10.1016/j.pain.2014.04.018
Vergoni, A. V., Scarano, A., and Bertolini, A. (1992). Pinacidil potentiates morphine analgesia. Life Sci. 50, L135–L138.
Vikman, K. S., Hill, R. H., Backstrom, E., Robertson, B., and Kristensson, K. (2003). Interferon-gamma induces characteristics of central sensitization in spinal dorsal horn neurons in vitro. Pain 106, 241–251. doi: 10.1016/s0304-3959(03)00262-8
von Hehn, C. A., Baron, R., and Woolf, C. J. (2012). Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73, 638–652. doi: 10.1016/j.neuron.2012.02.008
Vydyanathan, A., Wu, Z. Z., Chen, S. R., and Pan, H. L. (2005). A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J. Neurophysiol. 93, 3401–3409. doi: 10.1152/jn.01267.2004
Wagner, R., DeLeo, J. A., Coombs, D. W., and Myers, R. R. (1995). Gender differences in autotomy following sciatic cryoneurolysis in the rat. Physiol. Behav. 58, 37–41. doi: 10.1016/0031-9384(95)00037-j
Wagner, R., and Myers, R. R. (1996a). Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport 7, 2897–2901. doi: 10.1097/00001756-199611250-00018
Wagner, R., and Myers, R. R. (1996b). Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience 73, 625–629. doi: 10.1016/0306-4522(96)00127-3
Wall, P. D., and Devor, M. (1983). Sensory afferent impulses result from dorsal root ganglia as well as from the periphery in normal and nerve-injured rats. Pain 17, 321–339. doi: 10.1016/0304-3959(83)90164-1
Wall, P. D., Devor, M., Inbal, R., Scadding, J. W., Schonfeld, D., Seltzer, Z., et al. (1979). Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain 7, 103–113. doi: 10.1016/0304-3959(79)90002-2
Wall, P. D., and Gutnick, M. (1974). Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp. Neurol. 45, 580–593. doi: 10.1016/0014-4886(74)90197-6
Wang, X., Shen, X., Ma, S., Liu, Y., Xu, S., Wu, S., et al. (2017). Threshold Effect of G9a/Glp on Peripheral Nerve Injury Induced Hypersensitivity. Mol. Pain 13:1744806917729305.
Watkins, L. R., and Maier, S. F. (2002). Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 82, 981–1011. doi: 10.1152/physrev.00011.2002
Waxman, S. G. (2019). Peripheral afferents and the pain experience. Pain 160, 1487–1488. doi: 10.1097/j.pain.0000000000001527
Waxman, S. G., and Zamponi, G. W. (2014). Regulating excitability of peripheral afferents: emerging ion channel targets. Nat. Neurosci. 17, 153–163. doi: 10.1038/nn.3602
Wei, A. D., Gutman, G. A., Aldrich, R., Chandy, K. G., Grissmer, S., and Wulff, H. (2005). International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol. Rev. 57, 463–472. doi: 10.1124/pr.57.4.9
Weiser, M., Vega-Saenz de, M. E., Kentros, C., Moreno, H., Franzen, L., Hillman, D., et al. (1994). Differential expression of Shaw-related K+ channels in the rat central nervous system. J. Neurosci. 14, 949–972. doi: 10.1523/jneurosci.14-03-00949.1994
Welch, S. P., and Dunlow, L. D. (1993). Antinociceptive activity of intrathecally administered potassium channel openers and opioid agonists: a common mechanism of action? J. Pharmacol. Exp. Ther. 267, 390–399.
Willis, D. E., Wang, M., Brown, E., Fones, L., and Cave, J. W. (2016). Selective repression of gene expression in neuropathic pain by the neuron-restrictive silencing factor/repressor element-1 silencing transcription (NRSF/REST). Neurosci. Lett. 625, 20–25. doi: 10.1016/j.neulet.2015.12.003
Wolf, G., Gabay, E., Tal, M., Yirmiya, R., and Shavit, Y. (2006). Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain 120, 315–324. doi: 10.1016/j.pain.2005.11.011
Woolf, C. J. (1983). Evidence for a central component of post-injury pain hypersensitivity. Nature 306, 686–688. doi: 10.1038/306686a0
Wu, Q., Wei, G., Ji, F., Jia, S., Wu, S., Guo, X., et al. (2019). TET1 Overexpression Mitigates Neuropathic Pain Through Rescuing the Expression of ++-Opioid Receptor and Kv1.2 in the Primary Sensory Neurons. Neurotherapeutics 16, 491–504. doi: 10.1007/s13311-018-00689-x
Xie, Y. B., Zhao, H., Wang, Y., Song, K., Zhang, M., Meng, F. C., et al. (2016). Bilateral Neuropathy of Primary Sensory Neurons by the Chronic Compression of Multiple Unilateral DRGs. Neural Plast. 2016:2130901.
