
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci. , 02 August 2019
Sec. Cellular Neuropathology
Volume 13 - 2019 | https://doi.org/10.3389/fncel.2019.00353
This article is part of the Research Topic Mast Cells in Itch, Pain and Neuro-inflammation View all 18 articles
Fibromyalgia Syndrome (FMS) is a disorder of chronic, generalized muscular pain, accompanied by sleep disturbances, fatigue and cognitive dysfunction. There is no definitive pathogenesis except for altered central pain pathways. We previously reported increased serum levels of the neuropeptides substance P (SP) and its structural analogue hemokinin-1 (HK-1) together with the pro-inflammatory cytokines IL-6 and TNF in FMS patients as compared to sedentary controls. We hypothesize that thalamic mast cells contribute to inflammation and pain, by releasing neuro-sensitizing molecules that include histamine, IL-1β, IL-6 and TNF, as well as calcitonin-gene related peptide (CGRP), HK-1 and SP. These molecules could either stimulate thalamic nociceptive neurons directly, or via stimulation of microglia in the diencephalon. As a result, inhibiting mast cell stimulation could be used as a novel approach for reducing pain and the symptoms of FMS.
Fibromyalgia Syndrome is a disorder of chronic generalized muscular pain, stiffness, generalized fatigue, sleep abnormalities, (Clauw et al., 2011; Schmidt-Wilcke and Clauw, 2011; Clauw, 2014) and cognitive problems (Theoharides et al., 2015b; Hauser et al., 2019) assessed by the FSQ (Ferrari and Russell, 2013), which has about 93% sensitivity and 92% specificity (Clauw, 2014). FMS affects about 5% of adults, primarily women 20–60 years of age (Branco et al., 2010) and belongs to a family of overlapping painful conditions (Table 1) known as CSS (Yunus, 2007; Theoharides, 2013). Central sensitization is recognized as the main mechanism involved (Woodman, 2013) and is characterized by allodynia, pain from an otherwise non-painful stimulus, (Russell and Larson, 2009) and hyperalgesia (Staud et al., 2001) due to an exaggerated response to a painful stimulus (Woolf, 2011). The pathogenesis of FMS remains unknown and with no objective diagnostic criteria (McBeth and Mulvey, 2012; Wolfe and Walitt, 2013). FMS patients have reduced tolerance to pain, especially extremes of heat and cold (Desmeules et al., 2003). There is considerable evidence of altered circuity of pain networks and (Jensen et al., 2012; Flodin et al., 2014) abnormal pain processing in FMS (Staud, 2011).
The PubMed database was searched between 1960 and 2018 using the terms fatigue, fibromyalgia, hypothalamus, inflammation, mast cells, pain and stress. Only articles in English were included.
Here we discuss how brain mast cell release of neuro-sensitizing mediators in the thalamus leads to focal inflammation and contribute to the pathogenesis of FMS.
It was recently proposed that FMS may involve localized inflammation in the hypothalamus (Theoharides et al., 2015c). Elevations in pro-inflammatory chemokines/cytokines could negatively impact symptoms (Bazzichi et al., 2007; Carvalho et al., 2008; Nugraha et al., 2013) leading to sensitization of peripheral and central nociceptors (Uceyler et al., 2011; Behm et al., 2012; Hornig et al., 2015). Increased levels of the pro-inflammatory chemokine IL-8 (CXCL8) have been reported in the serum and CSF in patients with FMS (Ross et al., 2010; Kadetoff et al., 2012; Rodriguez-Pinto et al., 2014). Chemokines facilitate nociception by directly acting on chemokine receptors present along the pain pathway (Abbadie, 2005; Charo and Ransohoff, 2006).
The cytokines TNF and IL-17 greatly contribute to the inflammatory response (Romero-Sanchez et al., 2011; Griffin et al., 2012). Plasma levels of IL-17 were increased and correlated with levels of TNF in patients with FMS (Pernambuco et al., 2013). CSF and serum IL-17 also positively correlated with pain (Meng et al., 2013) and anxiety (Liu et al., 2012). Mast cells, themselves, can secrete IL-17; moreover, IL-6 and TGFβ from mast cells contribute to the development of Th-17 cells (Kenna and Brown, 2013).
Fibromyalgia syndrome worsen by stress, (Geenen et al., 2002) which augments pain responses (Bote et al., 2012, 2013). Plasma concentrations of cortisol are increased in the evening, suggesting disruption of the circadian rhythm (Crofford et al., 2004). Serum levels of CRH, which is secreted under stress, were increased in patients with FMS (Tsilioni et al., 2016). CRH was also increased in the CSF of such patients and correlated with severity of pain (McLean et al., 2006). Physiological stress was reported to be the most common trigger in patients with systemic mastocytosis (SM) (Jennings et al., 2014) who also commonly experience FMS (Theoharides et al., 2015d, 2019). We reported increased levels of CRH in the serum of one patient with indolent systemic mastocytosis (Theoharides et al., 2014). CRH can trigger human mast cells to release VEGF without histamine or tryptase (Cao et al., 2005). CRH also has synergistic action with NT stimulating VEGF release. As a result, there is increased vascular permeability in the skin and the blood-brain barrier (BBB) (Esposito et al., 2002; Donelan et al., 2006; Theoharides and Konstantinidou, 2007). Stress also disrupts the gut-blood barrier (Theoharides et al., 1999; Wallon et al., 2008) allowing for gut microbiome-associated molecules, such as propionate (Minerbi et al., 2019) to enter the brain and contribute to focal inflammation. These results have led to the conclusion that mast cells may serve as “immune gate to the brain” (Theoharides, 1990; Ribatti, 2015).
Levels of the neuropeptide SP (Russell, 1998) and NGF (Giovengo et al., 1999) are elevated in the CSF of FMS patients. NGF has been reported to increase nociception and hyperalgesia (Maren, 2017). The SP receptor NK-1 has been involved in the pathophysiology of pain (Greenwood-Van et al., 2014). We reported increased serum levels of SP, its structural analogue Hemokinin-1 (HK-1) and TNF in patients with FMS (Tsilioni et al., 2016). SP (Theoharides et al., 2010a,b) and NGF (Levi-Montalcini, 1987) can stimulate mast cells. Moreover, SP induced mast cell expression of CRHR-1 (Scholzen et al., 2001). Cerebrovascular mast cells were stimulated by CGRP, (Reynier-Rebuffel et al., 1994; Ottosson and Edvinsson, 1997) which is now well established to participate in the pathophysiology of headaches (Edvinsson, 2018). In addition to neuropeptides, sex hormones can also affect mast cell reactivity. For instance, estradiol augments immune (Kovats, 2015) and allergic (Hox et al., 2015) processes. In particular, we had reported expression of estrogen receptors on rodent mast cells (Pang et al., 1995). We also reported that 17β-estradiol further increased stimulation of mast cells by SP (Theoharides et al., 1993). Such findings may help explain why FMS is more common in women.
In addition to allergic reactions, mast cells contribute to innate immunity, (Galli et al., 2011) autoimmunity (Rottem and Mekori, 2005) and inflammation (Theoharides et al., 2010a).
