- 1Department of Histology and Embryology, Chongqing Key Laboratory of Neurobiology, Third Military Medical University, Chongqing, China
- 2Department of Radiology, Institute of Surgery Research, Daping Hospital, Third Military Medical University, Chongqing, China
- 3Robert Stone Dow Neurobiology Laboratories, Legacy Research Institute, Portland, OR, USA
- 4Department of Pharmacology, Emory University School of Medicine, Atlanta, GA, USA
Adolescence is the critical time for developing proper oligodendrocyte (OL)-neuron interaction and the peak of onset for many cognitive diseases, among which anxiety disorders display the highest prevalence. However, whether impairment of de novo OL development causes neuronal abnormalities and contributes to the early onset of anxiety phenotype in childhood still remains unexplored. In this study, we tested the hypothesis that defects in OL maturation manifests cortical neuron function and leads to anxiety-like behaviors in juvenile mice. We report here that conditional knockout of the Olig2 gene (Olig2 cKO) specifically in differentiating OLs in the mouse brain preferentially impaired OL maturation in the gray matter of cerebral cortex. Interestingly, localized proton magnetic resonance spectroscopy revealed that Olig2 cKO mice displayed abnormally elevated cortical glutamate levels. In addition, transmission electron microscopy demonstrated increased vesicle density in excitatory glutamatergic synapses in the cortex of the Olig2 cKO mice. Moreover, juvenile Olig2 cKO mice exhibited anxiety-like behaviors and impairment in behavioral inhibition. Taken together, our results suggest that impaired OL development affects glutamatergic neuron function in the cortex and causes anxiety-related behaviors in juvenile mice. These discoveries raise an intriguing possibility that OL defects may be a contributing mechanism for the onset of anxiety in childhood.
Introduction
Adolescence is a peak time for the onset of numerous mental disorders, represented by anxiety, impulse control disorders, and schizophrenia (Paus et al., 2008). One in five children and adolescents suffers from a mental illness that persists into adulthood. Among the numerous mental disorders, the prevalence of anxiety is the highest (Kessler et al., 2005). Consistent with the emotionality, risk-taking and impulsivity characters (Butters, 2011), anxiety phenotypes often begin in childhood and early adolescence, sometimes continue into the adulthood (Lee et al., 2014). However, molecular and cellular mechanisms that lead to anxiety in adolescence remain unknown, which is a prevailing issue in understanding the early onset of mental dysfunction.
In the adolescent brain, the structure of neuronal circuits and the functional properties of neurons are highly plastic (Tau and Peterson, 2010). Not only the number and morphology of synapses are dynamically altered (Penzes et al., 2011), the availability of neurotransmitters and corresponding receptors also undergo differential regulation in various brain regions (Lee et al., 2014). Conceivably, a transient interference of neurotransmission may markedly affect the functional balance of neuronal circuitry in the young brain, thus resulting in dysregulated emotions and actions (Casey et al., 2008). Moreover, recent clinical studies revealed that aberrantly elevated glutamate levels in the anterior cingulate cortex were positively correlated with clinical symptoms of anxiety and impulsivity in patients (Phan et al., 2005; Hoerst et al., 2010; Modi et al., 2014). These findings suggest that abnormal cortical glutamate homeostasis may contribute to the pathogenesis of anxiety disorders, while the underlying mechanisms remain undefined.
Synaptic transmission and cognitive ability are tightly regulated by neuron-glia interaction (de Hoz and Simons, 2015). Emerging evidence suggests key roles of oligodendroglia (OL) in brain function and psychiatric diseases (Fields, 2008). In particular, the clinical onset of anxiety peaks during vigorous OL and myelin development in the cerebral cortex (Kessler et al., 2005; Miller et al., 2012; Young et al., 2013). Besides the classical view of OL function in myelination that is essential for saltatory conductance, recent studies revealed that OL also provides trophic factors and metabolites that are critical for modulating neuronal function and plasticity (Du and Dreyfus, 2002; Funfschilling et al., 2012; Lee et al., 2012). In fact, essential role of OL is demonstrated in higher brain function, including learning motor skills, long distance connectivity and spiking-timing-dependent plasticity (McKenzie et al., 2014; Nave and Ehrenreich, 2014). Moreover, OL impairment is frequently observed in neuropsychiatric disorders, including schizophrenia, bipolar, and major depression (Fields, 2008; Edgar and Sibille, 2012; Cassoli et al., 2015). Nonetheless, whether defects in OL development during childhood and early adolescence may affect cortical neurons and contribute to the anxiety behaviors still remain unexplored.
In this study, we explored whether specific impairment of de novo OL maturation may affect cortical glutamate and result in maladaptive behaviors in juvenile mice. The Olig2 gene encodes a transcription factor essential for OL lineage specification, differentiation and myelination (Zhou et al., 2000; Lu et al., 2002; Ligon et al., 2006; Yue et al., 2006; Mei et al., 2013). We showed that conditional deletion of the Olig2 gene specifically in differentiated OLs resulted in preferential reduction of mature OL in the cerebral cortex. Interestingly, Olig2 cKO mice display abnormally elevated glutamate levels in the cortex and increased density of vesicles in cortical glutamatergic synapses in young mice. Moreover, juvenile Olig2 cKO mice showed anxiety and impulsivity-like behaviors. Taking together, our results indicate that impaired OL development in the cortex interferes with glutamate function and lead to anxiety- and impulsivity-like behaviors reminiscent of adolescent mental illnesses.
Materials and Methods
Animals
The Olig2-flox mice were previously described (Yue et al., 2006) in which the Olig2 coding region is flanked by two inserted loxp sites. The mice that express Cre recombinase under the control of the CNPase (CNP) promoter (CNP-Cre mice) were also previously described (Lappe-Siefke et al., 2003) where Cre recombinase was knocked into one allele of the CNP gene locus. To delete Olig2 in oligodendroglia lineage cells, Olig2loxp/loxp mice were crossed with the CNP-Cre+/- mice to generate both CNP-Cre+; Olig2loxp/loxp offspring (Olig2 cKO) and CNP-Cre-; Olig2loxp/loxp littermates (WT).
Ethics Statement
All animal experiments were performed according to an approved protocol from the Laboratory Animal Welfare and Ethics Committee of the Third Military Medical University.
Immunohistochemistry
At the age of postnatal day 21 (P21), mice (n = 6 for each group) were deeply anesthetized with 1% sodium pentobarbital and transcardially perfused with 4% paraformaldehyde in PBS. Brains were dehydrated in 10, 20, 30% sucrose in 4% paraformaldehyde for 12 h, respectively. The frozen brains were sectioned (20 μm) on a cryostat microtome (MS 1900, Leica). Free-floating sections were incubated with primary antibodies overnight at 4°C after blocking with PBS containing 0.3% TritonX-100 and 5% bovine serum at 37°C for 1 h. Then they were incubated with secondary antibodies for 3 h following by SABC regent (1:200; VECTASTAIN) for 1h at room temperature. The antigen-antibody complexes were visualized using DAB (Boster) as the chromogen. The primary antibodies included: mouse anti-CC1 (1:500; Millipore), rabbit anti-PDGFRα (1:200; Santa Cruz Biotechnology). Biotinylated secondary antibodies to rabbit (1:1000; VECTOR BA1000) or mouse (1:1000; VECTOR BA9200) were used as indicated in the legends.
Western Blotting
At the age of P21, mice (n = 7 in WT, n = 8 in Olig2 cKO) were anesthetized with 1% pentobarbital. Cerebral cortex (anterior cingulated area) and corpus callosum were rapidly removed and frozen. Frozen samples were homogenized and proteins were extracted using RIPA lyses buffer with protease inhibitors (Roche). Lysates containing 40 μg protein were denatured in gel-loading buffer, separated on 10% SDS-PAGE gels, transferred to PVDF membranes and visualized by chemiluminescence (ECL plus, GE Healthcare). Quantification of band intensity was analyzed using Image-Pro Plus software 5.0 (Media Cybernetics). The following primary antibodies were used: mouse anti-Olig2 (1:500; Millipore), mouse anti-MBP (1:1000; Santa Cruz Biotechnology) and mouse anti-β-actin (1:2000; Santa Cruz Biotechnology).
MRI and MRS
At the age of P21, MRI and MRS were performed, as recently described (Michaelis et al., 2009), on Olig2 cKO (n = 7) and WT control (n = 6). Mice were initially anesthetized with 5% isoflurane, subsequently incubated and kept under anesthesia with 1.75% isoflurane in ambient air. In vivo localized proton MRS (PRESS, TR/TE = 3,000/20 ms) in cerebral cortex (2.5 mm × 1 mm × 2.5 mm) was performed at 7.0T (Bruker BioSpec 70/20 USR). T2-weighted MRI (Turbo-RARE, TR/TE = 1,500/35ms, 8 echoes, slice thickness 500 μm) in axial and sagittal orientation served to ensure a proper position of volumes-of-interest. Metabolite quantification involved spectral evaluation by LCModel and calibration with brain water concentration (Provencher, 1993). Metabolites with Cramer-Rao lower bounds above 20% were excluded from further analyses.
