- Department of Marine Biology and Ecology, University of Miami Rosenstiel School, Miami, FL, United States
The transcription factor Aplysia CCAAT/enhancer binding protein (ApC/EBP) is expressed as an immediate early gene in the cAMP responsive element binding protein (CREB) mediated gene cascade, and it has essential functions in the synaptic consolidation of memory following a learning event. Synaptic consolidation primarily involves morphological changes at neuronal synapses, which are facilitated through the reorganization of the actin and microtubular cytoarchitecture of the cell. During early nervous system development, the transmembrane synaptic protein teneurin acts directly upon neuronal presynaptic microtubules and postsynaptic spectrin-based cytoskeletons to facilitate the creation of new synapses. It is reasonable to hypothesize that teneurin may also be linked to learning-induced synaptic changes and is a potential candidate to be a later gene expressed in the CREB-mediated gene cascade downstream of ApC/EBP. To assess the role of ApC/EBP and teneurin in learning and memory in the marine snail Aplysia californica, young (age 7–8 months) and aged (age 13–15 months; aging stage AII) siblings of Aplysia were trained in an operant conditioning paradigm—learning food is inedible (LFI)—over 2 days, during which they learned to modify the feeding reflex. Aged Aplysia had enhanced performance of the LFI task on the second day than younger siblings although far more aged animals were excluded from the analysis because of the initial failure in learning to recognize the inedible probe. After 2 days of training, ApC/EBP isoform X1 mRNA and teneurin mRNA were quantified in selected neurons of the buccal ganglia, the locus of neural circuits in LFI. Teneurin expression was elevated in aged Aplysia compared to young siblings regardless of training. ApC/EBP isoform X1 expression was significantly higher in untrained aged animals than in untrained young siblings but decreased in trained aged animals compared to untrained aged animals. Elevated levels of ApC/EBP isoform X1 and teneurin mRNA before training may have contributed to the enhancement of LFI performance in the aged animals that successfully learned.
Introduction
Consolidation of experience into long-term memory (LTM) requires RNA transcription, protein synthesis, and the transport of RNA and proteins to the synapse to either create a new synapse or effect changes in the existing ones (Goelet et al., 1986; Bailey and Chen, 1988a,b; Bailey and Kandel, 2008). In Aplysia californica (Aplysia), consolidation and the stable long-term synaptic changes that accompany it are achieved by the regulated expression of many genes. Of note are CREB and ApC/EBP (Alberini and Kandel, 2015), which encode transcription factors (Alberini, 2009a) that then regulate the expression of effector genes necessary for the modulation of neuronal synapses (Alberini, 2009b). Regulated gene expression is also necessary for memory reconsolidation after recall (Alberini, 2009b; Alberini and Kandel, 2015), as well as new protein synthesis at the site of previously altered synapses (Cai et al., 2012; Lee et al., 2012).
The neural circuit controlling the feeding reflex of Aplysia has been mapped and studied well (Cropper et al., 2004). This circuit contains neurons from both the buccal and cerebral ganglia. Previous studies have discovered molecular and morphological changes in this circuit, attributed to learning that food is inedible (LFI). These include induction of ApC/EBP in buccal ganglia mechanoafferent neurons that detect and transduce information about touch (Levitan et al., 2008, 2012), and changes in the number of synaptic connections to motoneurons (Tam et al., 2020), as well as in cerebral-buccal interneurons in the form of decreased responsiveness to acetylcholine (McManus et al., 2019). No such gene expression signatures have been recorded in the buccal ganglia motoneurons. Here, we studied gene expression in buccal ganglia neurons without the contribution of the sensory buccal S cluster (BSC) neurons in a test of the hypothesis posed by Levitan et al. (2012) that the CREB and C/EBP learning cascade may be key to formation of the memory of LFI. The case we studied was the next-day reconsolidated memory of LFI, after evidence that LFI occurred.
Most LFI studies focus on changes in presynaptic sensory and interneuron connections to motoneurons to explain behavioral changes even though both presynaptic and postsynaptic changes contribute to learning (Nargeot, 2001; Roberts and Glanzman, 2003; Momohara et al., 2022). Central pattern generator (CPG) neurons B31/B32 activate buccal ganglia motoneurons that innervate the I2 radular protraction muscle (Hurwitz et al., 1996, 2003, 2008). Since LFI results in a reduction of radular protractions and ingestion attempts in response to inedible food (Susswein et al., 1986), it was hypothesized that gene expression in buccal ganglia that includes the somas of neurons B31/B32 drives long-term synaptic changes after LFI training that result in reduced activity. To test this, targeted gene expression analysis was conducted on a subset of neurons from the buccal ganglia which included motoneurons B31/B32. ApC/EBP isoform X1 and teneurin expression were quantitated from this subset of neurons for their possible involvement in LFI reconsolidation.
Several studies suggest that increased expression of C/EBP might occur during reconsolidation. Levitan et al. (2012) cited evidence that the increased expression of C/EBPß in the mammalian brain, as in Aplysia, is necessary for LTM formation during consolidation (Taubenfeld et al., 2001) with the same C/EBPß isoform expression necessary for reconsolidation in a different part of the brain (Milekic et al., 2007). This suggests that an increased expression of ApC/EBP might occur during reconsolidation of LFI in neurons other than the sensory neurons of the buccal ganglia. Additionally, Hatakeyama et al. (2004) showed that LymC/EBP expression in the right pedal dorsal 1 (RPeD1) neuron of Lymnaea stagnalis is required for the reconsolidation of operant conditioning LTM in CPG neurons. Thus, ApC/EBP expression changes during the reconsolidation of LFI in postsynaptic neurons of the buccal ganglia were investigated.
ApC/EBP isoform X1, previously shown to behave identically to the more commonly studied ApC/EBP (Lee et al., 2001), was studied because highly specific primers that spanned an exon junction were possible. Teneurin, which is most studied in the developing nervous system has been implicated in all aspects of synaptogenesis (Sanes and Zipursky, 2020). After learning, synaptogenesis promotes memory consolidation, which involves axon guidance, synaptic partner matching, and the organization of components at the new synapse, in which teneurin plays a critical role during development (Hong et al., 2012; Mosca et al., 2012; Mosca, 2015; Vysokov et al., 2018; DePew et al., 2019; Toro et al., 2020). Teneurin protein and transcripts have also been shown to be elevated in activated astrocytes and in the cerebral cortex, respectively, of adult rats after central nervous system (CNS) injury, indicating a putative function in CNS repair (Tessarin et al., 2019). It is possible that teneurin is involved in other functions of synaptic plasticity such as memory consolidation/reconsolidation. Therefore, teneurin was investigated to learn whether a change in expression of this gene during reconsolidation may be driven by the CREB and C/EBP learning cascade. Gene expression was measured 2 h after testing for LFI recall.
Since learning capabilities in LFI may change with animal age and confound gene expression, we studied sibling animals at two distinct time periods: just prior to first sexual maturity and in aging stage AII (Kempsell and Fieber, 2014). To ensure changes in performance and gene expression were a result of LFI training and not some other factor, we selected only animals that learned in LFI and demonstrated LTM of LFI for comparative analyses.
Materials and methods
Animal husbandry and training
A cohort of Aplysia was raised in the National Resource for Aplysia at the Rosenstiel School at the University of Miami. The animals were fed an ad libitum mixed diet of Agardhiella subulata, the red alga normally fed at the resource, romaine lettuce, and Ulva lactuca, a green alga, readily taken by Aplysia that can be folded tightly for use in the food probes used as training tools in this experiment. The animals were reared in exercise regimes, in which seawater forcefully flowed into their aquaria every 5–7 min, which caused the animals to secure themselves to the substrate or be swept up in the turbulent flow as described in Fieber et al. (2018). This mimicked the animal’s natural habitat of the intertidal rocky shores of California and provided constant neural stimulation throughout the animal’s life. Examples abound on the benefits of rearing laboratory animals in enriched environments, demonstrating that stimulation increases neural connections compared to rearing in desolate conditions (West and Greenough, 1972; Turner and Greenough, 1985; Jones and Greenough, 1996). Additionally, enriched-environment animals have been shown to perform better in cognitive tests (Kobayashi et al., 2002).
Animals were taught to recognize an inedible food source in the training protocol LFI that was adapted from Susswein et al. (1986) study. Animals were trained for 2 consecutive days. They were fasted for 48 h before testing began on Day 1 and were placed individually in 50 cm × 50 cm × 25 cm plastic aquaria containing 8L of aerated seawater, one animal per aquarium. A probe consisting of U. lactuca wrapped in 75-micron Nitex netting was held in the jaws of a plastic hemostat, and netted algae were presented to the animal by holding the probe approximately 1 cm in front of the animal’s oral tentacles. An animal’s voluntary stimulation of its lips by moving against the probe initiated the feeding reflex that is inherent to LFI (Susswein et al., 1986).
The animal’s behavior was noted while using a pair of stopwatches monitored by an assistant that recorded behavior times and total elapsed time. Data recorded were the latency between when the probe was first offered and when the animal took it into its mouth, the time at which the probe entered the animal’s mouth, how long the probe stayed in the animal’s mouth each time it entered, and the time at which the animal ejected the probe from its mouth. Radula scrapes were recorded when felt by the experimenter as a vibration of the hemostat and tended to accompany swallowing attempts. Training continued until a time of 3 min since the probe last exited the animal’s mouth when training of that animal ended for that day. The total time in the mouth (TTIM) was then calculated by adding together the elapsed times the probe remained in the animal’s mouth for each ingestion attempt. If TTIM was greater than 100 s on Day 1 training, the animal was regarded as successfully trained and was returned to a holding cage containing a non-experimental companion animal overnight. Animals that did not attain a TTIM greater than 100 s on Day 1 training were given the label “dud” and not used in any subsequent training. The minimum required TTIM of 100 s followed previously published protocols (Levitan et al., 2008, 2012) and was chosen to stress the importance of the animal’s failed attempts at swallowing the food probe that is necessary to establish the LTM of LFI (Katzoff et al., 2006). Day 2 training followed 26 h after Day 1 training and used the same protocol.
The percentage of total time saved relearning inedibility on Day 2 compared to Day 1 (%SAV) was calculated using the following equation:
If TTIM on Day 2 training was less than TTIM on Day 1, it was assumed the animal had retained memory of the previous Day 1’s training and had learned that the food was inedible (positive %SAV; +%SAV). Animals that successfully trained on Day 1 but then attained a higher TTIM on Day 2 were characterized as non-learning individuals (non-learners). These animals earned negative %SAV (-%SAV).
