- 1Department of Neuropsychiatry, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- 2Brain Research Unit, Innovation Center for Medical Redox Navigation, Kyushu University, Fukuoka, Japan
Microglia, glial cells with immunological functions, have been implicated in various neurological diseases and psychiatric disorders in rodent studies, and human postmortem and PET studies. However, the deeper molecular implications of living human microglia have not been clarified. Here, we introduce a novel translational research approach focusing on human microglia. We have recently developed a new technique for creating induced microglia-like (iMG) cells from human peripheral blood. Two cytokines, GM-CSF and IL-34, converted human monocytes into the iMG cells within 14 days, which show various microglial characterizations; expressing markers, forming a ramified morphology, and phagocytic activity with various cytokine releases. We have already confirmed the applicability of this technique by analyzing iMG cells from a patient of Nasu-Hakola disease (NHD; Ohgidani et al., 2014). We herein show possible applications of the iMG cells in translational research. We believe that this iMG technique will open the door to explore various unknown dynamic aspects of human microglia in psychiatric disorders. This also opens new routes for psychopharmacological approach such as drug efficacy screening and personalized medicine.
Recently, the roles of microglia, immune cells in the brain, have been highlighted not only by neuroscientists but also by a variety of clinical researchers, especially in the field of neurology and psychiatry (Hughes, 2012). The pathophysiology of microglia has been suggested in various neuronal diseases and psychiatric disorders by human postmortem and positron emission tomography (PET) studies (Steiner et al., 2008; van Berckel et al., 2008; Takano et al., 2010; Gupta et al., 2014). Furthermore, microglial modulation has been proposed as an intervention in brain diseases including psychiatric disorders by recent clinical trials using minocycline, an antibiotic with microglial inhibitory effects (Miyaoka et al., 2008; Levkovitz et al., 2010; Chaudhry et al., 2012; Hayakawa et al., 2014). It has also been suggested that minocycline acts to modulate social interactions not only in psychiatric patients but also in healthy volunteers (Kato et al., 2012; Watabe et al., 2012, 2013). On the other hand, various psychotropic drugs have been revealed to inhibit microglial over-activation in in vitro experiments using rodent cells (Kato et al., 2007, 2008, 2011a,b; Bian et al., 2008; Seki et al., 2013).
The above reports have strongly suggested that maladaptive microglial activation may play a crucial role in various brain disorders (Block et al., 2007; Hanisch and Kettenmann, 2007; Kato et al., 2013). However, the deeper dynamic molecular actions of living human microglia in real patients have not been well clarified due to technical and ethical issues (such as difficulties involved in human brain biopsies). Until now, almost all human microglia research has been conducted using the postmortem brain or PET. These approaches have revealed important roles for activated microglia in psychiatric patients such as schizophrenia and autism (Steiner et al., 2008; van Berckel et al., 2008; Takano et al., 2010; Suzuki et al., 2013; Gupta et al., 2014). However, using these methods, it is difficult to ascertain the molecular pathological mechanisms such as the signaling pathway and gene expression pattern. On the other hand, animal (rodent) studies with cytological and histological analysis can reveal the various deeper dynamic actions of microglia at molecular levels (Nimmerjahn et al., 2005; Wake et al., 2009; Kettenmann et al., 2011). In fact, microglial activation has been shown in a variety of cytological and histological analyses using rodent models of brain diseases (Wu et al., 2002; Yoshiyama et al., 2007). Molecular biological analysis using rodent primary cultures and cell strains has been the standard method until now (Kato et al., 2007, 2008, 2011a,b; Bian et al., 2008; Seki et al., 2013; Mizoguchi et al., 2014a,b). In fact, rodent studies have been very vital in microglial research, however the question remains- how much does human pathology reflect in rodents? Can schizophrenia model mice have delusions and/or hallucinations? Even though a variety of rodent models of psychiatric disorders exist, it is extremely hard to validate deeper psychopathologies in rodents.
