- 1Laboratory of Neurophysiology, Department of Physiology and Biochemistry, Faculty of Medicine and Surgery, University of Malta, Msida, Malta
- 2Division of Neuroscience, School of Biosciences, Cardiff University, Cardiff, United Kingdom
Adolescence is a critical developmental period, concerning anatomical, neurochemical and behavioral changes. Moreover, adolescents are more sensitive to the long-term deleterious effects of drug abuse. Binge-like consumption of alcohol and marijuana, along with tobacco smoking, is a dangerous pattern often observed in adolescents during weekends. Nevertheless, the long-term effect of their adolescent co-exposure has not been yet experimentally investigated. Long-Evans adolescent male (n = 20) and female (n = 20) rats from postnatal day 30 (P30) until P60 were daily treated with nicotine (0.3 mg/kg, i.p.), and, on two consecutive ‘binging days’ per week (for a total of eight times), received an intragastric ethanol solution (3 g/kg) and an intraperitoneal (i.p.) dose of cannabinoid 1/2 receptor agonist WIN55,212-2 (1.2 mg/kg). These rats were tested after treatment discontinuation at > P90 for associative food-rewarded operant learning in the two-lever conditioning chambers for six consecutive days on a fixed ratio 1 (FR1) schedule followed by another six days of daily FR2 schedule testing, after 42 days rest. We found the main effects of sex x treatment interactions in FR1 but not in FR2 experiments. Treated females show attenuated operant responses for food pellets during all FR1 and the FR2 schedule, whilst the treated males show an impairment in FR2 but not in the FR1 schedule. Moreover, the treated females’ percentage of learners was significantly lower than female controls in FR1 while treated males were lower than controls in FR2. Our findings suggest that intermittent adolescent abuse of common drugs, such as alcohol and marijuana, and chronic tobacco exposure can cause significant long-term effects on motivation for natural reinforcers later in adulthood in both sexes. Females appear to be sensitive earlier to the deleterious effects of adolescent polydrug abuse, with both sexes having an increased likelihood of developing lifelong brain alterations.
Introduction
Adolescence is a critical period of brain development, concerning anatomical, neurochemical and behavioral changes. As the reward system has not yet matured, adolescents are particularly predisposed to risk- and novelties-seeking behavior that increases their likelihood of drug experimentation (Winters and Arria, 2011). Vulnerability to the effects of drugs of abuse during adolescence may be related to altered activity of the circuitry that mediates incentive processes and involves both dopamine (DA) and glutamate (GLU) (Burton et al., 2011). Compelling animal and human studies have shown that adolescent exposure to drugs, apart from acute damage (Sathanantham et al., 2021), may cause maladaptive changes in brain structures leading to the development of neuropsychiatric disorders such as anxiety, depression and substance use disorder in adult life (Salmanzadeh et al., 2020), that are different from those caused by adult drugs abuse.
The effects of drugs of abuse on the adolescent brain are so detrimental, that even if the exposure is limited to adolescence and then lifelong discontinued, it may still cause mental health disorders and alterations in adulthood supporting the theory of the presence of an age-dependent vulnerability of the brain to the drugs (Jordan and Andersen, 2017). Nevertheless, the long-term effects of drug abuse limited to the adolescent period have not been constantly observed in preclinical and clinical settings (Whyte et al., 2018).
Compelling evidence exists for adolescent binge drinking, i.e., consumption of a large quantity of alcohol (56-70 g) in a short period (≤ 2 h) (National Institute on Alcohol Abuse and Alcoholism [NIAAA], 2004), is capable of inducing long-lasting alterations in brain development, plasticity and behavior (Lannoy et al., 2019), and increasing risks of psychiatric disorders i.e., alcohol use disorders in adult life (Crews et al., 2016).
Similarly, smoking (Leslie, 2020) or, now more commonly, vaping tobacco (Jenssen and Boykan, 2019) and cannabis (Jager and Ramsey, 2008; Vivian Chiu et al., 2022) in adolescent life seem to induce similar detrimental alterations in the maturing teen brain and with cannabis inducing disorders specifically in reward and motivation system (Pacheco-Colón et al., 2018).
When taking into account the long-term impact of adolescent drug addiction, an important problem is that they are commonly co-abused and their deleterious effects may synergistically be potentiated (Pacheco-Colón et al., 2018; Singh, 2019). Indeed, adolescents often consume alcohol and cannabis in combination (United Nations on Drugs and Crime, 2018) at weekends, whilst tobacco is commonly daily abused.
Nevertheless, there is a lack of experimental research around the long-term effects of adolescent polydrug abuse, with cannabis “binging” abuse never being investigated yet. Moreover, it is important to study if these impairments are sex-dependent, considering the well know gender effect in substance use disorder (Brady and Randall, 1999) and the recent attention to the inclusion of both sexes in animal experimentation (Voelkl et al., 2020). With this aim, we have set up a combined chronic (for nicotine) and binging (for alcohol and cannabinoid agonist) polydrug abuse administration protocol to study the specific impairments induced in reward and motivation in adult male and female Long-Evans rats. Specifically, adolescent (P30-P60) rats were treated with daily nicotine (0.3 mg/kg, i.p.), and, on two consecutive “binging days” per week, with intragastric ethanol solution (3 g/kg) and the cannabinoid 1/2 receptor agonist WIN55, 212-2 (1.2 mg/kg, i.p.) (Howlett et al., 2002). The rats were tested at > P90 for associative food-rewarded operant learning in the two-lever conditioning chambers for six consecutive days on a fixed ratio 1 (FR1) schedule followed by an interval of rest (42 days) and then by another six days of daily FR2 schedule to test eventual reconsolidation/retention of the “instrumental memory” impairment.
We found the main effects of sex x treatment interactions in FR1 but not in FR2 experiments. Treated females show attenuated operant responses for banana-flavored food pellets during all FR1 and the FR2 schedule, whilst the treated males show an impairment in FR2 but not in the FR1 schedule. Moreover, the treated females’ percentage of learners was significantly lower than female controls in FR1 while treated males were lower than male controls in FR2.
Our findings suggest that adolescent polyabuse of common drugs such as tobacco and binge-like intoxication of alcohol and marijuana can cause significant long-term effects on motivation for natural reinforcers later in adulthood, especially in females.
