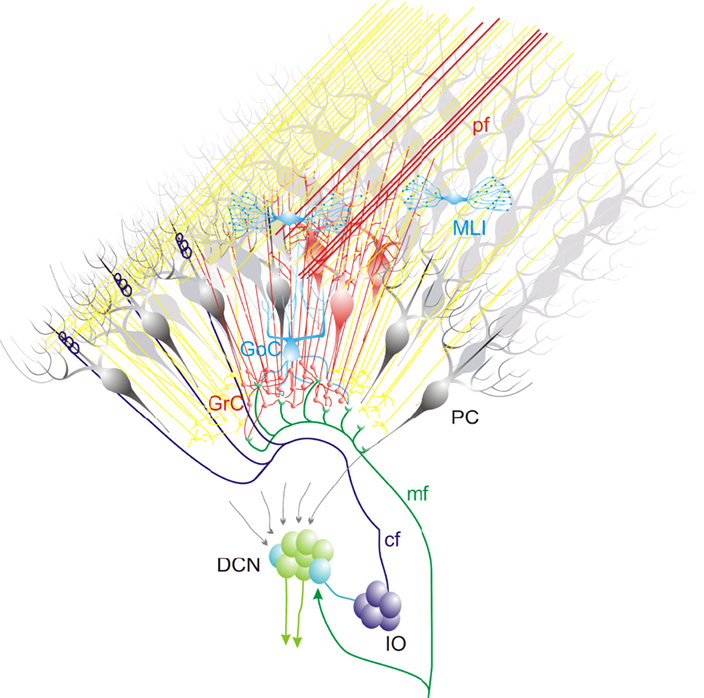
This schematic drawing shows the most relevant connections within a cerebellar module. The mossy fibers contact granule cells (GrC) and deep cerebellar nuclei (DCN) cells which, in turn, receive inhibition from the same common set of Purkinje cells (PC). Moreover, the interior olive (IO) cells emit climbing fibers that contact DCN cells and Purkinje cells (PC), which also project to the same DCN cells. An activate group of GrCs is in (red), while others (yellow) are laterally inhibited by the GoCs. The active GrCs excite the overlaying PCs (dark red) according to a vertical organization pattern (Bower and Woolston, 1983). The PCs inhibit DCN neurons which in turn inhibit the IO neurons. Note that, within a cerebellar module, different circuit elements communicate in closed loops. The mossy fibers contact granule cells and DCN cells which, in turn, receive inhibition from the same common set of Purkinje cells. Moreover, the IO cells emit climbing fibers that contact DCN and PC, which also project to the same DCN cells.
The cerebellum has traditionally provided an ideal case for investigating the relationship between cellular neurophysiology and circuit functions, because of the limited number of neuronal types and the regular organization of its internal network (Figure 1). The Motor Learning Theory (Marr, 1969; Albus, 1971), which proposed the first computational model of cerebellar function, was inspired by morphological determinations of the number of neurons and synapses but accounted for only very limited knowledge on functional properties of the cerebellar circuitry. In recent years, in association with remarkable developments of physiological technologies, important achievements at the cellular level have suggested that the original view needs to be revisited (Rokni et al., 2008). The papers in this special issue are focused on the relationship between cellular properties and circuit responses, which hold the key to control spike timing and long-term synaptic plasticity (Hansel et al., 2001; De Zeeuw and Yeo, 2005; D’Angelo and De Zeeuw, 2009; D’Angelo et al., 2009) and eventually cerebellar functioning.
In the cerebellum, inputs are conveyed through a double system formed by the mossy fibers and the climbing fibers. These inputs converge onto Purkinje cells, which eventually inhibit the DCN, representing the sole output of the circuit (Figure 1). Despite the wealth of available information, outstanding issues remain open about the spatial organization of granular layer activity, the discharge of Purkinje cells and deep cerebellar neurons, the mechanisms of circuit inhibition, the forms of long-term synaptic plasticity and their relationship with behavior. These aspects are covered by the papers in this special issue combining a careful literature review with significant original data.
