- 1CNC—Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal
- 2Institute for Interdisciplinary Research, University of Coimbra, Coimbra, Portugal
- 3Department of Life Sciences, University of Coimbra, Coimbra, Portugal
GABAA receptors (GABAAR) are the major players in fast inhibitory neurotransmission in the central nervous system (CNS). Regulation of GABAAR trafficking and the control of their surface expression play important roles in the modulation of the strength of synaptic inhibition. Different pieces of evidence show that alterations in the surface distribution of GABAAR and dysregulation of their turnover impair the activity of inhibitory synapses. A diminished efficacy of inhibitory neurotransmission affects the excitatory/inhibitory balance and is a common feature of various disorders of the CNS characterized by an increased excitability of neuronal networks. The synaptic pool of GABAAR is mainly controlled through regulation of internalization, recycling and lateral diffusion of the receptors. Under physiological condition these mechanisms are finely coordinated to define the strength of GABAergic synapses. In this review article, we focus on the alteration in GABAAR trafficking with an impact on the function of inhibitory synapses in various disorders of the CNS. In particular we discuss how similar molecular mechanisms affecting the synaptic distribution of GABAAR and consequently the excitatory/inhibitory balance may be associated with a wide diversity of pathologies of the CNS, from psychiatric disorders to acute alterations leading to neuronal death. A better understanding of the cellular and molecular mechanisms that contribute to the impairment of GABAergic neurotransmission in these disorders, in particular the alterations in GABAAR trafficking and surface distribution, may lead to the identification of new pharmacological targets and to the development of novel therapeutic strategies.
Introduction
The appropriate equilibrium between excitatory and inhibitory neurotransmission, which is mainly mediated by glutamate and γ-aminobutyric acid (GABA), respectively, is necessary for the correct function of neuronal circuits in the central nervous system (CNS; Smith and Kittler, 2010). Therefore, the control of GABAergic synaptic strength and transmission plays a crucial role in the maintenance of the excitatory/inhibitory synaptic balance (Smith and Kittler, 2010; Mele et al., 2016). An impairment of these mechanisms leading to neuronal hyperexcitability is a common and early event that characterizes several brain disorders (McCormick and Contreras, 2001; Saxena and Caroni, 2011).
The neurotransmitter GABA acts, in part, through activation of GABAA receptors (GABAAR), which are heteropentameric chloride channels, composed in most cases of 2α-, 2β-, and 1γ2-subunits (Rudolph and Möhler, 2004). GABAAR with different subunit compositions have different physiological and pharmacological properties, are differentially expressed throughout the brain and are targeted to different subcellular regions (Fritschy and Mohler, 1995; Nusser et al., 1998b). Receptors composed of α1, α2 or α3 subunits together with β and γ subunits are benzodiazepine-sensitive and largely synaptically located, mediating most phasic inhibition in the brain (Rudolph and Möhler, 2004). The synaptic localization of these receptors is determined by the direct interaction of the alpha subunits with the scaffold protein gephyrin (Tretter et al., 2008, 2011; Mukherjee et al., 2011). On the other hand, GABAAR composed of α4, α5 or α6 subunits, together with β and δ subunits, are predominantly extrasynaptic, mediate tonic inhibition resulting mainly from synaptic “spillover” and are insensitive to benzodiazepine modulation (Brünig et al., 2002; Glykys and Mody, 2007; Jacob et al., 2008). The tonic inhibition in CA1 and CA3 pyramidal neurons is mediated by α5 and δ subunit-containing GABAAR (Glykys and Mody, 2006) that detect low, ambient concentrations of GABA in the extracellular space and desensitize slowly. Accordingly, deletion of the α5 subunit eliminates about half of the tonic currents mediated by GABAAR in hippocampal CA1 and CA3 pyramidal neurons; the remaining current was found to be mediated by GABAAR containing δ subunits (Glykys and Mody, 2006). Moreover, studies using mice bearing a point mutation in position 105 of the GABAAR α5 subunit, which downregulates the expression of the receptors exclusively in hippocampal pyramidal neurons, showed an important role for these subunits in cognitive processes (Crestani et al., 2002).
Under normal physiological conditions GABAAR respond to the binding of GABA by opening an integral chloride channel and allowing chloride to enter the neuron. The result is a membrane hyperpolarization and neuronal inhibition. This mechanism of inhibition by GABAAR depends on the electrochemical potential for chloride. Therefore changes of the intracellular Cl− concentration ([Cl−]i) may regulate the response to the activation of GABAAR (Jedlicka et al., 2011). For example, in immature neurons GABAAR are mostly excitatory due to the fact that the intracellular chloride concentration is above the equilibrium. Maturation of the CNS is accompanied by a decrease of neuronal [Cl−]i, which accounts for the hyperpolarizing effect of the receptor (Watanabe and Fukuda, 2015).
Neuronal [Cl−]i is mostly regulated by two chloride cotransporters, KCC2 (K+-Cl− cotransporter; KCC type 2) and NKCC1 (the Na+-K+-2Cl− cotransporter type 1; Russell, 2000; Blaesse et al., 2009). KCC2 expression is neuronal specific and under normal physiological conditions the transporter extrudes Cl− out of the cell. NKCC1 is present in a variety of cells and generally loads cells with Cl−. Furthermore, the relative expression pattern of the two transporters differs across development (Russell, 2000; Ben-Ari, 2002). The NKCC1 transporter is more expressed earlier in development than KCC2, and this accounts for the high [Cl−]i observed in immature neurons. In the mature brain, the increased abundance of KCC2 contributes to a lower [Cl−]i when compared with the extracellular concentration, favoring the influx of Cl− through the GABAAR channel and consequent membrane hyperpolarization upon activation of the receptors (Kaila et al., 2014).
The activity of GABAAR is also regulated by “cross-talk” with other receptors (Shrivastava et al., 2011a). Since GABAAR can be found in heterologous synapses (Nusser et al., 1996; Renner et al., 2012; de Luca et al., 2017), such receptor cross-talk may be mediated by a direct interaction with other receptors or through activation of intracellular signaling pathways. For example, GABAARs have been demonstrated to heteromerize with GABABR (Balasubramanian et al., 2004), dopamine D5 receptors (Liu et al., 2000), purinergic P2X receptors (Jo et al., 2011; Shrivastava et al., 2011b), nicotinic acetylcholine receptors (Lee et al., 2010) and adenosine A1 receptors (Hu and Li, 1997). In particular, the cross-talk between GABABR/GABAAR may contribute to their regulation at pre- and postsynaptic levels. For instance, a direct interaction of GABAB1 subunits with γ2S subunits of GABAAR was observed in the rat brain, and co-expression of GABAB1 subunits with GABAAR increases the inhibitory responses mediated by the latter receptors (Balasubramanian et al., 2004). Of particular interest is the NMDA receptor (NMDAR) mediated modulation of GABAAR. It has been demonstrated that activation of NMDAR downregulates GABAAR function due to calcium dependent activation of phosphatase 2B/calcineurin followed by dephosphorylation of GABAAR (Stelzer and Shi, 1994; Chen and Wong, 1995; Marsden et al., 2007; Bannai et al., 2009). A recent study showed that GABAARs are trapped at glutamatergic synapses in response to glutamatergic stimulation, thereby limiting GABAAR inter-synaptic diffusion (de Luca et al., 2017). The evidence that a hetero-synaptic interaction is modulated by neuronal activity suggests that cross-talk between GABAAR and other receptors may be considered a mechanism for tuning inhibition in the CNS.
Deficits in the functional expression of GABAAR have been implicated in the pathogenesis of several neurological and psychiatric diseases (Schwartz-Bloom and Sah, 2001; Rudolph and Knoflach, 2011; Kaila et al., 2014). GABAAR are assembled within the endoplasmic (ER) and are then transported to the Golgi. In the ER, unassembled receptor subunits are subjected to poly-ubiquitination that targets them for proteasomal degradation (Kittler et al., 2002), a phenomenon that is dependent on the level of neuronal activity (Saliba et al., 2007). This process is negatively regulated by Plic-1 (the protein that links integrin-associated protein with the cytoskeleton-1; Bedford et al., 2001), which binds directly to the α- and β-subunits of the receptor, prolonging their residence times in the ER (Figure 1). Inside the Golgi, GABAAR receptors bind to GABAAR associated protein (GABARAP)/N-ethylmaleimide-sensitive factor (NSF) complexes, facilitating their transport to the plasma membrane (Leil et al., 2004). This mechanism mediates the increase in the exocytosis of GABAAR observed upon stimulation of cultured hippocampal neurons with N-Methyl-D-aspartate (NMDA; Marsden et al., 2007). The delivery of GABAAR to the plasma membrane is regulated by Golgi-specific DHHC (Asp-His-His-Cys) zinc finger protein (GODZ), a Golgi resident palmitoyltransferase responsible for the palmitoylation of γ subunits. GODZ interacts with the GABAAR γ2 subunit recognizing a 14-amino acid cysteine-rich domain conserved in the intracellular domain of γ1–3 subunits, NH2-terminal to the GABARAP binding site (Rathenberg et al., 2004). The γ2 subunit is palmitoylated at all four cysteines within the GODZ binding domain (Rathenberg et al., 2004; Vithlani et al., 2011). The ADP ribosylation factor (Arf) guanine nucleotide exchange factor (GEF) Big2 (brefeldin A-inhibited GDP/GTP exchange factor 2) also plays a role in the delivery of GABAAR from the Golgi to the plasma membrane by promoting the budding and trafficking of vesicles from this compartment (Charych et al., 2004b). This protein interacts with the intracellular loop of all GABAAR β2 subunits (Charych et al., 2004b). Additional proteins important in the trafficking of GABAAR from the Golgi to the plasma membrane are the glutamate receptor-interacting protein (GRIP; Charych et al., 2004b; Kittler et al., 2004a), the phospholipase C-related catalytically inactive proteins 1 and 2 (PRIP1/2; Kanematsu et al., 2002; Uji et al., 2002), the GABAAR-interacting factor (GRIF-1; Beck et al., 2002) and Maf1 interacting coiled-coil protein (Macoco; Smith et al., 2010). The insertion into the membrane of the vesicles containing GABAAR also depends on SNAP23-syntaxin1A/B-VAMP2 complexes (Gu et al., 2016).
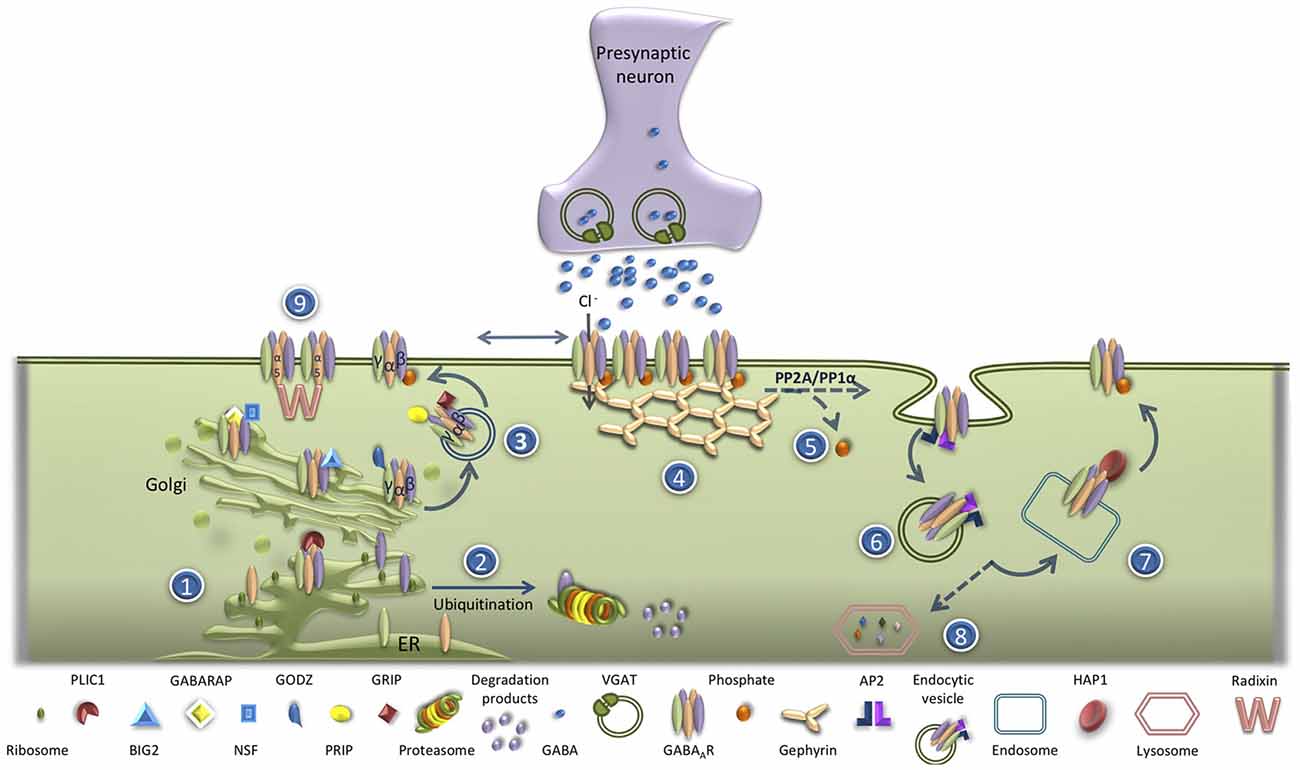
Figure 1. GABAA receptor (GABAAR) trafficking under physiologic condition. (1) GABAAR are assembled in the ER. (2) In the ER, unassembled receptor subunits are subjected to poly-ubiquitination and targeted for proteasomal degradation. (3) GABAAR transport to the Golgi is a process negatively regulated by Plic-1. Inside the Golgi, GABAAR bind to GABAAR associated protein (GABARAP)/N-ethylmaleimide-sensitive factor (NSF) complex that facilitates their transport to the plasma membrane. The delivery of GABAAR to the plasma membrane is also regulated by GODZ, Big2, glutamate receptor-interacting protein (GRIP) and PRIP. (4) At the plasma membrane, GABAAR quickly exchange between synaptic and extrasynaptic locations, and the accumulation of the receptor at the inhibitory synapses is regulated by its scaffold protein gephyrin. (5) The phosphorylation of β3 or γ2 GABAAR subunits on their intracellular loop negatively regulates GABAAR internalization. (6) The process of GABAAR endocytosis is AP2/clathrin/dynamin-mediated. (7) Most internalized GABAAR are rapidly recycled back to the plasma membrane by a mechanism dependent of the interaction with huntingtin-associated protein 1 (HAP1). (8) The non-recycled GABAAR are targeted for lysosomal degradation.
