- 1Department of Clinical and Biological Sciences, University of Turin, Turin, Italy
- 2Neuroscience Institute Cavalieri Ottolenghi Foundation (NICO), University of Turin, Turin, Italy
- 3Dipartimento di Chirurgia Generale e Specialistica, Azienda Ospedaliera Universitaria, Ancona, Italy
- 4UO Microchirurgia e Chirurgia della Mano, Ospedale Gaetano Pini, Milan, Italy
- 5Institute of Neuroanatomy and Cell Biology, Hannover Medical School, Hanover, Germany
- 6Center for Systems Neuroscience (ZSN) Hannover, Hanover, Germany
The successful introduction of innovative treatment strategies into clinical practise strongly depends on the availability of effective experimental models and their reliable pre-clinical assessment. Considering pre-clinical research for peripheral nerve repair and reconstruction, the far most used nerve regeneration model in the last decades is the sciatic nerve injury and repair model. More recently, the use of the median nerve injury and repair model has gained increasing attention due to some significant advantages it provides compared to sciatic nerve injury. Outstanding advantages are the availability of reliable behavioural tests for assessing posttraumatic voluntary motor recovery and a much lower impact on the animal wellbeing. In this article, the potential application of the median nerve injury and repair model in pre-clinical research is reviewed. In addition, we provide a synthetic overview of a variety of methods that can be applied in this model for nerve regeneration assessment. This article is aimed at helping researchers in adequately adopting this in vivo model for pre-clinical evaluation of peripheral nerve reconstruction as well as for interpreting the results in a translational perspective.
Introduction
Peripheral nerve injuries are commonly caused by motor vehicle, domestic, work or sport accidents or during surgeries (iatrogenic nerve injuries) (Jones et al., 2016). Nerve injuries can lead to motor and sensory deficits that may result in disabilities permanently compromising the patients’ quality of life.
The general ability of peripheral nerves to regenerate has been recognised more than a century ago, but until today functional recovery outcome after severe nerve injury and reconstructive surgery is often still poor in many patients. Nowadays, the “gold standard” reconstructive technique for bridging a nerve gap is autologous nerve grafting. This technique, however, is accompanied by important drawbacks such as the donor site morbidity, the need of additional surgery and the limited availability of graft material for extended repair (Konofaos and Ver Halen, 2013; Faroni et al., 2015).
Over the past decades, substantial effort has been made to identify new strategies to improve peripheral nerve regeneration after grafting and to substitute the autologous nerve graft. Advancements in biomedical methods, the tissue-engineered technology, gene therapy approaches, nanotechnology, biology, and microsurgical skills have opened new research fields in the nerve reconstruction area. Indeed, there is an exponential increase in the number of publications dealing with experimental nerve regeneration research over the years: a literature search with the PubMed search string (“Nerve-Regeneration”[Mesh] OR nerve-regenerat∗ OR nerve-repair∗) AND (rat∗ OR mouse OR mice OR rabbit∗ OR sheep), delivered 26 results in 1970 and 479 in 2018.
In the context of pre-clinical peripheral nerve regeneration research, the choice of the experimental animal model is of fundamental importance. When a researcher moves on to test a novel attempt in vivo, the animal model should be chosen according to the study aims [e.g., for studying the involvement of a specific molecule in the biological process of nerve regeneration, the most appropriate choice will most likely be different to the model chosen for evaluating the effectiveness of a nerve conduit for long gap (>50 mm) repair]. Obviously, the pros and cons of the different available options must also be taken into careful consideration.
The choice of the appropriate experimental nerve injury model is usually guided by several factors. For nerve repair studies, in particular, the size (diameter and length) of the model nerve is certainly one of the main aspects considered. Indeed, most nerve repair studies are conducted on the sciatic nerve especially because of its big dimension that facilitates experimental microsurgery (Varejao et al., 2004; Bozkurt et al., 2011; Sinis et al., 2011a). The sciatic nerve is the biggest nerve in the body and the choice among mouse, rat, rabbit, dog, or sheep already provides variability in nerve gap lengths to be applied (Angius et al., 2012).
