- 1Department of Spinal Cord Injuries, BG University Hospital Bergmannsheil, Bochum, Germany
- 2Department of General and Trauma Surgery, BG University Hospital Bergmannsheil, Bochum, Germany
- 3Institute for Medical Information Processing, Biometrics and Epidemiology, Ludwig-Maximilians-University Munich, Munich, Germany
This study aimed to assess the outcome of acute and chronic participants with spinal cord injury (SCI) after 12 weeks of bodyweight supported treadmill training (BWSTT) with a hybrid assistive limb exoskeleton (HAL). Acute participants were defined as ≤12 months between SCI and training, chronic participants >12 months between SCI and training. We assessed whether HAL-assisted BWSTT is advantageous for acute and chronic participants and if length of time post injury impacts the outcome of HAL-assisted BWSTT. As the primary outcome, we assessed the time needed for the 10 meter walk test (10MWT). Hundred and twenty-one individuals participated in a 12-week HAL-assisted BWSTT five times a week. We regularly conducted a 10MWT, a 6 minute walk test (6MWT), and assessed the walking index for spinal cord injury (WISCI II) and lower extremity motor score (LEMS) to evaluate the gait performance without the exoskeleton. Distance and time were recorded by the treadmill while the participant was walking with the exoskeleton. All participants benefit from the 12-week HAL-assisted BWSTT. A significant difference between acute and chronic participants' outcomes was found in 6MWT, LEMS, and WISCI II, though not in 10MWT. Although chronic participants improved significantly lesser than acute participants, they did improve their outcome significantly compared to the beginning. Hybrid assistive limb-assisted BWSTT in the rehabilitation of patients with SCI is advantageous for both acute and chronic patients. We could not define a time related cut-off threshold following SCI for effectiveness of HAL-assisted BWSTT.
Introduction
The incidence of spinal cord injury (SCI) is 54 per one million people in the USA. There are about 17.81 new cases each year. 66.8% of those are incomplete lesions (47.2% tetraplegic, 19.6% paraplegic) and therefore form the most frequent subgroup since 2015 (National Spinal Cord Injury Statistical Center, 2021). According to the US National Spinal Cord Statistical Center, “less than 1% of patients experienced complete neurological recovery by the time of hospital discharge” (National Spinal Cord Injury Statistical Center, 2021). The quality of life of those patients is strongly dependent on their ability to walk (Brown-Triolo et al., 2002; Estores, 2003; Ditunno et al., 2008). Therefore, functional and motor recovery is of great importance.
Physical activity enhancing motor and neurological recovery of patients with SCI is significantly associated with a higher quality of life, fewer depression symptoms, less SCI-related pain, and better cardiovascular health and fitness (Lam et al., 2014; Warburton et al., 2018; Mehta et al., 2019). Bodyweight supported treadmill training (BWSTT) has been an established method in rehabilitation post-SCI for decades with lasting effects noted for individuals with incomplete injuries (Wirz et al., 2001).
Therapies supporting motor and neurological recovery consist of numerous different approaches such as gait training, BWSTT, robotic-assisted BWSTT, and BWSTT with manual assistance and/ or functional electric stimulation. So far, no approach could prove superiority to the others (Lam et al., 2007, 2014; Schwartz et al., 2011; Mehrholz et al., 2012, 2017; Morawietz and Moffat, 2013).
In the 1980s, the first trials with robotic exoskeletons indicated that robotic-assisted locomotion training in rehabilitation was possible and could improve walking capabilities (van Vliet and Wing, 1991; Colombo et al., 2000). Compared to conventional BWSTT, robotic-assisted locomotion training needs less effort from physiotherapists, enables longer training sessions, and a reproducible gait pattern (Colombo et al., 2000, 2001). Robotic-assisted BWSTT proved several advantages such as improved gait pattern, cardiorespiratory fitness, urinary and bowel functions, and reduced pain and spasticity (Holanda et al., 2017; Brinkemper et al., 2021).
One of the robotic exoskeletons currently utilized in rehabilitation is the hybrid assistive limb (HAL), which uses minimal bioelectrical signals from the remaining motor function of the patients to transfer into movement. To assess patients' with SCI functional gait, the 10 meter walk test (10MWT), 6 minute walk test (6MWT), and walking index for spinal cord injury II (WISCI II) are established and valid measures (van Hedel et al., 2005). Significant improvements as a reduction in the number of steps and increased speed in the 10MWT, walking and cardiovascular endurance in the 6MWT, and lesser walking aids assessed in the WISCI II were reported after HAL exoskeleton training (Kubota et al., 2013; Aach et al., 2014, 2015; Cruciger et al., 2014; Sczesny-Kaiser et al., 2015; Wall et al., 2015; Grasmücke et al., 2017). Improved muscular strength, as measured by the lower extremity motor score (LEMS), has also been reported after HAL-assisted BWSTT (Aach et al., 2014; Sczesny-Kaiser et al., 2015).
Research about robotic assisted BWSTT for patients with SCI is growing, but due to the heterogeneity of different exoskeletons and broad field of applications, the effectiveness is not yet defined. According to a Cochrane review, robotic-locomotor training approaches should be further investigated, specifically which patients and at what stages of recovery the benefit from the intervention is highest (Mehrholz et al., 2012).