Signals coming into the cerebellum through the mossy fibers are first processed in the granular layer network. The mossy fibers show complex firing patterns, ranging from frequency-modulated discharges to short bursts (van Kan et al., 1993; Chadderton et al., 2004; Rancz et al., 2007; Arenz et al., 2008; Prsa et al., 2009). With the intervention of the inhibitory circuits and synaptic plasticity, mossy fiber activity is transformed into new spatio-temporally organized spike sequences for further processing in Purkinje cells (Mitchell and Silver, 2003; Nieus et al., 2006; Mapelli and D’Angelo, 2007; D’Angelo, 2008; D’Angelo et al., 2009). Then, granule cell spikes propagate through the ascending axon and along the parallel fibers. Despite the wealth of information on single cell properties, the spatio-temporal organization of activity in the granular layer network remains largely to be determined.
In this special issue, it is shown that afferent mossy fiber signals are differentially filtered and amplified depending on the intensity of local inhibition and on several receptor- and channel-dependent properties (Mapelli and D’Angelo, 2007; Mapelli et al., 2010a,b). These results lend support to the emerging concept that the granular layer performs complex transformations on the mossy fiber input by generating new spatio-temporal pattern with the aid of local circuitry and synaptic plasticity. These temporal patterns are likely to integrate with repetitive and coherent activity enhancing responses in the theta band (Pellerin and Lamarre, 1997; Hartmann and Bower, 1998; Courtemanche et al., 2009). These observations are integrated through the first realistic large-scale computational reconstruction of the granular layer providing a direct link between molecular, cellular, and network properties in the cerebellar network (Solinas et al., 2010).
These papers also contribute to sheds light on the mechanisms of transmission from granular layer to Purkinje cells and molecular layer interneurons. This process has been the object of debate, in which evidence for spots-like or beam-like activation has been contrasted (Rokni et al., 2007). Here, it is suggested that native bursts are amplified along the vertical transmission line, thereby generating activity spots (Mapelli et al., 2010b). Then, the effect of bursts along the parallel fibers is filtered, probably through molecular layer interneurons (Bower, 2010), generating weaker and frequency-independent responses. This effect was proposed to explain the “spot vs. stripe” controversy, since spots would easily emerge following burst transmission generated following punctuate stimulation.
The Purkinje cells receive inputs both from parallel fibers and climbing fibers originating from the inferior olive. The inferior olive itself is an oscillator (Llinas and Yarom, 1981a,b; Chorev et al., 2007; Khosrovani et al., 2007; Van Der Giessen et al., 2008), which can produce theta-frequency patterns influencing Purkinje cells and inhibitory interneurons of the molecular layer (Barmack and Yakhnitsa, 2008). Although much less numerous than parallel fibers, the climbing fibers exert a powerful effect on the Purkinje cells eliciting the complex spikes. The complex spike has been variously interpreted as a signal carrying either an error or an instruction for generating synaptic plasticity at the parallel fiber – Purkinje cell synapse (Ito and Kano, 1982; Ito et al., 1982). Moreover, it has recently been demonstrated that both the climbing fiber and the parallel fiber inputs may influence the bistable transition of Purkinje cells between UP and DOWN states (Loewenstein et al., 2005; Jacobson et al., 2008), at least in anesthetized animals (Schonewille et al., 2006). Both the mechanisms of olive activation and of climbing fiber control of plasticity are incompletely understood. Moreover, it is still debated which kind of coding is used by Purkinje cell and how molecular layer inhibition could control it.
In this special issue, the Purkinje cell processing mechanisms have been considered. Purkinje cells are spontaneously active and their discharge is modulated by the activity coming from the granular layer and the inferior olive. It was recently shown that the molecular layer can sustain synchronous gamma band (30–80 Hz) and high-frequency (100–200 Hz) oscillations entraining the Purkinje cells (de Solages et al., 2008; Middleton et al., 2008). Purkinje cells were proposed to act as perceptrons (Brunel et al., 2004) and to process spike pauses (Steuber et al., 2007), and may live in a bistable UP–DOWN state (Loewenstein et al., 2005). Here, it is proposed that, in Purkinje cells, regulation of firing precision seems a more creditable coding strategy than frequency modulation (Rokni et al., 2009). Moreover, superposition of UP/DOWN states can engage specific groups of Purkinje cells. Interestingly, the mossy fiber input can play a critical role in determining both the spike-to-spike variability and the UP/DOWN state of Purkinje cells, generating a complex blend of dynamics on multiple time-scales. Purkinje cell synchrony and the repercussion of PC firing on DCN neurons have been investigated in vivo (Jorntell and Ekerot, 2006; Baumel et al., 2009; Bengtsson and Jorntell, 2009) and novel information on PC functioning has been provided through the development of genetically expressed fluorescent proteins in these neurons (Akemann et al., 2009).