Thus, human studies using living brain cells derived from psychiatric patients have been warranted to evaluate the interactions of microglial activities and deeper psychopathology including psychiatric symptoms. A novel term “cellular model” has emerged in addition to “animal model” in the research of various physical diseases. The technology to obtain human neuronal cells from non-brain tissues (e.g., skin) by novel reproductive techniques such as induced pluripotent stem (iPS) cells (Takahashi et al., 2007) and directly induced neuronal (iN) cells (Pang et al., 2011; Liu et al., 2013) has emerged, and these tools have just recently been applied to psychiatric research (Kim, 2010; Brennand et al., 2011; Wen et al., 2014). Further, iPS technology can produce glial cells such as astrocytes (Juopperi et al., 2012) and progenitor of oligodendrocytes (Wang et al., 2013). However, to our knowledge, there exist no reports on the production of microglia by iPS technology. Very recently, we have developed a novel technique to create induced microglia-like cells (iMG) from human peripheral blood (Ohgidani et al., 2014).
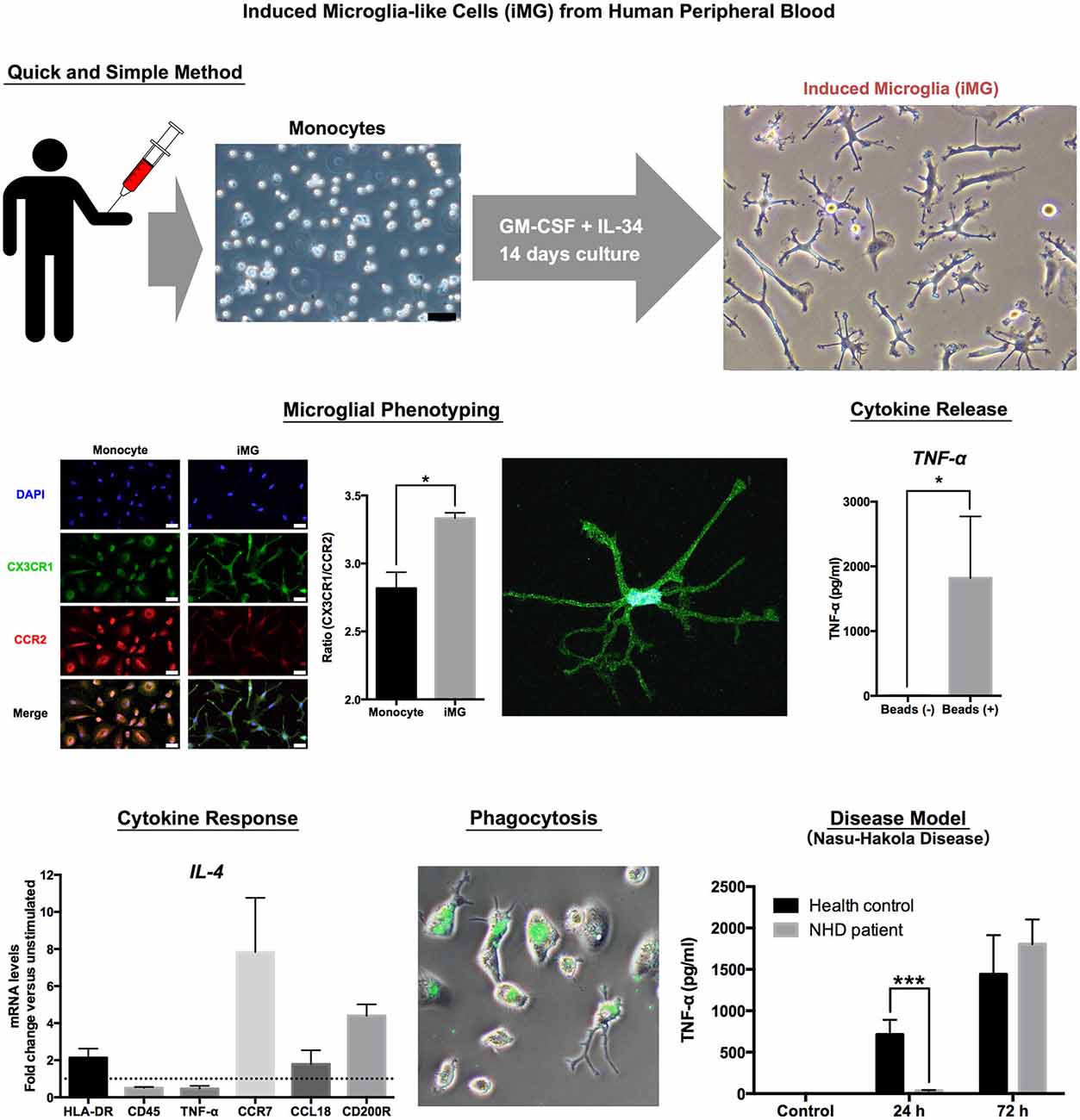
The brain is mostly composed of ectomorphic cells such as neurons, astrocytes and oligodendrocytes. Microglia is the only mesomorphic cell in the brain (Kettenmann et al., 2011). Therefore, we tried to induce microglia-like cells from peripheral monocytes, which have the same origin as mesomorphic cells. To determine what cytokines can induce iMG cells from human peripheral monocytes, we tested the effects of cytokines. Surprisingly, the cocktail of both granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL) −34 successfully induced small soma bodies bearing numerous branched collaterals, which expressed the specific morphology of ramified microglia-small soma with extensive radial ramifications. The iMG cells express the essential characteristics of human microglia such as surface markers and drug responses, phagocytosis and cytokine production (Figure 1).