The start-time threshold for BWSTT is another topic still to be investigated. The major recovery of patients with acute SCI takes place within the first 12 months of rehabilitation (Fawcett et al., 2007). Still, significant changes occur in chronic patients with SCI (trauma >12 months ago) with a small degree of neurologic recovery (Piepmeier and Jenkins, 1988; Kirshblum et al., 2004). According to Lam et al., bodyweight-supported training seems to be more effective in acute patients than in chronic patients (Lam et al., 2007). According to Grasmücke et al., patients with chronic SCI benefit from HAL-assisted BWSTT (Grasmücke et al., 2017). Patients with incomplete SCI have greater chances of neurological and functional recovery compared to patients with complete SCI (Fawcett et al., 2007; Curt et al., 2008; Lee et al., 2016).
The purpose of our study was to investigate the impact of HAL-assisted BWSTT on functional and motor recovery in post-acute phases of neurorehabilitation. We hypothesize that there will be significant improvements in gait and motor function after HAL BWSTT as measured using the 10MWT, 6MWT, WISCI II, and LEMS outcome measures for participants with both acute and chronic SCI. We also hypothesize that patients with acute injury will improve motor function to the same extent as patients with chronic injury.
Materials and Methods
Patient Population
Our cohort consists of participants that started HAL training between 02/2012 and 10/2018 at Bergmannsheil Hospital in Bochum, Germany.
Both acute and chronic participants with SCI were included, with time between trauma and therapy of 12 months marking the cut-off between acute and chronic using previous guidelines by Fawcett et al. (2007).
The data of chronic and acute participants have been described previously in our subgroup analysis and a paper currently under review (Grasmücke et al., 2017).
Inclusion criteria were defined as incomplete SCI (AIS C, D) or complete SCI from conus medullaris to cauda equine with zones of partial preservation (ZPP) (AIS A) and existing motor function of hip and knee extensor and flexor muscle groups to operate the exoskeleton (Frankel and Janda Grade 1/5 or 2/5) (Janda, 1983).
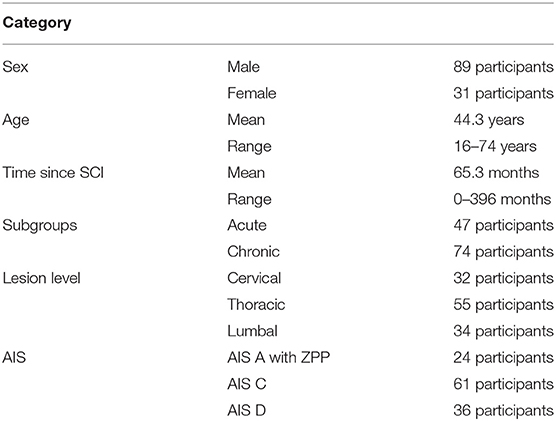
Exclusion criteria consisted of: inadequate attendance of training sessions such that participants were only present for ≤ 20, a severe joint contracture in hip or knee, bodyweight over 100 kg, pressure sores, non-consolidated fractures, epilepsy, cognitive impairment that makes the therapy substantially difficult or impossible, and severe heart insufficiency. All 121 participants were classified according to the International standards for neurological classification of spinal cord injury (ISNCSCI) by the American spinal cord injury association (ASIA) impairment scale (AIS) (Kirshblum et al., 2011).
The participants suffered spinal cord lesions between C2 and L4 (32 cervical, 55 thoracic, 34 lumbal). The mean time between SCI and the start of HAL training was 65.3 months (SD 89.5 months, range: 0–396 months).
All participants gave written informed consent to participate in this trial. They confirmed consent for anonymized data publishing. The ethics committee of BG University Hospital Bergmannsheil and the Ruhr University Bochum approved the study protocol. This study was conducted according to the principles expressed in the Declaration of Helsinki.
The Exoskeleton
The HAL (Cyberdyne Inc., Japan) exoskeleton used in this study acts on the lower limbs. Electrical motors support flexion and extension of the hip and knee joints. The robot suit is attached to the patient's legs and around the waistline. EMG electrodes detect voluntary minimal bioelectrical signals from extensor and flexor muscles of the hip and knee. Thereby, the gait pattern is controlled by the patient itself and allows an individual motion sequence (Suzuki et al., 2007). Hybrid assistive limb locomotion training is safe for patients with SCI and enhances the rehabilitation progress (Kubota et al., 2013; Aach et al., 2014, 2015; Cruciger et al., 2014; Sczesny-Kaiser et al., 2015; Wall et al., 2015; Grasmücke et al., 2017).
Intervention
All participants used the HAL robot suit exoskeleton under the supervision of a HAL-trained physiotherapist. The training took place five times a week for 90–120 min for 3 months. In addition to the HAL-based training, the participants regularly performed a 10MWT and 6MWT without the exoskeleton with individual walking aids. The treadmill-associated data, distance, and time were continuously recorded. The exoskeleton's weight (14–17 kg depending on the size) and an individual amount of the patient's body weight (0–20 kg) were compensated by the bodyweight support of the treadmill. The treadmill used is by Woodway, Inc.
Outcome Measures
The physiotherapists performing the examinations and training supervision did not participate in data analysis or study design.