Xu, X. J., Hao, J. X., Andell-Jonsson, S., Poli, V., Bartfai, T., and Wiesenfeld-Hallin, Z. (1997). Nociceptive responses in interleukin-6-deficient mice to peripheral inflammation and peripheral nerve section. Cytokine 9, 1028–1033. doi: 10.1006/cyto.1997.0243
Yang, E. K., Takimoto, K., Hayashi, Y., de Groat, W. C., and Yoshimura, N. (2004). Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience 123, 867–874. doi: 10.1016/j.neuroscience.2003.11.014
Yang, J., Xie, M. X., Hu, L., Wang, X. F., Mai, J. Z., Li, Y. Y., et al. (2018). Upregulation of N-type calcium channels in the soma of uninjured dorsal root ganglion neurons contributes to neuropathic pain by increasing neuronal excitability following peripheral nerve injury. Brain Behav. Immun. 71, 52–65. doi: 10.1016/j.bbi.2018.04.016
Yang, J. W., Vacher, H., Park, K. S., Clark, E., and Trimmer, J. S. (2007). Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc. Natl. Acad. Sci. U.S.A. 104, 20055–20060. doi: 10.1073/pnas.0708574104
Yang, Q., Wu, Z., Hadden, J. K., Odem, M. A., Zuo, Y., Crook, R. J., et al. (2014). Persistent pain after spinal cord injury is maintained by primary afferent activity. J. Neurosci. 34, 10765–10769. doi: 10.1523/jneurosci.5316-13.2014
Yatziv, S. L., and Devor, M. (2019). Suppression of neuropathic pain by selective silencing of dorsal root ganglion ectopia using nonblocking concentrations of lidocaine. Pain 160, 2105–2114. doi: 10.1097/j.pain.0000000000001602
Yekkirala, A. S., Roberson, D. P., Bean, B. P., and Woolf, C. J. (2017). Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 16, 545–564. doi: 10.1038/nrd.2017.87
Young, G. T., Emery, E. C., Mooney, E. R., Tsantoulas, C., and McNaughton, P. A. (2014). Inflammatory and neuropathic pain are rapidly suppressed by peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels. Pain 155, 1708–1719. doi: 10.1016/j.pain.2014.05.021
Yousuf, M. S., Noh, M. C., Friedman, T. N., Zubkow, K., Johnson, J. C., Tenorio, G., et al. (2019). Sensory neurons of the dorsal root ganglia become hyperexcitable in a T-Cell-Mediated MOG-EAE Model of Multiple Sclerosis. eNeuro 6:ENEURO.0024-19.2019.
Yousuf, M. S., Samtleben, S., Lamothe, S. M., Friedman, T. N., Catuneanu, A., Thorburn, K., et al. (2020). Endoplasmic reticulum stress in the dorsal root ganglia regulates large-conductance potassium channels and contributes to pain in a model of multiple sclerosis. FASEB J. doi: 10.1096/fj.202001163R [Epub ahead of print].
Yuan, A., Santi, C. M., Wei, A., Wang, Z. W., Pollak, K., Nonet, M., et al. (2003). The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 37, 765–773. doi: 10.1016/s0896-6273(03)00096-5
Yuan, Q., Liu, X., Xian, Y. F., Yao, M., Zhang, X., Huang, P., et al. (2020). Satellite glia activation in dorsal root ganglion contributes to mechanical allodynia after selective motor fiber injury in adult rats. Biomed. Pharmacother. 127:110187. doi: 10.1016/j.biopha.2020.110187
Yunoki, T., Takimoto, K., Kita, K., Funahashi, Y., Takahashi, R., Matsuyoshi, H., et al. (2014). Differential contribution of Kv4-containing channels to A-type, voltage-gated potassium currents in somatic and visceral dorsal root ganglion neurons. J. Neurophysiol. 112, 2492–2504. doi: 10.1152/jn.00054.2014
Zakrzewska, J. M., Palmer, J., Morisset, V., Giblin, G. M., Obermann, M., Ettlin, D. A., et al. (2017). Safety and efficacy of a Nav1.7 selective sodium channel blocker in patients with trigeminal neuralgia: a double-blind, placebo-controlled, randomised withdrawal phase 2a trial. Lancet Neurol. 16, 291–300. doi: 10.1016/s1474-4422(17)30005-4
Zemel, B. M., Muqeem, T., Brown, E. V., Goulao, M., Urban, M. W., Tymanskyj, S. R., et al. (2017). Calcineurin dysregulation underlies spinal cord injury-induced K(+) Channel Dysfunction in DRG Neurons. J. Neurosci. 37, 8256–8272. doi: 10.1523/jneurosci.0434-17.2017
Zemel, B. M., Ritter, D. M., Covarrubias, M., and Muqeem, T. (2018). A-Type KV channels in dorsal root ganglion neurons: diversity, function, and dysfunction. Front. Mol. Neurosci. 11:253. doi: 10.3389/fnmol.2018.00253
Zhang, F. X., Gadotti, V. M., Souza, I. A., Chen, L., and Zamponi, G. W. (2018). BK potassium channels suppress Cavalpha2delta subunit function to reduce inflammatory and neuropathic pain. Cell Rep. 22, 1956–1964. doi: 10.1016/j.celrep.2018.01.073
Zhang, J., Rong, L., Shao, J., Zhang, Y., Liu, Y., Zhao, S., et al. (2020). Epigeneticrestoration of voltage-gated potassium Channel Kv1.2 Alleviates Nerve Injury-induced Neuropathic Pain. J. Neurochem. 3. doi: 10.1111/jnc.15117 [Epub ahead of print].