Increasing evidence supports the involvement of mast cells in FMS (Lucas et al., 2006; Pollack, 2014) and comorbid disorders (Theoharides, 2013) as well as other inflammatory (Galli et al., 2008; Theoharides et al., 2010a) and painful conditions, (Heron and Dubayle, 2013; Chatterjea and Martinov, 2014) as well as neuroimmune interactions (Skaper et al., 2017) (Figure 1). Chronic urticaria, which involves stimulation of skin mast cells is more common in FMS (Torresani et al., 2009). Moreover, mast cells are significantly increased in the papillary dermis of FMS patients (Blanco et al., 2010). The chemokines monocyte chemoattractant protein-1 (MCP-1/CCL2) and eotaxin (CCL-11) are elevated in plasma of FMS patients (Zhang et al., 2008). MCP-1 is a strong mast cell chemoattractant (Conti et al., 1998) and also triggers mast cells in rodents (Conti and Theoharides, 1994). MCP-1 induced prolonged muscle hyperalgesia in rats via activation of its high-affinity receptor, CC Chemokine receptor 2 (CCR2), on the peripheral nerve terminals (Alvarez et al., 2014). Myoblasts treated with MCP-1 secreted significant amounts of the key pro-inflammatory cytokine IL-1β (Zhang et al., 2008). C-reactive protein (CRP) is now considered a marker of chronic inflammation. CRP may be useful in the diagnostic of FMS (and depression/anxiety that often accompany FMS), even though there is no direct correlation reported (De Berardis et al., 2006, 2017; Orsolini et al., 2018).
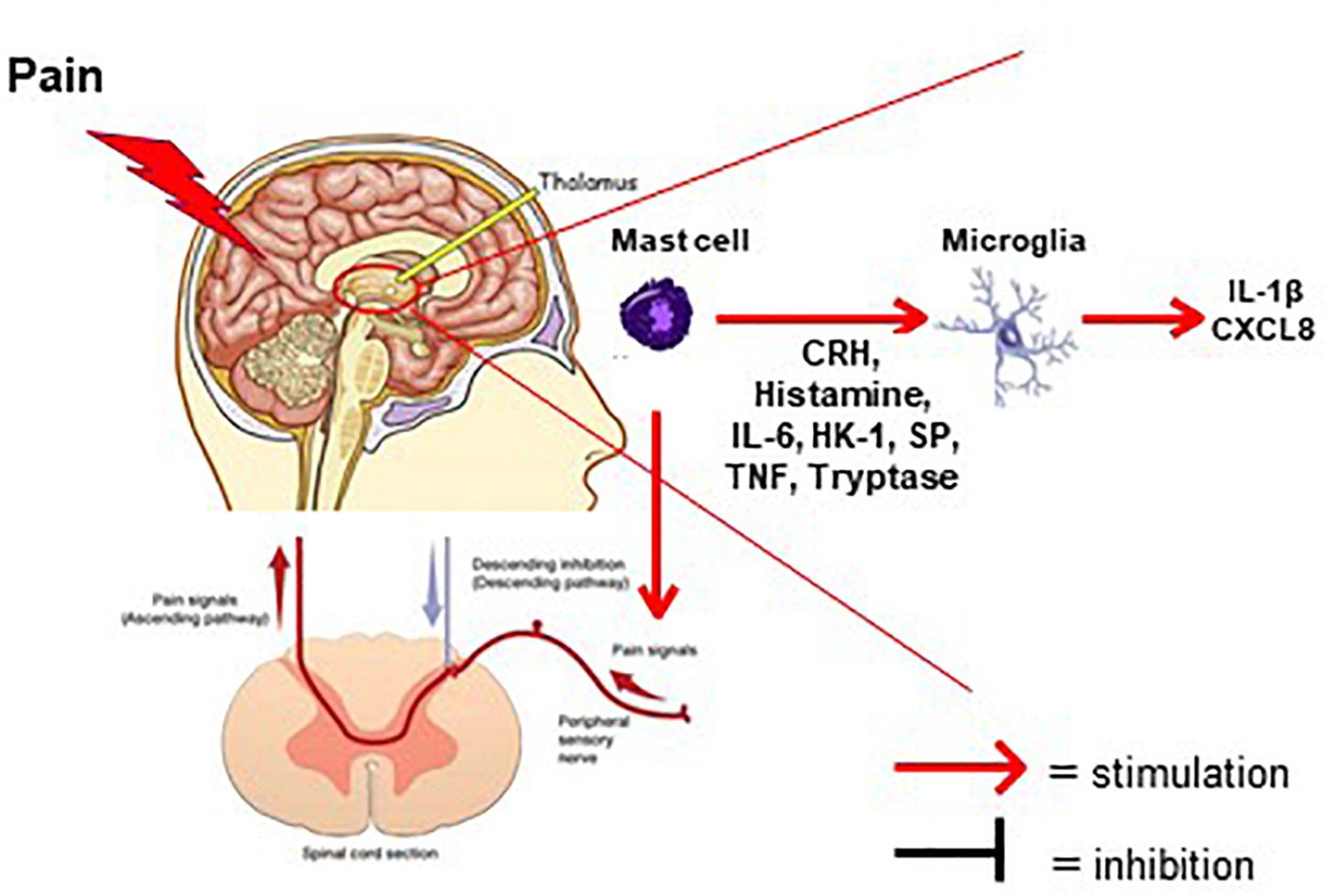
Figure 1. Diagram depicting the involvement of mast cells in the generation of pain in FMS. Mast cells (violet color) in the thalamus secrete pro-inflammatory and neuro-sensitizing mediators (CRH, histamine, IL-6, HK-1, SP, TNF, Tryptase). These mediators can then activate either microglia in thalamic nuclei or ascending nociceptive tracks creating the sensation of pain. Possible natural molecules to inhibit stimulated mast cells and/or microglia are flavonoids such as luteolin or tetramethoxyluteolin (Methlut).
Mast cells derive from the bone marrow and mature in response to SCF, which acts via the cell surface tyrosine kinase KIT receptor (Galli et al., 2011). Mast cell progenitors then migrate in all tissues. As a result, mast cell mediators can affect all organs and lead to multiple symptoms. Mast cells are found adjacent to blood vessels and nerve endings; in the brain, mast cells are located in the thalamus, hypothalamus and median eminence (Edvinsson et al., 1976; Lambracht-Hall et al., 1990; Theoharides et al., 2015d).
Mast cells are known to be stimulated by IgE, via activation of its unique surface receptors (FcεRI) (Rivera et al., 2008). Mast cells can also be stimulated via TLRs, (Abraham and St John, 2010; Zhang et al., 2010). Stimulated mast cells secrete multiple vasoactive, pro-inflammatory and neuro-sensitizing molecules (Galli and Tsai, 2008; Theoharides et al., 2010a). Stimulation of mast cells can be augmented by the cytokine IL-33, (Fux et al., 2014) which synergizes with SP to induce release of impressive amounts of VEGF, (Theoharides et al., 2010b) TNF (Taracanova et al., 2017) or IL-1β (Taracanova et al., 2018). As a result, mast cells can serve as “sensors of cell danger” (Theoharides, 1996; Enoksson et al., 2011; Theoharides et al., 2015a).
Mast cell secretory granules store many preformed pro-inflammatory and neuro-sensitizing mediators including bradykinin, histamine, TNF and tryptase (Nakae et al., 2005; Olszewski et al., 2007). Mast cells also release de novo synthesized molecules: (a) lipid mediators (leukotrienes, prostaglandins, and PAF), (b) cytokines (IL-6, IL-13, IL-33, TNF) and (c) chemokines (CXCL8, CCL2, CCL5), (Theoharides et al., 2015d; Mukai et al., 2018). Mast cell could often release mediators selectively without histamine or tryptase (Theoharides et al., 2007). Mast cells also release IL-31, which is important in the sensation of itching and pain, in response to IgE and SP, IL-33 and specifically their combination (Petra et al., 2018). We reported that mast cells can release mtDNA, which is mistaken as a pathogen and stimulates inflammatory responses (Zhang B. et al., 2012).
