
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Behav. Neurosci., 30 January 2025
Sec. Emotion Regulation and Processing
Volume 18 - 2024 | https://doi.org/10.3389/fnbeh.2024.1504229
Rationale: Music therapy has been in practice for years. However, the mechanism of action of music or music therapy is not well understood. It is only recently that the neuroendocrinological basis of therapeutic relationships has become the subject of growing research interest. The aim of this pilot study (Clinical Trial No: DRKS00035174) is to investigate whether oxytocin is usable and feasible as a biomarker of attachment to demonstrate the development of therapeutic alliance between therapist and patient in a dyadic music therapy setting.
Methods: In a single-measure crossover design, children aged 6–12 years from a special school for social and emotional disorders, were randomly with either music therapy followed by a waiting list control group that performed silent work, or vice versa. The respective interventions were conducted on the school premises on different days over a period of 1 month. The primary outcome was salivary oxytocin, with tests performed immediately before and after each 30-min intervention.
Results: Thirty-two children were included in the study, resulting in n = 16 children per allocation sequence. During the implementation of the study, difficulties were encountered with protocol adherence both in terms of the duration of the music therapy and the implementation of the silent work in the control group. There were no dropouts, however, only 28 children were included in the final data analysis as two participants in each group were excluded due to large fluctuations in oxytocin levels. Between-group comparison and within-group comparisons showed no significant changes in oxytocin levels. However, the music therapist showed a significant increase in oxytocin levels in the before after measurement. No side effects or adverse events were reported during the trial.
Conclusion: The findings indicated a responsiveness of oxytocin to musical stimulation. Although feasibility of oxytocin measurement was clearly demonstrated, evaluation of the results is difficult against the background of many remaining questions regarding individual and contextual factors influencing the oxytocinergic system. Moreover, the clinical significance of changes in oxytocin levels remains a topic for further research to better understand the role of oxytocin in the attachment formation between therapist and patient in music therapy.
The foundations of attachment theory were established in the 20th century by the pediatrician John Bowlby and the developmental psychologist Mary Ainsworth (Ainsworth and Bowlby, 1991; Bretherton, 1992). In their work, attachment is defined as an independent neurobiological system that develops through interaction between the child and the caregiver, providing the child with a sense of protection and a secure base from which to explore and return to (Benoit, 2004; Chambers, 2017). According to attachment theory, this creates an inner working model of relationships, which can result in four different attachment styles: secure, insecure-avoidant, insecure-resistant and insecure-disorganized (Archer et al., 2015; Shemmings, 2006).
Over the years, a large body of literature has accumulated investigating the link between attachment style and mental or physical health outcomes as well as social functioning and affect regulation (Groh et al., 2017; Pietromonaco and Beck, 2019; Widom et al., 2018). Compared to secure attachment styles, insecure attachment styles are associated with a range of emotional and behavioral problems, including depression (Simon et al., 2019; Warfa et al., 2014), anxiety (Manning et al., 2017; Nielsen et al., 2017), suicidal behavior (Falgares et al., 2017; Naifeh et al., 2024) as well as the risk and severity of drug addiction (Strathearn et al., 2019; Valizadeh et al., 2017). It is further a greater risk for the development of chronic pain (Romeo et al., 2017), increased pain perception (Peñacoba et al., 2018), and an overall reduced quality of life (Bodner and Cohen-Fridel, 2010; Karveli et al., 2023).
Attachment styles primarily develop in early childhood in relation to primary caregivers and often show a certain stability over time. However, they can also change during life in relation to subsequent attachment figures, thereby alter a persons’ sense of attachment security (Mikulincer et al., 2013; Pinquart et al., 2013). According to Bowlby, a variety of people can serve as attachment figures if they meet a number of conditions: 1. maintain proximity, 2. represent a safe haven physically and emotionally, and 3. provide a secure base from which to explore (Bowlby, 1982). In this way, a therapist can also provide a patient with a secure base and strengthen the feeling of attachment security (Mikulincer et al., 2013).
Although the concepts of ‘attachment’ and ‘bonding’ are often used interchangeably in the literature, the difference should be articulated at this juncture. While both theories deal with the formation of relationships, bonding primarily refers to physical closeness, especially skin-to-skin contact, during a certain period after birth, which is primarily intended to serve the acceptance of the child by the parents (Ettenberger et al., 2021).
Various methods have been used to measure attachment, including interviews like the Adult Attachment Interview (AAI) (George et al., 1985), self-reports (Hazan and Shaver, 1987), scales and questionnaires (Ravitz et al., 2010), behavioral observation as in the Strange Situation Test developed by Mary Ainsworth (Van Rosmalen et al., 2015) and, more recently, the collection of biomarkers such as oxytocin (Feldman, 2012; Tops et al., 2007). To date, self-reports have been used predominantly to determine therapy outcomes, but with large numbers of patients seeking psychotherapy, there is a growing demand to complement subjective reports with objective measures such as biomarkers (Atzil-Slonim et al., 2022). However, particularly the investigation of underlying mechanisms of psychotherapy and the neurobiological basis of human attachment, is still in its early stages (Zilcha-Mano et al., 2020).
Evidence of endocrine synchrony in periods of bond formation has previously been found between parents and their children as well romantic partners (Djalovski et al., 2021; Feldman et al., 2011; Schneiderman et al., 2014; Ulmer-Yaniv et al., 2016). This raises the question of whether such a synchronization process might also occur between a therapist and his patient and, moreover, whether this hormonal attunement might correlate with the success of a therapy (Zilcha-Mano et al., 2020).
In a meta-analysis, (Flückiger et al., 2018) showed that therapeutic alliance and thus psychotherapy as an attachment-based intervention is one of the most reliable predictors of therapeutic change. Therefore, linking attachment theory with oxytocin as a potential biomarker for attachment formation, i.e., the therapeutic alliance is ostensibly promising and of growing interest (Palmieri et al., 2021; Striepens et al., 2011).
Oxytocin is a peptide hormone synthesized in the supraoptic and paraventricular nuclei of the hypothalamus. It is released both centrally and peripherally (Harvey, 2020) in situations of sensory stimulation such as skin-to-skin contact, food intake, labor and breastfeeding (Uvnäs-Moberg et al., 2015). Increasing research in oxytocin is currently been driven by its effects on social interaction in humans (Bartz et al., 2011; Erdozain and Peñagarikano, 2020) in particular in psychotherapy research. According to Baskaran et al. (2017) oxytocin has an important mediating role in human social–emotional functioning.
To date, there are only a few studies in which oxytocin levels have been measured before and after a psychotherapy session, so that overall little evidence is available in this area (Zilcha-Mano et al., 2020). Previous studies have primarily investigated the value of oxytocin as a biomarker for treatment outcomes in patients with depression. However, these studies support the hypothesis that oxytocin reactivity es treatment response. In several studies, oxytocin levels were measured in patients only, therapists only, or patients and therapists before and after treatment. Results showed that oxytocin synchrony between therapist and patient was associated with effective treatment (Zilcha-Mano et al., 2021), oxytocin plasma levels at baseline predicted improvement in self-rated depression (BDI-II-scores) (Jobst et al., 2018), therapists’ oxytocin response predicted reduction in patients’ depressive symptoms in the next session (Fisher et al., 2023), and oxytocin reactivity was associated with improvement in depressive symptoms (Atzil-Slonim et al., 2022).
Thus, the question remains to what extent hormonal changes are predictive and indicative of psychotherapeutic changes (Fischer and Zilcha-Mano, 2022). It is hypothesized that baseline levels of oxytocin represent the trait-like effects and could therefore act as a biomarker for the extent to which people are receptive to a particular treatment, i.e., baseline levels may be predictive. On the other hand, changes in oxytocin levels, the state-like-effects, over the course of a treatment may function as a biomarker for the degree of synchronization between therapist and patient, i.e., changes in oxytocin levels may be indicative (Zilcha-Mano et al., 2020).
Beyond this, there is a growing field of research dedicated to oxytocin administration and psychotherapy. The rationale here is that exogenously supplied oxytocin may act as a therapeutic agent itself or as a catalyst improving therapeutic alliance and may thus be used as an augmenting agent in psychotherapy (Koch et al., 2014; Olff et al., 2010; Zilcha-Mano et al., 2020). Although a number of studies show promising preliminary results regarding reported improvements in therapeutic outcomes, the evidence is not yet conclusive and there is still a lack of large-scale studies to substantiate the results obtained so far (Ellenbogen et al., 2024; Grossman-Giron et al., 2023; Hertenstein et al., 2021; MacDonald et al., 2013).
Altered oxytocin levels have been previously reported in a number of psychiatric disorders, including autism spectrum disorder (Huang et al., 2021; John and Jaeggi, 2021), borderline personality disorder (Bertsch et al., 2013; Carrasco et al., 2020), post-traumatic stress disorder (Carmassi et al., 2021; Donadon et al., 2018), schizophrenia (Hernández-Díaz et al., 2021; Strauss et al., 2019), social anxiety (Schneider et al., 2021) as well as in children with adverse childhood experiences (Ellis et al., 2021). Nevertheless, a meta-analysis by (Ferreira and Osório, 2022) showed equivocal results regarding altered oxytocin levels in psychiatric patients compared to healthy controls. Yet difficulties in forming relationships are characteristic of these disorders, which may also result in a lower-quality therapeutic alliance (Fischer and Zilcha-Mano, 2022). In this regard, the stimulation of endogenous oxytocin release, as possibly in music therapy, or the exogenous supply of oxytocin, as in previous studies with nasally applied oxytocin, is a promising therapeutic approach in this area of medicine (Striepens et al., 2011).
Music therapy as defined by the American Music Therapy Association is “the clinical and evidence-based use of music interventions to achieve individual goals within a therapeutic relationship by a credentialed professional who has completed an approved music therapy program” (American Music Therapy Association, 2024). Therefore, music therapy uses various components of music such as melody, harmony, rhythm, timbre and pitch to support the development of a therapeutic relationship between therapist and patient (Hanson-Abromeit, 2015; Mössler et al., 2019). In this process, music therapy can consist of active or passive music interventions as well as a mixture of these qualities and may be provided both individually and in groups (Lynch et al., 2021; Wheeler et al., 2003). While music therapy requires extensive training and knowledge of musical skills on the part of the therapist, no prior musical training or musical skills are needed at the client’s site. Thus, music therapy can be used in a variety of clinical contexts for patients with physiological, psychological, spiritual, cognitive, social and behavioral challenges (Dileo and Bradt, 2009).