Quantification of Glutamate in Cerebral Cortex
At the age of P21, mice (n = 6 for each group) were initially anesthetized with 5% isoflurane. Cerebral cortex (anterior cingulate area) was removed and frozen at -80°C immediately. The brain tissues were homogenized with 100 μl glutamate assay buffer and followed the manufacturer’s instruction from Glutamate assay kit (Sigma). The 10KD spin filters (Biovision) were applied. The absorbance was measured at 450 nm with Model 680 microplate reader (Bio-Rad).
Electron Microscopy
Electron microscopy (EM) analysis was performed as previously described (Mei et al., 2013). At the age of P21, mice were initially anesthetized with 5% isoflurane. The anterior cingulate cortex of Olig2 cKO mice (n = 3) and WT controls (n = 3) were removed rapidly and fixed in fresh fixative overnight at 4°C. Tissue cubes (1 mm × 1 mm × 1 mm) were rinsed in PBS, postfixed in 1% OsO4 in PBS for 2 h, counterstained with uranyl acetate, dehydrated in a graded ethanol series, infiltrated with propylene oxide, and embedded in Epon. Ultrathin sections (∼60 nm) were generated by an ultramicrotome (LKB-V, LKB Produkter AB, Bromma) and were viewed with a transmission electron microscope (TECNAI10, Philips). Three sections from each mouse were investigated at a comparable location from Olig2 cKO and WT control mice under magnification of 60k. Digital images were acquired with an AMT XR-60 CCD Digital Camera System and analyzed using Image-Pro Plus software 5.0 (Media Cybernetics).
Behavioral Tests
Olig2 cKO mice and WT littermates were housed in a controlled environment (25°C) with free access to food and water and maintained on a 12 h/12 h day/night cycle. Behavioral tests were conducted on sex-balanced groups of experimentally naive mice at P21. All tests were done from 10:00 h to 18:00 h. One group of mice were tested in open-field test (between 10:00 h and 12:00 h) and elevated plus-maze test (between 15:00 h and 18:00 h) on the same day to measure anxiety. Another group of mice were only tested in cliff avoidance reaction (CAR) test (between 10:00 h and 18:00 h) to measure impulsivity. After each experiment, all the apparatuses were wiped clean with 70% ethanol to prevent a bias due to olfactory cues. For all behavioral experiments, investigators were blinded for genotype and mice were gently handled to avoid stress.
Open field test was performed using an open-field activity system (Biowill, Shanghai, China), as described (Wang et al., 2013). Briefly, mice (n = 26 in WT, n = 18 in Olig2 cKO) were placed in the center of the open-field box (50 cm × 50 cm × 50 cm), and activity was recorded during a period of 10 min. The total and center-area travel distances were measured and the time spent in the central area was recorded.
Elevated plus-maze test was conducted as previously described (Katayama et al., 2010). Briefly, mice (n = 24 in WT, n = 15 in Olig2 cKO) were tested on a plus-maze apparatus (Biowill, Shanghai, China) [closed arms, 25 × 5 × 15 (H) cm; open arms 25 × 5 × 0.3 (H) cm] arranged orthogonally 60 cm above the floor. Each mouse was initially placed in the center area facing an open arm, and then allowed to move freely in the maze for 5 min. The total travel distance on the maze, time spent on any arms and entries into any arms were recorded.
Cliff avoidance reaction test was conducted as previously described with slight modification (Yamashita et al., 2013). Mice (n = 14 in WT, n = 15 in Olig2 cKO) were assessed using a round platform (diameter, 16 cm; height, 50 cm). The test was initiated by gently placing an animal on the platform with the forelimbs approached the edge. If the animal fell from the platform during the 20 min test, it was judged to have impaired CAR. The latency from an initial placement on the platform until falling was recorded. The incidence of impaired CAR was calculated as a percentage index for each group: % (CAR) = (the number of intact CAR mice (which did not fall from platform)/total number of tested mice) × 100. Duration of each entry into the edge area, which was defined as an outer ring with a width of 2 cm, was recorded before the mouse fell off.
Statistical Analysis
We performed statistical analyses using the SPSS software version 13.0. All the data were confirmed to be normal distribution, as tested by the Kolmogorov–Smirnov test. For between-group comparisons, we used the Independent-Samples t-test with Welch correction, if the variance was unequal. We considered results to be significant at p < 0.05.
Results
The Loss of Olig2 Preferentially Impaired OL Maturation in the Cerebral Cortex
To explore the consequence of impaired OL development on brain function, conditional deletion of the Olig2 gene was achieved in differentiated OL during neonatal development by introducing expression of the cre recombinase under transcriptional control of the CNPase promoter (CNP-Cre) in the Olig2-loxp mice (Figure 1A). The offspring were genotyped by PCR at postnatal day 7 (P7) to identify Olig2loxp/loxp mice that carry the CNP-Cre allele (Olig2 cKO) and Olig2loxp/loxp littermates that lack the CNP-Cre allele (WT control) (Figure 1B). The knockout efficiency of Olig2 was confirmed by the diminished expression of the Olig2 protein in both the corpus callosum and cerebral cortex of the Olig2 cKO mice at P21 (Figure 1C). To investigate the effects of Olig2 loss on OL development, we quantified oligodendroglia progenitor cells (OPCs) marked by immunohistochemistry staining of PDGF receptor α (PDGFRα) and mature OL marked by CC1. As shown in Figure 2, the numbers of CC1+ mature OL in Olig2 cKO mice were significantly decreased by 85.6% in the cortex (250 ± 13 in WT vs. 36 ± 2.8 in Olig2 cKO) and 36.1% (521 ± 40 in WT vs. 333 ± 47 in Olig2 cKO) in the corpus callosum, respectively (Figures 2A,B). In contrast, the density of PDGFRα+ OPCs was not affected (Figures 2C,D). These results suggest that OL maturation is preferentially affected in the cerebral cortex of the Olig2 cKO. Such a conclusion is further supported by the observation that myelin basic protein (MBP) was reduced 94% in the Olig2 cKO cortex (100 ± 12.3% in WT vs. 6.6 ± 3.0% in Olig2 cKO), but only reduced 60% in the Olig2 cKO corpus callosum (100 ± 15.1% in WT vs. 40.0 ± 7.3% in Olig2 cKO) (Figures 2E,F).
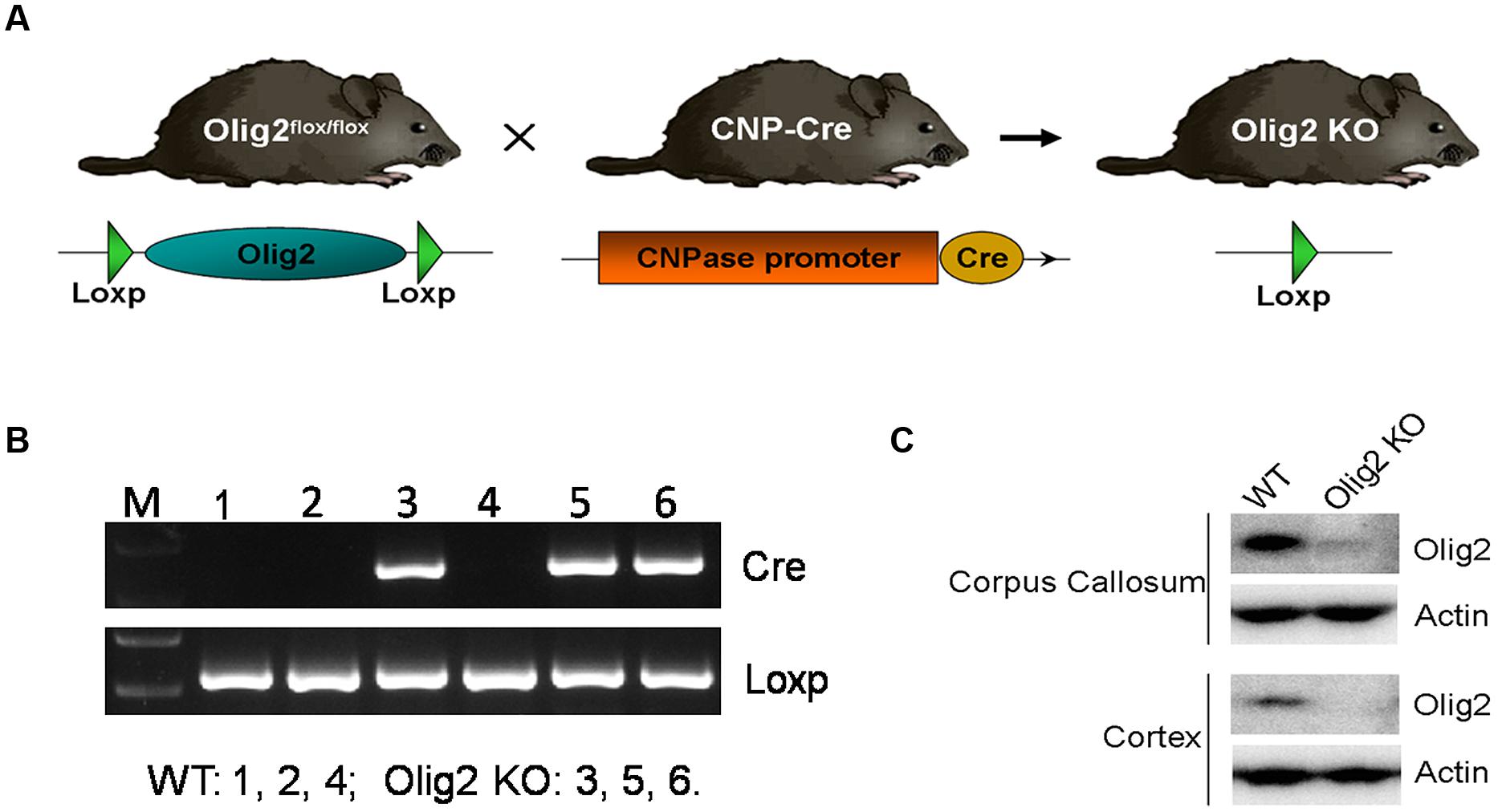
FIGURE 1. Generating Olig2 conditional knockout mice. (A) Diagram shows Olig2flox/flox mice were crossed with mice that express Cre recombinase under the control of the CNPase promoter to generate Olig2 cKO mice. (B) The offspring were genotyped by PCR for cnp-cre and floxed Olig2 alleles. (C) Western Blot analyses showed an absence of Olig2 protein in cortex and corpus callosum of Olig2 cKO mice.