Zhang, X. L., Mok, L. P., Katz, E. J., and Gold, M. S. (2010). BKCa currents are enriched in a subpopulation of adult rat cutaneous nociceptive dorsal root ganglion neurons. Eur. J. Neurosci. 31, 450–462. doi: 10.1111/j.1460-9568.2009.07060.x
Zhang, X. L., Mok, L. P., Lee, K. Y., Charbonnet, M., and Gold, M. S. (2012). Inflammation-induced changes in BK(Ca) currents in cutaneous dorsal root ganglion neurons from the adult rat. Mol. Pain 8:37.
Zhang, Y. H., Kays, J., Hodgdon, K. E., Sacktor, T. C., and Nicol, G. D. (2012). Nerve growth factor enhances the excitability of rat sensory neurons through activation of the atypical protein kinase C isoform, PKMzeta. J. Neurophysiol. 107, 315–335. doi: 10.1152/jn.00030.2011
Zhao, J. Y., Liang, L., Gu, X., Li, Z., Wu, S., Sun, L., et al. (2017). DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat. Commun. 8:14712.
Zhao, X., Tang, Z., Zhang, H., Atianjoh, F. E., Zhao, J. Y., Liang, L., et al. (2013). A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 16, 1024–1031. doi: 10.1038/nn.3438
Zheng, Q., Fang, D., Liu, M., Cai, J., Wan, Y., Han, J. S., et al. (2013). Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain 154, 434–448. doi: 10.1016/j.pain.2012.12.005
Zheng, Y., Liu, P., Bai, L., Trimmer, J. S., Bean, B. P., and Ginty, D. D. (2019). Deep sequencing of somatosensory neurons reveals molecular determinants of intrinsic physiological properties. Neuron 103, 598–616. doi: 10.1016/j.neuron.2019.05.039
Zhou, X. F., Deng, Y. S., Xian, C. J., and Zhong, J. H. (2000). Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur. J. Neurosci. 12, 100–105. doi: 10.1046/j.1460-9568.2000.00884.x
Zhu, Y., Colak, T., Shenoy, M., Liu, L., Mehta, K., Pai, R., et al. (2012a). Transforming growth factor beta induces sensory neuronal hyperexcitability, and contributes to pancreatic pain and hyperalgesia in rats with chronic pancreatitis. Mol. Pain 8:65.
Zhu, Y., Mehta, K., Li, C., Xu, G. Y., Liu, L., Colak, T., et al. (2012b). Systemic administration of anti-NGF increases A-type potassium currents and decreases pancreatic nociceptor excitability in a rat model of chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G176–G181.
Zhuang, Z. Y., Wen, Y. R., Zhang, D. R., Borsello, T., Bonny, C., Strichartz, G. R., et al. (2006). A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 26, 3551–3560. doi: 10.1523/jneurosci.5290-05.2006
Zoga, V., Kawano, T., Liang, M. Y., Bienengraeber, M., Weihrauch, D., McCallum, B., et al. (2010). KATP channel subunits in rat dorsal root ganglia: alterations by painful axotomy. Mol. Pain 6:6.
Keywords: neuropathic pain, nerve injury, neuroimmunology, allodynia, hyperalgesia, dorsal root ganglion, primary afferent, electrophysiology
Citation: Smith PA (2020) K+ Channels in Primary Afferents and Their Role in Nerve Injury-Induced Pain. Front. Cell. Neurosci. 14:566418. doi: 10.3389/fncel.2020.566418
Received: 27 May 2020; Accepted: 21 August 2020;
Published: 17 September 2020.
Edited by:
David J. Adams, University of Wollongong, AustraliaReviewed by:
Manuel L. Covarrubias, Thomas Jefferson University, United StatesCopyright © 2020 Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter A. Smith, cGFzM0B1YWxiZXJ0YS5jYQ==; cGV0ZXIuYS5zbWl0aEB1YWxiZXJ0YS5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.