The cells of DCN consists of diverse neuronal populations with distinct integrative properties (Uusisaari et al., 2007) and generate the sole cerebellar output. Both mossy fibers and to a lesser extent climbing fibers make collateral connections on to neurons of the DCN. DCN neurons also inhibit the IO cells regulating their coupling. IN DCN cells, intrinsic dynamics generate silent pauses, and possibly rebound excitation, producing alternating phases of activity. The DCN, in addition to act as a “relay station” between cerebellar mossy fiber input and cerebellar output to premotor areas, either directly or via the cerebellar cortex may also act as the substrate of motor memory storage (Raymond et al., 1996; Aizenman et al., 1998; Aizenman and Linden, 2000; Ito, 2006). It has been hypothesized that the synchronous oscillations in the Purkinje cell activities together with plasticity at the mossy fiber – DCN and the Purkinje cell – DCN synapses form the main mechanistic tools to control the activity in the DCN output neurons (De Zeeuw et al., 2008). A critical issue is that the convergence of Purkinje cell inhibition on DCN neurons remains to be demonstrated. Here, the discharge of DCN neurons in vivo is considered under the effect of harmaline (Baumel et al., 2009). Harmaline induced a rhythmic firing pattern of short bursts on a quiescent background at about 8 Hz, while other neurons become quiescent for long periods (seconds to minutes). The major effect harmaline was carried indirectly by the inhibitory Purkinje cells (PCs) activated by the IO, so that the DCN response profile was probably determined by the number of concurrently active PCs, their firing rate and the level of synchrony occurring in their transitions between continuous firing and quiescence.
These papers suggest an extension of anatomical observations toward a dynamic view of the cerebellar circuit. Figure 1 shows that the entire olivo-cerebellar system is organized in modules (Voogd et al., 2003; Apps and Hawkes, 2009; Glickstein et al., 2009). The intricate set of connections makes sense under specific hypothesis for network dynamics. Once a MF bundle discharges, it activates a groups of granule cells, while others are laterally inhibited by the Golgi cells. The active granule cells could be coordinated into low-frequency oscillations (Courtemanche et al., 2009; Solinas et al., 2010) and excite the overlaying PCs according to a vertical organization pattern upon arrival of specific afferent signals (Bower and Woolston, 1983). The parallel fibers can also activate PCs on the low-frequency band due to the intense low-pass filtering caused by molecular layer interneurons (Santamaria et al., 2007; Mapelli et al., 2010b). Eventually, the PCs can entrain the DCN-IO-PC system into coherent low-frequency oscillations (Jacobson et al., 2008; Rokni et al., 2009). The hypothesis is that certain inhibitory interneurons on the DCN are inhibited, releasing electrical coupling groups of IO neurons and causing their coherent subthreshold oscillation. The oscillations generated by the IO along the sagittal axis (climbing fiber-mediated) may collide with that conveyed by the cerebral cortex and diffusing along the transverse axis (parallel fiber-mediated), causing local resonance at the intersection of the parallel fiber and climbing fiber signals. DCN neurons may finally transfer temporal patterns resulting from strong correlations in PCs state transitions, while largely ignoring the timing of simple spikes from individual PCs (Baumel et al., 2009). PC correlations occur also in high-frequency bands (Akemann et al., 2009; Bengtsson and Jorntell, 2009). The Purkinje cell synapses are sites of plasticity, including the renown parallel fiber – Purkinje cell LTD (Hansel et al., 2001). At odd with the original predictions of the motor learning theory, the rearrangement of the network during learning may critically involve reversible LTP/LTD both at parallel fiber – Purkinje cell synapses (Ohtsuki et al., 2009) and at mossy fiber – granule cells synapses (Solinas et al., 2010). The impact of plasticity on behavior is addressed by reviewing the effect of mutations in the pf-PC mechanisms on VOR and eye-blink conditioning reflex (Boele et al., 2010). Finally, the impact of cerebellar learning and computation at the system level is critically considered by comparing the feed-forward and feed-back controller hypothesis (Frens and Donchin, 2009).