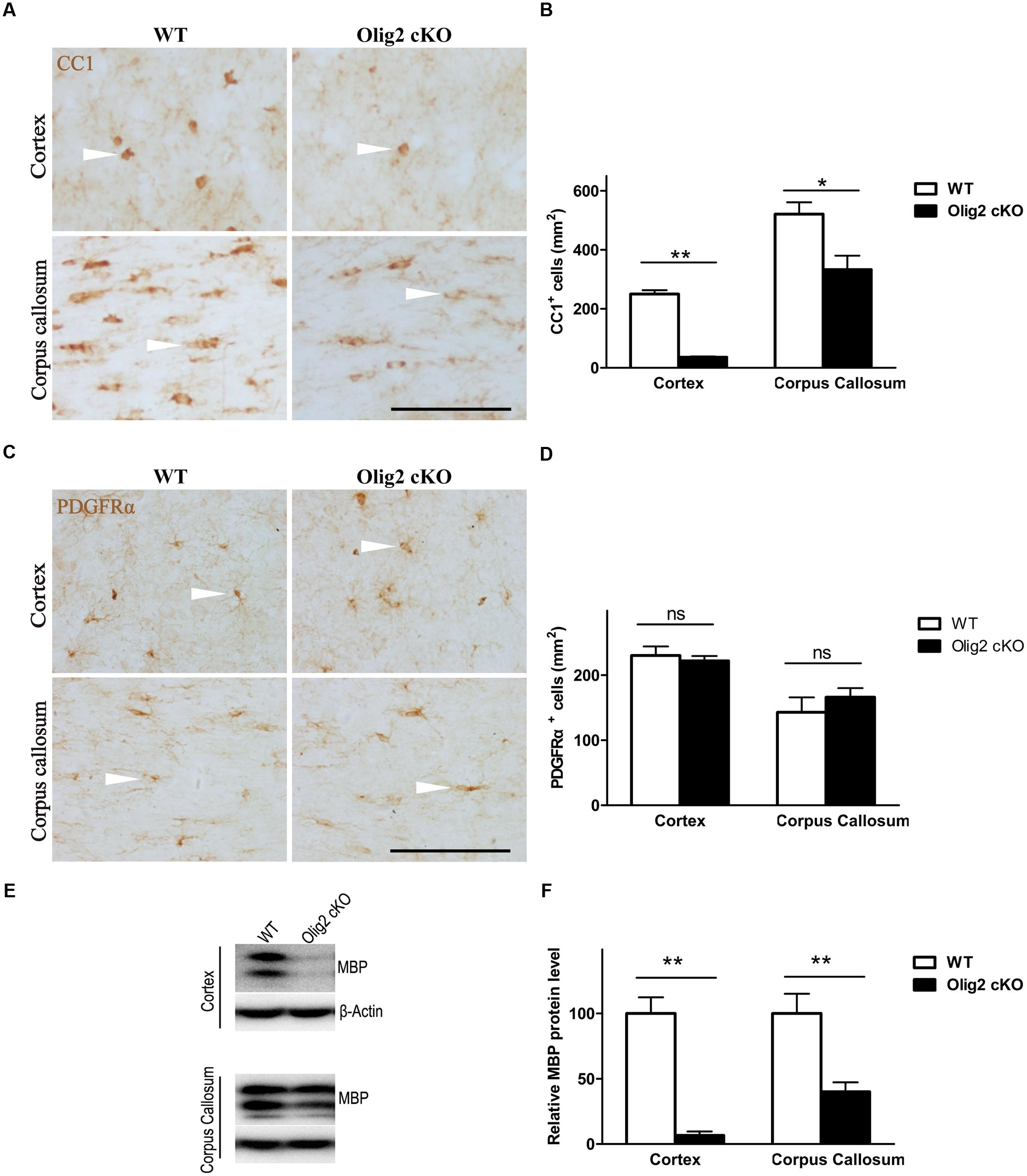
FIGURE 2. Preferential impairment of oligodendroglia maturation in the cortex of Olig2 cKO mice. (A) Representative images of immunohistochemistry of CC1 showing mature oligodendrocytes (OLs) (white arrow head) in the cortex and corpus callosum from WT and Olig2 cKO mice. (B) Quantification of the density of CC1+ cells in the cortex and corpus callosum from WT and Olig2 cKO mice. (C) Representative images of immunohistochemistry of PDGFRα+ showing oligodendroglia progenitor cells (OPCs) in the cortex and corpus callosum from WT and Olig2 cKO mice. (D) Quantification of the density of PDGFRα+ cells in the cortex and corpus callosum from WT and Olig2 cKO mice. (E) Representative Western blot of MBP expression in the cortex and corpus callosum from WT or Olig2 cKO mice. (F) Quantification of MBP signal on the immunoblot of the cortex and corpus callosum from WT or Olig2 cKO mice normalized to β-actin as a loading control. Scale bars = 100 μm. Data are presented as mean ± SEM. ns, no significant different; ∗P < 0.05; ∗∗P < 0.01.
The Cerebral Cortex of Olig2 cKO Mice Harbored Abnormally Higher Levels of Glutamate
To explore whether and how impaired OL maturation may interfere with neuronal function in the juvenile brain, we examined the spectrum of neurochemicals in the cortex of WT and Olig2 cKO mice at the age of P21 using proton magnetic resonance spectroscopy (1H MRS). T2-weighted MRI images in axial and sagittal orientation were used to ensure the volume-of-interest (Figure 3A). Representative MRS spectrums showed resonance of various neurochemicals (Figure 3B). N-acetylaspartate (NAA) and glutamate (Glu) are the two most abundant neurochemicals in the brain. The peak areas for NAA and Glu were normalized to that of creatine (Cr) for quantitative comparison between the genotypes. Interestingly, the Olig2 cKO cortex harbors a moderate yet statistically significant increase of Glu/Cr than WT littermates (0.83 ± 0.02 in WT vs. 0.93 ± 0.03 in Olig2 cKO, P = 0.01) (Figure 3D). In contrast, the ratio of NAA/Cr, which is thought to reflect neuronal health or viability (Urenjak et al., 1993), was not altered in the Olig2 cKO mutant (P = 0.379) (Figure 3C). In addition, choline (Cho) level showed a trend of decrease in the Olig2 cKO cortex, although no statistical significance was achieved (data not shown). The increase of glutamate in Olig2 cKO cortex was further validated by enzymatic assay, in which a significant increase of total glutamate in the cortex of Olig2 cKO mice was observed (1.32 ± 0.06 μg/mg in WT vs. 1.53 ± 0.06 μg/mg in Olig2 cKO, P = 0.029) (Figure 3E). These results demonstrated that impaired OL maturation specifically leads to an abnormal increase of glutamate in the cortex without altering neuronal viability.