Materials and methods
Animals
Twenty male and twenty female Long-Evans rats (about 28 day-olds) were obtained from a colony bred at the University of Malta. The drug treatment started a P30 and ended at P60 while they were tested at age > P90. Animals were housed in a 12:12 light cycle (lights on at 07.00 a.m. and off at 07.00 p.m.). Purified tap water and food chow were available ad libitum throughout the course of the study, except when the animals were exposed to food deprivation. All animal procedures were carried out under the University of Malta’s ethical guidelines and in conformity with Maltese and international laws and policies (EU Directive, 2010/63/EU for animal experiments). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Pharmacological treatment and experimental design
Drugs
Ethanol 95% (v/v) and (−)-nicotine hydrogen tartrate salt ((-)-1-Methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate salt were purchased by Sigma Aldrich (St. Louis, MO, USA). The CB1/2 receptor agonist (R)-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate, (R)-(+)-WIN 55,212-2) WIN 55,212-2 was purchased from Tocris Cookson Ltd. (Bristol, United Kingdom). Ethanol was intragastric (i.g.) administrated as a 25% (v/v) ethanol solution in water. WIN 55,212-2 was freshly dissolved in a vehicle solution (2 ml/kg) made of 5% PEG-400, 5% Tween 80 in saline and i.p. administered. Nicotine was dissolved in saline, with pH adjusted to about 7.4. and i.p., administered and the weight was given as free-base. In our study, we used the intermediate 0.3 mg/kg nicotine dose capable to enhance reward function and psychomotor performance in adolescent and adult male and female rats (Xue et al., 2020) and induce anxiety (Casarrubea et al., 2015, 2020, 2021) and dopamine cell excitation in adult rats (Pierucci et al., 2022). The dose of 3 g/kg alcohol, i.g., administered, was chosen because it is comparable to previously reported data (Lauing et al., 2008; Helfer et al., 2009; Maldonado-Devincci et al., 2010), and capable of inducing long-term alterations in memory and behavior (Mooney-Leber and Gould, 2018). Moreover, we used a dose of WIN55,212-2 (1.2 mg/kg), higher than the dose self-administered by rats (Fattore et al., 2007; Kirschmann et al., 2017), that was capable to induce deficits in short-term memory when chronically administered in adolescent male rats (Schneider and Koch, 2003; O’Shea et al., 2006) but not in female rats (Kirschmann et al., 2017) and impaired the dentate gyrus LTP in adult male rats (Colangeli et al., 2017). The dose of WIN55,212-2 used here can be considered equivalent to a cannabis cigarette (∼800 mg) containing a dose of 3 mg/kg THC (∼20% Δ9-THC) (Health Canada, 2013), considering that the synthetic cannabinoid agonist has a higher affinity for CBRs than THC (Lawston et al., 2000).
Experimental protocol
The experimental protocol was designed to mimic the polydrug-abuse pattern typical of teenagers/young adults (Figure 1A). In rodents, adolescence is typically defined as the entire postnatal period ranging from weaning (P21) to adulthood (P60) (Laviola et al., 2003). We choose, therefore, to exposed the Long-Evans rats to the polydrug treatment from early-adolescence (∼P30) through peri-adolescence (∼P40) until late adolescence (P60), in order to cover the entire developmental period.
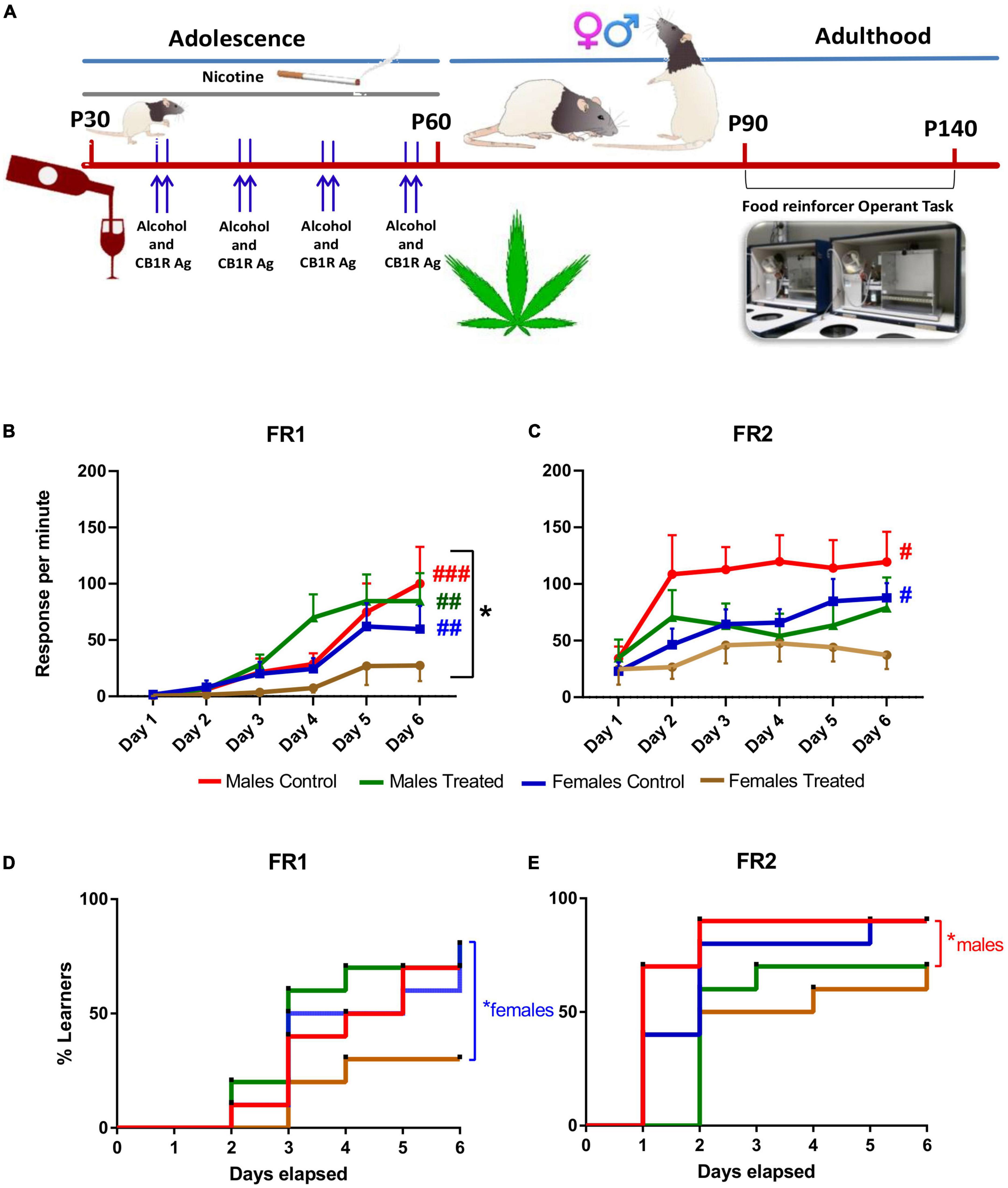
Figure 1. Long-term term effect of adolescent binging on alcohol and cannabinoid and daily exposure to nicotine on learning and motivation in rats. (A) The chronic treatment was initiated at P30 with daily nicotine administration (0.3 mg/kg., i.p.) for 30 days and two consecutive days once a week with ethanol (3 g/kg; i.g.) and 1.2 mg/kg CB 1/2 receptor agonist WIN 55,212-2. Rats were food restricted before each test until they reached 85% of their ad libitum body weight. Operant conditioning testing was performed at least after 30 days the end of the treatment (> P90). (B) Rats underwent the first 6 days of testing under a FR1 schedule. Female rats made fewer presses compared to males. Control males, treated males and control females improved throughout the FR1 schedule when compared to FR1 day 1, while treated females did not. (C) After 45 days, rats underwent to a FR2 schedule. Male and Female controls improved in response per min during the FR2 while both treated male and female groups did not show an improvement during the six days of the test. *p < 0.05, Two-way ANOVA; #p < 0.01; ##p < 0.005; ###p < 0.001 generalized Kruskal Wallis analysis. (D) Instrumental learning performance was significantly decreased in polydrug-treated females during FR1 and in (E) polydrug-treated males during FR2 compared to their respective control groups. *p < 0.05, log-rank Mantel-Cox test. i.p.: intraperitoneal; i.g.: intragastric; FR: fixed ratio.
