
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cell. Neurosci. , 24 May 2019
Sec. Cellular Neurophysiology
Volume 13 - 2019 | https://doi.org/10.3389/fncel.2019.00226
This article is part of the Research Topic Emerging Cellular Stress Sensors in Neurological Disorders: Closing in on the Nucleolus and the Primary Cilium View all 9 articles
 Micaela Lucarelli1,2
Micaela Lucarelli1,2 Chiara Di Pietro3
Chiara Di Pietro3 Gina La Sala3
Gina La Sala3 Maria Teresa Fiorenza1
Maria Teresa Fiorenza1 Daniela Marazziti3
Daniela Marazziti3 Sonia Canterini1*
Sonia Canterini1*The Niemann-Pick type C1 (NPC1) is a rare genetic disease characterized by the accumulation of endocytosed cholesterol and other lipids in the endosome/lysosome compartments. In the brain, the accumulation/mislocalization of unesterified cholesterol, gangliosides and sphingolipids is responsible for the appearance of neuropathological hallmarks, and progressive neurological decline in patients. The imbalance of unesterified cholesterol and other lipids, including GM2 and GM3 gangliosides, alters a number of signaling mechanisms impacting on the overall homeostasis of neurons. In particular, lipid depletion experiments have shown that lipid rafts regulate the cell surface expression of dopamine transporter (DAT) and modulate its activity. Dysregulated dopamine transporter’s function results in imbalanced dopamine levels at synapses and severely affects dopamine-induced locomotor responses and dopamine receptor-mediated synaptic signaling. Recent studies begin to correlate dopaminergic stimulation with the length and function of the primary cilium, a non-motile organelle that coordinates numerous signaling pathways. In particular, the absence of dopaminergic D2 receptor stimulation induces the elongation of dorso-striatal neuron’s primary cilia. This study has used a mouse model of the NPC1 disease to correlate cholesterol dyshomeostasis with dorso-striatal anomalies in terms of DAT expression and primary cilium (PC) length and morphology. We found that juvenile Npc1nmf164 mice display a reduction of dorso-striatal DAT expression, with associated alterations of PC number, length-frequency distribution, and tortuosity.
Niemann-Pick type C1 (NPC1) is a rare lysosomal lipid storage disorder caused by mutations in the NPC1 gene, whose protein mediates the egress of cholesterol from lysosomes/endosomes (Peake and Vance, 2010). NPC1 patients develop severe neurological-neurovisceral disorders, including cerebellar ataxia, dysarthria, dysphagia, seizures, and progressive dementia (Vanier, 2010, 2013).
Niemann-Pick type C1 cells display defective synthesis and mobilization of endocytosed cholesterol to the plasma membrane, which affects the functions of neurotransmitters, and their receptors (Fiorenza et al., 2013, 2018).
Dopamine transporter (DAT) regulates the spatio/temporal dynamics of dopamine (DA) neurotransmission by regulating the reuptake of extracellular DA into presynaptic terminals. DAT localization and function are directly regulated by cholesterol (Jones et al., 2012). For instance, cholesterol depletion studies have shown that lipid rafts regulate DAT cell-surface expression (Foster et al., 2008; Gabriel et al., 2013), and modulate its activity (Adkins et al., 2007). In addition, cholesterol has been shown to stabilize DAT conformation and DA binding (Hong and Amara, 2010). A number of G protein-coupled receptors including dopaminergic D2-receptors (D2R) are localized to the primary cilium (PC) of mammalian neurons and the lack of D2 dopaminergic input increases striatal PC length (Marley and von Zastrow, 2010; Miyoshi et al., 2014).
We have recently reported an impairment of Sonic hedgehog (Shh) signaling and PC density and length, in hippocampal neurons of Npc1−/− mice and fibroblasts of NPC1 patients (Canterini et al., 2017). PC is a non-motile organelle that plays critical roles in coordinating numerous neuronal/developmental signaling pathways. Alterations of PC morphology and localization are responsible for ciliopathies, disorders that manifest, like the NPC1 disease, a constellation of clinical features including ataxia, retinal degeneration, behavioral disturbance, and intellectual disability (Waters and Beales, 2011; Guo et al., 2015).
In this study we investigate DA signaling and reception in the striatum of mouse of the Npc1nmf164 strain, that bears a point mutation in the Npc1 gene (D1005G), resulting in a milder, and late-onset form comparable to the most part of human cases (Maue et al., 2012). In this study, we show that juvenile Npc1nmf164 mice display a reduction of DAT expression and alteration of PC number and length-frequency distribution in the dorsal striatum.