We first utilized the iMG cells as a “cellular disease model” focusing on one of the most famous primary microglia-oriented diseases, the Nasu-Hakola disease (NHD; Hakola, 1972; Nasu et al., 1973). NHD is a very rare autosomal recessive disease, and the responsible two genes, which are expressed in microglia in the brain, are DNAX-activation protein of 12 kDA (DAP12) and triggering receptor expressed on myeloid cells 2 (TREM2; Paloneva et al., 2000, 2002). The deeper pathophysiological roles of microglia have not been well understood. Thus, we investigated the pathophysiology of NHD using the iMG technique. In agreement with genetic diagnosis, the iMG cells from a NHD patient (141delG in DAP12 gene) showed significantly lower expression of DAP12 than those from a healthy control (HC). Interestingly, the response of producing pro-inflammatory cytokines (TNF-α and IL-6) was delayed in the iMG cells from the NHD patient as compared to those from HC. Furthermore, we have also confirmed the delayed cytokine productions in the NHD model of iMG cells which was prepared by siRNA (Ohgidani et al., 2014). These novel findings may help to understand the hitherto unknown pathophysiological aspects of NHD.
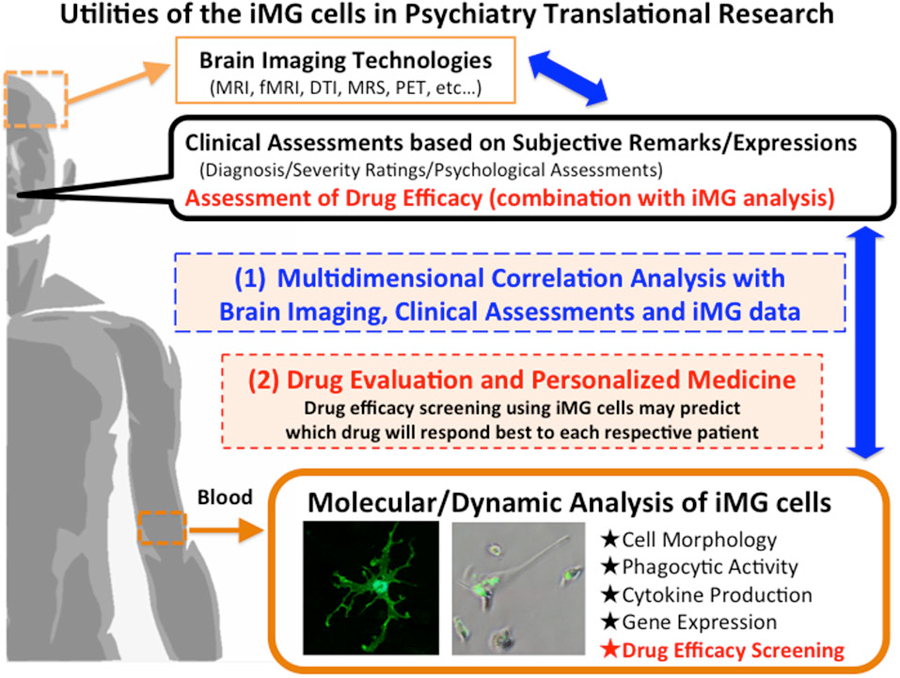
In this way, we believe that the iMG technique will enable the clarification of novel pathophysiological dysfunctions of human microglia as a translational research tool in various brain diseases including psychiatric disorders. We believe that the iMG technique will enable the exploration and development of psychiatric research especially to the following areas;
Multidimensional Correlation Analysis with Clinical Data, Brain Imaging Data and iMG
By combining clinical data, brain imaging data, and the iMG data from the same patient will be able to clarify the dynamic interaction between a specific psychopathology and a specific microglial activation (Figure 2-(1)). For example, the aberration of TREM, which is expressed in microglia, has recently been observed in psychiatric disorders such as bipolar disorder (Weigelt et al., 2011) and Alzheimer’s disease (Jonsson et al., 2013). Analyzing the TREM aberration of iMG cells from psychiatric patients can help to clarify the main role of TREM in psychopathology, which in turn may assist in the psychiatric evaluation of diagnosis and severity.
Drug Evaluation and Personalized Medicine