Finally, mast cells can release extracellular vesicles (exosomes) (Skokos et al., 2002, 2003) that could deliver regulatory molecules, including mtDNA and microRNAs (Kawikova and Askenase, 2014). Such microvesicles have been implicated in brain disorders (Tsilioni et al., 2014; Kawikova and Askenase, 2014) and pain disorders (Rafiee et al., 2018; Silva-Freire et al., 2019). We recently reported that extracellular vesicles are increased in the serum of children with ASD, contained mtDNA and stimulated cultured human microglia to secrete the pro-inflammatory molecules IL-1β and CXCL8 (Tsilioni and Theoharides, 2018).
Mast cells communicate with microglia (Skaper et al., 2012, 2014b). Mediators secreted from mast cells, (Zhang et al., 2016) such as histamine (Dong et al., 2014) and tryptase, (Zhang S. et al., 2012) can activate microglia leading to secretion of the pro-inflammatory cytokines IL-1β, IL-6 and TNF. Microglia can also be activated by CRH secreted from mast cells (Wang et al., 2002; Kempuraj et al., 2004). Stimulation of brain mast cells in mice led to activation of microglia, which was decreased by administration of a mast cell inhibitor (Dong et al., 2017).
Microglia are involved in synapse plasticity, (Shemer et al., 2015; Wu et al., 2015; Reu et al., 2017) but are responsible for innate immunity of the brain (Ransohoff and Brown, 2012; Aguzzi et al., 2013). Microglia contribute to brain inflammation (Hagberg et al., 2012; Aguzzi et al., 2013; Nakagawa and Chiba, 2016) and the pathogenesis of different brain disorders, (Takeda et al., 2014; Reus et al., 2015; Faden et al., 2016; Garden and Campbell, 2016; Groh and Martini, 2017; Koutsouras et al., 2017; Pennisi et al., 2017; Jiang et al., 2018; Thonhoff et al., 2018) especially ASD (Vargas et al., 2005; Morgan et al., 2010; Suzuki et al., 2013; Edmonson et al., 2014; Gupta et al., 2014). Microglia in the thalamus have been discussed in the context of pain, especially maintaining the pain sensation even after the original painful stimulus is not present (Banati, 2002; Hansson, 2010; Saghaei et al., 2013; Blaszczyk et al., 2018).
Mast cells have been implicated in headaches (Theoharides, 1983; Theoharides et al., 2005) and pain (Xanthos et al., 2011; Aich et al., 2015; Gupta and Harvima, 2018). Activation of the mast cell-specific receptor, MRGPRX2, (McNeil et al., 2015) and its mouse analogue, Mrgprb2, mediated inflammatory mechanical and thermal hyperalgesia (Green et al., 2019). Hence, mast cells are key players of neuroendocrine (Theoharides, 2017) and painful disorders (Theoharides et al., 2019).
In this context, inhibitors of mast cells (Harvima et al., 2014) would be useful in the treatment of FMS. Natural molecules could include the flavonoids, luteolin (Kempuraj et al., 2008; Theoharides et al., 2015c; Ashaari et al., 2018) and tetramethoxyluteolin, (Theoharides et al., 2017; Theoharides and Tsilioni, 2018) alone or in combination with other substances selected to reduce stress (Theoharides and Kavalioti, 2018). Other natural molecules could include palmitoylethanolamide, (Schweiger et al., 2019) which apparently inhibits neuro-inflammation (Skaper et al., 2013, 2015) and reduces pain (Skaper et al., 2014a; Impellizzeri et al., 2016).
Research should focus on identifying in serum of patients with FMS novel molecules that are involved in pain transmission such as bradykinin, CGRP and IL-31. Extracellular vesicles should also be isolated from the serum and CSF of FMS patients, their content identified, and their effect investigated on cultured human mast cells and microglia. Such possible interactions would serve as useful in vitro assays for the screening of potential novel treatment agents. Recent reports have also stressed the possible use of the cytokine IL-37, (Mastrangelo et al., 2018) which is known to have anti-inflammatory actions (Cavalli and Dinarello, 2018). It would be important to explore the possible use of IL-37 isoforms in the treatment of FMS.
TT, IT, and MB participated in searching the literature. TT and IT wrote or contributed to the writing of the manuscript. IT prepared the figure.
Some aspects of our work described were supported in part by the National Institutes of Health (NIH) (Grants NS38326 and AR47652), as well as the Michael and Katherine Johnson Family Fund to TT.
TT is the inventor of US patents No. 7,906,153 and No. 8,268,365 for the treatment of neuroinflammatory conditions.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACR, American College of Rheumatology; ASD, autism spectrum disorder; CGRP, calcitonin-gene related peptide; CIRS, chronic inflammatory response syndrome; CRH, corticotropin-releasing hormone; CSF, cerebrospinal fluid; CSS, central sensitivity syndromes; CXCL8, IL-8; FcεRI, high affinity surface receptors; FMS, fibromyalgia syndrome; FSQ, fibromyalgia survey questionnaire; IBS, irritable bowel syndrome; IC/BPS, interstitial cystitis/bladder pain syndrome; IgE, immunoglobulin E; MCP-1/CCL2, monocyte chemoattractant protein-1; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; MRGPRX2, mas-related G-protein coupled receptor member X2; (mt)DNA, mitochondrial; NGF, nerve growth factor; PAF, platelet activating factor; PTSD, post-traumatic stress disorder; SCF, stem cell factor; SP, substance P; TLRs, toll-like receptors; TMD, myogenic temporomandibular disorder.
Abbadie, C. (2005). Chemokines, chemokine receptors and pain. Trends Immunol. 26, 529–534. doi: 10.1016/j.it.2005.08.001
Abraham, S. N., and St John, A. L. (2010). Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10, 440–452. doi: 10.1038/nri2782
Aguzzi, A., Barres, B. A., and Bennett, M. L. (2013). Microglia: scapegoat, saboteur, or something else? Science 339, 156–161. doi: 10.1126/science.1227901
Aich, A., Afrin, L. B., and Gupta, K. (2015). Mast cell-mediated mechanisms of nociception. Int. J. Mol. Sci. 16, 29069–29092. doi: 10.3390/ijms161226151
Alvarez, P., Green, P. G., and Levine, J. D. (2014). Role for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the rat. Pain 155, 1161–1167. doi: 10.1016/j.pain.2014.03.004
Ashaari, Z., Hadjzadeh, M. A., Hassanzadeh, G., Alizamir, T., Yousefi, B., Keshavarzi, Z., et al. (2018). The flavone luteolin improves central nervous system disorders by different mechanisms: a review. J. Mol. Neurosci. 65, 491–506. doi: 10.1007/s12031-018-1094-2
Banati, R. B. (2002). Brain plasticity and microglia: is transsynaptic glial activation in the thalamus after limb denervation linked to cortical plasticity and central sensitisation? J. Physiol. Paris 96, 289–299. doi: 10.1016/s0928-4257(02)00018-9
Bazzichi, L., Rossi, A., Massimetti, G., Giannaccini, G., Giuliano, T., De Feo, F., et al. (2007). Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin. Exp. Rheumatol. 25, 225–230.