The binge-drinking alcohol (3 g/kg, intragastric, i.g.) with the contextual WIN 55,212-2 (1.2 mg/kg, i.p.) administration and daily nicotine administration (0.3 mg/kg, i.p.) mimicked the 2-day heavy drinking and moderate abuse of cannabis in light smoking teenagers at the weekend. Two groups of male and female adolescent rats (n = 40), from P30 to P60, were weekly exposed to a two-day binge of administration of 25% (v/v) ethanol solution or its vehicle for four consecutive weeks. On the same days, rats received an i.p. low/moderate dose of WIN 55,212,2. Moreover, for the period of observation (30 days) the rats received a daily low dose of nicotine administration (0.3 mg/kg, i.p.) (Matta et al., 2007; Figure 1A). To reduce the stress of the manipulations, rats were handled for a week before the experimentation.
Operant instrumental learning
Eight operant conditioning chambers (Lafayette Instrument, Lafayette, IN) were used, in a dedicated temperature and humidity control laboratory. In all operant conditioning sessions, one lever was designated as the active lever and remained active during all sessions and the second lever was designated as inactive and left retracted. The food reinforcer was a 45 mg appetitive pellet (Precision Sucrose pellet, Banana flavor- LBS LTD). Rats were tested at age > P90 and submitted to a one-time random feeding as a training session. All training sessions were performed one day prior to the FR1 test. These consisted of 25 randomly timed pellets that were dispensed from the roulette through the tube and into the feeder without the need for the rat to press the lever. The time, which was randomly chosen by the program, was not less than 2 seconds but no more than 60 sec, and with a 20 sec delay time. Rats were food restricted before each test until they reached 85% of their ad libitum body weight (Zellner and Ranaldi, 2006). The food restriction was maintained throughout the testing and the interval between FR1 and FR2. During the food deprivation period, the rats were handled daily for about 10 min. Rats were left in their cage for about ten min and placed in the operant conditioning chambers for another ten min as a habituation procedure before being tested. Rats underwent the first 6 days of testing under an FR1 schedule. The rats had 60-sec chance to press the level and if successful a food pellet was provided as a reward. A 20-sec delay followed each trial with the stimulus light on. This was repeated 25 times. After a resting period of 42 days, with their body weight to 85% of their ad libitum weight, the rats were tested for another 6 days of FR2 schedules. The same parameters were used as in the FR1 but with the need for two presses for one reward. The data collected were the responses of the rats on the lever to obtain food per 25 schedules and also the latency in seconds for the pressing of the lever. If the lever was not pressed in the permitted time interval, this was taken as a full latency of 60 seconds. For data processing, the response per min or rate of response was calculated from the two parameters obtained from the test by using the equation below (Beninger, 1991).
Differences between learners and non-learners between treatment groups of both female and male groups were also calculated. All the treatment groups during all test days were compared to identify if the treatment created any difference in associative learning. The analysis of this data also produced a distinction between the days of the test, and hence a measure of the progress achieved by rats. Learners were classified as such when they achieved a 100% response within the test schedule. One of the dependent variables was the learning outcome (Learners vs non-learners) while the independent variables were the day of the test, the sex and the treatment regime.
Statistical analysis of the data
ABET II software (Lafayette Instrument, Lafayette, IN) was used to control, monitor and record individually from all chambers via an independent interface.
A three-way ANOVA analysis was performed so that an interaction effect between the three independent variables on the continuous dependent variable could be determined if it exists. So here it was important to identify if there was an interacting effect of the drugs under study on the associative learning potential of the rats, together with sex and day of the test. So a possible three-way interaction effect could be determined between the three independent variables. A statistically significant three-way interaction was determined by a p < 0.05 criterion. A two-way ANOVA analysis followed, to compare the mean differences between groups (sex x day of the test), (sex x treatment) and (day of test x treatment) so that it could be understood if there was an interaction between all combinations of the two independent variables on the associative learning potential in rats.
Data that did not display equal variances were analyzed using nonparametric tests. Kruscal-Wallis non-parametric statistical test was performed to test the significant difference between the responses per min output of each day when compared with its starting point, for each sex under the two different treatment conditions.
The performance of each rat to reach the learning criterion was analyzed using Kaplan-Meier event analysis over the instrumental learning period, and the resulting curves were compared by employing the log-rank Mantel-Cox test. Statistical analysis was carried out using GraphPad Prism v. 9 (GraphPad Software, Inc., San Diego, CA). All values represent the mean ± standard error of the mean. A P-value lower than 0.05 level was considered significant.
Results
Effect of the adolescent polydrug treatment on weight change
All rats were housed in the same room with good and adequate environmental conditions with food and water ad libitum. The weight of each individual rat was recorded regularly from P30 to P90. The weight gain was smaller for the treated group compared to the control group for both males (-54.5 ± 13.36 g; F(18) = 0.715, p = 0.001) and females (−22.8 ± 9.56 g; F(18) = 0.166, p = 0.028; not shown). No difference instead was observed between the weight gain between males and females (p > 0.5; not shown).
Long-term effect of the adolescent polydrug treatment on operant food-conditioning acquisition
The effects of adolescent exposure to our polydrug treatment on the adults’ instrumental learning were assessed by evaluating the animal’s ability to acquire simple instrumental tasks FR1 and, after 42 days interval, FR2 (Figure 1).
First, we compared the performance of the 4 groups considering the response, that it is equal to the active lever presses per minute Three-way ANOVA analysis did not show significant three-way interaction between sex, day of test and treatment for the associative learning potential during FR1 (F(5, 216) = 0.569, p > 0.05). A significant two-way interaction was found for sex x treatment (F(1, 216) = 4.384, p < 0.05). No significant two-way interaction was found for the day of test x treatment (F(5, 216) = 0.671, p > 0.05) and for sex x day of the test (F(5, 216) = 1.819, p > 0.05) (Figure 1B). No statistically significant three-way interaction between Sex, day of test and treatment was found for the associative learning potential during FR2 (F(5, 216) = 0.300, p > 0.05). No significant two-way interaction was found for sex x treatment (F(1, 216) = 1.111, p > 0.05). No significant two-way interaction was found on the day of test x treatment (F(5, 216) = 0.897, p > 0.05). No significant two-way interaction was found for sex x day of the test (F(5, 216) = 0.553, p > 0.05) (Figure 1C).