Different downsides resulting from experimental injury to the sciatic nerve have, however, led to increasing interest in the median nerve as alternative model nerve (Bertelli et al., 2004). At first, injury to the sciatic nerve results in a paralysis of the hind limb and, often, in automutilation behaviour, such as biting and self-amputation of denervated toes and paw areas by the subjected animal. Longer lasting paralysis (>4 weeks in the rat) often leads to joint contractures and stiffness. Automutilation behaviour and joint contractures reduce the reliability of functional tests, such as estimation of the sciatic function index or, in severe cases, will lead to exclusion of the respective animal from a study for ethical and animal welfare reasons. Furthermore, possibilities for evaluation functional recovery of motor skills after sciatic nerve lesion in the awaken animal are rather limited or need considerable efforts to be realised (Navarro, 2016).
In the recent years employment of the median nerve injury and repair model in the experimental research has increased (Papalia et al., 2003b; Ronchi et al., 2009) because of several advantages. Transection injury of the rodent median nerve, results in only partial impairment of the upper limb function (Bertelli et al., 1995). Incidence of automutilation is significantly lower in comparison to the sciatic nerve model, ulcerations are fewer and no joint contractures can be seen. This milder phenotype results from the fact that after median nerve injury, the ulnar and radial nerves still preserve sensitivity and motor function in the forearm (Sinis et al., 2006). An additional advantage of the rodent median nerve injury and repair model is that positively evaluated attempts are more likely to be translated into clinical practise, since surgical interventions for the repair of a damaged human nerve are very often performed at the upper limb level. In addition, the hand functions require a fine finger movement that is quite similar between rodents and humans (Whishaw et al., 1992). From this perspective, the possibility to apply specific and precise functional tests for motor recovery evaluation following median nerve reconstruction is a further pro of this model in pre-clinical research.
This review provides an overview on the use of the median nerve injury and repair experimental model in pre-clinical research. The different animal species (not only mouse and rat but also larger animals such as rabbit, sheep, and monkey) are taken into account as are the different options these species provide with regard to comprehensive analysis of the regeneration outcome.
The Pre-Clinical Median Nerve Injury and Repair Model in Different Animal Species
The use of the median nerve injury and repair model as pre-clinical model has progressively increased in the last years, but the sciatic nerve injury and repair model is still more often employed [in PubMed a research on (median-nerv∗) AND (regenerat∗ OR repair) yielded 1002 papers, while (sciatic-nerv∗) yielded 6612 papers].
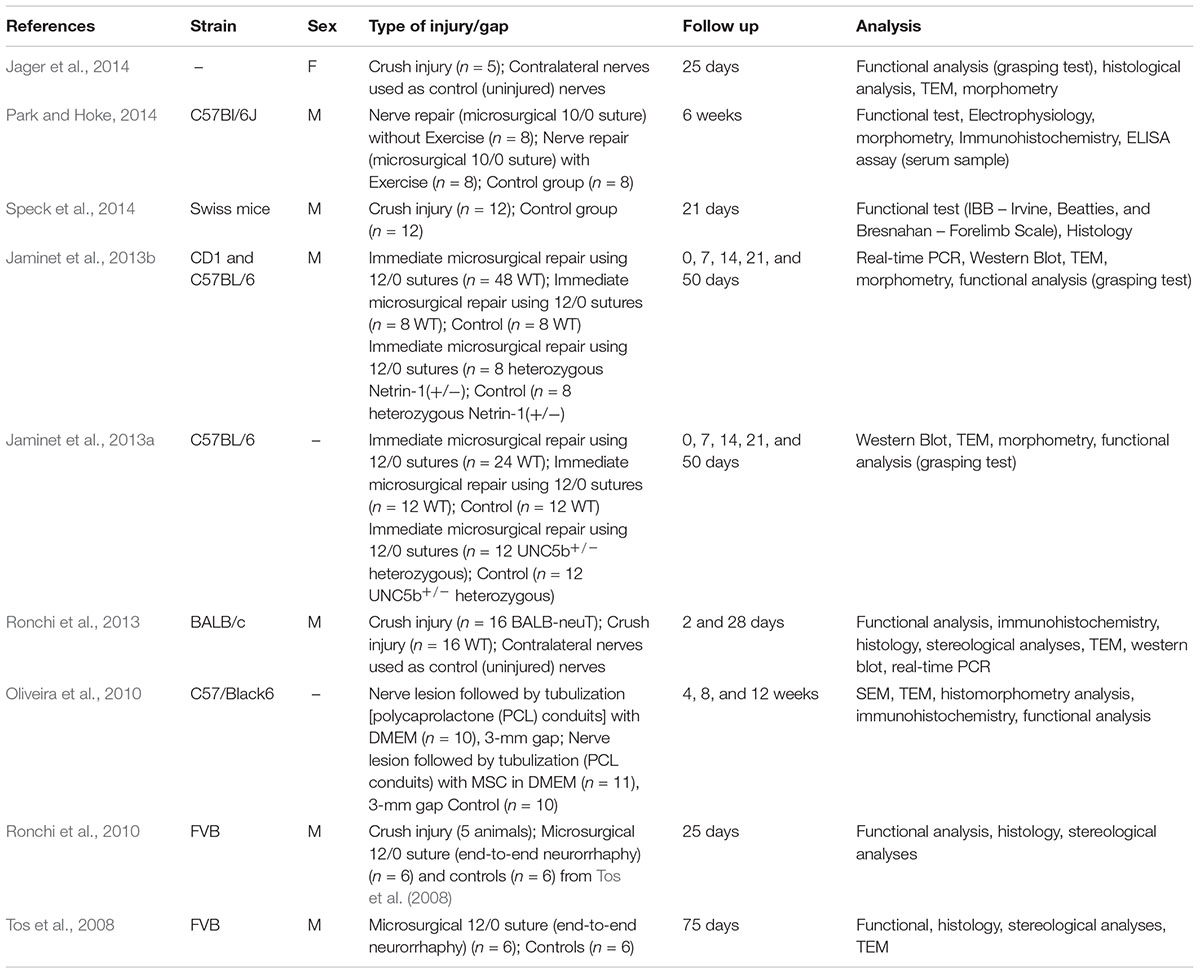
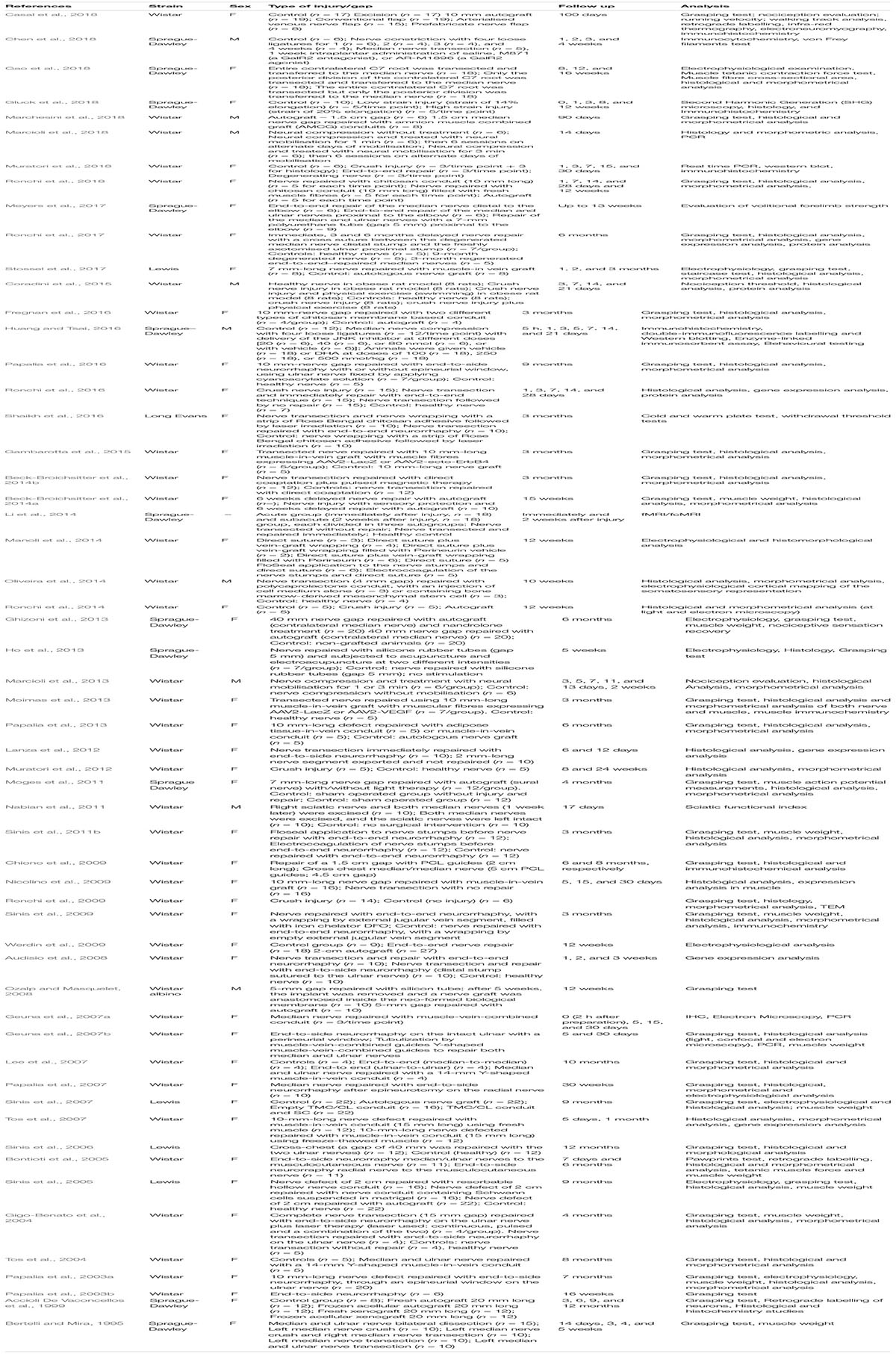
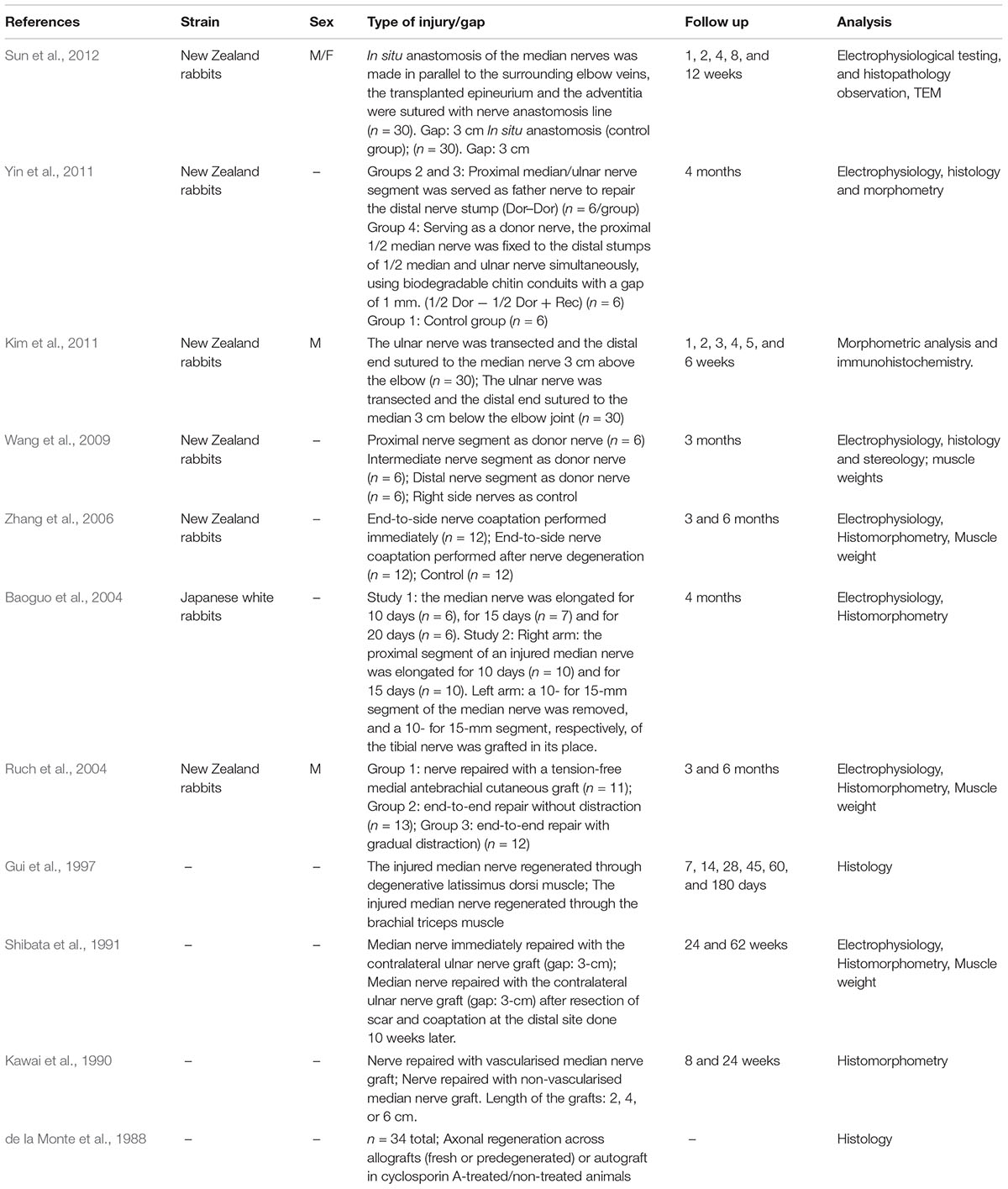
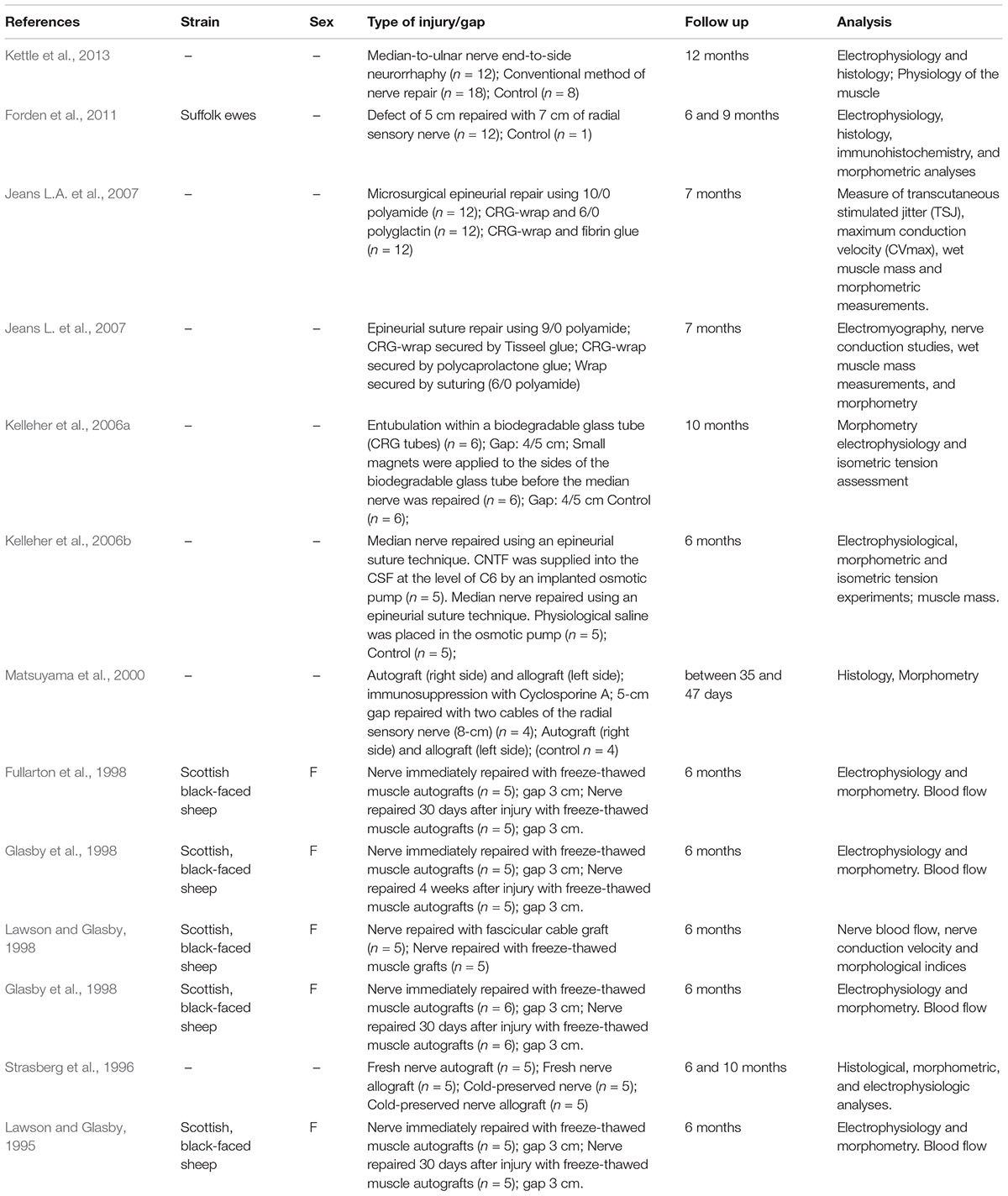
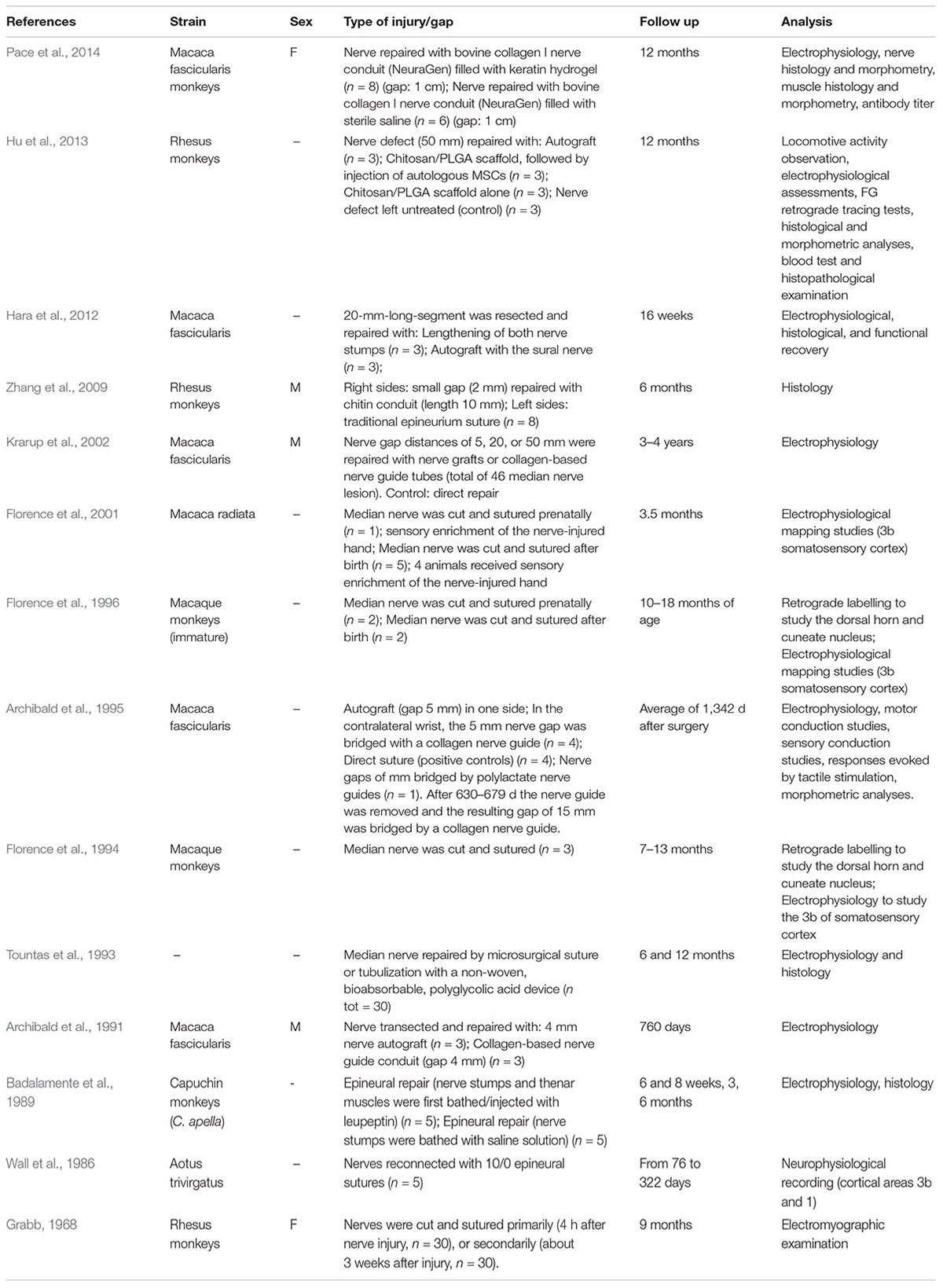
Information from our literature search is presented in Tables 1–5. The tables summarise peripheral nerve regeneration studies after median nerve injury and repair in the different animal models. In addition to the specific reference, the tables list animal strain and sex, type of injury/gap, follow up periods and type of analyses conducted. We took our best efforts to include all articles available until end of 2018; nevertheless, inadvertently, we could have missed some papers and apologise in advance with their authors.
In the following paragraph specificities of the different models listed in the tables will be reviewed in more detail.
Mouse Model
Since most of the available transgenic animal models are mice, they are often used for studying the role of specific genes in the peripheral nerve regeneration process. For this purpose, genes of interest are knocked-out, mutated or over-expressed. Moreover, mice – especially wild type strains – are economical in their keeping, simple to handle and to care for and can therefore be studied in large groups. Mice nerves can be subjected to different types of injury and repair and analysed with all kinds of functional, morphological and biomolecular assays. The most simple crush injury can be easily used and standardised. On the other hand, peripheral nerves in mice are rather small and experimental in vivo work employing more complex surgeries on them, such as end-to-end repair, requires advanced microsurgical skills. These skills are provided by many clinical researchers from disciplines routinely performing nerve surgeries but are less prevalent in the basic researcher community. Furthermore, when it comes to studies evaluating new developments for bio-artificial nerve guides, the much smaller diameter of mouse peripheral nerves is not fitting the larger one of nerve guides that are primarily commonly designed to fit human digital nerves.