Behm, F. G., Gavin, I. M., Karpenko, O., Lindgren, V., Gaitonde, S., Gashkoff, P. A., et al. (2012). Unique immunologic patterns in fibromyalgia. BMC Clin. Pathol. 12:25. doi: 10.1186/1472-6890-12-25
Blanco, I., Beritze, N., Arguelles, M., Carcaba, V., Fernandez, F., Janciauskiene, S., et al. (2010). Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin. Rheumatol. 29, 1403–1412. doi: 10.1007/s10067-010-1474-7
Blaszczyk, L., Maitre, M., Leste-Lasserre, T., Clark, S., Cota, D., Oliet, S. H. R., et al. (2018). Sequential alteration of microglia and astrocytes in the rat thalamus following spinal nerve ligation. J. Neuroinflammation 15:349. doi: 10.1186/s12974-018-1378-z
Bote, M. E., Garcia, J. J., Hinchado, M. D., and Ortega, E. (2012). Inflammatory/stress feedback dysregulation in women with fibromyalgia. Neuroimmunomodulation 19, 343–351. doi: 10.1159/000341664
Bote, M. E., Garcia, J. J., Hinchado, M. D., and Ortega, E. (2013). Fibromyalgia: anti-inflammatory and stress responses after acute moderate exercise. PLoS One 8:e74524. doi: 10.1371/journal.pone.0074524
Branco, J. C., Bannwarth, B., Failde, I., Abello, C. J., Blotman, F., Spaeth, M., et al. (2010). Prevalence of fibromyalgia: a survey in five European countries. Semin. Arthritis Rheum. 39, 448–453. doi: 10.1016/j.semarthrit.2008.12.003
Cao, J., Papadopoulou, N., Kempuraj, D., Boucher, W. S., Sugimoto, K., Cetrulo, C. L., et al. (2005). Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J. Immunol. 174, 7665–7675. doi: 10.4049/jimmunol.174.12.7665
Carvalho, L. S., Correa, H., Silva, G. C., Campos, F. S., Baiao, F. R., Ribeiro, L. S., et al. (2008). May genetic factors in fibromyalgia help to identify patients with differentially altered frequencies of immune cells? Clin. Exp. Immunol. 154, 346–352. doi: 10.1111/j.1365-2249.2008.03787.x
Cavalli, G., and Dinarello, C. A. (2018). Suppression of inflammation and acquired immunity by IL-37. Immunol. Rev. 281, 179–190. doi: 10.1111/imr.12605
Charo, I. F., and Ransohoff, R. M. (2006). The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621. doi: 10.1056/nejmra052723
Chatterjea, D., and Martinov, T. (2014). Mast cells: versatile gatekeepers of pain. Mol. Immunol. 63, 38–44. doi: 10.1016/j.molimm.2014.03.001
Clauw, D. J. (2014). Fibromyalgia: a clinical review. JAMA 311, 1547–1555. doi: 10.1001/jama.2014.3266
Clauw, D. J., Arnold, L. M., and McCarberg, B. H. (2011). The science of fibromyalgia. Mayo Clin. Proc. 86, 907–911. doi: 10.4065/mcp.2011.0206
Conti, P., Reale, M., Barbacane, R. C., Letourneau, R., and Theoharides, T. C. (1998). Intramuscular injection of hrRANTES causes mast cell recruitment and increased transcription of histidine decarboxylase: lack of effects in genetically mast cell-deficient W/Wv mice. FASEB J. 12, 1693–1700. doi: 10.1096/fasebj.12.15.1693
Conti, P., and Theoharides, T. C. (1994). Monocyte chemotactic Protein-1 (MCP-1) is active on mast cells and causes clump formation. Int. J. Immunopathol. Pharmacol. 7, 149–151.
Crofford, L. J., Young, E. A., Engleberg, N. C., Korszun, A., Brucksch, C. B., McClure, L. A., et al. (2004). Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav. Immun. 18, 314–325. doi: 10.1016/s0889-1591(04)00021-2
De Berardis, D., Campanella, D., Gambi, F., La, R. R., Carano, A., Conti, C. M., et al. (2006). The role of C-reactive protein in mood disorders. Int. J. Immunopathol. Pharmacol. 19, 721–725.
De Berardis, D., Serroni, N., Campanella, D., Marini, S., Rapini, G., Valchera, A., et al. (2017). Alexithymia, suicide ideation, C-Reactive Protein, and serum lipid levels among outpatients with generalized anxiety disorder. Arch. Suicide Res. 21, 100–112. doi: 10.1080/13811118.2015.1004485
Desmeules, J. A., Cedraschi, C., Rapiti, E., Baumgartner, E., Finckh, A., Cohen, P., et al. (2003). Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 48, 1420–1429. doi: 10.1002/art.10893
Donelan, J., Boucher, W., Papadopoulou, N., Lytinas, M., Papaliodis, D., and Theoharides, T. C. (2006). Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc. Natl. Acad. Sci. U.S.A. 103, 7759–7764. doi: 10.1073/pnas.0602210103
Dong, H., Zhang, W., Zeng, X., Hu, G., Zhang, H., He, S., et al. (2014). Histamine induces upregulated expression of histamine receptors and increases release of inflammatory mediators from microglia1. Mol. Neurobiol. 49, 1487–1500. doi: 10.1007/s12035-014-8697-6
Dong, H., Zhang, X., Wang, Y., Zhou, X., Qian, Y., and Zhang, S. (2017). Suppression of brain mast cells degranulation inhibits microglial activation and central nervous system inflammation. Mol. Neurobiol. 54, 997–1007. doi: 10.1007/s12035-016-9720-x
Edmonson, C., Ziats, M. N., and Rennert, O. M. (2014). Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol. Autism 5:3. doi: 10.1186/2040-2392-5-3
Edvinsson, L. (2018). The CGRP pathway in migraine as a viable target for therapies. Headache 58(Suppl. 1), 33–47. doi: 10.1111/head.13305
Edvinsson, L., Owman, C., and Sjöberg, N. O. (1976). Autonomic nerves, mast cells and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 115, 377–393. doi: 10.1016/0006-8993(76)90356-5
Enoksson, M., Lyberg, K., Moller-Westerberg, C., Fallon, P. G., Nilsson, G., and Lunderius-Andersson, C. (2011). Mast cells as sensors of cell injury through IL-33 recognition. J. Immunol. 186, 2523–2528. doi: 10.4049/jimmunol.1003383
Esposito, P., Chandler, N., Kandere-Grzybowska, K., Basu, S., Jacobson, S., Connolly, R., et al. (2002). Corticotropin-releasing hormone (CRH) and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J. Pharmacol. Exp. Ther. 303, 1061–1066. doi: 10.1124/jpet.102.038497
Faden, A. I., Wu, J., Stoica, B. A., and Loane, D. J. (2016). Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br. J. Pharmacol. 173, 681–691. doi: 10.1111/bph.13179
Ferrari, R., and Russell, A. S. (2013). A questionnaire using the modified 2010 American College of rheumatology criteria for fibromyalgia: specificity and sensitivity in clinical practice. J. Rheumatol. 40, 1590–1595. doi: 10.3899/jrheum.130367
Flodin, P. D., Martinsen, S., Lofgren, M., Bileviciute-Ljungar, I., Kosek, E., and Fransson, P. (2014). Fibromyalgia is associated with decreased connectivity between pain- and sensorimotor brain areas. Brain Connect. 4, 587–594. doi: 10.1089/brain.2014.0274
Fux, M., Pecaric-Petkovic, T., Odermatt, A., Hausmann, O. V., Lorentz, A., Bischoff, S. C., et al. (2014). IL-33 is a mediator rather than a trigger of the acute allergic response in humans. Allergy 69, 216–222. doi: 10.1111/all.12309
Galli, S. J., Borregaard, N., and Wynn, T. A. (2011). Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 12, 1035–1044. doi: 10.1038/ni.2109
Galli, S. J., and Tsai, M. (2008). Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 49, 7–19. doi: 10.1016/j.jdermsci.2007.09.009
Galli, S. J., Tsai, M., and Piliponsky, A. M. (2008). The development of allergic inflammation. Nature 454, 445–454. doi: 10.1038/nature07204
Garden, G. A., and Campbell, B. M. (2016). Glial biomarkers in human central nervous system disease. Glia 64, 1755–1771. doi: 10.1002/glia.22998
Geenen, R., Jacobs, J. W., and Bijlsma, J. W. (2002). Evaluation and management of endocrine dysfunction in fibromyalgia. Rheum. Dis. Clin. North Am. 28, 389–404. doi: 10.1016/s0889-857x(01)00009-6
Giovengo, S. L., Russell, I. J., and Larson, A. A. (1999). Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J. Rheumatol. 26, 1564–1569.