The functional outcome and walking capability of the participants were measured at the beginning, after 6 and 12 weeks of HAL training. For the 10MWT, the seconds needed for walking a 10 m distance were recorded (van Hedel et al., 2005, 2006). The participants were advised to walk the 10 m at their own preferred pace. The required assistance in the 10MWT was monitored by the WISCI II (Ditunno et al., 2000; Ditunno and Dittuno, 2001). Through 6MWT, walking and cardiovascular endurance was assessed by the distance participants walked in 6 min. Participants chose their speed and were advised to pause if they felt unable to continue (Harada et al., 1999; van Hedel et al., 2006). The LEMS was recorded before and after the 12 weeks of training to evaluate the motor function and gait performance (Waters et al., 1994).
The parameters of walking time and distance were recorded by the treadmill during each training session while wearing the exoskeleton. The velocity of the treadmill was settled individually among comfortable and maximum speed tolerated by the participants.
Statistical Analysis
Descriptive statistics of age, injury characteristics, and sex were calculated by frequency distributions for categorical data and means for continuous variables.
All outcomes were tested for normal distribution using the Shapiro-Wilk Test and visibly through distribution plots for all three time points. Time trends over all measurements were visualized for all the outcomes WISCI II, 10MWT, 6MWT, and LEMS. To analyze the differences between chronic and acute participants and the differences over the three time points of evaluation (start of training, after 6 weeks, and after 12 weeks), a repeated measures ANOVA was performed for each outcome (testing two groups over three time-points). For the repeated measures ANOVA, cases were only included if they had valid measures for all three timepoints. Equal group variances were tested with the Levene's test. The alpha level was set to 0.05.
Differences within and between groups have been estimated with t-tests and with repeated measure ANOVA, assuming normal distribution of the continuous variable. Data was lightly skewed at some timepoints; therefore tests have been repeated with a Wilcoxon test (for non-parametric data). Results from the Wilcoxon test have confirmed the results of the t-test. Levene's tests for the repeated measures ANOVA were all non-significant.
All statistical analyses have been performed with SAS for Windows (Vers. 9.4).
Results
A total of 137 patients were screened for the study. Ten patients were excluded after the first screening, and six participants were eliminated for evaluation because they had participated in <20 sessions. The 121 participants included in the analyses are presented in Table 1.
The participants were divided into two subgroups (acute, chronic) according to the time between the date of SCI and the point of time training started. The acute group consisted of participants with a SCI duration under 12 months at the beginning of the intervention (n = 47). In the chronic group were 74 participants with the SCI being more than 1 year ago (13 months to 33 years).
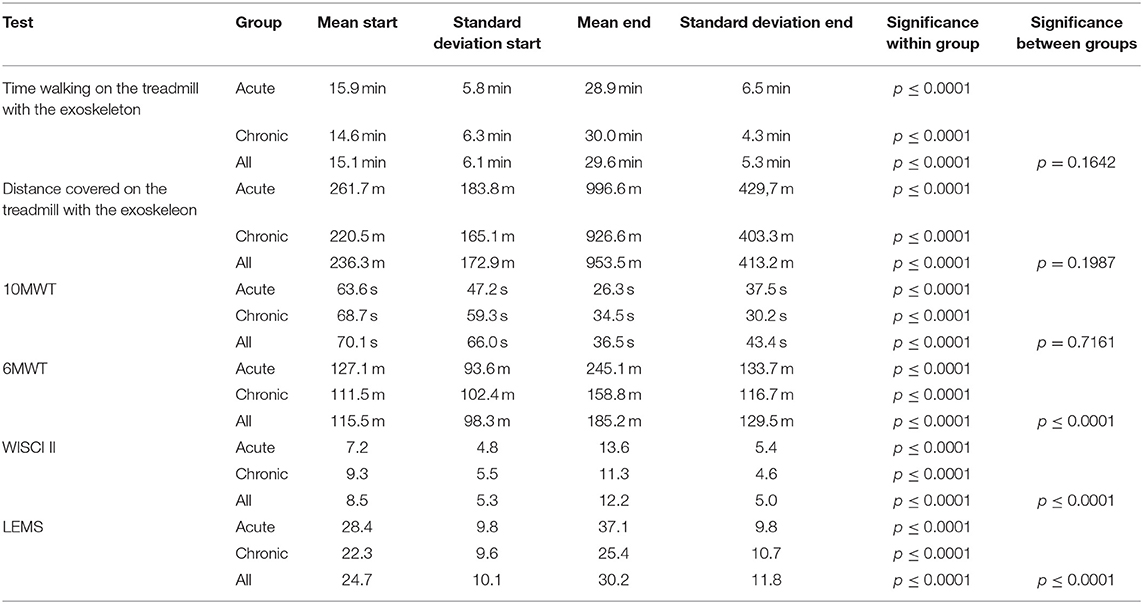
Within the time of training (02/12 to 10/18), no adverse events happened. There were no falls or discontinuation due to HAL-assisted BWSTT. All Means and standard deviations are displayed in Table 2.
HAL Associated Outcomes
All participants improved treadmill performance within the 12 weeks of training.
Participants could significantly extend walking time with the exoskeleton on the treadmill from 15.1 min (±6.1 min) baseline to 29.6 min (±5.3 min) after 12 weeks (p ≤ 0.0001). Acute participants walked from 15.9 min (±5.8 min) baseline to 28.9 min (±6.5 min) after 12 weeks (p ≤ 0.0001). Chronic participants walked from 14.6 min (±6.3 min) baseline to 30.0 min (±4.3 min) after 12 weeks (p ≤ 0.0001). Due to the limited session time, participants were not allowed to walk longer than 30 min. No significant difference in walking time could be observed between the subgroups (p = 0.1642).