Once in the membrane, GABAAR are very dynamic, exchanging between synaptic and extrasynaptic locations (Jacob et al., 2005; Thomas et al., 2005; Bogdanov et al., 2006), being the accumulation of the receptors at the inhibitory synapses regulated by the scaffold protein gephyrin (Fritschy et al., 2008; Tyagarajan and Fritschy, 2014). Gephyrin recruitment to inhibitory synapses is a fundamental phenomenon for their long-term potentiation (iLTP). Studies using a chemical protocol to induce iLTP in cultured hippocampal neurons, consisting in a moderate activation of NMDARs, showed an increased synaptic clustering of GABAAR by a mechanism involving a CaMKII-dependent phosphorylation of GABAAR β3 subunits on S383 (Petrini et al., 2014). Potentiation of inhibitory synapses in the same model was found to be mediated by recruitment of gephyrin from extrasynaptic regions, downstream of GABAAR phosphorylation, as shown by single-particle tracking (SPT) analysis (Petrini et al., 2014). Recent studies using single-molecule super-resolution imaging with a novel clustering analysis, showed a rearrangement of synaptic gephyrin molecules during iLTP, with the formation of gephyrin nanodomains within the synaptic area (Pennacchietti et al., 2017).
GABAAR are in a continuous cycle between the plasma membrane and the intracellular compartments (Jacob et al., 2008; Mele et al., 2016). Regulation of the total GABAAR surface expression plays a key role in the control of the postsynaptic receptor pool size and the strength of synaptic inhibition (Mele et al., 2016). The process of GABAAR endocytosis occurs mainly via clathrin- and dynamin-dependent mechanisms upon interaction of GABAAR β and γ subunits with the adaptor protein 2 (AP2) clathrin adaptor protein complex (Kittler et al., 2000, 2005, 2008). In the brain, GABAAR interact with AP2 through a direct binding of the β1–3 and γ2 GABAAR subunits (Kittler et al., 2000). The first sequence motif important for AP2/clathrin/dynamin-mediated endocytosis of GABAAR was identified in an heterologous system and corresponds to a di-leucine motif present in β subunits (Herring et al., 2003, 2005). Additional studies performed in neurons, identified an amino acid sequence motif (KTHLRRRSSQLK in the β3 subunit), which includes a major phosphorylation site conserved in the cytoplasmic loop region of β1–3 subunits (Ser408, Ser409 in β3), as an important motif for AP2/clathrin/dynamin-mediated GABAAR internalization (Kittler et al., 2005, 2008). This motif also contains the major sites of phosphorylation by cAMP-dependent protein kinase A (PKA) and calcium/phospholipid-dependent PKC within this class of receptor subunits: Ser409 in β1, Ser410 in β2, and Ser408/9 in β3 (McDonald et al., 1998; Brandon et al., 2002, 2003; Kittler et al., 2005; Smith et al., 2008). Furthermore, a sequence of three arginine residues (405RRR407) was identified within the β3 subunit that is responsible for the interaction of GABAAR with AP2 and in the stabilization of the receptors at dendritic endocytic zones where they are internalized (Smith et al., 2012). The GABAAR internalization rate is negatively regulated by phosphorylation of β3 or γ2 GABAAR subunits on their intracellular loop. Thus, NMDAR signaling is known to control the stability of synaptic GABAAR via calcineurin-mediated dephosphorylation of the receptors (Muir et al., 2010). Moreover, a tyrosine-based AP2-μ2 adaptin-binding motif (Y365GY367ECL) was identified in the GABAAR γ2 subunit, which is also conserved in the γ1 and γ3 subunits (Moss et al., 1995; Kittler et al., 2008). These tyrosine residues are the major sites for phosphorylation by Fyn and Src kinases (Nishikawa et al., 2002; Jacob et al., 2005; Bogdanov et al., 2006), and their phosphorylation reduces AP2 binding (Kittler et al., 2008).
The internalized GABAAR may be rapidly recycled back to the neuronal plasma membrane or targeted for lysosomal degradation. The destiny of receptors following endocytosis is determinant for the regulation of surface/synaptic receptor abundance. The interaction of GABAAR β1–3 subunits with huntingtin-associated protein 1 (HAP1) determines whether endocytosed GABAAR are recycled (Kittler et al., 2004b). HAP1 is a GABAAR associated protein that binds the intracellular loop of β subunits in vitro and in vivo (Kittler et al., 2004b). Overexpression of HAP1 in neurons inhibits GABAAR degradation and consequently increases receptor recycling (Kittler et al., 2004b). Furthermore, HAP1 overexpression was shown to increase surface levels of GABAAR and miniature inhibitory postsynaptic current (mIPSC) amplitude in cultured hippocampal neurons (Kittler et al., 2004b).
The balance between the insertion, lateral diffusion, internalization and recycling of GABAAR in the neuronal plasma membrane determines the strength of GABAergic synapses. Defects in GABAAR trafficking have been reported as triggers of GABAergic dysfunction in a number of brain pathological conditions (Hines et al., 2012). The following sections will address the alterations in GABAAR trafficking, in acute brain disorders, as well as in neuropsychiatric and neurodegenerative diseases (Figure 2).
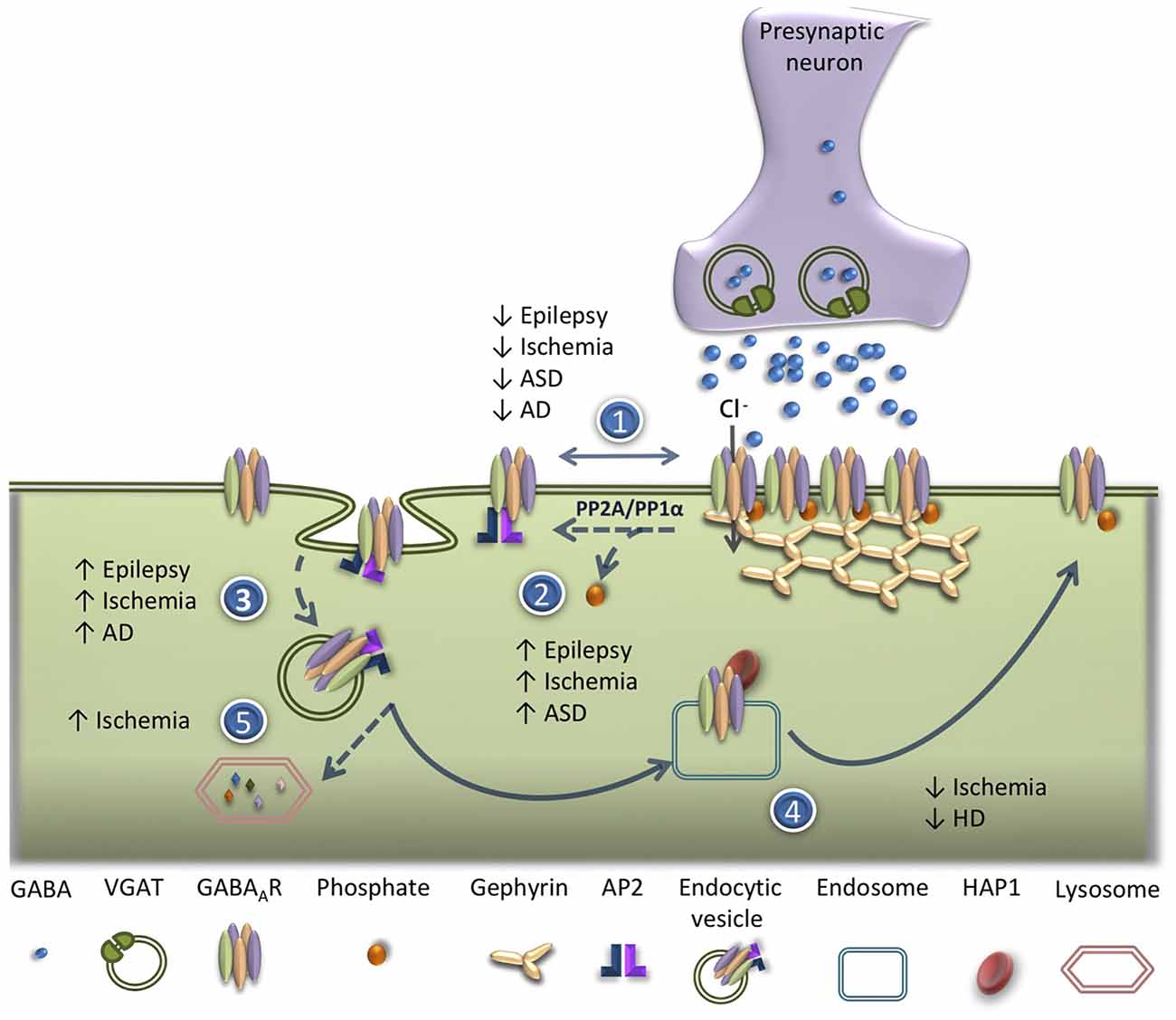
Figure 2. Alterations of GABAAR trafficking in brain disorders. Deficits in GABAAR trafficking have been reported in different pathological conditions in the central nervous system (CNS). (1) Reduced synaptic clustering of GABAAR has been observed in epilepsy, ischemia, autism spectrum disorders (ASDs) and Alzheimer’s disease (AD). (2) Increased dephosphorylation of GABAAR β3 subunit on serine residues 408/9 (Ser408/409) has been reported in epilepsy, ischemic condition and ASD. (3) An increase in AP2/clathrin/dynamin-mediated endocytosis of GABAAR occurs in epileptic conditions, ischemia, ASD and AD. (4) Impairment in GABAAR recycling has been shown in ischemic conditions and in Huntington’s disease (HD). (5) Enhanced lysosomal degradation of GABAAR due to ubiquitination was detected after an ischemic insult.
Alterations in the Rate of Constitutive Degradation and on the Trafficking of GABAAR in Epilepsy
Epilepsy is a chronic disorder of the brain characterized by the presence of recurrent spontaneous seizures. The disease affects approximately 65 million people worldwide, from all ages and both genders (Jacobs et al., 2009; Hesdorffer et al., 2013). In temporal lobe epilepsy, the most common form of partial epilepsy in humans, an initial insult is followed by a seizure-free period before the development of spontaneous seizures. The process by which the brain become hyperexcitable and prone to generate seizures is defined as epileptogenesis (Sharma et al., 2007; Curia et al., 2008). During the latent (seizure-free) period there is a complex reorganization of neuronal networks, which has been characterized in more detail in the hippocampus (Goldberg and Coulter, 2013). An increase in neuronal excitability may contribute to the genesis and/or propagation of epileptic seizures, and several cellular and molecular changes are thought to be involved in the development of spontaneous seizures following a brain insult (Loscher and Brandt, 2010; Goldberg and Coulter, 2013; Staley, 2015).
Studies in animal models have shown that the pathophysiology related with the appearance of seizures is associated with a dysfunction of GABAergic neurotransmission (El-Hassar et al., 2007). Accordingly, several antiepileptic drugs act as agonists of GABAAR (Czuczwar and Patsalos, 2001) and a dysfunction of GABAAR has been proposed to be involved in the etiology of epilepsy. In fact, mutations or genetic variants of the genes encoding the α1, α6, β2, β3, γ2, or δ subunits have been associated with human epilepsy (reviewed by Hirose, 2014). Also, mutations in GABAAR that enhance the constitutive ER-associated degradation (ERAD) of the receptors have been associated with genetically determined epilepsies, as well as, with idiopathic generalized epilepsies (Cossette et al., 2002; Huang et al., 2014). Furthermore, multiple GABAAR mutations associated with epilepsy result in the abnormal trafficking of the receptors (Kang et al., 2015), perturbing their expression on the plasma membrane and synaptic clustering (Han et al., 2015; Huang et al., 2017; Ishii et al., 2017).