Green, D. P., Limjunyawong, N., Gour, N., Pundir, P., and Dong, X. (2019). A Mast-Cell-Specific receptor mediates neurogenic inflammation and pain. Neuron 101, 412–420. doi: 10.1016/j.neuron.2019.01.012
Greenwood-Van, M. B., Mohammadi, E., Tyler, K., Pietra, C., Bee, L. A., and Dickenson, A. (2014). Synergistic effect of 5-hydroxytryptamine 3 and neurokinin 1 receptor antagonism in rodent models of somatic and visceral pain. J. Pharmacol. Exp. Ther. 351, 146–152. doi: 10.1124/jpet.114.216028
Griffin, G. K., Newton, G., Tarrio, M. L., Bu, D. X., Maganto-Garcia, E., Azcutia, V., et al. (2012). IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 188, 6287–6299. doi: 10.4049/jimmunol.1200385
Groh, J., and Martini, R. (2017). Neuroinflammation as modifier of genetically caused neurological disorders of the central nervous system: understanding pathogenesis and chances for treatment. Glia 65, 1407–1422. doi: 10.1002/glia.23162
Gupta, K., and Harvima, I. T. (2018). Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 282, 168–187. doi: 10.1111/imr.12622
Gupta, S., Ellis, S. E., Ashar, F. N., Moes, A., Bader, J. S., Zhan, J., et al. (2014). Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 5:5748. doi: 10.1038/ncomms6748
Hagberg, H., Gressens, P., and Mallard, C. (2012). Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 71, 444–457. doi: 10.1002/ana.22620
Hansson, E. (2010). Long-term pain, neuroinflammation and glial activation. Scand. J. Pain 1, 67–72. doi: 10.1016/j.sjpain.2010.01.002
Harvima, I. T., Levi-Schaffer, F., Draber, P., Friedman, S., Polakovicova, I., Gibbs, B. F., et al. (2014). Molecular targets on mast cells and basophils for novel therapies. J. Allergy Clin. Immunol. 134, 530–544. doi: 10.1016/j.jaci.2014.03.007
Hauser, W., Sarzi-Puttini, P., and Fitzcharles, M. A. (2019). Fibromyalgia syndrome: under-, over- and misdiagnosis. Clin. Exp. Rheumatol. 37(Suppl. 116), 90–97.
Heron, A., and Dubayle, D. (2013). A focus on mast cells and pain. J. Neuroimmunol. 264, 1–7. doi: 10.1016/j.jneuroim.2013.09.018
Hornig, M., Montoya, J. G., Klimas, N. G., Levine, S., Felsenstein, D., Bateman, L., et al. (2015). Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci. Adv. 1:e1400121. doi: 10.1126/sciadv.1400121
Hox, V., Desai, A., Bandara, G., Gilfillan, A. M., Metcalfe, D. D., and Olivera, A. (2015). Estrogen increases the severity of anaphylaxis in female mice through enhanced endothelial nitric oxide synthase expression and nitric oxide production. J. Allergy Clin. Immunol. 135, 729–736. doi: 10.1016/j.jaci.2014.11.003
Impellizzeri, D., Di, P. R., Cordaro, M., Gugliandolo, E., Casili, G., Morittu, V. M., et al. (2016). Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 119, 27–41. doi: 10.1016/j.bcp.2016.09.001
Jennings, S., Russell, N., Jennings, B., Slee, V., Sterling, L., Castells, M., et al. (2014). The mastocytosis society survey on mast cell disorders: patient experiences and perceptions. J. Allergy Clin. Immunol. Pract. 2, 70–76. doi: 10.1016/j.jaip.2013.09.004
Jensen, K. B., Loitoile, R., Kosek, E., Petzke, F., Carville, S., Fransson, P., et al. (2012). Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol. Pain 8:32. doi: 10.1186/1744-8069-8-32
Jiang, N. M., Cowan, M., Moonah, S. N., and Petri, W. A. Jr. (2018). The impact of systemic inflammation on neurodevelopment. Trends Mol. Med. 24, 794–804. doi: 10.1016/j.molmed.2018.06.008
Kadetoff, D., Lampa, J., Westman, M., Andersson, M., and Kosek, E. (2012). Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 242, 33–38. doi: 10.1016/j.jneuroim.2011.10.013
Kawikova, I., and Askenase, P. W. (2014). Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res. 1617, 63–71. doi: 10.1016/j.brainres.2014.09.070
Kempuraj, D., Papadopoulou, N. G., Lytinas, M., Huang, M., Kandere-Grzybowska, K., Madhappan, B., et al. (2004). Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology 145, 43–48. doi: 10.1210/en.2003-0805
Kempuraj, D., Tagen, M., Iliopoulou, B. P., Clemons, A., Vasiadi, M., Boucher, W., et al. (2008). Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell dependent stimulation of Jurkat T cells. Br. J. Pharmacol. 155, 1076–1084. doi: 10.1038/bjp.2008.356
Kenna, T. J., and Brown, M. A. (2013). The role of IL-17-secreting mast cells in inflammatory joint disease. Nat. Rev. Rheumatol. 9, 375–379. doi: 10.1038/nrrheum.2012.205
Koutsouras, G. W., Ramos, R. L., and Martinez, L. R. (2017). Role of microglia in fungal infections of the central nervous system. Virulence 8, 705–718. doi: 10.1080/21505594.2016.1261789
Kovats, S. (2015). Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 294, 63–69. doi: 10.1016/j.cellimm.2015.01.018
Lambracht-Hall, M., Dimitriadou, V., and Theoharides, T. C. (1990). Migration of mast cells in the developing rat brain. Dev. Brain Res. 56, 151–159. doi: 10.1016/0165-3806(90)90077-c
Levi-Montalcini, R. (1987). The nerve growth factor 35 years later. Science 237, 1154–1162. doi: 10.1126/science.3306916
Liu, Y., Ho, R. C., and Mak, A. (2012). The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int. J. Rheum. Dis. 15, 183–187. doi: 10.1111/j.1756-185X.2011.01673.x
Lucas, H. J., Brauch, C. M., Settas, L., and Theoharides, T. C. (2006). Fibromyalgia–new concepts of pathogenesis and treatment. Int. J. Immunopathol. Pharmacol. 19, 5–10.
Maren, S. (2017). Synapse-Specific encoding of fear memory in the amygdala. Neuron 95, 988–990. doi: 10.1016/j.neuron.2017.08.020
Mastrangelo, F., Frydas, I., Ronconi, G., Kritas, S. K., Tettamanti, L., Caraffa, A., et al. (2018). Low-grade chronic inflammation mediated by mast cells in fibromyalgia: role of IL-37. J. Biol. Regul. Homeost Agents 32, 195–198.