Ambulated distance increased significantly in all participants from 236.3 m (±172.9 m) baseline to 953.5 m (±413.2 m) after 12 weeks (p ≤ 0.0001). Acute participants improved from 261.7 m (±183.8 m) baseline to 996.6 m (±429,7 m) after 12 weeks (p ≤ 0.0001). Chronic participants improved from 220.5 m (±165.1 m) baseline to 926.6 m (±403.3 m) after 12 weeks (p ≤ 0.0001). Concerning walking distance, no significant difference was measured between the subgroups (p = 0.1987).
Functional Outcomes
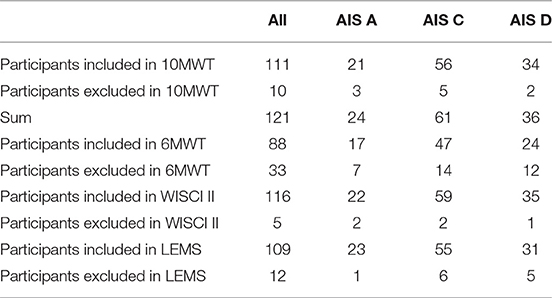
All participants significantly improved in the functional assessments performed without the exoskeleton. Table 3 demonstrates the included and excluded participants for all functional tests due to AIS levels.
10MWT
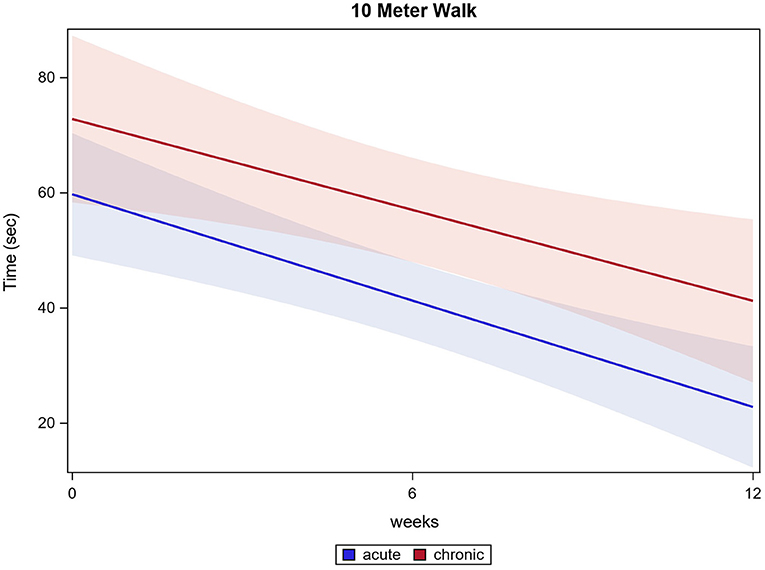
Of all 121 participants, 111 participants were able to conduct the 10MWT at the first training session, 117 participants completed the 10MWT after 12 weeks of training. Participants significantly accelerated in 10MWT from 70.1 s (±66.0 s) baseline to 36.5 s (±43.4 s) after 12 weeks (p ≤ 0.0001). Acute participants improved from 63.6 s (±47.2 s) baseline to 26.3 s (±37.5 s) after 12 weeks and chronic participants improved from 68.7 s (±59.3 s) baseline to 34.5 s (±30.2 s) after 12 weeks (both p ≤ 0.0001). There were no significant differences between the two groups in the 10MWT (p = 0.7161). 10MWT results are shown in Figure 1.
6MWT
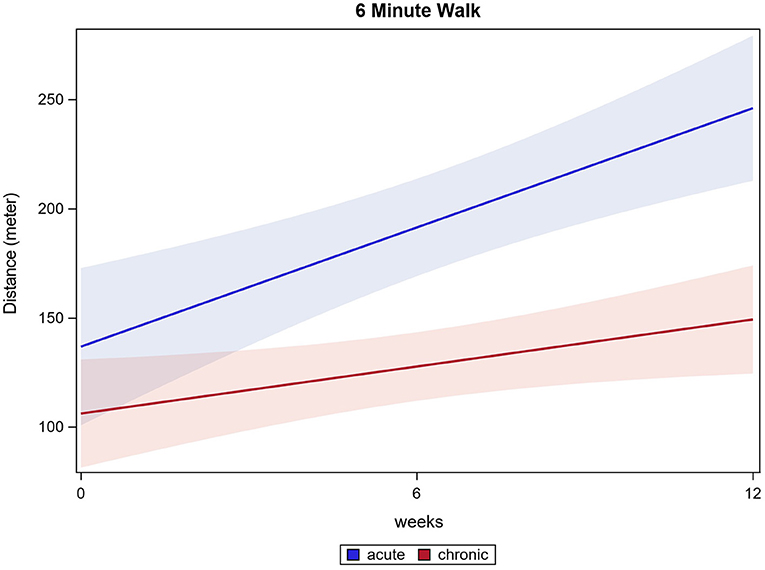
Eighty-eight participants had fully documented 6MWT values for all time points and were included in the analysis. The distance ambulated in 6 min increased significantly in all participants from 115.5 m (±98.3 m) baseline to 185.2 m (±129.5 m) after 12 weeks (p ≤ 0.0001). Acute participants improved from 127.1 m (±93.6 m) baseline to 245.1 m (±133.7 m) after 12 weeks. Chronic participants improved from 111.5 m (±102.4 m) baseline to 158.8 m (±116.7 m) after 12 weeks (both p ≤ 0.0001). Acute participants improved significantly more than chronic participants, while both groups enhanced the performance in 6MWT (p ≤ 0.0001). 6MWT results are shown in Figure 2.