When considering the learning performance, generalized Kruskal Wallis analysis showed that control males improve throughout the FR1 schedule when compared to FR1 day 1 (χ2(11) = 43.980, p < 0.001). This improvement was also seen during the whole FR2 schedule (χ2(5) = 11.778, p = 0.038). Treated males showed an improvement during the FR1 schedule (χ2(5) = 15.658, p = 0.008) Of note, treated males showed no improvement during the FR2 schedule (χ2(5) = 1.698, p = 0.889). Control females showed a generalized improvement during FR1 schedule (χ2(5) = 17.614, p = 0.003) and FR2 schedule (χ2(5) = 12.984, p = 0.024). Treated females showed no improvement in response per min during the FR1 schedule (χ2(5) = 0.545, p = 0.990) and the FR2 schedule (χ2(5) = 2.093, p = 0.836).
Remarkably, the memory retention (expressed as the difference in active lever responses between FR1 day 6 and FR2 day 1) after the interval period (7 weeks) between the two schedules was similar for all the groups, except the treated females who showed a very similar value (Treated female FR1 day six 27.39 ± 13.78 and 24.79 ± 13.71 (χ2(1) = 0.073, p = 0.787). This was because treated female rats ended at a very low level of associative learning on the 6th day of FR1. Therefore, the present findings indicate that the acquisition of the present task was so impaired in female-treated rats that no retention was possible.
Moreover, we analyzed the instrumental learning performance in terms of days elapsed to reach the learning criterion for each subject within the 4 groups for both FR1 and FR2 schedules. Kaplan-Meier analysis showed for FR1 that median learning times were three days for all the groups apart from female polydrug-treated rats. Indeed, 30% of male and female control rats and 20% of males and 70% of females of the polydrug-treated rats failed to reach the criterion within 6 days. The log-rank Mantel-Cox test for comparison of survival curves indicated that FR1 learning performance was significantly decreased in polydrug-treated females compared to their control rats (χ2 = 4.39, df = 1, p = 0.0360; Figure 1D). Kaplan-Meier analysis for FR2 showed that median learning times were one day and two days for male and female controls, respectively, two days and one day for polydrug-treated males and females, respectively. In addition, 10% of male and female control rats and 30% of males and females of the polydrug-treated rats failed to reach the criterion within 6 days. The log-rank Mantel-Cox test for comparison of survival curves indicated that FR2 learning performance was significantly decreased only in polydrug-treated males compared to control rats (χ2 = 6.12, df = 1, p = 0.0134; Figure 1E).
Discussion
Nicotine, alcohol and cannabis are the most commonly abused substances among youth (World Drug Report, 2022), most likely due to their easy obtainment. Although these substances are generally co-abused by adolescents, little is known about their combined long-term effects on the brain and health in general. Indeed, most of the studies have taken into consideration the short- and long-term effects of a single drug administered, prevalently chronically, during adolescence [see for a recent review (Mooney-Leber and Gould, 2018)]. Moreover, alcohol and cannabis are frequently used by teenagers and young adults in a binge pattern, particularly during the weekend (United Nations on Drugs and Crime, 2018). While the short and long-term effect of binge drinking during adolescence has been intensively investigated on brain functions (Pérez-García et al., 2022), the consequences of cannabis binges, typical of social situations where cannabis is readily available, remain unknown.
The first outcome of this study is about the short and long-term effects of the polydrug treatment on the body weight of the rats. It is well known that drug abuse affects food intake and body weight (Mohs et al., 1990), but conflicting results exist instead about the effect of their adolescent administration. For instance, adolescent nicotine exposure does not affect adolescent and adult weight gain (Pushkin et al., 2019) but reduced the nicotine anorectic effect (Natividad et al., 2013) in adulthood. On the other hand, binging on 3 g/kg alcohol for 4 weeks produced a reduction (Lauing et al., 2008) or no change (Helfer et al., 2009) in the post-treatment body weight in rats. Conflicting results with lack of effect or decrease of body weight have been obtained with adolescent cannabis smoke exposure from P35-P45 (Bruijnzeel et al., 2019; Hernandez et al., 2020), or to WIN55,212-2 (P30-P43) (Schoch et al., 2018; Pushkin et al., 2019). Conversely, in the current study, the rat body weight gain during the polydrug treatment at P30-P60 but also a P90 was, as expected, higher in males compared to females but independently from the adolescent exposure to the drugs.
The lack of the anorectic effect of the polydrug treatment used here on body weight gain is probably due to either the particular binging paradigm of administration and/or the counteracting effects of the single drugs. In support of the latter hypotheses, a slightly higher dose of WIN55,212-2 (2 mg/kg) produced a decrease in body weight gain, although only in female adolescent mice, that was prevented by the co-exposure with 0.36 mg/kg nicotine (Pushkin et al., 2019).
As far as our findings about associative food-rewarded operant learning are concerned, they are in line with a large body of evidence showing a sex-dependent alteration of the brain reward system (Walker et al., 2017) and related learning, induced by adolescent exposure to drugs of abuse, with the females being the most affected (Mooney-Leber and Gould, 2018). Indeed, we found a decreased effect of natural rewards (i.e., the palatable banana-flavored pellets) on inducing instrumental learning that was sex-dependent although both sexes were differently affected. After a period of 30-day washout from the last polydrug treatment (at > P90), over the FR1 schedule of reinforcement, only treated females were not able to acquire and maintain the instrumental conditioning task (i.e., lever pressing). It is interesting to note, there was an expected decrease in level-pressing values of FR2 Day 1 compared to FR1 Day 6 due to the interval (42 days) between the two tests, an expression of the normal strength reduction of memory retention which facilitates re-acquisition of a similar already acquired task [see (Ohta et al., 1993; Exton-McGuinness et al., 2014)], which showed similar results for all groups, with the exception of the females showing no change. Indeed, the polydrug treatment completely hindered female rats’ acquisition of FR1 and contextual instrumental memory of the task acting on the brain circuitry sustaining instrumental learning (Balleine and Dickinson, 1998). This includes the dopamine (DA)-containing neurons of the ventral tegmental area (VTA) projecting to the core of the nucleus accumbens (NAc) (Chase et al., 2015; Heymann et al., 2020) and other areas receiving DAergic innervation i.e., dorsal medial striatum (Yin et al., 2005), amygdala (Andrzejewski et al., 2005), mPFC and anterior cingulate cortex (ACC) (Baldwin et al., 2002; Caballero et al., 2019). Moreover, another neurotransmitter system impacted by the polydrug treatment is glutamate (GLU) and its NMDA receptors which have a clear role in the acquisition of instrumental learning (Freed and Wyatt, 1981) and its reconsolidation (Quick et al., 2014). GLU and DA interact as, for instance, the genetic deletion of NMDA receptors in VTA DA neurons slowed instrumental learning (James et al., 2015).