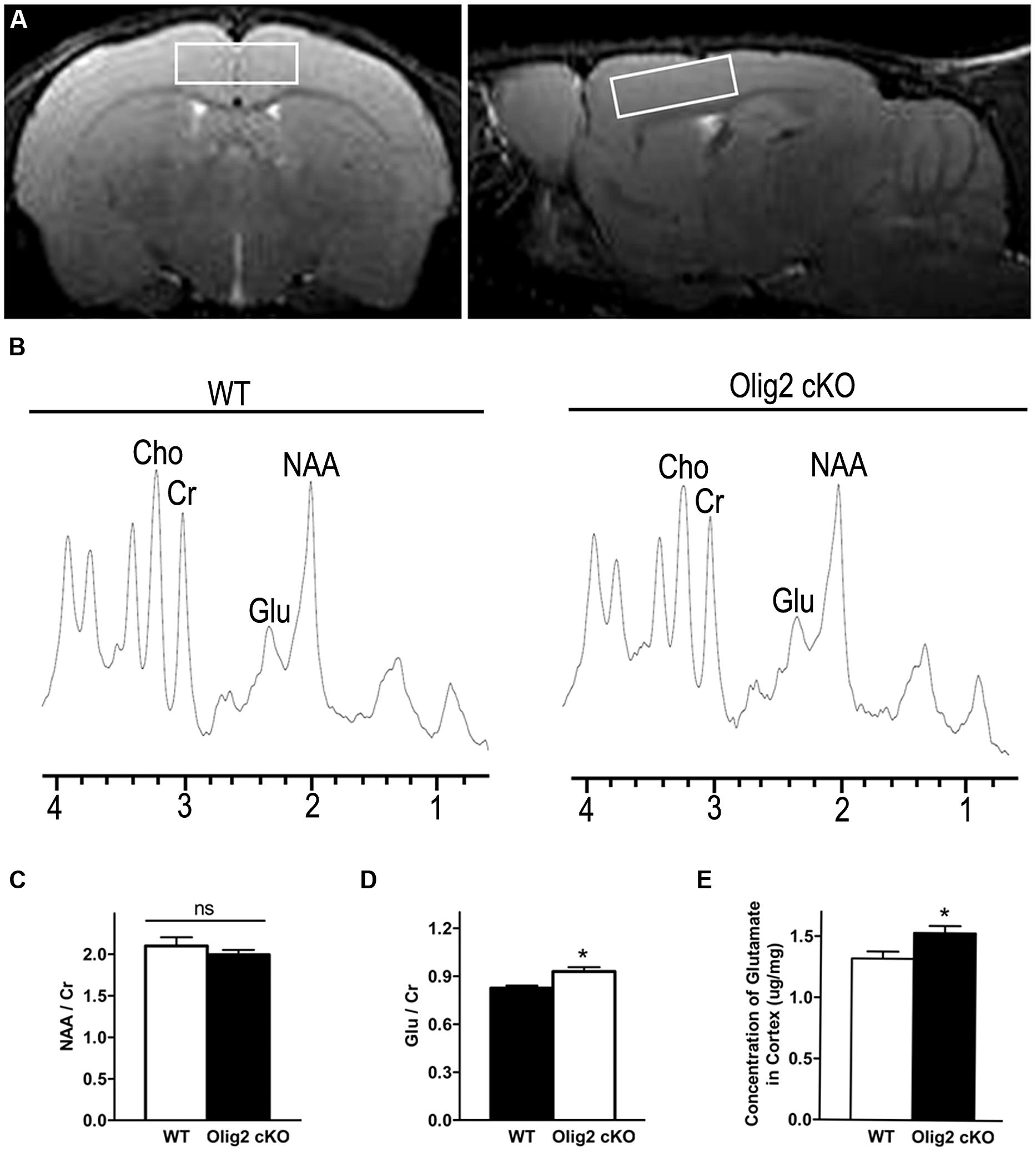
FIGURE 3. Aberrantly increased glutamate level in the cortex of Olig2 cKO mice. (A) Representative axial and sagittal T2-weighted MR images indicate the position and orientation of point-resolved spectroscopy voxel in the cortex (white rectangle). (B) Localized proton magnetic resonance spectra of the cortex from WT or Olig2 cKO mice, respectively. NAA, N-acetylaspartate; Glu, Glutamate; Cr, total creatine; ppm, parts per million. (C) Quantification of the ratio of NAA/Cr in the cortex of WT or Olig2 cKO mice. (D) Quantification of the ratio of Glu/Cr in the cortex of WT or Olig2 cKO mice. (E) Quantification of glutamate concentration in the cortex by the enzymatic assay from WT or Olig2 cKO mice. Data are mean ± SEM. ns, no significant difference. ∗P < 0.05.
Glutamatergic Synapses of Olig2 cKO Cortical Neurons Contained Higher Density of Synaptic Vehicles
Glutamate is the primary excitatory neurotransmitter, which is largely stored in synaptic vesicles in pyramidal neurons and released upon synaptic stimulation in the cortex (Fonnum, 1984). The long axonal projections of cortical glutamatergic neurons are the primary target for myelination (Tomassy et al., 2014). Moreover, most non-myelinating cortical OLs form contacts with the soma of glutamatergic neurons (Takasaki et al., 2010). Thus, we next questioned whether OL impairment caused by the loss of Olig2 may affect glutamatergic synapses. Transmission EM images were captured in randomly selected sections and subjected to double blind analysis. The vesicles in each asymmetric synapse with a prominent postsynaptic density (PSD), which is a hall mark of excitatory glutamatergic synapses, were counted in all EM images. Consistent with the increase of overall glutamate levels in the cortex of Olig2 cKO mice (Figure 3), the density of synaptic vesicles within the excitatory presynaptic boutons in the cotex of Olig2 cKO mice was significantly increased as compared to WT controls (97.2 ± 4.1 in WT vs. 125.2 ± 4.5 in Olig2 cKO) (Figures 4A,B). We also observed a trend of increase in the numbers of docked vesicles at the active zone of Olig2 cKO boutons (1.53 ± 0.18 in WT vs. 1.97 ± 0.18 in Olig2 cKO, P = 0.096) (Figure 4C). No significant difference was detected in the length of PSD between WT and Olig2 cKO (282.5 ± 17.7 in WT vs. 260.2 ± 12.5 in Olig2 cKO, P = 0.293) (Figure 4D). These results suggest that OL impairment caused by the loss of Olig2 may lead to aberrantly increased glutamate vesicles within synapses and likely increased glutamate release upon synaptic activation.
