
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurorobot., 18 February 2022
Volume 16 - 2022 | https://doi.org/10.3389/fnbot.2022.775724
This article is part of the Research TopicRehabilitation Robotics: Challenges in Design, Control, and Real ApplicationsView all 16 articles
 Soichiro Koyama1
Soichiro Koyama1 Shigeo Tanabe1
Shigeo Tanabe1 Takeshi Gotoh2
Takeshi Gotoh2 Yuta Taguchi2
Yuta Taguchi2 Masaki Katoh2
Masaki Katoh2 Eiichi Saitoh3
Eiichi Saitoh3 Yohei Otaka3
Yohei Otaka3 Satoshi Hirano3*
Satoshi Hirano3*Wearable robotic exoskeletons (WREs) have been developed from orthoses as assistive devices for gait reconstruction in patients with spinal cord injury. They can solve some problems encountered with orthoses, such as difficulty in independent walking and standing up and high energy consumption during walking. The Wearable Power-Assist Locomotor (WPAL), a WRE, was developed based on a knee–ankle–foot orthosis with a single medial hip joint. The WPAL has been updated seven times during the period from the beginning of its development, in 2005, to 2020. The latest version, launched as a commercialized model in 2016, is available for medical facilities. In this retrospective study, which included updated results from previous reports, all data were extracted from development research records from July 2007 to December 2020. The records were as follows: patient characteristics [the number of participants, injury level, and the American Spinal Injury Association Impairment Scale (AIS) score], the total number of WPAL trials when aggregating the cases with all the versions or only the latest version of the WPAL, and maximum walking performance (functional ambulation category [FAC], distance, and time of continuous walking). Thirty-one patients participated in the development research. The levels of spinal cord injury were cervical (C5–C8), upper thoracic (T3–T6), lower thoracic (T7–T12), and lumbar (L1) in 10, 5, 15, and 1 of the patients, respectively. The numbers of patients with AIS scores of A, B, C, and D were 20, 7, 4, and 0, respectively. The total number of WPAL trials was 1,785, of which 1,009 were used the latest version of the WPAL. Twenty of the patients achieved an FAC score of 4 after an average of 9 (median 8, range 2–22) WPAL trials. The continuous walking distance and time improved with the WPAL were compared to the orthosis. We confirmed that the WPAL improves walking independence in people with a wide range of spinal cord injuries, such as cervical spinal cord injuries. Further refinement of the WPAL will enable its long-term use at home.
Traumatic spinal cord injury (SCI) is one of the most devastating events that occur after various accidents. Long-term motor, sensory, and autonomic dysfunction caused by traumatic SCI have a tremendous effect on the daily life of patients and their families. The incidence rates of traumatic SCI per 1 million people per year were 14 cases in Austria (Majdan et al., 2016), 18 cases in Switzerland (Chamberlain et al., 2015), and 10 cases in Denmark (Bjornshave Noe et al., 2015), representing almost 180,000 cases annually worldwide in 2014 (Lee et al., 2014). Similarly, the annual incidence of SCI is approximately 54 cases per 1 million people in the United States, which is approximately 17,900 new SCI cases in 2021 (NSCIS Center, 2021). In Japan, the estimated incidence of traumatic SCI, excluding the grade E in the Frankel scale, that is, defined as no neurological deficit/complete recovery, was 49 cases per 1 million people annually in 2010 (Miyakoshi et al., 2020).
The majority of patients with motor-complete SCI often rely on a wheelchair as a mobility device in activities of daily living because a wheelchair is energy efficient and enables patients to safely perform their daily activities. However, long-term inactivity due to wheelchair use results in various medical problems [e.g., joint contraction (Kunkel et al., 1993), pressure sores (Verschueren et al., 2011), osteoporosis (Varacallo et al., 2021), and psychosocial problems as a result of a relatively low eye level (Dijkers, 1999; Levins et al., 2004)]. For patients with SCI, the opportunity to stand and walk occasionally is important from both physical and psychosocial perspectives.
In the past few decades, various wearable robotic exoskeletons (WREs) have been developed for stand and gait reconstruction in patients with motor-complete SCI. They offer the opportunity to walk in home and community environments by moving the paretic legs of patients with partial or complete SCI in a reciprocal stepping pattern (Fisahn et al., 2016; Miller et al., 2016; Palermo et al., 2017; Tan et al., 2021). Arazpour et al. (2013) reported that the gait, speed, and endurance of patients with SCI using WREs are superior to those of patients using either reciprocating gait or hip–knee–ankle–foot orthoses (Arazpour et al., 2013). The oxygen consumption and heart rate during gait training with WRE are slightly increased compared with that during sitting and standing, and the load during gait training with WRE is less than that with conventional orthoses (Asselin et al., 2015; Yatsuya et al., 2018).
We have previously reported the effects of the Wearable Power-Assist Locomotor (WPAL) on walking ability and gait pattern in patients with various levels of SCI. The first report shared the basic concept of WPAL development and a comparison of walking performance between the WPAL and the conventional Primewalk orthosis (Tanabe et al., 2013b). The report showed that the WPAL has a lower physiological cost index and involves less muscle activity in the upper limbs during walking, compared with the Primewalk orthosis (Tanabe et al., 2013b). We also reported gait pattern, the basic training procedure, and gait performance of the WPAL in seven patients with SCI at the thoracic level of injury (T6–T12) (Tanabe et al., 2013a). In addition, we found that continuous walking time and distance were prolonged with the WPAL compared with orthotic walking in 12 patients with SCI (Hirano et al., 2015). The WPAL significantly decreased the physiological cost index, heart rate, and modified Borg score during the 6-min walking test compared with conventional knee–ankle–foot orthoses in six other patients with SCI (Yatsuya et al., 2018). Recently, we have reported that the WPAL improves walking ability more than conventional orthoses in patients with cervical SCI (Fuse et al., 2019).
The objective of this study was to summarize all data that include updated results from previous reports, about physical characteristics, and walking abilities of patients with SCI, from the development research records in clinical practice from July 2007 to December 2020. The present findings are potentially useful for gait performance comparison with other robots, a meta-analysis of the effects of WREs in patients with SCI, and evidence-based selection of WREs for patients with SCI.