Indeed, adolescent exposure to chronic alcohol (Sircar and Sircar, 2006) or binge drinking (Obray et al., 2022) and Δ9-THC (Rubino et al., 2015) depress D1 and NMDA receptor signals in adults. A reduction of the DA D1 and NMDA receptor function in the NAc in females compared to males could explain our findings in animals prior exposed to polydrugs. Consistently, D1 receptor antagonist SCH23390 (Smith-Roe and Kelley, 2000; Hernandez et al., 2005) and NMDA receptors antagonist AP-5 (Kelley et al., 1997) infusion in the NAc core impaired both instrumental learning and performance but did not affect memory consolidation and retrieval (Hernandez et al., 2005). Similar results were obtained with the blockade of NMDA receptors by AP-5 infusion in the basolateral amygdala (BLA), mPFC (Baldwin et al., 2000) and ACC (McKee et al., 2010) completely abolished acquisition while the retention of lever pressing for food response was unaffected. A rise of accumbal DA release occurs during the initial acquisition of the task (Segovia et al., 2011), proportionally to the rates of lever-pressing (Salamone et al., 1994). The alteration of the acquisition of FR1 and the maintenance of the instrumental response in female rats may depend on the lack of selective increase in the firing activity of VTA neurons projecting to the core NAc (Heymann et al., 2020). Consistently, adolescent Δ9-THC (Scherma et al., 2016) WIN55,212-2 (Pistis et al., 2004) exposure decreased the VTA DA neurons’ firing activation and NAc DA release induced by WIN55,212-2 administration in adult rats (Scherma et al., 2016) and induced a functional tolerance of VTA DA neurons to WIN55,212-2, morphine, amphetamine and cocaine administration on the DA neuron firing rate (Pistis et al., 2004).
Late adolescent (P40-P65) 1.2 mg/kg WIN55, 212-2 treatment decreased palatable food intake and breakpoint under progressive ratio (PR) in adulthood after a wash-out of 20 days (Schneider and Koch, 2003). Contrastingly, early adolescent (P30-43) exposure to WIN55,212-2 (Schoch et al., 2018) or cannabis smoke (Hernandez et al., 2020) did not affect food motivation under a PR schedule of instrumental responding for food reward at > P70. As far as nicotine, a similar dose (0.35 mg/kg) to that used in the current study daily administered to adolescent rats (P35-50) was found to induce better reward-related learning in adult males while impairment in female rats (Quick et al., 2014), but resulted ineffective when administered in adult mice (Pushkin et al., 2019). Finally, alcohol abuse during adolescence is known to cause impairment in learning and memory in adult life in rats (White et al., 2000) and humans (Crews et al., 2016, 2019) but initial instrumental learning is generally unaffected (Risher et al., 2013).
The only available evidence of adolescent drug abuse co-exposure on instrumental learning showed that prior 0.36 mg/kg nicotine and 2 mg/kg WIN55,212-2 treatment did not change the ability of both sexes to learn an operant task to obtain food reward in adult mice (Pushkin et al., 2019). Our polydrug treatment also included binge drinking, and this might be responsible for the diverse outcome.
Our findings indicate that control male and female rats showed good performance when retested in the FR2 schedule, while both treated males and females exhibited impaired learning and consequently did not improve their performance of the represented task. Consistently, the percentage of the learners (those who meet the criterion) observed in the control males in FR2 was higher than in FR1, reaching the maximum of the learners on Day 2 FR2. The male-treated FR2 learners were lower than controls while instead control and treated females behaved in the same way in FR2. Summing up, female-treated rats showed deficits in the acquisition, performance (FR1), and consequently in the consolidation of the reference memory (FR2), while treated males have a selective deficit of memory reconsolidation/retrival and performance in FR2 with a normal memory process in FR1. Change in anxiety-like or motor behavior cannot account for the results obtained in this study since anxiety-like and motor behavior were not affected in P90 rats (unpublished observations).
The reason for this selectively delayed male deficit on FR2 induced by the polydrug treatment compared to females is not evident. It might be related to the different sex-dependent neuronal reorganization and DA and GLU receptor pruning that occur during adolescence (Walker et al., 2017) or aging (FR2 occurred after 42 days) (Ohta et al., 1993) and/or a specific learning deficit upon retrieval in the reconsolidation (Milekic and Alberini, 2002). Other neurotransmitter systems cannot be ruled out, for instance, serotonin (5-HT) for its implication in memory and reward (De Deurwaerdere and Di Giovanni, 2020; Bombardi et al., 2021b) and nicotine (Bombardi and Di Giovanni, 2013; Bombardi et al., 2020, 2021a), cannabinoids (Colangeli et al., 2021) and alcohol (Sari et al., 2011) addiction. These possibilities urge experimental validation.
It should be noted that, in the present preliminary study, the polydrugs were experimenter-administered and not all the possible combinations of nicotine, cannabinoid and alcohol and their different vehicles were tested.
Notwithstanding these limitations, this is the first evidence showing that smoking and having a few drinks and a joint at the weekend, which is common and considered not particularly harmful by adolescents/young adults, has instead long-term gender-dependent effects on the learning and reward system. The extrapolation of our data to humans further supports the concept of adolescence as an optimal time to employ gender-targeted preventive and meliorative (or primary and secondary preventive) strategies, instead of relying on tertiary prevention in adulthood. Considering that adolescence has been referred to as the gateway to adult health outcomes (Raphael, 2013) it would be pivotal to act fast on prevention.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Use and Care Committee (IAUCC).
Author contributions
GDG: conceptualization, supervision, project administration, resources, and funding acquisition. NA and GDG: methodology, formal analysis, and data curation. KH and NA: investigation. GDG, KH, and NA: writing—original draft preparation and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the University of Malta, Faculty of Medicine Research Support Grant NA and an Erasmus fellowship KH. The assistance provided by Dr. Massimo Pierucci and Ms. Maria Vella was greatly appreciated.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andrzejewski, M. E., Spencer, R. C., and Kelley, A. E. (2005). Instrumental learning, but not performance, requires dopamine D1-receptor activation in the amygdala. Neuroscience 135, 335–345.
Baldwin, A. E., Holahan, M. R., Sadeghian, K., and Kelley, A. E. (2000). N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav. Neurosci. 114:84. doi: 10.1037//0735-7044.114.1.84
Baldwin, A. E., Sadeghian, K., and Kelley, A. E. (2002). Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J. Neurosci. 22, 1063–1071. doi: 10.1523/JNEUROSCI.22-03-01063.2002
Balleine, B. W., and Dickinson, A. (1998). Goal-directed instrumental action: Contingency and incentive learning and their cortical substrates. Neuropharmacology 37, 407–419.