The occurrence of +%SAV was recorded as evidence that an animal had learned (Schwarz et al., 1991; Levitan et al., 2012). Animals with +%SAV were placed back into a holding cage for 2 h following Day 2 training and then anesthetized and dissected. Statistical analysis of learning was performed on the subset of all animals that were successfully trained in LFI and had a +%SAV.
Learning food is inedible training began in animals of the cohort at the age of 7 months. This age was chosen to precede sexual maturity in the stages of aging (Kempsell and Fieber, 2014), so that animals would be focused on feeding and not on mating; these animals were designated young in the data that follows. They had been reared in exercise for 4 months. Training in young animals occurred over the course of 42 days with groups of approximately six animals undergoing training at a time, and those achieving +%SAV were subsequently sacrificed to collect the ganglia. Young untrained control animals were sacrificed alongside their LFI-trained siblings. To test the effects of age on LFI, training and ganglia collection were repeated in sibling animals at the age of 13 months when animals were in aging stage AII (Kempsell and Fieber, 2014). Aged animals were in exercise for 9 months, and their training continued over the course of 93 days. Aged untrained control animals were sacrificed alongside their LFI-trained siblings.
A far greater number of aged animals were trained compared to their young siblings to attain a comparable sample size of +%SAV animals. Twenty young animals and fifty aged animals were trained in LFI. In the study, 10% of young and 36% of aged siblings did not attain 100 s in the mouth during Day 1 training (duds) and were excluded from further analysis. A -%SAV, where Day 2 TTIM exceeded Day 1’s TTIM of >100 s, occurred in a separate 17% and 31% of young and aged siblings, respectively. As a result, the subset of animals with +%SAV used for comparisons was 15 young and 22 aged animals for behavioral comparisons, and 7 young and 18 aged animals for gene expression comparisons because some of the young samples were lost during the RNA extraction process.
Untrained control sibling animals were reared at the same time and under the same conditions as the LFI-trained animals. Untrained control animals were not trained in LFI, so there are no behavioral data to report on these animals; however, they were used in gene expression analyses. Untrained controls fasted for 76 h before sacrifice to conform to the feeding conditions of LFI-trained animals.
The Aplysia stages of aging were previously established in Kempsell and Fieber (2014) and were determined in this study by the onset of sexual maturity at the age of 10 months as well as the eventual significant slowing of the righting and tail withdrawal reflexes at the age of 12 months. No fewer than 30 animals were tested in the righting reflex, and no fewer than 17 animals were tested in the tail withdrawal reflex each month. Animals tested in reflex behaviors were selected at random from the cohort. Therefore, the results include reflex times from animals that were later trained in LFI and those that remained untrained and were used as controls.
Quantitative gene expression
After a 76 h fast and 2 h after Day 2 training, untrained animals or LFI-trained animals were anesthetized by injecting chilled isotonic magnesium chloride at a volume of 1/6th of their body weight into their posterior sinus. The buccal ganglia were then removed under a dissecting microscope, and conspicuous neurons B2 and B1 of the right hemiganglion were located and used as a reference for the location of nearby target neurons B31/B32 (Figure 1). A straight line cut was made next to neuron B2, and only the section of the hemiganglion containing B31/B32 minus the connectives was saved. This trimmed buccal ganglia preparation eliminated from gene expression analysis the buccal S clusters and many moto- and interneurons. Previous studies demonstrated no changes in ApC/EBP expression after LFI when testing buccal ganglia without BSCs (Levitan et al., 2012); thus, additional neurons were trimmed to attempt to boost the signal from moto- and interneurons associated with the feeding reflex such as B20, B51, B52, and B34. A single experimenter performed all dissections to attempt to make them as uniform as possible.
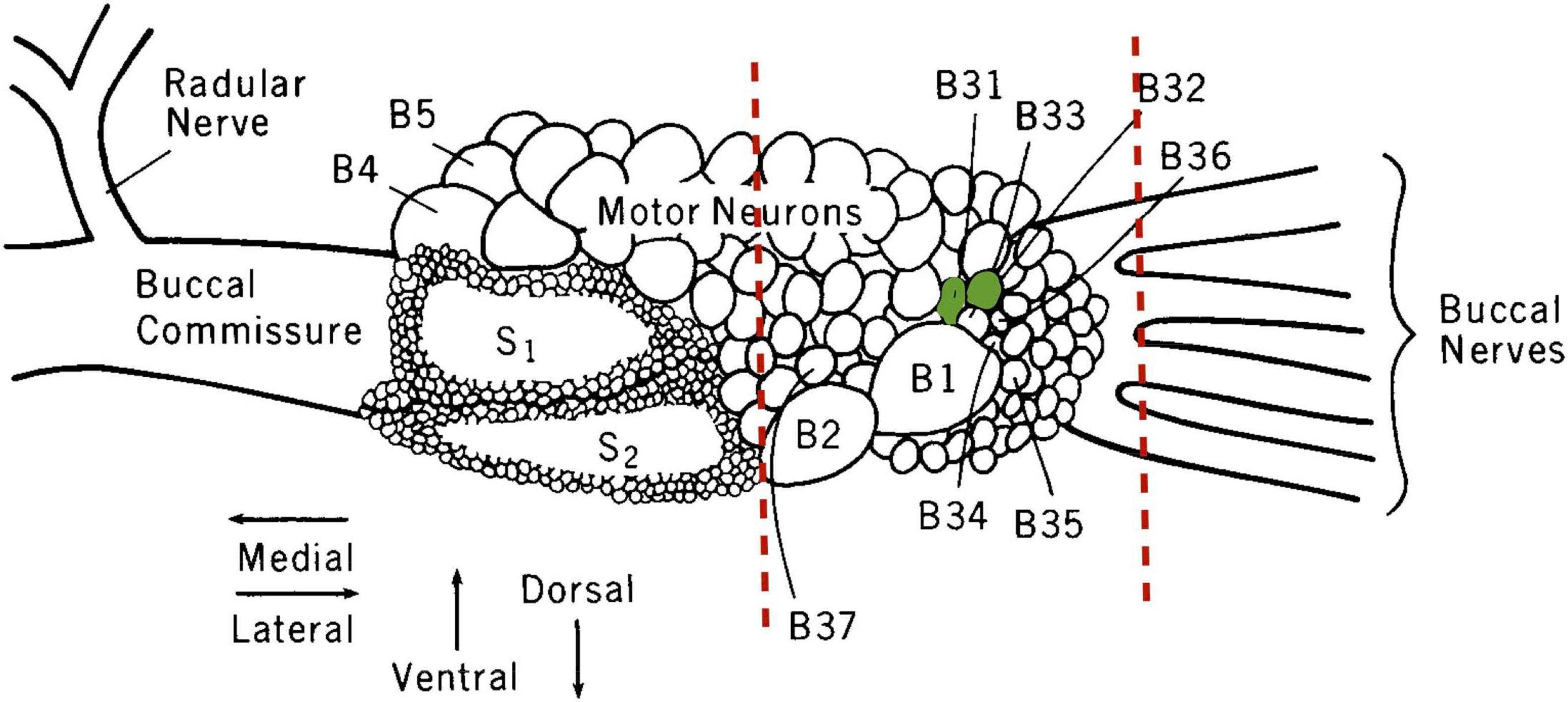
Figure 1. Illustration of the right buccal hemiganglion. The sites where buccal ganglia elements were trimmed away to form the B31/B32-inclusive fragment used for gene expression analysis are indicated by the dashed red lines. Neurons B31/B32 are colored green. S1 and S2 denote the buccal S cluster neurons excluded from the preparation. Modified from Susswein and Byrne (1988), with permission from Journal of Neuroscience © 1988 Society for Neuroscience.
Trimmed buccal ganglia samples were placed in 1.5 mL microcentrifuge tubes containing 300 μL RNAProtect. RNA was extracted using the RNeasy Micro Kit (Qiagen RNeasy Micro Kit). The concentration and purity of RNA were determined by Nanodrop analysis of 1.5 μL of RNA (NanoDrop Technologies Model ND-1000). A total of 100 ng RNA was reverse-transcribed into cDNA using SuperScript III (Invitrogen SuperScript III First-Strand).
ApC/EBP isoform X1, previously shown to behave identically to the more commonly studied ApC/EBP (Lee et al., 2001), was studied because highly specific primers were produced that spanned an exon junction. This was impossible for ApC/EBP as its gene is intronless and using previously published primers for ApC/EBP resulted in some non-specific binding. ApC/EBP isoform X1 is a truncated protein of ApC/EBP. Both ApC/EBP and its isoform X1 are functional transcription factors capable of binding to and activating enhancer response element (ERE) promoters. Lee et al. (2001) demonstrated a 16-fold increase in ERE-luciferase reporter expression in Aplysia neurons 24 h after DNA microinjection of ApC/EBP or its isoform X1. This indicated that ApC/EBP and its isoform X1 do not differ in function or effect. ApC/EBP isoform X1 was used here as the proxy for ApC/EBP. In contrast, Aplysia has a single teneurin paralog, unlike Drosophila, with 2, ten-a and ten-m (Wides, 2019).
SYBR Green (Applied Biosystem PowerSYBR Green PCR Master Mix) qPCR in a 96-well plate was conducted using a Stratagene thermocycler (Stratagene Mx3005P) under the following cycle parameters: 10 min initial denaturation at 95°C followed by 40 cycles of 30 s at 95°C, 1 min at 55°C, and 1 min at 72°C with fluorescence measured at the end of each cycle, and followed by a melt curve for 1 min at 95°C, 30 s at 55°C, and 30 s at 95°C. In this study, 2 μL of cDNA was used in each qPCR reaction which is equivalent to 10 ng of starting RNA. Gene specific primers for teneurin, i.e., forward primer, 5′-TCAACAGGATCCGAGTGGTCAGTA-3′, reverse primer, 5′TGCTACGACCCTCACGAGACA-3′ and ApC/EBP isoform X1: forward primer, 5′GCACAAACAAAGATCCCACGG-3′ reverse primer, 5′-CGGACGTGACGAGCTACTAC-3′, were used. Samples and standard curves were run in triplicate.
Standards for the absolute quantification of ApC/EBP isoform X1 and teneurin transcripts were created by cloning PCR products using a TOPO II cloning kit (Thermo Fisher Scientific TOPO TA Cloning Kit, Dual Promoter, without competent cells) and then quantifying plasmid levels. Copy numbers were estimated by comparison with a standard curve (teneurin m = −3.36, efficiency = 98.4%; ApC/EBP isoform X1 m = −3.54, efficiency = 91.7%).