Our findings identify early and subtle anomalies in striatal dopaminergic neurotransmission that might contribute to the subsequent appearance of NPC1 disease manifestations.
Homozygous Npc1nmf164 mice maintained on BALB/cJ background were derived from heterozygous matings. Genotypes were identified by PCR analysis of tail DNA (Palladino et al., 2015).
Animal experimental protocols and related procedures were approved by the Italian Ministry of Health-General Directorate of Animal Health (995/2016; D.Igs. 26/2014). All efforts were made to minimize animal suffering, according to European Directive 2010/63/EU.
Brains of postnatal (PN) day 30 Npc1nmf164 mice and wild-type (wt) littermates (5 mice/genotype) (Supplementary Table S1) were collected on ice-cold PBS and cut along the mid-sagittal plane. For Western Blotting, punches of the dorsal striatum (DS) were obtained from coronal slices of one hemisphere using a steel needle (1.5 mm diameter) (Colelli et al., 2010; Campus et al., 2017). The other hemisphere was fixed overnight in 4% paraformaldehyde and cut on Leica-Vibratome (S1000, Leica), for immunohistochemistry (IHC).
Tissue punches of DS were processed for protein extraction and Western blot analysis as previously described (Di Pietro et al., 2017). Primary and secondary antibodies used are listed in Supplementary Table S2. Band intensity was normalized to α-tubulin signal. The average values were expressed in arbitrary units, as a ratio to wt mean values.
Double IHC staining on free-floating sections (30 μm) was performed as previously described (Canterini et al., 2012). Primary and secondary antibodies data are listed in Supplementary Table S2.
Only striatal PC “clearly” double-stained for γ-tubulin (basal-body) and ACIII (PC-shaft) were selected for morphological analysis using Neurolucida analysis system (MBF Bioscience, Williston, VT, United States), connected to Olympus BX53 microscope (100X/1.25 numerical aperture) with 40X/100X immersion objective lens. An average of 6 PC was measured from 10 random fields per mice (n = 318 PC/genotype).
A Mann-Whitney U-test was used to determine the difference in protein levels (GraphPad Software, Inc). The D’Agostino & Pearson omnibus normality test was used to assess the distribution of values. Differences in PC distribution were determined by Chi-squared test, whereas PC lengths and related parameters were analyzed by non-parametric Mann-Withney U-test and Spearman’s correlation analysis. P-values < 0.05 were considered significant.
It is know the functional relation between DAT and TH expression in the striatum (Salvatore et al., 2016) and the physical association between D2R and DAT proteins at striatal presynaptic terminals (Lee et al., 2007). To examine whether the dysregulation of cholesterol homeostasis in Npc1-mutant mice affects dopaminergic signaling in DS, Western blot analysis for the mature form of DAT (mDAT), D2R, TH was performed on protein extracted from tissue punches of PN30 Npc1nmf164 mice, and wt littermates (Figure 1A). Only striatal mDAT levels were found significantly reduced in Npc1nmf164 mice compared to control littermates (P < 0.01; Figure 1B).