WISCI II
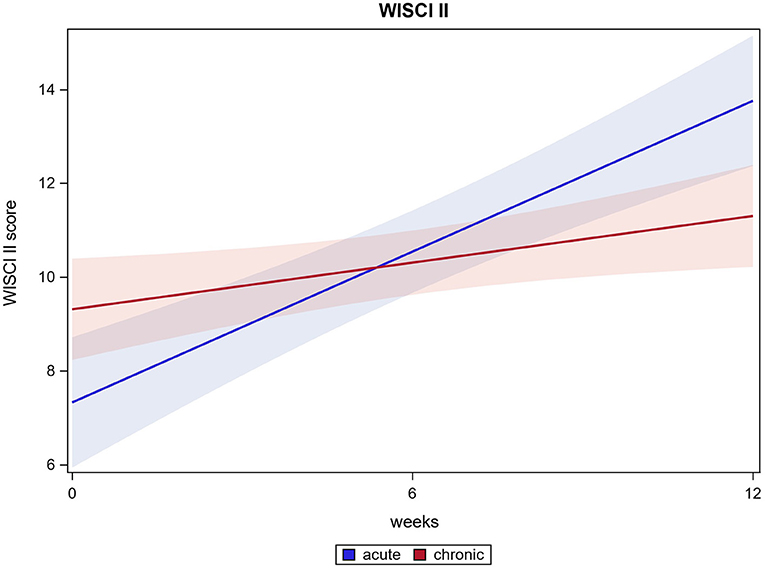
For 116 out of 121 participants the WISCI II could be measured after HAL training. The Mean score from 8.5 (±5.3) at baseline improved to 12.2 (±5.0) after 12 weeks (p ≤ 0.0001). Acute participants' WISCI II scores changed from 7.2 (±4.8) at baseline to 13.6 (±5.4) after 12 weeks, chronic participants' WISCI II scores from 9.3 (±5.5) at baseline to 11.3 (±4.6) after 12 weeks (both p ≤ 0.0001). There was a significant difference between acute and chronic participants' results. Whereas, both groups improved, acute participants' improvement is significantly higher than chronic patient's improvement. (p ≤ 0.0001). WISCI II score results are shown in Figure 3.
LEMS
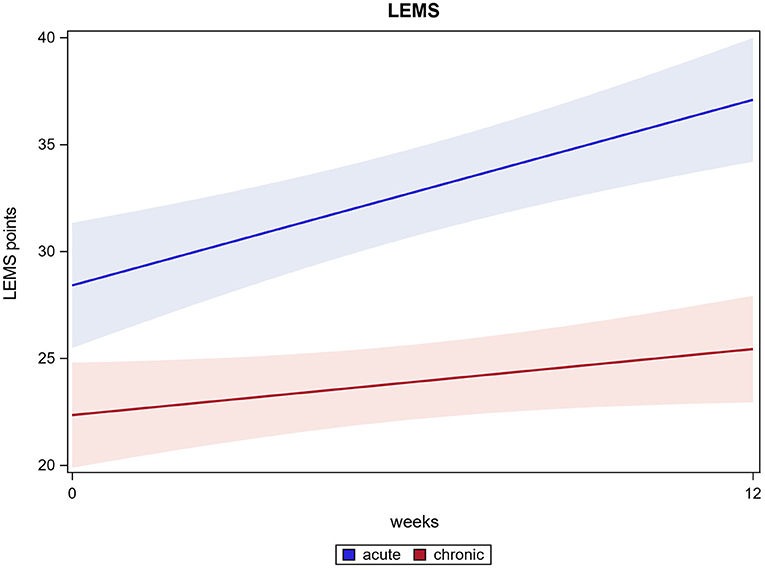
In 109 out of 121 participants, an enhanced LEMS could be examined at two time points (at baseline and after 12 weeks). The scores improved significantly from 24.7 (±10.1) at baseline to 30.2 (±11.8) after 12 weeks (p ≤ 0.0001). Acute participants improved significantly from 28.4 (±9.8) at baseline to 37.2 (±9.8) after 12 weeks, chronic participants improved significantly from 22.3 (±9.6) at baseline to 25.4 (±10.7) after 12 weeks (both p ≤ 0.0001). There was a significant difference found between acute and chronic participants' improvement, while both groups improved significantly from the start of the intervention to the end (p ≤ 0.0001). LEMS results are shown in Figure 4.
Discussion
This study investigated whether HAL-assisted BWSTT is advantageous for acute and chronic patients with SCI and if there was a cut-off threshold for effectiveness.
We investigated 121 acute vs. chronic participants performing a 12-week HAL-supported BWSTT.