A time course study was conducted to pinpoint teneurin’s optimal gene expression window using age 13-month Aplysia from a separate cohort. LFI training was followed by trimmed buccal ganglia dissections at 1, 2, and 3 h after Day 2 recall testing. Two +%SAV animals for each timepoint were used to determine teneurin expression via qPCR.
Statistical analysis
The Shapiro–Wilk test was used to test for normality. A Student’s t-test was used to determine significant differences in percent savings. The Wilcoxon signed-rank test was used to determine significant differences in TTIM within each age group, comparing TTIM on Day 1 to TTIM on Day 2, while multiple Wilcoxon rank-sum tests followed by a Bonferroni’s correction was used to determine significant differences in TTIM on Day 1 and on Day 2 between young and aged. Significant differences in bite frequency were determined through a chi-square test. Multiple chi-square tests followed by a Bonferroni’s correction were used to determine significant differences in bite frequency.
When comparing gene expression differences within an age class, a one-way ANOVA was used for teneurin expression, and a Kruskal–Wallis rank-sum test followed by a post-hoc Dunn test was used for ApC/EBP isoform X1 expression. Multiple student’s t-tests followed by a Bonferroni’s multiple test correction were used to determine significant differences in teneurin expression across age groups. Multiple non-parametric Kruskal–Wallis rank-sum tests followed by a Bonferroni’s multiple test correction were used to determine significant differences in ApC/EBP isoform X1 expression.
Pearson’s correlation coefficient was used for correlation analyses. Significant differences between months in reflex behaviors were determined by a Kruskal–Wallis rank-sum test and post-hoc Dunn test. Differences were considered significant at a p-value of ≤ 0.05 unless an adjustment was needed after performing a Bonferroni’s multiple test correction on the p-value.
Results
Age determination
Animal age in this study was determined by the chronological age as well as the significant decline in performance of reflex behaviors beginning at the age of 12 months (Figure 2). Our laboratory demonstrated that performance in these behaviors declines in a predictable manner with age (Kempsell and Fieber, 2014) and that declining performance is accompanied by a decline in excitability of PVC sensory neurons that control tail withdrawal, decreased short-term facilitation (STF) between tail sensory and motor neurons (Kempsell and Fieber, 2015), decreased expression of ionotropic glutamate receptor subunits (Greer et al., 2019), and other changes in gene expression that alter proteostasis and increase neuro-inflammation (Kron et al., 2020; Kron and Fieber, 2021).
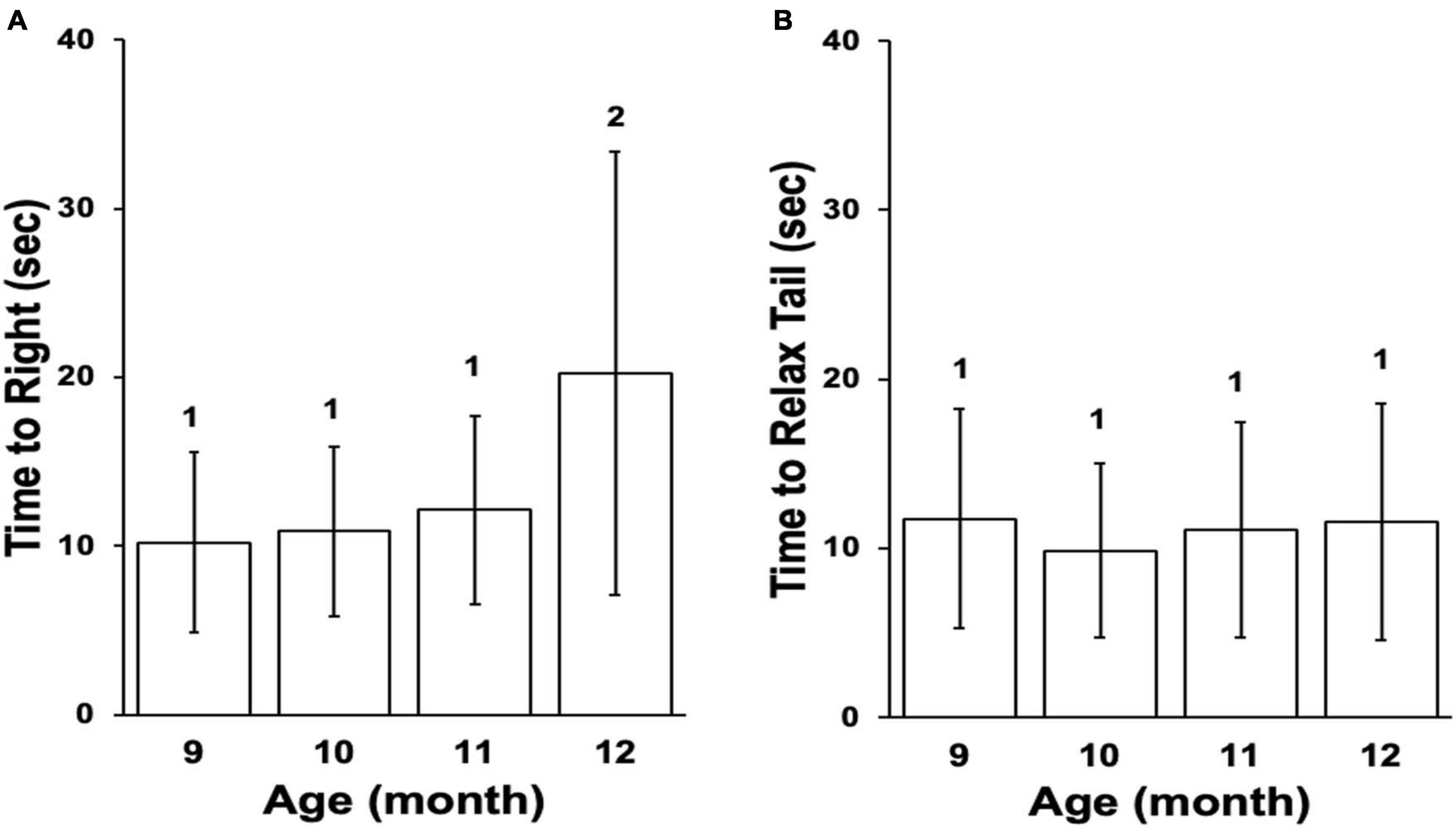
Figure 2. Reflex behaviors for the cohort. (A) Mean time to right in righting reflex, ± SD. (B) Mean time to relax tail in tail withdrawal reflex, ± SD. Differences in numbers above columns denote significance differences between months (Time to Right, Kruskal–Wallis rank sum test p ≤ 0.001; Dunn’s test 12 vs. 9 padj ≤ 0.001, 12 vs. 10 padj ≤ 0.01, 12 vs. 11 padj ≤ 0.05). The cohort entered stage Aged II at age 12 months based on the righting reflex.
Behavioral results
Upon initial presentation of the probe in LFI, an animal advanced toward the netted algae, and once the probe touched the lips, it attempted to ingest it, likely due to hunger. It pulled the probe into its buccal cavity (biting) and then attempted to move the probe into its crop (swallow), which the probe was designed to thwart. Often during swallowing attempts, the animal’s radula could be felt scraping the probe as a vibration through the hemostat. Eventually, the animal pushed the probe out of its buccal cavity (exit). As training progressed, the duration of time the probe was held in the mouth decreased as interest in attempted consumption waned and then ceased altogether. Consistent with Susswein et al. (1986) findings, the relationship between attempts at ingestion on Day 1 and the time the probe remained in the mouth during that ingestion attempt were inversely correlated (Figure 3). Especially during the first five bites, the probe exited from the buccal cavity more quickly with each successive ingestion attempt.
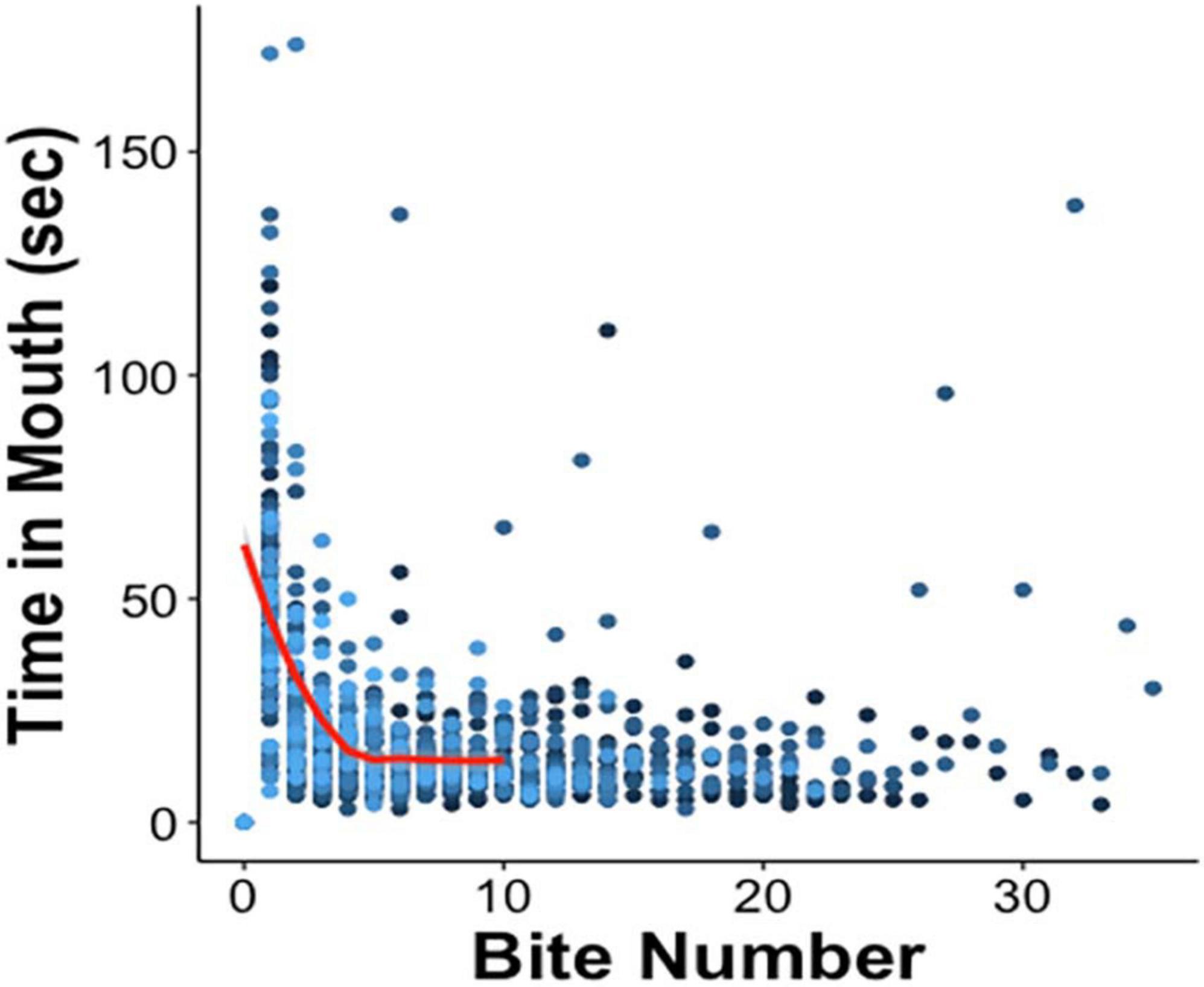
Figure 3. Negative correlation between time spent attempting to ingest the probe and the first five ingestion attempts on Day 1 [red curve; Pearson’s correlation coefficient r(230) = –0.57, p ≤ 0.001], after which the data begin to plateau as animals successfully complete training for that day. The amount of time the probe was held in the mouth was recorded for each bite. Each bite number along the x-axis records Day 1 tests of all 70 animals, in 70 different shades of blue.