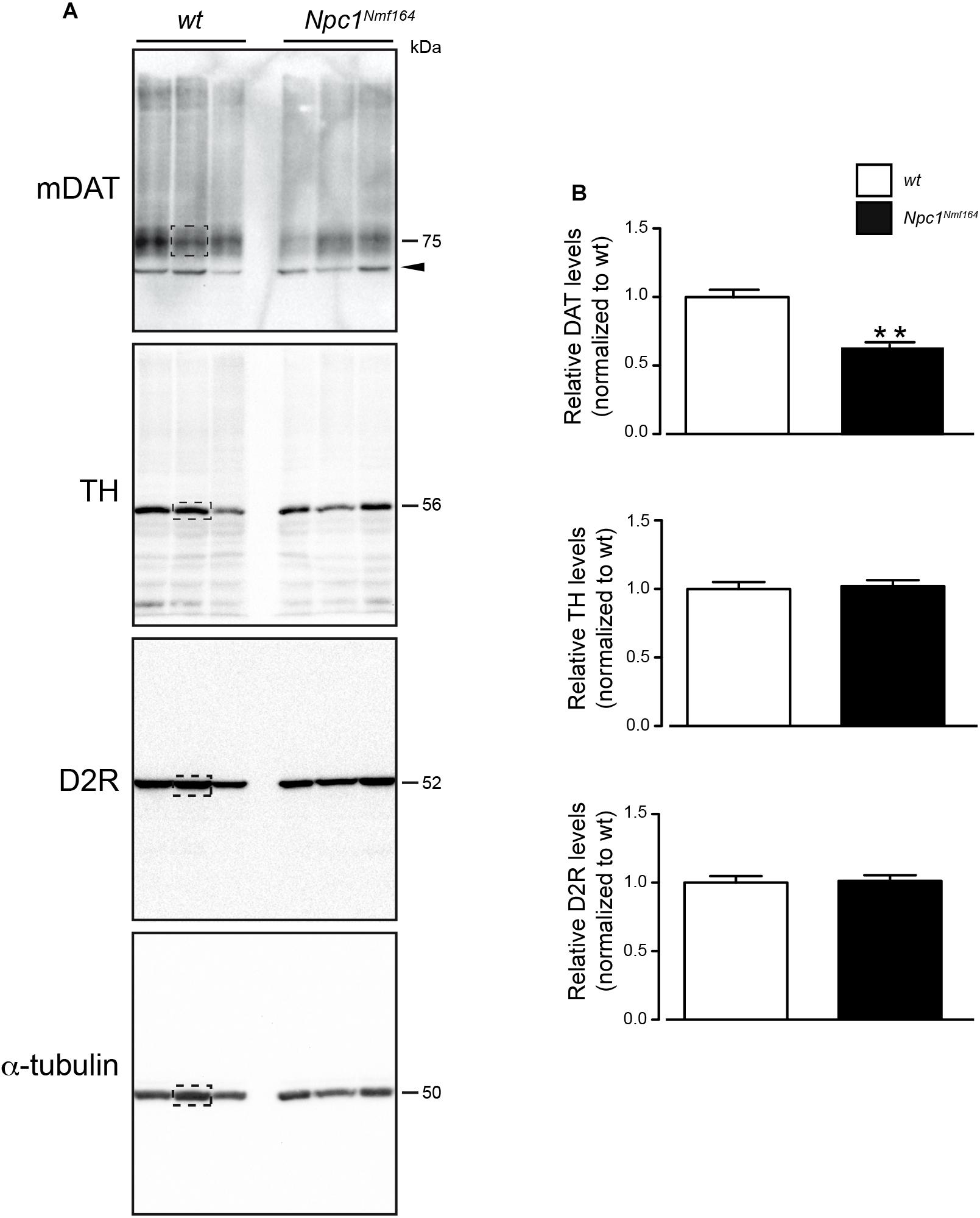
Figure 1. Npc1nmf164 mice display reduction of striatal mDAT expression. (A) Western blot analysis of DAT, D2R, TH, and α-tubulin in representative dorsal striatum (DS) samples of wt or Npc1nmf164 juvenile littermate mice. Boxed areas highlight bands of interest. (B) Densitometric quantification of immunostained mDAT, D2R, and TH proteins in DS extracts prepared from juvenile wt or Npc1nmf164 littermate mice are shown as median ± SEM (n = 5 mice per group), ∗∗P < 0.01, Mann-Withney U-test. Arrowhead indicates likely immature form of DAT.
Npc1nmf164 mice show a reduction of mDAT expression that is likely associated to increased dopaminergic stimulation, which controls PC length through the cAMP pathway (Neve et al., 2004; Ou et al., 2009). To study possible variations of PC morphology in striatal sections from Npc1nmf164 and wt mice, we performed a double IHC labeling with antibodies against γ-tubulin (as basal body marker) and ACIII (as neuronal ciliary shaft marker) (Figure 2A) coupled to the Neurolucida acquisition system. The latter allows the application to PC analysis of standard tools used for neuron tracing, as simultaneous and precise 3D measurements (±0.5 μm) of length, diameter, and tortuosity.