In this retrospective study, we included 31 patients with SCI in our university from 2007 to 2020, regardless of being inpatients or outpatients. The causes of spinal cord injury were traumatic spinal cord injury in 24 patients, spinal cord infarction in 3 patients, encephalomyelitis in 1 patient, radiation myelitis in 1 patient, hemorrhage from the thoracic spinal cord cavernous hemangioma in 1 patient, and acute thoracic spinal epidural hematomas in 1 patient. The principal inclusion criteria for participation were as follows: (1) patients with motor paralysis [American Spinal Injury Association Impairment Scale (AIS) classification A–C] who had a neurological level of injury from C3 to L1; (2) a height of 155–180 cm; (3) a weight <80 kg; and (4) sufficient upper limb muscle strength (to the extent that the patient can transfer independently). All patients provided written informed consent prior to study participation. This study was performed in accordance with the principles of the Declaration of Helsinki and conducted after receiving approval from the ethics committee of our university (approval number: CR18-035).
The exclusion criteria were as follows: (1) progressive disease (excluding disuse syndrome); (2) difficulty in communication due to dementia or impaired consciousness; (3) high risk of fracture of the lower limbs or spine (e.g., severe osteoporosis); (4) uncontrolled hypertension (resting systolic blood pressure >180 mmHg and diastolic blood pressure >120 mmHg); (5) uncontrolled tachycardia (ventricular rate >120 beats per min); and (6) limitation of movement due to impaired cardiac or respiratory function (e.g., shortness of breath when using a wheelchair).
A detailed basic design of the WPAL has been published previously (Tanabe et al., 2013a,b, 2017). The main structures, such as frames and motors, were placed between the lower limbs. The frame was connected by a single mechanical hip joint medially under the perineum. The mechanical hip joint had a sliding structure that curves anterior-posteriorly based on the structure of Primewalk orthosis (Suzuki et al., 2005). The sliding structure enables the virtual center of rotation of the robotic hip joint to be closer to the physiological center of the hip joint. Six motors were located in the hip, knee, and ankle joints. The ranges of motion were as follows: hip, 40° (flexion 25°-extension 15°); knee, 120° (flexion 120°-extension 0°); and ankle, 50° (dorsiflexion 35°-plantar flexion 15°). Each joint used an individual custom-made brushless DC servomotor, which was compact to fit between the legs (24 V, 78 W, peak torque of 4 Nm, speed range from 0 to 1,000 deg/s). The weight of the WPAL was approximately 13 kg; however, the patient did not feel the weight because one foot was always on the ground. The use of a customized walker with motor control circuitry and batteries ensured safety and eliminated the need for the patients to carry the device themselves. Two lever switches and two button switches were installed on both handgrips of the walker to enable the patients to operate it themselves. The WPAL could also be put on and removed by the patient; a skilled patient could do this within approximately 2 min.
The WPAL is equipped with the following five modes: (1) standing-up mode, (2) adjustment mode for the ankle joint angle during standing, (3) walking mode, (4) sitting-down mode, and (5) manipulation mode for the knee joint while putting on or removing the WPAL. Each mode is selected by pressing the button attached to the left grip of the walker. When the WPAL user presses a button, only the indicator lamp for the currently selected mode lights up on the control panel mounted on the front bar of the walker to indicate the currently selected mode. Figure 1 shows a state transition diagram for the WPAL operation and control.
In the standing-up mode, pressing the button attached to the right grip of the walker for 0.5 s will start the WPAL in motion from the sitting position. The user stands up by moving the body's center of gravity slightly forward and pushes the walker slightly downward and backward using residual functions in accordance with the movement of the WPAL. In the adjustment mode for the ankle joint angle during standing, the user can adjust the ankle joint angle for a stable standing posture by pulling the lever attached below the grip of the walker. Pulling the lever on the left side increases the dorsiflexion angle of the ankle joint, and pulling the lever on the right side increases the plantar flexion angle of the ankle joint. This function is necessary because each patient with SCI has a different ankle joint angle for a stable standing posture. In the walking mode, the WPAL motion is started by pressing the button attached to the right grip of the walker for 0.5 s. In this mode, the user is able to select the first step of walking (left or right). If the user selects the left side for the first step, the user has to shift the center of gravity to the right side to start walking smoothly. The WPAL moves both lower limbs alternately and constantly. WPAL users have to move their center of gravity laterally rhythmically with the WPAL motion using residual upper limb and trunk muscles and move the walker forward at the appropriate time in the gait cycle of the WPAL. WPAL motion is stopped when the user pulls the levers on both sides simultaneously for a few seconds. In the sitting-down mode, the WPAL motion is started by pressing the right button for 0.5 s in the standing position. The sitting-down motion of the WPAL is caused first by plantar flexion of the ankle joint, followed by flexion of the knee joint. The user needs to lean forward with their trunk and maintain the posture until full flexion to the pre-programmed angle of the knee joint of the WPAL for sitting in the wheelchair. In the manipulation mode of the knee joint for putting on and removing the WPAL, the user can manipulate the knee joint of the WPAL by using the lever attached under the grip of the walker. The user flexes the knee joint of the WPAL by pulling the lever under the left grip. This action is used when the user wants to put on the WPAL from a wheelchair. In contrast, pulling the right lever extends the knee joint of the WPAL. This action enables the user to remove the WPAL while on the wheelchair after the sitting-down motion.
The WPAL moves both lower limbs in a constant rhythm. WPAL users need to shift their lateral weight rhythmically in accordance with the WPAL motions. We have recommended five stages of gait training to achieve independent walking with the WPAL and a specialized wheeled walker (Tanabe et al., 2013a,b, 2017). In the initial four stages of gait training, the exercises are performed under the suspension system to prevent falls and reduce excessive fear of falling. The harness of the suspension system is set to slack without partially supporting the body weight. The first stage is a stepping exercise in the parallel bars. Before walking with the WPAL, the user learns to use the upper limbs and trunk muscles to perform a lateral weight shift with appropriate timing while keeping their center of gravity forward and backward, referring to a beeping sound produced in time with the walking rhythm. Subsequently, the user performs the same motions as the driving WPAL. The second stage is walking in the parallel bars. The step time is gradually shortened to approximately 1.5 s. The stride length is gradually increased to approximately 200 mm. The third stage is a walking exercise on a treadmill with a slow speed (approximately 0.1–0.3 km/h). Through continuous walking for a long period, the WPAL user can learn stable and rhythmic lateral weight shifts. The fourth stage is a walking exercise with a specialized wheeled walker. The user operates the WPAL using buttons or triggers installed on the grip of the walker. The final stage is a walking exercise without suspension using a specialized wheeled walker. Consensus decision-making is made between a certified physical therapist and a rehabilitation physician as to whether to proceed to the next stage. Patients also practice standing up and sitting down and donning/doffing of the WPAL during trials.