Among +%SAV animals, there was no significant difference in the average TTIM on Day 1 between age groups; however, the mean Day 2 TTIM was significantly less than Day 1 in both young and aged animals (Figure 4A). Day 2 aged TTIM, in addition, was significantly less than young Day 2 TTIM, and thus, the aged animals that learned had significantly higher %SAV than their young siblings (Figure 4B). Biting followed a similar trend, with no significant difference between age groups in the distribution of biting occurrences on Day 1 and a significant reduction in biting occurrences on Day 2 in both young and aged animals (Figure 4C). Aged +%SAV siblings made significantly fewer biting attempts on Day 2 compared to young siblings, with an additional metric leading to higher %SAV in aged animals.
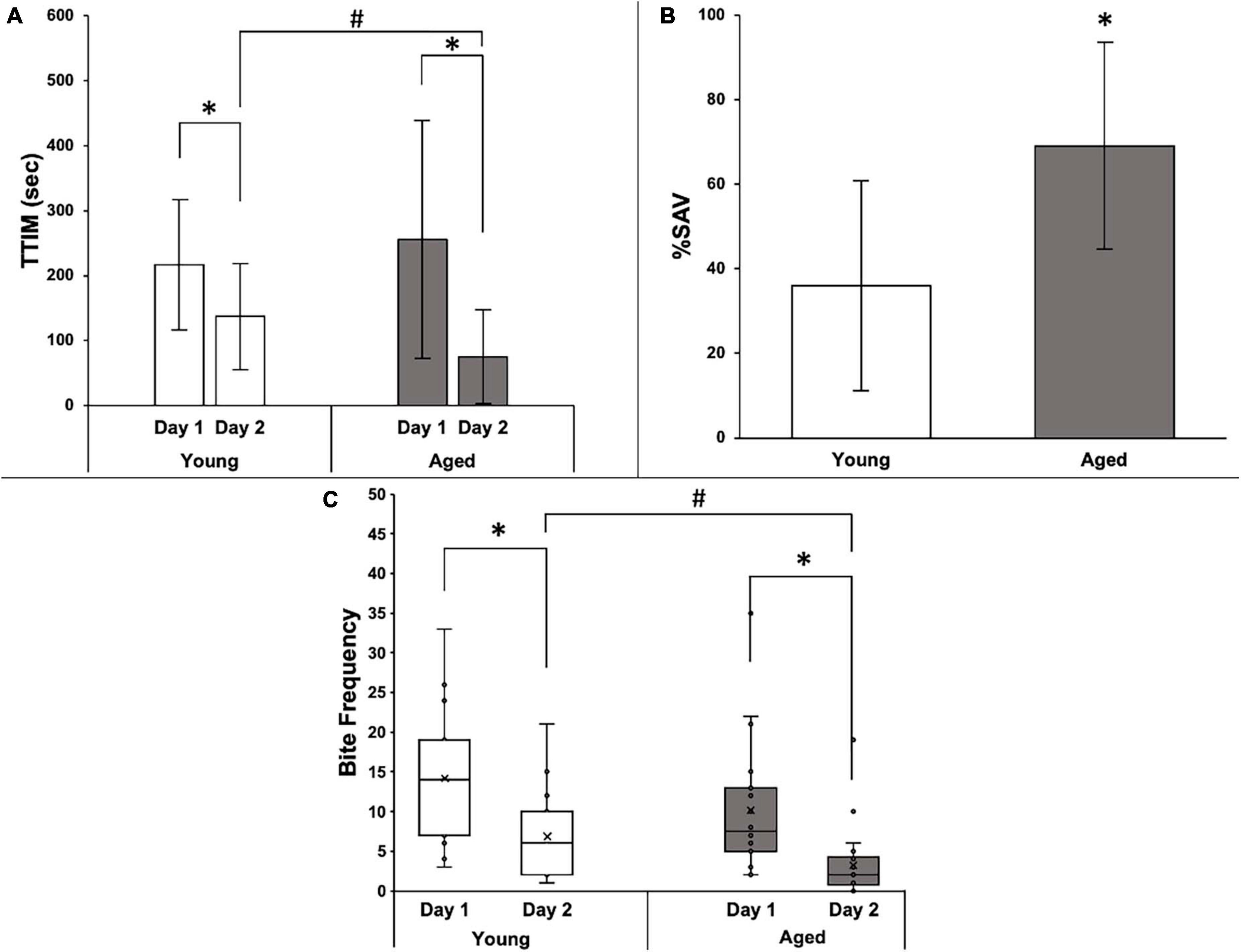
Figure 4. Behavioral data for 15 young and 22 aged sibling Aplysia. (A) Mean TTIM of young and aged sibling Aplysia, ± SD, on Day 1 and Day 2 training. *Denotes significant difference via Wilcoxon signed-rank (p ≤ 0.05: young p ≤ 0.001; aged p ≤ 0.001). #Denotes a significant difference via the Wilcoxon rank-sum test and Bonferroni’s correction (padj ≤ 0.05). (B) Mean %SAV in young and aged sibling Aplysia, ± SD. *Denotes a significant difference via Student’s t-test (p ≤ 0.01). (C) Distribution of bite frequency of young and aged sibling Aplysia on Day 1 and Day 2 of LFI re-training. *Denotes significance difference via chi-square (p ≤ 0.001). #Denotes significance difference via chi-square test and Bonferroni’s correction (padj ≤ 0.05).
Five aged +%SAV Aplysia who qualified for Day 2 re-training showed no interest in the probe on Day 2. These animals were designated as 100% learners, with perfect recall that the probe was inedible on Day 2. They are included in the analyses of Figures 3, 4. An alternative explanation, however, is that they simply failed Day 2 training, a behavior that would have classified them as duds if it occurred on Day 1. This ambiguity warranted a separate analysis of the data in which these individuals were excluded. The results with their exclusion were largely unchanged, with the only difference being a loss in significance when comparing TTIM on Day 2 between the two +%SAV age groups (data not shown). Importantly, however, exclusion of the 100% learners resulted in a significantly higher %SAV compared to young siblings. Since the exclusion of 100% SAV individuals did not markedly alter the results, they were included in the final behavioral and gene expression analyses.
Gene expression
Teneurin expression was significantly elevated in aged animals in untrained and trained +%SAV categories compared to their young sibling counterparts (Figure 5) but did not differ among the aged categories. Similarly, the young animals in all training categories showed no significant differences in teneurin expression.
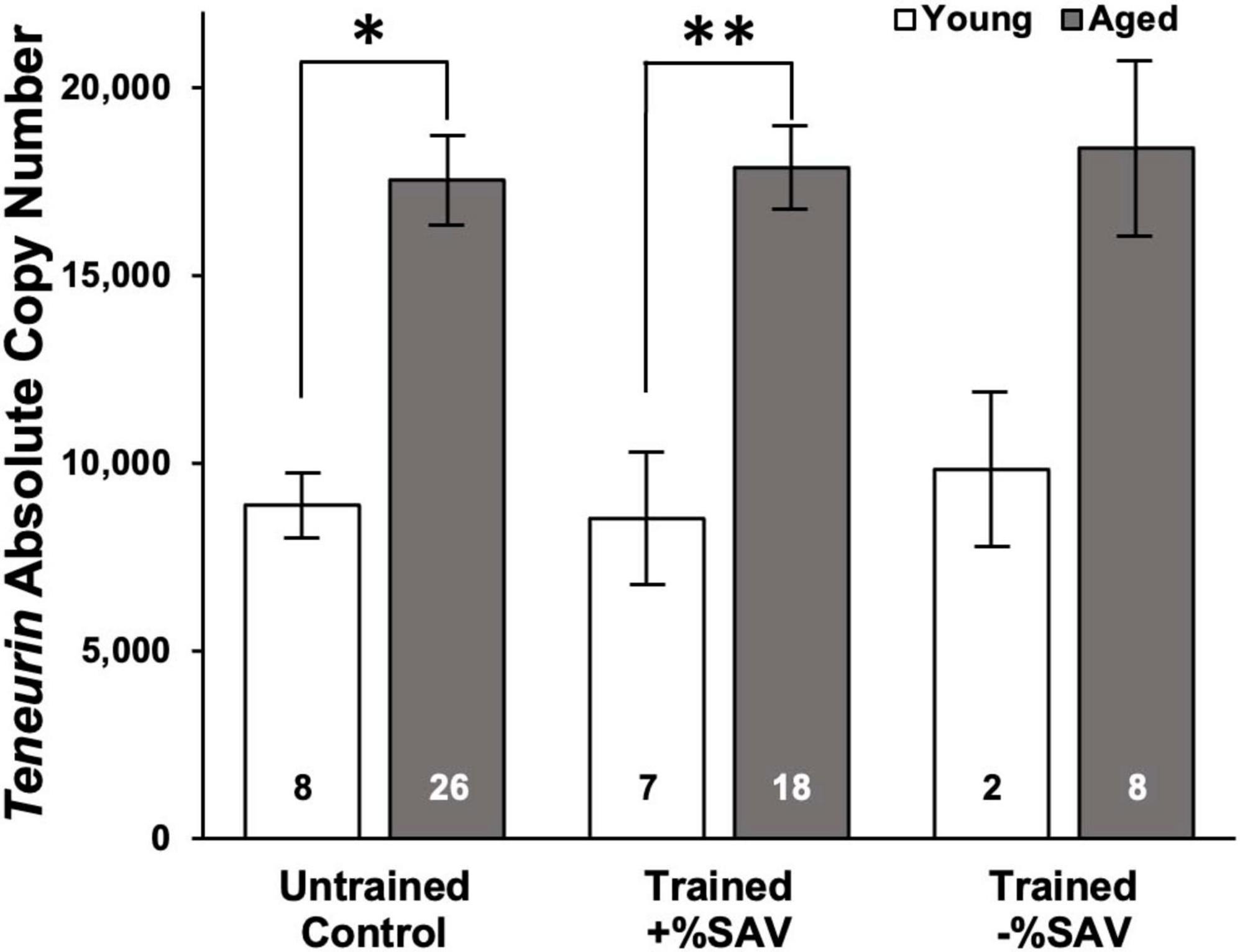
Figure 5. Mean teneurin copy number ± SE in young (white bars) and aged (gray bars) animals. ANOVA in young and aged animals, all categories, showed no significant differences. Multiple student’s t-tests with a Bonferroni’s correction were then used to test for significant differences between ages. *Denotes a significant difference via Student’s t-test (p ≤ 0.001). **Denotes significance difference via Student’s t-test (p ≤ 0.001). Sample size noted within bars. Teneurin expression for young animals was quantified over 2 qPCR plates with the following standard curve slopes and primer efficiencies: Plate 1 m = –3.40, efficiency = 96.7%; Plate 2 m = –3.53, efficiency = 92.0%. Teneurin expression for aged animals was quantified over three qPCR plates with the following standard curve slopes and primer efficiencies: Plate 1 m = –3.46, efficiency = 94.5%; Plate 2 m = –3.56, efficiency = 90.8%; Plate 3 m = –3.47, efficiency = 94.3%.