Beninger, R. J. (1991). “Receptor subtype-specific dopamine agonists and antagonists and conditioned behaviour,” in The mesolimbic dopamine system: From motivation to action, eds P. Willner and J. Scheel-Krüger (Chichester: John Wiley & Sons), 273–299.
Bombardi, C., and Di Giovanni, G. (2013). Functional anatomy of 5-HT2A receptors in the amygdala and hippocampal complex: Relevance to memory functions. Exp. Brain Res. 230, 427–439. doi: 10.1007/s00221-013-3512-6
Bombardi, C., Grandis, A., Pivac, N., Sagud, M., Lucas, G., Chagraoui, A., et al. (2021b). Serotonin modulation of hippocampal functions: From anatomy to neurotherapeutics. Prog. Brain Res. 261, 83–158. doi: 10.1016/bs.pbr.2021.01.031
Bombardi, C., Delicata, F., Tagliavia, C., Grandis, A., Pierucci, M., Marino Gammazza, A., et al. (2021a). Lateral habenula 5-HT(2C) receptor function is altered by acute and chronic nicotine exposures. Int. J. Mol. Sci. 22:4775. doi: 10.3390/ijms22094775
Bombardi, C., Delicata, F., Tagliavia, C., Pierucci, M., Deidda, G., Casarrubea, M., et al. (2020). Acute and chronic nicotine exposures differentially affect central serotonin 2a receptor function: Focus on the lateral habenula. Int. J. Mol. Sci. 21:1873. doi: 10.3390/ijms21051873
Brady, K. T., and Randall, C. L. (1999). Gender differences in substance use disorders. Psychiatr. Clin. N. Am. 22, 241–252.
Bruijnzeel, A. W., Knight, P., Panunzio, S., Xue, S., Bruner, M. M., Wall, S. C., et al. (2019). Effects in rats of adolescent exposure to cannabis smoke or THC on emotional behavior and cognitive function in adulthood. Psychopharmacology 236, 2773–2784.
Burton, C. L., Noble, K., and Fletcher, P. J. (2011). Enhanced incentive motivation for sucrose-paired cues in adolescent rats: Possible roles for dopamine and opioid systems. Neuropsychopharmacology 36, 1631–1643. doi: 10.1038/npp.2011.44
Caballero, J. P., Scarpa, G. B., Remage-Healey, L., and Moorman, D. E. (2019). Differential effects of dorsal and ventral medial prefrontal cortex inactivation during natural reward seeking, extinction, and cue-induced reinstatement. eNeuro 6:ENEURO.0296-19.2019. doi: 10.1523/ENEURO.0296-19.2019
Casarrubea, M., Davies, C., Faulisi, F., Pierucci, M., Colangeli, R., Partridge, L., et al. (2015). Acute nicotine induces anxiety and disrupts temporal pattern organization of rat exploratory behavior in hole-board: A potential role for the lateral habenula. Front. Cell. Neurosci. 9:197. doi: 10.3389/fncel.2015.00197
Casarrubea, M., Davies, C., Pierucci, M., Colangeli, R., Deidda, G., Santangelo, A., et al. (2021). The impact of chronic daily nicotine exposure and its overnight withdrawal on the structure of anxiety-related behaviors in rats: Role of the lateral habenula. Prog. Neuropsychopharmacol. Biol. Psychiatry 105:110131. doi: 10.1016/j.pnpbp.2020.110131
Casarrubea, M., Pierucci, M., Aiello, S., Cassar, D., Deidda, G., Crescimanno, G., et al. (2020). Effects of chronic nicotine on the temporal structure of anxiety-related behavior in rats tested in hole-board. Prog. Neuropsychopharmacol. Biol. Psychiatry 96:109731. doi: 10.1016/j.pnpbp.2019.109731
Chase, H. W., Kumar, P., Eickhoff, S. B., and Dombrovski, A. Y. (2015). Reinforcement learning models and their neural correlates: An activation likelihood estimation meta-analysis. Cogn. Affect. Behav. Neurosci. 15, 435–459.
Colangeli, R., Pierucci, M., Benigno, A., Campiani, G., Butini, S., and Di Giovanni, G. (2017). The FAAH inhibitor URB597 suppresses hippocampal maximal dentate afterdischarges and restores seizure-induced impairment of short and long-term synaptic plasticity. Sci. Rep. 7:11152.
Colangeli, R., Teskey, G. C., and Di Giovanni, G. (2021). Endocannabinoid-serotonin systems interaction in health and disease. Prog. Brain Res. 259, 83–134. doi: 10.1016/bs.pbr.2021.01.003
Crews, F. T., Robinson, D. L., Chandler, L. J., Ehlers, C. L., Mulholland, P. J., Pandey, S. C., et al. (2019). Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcohol. Clin. Exp. Res. 43, 1806–1822. doi: 10.1111/acer.14154
Crews, F. T., Vetreno, R. P., Broadwater, M. A., and Robinson, D. L. (2016). Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol. Rev. 68, 1074–1109.
De Deurwaerdere, P., and Di Giovanni, G. (2020). Serotonin in health and disease. Int. J. Mol. Sci. 21:3500.
Exton-McGuinness, M. T., Patton, R. C., Sacco, L. B., and Lee, J. L. (2014). Reconsolidation of a well-learned instrumental memory. Learn. Mem. 21, 468–477.
Fattore, L., Spano, M. S., Altea, S., Angius, F., Fadda, P., and Fratta, W. (2007). Cannabinoid self-administration in rats: Sex differences and the influence of ovarian function. Br. J. Pharmacol. 152, 795–804.
Freed, W. J., and Wyatt, R. J. (1981). Impairment of instrumental learning in rats by glutamic acid diethyl ester. Pharmacol. Biochem. Behav. 14, 223–226. doi: 10.1016/0091-3057(81)90247-1
Health Canada (2013). Information for health care professionals: Cannabis (marihuana, marijuana) and the cannabinoids. Ottawa, ON: Health Canada.
Helfer, J. L., Goodlett, C. R., Greenough, W. T., and Klintsova, A. Y. (2009). The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Res. 1294, 1–11. doi: 10.1016/j.brainres.2009.07.090
Hernandez, C. M., Orsini, C. A., Blaes, S. L., Bizon, J. L., Febo, M., Bruijnzeel, A. W., et al. (2020). Effects of repeated adolescent exposure to cannabis smoke on cognitive outcomes in adulthood. J. Psychopharmacol. 35, 848–863. doi: 10.1177/0269881120965931
Hernandez, P. J., Andrzejewski, M. E., Sadeghian, K., Panksepp, J. B., and Kelley, A. E. (2005). AMPA/kainate, NMDA, and dopamine D1 receptor function in the nucleus accumbens core: A context-limited role in the encoding and consolidation of instrumental memory. Learn. Mem. 12, 285–295. doi: 10.1101/lm.93105
Heymann, G., Jo, Y. S., Reichard, K. L., McFarland, N., Chavkin, C., Palmiter, R. D., et al. (2020). Synergy of Distinct Dopamine Projection Populations in Behavioral Reinforcement. Neuron 105, 909–920.e905. doi: 10.1016/j.neuron.2019.11.024
Howlett, A., Barth, F., Bonner, T., Cabral, G., Casellas, P., Devane, W., et al. (2002). International union of pharmacology. XXVII. classification of cannabinoid receptors. Pharmacol. Rev. 54, 161–202.