The development history of the WPAL was obtained from research records and interviews with members of the WPAL development team (rehabilitation physician, physical therapist, prosthetist and orthotist, and engineer). In the research records, medical doctors or physical therapists in the team recorded, on a daily basis, the details of training, any troubles related to the training as well as the robots, and all other necessary information, such as the version of the robots. Interviews were conducted to confirm the contents of the research records if needed. The number of WPAL trials was calculated from the detailed trial data from 2007 to 2020 in the development research records. However, the WPAL trials included a few exceptional cases where the trial did not contain gait training with the WPAL; instead, the trial was used for fine-tuning the WPAL motion and three-dimensional gait analysis with the WPAL and/or the orthosis only. If the name of the patient was not recorded, we included only the total number of WPAL trials. The number of WPAL trials for each patient was calculated for those whose names were listed in the detailed trial data. The number of WPAL trials to reach a functional ambulation category (FAC) score of 4 was also counted from the development research records. The reasons for the discontinuation of the WPAL trials were also collected from the development research records. Motor function was assessed using the AIS score. The evaluation was performed by a rehabilitation physician and the physical therapist in charge, and the score was agreed upon by both parties. Gait performance using the WPAL was measured in terms of the maximum continuous walking distance and time. However, the maximum run time of the WPAL is 120 min owing to the battery capacity. The Kolmogorov-Smirnov test was used to evaluate the pattern of data distribution. Since the normality of the data was not confirmed, the FAC score and maximal continuous walking distance and time were compared between the orthosis and WPAL using the Wilcoxon signed-rank test. The FAC score was used for all patients with both orthotic and WPAL records, and the maximum continuous walking distance and time were used for patients who reached an FAC score of 4 only. Adverse events, such as falls, were recorded by a certified physiotherapist who supervised the patient while walking with the WPAL. The patients were divided into four groups according to the level of injury: cervical (C5–C8), upper thoracic (T1–T6), lower thoracic (T7–T12), and lumbar. If the SCI level of the patient was different between the left and right sides, the higher level was defined as the injury level. The practice was performed for 1 or 1.5 h per day, i.e., preparatory exercises. All statistical analyses were performed with SPSS version 25 (SPSS Inc., Chicago, IL, USA). Any values of p < 0.05 were considered statistically significant.
The WPAL, developed in collaboration with Aska Corporation and Tomei Brace Co. in 2005, is a walking-assist robot for people with SCI, based on the design concept of a knee–ankle–foot orthosis with a single medial hip joint (Primewalk) (Suzuki et al., 2005) (Table 1 and Figure 2). From the Primewalk, which was the basis of the WPAL, to the commercialized current model, seven major updates were implemented to improve the design and control system. The first version was a Primewalk with a total of five motors attached one to the hip joint, two to both knee joints, and two to both ankle joints. Six months later, a hip joint motor was mounted on both sides for a total of six motors in the second version. In 2007, the motors were newly developed with the support of the New Energy and Industrial Technology Development Organization in the third version. In 2008, the thigh and lower leg cuffs were made smaller and lighter. The orthotic parts were improved so that the thigh and lower leg cuffs were independent in the fourth version. When patients with SCI used the WPAL to walk, the orthotic parts were first attached to the thigh and lower leg cuffs; then, they were connected to the robotic part by themselves. This connecting procedure between the cuff and robotic part was the same as that in the current system. The shape of the footplate was also improved. In 2010, in the fifth version, the motor was improved by attaching a cover. In 2011, we developed a universal cuff and improved the positioning of the robot and the cuff fixation part in the sixth version. In 2016, the seventh and current WPAL model began commercialization for medical and social service organizations. The touchscreen tablet PC for control and settings was placed in front of the walker. Until August 2007, we used the direct current (DC) brush motors manufactured by Maxon of Switzerland. Then, we developed a new brushless servo motor that had two advantages for our robot. First, the motor allows long-term use because of better heat dissipation and lower friction. Second, it can be installed between both the lower limbs. Consequently, we introduced minor updates (mainly improvement in heat resistance) in the motor. The currently used dedicated motor can never be damaged by heat.