McBeth, J., and Mulvey, M. R. (2012). Fibromyalgia: mechanisms and potential impact of the ACR 2010 classification criteria. Nat. Rev. Rheumatol. 8, 108–116. doi: 10.1038/nrrheum.2011.216
McLean, S. A., Williams, D. A., Stein, P. K., Harris, R. E., Lyden, A. K., Whalen, G., et al. (2006). Cerebrospinal fluid corticotropin-releasing factor concentration is associated with pain but not fatigue symptoms in patients with fibromyalgia. Neuropsychopharmacology 31, 2776–2782. doi: 10.1038/sj.npp.1301200
McNeil, B. D., Pundir, P., Meeker, S., Han, L., Undem, B. J., Kulka, M., et al. (2015). Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241. doi: 10.1038/nature14022
Meng, X., Zhang, Y., Lao, L., Saito, R., Li, A., Backman, C. M., et al. (2013). Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain 154, 294–305. doi: 10.1016/j.pain.2012.10.022
Minerbi, A., Gonzalez, E., Brereton, N. J. B., Anjarkouchian, A., Dewar, K., Fitzcharles, M. A., et al. (2019). Altered microbiome composition in individuals with fibromyalgia. Pain doi: 10.1097/j.pain.0000000000001640 [Epub ahead of print].
Morgan, J. T., Chana, G., Pardo, C. A., Achim, C., Semendeferi, K., Buckwalter, J., et al. (2010). Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry 68, 368–376. doi: 10.1016/j.biopsych.2010.05.024
Mukai, K., Tsai, M., Saito, H., and Galli, S. J. (2018). Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 282, 121–150. doi: 10.1111/imr.12634
Nakae, S., Suto, H., Kakurai, M., Sedgwick, J. D., Tsai, M., and Galli, S. J. (2005). Mast cells enhance T cell activation: importance of mast cell-derived TNF. Proc. Natl. Acad. Sci. U.S.A. 102, 6467–6472. doi: 10.1073/pnas.0501912102
Nakagawa, Y., and Chiba, K. (2016). Involvement of neuroinflammation during brain development in social cognitive deficits in autism spectrum disorder and schizophrenia. J. Pharmacol. Exp. Ther. 358, 504–515. doi: 10.1124/jpet.116.234476
Nugraha, B., Korallus, C., Kielstein, H., and Gutenbrunner, C. (2013). CD3+CD56+natural killer T cells in fibromyalgia syndrome patients: association with the intensity of depression. Clin. Exp. Rheumatol. 31, S9–S15.
Olszewski, M. B., Groot, A. J., Dastych, J., and Knol, E. F. (2007). TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J. Immunol. 178, 5701–5709. doi: 10.4049/jimmunol.178.9.5701
Orsolini, L., Sarchione, F., Vellante, F., Fornaro, M., Matarazzo, I., Martinotti, G., et al. (2018). Protein-C reactive as biomarker predictor of schizophrenia phases of illness? A systematic review. Curr. Neuropharmacol. 16, 583–606. doi: 10.2174/1570159X16666180119144538
Ottosson, A., and Edvinsson, L. (1997). Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia 17, 166–174. doi: 10.1046/j.1468-2982.1997.1703166.x
Pang, X., Cotreau-Bibbo, M. M., Sant, G. R., and Theoharides, T. C. (1995). Bladder mast cell expression of high affinity estrogen receptors in patients with interstitial cystitis. Br. J. Urol. 75, 154–161. doi: 10.1111/j.1464-410x.1995.tb07303.x
Pennisi, M., Crupi, R., Di, P. R., Ontario, M. L., Bella, R., Calabrese, E. J., et al. (2017). Inflammasomes, hormesis, and antioxidants in neuroinflammation: role of NRLP3 in Alzheimer disease. J. Neurosci. Res. 95, 1360–1372. doi: 10.1002/jnr.23986
Pernambuco, A. P., Schetino, L. P., Alvim, C. C., Murad, C. M., Viana, R. S., Carvalho, L. S., et al. (2013). Increased levels of IL-17A in patients with fibromyalgia. Clin. Exp. Rheumatol. 31, S60–S63.
Petra, A. I., Tsilioni, I., Taracanova, A., Katsarou-Katsari, A., and Theoharides, T. C. (2018). Interleukin 33 and interleukin 4 regulate interleukin 31 gene expression and secretion from human laboratory of allergic diseases 2 mast cells stimulated by substance P and/or immunoglobulin E. Allergy Asthma Proc. 39, 153–160. doi: 10.2500/aap.2018.38.4105
Rafiee, Z. A., Falahatian, M., and Alsahebfosoul, F. (2018). Serum levels of histamine and diamine oxidase in multiple sclerosis. Am. J. Clin. Exp. Immunol. 7, 100–105.
Ransohoff, R. M., and Brown, M. A. (2012). Innate immunity in the central nervous system. J. Clin. Invest. 122, 1164–1171. doi: 10.1172/JCI58644
Reu, P., Khosravi, A., Bernard, S., Mold, J. E., Salehpour, M., Alkass, K., et al. (2017). The lifespan and turnover of microglia in the human brain. Cell Rep. 20, 779–784. doi: 10.1016/j.celrep.2017.07.004
Reus, G. Z., Fries, G. R., Stertz, L., Badawy, M., Passos, I. C., Barichello, T., et al. (2015). The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300, 141–154. doi: 10.1016/j.neuroscience.2015.05.018
Reynier-Rebuffel, A.-M., Mathiau, P., Callebert, J., Dimitriadou, V., Farjaudon, N., Kacem, K., et al. (1994). Substance P, calcitonin gene-related peptide, and capsaicin release serotonin from cerebrovascular mast cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 267, R1421–R1429.
Ribatti, D. (2015). The crucial role of mast cells in blood-brain barrier alterations. Exp. Cell Res. 338, 119–125. doi: 10.1016/j.yexcr.2015.05.013
Rivera, J., Fierro, N. A., Olivera, A., and Suzuki, R. (2008). New insights on mast cell activation via the high affinity receptor for IgE. Adv. Immunol. 98, 85–120. doi: 10.1016/S0065-2776(08)00403-3
Rodriguez-Pinto, I., Agmon-Levin, N., Howard, A., and Shoenfeld, Y. (2014). Fibromyalgia and cytokines. Immunol. Lett. 161, 200–203. doi: 10.1016/j.imlet.2014.01.009
Romero-Sanchez, C., Jaimes, D. A., Londono, J., De Avila, J., Castellanos, J. E., Bello, J. M., et al. (2011). Association between Th-17 cytokine profile and clinical features in patients with spondyloarthritis. Clin. Exp. Rheumatol. 29, 828–834.