Rat Model
Among rodents, rats are the most commonly used in vivo model in peripheral nerve regeneration research. This relates mainly to the dimension of their nerves. Surgery on rat peripheral nerves requires less microsurgical skills than needed for mouse models and rat limbs and nerves are long enough to allow 1.5–2 cm gap repair to be studied at least in the sciatic nerve. Moreover, rats can be investigated in large groups because their keeping is economical, and they are mostly simple to handle and to care for. Transgenic rat models are, however, less available than mouse transgenic models and also the availability of rat specific antibodies for molecular and histological examination is rather limited. But due to their prevalent employment, functional tests for evaluating motor or sensory recovery in rats are more standardised (Navarro, 2016) and comparable among different research groups. Rat nerves can be subjected to different types of injury and repair and analysed in most comprehensive functional, morphological and biomolecular assays (Ronchi et al., 2009; Navarro, 2016; Ronchi et al., 2016). Different rat strains can be utilised, for which varying willingness to enrol in specific functional tests is reported (Nikkhah et al., 1998; Galtrey and Fawcett, 2007), but so no comparative studies investigating differences in their ability to regenerate have been published.
When using rodents (mice and rats) as animal models for peripheral nerve regeneration, researchers must finally be aware of the following immanent differences to mankind: (1) gaps that can be produced are shorter than those commonly found in human nerve lesions; (2) axonal regeneration rate is faster than in humans; (3) recovery is often complete, while in humans it is often incomplete (Kaplan et al., 2015).
Rabbit Model
In peripheral nerve regeneration research, rabbits offer the possibility to study regeneration across gap lengths of up to 6 cm. Rabbits are, however, expensive to purchase and maintain, and difficult to care for. Rabbits are more delicate and less resilient than rats and mice, and their occurrence as pet animals probably creates ethical problems for animal care takers and researchers. Also, there are almost no valid functional assays that can be applied in rabbit models, besides electrodiagnostic evaluation. Finally, very few specific antibodies are available to be used on rabbit tissue samples, so that conclusions on the regeneration outcome can mainly only be based on nerve morphometry studies.
Sheep Model
The sheep as an animal model is useful when nerve regeneration across very long gaps should be evaluated. An ethical advantage of this animal is provided by the fact that a median nerve transection injury does not result in serious impairment of the limb usage ability and functional read-outs have been described in the recent years. To establish the model and to provide adequate housing conditions is, however, a considerable challenge, and research in this model will often only be realised through collaborative work.
Monkey Model
Although non-human primates could be useful to test safety and efficacy of synthetic nerve conduits – because of the similarity of non-human primates with human beings – their use is considerably limited for ethical reasons. Monkeys are considered as animal models mainly to study neuronal plasticity occurring in the brain following peripheral nerve injury and repair. Again, studies in this model will mainly be subject of collaborative work and not appropriate for early stage pre-clinical research.
Other Animals
Other large animals can be used as models to study median nerve injury and regeneration, such as pigs (Ochoa and Marotte, 1973; Marotte, 1974), dogs (Lee et al., 1999), and cats (Murray et al., 1997), but their use is limited for three main reasons: (1) animal care for large species is considerably expensive; (2) for some species the possibilities for functional testing are limited or require complex training, therefore, nerve regeneration may only be assessed with nerve morphometry; (3) dogs and cats are domestic animals, and their use in research is more restricted for ethical reasons.
Methodological Considerations – Approaches for the Treatment of Nerve Injuries
The following criteria should be considered for selecting the most suitable animal model for pre-clinical research on peripheral nerve regeneration: (1) costs to purchase and house the animals; (2) ease of handling, such as tolerance to captivity; (3) tolerance for surgery and eventual repetitive anaesthesia, as well as resistance to infection; (4) compliance with national policies and with ethical principles; (5) inter-animal uniformity, life span of the species, biological information and available tools; (6) overall experimental plan (Angius et al., 2012). With regard to the latter, especially when several time-points are to be analysed, rodents represent the best choice. On the contrary, rodent life span is short, therefore for long experiments (>1–1.5 years), it becomes necessary to use lager animals as model organism.
In the last years, several approaches have been developed and tested in pre-clinical animal models to improve peripheral nerve regeneration. All these approaches can be applied on different nerves and are not median-nerve specific. In this paragraph we will present an overview of different techniques, including the use of different conduits to guide regenerating axons, the use of stem cell transplantation, the application of physical therapies and optogenetics, all of which have been demonstrating variable positive effects on nerve regeneration irrespectively of the model they were investigated in.
Nerve Guidance Conduits
The major disadvantage of the autologous nerve graft technique is the remarkable sensitivity loss (mainly sural nerve grafts are harvested for this) and the limited availability of donor tissue (Ray and Mackinnon, 2010). Reconstruction of a nerve with artificial or non-artificial nerve conduit grafts is particularly indicated in case of extensive nerve tissue loss. Nerve guidance conduits made of several materials, artificial or of natural origin, are available; most common and FDA approved for clinical use are conduits made of collagen, chitosan, or poly (DL-lactide-ε-caprolactone) (Kornfeld et al., 2018). All devices currently on the market and FDA-approved have proven good support for the promotion of peripheral nerve regeneration in pre-clinical models. Good clinical results, however, are obtained only for lesions with a substance loss inferior to 3 cm in length, while severe and enlarged injuries remain a critical condition (Kaplan et al., 2015). For this reason, research in the field of novel nerve conduit functionalisation strategies is still highly vivid.
Application of Stem Cells and Their Secretome
In regenerative medicine and tissue engineering, the use of Stem Cells and their secretome is fast expanding with the aim to develop innovative therapeutic strategies for the treatment of peripheral nerve injuries (Caplan, 2015; Caseiro et al., 2016; Busuttil et al., 2017; Sayad-Fathi et al., 2019). In particular, Mesenchymal Stem Cells (MSCs) present relevant key features: they can be easily expanded, they can differentiate into different cell types, they are immune-privileged and immune-modulatory, they show preferential homing to injured sites (Frausin et al., 2015; Sullivan et al., 2016; Jiang et al., 2017). Moreover, the MSC secretome contains trophic mediators (Meirelles Lda et al., 2009; Fu et al., 2017), modulating the function of several tissues, including the skeletal muscle (Pereira et al., 2014) and the peripheral nervous system (Lopatina et al., 2011; Gartner et al., 2012, 2014).
The most widely source of MSCs for therapeutic purposes is the bone marrow; as good alternative other sources are: the umbilical cord blood, the stromal tissue of the umbilical cord, the dental pulp, the adipose tissue (Jin et al., 2013).
Transgenic Models to Promote Peripheral Nerve Regeneration
To study the biology of peripheral nerve regeneration, different transgenic models can be used (Magill et al., 2008). Most of the available transgenic animals are mice and they represent a powerful tool to study the influence of over-expression or depletion or mutation of a specific gene in a specific cell type, using inducible systems, but it must be kept in mind that mice are difficult subjects for microsurgical models due to the small size of their nerves, as discussed above.
To investigate the function of specific genes in nerve regeneration discriminating between motor and sensitive neurons, transgenic mice over-expressing the gene of interest in postnatal motoneurons or dorsal root ganglion neurons can be obtained using Thy1 or NSE (neuron specific enolase) promoters (Michailov et al., 2004; Gomez-Sanchez et al., 2009; Velanac et al., 2012).
To obtain tissue specific expression or depletion of specific proteins, the inducible cre-lox system can be applied: transgenic mice driving motor neuron specific expression of cre recombinase with the promoter of Mnx1 (motor neuron and pancreas homeobox 1) gene can be used to specifically in/activate the expression of floxed genes in motor neurons, while specific in/activation in Schwann cells can be obtained using the promoter of Mpz (myelin protein zero) gene (La Marca et al., 2011).
Transgenic animals can also be developed as advanced experimental models to study genetic diseases giving rise to peripheral neuropathies (Hoke, 2012; Juneja et al., 2018), and they can be studied to investigate their ability to regenerate injured peripheral nerves.
Finally, the expression of neurotrophic factors or other potentially therapeutic proteins in Schwann cells or in neurons can be obtained through the use of different viral vectors (Tannemaat et al., 2008).
Physical Therapies
The efficacy of brief Electric Stimulation (ES) of the proximal stump of an injured nerve in promoting nerve regeneration in animal models has been verified in several independent studies reviewed by Gordon (2016) and Gordon and English (2016). In particular, a 14 days period of ES was chosen (Al-Majed et al., 2000) to accelerate the regenerative process and the effect was dramatic: preferential motor reinnervation of motor pathways was evident at 21 days rather than at 42 days, and, importantly, all of the motoneurons had regenerated into the motor nerve branch.
Another interesting study was aiming at evaluating the value of electromagnetic stimulation for the neural regenerative process of the rat median nerve after transection and end-to-end repair (Beck-Broichsitter et al., 2014b). From the 1st day after surgery a pulsed magnetic therapy was daily applied in the experimental group. Magnetic stimulation was positively influencing the functional regeneration in terms of grasping force and reduced muscular atrophy.
Optogenetics
Axonal regeneration and functional recovery are enhanced by activity-related therapies, such as exercise and electrical stimulation (Gordon, 2016). Unlike electrical stimulation, optogenetics allows to selectively activate or inactivate specific neurons: for example, selective expression of the light-sensitive cation channel channelrhodopsin-2 (ChR2), that is maximally activated by blue light, can be used to depolarise neurons, thereby driving action potentials. Conversely, selective expression of the light-sensitive inward chloride pump halorhodopsin (Halo), that is maximally activated by amber light, can be used to obtain neuron hyperpolarisation, thereby inhibiting action potentials (Montgomery et al., 2016). Optically induced neuronal activity has been shown to be sufficient to promote functional motor axon regeneration in vivo (Ward et al., 2016). Moreover, through the selective expression of opsins in sensory neurons or motoneurons, it was possible to investigate the effect of system-specific neuronal activation on axonal regeneration, thus demonstrating that acute activation is sufficient to enhance regeneration of both motor and sensory axons (Ward et al., 2018).
Until now, in the peripheral nervous system, optogenetics has been applied mainly to sciatic nerves in transgenic mice expressing different opsins, but given its therapeutic potential it will be certainly applied also to median nerves and in other animal models, by injection of optogenetic constructs to transduce opsin expression in peripheral nerves in the future.
Immunomodulation
The early inflammatory reactions undergoing in the course of Wallerian Degeneration of the distal nerve, comprise the activation of the complement system, arachidonic acid metabolites, and inflammatory mediators involved in myelin fragmentation and activation of repair Schwann cells. Fine-tuned upregulation of the cytokine/chemokine network by repair Schwann cells activates resident and hematogenous macrophages to complete the clearance of axonal and myelin debris and stimulate regrowth of axonal sprouts (Yona and Jung, 2010; Cortez-Retamozo et al., 2012; Dubovy et al., 2013; Jessen and Mirsky, 2016). An innovative approach in the field of peripheral nerve regeneration is exploiting the endogenous capacity of the body to repair itself through immune cells. In very promising studies, starting from the known different pro-inflammatory and pro-regenerative macrophage phenotypes, they were modulated through their response to different IFN-γ or IL-4 cytokines and studied in their ability to influence nerve regeneration in a critically sized, 15 mm rat sciatic nerve gap. The results of this research have shown that the administration of IL-4 at the injury site increased the pro-regenerative effect and therefore that the regenerative outcomes appeared to be influenced not only by the macrophage presence, but by their specific phenotype at the site of injury (Mokarram et al., 2012). A similar approach was conducted later by the same authors, through early stage administration of fractalkine, a chemokine able to control the phenotype in monocyte recruitment and to increase the regenerative potential. The pharmaceutical approach was evaluated from a morphological and functional point of view (Mokarram et al., 2017).
Methodological Considerations – Techniques to Investigate Peripheral Nerve Regeneration
A number of different techniques have been developed to investigate the degree and the accuracy of nerve regeneration. While functional tests must be nerve-specific, all other methods can be applied to all types of peripheral nerves. In this paragraph we describe in detail different functional tests that are used to study the functional recovery of the median nerve and, in addition, we present an overview of other methods used for the investigation of nerve regeneration, including morphological and morphometrical analysis, gene expression analysis and fluorescent transgenic animal models.
Functional Evaluation
Several functional tests are available for rodents, both rats and mice (Galtrey and Fawcett, 2007). Some have been designated ad hoc to evaluate functional recovery following median nerve repair (e.g., grasping test), others have been adapted from tests normally used following other lesion types (injuries to the spinal cord or sciatic nerves).
The tests described below refer to the rat, but most of them are adaptable also to mice.
• The grasping test is a simple method to assess the flexor function, first introduced by Bertelli and Mira (1995). The modified method (Papalia et al., 2003a) consists in presenting a small tower with only three bars forming a triangle on its top instead of a grid for grasping. With this modification the tendency to walk on the grid is avoided and the presence of a band put just below the three bars avoids that the rat employs the wrist flexion to hold the bars. This device is connected to a precision dynamometer. The animal is hold by its tail and allowed to grasp the grid. Then, the animal is pulled upward until it loses its grip. The balance records the maximum weight that the animal managed to hold before losing the grip.