Among genetic epilepsies displaying abnormal GABAergic neurotransmission, a group of pediatric monogenic epilepsies was characterized in patients with the Dravet and Rett syndromes (Ali Rodriguez et al., 2018; Gataullina et al., 2019). These disorders are associated with neurodevelopmental complications, and autism spectrum disorders (ASD)-like features are common in patients with both syndromes, suggesting a link between epilepsy and ASD (Ali Rodriguez et al., 2018). In fact, epilepsy is quite common in patients with ASD and therefore the association between epilepsy and autism is receiving growing interest (Deykin and MacMahon, 1979; Olsson et al., 1988; Galanopoulou et al., 2000; Giovanardi Rossi et al., 2000; Besag, 2004; Hughes and Melyn, 2005; Kosinovsky et al., 2005). In addition to the most common mutation in the SCN1A gene affecting the α1 subunit of voltage-gated sodium channels (Wu et al., 2015), Dravet syndrome may also result from mutations in genes that alter GABAergic transmission, such as GABRA1, GABRB2, GABRB3, and GABRG2, encoding the corresponding subunits of GABAAR (α1, β1, β2 and γ2 subunits, respectively). Moreover, a recent study identified a de novo heterozygous missense mutation in GPHN, which encodes for gephyrin, in a patient with Dravet-like syndrome (Dejanovic et al., 2017). Human mutations in the protocadherin-19 (PCDH19) gene, which encodes for the PCDH19 protein, also cause early infantile epileptic encephalopathy, associated with intellectual disability and autistic features (Kolc et al., 2019), similar to Dravet syndrome. PCDH19 cytoplasmic region binds to the α subunits of GABAAR thereby regulating the receptor surface expression, suggesting that PCDH19 might be involved in the regulation of GABAAR intracellular trafficking (Bassani et al., 2018). Furthermore, PCDH19 downregulation in hippocampal neurons causes a reduced frequency of mIPSCs (Bassani et al., 2018).
The primary cause of Rett syndrome is a mutation of the gene encoding the transcriptional repressor methyl-CpG-binding protein 2 (MeCP2; Kozinetz et al., 1993). Between 60 and 80% of females with Rett syndrome suffer from epilepsy (Vignoli et al., 2017). Studies performed in the Mecp2 KO animal model of Rett syndrome, showed a dramatic loss of GABAergic neurons (Chao et al., 2010). Moreover, recent evidence demonstrated that Mecp2 targets KCC2, and neurons differentiated from induced pluripotent stem cells from patients with Rett syndrome showed a reduced expression of KCC2 and a delayed switch in the excitatory to inhibitory responses to GABA during development (Tang et al., 2016).
The major problem in the therapy of status epilepticus (SE), and recurrent epileptiform discharges, is the time-dependent pharmacoresistance; about 30% of the patients become resistant to the treatment (Regesta and Tanganelli, 1999; French, 2007). A potential mechanism accounting for the impairment of inhibitory neurotransmission, characteristic of SE, and for the development of pharmacoresistance to benzodiazepines (De Koninck, 2007), is a reduction in the availability of functional GABAARs associated with the plasma membrane, which may arise from an altered pattern of receptor trafficking (Figure 2). Accordingly, in vitro studies performed in hippocampal neurons exposed to a medium lacking Mg2+, to induce epileptiform discharges, showed a reduction of about 50% in the surface expression of GABAAR after 1 h of SE, as demonstrated by a biotinylation assay (Cho et al., 2017). Furthermore, experiments using cultured hippocampal neurons incubated in a medium lacking Mg2+, an in vitro model of SE, showed a reduction in the surface stability of GABAAR as determined by live-cell imaging of SE pHluorin (SEP)-tagged α2 subunits. The observed decrease in the surface expression of GABAAR was mediated by activation of NMDARs for glutamate and was sensitive to inhibition of the phosphatase calcineurin (Eckel et al., 2015). Additional studies using the same in vitro model of SE combined with electrophysiological and cellular imaging techniques, showed that prolonged epileptiform bursting leads to a reduction of GABA-mediated synaptic inhibition; the constitutive internalization of GABAAR accelerated by the increased neuronal activity was associated with seizure activity. Moreover, inhibition of neuronal activity reduced the effect of SE on the rate of GABAAR internalization as well as the downstream reduction in the surface expression of the receptors that may contribute to the downregulation of inhibitory neurotransmission observed during seizures (Goodkin et al., 2005). This model is supported by evidence obtained in in vivo studies using the lithium-pilocarpine model of TLE, which showed a reduction in the amplitude of mIPSCs mediated by postsynaptic GABAAR when tested in dentate gyrus granule cells (Naylor et al., 2005). In contrast, the amplitude of extrasynaptic GABAAR tonic currents was increased during SE (Naylor et al., 2005). These results also suggests a possible increase in extracellular GABA concentration during SE, which may be coupled to an upregulation of extrasynaptic tonic currents, while synaptic currents may be decreased under the same conditions due to desensitization and internalization of GABAAR (Naylor et al., 2005). In fact, inhibition of GABAAR endocytosis in epileptic cultures resulted in both a recovery of the levels of membrane associated GABAAR and a total blockade of spontaneous recurrent epileptiform discharges (Blair et al., 2004).
In accordance with the role of GABAAR phosphorylation in the regulation of their surface expression (see above), SE reduces PKC-dependent phosphorylation of GABAAR β3 subunit on the serine residues 408/9 (Ser408/409; Terunuma et al., 2008). These residues contain a binding motif for the clathrin AP AP2, being a critical regulator of GABAAR endocytosis (Nakamura et al., 2015). Pharmacological activation of PKC or the specific blockade of GABAAR binding to AP2, during SE, restores the surface expression of the receptors, re-establishing the efficacy of synaptic inhibition (Terunuma et al., 2008).
The proper trafficking of GABAAR required to maintain the number and localization of the receptors at the neuronal surface is also dependent on the function of different proteins that interact with GABAAR directly or through adaptor proteins linked with microtubules (Mele et al., 2016). The expression of key scaffolding proteins associated with GABAAR is altered during epileptogenesis. For example, SE downregulates the expression of gephyrin and GRIP in the hippocampal CA1 region 4–8 days after the insult (pilocarpine injection; González et al., 2013). These alterations are correlated with changes in the plasma membrane expression and assembly of GABAAR (González et al., 2013). To what extent the downregulation of GRIP contributes to the observed reduction in the surface expression of GABAAR remains to be investigated. In fact, GRIP interacts with GABARAP (Kittler et al., 2004b) and is expressed at inhibitory postsynapses (Dong et al., 1999; Charych et al., 2004a; Li et al., 2005). Therefore, the SE-induced decrease in GRIP protein levels may impair the GABARAP-mediated delivery of GABAAR to the plasma membrane (Marsden et al., 2007).
Alterations in gephyrin clustering and expression during epileptogenesis were also detected in the hippocampus and in the cerebral cortex (Thind et al., 2010; Fang et al., 2011). The epileptogenic period is characterized by a reduction in the number of gephyrin puncta and GABAergic synapses in dentate gyrus, while an increased number of gephyrin clusters was detected during the chronic period (Thind et al., 2010). Moreover, studies performed in the neocortex showed that gephyrin expression gradually decreases during the epileptogenic period and returns to basal levels during the chronic phase (Fang et al., 2011). Thus, gephyrin downregulation may contribute to the instability of GABAAR clustering, amplifying the deficit in GABAergic neurotransmission observed in epileptic condition.
The ezrin/radixin/moesin (ERM) family protein radixin acts a scaffold to anchor α5βγ2 GABAAR to the actin cytoskeleton at extrasynaptic sites (Loebrich et al., 2006). This interaction is regulated by an activity-dependent manner through the RhoA-ROCK pathway (Hausrat et al., 2015). The dissociation of the receptors from the radixin anchor allows the lateral diffusion of GABAAR to increase their synaptic expression (Hausrat et al., 2015). However, whether this type of mechanism regulates the surface expression of GABAAR containing α5 subunits remains to be investigated.
Recent evidence indicates that alterations in chloride homeostasis may also contribute to the impairment of the GABA inhibitory activity (Rivera et al., 2004). These alterations have been attributed to a downregulation of the K+-Cl− cotransporter KCC2. The resulting increase in the intracellular Cl− concentration may account for the positive shift of the GABAAR reversal potential, and the consequent depolarizing effects of GABA, observed in hippocampal slices exposed to conditions mimicking status epilepticus (Coull et al., 2003). Interestingly, two independent studies reported that rare variants of KCC2 confer an increased risk of epilepsy in humans (Kahle et al., 2014; Puskarjov et al., 2014). However, whether the SE-induced alteration in GABAAR trafficking depends on the alteration in Cl– gradient was not yet confirmed.
Taken together, the studies mentioned above indicate that during seizures, the persistent cell firing and GABA release may lead to the extracellular accumulation of GABA, causing desensitization and internalization of postsynaptic GABAAR. Moreover, alterations of scaffolding proteins associated with GABAAR, mainly gephyrin, contribute to the ultimate failure of inhibition observed in epilepsy. These mechanisms could account for the maintenance of recurrent seizure activity and benzodiazepine pharmacoresistance.
A Decrease in GABAAR Anchoring at the Synapse and in Receptor Recycling Impair Inhibitory Synapses in Brain Ischemia
Cerebral ischemia is a pathological condition caused by insufficient blood supply to the brain, which leads to an increase in glutamatergic neurotransmission coupled to excitotoxic neuronal death. The down-regulation of GABAergic synapses in brain ischemia resulting from GABAAR desensitization (Gyenes et al., 1994) and a reduction of cell surface density of GABAAR (Nusser et al., 1997, 1998a), is one of the major factors contributing to excitotoxicity (Mele et al., 2014).
One of the first direct evidence suggesting that ischemic insults decrease the cell surface expression of GABAAR through an increase in receptor internalization (Figure 2) came from in vitro studies using ELISA, as a cell surface receptor assay (Mielke and Wang, 2005). These studies showed that transient incubation of cultured cortical neurons in the absence of oxygen and glucose to mimic global ischemia decreases cell surface GABAAR without altering the total expression of receptors. In fact, inhibition of receptor endocytosis with hypertonic sucrose treatment prevented receptor internalization. In the same study, the authors suggested that GABAAR internalization could contribute to neuronal death (Mielke and Wang, 2005). Similarly, studies using quantitative membrane protein biotinylation assays and immunocytochemistry confirmed that the abundance of plasma membrane-associated GABAAR was significantly decreased in cortical and hippocampal neurons exposed to oxygen and glucose deprivation (OGD). In this set of experiments the activation of phosphatidylinositol 3-kinase/Akt-dependent signaling pathway, through PTEN downregulation, was shown to protect neurons from the toxic effects of OGD by preventing the reduction in the surface expression of GABAAR (Smith et al., 2012). Results obtained with antibody feeding assay also showed that OGD-induces the internalization of GABAAR-α1 and β3 subunits in cultured hippocampal neurons by a dynamin-dependent mechanism (Mele et al., 2014). Additionally, it was reported that the down-modulation of GABAAR from dendritic clusters during OGD is dependent on the AP2 pathway for cell surface removal of the receptors. Moreover, blockade of this pathway reduced the neuronal death induced by OGD (Kittler et al., 2008).
The interaction between β3-subunit and AP2 seems to be critical for GABAAR reduction in synapses during ischemic insult (Smith et al., 2012). The identification of the intracellular domains (ICD) region of the β3-subunit that mediates the interaction with the clathrin adaptor AP2 also revealed the presence of three arginine residues (405RRR407) within this binding motif that are essential for the interaction with μ2–AP2; mutation of these residues impairs receptor recruitment to clathrin-coated pits, significantly reducing receptor endocytosis (Smith et al., 2012). Studies performed with a β3-subunit RRR motif mutant with a deficient AP2 binding site showed that the acute loss of synaptic GABAAR during OGD is mediated by an AP2/β3 interaction. Furthermore, blocking the internalization of GABAAR using a peptide competing with β3 for the binding to AP2 reduces OGD-induced cell death (Smith et al., 2012).
Interestingly, the β3-subunit RRR motif is located adjacent to a phosphorylation site, Ser408/Ser409, which is known to negatively regulate the internalization of the receptor when phosphorylated (Kittler et al., 2005, 2008). These phosphorylation sites are also regulated during an ischemic insult, both in vivo (using the transient middle cerebral artery occlusion—MCAO, a model of focal ischemia) and in vitro (OGD). In particular, it was found that brain ischemia induces the dephosphorylation of GABAAR β3-subunit (Ser408/Ser409) in vitro and in vivo (Mele et al., 2014). Studies with cultured hippocampal neurons subjected to OGD confirmed that the dephosphorylation of this domain is responsible for the observed increase in receptor internalization (Mele et al., 2014). Again, the consequent reduction in the surface expression of GABAAR was correlated with ischemia-induced cell death, since the transfection of hippocampal neuron with a phospho-mimetic mutant of GABAAR β3 subunit (SS408/409AA), which does not undergo internalization, reduced significantly the OGD-induced apoptotic neuronal death (Mele et al., 2014).
