Corrigendum: VIP Modulation of Hippocampal Synaptic Plasticity: A Role for VIP Receptors as Therapeutic Targets in Cognitive Decline and Mesial Temporal Lobe Epilepsy
- 1BioISI - Biosystems and Integrative Sciences Institute, Faculdade de Ciências, Universidade de Lisboa, Lisbon, Portugal
- 2Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, Lisbon, Portugal
Vasoactive intestinal peptide (VIP) is an important modulatory peptide throughout the CNS acting as a neurotransmitter, neurotrophic or neuroprotective factor. In the hippocampus, a brain area implicated in learning and memory processes, VIP has a crucial role in the control of GABAergic transmission and pyramidal cell activity in response to specific network activity by either VIP-containing basket cells or interneuron-selective (IS) interneurons and this appears to have a differential impact in hippocampal-dependent cognition. At the cellular level, VIP regulates synaptic transmission by either promoting disinhibition, through activation of VPAC1 receptors, or enhancing pyramidal cell excitability, through activation of VPAC2 receptors. These actions also control several important synaptic plasticity phenomena such as long-term potentiation (LTP) and long-term depression (LTD). This paper reviews the current knowledge on the activation and multiple functions of VIP expressing cells in the hippocampus and their role in controlling synaptic transmission, synaptic plasticity and learning and memory processes, discussing also the role of VPAC1 and VPAC2 VIP receptors in the regulation of these different processes. Furthermore, we address the current knowledge regarding changes in VIP mediated neurotransmission in epileptogenesis and mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS), and discuss the therapeutic opportunities of using selective VIP receptor ligands to prevent epileptogenesis and cognitive decline in MTLE-HS.
Introduction
Vasoactive intestinal peptide (VIP), a 28 amino-acid residue peptide originally isolated from porcine duodenum by Mutt and Said (1974), owes its name to its powerful ability to cause vasodilatation (Said and Mutt, 1970), by promoting vascular smooth muscle relaxation in the gastrointestinal tract when released by peripheral nerves of the sympathetic nervous system (Said and Rosenberg, 1976). In subsequent years, VIP was described in multiple peripheral and central neuronal control systems, where it acts as neurotransmitter, neurotrophic or neuroprotective factor (Borbély et al., 2013; Deng and Jin, 2017). Discovery of pituitary adenylate cyclase-activating polypeptide (PACAP) in the ovine hypothalamus (Miyata et al., 1989), where it acts as an endocrine regulator, brought additional complexity to the understanding of the actions of VIP, since these two peptides share common receptors and are often present together in the same brain regions (see below). The actions of PACAP on synaptic transmission, plasticity and cognition are reviewed in another paper in this research topic (Ciranna and Costa, 2019) and will be discussed here only when clarifying the duality of VIP vs. PACAP signaling. VIP is nowadays recognized as an important modulator of synaptic transmission and plasticity, network excitability as well as of learning and memory processes and has been associated with cognitive deficits in several central nervous system (CNS) diseases. This paper reviews the multiple roles of VIP in synaptic transmission, synaptic plasticity and hippocampal-dependent learning and memory processes, the role of VIP in hippocampal and cognitive disfunction in mesial temporal lobe epilepsy (MTLE) and the therapeutic opportunities that this presents.
VIP in the Hippocampus
Upon its discovery, VIP expression was reported in the human hippocampus and the hippocampus of animal models (Emson et al., 1979; Lorén et al., 1979; Besson et al., 1984), where VIP was also shown to bind to hippocampal membranes (Taylor and Pert, 1979; Besson et al., 1984). Shortly after, it became evident that VIP expression was predominant in hippocampal GABAergic interneurons (Köhler, 1982, 1983; Léránth et al., 1984; Kosaka et al., 1985) and that modulation of GABAergic transmission was likely an important target for VIP action. VIP was also early recognized to have a crucial role in mnemonic processes and particularly in hippocampal-dependent memory traits (Cottrell et al., 1984; Flood et al., 1990; Glowa et al., 1992). Nevertheless, the first report of its physiological actions in the CNS described VIP excitation of hippocampal CA1 neurones (Dodd et al., 1979). This enhancement in pyramidal cell excitability was latter shown to occur essentially through reduction of the Ca2+- and cAMP-dependent K+-conductance, leading to a decrease of the long-lasting afterhyperpolarization (sAHP) and a reduction of the accommodation of firing (Haas and Gähwiler, 1992). This action was postsynaptic since it prevailed in low Ca2+ – high Mg2+ medium and was later demonstrated to depend on protein kinase A (PKA) activity (Haug and Storm, 2000). Later, the actions of VIP on hippocampal GABAergic transmission were described showing that VIP increases the frequency of miniature IPSCs in cultured pyramidal neurones without affecting their amplitude (Wang et al., 1997), which suggests a presynaptic facilitation of GABA release by VIP. This appeared contradictory since VIP actions would lead to opposing effects on pyramidal cell excitability. All these findings are summarized in Table 1. When the anatomy of VIP-expressing interneurons (VIP+ INs) in the hippocampus was elucidated (Acsády et al., 1996a, b; Hájos et al., 1996) the different roles of VIP in modulation of hippocampal GABAergic transmission and regulation of pyramidal cell excitability began to be clarified.
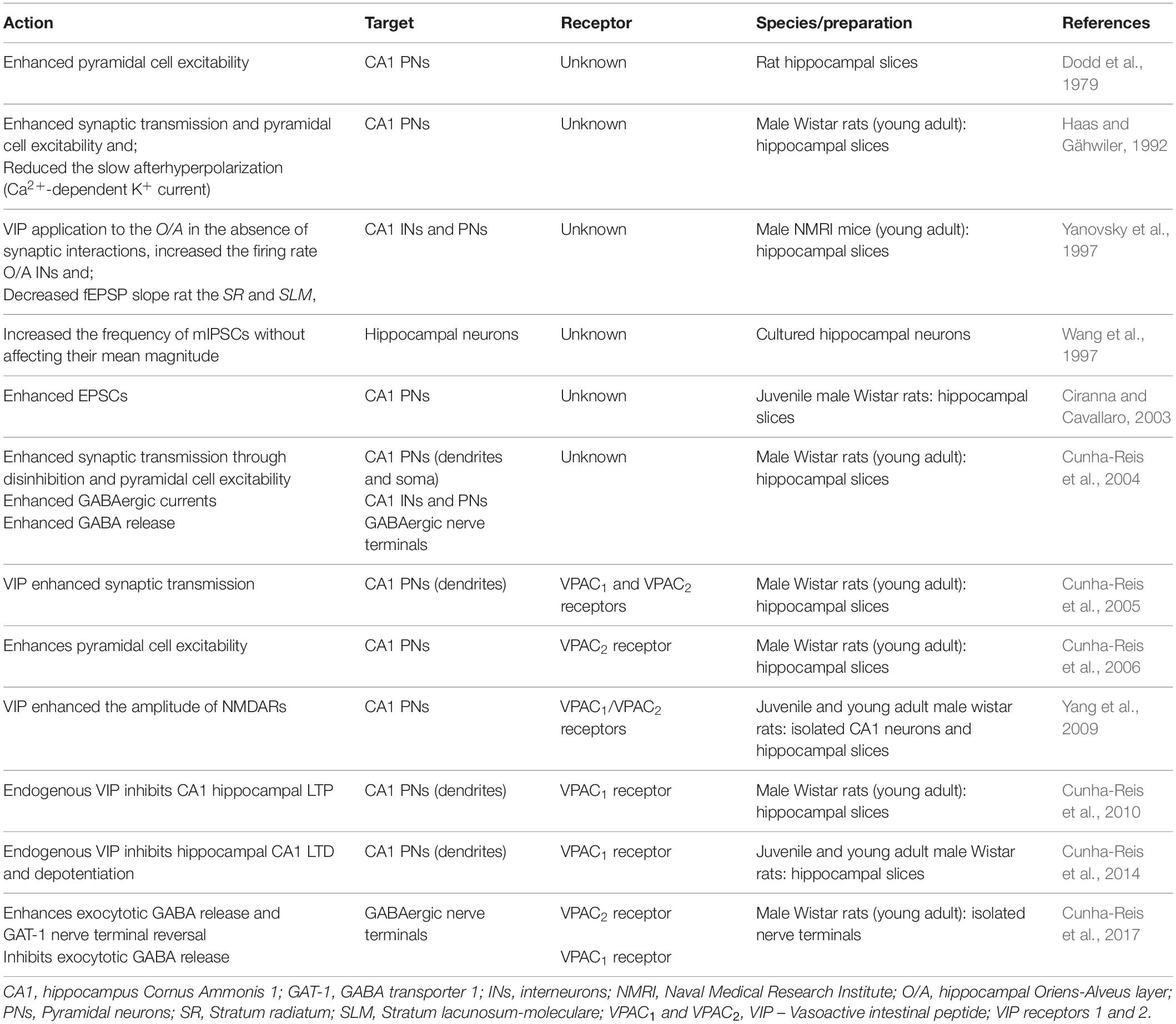
Table 1. Effects of VIP on hippocampal excitatory and inhibitory networks and VIP receptors involved.