• The staircase test is a functional test which assesses skilled forepaw reaching and grasping (Montoya et al., 1991). Two/three food pellets are placed on each step of two staircases located one on either side of a central platform. Both stairs are composed of seven steps. The animal is placed in a box and can only reach the pellets from the left staircase with its left paw and those from the right staircase with its right paw. The rat can grasp, lift, and retrieve food pellets from the steps of the staircase. The number of pellets completely removed from the staircase box provides a quantifiable measure of the distance and efficiency of fine motor reaching skills. Rats need to be pre-trained in the staircase test before surgery, put on restrictive diet before testing and need to be accomodated again to the test conditions for some days before the following evaluation. Therefore, this test is more complex to be applied than the grasping test described above.
• The walking track analysis is used to evaluate forelimb motor recovery (Ozmen et al., 2002; Galtrey and Fawcett, 2007). The rat forepaw is dipped in an ink solution and the animal is allowed to walk down the track upon a strip of white or graph paper. The prints by the ink are left to dry and then analysed. Different parameters can be analysed (longest length and widest width of the paw impression, widest width between the second and third fingers, distance between homologous points of sequential paw impressions on a given side, perpendicular distance between the central portion of the paw impression and the direction of movement). Moreover, walking track analysis can be performed by 2D digital video motion analysis, which allows also to quantify the movement of the wrist and the metacarpophalangeal joint (Wang et al., 2008).
• The Von Frey filament test is used to evaluate mechanical allodynia (Galtrey and Fawcett, 2007). The animal is placed in a box on a wire mesh floor. Von Frey filaments of different bending forces are used to examine the mechanical threshold of the rat forepaws. The test starts with the smallest bending force and continues in increasing order. Each filament is inserted through the mesh and applied in the medial surface of the forepaw. To perform the test, the rat must be stationary and standing on the four paws. The first filament in the series that evoked withdrawal three times is regarded as the paw withdrawal threshold.
• The Irvine, Beattie, and Bresnahan (IBB) scale test (Irvine et al., 2014) is used for the assessment of fine control of the forelimb and digit movements. Spherical- and donut-shaped pieces of cereal are given to the rat. The forelimb behaviour (joint position, object support, wrist and digit movement, and grasping method) used while eating both cereal shapes is analysed. An IBB score is assigned using the 10-point (0–9) ordinal scale for each shape, and the highest score reflects the greatest amount of forelimb recovery.
• The ladder rung walking test is used to assess forelimb strength, stepping, placing, and co-ordination during skilled locomotion (Metz and Whishaw, 2002; Galtrey and Fawcett, 2007). The apparatus consists of two side walls with rungs inserted into the walls to create a ladder. The ladder is elevated and can also be inclined. The animal is conditioned to run on the ladder on several training sessions. Performance is scored (successful steps/total steps).
• The Randall-Selitto test is used to assess the nociceptive withdrawal threshold (Galtrey and Fawcett, 2007). The test consists in the application of an increasing mechanical force with the tip of an algesimeter on the medial portion of the forepaw until a withdrawal response results.
• For the cold sensory test an ice probe is made by freezing water in a 1.5-ml tube (Lindsey et al., 2000; Galtrey and Fawcett, 2007). When the rat is drinking from the water bottle, the ice probe is applied to the glabrous skin of the forepaw. The withdrawal latency is measured. At least 1 min between trials is needed to allow the skin to return to body temperature and prevent sensitisation. If the rat does not withdraw the paw after 10 s, the probe is removed.
• The cold and hot plate test is used to assess temperature sensation. The rat is placed in a Plexiglas chamber where the metal base temperature is 25°C. For cold plate testing, the temperature is rapidly lowered and the animal behaviour is observed for signs of pain-like behaviour (avoiding contact with the cold plate, suspension of the affected forelimb, licking of the paw, lack of grooming and exploration vocalisation, or freezing behaviour). Once pain-like behaviour is observed, the temperature is increased to a higher temperature. For hot plate testing, the temperature is raised and the behaviour is assessed as described above (Shaikh et al., 2016).
• The CatWalk automated quantitative gait analysis is a computer-assisted method that can simultaneously measure dynamic as well as static gait parameters, including duration of different phases of the step cycle and pressure applied during locomotion (Bozkurt et al., 2008). The animal is placed in the CatWalk walkway, which is comprised of a glass plate with two Plexiglas walls, a high-speed colour camera, and recording and analysis software. The animal walks voluntarily from one side of the glass plate to the other. Its footprints are captured. The intensity of the signal depends on the degree of paw floor contact and increases with pressure applied. The more pressure is exerted, the larger the total area of skin–floor contact and thus the brighter the pixel. An appropriate software visualises the prints and calculates statistics related to print dimensions and the time and distance relationships between footfalls (Chen et al., 2012a,b).
• Electrophysiology is often used in rat, while in the mouse model it is less used probably for the small size. The maximum amplitude and latency of evoked compound muscle action potentials recorded from the thenar muscles are usually evaluated (Werdin et al., 2009).
In conclusion, the list provided above clearly demonstrates that a large variety of tests can be used to evaluate the functional recovery after median nerve transection and repair in rodents.
From a translational point of view, tests should be selected in way to model as closely as possible the course of functional recovery as it is observed in human patients. Recently, the combination of electrodiagnostic evaluation, with the commonly used grasping test (reflex-based gross motor function) and the staircase test (skilled forelimb reaching) has been described to produce results with high translatability (Stossel et al., 2017).
A final comment needs to be put on the fact that different rat strains have been described to demonstrate different motivation degrees to participate in more complex tasks, like, e.g., the staircase test. Especially, Lewis rats have been described to be less motivated and also to eventually be less capable of learning how to perform more complex motor tasks (Nikkhah et al., 1998; Galtrey and Fawcett, 2007).
In the other animals, the functional recovery is assessed mainly by electrophysiology. Indeed, while in rats and mice the fingers do a fine movement quite similar to humans and their functionality can be assessed by different suitable and specific tests, the other animals can do gross movements only.
Morphology and Morphometry (Stereology)
Regardless of the animal model used, nerve regeneration assessment must necessarily have an accurate morphological and morphometrical evaluation (Geuna and Herrera-Rincon, 2015). Among the techniques that allow this type of analysis, immuno-histochemistry offers the possibility to specifically identify the different structures of the regenerating nerve, such as Schwann cells, motor or sensory axons, blood vessels, and other cell types, including macrophages, fibroblast-like cells, perineurial cells, endothelial cells (Carriel et al., 2014b). Moreover, immunofluorescence or immunohistochemical techniques allow to accurately quantify the fraction area and the intensity of the expression of specific proteins which are correlated with regenerative processes. For example, the expression of markers such as Neurofilament in neurons or S-100 in Schwann cells are indicative of an excellent regenerative process, when these levels reach the values of the control nerves (Carriel et al., 2014a).
To quantify the number of sensitive and motor neurons which were able to regenerate axons across a nerve gap, the retrograde-labelling technique can be applied. A dye will be applied into the distal nerve and taken up by regenerated axons and retrogradely transported into the neuronal soma of sensory neurons in the dorsal root ganglia or motor-neurons in the ventral horn of the spinal cord (Hayashi et al., 2007; Kemp et al., 2017).
Together with functional assessment, quantitative estimation of regenerated nerve fibres is a key investigation tool in nerve regeneration research (Kanaya et al., 1996; Geuna et al., 2004). The toluidine blue staining of resin-embedded semithin sections allows to clearly identify most of the myelinated axons and their myelin organisation thanks to the post-fixation with OsO4 (Raimondo et al., 2009). Usually, morpho-quantitative analysis is performed on one randomly selected toluidine blue semi-thin transverse nerve section. The total cross-sectional area of the nerve is measured. Then, an adequate number of fields of interest (according to the size of the nerve) is randomly selected following a systematic random protocol and analysed (Raimondo et al., 2009). The parameters used as nerve regeneration indicators are myelinated fibre number and density, fibre and axon diameter, myelin thickness and g-ratio (axon-diameter/fibre-diameter) (Geuna, 2000, 2005).
In order to compare results obtained by different research groups, different potential sources of bias should be considered.
First of all, the strain, the gender and the age of experimental animals, that can affect the quantification outcome.
The second aspect that can influence the results of a stereological analysis is the level at which it is conducted and, obviously, the different investigation time points. The analysed parameters can vary significantly depending on the distance from the lesion point, especially in the early time points after injury, considering that a nerve can grow approximately 1 mm/day (Santos et al., 2007). Therefore, only quantitative data taken at the same location along the nerves can actually be compared (e.g., 5 mm distal to the lesion site). Obviously, also the time point analysed gives different results and should always be considered (with the same lesion type, a 3-month regenerated nerve will be different from a 6-month regenerated nerve). Finally, it must possibly be considered to analyse all branches of a nerve. The portion of the median nerve that is usually injured and repaired in experimental studies goes from the axillary region to the elbow. In this tract, the median nerve is unifascicular, but for more distal investigation sites the anatomy of the nerve needs to be considered. The median nerve gives off three palmar digital branches more distally, at the level of the carpal bones, that in turn bifurcate between the 1st, 2nd, and 3rd digit (Barton et al., 2016). Other nerves (i.e., the sciatic nerve) release branches in the tract that is commonly investigated. Therefore, if the morphological/morphometrical analysis requires the nerve cross section (for example to estimate the total number of nerve fibres), all branches must be analysed.
The third aspect is represented by the chosen method for measuring the selected size parameters (computerised or manual analysis). It is important to note how computers can certainly make quantitative morphology easier and faster (Williams and Rakic, 1988; Dolapchieva et al., 2000), but a comparison of the performance of automated cell detection revealed that a manual approach is still the most appropriate method for stereological counting (Schmitz et al., 2014).
Gene Expression Analysis
Injured median nerve gene expression analysis can be carried out both at mRNA and protein level. To reduce the number of animals and comply with the 3R’ Principle (Replace, Reduce, and Refine) (Tannenbaum and Bennett, 2015) a good strategy is to extract from the same nerve sample both total RNA and proteins, using commercially available kits.
The first point that should be considered when analysing a nerve sample is that protein extraction involves all nerve components, neuronal axons and peripheral cells (Schwann cells, fibroblasts, macrophages, and so on). RNA extraction mostly encloses peripheral cells, because neuronal RNA is mainly localised in the cell bodies of sensory neurons (in the dorsal root ganglia) or motor neurons (in the ventral horn of the spinal cord), with only few mRNAs locally translated in the axon after nerve injury (Terenzio et al., 2018).
The second point that should be carefully considered is the portion of the injured nerve to be analysed and the time window for the analysis. Indeed, in the 1st days after injury, regenerating axons start to colonise the proximal portion of the repaired nerve, while in the distal portion axons are still undergoing Wallerian degeneration (Girouard et al., 2018). In the following days, regeneration occurs also in the distal stump. Therefore, gene expression analysis will give information about regeneration or Wallerian degeneration taking place according to the region and the time point analysed: for gene expression analysis, like previously discussed for morphology and morphometry, the region and the time point analysed must be the same for all samples and for comparison with other studies.
For mRNA analysis, quantitative real time PCR can be carried under paying attention to the housekeeping genes used for normalisation: indeed, it is really important to normalise data to genes whose expression is not affected by nerve injury (Vandesompele et al., 2002). To this aim, stable housekeeping genes suitable for gene expression analysis were identified (Gambarotta et al., 2014; Wang et al., 2017). To avoid amplification of contaminant genomic DNA, a good strategy is to design primers on different exons, possibly separated by introns larger than 1,000 bp, or straddling two contiguous exons. For protein analysis, western blots can be carried out using the stain-free technology, which is a good strategy, because protein expression is normalised to the total protein content, bypassing the problem of the choice of suitable housekeeping genes (Gurtler et al., 2013).
Transgenic Models to Evaluate Peripheral Nerve Regeneration
A transgenic model that can be used for evaluating and monitoring peripheral nerve regeneration in mice and rats is Thy1-GFP, in which Thy1 promoter drives neuron specific expression of GFP, allowing imaging of nerve regeneration following nerve injuries (Porrero et al., 2010; Moore et al., 2012; Kemp et al., 2013).
Conclusion
In this review we emphasised the use of the median nerve as pre-clinical experimental model to study nerve regeneration in vivo. Accordingly, in the last years the median nerve model is increasingly used due to different reasons, including a better animal well-being, and availability of different functional tests which, specifically in rodents, allow testing the digital fine movement, making the results obtained with this model more translatable into the clinic.
With regard to the animal choice, the rat definitely represents the easiest and more translatable model, especially for studies evaluating regeneration across short nerve gaps, while for large nerve gaps the use of other, larger, animals would be recommended. In case large animal facilities are not available, an alternative and very interesting approach might be the cross-chest median nerve transfer in the rat animal model (Sinis et al., 2006). Indeed, this method would allow the use of rodents, which are less expensive and easier to handle compared to large animals, but at the same time would allow studying nerve regeneration across long gaps (up to 40 mm).
Author Contributions
GR, GG, SG, and KH-T organised the manuscript. GR, GG, and MM prepared all the tables. GR, GG, MM, FF, SG, and KH-T wrote different sections of the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
Funding
This study was supported by the European Community’s Seventh Framework Programme (FP7-HEALTH-2011) (Grant No. 278612; BIOHYBRID).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor is currently co-organizing a Research Topic with two of the authors KH-T and GG, and confirms the absence of any other collaboration.