Jager, G., and Ramsey, N. F. (2008). Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: An overview of animal and human research. Curr. Drug Abuse Rev. 1, 114–123. doi: 10.2174/1874473710801020114
James, A. S., Pennington, Z. T., Tran, P., and Jentsch, J. D. (2015). Compromised NMDA/glutamate receptor expression in dopaminergic neurons impairs instrumental learning, but not pavlovian goal tracking or sign tracking. eNeuro 2:ENEURO.0040-14.2015. doi: 10.1523/ENEURO.0040-14.2015
Jenssen, B. P., and Boykan, R. (2019). Electronic cigarettes and youth in the United States: A call to action (at the local, national and global levels). Children 6:30. doi: 10.3390/children6020030
Jordan, C. J., and Andersen, S. L. (2017). Sensitive periods of substance abuse: Early risk for the transition to dependence. Dev. Cogn. Neurosci. 25, 29–44.
Kelley, A. E., Smith-Roe, S. L., and Holahan, M. R. (1997). Response-reinforcement learning is dependent on N-methyl-<span class=”smallcaps smallerCapital”>d</span>-aspartate receptor activation in the nucleus accumbens core. Proc. Natl. Acad. Sci. U.S.A. 94, 12174–12179.
Kirschmann, E. K., McCalley, D. M., Edwards, C. M., and Torregrossa, M. M. (2017). Consequences of adolescent exposure to the cannabinoid receptor agonist WIN55,212-2 on working memory in female rats. Front. Behav. Neurosci. 11:137. doi: 10.3389/fnbeh.2017.00137
Lannoy, S., Billieux, J., Dormal, V., and Maurage, P. (2019). Behavioral and cerebral impairments associated with binge drinking in youth: A critical review. Psychol. Belg. 59, 116–155.
Lauing, K., Himes, R., Rachwalski, M., Strotman, P., and Callaci, J. J. (2008). Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol 42, 649–656. doi: 10.1016/j.alcohol.2008.08.005
Laviola, G., MacrıÌ, S., Morley-Fletcher, S., and Adriani, W. (2003). Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 27, 19–31. doi: 10.1016/s0149-7634(03)00006-x
Lawston, J., Borella, A., Robinson, J. K., and Whitaker-Azmitia, P. M. (2000). Changes in hippocampal morphology following chronic treatment with the synthetic cannabinoid WIN 55,212-2. Brain Res. 877, 407–410.
Leslie, F. M. (2020). Unique, long-term effects of nicotine on adolescent brain. Pharmacol. Biochem. Behav. 197:173010.
Maldonado-Devincci, A. M., Alipour, K. K., Michael, L. A., and Kirstein, C. L. (2010). Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol. Biochem. Behav. 96, 476–487. doi: 10.1016/j.pbb.2010.07.008
Matta, S. G., Balfour, D. J., Benowitz, N. L., Boyd, R. T., Buccafusco, J. J., Caggiula, A. R., et al. (2007). Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190, 269–319.
McKee, B. L., Kelley, A. E., Moser, H. R., and Andrzejewski, M. E. (2010). Operant learning requires NMDA-receptor activation in the anterior cingulate cortex and dorsomedial striatum, but not in the orbitofrontal cortex. Behav. Neurosci. 124, 500–509. doi: 10.1037/a0020270
Milekic, M. H., and Alberini, C. M. (2002). Temporally graded requirement for protein synthesis following memory reactivation. Neuron 36, 521–525. doi: 10.1016/s0896-6273(02)00976-5
Mohs, M. E., Watson, R. R., and Leonard-Green, T. (1990). Nutritional effects of marijuana, heroin, cocaine, and nicotine1. J. Am. Dietetic Assoc. 90, 1261–1267.
Mooney-Leber, S. M., and Gould, T. J. (2018). The long-term cognitive consequences of adolescent exposure to recreational drugs of abuse. Learn. Mem. 25, 481–491.
National Institute on Alcohol Abuse and Alcoholism [NIAAA] (2004). NIAAA council approves definition of binge drinking. NIAAA newsletter 3. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism.
Natividad, L. A., Torres, O. V., Friedman, T. C., and O’Dell, L. E. (2013). Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behavi. Brain Res. 257, 275–285. doi: 10.1016/j.bbr.2013.10.003
Obray, J. D., Landin, J. D., Vaughan, D. T., Scofield, M. D., and Chandler, L. J. (2022). Adolescent alcohol exposure reduces dopamine 1 receptor modulation of prelimbic neurons projecting to the nucleus accumbens and basolateral amygdala. Addict. Neurosci. 4:100044. doi: 10.1016/j.addicn.2022.100044
Ohta, H., Matsumoto, K., and Watanabe, H. (1993). Impairment of acquisition but not retention of a simple operant discrimination performance in aged Fischer 344 rats. Physiol. Behav. 54, 443–448.