Detailed immunohistochemistry studies fully characterized hippocampal VIP+ INs dendritic trees and axon projections (Acsády et al., 1996a, b), allowing the classification of VIP+ INs into two fundamental groups according to their targets: VIP+ basket cells are responsible for somatic inhibition of pyramidal cells, are also immunoreactive for cholecystokinin (VIP+-CCK+ BCs, Figure 1) and do not express parvalbumin, as most BCs in the hippocampus. VIP+ INs that selectively innervate other interneurons (VIP+ IS INs) include two subtypes: (a) interneurons with cell bodies located at the stratum pyramidale (SP) or near and projecting to the stratum Oriens/Alveus border (VIP+ IS O/A INs or type III IS cells, Figure 1), that also express the interneuron marker calretinin and target mostly somatostatin-expressing (SOM+) oriens lacunosum-moleculare (OLM) interneurons innervating the distal dendrites of pyramidal cells at the stratum lacunosum-moleculare (SLM) and (b) VIP+ INs that project their axons to the stratum radiatum (SR, VIP+ IS SR INs, Figure 1), with cell bodies located either at the SR/SLM border (type II IS cells) or at SR/SP and targeting interneurons controlling synaptic transmission to proximal dendrites of pyramidal cells in the SR (Acsády et al., 1996a, b; Klausberger and Somogyi, 2008). In genetically modified VIP-eGFP mice, additional targets of VIP+ IS O/A INs in the O/A, including bistratified cells and oriens–oriens INs, have been described and recently a new VIP expressing interneuron population located at the O/A (VIP+ long-range projecting INs, VIP+ LRP INs) was described targeting INs within the O/A in CA1 but also both INs and pyramidal cells within the subiculum (Francavilla et al., 2018). It is not clear if it is also present in the rat hippocampus.
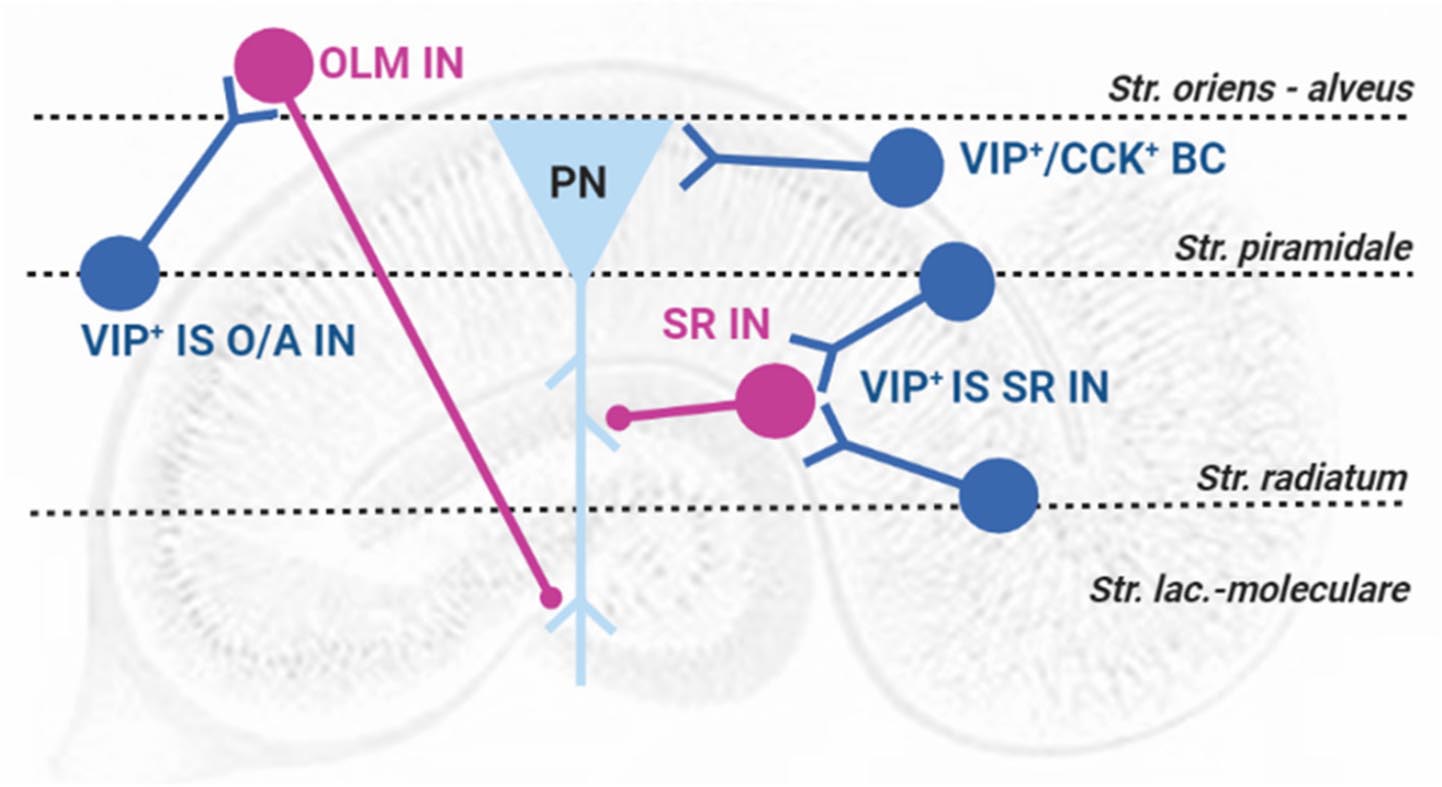
Figure 1. Representation of VIP-containing interneurons in the rat hippocampus: layer location and target selectivity. PN, pyramidal neuron (triangle, light blue); Interneurons (circles, pink); VIP-containing interneurons (circles, blue); VIP+-CCK+ BCs: VIP-containing basket cells; VIP+ IS O/A IN: VIP-containing interneuron-selective interneuron targeting the stratum oriens/Alveus and VIP+ IS SR IN: VIP-containing interneuron-selective interneuron targeting the stratum radiatum; OLM IN – Stratum oriens interneuron projecting to the Stratum lacunosum-moleculare; SR IN – Stratum radiatum local interneurons. Str.: stratum.
Considering the early acquired knowledge (Acsády et al., 1996a, b) on the target selectivity of VIP-IS hippocampal interneurons in the rat, Yanovsky et al. (1997) studied the influence of VIP application to the Oriens/Alveus border and showed that in the absence of synaptic interactions, VIP increased the firing rate of these interneurons and decreased the slope of the fEPSPs recorded at the SR and SLM, thus decreasing excitatory synaptic transmission through an increase in inhibitory transmission (Yanovsky et al., 1997). These mechanisms could not account for the previously observed increase in synaptic transmission and pyramidal cell firing (Haas and Gähwiler, 1992). In fact, VIP-mediated concomitant pre and post-synaptic enhancement of GABAergic transmission generating disinhibition of synaptic transmission to pyramidal cell dendrites (Cunha-Reis et al., 2004) appears to coexist with direct VIP mediated actions on pyramidal cell bodies either promoting enhancement glutamatergic EPSCs (Ciranna and Cavallaro, 2003) or GABAergic currents (Cunha-Reis et al., 2004) in rat hippocampal slices. The physiological relevance of these conflicting observations remains poorly understood but may be relevant in distinct physiological conditions, depending on network and behavioral state-dependent activation of different interneuron populations (Tyan et al., 2014; Artinian and Lacaille, 2018; Francavilla et al., 2018; Turi et al., 2019; Luo et al., 2020).