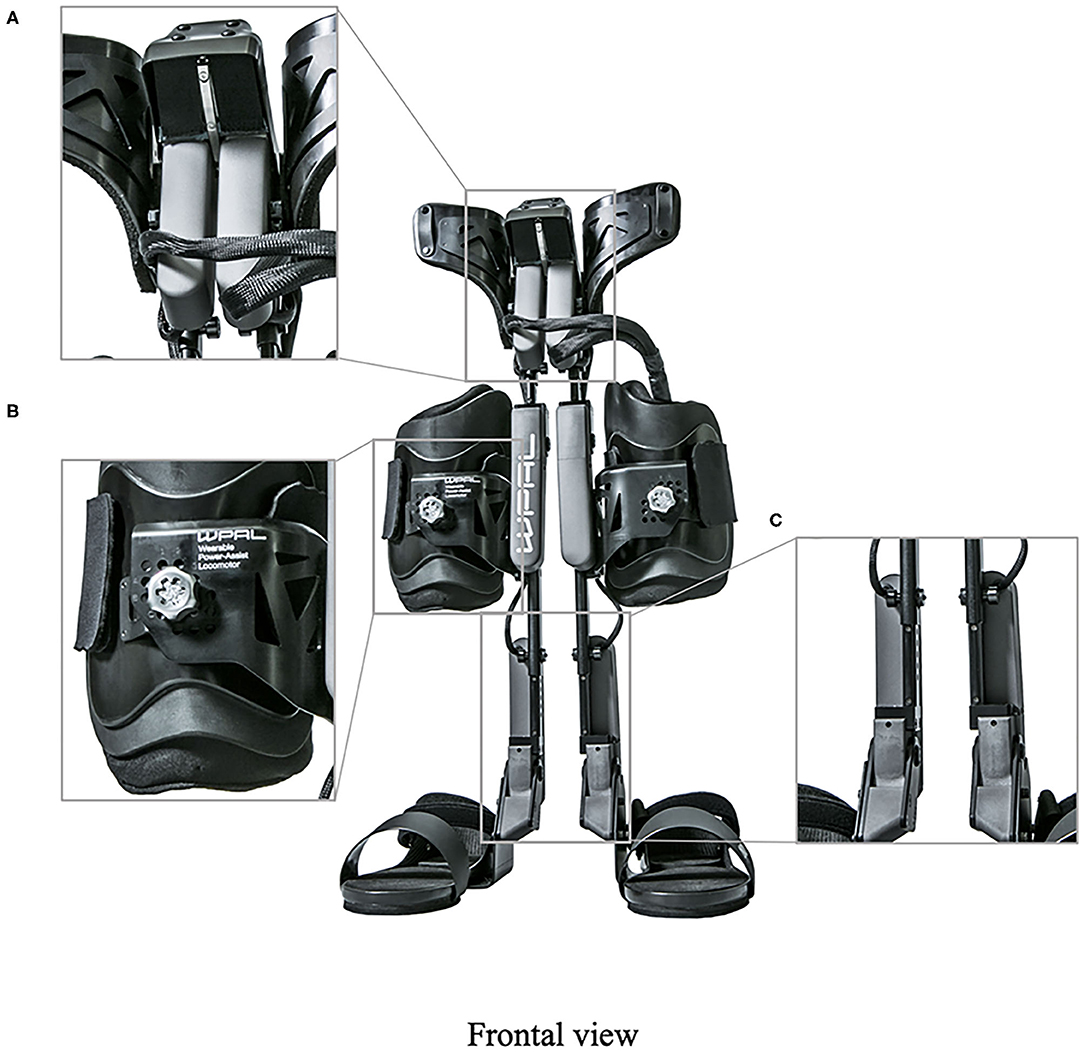
Figure 2. The structure of the latest WPAL in detail. (A) The hip joint in frontal view, (B) the knee joint in frontal view, and (C) the ankle joint in frontal view. WPAL, Wearable Power-Assist Locomotor.
The SCI level was cervical (C5–C8) in 10 patients, upper thoracic (T3–T6) in 5 patients, lower thoracic (T7–T12) in 15 patients, and lumbar (L1) in one patient. The proportions of patients with AIS scores of A, B, C, and D were 20, 7, 4, and 0, respectively (Table 2). The total number of WPAL trials was 1,785, of which 1,009 used the latest WPAL version, and the names of the patients were not recorded in the 88 development research records. Three falls due to mechanical errors in the battery control system and the servo motor system associated with lower limb spasticity in patients with SCI were observed. No severe incidents, such as bone fractures and skin injuries, that require unusual treatment were observed.
The mean number of WPAL trials for each patient was 55 (range 2–252) from 2007 to 2020. For the latest WPAL version, the mean number of WPAL trials for each patient was 51 (range 1–181). The person with the highest number of WPAL trials had an injury at T12 (AIS, A) and had the longest continuous walking distance and time. The most frequent reasons for discontinuation of WPAL gait training were social reasons unrelated to WPAL trials (10 patients), such as job-hunting and being busy with work, and discontinuation due to medical problems (5 patients). Twenty patients reached an FAC score of 4. The mean number of WPAL trials to reach an FAC score of 4 was 9 (median 8, range 2–22) in all patients and 13 (median 13, range 8–17) in the cervical group, 12 (median 12, range 8–16) in the upper thoracic group, 8 (median 8, range 2–22) in the lower thoracic group, and 6 in the lumbar group. However, 2 patients were excluded from these analyses due to missing data. The mean number of days taken to achieve an FAC score of 4 under the WPAL usage condition was 143 days (median 126, 16–555). Patients who reached an FAC score of 4 continued the WPAL trials. The mean number of continued WPAL trials was 66 (median 39, range 6–247). The number of continued WPAL trials varied among the patients due to factors unrelated to the WPAL trials, such as work. The mean duration following achievement of an FAC score of 4 was 1,221 days (median 763, 65–4,058) in December 2020.
Table 3 and Figure 3 show the results of the gait performance in terms of the FAC scores and continuous walking distance and time when using the WPAL and the orthosis. Thirteen patients had improved FAC scores using the WPAL compared with those using the orthosis, whereas two patients had a decreased FAC score using the WPAL compared with those using the orthosis. In all patients using the WPAL, the continuous walking distance and time ranged from 5.0 to 2375.0 m (median 80 m) and from 0.5 to 120.0 min (median 10 min), respectively. Contrarily, when using the orthosis, the continuous walking distance and time ranged from 3.5 to 879.0 m (median 40 m) and from 1.9 to 61.0 min (median 6 min), respectively. For the 20 patients who achieved an FAC score of 4 using the WPAL, the continuous walking distance and time ranged from 20 to 2,375 m (median 99 m) and from 3 to 120 min (median 10 min), respectively. For using orthosis in the patients who achieved an FAC score of 4 using the WPAL, the continuous walking distance and time ranged from 9 to 879 m (median 40 m) and from 2 to 61 min (median 6 min), respectively. The FAC scores in the 26 patients in whom measurements were made for both the orthosis and WPAL, and the maximal continuous walking distance in eight patients who achieved an FAC score of 4 using both devices was significantly higher for the WPAL than for the orthosis (p = 0.005 and 0.012, respectively). However, the difference in the maximal continuous walking time in the eight patients who achieved an FAC score of 4 was not statistically significant between the WPAL and the orthosis (p = 0.091).