References
Accioli De Vaconcellos, Z. A., Duchossoy, Y., Kassar-Duchossoy, L., and Mira, J. C. (1999). Experimental median nerve repair by fresh or frozen nerve autografts and xenografts. Ann. Chir. Main Memb. Super 18, 74–84. doi: 10.1016/s0753-9053(99)80059-3
Al-Majed, A. A., Brushart, T. M., and Gordon, T. (2000). Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 12, 4381–4390. doi: 10.1111/j.1460-9568.2000.01341.x
Angius, D., Wang, H., Spinner, R. J., Gutierrez-Cotto, Y., Yaszemski, M. J., and Windebank, A. J. (2012). A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials 33, 8034–8039. doi: 10.1016/j.biomaterials.2012.07.056
Archibald, S. J., Krarup, C., Shefner, J., Li, S. T., and Madison, R. D. (1991). A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J. Comp. Neurol. 306, 685–696. doi: 10.1002/cne.903060410
Archibald, S. J., Shefner, J., Krarup, C., and Madison, R. D. (1995). Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J. Neurosci. 15(5 Pt 2), 4109–4123. doi: 10.1523/jneurosci.15-05-04109.1995
Audisio, C., Nicolino, S., Scevola, A., Tos, P., Geuna, S., Battiston, B., et al. (2008). ErbB receptors modulation in different types of peripheral nerve regeneration. Neuroreport. 19, 1605–1609. doi: 10.1097/WNR.0b013e32831313ef00001756-200810290-00010
Badalamente, M. A., Hurst, L. C., and Stracher, A. (1989). Neuromuscular recovery using calcium protease inhibition after median nerve repair in primates. Proc. Natl. Acad. Sci. U.S.A. 86, 5983–5987. doi: 10.1073/pnas.86.15.5983
Baoguo, J., Shibata, M., Matsuzaki, H., and Takahashi, H. E. (2004). Proximal nerve elongation vs nerve grafting in repairing segmental nerve defects in rabbits. Microsurgery 24, 213–217. doi: 10.1002/micr.20039
Barton, M. J., StJohn, J., Tatian, A., Riches, J. D., Mograby, O., and Mahns, D. A. (2016). Morphological and morphometric analysis of the distal branches of the rat brachial plexus. Italian J. Anat. Embryol. 121, 13. doi: 10.13128/IJAE-20273
Beck-Broichsitter, B. E., Becker, S. T., Lamia, A., Fregnan, F., Geuna, S., and Sinis, N. (2014a). Sensoric protection after median nerve injury: babysitter-procedure prevents muscular atrophy and improves neuronal recovery. Biomed. Res. Int. 2014:724197. doi: 10.1155/2014/724197
Beck-Broichsitter, B. E., Lamia, A., Geuna, S., Fregnan, F., Smeets, R., Becker, S. T., et al. (2014b). Does pulsed magnetic field therapy influence nerve regeneration in the median nerve model of the rat? Biomed. Res. Int. 2014:401760. doi: 10.1155/2014/401760
Bertelli, J. A., dos Santos, A. R., Taleb, M., Calixto, J. B., Mira, J. C., and Ghizoni, M. F. (2004). Long interpositional nerve graft consistently induces incomplete motor and sensory recovery in the rat. An experimental model to test nerve repair. J. Neurosci. Methods 134, 75–80. doi: 10.1016/j.jneumeth.2003.11.002
Bertelli, J. A., and Mira, J. C. (1995). The grasping test: a simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the rat. J. Neurosci. Methods 58, 151–155. doi: 10.1016/0165-0270(94)00169-h
Bertelli, J. A., Taleb, M., Saadi, A., Mira, J. C., and Pecot-Dechavassine, M. (1995). The rat brachial plexus and its terminal branches: an experimental model for the study of peripheral nerve regeneration. Microsurgery 16, 77–85. doi: 10.1002/micr.1920160207
Bontioti, E., Kanje, M., Lundborg, G., and Dahlin, L. B. (2005). End-to-side nerve repair in the upper extremity of rat. J. Peripher. Nerv. Syst. 10, 58–68. doi: 10.1111/j.1085-9489.2005.10109.x
Bozkurt, A., Deumens, R., Scheffel, J., O’Dey, D. M., Weis, J., Joosten, E. A., et al. (2008). CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. J. Neurosci. Methods 173, 91–98. doi: 10.1016/j.jneumeth.2008.05.020
Bozkurt, A., Scheffel, J., Brook, G. A., Joosten, E. A., Suschek, C. V., O’Dey, D. M., et al. (2011). Aspects of static and dynamic motor function in peripheral nerve regeneration: SSI and CatWalk gait analysis. Behav. Brain Res. 219, 55–62. doi: 10.1016/j.bbr.2010.12.018
Busuttil, F., Rahim, A. A., and Phillips, J. B. (2017). Combining gene and stem cell therapy for peripheral nerve tissue engineering. Stem Cells Dev. 26, 231–238. doi: 10.1089/scd.2016.0188
Caplan, A. I. (2015). Adult mesenchymal stem cells: when, where, and how. Stem Cells Int. 2015:628767. doi: 10.1155/2015/628767
Carriel, V., Alaminos, M., Garzon, I., Campos, A., and Cornelissen, M. (2014a). Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 14, 301–318. doi: 10.1586/14737175.2014.887444
Carriel, V., Garzon, I., Alaminos, M., and Cornelissen, M. (2014b). Histological assessment in peripheral nerve tissue engineering. Neural Regen. Res. 9, 1657–1660. doi: 10.4103/1673-5374.141798
Casal, D., Mota-Silva, E., Iria, I., Alves, S., Farinho, A., Pen, C., et al. (2018). Reconstruction of a 10-mm-long median nerve gap in an ischemic environment using autologous conduits with different patterns of blood supply: a comparative study in the rat. PLoS One 13:e0195692. doi: 10.1371/journal.pone.0195692
Caseiro, A. R., Pereira, T., Ivanova, G., Luis, A. L., and Mauricio, A. C. (2016). Neuromuscular regeneration: perspective on the application of mesenchymal stem cells and their secretion products. Stem Cells Int. 2016:9756973. doi: 10.1155/2016/9756973
Chen, S. H., Lue, J. H., Hsiao, Y. J., Lai, S. M., Wang, H. Y., Lin, C. T., et al. (2018). Elevated galanin receptor type 2 primarily contributes to mechanical hypersensitivity after median nerve injury. PLoS One 13:e0199512. doi: 10.1371/journal.pone.0199512
Chen, S. H., Tsai, Y. J., Lin, C. T., Wang, H. Y., Li, S. F., and Lue, J. H. (2012a). Changes in GABA and GABA(B) receptor expressions are involved in neuropathy in the rat cuneate nucleus following median nerve transection. Synapse 66, 561–572. doi: 10.1002/syn.21539
Chen, S. H., Tsai, Y. J., Wang, H. Y., Lin, C. T., Li, S. F., and Lue, J. H. (2012b). Decreases of glycine receptor expression induced by median nerve injury in the rat cuneate nucleus contribute to NPY release and c-Fos expression. Life Sci. 90, 278–288. doi: 10.1016/j.lfs.2011.11.014
Chiono, V., Vozzi, G., Vozzi, F., Salvadori, C., Dini, F., Carlucci, F., et al. (2009). Melt-extruded guides for peripheral nerve regeneration. Part I: poly(epsilon-caprolactone). Biomed. Microdev. 11, 1037–1050. doi: 10.1007/s10544-009-9321-9329
Coradini, J. G., Kunz, R. I., Kakihata, C. M., Errero, T. K., Bonfleur, M. L., Ribeiro Lde, F., et al. (2015). Swimming does not alter nociception threshold in obese rats submitted to median nerve compression. Neurol. Res. 37, 1118–1124. doi: 10.1080/01616412.2015.1114742
Cortez-Retamozo, V., Etzrodt, M., and Pittet, M. J. (2012). Regulation of macrophage and dendritic cell responses by their lineage precursors. J. Innate Immun. 4, 411–423. doi: 10.1159/000335733
de la Monte, S. M., Bour, C., Radhakrishnan, V. V., Jupiter, J. B., Smith, R. J., and Hedley-Whyte, E. T. (1988). Effects of cyclosporin A and predegeneration on survival and regeneration of peripheral nerve allografts in rabbits. Surg. Neurol. 29, 95–100. doi: 10.1016/0090-3019(88)90064-x
Dolapchieva, S., Eggers, R., and Kuhnel, W. (2000). Automatic image analysis of the postnatal growth of axons and myelin sheaths in the tibial and peroneal nerves of the rabbit. Ann. Anat. 182, 133–142. doi: 10.1016/s0940-9602(00)80072-80072
Dubovy, P., Jancalek, R., and Kubek, T. (2013). Role of inflammation and cytokines in peripheral nerve regeneration. Int. Rev. Neurobiol. 108, 173–206. doi: 10.1016/b978-0-12-410499-0.00007-1
Faroni, A., Mobasseri, S. A., Kingham, P. J., and Reid, A. J. (2015). Peripheral nerve regeneration: experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 82-83, 160–167. doi: 10.1016/j.addr.2014.11.010
Florence, S. L., Boydston, L. A., Hackett, T. A., Lachoff, H. T., Strata, F., and Niblock, M. M. (2001). Sensory enrichment after peripheral nerve injury restores cortical, not thalamic, receptive field organization. Eur. J. Neurosci. 13, 1755–1766. doi: 10.1046/j.0953-816x.2001.01555.x
Florence, S. L., Garraghty, P. E., Wall, J. T., and Kaas, J. H. (1994). Sensory afferent projections and area 3b somatotopy following median nerve cut and repair in macaque monkeys. Cereb. Cortex 4, 391–407. doi: 10.1093/cercor/4.4.391
Florence, S. L., Jain, N., Pospichal, M. W., Beck, P. D., Sly, D. L., and Kaas, J. H. (1996). Central reorganization of sensory pathways following peripheral nerve regeneration in fetal monkeys. Nature 381, 69–71. doi: 10.1038/381069a0
Forden, J., Xu, Q. G., Khu, K. J., and Midha, R. (2011). A long peripheral nerve autograft model in the sheep forelimb. Neurosurgery 68, 1354–1362. doi: 10.1227/NEU.0b013e31820c08de
Frausin, S., Viventi, S., Verga Falzacappa, L., Quattromani, M. J., Leanza, G., Tommasini, A., et al. (2015). Wharton’s jelly derived mesenchymal stromal cells: biological properties, induction of neuronal phenotype and current applications in neurodegeneration research. Acta Histochem. 117, 329–338. doi: 10.1016/j.acthis.2015.02.005
Fregnan, F., Ciglieri, E., Tos, P., Crosio, A., Ciardelli, G., Ruini, F., et al. (2016). Chitosan crosslinked flat scaffolds for peripheral nerve regeneration. Biomed. Mater. 11:045010. doi: 10.1088/1748-6041/11/4/045010
Fu, Y., Karbaat, L., Wu, L., Leijten, J., Both, S. K., and Karperien, M. (2017). Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng. Part B Rev. 23, 515–528. doi: 10.1089/ten.TEB.2016.0365
Fullarton, A. C., Glasby, M. A., and Lawson, G. M. (1998). Immediate and delayed nerve repair using freeze-thawed muscle allografts. associated long-bone fracture. J. Hand. Surg. Br. 23, 360–364. doi: 10.1016/s0266-7681(98)80058-2
Galtrey, C. M., and Fawcett, J. W. (2007). Characterization of tests of functional recovery after median and ulnar nerve injury and repair in the rat forelimb. J. Peripher. Nerv. Syst. 12, 11–27. doi: 10.1111/j.1529-8027.2007.00113.x
Gambarotta, G., Pascal, D., Ronchi, G., Morano, M., Jager, S. B., Moimas, S., et al. (2015). Local delivery of the neuregulin1 receptor ecto-domain (ecto-ErbB4) has a positive effect on regenerated nerve fiber maturation. Gene Ther. 22, 901–907. doi: 10.1038/gt.2015.46gt201546
Gambarotta, G., Ronchi, G., Friard, O., Galletta, P., Perroteau, I., and Geuna, S. (2014). Identification and validation of suitable housekeeping genes for normalizing quantitative real-time PCR assays in injured peripheral nerves. PLoS One 9:e105601. doi: 10.1371/journal.pone.0105601PONE-D-14-24896
Gao, K. M., Lao, J., Guan, W. J., and Hu, J. J. (2018). Is it necessary to use the entire root as a donor when transferring contralateral C7 nerve to repair median nerve? Neural. Regen. Res. 13, 94–99. doi: 10.4103/1673-5374.224376
Gartner, A., Pereira, T., Alves, M. G., Armada-da-Silva, P. A., Amorim, I., Gomes, R., et al. (2012). Use of poly(DL-lactide-epsilon-caprolactone) membranes and mesenchymal stem cells from the Wharton’s jelly of the umbilical cord for promoting nerve regeneration in axonotmesis: in vitro and in vivo analysis. Differentiation 84, 355–365. doi: 10.1016/j.diff.2012.10.001
Gartner, A., Pereira, T., Armada-da-Silva, P., Amado, S., Veloso, A., Amorim, I., et al. (2014). Effects of umbilical cord tissue mesenchymal stem cells (UCX(R)) on rat sciatic nerve regeneration after neurotmesis injuries. J. Stem Cells Regen. Med. 10, 14–26.