The present study demonstrated that acute and chronic participants benefit in all conducted tests after 12 weeks of HAL-assisted BWSTT. For 6MWT, WISCI II, and LEMS, test results show a significant difference between the acute and the chronic participants' outcomes. For the 6MWT, acute participants improved distance walked by 93% and chronic participants improved in means by 42% (equates 47 m). Bohannon et al. state that a clinically relevant change in 6MWT is considered as at least 14 to 30.5 m gain in 6MWT (Bohannon and Crouch, 2017). Following this review, acute and chronic participants outcomes could be considered clinically relevant, although acute participants improved to a greater extend. Most of our participants are wheelchair users. The preserved motor function enables them to ambulate short distances, they would, however, use a wheelchair for long distances to save time. Therefore, the 6MWT is measured as an objective means to assess a patient's walking, muscular and cardiovascular endurance although the everyday relevance is limited. Ten meters are a distance likelier to be ambulated by foot. Therefore, the 10MWT presents a more clinically relevant outcome concerning speed of ambulation. The 6MWT is described to be potentially biased as not all participating individuals could perform the test. Therefore, a smaller number of participants are considered. In our data, 33 participants did not perform the 6MWT at a minimum of one time point. Generally, it must be stated that acute participants started training with overall better mean scores at most tests. Moreover, one should assess the outcome of acute participants considering spontaneous recovery. Still, improvement was visible in both chronic and acute participants for all functional tests.
Both, acute and chronic participants significantly improved their test values in 10MWT. Surprisingly, there was no significant difference between acute participants' and chronic participants' outcomes in 10MWT and all treadmill-associated data.
Commencing HAL-associated BWSTT seems to be of advantage in the acute phase after SCI and after several years. As described in our previous paper, chronic patients benefit from HAL-associated BWSTT with improved overground walking and mobility, and no adverse events were recorded (Grasmücke et al., 2017). Whilst feasibility was proved for chronic participants, there is to the best of our knowledge no study comparing acute and chronic participant's outcomes after HAL-assisted training.
Concerning the limitations of this study, we did not record additional treatments or medication for our participants. Most acute participants attended physiotherapy five times a week, some including gait training and some without. Chronic participants attended between zero and three times a week of physiotherapy. Some participants attended regular wheelchair sports groups. The spectrum of different additional treatments is so wide, that standardization and comparison were not possible. As the potential for rehabilitation is highest in the first year after SCI, it would be ethically unjustifiable to prohibit additional treatments to acute participants in particular. Participants were asked to continue any established treatments and alter medication only in case of need.
Another limitation is, that a ceiling effect has been described for higher AIS graded patients (C, D). That may cause inaccuracies in measuring the effect of the treatment. It can be balanced by the functional tests WISCI II, 10MWT, and 6MWT we conducted (Steeves et al., 2007).
The lack of a control group is a limitation of this study. A randomized controlled trial to further assess and compare the improvements in motor function with HAL training is planned for future studies. This should help to eliminate potential biases like placebo effect or investigator bias (Lammertse et al., 2007). Hybrid assistive limb training should be compared to conventional training and gait machines with other means of control, such as the Lokomat (Wall et al., 2015).
Conclusion
This study provides evidence that a training protocol with BWSTT with assisted HAL training improves functional ambulation in all participants. It indicates, that acute as well as chronic participants with SCI enhance their functional ambulation without the exoskeleton. While further investigation is needed to compare such training to a control group using randomization, these findings are a first step toward discovering a walking protocol for individuals with SCI regardless of chronicity.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of BG University Hospital Bergmannsheil and the Ruhr University Bochum. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
AZ: conceptualization, methodology, investigation, writing—original draft, visualization, and project administration. MA: conceptualization, methodology, investigation, reviewing and editing, visualization, and project administration. AB: conceptualization, methodology, and writing—reviewing and editing. DK: formal analysis, data curation, writing—reviewing and editing, and visualization. TS: conceptualization, writing—reviewing and editing, and supervision. DG: conceptualization, methodology, writing—reviewing and editing, and supervision. All authors have read and approved the final manuscript.
Funding
This work was supported by the New Energy and Industrial Technology Development Organization, Japan (NEDO) and a governmental grant (I&K-Gender-Study, European-Union, and NRW, Germany under Grant number 005-GW02-069A). The trial has been exclusively performed and supervised by the staff of BG University Hospital Bergmannsheil Bochum.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge support by the Open Access Publication Funds of the Ruhr-Universität Bochum.
Abbreviations
SCI, spinal cord injury; HAL, hybrid assistive limb exoskeleton; BWSTT, bodyweight supported treadmill training; 10MWT, 10 meter walk test; 6MWT, 6 minute walk test; LEMS, lower extremity motor score; WISCI II, walking index for spinal cord injury II; ASIA/AIS, American spinal injury association/Asia impairment scale; ZPP, zones of partial preservation.