VIP and VIP Receptors
VIP shows structural similarity to other neuroendocrine peptides, including secretin, glucagon, gastric inhibitory peptide, growth hormone releasing factor and PACAP (Deng and Jin, 2017); and belongs glucagon–secretin–VIP family of peptides targets (Clynen et al., 2014). VIP acts trough two high affinity receptors (VPAC1 and VPAC2) that belong to Group II receptor (GPCR) family and are encoded by two different genes sharing only 55% similarity. These have nearly the same affinity for VIP (in the low nanomolar range) (Yang et al., 2010; Harmar et al., 2012) and bind also PACAP with similar affinity, hence the name VPAC given to VIP receptors (Laburthe et al., 2002). The VIP receptor subfamily also includes a third receptor, PAC1 (PACAP specific receptor), which binds VIP with low affinity (in the micromolar range) (Harmar et al., 2012). VPAC receptors exhibit multiple consensus sites for phosphorylation by intracellular kinases and N-glycosylation, but differences in the N-glycosylation are observed according to tissue and/or species (Laburthe et al., 2002).
Both VPAC and PAC1 receptors are positively coupled to Gαs and stimulate the cAMP/PKA signaling pathway (see Laburthe et al., 2002; Harmar et al., 2012 for review). However, PAC1 receptors additionally strongly stimulate Gαq and the phospholipase C (PLC)/PKC signaling pathway, while VPAC receptors activate it weakly (Harmar et al., 1998; Yang et al., 2010). However, VPAC1 receptors can couple to Gi/o proteins in the hippocampus (Shreeve, 2002) and VIP enhancement of [Ca2+]i in astrocyte cultures depends on IP3 turnover (Fatatis et al., 1994).
VIP Receptors and Hippocampal Neurotransmission
Although VIP and PACAP receptors have a widespread expression in the brain, VPAC1 receptors are predominantly found in the hippocampus and cerebral cortex, while VPAC2 receptors prevail in the thalamus and suprachiasmatic nucleus, showing lower expression in the hippocampus, spinal cord, dorsal root ganglia and brainstem (Harmar et al., 2012; Borbély et al., 2013). Not surprisingly, VIP and VIP receptors are involved in learning and memory processes (Yang et al., 2010; Borbély et al., 2013), yet, elucidating the differential involvement of each VIP receptor in the actions of VIP has proven very difficult until ligands with enough selectivity to discriminate between VPAC1 and the VPAC2 receptor were developed (Gourlet et al., 1997a,b,c; Moreno et al., 2000). This is particularly important in the hippocampus, where both receptors are expressed (Vertongen et al., 1997; Joo et al., 2004).
VIP receptors are unevenly distributed in different hippocampal layers. VPAC2 receptors are more expressed in SP of the Ammon’s Horn implying a key role in the modulation of hippocampal pyramidal cell activity, whereas VPAC1 receptors are preferentially located in the SO and SR and partially co-localized with glial markers (Acsády et al., 1996a; Joo et al., 2004). No study has to date identified VPAC1 receptors in hippocampal interneurons, yet the fact that VIP enhancement of synaptic transmission to CA1 pyramidal cells involves inhibition of GABAergic interneurons that control pyramidal cell dendrites, leading to disinhibition (Cunha-Reis et al., 2004), an action mediated by activation of VPAC1 receptors (Cunha-Reis et al., 2005) preferentially located in the SO, SR or O/A (Vertongen et al., 1997; Joo et al., 2004) suggests VPAC1 receptors are in fact responsible for VIP actions on hippocampal interneurons. VPAC2 receptors are the main mediators of VIP enhancement of pyramidal cell excitability (Cunha-Reis et al., 2006), and likely mediators of VIP enhancement of NMDA receptor currents in pyramidal cells (Yang et al., 2009), effects that are mostly post-synaptic and independent of GABAergic transmission (Ciranna and Cavallaro, 2003; Cunha-Reis et al., 2004), and that likely involve inhibition of the sAHP (Haas and Gähwiler, 1992) (see Table 1).
VIP modulation of hippocampal GABAergic transmission involves both presynaptic enhancement of GABA release and postsynaptic facilitation of GABAergic currents in interneurons (Wang et al., 1997; Cunha-Reis et al., 2004). We recently reported a dual opposing regulation of GABA release by VPAC receptors in isolated hippocampal nerve terminals (Cunha-Reis et al., 2017): VPAC1 receptors inhibit and VPAC2 receptors enhance GABA release. VPAC1 receptor activation inhibits voltage-gated calcium channel (VGCC)-dependent GABA exocytosis through a Gi/o and PKA-independent and partially PKC-dependent mechanism (Cunha-Reis et al., 2017). VPAC2 receptor activation enhances VGCC-dependent GABA exocytosis by a Gs/PKA/PKC-dependent mechanism but also enhances GAT-1 carrier-mediated GABA outflow through a Gs/PKC-dependent mechanism. Given the asymmetry in VPAC1 and VPAC2 receptor location in different layers of Ammon’s horn, VIP may differentially modulate GABA release to pyramidal cells and INs, and thus have distinct consequences on synaptic transmission to pyramidal cell dendrites and pyramidal cell activity, suggesting several possible therapeutic applications.
VIP and Synaptic Plasticity
Synaptic plasticity relies on long-lasting, activity-dependent bidirectional changes in the strength of synaptic communication leading to long-term potentiation (LTP) and long-term depression (LTD) (Mellor, 2018) and is widely accepted as the cellular mechanism underlying memory storage (Bliss and Collingridge, 2019). LTP can be triggered by a single episode of high frequency stimulations (HFS), such as a tetanus or theta burst (Albensi et al., 2007; Larson and Munkácsy, 2015; Bliss and Collingridge, 2019), mimicking the firing of hippocampal principal cells during learning tasks, and was the first synaptic plasticity mode to be associated with hippocampal-dependent memory formation (Bliss and Collingridge, 2019). LTD can be elicited by low-frequency stimulation (LFS), mimicking hippocampal activity during delta waves, and is involved in hippocampal-dependent memory processes associated with behavioral flexibility like memory extinction, reversal learning, reformulation of previously formed memories, terminating/shifting attention and in stabilizing the effects of learning (Kitchigina et al., 1999; Albensi et al., 2007; Kemp and Manahan-Vaughan, 2007; Collingridge et al., 2010). Both LTP and LTD require the activation of NMDA receptors, and their stability or long-lasting expression is dependent on subsequent activation of multiple intracellular cascades (Collingridge et al., 2010; Bliss and Collingridge, 2019). LTP of glutamatergic transmission requires activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and recruitment and insertion of AMPA receptors into the postsynaptic membrane (early-LTP) (Park et al., 2018; Benke and Traynelis, 2019). Endurance and stability of LTP is believed to require synaptic contact enlargement and both PKA activity and de novo protein synthesis (late-LTP) (Park et al., 2018; Bliss and Collingridge, 2019).