Music therapists use a broad repertoire of musical interventions and cover a wide range of indications across the entire lifespan of people in numerous outpatient and inpatient facilities (Kern and Tague, 2017). Target groups for music therapy include among others premature infants (Loewy et al., 2013; Standley, 2002), patients with depression and/or anxiety (Belski et al., 2022; Jasemi et al., 2016; Zhao et al., 2016), sleep disorders (Tang et al., 2022; Wang et al., 2014), dementia (Lam et al., 2020; Ueda et al., 2013), stroke (Liu et al., 2022; Wang et al., 2021), mental disorders (Gold et al., 2009), post-traumatic stress disorder (Baker et al., 2018), chronic pain (Hsu et al., 2022; Korhan et al., 2014) as well patients with cancer or in palliative care (Köhler et al., 2020; Pérez-Eizaguirre and Vergara-Moragues, 2021).
The benefits of music therapy and music’s effect on mood (Dóro et al., 2017; Raglio et al., 2015), language (Liu et al., 2022), communication and social skills (Shi et al., 2024) as well as motor, cognitive, psychological and emotional functioning (Fujioka et al., 2018; Weller and Baker, 2011) have been the subject of considerable research. And although the psychophysiology of music therapy has been examined, e.g., on its impact on the experience of pain (Arnold et al., 2024), the full landscape of mechanism of action of music therapy responsible for these outcomes is not well understood (Brancatisano et al., 2020). Indeed, music therapy has long lacked standard research tools and a common methodology, and in the past only gradually the way has been paved for evidence-based music therapy (Aldridge, 1994; Hillecke et al., 2005). Today, evidence based music therapy research includes translational research approaches such as investigations of neural mechanism and activities involved in musical perception (Thaut et al., 2021; Chen et al., 2022).
In this respect, neuroendocrinology holds great potential for enabling scientific progress in the research field of music therapy as it provides objective and reproducible results.
To a considerable extent, the brain regions responding to interactive prosocial tasks overlap with those activated when making or listening to music (Harvey, 2020). It is therefore possible that the biology of oxytocin mirrors at least parts of the positive and diverse effects of music (Keeler et al., 2015).
Overall, only a few studies have investigated the relationship between oxytocin and music. In a systematic review, (Busse et al., 2024) examined studies that measured oxytocin levels before and after a music intervention. Due to highly differing study designs, study quality, sample sizes and music interventions, comparability between the studies was limited, making further studies with comparable music interventions necessary to obtain clear results. Inconclusive findings suggest that the change in oxytocin levels depends on various individual and contextual factors such as music preferences and social environment, which need to be controlled for in further studies. There was only one study included in the review involving music therapy as a music intervention. Neither the within-subject comparisons nor the comparisons between groups, with the control group undergoing a home exercise program, showed significant changes in oxytocin levels (Palumbo et al., 2022).
Combining the previously described three elements of therapy, oxytocin and music, this pilot study investigates the mediating role of oxytocin in the therapeutic synchronization process in a music therapy setting. The aim of this study is therefore to examine whether the responsiveness of biomarkers to musical stimulation can be shown in music therapy, i.e., an increase in oxytocin levels in both therapist and patient. In this way, the development of a therapeutic alliance via the biomarker oxytocin is to be demonstrated.
The study was approved by the ethics committee of the Witten/Herdecke University (No. 122/2023) and is reported based on the CONSORT 2010 guidelines and the extension for randomized crossover trials. The music therapy intervention was described according to the reporting guidelines for music interventions (Robb et al. 2011).
Twenty-nine boys and three girls were recruited from the Old Vicarage school (“Altes Pfarrhaus”), Herdecke, Germany, a special school for children with social and emotional disorders.
The inclusion criteria for the study consisted of.
1. Children aged 6 to 12 years with social–emotional disorders and
2. The presence of a confirmed special educational need in social–emotional development based on the school assessment procedure.
The exclusion criteria were as follows.
1. The presence of cognitive, intellectual, or physical impairments that could be the cause of the social disorders,
2. A causality of the social problems in a circumscribed partial performance disorder and.
3. A concurrent participation in another clinical study or completion of participation in such a study less than 6 months ago.
The parents or legal representatives gave informed consent for their children to be involved in the study, so that ultimately all students (n = 32) from first to fourth grade who were attending the special school at the given time were recruited for participation.
A single-measure cross-over design was used for both within-subject and between-condition comparisons.
We opted for a crossover design as it allowed a small sample size to demonstrate an effect and, due to its very short half-life of <10 min in the blood (Chard et al., 1970; Leng and Sabatier, 2016), oxytocin was very well suited to carry out the two interventions in the same collective. Another consideration in favor of a crossover design was the fact that the primary therapeutic goal of music therapy for social and emotional disorders was to improve symptomatology, i.e., social functioning, so no causal change was anticipated between interventions. Further, no short-term change in the characteristic of the known social and emotional disorders was expected after a single exposure to either of the interventions, which potentially could have led to side effects, withdrawal from the study or confounding of the results. A disadvantage inherent in the design of the intervention groups was the impossibility of blinding, which had to be made an allowance for, assuming it would not have a decisive effect on the biomarker collected.
As we had no data on the expected difference between the groups, and, in addition, no estimates of within-participant variability were available in the literature for this particular study population, we used a moderate effect size of d = 0.55 to calculate the sample size. With an alpha error of 5% and a power of 80%, this resulted in a sample size of n = 28.
Before school started, participants of each class were randomly assigned to an allocation sequence by drawing lots: sequence one had music therapy sessions first and silent work sessions after, while sequence two had silent work sessions first followed by the music therapy sessions. The sequence was generated upon commencement of the study by the music therapist. Thus, each participant received a 30-min music therapy session as well as a 30-min silence work session, resulting in an allocation ratio of 1:1. There was at least 1 day, i.e., 24 h, between the two interventions, so that the washout phase can be considered long enough, and carryover effects can be safely excluded due to the short half-life of oxytocin.
All children were already familiar with music therapy from group and individual settings in their everyday school life as music therapy is an integral part of the school program.
Music therapy was provided in the school hall which is a rectangular room with an area of 50 m2. As it is architecturally located in a corner of the school, it has five large window panels on two walls, flooding the room with natural daylight. The instruments are positioned so that the children face diagonally into the room while playing, with their backs to the windows. This arrangement avoids visual distractions. To match the historical building ensemble (constructed in 1820), the walls are finished with clay plaster, which creates a pleasant indoor climate and prevents temperatures from exceeding 21°C, even during warm outdoor conditions. The room is fitted with a parquet floor and the walls are painted in a pastel terracotta tone. An acoustic ceiling and curtains on the windows help prevent excessive reverberation.
The music therapy sessions are based on the Nordoff-Robbins approach (Aigen, 2014) and were always provided by the same music therapist, who was already known to the children in advance. The room is equipped with a grand piano and instruments from the orchestra percussion section such as drums. All instruments can be played by children without prior knowledge or instrumental training. They can improvise freely, setting essential musical parameters such as tempo, rhythm, volume, intensity and timbre (Neugebauer and Ostermann, 2024). Each improvisation included a four-hand piano improvisation (sometimes with spontaneous singing) and a joint improvisation by the piano (therapist) and drum cymbal (played by the child with sticks or mallets). During the piano improvisation, the child sits in the treble, while the drum and cymbal are positioned to the right in extension of the keyboard at a distance of about 1 meter from the piano. This allows eye contact during the entire improvisation.
In the control condition, the children were located in a room on their own and could choose to either paint, read, write or play in silence.
Saliva samples were taken by a medically trained person before and after each session on the premises of the special school. In addition to the students, saliva samples were also taken from the music therapist before and after each music therapy session (n = 32). The interventions and sampling were conducted during regular school hours at identical times between 8 and 10 a.m. in order to avoid a potential distortion due to diurnal cycling of oxytocin (Forsling, 2000; Kerem and Lawson, 2021) and thus allowing the total data collection to be completed within a month.
As primary and exclusive outcome measure in this trial, salivary oxytocin levels were measured before and after music therapy or the control group. After collection with the Salivette® Cortisol from Sarstedt (item no. 51.1534.500), the saliva samples were frozen and stored at −20°C in a designated cooling device in the special school until analysis. Once all samples had been taken, they were transported on dry ice to the biopsychological laboratory in Dresden to ensure maintenance of the cold chain. Salivary oxytocin levels were measured using a commercially available ELISA with high sensitivity (Tecan, Hamburg, Germany; catalog number RE52331) without prior extraction. Following the laboratory’s recommendations, a total of 20% of the measurements were performed in duplicate to verify analytical quality. As reported by the laboratory, the intra- and interassay coefficients of variance were below 9%. Salivary oxytocin levels were excluded from data analysis, if differences of pre and post measures exceeded 30 pg./mL.
In this study, demographic data were routinely analyzed using descriptive statistics to summarize the key characteristics of the two groups. Comparisons between groups were performed using chi-square tests for categorical variables and t-tests or Mann–Whitney U tests for continuous variables, depending on the data distribution. These analyses ensured that any baseline differences between the groups were accounted for, minimizing potential confounding factors.
Between-group treatment outcomes were evaluated using a two-factor analysis of variance (ANOVA) to assess differences between the groups. An effect size estimate (Hedge’s g) was calculated to determine the clinical relevance of changes in oxytocin levels.
Within-group comparisons, subgroup analyses, and the evaluation of the music therapist’s oxytocin levels were performed using paired t-tests. Means, standard-deviations and corresponding 95%-confidence intervals were calculated. Additionally, assumptions for normality and homogeneity of variances were rigorously tested using Shapiro–Wilk and Levene’s tests, respectively, to ensure the validity of the statistical tests applied. In cases where these assumptions were violated, non-parametric alternatives were considered to maintain the robustness of the findings. All statistical analyses were conducted with a significance level set at p < 0.05, with adjustments made for multiple comparisons using the Bonferroni correction to control for Type I error. Data were analyzed with SPSS software version 28.
Of the 32 children who participated in the study, the results of 28 children were included in the final data analysis, as two participants in each group were excluded due to large fluctuations in oxytocin levels (Delta Ox >30 pg./mL) (Figure 1).
The remaining 28 participants had a mean chronological age of 8.7 years (SD = 1.3, range 6–11 years), including 25 boys and 3 girls. Most of the children included in the study were pre-diagnosed with multiple developmental and behavioral disorders, the most common being pervasive developmental disorders (F84) (n = 14), hyperkinetic disorders (F90) (n = 12), specific developmental disorders of speech and language (F80) (n = 8,), specific developmental disorder of motor function (F82) (n = 7), emotional disorders with onset specific to childhood (F93) (n = 5), disorders of social functioning with onset specific to childhood and adolescence (F94) (n = 5) and conduct disorders (F91) (n = 3). In the number of diagnoses given here, a child may have several diagnoses within one category, e.g., both an expressive and a receptive language disorder (F80.1 and F80.2). To ensure clarity, these diagnoses have been combined into superordinate groups. Concomitant pharmacological treatment with one or more medications was present in in overall nine children, including methylphenidate (n = 6, 18.8%), lisdexamphetamine (n = 3, 9.4%), risperidone (n = 3, 9.4%) and aripiprazole (n = 1, 3.1%). The respective diagnoses and medications for each child were made available from the school files. Sociodemographic and clinical characteristics are shown in Table 1.