References
Aach, M., Cruciger, O., Sczesny-Kaiser, M., Höffken, O., Meindl, R. C., Tegenthoff, M., et al. (2014). Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: a pilot study. Spine J. 14, 2847–2853. doi: 10.1016/j.spinee.2014.03.042
Aach, M., Meindl, R. C., Geßmann, J., Schildhauer, T. A., Citak, M., and Cruciger, O. (2015). Exoskelette in der rehabilitation querschnittgelähmter. Unfallchirurg 118, 130–137. doi: 10.1007/s00113-014-2616-1
Bohannon, R. W., and Crouch, R. (2017). Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J. Eval. Clin. Pract. 23, 377–381. doi: 10.1111/jep.12629
Brinkemper, A., Grasmücke, D., Yilmaz, E., Reinecke, F., Schildhauer, T. A., and Aach, M. (2021). Influence of locomotion therapy with the wearable cyborg HAL on bladder and bowel function in acute and chronic SCI patients. Glob. Spine J. 2021, 219256822110038. doi: 10.1177/21925682211003851
Brown-Triolo, D. L., Roach, M. J., Nelson, K., and Triolo, R. J. (2002). Consumer perspectives on mobility: implications for neuroprosthesis design. J. Rehabil. Res. Dev. 39, 659–669.
Colombo, G., Joerg, M., Schreier, R., and Dietz, V. (2000). Treadmill training of paraplegic patients using a robotic orthosis. J. Rehabil. Res. Dev. 37, 693–700.
Colombo, G., Wirz, M., and Dietz, V. (2001). Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal Cord. 39, 252. doi: 10.1038/sj.sc.3101154
Cruciger, O., Tegenthoff, M., Schwenkreis, P., Schildhauer, T. A., and Aach, M. (2014). Locomotion training using voluntary driven exoskeleton (HAL) in acute incomplete SCI. Neurology 83, 474. doi: 10.1212/WNL.0000000000000645
Curt, A., van Hedel, H. J. A., Klaus, D., and Dietz, V. (2008). Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J. Neurotrauma 25, 677–685. doi: 10.1089/neu.2007.0468
Ditunno, J. F., Ditunno, P. L., Graziani, V., Scivoletto, G., Bernardi, M., Castellano, V., et al. (2000). Walking index for spinal cord injury (WISCI): an international multicenter validity and reliability study. Spinal Cord 38, 234–243. doi: 10.1038/sj.sc.3100993
Ditunno, P. L., and Dittuno, J. F. Jr. (2001). Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord 39, 654–656. doi: 10.1038/sj.sc.3101223
Ditunno, P. L., Patrick, M., Stineman, M., and Ditunno, J. F. (2008). Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord 46, 500–506. doi: 10.1038/sj.sc.3102172
Estores, I. M. (2003). The consumer's perspective and the professional literature: what do persons with spinal cord injury want? J. Rehabil. Res. Dev. 40(4 Suppl. 1), 93–98. doi: 10.1682/jrrd.2003.08.0093
Fawcett, J. W., Curt, A., Steeves, J. D., Coleman, W. P., Tuszynski, M. H., Lammertse, D. P., et al. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45, 190. doi: 10.1038/sj.sc.3102007
Grasmücke, D., Zieriacks, A., Jansen, O., Fisahn, C., Sczesny-Kaiser, M., Wessling, M., et al. (2017). Against the odds: what to expect in rehabilitation of chronic spinal cord injury with a neurologically controlled hybrid assistive limb exoskeleton. A subgroup analysis of 55 patients according to age and lesion level. Neurosurg. Focus 42, E15. doi: 10.3171/2017.2.FOCUS171
Harada, N. D., Chiu, V., and Stewart, A. L. (1999). Mobility-related function in older adults: assessment with a 6-minute walk test. Arch. Phys. Med. Rehabil. 80, 837–841. doi: 10.1016/s0003-9993(99)90236-8
Holanda, L. J., Silva, P. M. M., Amorim, T. C., Lacerda, M. O., Simão, C. R., and Morya, E. (2017). Robotic assisted gait as a tool for rehabilitation of individuals with spinal cord injury: a systematic review. J. Neuroeng. Rehabil. 14, 126. doi: 10.1186/s12984-017-0338-7
Kirshblum, S. C., Burns, S. P., Biering-Sorensen, F., Donovan, W. H., Graves, D. E., Jha, A., et al. (2011). International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 34, 535–546. doi: 10.1179/204577211X13207446293695
Kirshblum, S. C., Millis, S., McKinley, W., and Tulsky, D. (2004). Late neurologic recovery after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1811–1817. doi: 10.1016/j.apmr.2004.03.015
Kubota, S., Nakata, Y., Eguchi, K., Kawamoto, H., Kamibayashi, K., Sakane, M., et al. (2013). Feasibility of rehabilitation training with a newly developed wearable robot for patients with limited mobility. Arch. Phys. Med. Rehabil. 94, 1080–1087. doi: 10.1016/j.apmr.2012.12.020
Lam, T., Eng, J. J., Wolfe, D. L., Hsieh, J. T., and Whittaker, M. (2007). A systematic review of the efficacy of gait rehabilitation strategies for spinal cord injury. Top. Spinal Cord Inj. Rehabil. 13, 32–57. doi: 10.1310/sci1301-32
Lam, T., Wolfe, D. L., Domingo, A., Eng, J. J., and Sproule, S. (2014). Lower Limb Rehabilitation Following Spinal Cord Injury: Gait Retraining Strategies to Enhance Functional Ambulation. Available online at: https://scireproject.com/evidence/rehabilitation-evidence/lower-limb/ (accessed February 11, 2019).