Recent evidence supports the view that disinhibition plays a crucial role in regulating hippocampal synaptic plasticity (Artinian and Lacaille, 2018). Furthermore, Yang et al. (2009) showed that exogenously applied VIP enhances NMDA currents in CA1 pyramidal cells, an effect mimicked by VPAC2 and to a lesser extent by VPAC1 selective agonists. This suggests that either endogenous VIP or PACAP, the two endogenous agonists of this receptor, could contribute to NMDA-dependent hippocampal synaptic plasticity such as LTP, LTD and depotentiation. We recently described that endogenous VIP, through VPAC1 receptor activation, modulates the NMDA receptor-dependent LTD and depotentiation in the CA1 area of the hippocampus (Cunha-Reis et al., 2014). Furthermore, disinhibition achieved through inhibition of VPAC1 receptors was more efficient than blockade of GABAA–mediated transmission in revealing LTD, suggesting that SR interneurons are fundamental in restraining synaptic adaptations underlying expression of LTD. VPAC1 receptor activation by endogenous VIP also enhances hippocampal LTP induced by TBS, an action that is dependent on GABAergic transmission and involves phosphorylation of GluA1 AMPA subunit by CamKII, a fundamental mechanism for receptor synaptic recruitment (Cunha-Reis et al., 2010; Carmo and Cunha-Reis, 2011; Cunha-Reis and Carmo, 2011) (see Table 1). Activation of hippocampal VPAC2 (but not VPAC1) receptors also promotes phosphorylation of GluA1 at Ser845 (Toda and Huganir, 2015), a PKA target site that is implicated in LTP maintenance and late-LTP (Benke and Traynelis, 2019).
VIP and PACAP modulation of hippocampal principal cell activity targets (directly or indirectly) both the dendritic and somatic compartments implicating these peptides in regulation of both Hebbian and homeostatic plasticity (Wefelmeyer et al., 2016; Yee et al., 2017; Foncelle et al., 2018), yet the physiological and behaviorally relevant stimuli for this modulation are still largely uncovered. Recently, it was described that VIP+ IS INs are activated by both Schaffer collateral and commissural excitatory fibers, being recruited fundamentally during theta oscillations but not during fast ripples (Luo et al., 2020), suggesting a fundamental role in information gating during spatial navigation and memory encoding. Accordingly, VIP+ IS INs are targeted by medium raphe serotonergic and GABAergic projections and septal cholinergic fibers, fundamental for the pacing, engagement and suppression of hippocampal theta rhythm (Vinogradova et al., 1999; Borhegyi et al., 2004; Vandecasteele et al., 2014).
Release of large dense core vesicles containing neuropeptides is known to require high-intensity repetitive stimulation, unlike release of small synaptic vesicles containing fast transmitters such as glutamate or GABA (Ghijsen and Leenders, 2005). Firing of VIP-containing interneurons locked with theta rhythm may suffice to release endogenous VIP from hippocampal nerve terminals.
VIP in Cognitive Processes
Early from its discovery, VIP was described to have a crucial role in mnemonic processes and particularly in hippocampal-dependent memory traits. In particular, endogenous VIP was implicated in spatial learning in the Morris water maze (Glowa et al., 1992; Takashima et al., 1993a; Itoh et al., 1994), avoidance learning in the T-maze (Flood et al., 1990) or the shuttle box, together with reduced rearing exploratory behavior (Cottrell et al., 1984; Takashima et al., 1993b), suggesting that VIP is mainly involved in regulating motivated learning behavior. VIP has lateralized effects on the modulation of exploratory behavior and passive avoidance learning (Ivanova et al., 2008, 2009) and anxiolytic and anti-depressive effects (Ivanova et al., 2014), and rescues deficits in hippocampal-dependent passive avoidance learning tasks in a rat model of depression. Recently, VIP-mediated hippocampal disinhibition of pyramidal cell activity was shown to play a crucial role in goal-directed spatial learning tasks (Turi et al., 2019).
VIP-KO mice show decreased expression of VPAC2 and to a lesser extent VPAC1 receptors together with strong circadian rhythm disruption and enhanced arousal and hyperactivity in the open-field test (Girard et al., 2006). Furthermore, VIP-deficient mice shows impaired recall and reversal learning in a fear conditioning test and deficits in social behavior (Chaudhury et al., 2008; Stack et al., 2008). VPAC2-KO mice display normal acquisition of fear conditioning, contextual and cued fear memory, but impaired extinction of cued fear memory (Ago et al., 2017).
VIP participates in the pathophysiology of several neurological disorders associated with cognitive disfunction, like depression (Ivanova et al., 2012), autism spectrum disorders, Alzheimer’s disease (AD), Parkinson’s disease (PD) and epilepsy (de Lanerolle et al., 1995; Hill, 2007; White et al., 2010). Due to its anti-apoptotic, anti-inflammatory and neuroprotective actions, VIP and its receptors constitute promising therapeutic targets in many of these pathologies (Gozes, 2001; Delgado et al., 2002; Yu et al., 2017).
VIP, Seizures, and Epilepsy
Epilepsy is the most common, chronic neurological disease (Devinsky et al., 2018) and is characterized by the incidence of recurrent, unprovoked seizures with associated cognitive, psychological and social disturbances (Clynen et al., 2014; Devinsky et al., 2018). According to its underlying causes epilepsy is classified into genetic or idiopathic. More than 500 genes are associated with predisposition to develop epilepsy (Devinsky et al., 2018). Idiopathic epilepsy, has unknown causes but often follows several possible precipitating events such as head trauma, stroke, brain hypoxia, infectious/autoimmune diseases, tumors or childhood febrile seizures (Clynen et al., 2014).
Mesial temporal-lobe epilepsy with hippocampal sclerosis (MTLE-HS), the most prevalent form of symptomatic focal epilepsy, is a heavy burden for the healthcare system. Many MTLE-HS patients are refractory to treatment with multiple anti-epileptic drugs, and amygdalohippocampectomy surgery is the last intervention to prevent complex partial seizures (Kuang et al., 2014). Declarative memory deficits (Helmstaedter and Kockelmann, 2006) are also a hallmark of MTLE-HS, that can be further aggravated by hippocampal removal. Most MTLE cases are idiopathic and evidence suggests that precipitating events trigger epileptogenesis by generating aberrant synaptic plasticity/neuronal excitability, excitotoxicity, secondary non-convulsive status epilepticus, inflammation and generation of reactive oxygen species (ROS) (Devinsky et al., 2018; Rana and Musto, 2018), ultimately leading to occurrence of spontaneous recurrent seizures. MTLE-HS is characterized by hippocampal sclerosis, massive neuronal loss and severe astrogliosis (Thom, 2014). Enhanced neurogenesis initially drives formation of new neural pathways in epileptogenesis (Beck and Yaari, 2008), but is impaired in MTLE-HS chronic phase (Zhong et al., 2016). Impaired LTP, due to pathological saturation (Beck et al., 2000), is a major cause for cognitive impairment in MTLE-HS, but changes in input/output neuronal electrical properties (El-Hassar et al., 2007a) and inhibitory/excitatory balance also occur from early in epileptogenesis (El-Hassar et al., 2007b). New drug targets able to control seizures or preventing epileptogenesis are an urgent need (Clynen et al., 2014).
Neuropeptides, such as VIP, are stored in large dense-core granules and are released during the sustained high-frequency activity (5–40 Hz) occurring during epileptiform activity, being implicated in regulation of seizure susceptibility, constituting appealing targets for the development of new AEDs, potentially less susceptible to side-effects (Clynen et al., 2014).
VIP is an important regulator of hippocampal activity through both direct actions on pyramidal cell excitability (Haas and Gähwiler, 1992) and by regulating synaptic transmission and synaptic plasticity to pyramidal cell dendrites through disinhibition (Cunha-Reis et al., 2004, 2010, 2014; Cunha-Reis and Carmo, 2011; Luo et al., 2020), actions that have a major impact on hippocampal-dependent learning and memory formation (Turi et al., 2019).
In human MTLE-HS, an up-regulation in VIP receptors in the seizure focus (hippocampus) was linked to the loss of principal neurons (i.e., granule cells and pyramidal neurons) without changes in the pattern and distribution of VIP+ INs (de Lanerolle et al., 1995). Accordingly, an enhancement in VIP+ INs has been described in a mouse model of temporal lobe epilepsy (TLE) (King and LaMotte, 1988) and decreased dendritic but not somatic GABAergic inhibition has been implicated in different animal models of experimental TLE (Sloviter, 1987; Cossart et al., 2001). Although an enhancement in disinhibition caused by VIP could be implicated in reduced seizure threshold in MTLE-HS, enhancement in VIP expression is more likely a compensatory mechanism for the selective loss of OLM interneurons in TLE, the main targets of VIP+ IS O/A INs. Recently, it was described that while the overall density of the VIP+ IS O/A INs was preserved, the number of their synaptic contacts in CA1 O/A was reduced in the pilocarpine model of TLE and was accompanied by significant alterations in their dendritic morphology and passive membrane properties (David and Topolnik, 2017).