The destiny of GABAAR after endocytosis depends on their interaction with HAP1 (Kittler et al., 2004b). Under physiologic conditions most internalized GABAAR are rapidly recycled back to the plasma membrane, by a mechanism dependent of HAP1, while the remaining pool of receptors undergoes lysosomal degradation (Kittler et al., 2004b). Cultured hippocampal neurons subjected to OGD showed an impairment in receptor recycling that was correlated with a decrease in the interaction of the receptor with HAP1. This protein is indeed downregulated during OGD condition by a calpain mediated mechanism. When overexpressed, HAP1 protected hippocampal neurons from OGD-induced cell death (Mele et al., 2017).
The reported reduction in the number of synaptic GABAAR observed in brain ischemia may also be directly related with the ubiquitination-dependent degradation of the receptors. In particular the ubiquitination of lysine residues between amino acids 317–328 within the intracellular domain of the GABAAR γ2 subunit modulates the lysosomal targeting of the receptor. This process controls the efficacy of neuronal inhibition under basal conditions by regulating the accumulation of GABAAR at inhibitory synapses (Arancibia-Cárcamo et al., 2009). The deficit in neuronal inhibition under conditions of OGD also involves an enhanced degradation of GABAAR due to ubiquitination of a motif located within the intracellular domain of the γ2 subunit, with a consequent deficit in the cell surface stability of the receptors (Arancibia-Cárcamo et al., 2009).
Together, these studies point to postsynaptic alterations of GABAergic synapses as central players in synaptic dysfunction induced by brain ischemia. The internalization of GABAAR that accounts for the impairment in inhibitory neurotransmission may also be related with the synaptic instability of the receptor. Indeed, the gephyrin scaffold protein was found to be cleaved in cultured hippocampal neurons subjected to OGD, by a calpain-dependent mechanism. The resulting disassembly of the gephyrin lattice underneath the plasma membrane is likely to cause an inefficient synaptic anchoring of GABAAR (Costa et al., 2016). OGD also decreases GABAAR/gephyrin interaction, as shown in experiments of surface co-immunoprecipitation of GABAAR α1 subunits and gephyrin (Mele et al., 2014). The decrease in the interaction between GABAAR and its scaffold protein gephyrin suggests a possible alteration in the membrane dynamics of the receptor. An increased mobility of the receptors at the synapse may make them less confined within this compartment, and these receptors would become more prone to be internalized. However, further experiments are needed to better understand the alteration induced by ischemic insults on the lateral diffusion of GABAAR, and the signaling mechanisms involved, contributing to the impairment of GABAergic synapse strength. The internalization of GABAAR after an ischemic injury may explain, at least in part, the failure of receptor agonists or modulators in clinical trials for stroke (Amantea and Bagetta, 2017).
Alteration of the electrochemical gradient may also contribute to the impairment of GABAergic neurotransmission in brain ischemia. Several studies reported a decrease in KCC2 expression in brain ischemia (Galeffi et al., 2004; Papp et al., 2008; Jaenisch et al., 2010). Transient MCAO was found to decrease KCC2 mRNA levels, 1 day after reperfusion, and a consequent downregulation in the protein levels of the transporter was detected 7 days after reperfusion (Jaenisch et al., 2010). An attenuated expression of KCC2 in neurons subjected to an ischemic insult may trigger GABA-evoked depolarizing responses, thereby influencing plasticity and damage induced by stroke.
Animal Models of Autism Spectrum Disorders (ASD) Are Characterized by a Downregulation of GABAAR and Alteration in Their Synaptic Distribution
ASD is a group of early-onset developmental disorders characterized by a variety of behavioral deficits and intellectual disability (Mattina et al., 2009). More than 80% of ASD cases are caused by genetic alterations (Rosenberg et al., 2009; Frazier et al., 2014; Baio et al., 2018). However, a huge number of genes have been identified associated to ASD, making difficult the study of the physiological pathways affected by these conditions.
The imbalance between neuronal excitation and inhibition within cortical circuits has been suggested as a cellular mechanism accounting for the behavioral and cognitive symptoms of ASD (Jenks and Volkers, 1992; Ramamoorthi and Lin, 2011; Yizhar et al., 2011). Although the neurobiological bases of ASD have not been clearly established, several genes related to autism were shown to encode synaptic proteins. Accordingly, an aberrant synaptic activity is characteristic of ASD patients (Howell and Smith, 2019). In particular, a dysfunction in the GABAergic system has been suggested to play an important role in the pathogenesis of ASD (Nielsen, 1990; Dhossche et al., 2002; Pizzarelli and Cherubini, 2011; Figure 2).
A recent study reported a decreased expression of membrane associated GABAAR-β3 subunits, as well as a downregulation of the phosphorylated form of the receptor subunit, in the sodium valproate (VPA)-induced rat model of ASD. The reduced phosphorylation levels of GABAAR-β3 subunit suggests alterations in the trafficking of the receptor, namely an increase in receptor internalization. The changes in GABAergic neurotransmission induced by prenatal exposure to VPA were also associated to impaired spatial memory, limited exploration, increased anxiety, and reduced sociability (Li et al., 2017b).
Alterations in the phosphorylation of GABAAR γ2 subunits may also be relevant for the ASD phenotype as shown in studies using the Ser408/409Ala homozygous mice, in which the receptor subunit shows a low interaction with the AP2 complex which decreases internalization, similarly to the behavior of phosphorylated receptors. These animals are characterized by an increase in the activity of synaptic GABAAR, together with a reduction in the extrasynaptic inhibitory currents, and exhibit the core phenotypes of ASD (Vien et al., 2015). The fmr1 KO mice which are commonly used as a model to study the fragile X syndrome and ASD also display an increased phosphorylation of GABAAR γ2 on Ser408/409 (Vien et al., 2015), further pointing to a role for alterations in the phosphorylation state of this subunit in neuropsychiatric disorders.
Deficits in GABAAR surface expression were also detected in mice with a loss-of-function of PX-RICS that results in ASD-like behaviors (Nakamura et al., 2016). These mice recapitulate the pathogenic process of ASD-like behavior characteristic of Jacobsen syndrome (JBS) patients (Mattina et al., 2009). PX-RICS−/− mice exhibit a dysfunction of the postsynaptic mechanism for GABAAR trafficking. Cell surface labeling and biotinylation assays revealed that GABAAR γ2 surface expression is significantly reduced in PX-RICS−/− hippocampal neurons and in cerebellar granule neurons (CGNs). Moreover, whole-cell patch–clamp experiments detected a reduction in the amplitude of mIPSCs with no significant differences in their frequency, suggesting that the postsynaptic responsiveness to inhibitory input is impaired without alteration in the presynaptic release of the neurotransmitter. Interestingly, stimulation with a GABAAR agonist improved some autistic-like phenotypes of PX-RICS−/− mice (Nakamura et al., 2016). This suggests that a potentiation of postsynaptic GABAergic signaling could be a possible therapeutic strategy for ASD-like behavior.
The impairment of GABAergic neurotransmission in patients with ASD is further supported by evidence showing increased levels of Hrd1 in the middle frontal cortex of patients with ASD (Crider et al., 2014). This E3 ligase ubiquitinates misfolded GABAAR α1 subunits before ERAD in HEK293 cells (Di et al., 2016). Interestingly, a downregulation of GABAAR α1 subunits was also detected in the middle frontal cortex of ASD patients (Crider et al., 2014).
Mutations in several proteins associated with the postsynaptic density (PSD) of excitatory synapses have been associated with neuropsychiatric disorders (Volk et al., 2015; Li et al., 2017a; Gandal et al., 2018). The growing interest in the characterization of the inhibitory PSD (Tyagarajan and Fritschy, 2014) may shed light into the complexity of the mechanisms involved in the regulation of GABAergic neurotransmission and may show novel molecular players involved in the regulation of the surface dynamics of GABAAR with a role in neuropsychiatric disorders, including ASD.
GABAA-Receptor Trafficking Involvement in Neurodegenerative Disorders
Alterations in GABAAR trafficking coupled to the dysregulation of the synaptic excitatory/inhibitory balance are also a common feature of several neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and Huntington’s disease (HD; Figure 2). These alterations might induce changes in synaptic strength and ultimately lead to excitotoxicity and consequent neuronal cell death.
AD is a chronic and progressive neurodegenerative disease characterized by memory deficits and cognitive decline owing to synaptic and neuronal loss in the hippocampus and cerebral cortex. The abnormal deposition of amyloid-β (Aβ) in these brain regions suggests that this peptide plays an essential role in AD pathogenesis (Karran and De Strooper, 2016). In fact, the observed deleterious effects of Aβ were shown to arise, in part, from the interaction of the peptide with NMDAR, causing excitotoxity and neuronal dysfunction (Costa et al., 2012).
GABAergic signaling was also demonstrated to be profoundly altered in the AD brain (Limon et al., 2012). Indeed, GABA currents were shown to desensitize faster and the GABAAR were found to be less sensitive to GABA after micro-transplantation of membranes from the temporal cortex of AD patients into Xenopus oocytes (Limon et al., 2012). Aβ was also shown to weaken synaptic inhibition through downregulation of GABAAR via receptor endocytosis (Ulrich, 2015). Accordingly, Aβ induced a decline in mIPSCs in layer V pyramidal neurons, an effect that was prevented using an inhibitor of the dynamin-mediated internalization of GABAAR (Ulrich, 2015). This result indicates that the observed hyperexcitability characteristic of AD could be partly related with the loss of functional GABAAR observed in the AD brain (Limon et al., 2012) and with the loss of synaptic inhibitory strength induced by Aβ (Ulrich, 2015).
In the context of AD, GABAAR were also show to suffer several consistent alterations in their subunit composition (e.g., α1, α2, α5, β2, β3 and γ2), in different brain regions, namely in the hippocampus (Kwakowsky et al., 2018). The complexity of these alterations is not compatible with simple compensatory mechanisms, but may reflect instead the reorganization of defined neuronal circuits (Kwakowsky et al., 2018). Despite these results, the effects of Aβ on inhibitory synapses are still poorly understood as most studies have focused on the impairment of excitatory synaptic transmission. In particular, the signaling pathways by which Aβ induce GABAAR endocytosis remain to be investigated. Since Aβ enhances neuronal excitability though NMDA activation and synaptic plasticity (Parihar and Brewer, 2010; Costa et al., 2012; Varga et al., 2014), this may constitute the signal to induce the internalization of GABAAR. Future studies should also address a possible direct interaction of Aβ with GABAAR or with proteins associated with the inhibitory PSD. Whether the tau pathology in AD is also somehow related with alterations in GABAAR traffic also remains to be investigated. Furthermore, the implications of the alteration in GABAAR trafficking in AD progression are still unclear. Several studies suggested that part of the symptoms associated to this disorder might be caused by the loss of the synaptic excitatory/inhibitory balance (Michels and Moss, 2007; McDade et al., 2009; Ulrich, 2015).
Alterations in GABAAR trafficking have also been associated with PD. This long-term neurodegenerative disorder mainly affects the motor system and causes a characteristic combination of motor symptoms (e.g., hypertonia) due to progressive neurodegeneration of dopaminergic neurons (Gilbert et al., 2006; Meder et al., 2018). The symptomatic treatment of hypertonia can be achieved by enhancing GABAergic transmission. Indeed, the regulation of GABAAR homeostasis was reported to be disrupted in a hypertonic mouse model bearing a mutation in the hyrt gene, which codes for the trafficking protein kinesin binding 1 (Trak1). This study showed a marked reduction in the levels GABAAR in the CNS, particularly in the lower motor neurons, and, interestingly, Trak1 was found to interact with GABAAR (Gilbert et al., 2006). Trak1 (and Trak2) shares some homology with HAP1 (Li et al., 1995), which has been implicated in intracellular trafficking and transport of GABAAR (Kittler et al., 2004b; Gilbert et al., 2006). In contrast with the effect on the expression of GABAAR, the distribution of the GABAAR anchoring protein gephyrin was not altered in hyrt mice. Therefore, the reduction in GABAAR in hyrt mice may be due to the dysregulation of GABAAR endocytic trafficking rather than to the destabilization of the plasma membrane complex that stabilizes the receptors at the synapse (Gilbert et al., 2006). Thus, it can be hypothesized that Trak1 may facilitate the targeting of endocytosed receptors back to the membrane or it may block their degradation. Interestingly, no significant degeneration of GABAergic neurons was observed in hyrt mice despite the reduction in the levels of GABAAR subunits in this hypertonic mouse model (Gilbert et al., 2006), as described for AD (Ulrich, 2015).
Other proteins have been associated with the reduction of GABAAR surface expression in PD. GABARAPs are a family of proteins that play a role in vesicle and receptor trafficking (Kittler et al., 2001), and in particular they were shown to interact and regulate the intracellular trafficking of GABAAR (Wang et al., 1999; Chen et al., 2001; Chen and Olsen, 2007). Furthermore, members of this protein family have been implicated in autophagy (Rowland et al., 2006; Schwarten et al., 2009), a mechanism involved in GABAAR clearance (Rowland et al., 2006). A recent study showed that GABARAPs also bind the parkin-associated endothelin-like receptor (PAELR; Dutta et al., 2018), which is localized in the core of Lewy bodies, a PD hallmark (Murakami et al., 2004). Furthermore, PAELR interacts with the GABAAR binding site of GABARAPL2, and this protein together with Parkin and PICK1 are most likely involved in the regulation of PAELR protein levels. This occurs via autophagy, ubiquitination and proteasomal degradation (Dutta et al., 2018), which ultimately might lead to the regulation of GABAAR trafficking. However, additional studies are required to establish a role for GABARAPs in PD.