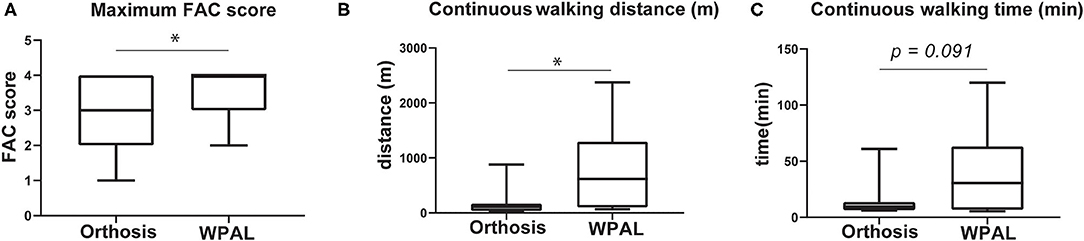
Figure 3. Comparisons of gait performance between the conventional orthosis and the WPAL. (A) Maximum FAC score, (B) continuous walking distance, and (C) continuous walking time. Each box represents 25–75% percentile and whiskers represent 5–95 percentile. Asterisk indicates statistically significant differences (p < 0.05). FAC, functional ambulation category; WPAL, Wearable Power-Assist Locomotor.
In this retrospective study that includes updated results from previous reports, all data were extracted from development research records from 2007 to 2020. The present findings confirm that the WPAL improves walking ability in patients with a wide range of SCIs compared with the orthosis, such as cervical SCI.
For the first time, we summarized the development history of the WPAL in this study. During this study period, the WPAL was updated seven times. The main updates were related to the safety and appearance of the device for the purpose of improved usability. Contrarily, the medial-type hip joint system without the trunk orthosis has not changed. Additionally, the location and number of motors have not changed since 2006. We believe that the development history would be helpful for devising a new gait-assisted robot (e.g., development order and required period of development). To the best of our knowledge, there is one report about the differences in the updates of the robots. Guanziroli et al. compared two different gait patterns using the Rewalk™ (ReWalk Robotics Ltd., Yokneam, Israel) between first- and second-generation software control; the latter had better synchronization between the hip and knee kinematics based on healthy kinematics and kinetics profiles for improvement of the quality of the gait pattern (Guanziroli et al., 2019). They reported an extension of the 6-min walking test and an improvement in the 10-m walking test.
The mean number of WPAL trials to reach an FAC score of 4 using a rolling walker was 9 in all patients and 13 in the cervical group, 12 in the upper thoracic group, 8 in the lower thoracic group, and 6 in the lumbar group. Some studies have reported the number of WRE trials required to walk with a walking aid and physical assistance (Esquenazi et al., 2012; Hartigan et al., 2015; Kozlowski et al., 2015; Guanziroli et al., 2019; Tsai et al., 2021) (Table 4). Esquenazi et al. reported that all the 12 patients with a motor-complete SCI (T3–T12 injury level) were able to walk independently without physical assistance using the ReWalk™ system (ReWalk Robotics Ltd., Yokneam, Israel) for at least 50–100 m continuously, for a period of at least 5–10 min continuously, after up to 24 trials (Esquenazi et al., 2012). Guanziroli et al. reported that 13 patients with a motor-complete SCI (T4–L4 injury level) required a mean of 22 trials to achieve independent walking using ReWalk™ with crutches (Guanziroli et al., 2019). Tsai et al. reported that eight patients with motor-complete SCIs (T1–T11 injury level) required a median of 30 (range 7–90) trials to achieve independent walking using ReWalk™ with crutches within a median of 111 days (range 87–210 days) (Tsai et al., 2021). Kozlowski et al. reported that 6 of 7 patients with motor-complete (4) or incomplete (3) SCIs (C4–T10 injury level) achieved walking using the Exso system (Exso Bionics, Richmond, CA, USA) with a front-wheeled walker or Lofstrand crutches and minimal assistance in a median of 8 trials, and 5 of them achieved walking with contact guard and close supervision assistance in a median of 15 trials (Kozlowski et al., 2015). Hartigan et al. reported three patients with motor-complete tetraplegia (C5–C7 injury level) who were able to walk after 5 WRE trials using a bilateral platform rolling walker with the minimal or moderate assistance of one therapist (Hartigan et al., 2015). In addition, six of the eight patients with motor-complete SCIs (T9–L1 injury level) were able to walk with supervised assistance using forearm crutches or a rolling walker (Hartigan et al., 2015). Regarding the level of injury, the WPAL was able to achieve walking independence with a rolling walker within a relatively small number of trials.
The maximum continuous walking time and distance using the WPAL were 120 min and 2,375 m, respectively. The maximum run time of WPAL is 120 min owing to the battery capacity. This result suggests that the WPAL has sufficient walking performance for it to be utilized at home and in the community. van Dijsseldonk et al. (2020) investigated the number of WREs used in home and community environments in 14 patients with SCIs at an injury level of T4 to L1 (van Dijsseldonk et al., 2020). This previous study reported that the estimated median active time is 46 (range 19–84) min, during which the median estimated total distance covered is 243 (range 22–1,367) m, and the median estimated maximal distance covered without rest is 120 (range 12–1,125) m (van Dijsseldonk et al., 2020).