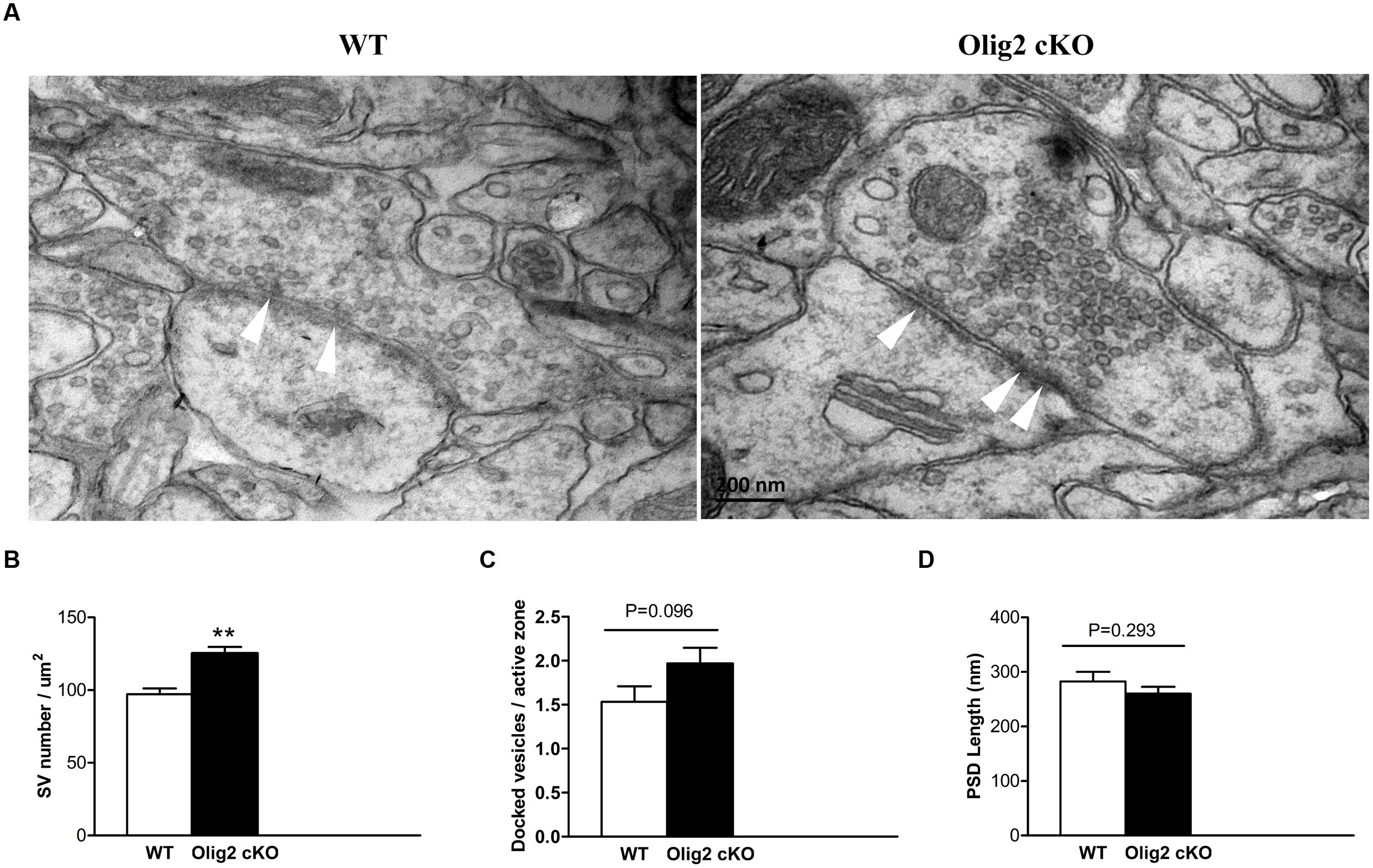
FIGURE 4. Electronmicrocopy analysis of glutamatergic synapses and synaptic vesicles in the cortex of WT and Olig2 cKO mice. (A) Representative electron microscopy micrographs show an excitatory synapse in the WT and Olig2 cKO cortices. White arrows indicate docked vesicles. (B) Quantification of the density of synaptic vesicles in cortical glutamatergic presynaptic boutons from EM images of WT or Olig2 cKO mice. (C) Quantification of the number of docked vesicles per active zone in WT or Olig2 cKO excitatory synapses. (D) Quantification of the length of the postsynaptic density (PSD) in WT or Olig2 cKO excitatory synapses. Scale bar = 200 nm, Data are given as mean ± SEM, ∗∗P < 0.01.
Olig2 cKO Mice Displayed Anxiety-like Behaviors and Deficits in Behavioral Inhibition
We next tested whether OL impairment and glutamate abnormalities may lead to maladaptive behaviors in juvenile mice. We first chose the open field test and elevated plus maze test, which are non-conditioned procedures commonly used for assessing anxiety-like behaviors in rodents (Bourin et al., 2007). In the open field test, juvenile Olig2 cKO mice showed a significantly lower ratio of travel distance within central area to total travel distance, as compared with WT controls (13.8 ± 1.2% in WT vs. 9.0 ± 0.8% in Olig2 cKO, P = 0.004) (Figure 5A) and spent more time in the central area (30.8 ± 3.6 sec in WT vs. 51.1 ± 4.1 sec in Olig2 cKO, P = 0.001) (Figure 5B) during the 10-min test, indicating an anxious phenotype. In addition, in the elevated plus maze test, the numbers of entry into the open arms and time spent on the open arms were previously shown to be inversely related with the anxiety level (Xu et al., 2014), hence used to identify anxiety-like behaviors (Brunner et al., 2014). The time spent on the open arms and entries into the open arms were expressed as a percentage of the total time on any arms and entries into any arms during the test. We found that Olig2 cKO mice showed less entries into the open arms (51.9 ± 2.0% in WT vs. 43.4 ± 3.1% in Olig2 cKO, P = 0.021) (Figure 5C) and spent less time on open arms (34.1 ± 3.2% in WT vs. 22.2 ± 2.3% in Olig2 cKO, P = 0.011) (Figure 5D), again indicating a more anxious behavior. We also assessed locomotor activity of the animals on the maze by the total distance traveled on both open and closed arms. We found that Olig2 cKO mice traveled a significantly longer distance than WT controls (6663 ± 404.5 cm in WT vs. 8264 ± 444.3 cm in Olig2 cKO, P = 0.014) (Figure 5E), suggesting a higher locomotor activity of Olig2 cKO mice on the maze. Together, all the aforementioned behavior tests consistently demonstrated anxiety-like behaviors in juvenile Olig2 cKO mice.
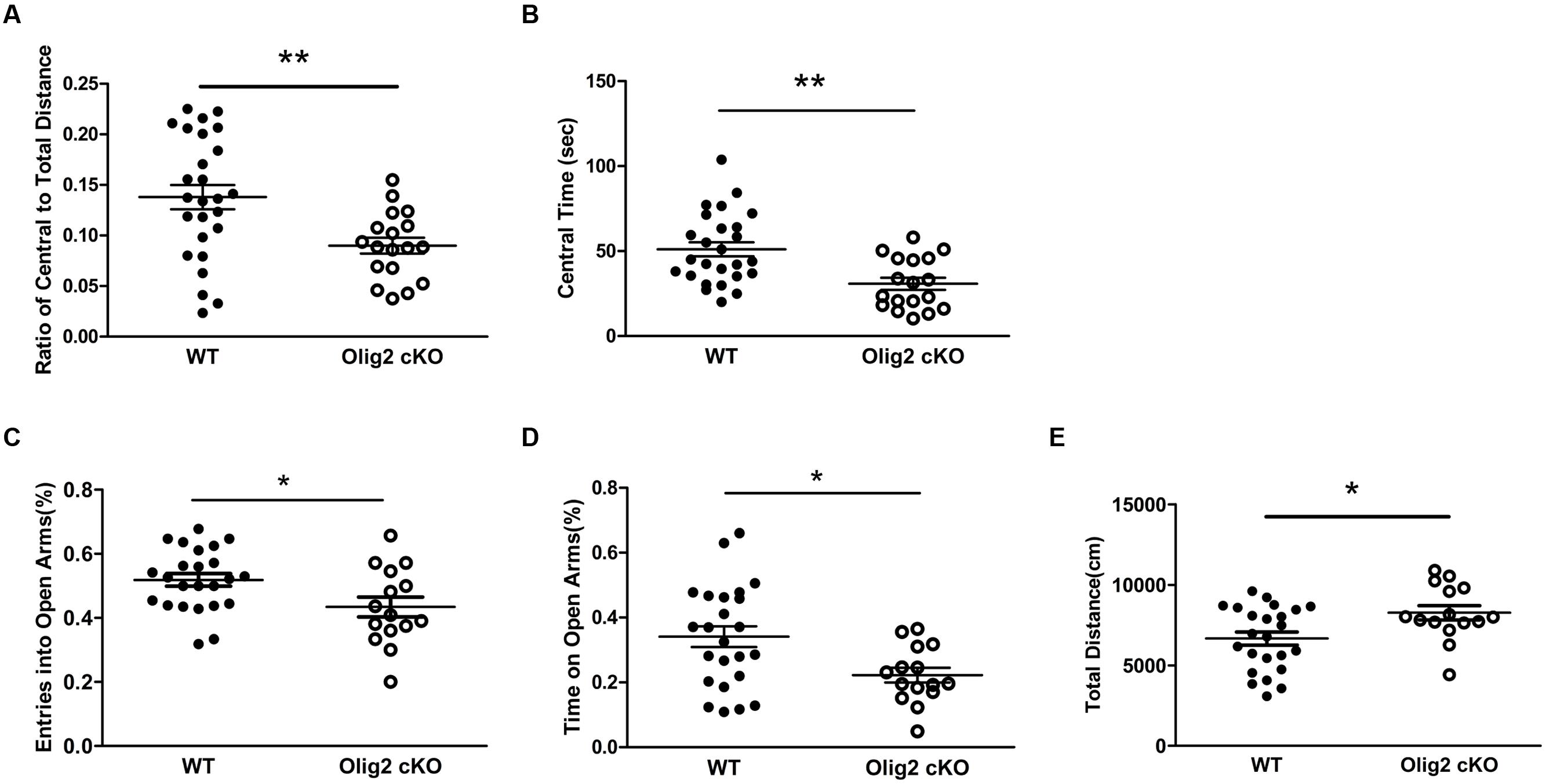
FIGURE 5. Anxiety-like behavior tests on WT and Olig2 cKO mice. Open field test (A,B): Graphs showed ratio of central to total distance (A) and center time (B) in WT and Olig2 cKO mice. Elevated plus maze test (C–E): Graphs showed number of entries into open arms (C), time spent on the open arms (D) and total distance traveled on the arms (E), Data are given as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01.
