
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Behav. Neurosci., 15 June 2023
Sec. Emotion Regulation and Processing
Volume 17 - 2023 | https://doi.org/10.3389/fnbeh.2023.1124940
This article is part of the Research TopicThe Science and Art of Mood - The Neural Basis of Mood Disorders and Strategies for Mood ManagementView all 4 articles
 Niamh MacSweeney1,2,3*
Niamh MacSweeney1,2,3* Perrine Louvet1†
Perrine Louvet1† Simal Zafar4†
Simal Zafar4† Stella W. Y. Chan5
Stella W. Y. Chan5 Alex S. F. Kwong1,6,7
Alex S. F. Kwong1,6,7 Stephen M. Lawrie1
Stephen M. Lawrie1 Liana Romaniuk1
Liana Romaniuk1 Heather C. Whalley1,8
Heather C. Whalley1,8Irritability is a core symptom of adolescent depression, characterized by an increased proneness to anger or frustration. Irritability in youth is associated with future mental health problems and impaired social functioning, suggesting that it may be an early indicator of emotion regulation difficulties. Adolescence is a period during which behavior is significantly impacted by one’s environment. However, existing research on the neural basis of irritability typically use experimental paradigms that overlook the social context in which irritability occurs. Here, we bring together current findings on irritability in adolescent depression and the associated neurobiology and highlight directions for future research. Specifically, we emphasize the importance of co-produced research with young people as a means to improve the construct and ecological validity of research within the field. Ensuring that our research design and methodology accurately reflect to lives of young people today lays a strong foundation upon which to better understand adolescent depression and identify tractable targets for intervention.
Adolescence, a life phase spanning the ages 10–24 years, is a time of increased risk for the emergence of depressive disorders, which have a peak onset age of 19.5 years (Sawyer et al., 2018; Solmi et al., 2021). Importantly, rates of adolescent depression are rising. In the US, rates increased from 8.1% in 2009 to 15.8% in 2019 (Daly, 2022). Compared to adult-onset depression, adolescent-onset depression is associated with a more recurrent illness course and a host of physical and psycho-social difficulties with longer term consequences (Thapar et al., 2012; Malhi and Mann, 2018). It is therefore unsurprising that it is a leading cause of illness and disability for this age group (James et al., 2018). Taken together, these findings suggest that there are increasing unmet needs of adolescents with mental health difficulties (Wilson and Dumornay, 2022).
Unlike major depressive disorder (MDD) in adults, where low mood and anhedonia are primary diagnostic symptoms, irritability is considered an additional cardinal symptom specific to MDD in adolescence (American Psychiatric Association, 2013). Irritability can be defined as low frustration tolerance and an overreaction to blocked goal attainment relative to same-age peers (Stringaris et al., 2013; Avenevoli et al., 2015). While this can represent a normative behavior in adolescence, it becomes a pathological feature when associated with persistent functional impairment. Several behavioral studies demonstrate that high irritability in childhood and youth predicts later depression, suicidality, and impaired social functioning in adulthood (Leibenluft and Stoddard, 2013; Stringaris et al., 2013). This suggests that irritability may be an early indicator of emotion regulation difficulties and an actionable target for the prevention of downstream mental illness.
Existing definitions of irritability (i.e., proneness to anger/frustration) have shaped the primary experimental paradigms used in neuroimaging research on irritability, typically using frustrative non-reward and threat response tasks. Although these methodological approaches likely induce an irritable mood, the question remains as to whether they sufficiently tap into the broader social context in which the irritable mood occurs. This question is particularly pertinent when studying irritability in adolescence, a period during which mood and behavior are heavily impacted by one’s social environment (Blakemore and Mills, 2014; Sawyer et al., 2018). Here, we therefore seek to draw together current findings on adolescent irritability and its underlying neurobiology, not as a formal literature review (available elsewhere, see Nielsen et al., 2021 and Lee et al., 2022) but as a means to discuss opportunities for future directions in this field. Specifically, we highlight the value of co-produced research with young people as a way to ensure that our research design and methodology accurately reflect the lives of adolescents (MacSweeney et al., 2019; Whitmore and Mills, 2021). Improving the ecological validity of our research will help maximize the chances of identifying tractable targets for intervention that are appropriate for today’s youth.