Following kainic acid and pentylenetetrazole-induced seizures in rodents, an early short-term decrease in hippocampal VIP levels following the initial (precipitating) seizures was described (Marksteiner et al., 1989; Romualdi et al., 1992), suggesting that transient changes in VIP expression either contribute or counteract selective interneuron loss and plasticity changes during latent-period epileptogenesis. Preliminary studies in vitro show that changes in synaptic plasticity and synaptic plasticity markers following brief insults like hypoxia, bicuculine-induced seizures or inter-ictal like activity are either prevented or enhanced by a VPAC1 receptor antagonist, suggesting that different epileptogenic events are differentially regulated by VPAC1 receptor activity (Cunha-Reis, 2013; Carvalho-Rosa and Cunha-Reis, 2019).
In MTLE patients, the up-regulation of VIP receptors observed chronically is consistent with an increase in surviving neurons and levels of reactive glia (de Lanerolle et al., 1995; Clynen et al., 2014), suggesting that VPAC receptors (especially VPAC1) are promising targets for preventing epileptogenesis, a process that extends beyond the initial latent period (Devinsky et al., 2018). Given their role in the control of hippocampal synaptic plasticity they constitute also excellent candidates for prevention or attenuation of cognitive decline in MTLE. Furthermore, the dual role of VPAC1 and VPAC2 receptors in the control of hippocampal GABA release makes them the perfect targets for development of drugs aiming to control the imbalance in GABAergic and glutamatergic transmission associated with TLE (Schousboe et al., 2014; Cunha-Reis et al., 2017).
Concluding Remarks
In conclusion, the importance of VIP, acting through VPAC1 or VPAC2 Rs, either to the control of hippocampal disinhibition leading to enhanced synaptic transmission or promoting a direct enhancement of pyramidal cell excitability suggests that VIP can have a differential impact in hippocampal-dependent cognition, and its possible therapeutic applications should be explored. The up-regulation of VIP receptors observed in MTLE patients and the finding obtained in animal models that the interneuron targets of VIP-containing interneurons are particularly susceptible to epileptic damage, suggest that VPAC receptors (especially VPAC1) are promising targets for epileptogenesis prevention and for prevention or attenuation of cognitive decline in MTLE.
Author Contributions
AC-R: writing – review and editing. DC-R: resources, supervision, funding acquisition, project administration, and writing – original draft, review and editing.
Funding
This work was supported national and international funding managed by the Fundação para a Ciência e a Tecnologia (FCT, IP), Portugal. Grants: UIDB/04046/2020 and UIDP/04046/2020 centre grants (to BioISI) and research grant FCT/POCTI (PTDC/SAUPUB/28311/2017) EPIRaft grant (to DC-R). Researcher contract: Norma Transitória – DL57/2016/CP1479/CT0044 to DC-R.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Acsády, L., Arabadzisz, D., and Freund, T. F. (1996a). Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience 73, 299–315. doi: 10.1016/0306-4522(95)00610-9
Acsády, L., Görcs, T. J., and Freund, T. F. (1996b). Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience 73, 317–334. doi: 10.1016/0306-4522(95)00609-5
Ago, Y., Hayata-Takano, A., Kawanai, T., Yamauchi, R., Takeuchi, S., Cushman, J. D., et al. (2017). Impaired extinction of cued fear memory and abnormal dendritic morphology in the prelimbic and infralimbic cortices in VPAC2 receptor (VIPR2)-deficient mice. Neurobiol. Learn. Mem. 145, 222–231. doi: 10.1016/j.nlm.2017.10.010
Albensi, B. C., Oliver, D. R., Toupin, J., and Odero, G. (2007). Electrical stimulation protocols for hippocampal synaptic plasticity and neuronal hyper-excitability: are they effective or relevant? Exp. Neurol. 204, 1–13. doi: 10.1016/j.expneurol.2006.12.009
Artinian, J., and Lacaille, J. C. (2018). Disinhibition in learning and memory circuits: new vistas for somatostatin interneurons and long-term synaptic plasticity. Brain Res. Bull. 141, 20–26. doi: 10.1016/j.brainresbull.2017.11.012
Beck, H., Goussakov, I. V., Lie, A., Helmstaedter, C., and Elger, C. E. (2000). Synaptic plasticity in the human dentate gyrus. J. Neurosci. 20, 7080–7086. doi: 10.1523/jneurosci.20-18-07080.2000
Beck, H., and Yaari, Y. (2008). Plasticity of intrinsic neuronal properties in CNS disorders. Nat. Rev. Neurosci. 9, 357–369. doi: 10.1038/nrn2371
Benke, T., and Traynelis, S. F. (2019). AMPA-type glutamate receptor conductance changes and plasticity: still a lot of noise. Neurochem. Res. 44, 539–548. doi: 10.1007/s11064-018-2491-1
Besson, J., Dussaillant, M., Marie, J. C., Rostene, W., and Rosselin, G. (1984). In vitro autoradiographic localization of vasoactive intestinal peptide (VIP) binding sites in the rat central nervous system. Peptides 5, 339–340. doi: 10.1016/0196-9781(84)90231-6
Bliss, T., and Collingridge, G. (2019). Persistent memories of long-term potentiation and the N -methyl-d-aspartate receptor. Brain Neurosci. Adv. 3:239821281984821. doi: 10.1177/2398212819848213
Borbély, É, Scheich, B., and Helyes, Z. (2013). Neuropeptides in learning and memory. Neuropeptides 47, 439–450. doi: 10.1016/j.npep.2013.10.012
Borhegyi, Z., Varga, V., Szilágyi, N., Fabo, D., and Freund, T. F. (2004). Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J. Neurosci. 24, 8470–8479. doi: 10.1523/JNEUROSCI.1413-04.2004
Carmo, A. J., and Cunha-Reis, D. (2011). Evidence of emergent patterns in GluR1 phosphorylation in theta-burst LTP experiments with data mining tools. Amino Acids 41:S44.
Carvalho-Rosa, J. D., and Cunha-Reis, D. (2019). Endogenous VIP VPAC1 receptor activation during ictal and interictal-like activity induced in vitro by biccuculine and 0-Mg2+ modulates subsequent LTP expression in the rat hippocampus. Front. Cell. Neurosci. doi: 10.3389/conf.fncel.2019.01.00028
Chaudhury, D., Loh, D. H., Dragich, J. M., Hagopian, A., and Colwell, C. S. (2008). Select cognitive deficits in vasoactive intestinal peptide deficient mice. BMC Neurosci. 9:63. doi: 10.1186/1471-2202-9-63
Ciranna, L., and Cavallaro, S. (2003). Opposing effects by pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide on hippocampal synaptic transmission. Exp. Neurol. 184, 778–784. doi: 10.1016/S0014-4886(03)00300-5
Ciranna, L., and Costa, L. (2019). Pituitary adenylate cyclase-activating polypeptide modulates hippocampal synaptic transmission and plasticity: new therapeutic suggestions for fragile X syndrome. Front. Cell. Neurosci. 13:524. doi: 10.3389/fncel.2019.00524
Clynen, E., Swijsen, A., Raijmakers, M., Hoogland, G., and Rigo, J. M. (2014). Neuropeptides as targets for the development of anticonvulsant drugs. Mol. Neurobiol. 50, 626–646. doi: 10.1007/s12035-014-8669-x
Collingridge, G. L., Peineau, S., Howland, J. G., and Wang, Y. T. (2010). Long-term depression in the CNS. Nat. Rev. Neurosci. 11, 459–473. doi: 10.1038/nrn2867
Cossart, R., Dinocourt, C., Hirsch, J. C., Merchan-Perez, A., De Felipe, J., Ben-Ari, Y., et al. (2001). Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat. Neurosci. 4, 52–62. doi: 10.1038/82900
Cottrell, G. A., Veldhuis, H. D., Rostene, W. H., and de Kloet, E. R. (1984). Behavioral actions of vasoactive intestinal peptide (VIP). Neuropeptides 4, 331–341. doi: 10.1016/0143-4179(84)90008-8
Cunha-Reis, D. (2013). “Contribution of endogenous VIP VPAC1 receptor activation during hypoxia and interictal-like activity induced in vitro by 0-Mg2+ to subsequent changes in LTP expression in the rat hippocampus,” in Proceedings of the 37th Congress of the International Union of Physiological Sciences IUPS 2013 - PCB102, Birmingham, 474.