Impulsivity is defined as a failure in controlling and inhibiting the emotion for appropriate actions and behaviors (Dalley et al., 2008), which is also a typical tendency in adolescence. Recent studies suggest that certain types of functional impulsivity may be linked with anxiety (Taylor et al., 2008). Thus, we explored whether the loss of Olig2 may also lead to impulsivity-like behavior in juvenile mice. CAR impairment is thought to represent impulsivity-like behaviors in rodents (Matsuoka et al., 2005). Unlike many classical mouse impulsivity tests that require lengthy training, the CAR test can be readily applied to juvenile mice. We found Olig2 cKO mice traveled more and spent longer time than WT control in the edge area in the CAR test (17.7 ± 3.1 s in WT vs. 49.4 ± 10.2 s in Olig2 cKO, P = 0.007) (Figures 6A,B). Moreover, 60% of Olig2 cKO mice failed to avoid a potential fall from a height, whereas only 14.3% of WT controls fell (Figure 6C). These results suggest that the defects in OL maturation due to the loss of Olig2 also lead to impulsivity-like behaviors.
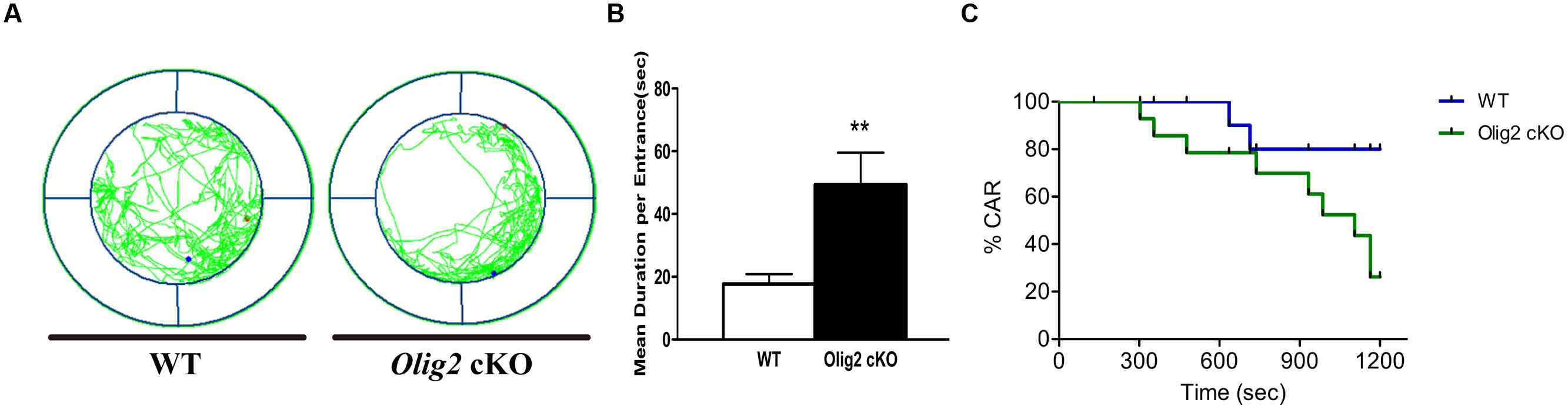
FIGURE 6. Impulsivity-like behavior analyses on WT and Olig2 cKO mice. Cliff avoidance reaction test (A–C): an example of the travel pathway of WT and Olig2 cKO on the elevated platform (A). Olig2 cKO mice failed to avoid the cliff actively by spending significantly more time around the cliff (B). Graphs represent the time course of CAR measurement in WT and Olig2 cKO mice (C). Data are given as mean ± SEM. ∗∗P < 0.01.
Discussion
Using the Olig2 cKO mouse model, our studies demonstrated that impaired OL maturation can affect glutamate levels in the cerebral cortex and synaptic vesicle density in glutamatergic presynaptic boutons. Furthermore, Olig2 cKO mice display anxiety-related maladaptive behaviors. To our knowledge, it is the first evidence that demonstrates behavioral abnormalities of Olig2 cKO mice. These results suggests that OL-neuron interaction in the cortex plays important roles in governing synaptic function and raises an intriguing possibility that impaired de novo OL maturation in adolescence may contribute to the early onset of anxiety.
Proton magnetic resonance spectroscopy (1H MRS) is a non-invasive technique that provides great advantages in studying biochemical concentrations of neurotransmitters in vivo (Soares and Law, 2009). Specifically, glutamate, which is measured by 1H MRS, has been suggested as an index of cortical excitability (Stagg et al., 2009, 2011). In multiple clinical MRS studies, increased cortical glutamate levels were positively correlated with anxiety symptoms in patients (Phan et al., 2005; Modi et al., 2014). These observations suggest that hyperfunction of cortical glutamatergic neurons may be an important contributing factor for the etiology of anxiety and related disorders. Extensive studies have demonstrated that a wide variety of molecular and cellular mechanisms in neurons and nearby glia cells tightly control glutamate homeostasis and synaptic release (Deitmer et al., 2003; DeSilva et al., 2009). However, which mechanism(s) is dysregulated that underlie the increased glutamate in the cortex of anxiety disorder patients still remains elusive.
Cortical OL maturation occurs concurrently with the most frequent onset of anxiety disorders (Kessler et al., 2005; Miller et al., 2012; Young et al., 2013). In addition, OL and myelin development overlaps with the time for peak binding of cortical glutamate to NMDA receptors in early adolescence (Kornhuber et al., 1988). Therefore, we explored whether selective impairment of OL maturation may affect glutamatergic neurons and behavior in juvenile mice. The CNP-Cre mouse line was used in previous studies for specific deletion of genes in mature OL (Wahl et al., 2014; Zou et al., 2014). We employed this mouse line for conditionally deleting the Olig2 gene that encodes a transcription factor critical for OL development (Zhou et al., 2000; Lu et al., 2002; Ligon et al., 2006; Yue et al., 2006; Mei et al., 2013). Interestingly, Olig2 cKO mice displayed preferential reduction of cortical mature OLs at P21, whereas OLs in the corpus callosum were much less severely affected. Hence, despite the undefined mechanism that underlies the preferential impairment of OL maturation in the cortex of Olig2 cKO mouse, this genetic model provides a reasonable tool for dissecting the role of OL maturation in modulating cortical neuronal function during juvenile age. Importantly, using 1H MRS, we detected an overall increase of glutamate in the cortex of Olig2 cKO mice, suggesting elevated cortical excitability. In addition, we detected increased vesicles in glutamatergic synapses, which further supported glutamatergic hyperfunction as a result of defects in OL maturation. Moreover, juvenile Olig2 cKO mice displayed anxiety-related behaviors. Together, these observations suggest that normal OL development plays essential roles in governing glutamate signaling and adolescence behavior, and defects in de novo OL maturation in early postnatal life could cause aberrant glutamate function and maladaptive behaviors.
Oligodendroglia impairment may affect cortical neurons through multiple distinct mechanisms that still remain elusive. Because the loss of Olig2 could affect myelination and axonal conductance (Baumann and Pham-Dinh, 2001), neurons in Olig2 cKO cortex may increase synaptic vesicles as a compensatory adaptation. This may especially affect glutamatergic neurons, because their axons are mostly myelinated by OLs (Tomassy et al., 2014). However, several lines of evidence argue that myelination defects alone do not necessarily cause glutamatergic hyperfunction. For instance, cortical glutamate levels were maintained normal in the heterozygous shiverer mutant mouse that lacked one copy of the MBP gene thus displaying hypomyelination without affecting mature OL density (Takanashi et al., 2014). Thus, the preferential impairment of de novo OL maturation in the cortex may be a specific mechanism that underlies the aberrant increase of cortical glutamate.
Unlike white matter OLs that perform primary roles in myelin formation, cortical OLs display a radial gradient distribution and differentially myelinate the long-range projections of glutamatergic pyramidal neurons in distinct cortical layers (Tomassy et al., 2014). Besides myelination, the majority of mature OLs reside in deep layers of cerebral cortex are attached with the soma of pyramidal neurons, rather than GABAergic neurons (Takasaki et al., 2010). Although the function of these perineuronal OLs in the cortex still remains unknown, accumulating evidence suggest that OLs can provide metabolic and neurotrophic support (Du and Dreyfus, 2002). In addition, OLs are known to express several glutamate transporters (Domercq et al., 1999; Karadottir et al., 2005), and can uptake glutamate in the gray matter of the developing brain (DeSilva et al., 2009). More importantly, cortical perineuronal oligodendroglia, but not white matter oligodendroglia, express glutamine synthetase (GS) for converting glutamate to glutamine (Damelio et al., 1990). It is well established that glutamine produced by astrocyte GS from the up-taken glutamate can be recycled into neurons, which plays key roles in controlling glutamate recycling (Deitmer et al., 2003). In this regard, cortical gray matter OLs may play important roles in controlling glutamate homeostasis, in parallel with the known function of astrocytes in modulating glutamatergic function.