The teneurin expression was not correlated to individual animals’ %SAV (data not shown). A time course experiment on aged animals to determine whether the time of sampling after LFI training affected teneurin expression showed that time of sampling at 1, 2, or 3 h after recall testing did not significantly influence teneurin gene expression (data not shown).
In aged animals, there was a significant reduction in ApC/EBP isoform X1 expression in +%SAV animals compared to untrained controls (Figure 6). Expression was significantly elevated in aged untrained controls compared to their younger siblings. Young animals in all training categories showed no significant differences in expression. Individual animals’ ApC/EBP isoform X1 expression and %SAV showed a negative correlation only in the aged animals (Figure 7). Finally, ApC/EBP isoform X1 expression and teneurin expression were significantly positively correlated only in the young animals (Figure 8).
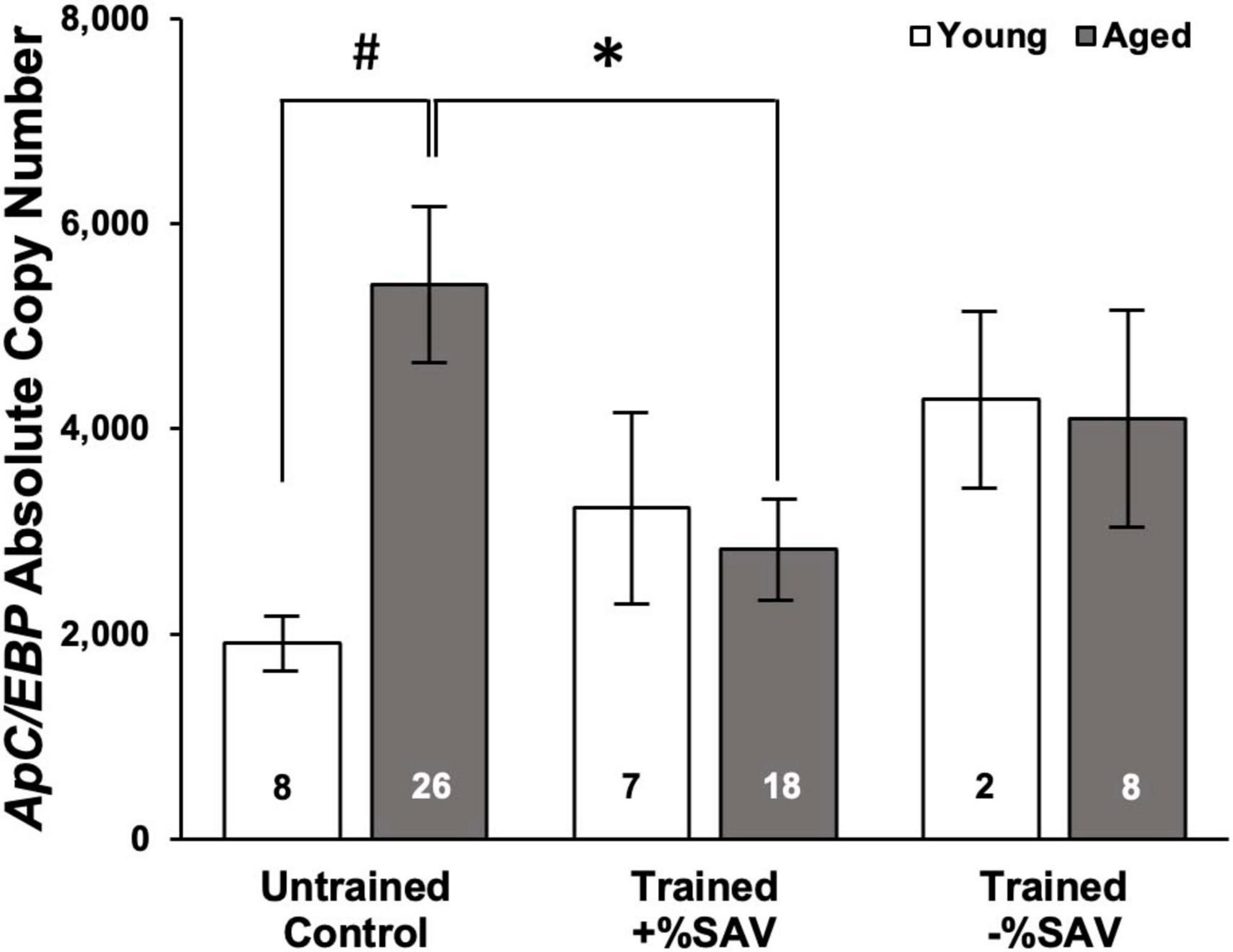
Figure 6. Mean ApC/EBP isoform X1 copy number, ± SE in young (white bars) and aged (gray bars) animals. Multiple Kruskal–Wallis rank sum tests with a Bonferroni’s correction were used to test for significant differences. *Denotes a significant difference (p ≤ 0.05) via Kruskal–Wallis rank sum test (padj ≤ 0.05 via Dunn’s test). #Denotes significance difference via Kruskal–Wallis rank sum test (p ≤ 0.01). ApC/EBP isoform X1 expression for both young and aged animals was quantified over four qPCR plates with the following standard curve slopes and primer efficiencies: Plate 1 m = –3.72, efficiency = 85.8%; Plate 2 m = –3.61, efficiency = 89.2%; Plate 3 m = –3.66, efficiency = 87.5%; Plate 4 m = –3.54, efficiency = 91.7%.
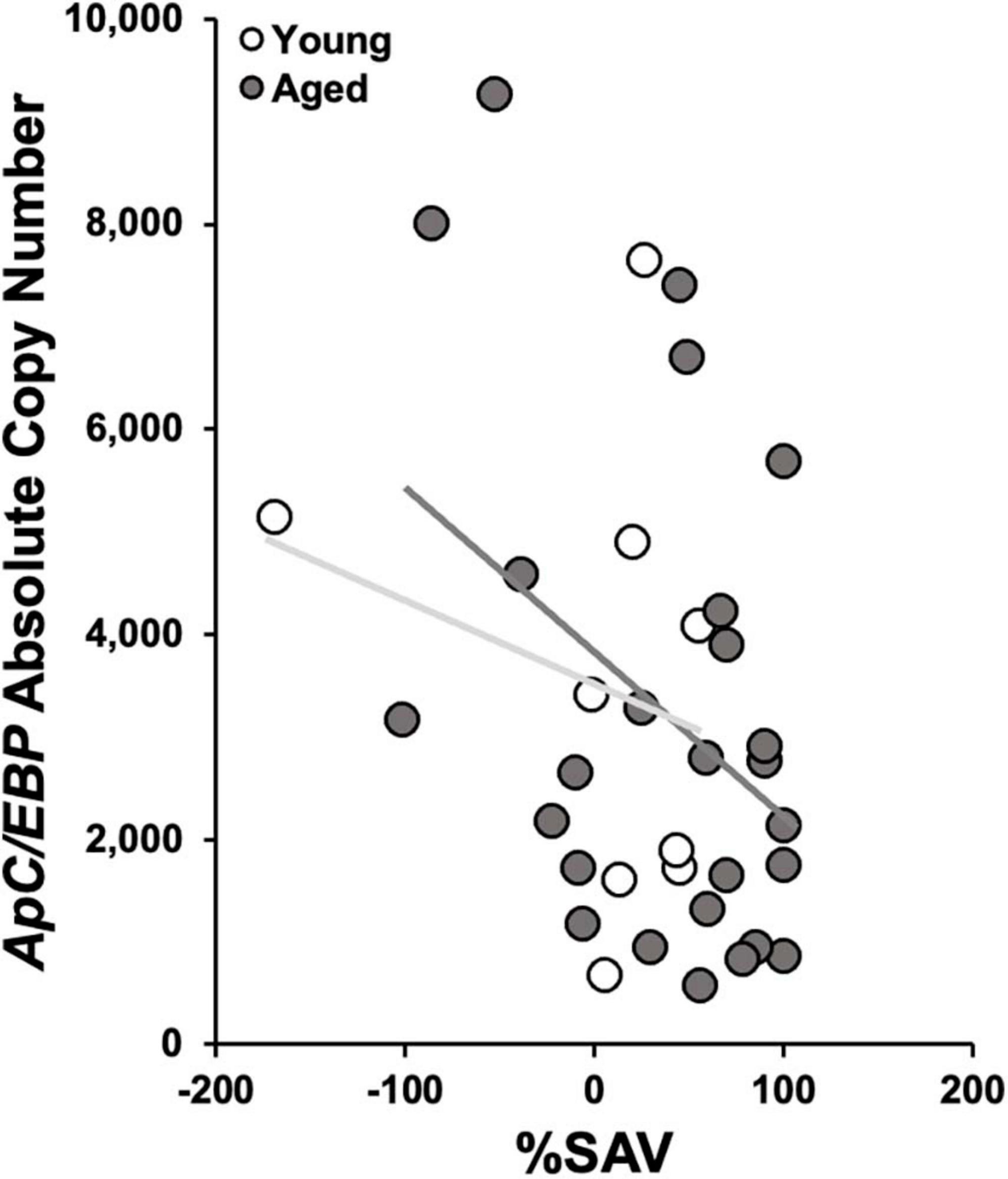
Figure 7. ApC/EBP isoform X1 copy number as a function of %SAV. Aged animal expression was negatively correlated to performance via Pearson’s correlation coefficient [gray; r(24) = –0.39, p ≤ 0.05; regression line in dark gray]. No significant correlation was found in the young samples (white; regression line light gray).