In the present study, 10 patients with cervical SCIs practiced the WPAL gait. All patients with cervical SCIs who reached an FAC score of 4 in the WPAL gait had longer continuous walking distances; however, two patients had shorter continuous walking time compared with those using an orthosis. The results of the longer walking distance with shorter walking time for WPAL than for orthosis suggests that WPAL walking requires a more quick and constant lateral weight shift than orthotic walking. A previous study reported that a patient is required to make a quick and constantly lateral weight shift with WPAL motion during the WPAL gait. This rhythmic lateral weight shift is produced by the lateral force using the upper limbs (Tanabe et al., 2017). Even with other WREs, independent rhythmic lateral weight shifts during walking are difficult for patients with cervical SCIs. Many previous studies reported that other WREs require assistance for walking in patients with cervical SCIs (Hartigan et al., 2015; Kozlowski et al., 2015; Benson et al., 2016; Birch et al., 2017). A previous study reported that two patients with cervical SCIs (a motor-complete C8 and a motor-incomplete C4) were able to walk over 100 m using an Ekso powered exoskeleton with supervision or minimal assistance (Kozlowski et al., 2015). Benson et al. (2016) reported that one patient with motor-incomplete C7 lesions, who could walk without using an exoskeleton, was able to walk up to 91 m in a 6-min walk test using the ReWalk™ (Benson et al., 2016). Hartigan et al. (2015) reported that patients with three motor-complete cervical SCIs (one C5 and two C6 lesions) were able to walk an average distance of 64 m in a 6-min walk test using an Indego exoskeleton (Parker Hannifin Corporation) and a bilateral platform rolling walker with minimal or moderate assistance from one therapist (Hartigan et al., 2015). Birch et al. (2017) reported that five patients with cervical SCIs with motor-incomplete injury (three C4 and two C6 lesions) were able to perform a timed up and go test in a mean of 302 s (95% CI ± 49.6 s) with one assistant for three patients and two assistants for two patients using the REX robotic exoskeleton (Rex Bionics) (Birch et al., 2017). In the present study, patients with C5–C6 lesions did not reach an FAC score of 4 using the WPAL, on equality with using other WREs. However, patients with C7 lesions, even with motor-complete SCIs, reached an FAC score of 4 even in patients with motor-complete because the WPAL has a high standing stability, which is a structural characteristic similar to medial-type orthoses (Saitoh et al., 1996; Tanabe et al., 2013b; Koyama et al., 2016).
For using WPAL, the number of gait training to achieve independent gait was tended to less than other robots. In addition, WPAL tended to achieve independent gait in patients with cervical SCI compared with other robots. However, previous studies determined the number of gait training in advance during study periods and reported the degree of gait independence after completing total sessions of gait training. In future studies, it is necessary to compare the number of gait training to acquire independent walking with the gait-assisted robot among different robots in the same patients with SCI, rather than studies with a predetermined number of gait training.
In this study, the number and frequency of WPAL trials differed among patients. A previous study suggested that the time required to learn to safely walk with WREs is affected by the learning capacity, level of injury and completeness of SCI, and the user's strength and endurance levels (Kandilakis and Sasso-Lance, 2021). In the future, we need to clarify the rate of achievement of independent walking by configuring the previous number and frequency of WPAL trials. Moreover, the accumulation of clinical trials using WREs helps to elucidate the optimal number of training sessions and the frequency per week.
In this study, patients were able to continue the WPAL trail even after reaching an FAC score of 4 if they wished. In the future, the long-term effects of habitual walking using the WPAL on physical functions should be examined. Prolonged sitting in a wheelchair is associated with an increased risk of all-cause mortality (Rezende et al., 2016). Patients with SCIs commonly engage in less physical activity compared with healthy adults (Haisma et al., 2006; van den Berg-Emons et al., 2010). According to the physical activity guidelines, patients with SCIs should engage in at least 30 min of moderate- to vigorous-intensity aerobic exercise three times per week for cardiometabolic health benefits (Martin Ginis et al., 2018). Some studies reported that walking with WREs has numerous beneficial effects on pulmonary function (Xiang et al., 2021), bladder function (Chun et al., 2020), and sitting balance (Tsai et al., 2021). Habitual walking exercises using the WPAL three times a week for a long time may provide health benefits for patients with SCIs.
Long-term use in the community and its effects should be examined. Another robot is already being used in daily life situations (van Dijsseldonk et al., 2020). Further refinement of the WPAL will enable its long-term use in homes. The common prevent factor for long-term use is that a wheelchair is a very safe and comfortable mobility device for daily transportation in patients with SCI. Because the homes of wheelchair users are remodeled to make them accessible for wheelchair users, they can live without walking or standing. However, the robots have the advantage when patients with SCI perform cooking, washing, and cleaning (windows and shelves that cannot be reached on the wheelchair). Kandilakis et al. reported that the goal of robotic exoskeleton use is not to replace a wheelchair, but to create a supplemental means of mobility, exercise, or activities of daily living (Kandilakis and Sasso-Lance, 2021). We also believe that the alternate usage between robots and wheelchairs is the goal in the near future. A beneficial point of the WPAL is that it is possible to move using a wheelchair with the WPAL attached to their lower limbs because the WPAL has motors installed between both the lower limbs and no trunk support orthosis.
Further important development points for all robots, such as the WPAL, are the stair climbing, the lateral movement in a standing position, the ease of transporting the robots and the walking aids, the lightweight of the robots, the ease of donning/doffing them, the easy maintenance, high sound/waterproofing property, and fall prevention or detection system. For these, it is necessary to improve elemental technologies, such as the motors and the materials used for the robots. Lastly, we should examine whether the WPAL can improve or recover motor function in patients with motor-incomplete injury by partial motor assistance rather than complete motor assistance.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board of Fujita Health University. The patients/participants provided their written informed consent to participate in this study.
SK performed the data collection, analysis, writing original draft and review, editing, designing the research, and project administration. ST designed the research, writing—original draft, writing—review, and editing. TG and YT participated in data collection, analysis, and writing original draft. MK, ES, YO, and SH participated in data collection, writing original draft, writing review and editing, directed the research, project administration, and supervision. All authors contributed to the article and approved the submitted version.
This work was supported by a grant-in-aid of Basic Technology Development for Practical Application of Human Support Robots [8068149], Practical Development of Industrial Technology [8080694] by New Energy and Industrial Technology Development Organization (NEDO), and the JSPS KAKENI, Grant Number 20K11223.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AIS, American Spinal Injury Association Impairment Scale; FAC, functional ambulation category; SCI, spinal cord injury; WPAL, Wearable Power-Assist Locomotor; WRE, wearable robotic exoskeletons.