Cunha-Reis, D., Aidil-Carvalho, F., and Ribeiro, J. A. (2014). Endogenous inhibition of hippocampal LTD and depotentiation by vasoactive intestinal peptide VPAC1 receptors. Hippocampus 24, 1353–1363. doi: 10.1002/hipo.22316
Cunha-Reis, D., and Carmo, A. J. (2011). Correlation of VIP VPAC(1) modulation of GluR1 phosphorylation and inhibition of protein kinases in theta-burst LTP experiments with data mining tools. Amino Acids 41:S43.
Cunha-Reis, D., Ribeiro, J. A., de Almeida, R. F. M. M., and Sebastião, A. M. (2017). VPAC1 and VPAC2 receptor activation on GABA release from hippocampal nerve terminals involve several different signalling pathways. Br. J. Pharmacol. 174, 4725–4737. doi: 10.1111/bph.14051
Cunha-Reis, D., Ribeiro, J. A., and Sebastião, A. M. (2005). VIP enhances synaptic transmission to hippocampal CA1 pyramidal cells through activation of both VPAC1 and VPAC2 receptors. Brain Res. 1049, 52–60. doi: 10.1016/j.brainres.2005.04.077
Cunha-Reis, D., Ribeiro, J. A., and Sebastião, A. M. (2006). VPAC2 receptor activation mediates VIP enhancement of population spikes in the CA1 area of the hippocampus. Ann. N. Y. Acad. Sci. 1070, 210–214. doi: 10.1196/annals.1317.016
Cunha-Reis, D., Rodrigues, N. C., and Ribeiro, J. A. (2010). On the cellular and molecular pathways involved in the inhibition of LTP in the CA1 area of the hippocampus. J. Mol. Neurosci. 42:278.
Cunha-Reis, D., Sebastião, A. M., Wirkner, K., Illes, P., and Ribeiro, J. A. (2004). VIP enhances both pre-and postsynaptic GABAergic transmission to hippocampal interneurones leading to increased excitatory synaptic transmission to CA1 pyramidal cells. Br. J. Pharmacol. 143, 733–744. doi: 10.1038/sj.bjp.0705989
David, L. S., and Topolnik, L. (2017). Target-specific alterations in the VIP inhibitory drive to hippocampal GABAergic cells after status epilepticus. Exp. Neurol. 292, 102–112. doi: 10.1016/j.expneurol.2017.03.007
de Lanerolle, N. C., Gunel, M., Sundaresan, S., Shen, M. Y., Brines, M. L., and Spencer, D. D. (1995). Vasoactive intestinal polypeptide and its receptor changes in human temporal lobe epilepsy. Brain Res. 686, 182–193. doi: 10.1016/0006-8993(95)00365-w
Delgado, M., Jonakait, G. M., and Ganea, D. (2002). Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit chemokine production in activated microglia. Glia 39, 148–161. doi: 10.1002/glia.10098
Deng, G., and Jin, L. (2017). The effects of vasoactive intestinal peptide in neurodegenerative disorders. Neurol. Res. 39, 65–72. doi: 10.1080/01616412.2016.1250458
Devinsky, O., Vezzani, A., O’Brien, T. J., Jette, N., Scheffer, I. E., De Curtis, M., et al. (2018). Epilepsy. Nat. Rev. Dis. Primers 4:18024. doi: 10.1038/nrdp.2018.24
Dodd, J., Kelly, J. S., and Said, S. I. (1979). Excitation of CA1 neurones of the rat hippocampus by the octacosapeptide, vasoactive intestinal polypeptide (VIP). Br. J. Pharmacol. 66:125. doi: 10.1111/j.1748-1716.1983.tb07297.x
El-Hassar, L., Esclapez, M., and Bernard, C. (2007a). Hyperexcitability of the CA1 hippocampal region during epileptogenesis. Epilepsia 48(Suppl. 5) 131–139. doi: 10.1111/j.1528-1167.2007.01301.x
El-Hassar, L., Milh, M., Wendling, F., Ferrand, N., Esclapez, M., and Bernard, C. (2007b). Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J. Physiol. 578, 193–211. doi: 10.1113/jphysiol.2006.119297
Emson, P. C., Fahrenkrug, J., and Spokes, E. G. S. (1979). Vasoactive intestinal polypeptide (VIP): distribution in normal human brain and in Huntington’s disease. Brain Res. 173, 174–178. doi: 10.1016/0006-8993(79)91109-0
Fatatis, A., Holtzclaw, L. A., Avidor, R., Brenneman, D. E., and Russell, J. T. (1994). Vasoactive intestinal peptide increases intracellular calcium in astroglia: synergism with alpha-adrenergic receptors. Proc. Natl. Acad. Sci. U.S.A. 91, 2036–2040. doi: 10.1073/pnas.91.6.2036
Flood, J. F., Garland, J. S., and Morley, J. E. (1990). Vasoactive intestinal peptide (VIP): an amnestic neuropeptide. Peptides 11, 933–938. doi: 10.1016/0196-9781(90)90012-t
Foncelle, A., Mendes, A., Jȩdrzejewska-Szmek, J., Valtcheva, S., Berry, H., Blackwell, K. T., et al. (2018). Modulation of spike-timing dependent plasticity: towards the inclusion of a third factor in computational models. Front. Comput. Neurosci. 12:49. doi: 10.3389/fncom.2018.00049
Francavilla, R., Villette, V., Luo, X., Chamberland, S., Muñoz-Pino, E., Camiré, O., et al. (2018). Connectivity and network state-dependent recruitment of long-range VIP-GABAergic neurons in the mouse hippocampus. Nat. Commun. 9:5043. doi: 10.1038/s41467-018-07162-5
Ghijsen, W. E., and Leenders, A. G. (2005). Differential signaling in presynaptic neurotransmitter release. Cell. Mol. Life Sci. 62, 937–954. doi: 10.1007/s00018-004-4525-0
Girard, B. A., Lelievre, V., Braas, K. M., Razinia, T., Vizzard, M. A., Ioffe, Y., et al. (2006). Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J. Neurochem. 99, 499–513. doi: 10.1111/j.1471-4159.2006.04112.x
Glowa, J. R., Panlilio, L. V., Brenneman, D. E., Gozes, I., Fridkin, M., and Hill, J. M. (1992). Learning impairment following intracerebral administration of the HIV envelope protein gp120 or a VIP antagonist. Brain Res. 570, 49–53. doi: 10.1016/0006-8993(92)90562-n
Gourlet, P., De Neef, P., Cnudde, J., Waelbroeck, M., and Robberecht, P. (1997a). In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides 18, 1555–1560. doi: 10.1016/s0196-9781(97)00230-1
Gourlet, P., Vandermeers, A., Vertongen, P., Rathe, J., De Neef, P., Cnudde, J., et al. (1997b). Development of high affinity selective VIP1 receptor agonists. Peptides 18, 1539–1545. doi: 10.1016/s0196-9781(97)00228-3
Gourlet, P., Vertongen, P., Vandermeers, A., Vandermeers-Piret, M. C., Rathe, J., De Neef, P., et al. (1997c). The long-acting vasoactive intestinal polypeptide agonist RO 25-1553 is highly selective of the VIP2 receptor subclass. Peptides 18, 403–408. doi: 10.1016/s0196-9781(96)00322-1
Gozes, I. (2001). Neuroprotective peptide drug delivery and development: potential new therapeutics. Trends Neurosci. 24, 700–705. doi: 10.1016/S0166-2236(00)01931-7
Haas, H. L., and Gähwiler, B. H. (1992). Vasoactive intestinal polypeptide modulates neuronal excitability in hippocampal slices of the rat. Neuroscience 47, 273–277. doi: 10.1016/0306-4522(92)90243-u
Hájos, N., Acsády, L., and Freund, T. F. (1996). Target selectivity and neurochemical characteristics of VIP-immunoreactive interneurons in the rat dentate gyrus. Eur. J. Neurosci. 8, 1415–1431. doi: 10.1111/j.1460-9568.1996.tb01604.x
Harmar, A. J., Arimura, A., Gozes, I., Journot, L., Laburthe, M., Pisegna, J. R., et al. (1998). International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 50, 265–270. doi: 10.1042/bj2180893
Harmar, A. J., Fahrenkrug, J., Gozes, I., Laburthe, M., May, V., Pisegna, J. R., et al. (2012). Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR Review 1. Br. J. Pharmacol. 166, 4–17. doi: 10.1111/j.1476-5381.2012.01871.x
Haug, T., and Storm, J. F. (2000). Protein kinase a mediates the modulation of the slow Ca2 +-dependent K+ current, IsAHP, by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J. Neurophysiol. 83, 2071–2079. doi: 10.1152/jn.2000.83.4.2071
Helmstaedter, C., and Kockelmann, E. (2006). Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia 47(Suppl. 2) 96–98. doi: 10.1111/j.1528-1167.2006.00702.x
Hill, J. M. (2007). Vasoactive intestinal peptide in neurodevelopmental disorders: therapeutic potential. Curr. Pharm. Des. 13, 1079–1089. doi: 10.2174/138161207780618975
Itoh, S., Takashima, A., and Morimoto, T. (1994). Impaired spatial learning by vasoactive intestinal peptide in Morris water maze task in the rat. Can. J. Physiol. Pharmacol. 72, 25–29. doi: 10.1139/y94-005
Ivanova, M., Belcheva, S., Belcheva, I., Negrev, N., and Tashev, R. (2012). Lateralized hippocampal effects of vasoactive intestinal peptide on learning and memory in rats in a model of depression. Psychopharmacology (Berl). 221, 561–574. doi: 10.1007/s00213-011-2600-1
Ivanova, M., Belcheva, S., Belcheva, I., Stoyanov, Z., and Tashev, R. (2014). Modulatory effect of VIP injected into hippocampal CA1 area on anxiety in olfactory bulbectomized rats. Acta Neurobiol. Exp. 74, 317–327.