According to current psychiatric nosology, irritability can be categorized as being chronic or episodic (Leibenluft et al., 2006). Chronic irritability in adolescence represents a young person’s baseline mood. Chronic irritability is considered a defining feature of disruptive mood dysregulation disorder (DMDD), whereby clinically significant irritability must have been present for at least 12 months (American Psychiatric Association, 2013). Conversely, episodic irritability, which refers to changes from baseline mood, is more often seen in mood disorders such as depression and bipolar disorder (American Psychiatric Association, 2013). The Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) (DSM-5) further distinguishes irritability into phasic and tonic. The former referring to behavioral outbursts of extreme anger from a high baseline, while the latter relates to angry mood lasting several days, weeks, or months. Although typically described in chronic irritability, phasic and tonic irritability may also be present within episodic irritability for the duration of the irritable mood (Vidal-Ribas and Stringaris, 2021). In terms of links between irritability and depression (see Vidal-Ribas and Stringaris, 2021 for further detail), the model with the most support is that of “shared risk factors.” That is, shared risk factors including genetic risk, family history of depression, temperament characteristics, and negative parenting styles, influence both outcomes (Vidal-Ribas and Stringaris, 2021).
While theoretical models of irritability can help contextualize factors associated with the emergence and development of irritability, identifying tractable targets for intervention requires an understanding of the neural mechanisms underpinning this salient and transdiagnostic marker of mental illness. The growing emphasis on adopting a translational neuroscience perspective is reflected in the significant increase in the number of studies published on the neural basis of irritability over the past decade (Nielsen et al., 2021; Lee et al., 2022). These studies have largely focused on exploring how irritability in childhood and adolescence, typically in clinical samples (e.g., youth with DMDD, attention deficit hyperactivity disorder (ADHD), internalizing difficulties), relates to changes in blood-oxygen-level-dependent (BOLD) signal, measured via functional magnetic resonance imaging (fMRI).
Task-based fMRI studies make up much of this relatively nascent field of research, which pivots upon three neurocognitive domains: threat processing/emotional reactivity, reward processing, and cognitive control. Threat processing and reward processing constitute the two brain/behavior pathways proposed by Brotman et al. (2017) in their translational neuroscience model of irritability (Brotman et al., 2017). Evidence for the threat processing pathway emerges from research which suggests that increased irritability is associated with an aberrant neural response in the medial (e.g., parahippocampal gyrus) and lateral (e.g., superior temporal gyrus) temporal regions, and the lateral prefrontal cortex (lPFC) (e.g., middle and inferior frontal gyri) when youth are presented with emotionally threatening stimuli, such as angry or fearful faces (Tseng et al., 2016; Wiggins et al., 2016; Kryza-Lacombe et al., 2020). Moreover, higher levels of irritability were found to be associated with more pronounced fluctuations in neural activation across task conditions (e.g., from congruent to incongruent trials), which may represent the additional effort required by youth with high irritability levels to process and respond to emotional stimuli in their environment. Despite the amygdala being the most commonly studied brain region in threat-processing studies on irritability, a recent review and meta-analysis by Lee et al. (2022) found that only 2 out of 12 studies reported increased amygdala activation in youth with higher levels of irritability (Lee et al., 2022).
Research supporting the reward processing pathway of irritability centers on frustrative non-reward tasks, whereby a frustrated psychological state is induced when the participant fails to receive a reward they have been conditioned to expect. Thus, the neural mechanisms of irritability are examined by inducing a frustrated state in real time and studying the associated neural correlates. Using this rigged reward paradigm, research has shown that youth with high irritability exhibit aberrant neural responses in fronto-striatal regions, such as the medial prefrontal cortex (mPFC), cingulate, caudate, and parietal regions, including the cuneus and precuneus, and inferior parietal gyrus, compared to typically developing youth (Deveney et al., 2013; Perlman et al., 2015; Tseng et al., 2019). Further, a recent study by Scheinost and colleagues used connectome-based predictive modeling and found that during frustration trials, functional connectivity within motor-sensory, subcortical and salience networks, and between these networks and fronto-parietal networks, was associated with increased levels of irritability (Scheinost et al., 2021).
Studies that have examined the neural basis of irritability via cognitive control fMRI tasks, such as inhibitory control paradigms (e.g., stop signal task, Flanker task), suggest that youth with high levels of irritability may exhibit inhibitory control deficits. For example, higher levels of irritability have been associated with aberrant patterns of neural activation in the superior temporal gyrus and pre- and post-central gyri (Chaarani et al., 2020), as well as the middle frontal gyrus, anterior cingulate, and striatum (Liuzzi et al., 2020). An illustrative summary of the brain regions associated with irritability in task-based fMRI research is shown in Figure 1.