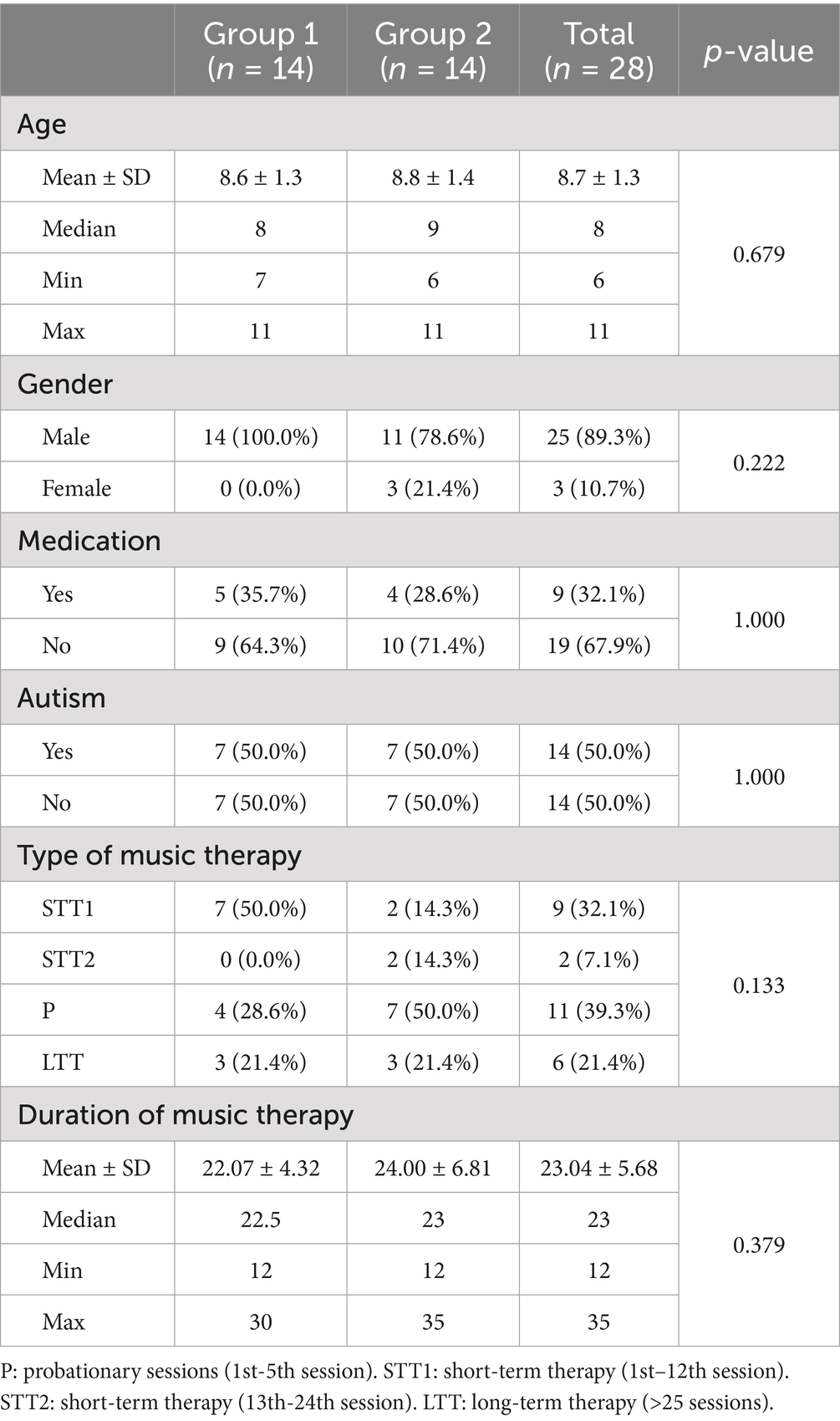
Table 1. Sociodemographic and clinical data of participants (Group 1: MT first, Group 2: WLC first).
At the beginning of the study, the children had already participated in music therapy to varying degrees as part of the school program. Accordingly, 11 children had already had probationary sessions, a further 11 children were in short-term therapy (STT1/STT2) and the remaining 6 children were in long-term therapy (> 25 sessions).
The duration of the music therapy varied between 12 and 35 min, as the children could not always be encouraged to participate for the entire duration of the therapy.
The treatment outcome was calculated using a two-factor analysis of variance (ANOVA), with the between-group comparison, namely music therapy and control group, showing no significant changes in oxytocin levels (p = 0.306, F = 1.089). However, the changes in oxytocin levels in the music therapy group showed a non-significant increase in oxytocin levels, while the control group showed no change in oxytocin levels in the before-after measurement. Figure 2 shows the oxytocin levels for the music therapy group compared to the waiting list control group for the total sample; Table 2 provides a list of the exact values.
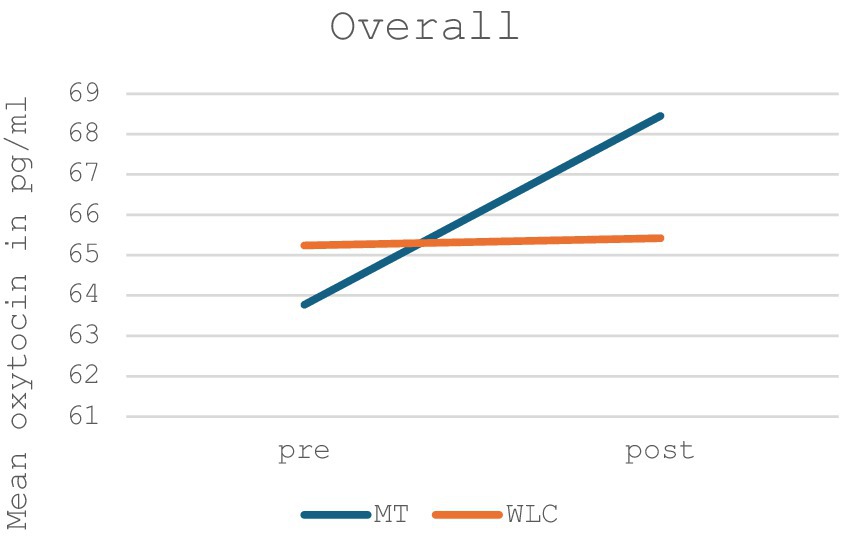
Figure 2. Mean oxytocin levels in music therapy [MT] compared to the waiting list control group [WLC] in the pre-post measurement for the total sample (n = 28).
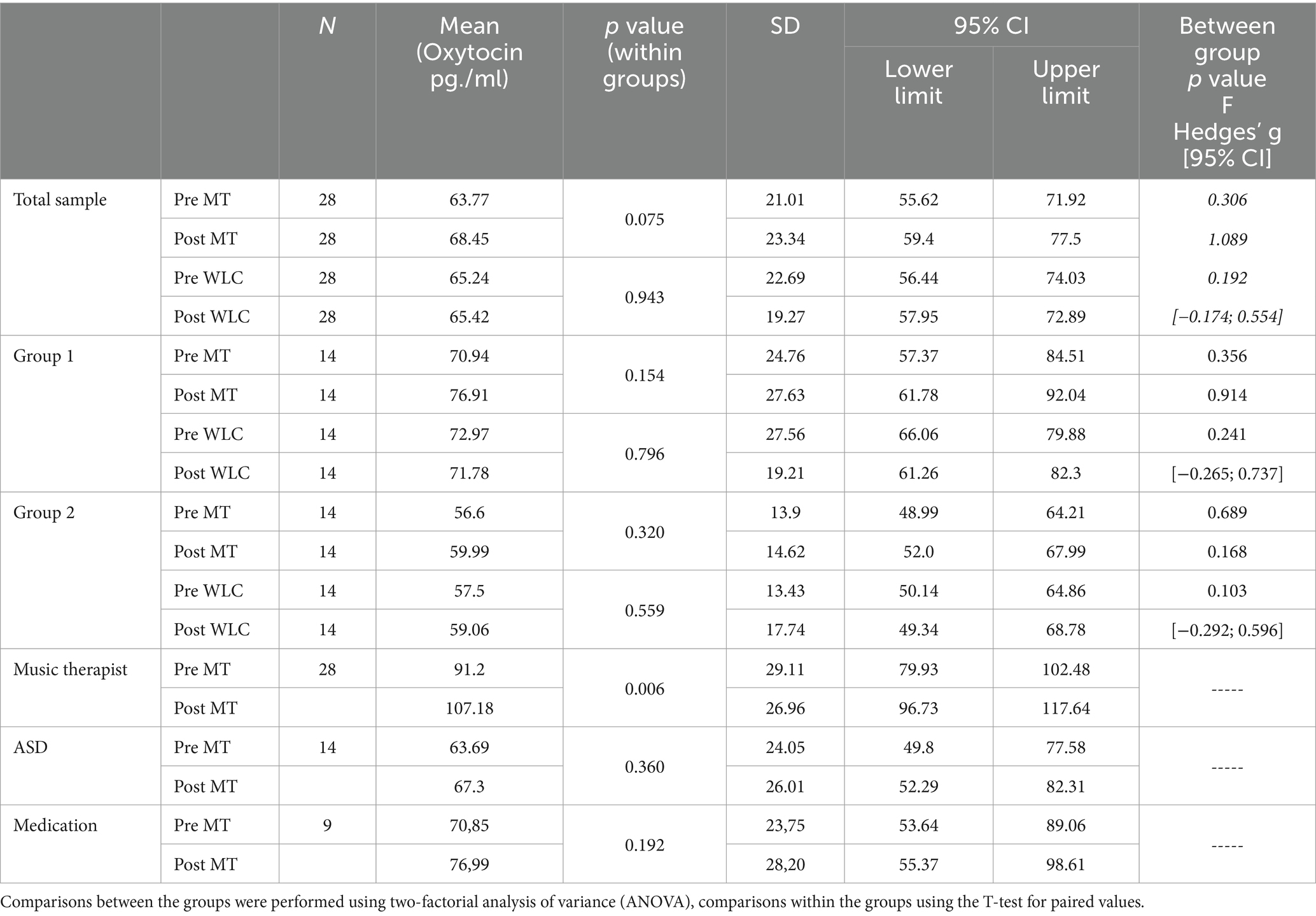
Table 2. Shown are the measured mean oxytocin levels with standard deviation, 95% confidence intervals, p-values for comparisons within and between the groups, F-values and the effect size (Hedges’ g) before and after music therapy [MT] or the waiting list control group [WLC] for the total sample, group 1, group 2, the music therapist and the subgroups of children with autism spectrum disorder [ASD] and those with medication.
When looking at Group 1, there was a non-significant increase in oxytocin levels in the music therapy group and an equally non-significant decrease in oxytocin levels in the control group (p = 0.356, F = 0.914). A graphical depiction of Group 1 can be found in Figure 3, the detailed values in Table 2.