HD is an autosomal dominant progressive neurodegenerative disorder caused by the mutant huntingtin (Htt), with an expanded polyglutamine (polyQ) repeat (McClory et al., 2014). This disorder is characterized by progressive involuntary choreiform movements, emotional disturbances and cognitive decline (Pinborg et al., 2001), associated with degeneration of GABAergic neurons (Fritschy and Brünig, 2003).
An early study using emission tomography methods (PET and SPECT) showed a reduction in the abundance of benzodiazepine receptors in the striatum (but not in the cortex) of HD patients (Pinborg et al., 2001). These binding sites are present in GABAAR containing, for example, α1, α2, α3, or α5 subunits, together with β and γ subunits, and are mainly located at the synapse where they mediate most phasic inhibition in the brain (Jacob et al., 2008). This contrasts with the extrasynaptic GABAAR that mediate tonic inhibition, which are insensitive to benzodiazepines (Jacob et al., 2008). The putative alterations in the expression of GABAAR in HD requires further investigation since immunohistochemistry experiments showed an increase in the abundance of the α1 and γ2 receptor subunits in the globus pallidus of patients with the disease, while the levels of gephyrin were not changed (Thompson-Vest et al., 2003). The discrepancy between the results obtained in the analysis of benzodiazepine receptors and expression of GABAAR subunits may be due to differences in the brain regions analyzed, which was more restricted in the latter case.
In contrast with the evidence showing changes in the abundance of GABAAR in certain brain regions of HD patients, the alterations in receptor trafficking in the disease have been poorly investigated. As mentioned before, HAP1 interacts directly with GABAAR and regulates inhibitory synaptic transmission by modulating GABAAR recycling (Kittler et al., 2004b). GABAAR are trafficked to synapses by the kinesin family motor protein 5 (KIF5), which mediates the insertion of GABAAR into the plasma membrane, and HAP1, the adaptor that links the motor protein to the receptors. Accordingly, HAP1-KIF5 dependent GABAAR trafficking was reported as a fundamental mechanism controlling the strength of synaptic inhibition in the brain (Twelvetrees et al., 2010). Mutant huntingtin containing a polyQ expansion disrupts the HAP1-KIF5 GABAAR trafficking and synaptic delivery (Twelvetrees et al., 2010). Thus, the disruption of this complex by mutant huntingtin may lead to altered synaptic inhibition and increased neuronal excitability in HD (Twelvetrees et al., 2010).
The disruption of GABAAR trafficking and synaptic inhibition was also observed in a mouse model of HD (Yuen et al., 2012). In the latter study, GABAAR-mediated inhibitory transmission was found to be disrupted in the HD at the symptomatic stage, a consequence of a diminished surface GABAAR expression, which may underlie the impaired GABAergic transmission (Yuen et al., 2012). Furthermore, the KIF5-mediated microtubule-based transport of GABAAR was confirmed to be impaired in HD, which may underlie the disruption of GABAAR trafficking to the synaptic membrane. Therefore, the interference in the effect of polyQ-Htt on the HAP1/KIF5-mediated trafficking of GABAAR to synapses may constitute a therapeutic approach for HD, by restoring synaptic function (Yuen et al., 2012).
The chronic neuroinflammation observed in these neurodegenerative disorders induces the upregulation of tumor necrosis factor-α (TNF-α), which might play a role in the observed synaptic excitatory/inhibitory unbalance (Frankola et al., 2011). TNF-α was already described as an important mediator of homeostatic synaptic plasticity (Stellwagen and Malenka, 2006), and, interestingly, it was shown to modulate GABAAR trafficking, thereby downregulating the inhibitory neurotransmission. Indeed, TNF-α enhances the association of protein phosphatase 1 (PP1) with GABAAR β3 subunits and dephosphorylates the amino acid residue of the β3 subunit responsible for the regulation of the phospho-dependent interactions with the endocytic machinery (Pribiag and Stellwagen, 2013).
Final Remarks
Aberrant excitability is a common feature of numerous disorders of the CNS. Dysfunction of GABAergic synapses and in particular alterations of postsynaptic GABAAR trafficking have been reported as a key mechanism that contributes to the unbalance between excitation an inhibition, which ultimately will lead to neuronal hyperexcitability. Interestingly, similar alterations in the mechanism coupled to an increased internalization of GABAAR result in distinct outcomes/symptoms associated to different pathologies of the CNS. Depending on the circuits, the brain region and the developmental stage in which the postsynaptic alteration of GABAergic system is initiated, different structural and molecular modifications of the involved neurons may occur, triggering distinct pathologic responses. However, the disruption of the GABAergic neurotransmission characteristic of various illnesses may partly account for some common symptoms. For example, patients with cerebral ischemia, as well as certain cases of ASD or HD (Gambardella et al., 2001), may present seizures that are a hallmark of epilepsy. The reviewed studies indicate that the mechanisms involved in the control of plasma membrane and synaptic expression of GABAAR are key players in the modulation of neuronal excitability. However, considering the recent findings showing that the nanoscale redistribution of the scaffold protein gephyrin is a key event in the potentiation of inhibitory synapses, additional studies are required to evaluate the alterations in GABAAR and gephyrin nanoscale redistribution induced by hyperexcitability in pathological conditions. The outcome of this type of studies may contribute to the identification of novel therapeutic targets for various brain disorders characterized by an impaired regulation of the excitation/inhibition balance.
Author Contributions
MM, RC and CD wrote the manuscript.
Funding
This work was financed by the European Regional Development Fund (ERDF), through the Centro 2020 Regional Operational Programme under project CENTRO-01-0145-FEDER-000008:BrainHealth 2020, and through the COMPETE 2020—Operational Programme for Competitiveness and Internationalisation and Portuguese national funds via FCT—Fundação para a Ciência e a Tecnologia, I.P., under projects POCI-01-0145-FEDER-007440 and CENTRO-01-0145-FEDER-030659. FCT also supported the following individual grants: SFRH/BPD/115546/2016 (MM) and SFRH/BPD/84593/2012 (RC).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ali Rodriguez, R., Joya, C., and Hines, R. M. (2018). Common ribs of inhibitory synaptic dysfunction in the umbrella of neurodevelopmental disorders. Front. Mol. Neurosci. 11:132. doi: 10.3389/fnmol.2018.00132
Amantea, D., and Bagetta, G. (2017). Excitatory and inhibitory amino acid neurotransmitters in stroke: from neurotoxicity to ischemic tolerance. Curr. Opin. Pharmacol. 35, 111–119. doi: 10.1016/j.coph.2017.07.014
Arancibia-Cárcamo, I. L., Yuen, E. Y., Muir, J., Lumb, M. J., Michels, G., Saliba, R. S., et al. (2009). Ubiquitin-dependent lysosomal targeting of GABAA receptors regulates neuronal inhibition. Proc. Natl. Acad. Sci. U S A 106, 17552–17557. doi: 10.1073/pnas.0905502106
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., Warren, Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, united states, 2014. MMWR Surveill. Summ. 67, 1–23. doi: 10.15585/mmwr.ss6706a1
Balasubramanian, S., Teissére, J. A., Raju, D. V., and Hall, R. A. (2004). Hetero-oligomerization between GABAA and GABAB receptors regulates GABAB receptor trafficking. J. Biol. Chem. 279, 18840–18850. doi: 10.1074/jbc.M313470200
Bannai, H., Lévi, S., Schweizer, C., Inoue, T., Launey, T., Racine, V., et al. (2009). Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron 62, 670–682. doi: 10.1016/j.neuron.2009.04.023
Bassani, S., Cwetsch, A. W., Gerosa, L., Serratto, G. M., Folci, A., Hall, I. F., et al. (2018). The female epilepsy protein PCDH19 is a new GABAAR-binding partner that regulates GABAergic transmission as well as migration and morphological maturation of hippocampal neurons. Hum. Mol. Genet. 27, 1027–1038. doi: 10.1093/hmg/ddy019
Beck, M., Brickley, K., Wilkinson, H. L., Sharma, S., Smith, M., Chazot, P. L., et al. (2002). Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J. Biol. Chem. 277, 30079–30090. doi: 10.1074/jbc.M200438200
Bedford, F. K., Kittler, J. T., Muller, E., Thomas, P., Uren, J. M., Merlo, D., et al. (2001). GABAA receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat. Neurosci. 4, 908–916. doi: 10.1038/nn0901-908
Ben-Ari, Y. (2002). Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739. doi: 10.1038/nrn920
Besag, F. M. (2004). Behavioral aspects of pediatric epilepsy syndromes. Epilepsy Behav. 5, S3–S13. doi: 10.1016/j.yebeh.2003.11.002
Blaesse, P., Airaksinen, M. S., Rivera, C., and Kaila, K. (2009). Cation-chloride cotransporters and neuronal function. Neuron 61, 820–838. doi: 10.1016/j.neuron.2009.03.003
Blair, R. E., Sombati, S., Lawrence, D. C., McCay, B. D., and DeLorenzo, R. J. (2004). Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J. Pharmacol. Exp. Ther. 310, 871–880. doi: 10.1124/jpet.104.068478
Bogdanov, Y., Michels, G., Armstrong-Gold, C., Haydon, P. G., Lindstrom, J., Pangalos, M., et al. (2006). Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 25, 4381–4389. doi: 10.1038/sj.emboj.7601309
Brandon, N. J., Jovanovic, J. N., Colledge, M., Kittler, J. T., Brandon, J. M., Scott, J. D., et al. (2003). A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABAA receptors by cAMP-dependent protein kinase via selective interaction with receptor β subunits. Mol. Cell. Neurosci. 22, 87–97. doi: 10.1016/s1044-7431(02)00017-9
Brandon, N., Jovanovic, J., and Moss, S. (2002). Multiple roles of protein kinases in the modulation of γ-aminobutyric acidA receptor function and cell surface expression. Pharmacol. Ther. 94, 113–122. doi: 10.1016/s0163-7258(02)00175-4
Brünig, I., Scotti, E., Sidler, C., and Fritschy, J. M. (2002). Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 443, 43–55. doi: 10.1002/cne.10102
Chao, H. T., Chen, H., Samaco, R. C., Xue, M., Chahrour, M., Yoo, J., et al. (2010). Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468, 263–269. doi: 10.1038/nature09582
Charych, E. I., Yu, W., Li, R., Serwanski, D. R., Miralles, C. P., Li, X., et al. (2004a). A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain. J. Biol. Chem. 279, 38978–38990. doi: 10.1074/jbc.m405786200
Charych, E. I., Yu, W., Miralles, C. P., Serwanski, D. R., Li, X., Rubio, M., et al. (2004b). The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the β subunits of the GABA receptors. J. Neurochem. 90, 173–189. doi: 10.1111/j.1471-4159.2004.02481.x
Chen, Z. W., and Olsen, R. W. (2007). GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J. Neurochem. 100, 279–294. doi: 10.1111/j.1471-4159.2006.04206.x
Chen, Q. X., and Wong, R. K. (1995). Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J. Physiol. 482, 353–362. doi: 10.1113/jphysiol.1995.sp020522
Chen, Z., Xin, Y. R., Jiang, Y., and Jiang, J. X. (2001). Cloning a novel mouse Gabarapl2 cDNA and its characterization. Acta Pharmacol. Sin. 22, 751–755.