Arazpour, M., Bani, M. A., Hutchins, S. W., and Jones, R. K. (2013). The physiological cost index of walking with mechanical and powered gait orthosis in patients with spinal cord injury. Spinal Cord. 51, 356–359. doi: 10.1038/sc.2012.162
Asselin, P., Knezevic, S., Kornfeld, S., Cirnigliaro, C., Agranova-Breyter, I., Bauman, W. A., et al. (2015). Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J. Rehabil. Res. Dev. 52, 147–158. doi: 10.1682/JRRD.2014.02.0060
Benson, I., Hart, K., Tussler, D., and van Middendorp, J. J. (2016). Lower-limb exoskeletons for individuals with chronic spinal cord injury: findings from a feasibility study. Clin. Rehabil. 30, 73–84. doi: 10.1177/0269215515575166
Birch, N., Graham, J., Priestley, T., Heywood, C., Sakel, M., Gall, A., et al. (2017). Results of the first interim analysis of the RAPPER II trial in patients with spinal cord injury: ambulation and functional exercise programs in the REX powered walking aid. J. Neuroeng. Rehabil. 14, 60. doi: 10.1186/s12984-017-0274-6
Bjornshave Noe, B., Mikkelsen, E. M., Hansen, R. M., Thygesen, M., and Hagen, E. M. (2015). Incidence of traumatic spinal cord injury in Denmark, 1990-2012: a hospital-based study. Spinal Cord. 53, 436–440. doi: 10.1038/sc.2014.181
Chamberlain, J. D., Deriaz, O., Hund-Georgiadis, M., Meier, S., Scheel-Sailer, A., Schubert, M., et al. (2015). Epidemiology and contemporary risk profile of traumatic spinal cord injury in Switzerland. Inj. Epidemiol. 2, 28. doi: 10.1186/s40621-015-0061-4
Chun, A., Asselin, P. K., Knezevic, S., Kornfeld, S., Bauman, W. A., Korsten, M. A., et al. (2020). Changes in bowel function following exoskeletal-assisted walking in persons with spinal cord injury: an observational pilot study. Spinal Cord. 58, 459–466. doi: 10.1038/s41393-019-0392-z
Dijkers, M. P. (1999). Correlates of life satisfaction among persons with spinal cord injury. Arch. Phys. Med. Rehabil. 80, 867–876. doi: 10.1016/S0003-9993(99)90076-X
Esquenazi, A., Talaty, M., Packel, A., and Saulino, M. (2012). The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am. J. Phys. Med. Rehabil. 91, 911–921. doi: 10.1097/PHM.0b013e318269d9a3
Fisahn, C., Aach, M., Jansen, O., Moisi, M., Mayadev, A., Pagarigan, K. T., et al. (2016). The effectiveness and safety of exoskeletons as assistive and rehabilitation devices in the treatment of neurologic gait disorders in patients with spinal cord injury: a systematic review. Global Spine J. 6, 822–841. doi: 10.1055/s-0036-1593805
Fuse, I., Hirano, S., Saitoh, E., Otaka, Y., Tanabe, S., Katoh, M., et al. (2019). Gait reconstruction using the gait assist robot WPAL in patients with cervical spinal cord injury. Jap. J. Compr. Rehabil. Sci. 10, 88–95. doi: 10.11336/jjcrs.10.88
Guanziroli, E., Cazzaniga, M., Colombo, L., Basilico, S., Legnani, G., and Molteni, F. (2019). Assistive powered exoskeleton for complete spinal cord injury: correlations between walking ability and exoskeleton control. Eur. J. Phys. Rehabil. Med. 55, 209–216. doi: 10.23736/S1973-9087.18.05308-X
Haisma, J. A., van der Woude, L. H., Stam, H. J., Bergen, M. P., Sluis, T. A., and Bussmann, J. B. (2006). Physical capacity in wheelchair-dependent persons with a spinal cord injury: a critical review of the literature. Spinal Cord. 44, 642–652. doi: 10.1038/sj.sc.3101915
Hartigan, C., Kandilakis, C., Dalley, S., Clausen, M., Wilson, E., Morrison, S., et al. (2015). Mobility outcomes following five training sessions with a powered exoskeleton. Top. Spinal Cord Inj. Rehabil. 21, 93–99. doi: 10.1310/sci2102-93
Hirano, S., Saitoh, E., Tanabe, S., Katoh, M., Shimizu, Y., Yatsuya, K., et al. (2015). Comparison between gait-assisting robot (WPAL) and bilateral knee-ankle-foot orthoses with a medial single hip joint in gait reconstruction for patients with paraplegia. Jap. J. Compr. Rehabil. Sci. 6, 21–26. doi: 10.11336/jjcrs.6.21
Kandilakis, C., and Sasso-Lance, E. (2021). Exoskeletons for personal use after spinal cord injury. Arch. Phys. Med. Rehabil. 102, 331–337. doi: 10.1016/j.apmr.2019.05.028
Koyama, S., Tanabe, S., Saitoh, E., Hirano, S., Shimizu, Y., Katoh, M., et al. (2016). Characterization of unexpected postural changes during robot-assisted gait training in paraplegic patients. Spinal Cord. 54, 120–125. doi: 10.1038/sc.2015.138
Kozlowski, A. J., Bryce, T. N., and Dijkers, M. P. (2015). Time and effort required by persons with spinal cord injury to learn to use a powered exoskeleton for assisted walking. Top. Spinal Cord Inj. Rehabil. 21, 110–121. doi: 10.1310/sci2102-110
Kunkel, C. F., Scremin, A. M., Eisenberg, B., Garcia, J. F., Roberts, S., and Martinez, S. (1993). Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch. Phys. Med. Rehabil. 74, 73–78.
Lee, B. B., Cripps, R. A., Fitzharris, M., and Wing, P. C. (2014). The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 52, 110–116. doi: 10.1038/sc.2012.158
Levins, S. M., Redenbach, D. M., and Dyck, I. (2004). Individual and societal influences on participation in physical activity following spinal cord injury: a qualitative study. Phys. Ther. 84, 496–509. doi: 10.1093/ptj/84.6.496
Majdan, M., Brazinova, A., and Mauritz, W. (2016). Epidemiology of traumatic spinal cord injuries in Austria 2002-2012. Eur. Spine J. 25, 62–73. doi: 10.1007/s00586-015-3985-z
Martin Ginis, K. A., van der Scheer, J. W., Latimer-Cheung, A. E., Barrow, A., Bourne, C., Carruthers, P., et al. (2018). Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 56, 308–321. doi: 10.1038/s41393-017-0017-3
Miller, L. E., Zimmermann, A. K., and Herbert, W. G. (2016). Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: systematic review with meta-analysis. Med. Devices 9, 455–466. doi: 10.2147/MDER.S103102
Miyakoshi, N., Suda, K., Kudo, D., Sakai, H., Nakagawa, Y., Mikami, Y., et al. (2020). A nationwide survey on the incidence and characteristics of traumatic spinal cord injury in Japan in 2018. Spinal Cord. 59, 626–634. doi: 10.1038/s41393-020-00533-0
NSCIS Center (2021). Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham.