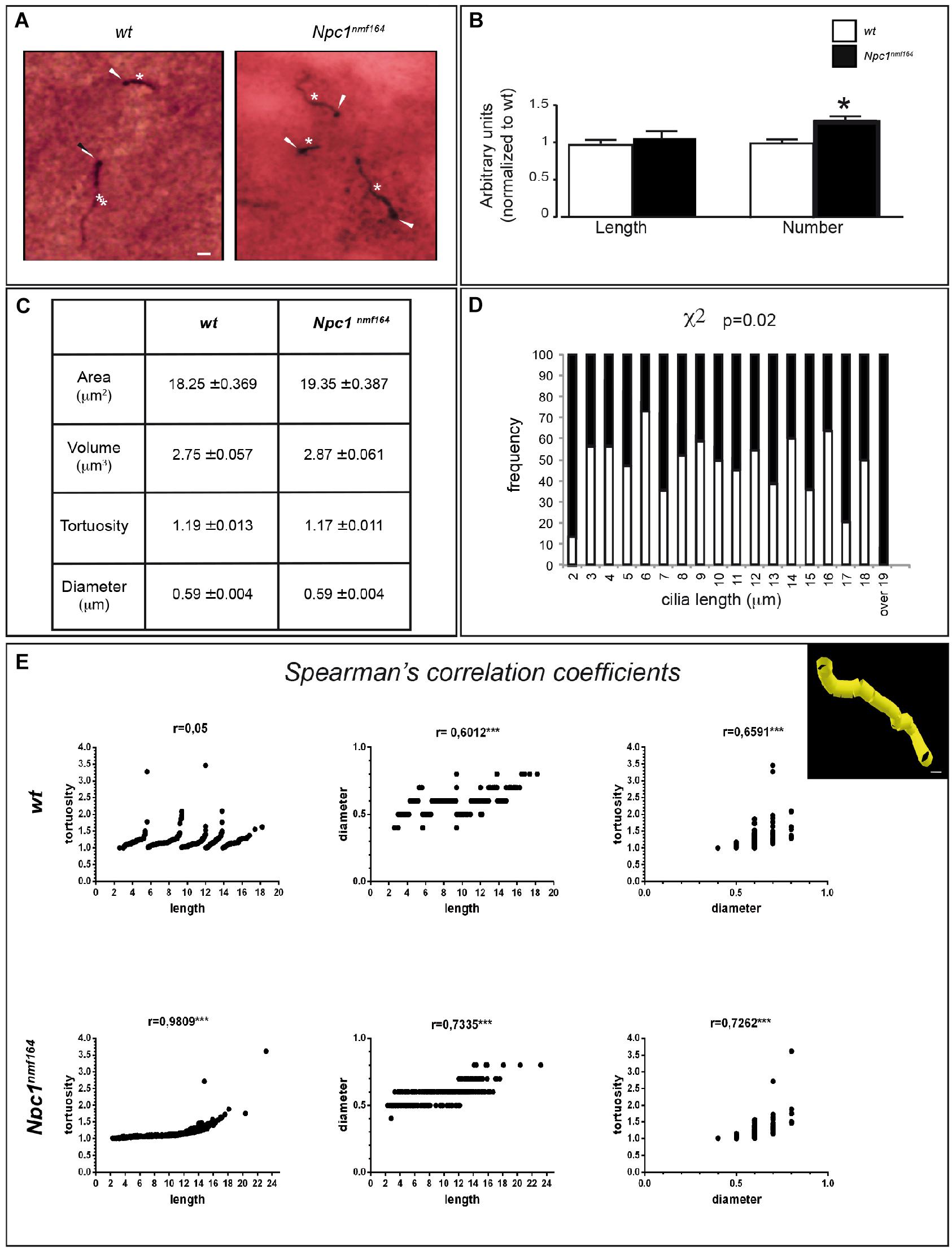
Figure 2. Npc1nmf164 mice show an altered number and length distribution of striatal neuronal primary cilia. (A) Detection of primary cilia (PC) in the DS of PN30 wt and Npc1nmf164 mouse by double IHC with antibodies against γ-tubulin (basal body) and adenylyl cyclase III (ACIII, PC shaft) as indicated by arrowheads and asterisks, respectively. Scale bar: 5 μm. (B) Histograms represent (median ± SE) the quantification of PC length and number in wt (empty bars) and Npc1nmf164 (full bars) mice (n = 5 animals/genotype). Asterisks indicate statistically significant differences (∗P < 0.05, Mann-Withney U-test and Student’s t-tests). (C) Summary of ciliary morphological features and corresponding average values, indicated as median ± SE. No significant differences were found. (D) Histograms show the significant difference in the distributions of ACIII-positive ciliary length values in the dorsal striatum, between wt and Npc1nmf164 mice (P = 0.02). (E) Scatter plot and Spearman’s correlation coefficients between three morphological features (ciliary length, tortuosity and diameter) for each genotype (∗∗∗P < 0.0005). The upper right panel shows a representative 3D Neurolucida reconstruction of dorsal striatal PC. Scale bar: 1 μm.