We previously reported the neuroprotective effects of psychotropic drugs via suppressing microglial activation using rodent microglial cells (Kato et al., 2007, 2008, 2011a,b; Bian et al., 2008; Seki et al., 2013). The iMG technique may help to predict drug responses before treating patients. Drug efficacy screening using iMG cells can predict which drug will respond best to each respective patient, and the technique may be applied as a companion diagnostic tool, which has raised expectations for the application of “order-made” medicine with a reduction in side effects and a shortening of treatment period (Figure 2-(2)).
Conclusion
We introduced a novel translational research approach focusing on human microglia-like cells using the iMG technique. Before reaching a conclusion, we need to mention a recent considerable discussion regarding functional differences between yolk sac derived microglia and monocyte derived microglia (Ginhoux et al., 2010; Katsumoto et al., 2014). We should keep it in our mind that the iMG cells are originated from monocytes, which may be different from the functions of yolk sac derived microglia. In addition, because IL-34 is a tissue-restricted ligand of CSF1R and this cytokine is associated with the development of other types of monocyte-derived cells such as Langerhans cells and possibly dendritic cells (Wang et al., 2012), which should also be considered. Despite these propositions, we believe that the iMG technique, at least to some extent, can analyze human microglial pathology in a living state, which had been impossible until recently. We hope that this translational method will open the door to explore various unknown dynamic aspects of human microglia in brain diseases, especially psychiatric disorders. This opens new routes for not only understanding the psychopathological mechanism of psychiatric disorders but also psychopharmacological approach such as drug efficacy screening and personalized medicine.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by the Japanese Ministry of Education, Culture, Sports, Science, and Technology KAKENHI (Grant-in-Aid 26713039 for Young Scientists (A) to TAK, Grant-in-Aid 26860933 for Young Scientists (B) to MO, and Grant-in-Aid 25117011 for Scientific Research on Innovative Areas [Glia assembly] to SK); Japan Agency for Medical Research and Development (AMED)-The Japanese Ministry of Health, Labour and Welfare (H27 - Seishin-Syogai Taisaku-Jigyo to SK); Takeda Science Foundation (to TAK); and SENSHIN Medical Research Foundation (to TAK, MO, and SK).