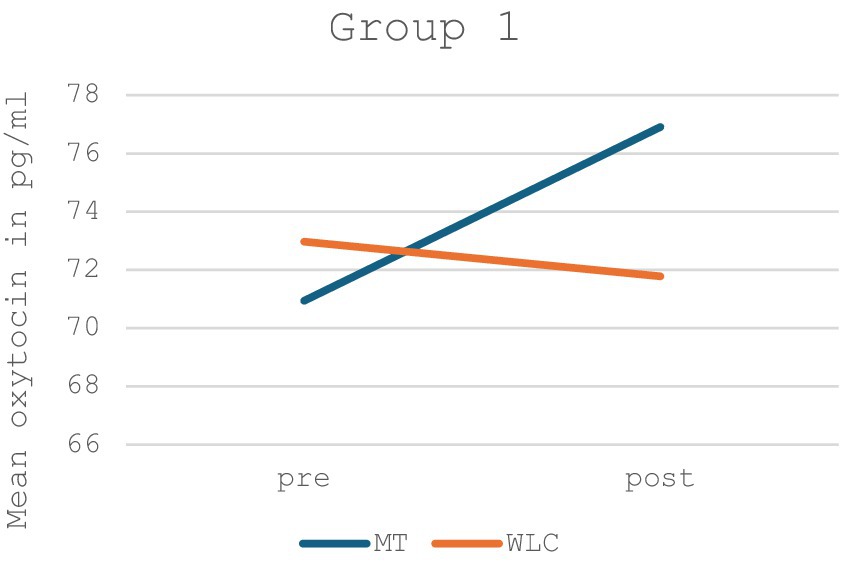
Figure 3. Mean oxytocin levels in music therapy [MT] compared to the waiting list control group [WLC] in the pre-post measurement for Group 1 (n = 14).
In contrast, Group 2 likewise showed a non-significant increase in oxytocin levels in the music therapy group, however, there was also an increase in oxytocin levels in the control group (p = 0.689, F = 0.168). An illustration and values for Group 2 can be found in Figure 4 and Table 2.
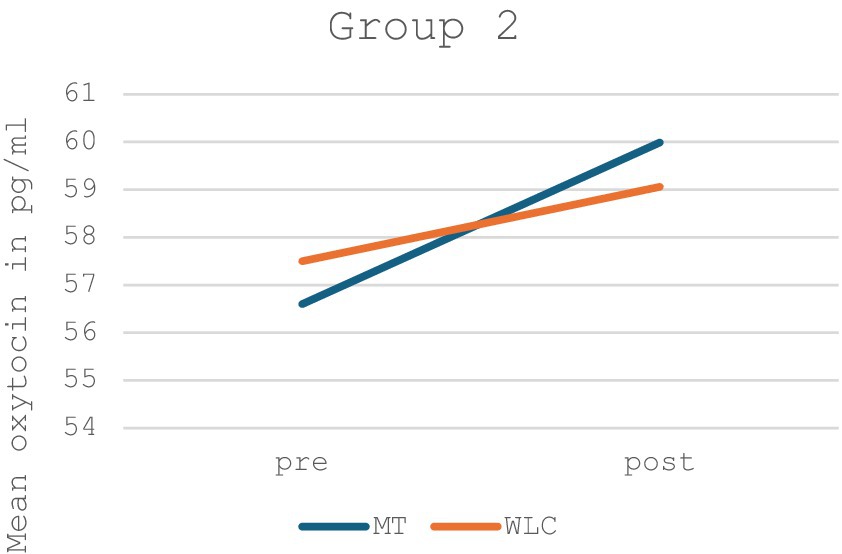
Figure 4. Mean oxytocin levels in music therapy [MT] compared to the waiting list control group [WLC] in the pre-post measurement for Group 2 (n = 14).
When comparing within groups using a paired-samples t-test, also no significant changes in oxytocin levels were found in either music therapy [p = 0.075, Hedges’ g = −0.340 CI95% (−0.708; 0.034)] nor the control group [p = 0.943, Hedges’ g = −0.013 CI95% (−0.373; 0.347)].
Nonetheless, the assessment of the within-group comparisons shows that the one-tailed p-value (one-tailed p = 0.038), in contrast to the two-tailed p-value (p = 0.075) for the music therapy group, is indeed significant.
In addition the effect of potentially oxytocin-level altering medication such as methylphenidate (n = 6, 18.8%) and lisdexamphetamine (n = 3, 9.4%) as described by Levi-Shachar et al. (2020) was analyzed. And although a moderately higher effect within this group was found [Hedges’ g = −0.429; CI95% (−1.042; 0.208); p = 0.192], it failed being significant between the groups when controlling for it (F = 0.635; p = 0.433). Moreover, there is evidence from a recent meta analysis, that children with autism have altered oxytocin levels (John and Jaeggi, 2021). As half of the children were diagnoses with Autism Spectrum Disorders (ASD), this could also have affected the results. Again, however, post hoc analysis of the results found an effect within the group [Hedges’ g = −0.239; CI95% (−0.735; 0.267); p = 0.360] but no significant difference between the groups when controlled for autism (F = 0.42; p = 0.840). All results of within-group comparisons are listed in Table 2.
As shown in Figure 5, the duration of music therapy had no effect on the measured oxytocin difference.
Finally, the analysis of the music therapist’s oxytocin values, i.e., the 28 averaged individual measurements, revealed a significant increase in the oxytocin levels [p = 0.006, Hedges’ g = −0.543 CI95% (−0.926; −0.150)].
With respect to the reliability of the results, the analysis of the 20% of samples measured in duplicate (n = 38) using a t-test for paired values showed a significant difference between the initial measurement and the control measurement (p = 0.007), as shown in Table 3. Even after excluding the four outliers (delta oxygen >30 pg./mL), there was still a significant difference between the first and second measurement. These measurement inaccuracies already indicate difficulties in using oxytocin as a biomarker in studies.
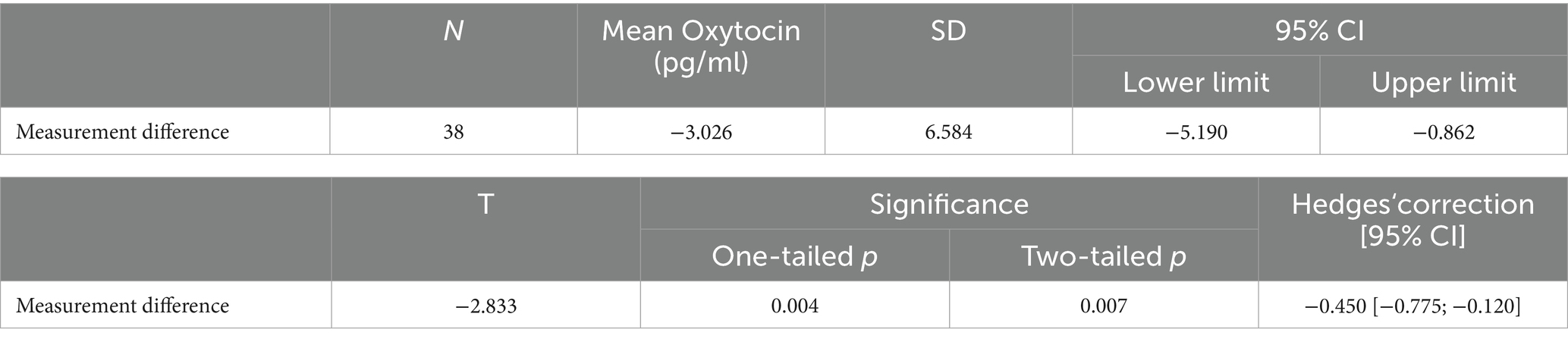
Table 3. Analysis of the 20% duplicated measurements (n = 38) using a paired samples t-test (total measurements: n = 192, including 32 children and the music therapist).
A total of 2 months recruitment time was required for this study and, after providing detailed information to parents and children, a consent rate of 100% (n = 32) was achieved for participation. There were no dropouts during the study, allowing it to be conducted as scheduled within 1 month.
However, there were some difficulties with protocol adherence in implementing the waiting list control group, as the children often had difficulty spending the full 30 min alone in a room. Consequently, several children occasionally left the room and interacted with other students and teaching staff during the time of the actual silent work. In this respect, a bias in the results of the control group cannot be ruled out. Similarly, the music therapy sessions could not always be carried out to its full extent, as the children did not always engage with it for the entire 30 min, so that the duration of the music therapy varied considerably (Table 1; Figure 5). Nevertheless, the study was well received by the students, and they cooperated to the best of their ability.
Overall, no significant change in oxytocin levels was measured either between groups, namely the music therapy and the waiting list control group, nor within groups. The non-significant increase in oxytocin concentration in the music therapy group, however, in line with previous literature (Fancourt et al., 2014; Grape et al., 2002; Kreutz, 2014), suggests a responsiveness of oxytocin to musical stimulation. In contrast, oxytocin levels remained unchanged in the waiting list control group.
When looking at Group 1 and Group 2 separately, music therapy showed a non-significant increase in oxytocin concentration in each respective group, whereas a slight decrease in oxytocin was measured in the control group of Group 1 and a slight increase in oxytocin in the control group of Group 2. Yet, a bias in the results of the control group cannot be ruled out with certainty due to the above-mentioned difficulties in the conduct of the silent work with these children. A similar problem was also evident in the implementation of the music therapy group, however, the evaluation showed that the considerably varying duration of the music therapy had no significant effect on the measured oxytocin difference. Nevertheless, an effect of the order of interventions is highly unlikely due to randomization to an allocation sequence. Further, a carryover effect is also not to be expected due to the short half-life of oxytocin and a washout phase of >24 h.
In the subgroup analysis, the results of the children with an autism spectrum disorder, who made up exactly half of the study population, were examined. No differences were found compared to children without autism spectrum disorder, so that no evidence of a specific response in oxytocin levels was found in the subgroup of children with autism spectrum disorder.
The music therapist showed a significant increase in oxytocin levels in the before-after comparison. This also demonstrated a clear responsiveness of the biomarker oxytocin to musical stimulation. Furthermore, the question arises as to whether repeated music therapy in particular is associated with an increase in levels and to what extent the perception of the situation, i.e., the feeling of well-being or discomfort during music therapy, has an influence on changes in oxytocin levels.
As far as we know, there is only one other study that measured oxytocin before and after music therapy, so there is little comparable data on this subject. Nevertheless, in this study by (Palumbo et al., 2022), also no significant changes in oxytocin levels before and after music therapy were reported. However, when looking at a wider range of studies, where oxytocin levels were measured before and after various types of music interventions, findings showed a responsiveness of oxytocin to music, but with contradictory results regarding an increase, decrease or unchanged oxytocin levels. The authors of the respective studies attribute the ambiguous results primarily to individual factors and contextual factors influencing the oxytocinergic system that were not sufficiently controlled or comparable in these particular studies (Bowling et al., 2022; Busse et al., 2024; Fancourt et al., 2016; Keeler et al., 2015; Schladt et al., 2017).