Geuna, S. (2000). Appreciating the difference between design-based and model-based sampling strategies in quantitative morphology of the nervous system. J. Comp. Neurol. 427, 333–339. doi: 10.1002/1096-9861(20001120)427:3<333::aid-cne1>3.3.co;2-k
Geuna, S. (2005). The revolution of counting “tops”: two decades of the disector principle in morphological research. Microsc. Res. Tech. 66, 270–274. doi: 10.1002/jemt.20167
Geuna, S., Gigo-Benato, D., and Rodrigues Ade, C. (2004). On sampling and sampling errors in histomorphometry of peripheral nerve fibers. Microsurgery 24, 72–76. doi: 10.1002/micr.10199
Geuna, S., and Herrera-Rincon, C. (2015). Update on stereology for light microscopy. Cell Tissue Res. 360, 5–12. doi: 10.1007/s00441-015-2143-2146
Geuna, S., Nicolino, S., Raimondo, S., Gambarotta, G., Battiston, B., Tos, P., et al. (2007a). Nerve regeneration along bioengineered scaffolds. Microsurgery 27, 429–438. doi: 10.1002/micr.20383
Geuna, S., Tos, P., Raimondo, S., Lee, J. M., Gambarotta, G., Nicolino, S., et al. (2007b). Functional, morphological and biomolecular assessment of posttraumatic neuro-muscular recovery in the rat forelimb model. Acta Neurochir. Suppl. 100, 173–177. doi: 10.1007/978-3-211-72958-8_36
Ghizoni, M. F., Bertelli, J. A., Grala, C. G., and da Silva, R. M. (2013). The anabolic steroid nandrolone enhances motor and sensory functional recovery in rat median nerve repair with long interpositional nerve grafts. Neurorehabil. Neural. Repair 27, 269–276. doi: 10.1177/1545968312465190
Gigo-Benato, D., Geuna, S., de Castro Rodrigues, A., Tos, P., Fornaro, M., Boux, E., et al. (2004). Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: a double-blind randomized study in the rat median nerve model. Lasers Med. Sci. 19, 57–65. doi: 10.1007/s10103-004-0300-303
Girouard, M. P., Bueno, M., Julian, V., Drake, S., Byrne, A. B., and Fournier, A. E. (2018). The molecular interplay between axon degeneration and regeneration. Dev. Neurobiol. 78, 978–990 doi: 10.1002/dneu.22627
Glasby, M. A., Fullerton, A. C., and Lawson, G. M. (1998). Immediate and delayed nerve repair using freeze-thawed muscle autografts in complex nerve injuries. associated arterial injury. J. Hand. Surg. Br. 23, 354–359. doi: 10.1016/s0266-7681(98)80057-0
Gluck, M. J., Vijayaraghavan, S., Sinclair, E. B., Ashraf, A., Hausman, M. R., and Cagle, P. J. (2018). Detecting structural and inflammatory response after in vivo stretch injury in the rat median nerve via second harmonic generation. J. Neurosci. Methods 303, 68–80. doi: 10.1016/j.jneumeth.2018.02.006
Gomez-Sanchez, J. A., Lopez de Armentia, M., Lujan, R., Kessaris, N., Richardson, W. D., and Cabedo, H. (2009). Sustained axon-glial signaling induces Schwann cell hyperproliferation, remak bundle myelination, and tumorigenesis. J. Neurosci. 29, 11304–11315. doi: 10.1523/JNEUROSCI.1753-09.2009
Gordon, T. (2016). Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics 13, 295–310. doi: 10.1007/s13311-015-0415-411
Gordon, T., and English, A. W. (2016). Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur. J. Neurosci. 43, 336–350. doi: 10.1111/ejn.13005
Grabb, W. C. (1968). Median and ulnar nerve suture. An experimental study comparing primary and secondary repair in monkeys. J. Bone Joint Surg. Am. 50, 964–972. doi: 10.2106/00004623-196850050-00008
Gui, L. A., Xin, C. T., Xue, C. S., Lin, F. C., Yu, W., Li, N. W., et al. (1997). Regenerative changes in median nerve defects using various rabbit skeletal muscles. Nihon Hansenbyo Gakkai Zasshi 66, 207–213. doi: 10.5025/hansen.66.207
Gurtler, A., Kunz, N., Gomolka, M., Hornhardt, S., Friedl, A. A., McDonald, K., et al. (2013). Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 433, 105–111. doi: 10.1016/j.ab.2012.10.010
Hara, Y., Nishiura, Y., Ochiai, N., Sharula, Nakajima, Y., Kubota, S., et al. (2012). New treatment for peripheral nerve defects: reconstruction of a 2 cm, monkey median nerve gap by direct lengthening of both nerve stumps. J. Orthop. Res. 30, 153–161. doi: 10.1002/jor.21476
Hayashi, A., Moradzadeh, A., Hunter, D. A., Kawamura, D. H., Puppala, V. K., Tung, T. H., et al. (2007). Retrograde labeling in peripheral nerve research: it is not all black and white. J. Reconstr. Microsurg. 23, 381–389. doi: 10.1055/s-2007-992344
Ho, C. Y., Yao, C. H., Chen, W. C., Shen, W. C., and Bau, D. T. (2013). Electroacupuncture and acupuncture promote the rat’s transected median nerve regeneration. Evid. Based Comp. Alternat. Med. 2013:514610. doi: 10.1155/2013/514610
Hoke, A. (2012). Animal models of peripheral neuropathies. Neurotherapeutics 9, 262–269. doi: 10.1007/s13311-012-0116-y
Hu, N., Wu, H., Xue, C., Gong, Y., Wu, J., Xiao, Z., et al. (2013). Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials 34, 100–111. doi: 10.1016/j.biomaterials.2012.09.020
Huang, C. T., and Tsai, Y. J. (2016). Docosahexaenoic acid confers analgesic effects after median nerve injury via inhibition of c-Jun N-terminal kinase activation in microglia. J. Nutr. Biochem. 29, 97–106. doi: 10.1016/j.jnutbio.2015.11.009
Irvine, K. A., Ferguson, A. R., Mitchell, K. D., Beattie, S. B., Lin, A., Stuck, E. D., et al. (2014). The irvine, beatties, and bresnahan (IBB) forelimb recovery scale: an assessment of reliability and validity. Front. Neurol. 5:116. doi: 10.3389/fneur.2014.00116
Jager, S. B., Ronchi, G., Vaegter, C. B., and Geuna, S. (2014). The mouse median nerve experimental model in regenerative research. Biomed. Res. Int. 2014:701682. doi: 10.1155/2014/701682
Jaminet, P., Kohler, D., Rahmanian-Schwarz, A., Lotter, O., Mager, A., Fornaro, M., et al. (2013a). Expression patterns and functional evaluation of the UNC5b receptor during the early phase of peripheral nerve regeneration using the mouse median nerve model. Microsurgery 33, 216–222. doi: 10.1002/micr.22059
Jaminet, P., Kohler, D., Schaufele, M., Rahmanian-Schwarz, A., Lotter, O., Fornaro, M., et al. (2013b). Evaluating the role of Netrin-1 during the early phase of peripheral nerve regeneration using the mouse median nerve model. Restor. Neurol. Neurosci. 31, 337–345. doi: 10.3233/rnn-120277
Jeans, L., Healy, D., and Gilchrist, T. (2007). An evaluation using techniques to assess muscle and nerve regeneration of a flexible glass wrap in the repair of peripheral nerves. Acta Neurochir. Suppl. 100, 25–28. doi: 10.1007/978-3-211-72958-8_5
Jeans, L. A., Gilchrist, T., and Healy, D. (2007). Peripheral nerve repair by means of a flexible biodegradable glass fibre wrap: a comparison with microsurgical epineurial repair. J. Plast Reconstr. Aesthet. Surg. 60, 1302–1308. doi: 10.1016/j.bjps.2006.06.014
Jessen, K. R., and Mirsky, R. (2016). The repair Schwann cell and its function in regenerating nerves. J. Physiol. 594, 3521–3531 doi: 10.1113/jp270874
Jiang, L., Jones, S., and Jia, X. (2017). Stem cell transplantation for peripheral nerve regeneration: current options and opportunities. Int. J. Mol. Sci. 18:E94
Jin, H. J., Bae, Y. K., Kim, M., Kwon, S. J., Jeon, H. B., Choi, S. J., et al. (2013). Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 14, 17986–18001. doi: 10.3390/ijms140917986
Jones, S., Eisenberg, H. M., and Jia, X. (2016). Advances and future applications of augmented peripheral nerve regeneration. Int. J. Mol. Sci. 17:E1494
Juneja, M., Burns, J., Saporta, M. A., and Timmerman, V. (2018). Challenges in modelling the charcot-marie-tooth neuropathies for therapy development. J. Neurol. Neurosurg. Psychiatry 90, 58–67. doi: 10.1136/jnnp-2018-318834
Kanaya, F., Firrell, J. C., and Breidenbach, W. C. (1996). Sciatic function index, nerve conduction tests, muscle contraction, and axon morphometry as indicators of regeneration. Plast. Reconstr. Surg. 98, 1264–1271. doi: 10.1097/00006534-199612000-00023
Kaplan, H. M., Mishra, P., and Kohn, J. (2015). The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J. Mater. Sci. Mater. Med. 26:226. doi: 10.1007/s10856-015-5558-5554
Kawai, H., Baudrimont, M., Travers, V., and Sedel, L. (1990). A comparative experimental study of vascularized and nonvascularized nerve grafts. J. Reconstr. Microsurg. 6, 255–259. doi: 10.1055/s-2007-1006827
Kelleher, M. O., Al-Abri, R. K., Lenihan, D. V., and Glasby, M. A. (2006a). Use of a static magnetic field to promote recovery after peripheral nerve injury. J. Neurosurg. 105, 610–615. doi: 10.3171/jns.2006.105.4.610
Kelleher, M. O., Myles, L. M., Al-Abri, R. K., and Glasby, M. A. (2006b). The use of ciliary neurotrophic factor to promote recovery after peripheral nerve injury by delivering it at the site of the cell body. Acta Neurochir. 148, 55–60. doi: 10.1007/s00701-005-0631-632
Kemp, S. W., Cederna, P. S., and Midha, R. (2017). Comparative outcome measures in peripheral regeneration studies. Exp. Neurol. 287(Pt 3), 348–357. doi: 10.1016/j.expneurol.2016.04.011
Kemp, S. W., Phua, P. D., Stanoulis, K. N., Wood, M. D., Liu, E. H., Gordon, T., et al. (2013). Functional recovery following peripheral nerve injury in the transgenic Thy1-GFP rat. J. Peripher. Nerv. Syst. 18, 220–231. doi: 10.1111/jns5.12035
Kettle, S. J., Starritt, N. E., Glasby, M. A., and Hems, T. E. (2013). End-to-side nerve repair in a large animal model: how does it compare with conventional methods of nerve repair? J. Hand. Surg. Eur. 38, 192–202. doi: 10.1177/1753193412445119
Kim, J. K., Chung, M. S., and Baek, G. H. (2011). The origin of regenerating axons after end-to-side neurorrhaphy without donor nerve injury. J. Plast. Reconstr. Aesthet. Surg. 64, 255–260. doi: 10.1016/j.bjps.2010.04.033
Konofaos, P., and Ver Halen, J. P. (2013). Nerve repair by means of tubulization: past, present, future. J. Reconstr. Microsurg. 29, 149–164. doi: 10.1055/s-0032-1333316
Kornfeld, T., Vogt, P. M., and Radtke, C. (2018). Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien Med. Wochenschr. 169, 240–251 doi: 10.1007/s10354-018-0675-6
Krarup, C., Archibald, S. J., and Madison, R. D. (2002). Factors that influence peripheral nerve regeneration: an electrophysiological study of the monkey median nerve. Ann. Neurol. 51, 69–81. doi: 10.1002/ana.10054
La Marca, R., Cerri, F., Horiuchi, K., Bachi, A., Feltri, M. L., Wrabetz, L., et al. (2011). TACE (ADAM17) inhibits schwann cell myelination. Nat. Neurosci. 14, 857–865. doi: 10.1038/nn.2849
Lanza, C., Raimondo, S., Vergani, L., Catena, N., Senes, F., Tos, P., et al. (2012). Expression of antioxidant molecules after peripheral nerve injury and regeneration. J. Neurosci. Res. 90, 842–848. doi: 10.1002/jnr.22778
Lawson, G. M., and Glasby, M. A. (1995). A comparison of immediate and delayed nerve repair using autologous freeze-thawed muscle grafts in a large animal model. The simple injury. J. Hand. Surg. Br. 20, 663–700. doi: 10.1016/s0266-7681(05)80131-7
Lawson, G. M., and Glasby, M. A. (1998). Peripheral nerve reconstruction using freeze-thawed muscle grafts: a comparison with group fascicular nerve grafts in a large animal model. J. R. Coll. Surg. Edinb. 43, 295–302.