References
Bian, Q., Kato, T., Monji, A., Hashioka, S., Mizoguchi, Y., Horikawa, H., et al. (2008). The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 42–48. doi: 10.1016/j.pnpbp.2007.06.031
Block, M. L., Zecca, L., and Hong, J. S. (2007). Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69. doi: 10.1038/nrn2038
Brennand, K. J., Simone, A., Jou, J., Gelboin-Burkhart, C., Tran, N., Sangar, S., et al. (2011). Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225. doi: 10.1038/nature09915
Chaudhry, I. B., Hallak, J., Husain, N., Minhas, F., Stirling, J., Richardson, P., et al. (2012). Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J. Psychopharmacol. 26, 1185–1193. doi: 10.1177/0269881112444941
Ginhoux, F., Greter, M., Leboeuf, M., Nandi, S., See, P., Gokhan, S., et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. doi: 10.1126/science.1194637
Gupta, S., Ellis, S. E., Ashar, F. N., Moes, A., Bader, J. S., Zhan, J. N., et al. (2014). Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 5:5748. doi: 10.1038/ncomms6748
Hakola, H. P. A. (1972). Neuropsychiatric and genetic aspects of a new hereditary disease characterized by progressive dementia and lipomembranous polycystic osteodysplasia. Acta Psychiat. Scand. Suppl. 232, 1–173.
Hanisch, U. K., and Kettenmann, H. (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394. doi: 10.1038/nn1997
Hayakawa, K., Kato, T. A., Kohjiro, M., Monji, A., and Kanba, S. (2014). Minocycline, a microglial inhibitor, diminishes terminal patients’ delirium? Am. J. Geriatr. Psychiatry 22, 314–315. doi: 10.1016/j.jagp.2013.11.003
Jonsson, T., Stefansson, H., Steinberg, S., Jonsdottir, I., Jonsson, P. V., Snaedal, J., et al. (2013). Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116. doi: 10.1056/NEJMoa1211103
Juopperi, T. A., Kim, W. R., Chiang, C. H., Yu, H. M., Margolis, R. L., Ross, C. A., et al. (2012). Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol. Brain 5:17. doi: 10.1186/1756-6606-5-17
Kato, T., Mizoguchi, Y., Monji, A., Horikawa, H., Suzuki, S. O., Seki, Y., et al. (2008). Inhibitory effects of aripiprazole on interferon-gamma-induced microglial activation via intracellular Ca2+ regulation in vitro. J. Neurochem. 106, 815–825. doi: 10.1111/j.1471-4159.2008.05435.x
Kato, T., Monji, A., Hashioka, S., and Kanba, S. (2007). Risperidone significantly inhibits interferon-gamma-induced microglial activation in vitro. Schizophr. Res. 92, 108–115. doi: 10.1016/j.schres.2007.01.019
Kato, T. A., Monji, A., Mizoguchi, Y., Hashioka, S., Horikawa, H., Seki, Y., et al. (2011a). Anti-inflammatory properties of antipsychotics via microglia modulations: are antipsychotics a ‘fire extinguisher’ in the brain of schizophrenia? Mini Rev. Med. Chem. 11, 565–574. doi: 10.2174/138955711795906941
Kato, T. A., Monji, A., Yasukawa, K., Mizoguchi, Y., Horikawa, H., Seki, Y., et al. (2011b). Aripiprazole inhibits superoxide generation from phorbol-myristate-acetate (PMA)-stimulated microglia in vitro: implication for antioxidative psychotropic actions via microglia. Schizophr. Res. 129, 172–182. doi: 10.1016/j.schres.2011.03.019
Kato, T. A., Watabe, M., Tsuboi, S., Ishikawa, K., Hashiya, K., Monji, A., et al. (2012). Minocycline modulates human social decision-making: possible impact of microglia on personality-oriented social behaviors. PLoS One 7:e40461. doi: 10.1371/journal.pone.0040461
Kato, T. A., Yamauchi, Y., Horikawa, H., Monji, A., Mizoguchi, Y., Seki, Y., et al. (2013). Neurotransmitters, psychotropic drugs and microglia: clinical implications for psychiatry. Curr. Med. Chem. 20, 331–344. doi: 10.2174/0929867311320030003