Ivanova, M., Ternianov, A., Belcheva, S., Tashev, R., Negrev, N., and Belcheva, I. (2008). Hippocampal asymmetry in exploratory behavior to vasoactive intestinal polypeptide. Peptides 29, 940–947. doi: 10.1016/j.peptides.2008.01.016
Ivanova, M., Ternianov, A., Tashev, R., Belcheva, S., and Belcheva, I. (2009). Lateralized learning and memory effects of vasoactive intestinal peptide infused into the rat hippocampal CA1 area. Regul. Pept. 156, 42–46. doi: 10.1016/j.regpep.2009.05.009
Joo, K. M., Chung, Y. H., Kim, M. K., Nam, R. H., Lee, B. L., Lee, K. H., et al. (2004). Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J. Comp. Neurol. 476, 388–413. doi: 10.1002/cne.20231
Kemp, A., and Manahan-Vaughan, D. (2007). Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 30, 111–118. doi: 10.1016/j.tins.2007.01.002
King, J. T., and LaMotte, C. C. (1988). VIP-, SS-, and GABA-like immunoreactivity in the mid-hippocampal region of El (epileptic) and C57BL/6 mice. Brain Res. 475, 192–197. doi: 10.1016/0006-8993(88)90218-1
Kitchigina, V. F., Kudina, T. A., Kutyreva, E. V., and Vinogradova, O. S. (1999). Neuronal activity of the septal pacemaker of theta rhythm under the influence of stimulation and blockade of the median raphe nucleus in the awake rabbit. Neuroscience 94, 453–463. doi: 10.1016/s0306-4522(99)00258-4
Klausberger, T., and Somogyi, P. (2008). Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. doi: 10.1126/science.1149381
Köhler, C. (1982). Distribution and morphology of vasoactive intestinal polypeptide-like immunoreactive neurons in regio superior of the rat hippocampal formation. Neurosci. Lett. 33, 265–270. doi: 10.1016/0304-3940(82)90382-2
Köhler, C. (1983). A morphological analysis of vasoactive intestinal polypeptide (VIP)−like immunoreactive neurons in the area dentata of the rat brain. J. Comp. Neurol. 221, 247–262. doi: 10.1002/cne.902210302
Kosaka, T., Kosaka, K., Tateishi, K., Hamaoka, Y., Yanaihara, N., Wu, J. Y., et al. (1985). GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J. Comp. Neurol. 239, 420–430. doi: 10.1002/cne.902390408
Kuang, Y., Yang, T., Gu, J., Kong, B., and Cheng, L. (2014). Comparison of therapeutic effects between selective amygdalohippocampectomy and anterior temporal lobectomy for the treatment of temporal lobe epilepsy: a meta-analysis. Br. J. Neurosurg. 28, 374–377. doi: 10.3109/02688697.2013.841854
Laburthe, M., Couvineau, A., and Marie, J. C. (2002). VPAC receptors for VIP and PACAP. Receptos Channels 8, 137–153. doi: 10.1080/10606820213680
Larson, J., and Munkácsy, E. (2015). Theta-burst LTP. Brain Res. 1621, 38–50. doi: 10.1016/j.brainres.2014.10.034
Léránth, C., Frotscher, M., Tömböl, T., and Palkovits, M. (1984). Ultrastructure and synaptic connections of vasoactive intestinal polypeptide-like immunoreactive non-pyramidal neurons and axon terminals in the rat hippocampus. Neuroscience 12, 531–542. doi: 10.1016/0306-4522(84)90071-x
Lorén, I., Emson, P. C., Fahrenkrug, J., Björklund, A., Alumets, J., Håkanson, R., et al. (1979). Distribution of vasoactive intestinal polypeptide in the rat and mouse brain. Neuroscience 4, 1953–1976. doi: 10.1016/0306-4522(79)90068-x
Luo, X., Guet-McCreight, A., Villette, V., Francavilla, R., Marino, B., Chamberland, S., et al. (2020). Synaptic mechanisms underlying the network state-dependent recruitment of VIP-expressing interneurons in the CA1 hippocampus. Cereb. Cortex 20:bhz334. doi: 10.1093/cercor/bhz334
Marksteiner, J., Sperk, G., and Maas, D. (1989). Differential increases in brain levels of neuropeptide Y and vasoactive intestinal polypeptide after kainic acid-induced seizures in the rat. Naunyn. Schmiedebergs Arch. Pharmacol. 339, 173–177. doi: 10.1007/BF00165140
Mellor, J. (2018). “Synaptic plasticity at hippocampal synapses: experimental background,” in Hippocampal Microcircuits. Springer Series in Computational Neuroscience, eds V. Cutsuridis, B. Graham, S. Cobb, and I. Vida, (Cham: Springer), 201–226. doi: 10.1007/978-3-319-99103-0_6
Miyata, A., Arimura, A., Dahl, R. R., Minamino, N., Uehara, A., Jiang, L., et al. (1989). Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 164, 567–574. doi: 10.1016/0006-291X(89)91757-9
Moreno, D., Gourlet, P., De Neef, P., Cnudde, J., Waelbroeck, M., and Robberecht, P. (2000). Development of selective agonists and antagonists for the human vasoactive intestinal polypeptide VPAC(2) receptor. Peptides 21, 1543–1549. doi: 10.1016/s0196-9781(00)00309-0
Mutt, V., and Said, S. I. (1974). Structure of the porcine vasoactive intestinal octacosapeptide: the amino−acid sequence. use of kallikrein in its determination. Eur. J. Biochem. 42, 581–589. doi: 10.1111/j.1432-1033.1974.tb03373.x
Park, P., Kang, H., Sanderson, T. M., Bortolotto, Z. A., Georgiou, J., Zhuo, M., et al. (2018). The role of calcium-permeable AMPARs in long-term potentiation at principal neurons in the rodent hippocampus. Front. Synaptic Neurosci. 10:42. doi: 10.3389/fnsyn.2018.00042
Rana, A., and Musto, A. E. (2018). The role of inflammation in the development of epilepsy. J. Neuroinflammation 15:144. doi: 10.1186/s12974-018-1192-7
Romualdi, P., Lesa, G., Donatini, A., Balboni, G., Tomatis, R., and Ferri, S. (1992). Alterations in vasoactive intestinal polypeptide-related peptides after pentylenetetrazole-induced seizures in rat brain. Eur. J. Pharmacol. 229, 149–153. doi: 10.1016/0014-2999(92)90549-J
Said, S. I., and Mutt, V. (1970). Polypeptide with broad biological activity: isolation from small intestine. Science 169, 1217–1218. doi: 10.1126/science.169.3951.1217
Said, S. I., and Rosenberg, R. N. (1976). Vasoactive intestinal polypeptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science 192, 907–908. doi: 10.1126/science.1273576