Lee, J. M., Tos, P., Raimondo, S., Fornaro, M., Papalia, I., Geuna, S., et al. (2007). Lack of topographic specificity in nerve fiber regeneration of rat forelimb mixed nerves. Neuroscience 144, 985–990. doi: 10.1016/j.neuroscience.2006.11.001
Lee, W. P., Constantinescu, M. A., and Butler, P. E. (1999). Effect of early mobilization on healing of nerve repair: histologic observations in a canine model. Plast Reconstr. Surg 104, 1718–1725. doi: 10.1097/00006534-199911000-00016
Li, R., Hettinger, P. C., Liu, X., Machol, J. T., Yan, J. G., Matloub, H. S., et al. (2014). Early evaluation of nerve regeneration after nerve injury and repair using functional connectivity MRI. Neurorehabil. Neural. Repair. 28, 707–715. doi: 10.1177/1545968314521002
Lindsey, A. E., LoVerso, R. L., Tovar, C. A., Hill, C. E., Beattie, M. S., and Bresnahan, J. C. (2000). An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil. Neural. Repair. 14, 287–300. doi: 10.1177/154596830001400405
Lopatina, T., Kalinina, N., Karagyaur, M., Stambolsky, D., Rubina, K., Revischin, A., et al. (2011). Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One 6:e17899. doi: 10.1371/journal.pone.0017899
Magill, C., Whitlock, E., Solowski, N., and Myckatyn, T. (2008). Transgenic models of nerve repair and nerve regeneration. Neurol. Res. 30, 1023–1029. doi: 10.1179/174313208x362497
Manoli, T., Werdin, F., Gruessinger, H., Sinis, N., Schiefer, J. L., Jaminet, P., et al. (2014). Correlation analysis of histomorphometry and motor neurography in the median nerve rat model. Eplasty 14:e17.
Marchesini, A., Raimondo, S., Zingaretti, N., Riccio, V., Battiston, B., Provinciali, M., et al. (2018). The amnion muscle combined graft (AMCG) conduits in nerves repair: an anatomical and experimental study on a rat model. J. Mater. Sci. Mater. Med. 29:120. doi: 10.1007/s10856-018-6126-6125
Marcioli, M. A., Coradini, J. G., Kunz, R. I., Ribeiro Lde, F., Brancalhao, R. M., and Bertolini, G. R. (2013). Nociceptive and histomorphometric evaluation of neural mobilization in experimental injury of the median nerve. Sci. World J. 2013:476890. doi: 10.1155/2013/476890
Marcioli, M. A. R., Silva, J., Ribeiro, L. F. C., Brancalhao, R. M. C., and Bertolini, G. R. F. (2018). Neurotrophin expression and histomorphometric evaluation in Wistar rats subjected to neural mobilization after compression of the median nerve. Rev. Bras. Ortop. 53, 276–280. doi: 10.1016/j.rboe.2018.03.006
Marotte, L. R. (1974). An electron microscope study of chronic median nerve compression in the guinea pig. Acta Neuropathol. 27, 69–82. doi: 10.1007/bf00687242
Matsuyama, T., Midha, R., Mackinnon, S. E., Munro, C. A., Wong, P. Y., and Ang, L. C. (2000). Long nerve allografts in sheep with cyclosporin A immunosuppression. J. Reconstr. Microsurg. 16, 219–225.
Meirelles Lda, S., Fontes, A. M., Covas, D. T., and Caplan, A. I. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 20, 419–427. doi: 10.1016/j.cytogfr.2009.10.002
Metz, G. A., and Whishaw, I. Q. (2002). Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods 115, 169–179. doi: 10.1016/s0165-0270(02)00012-2
Meyers, E. C., Granja, R., Solorzano, B. R., Romero-Ortega, M., Kilgard, M. P., Rennaker, R. L., et al. (2017). Median and ulnar nerve injuries reduce volitional forelimb strength in rats. Muscle Nerve 56, 1149–1154. doi: 10.1002/mus.25590
Michailov, G. V., Sereda, M. W., Brinkmann, B. G., Fischer, T. M., Haug, B., Birchmeier, C., et al. (2004). Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700–703. doi: 10.1126/science.1095862
Moges, H., Wu, X., McCoy, J., Vasconcelos, O. M., Bryant, H., Grunberg, N. E., et al. (2011). Effect of 810 nm light on nerve regeneration after autograft repair of severely injured rat median nerve. Lasers Surg. Med. 43, 901–906. doi: 10.1002/lsm.21117
Moimas, S., Novati, F., Ronchi, G., Zacchigna, S., Fregnan, F., Zentilin, L., et al. (2013). Effect of vascular endothelial growth factor gene therapy on post-traumatic peripheral nerve regeneration and denervation-related muscle atrophy. Gene Ther. 20, 1014–1021. doi: 10.1038/gt.2013.26
Mokarram, N., Dymanus, K., Srinivasan, A., Lyon, J. G., Tipton, J., Chu, J., et al. (2017). Immunoengineering nerve repair. Proc. Natl. Acad. Sci. U.S.A. 114, E5077–E5084. doi: 10.1073/pnas.1705757114
Mokarram, N., Merchant, A., Mukhatyar, V., Patel, G., and Bellamkonda, R. V. (2012). Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 33, 8793–8801. doi: 10.1016/j.biomaterials.2012.08.050
Montgomery, K. L., Iyer, S. M., Christensen, A. J., Deisseroth, K., and Delp, S. L. (2016). Beyond the brain: optogenetic control in the spinal cord and peripheral nervous system. Sci. Transl. Med. 8:337rv335. doi: 10.1126/scitranslmed.aad7577
Montoya, C. P., Campbell-Hope, L. J., Pemberton, K. D., and Dunnett, S. B. (1991). The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J. Neurosci. Methods 36, 219–228. doi: 10.1016/0165-0270(91)90048-5
Moore, A. M., Borschel, G. H., Santosa, K. A., Flagg, E. R., Tong, A. Y., Kasukurthi, R., et al. (2012). A transgenic rat expressing green fluorescent protein (GFP) in peripheral nerves provides a new hindlimb model for the study of nerve injury and regeneration. J. Neurosci. Methods 204, 19–27. doi: 10.1016/j.jneumeth.2011.10.011
Muratori, L., Gnavi, S., Fregnan, F., Mancardi, A., Raimondo, S., Perroteau, I., et al. (2018). Evaluation of vascular endothelial growth factor (VEGF) and its family member expression after peripheral nerve regeneration and denervation. Anat. Rec. 301, 1646–1656. doi: 10.1002/ar.23842
Muratori, L., Ronchi, G., Raimondo, S., Giacobini-Robecchi, M. G., Fornaro, M., and Geuna, S. (2012). Can regenerated nerve fibers return to normal size? a long-term post-traumatic study of the rat median nerve crush injury model. Microsurgery 32, 383–387. doi: 10.1002/micr.21969
Murray, G. M., Taub, D. R., Mackie, P. D., Zhang, H. Q., Ghosh, S., and Rowe, M. J. (1997). The effects of neonatal median nerve injury on the responsiveness of tactile neurones within the cuneate nucleus of the cat. J. Physiol. 505 (Pt 3), 759–768. doi: 10.1111/j.1469-7793.1997.759ba.x
Nabian, M. H., Nadji-Tehrani, M., Zanjani, L. O., Kamrani, R. S., Rahimi-Movaghar, V., and Firouzi, M. (2011). Effect of bilateral median nerve excision on sciatic functional index in rat: an applicable animal model for autologous nerve grafting. J. Reconstr. Microsurg. 27, 5–10. doi: 10.1055/s-0030-1267387
Navarro, X. (2016). Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: a critical overview. Eur. J. Neurosci. 43, 271–286. doi: 10.1111/ejn.13033
Nicolino, S., Panetto, A., Raimondo, S., Gambarotta, G., Guzzini, M., Fornaro, M., et al. (2009). Denervation and reinnervation of adult skeletal muscle modulate mRNA expression of neuregulin-1 and ErbB receptors. Microsurgery 29, 464–472. doi: 10.1002/micr.20636
Nikkhah, G., Rosenthal, C., Hedrich, H. J., and Samii, M. (1998). Differences in acquisition and full performance in skilled forelimb use as measured by the ’staircase test’ in five rat strains. Behav. Brain Res. 92, 85–95. doi: 10.1016/s0166-4328(97)00128-9
Ochoa, J., and Marotte, L. (1973). The nature of the nerve lesion caused by chronic entrapment in the guinea-pig. J. Neurol. Sci. 19, 491–495. doi: 10.1016/0022-510x(73)90045-2
Oliveira, J. T., Almeida, F. M., Biancalana, A., Baptista, A. F., Tomaz, M. A., Melo, P. A., et al. (2010). Mesenchymal stem cells in a polycaprolactone conduit enhance median-nerve regeneration, prevent decrease of creatine phosphokinase levels in muscle, and improve functional recovery in mice. Neuroscience 170, 1295–1303. doi: 10.1016/j.neuroscience.2010.08.042
Oliveira, J. T., Bittencourt-Navarrete, R. E., de Almeida, F. M., Tonda-Turo, C., Martinez, A. M., and Franca, J. G. (2014). Enhancement of median nerve regeneration by mesenchymal stem cells engraftment in an absorbable conduit: improvement of peripheral nerve morphology with enlargement of somatosensory cortical representation. Front. Neuroanat. 8:111. doi: 10.3389/fnana.2014.00111
Ozalp, T., and Masquelet, A. C. (2008). The role of creating a biological membrane in expediting nerve regeneration for peripheral nerve repairs. Acta Orthop. Traumatol. Turc. 42, 130–134. doi: 10.3944/aott.2008.42.2.130
Ozmen, S., Ayhan, S., Latifoglu, O., and Siemionow, M. (2002). Stamp and paper method: a superior technique for the walking track analysis. Plast Reconstr. Surg. 109, 1760–1761. doi: 10.1097/00006534-200204150-00065
Pace, L. A., Plate, J. F., Mannava, S., Barnwell, J. C., Koman, L. A., Li, Z., et al. (2014). A human hair keratin hydrogel scaffold enhances median nerve regeneration in nonhuman primates: an electrophysiological and histological study. Tissue Eng. Part A 20, 507–517. doi: 10.1089/ten.TEA.2013.0084
Papalia, I., Cardaci, A., d’Alcontres, F. S., Lee, J. M., Tos, P., and Geuna, S. (2007). Selection of the donor nerve for end-to-side neurorrhaphy. J. Neurosurg. 107, 378–382. doi: 10.3171/jns-07/08/0378
Papalia, I., Geuna, S., Tos, P. L., Boux, E., Battiston, B., and Stagno D’Alcontres, F. (2003a). Morphologic and functional study of rat median nerve repair by terminolateral neurorrhaphy of the ulnar nerve. J. Reconstr. Microsurg. 19, 257–264. doi: 10.1055/s-2003-40582
Papalia, I., Magaudda, L., Righi, M., Ronchi, G., Viano, N., Geuna, S., et al. (2016). Epineurial window is more efficient in attracting axons than simple coaptation in a sutureless (Cyanoacrylate-Bound) model of end-to-side nerve repair in the rat upper limb: functional and morphometric evidences and review of the literature. PLoS One 11:e0148443. doi: 10.1371/journal.pone.0148443
Papalia, I., Raimondo, S., Ronchi, G., Magaudda, L., Giacobini-Robecchi, M. G., and Geuna, S. (2013). Repairing nerve gaps by vein conduits filled with lipoaspirate-derived entire adipose tissue hinders nerve regeneration. Ann. Anat. 195, 225–230. doi: 10.1016/j.aanat.2012.10.012
Papalia, I., Tos, P., Stagno d’Alcontres, F., Battiston, B., and Geuna, S. (2003b). On the use of the grasping test in the rat median nerve model: a re-appraisal of its efficacy for quantitative assessment of motor function recovery. J. Neurosci. Methods 127, 43-47.