The experimental results revealed no significant difference between the two genotypes in the mean length of neuronal PC (Figure 2B), as well as in other parameters, including ciliary area, volume, tortuosity, and diameter (Figure 2C). In Npc1nmf164 mice, however, significant differences were observed in the number of ACIII-positive PC (P = 0.03) and distribution of PC lengths (P = 0.02), with an increment of PC with very short (2 μm), or very long length (17 μm and over), compared to control mice (Figure 2D).
Finally, Spearman’s correlation analysis was used to investigate the relationships between three major PC morphological parameters: length, diameter, and tortuosity. The analysis between ACIII-positive PC length and tortuosity, defined as greater bends or kinks in the ciliary axoneme, surprisingly demonstrated a perfect Spearmann’s correlation in Npc1nmf164 mice, in contrast to wt mice. In mutant mice, in fact, the tortuosity progressively rises with increasing length, whereas in wt mice a considerable tortuosity variability is observed both in long and short cilia. Concerning length-diameter and diameter-tortuosity relationships, similar positive Spearman coefficients were found in both wt and mutant samples (Figure 2E).
The prominent feature of the NPC1 disease is a distinctive progressive neurodegeneration, with cerebellum and Purkinje neurons being particularly vulnerable (Higashi et al., 1993; Nusca et al., 2014). Some clinical features, however, overlap between lysosomal storage disorders as NPC1 and Parkinson’s disease, suggesting that the two disorders may be pathogenically linked (Storch et al., 2004; Deng et al., 2015).
To characterize the likely contribution of striatal component in the etiology of NPC1 disease, we analyzed the expression of effectors of DA signaling, including mDAT, D2R, and TH by Western Blot analysis of striatal samples from Npc1nmf164 mice at a juvenile, asymptomatic age in comparison to wt littermates. The reduction of mDAT protein levels in PN30 Npc1nmf164 mice is in agreement with the marked symmetrical loss of striatal DAT, especially in the putamen, observed in NPC1 patients by DAT-scan analysis (Terbeek et al., 2017; Tomic, 2018).
The absence of D2R-mediated stimulation increases cAMP level, which in turn leads to neuronal PC elongation (Besschetnova et al., 2010; Miyoshi et al., 2014). It is also known that PC length and density exhibit brain region-specific changes (Sipos et al., 2018) and ciliary D1-receptor translocates to and from cilia in response to environmental cues (Domire et al., 2011). In addition, DAT, TH, and D2R proteins colocalize in nigrostriatal terminals and their expression levels are often affected in neurological/ neurodegenerative disorders. The distribution of D1R and D2R varies along the rostro-caudal axis of the DS (Gangarossa et al., 2013), whereas DAT and D2R directly interact to facilitate the recruitment of DAT to the plasma membrane (Lee et al., 2007).
We have recently reported that there is a reduction of PC density and length in hippocampal neurons of Npc1−/− mice as well as in fibroblasts of NPC1 patients, with associated dysregulation of expression/subcellular localization of Shh pathway components (Canterini et al., 2017). As no previous information was available on Npc1 deficiency-dependent morphological changes of striatal PC, we performed a 3D analysis of ciliary images for understanding structural determinants of normal and pathological PC function.
The remarkable length of PC of striatal neurons of either wt or Npc1nmf164 mice is in agreement with a previous study that reported the presence in the striatum of a large number of long ACIII-positive PC (Bishop et al., 2007). The absence of statistically significant differences between the two genotypes in the average values of ACIII-, γ tubulin-positive PC length, and related parameters indicates that the mild alteration of DAT expression that we found does not lead to a structural remodeling of dorsal striatal PC in mutant mice. However, a more detailed analysis showed that Npc1nmf164 mice display an increased number of ACIII-, γ-tubulin-positive PC and a different distribution of their lengths, together with increased tortuosity in a length-dependent manner, suggesting anomalies of ciliary functions.
The wider range of lengths and the positive correlation between length and tortuosity observed in Npc1nmf164 suggest that mutant cilia are “unstable.” Such instability possibly reflects a mis-regulation of axonemal length. Mutant cilia could undergo excessive elongation and fragmentation that would explain the increment of either very short or very long PC, which is observed in mutant mice. Similar ciliary instability was reported in Kdm3a mutants (Yeyati et al., 2017).
The reason of regional difference of PC expression in NPC1 disease is still unclear. It could be attributable to multiple factors such as regional changes in dopaminergic signaling or projections, spatial regulation of Shh released from dendrites and axons of dopaminergic neurons or to differences in intracellular cAMP levels that positively regulate the length of PC through the modulation of protein kinase A activity.