Palermo, A. E., Maher, J. L., Baunsgaard, C. B., and Nash, M. S. (2017). Clinician-focused overview of bionic exoskeleton use after spinal cord injury. Top. Spinal Cord Inj. Rehabil. 23, 234–244. doi: 10.1310/sci2303-234
Rezende, L. F. M., Sa, T. H., Mielke, G. I., Viscondi, J. Y. K., Rey-Lopez, J. P., and Garcia, L. M. T. (2016). All-cause mortality attributable to sitting time: analysis of 54 countries worldwide. Am. J. Prev. Med. 51, 253–263. doi: 10.1016/j.amepre.2016.01.022
Saitoh, E., Suzuki, T., Sonoda, S., Fujitani, J., Tomita, Y., and Chino, N. (1996). Clinical experience with a new hip-knee-ankle-foot orthotic system using a medial single hip joint for paraplegic standing and walking. Am. J. Phys. Med. Rehabil. 75, 198–203. doi: 10.1097/00002060-199605000-00010
Suzuki, T., Sonoda, S., Saitoh, E., Murata, M., Uno, A., Shimizu, Y., et al. (2005). Development of a novel type of shoe to improve the efficiency of knee-ankle-foot orthoses with a medial single hip joint (Primewalk orthoses): a novel type of shoe for Primewalk orthosis. Prosthet. Orthot. Int. 29, 303–311. doi: 10.1080/03093640500465195
Tan, K., Koyama, S., Sakurai, H., Teranishi, T., Kanada, Y., and Tanabe, S. (2021). Wearable robotic exoskeleton for gait reconstruction in patients with spinal cord injury: a literature review. J. Orthop. Translat. 28, 55–64. doi: 10.1016/j.jot.2021.01.001
Tanabe, S., Hirano, S., and Saitoh, E. (2013a). Wearable Power-Assist Locomotor (WPAL) for supporting upright walking in persons with paraplegia. NeuroRehabilitation. 33, 99–106. doi: 10.3233/NRE-130932
Tanabe, S., Koyama, S., Saitoh, E., Hirano, S., Yatsuya, K., Tsunoda, T., et al. (2017). Clinical feasibility of gait training with a robotic exoskeleton (WPAL) in an individual with both incomplete cervical and complete thoracic spinal cord injury: a case study. NeuroRehabilitation. 41, 85–95. doi: 10.3233/NRE-171460
Tanabe, S., Saitoh, E., Hirano, S., Katoh, M., Takemitsu, T., Uno, A., et al. (2013b). Design of the Wearable Power-Assist Locomotor (WPAL) for paraplegic gait reconstruction. Disabil. Rehabil. Assist. Technol. 8, 84–91. doi: 10.3109/17483107.2012.688238
Tsai, C. Y., Asselin, P. K., Hong, E., Knezevic, S., Kornfeld, S. D., Harel, N. Y., et al. (2021). Exoskeletal-assisted walking may improve seated balance in persons with chronic spinal cord injury: a pilot study. Spinal Cord Ser. Cases 7, 20. doi: 10.1038/s41394-021-00384-8
van den Berg-Emons, R. J., Bussmann, J. B., and Stam, H. J. (2010). Accelerometry-based activity spectrum in persons with chronic physical conditions. Arch. Phys. Med. Rehabil. 91, 1856–1861. doi: 10.1016/j.apmr.2010.08.018
van Dijsseldonk, R. B., van Nes, I. J. W., Geurts, A. C. H., and Keijsers, N. L. W. (2020). Exoskeleton home and community use in people with complete spinal cord injury. Sci. Rep. 10, 15600. doi: 10.1038/s41598-020-72397-6
Varacallo, M., Davis, D. D., and Pizzutillo, P. (2021). Osteoporosis in Spinal Cord Injuries, [Updated 2021 June 3] in StatPearls. Treasure Island, FL: StatPearls Publishing. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK526109/
Verschueren, J. H., Post, M. W., de Groot, S., van der Woude, L. H., van Asbeck, F. W., and Rol, M. (2011). Occurrence and predictors of pressure ulcers during primary in-patient spinal cord injury rehabilitation. Spinal Cord. 49, 106–112. doi: 10.1038/sc.2010.66
Xiang, X. N., Zong, H. Y., Ou, Y., Yu, X., Cheng, H., Du, C. P., et al. (2021). Exoskeleton-assisted walking improves pulmonary function and walking parameters among individuals with spinal cord injury: a randomized controlled pilot study. J. Neuroeng. Rehabil. 18, 86. doi: 10.1186/s12984-021-00880-w
Yatsuya, K., Hirano, S., Saitoh, E., Tanabe, S., Tanaka, H., Eguchi, M., et al. (2018). Comparison of energy efficiency between Wearable Power-Assist Locomotor (WPAL) and two types of knee-ankle-foot orthoses with a medial single hip joint (MSH-KAFO). J. Spinal Cord Med. 41, 48–54. doi: 10.1080/10790268.2016.1226701
Keywords: wearable robotic exoskeleton, gait, paraplegia, tetraplegia, clinical experience
Citation: Koyama S, Tanabe S, Gotoh T, Taguchi Y, Katoh M, Saitoh E, Otaka Y and Hirano S (2022) Wearable Power-Assist Locomotor for Gait Reconstruction in Patients With Spinal Cord Injury: A Retrospective Study. Front. Neurorobot. 16:775724. doi: 10.3389/fnbot.2022.775724
Received: 14 September 2021; Accepted: 11 January 2022;
Published: 18 February 2022.
Edited by:
Massimo Sartori, University of Twente, NetherlandsReviewed by:
Nicola Lotti, Heidelberg University, GermanyCopyright © 2022 Koyama, Tanabe, Gotoh, Taguchi, Katoh, Saitoh, Otaka and Hirano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoshi Hirano, c3NoaXJhbm9AZnVqaXRhLWh1LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.