Figure 8. Teneurin copy number as a function of ApC/EBP isoform X1 copy number. Young animal expression was significantly positively correlated via Pearson’s correlation coefficient [white; r(7) = 0.78, p ≤ 0.05]. No significant correlation was found in aged samples (gray). Regression lines as in Figure 7.
Discussion
Young and aged Aplysia both demonstrated learning and recall in LFI. Some failed the Day 1 learning protocol by not attaining the TTIM threshold of 100 s common to these studies (Levitan et al., 2012; Tam et al., 2020), while others failed Day 2 recall testing when their TTIM was greater than that on Day 1. Aged animals failed at a greater proportion on both Day 1 and Day 2. This resulted in more aged animals being tested to achieve a sample size of good performers (+%SAV) in age to compare to young animals. It was particularly important to have a standardized learning threshold for gene expression analysis linked to learning performance. The necessity to test more aged animals is a common dynamic in aged learning (Gage et al., 1984; Lee et al., 1994; Maliković et al., 2019); however, it may have resulted in selection bias in its focus solely on the animals from each age group that learned on Day 1 and demonstrated recall on Day 2. The aged animals that learned had significantly lower TTIM on Day 2, resulting in significantly higher +%SAV than their younger siblings.
There have been few studies that demonstrated increased cognitive performance in age, always specific to operant conditioning (Samson et al., 2014). LFI, as an operant paradigm (Michel et al., 2011), is consistent with this pattern. In contrast, and more commonly reported, there are many other studies that assert cognition declines with age, including spatial learning in rats (Adams et al., 2008); spatial, visual, and temporal learning in fish (Yu et al., 2006; Terzibasi et al., 2008); sensitization and habituation in Aplysia (Kempsell and Fieber, 2015, 2016); electric shock avoidance, locomotion, geotaxis, and phototaxis in Drosophila (Simon et al., 2006); and isothermal tracking in C. elegans (Murakami and Murakami, 2005).
In operant conditioning, organisms will learn to change behavior to receive rewards or avoid punishments (Skinner, 1938). In LFI, the negative reinforcement (Michel et al., 2011) of inedibility, culminating in ceasing attempts to ingest the probe (Schwarz and Susswein, 1986), was better avoided in aged animals compared to their younger siblings. This is similar to a phenomenon observed in humans who commonly shift away from reward-based and toward loss-avoidance operant learning during aging (Freund and Ebner, 2005; Frank and Kong, 2008; Horn and Freund, 2021).
Another interpretation is that aged Aplysia are more selective eaters than their younger siblings. Wild A. californica has few natural predators (Moroz, 2011) and an abundant food supply, which allows it to be a preferential eater (Kupfermann and Carew, 1974). Preferential eating has been observed in the laboratory (Carefoot, 1967), and firsthand while rearing Aplysia in this study, where hungry animals refused U. lactuca, fasting until the preferred A. subulata was offered. Aged Aplysia may have been faster to refuse the Ulva food probe, potentially waiting for the preferred Agardhiella to be offered.
A plausible interpretation of aged animal performance in LFI is that aged Aplysia that learned did not necessarily learn better in LFI, but rather they remembered their training better on Day 2, signifying that a memory was formed. This would place Day 2 of LFI not as a day of re-training but as a test of the animal’s LTM of Day 1’s training (Schwarz et al., 1991; Botzer et al., 1998; Katzoff et al., 2002; Michel et al., 2011; Krishnan et al., 2016). Trained animals on Day 1 learned to recognize the inedible probe in an approximately equal time and number of ingestion attempts, and it was only on Day 2 that age benefitted recognition of the probe as inedible. This behavior was also observed in aged rats during extinction training, in which Day 1 performance was no different, but on Day 2, aged rats performed significantly better than their younger counterparts (Samson et al., 2014). In this view, data suggest that the aged animals that learned had a better ability to store and recall LTM of LFI than younger siblings.
ApC/EBP isoform X1 expression was either unchanged (young animals) or decreased (aged animals) after 2 days of LFI training in trimmed buccal ganglia neurons. This was unexpected; ApC/EBP’s role in LFI necessitates that it rises in expression to facilitate long-term facilitation (LTF). The explanation may lie in timing of the qPCR analysis of ApC/EBP isoform X1 transcripts as well as the trimmed buccal ganglia sample preparation.
Learning food is inedible training was shown to induce ApC/EBP expression 15 min, 1 h, and 2 h after Day 1 LFI training in isolated buccal S cluster (BSC) sensory neurons of the buccal ganglia but not elsewhere (Levitan et al., 2008, 2012; Briskin-Luchinsky et al., 2018), implying that the memory for LFI was stored in the BSC. It was later discovered that LFI facilitates functional rewiring between BSC neuronal processes and their postsynaptic motoneurons, resulting in changes in synaptic strength and number of synaptic connections and culminating in the flipping of postsynaptic potentials between excitatory and inhibitory (Tam et al., 2020). However, this still did not explain the physiological changes that accompany LFI, suggesting other synaptic modifications must be involved.
For example, cerebral-buccal interneurons (CBIs) of the cerebral ganglia were shown to decrease their responsiveness to the neurotransmitter acetylcholine (ACh) after LFI (McManus et al., 2019). ACh, released from sensory afferent neurons after the animal is stimulated by food, depolarized CBIs (Susswein et al., 1996), which then activates central pattern generators of the buccal ganglia that control the animal’s feeding reflexes (Hurwitz et al., 2003; Jing et al., 2003). Unresponsiveness in CBI resulted in fewer biting responses and a shortened duration of ingestion attempts (McManus et al., 2019), illustrating that memory for LFI is distributed among multiple sites within the neural circuit controlling the reflex and not confined to BSC neurons as previously thought.
Here, BSC neurons were removed from the buccal ganglia to enhance the detection of ApC/EBP isoform X1 and teneurin expression changes in a subset of inter- and motoneurons of the buccal ganglia, suggested to be important in the freshwater pond snail Lymnaea stagnalis (Perry et al., 1998; Hatakeyama et al., 2006), where C/EBP mRNA expression decreased after conditioned taste aversion (CTA) training and C/EBP protein production and phosphorylation increased. This suggested that training rapidly induced the translation and then immediate degradation of available C/EBP transcripts in postsynaptic motoneurons, a pattern that may have played out here in trimmed buccal ganglia samples after LFI.
One of the outcomes of LFI is a reduction in radula protractions. Changes in B31/B32 activity were hypothesized to be implicated and to experience changes in expression of ApC/EBP isoform X1. This either was not observed or not measured accurately as trimmed buccal ganglia samples included other inter- and motoneurons along with B31/B32. While the goal of trimming the buccal ganglia was to boost the signal coming from B31/B32 by eliminating signals from the BSCs along with a great proportion of inter- and motoneurons that lie superior to the BSCs, B31/B32 may have remained only minor contributors to the overall expression signal coming from the sample. Levitan et al. (2012) hypothesized that the inclusion of superfluous cells retards the detection of even large changes in ApC/EBP expression. It may not be possible to accurately infer LFI-related changes in ApC/EBP isoform X1 in this study for this reason.
Another possible reason for failure to detect changes in ApC/EBP isoform X1 expression in this study is sampling may have occurred outside the appropriate time window. Levitan et al. (2012) noticed that ApC/EBP expression changes were undetectable in BSCs 24 h after LFI. Therefore, ApC/EBP isoform X1-induced memory consolidation may have already taken place by the start of Day 2 recall testing, and gene expression levels returned to normal. Detectable after Day 2 of LFI would instead be genes involved in the reconsolidation of LFI memory, and it is possible that ApC/EBP isoform X1 plays no role in this process (Taubenfeld et al., 2001).
A significantly higher ApC/EBP isoform X1 expression was noted in aged untrained Aplysia compared to young siblings. While in the absence of a learning component, this increase is common in neurons as animals age, for example, human and rat (Wang et al., 2018), and has been suggested to reflect a role in inflammation, the overexpression of ApC/EBP by DNA microinjection has also been shown to increase the efficiency of LTF induction after a single pulse of 5-HT (Lee et al., 2001), demonstrating that the more ApC/EBP available at the time of training, the faster LTF occurs and in response to fewer repeated stimuli. The significant reduction in ApC/EBP isoform X1 in aged trained Aplysia compared to their untrained siblings and the negative correlation of ApC/EBP isoform X1 to increased +%SAV suggest that the neurons are rapidly translating ApC/EBP isoform X1 protein from the larger reservoir of ApC/EBP isoform X1 mRNA in aged neurons, ApC/EBP isoform X1 mRNA is then being rapidly degraded as was shown in L. stagnalis after CTA (Hatakeyama et al., 2006), and this process may be aiding the performance of LFI in aged Aplysia.
Teneurin expression was elevated in aged animals, yet after testing for recall of LFI, there was no effect on its expression in either young or aged animals. Its failure to increase may indicate that teneurin expression is not affected by learning. Gene expression results did not corroborate the hypothesis that teneurin is involved in learning-induced synaptic modifications because changes in teneurin expression in +%SAV animals were not detected. This may be due to the goal of testing gene expression during the reconsolidation of memory and not during the consolidation process, which can initiate different transcriptional pathways (Hertzen and Giese, 2005), resulting in either enhancement or reduction of the memory (Lee et al., 2017).
Much similar to the results for ApC/EBP isoform X1, qPCR analysis may have taken place long after teneurin expression was expected to be affected by learning. Samples were taken 2 h after animals were showing signs of LTM from the previous day’s training. This indicated that synaptic connections had already been altered during the consolidation of LFI. If no longer needed for the consolidation of memory, teneurin expression levels may have receded back to normal. As for ApC/EBP isoform X1, dilution also was possible, in a preparation designed to bolster the contribution of neurons B31/B32 which are small and anonymous absent electrophysiological recording (Susswein and Byrne, 1988; Dembrow et al., 2004). It is therefore not possible to accurately conclude that teneurin is not involved in memory consolidation based on these results.