References
Aizenman, C. D., and Linden, D. J. (2000). Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat. Neurosci. 3, 109–111.
Aizenman, C. D., Manis, P. B., and Linden, D. J. (1998). Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron 21, 827–835.
Akemann, W., Middleton, S. J., and Knopfel, T. (2009). Optical imaging as a link between cellular neurophysiology and circuit modeling. Front. Cell. Neurosci. 3:5. doi: 10.3389/neuro.03.005.2009.
Apps, R., and Hawkes, R. (2009). Cerebellar cortical organization: a one-map hypothesis. Nat. Rev. Neurosci. 10, 670–681.
Arenz, A., Silver, R. A., Schaefer, A. T., and Margrie, T. W. (2008). The contribution of single synapses to sensory representation in vivo. Science 321, 977–980.
Barmack, N. H., and Yakhnitsa, V. (2008). Functions of interneurons in mouse cerebellum. J. Neurosci. 28, 1140–1152.
Baumel, Y., Jacobson, G. A., and Cohen, D. (2009). Implications of functional anatomy on information processing in the deep cerebellar nuclei. Front. Cell. Neurosci. 3:14. doi: 10.3389/neuro.03.014.2009.
Bengtsson, F., and Jorntell, H. (2009). Climbing fiber coupling between adjacent purkinje cell dendrites in vivo. Front. Cell. Neurosci. 3:7. doi: 10.3389/neuro.03.007.2009.
Boele, H. J., Koekkoek, S. K., and De Zeeuw, C. I. (2010). Cerebellar and extracerebellar involvement in mouse eyeblink conditioning: the ACDC model. Front. Cell. Neurosci. 3:19. doi: 10.3389/neuro.03.019.2009.
Bower, J. M. (2010). Model-founded explorations of the roles of molecular layer inhibition in regulating purkinje cell responses in cerebellar cortex: more trouble for the beam hypothesis. Front. Cell Neurosci. 4: 27. doi: 10.3389/fncel.2010.00027.
Bower, J. M., and Woolston, D. C. (1983). Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J. Neurophysiol. 49, 745–766.
Brunel, N., Hakim, V., Isope, P., Nadal, J. P., and Barbour, B. (2004). Optimal information storage and the distribution of synaptic weights: perceptron versus Purkinje cell. Neuron 43, 745–757.
Chadderton, P., Margrie, T. W., and Hausser, M. (2004). Integration of quanta in cerebellar granule cells during sensory processing. Nature 428, 856–860.
Chorev, E., Yarom, Y., and Lampl, I. (2007). Rhythmic episodes of subthreshold membrane potential oscillations in the rat inferior olive nuclei in vivo. J. Neurosci. 27, 5043–5052.
Courtemanche, R., Chabaud, P., and Lamarre, Y. (2009). Synchronization in primate cerebellar granule cell layer local field potentials: basic anisotropy and dynamic changes during active expectancy. Front. Cell Neurosci. 3:6. doi: 10.3389/neuro.03.006.2009.
D’Angelo, E. (2008). The critical role of Golgi cells in regulating spatio-temporal integration and plasticity at the cerebellum input stage. Front. Neurosci 2, 35–46. doi: 10.3389/neuro.01.008.2008.
D’Angelo, E., and De Zeeuw, C. I. (2009). Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci. 32, 30–40.