In conclusion, our findings identify for the first time subtle changes occurring in the striatum of juvenile asymptomatic Npc1nmf164 mice that could contribute to NPC1 disease neurological manifestations. This is in agreement with: (i) our previous studies that demonstrated early developmental defects which occur postnatally in the cerebellum of Npc1-deficient mice and largely anticipate motor deficits, typically observed during adulthood (Nusca et al., 2014; Caporali et al., 2016); (ii) reported embryonic abnormalities in the metabolism of cholesterol in striatal neurons of Npc1-deficient mice (Henderson et al., 2000); (iii) DAT KO-mice display ataxic symptoms, tremors, dystonia and saccade-failure (Cyr et al., 2003), typical of related-dopamine-transporter-deficiency syndrome (Ng et al., 2014) and late-onset NPC1 disease (Vanier, 2010).
Although the anomalies in DAT expression and PC of Pn30 Npc1 mice we report in this study appear mild, we expect later stages of the disease to be landmarked by more robust alterations, with a decrement of DAT expression and PC length, as consequence of a progressive worsening of the perturbations of plasma membrane lipid content (Peake and Vance, 2010).
Animal experimental protocols and related procedures were approved by the Italian Ministry of Health-General Directorate of Animal Health (995/2016; D.Igs. 26/2014). All efforts were made to minimize animal suffering, according to European Directive 2010/63/EU.
ML performed IHC, image acquisition, and statistical data-analysis. CDP and GLS performed Western Blot and statistical data-analysis. MF contributed with advise and discussion. SC conceived the project. SC and DM directed the project, prepared the figures, and wrote the manuscript.
This study was supported by FFABR to SC and Ateneo La Sapienza (RM11615501ED6577) to MF.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Raffaele Matteoni for critically reading the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2019.00226/full#supplementary-material
Adkins, E. M., Samuvel, D. J., Fog, J. U., Eriksen, J., Jayanthi, L. D., Vaegter, C. B., et al. (2007). Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry 46, 10484–10497. doi: 10.1021/bi700429z
Besschetnova, T. Y., Kolpakova-Hart, E., Guan, Y., Zhou, J., Olsen, B. R., and Shah, J. V. (2010). Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr. Biol. 20, 182–187. doi: 10.1016/j.cub.2009.11.072
Bishop, G. A., Berbari, N. F., Lewis, J., and Mykytyn, K. (2007). Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J. Comp. Neurol. 505, 562–571. doi: 10.1002/cne.21510
Campus, P., Canterini, S., Orsini, C., Fiorenza, M. T., Puglisi-Allegra, S., and Cabib, S. (2017). Stress-induced reduction of dorsal striatal D2 dopamine receptors prevents retention of a newly acquired adaptive coping strategy. Front. Pharmacol. 8:621. doi: 10.3389/fphar.2017.00621
Canterini, S., Bosco, A., Carletti, V., Fuso, A., Curci, A., Mangia, F., et al. (2012). Subcellular TSC22D4 localization in cerebellum granule neurons of the mouse depends on development and differentiation. Cerebellum 11, 28–40. doi: 10.1007/s12311-010-0211-8
Canterini, S., Dragotto, J., Dardis, A., Zampieri, S., De Stefano, M. E., Mangia, F., et al. (2017). Shortened primary cilium length and dysregulated sonic hedgehog signaling in niemann-pick C1 disease. Hum. Mol. Genet. 26, 2277–2289. doi: 10.1093/hmg/ddx118
Caporali, P., Bruno, F., Palladino, G., Dragotto, J., Petrosini, L., Mangia, F., et al. (2016). Developmental delay in motor skill acquisition in niemann-pick C1 mice reveals abnormal cerebellar morphogenesis. Acta Neuropath. Commun. 4:94. doi: 10.1186/s40478-016-0370-z
Colelli, V., Fiorenza, M. T., Conversi, D., Orsini, C., and Cabib, S. (2010). Strain-specific proportion of the two isoforms of the dopamine D2 receptor in the mouse striatum: associated neural and behavioral phenotypes. Genes Brain Behav. 9, 703–711. doi: 10.1111/j.1601-183X.2010.00604.x
Cyr, M., Beaulieu, J. M., Laakso, A., Sotnikova, T. D., Yao, W. D., Bohn, L. M., et al. (2003). Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc. Natl. Acad. Sci. U.S.A. 16, 11035–11040. doi: 10.1073/pnas.1831768100
Deng, H., Xiu, X., and Jankovic, J. (2015). Genetic convergence of Parkinson’s disease and lysosomal storage disorders. Mol. Neurobiol. 51, 1554–1568. doi: 10.1007/s12035-014-8832-4
Di Pietro, C., Marazziti, D., La Sala, G., Abbaszadeh, Z., Golini, E., Matteoni, R., et al. (2017). Primary cilia in the murine cerebellum and in mutant models of medulloblastoma. Cell. Mol. Neurobiol. 37, 145–154. doi: 10.1007/s10571-016-0354-3
Domire, J. S., Green, J. A., Lee, K. G., Johnson, A. D., Askwith, C. C., and Mykytyn, K. (2011). Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell. Mol. Life Sci. 68, 2951–2960. doi: 10.1007/s00018-010-0603-4
Fiorenza, M. T., Dardis, A., Canterini, S., and Erickson, R. P. (2013). Cholesterol metabolism associated molecules in late onset Alzheimer’s disease. J. Biol. Regul. Homeost. Agents 27, 23–35.