In the Drosophila mutant central body defect (cbd) which is deficient in teneurin isoform, ten-a, development in regions of the brain important to visual pattern memory is disrupted (Pan et al., 2009; Cheng et al., 2013), and olfactory learning is impacted (Heisenberg et al., 1985). The overexpression of ten-a restores normal development and learning, while downregulation through RNAi disrupts them (Cheng et al., 2013). A similar causal link between teneurin expression and learning in Aplysia remains undemonstrated; however, higher teneurin levels may result in better learners or a stronger consolidation of memory. Aged animals that learned in LFI had elevated teneurin levels and were significantly better performers.
Conclusion
Young and aged Aplysia that demonstrated learning and recall in LFI were compared for behavioral and transcriptional differences. Aged Aplysia selected for their success in the protocol, which occurred less frequently in age than in youth, demonstrated recall superior to their younger siblings. This coincided with differences in gene expression underlying the feeding reflex between the two age groups. Neurons from naive aged Aplysia showed increased transcript levels of two genes that are believed to be involved in learning, which may have benefitted the aged Aplysia that learned in their increased ability to recall LFI. It is also possible that aged animals that learned in the protocol experienced a shift in learning performance from reward-based to loss avoidance captured in the negative operant conditioning of LFI.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study of animals in accordance with the local legislation and institutional requirements.
Author contributions
ER and LF conceived the research executed by ER and summarized the data. ER executed statistical analysis. Both authors co-wrote the manuscript and approved the submitted version.
Funding
This study was funded by the National Institutes of Health Grant P40OD010952.
Acknowledgments
We thank the University of Miami Resource for Aplysia staff for their expertise in animal care, and Mia DiCaprio, Faith Bellas, Kasandra Scholz, Alexis Pupo, Seré Politano, and Jackson Fiorini for assistance. We are grateful to Dr. Abraham J. Susswein for inspiration, and Patrick D. L. Gibbs for guidance in creating qPCR standards. We also thank Dr. Nicholas S. Kron for constant moral support and advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, M., Shi, L., Linville, M., Forbes, M., Long, A., Bennett, C., et al. (2008). Caloric restriction and age affect synaptic proteins in the hippocampal CA3 and spatial learning ability. Exp. Neurol. 211, 141–149. doi: 10.1016/j.expneurol.2008.01.016
Alberini, C. (2009a). Transcription factors in synaptic plasticity and learning and memory. Encyclopedia Neurosci. 9, 1081–1092.
Alberini, C. (2009b). Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 89, 121–145.
Alberini, C., and Kandel, E. (2015). The regulation of transcription in memory consolidation. Cold Spring Harbor Perspect. Biol. 7:a021741.
Bailey, C., and Chen, M. (1988a). Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 85, 2373–2377. doi: 10.1073/pnas.85.7.2373
Bailey, C., and Chen, M. (1988b). Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7. Proc. Natl. Acad. Sci. U.S.A. 85, 9356–9359. doi: 10.1073/pnas.85.23.9356
Bailey, C., and Kandel, E. (2008). Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia. Prog. Br. Res. 169, 179–198. doi: 10.1016/S0079-6123(07)00010-6
Botzer, D., Markovich, S., and Susswein, A. (1998). Multiple memory processes following training that a food is inedible in Aplysia. Learn. Mem. 5, 204–219.
Briskin-Luchinsky, V., Roi, L., Halfon, M., and Susswein, A. (2018). Molecular correlates of separate components of training that contribute to long-term memory formation after learning that food is inedible in Aplysia. Learn. Mem. 25, 90–99. doi: 10.1101/lm.046326.117
Cai, D., Pearce, K., Chen, S., and Glanzman, D. (2012). Reconsolidation of long-term memory in Aplysia. Curr. Biol. 22, 1783–1788.
Carefoot, T. (1967). Growth and nutrition of Aplysia punctata feeding on a variety of marine algae. J. Mar. Biol. Assoc. U.K. 47, 565–589.
Cheng, X., Jiang, H., Li, W., Lv, H., Gong, Z., and Liu, L. (2013). Ten-a affects the fusion of central complex primordia in Drosophila. PLoS One 8:e57129. doi: 10.1371/journal.pone.0057129
Cropper, E., Evans, C., Hurwitz, I., Jing, J., Proekt, A., Romero, A., et al. (2004). Feeding neural networks in the mollusc Aplysia. Neurosignals 13, 70–86. doi: 10.1159/000076159
Dembrow, N., Jing, J., Brezina, V., and Weiss, K. (2004). A Specific synaptic pathway activates a conditional plateau potential underlying protraction phase in the Aplysia feeding central pattern generator. J. Neurosci. 24, 5230–5238. doi: 10.1523/JNEUROSCI.5649-03.2004
DePew, A., Aimino, M., and Mosca, T. (2019). The tenets of teneurin: conserved mechanisms regulate diverse development processes in the Drosophila nervous system. Front. Neurosci. 13:27. doi: 10.3389/fnins.2019.00027
Fieber, L., Kron, N., Greer, J., Rooney, H., Prostko, R., Stieglitz, J., et al. (2018). A comparison of hatchery-rearing in exercise to wild animal physiology and reflex behavior in Aplysia californica. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 221, 24–31. doi: 10.1016/j.cbpa.2018.03.006
Freund, A., and Ebner, N. (2005). “The aging self: Shifting from promoting gains to balancing losses,” in The adaptive self: Personal continuity and intentional self-development, eds W. Greve, K. Rothermund, and D. Wentura (Hogrefe: Göttingen), 185–202.
Gage, F., Dunnett, S., and Björklund, A. (1984). Spatial learning and motor deficits in aged rats. Neurobiol. Aging 5, 43–48.
Goelet, P., Castellucci, V. F., Schacher, S., and Kandel, E. R. (1986). The long and the short of long-term memory – a molecular framework. Nature 322, 419–422.
Greer, J. B., Mager, E. M., and Fieber, L. A. (2019). Altered expression of ionotropic L-glutamate receptors in ages sensory neurons of Aplysia californica. PLoS One 14:e0217300. doi: 10.1371/journal.pone.0217300
Hatakeyama, D., Fujito, Y., Sakakibara, M., and Ito, E. (2004). Expression and distribution of transcription factor CCAAT/enhancer-binding protein in the central nervous system of Lymnaea stagnalis. Cell Tissue Res. 318, 631–641. doi: 10.1007/s00441-004-0965-8
Hatakeyama, D., Sabamoto, H., Watanabe, T., Wagatsuma, A., Kobayashi, S., Fujito, Y., et al. (2006). Requirement of new protein synthesis of a transcription factor for memory consolidation: paradoxical changes in mrna and protein levels of C/EBP. J. Mol. Biol. 356, 569–577. doi: 10.1016/j.jmb.2005.12.009
Heisenberg, M., Borst, A., Wagner, S., and Byers, D. (1985). Drosophila mushroom body mutants and deficient in olfactory learning. J. Neurogen. 2, 1–30. doi: 10.3109/01677068509100140
Hertzen, L., and Giese, K. (2005). Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J. Neurosci. 25, 1935–1942. doi: 10.1523/JNEUROSCI.4707-04.2005
Hong, W., Mosca, T. J., and Luo, L. (2012). Teneurins instruct synaptic partner matching in an olfactory map. Nature 484, 201–207.
Horn, S., and Freund, A. (2021). Adult age differences in remembering gain- and loss-related intentions. Cogn. Emot. 35, 1652–1669. doi: 10.1080/02699931.2021.1986375
Hurwitz, I., Kupfermann, I., and Weiss, K. (2003). Fast synaptic connections from CBIs to pattern-generating neurons in Aplysia: initiation and modification of motor programs. J. Neurophysiol. 89, 2120–2136. doi: 10.1152/jn.00497.2002
Hurwitz, I., Neustadter, D., Morton, D., Chiel, H., and Susswein, A. (1996). Activity patterns of the B31/B32 pattern initiators innervating the i2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J. Neurophysiol. 75, 1309–1326. doi: 10.1152/jn.1996.75.4.1309
Hurwitz, I., Ophir, A., Korngreen, A., Koester, J., and Susswein, A. (2008). Currents contributing to decision making in neurons B31/B32 of Aplysia. J. Neurophysiol 99, 814–830. doi: 10.1152/jn.00972.2007
Jing, J., Vilim, F., Wu, J., Park, J., and Weiss, K. (2003). Concerted GABAergic actions of Aplysia feeding interneurons in motor program specification. J. Neurosci. 23, 5283–5294. doi: 10.1523/JNEUROSCI.23-12-05283.2003
Jones, T. A., and Greenough, W. T. (1996). Ultrastructural evidence for increased contact between astrocytes and synapses in rats reared in a complex environment. Neurobiol. Learn. Mem. 65, 48–56.
Katzoff, A., Ben-Gedalya, T., Hurwitz, I., Miller, N., Susswein, Y., and Susswein, A. (2006). Nitric oxide signals that aplysia have attempted to eat, a necessary component of memory formation after learning that food is inedible. J. Neurophysiol. 96, 1247–1257. doi: 10.1152/jn.00056.2006
Katzoff, A., Ben-Gedalya, T., and Susswein, A. (2002). Nitric oxide is necessary for multiple memory processes after leraning that a food is inedible in Aplysia. J. Neurosci. 22, 9581–9594.