O’Shea, M., McGregor, I. S., and Mallet, P. E. (2006). Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J. Psychopharmacol. 20, 611–621. doi: 10.1177/0269881106065188
Pacheco-Colón, I., Limia, J. M., and Gonzalez, R. (2018). Nonacute effects of cannabis use on motivation and reward sensitivity in humans: A systematic review. Psychol. Addict. Behav. 32:497. doi: 10.1037/adb0000380
Pérez-García, J. M., Suárez-Suárez, S., Doallo, S., and Cadaveira, F. (2022). Effects of binge drinking during adolescence and emerging adulthood on the brain: A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 137:104637. doi: 10.1016/j.neubiorev.2022.104637
Pierucci, M., Delicata, F., Colangeli, R., Marino Gammazza, A., Pitruzzella, A., Casarrubea, M., et al. (2022). Nicotine modulation of the lateral habenula/ventral tegmental area circuit dynamics: An electrophysiological study in rats. Neuropharmacology 202:108859. doi: 10.1016/j.neuropharm.2021.108859
Pistis, M., Perra, S., Pillolla, G., Melis, M., Muntoni, A. L., and Gessa, G. L. (2004). Adolescent exposure to cannabinoids induces long-Lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol. Psychiatry 56, 86–94. doi: 10.1016/j.biopsych.2004.05.006
Pushkin, A. N., Eugene, A. J., Lallai, V., Torres-Mendoza, A., Fowler, J., Chen, E., et al. (2019). Cannabinoid and nicotine exposure during adolescence induces sex-specific effects on anxiety-and reward-related behaviors during adulthood. PLoS One 14:e0211346. doi: 10.1371/journal.pone.0211346
Quick, S. L., Olausson, P., Addy, N. A., and Taylor, J. R. (2014). Repeated nicotine exposure during adolescence alters reward-related learning in male and female rats. Behav. Brain Res. 261, 171–176. doi: 10.1016/j.bbr.2013.12.001
Risher, M.-L., Fleming, R. L., Boutros, N., Semenova, S., Wilson, W. A., Levin, E. D., et al. (2013). Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: Radial-arm maze performance and operant food reinforced responding. PLoS One 8:e62940. doi: 10.1371/journal.pone.0062940
Rubino, T., Prini, P., Piscitelli, F., Zamberletti, E., Trusel, M., Melis, M., et al. (2015). Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol. Dis. 73, 60–69. doi: 10.1016/j.nbd.2014.09.015
Salamone, J. D., Cousins, M. S., McCullough, L. D., Carriero, D. L., and Berkowitz, R. J. (1994). Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol. Biochem. Behav. 49, 25–31. doi: 10.1016/0091-3057(94)90452-9
Salmanzadeh, H., Ahmadi-Soleimani, S. M., Pachenari, N., Azadi, M., Halliwell, R. F., Rubino, T., et al. (2020). Adolescent drug exposure: A review of evidence for the development of persistent changes in brain function. Brain Res. Bull. 156, 105–117.
Sari, Y., Johnson, V. R., and Weedman, J. M. (2011). Role of the serotonergic system in alcohol dependence: From animal models to clinics. Prog. Mol. Biol. Transl. Sci. 98, 401–443.
Sathanantham, S., Dayasiri, K., and Thadchanamoorthy, V. (2021). Approach to the adolescent with substance use in the acute setting. Cureus 13, e16309–e16309. doi: 10.7759/cureus.16309
Scherma, M., Dessì, C., Muntoni, A. L., Lecca, S., Satta, V., Luchicchi, A., et al. (2016). Adolescent Δ(9)-tetrahydrocannabinol exposure alters WIN55,212-2 Self-administration in adult rats. Neuropsychopharmacology 41, 1416–1426. doi: 10.1038/npp.2015.295
Schneider, M., and Koch, M. (2003). Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28, 1760–1769.
Schoch, H., Huerta, M. Y., Ruiz, C. M., Farrell, M. R., Jung, K. M., Huang, J. J., et al. (2018). Adolescent cannabinoid exposure effects on natural reward seeking and learning in rats. Psychopharmacology 235, 121–134. doi: 10.1007/s00213-017-4749-8
Segovia, K. N., Correa, M., and Salamone, J. D. (2011). Slow phasic changes in nucleus accumbens dopamine release during fixed ratio acquisition: A microdialysis study. Neuroscience 196, 178–188. doi: 10.1016/j.neuroscience.2011.07.078
Singh, A. K. (2019). Alcohol interaction with cocaine, methamphetamine, opioids, nicotine, cannabis, and γ-hydroxybutyric acid. Biomedicines 7:16. doi: 10.3390/biomedicines7010016
Sircar, R., and Sircar, D. (2006). Repeated ethanol treatment in adolescent rats alters cortical NMDA receptor. Alcohol 39, 51–58.
Smith-Roe, S. L., and Kelley, A. E. (2000). Coincident activation of NMDA and dopamine D1Receptors within the nucleus accumbens core is required for appetitive instrumental learning. J. Neurosci. 20, 7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000
United Nations on Drugs and Crime (2018). The world drug report 2018. Vienna: United Nations on Drugs and Crime Vienna.
Vivian Chiu, M., Stjepanoviæ, D., and Chan, G. C. (2022). Prevalence of adolescent cannabis vaping: A systematic review and meta-analysis of US and Canadian studies. JAMA Pediatr. 176, 42–51. doi: 10.1001/jamapediatrics.2021.4102
Voelkl, B., Altman, N. S., Forsman, A., Forstmeier, W., Gurevitch, J., Jaric, I., et al. (2020). Reproducibility of animal research in light of biological variation. Nat. Rev. Neurosci. 21, 384–393.
Walker, D. M., Bell, M. R., Flores, C., Gulley, J. M., Willing, J., and Paul, M. J. (2017). Adolescence and reward: Making sense of neural and behavioral changes amid the chaos. J. Neurosci. 37, 10855–10866. doi: 10.1523/JNEUROSCI.1834-17.2017
White, A. M., Ghia, A. J., Levin, E. D., and Swartzwelder, H. S. (2000). Binge pattern ethanol exposure in adolescent and adult rats: Differential impact on subsequent responsiveness to ethanol. Alcohol. Clin.Exp. Res. 24, 1251–1256.
Whyte, A. J., Torregrossa, M. M., Barker, J. M., and Gourley, S. L. (2018). Editorial: Long-term consequences of adolescent drug use: Evidence from pre-clinical and clinical models. Front. Behav. Neurosc. 12:83. doi: 10.3389/fnbeh.2018.00083
Xue, S., Behnood-Rod, A., Wilson, R., Wilks, I., Tan, S., and Bruijnzeel, A. W. (2020). Rewarding effects of nicotine in adolescent and adult male and female rats as measured using intracranial self-stimulation. Nicotine Tob. Res. 22, 172–179. doi: 10.1093/ntr/nty249
Yin, H. H., Ostlund, S. B., Knowlton, B. J., and Balleine, B. W. (2005). The role of the dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 22, 513–523.
Keywords: marijuana abuse, smoking, adolescent drug abuse, learning, cannabinoids, nicotine
Citation: Abela N, Haywood K and Di Giovanni G (2023) Alcohol and cannabinoid binges and daily exposure to nicotine in adolescent/young adult rats induce sex-dependent long-term appetitive instrumental learning impairment. Front. Behav. Neurosci. 17:1129866. doi: 10.3389/fnbeh.2023.1129866
Received: 22 December 2022; Accepted: 16 January 2023;
Published: 06 February 2023.
Edited by:
Liana Fattore, CNR Neuroscience Institute (IN), ItalyReviewed by:
Magor László Lõrincz, University of Szeged, HungarySandra Henriques Vaz, Universidade de Lisboa, Portugal
Copyright © 2023 Abela, Haywood and Di Giovanni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Di Giovanni,  Z2l1c2VwcGUuZGlnaW92YW5uaUB1bS5lZHUubXQ=;
Z2l1c2VwcGUuZGlnaW92YW5uaUB1bS5lZHUubXQ=;  ZGlnaW92YW5uaWdAY2FyZGlmZi5hYy51aw==
ZGlnaW92YW5uaWdAY2FyZGlmZi5hYy51aw==
 Norbert Abela1
Norbert Abela1 Giuseppe Di Giovanni
Giuseppe Di Giovanni