Fiorenza, M. T., Moro, E., and Erickson, R. P. (2018). The pathogenesis of lysosomal storage disorders: beyond the engorgement of lysosomes to abnormal development and neuroinflammation. Hum. Mol. Genet. 27, R119–R129. doi: 10.1093/hmg/ddy155
Foster, J. D., Adkins, S. D., Lever, J. R., and Vaughan, R. A. (2008). Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J. Neurochem. 105, 1683–1699. doi: 10.1111/j.1471-4159.2008.05262
Gabriel, L. R., Wu, S., Kearney, P., Bellvé, K. D., Standley, C., Fogarty, K. E., et al. (2013). Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: differential dependence on dynamin and the actin cytoskeleton. J. Neurosci. 33, 17836–17846. doi: 10.1523/JNEUROSCI.3284-13.2013
Gangarossa, G., Espallergues, J., de Kerchove d’Exaerde, A., El Mestikawy, S., Gerfen, C. R., Hervé, D., et al. (2013). Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front. Neural. Circ. 7:22. doi: 10.3389/fncir.2013.00022
Guo, J., Higginbotham, H., Li, J., Nichols, J., Hirt, J., Ghukasyan, V., et al. (2015). Developmental disruptions underlying brain abnormalities in ciliopathies. Nat. Commun. 6, 7857–7870. doi: 10.1038/ncomms8857
Henderson, L. P., Lin, L., Prasad, A., Paul, C. A., Chang, T. Y., and Maue, R. A. (2000). Embryonic striatal neurons from niemann-pick type C mice exhibit defects in cholesterol metabolism and neurotrophin responsiveness. J. Biol. Chem. 275, 20179–20187. doi: 10.1074/jbc.M001793200
Higashi, Y., Murayama, S., Pentchev, P. G., and Suzuki, K. (1993). Cerebellar degeneration in the niemann-pick type C mouse. Acta Neuropathol. 85, 175–184.
Hong, W. C., and Amara, S. G. (2010). Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J. Biol. Chem. 285, 32616–32626. doi: 10.1074/jbc.M110.150565
Jones, K. T., Zhen, J., and Reith, M. E. (2012). Importance of cholesterol in dopamine transporter function. J. Neurochem. 123, 700–715. doi: 10.1111/jnc.12007
Lee, F. J. S., Pei, L., Moszczynska, A., Vukusic, B., Fletcher, P. J., and Liu, F. (2007). Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 26, 2127–2136. doi: 10.1038/sj.emboj.7601656
Marley, A., and von Zastrow, M. (2010). DISC1 regulates primary cilia that display specific dopamine receptors. PLoS One 5:e10902. doi: 10.1371/journal.pone.0010902
Maue, R. A., Burgess, R. W., Wang, B., Wooley, C. M., Seburn, K. L., Vanier, M. T., et al. (2012). A novel mouse model of niemann-pick type C disease carrying a D1005G-Npc1 mutation comparable to commonly observed human mutations. Hum. Mol. Genet. 21, 730–750. doi: 10.1093/hmg/ddr505
Miyoshi, K., Kasahara, K., Murakami, S., Takeshima, M., Kumamoto, N., Sato, A., et al. (2014). Lack of dopaminergic inputs elongates the primary cilia of striatal neuron. PLoS One 9:e97918. doi: 10.1371/journal.pone.0097918
Neve, K. A., Seamans, J. K., and Trantham-Davidson, H. (2004). Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 24, 165–205. doi: 10.1081/rrs-200029981
Ng, J., Zhen, J., Meyer, E., Erreger, K., Li, Y., Kakar, N., et al. (2014). Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood. Brain 137, 1107–1119. doi: 10.1093/brain/awu022