Katsumoto, A., Lu, H., Miranda, A. S., and Ransohoff, R. M. (2014). Ontogeny and functions of central nervous system macrophages. J. Immunol. 193, 2615–2621. doi: 10.4049/jimmunol.1400716
Kettenmann, H., Hanisch, U. K., Noda, M., and Verkhratsky, A. (2011). Physiology of microglia. Physiol. Rev. 91, 461–553. doi: 10.1152/physrev.00011.2010
Kim, K. S. (2010). Induced pluripotent stem (iPS) cells and their future in psychiatry. Neuropsychopharmacology 35, 346–348. doi: 10.1038/npp.2009.108
Levkovitz, Y., Mendlovich, S., Riwkes, S., Braw, Y., Levkovitch-Verbin, H., Gal, G., et al. (2010). A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry 71, 138–149. doi: 10.4088/JCP.08m04666yel
Liu, M. L., Zang, T., Zou, Y. H., Chang, J. C., Gibson, J. R., Huber, K. M., et al. (2013). Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat. Commun. 4:2183. doi: 10.1038/ncomms3183
Miyaoka, T., Yasukawa, R., Yasuda, H., Hayashida, M., Inagaki, T., and Horiguchi, J. (2008). Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clin. Neuropharmacol. 31, 287–292. doi: 10.1097/WNF.0b013e3181593d45
Mizoguchi, Y., Kato, T. A., Horikawa, H., and Monji, A. (2014a). Microglial intracellular Ca2+ signaling as a target of antipsychotic actions for the treatment of schizophrenia. Front. Cell. Neurosci. 8:370. doi: 10.3389/fncel.2014.00370
Mizoguchi, Y., Kato, T. A., Seki, Y., Ohgidani, M., Sagata, N., Horikawa, H., et al. (2014b). Brain-derived neurotrophic factor (BDNF) induces sustained intracellular Ca2+ elevation through the up-regulation of surface transient receptor potential 3 (TRPC3) channels in rodent microglia. J. Biol. Chem. 289, 18549–18555. doi: 10.1074/jbc.M114.555334
Nasu, T., Tsukahar, Y., and Terayama, K. (1973). A lipid metabolic disease-“membranous lipodystrophy”-an autopsy case demonstrating numerous peculiar membrane-structures composed of compound lipid in bone and bone marrow and various adipose tissues. Acta Pathol. Jpn. 23, 539–558. doi: 10.1111/j.1440-1827.1973.tb01223.x
Nimmerjahn, A., Kirchhoff, F., and Helmchen, F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. doi: 10.1126/science.1110647
Ohgidani, M., Kato, T. A., Setoyama, D., Sagata, N., Hashimoto, R., Shigenobu, K., et al. (2014). Direct induction of ramified microglia-like cells from human monocytes: dynamic microglial dysfunction in Nasu-Hakola disease. Sci. Rep. 4:4957. doi: 10.1038/srep04957
Paloneva, J., Kestilä, M., Wu, J., Salminen, A., Bohling, T., Ruotsalainen, V., et al. (2000). Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 25, 357–361. doi: 10.1038/77153
Paloneva, J., Manninen, T., Christman, G., Hovanes, K., Mandelin, J., Adolfsson, R., et al. (2002). Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 71, 656–662. doi: 10.1086/342259
Pang, Z. P. P., Yang, N., Vierbuchen, T., Ostermeier, A., Fuentes, D. R., Yang, T. Q., et al. (2011). Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223. doi: 10.1038/nature10202
Seki, Y., Kato, T. A., Monji, A., Mizoguchi, Y., Horikawa, H., Sato-Kasai, M., et al. (2013). Pretreatment of aripiprazole and minocycline, but not haloperidol, suppresses oligodendrocyte damage from interferon-gamma-stimulated microglia in co-culture model. Schizophr. Res. 151, 20–28. doi: 10.1016/j.schres.2013.09.011
Steiner, J., Bielau, H., Brisch, R., Danos, P., Ullrich, O., Mawrin, C., et al. (2008). Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 42, 151–157. doi: 10.1016/j.jpsychires.2006.10.013