Lammertse, D. P., Tuszynski, M. H., Steeves, J. D., Curt, A., Fawcett, J. W., Rask, C., et al. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord 45, 232–242. doi: 10.1038/sj.sc.3102010
Lee, B. A., Leiby, B. E., and Marino, R. J. (2016). Neurological and functional recovery after thoracic spinal cord injury. J. Spinal Cord Med. 39, 67–76. doi: 10.1179/2045772314Y.0000000280
Mehrholz, J., Harvey, L. A., Thomas, S., and Elsner, B. (2017). Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord 55, 722–729. doi: 10.1038/sc.2017.31
Mehrholz, J., Kugler, J., and Pohl, M. (2012). Locomotor training for walking after spinal cord injury. Cochrane Database Syst. Rev. 11, CD006676. doi: 10.1002/14651858.CD006676.pub3
Mehta, S., Teasell, R. W., Loh, E., Short, C., Wolfe, D. L., Benton, B., et al. (2019). Pain Following Spinal Cord Injury. Available online at: https://scireproject.com/evidence/rehabilitation-evidence/pain-management/ (accessed February 12, 2019).
Morawietz, C., and Moffat, F. (2013). Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch. Phys. Med. Rehabil. 94, 2297–2308. doi: 10.1016/j.apmr.2013.06.023
National Spinal Cord Injury Statistical Center. (2021). Facts and Figures at a Glance. Available online at: https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%202020.pdf (accessed June 1, 2021).
Piepmeier, J. M., and Jenkins, N. R. (1988). Late neurological changes following traumatic spinal cord injury. J. Neurosurg. 69, 399–402. doi: 10.3171/jns.1988.69.3.0399
Schwartz, I., Sajina, A., Neeb, M., Fisher, I., Katz-Luerer, M., and Meiner, Z. (2011). Locomotor training using a robotic device in patients with subacute spinal cord injury. Spinal Cord 49, 1062–1067. doi: 10.1038/sc.2011.59
Sczesny-Kaiser, M., Höffken, O., Aach, M., Cruciger, O., Grasmücke, D., Meindl, R. C., et al. (2015). HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J. Neuroeng. Rehabil. 12, 68. doi: 10.1186/s12984-015-0058-9
Steeves, J. D., Lammertse, D. P., Curt, A., Fawcett, J. W., Tuszynski, M. H., Ditunno, J. F., et al. (2007). Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 45, 206–221. doi: 10.1038/sj.sc.3102008
Suzuki, K., Mito, G., Kawamoto, H., Hasegawa, Y., and Sankai, Y. (2007). Intention-based walking support for paraplegia patients with robot suit HAL. Adv. Robot. 21, 1441–1469. doi: 10.1163/156855307781746061
van Hedel, H. J. A., Wirz, M., and Curt, A. (2006). Improving walking assessment in subjects with an incomplete spinal cord injury: responsiveness. Spinal Cord 44, 352–356. doi: 10.1038/sj.sc.3101853
van Hedel, H. J. A., Wirz, M., and Dietz, V. (2005). Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch. Phys. Med. Rehabil. 86, 190–196. doi: 10.1016/j.apmr.2004.02.010
van Vliet, P., and Wing, A. M. (1991). A new challenge–robotics in the rehabilitation of the neurologically motor impaired. Phys. Ther. 71, 39–47. doi: 10.1093/ptj/71.1.39
Wall, A., Borg, J., and Palmcrantz, S. (2015). Clinical application of the hybrid assistive limb (HAL) for gait training-a systematic review. Front. Syst. Neurosci. 9, 48. doi: 10.3389/fnsys.2015.00048
Warburton, D. E. R., Krassioukov, A., Sproule, S., and Eng, J. J. (2018). “Cardiovascular health and exercise following spinal cord injury,” Spinal Cord Injury Rehabilitation Evidence, eds J. J. Eng, R. W. Teasell, W. C. Miller, D. L. Wolfe, A. F. Townson, J. T. C. Hsieh, S. J. Connolly, S. Mehta, B. M. Sakakibara (Vancouver, CA), 1–68.
Waters, R. L., Adkins, R., Yakura, J., and Vigil, D. (1994). Prediction of ambulatory performance based on motor scores derived from standards of the American Spinal Injury Association. Arch. Phys. Med. Rehabil. 75, 756–760.
Keywords: spinal cord injury, training, exoskeleton, hybrid assistive limb, rehabilitation
Citation: Zieriacks A, Aach M, Brinkemper A, Koller D, Schildhauer TA and Grasmücke D (2021) Rehabilitation of Acute Vs. Chronic Patients With Spinal Cord Injury With a Neurologically Controlled Hybrid Assistive Limb Exoskeleton: Is There a Difference in Outcome? Front. Neurorobot. 15:728327. doi: 10.3389/fnbot.2021.728327
Received: 21 June 2021; Accepted: 04 October 2021;
Published: 27 October 2021.
Edited by:
Shih-Chiao Tseng, University of Texas Medical Branch at Galveston, United StatesReviewed by:
Taimoor Afzal, Northwestern University, United StatesMichelle Sawtelle, University of St. Augustine for Health Sciences, United States
Copyright © 2021 Zieriacks, Aach, Brinkemper, Koller, Schildhauer and Grasmücke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexis Brinkemper, YWxleGlzLmJyaW5rZW1wZXJAYmVyZ21hbm5zaGVpbC5kZQ==
 Amrei Zieriacks
Amrei Zieriacks Mirko Aach1
Mirko Aach1 Alexis Brinkemper
Alexis Brinkemper Dennis Grasmücke
Dennis Grasmücke