Nusca, S., Canterini, S., Palladino, G., Bruno, F., Mangia, F., Erickson, R. P., et al. (2014). A marked paucity of granule cells in the developing cerebellum of the Npc1(-/-) mouse is corrected by a single injection of hydroxypropyl-β-cyclodextrin. Neurobiol. Dis. 70, 117–126. doi: 10.1016/j.nbd.2014.06.012
Ou, Y., Ruan, Y., Cheng, M., Moser, J. J., Rattner, J. B., and van der Hoorn, F. A. (2009). Adenylate cyclase regulates elongation of mammalian primary cilia. Exp. Cell Res. 315, 2802–2817. doi: 10.1016/j.yexcr.2009.06.028
Palladino, G., Loizzo, S., Fortuna, A., Canterini, S., Palombi, F., Erickson, R. P., et al. (2015). Visual evoked potentials of Niemann-Pick type C1 mice reveal an impairment of the visual pathway that is rescued by 2-hydroxypropyl-ß-cyclodextrin. Orphanet J. Rare Dis. 10, 133–144. doi: 10.1186/s13023-015-0348-0
Peake, K. B., and Vance, J. E. (2010). Defective cholesterol trafficking in niemann-pick C-deficient cells. FEBS Lett. 584, 2731–2739. doi: 10.1016/j.febslet.2010.04.047
Salvatore, M. F., Calipari, E. S., and Jones, S. R. (2016). Regulation of tyrosine hydroxylase expression and phosphorylation in dopamine transporter-deficient mice. ACS Chem. Neurosci. 7, 941–951. doi: 10.1021/acschemneuro.6b00064
Sipos, É, Komoly, S., and Ács, P. (2018). Quantitative comparison of primary cilia marker expression and length in the mouse brain. J. Mol. Neurosci. 64, 1–13. doi: 10.1007/s12031-018-1036-z
Storch, A., Ludolph, A. C., and Schwarz, J. (2004). Dopamine transporter: involvement in selective dopaminergic neurotoxicity and degeneration. J. Neural. Transm. 11, 1267–1286. doi: 10.1007/s00702-004-0203-2
Terbeek, J., Latour, P., Van Laere, K., and Vandenberghe, W. (2017). Abnormal dopamine transporter imaging in adult-onset niemann-pick disease type C. Parkinsonism. Relat. Disord. 36, 107–108. doi: 10.1016/j.parkreldis.2016.12.029
Tomic, S. (2018). Dopamine transport system imaging is pathologic in niemann-pick type C-case report. Neurol. Sci. 39, 1139–1140. doi: 10.1007/s10072-018-3269-6
Vanier, M. T. (2010). Niemann-pick disease type C. Orphanet J. Rare Dis. 5, 16–33. doi: 10.1186/1750-1172-5-16
Vanier, M. T. (2013). Niemann–pick diseases. Handb. Clin. Neurol. 113, 1717–1721. doi: 10.1016/B978-0-444-59565-2.00041-1
Waters, A. M., and Beales, P. L. (2011). Ciliopathies: an expanding disease spectrum. Pediatr. Nephrol. 26, 1039–1056. doi: 10.1007/s00467-010-1731-7
Keywords: Niemann-Pick C1, mouse model, striatum, primary cilium, dopamine
Citation: Lucarelli M, Di Pietro C, La Sala G, Fiorenza MT, Marazziti D and Canterini S (2019) Anomalies in Dopamine Transporter Expression and Primary Cilium Distribution in the Dorsal Striatum of a Mouse Model of Niemann-Pick C1 Disease. Front. Cell. Neurosci. 13:226. doi: 10.3389/fncel.2019.00226
Received: 23 January 2019; Accepted: 06 May 2019;
Published: 24 May 2019.
Edited by:
Rosanna Parlato, Ulm University, GermanyReviewed by:
Martin Witt, Rostock University Hospital, GermanyCopyright © 2019 Lucarelli, Di Pietro, La Sala, Fiorenza, Marazziti and Canterini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sonia Canterini, c29uaWEuY2FudGVyaW5pQHVuaXJvbWExLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.