Cho, Y. J., Kim, H., Kim, W. J., Chung, S., Kim, Y. H., Cho, I., et al. (2017). Trafficking patterns of NMDA and GABAA receptors in a Mg2+-free cultured hippocampal neuron model of status epilepticus. Epilepsy Res. 136, 143–148. doi: 10.1016/j.eplepsyres.2017.08.003
Cossette, P., Liu, L., Brisebois, K., Dong, H., Lortie, A., Vanasse, M., et al. (2002). Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat. Genet. 31, 184–189. doi: 10.1038/ng885
Costa, R. O., Lacor, P. N., Ferreira, I. L., Resende, R., Auberson, Y. P., Klein, W. L., et al. (2012). Endoplasmic reticulum stress occurs downstream of GluN2B subunit of N-methyl-d-aspartate receptor in mature hippocampal cultures treated with amyloid-β oligomers. Aging Cell 11, 823–833. doi: 10.1111/j.1474-9726.2012.00848.x
Costa, J. T., Mele, M., Baptista, M. S., Gomes, J. R., Ruscher, K., Nobre, R. J., et al. (2016). Gephyrin cleavage in in vitro brain ischemia decreases GABAA receptor clustering and contributes to neuronal death. Mol. Neurobiol. 53, 3513–3527. doi: 10.1007/s12035-015-9283-2
Coull, J. A., Boudreau, D., Bachand, K., Prescott, S. A., Nault, F., Sik, A., et al. (2003). Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942. doi: 10.1038/nature01868
Crestani, F., Keist, R., Fritschy, J. M., Benke, D., Vogt, K., Prut, L., et al. (2002). Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc. Natl. Acad. Sci. U S A 99, 8980–8985. doi: 10.1073/pnas.142288699
Crider, A., Pandya, C. D., Peter, D., Ahmed, A. O., and Pillai, A. (2014). Ubiquitin-proteasome dependent degradation of GABAA α1 in autism spectrum disorder. Mol. Autism 5:45. doi: 10.1186/2040-2392-5-45
Curia, G., Longo, D., Biagini, G., Jones, R. S., and Avoli, M. (2008). The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 172, 143–157. doi: 10.1016/j.jneumeth.2008.04.019
Czuczwar, S. J., and Patsalos, P. N. (2001). The new generation of GABA enhancers. Potential in the treatment of epilepsy. CNS Drugs 15, 339–350. doi: 10.2165/00023210-200115050-00001
De Koninck, Y. (2007). Altered chloride homeostasis in neurological disorders: a new target. Curr. Opin. Pharmacol. 7, 93–99. doi: 10.1016/j.coph.2006.11.005
de Luca, E., Ravasenga, T., Petrini, E. M., Polenghi, A., Nieus, T., Guazzi, S., et al. (2017). Inter-synaptic lateral diffusion of GABAA receptors shapes inhibitory synaptic currents. Neuron 95, 63.e5–69.e5. doi: 10.1016/j.neuron.2017.06.022
Dejanovic, B., Djémié, T., Grünewald, N., Suls, A., Kress, V., Hetsch, F., et al. (2017). Simultaneous impairment of neuronal and metabolic function of mutated gephyrin in a patient with epileptic encephalopathy. EMBO Mol. Med. 9:1764. doi: 10.15252/emmm.201708525
Deykin, E. Y., and MacMahon, B. (1979). The incidence of seizures among children with autistic symptoms. Am. J. Psychiatry 136, 1310–1312. doi: 10.1176/ajp.136.10.1310
Dhossche, D., Applegate, H., Abraham, A., Maertens, P., Bland, L., Bencsath, A., et al. (2002). Elevated plasma γ-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Med. Sci. Monit. 8, PR1–6.
Di, X. J., Wang, Y. J., Han, D. Y., Fu, Y. L., Duerfeldt, A. S., Blagg, B. S., et al. (2016). Grp94 protein delivers γ-aminobutyric acid type A (GABAA) receptors to Hrd1 protein-mediated endoplasmic reticulum-associated degradation. J. Biol. Chem. 291, 9526–9539. doi: 10.1074/jbc.M115.705004
Dong, H., Zhang, P., Song, I., Petralia, R. S., Liao, D., and Huganir, R. L. (1999). Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. J. Neurosci. 19, 6930–6941. doi: 10.1523/JNEUROSCI.19-16-06930.1999
Dutta, P., Dargahi, L., O’Connell, K. E., Bolia, A., Ozkan, B., Sailer, A. W., et al. (2018). A novel modelling mechanism of PAEL receptor and GABARAPL2 interaction involved in Parkinson’s disease. Neurosci. Lett. 673, 12–18. doi: 10.1016/j.neulet.2018.02.055
Eckel, R., Szulc, B., Walker, M. C., and Kittler, J. T. (2015). Activation of calcineurin underlies altered trafficking of α2 subunit containing GABAA receptors during prolonged epileptiform activity. Neuropharmacology 88, 82–90. doi: 10.1016/j.neuropharm.2014.09.014
El-Hassar, L., Esclapez, M., and Bernard, C. (2007). Hyperexcitability of the CA1 hippocampal region during epileptogenesis. Epilepsia 48, 131–139. doi: 10.1111/j.1528-1167.2007.01301.x
Fang, M., Shen, L., Yin, H., Pan, Y. M., Wang, L., Chen, D., et al. (2011). Downregulation of gephyrin in temporal lobe epilepsy neurons in humans and a rat model. Synapse 65, 1006–1014. doi: 10.1002/syn.20928
Frankola, K. A., Greig, N. H., Luo, W., and Tweedie, D. (2011). Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 10, 391–403. doi: 10.2174/187152711794653751
Frazier, T. W., Thompson, L., Youngstrom, E. A., Law, P., Hardan, A. Y., Eng, C., et al. (2014). A twin study of heritable and shared environmental contributions to autism. J. Autism Dev. Disord. 44, 2013–2025. doi: 10.1007/s10803-014-2081-2
French, J. A. (2007). Refractory epilepsy: clinical overview. Epilepsia 48, 3–7. doi: 10.1111/j.1528-1167.2007.00992.x
Fritschy, J. M., and Brünig, I. (2003). Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol. Ther. 98, 299–323. doi: 10.1016/s0163-7258(03)00037-8
Fritschy, J. M., Harvey, R. J., and Schwarz, G. (2008). Gephyrin: where do we stand, where do we go? Trends Neurosci. 31, 257–264. doi: 10.1016/j.tins.2008.02.006
Fritschy, J. M., and Mohler, H. (1995). GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 359, 154–194. doi: 10.1002/cne.903590111
Galanopoulou, A. S., Bojko, A., Lado, F., and Moshé, S. L. (2000). The spectrum of neuropsychiatric abnormalities associated with electrical status epilepticus in sleep. Brain Dev. 22, 279–295. doi: 10.1016/s0387-7604(00)00127-3
Galeffi, F., Sah, R., Pond, B. B., George, A., and Schwartz-Bloom, R. D. (2004). Changes in intracellular chloride after oxygen-glucose deprivation of the adult hippocampal slice: effect of diazepam. J. Neurosci. 24, 4478–4488. doi: 10.1523/JNEUROSCI.0755-04.2004
Gambardella, A., Muglia, M., Labate, A., Magariello, A., Gabriele, A. L., Mazzei, R., et al. (2001). Juvenile Huntington’s disease presenting as progressive myoclonic epilepsy. Neurology 57, 708–711. doi: 10.1212/wnl.57.4.708
Gandal, M. J., Haney, J. R., Parikshak, N. N., Leppa, V., Ramaswami, G., Hartl, C., et al. (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359, 693–697. doi: 10.1126/science.aad6469
Gataullina, S., Bienvenu, T., Nabbout, R., Huberfeld, G., and Dulac, O. (2019). Gene mutations in paediatric epilepsies cause NMDA-pathy and phasic and tonic GABA-pathy. Dev. Med. Child Neurol. doi: 10.1111/dmcn.14152 [Epub ahead of print].
Gilbert, S. L., Zhang, L., Forster, M. L., Anderson, J. R., Iwase, T., Soliven, B., et al. (2006). Trak1 mutation disrupts GABAA receptor homeostasis in hypertonic mice. Nat. Genet. 38, 245–250. doi: 10.1038/ng1715
Giovanardi Rossi, P., Posar, A., and Parmeggiani, A. (2000). Epilepsy in adolescents and young adults with autistic disorder. Brain Dev. 22, 102–106. doi: 10.1016/s0387-7604(99)00124-2
Glykys, J., and Mody, I. (2006). Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit-deficient mice. J. Neurophysiol. 95, 2796–2807. doi: 10.1152/jn.01122.2005
Glykys, J., and Mody, I. (2007). The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J. Physiol. 582, 1163–1178. doi: 10.1113/jphysiol.2007.134460
Goldberg, E. M., and Coulter, D. A. (2013). Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat. Rev. Neurosci. 14, 337–349. doi: 10.1038/nrn3482
González, M. I., Cruz Del Angel, Y., and Brooks-Kayal, A. (2013). Down-regulation of gephyrin and GABAA receptor subunits during epileptogenesis in the CA1 region of hippocampus. Epilepsia 54, 616–624. doi: 10.1111/epi.12063
Goodkin, H. P., Yeh, J. L., and Kapur, J. (2005). Status epilepticus increases the intracellular accumulation of GABAA receptors. J. Neurosci. 25, 5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005
Gu, Y., Chiu, S. L., Liu, B., Wu, P. H., Delannoy, M., Lin, D. T., et al. (2016). Differential vesicular sorting of AMPA and GABAA receptors. Proc. Natl. Acad. Sci. U S A 113, E922–E931. doi: 10.1073/pnas.1525726113
Gyenes, M., Wang, Q., Gibbs, T. T., and Farb, D. H. (1994). Phosphorylation factors control neurotransmitter and neuromodulator actions at the γ-aminobutyric acid type A receptor. Mol. Pharmacol. 46, 542–549.
Han, D. Y., Guan, B. J., Wang, Y. J., Hatzoglou, M., and Mu, T. W. (2015). L-type calcium channel blockers enhance trafficking and function of epilepsy-associated α1 (D219N) subunits of GABAA receptors. ACS Chem. Biol. 10, 2135–2148. doi: 10.1021/acschembio.5b00479
Hausrat, T. J., Muhia, M., Gerrow, K., Thomas, P., Hirdes, W., Tsukita, S., et al. (2015). Radixin regulates synaptic GABAA receptor density and is essential for reversal learning and short-term memory. Nat. Commun. 6:6872. doi: 10.1038/ncomms7872
Herring, D., Huang, R., Singh, M., Dillon, G. H., and Leidenheimer, N. J. (2005). PKC modulation of GABAA receptor endocytosis and function is inhibited by mutation of a dileucine motif within the receptor β2 subunit. Neuropharmacology 48, 181–194. doi: 10.1016/j.neuropharm.2004.09.015
Herring, D., Huang, R., Singh, M., Robinson, L. C., Dillon, G. H., and Leidenheimer, N. J. (2003). Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the β2 subunit of the receptor. J. Biol. Chem. 278, 24046–24052. doi: 10.1074/jbc.M301420200
Hesdorffer, D. C., Beck, V., Begley, C. E., Bishop, M. L., Cushner-Weinstein, S., Holmes, G. L., et al. (2013). research implications of the institute of medicine report, epilepsy across the spectrum: promoting health and understanding. Epilepsia 54, 207–216. doi: 10.1111/epi.12056
Hines, R. M., Davies, P. A., Moss, S. J., and Maguire, J. (2012). Functional regulation of GABAA receptors in nervous system pathologies. Curr. Opin. Neurobiol. 22, 552–558. doi: 10.1016/j.conb.2011.10.007
Hirose, S. (2014). Mutant GABAA receptor subunits in genetic (idiopathic) epilepsy. Prog. Brain Res. 213, 55–85. doi: 10.1016/b978-0-444-63326-2.00003-x
Howell, B. W., and Smith, K. M. (2019). Synaptic structural protein dysfunction leads to altered excitation inhibition ratios in models of autism spectrum disorder. Pharmacol. Res. 139, 207–214. doi: 10.1016/j.phrs.2018.11.019
Hu, H. Z., and Li, Z. W. (1997). Modulation by adenosine of GABA-activated current in rat dorsal root ganglion neurons. J. Physiol. 501, 67–75. doi: 10.1111/j.1469-7793.1997.067bo.x
Huang, X., Hernandez, C. C., Hu, N., and Macdonald, R. L. (2014). Three epilepsy-associated GABRG2 missense mutations at the γ+/β− interface disrupt GABAA receptor assembly and trafficking by similar mechanisms but to different extents. Neurobiol. Dis. 68, 167–179. doi: 10.1016/j.nbd.2014.04.015
Huang, X., Zhou, C., Tian, M., Kang, J. Q., Shen, W., Verdier, K., et al. (2017). Overexpressing wild-type γ2 subunits rescued the seizure phenotype in Gabrg2+/Q390X Dravet syndrome mice. Epilepsia 58, 1451–1461. doi: 10.1111/epi.13810
Hughes, J. R., and Melyn, M. (2005). EEG and seizures in autistic children and adolescents: further findings with therapeutic implications. Clin. EEG Neurosci. 36, 15–20. doi: 10.1177/155005940503600105
Ishii, A., Kang, J. Q., Schornak, C. C., Hernandez, C. C., Shen, W., Watkins, J. C., et al. (2017). A de novo missense mutation of GABRB2 causes early myoclonic encephalopathy. J. Med. Genet. 54, 202–211. doi: 10.1136/jmedgenet-2016-104083