When considering oxytocin as a biomarker for attachment, there are therefore a series of challenges in interpreting the results. First of all, there are no standard values for oxytocin in a healthy normal population available in the literature (Martins et al., 2020), rather, human studies rely on the repeated assessment of oxytocin levels as pre-post measurements of an intervention, i.e., baseline values and changes in oxytocin levels, so that primarily an activity or reactivity of the oxytocinergic system to stimuli is investigated (Tabak et al., 2023). Further, against the background of contradictory results in oxytocin research the dependence of oxytocin on individual factors such as age, sex, female cycle as well as context is repeatedly pointed out (Bartz et al., 2011; Engel et al., 2019; Marazziti et al., 2019; Olff et al., 2013; Zak et al., 2022). Finally, interactions of oxytocin with other neuromodulator systems including dopamine, endorphine and serotonin makes the field even more complex and thus complicates the identification of neurobiological signaling pathways (Iovino et al., 2021).
In view of the growing literature on the topic of potentially reduced oxytocin levels in psychiatric patients, studies in which precisely this patient collective of children with adverse childhood experiences was examined with regard to oxytocin levels are of particular interest for the interpretation of the present study results. For example, in a study from Japan, Suzuki et al. (2020) showed that maltreated children had lower oxytocin levels compared to the healthy control group. In addition, Ellis et al. (2021) showed in a series of meta-analyses that adverse childhood experiences predicted lower oxytocin levels. Further research will thus need to address the role of adaptations of the oxtocinergic system in the context of early childhood experiences, as well as the potential for prevention and intervention.
Another field of oxytocin research is concerned with the genetics and epigenetics of the oxytocinergic system, including oxytocin receptor polymorphisms and methylation status (Harvey, 2020; Chen et al., 2020). However, the effects of oxytocin receptor alleles on social behavior are complex, assessments across studies are not always consistent, and the extent to which these inter-individual differences play a role in empathy, stress regulation and social behavior (Rodrigues et al., 2009; Sicorello et al., 2020) is still the subject of research.
Nevertheless, initial results are available with regard to the patient population investigated in this pilot study. For instance, in a meta-analysis, Poore and Waldman (2020) showed an association of oxytocin receptor polymorphisms and antisocial behavior. In addition, Moerkerke et al. (2021) in a systematic review showed that hypermethylation of the oxytocin receptor is associated with higher quantitative autism traits in adults. The transfer and inclusion of these results in the interpretation of the results of this pilot study is still challenging at this stage. Nevertheless, the field of genetics and epigenetics will be an integral part of oxytocin research in order to draw a comprehensive portrait of the complexity of the oxytocinergic system.
Taken together, this makes it particularly difficult to interpret results, namely the changes in oxytocin levels, of patients with different diseases or healthy controls and to classify their clinical relevance.
In addition, the measurement of oxytocin in different compartments of the body, namely CSF, plasma, saliva and urine, also poses a challenge, as various studies report correlations between the compartments (Valstad et al., 2017) and possible diurnal cycling of oxytocin levels, but there is no clear scientific consensus to date (Carson et al., 2015; Kagerbauer et al., 2019). Further, a lack of standardization in the measurement and evaluation of oxytocin samples is also repeatedly a subject of criticism, since results are often only comparable to a limited extent (Tabak et al., 2023).
Therefore, conclusions of the results should only be drawn with caution and against the background of the current state of knowledge in oxytocin research.
In terms of the limitations of this pilot study, the significance of the results obtained is limited due to the relatively small number of cases and should be considered preliminary. Due to the nature of the interventions, blinding was precluded and as the study design did not include a control group of healthy children, comparability with a healthy population is limited.
Finally, study participants were familiar with music therapy prior to the study and most of them already had received music therapy. This might have an impact on oxytocin measures given the potential for an already developed therapeutic relationship. However the study sample was too small to go into a detailed subgroup analysis including the participant’s history of music therapy. It is also unclear whether long-term music therapy modulates oxytocin response in humans or whether it remains to occur spontaneously, which is also discussed in a previously conducted systematic review on the role of oxytocin in music interventions (Busse et al., 2024). On the other hand, given these are children with social and emotional disorders, the struggle to develop attachment, a significant change in oxytocin within the timeframe of the study might also be questioned.
As already reported by (Chanda and Levitin, 2013), field studies such as the one presented here, suffer from confounding factors that relate primarily to the control intervention. In this study, too, adherence to the protocol was only partially possible in the waiting list control group as the study participants still acted with other children, which may have led to the non-significant results between the groups. On the other hand having children being alone in the control group makes the two conditions not comparable. A three-armed study containing these interventions (music therapy, social interaction, no intervention) would provide information on the corresponding effects, but was not feasible in the present setting.
Moreover, there is evidence from a recent meta analysis, that children with autism have altered oxytocin levels (John and Jaeggi, 2021). As half of the children were diagnoses with autism, this could this have affected the results. A post hoc analysis of the results however found no differences.
Another limitation is given by using salivary oxytocin levels as the primary and exclusive outcome measure. Therefore, the evaluation lacks data that take into account contextual aspects of the interventions that may influence the perception of the respective situation and thus the oxytocinergic system, e.g., the measurement of cortisol to control HPA axis activity (Schladt et al., 2017) or questionnaires on the children’s sense of well-being during music therapy and the control condition. In addition, due to the small sample size, the effect of potentially oxytocin-level altering medication such as methylphenidate (Levi-Shachar et al., 2020) was not taken into account in the present statistical analysis.
Overall, the particularity of the study population as well as the lack of control for contextual factors may therefore impede the generalizability of the results and the synthesis of findings across studies.
In addition, the evaluation of the 20% duplicate measurements in this pilot study revealed an inaccuracy in the measured values that proved to be significant. This demonstrates the importance of a larger study population for further research to detect an effect of music therapy on the oxytocinergic system.
Nevertheless, the use of saliva samples as a low-risk and cost-effective test offers a great opportunity to investigate the neuroendocrinological basis of music therapy non-invasively and without interrupting the therapeutic process. Future research will require larger studies including healthy controls to better investigate the neuroendocrinology of therapeutic alliance and thus attachment formation in music therapy. It will also require continued research into the oxytocinergic system to better understand individual and contextual factors influencing the oxytocinergic system. This combined knowledge may facilitate the interpretation of the reported changes in oxytocin levels and their clinical relevance, as well as enable the synthesis of findings across studies.
Overall, the importance of the oxytocinergic system for social behavior and its role in the development of diseases, but also as a target for therapy, is increasingly being recognized. The present study therefore aims to contribute by presenting music therapy as an attachment-based intervention that may influence the endogenous release of oxytocin and thus have an impact on social behavior.
Ultimately, it remains a difficult yet promising task to provide scientific neuroendocrinological evidence for music therapy.
This pilot study described the basic feasibility of this study design and discussed the difficulties of conducting it with children with social and emotional disorders.
Although we found a difference between the music therapy group and the waiting list control group, this difference was not significant. The within group comparisons, namely the music therapy group, the waiting list control group and the autism spectrum disorder subgroup, also showed no significant changes in oxytocin levels. The music therapist, however, showed a significant increase in oxytocin levels in the before and after comparison. Yet, it remains unclear whether and to what extent changes in oxytocin levels are associated with clinical benefits. Larger studies with healthy controls and a better understanding of the oxytocinergic system as well as the role of individual and contextual factors for changes in oxytocin levels will be essential to better interpret the results obtained to date and their clinical relevance.
The raw data supporting the conclusions of this article will be made available by the authors upon request.
The studies involving humans were approved by Ethical commitee of the Univeristy of Witten/Herdecke. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
PB: Conceptualization, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. LN: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. GK: Resources, Supervision, Writing – original draft, Writing – review & editing. DA: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. TO: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Andreas Tobias Kind Foundation and the Kämpgen Foundation to cover the costs of conducting the research and analyzing the data obtained.
We thank Dresden Lab Service GmbH for analyzing the saliva samples.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2024.1504229/full#supplementary-material
Aigen, K. (2014). Music-centered dimensions of Nordoff-Robbins music therapy. Music. Ther. Perspect. 32, 18–29. doi: 10.1093/mtp/miu006
Ainsworth, M. S., and Bowlby, J. (1991). An ethological approach to personality development. Am. Psychol. 46, 333–341. doi: 10.1037/0003-066X.46.4.333
Aldridge, D. (1994). An overview of music therapy research. Complement. Ther. Med. 2, 204–216. doi: 10.1016/0965-2299(94)90021-3
American Music Therapy Association. (2024). What is music therapy? Available at: http://www.musictherapy.org/about/musictherapy (Accessed December 09, 2024).