Ross, R. L., Jones, K. D., Bennett, R. M., Ward, R. L., Druker, B. J., and Wood, L. J. (2010). Preliminary evidence of increased pain and elevated cytokines in fibromyalgia patients with defective growth hormone response to exercise. Open Immunol. J. 3, 9–18. doi: 10.2174/1874226201003010009
Rottem, M., and Mekori, Y. A. (2005). Mast cells and autoimmunity. Autoimmun. Rev. 4, 21–27. doi: 10.1016/j.autrev.2004.05.001
Russell, I. J. (1998). Advances in fibromyalgia: possible role for central neurochemicals. Am. J. Med. Sci. 315, 377–384. doi: 10.1016/s0002-9629(15)40355-6
Russell, I. J., and Larson, A. A. (2009). Neurophysiopathogenesis of fibromyalgia syndrome: a unified hypothesis. Rheum. Dis. Clin. North Am. 35, 421–435. doi: 10.1016/j.rdc.2009.06.005
Saghaei, E., Abbaszadeh, F., Naseri, K., Ghorbanpoor, S., Afhami, M., Haeri, A., et al. (2013). Estradiol attenuates spinal cord injury-induced pain by suppressing microglial activation in thalamic VPL nuclei of rats. Neurosci. Res. 75, 316–323. doi: 10.1016/j.neures.2013.01.010
Schmidt-Wilcke, T., and Clauw, D. J. (2011). Fibromyalgia: from pathophysiology to therapy. Nat. Rev. Rheumatol. 7, 518–527. doi: 10.1038/nrrheum.2011.98
Scholzen, T. E., Steinhoff, M., Bonaccorsi, P., Klein, R., Amadesi, S., Geppetti, P., et al. (2001). Neutral endopeptidase terminates substance P-induced inflammation in allergic contact dermatitis. J. Immunol. 166, 1285–1291. doi: 10.4049/jimmunol.166.2.1285
Schweiger, V., Martini, A., Bellamoli, P., Donadello, K., Schievano, C., Del, B. G., et al. (2019). Ultramicronized palmitoylethanolamide (um-PEA) as add-on treatment in fibromyalgia syndrome (FMS): retrospective observational study on 407 patients. CNS Neurol. Disord. Drug Targets doi: 10.2174/1871527318666190227205359 [Epub ahead of print].
Shemer, A., Erny, D., Jung, S., and Prinz, M. (2015). Microglia plasticity during health and disease: an immunological perspective. Trends Immunol. 36, 614–624. doi: 10.1016/j.it.2015.08.003
Silva-Freire, N., Mayado, A., Teodosio, C., Jara-Acevedo, M., Varez-Twose, I., Matito, A., et al. (2019). Bone marrow mast cell antibody-targetable cell surface protein expression profiles in systemic mastocytosis. Int. J. Mol. Sci. 20:E552. doi: 10.3390/ijms20030552
Skaper, S. D., Facci, L., Barbierato, M., Zusso, M., Bruschetta, G., Impellizzeri, D., et al. (2015). N-Palmitoylethanolamine and neuroinflammation: a novel therapeutic strategy of resolution. Mol. Neurobiol. 52, 1034–1042. doi: 10.1007/s12035-015-9253-8
Skaper, S. D., Facci, L., Fusco, M., la Valle, M. F., Zusso, M., Costa, B., et al. (2014a). Palmitoylethanolamide, a naturally occurring disease-modifying agent in neuropathic pain. Inflammopharmacology 22, 79–94. doi: 10.1007/s10787-013-0191-7
Skaper, S. D., Facci, L., and Giusti, P. (2014b). Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS Neurol. Disord. Drug Targets 13, 1654–1666. doi: 10.2174/1871527313666141130224206
Skaper, S. D., Facci, L., and Giusti, P. (2013). Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol. Neurobiol. 48, 340–352. doi: 10.1007/s12035-013-8487-6
Skaper, S. D., Facci, L., Zusso, M., and Giusti, P. (2017). Neuroinflammation, mast cells, and glia: dangerous liaisons. Neuroscientist 23, 478–498. doi: 10.1177/1073858416687249
Skaper, S. D., Giusti, P., and Facci, L. (2012). Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J. 26, 3103–3117. doi: 10.1096/fj.11-197194
Skokos, D., Botros, H. G., Demeure, C., Morin, J., Peronet, R., Birkenmeier, G., et al. (2003). Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J. Immunol. 170, 3037–3045. doi: 10.4049/jimmunol.170.6.3037
Skokos, D., Goubran-Botros, H., Roa, M., and Mecheri, S. (2002). Immunoregulatory properties of mast cell-derived exosomes. Mol. Immunol. 38, 1359–1362. doi: 10.1016/s0161-5890(02)00088-3
Staud, R., Vierck, C. J., Cannon, R. L., Mauderli, A. P., and Price, D. D. (2001). Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 91, 165–175. doi: 10.1016/s0304-3959(00)00432-2
Suzuki, K., Sugihara, G., Ouchi, Y., Nakamura, K., Futatsubashi, M., Takebayashi, K., et al. (2013). Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 70, 49–58.
Takeda, S., Sato, N., and Morishita, R. (2014). Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front. Aging Neurosci. 6:171. doi: 10.3389/fnagi.2014.00171
Taracanova, A., Alevizos, M., Karagkouni, A., Weng, Z., Norwitz, E., Conti, P., et al. (2017). SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc. Natl. Acad. Sci. U.S.A. 114, E4002–E4009. doi: 10.1073/pnas.1524845114
Taracanova, A., Tsilioni, I., Conti, P., Norwitz, E. R., Leeman, S. E., and Theoharides, T. C. (2018). Substance P and IL-33 administered together stimulate a marked secretion of IL-1beta from human mast cells, inhibited by methoxyluteolin. Proc. Natl. Acad. Sci. U.S.A 115, E9381–E9390. doi: 10.1073/pnas.1810133115
Theoharides, T. C. (1983). Mast cells and migraines. Perspect. Biol. Med. 26, 672–675. doi: 10.1353/pbm.1983.0028
Theoharides, T. C. (1990). Mast cells: the immune gate to the brain. Life Sci. 46, 607–617. doi: 10.1016/0024-3205(90)90129-f
Theoharides, T. C. (1996). Mast cell: a neuroimmunoendocrine master player. Int. J. Tissue React. 18, 1–21.
Theoharides, T. C. (2013). Atopic conditions in search of pathogenesis and therapy. Clin. Ther. 35, 544–547. doi: 10.1016/j.clinthera.2013.04.002
Theoharides, T. C. (2017). Neuroendocrinology of mast cells: challenges and controversies. Exp. Dermatol. 26, 751–759. doi: 10.1111/exd.13288
Theoharides, T. C., Alysandratos, K. D., Angelidou, A., Delivanis, D. A., Sismanopoulos, N., Zhang, B., et al. (2010a). Mast cells and inflammation. Biochim. Biophys. Acta 1822, 21–33.
Theoharides, T. C., Zhang, B., Kempuraj, D., Tagen, M., Vasiadi, M., Angelidou, A., et al. (2010b). IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. U.S.A. 107, 4448–4453. doi: 10.1073/pnas.1000803107
Theoharides, T. C., Dimitriadou, V., Letourneau, R. J., Rozniecki, J. J., Vliagoftis, H., and Boucher, W. S. (1993). Synergistic action of estradiol and myelin basic protein on mast cell secretion and brain demyelination: changes resembling early stages of demyelination. Neuroscience 57, 861–871. doi: 10.1016/0306-4522(93)90030-j
Theoharides, T. C., Donelan, J., Kandere-Grzybowska, K., and Konstantinidou, A. (2005). The role of mast cells in migraine pathophysiology. Brain Res. Brain Res. Rev. 49, 65–76. doi: 10.1016/j.brainresrev.2004.11.006
Theoharides, T. C., and Kavalioti, M. (2018). Stress, inflammation and natural treatments. J. Biol. Regul. Homeost Agents 32, 1345–1347.