D’Angelo, E., Koekkoek, S. K., Lombardo, P., Solinas, S., Ros, E., Garrido, J., Schonewille, M., and De Zeeuw, C. I. (2009). Timing in the cerebellum: oscillations and resonance in the granular layer. Neuroscience 162, 805–815.
de Solages, C., Szapiro, G., Brunel, N., Hakim, V., Isope, P., Buisseret, P., Rousseau, C., Barbour, B., and Lena, C. (2008). High-frequency organization and synchrony of activity in the purkinje cell layer of the cerebellum. Neuron 58, 775–788.
De Zeeuw, C. I., Hoebeek, F. E., and Schonewille, M. (2008). Causes and consequences of oscillations in the cerebellar cortex. Neuron 58, 655–658.
De Zeeuw, C. I., and Yeo, C. H. (2005). Time and tide in cerebellar memory formation. Curr. Opin. Neurobiol. 15, 667–674.
Frens, M. A., and Donchin, O. (2009). Forward models and state estimation in compensatory eye movements. Front. Cell. Neurosci. 3:13. doi: 10.3389/neuro.03.013.2009.
Glickstein, M., Sultan, F., and Voogd, J. (2009). Functional localization in the cerebellum. Cortex.
Hansel, C., Linden, D. J., and D’Angelo, E. (2001). Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 4, 467–475.
Hartmann, M. J., and Bower, J. M. (1998). Oscillatory activity in the cerebellar hemispheres of unrestrained rats. J. Neurophysiol. 80, 1598–1604.
Ito, M., and Kano, M. (1982). Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci. Lett. 33, 253–258.
Ito, M., Sakurai, M., and Tongroach, P. (1982). Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J. Physiol. 324, 113–134.
Jacobson, G. A., Rokni, D., and Yarom, Y. (2008). A model of the olivo-cerebellar system as a temporal pattern generator. Trends Neurosci. 31, 617–625.
Jorntell, H., and Ekerot, C. F. (2006). Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J. Neurosci. 26, 11786–11797.
Khosrovani, S., Van Der Giessen, R. S., De Zeeuw, C. I., and De Jeu, M. T. (2007). In vivo mouse inferior olive neurons exhibit heterogeneous subthreshold oscillations and spiking patterns. Proc. Natl. Acad. Sci. U.S.A. 104, 15911–15916.
Llinas, R., and Yarom, Y. (1981a). Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J. Physiol. 315, 549–567.
Llinas, R., and Yarom, Y. (1981b). Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J. Physiol. 315, 569–584.
Loewenstein, Y., Mahon, S., Chadderton, P., Kitamura, K., Sompolinsky, H., Yarom, Y., and Hausser, M. (2005). Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat. Neurosci. 8, 202–211.
Mapelli, J., and D’Angelo, E. (2007). The spatial organization of long-term synaptic plasticity at the input stage of cerebellum. J. Neurosci. 27, 1285–1296.
Mapelli, J., Gandolfi, D., and D’Angelo, E. (2010a). Combinatorial responses controlled by synaptic inhibition in the cerebellum granular layer. J. Neurophysiol. 103, 250–261.
Mapelli, J., Gandolfi, D., and D’Angelo, E. (2010b). High-pass filtering and dynamic gain regulation enhance vertical bursts transmission along the mossy fiber pathway of cerebellum. Front. Cell. Neurosci. 4:14. doi: 10.3389/fncel.2010.00014.
Middleton, S. J., Racca, C., Cunningham, M. O., Traub, R. D., Monyer, H., Knopfel, T., Schofield, I. S., Jenkins, A., and Whittington, M. A. (2008). High-frequency network oscillations in cerebellar cortex. Neuron 58, 763–774.
Mitchell, S. J., and Silver, R. A. (2003). Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38, 433–445.
Nieus, T., Sola, E., Mapelli, J., Saftenku, E., Rossi, P., and D’Angelo, E. (2006). LTP regulates burst initiation and frequency at mossy fiber-granule cell synapses of rat cerebellum: experimental observations and theoretical predictions. J. Neurophysiol. 95, 686–699.
Ohtsuki, G., Piochon, C., and Hansel, C. (2009). Climbing fiber signaling and cerebellar gain control. Front. Cell. Neurosci. 3:4. doi: 10.3389/neuro.03.004.2009.