Kempsell, A., and Fieber, L. (2014). Behavioral aging is associated with reduced sensory neuron excitability in Aplysia californica. Front. Ag. Neurosci. 6:84. doi: 10.3389/fnagi.2014.00084
Kempsell, A., and Fieber, L. (2015). Aging in sensory and motor neurons results in learning failure in Aplysia californica. PLoS One 10:e0127056. doi: 10.1371/journal.pone.0127056
Kempsell, A., and Fieber, L. (2016). Habituation in the tail withdrawal reflex circuit is impaired during aging in Aplysia californica. Front. Aging Neurosci. 8:24. doi: 10.3389/fnagi.2016.00024
Kobayashi, S., Ohashi, Y., and Ando, S. (2002). Effects of enriched environments with different durations and starting times on learning capacity during aging in rats assessed by a refined procedure of the hebb-williams maze task. J. Neurosci. Res. 70, 340–346. doi: 10.1002/jnr.10442
Krishnan, H., Gandour, C., Ramos, J., Wrinkle, M., Sanchez-Pacheco, J., and Lyons, L. (2016). Acute sleep deprivation blocks short- and long-term operant memory in Aplysia. Sleep 39, 2161–2171. doi: 10.5665/sleep.6320
Kron, N., and Fieber, L. (2021). Co-expression analysis identifies neuro-inflammation as a driver of sensory neuron aging in Aplysia californica. PLoS One 16:e0252647. doi: 10.1371/journal.pone.0252647
Kron, N., Schmale, M., and Fieber, L. (2020). Changes in metabolism and proteostasis drive aging phenotype in Aplysia californica sensory neurons. Front. Aging Neurosci. 12:280. doi: 10.3389/fnagi.2020.573764
Kupfermann, I., and Carew, T. (1974). Behavior Patterns of Aplysia californica in its natural environment. Beh. Biol. 12, 317–337. doi: 10.1016/s0091-6773(74)91503-x
Lee, J., Kim, H., Kim, K., Han, J., Lee, Y., Lim, C., et al. (2001). Overexpression of and RNA Interference with the CCAAT enhancer-binding protein on long-term facilitation of aplysia sensory to motor synapses. Learn. Mem. 8, 220–226. doi: 10.1101/lm.40201
Lee, J., Nader, K., and Schiller, D. (2017). An update on memory reconsolidation updating. Trends Cogn. Sci. 21, 531–545.
Lee, J., Ross, E., Gower, A., Paris, J., Martensson, R., and Lorens, S. (1994). Spatial learning deficits in the aged rat: neuroanatomical and neurochemical correlates. Br. Res. Bull. 33, 489–500. doi: 10.1016/0361-9230(94)90073-6
Lee, S., Kwak, C., Shim, J., and Kaang, B. (2012). A cellular model of memory reconsolidation involves reactivation-induced destabilization and restabilization at the sensorimotor synapse in Aplysia. PNAS 109, 14200–14205. doi: 10.1073/pnas.1211997109
Levitan, D., Lyons, L., Perelman, A., Green, C., Motro, B., Eskin, A., et al. (2008). Training with inedible food in Aplysia causes expression of C/EBP in the buccal but not cerebral ganglion. Learn. Mem. 15, 412–416. doi: 10.1101/lm.970408
Levitan, D., Saada-Madar, R., Teplinsky, A., and Susswein, A. (2012). Localization of molecular correlates of memory consolidation to the buccal ganglia mechanoafferent neurons after learning that food is inedible in Aplysia. Learn. Mem. 19, 503–512. doi: 10.1101/lm.026393.112
Maliković, J., Feyissa, D., Kalaba, P., Marouf, B., Höger, H., Hartmann, M., et al. (2019). Age and cognitive status dependent differences in blood steroid and thyroid hormone concentrations in intact male rats. Behav. Brain Funct. 15:10. doi: 10.1186/s12993-019-0161-3
McManus, J., Chiel, H., and Susswein, A. (2019). Successful and unsuccessful attempts to swallow in a reduced Aplysia preparation regulate feeding responses and produce memory at different neural sites. Learn. Mem. 26, 151–165. doi: 10.1101/lm.048983.118
Michel, M., Green, C., and Lyons, L. (2011). PKA and PKC are required for long-term but not short-term in vivo operant memory in Aplysia. Learn. Mem. 18, 19–23.
Milekic, M., Pollonini, G., and Alberini, C. (2007). Temporal requirement of C/EBPß in the amygdala following reactivation but not acquisition of inhibitory avoidance. Learn. Mem. 14, 504–511. doi: 10.1101/lm.598307
Momohara, Y., Neveu, C., Chen, H., Baxter, D., and Byrne, J. (2022). Specific plasticity loci and their synergism mediate operant conditioning. J. Neurosci. 42, 1211–1223.
Mosca, T. (2015). On the teneurin track: a new synaptic organization molecule emerges. Front. Cell Neurosci. 9:204. doi: 10.3389/fncel.2015.00204
Mosca, T., Hong, W., Dani, V., Favaloro, V., and Luo, L. (2012). Trans-synaptic teneurin signaling in neuromuscular synapse organization and target choice. Nature 484, 237–241. doi: 10.1038/nature10923
Murakami, S., and Murakami, H. (2005). The effects of aging and oxidative stress on learning behavior in C. elegans. Neurobiol. Aging 26, 899–905. doi: 10.1016/j.neurobiolaging.2004.08.007
Nargeot, R. (2001). Long-lasting reconfiguration of two interacting networks by a cooperation of presynaptic and postsynaptic plasticity. J. Neurosci. 21, 3282–3294. doi: 10.1523/JNEUROSCI.21-09-03282.2001
Pan, Y., Zhou, Y., Guo, C., Gong, H., Gong, Z., and Liu, L. (2009). Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn. Mem. 16, 289–295. doi: 10.1101/lm.1331809
Perry, S., Straub, V., Kemenes, G., Santama, N., Worster, B., Burke, J., et al. (1998). Neural modulation of gut motility by myomodulin peptides and acetylcholine in the snail Lymnaea. J. Neurophsiol. 79, 2460–2474. doi: 10.1152/jn.1998.79.5.2460
Roberts, A., and Glanzman, D. (2003). Learning in Aplysia: looking at synaptic plasticity from both sides. Tr. Neurosci. 26, 662–670. doi: 10.1016/j.tins.2003.09.014
Samson, R., Venkatesh, A., Patel, D., Lipa, P., and Barnes, C. (2014). Enhanced performance of aged rats in contingency degradation and instrumental extinction tasks. Behav. Neurosci. 128, 122–133. doi: 10.1037/a0035986
Sanes, J., and Zipursky, S. (2020). Synaptic specificity, recognition molecules, and assembly of neural circuits. Cell 181, 536–556.
Schwarz, M., Feldman, E., and Susswein, A. (1991). Variables affecting long-term memory of learning that a food is inedible in Aplysia. Behav. Neurosci. 105, 193–201. doi: 10.1037//0735-7044.105.1.193
Schwarz, M., and Susswein, A. (1986). Identification of the neural pathway for reinforcement of feeding when Aplysia learn that food is inedible. J. Neurosci. 6, 1528–1536. doi: 10.1523/JNEUROSCI.06-05-01528.1986
Simon, A., Liang, D., and Krantz, D. (2006). Differential decline in behavioral performance of Drosophila melanogaster with age. Mech. Ag. Dev. 127, 647–651. doi: 10.1016/j.mad.2006.02.006
Skinner, B. (1938). The behavior of organisms: An experimental analysis. New York, NY: Appleton-Century-Crofts.
Susswein, A., and Byrne, J. (1988). Identification and characterization of neurons initiating patterned neural activity in the buccal ganglia of Aplysia. J. Neurosci. 8, 2049–2061.
Susswein, A., Rosen, S., Gapon, S., and Kupfermann, I. (1996). Characterization of buccal motor programs elicited by a cholinergic agonist applied to the cerebral ganglion of Aplysia californica. J. Comp. Physiol. A 179, 509–524. doi: 10.1007/BF00192317
Susswein, A., Schwarz, M., and Feldman, E. (1986). Learned Changes of Feeding Behavior in Aplysia in Response to Edible and Inedible Foods. J. Neurosci. 6, 1513–1527. doi: 10.1523/JNEUROSCI.06-05-01513.1986
Tam, S., Hurwitz, I., Chiel, H., and Susswein, A. (2020). Multiple local synaptic modifications at specific sensorimotor connections after learning are associated with behavioral adaptations that are components of a global response change. J. Neurosci. 40, 4363–4371. doi: 10.1523/JNEUROSCI.2647-19.2020
Taubenfeld, S., Milekic, M., Monti, B., and Alberini, C. (2001). The consolidation of new but not reactivated memory requires hippocampal C/EBPß. Nat. Neurosci. 4, 813–818.
Terzibasi, E., Valenzano, D., Benedetti, M., Roncaglia, P., Cattaneo, A., Domenici, L., et al. (2008). Large differences in aging phenotype between strains of the short-lived annual fish Nothobranchius furzeri. PLoS One 3:e3866. doi: 10.1371/journal.pone.0003866
Tessarin, G., Michalec, O., Torres-da-Silva, K., Da Silva, A., Cruz-Rizzolo, R., Gonçalves, A., et al. (2019). A putative role of teneurin-2 and its related proteins in astrocytes. Front. Neurosci. 13:655. doi: 10.3389/fnins.2019.00655
Toro, D., Carrasquero-Ordaz, M., Chu, A., Ruff, T., Shahin, M., Jackson, V., et al. (2020). Structural Basis of Teneurin-Latrophilin Interaction in repulsive guidance of migrating neurons. Cell 180, 323–339. doi: 10.1016/j.cell.2019.12.014
Turner, A., and Greenough, W. (1985). Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 329, 195–203.
Vysokov, N., Silva, J., Lelianova, V., Suckling, J., Cassidy, J., Blckburn, J., et al. (2018). Proteolytically released Lasso/teneurin-2 induces axonal attraction by interacting with latrophilin-1 on axonal growth cones. eLife 7:e37935. doi: 10.7554/eLife.37935
Wang, Z., Gong, K., Liu, X., Zhang, Z., Sun, X., Wei, Z., et al. (2018). C/EBPß regulates delta-secretase expression and mediates pathogenesis in mouse models of Alzheimer’s disease. Nat. Commun. 9:1784.
West, R., and Greenough, W. (1972). Effect of environmental complexity on cortical synapses of rats: preliminary results. Behav. Biol. 7, 279–284. doi: 10.1016/s0091-6773(72)80207-4
Wides, R. (2019). The natural history of teneurins: a billion years of evolution in three key steps. Front. Neurosci. 13:109. doi: 10.3389/fnins.2019.00109
Keywords: marine model, invertebrate, mollusk, neuron, transcriptomics, long term potentiation
Citation: Randolph EC and Fieber LA (2023) Improvements in operant memory of Aplysia are correlated with age and specific gene expression. Front. Behav. Neurosci. 17:1221794. doi: 10.3389/fnbeh.2023.1221794
Received: 12 May 2023; Accepted: 03 October 2023;
Published: 23 October 2023.
Edited by:
Yun-Beom Choi, United States Department of Veterans Affairs, United StatesReviewed by:
Bong-Kiun Kaang, Seoul National University, Republic of KoreaWayne S. Sossin, McGill University, Canada
Copyright © 2023 Randolph and Fieber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lynne A. Fieber, bGZpZWJlckBtaWFtaS5lZHU=
 Eric C. Randolph
Eric C. Randolph Lynne A. Fieber
Lynne A. Fieber