Jacob, T. C., Bogdanov, Y. D., Magnus, C., Saliba, R. S., Kittler, J. T., Haydon, P. G., et al. (2005). Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J. Neurosci. 25, 10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005
Jacob, T. C., Moss, S. J., and Jurd, R. (2008). GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 9, 331–343. doi: 10.1038/nrn2370
Jacobs, M. P., Leblanc, G. G., Brooks-Kayal, A., Jensen, F. E., Lowenstein, D. H., Noebels, J. L., et al. (2009). Curing epilepsy: progress and future directions. Epilepsy Behav. 14, 438–445. doi: 10.1016/j.yebeh.2009.02.036
Jaenisch, N., Witte, O. W., and Frahm, C. (2010). Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke 41, e151–e159. doi: 10.1161/STROKEAHA.109.570424
Jedlicka, P., Deller, T., Gutkin, B. S., and Backus, K. H. (2011). Activity-dependent intracellular chloride accumulation and diffusion controls GABAA receptor-mediated synaptic transmission. Hippocampus 21, 885–898. doi: 10.1002/hipo.20804
Jenks, S., and Volkers, N. (1992). Razors and refrigerators and reindeer—oh my! J. Natl. Cancer Inst. 84:1863. doi: 10.1093/jnci/84.24.1863
Jo, Y. H., Donier, E., Martinez, A., Garret, M., Toulmé, E., and Boué-Grabot, E. (2011). Cross-talk between P2X4 and γ-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. J. Biol. Chem. 286, 19993–20004. doi: 10.1074/jbc.m111.231324
Kahle, K. T., Merner, N. D., Friedel, P., Silayeva, L., Liang, B., Khanna, A., et al. (2014). Genetically encoded impairment of neuronal KCC2 cotransporter function in human idiopathic generalized epilepsy. EMBO Rep. 15, 766–774. doi: 10.15252/embr.201438840
Kaila, K., Price, T. J., Payne, J. A., Puskarjov, M., and Voipio, J. (2014). Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 15, 637–654. doi: 10.1038/nrn3819
Kanematsu, T., Jang, I. S., Yamaguchi, T., Nagahama, H., Yoshimura, K., Hidaka, K., et al. (2002). Role of the PLC-related, catalytically inactive protein p130 in GABAA receptor function. EMBO J. 21, 1004–1011. doi: 10.1093/emboj/21.5.1004
Kang, J. Q., Shen, W., Zhou, C., Xu, D., and Macdonald, R. L. (2015). The human epilepsy mutation GABRG2(Q390X) causes chronic subunit accumulation and neurodegeneration. Nat. Neurosci. 18, 988–996. doi: 10.1038/nn.4024
Karran, E., and De Strooper, B. (2016). The amyloid cascade hypothesis: are we poised for success or failure? J. Neurosurg. 139, 237–252. doi: 10.1111/jnc.13632
Kittler, J. T., Arancibia-Carcamo, I. L., and Moss, S. J. (2004a). Association of GRIP1 with a GABAA receptor associated protein suggests a role for GRIP1 at inhibitory synapses. Biochem. Pharmacol. 68, 1649–1654. doi: 10.1016/j.bcp.2004.07.028
Kittler, J. T., Thomas, P., Tretter, V., Bogdanov, Y. D., Haucke, V., Smart, T. G., et al. (2004b). Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating γ-aminobutyric acid type A receptor membrane trafficking. Proc. Natl. Acad. Sci. U S A 101, 12736–12741. doi: 10.1073/pnas.0401860101
Kittler, J. T., Chen, G., Honing, S., Bogdanov, Y., McAinsh, K., Arancibia-Carcamo, I. L., et al. (2005). Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc. Natl. Acad. Sci. U S A 102, 14871–14876. doi: 10.1073/pnas.0506653102
Kittler, J. T., Chen, G., Kukhtina, V., Vahedi-Faridi, A., Gu, Z., Tretter, V., et al. (2008). Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor γ2 subunit. Proc. Natl. Acad. Sci. U S A 105, 3616–3621. doi: 10.1073/pnas.0707920105
Kittler, J. T., Delmas, P., Jovanovic, J. N., Brown, D. A., Smart, T. G., and Moss, S. J. (2000). Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J. Neurosci. 20, 7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000
Kittler, J. T., McAinsh, K., and Moss, S. J. (2002). Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol. Neurobiol. 26, 251–268. doi: 10.1385/mn:26:2-3:251
Kittler, J. T., Rostaing, P., Schiavo, G., Fritschy, J. M., Olsen, R., Triller, A., et al. (2001). The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABAA receptors. Mol. Cell. Neurosci. 18, 13–25. doi: 10.1006/mcne.2001.1005
Kolc, K. L., Sadleir, L. G., Scheffer, I. E., Ivancevic, A., Roberts, R., Pham, D. H., et al. (2019). A systematic review and meta-analysis of 271 PCDH19-variant individuals identifies psychiatric comorbidities and association of seizure onset and disease severity. Mol. Psychiatry 24, 241–251. doi: 10.1038/s41380-018-0066-9
Kosinovsky, B., Hermon, S., Yoran-Hegesh, R., Golomb, A., Senecky, Y., Goez, H., et al. (2005). The yield of laboratory investigations in children with infantile autism. J. Neural Transm. 112, 587–596. doi: 10.1007/s00702-004-0198-8
Kozinetz, C. A., Skender, M. L., MacNaughton, N., Almes, M. J., Schultz, R. J., Percy, A. K., et al. (1993). Epidemiology of Rett syndrome: a population-based registry. Pediatrics 91, 445–450.
Kwakowsky, A., Calvo-Flores Guzman, B., Govindpani, K., Waldvogel, H. J., and Faull, R. L. (2018). γ-aminobutyric acid A receptors in Alzheimer’s disease: highly localized remodeling of a complex and diverse signaling pathway. Neural Regen. Res. 13, 1362–1363. doi: 10.4103/1673-5374.235240
Lee, S., Kim, K., and Zhou, Z. J. (2010). Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron 68, 1159–1172. doi: 10.1016/j.neuron.2010.11.031
Leil, T. A., Chen, Z. W., Chang, C. S., and Olsen, R. W. (2004). GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J. Neurosci. 24, 11429–11438. doi: 10.1523/JNEUROSCI.3355-04.2004
Li, X. J., Li, S. H., Sharp, A. H., Nucifora, F. C. Jr., Schilling, G., Lanahan, A., et al. (1995). A huntingtin-associated protein enriched in brain with implications for pathology. Nature 378, 398–402. doi: 10.1038/378398a0
Li, R. W., Serwanski, D. R., Miralles, C. P., Li, X., Charych, E., Riquelme, R., et al. (2005). GRIP1 in GABAergic synapses. J. Comp. Neurol. 488, 11–27. doi: 10.1002/cne.20566
Li, J., Zhang, W., Yang, H., Howrigan, D. P., Wilkinson, B., Souaiaia, T., et al. (2017a). Spatiotemporal profile of postsynaptic interactomes integrates components of complex brain disorders. Nat. Neurosci. 20, 1150–1161. doi: 10.1038/nn.4594
Li, Y., Zhou, Y., Peng, L., and Zhao, Y. (2017b). Reduced protein expressions of cytomembrane GABAARβ3 at different postnatal developmental stages of rats exposed prenatally to valproic acid. Brain Res. 1671, 33–42. doi: 10.1016/j.brainres.2017.06.018
Limon, A., Reyes-Ruiz, J. M., and Miledi, R. (2012). Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc. Natl. Acad. Sci. U S A 109, 10071–10076. doi: 10.1073/pnas.1204606109
Liu, F., Wan, Q., Pristupa, Z. B., Yu, X.-M., Wang, Y. T., and Niznik, H. B. (2000). Direct protein-protein coupling enables cross-talk between dopamine D5 and γ-aminobutyric acid A receptors. Nature 403, 274–280. doi: 10.1038/35002014
Loebrich, S., Bähring, R., Katsuno, T., Tsukita, S., and Kneussel, M. (2006). Activated radixin is essential for GABAA receptor α5 subunit anchoring at the actin cytoskeleton. EMBO J. 25, 987–999. doi: 10.1038/sj.emboj.7600995
Loscher, W., and Brandt, C. (2010). Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol. Rev. 62, 668–700. doi: 10.1124/pr.110.003046
Marsden, K. C., Beattie, J. B., Friedenthal, J., and Carroll, R. C. (2007). NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABAA receptors. J. Neurosci. 27, 14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007
Mattina, T., Perrotta, C. S., and Grossfeld, P. (2009). Jacobsen syndrome. Orphanet J. Rare Dis. 4:9. doi: 10.1186/1750-1172-4-9
McClory, H., Williams, D., Sapp, E., Gatune, L. W., Wang, P., DiFiglia, M., et al. (2014). Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington’s disease mice. Acta Neuropathol. Commun. 2:179. doi: 10.1186/s40478-014-0178-7
McCormick, D. A., and Contreras, D. (2001). On the cellular and network bases of epileptic seizures. Annu. Rev. Physiol. 63, 815–846. doi: 10.1146/annurev.physiol.63.1.815
McDade, D. M., Conway, A. M., James, A. B., and Morris, B. J. (2009). Activity-dependent gene transcription as a long-term influence on receptor signalling. Biochem. Soc. Trans. 37, 1375–1377. doi: 10.1042/bst0371375
McDonald, B. J., Amato, A., Connolly, C. N., Benke, D., Moss, S. J., and Smart, T. G. (1998). Adjacent phosphorylation sites on GABAA receptor β subunits determine regulation by cAMP-dependent protein kinase. Nat. Neurosci. 1, 23–28. doi: 10.1038/223
Meder, D., Herz, D. M., Rowe, J. B., Lehericy, S., and Siebner, H. R. (2018). The role of dopamine in the brain—Lessons learned from Parkinson’s disease. Neuroimage doi: 10.1016/j.neuroimage.2018.11.021 [Epub ahead of print].
Mele, M., Aspromonte, M. C., and Duarte, C. B. (2017). Downregulation of GABAA receptor recycling mediated by HAP1 contributes to neuronal death in in vitro brain ischemia. Mol. Neurobiol. 54, 45–57. doi: 10.1007/s12035-015-9661-9
Mele, M., Leal, G., and Duarte, C. B. (2016). Role of GABAAR trafficking in the plasticity of inhibitory synapses. J. Neurochem. 139, 997–1018. doi: 10.1111/jnc.13742
Mele, M., Ribeiro, L., Inácio, A. R., Wieloch, T., and Duarte, C. B. (2014). GABAA receptor dephosphorylation followed by internalization is coupled to neuronal death in in vitro ischemia. Neurobiol. Dis. 65, 220–232. doi: 10.1016/j.nbd.2014.01.019
Michels, G., and Moss, S. J. (2007). GABAA receptors: properties and trafficking. Crit. Rev. Biochem. Mol. Biol. 42, 3–14. doi: 10.1080/10409230601146219
Mielke, J. G., and Wang, Y. T. (2005). Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABA receptors following oxygen-glucose deprivation in cultured cortical neurons. J. Neurochem. 92, 103–113. doi: 10.1111/j.1471-4159.2004.02841.x
Moss, S. J., Gorrie, G. H., Amato, A., and Smart, T. G. (1995). Modulation of GABAA receptors by tyrosine phosphorylation. Nature 377, 344–348. doi: 10.1038/377344a0
Muir, J., Arancibia-Carcamo, I. L., MacAskill, A. F., Smith, K. R., Griffin, L. D., and Kittler, J. T. (2010). NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the γ2 subunit. Proc. Natl. Acad. Sci. U S A 107, 16679–16684. doi: 10.1073/pnas.1000589107
Mukherjee, J., Kretschmannova, K., Gouzer, G., Maric, H. M., Ramsden, S., Tretter, V., et al. (2011). The residence time of GABAARs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin. J. Neurosci. 31, 14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011
Murakami, T., Shoji, M., Imai, Y., Inoue, H., Kawarabayashi, T., Matsubara, E., et al. (2004). Pael-R is accumulated in Lewy bodies of Parkinson’s disease. Ann. Neurol. 55, 439–442. doi: 10.1002/ana.20064
Nakamura, T., Arima-Yoshida, F., Sakaue, F., Nasu-Nishimura, Y., Takeda, Y., Matsuura, K., et al. (2016). PX-RICS-deficient mice mimic autism spectrum disorder in Jacobsen syndrome through impaired GABAA receptor trafficking. Nat. Commun. 7:10861. doi: 10.1038/ncomms10861
Nakamura, Y., Darnieder, L. M., Deeb, T. Z., and Moss, S. J. (2015). Regulation of GABAARs by phosphorylation. Adv. Pharmacol. 72, 97–146. doi: 10.1016/bs.apha.2014.11.008
Naylor, D. E., Liu, H., and Wasterlain, C. G. (2005). Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J. Neurosci. 25, 7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005
Nielsen, L. A. (1990). Plaque and gingivitis in children with cerebral palsy: relation to CP-diagnosis, mental and motor handicap. Tandlaegernes Tidsskr. 5, 316–320.