Archer, M., Steele, M., Lan, J., Jin, X., Herreros, F., and Steele, H. (2015). Attachment between infants and mothers in China:Strange situation procedure findings to date and a new sample. Int. J. Behav. Dev. 39, 485–491. doi: 10.1177/0165025415575765
Arnold, C. A., Bagg, M. K., and Harvey, A. R. (2024). The psychophysiology of music-based interventions and the experience of pain. Front. Psychol. 15:1361857. doi: 10.3389/fpsyg.2024.1361857
Atzil-Slonim, D., Stolowicz-Melman, D., Bar-Kalifa, E., Gilboa-Schechtman, E., Paz, A., Wolff, M., et al. (2022). Oxytocin reactivity to the therapeutic encounter as a biomarker of change in the treatment of depression. J. Couns. Psychol. 69, 755–760. doi: 10.1037/cou0000617
Baker, F. A., Metcalf, O., Varker, T., and O'Donnell, M. (2018). A systematic review of the efficacy of creative arts therapies in the treatment of adults with PTSD. Psychol. Trauma Theory Res. Pract. Policy 10, 643–651. doi: 10.1037/tra0000353
Bartz, J. A., Zaki, J., Bolger, N., and Ochsner, K. N. (2011). Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. 15, 301–309. doi: 10.1016/j.tics.2011.05.002
Baskaran, C., Plessow, F., Silva, L., Asanza, E., Marengi, D., Eddy, K. T., et al. (2017). Oxytocin secretion is pulsatile in men and is related to social-emotional functioning. Psychoneuroendocrinology 85, 28–34. doi: 10.1016/j.psyneuen.2017.07.486
Belski, N., Abdul-Rahman, Z., Youn, E., Balasundaram, V., and Diep, D. (2022). Review: the effectiveness of musical therapy in improving depression and anxiety symptoms among children and adolescents – a systematic review. Child Adolesc. Mental Health 27, 369–377. doi: 10.1111/camh.12526
Benoit, D. (2004). Infant-parent attachment: definition, types, antecedents, measurement and outcome. Paediatr. Child Health 9, 541–545. doi: 10.1093/pch/9.8.541
Bertsch, K., Schmidinger, I., Neumann, I. D., and Herpertz, S. C. (2013). Reduced plasma oxytocin levels in female patients with borderline personality disorder. Horm. Behav. 63, 424–429. doi: 10.1016/j.yhbeh.2012.11.013
Bodner, E., and Cohen-Fridel, S. (2010). Relations between attachment styles, ageism and quality of life in late life. Int. Psychogeriatr. 22, 1353–1361. doi: 10.1017/s1041610210001249
Bowling, D. L., Gahr, J., Ancochea, P. G., Hoeschele, M., Canoine, V., Fusani, L., et al. (2022). Endogenous oxytocin, cortisol, and testosterone in response to group singing. Horm. Behav. 139:105105. doi: 10.1016/j.yhbeh.2021.105105
Brancatisano, O., Baird, A., and Thompson, W. F. (2020). Why is music therapeutic for neurological disorders? The therapeutic music capacities model. Neurosci. Biobehav. Rev. 112, 600–615. doi: 10.1016/j.neubiorev.2020.02.008
Bretherton, I. (1992). The origins of attachment theory: John Bowlby and Mary Ainsworth. Dev. Psychol. 28, 759–775. doi: 10.1037/0012-1649.28.5.759
Busse, P., Anheyer, D., Stronski, J., and Ostermann, T. (2024). The role of oxytocin in music interventions: a systematic review. Ger. Med. Sci. 2024:12. doi: 10.3205/23wfkt12
Carmassi, C., Marazziti, D., Mucci, F., Della Vecchia, A., Barberi, F. M., Baroni, S., et al. (2021). Decreased plasma oxytocin levels in patients with PTSD. Front. Psychol. 12:338. doi: 10.3389/fpsyg.2021.612338
Carrasco, J. L., Buenache, E., MacDowell, K. S., De la Vega, I., López-Villatoro, J. M., Moreno, B., et al. (2020). Decreased oxytocin plasma levels and oxytocin receptor expression in borderline personality disorder. Acta Psychiatr. Scand. 142, 319–325. doi: 10.1111/acps.13222
Carson, D. S., Berquist, S. W., Trujillo, T. H., Garner, J. P., Hannah, S. L., Hyde, S. A., et al. (2015). Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol. Psychiatry 20, 1085–1090. doi: 10.1038/mp.2014.132
Chambers, J. (2017). The neurobiology of attachment: from infancy to clinical outcomes. Psychodynamic Psychiatry 45, 542–563. doi: 10.1521/pdps.2017.45.4.542
Chanda, M. L., and Levitin, D. J. (2013). The neurochemistry of music. Trends Cogn. Sci. 17, 179–193. doi: 10.1016/j.tics.2013.02.007
Chard, T., Boyd, N. R. H., Forsling, M. L., McNeilly, A. S., and Landon, J. (1970). The development of a radioimmunoassay for oxytocin: the extraction of oxytocin from plasma, and its measurement during parturition in human and goat blood. J. Endocrinol. 48, 223–234. doi: 10.1677/joe.0.0480223
Chen, W. G., Iversen, J. R., Kao, M. H., Loui, P., Patel, A. D., Zatorre, R. J., et al. (2022). Music and brain circuitry: strategies for strengthening evidence-based research for music-based interventions. J. Neurosci. 42, 8498–8507. doi: 10.1523/JNEUROSCI.1135-22.2022
Chen, X., Nishitani, S., Haroon, E., Smith, A. K., and Rilling, J. K. (2020). OXTR methylation modulates exogenous oxytocin effects on human brain activity during social interaction. Genes Brain Behav. 19:e12555. doi: 10.1111/gbb.12555
Dileo, C., and Bradt, J. (2009). “Medical music therapy: evidence-based principles and practices” in International handbook of occupational therapy interventions. ed. I. Söderback (New York: Springer), 445–451.
Djalovski, A., Kinreich, S., Zagoory-Sharon, O., and Feldman, R. (2021). Social dialogue triggers biobehavioral synchrony of partners' endocrine response via sex-specific, hormone-specific, attachment-specific mechanisms. Sci. Rep. 11:12421. doi: 10.1038/s41598-021-91626-0
Donadon, M. F., Martin-Santos, R., and Osório, F. D. L. (2018). The associations between oxytocin and trauma in humans: a systematic review. Front. Pharmacol. 9:154. doi: 10.3389/fphar.2018.00154
Dóro, C. A., Neto, J. Z., Cunha, R., and Dóro, M. P. (2017). Music therapy improves the mood of patients undergoing hematopoietic stem cells transplantation (controlled randomized study). Support. Care Cancer 25, 1013–1018. doi: 10.1007/s00520-016-3529-z
Ellenbogen, M. A., Cardoso, C., Serravalle, L., Vadaga, K., and Joober, R. (2024). The effects of intranasal oxytocin on the efficacy of psychotherapy for major depressive disorder: a pilot randomized controlled trial. Psychol. Med. 54, 2122–2132. doi: 10.1017/S0033291724000217
Ellis, B. J., Horn, A. J., Carter, C. S., van Ijzendoorn, M. H., and Bakermans-Kranenburg, M. J. (2021). Developmental programming of oxytocin through variation in early-life stress: four meta-analyses and a theoretical reinterpretation. Clin. Psychol. Rev. 86:101985. doi: 10.1016/j.cpr.2021.101985
Engel, S., Klusmann, H., Ditzen, B., Knaevelsrud, C., and Schumacher, S. (2019). Menstrual cycle-related fluctuations in oxytocin concentrations: a systematic review and meta-analysis. Front. Neuroendocrinol. 52, 144–155. doi: 10.1016/j.yfrne.2018.11.002
Erdozain, A. M., and Peñagarikano, O. (2020). Oxytocin as treatment for social cognition, not there yet [Mini review]. Front. Psych. 10:930. doi: 10.3389/fpsyt.2019.00930
Ettenberger, M., Bieleninik, Ł., Epstein, S., and Elefant, C. (2021). Defining attachment and bonding: overlaps, differences and implications for music therapy clinical practice and research in the neonatal intensive care unit (NICU). Int. J. Environ. Res. Public Health 18:1733. doi: 10.3390/ijerph18041733
Falgares, G., Marchetti, D., De Santis, S., Carrozzino, D., Kopala-Sibley, D. C., Fulcheri, M., et al. (2017). Attachment styles and suicide-related behaviors in adolescence: the mediating role of self-criticism and dependency. Front. Psych. 8:36. doi: 10.3389/fpsyt.2017.00036
Fancourt, D., Ockelford, A., and Belai, A. (2014). The psychoneuroimmunological effects of music: a systematic review and a new model. Brain Behav. Immun. 36, 15–26. doi: 10.1016/j.bbi.2013.10.014
Fancourt, D., Williamon, A., Carvalho, L. A., Steptoe, A., Dow, R., and Lewis, I. (2016). Singing modulates mood, stress, cortisol, cytokine and neuropeptide activity in cancer patients and carers. Ecancermedicalscience 10:631. doi: 10.3332/ecancer.2016.631
Feldman, R. (2012). Oxytocin and social affiliation in humans. Horm. Behav. 61, 380–391. doi: 10.1016/j.yhbeh.2012.01.008
Feldman, R., Gordon, I., and Zagoory-Sharon, O. (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev. Sci. 14, 752–761. doi: 10.1111/j.1467-7687.2010.01021.x
Ferreira, A. C., and Osório, F. D. L. (2022). Peripheral oxytocin concentrations in psychiatric disorders – a systematic review and methanalysis: further evidence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 117:110561. doi: 10.1016/j.pnpbp.2022.110561
Fischer, S., and Zilcha-Mano, S. (2022). Why does psychotherapy work and for whom? Hormonal answers. Biomedicines 10:1361. doi: 10.3390/biomedicines10061361
Fisher, H., Solomonov, N., Falkenström, F., Shahar, B., Shamay-Tsoory, S., and Zilcha-Mano, S. (2023). Therapists' oxytocin response mediates the association between patients' negative emotions and psychotherapy outcomes. J. Affect. Disord. 338, 163–170. doi: 10.1016/j.jad.2023.06.013
Flückiger, C., Del Re, A. C., Wampold, B. E., and Horvath, A. O. (2018). The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic.) 55, 316–340. doi: 10.1037/pst0000172
Forsling, M. L. (2000). Diurnal rhythms in neurohypophysial function. Exp. Physiol. 85, 179s–186s. doi: 10.1111/j.1469-445X.2000.tb00022.x
Fujioka, T., Dawson, D. R., Wright, R., Honjo, K., Chen, J. L., Chen, J. J., et al. (2018). The effects of music-supported therapy on motor, cognitive, and psychosocial functions in chronic stroke. Ann. N. Y. Acad. Sci. 1423, 264–274. doi: 10.1111/nyas.13706
Gold, C., Solli, H. P., Krüger, V., and Lie, S. A. (2009). Dose–response relationship in music therapy for people with serious mental disorders: systematic review and meta-analysis. Clin. Psychol. Rev. 29, 193–207. doi: 10.1016/j.cpr.2009.01.001
Grape, C., Sandgren, M., Hansson, L.-O., Ericson, M., and Theorell, T. (2002). Does singing promote well-being?: an empirical study of professional and amateur singers during a singing lesson. Integr. Physiol. Behav. Sci. 38, 65–74. doi: 10.1007/BF02734261