Pellerin, J. P., and Lamarre, Y. (1997). Local field potential oscillations in primate cerebellar cortex during voluntary movement. J. Neurophysiol. 78, 3502–3507.
Prsa, M., Dash, S., Catz, N., Dicke, P. W., and Thier, P. (2009). Characteristics of responses of Golgi cells and mossy fibers to eye saccades and saccadic adaptation recorded from the posterior vermis of the cerebellum. J. Neurosci. 29, 250–262.
Rancz, E. A., Ishikawa, T., Duguid, I., Chadderton, P., Mahon, S., and Hausser, M. (2007). High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature 450, 1245–1248.
Raymond, J. L., Lisberger, S. G., and Mauk, M. D. (1996). The cerebellum: a neuronal learning machine? Science 272, 1126–1131.
Rokni, D., Llinas, R., and Yarom, Y. (2007). Stars and stripes in the cerebellar cortex: a voltage sensitive dye study. Front. Syst. Neurosci. 1:1. doi: 10.3389/neuro.06/001.2007.
Rokni, D., Llinas, R., and Yarom, Y. (2008). The morpho/functional discrepancy in the cerebellar cortex: looks alone are deceptive. Front. Neurosci. 2, 192–198. doi: 10.3389/neuro.01.036.2008.
Rokni, D., Tal, Z., Byk, H., and Yarom, Y. (2009). Regularity, variability and bi-stability in the activity of cerebellar purkinje cells. Front. Cell. Neurosci. 3:12. doi: 10.3389/neuro.03.012.2009.
Santamaria. F., Tripp, P. G., and Bower, J. M. (2007). Feedforward inhibition controls the spread of granule cell-induced Purkinje cell activity in the cerebellar cortex. J. Neurophysiol. 97, 248–263.
Schonewille, M., Khosrovani, S., Winkelman, B. H., Hoebeek, F. E., De Jeu, M. T., Larsen, I. M., Van der Burg, J., Schmolesky, M. T., Frens, M. A., and De Zeeuw, C. I. (2006). Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat. Neurosci. 9, 459–461; author reply 461.
Solinas, S., Nieus, T., and D’Angelo, E. (2010). A realistic large-scale model of the cerebellum granular layer predicts circuit spatio-temporal filtering properties. Front. Cell Neurosci. 4:12. doi: 10.3389/fncel.2010.00012.
Steuber, V., Mittmann, W., Hoebeek, F. E., Silver, R. A., De Zeeuw, C. I., Hausser, M., and De Schutter, E. (2007). Cerebellar LTD and pattern recognition by Purkinje cells. Neuron 54, 121–136.
Uusisaari, M., Obata, K., and Knopfel, T. (2007). Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J. Neurophysiol. 97, 901–911.
Van Der Giessen, R. S., Koekkoek, S. K., van Dorp, S., De Gruijl, J. R., Cupido, A., Khosrovani, S., Dortland, B., Wellershaus, K., Degen, J., Deuchars, J., Fuchs, E. C., Monyer, H., Willecke, K., De Jeu, M. T., and De Zeeuw, C. I. (2008). Role of olivary electrical coupling in cerebellar motor learning. Neuron 58, 599–612.
van Kan, P. L., Gibson, A. R., and Houk, J. C. (1993). Movement-related inputs to intermediate cerebellum of the monkey. J. Neurophysiol. 69, 74–94.
Voogd, J., Pardoe, J., Ruigrok, T. J., and Apps, R. (2003). The distribution of climbing and mossy fiber collateral branches from the copula pyramidis and the paramedian lobule: congruence of climbing fiber cortical zones and the pattern of zebrin banding within the rat cerebellum. J. Neurosci. 23, 4645–4656.
Citation: D‘angelo E (2010). Rebuilding cerebellar network computations from cellular neurophysiology. Front. Cell. Neurosci. 4:131. doi: 10.3389/fncel.2010.00131
Received: 24 September 2010;
Accepted: 27 September 2010;
Published online: 04 November 2010.
Copyright: © 2010 D‘angelo. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence:ZGFuZ2Vsb0B1bmlwdi5pdA==