Suzuki, K., Sugihara, G., Ouchi, Y., Nakamura, K., Futatsubashi, M., Takebayashi, K., et al. (2013). Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 70, 49–58. doi: 10.1001/jamapsychiatry.2013.272
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. doi: 10.1016/j.cell.2007.11.019
Takano, A., Arakawa, R., Ito, H., Tateno, A., Takahashi, H., Matsumoto, R., et al. (2010). Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [C-11]DAA1106. Int. J. Neuropsychopharmacol. 13, 943–950. doi: 10.1017/s1461145710000313
van Berckel, B. N., Bossong, M. G., Boellaard, R., Kloet, R., Schuitemaker, A., Caspers, E., et al. (2008). Microglia activation in recent-onset schizophrenia: a quantitative (R)-[C-11]PK11195 positron emission tomography study. Biol. Psychiatry 64, 820–822. doi: 10.1016/j.biopsych.2008.04.025
Wake, H., Moorhouse, A. J., Jinno, S., Kohsaka, S., and Nabekura, J. (2009). Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009
Wang, S., Bates, J., Li, X. J., Schanz, S., Chandler-Militello, D., Levine, C., et al. (2013). Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12, 252–264. doi: 10.1016/j.stem.2012.12.002
Wang, Y., Szretter, K. J., Vermi, W., Gilfillan, S., Rossini, C., Cella, M., et al. (2012). IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13, 753–760. doi: 10.1038/ni.2360
Watabe, M., Kato, T. A., Monji, A., Horikawa, H., and Kanba, S. (2012). Does minocycline, an antibiotic with inhibitory effects on microglial activation, sharpen a sense of trust in social interaction? Psychopharmacology (Berl) 220, 551–557. doi: 10.1007/s00213-011-2509-8
Watabe, M., Kato, T. A., Tsuboi, S., Ishikawa, K., Hashiya, K., Monji, A., et al. (2013). Minocycline, a microglial inhibitor, reduces ‘honey trap’ risk in human economic exchange. Sci. Rep. 3:1685. doi: 10.1038/srep01685
Weigelt, K., Carvalho, L. A., Drexhage, R. C., Wijkhuijs, A., de Wit, H., van Beveren, N. J. M., et al. (2011). TREM-1 and DAP12 expression in monocytes of patients with severe psychiatric disorders. EGR3, ATF3 and PU.1 as important transcription factors. Brain Behav. Immun. 25, 1162–1169. doi: 10.1016/j.bbi.2011.03.006
Wen, Z. X., Nguyen, H. N., Guo, Z. Y., Lalli, M. A., Wang, X. Y., Su, Y. J., et al. (2014). Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 515, 414–418. doi: 10.1038/nature13716
Wu, D. C., Jackson-Lewis, V., Vila, M., Tieu, K., Teismann, P., Vadseth, C., et al. (2002). Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 22, 1763–1771.
Keywords: microglia, regenerative medicine, translational research, psychiatric disorders, schizophrenia, mood disorders, GM-CSF, IL-34
Citation: Ohgidani M, Kato TA and Kanba S (2015) Introducing directly induced microglia-like (iMG) cells from fresh human monocytes: a novel translational research tool for psychiatric disorders. Front. Cell. Neurosci. 9:184. doi: 10.3389/fncel.2015.00184
Received: 24 March 2015; Accepted: 27 April 2015;
Published online: 27 May 2015.
Edited by:
Thomas Knöpfel, Imperial College London, UKReviewed by:
Hiroshi Kiyama, Nagoya University, Graduate School of Medicine, JapanYing Wang, City of Hope National Medical Center, USA
Alexej Verkhratsky, University of Manchester, UK
Copyright © 2015 Ohgidani, Kato and Kanba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takahiro A. Kato, Department of Neuropsychiatry, Graduate School of Medical Sciences, Kyushu University and Brain Research Unit, Innovation Center for Medical Redox Navigation, Kyushu University, 3-1-1 Maidashi Higashi-ku, Fukuoka 812-8582, Japan,dGFrYWhpcm9AbnBzeWNoLm1lZC5reXVzaHUtdS5hYy5qcA==
 Masahiro Ohgidani
Masahiro Ohgidani Takahiro A. Kato
Takahiro A. Kato Shigenobu Kanba1
Shigenobu Kanba1