Groh, A. M., Fearon, R. M. P., van IJzendoorn, M. H., Bakermans-Kranenburg, M. J., and Roisman, G. I. (2017). Attachment in the early life course: Meta-analytic evidence for its role in socioemotional development. Child Dev. Perspect. 11, 70–76. doi: 10.1111/cdep.12213
Grossman-Giron, A., Fisher, H., Atzil-Slonim, D., Maoz, H., Nitzan, U., and Tzur Bitan, D. (2023). The effect of oxytocin administration on patient-therapist alliance congruence: results from a randomized controlled trial. Psychother. Res. 34, 1092–1102. doi: 10.1080/10503307.2023.2269300
Hanson-Abromeit, D. (2015). A conceptual methodology to define the therapeutic function of music. Music. Ther. Perspect. 33, 25–38. doi: 10.1093/mtp/miu061
Harvey, A. R. (2020). Links between the neurobiology of oxytocin and human musicality. Front. Hum. Neurosci. 14:350. doi: 10.3389/fnhum.2020.00350
Hazan, C., and Shaver, P. (1987). Romantic love conceptualized as an attachment process. J. Pers. Soc. Psychol. 52, 511–524. doi: 10.1037/0022-3514.52.3.511
Hernández-Díaz, Y., González-Castro, T. B., Tovilla-Zárate, C. A., López-Narváez, M. L., Genis-Mendoza, A. D., Castillo-Avila, R. G., et al. (2021). Oxytocin levels in individuals with schizophrenia are high in cerebrospinal fluid but low in serum: a systematic review and meta-analysis. Metab. Brain Dis. 36, 2415–2424. doi: 10.1007/s11011-021-00836-y
Hertenstein, E., Trinca, E., Schneider, C. L., Wunderlin, M., Fehér, K., Riemann, D., et al. (2021). Augmentation of psychotherapy with neurobiological methods: current state and future directions. Neuropsychobiology 80, 437–453. doi: 10.1159/000514564
Hillecke, T., Nickel, A., and Bolay, H. V. (2005). Scientific perspectives on music therapy. Ann. N. Y. Acad. Sci. 1060, 271–282. doi: 10.1196/annals.1360.020
Hsu, H.-F., Chen, K.-M., and Belcastro, F. (2022). The effect of music interventions on chronic pain experienced by older adults: a systematic review. J. Nurs. Scholarsh. 54, 64–71. doi: 10.1111/jnu.12712
Huang, M., Liu, K., Wei, Z., Feng, Z., Chen, J., Yang, J., et al. (2021). Serum oxytocin level correlates with gut microbiome Dysbiosis in children with autism Spectrum disorder. Front. Neurosci. 15:1884. doi: 10.3389/fnins.2021.721884
Iovino, M., Messana, T., Tortora, A., Giusti, C., Lisco, G., Giagulli, V. A., et al. (2021). Oxytocin signaling pathway: from cell biology to clinical implications. Endocr Metab Immune Disord Drug Targets 21, 91–110. doi: 10.2174/1871530320666200520093730
Jasemi, M., Aazami, S., and Zabihi, R. E. (2016). The effects of music therapy on anxiety and depression of Cancer patients. Indian J. Palliat. Care 22, 455–458. doi: 10.4103/0973-1075.191823
Jobst, A., Sabaß, L., Hall, D., Brücklmeier, B., Buchheim, A., Hall, J., et al. (2018). Oxytocin plasma levels predict the outcome of psychotherapy: a pilot study in chronic depression. J. Affect. Disord. 227, 206–213. doi: 10.1016/j.jad.2017.10.037
John, S., and Jaeggi, A. V. (2021). Oxytocin levels tend to be lower in autistic children: a meta-analysis of 31 studies. Autism 25, 2152–2161. doi: 10.1177/13623613211034375
Kagerbauer, S. M., Debus, J. M., Martin, J., Gempt, J., Jungwirth, B., Hapfelmeier, A., et al. (2019). Absence of a diurnal rhythm of oxytocin and arginine-vasopressin in human cerebrospinal fluid, blood and saliva. Neuropeptides 78:101977. doi: 10.1016/j.npep.2019.101977
Karveli, S., Galanis, P., Mitropoulou, E. M., Karademas, E., and Markopoulos, C. (2023). The role of attachment styles on quality of life and distress among early-stage female breast Cancer patients: a systematic review. J. Clin. Psychol. Med. Settings 30, 724–739. doi: 10.1007/s10880-023-09940-w
Keeler, J. R., Roth, E. A., Neuser, B. L., Spitsbergen, J. M., Waters, D. J., and Vianney, J. M. (2015). The neurochemistry and social flow of singing: bonding and oxytocin. Front. Hum. Neurosci. 9:518. doi: 10.3389/fnhum.2015.00518
Kerem, L., and Lawson, E. A. (2021). The effects of oxytocin on appetite regulation, food intake and metabolism in humans. Int. J. Mol. Sci. 22:7737. doi: 10.3390/ijms22147737
Kern, P., and Tague, D. B. (2017). Music therapy practice status and trends worldwide: an international survey study. J. Music Ther. 54, 255–286. doi: 10.1093/jmt/thx011
Koch, S. B. J., van Zuiden, M., Nawijn, L., Frijling, J. L., Veltman, D. J., and Olff, M. (2014). Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: salience processing and fear inhibition processes. Psychoneuroendocrinology 40, 242–256. doi: 10.1016/j.psyneuen.2013.11.018
Köhler, F., Martin, Z.-S., Hertrampf, R.-S., Gäbel, C., Kessler, J., Ditzen, B., et al. (2020). Music therapy in the psychosocial treatment of adult Cancer patients: a systematic review and Meta-analysis [systematic review]. Front. Psychol. 11:651. doi: 10.3389/fpsyg.2020.00651
Korhan, E. A., Uyar, M., Eyigör, C., Hakverdioğlu Yönt, G., Çelik, S., and Khorshıd, L. (2014). The effects of music therapy on pain in patients with neuropathic pain. Pain Manag. Nurs. 15, 306–314. doi: 10.1016/j.pmn.2012.10.006
Kreutz, G. (2014). Does singing facilitate social bonding? Music Med. 6, 51–60. doi: 10.47513/mmd.v6i2.180
Lam, H. L., Li, W. T. V., Laher, I., and Wong, R. Y. (2020). Effects of music therapy on patients with dementia—a systematic review. Geriatrics 5:62. doi: 10.3390/geriatrics5040062
Leng, G., and Sabatier, N. (2016). Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J. Neuroendocrinol. 28:12413. doi: 10.1111/jne.12413
Levi-Shachar, O., Gvirts, H. Z., Goldwin, Y., Bloch, Y., Shamay-Tsoory, S., Zagoory-Sharon, O., et al. (2020). The effect of methylphenidate on social cognition and oxytocin in children with attention deficit hyperactivity disorder. Neuropsychopharmacology 45, 367–373. doi: 10.1038/s41386-019-0522-5
Liu, Q., Li, W., Yin, Y., Zhao, Z., Yang, Y., Zhao, Y., et al. (2022). The effect of music therapy on language recovery in patients with aphasia after stroke: a systematic review and meta-analysis. Neurol. Sci. 43, 863–872. doi: 10.1007/s10072-021-05743-9
Loewy, J., Stewart, K., Dassler, A.-M., Telsey, A., and Homel, P. (2013). The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pediatrics 131, 902–918. doi: 10.1542/peds.2012-1367
Lynch, K. A., Emard, N., Liou, K. T., Popkin, K., Borten, M., Nwodim, O., et al. (2021). Patient perspectives on active vs. passive music therapy for Cancer in the inpatient setting: a qualitative analysis. J. Pain Symptom Manag. 62, 58–65. doi: 10.1016/j.jpainsymman.2020.11.014
MacDonald, K., MacDonald, T. M., Brüne, M., Lamb, K., Wilson, M. P., Golshan, S., et al. (2013). Oxytocin and psychotherapy: a pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology 38, 2831–2843. doi: 10.1016/j.psyneuen.2013.05.014
Manning, R. P., Dickson, J. M., Palmier-Claus, J., Cunliffe, A., and Taylor, P. J. (2017). A systematic review of adult attachment and social anxiety. J. Affect. Disord. 211, 44–59. doi: 10.1016/j.jad.2016.12.020
Marazziti, D., Baroni, S., Mucci, F., Piccinni, A., Moroni, I., Giannaccini, G., et al. (2019). Sex-related differences in plasma oxytocin levels in humans. Clin. Pract. Epidemiol. Ment. Health 15, 58–63. doi: 10.2174/1745017901915010058
Martins, D., Gabay, A. S., Mehta, M., and Paloyelis, Y. (2020). Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans. eLife 9:e62456. doi: 10.7554/eLife.62456
Mikulincer, M., Shaver, P. R., and Berant, E. (2013). An attachment perspective on therapeutic processes and outcomes. J. Pers. 81, 606–616. doi: 10.1111/j.1467-6494.2012.00806.x
Moerkerke, M., Bonte, M. L., Daniels, N., Chubar, V., Alaerts, K., Steyaert, J., et al. (2021). Oxytocin receptor gene (OXTR) DNA methylation is associated with autism and related social traits–a systematic review. Res. Autism Spectr. Disord. 85:101785. doi: 10.1016/j.rasd.2021.101785
Mössler, K., Gold, C., Aßmus, J., Schumacher, K., Calvet, C., Reimer, S., et al. (2019). The therapeutic relationship as predictor of change in music therapy with young children with autism Spectrum disorder. J. Autism Dev. Disord. 49, 2795–2809. doi: 10.1007/s10803-017-3306-y
Naifeh, J. A., Ursano, R. J., Stein, M. B., Wang, J., Mash, H. B. H., Aliaga, P. A., et al. (2024). Prospective association of attachment style with suicide attempts among US Army soldiers. Psychol. Med. 54, 785–793. doi: 10.1017/S0033291723002489
Neugebauer, L., and Ostermann, T. (2024). Musiktherapeutische Improvisation–Definition, Kontext und Wirksamkeit. Z. Komplementärmed. 16, 36–41. doi: 10.1055/a-2234-8917
Nielsen, S. K. K., Lønfeldt, N., Wolitzky-Taylor, K. B., Hageman, I., Vangkilde, S., and Daniel, S. I. F. (2017). Adult attachment style and anxiety – the mediating role of emotion regulation. J. Affect. Disord. 218, 253–259. doi: 10.1016/j.jad.2017.04.047
Olff, M., Frijling, J. L., Kubzansky, L. D., Bradley, B., Ellenbogen, M. A., Cardoso, C., et al. (2013). The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 38, 1883–1894. doi: 10.1016/j.psyneuen.2013.06.019
Olff, M., Langeland, W., Witteveen, A., and Denys, D. (2010). A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectr. 15, 522–530. doi: 10.1017/S109285290000047X
Palmieri, A., Pick, E., Grossman-Giron, A., and Tzur Bitan, D. (2021). Oxytocin as the neurobiological basis of synchronization: a research proposal in psychotherapy settings [opinion]. Front. Psychol. 12:11. doi: 10.3389/fpsyg.2021.628011
Palumbo, A., Aluru, V., Battaglia, J., Geller, D., Turry, A., Ross, M., et al. (2022). Music upper limb therapy-integrated provides a feasible enriched environment and reduces post-stroke depression: a pilot randomized controlled trial. Am. J. Phys. Med. Rehabil. 101, 937–946. doi: 10.1097/phm.0000000000001938