Park, J. S., and Hoke, A. (2014). Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PLoS One 9:e90245. doi: 10.1371/journal.pone.0090245
Pereira, T., Armada-da Silva, P. A., Amorim, I., Rema, A., Caseiro, A. R., Gartner, A., et al. (2014). Effects of human mesenchymal stem cells isolated from wharton’s jelly of the umbilical cord and conditioned media on skeletal muscle regeneration using a myectomy model. Stem Cells Int. 2014:376918. doi: 10.1155/2014/376918
Porrero, C., Rubio-Garrido, P., Avendano, C., and Clasca, F. (2010). Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice. Brain Res. 1345, 59–72. doi: 10.1016/j.brainres.2010.05.061
Raimondo, S., Fornaro, M., Di Scipio, F., Ronchi, G., Giacobini-Robecchi, M. G., and Geuna, S. (2009). Chapter 5: methods and protocols in peripheral nerve regeneration experimental research: part II-morphological techniques. Int. Rev. Neurobiol. 87, 81–103. doi: 10.1016/S0074-7742(09)87005-87000
Ray, W. Z., and Mackinnon, S. E. (2010). Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 223, 77–85. doi: 10.1016/j.expneurol.2009.03.031
Ronchi, G., Cillino, M., Gambarotta, G., Fornasari, B. E., Raimondo, S., Pugliese, P., et al. (2017). Irreversible changes occurring in long-term denervated Schwann cells affect delayed nerve repair. J. Neurosurg. 127, 843–856. doi: 10.3171/2016.9.jns16140
Ronchi, G., Fornasari, B. E., Crosio, A., Budau, C. A., Tos, P., Perroteau, I., et al. (2018). Chitosan tubes enriched with fresh skeletal muscle fibers for primary nerve repair. Biomed. Res. Int. 2018:9175248. doi: 10.1155/2018/9175248
Ronchi, G., Gambarotta, G., Di Scipio, F., Salamone, P., Sprio, A. E., Cavallo, F., et al. (2013). ErbB2 receptor over-expression improves post-traumatic peripheral nerve regeneration in adult mice. PLoS One 8:e56282. doi: 10.1371/journal.pone.0056282PONE-D-12-18824
Ronchi, G., Haastert-Talini, K., Fornasari, B. E., Perroteau, I., Geuna, S., and Gambarotta, G. (2016). The Neuregulin1/ErbB system is selectively regulated during peripheral nerve degeneration and regeneration. Eur. J. Neurosci. 43, 351–364. doi: 10.1111/ejn.12974
Ronchi, G., Jager, S. B., Vaegter, C. B., Raimondo, S., Giacobini-Robecchi, M. G., and Geuna, S. (2014). Discrepancies in quantitative assessment of normal and regenerated peripheral nerve fibers between light and electron microscopy. J. Peripher. Nerv. Syst. 19, 224–233. doi: 10.1111/jns.12090
Ronchi, G., Nicolino, S., Raimondo, S., Tos, P., Battiston, B., Papalia, I., et al. (2009). Functional and morphological assessment of a standardized crush injury of the rat median nerve. J. Neurosci. Methods 179, 51–57. doi: 10.1016/j.jneumeth.2009.01.011
Ronchi, G., Raimondo, S., Varejao, A. S., Tos, P., Perroteau, I., and Geuna, S. (2010). Standardized crush injury of the mouse median nerve. J. Neurosci. Methods 188, 71–75. doi: 10.1016/j.jneumeth.2010.01.024
Ruch, D. S., Deal, D. N., Ma, J., Smith, A. M., Castle, J. A., Walker, F. O., et al. (2004). Management of peripheral nerve defects: external fixator-assisted primary neurorrhaphy. J. Bone Joint Surg. Am. 86-A, 1405–1413. doi: 10.2106/00004623-200407000-00007
Santos, A. P., Suaid, C. A., Fazan, V. P., and Barreira, A. A. (2007). Microscopic anatomy of brachial plexus branches in Wistar rats. Anat. Rec. 290, 477–485. doi: 10.1002/ar.20519
Sayad-Fathi, S., Nasiri, E., and Zaminy, A. (2019). Advances in stem cell treatment for sciatic nerve injury. Expert Opin. Biol. Ther. 19, 301–311. doi: 10.1080/14712598.2019.1576630
Schmitz, C., Eastwood, B. S., Tappan, S. J., Glaser, J. R., Peterson, D. A., and Hof, P. R. (2014). Current automated 3D cell detection methods are not a suitable replacement for manual stereologic cell counting. Front. Neuroanat. 8:27. doi: 10.3389/fnana.2014.00027
Shaikh, S., Shortland, P., Lauto, A., Barton, M., Morley, J. W., and Mahns, D. A. (2016). Sensory perturbations using suture and sutureless repair of transected median nerve in rats. Somatosens Mot. Res. 33, 20–28. doi: 10.3109/08990220.2016.1142438
Shibata, M., Breidenbach, W. C., Ogden, L., and Firrell, J. (1991). Comparison of one- and two-stage nerve grafting of the rabbit median nerve. J. Hand. Surg. Am. 16, 262–268. doi: 10.1016/s0363-5023(10)80107-8
Sinis, N., Di Scipio, F., Schonle, P., Werdin, F., Kraus, A., Koopmanns, G., et al. (2009). Local administration of DFO-loaded lipid particles improves recovery after end-to-end reconstruction of rat median nerve. Restor. Neurol. Neurosci. 27, 651–662. doi: 10.3233/rnn-2009-2517
Sinis, N., Kraus, A., Drakotos, D., Doser, M., Schlosshauer, B., Muller, H. W., et al. (2011a). Bioartificial reconstruction of peripheral nerves using the rat median nerve model. Ann. Anat. 193, 341–346. doi: 10.1016/j.aanat.2011.02.018
Sinis, N., Manoli, T., Schiefer, J. L., Werdin, F., Jaminet, P., Kraus, A., et al. (2011b). Application of two different hemostatic procedures during microsurgical median nerve reconstruction in the rat does not hinder axonal regeneration. Neurosurgery 68, 1399–1403. doi: 10.1227/NEU.0b013e3182127bc4
Sinis, N., Schaller, H. E., Becker, S. T., Lanaras, T., Schulte-Eversum, C., Muller, H. W., et al. (2006). Cross-chest median nerve transfer: a new model for the evaluation of nerve regeneration across a 40 mm gap in the rat. J. Neurosci. Methods 156, 166–172. doi: 10.1016/j.jneumeth.2006.02.022
Sinis, N., Schaller, H. E., Schulte-Eversum, C., Lanaras, T., Schlosshauer, B., Doser, M., et al. (2007). Comparative neuro tissue engineering using different nerve guide implants. Acta Neurochir. Suppl. 100, 61–64. doi: 10.1007/978-3-211-72958-8_13
Sinis, N., Schaller, H. E., Schulte-Eversum, C., Schlosshauer, B., Doser, M., Dietz, K., et al. (2005). Nerve regeneration across a 2-cm gap in the rat median nerve using a resorbable nerve conduit filled with Schwann cells. J. Neurosurg. 103, 1067–1076. doi: 10.3171/jns.2005.103.6.1067
Speck, A. E., Ilha, J., do Espirito Santo, C. C., Aguiar, A. S., Jr., Dos Santos, A. R., et al. (2014). The IBB forelimb scale as a tool to assess functional recovery after peripheral nerve injury in mice. J. Neurosci. Methods 226, 66–72. doi: 10.1016/j.jneumeth.2014.01.007
Stossel, M., Rehra, L., and Haastert-Talini, K. (2017). Reflex-based grasping, skilled forelimb reaching, and electrodiagnostic evaluation for comprehensive analysis of functional recovery-The 7-mm rat median nerve gap repair model revisited. Brain Behav. 7:e00813. doi: 10.1002/brb3.813
Strasberg, S. R., Mackinnon, S. E., Genden, E. M., Bain, J. R., Purcell, C. M., Hunter, D. A., et al. (1996). Long-segment nerve allograft regeneration in the sheep model: experimental study and review of the literature. J. Reconstr. Microsurg. 12, 529–537. doi: 10.1055/s-2007-1006625
Sullivan, R., Dailey, T., Duncan, K., Abel, N., and Borlongan, C. V. (2016). Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int. J. Mol. Sci. 17:E2101
Sun, Q., Zheng, J., and Zhao, C. (2012). Nerve transplantation and accompanying peripheral vessels for repair of long nerve defect. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 26, 832–836.
Tannemaat, M. R., Verhaagen, J., and Malessy, M. (2008). The application of viral vectors to enhance regeneration after peripheral nerve repair. Neurol. Res. 30, 1039–1046. doi: 10.1179/174313208x362514
Tannenbaum, J., and Bennett, B. T. (2015). Russell and Burch’s 3Rs then and now: the need for clarity in definition and purpose. J. Am. Assoc. Lab. Anim. Sci. 54, 120–132.
Terenzio, M., Koley, S., Samra, N., Rishal, I., Zhao, Q., Sahoo, P. K., et al. (2018). Locally translated mTOR controls axonal local translation in nerve injury. Science 359, 1416–1421. doi: 10.1126/science.aan1053
Tos, P., Battiston, B., Nicolino, S., Raimondo, S., Fornaro, M., Lee, J. M., et al. (2007). Comparison of fresh and predegenerated muscle-vein-combined guides for the repair of rat median nerve. Microsurgery 27, 48–55. doi: 10.1002/micr.20306
Tos, P., Calcagni, M., Gigo-Benato, D., Boux, E., Geuna, S., and Battiston, B. (2004). Use of muscle-vein-combined Y-chambers for repair of multiple nerve lesions: experimental results. Microsurgery 24, 459–464. doi: 10.1002/micr.20064
Tos, P., Ronchi, G., Nicolino, S., Audisio, C., Raimondo, S., Fornaro, M., et al. (2008). Employment of the mouse median nerve model for the experimental assessment of peripheral nerve regeneration. J. Neurosci. Methods 169, 119–127. doi: 10.1016/j.jneumeth.2007.11.030
Tountas, C. P., Bergman, R. A., Lewis, T. W., Stone, H. E., Pyrek, J. D., and Mendenhall, H. V. (1993). A comparison of peripheral nerve repair using an absorbable tubulization device and conventional suture in primates. J. Appl. Biomater. 4, 261–268. doi: 10.1002/jab.770040308
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:RESEARCH0034.
Varejao, A. S., Melo-Pinto, P., Meek, M. F., Filipe, V. M., and Bulas-Cruz, J. (2004). Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol. Res. 26, 186–194. doi: 10.1179/016164104225013833
Velanac, V., Unterbarnscheidt, T., Hinrichs, W., Gummert, M. N., Fischer, T. M., Rossner, M. J., et al. (2012). Bace1 processing of NRG1 type III produces a myelin-inducing signal but is not essential for the stimulation of myelination. Glia 60, 203–217. doi: 10.1002/glia.21255
Wall, J. T., Kaas, J. H., Sur, M., Nelson, R. J., Felleman, D. J., and Merzenich, M. M. (1986). Functional reorganization in somatosensory cortical areas 3b and 1 of adult monkeys after median nerve repair: possible relationships to sensory recovery in humans. J. Neurosci. 6, 218–233. doi: 10.1523/jneurosci.06-01-00218.1986
Wang, H., Spinner, R. J., Sorenson, E. J., and Windebank, A. J. (2008). Measurement of forelimb function by digital video motion analysis in rat nerve transection models. J. Peripher. Nerv. Syst. 13, 92–102. doi: 10.1111/j.1529-8027.2008.00162.x
Wang, Y., Shan, Q., Meng, Y., Pan, J., and Yi, S. (2017). Mrpl10 and Tbp are suitable reference genes for peripheral nerve crush injury. Int. J. Mol. Sci. 18:E263
Wang, Y. H., Zhang, D. Y., Zhang, P. X., Yin, X. F., Kou, Y. H., Wang, J., et al. (2009). Amplification effects in nerve regeneration after different segment injury: experiment with rabbit median nerve. Zhonghua Yi Xue Za Zhi 89, 1645–1649.
Ward, P. J., Clanton, S. L., II, and English, A. W. (2018). Optogenetically enhanced axon regeneration: motor versus sensory neuron-specific stimulation. Eur. J. Neurosci. 47, 294–304. doi: 10.1111/ejn.13836
Ward, P. J., Jones, L. N., Mulligan, A., Goolsby, W., Wilhelm, J. C., and English, A. W. (2016). Optically-induced neuronal activity is sufficient to promote functional motor axon regeneration in vivo. PLoS One 11:e0154243. doi: 10.1371/journal.pone.0154243
Werdin, F., Grussinger, H., Jaminet, P., Kraus, A., Manoli, T., Danker, T., et al. (2009). An improved electrophysiological method to study peripheral nerve regeneration in rats. J. Neurosci. Methods 182, 71–77. doi: 10.1016/j.jneumeth.2009.05.017
Whishaw, I. Q., Pellis, S. M., and Gorny, B. P. (1992). Skilled reaching in rats and humans: evidence for parallel development or homology. Behav. Brain Res. 47, 59–70. doi: 10.1016/s0166-4328(05)80252-9
Williams, R. W., and Rakic, P. (1988). Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J. Comp. Neurol. 278, 344–352. doi: 10.1002/cne.902780305
Yin, X. F., Kou, Y. H., Wang, Y. H., Zhang, P., Zhang, H. B., and Jiang, B. G. (2011). Portion of a nerve trunk can be used as a donor nerve to reconstruct the injured nerve and donor site simultaneously. Artif. Cells Blood Substit. Immobil. Biotechnol. 39, 304–309. doi: 10.3109/10731199.2011.574636
Yona, S., and Jung, S. (2010). Monocytes: subsets, origins, fates and functions. Curr. Opin. Hematol. 17, 53–59. doi: 10.1097/MOH.0b013e3283324f80
Zhang, P., Zhang, C., Kou, Y., Yin, X., Zhang, H., and Jiang, B. (2009). The histological analysis of biological conduit sleeve bridging rhesus monkey median nerve injury with small gap. Artif Cells Blood Substit. Immobil. Biotechnol. 37, 101–104. doi: 10.1080/10731190902742620
Keywords: median nerve, injury, animal experimental model, repair, regeneration, translational research
Citation: Ronchi G, Morano M, Fregnan F, Pugliese P, Crosio A, Tos P, Geuna S, Haastert-Talini K and Gambarotta G (2019) The Median Nerve Injury Model in Pre-clinical Research – A Critical Review on Benefits and Limitations. Front. Cell. Neurosci. 13:288. doi: 10.3389/fncel.2019.00288
Received: 08 January 2019; Accepted: 13 June 2019;
Published: 28 June 2019.
Edited by:
Esther Udina, Autonomous University of Barcelona, SpainReviewed by:
Arthur W. English, Emory University, United StatesGuilherme Lucas, University of São Paulo, Brazil
Copyright © 2019 Ronchi, Morano, Fregnan, Pugliese, Crosio, Tos, Geuna, Haastert-Talini and Gambarotta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulia Ronchi, Z2l1bGlhLnJvbmNoaUB1bml0by5pdA==
 Giulia Ronchi
Giulia Ronchi Michela Morano
Michela Morano Federica Fregnan
Federica Fregnan Pierfrancesco Pugliese3
Pierfrancesco Pugliese3 Stefano Geuna
Stefano Geuna Kirsten Haastert-Talini
Kirsten Haastert-Talini Giovanna Gambarotta
Giovanna Gambarotta