Schousboe, A., Scafidi, S., Bak, L. K., Waagepetersen, H. S., and McKenna, M. C. (2014). “Glutamate metabolism in the brain focusing on astrocytes,” in Glutamate and ATP at the Interface of Metabolism and Signaling in the Brain. Advances in Neurobiology, Vol. 11, eds V. Parpura, A. Schousboe, and A. Verkhratsky, (Cham: Springer), 13–30. doi: 10.1007/978-3-319-08894-5_2
Shreeve, S. M. (2002). Identification of G-proteins coupling to the vasoactive intestinal peptide receptor VPAC(1) using immunoaffinity chromatography: evidence for precoupling. Biochem. Biophys. Res. Commun. 290, 1300–1307. doi: 10.1006/bbrc.2002.6342
Sloviter, R. S. (1987). Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235, 73–76. doi: 10.1126/science.2879352
Stack, C. M., Lim, M. A., Cuasay, K., Stone, M. M., Seibert, K. M., Spivak-Pohis, I., et al. (2008). Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp. Neurol. 211, 67–84. doi: 10.1016/j.expneurol.2008.01.003
Takashima, A., Maeda, Y., and Itoh, S. (1993a). Influence of chronic intracerebroventricular infusion of vasoactive intestinal peptide (VIP) on memory processes in Morris water pool test in the rat. Peptides 14, 1073–1078. doi: 10.1016/0196-9781(93)90089-y
Takashima, A., Maeda, Y., and Itoh, S. (1993b). Vasoactive intestinal peptide (VIP) causes memory impairment in passive avoidance responding of the rat. Peptides 14, 1067–1071. doi: 10.1016/0196-9781(93)90088-x
Taylor, D. P., and Pert, C. B. (1979). Vasoactive intestinal polypeptide: Specific binding to rat brain membranes. Proc. Natl. Acad. Sci. U.S.A. 76, 660–664. doi: 10.1073/pnas.76.2.660
Thom, M. (2014). Review: hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol. Appl. Neurobiol. 40, 520–543. doi: 10.1111/nan.12150
Toda, A. M. A., and Huganir, R. L. (2015). Regulation of AMPA receptor phosphorylation by the neuropeptide PACAP38. Proc. Natl. Acad. Sci. U.S.A. 25, 6712–6717. doi: 10.1073/pnas.1507229112
Turi, G. F., Li, W. K., Chavlis, S., Pandi, I., O’Hare, J., Priestley, J. B., et al. (2019). Vasoactive intestinal polypeptide-expressing interneurons in the hippocampus support goal-oriented spatial learning. Neuron 101, 1150–1165.e8. doi: 10.1016/j.neuron.2019.01.009
Tyan, L., Chamberland, S., Magnin, E., Camiré, O., Francavilla, R., Suzanne David, L., et al. (2014). Dendritic inhibition provided by interneuron-specific cells controls the firing rate and timing of the hippocampal feedback inhibitory circuitry. J. Neurosci. 34, 4534–4547. doi: 10.1523/JNEUROSCI.3813-13.2014
Vandecasteele, M., Varga, V., Berényi, A., Papp, E., Barthó, P., Venance, L., et al. (2014). Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 111, 13535–13540. doi: 10.1073/pnas.1411233111
Vertongen, P., Schiffmann, S. N., Gourlet, P., and Robberecht, P. (1997). Autoradiographic visualization of the receptor subclasses for vasoactive intestinal polypeptide (VIP) in rat brain. Peptides 18, 1547–1554. doi: 10.1016/s0196-9781(97)00229-5
Vinogradova, O. S., Kitchigina, V. F., Kudina, T. A., and Zenchenko, K. I. (1999). Spontaneous activity and sensory responses of hippocampal neurons during persistent theta-rhythm evoked by median raphe nucleus blockade in rabbit. Neuroscience 94, 745–753. doi: 10.1016/s0306-4522(99)00253-5
Wang, H.-L., Li, A., and Wu, T. (1997). Vasoactive intestinal polypeptide enhances the GABAergic synaptic transmission in cultured hippocampal neurons. Brain Res. 746, 294–300. doi: 10.1016/S0006-8993(96)00772-X
Wefelmeyer, W., Puhl, C. J., and Burrone, J. (2016). Homeostatic plasticity of subcellular neuronal structures: from inputs to outputs. Trends Neurosci. 39, 656–667. doi: 10.1016/j.tins.2016.08.004
White, M., Ji, S., Cai, H., Maudsley, S., and Martin, B. (2010). Therapeutic potential of vasoactive intestinal peptide and its receptors in neurological disorders. CNS Neurol. Disord. Drug Targets 9, 661–666. doi: 10.2174/187152710793361595
Yang, K., Lei, G., Jackson, M. F., and MacDonald, J. F. (2010). The involvement of PACAP/VIP system in the synaptic transmission in the hippocampus. J. Mol. Neurosci. 42, 319–326. doi: 10.1007/s12031-010-9372-7
Yang, K., Trepanier, C. H., Li, H., Beazely, M. A., Lerner, E. A., Jackson, M. F., et al. (2009). Vasoactive intestinal peptide acts via multiple signal pathways to regulate hippocampal NMDA receptors and synaptic transmission. Hippocampus 19, 779–789. doi: 10.1002/hipo.20559
Yanovsky, Y., Sergeeva, O. A., Freund, T. F., and Haas, H. L. (1997). Activation of interneurons at the stratum oriens/alveus border suppresses excitatory transmission to apical dendrites in the CA1 area of the mouse hippocampus. Neuroscience 77, 87–96. doi: 10.1016/s0306-4522(96)00461-7
Yee, A. X., Hsu, Y. T., and Chen, L. (2017). A metaplasticity view of the interaction between homeostatic and hebbian plasticity. Philos. Trans. R. Soc. B Biol. Sci. 372:20160155. doi: 10.1098/rstb.2016.0155
Yu, R., Liu, H., Peng, X., Cui, Y., Song, S., Wang, L., et al. (2017). The palmitoylation of the N-terminal extracellular Cys37 mediates the nuclear translocation of VPAC1 contributing to its antiapoptotic activity. Oncotarget 8, 42728–42741. doi: 10.18632/oncotarget.17449
Keywords: VIP, synaptic plasticity, interneurons, hippocampus, MTLE, cognition, VPAC1 receptors
Citation: Cunha-Reis D and Caulino-Rocha A (2020) VIP Modulation of Hippocampal Synaptic Plasticity: A Role for VIP Receptors as Therapeutic Targets in Cognitive Decline and Mesial Temporal Lobe Epilepsy. Front. Cell. Neurosci. 14:153. doi: 10.3389/fncel.2020.00153
Received: 30 March 2020; Accepted: 11 May 2020;
Published: 12 June 2020.
Edited by:
Lucia Ciranna, University of Catania, ItalyCopyright © 2020 Cunha-Reis and Caulino-Rocha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diana Cunha-Reis, ZGNyZWlzQGNpZW5jaWFzLnVsaXNib2EucHQ=
 Diana Cunha-Reis
Diana Cunha-Reis Ana Caulino-Rocha
Ana Caulino-Rocha