Peñacoba, C., Perez-Calvo, S., Blanco, S., and Sanroman, L. (2018). Attachment styles, pain intensity and emotional variables in women with fibromyalgia. Scand. J. Caring Sci. 32, 535–544. doi: 10.1111/scs.12477
Pérez-Eizaguirre, M., and Vergara-Moragues, E. (2021). Music therapy interventions in palliative care: a systematic review. J. Palliat. Care 36, 194–205. doi: 10.1177/0825859720957803
Pietromonaco, P. R., and Beck, L. A. (2019). Adult attachment and physical health. Curr. Opin. Psychol. 25, 115–120. doi: 10.1016/j.copsyc.2018.04.004
Pinquart, M., Feußner, C., and Ahnert, L. (2013). Meta-analytic evidence for stability in attachments from infancy to early adulthood. Attach Hum. Dev. 15, 189–218. doi: 10.1080/14616734.2013.746257
Poore, H. E., and Waldman, I. D. (2020). The association of oxytocin receptor gene (OXTR) polymorphisms antisocial behavior: a meta-analysis. Behav. Genet. 50, 161–173. doi: 10.1007/s10519-020-09996-6
Raglio, A., Attardo, L., Gontero, G., Rollino, S., Groppo, E., and Granieri, E. (2015). Effects of music and music therapy on mood in neurological patients. World J. Psychiatry 5, 68–78. doi: 10.5498/wjp.v5.i1.68
Ravitz, P., Maunder, R., Hunter, J., Sthankiya, B., and Lancee, W. (2010). Adult attachment measures: a 25-year review. J. Psychosom. Res. 69, 419–432. doi: 10.1016/j.jpsychores.2009.08.006
Robb, S. L., Carpenter, J. S., and Burns, D. S. (2011). Reporting guidelines for music-based interventions. J. Health Psychol. 16, 342–352. doi: 10.1177/1359105310374781
Rodrigues, S. M., Saslow, L. R., Garcia, N., John, O. P., and Keltner, D. (2009). Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc. Natl. Acad. Sci. 106, 21437–21441. doi: 10.1073/pnas.0909579106
Romeo, A., Tesio, V., Castelnuovo, G., and Castelli, L. (2017). Attachment style and chronic pain: toward an interpersonal model of pain [Mini review]. Front. Psychol. 8:284. doi: 10.3389/fpsyg.2017.00284
Schladt, T. M., Nordmann, G. C., Emilius, R., Kudielka, B. M., de Jong, T. R., and Neumann, I. D. (2017). Choir versus solo singing: effects on mood, and salivary oxytocin and cortisol concentrations. Front. Hum. Neurosci. 11:430. doi: 10.3389/fnhum.2017.00430
Schneider, E., Müller, L. E., Ditzen, B., Herpertz, S. C., and Bertsch, K. (2021). Oxytocin and social anxiety: interactions with sex hormones. Psychoneuroendocrinology 128:105224. doi: 10.1016/j.psyneuen.2021.105224
Schneiderman, I., Kanat-Maymon, Y., Zagoory-Sharon, O., and Feldman, R. (2014). Mutual influences between partners’ hormones shape conflict dialog and relationship duration at the initiation of romantic love. Soc. Neurosci. 9, 337–351. doi: 10.1080/17470919.2014.893925
Shemmings, D. (2006). Using adult attachment theory to differentiate adult children's internal working models of later life filial relationships. J. Aging Stud. 20, 177–191. doi: 10.1016/j.jaging.2005.12.001
Shi, Z., Wang, S., Chen, M., Hu, A., Long, Q., and Lee, Y. (2024). The effect of music therapy on language communication and social skills in children with autism spectrum disorder: a systematic review and meta-analysis. Front. Psychol. 15:1336421. doi: 10.3389/fpsyg.2024.1336421
Sicorello, M., Dieckmann, L., Moser, D., Lux, V., Luhmann, M., Schlotz, W., et al. (2020). Oxytocin and the stress buffering effect of social company: a genetic study in daily life. Soc. Cogn. Affect. Neurosci. 15, 293–301. doi: 10.1093/scan/nsaa034
Simon, H. L. M., DiPlacido, J., and Conway, J. M. (2019). Attachment styles in college students and depression: the mediating role of self differentiation. Ment. Health Prev. 13, 135–142. doi: 10.1016/j.mhp.2019.01.011
Standley, J. M. (2002). A meta-analysis of the efficacy of music therapy for premature infants. J. Pediatr. Nurs. 17, 107–113. doi: 10.1053/jpdn.2002.124128
Strathearn, L., Mertens, C. E., Mayes, L., Rutherford, H., Rajhans, P., Xu, G., et al. (2019). Pathways relating the neurobiology of attachment to drug addiction. Front. Psych. 10:737. doi: 10.3389/fpsyt.2019.00737
Strauss, G. P., Chapman, H. C., Keller, W. R., Koenig, J. I., Gold, J. M., Carpenter, W. T., et al. (2019). Endogenous oxytocin levels are associated with impaired social cognition and neurocognition in schizophrenia. J. Psychiatr. Res. 112, 38–43. doi: 10.1016/j.jpsychires.2019.02.017
Striepens, N., Kendrick, K. M., Maier, W., and Hurlemann, R. (2011). Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front. Neuroendocrinol. 32, 426–450. doi: 10.1016/j.yfrne.2011.07.001
Suzuki, S., Fujisawa, T. X., Sakakibara, N., Fujioka, T., Takiguchi, S., and Tomoda, A. (2020). Development of social attention and oxytocin levels in maltreated children. Sci. Rep. 10:7407. doi: 10.1038/s41598-020-64297-6
Tabak, B. A., Leng, G., Szeto, A., Parker, K. J., Verbalis, J. G., Ziegler, T. E., et al. (2023). Advances in human oxytocin measurement: challenges and proposed solutions. Mol. Psychiatry 28, 127–140. doi: 10.1038/s41380-022-01719-z
Tang, Y. W., Teoh, S. L., Yeo, J. H. H., Ngim, C. F., Lai, N. M., Durrant, S. J., et al. (2022). Music-based intervention for improving sleep quality of adults without sleep disorder: a systematic review and Meta-analysis. Behav. Sleep Med. 20, 241–259. doi: 10.1080/15402002.2021.1915787
Thaut, M. H., Francisco, G., and Hoemberg, V. (2021). The clinical neuroscience of music: evidence based approaches and neurologic music therapy. Front. Neurosci. 15:740329. doi: 10.3389/fnins.2021.740329
Tops, M., Van Peer, J. M., Korf, J., Wijers, A. A., and Tucker, D. M. (2007). Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology 44, 444–449. doi: 10.1111/j.1469-8986.2007.00510.x
Ueda, T., Suzukamo, Y., Sato, M., and Izumi, S.-I. (2013). Effects of music therapy on behavioral and psychological symptoms of dementia: a systematic review and meta-analysis. Ageing Res. Rev. 12, 628–641. doi: 10.1016/j.arr.2013.02.003
Ulmer-Yaniv, A., Avitsur, R., Kanat-Maymon, Y., Schneiderman, I., Zagoory-Sharon, O., and Feldman, R. (2016). Affiliation, reward, and immune biomarkers coalesce to support social synchrony during periods of bond formation in humans. Brain Behav. Immun. 56, 130–139. doi: 10.1016/j.bbi.2016.02.017
Uvnäs-Moberg, K., Handlin, L., and Petersson, M. (2015). Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Front. Psychol. 5:1529. doi: 10.3389/fpsyg.2014.01529
Valizadeh, M., Motazedian, S., Kuchi, M., and Alipoor, R. (2017). Investigating the relationship between attachment styles and addiction severity. Bali Med. J. 6, 304–374. doi: 10.15562/bmj.v6i2.546
Valstad, M., Alvares, G. A., Egknud, M., Matziorinis, A. M., Andreassen, O. A., Westlye, L. T., et al. (2017). The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 78, 117–124. doi: 10.1016/j.neubiorev.2017.04.017
Van Rosmalen, L., Van der Veer, R., and Van der Horst, F. (2015). AINSWORTH'S STRANGE situation procedure: the origin of an instrument. J. Hist. Behav. Sci. 51, 261–284. doi: 10.1002/jhbs.21729
Wang, Y., Pan, W.-Y., Li, F., Ge, J.-S., Zhang, X., Luo, X., et al. (2021). Effect of rhythm of music therapy on gait in patients with stroke. J. Stroke Cerebrovasc. Dis. 30:105544. doi: 10.1016/j.jstrokecerebrovasdis.2020.105544
Wang, C.-F., Sun, Y.-L., and Zang, H.-X. (2014). Music therapy improves sleep quality in acute and chronic sleep disorders: a meta-analysis of 10 randomized studies. Int. J. Nurs. Stud. 51, 51–62. doi: 10.1016/j.ijnurstu.2013.03.008
Warfa, N., Harper, M., Nicolais, G., and Bhui, K. (2014). Adult attachment style as a risk factor for maternal postnatal depression: a systematic review. BMC Psychol. 2:56. doi: 10.1186/s40359-014-0056-x
Weller, C. M., and Baker, F. A. (2011). The role of music therapy in physical rehabilitation: a systematic literature review. Nord. J. Music. Ther. 20, 43–61. doi: 10.1080/08098131.2010.485785
Wheeler, B. L., Shiflett, S. C., and Nayak, S. (2003). Effects of number of sessions and group or individual music therapy on the mood and behavior of people who have had strokes or traumatic brain injuries. Nord. J. Music. Ther. 12, 139–151. doi: 10.1080/08098130309478084
Widom, C. S., Czaja, S. J., Kozakowski, S. S., and Chauhan, P. (2018). Does adult attachment style mediate the relationship between childhood maltreatment and mental and physical health outcomes? Child Abuse Negl. 76, 533–545. doi: 10.1016/j.chiabu.2017.05.002
Zak, P. J., Curry, B., Owen, T., and Barraza, J. A. (2022). Oxytocin release increases with age and is associated with life satisfaction and prosocial behaviors. Front. Behav. Neurosci. 16:234. doi: 10.3389/fnbeh.2022.846234
Zhao, K., Bai, Z. G., Bo, A., and Chi, I. (2016). A systematic review and meta-analysis of music therapy for the older adults with depression. Int. J. Geriatr. Psychiatry 31, 1188–1198. doi: 10.1002/gps.4494
Zilcha-Mano, S., Goldstein, P., Dolev-Amit, T., and Shamay-Tsoory, S. (2021). Oxytocin synchrony between patients and therapists as a mechanism underlying effective psychotherapy for depression. J. Consult. Clin. Psychol. 89, 49–57. doi: 10.1037/ccp0000619
Keywords: attachment, music, music therapy, oxytocin, psychotherapy, pilot study
Citation: Busse PK, Neugebauer L, Kaschubowski G, Anheyer D and Ostermann T (2025) Oxytocin as a physiological correlate of dyadic music therapy relationships — a randomized crossover pilot study. Front. Behav. Neurosci. 18:1504229. doi: 10.3389/fnbeh.2024.1504229
Received: 30 September 2024; Accepted: 23 December 2024;
Published: 30 January 2025.
Edited by:
Juan Francisco Rodríguez-Landa, Universidad Veracruzana, MexicoReviewed by:
Annie L. Heiderscheit, Anglia Ruskin University, United KingdomCopyright © 2025 Busse, Neugebauer, Kaschubowski, Anheyer and Ostermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Ostermann, VGhvbWFzLk9zdGVybWFubkB1bmktd2guZGU=
†ORCID: Dennis Anheyer, orcid.org/0000-0001-9310-8077
Thomas Ostermann, orcid.org/0000-0003-2695-0701
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.