Nishikawa, K., Jenkins, A., Paraskevakis, I., and Harrison, N. L. (2002). Volatile anesthetic actions on the GABAA receptors: contrasting effects of α 1(S270) and β 2(N265) point mutations. Neuropharmacology 42, 337–345. doi: 10.1016/s0028-3908(01)00189-7
Nusser, Z., Cull-Candy, S., and Farrant, M. (1997). Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron 19, 697–709. doi: 10.1016/s0896-6273(00)80382-7
Nusser, Z., Hájos, N., Somogyi, P., and Mody, I. (1998a). Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature 395, 172–177. doi: 10.1038/25999
Nusser, Z., Sieghart, W., and Somogyi, P. (1998b). Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 18, 1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998
Nusser, Z., Sieghart, W., Stephenson, F. A., and Somogyi, P. (1996). The α 6 subunit of the GABAA receptor is concentrated in both inhibitory and excitatory synapses on cerebellar granule cells. J. Neurosci. 16, 103–114. doi: 10.1523/JNEUROSCI.16-01-00103.1996
Olsson, I., Steffenburg, S., and Gillberg, C. (1988). Epilepsy in autism and autisticlike conditions. A population-based study. Arch. Neurol. 45, 666–668. doi: 10.1001/archneur.1988.00520300086024
Papp, E., Rivera, C., Kaila, K., and Freund, T. F. (2008). Relationship between neuronal vulnerability and potassium-chloride cotransporter 2 immunoreactivity in hippocampus following transient forebrain ischemia. Neuroscience 154, 677–689. doi: 10.1016/j.neuroscience.2008.03.072
Parihar, M. S., and Brewer, G. J. (2010). Amyloid-β as a modulator of synaptic plasticity. J. Alzheimers Dis. 22, 741–763. doi: 10.3233/jad-2010-101020
Pennacchietti, F., Vascon, S., Nieus, T., Rosillo, C., Das, S., Tyagarajan, S. K., et al. (2017). Nanoscale molecular reorganization of the inhibitory postsynaptic density is a determinant of GABAergic synaptic potentiation. J. Neurosci. 37, 1747–1756. doi: 10.1523/JNEUROSCI.0514-16.2016
Petrini, E. M., Ravasenga, T., Hausrat, T. J., Iurilli, G., Olcese, U., Racine, V., et al. (2014). Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 5:3921. doi: 10.1038/ncomms4921
Pinborg, L. H., Videbaek, C., Hasselbalch, S. G., Sorensen, S. A., Wagner, A., Paulson, O. B., et al. (2001). Benzodiazepine receptor quantification in Huntington’s disease with [(123)I]omazenil and SPECT. J. Neurol. Neurosurg. Psychiatry 70, 657–661. doi: 10.1136/jnnp.70.5.657
Pizzarelli, R., and Cherubini, E. (2011). Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011:297153. doi: 10.1155/2011/297153
Pribiag, H., and Stellwagen, D. (2013). TNF-α downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABAA receptors. J. Neurosci. 33, 15879–15893. doi: 10.1523/JNEUROSCI.0530-13.2013
Puskarjov, M., Seja, P., Heron, S. E., Williams, T. C., Ahmad, F., Iona, X., et al. (2014). A variant of KCC2 from patients with febrile seizures impairs neuronal Cl- extrusion and dendritic spine formation. EMBO Rep. 15, 723–729. doi: 10.1002/embr.201438749
Ramamoorthi, K., and Lin, Y. (2011). The contribution of GABAergic dysfunction to neurodevelopmental disorders. Trends Mol. Med. 17, 452–462. doi: 10.1016/j.molmed.2011.03.003
Rathenberg, J., Kittler, J. T., and Moss, S. J. (2004). Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol. Cell. Neurosci. 26, 251–257. doi: 10.1016/j.mcn.2004.01.012
Regesta, G., and Tanganelli, P. (1999). Clinical aspects and biological bases of drug-resistant epilepsies. Epilepsy Res. 34, 109–122. doi: 10.1016/s0920-1211(98)00106-5
Renner, M., Schweizer, C., Bannai, H., Triller, A., and Levi, S. (2012). Diffusion barriers constrain receptors at synapses. PLoS One 7:e43032. doi: 10.1371/journal.pone.0043032
Rivera, C., Voipio, J., Thomas-Crusells, J., Li, H., Emri, Z., Sipila, S., et al. (2004). Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 24, 4683–4691. doi: 10.1523/jneurosci.5265-03.2004
Rosenberg, R. E., Law, J. K., Yenokyan, G., McGready, J., Kaufmann, W. E., and Law, P. A. (2009). Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch. Pediatr. Adolesc. Med. 163, 907–914. doi: 10.1001/archpediatrics.2009.98
Rowland, A. M., Richmond, J. E., Olsen, J. G., Hall, D. H., and Bamber, B. A. (2006). Presynaptic terminals independently regulate synaptic clustering and autophagy of GABAA receptors in Caenorhabditis elegans. J. Neurosci. 26, 1711–1720. doi: 10.1523/jneurosci.2279-05.2006
Rudolph, U., and Knoflach, F. (2011). Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug. Discov. 10, 685–697. doi: 10.1038/nrd3502
Rudolph, U., and Möhler, H. (2004). Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharmacol. Toxicol. 44, 475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429
Russell, J. M. (2000). Sodium-potassium-chloride cotransport. Physiol. Rev. 80, 211–276. doi: 10.1152/physrev.2000.80.1.211
Saliba, R. S., Michels, G., Jacob, T. C., Pangalos, M. N., and Moss, S. J. (2007). Activity-dependent ubiquitination of GABAA receptors regulates their accumulation at synaptic sites. J. Neurosci. 27, 13341–13351. doi: 10.1523/jneurosci.3277-07.2007
Saxena, S., and Caroni, P. (2011). Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron 71, 35–48. doi: 10.1016/j.neuron.2011.06.031
Schwarten, M., Mohrluder, J., Ma, P., Stoldt, M., Thielmann, Y., Stangler, T., et al. (2009). Nix directly binds to GABARAP: a possible crosstalk between apoptosis and autophagy. Autophagy 5, 690–698. doi: 10.4161/auto.5.5.8494
Schwartz-Bloom, R. D., and Sah, R. (2001). γ-Aminobutyric acidA neurotransmission and cerebral ischemia. J. Neurochem. 77, 353–371. doi: 10.1046/j.1471-4159.2001.00274.x
Sharma, A. K., Reams, R. Y., Jordan, W. H., Miller, M. A., Thacker, H. L., and Snyder, P. W. (2007). Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol. Pathol. 35, 984–999. doi: 10.1080/01926230701748305
Shrivastava, A. N., Triller, A., and Sieghart, W. (2011a). GABAA receptors: post-synaptic co-localization and cross-talk with other receptors. Front. Cell. Neurosci. 5:7. doi: 10.3389/fncel.2011.00007
Shrivastava, A. N., Triller, A., Sieghart, W., and Sarto-Jackson, I. (2011b). Regulation of GABAA receptor dynamics by interaction with purinergic P2X2 receptors. J. Biol. Chem. 286, 14455–14468. doi: 10.1074/jbc.M110.165282
Smith, K. R., and Kittler, J. T. (2010). The cell biology of synaptic inhibition in health and disease. Curr. Opin. Neurobiol. 20, 550–556. doi: 10.1016/j.conb.2010.06.001
Smith, K. R., McAinsh, K., Chen, G., Arancibia-Carcamo, I. L., Haucke, V., Yan, Z., et al. (2008). Regulation of inhibitory synaptic transmission by a conserved atypical interaction of GABAA receptor β- and γ-subunits with the clathrin AP2 adaptor. Neuropharmacology 55, 844–850. doi: 10.1016/j.neuropharm.2008.06.072
Smith, K. R., Muir, J., Rao, Y., Browarski, M., Gruenig, M. C., Sheehan, D. F., et al. (2012). Stabilization of GABAA receptors at endocytic zones is mediated by an AP2 binding motif within the GABAA receptor β3 subunit. J. Neurosci. 32, 2485–2498. doi: 10.1523/jneurosci.1622-11.2011
Smith, K. R., Oliver, P. L., Lumb, M. J., Arancibia-Carcamo, I. L., Revilla-Sanchez, R., Brandon, N. J., et al. (2010). Identification and characterisation of a Maf1/Macoco protein complex that interacts with GABAA receptors in neurons. Mol. Cell. Neurosci. 44, 330–341. doi: 10.1016/j.mcn.2010.04.004
Staley, K. (2015). Molecular mechanisms of epilepsy. Nat. Neurosci. 18, 367–372. doi: 10.1038/nn.3947
Stellwagen, D., and Malenka, R. C. (2006). Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059. doi: 10.1038/nature04671
Stelzer, A., and Shi, H. (1994). Impairment of GABAA receptor function by N-methyl-D-aspartate-mediated calcium influx in isolated CA1 pyramidal cells. Neuroscience 62, 813–828. doi: 10.1016/0306-4522(94)90479-0
Tang, X., Kim, J., Zhou, L., Wengert, E., Zhang, L., Wu, Z., et al. (2016). KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc. Natl. Acad. Sci. U S A 113, 751–756. doi: 10.1073/pnas.1524013113
Terunuma, M., Xu, J., Vithlani, M., Sieghart, W., Kittler, J., Pangalos, M., et al. (2008). Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J. Neurosci. 28, 376–384. doi: 10.1523/jneurosci.4346-07.2008
Thind, K. K., Yamawaki, R., Phanwar, I., Zhang, G., Wen, X., and Buckmaster, P. S. (2010). Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J. Comp. Neurol. 518, 647–667. doi: 10.1002/cne.22235
Thomas, P., Mortensen, M., Hosie, A. M., and Smart, T. G. (2005). Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat. Neurosci. 8, 889–897. doi: 10.1038/nn1483
Thompson-Vest, N. M., Waldvogel, H. J., Rees, M. I., and Faull, R. L. (2003). GABAA receptor subunit and gephyrin protein changes differ in the globus pallidus in Huntington’s diseased brain. Brain Res. 994, 265–270. doi: 10.1016/j.brainres.2003.09.051
Tretter, V., Jacob, T. C., Mukherjee, J., Fritschy, J. M., Pangalos, M. N., and Moss, S. J. (2008). The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor α2 subunits to gephyrin. J. Neurosci. 28, 1356–1365. doi: 10.1523/jneurosci.5050-07.2008
Tretter, V., Kerschner, B., Milenkovic, I., Ramsden, S. L., Ramerstorfer, J., Saiepour, L., et al. (2011). Molecular basis of the γ-aminobutyric acid A receptor α3 subunit interaction with the clustering protein gephyrin. J. Biol. Chem. 286, 37702–37711. doi: 10.1074/jbc.m111.291336
Twelvetrees, A. E., Yuen, E. Y., Arancibia-Carcamo, I. L., MacAskill, A. F., Rostaing, P., Lumb, M. J., et al. (2010). Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron 65, 53–65. doi: 10.1016/j.neuron.2009.12.007
Tyagarajan, S. K., and Fritschy, J. M. (2014). Gephyrin: a master regulator of neuronal function? Nat. Rev. Neurosci. 15, 141–156. doi: 10.1038/nrn3670
Uji, A., Matsuda, M., Kukita, T., Maeda, K., Kanematsu, T., and Hirata, M. (2002). Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: comparison with PRIP-1. Life Sci. 72, 443–453. doi: 10.1016/s0024-3205(02)02275-0
Ulrich, D. (2015). Amyloid-β impairs synaptic inhibition via GABAA receptor endocytosis. J. Neurosci. 35, 9205–9210. doi: 10.1523/jneurosci.0950-15.2015
Varga, E., Juhász, G., Bozsó, Z., Penke, B., Fülöp, L., and Szegedi, V. (2014). Aβ(1–42) enhances neuronal excitability in the CA1 via NR2B subunit-containing NMDA receptors. Neural Plast. 2014:584314. doi: 10.1155/2014/584314
Vien, T. N., Modgil, A., Abramian, A. M., Jurd, R., Walker, J., Brandon, N. J., et al. (2015). Compromising the phosphodependent regulation of the GABAAR β3 subunit reproduces the core phenotypes of autism spectrum disorders. Proc. Natl. Acad. Sci. U S A 112, 14805–14810. doi: 10.1073/pnas.1514657112
Vignoli, A., Savini, M. N., Nowbut, M. S., Peron, A., Turner, K., La Briola, F., et al. (2017). Effectiveness and tolerability of antiepileptic drugs in 104 girls with Rett syndrome. Epilepsy Behav. 66, 27–33. doi: 10.1016/j.yebeh.2016.10.006
Vithlani, M., Terunuma, M., and Moss, S. J. (2011). The dynamic modulation of GABAA receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol. Rev. 91, 1009–1022. doi: 10.1152/physrev.00015.2010
Volk, L., Chiu, S. L., Sharma, K., and Huganir, R. L. (2015). Glutamate synapses in human cognitive disorders. Annu. Rev. Neurosci. 38, 127–149. doi: 10.1146/annurev-neuro-071714-033821
Wang, H., Bedford, F. K., Brandon, N. J., Moss, S. J., and Olsen, R. W. (1999). GABAA-receptor-associated protein links GABAA receptors and the cytoskeleton. Nature 397, 69–72. doi: 10.1038/16264
Watanabe, M., and Fukuda, A. (2015). Development and regulation of chloride homeostasis in the central nervous system. Front. Cell. Neurosci. 9:371. doi: 10.3389/fncel.2015.00371
Wu, Y. W., Sullivan, J., McDaniel, S. S., Meisler, M. H., Walsh, E. M., Li, S. X., et al. (2015). Incidence of dravet syndrome in a US population. Pediatrics 136, e1310–e1315. doi: 10.1542/peds.2015-1807
Yizhar, O., Fenno, L. E., Prigge, M., Schneider, F., Davidson, T. J., O’Shea, D. J., et al. (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178. doi: 10.1038/nature10360
Keywords: GABAA receptor trafficking, epilepsy, brain ischemia, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease
Citation: Mele M, Costa RO and Duarte CB (2019) Alterations in GABAA-Receptor Trafficking and Synaptic Dysfunction in Brain Disorders. Front. Cell. Neurosci. 13:77. doi: 10.3389/fncel.2019.00077
Received: 29 November 2018; Accepted: 15 February 2019;
Published: 07 March 2019.
Edited by:
David Perrais, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Luca Murru, Italian National Research Council (CNR), ItalyThierry Ralph Nieus, Luigi Sacco Hospital, Italy
Copyright © 2019 Mele, Costa and Duarte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos B. Duarte, Y2JkdWFydGVAY2kudWMucHQ=
 Miranda Mele
Miranda Mele Rui O. Costa
Rui O. Costa Carlos B. Duarte
Carlos B. Duarte