It is also important to note that besides aberrant glutamate, abnormalities in other neurotransmitters, including GABA, dopamine, serotonin and norepinephrine, are also thought to contribute to anxiety (Kent et al., 2002). Because our understanding of the functional interplay between OLs and neurons is still at its infancy, the types of neurons affected by OL defects still remain undefined. It is conceivable that OL defects in Olig2 cKO mice could also affect the function of other neuronal cell types in addition to glutamatergic pyramidal neurons, which could contribute to the anxiety-like behaviors we observed in this study. In fact, dopamine transporter knockout mice are severely impaired in CAR test (Yamashita et al., 2013). In addition, disturbing neuregulin-1-ErbB4 signaling in OLs can also lead to increased dopamine receptors and transporters and anxiety-like behaviors in mice (Roy et al., 2007). Whether and how OL maturation may modulate dopaminergic function is a challenging question for future studies. Moreover, the symptoms of anxiety are shared by some other psychiatric disorders, like schizophrenia (Buckley et al., 2009), suggesting that the anxiety like behavior found in Olig2 cKO mice may also contribute to other psychiatric disorders. In fact, ErbB signaling pathway has been considered as a potential therapeutic target for schizophrenia (Deng et al., 2013), which conceivably modulates OL function. However, many well-established behavioral tests for cognitive abnormalities require lengthy training hence could not be applied to juvenile mice. This limits our ability in the current study that aims to identify juvenile abnormalities caused by defects of OL maturation. Future studies may need more specific behavior tests utilized in both young and adult mice to explore whether OL maturation affects higher cognitive function.
Conclusion
Our studies demonstrate that impaired OL maturation by conditional deleting the Olig2 gene in differentiated OL can aberrantly increase cortical glutamate, which likely leads to glutamatergic hyperfunction. Moreover, the defects in de novo OL maturation clearly result in anxiety-like behaviors and deficits of behavioral inhibition in juvenile mice. Our findings prompt future studies to investigate the intriguing possibility whether defects in OL and/or myelin development in childhood may contribute to the pathogenesis of anxiety in humans.
Author Contributions
XC, LX, and YF designed the study. XC, WZ and YG acquired and analyzed the data. TL, YT and SL also acquired the data. H-YS analyzed the data. XC, YF and LX wrote the article, which all other authors reviewed. All authors approved the final version for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (NSCF 81471297), and Chongqing Science Foundation to LX. We thank Mrs. Yu Sun for her assistance in ultrathin sections.
References
Baumann, N., and Pham-Dinh, D. (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 81, 871–927.
Bourin, M., Petit-Demouliere, B., Dhonnchadha, B. N., and Hascoet, M. (2007). Animal models of anxiety in mice. Fundam. Clin. Pharmacol. 21, 567–574. doi: 10.1111/j.1472-8206.2007.00526.x
Brunner, S. M., Farzi, A., Locker, F., Holub, B. S., Drexel, M., Reichmann, F., et al. (2014). GAL3 receptor KO mice exhibit an anxiety-like phenotype. Proc. Natl. Acad. Sci. U.S.A. 111, 7138–7143. doi: 10.1073/pnas.1318066111
Buckley, P. F., Miller, B. J., Lehrer, D. S., and Castle, D. J. (2009). Psychiatric comorbidities and schizophrenia. Schizophr. Bull. 35, 383–402. doi: 10.1093/schbul/sbn135
Butters, R. P. (2011). The behavioral neuroscience of adolescence. Clin. Soc. Work J. 39, 315–317. doi: 10.1007/s10615-011-0320-y
Casey, B. J., Getz, S., and Galvan, A. (2008). The adolescent brain. Dev. Rev. 28, 62–77. doi: 10.1016/j.dr.2007.08.003
Cassoli, J. S., Guest, P. C., Malchow, B., Schmitt, A., Falkai, P., and Martins-de-Souza, D. (2015). Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: from structural findings to molecules. NPJ Schizophr. 1:15034. doi: 10.1038/npjschz.2015.34
Dalley, J. W., Mar, A. C., Economidou, D., and Robbins, T. W. (2008). Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 90, 250–260. doi: 10.1016/j.pbb.2007.12.021
Damelio, F., Eng, L. F., and Gibbs, M. A. (1990). Glutamine-synthetase immunoreactivity is present in oligodendroglia of various regions of the central-nervous-system. Glia 3, 335–341. doi: 10.1002/glia.440030504
de Hoz, L., and Simons, M. (2015). The emerging functions of oligodendrocytes in regulating neuronal network behaviour. Bioessays 37, 60–69. doi: 10.1002/bies.201400127
Deitmer, J. W., Broer, A., and Broer, S. (2003). Glutamine efflux from astrocytes is mediated by multiple pathways. J. Neurochem. 87, 127–135. doi: 10.1046/j.1471-4159.2003.01981.x
Deng, C., Pan, B., Engel, M., and Huang, X. F. (2013). Neuregulin-1 signalling and antipsychotic treatment: potential therapeutic targets in a schizophrenia candidate signalling pathway. Psychopharmacology (Berl) 226, 201–215. doi: 10.1007/s00213-013-3003-2
DeSilva, T. M., Kabakov, A. Y., Goldhoff, P. E., Volpe, J. J., and Rosenberg, P. A. (2009). Regulation of glutamate transport in developing rat oligodendrocytes. J. Neurosci. 29, 7898–7908. doi: 10.1523/Jneurosci.6129-08.2009
Domercq, M., Sanchez-Gomez, M. V., Areso, P., and Matute, C. (1999). Expression of glutamate transporters in rat optic nerve oligodendrocytes. Eur. J. Neurosci. 11, 2226–2236. doi: 10.1046/j.1460-9568.1999.00639.x
Du, Y. Z., and Dreyfus, C. F. (2002). Oligodendrocytes as providers of growth factors. J. Neurosci. Res. 68, 647–654. doi: 10.1002/jnr.10245
Edgar, N., and Sibille, E. (2012). A putative functional role for oligodendrocytes in mood regulation. Transl. Psychiatry 2:e102. doi: 10.1038/tp.2012.34
Fields, R. D. (2008). White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 31, 361–370. doi: 10.1016/j.tins.2008.04.001
Fonnum, F. (1984). Glutamate - a neurotransmitter in mammalian Brain. J. Neurochem. 42, 1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x
Funfschilling, U., Supplie, L. M., Mahad, D., Boretius, S., Saab, A. S., Edgar, J., et al. (2012). Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521. doi: 10.1038/nature11007
Hoerst, M., Weber-Fahr, W., Tunc-Skarka, N., Ruf, M., Bohus, M., Schmahl, C., et al. (2010). Correlation of glutamate levels in the anterior cingulate cortex with self-reported impulsivity in patients with borderline personality disorder and healthy controls. Arch. Gen. Psychiatry 67, 946–954. doi: 10.1001/archgenpsychiatry.2010.93
Karadottir, R., Cavelier, P., Bergersen, L. H., and Attwell, D. (2005). NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166. doi: 10.1038/nature04302
Katayama, K., Yamada, K., Ornthanalai, V. G., Inoue, T., Ota, M., Murphy, N. P., et al. (2010). Slitrk1-deficient mice display elevated anxiety-like behavior and noradrenergic abnormalities. Mol. Psychiatry 15, 177–184. doi: 10.1038/mp.2008.97
Kent, J. M., Mathew, S. J., and Gorman, J. M. (2002). Molecular targets in the treatment of anxiety. Biol. Psychiatry 52, 1008–1030. doi: 10.1016/S0006-3223(02)01672-4
Kessler, R. C., Berglund, P., Demler, O., Jin, R., and Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions’ of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 593–602. doi: 10.1001/archpsyc.62.6.593
Kornhuber, J., Retz, W., Riederer, P., Heinsen, H., and Fritze, J. (1988). Effect of antemortem and postmortem factors on [3H] glutamate binding in the human brain. Neurosci. Lett. 93, 312–317. doi: 10.1016/0304-3940(88)90101-2
Lappe-Siefke, C., Goebbels, S., Gravel, M., Nicksch, E., Lee, J., Braun, P. E., et al. (2003). Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 33, 366–374. doi: 10.1038/ng1095