Theoharides, T. C., Kempuraj, D., Tagen, M., Conti, P., and Kalogeromitros, D. (2007). Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 217, 65–78. doi: 10.1111/j.1600-065x.2007.00519.x
Theoharides, T. C., and Konstantinidou, A. (2007). Corticotropin-releasing hormone and the blood-brain-barrier. Front. Biosci. 12:1615–1628. doi: 10.2741/2174
Theoharides, T. C., Letourneau, R., Patra, P., Hesse, L., Pang, X., Boucher, W., et al. (1999). Stress-induced rat intestinal mast cell intragranular activation and inhibitory effect of sulfated proteoglycans. Dig. Dis. Sci. 44, 87S–93S.
Theoharides, T. C., Petra, A. I., Stewart, J. M., Tsilioni, I., Panagiotidou, S., and Akin, C. (2014). High serum corticotropin-releasing hormone (CRH) and bone marrow mast cell CRH receptor expression in a mastocytosis patient. J. Allergy Clin. Immunol. 134, 1197–1199. doi: 10.1016/j.jaci.2014.05.023
Theoharides, T. C., Petra, A. I., Taracanova, A., Panagiotidou, S., and Conti, P. (2015a). Targeting IL-33 in autoimmunity and inflammation. J. Pharmacol. Exp. Ther. 354, 24–31. doi: 10.1124/jpet.114.222505
Theoharides, T. C., Stewart, J. M., Hatziagelaki, E., and Kolaitis, G. (2015b). Brain “fog,” inflammation and obesity: key aspects of neuropsychiatric disorders improved by luteolin. Front. Neurosci. 9:225. doi: 10.3389/fnins.2015.00225
Theoharides, T. C., Tsilioni, I., Arbetman, L., Panagiotidou, S., Stewart, J. M., Gleason, R. M., et al. (2015c). Fibromyalgia, a syndrome in search of pathogenesis and therapy. J. Pharmacol. Exp. Ther. 355, 255–263.
Theoharides, T. C., Valent, P., and Akin, C. (2015d). Mast cells, mastocytosis, and related disorders. N. Engl. J. Med. 373, 163–172. doi: 10.1056/nejmra1409760
Theoharides, T. C., Stewart, J. M., and Tsilioni, I. (2017). Tolerability and benefit of a tetramethoxyluteolin-containing skin lotion. Int. J. Immunopathol. Pharmacol. 30, 146–151. doi: 10.1177/0394632017707610
Theoharides, T. C., and Tsilioni, I. (2018). Tetramethoxyluteolin for the treatment of neurodegenerative diseases. Curr. Top. Med. Chem. 18, 1872–1882. doi: 10.2174/1568026617666181119154247
Theoharides, T. C., Tsilioni, I., and Ren, H. (2019). Recent advances in our understanding of mast cell activation - or should it be mast cell mediator disorders? Expert. Rev. Clin. Immunol. 15, 639–656. doi: 10.1080/1744666X.2019.1596800
Thonhoff, J. R., Simpson, E. P., and Appel, S. H. (2018). Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis. Curr. Opin. Neurol. 31, 635–639. doi: 10.1097/WCO.0000000000000599
Torresani, C., Bellafiore, S., and De Panfilis, G. (2009). Chronic urticaria is usually associated with fibromyalgia syndrome. Acta Derm. Venereol. 89, 389–392. doi: 10.2340/00015555-0653
Tsilioni, I., Panagiotidou, S., and Theoharides, T. C. (2014). Exosomes in neurologic and psychiatric disorders. Clin. Ther. 36, 882–888. doi: 10.1016/j.clinthera.2014.05.005
Tsilioni, I., Russell, I. J., Stewart, J. M., Gleason, R. M., and Theoharides, T. C. (2016). Neuropeptides CRH, SP, HK-1, and inflammatory cytokines IL-6 and TNF are increased in serum of patients with fibromyalgia syndrome, implicating mast cells. J. Pharmacol. Exp. Ther. 356, 664–672. doi: 10.1124/jpet.115.230060
Tsilioni, I., and Theoharides, T. C. (2018). Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1beta. J. Neuroinflammation 15:239. doi: 10.1186/s12974-018-1275-5
Uceyler, N., Hauser, W., and Sommer, C. (2011). Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord. 12:245. doi: 10.1186/1471-2474-12-245
Vargas, D. L., Nascimbene, C., Krishnan, C., Zimmerman, A. W., and Pardo, C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 57, 67–81. doi: 10.1002/ana.20315
Wallon, C., Yang, P., Keita, A. V., Ericson, A. C., McKay, D. M., Sherman, P. M., et al. (2008). Corticotropin releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 57, 50–58. doi: 10.1136/gut.2006.117549
Wang, W., Ji, P., Riopelle, R. J., and Dow, K. E. (2002). Functional expression of corticotropin-releasing hormone (CRH) receptor 1 in cultured rat microglia. J. Neurochem. 80, 287–294. doi: 10.1046/j.0022-3042.2001.00687.x
Wolfe, F., and Walitt, B. (2013). Culture, science and the changing nature of fibromyalgia. Nat. Rev. Rheumatol. 9, 751–755. doi: 10.1038/nrrheum.2013.96
Woodman, I. (2013). Fibromyalgia: fibromyalgia-all in the brain? Nat. Rev. Rheumatol. 9:565. doi: 10.1038/nrrheum.2013.137
Woolf, C. J. (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain 152, S2–S15. doi: 10.1016/j.pain.2010.09.030
Wu, Y., Dissing-Olesen, L., MacVicar, B. A., and Stevens, B. (2015). Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 36, 605–613. doi: 10.1016/j.it.2015.08.008
Xanthos, D. N., Gaderer, S., Drdla, R., Nuro, E., Abramova, A., Ellmeier, W., et al. (2011). Central nervous system mast cells in peripheral inflammatory nociception. Mol. Pain 7:42. doi: 10.1186/1744-8069-7-42
Yunus, M. B. (2007). Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin. Arthritis Rheum. 36, 339–356. doi: 10.1016/j.semarthrit.2006.12.009
Zhang, B., Asadi, S., Weng, Z., Sismanopoulos, N., and Theoharides, T. C. (2012). Stimulated human mast cells secrete mitochondrial components that have autocrine and paracrine inflammatory actions. PLoS One 7:e49767. doi: 10.1371/journal.pone.0049767
Zhang, S., Zeng, X., Yang, H., Hu, G., and He, S. (2012). Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell Physiol. Biochem. 29, 931–940. doi: 10.1159/000171029
Zhang, Q., Raoof, M., Chen, Y., Sumi, Y., Sursal, T., Junger, W., et al. (2010). Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107. doi: 10.1038/nature08780
Zhang, X., Wang, Y., Dong, H., Xu, Y., and Zhang, S. (2016). Induction of microglial activation by mediators released from mast cells. Cell Physiol. Biochem. 38, 1520–1531. doi: 10.1159/000443093
Keywords: mast cells, pain, neuroinflammation, fibromyalgia syndrome, proinflammatory cytokines (TNF-alpha, IL-1 beta, IL-6)
Citation: Theoharides TC, Tsilioni I and Bawazeer M (2019) Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 13:353. doi: 10.3389/fncel.2019.00353
Received: 20 May 2019; Accepted: 16 July 2019;
Published: 02 August 2019.
Edited by:
Kempuraj Duraisamy, University of Missouri, United StatesReviewed by:
Domenico De Berardis, Azienda Usl Teramo, ItalyCopyright © 2019 Theoharides, Tsilioni and Bawazeer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theoharis C. Theoharides, dGhlb2hhcmlzLnRoZW9oYXJpZGVzQHR1ZnRzLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.