Lee, F. S., Heimer, H., Giedd, J. N., Lein, E. S., Sestan, N., Weinberger, D. R., et al. (2014). Adolescent mental health-Opportunity and obligation. Science 346, 547–549. doi: 10.1126/science.1260497
Lee, Y. J., Morrison, B. M., Li, Y., Lengacher, S., Farah, M. H., Hoffman, P. N., et al. (2012). Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448. doi: 10.1038/nature11314
Ligon, K. L., Kesari, S., Kitada, M., Sun, T., Arnett, H. A., Alberta, J. A., et al. (2006). Development of NG2 neural progenitor cells requires Olig gene function. Proc. Natl. Acad. Sci. U.S.A. 103, 7853–7858. doi: 10.1073/pnas.0511001103
Lu, Q. R., Sun, T., Zhu, Z. M., Ma, N., Garcia, M., Stiles, C. D., et al. (2002). Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86. doi: 10.1016/S0092-8674(02)00678-5
Matsuoka, Y., Furuyashiki, T., Yamada, K., Nagai, T., Bito, H., Tanaka, Y., et al. (2005). Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc. Natl. Acad. Sci. U.S.A. 102, 16066–16071. doi: 10.1073/pnas.0504908102
McKenzie, I. A., Ohayon, D., Li, H. L., de Faria, J. P., Emery, B., Tohyama, K., et al. (2014). Motor skill learning requires active central myelination. Science 346, 318–322. doi: 10.1126/science.1254960
Mei, F., Wang, H. K., Liu, S. B., Niu, J. Q., Wang, L. Y., He, Y. T., et al. (2013). Stage-Specific deletion of olig2 conveys opposing functions on differentiation and maturation of oligodendrocytes. J. Neurosci. 33, 8454–8462. doi: 10.1523/Jneurosci.2453-12.2013
Michaelis, T., Boretius, S., and Frahm, J. (2009). Localized proton MRS of animal brain in vivo: models of human disorders. Progr. Nuclear Magn. Reson. Spectrosc. 55, 1–34. doi: 10.1016/j.pnmrs.2008.11.001
Miller, D. J., Duka, T., Stimpson, C. D., Schapiro, S. J., Baze, W. B., McArthur, M. J., et al. (2012). Prolonged myelination in human neocortical evolution. Proc. Natl. Acad. Sci. U.S.A. 109, 16480–16485. doi: 10.1073/pnas.1117943109
Modi, S., Rana, P., Kaur, P., Rani, N., and Khushu, S. (2014). Glutamate level in anterior cingulate predicts anxiety in healthy humans: a magnetic resonance spectroscopy study. Psychiatry Res. 224, 34–41. doi: 10.1016/j.pscychresns.2014.03.001
Nave, K. A., and Ehrenreich, H. (2014). Myelination and Oligodendrocyte functions in psychiatric diseases. JAMA Psychiatry 71, 582–584. doi: 10.1001/jamapsychiatry.2014.189
Paus, T., Keshavan, M., and Giedd, J. N. (2008). OPINION why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 9, 947–957. doi: 10.1038/nrn2513
Penzes, P., Cahill, M. E., Jones, K. A., VanLeeuwen, J. E., and Woolfrey, K. M. (2011). Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 14, 285–293. doi: 10.1038/nn.2741
Phan, K. L., Fitzgerald, D. A., Cortese, B. M., Seraji-Bozorgzad, N., Tancer, M. E., and Moore, G. J. (2005). Anterior cingulate neurochemistry in social anxiety disorder: 1H-MRS at 4 Tesla. Neuroreport 16, 183–186. doi: 10.1097/00001756-200502080-00024
Provencher, S. W. (1993). Estimation of metabolite concentrations from localized in-vivo proton nmr-spectra. Magn. Reson. Med. 30, 672–679. doi: 10.1002/mrm.1910300604
Roy, K., Murtie, J. C., El Khodort, B. F., Edgar, N., Sardi, S. P., Hooks, B. M., et al. (2007). Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl. Acad. Sci. U.S.A. 104, 8131–8136. doi: 10.1073/pnas.0702157104
Soares, D. P., and Law, M. (2009). Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin. Radiol. 64, 12–21. doi: 10.1016/j.crad.2008.07.002
Stagg, C. J., Bestmann, S., Constantinescu, A. O., Moreno, L. M., Allman, C., Mekle, R., et al. (2011). Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. Lond. 589, 5845–5855. doi: 10.1113/jphysiol.2011.216978
Stagg, C. J., Wylezinska, M., Matthews, P. M., Johansen-Berg, H., Jezzard, P., Rothwell, J. C., et al. (2009). Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J. Neurophysiol. 101, 2872–2877. doi: 10.1152/jn.91060.2008
Takanashi, J., Nitta, N., Iwasaki, N., Saito, S., Tanaka, R., Barkovich, A. J., et al. (2014). Neurochemistry in shiverer mouse depicted on MR spectroscopy. J. Magn. Reson. Imaging 39, 1550–1557. doi: 10.1002/jmri.24306
Takasaki, C., Yamasaki, M., Uchigashima, M., Konno, K., Yanagawa, Y., and Watanabe, M. (2010). Cytochemical and cytological properties of perineuronal oligodendrocytes in the mouse cortex. Eur. J. Neurosci. 32, 1326–1336. doi: 10.1111/j.1460-9568.2010.07377.x
Tau, G. Z., and Peterson, B. S. (2010). Normal development of brain circuits. Neuropsychopharmacology 35, 147–168. doi: 10.1038/npp.2009.115
Taylor, C. T., Hirshfeld-Becker, D. R., Ostacher, M. J., Chow, C. W., LeBeau, R. T., Pollack, M. H., et al. (2008). Anxiety is associated with impulsivity in bipolar disorder. J. Anxiety Disord. 22, 868–876. doi: 10.1016/j.janxdis.2007.09.001
Tomassy, G. S., Berger, D. R., Chen, H. H., Kasthuri, N., Hayworth, K. J., Vercelli, A., et al. (2014). Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324. doi: 10.1126/science.1249766
Urenjak, J., Williams, S. R., Gadian, D. G., and Noble, M. (1993). Proton nuclear-magnetic-resonance spectroscopy unambiguously identifies different neural cell-types. J. Neurosci. 13, 981–989.
Wahl, S. E., McLane, L. E., Bercury, K. K., Macklin, W. B., and Wood, T. L. (2014). Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. J. Neurosci. 34, 4453–4465. doi: 10.1523/JNEUROSCI.4311-13.2014
Wang, H. K., Li, C. R., Wang, H. Z., Mei, F., Liu, Z., Shen, H. Y., et al. (2013). Cuprizone-induced demyelination in mice: age-related vulnerability and exploratory behavior deficit. Neurosci. Bull. 29, 251–259. doi: 10.1007/s12264-013-1323–1321
Xu, P., Xu, H., Tang, X., Xu, L., Wang, Y., Guo, L., et al. (2014). Liver X receptor beta is essential for the differentiation of radial glial cells to oligodendrocytes in the dorsal cortex. Mol. Psychiatry 19, 947–957. doi: 10.1038/mp.2014.60
Yamashita, M., Sakakibara, Y., Hall, F. S., Numachi, Y., Yoshida, S., Kobayashi, H., et al. (2013). Impaired cliff avoidance reaction in dopamine transporter knockout mice. Psychopharmacology (Berl) 227, 741–749. doi: 10.1007/s00213-013-3009
Young, K. M., Psachoulia, K., Tripathi, R. B., Dunn, S. J., Cossell, L., Attwell, D., et al. (2013). Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885. doi: 10.1016/j.neuron.2013.01.006
Yue, T., Xian, K., Hurlock, E., Xin, M., Kernie, S. G., Parada, L. F., et al. (2006). A critical role for dorsal progenitors in cortical myelination. J. Neurosci. 26, 1275–1280. doi: 10.1523/Jneurosci.4717-05.2006
Zhou, Q., Wang, S. L., and Anderson, D. J. (2000). Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron 25, 331–343. doi: 10.1016/S0896-6273(00)80898-3
Keywords: oligodendrocyte, brain development, Olig2 knockout, cortical neurons, glutamate, anxiety behavior
Citation: Chen X, Zhang W, Li T, Guo Y, Tian Y, Wang F, Liu S, Shen H-Y, Feng Y and Xiao L (2015) Impairment of Oligodendroglia Maturation Leads to Aberrantly Increased Cortical Glutamate and Anxiety-Like Behaviors in Juvenile Mice. Front. Cell. Neurosci. 9:467. doi: 10.3389/fncel.2015.00467
Received: 25 September 2015; Accepted: 16 November 2015;
Published: 15 December 2015.
Edited by:
Johann Steiner, University of Magdeburg, GermanyReviewed by:
Daniel Martins-de-Souza, University of Campinas, BrazilAndrea Schmitt, Ludwig-Maximilians-University Munich, Germany
Copyright © 2015 Chen, Zhang, Li, Guo, Tian, Wang, Liu, Shen, Feng and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Xiao, eGlhb2xhbjM1QGhvdG1haWwuY29t; Yue Feng, eWZlbmdAZW1vcnkuZWR1
 Xianjun Chen
Xianjun Chen Weiguo Zhang2
Weiguo Zhang2 Hai-Ying Shen
Hai-Ying Shen Lan Xiao
Lan Xiao