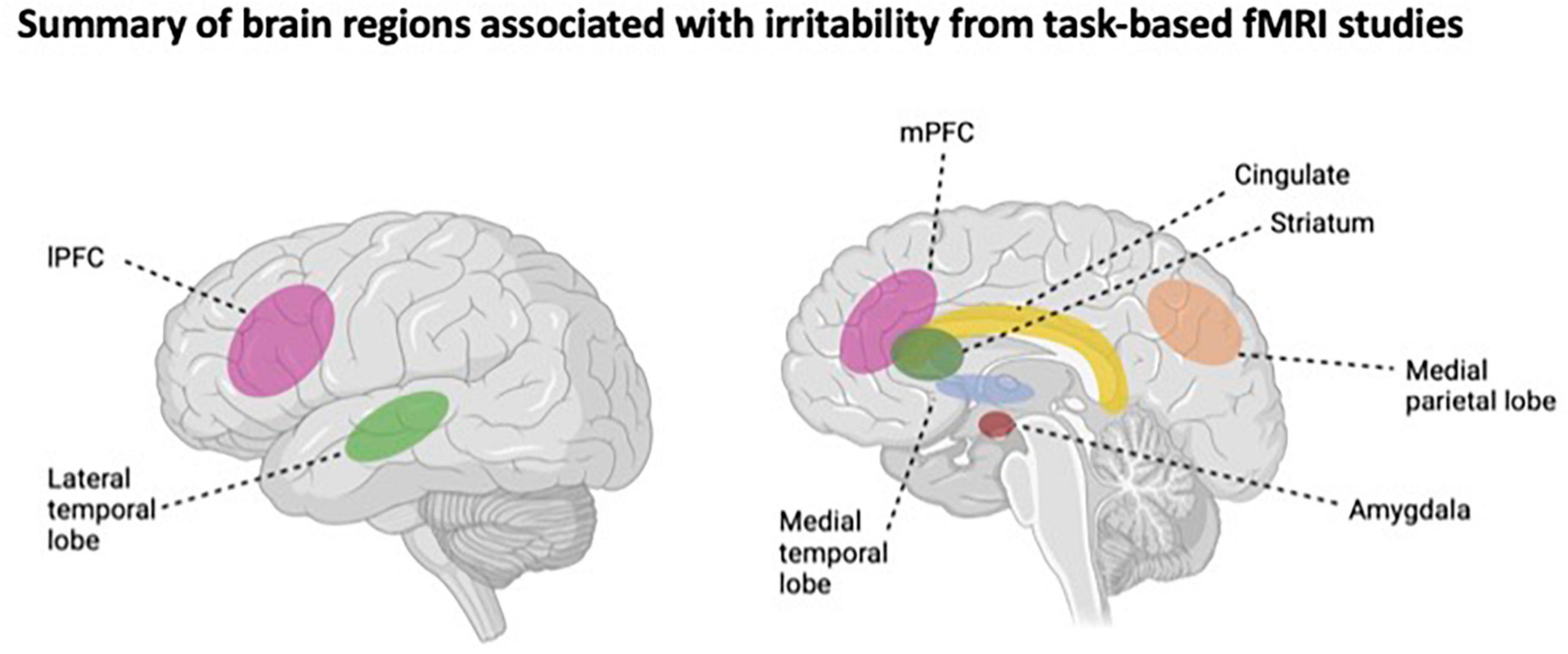
Figure 1. Illustrative summary of the brain regions associations with irritability from task-based fMRI studies. IPFC, lateral prefrontal cortex; mPFC, medial prefrontal cortex. This figure was made using BioRender.com.
Taken together, current paradigms used to examine irritability may be more likely to elicit fear or stress responses than more genuinely irritable ones, which highlights the need for tasks that aim to induce irritable mood specifically. Improving the construct validity of existing paradigms will have downstream effects on the ecological validity of the field by ensuring that our research methods capture the experience of youth irritability in the present day as accurately as possible.
There are a limited number of resting state studies that have examined the neural correlates of irritability, the majority of which focus on the amygdala and its functional connectivity with other brain networks. In these studies, a questionnaire-based measure of irritability [e.g., the Affective Reactivity Index (ARI); Stringaris et al., 2012] is collected outside the scanner and then these behavioral measures are related to resting state imaging features. Most of the existing resting state studies focus on chronic irritability within the context of aggressive behavior/temper outbursts in childhood-onset disorders such as ADHD, oppositional defiant disorder (ODD), and autism spectrum disorder (ASD) (Bennett et al., 2017; Roy et al., 2018; Weathersby et al., 2019; Gaffrey et al., 2021), as well as some examining irritability in DMDD and bipolar disorder (Stoddard et al., 2015). Similar to the task-fMRI irritability literature, these studies suggest that the neural correlates of irritability comprise a diverse set of functional networks such as the default mode network (DMN), fronto-parietal network (FPN), executive control, sensory-motor, and visual networks (Nielsen et al., 2021). These networks support and coordinate cognitive processes associated with irritable mood, including self-referential behavior (DMN), reward processing and emotion regulation (FPN, executive control network), and motor response (sensory motor network). A summary of the brain networks associated with irritability are illustrated in Figure 2.
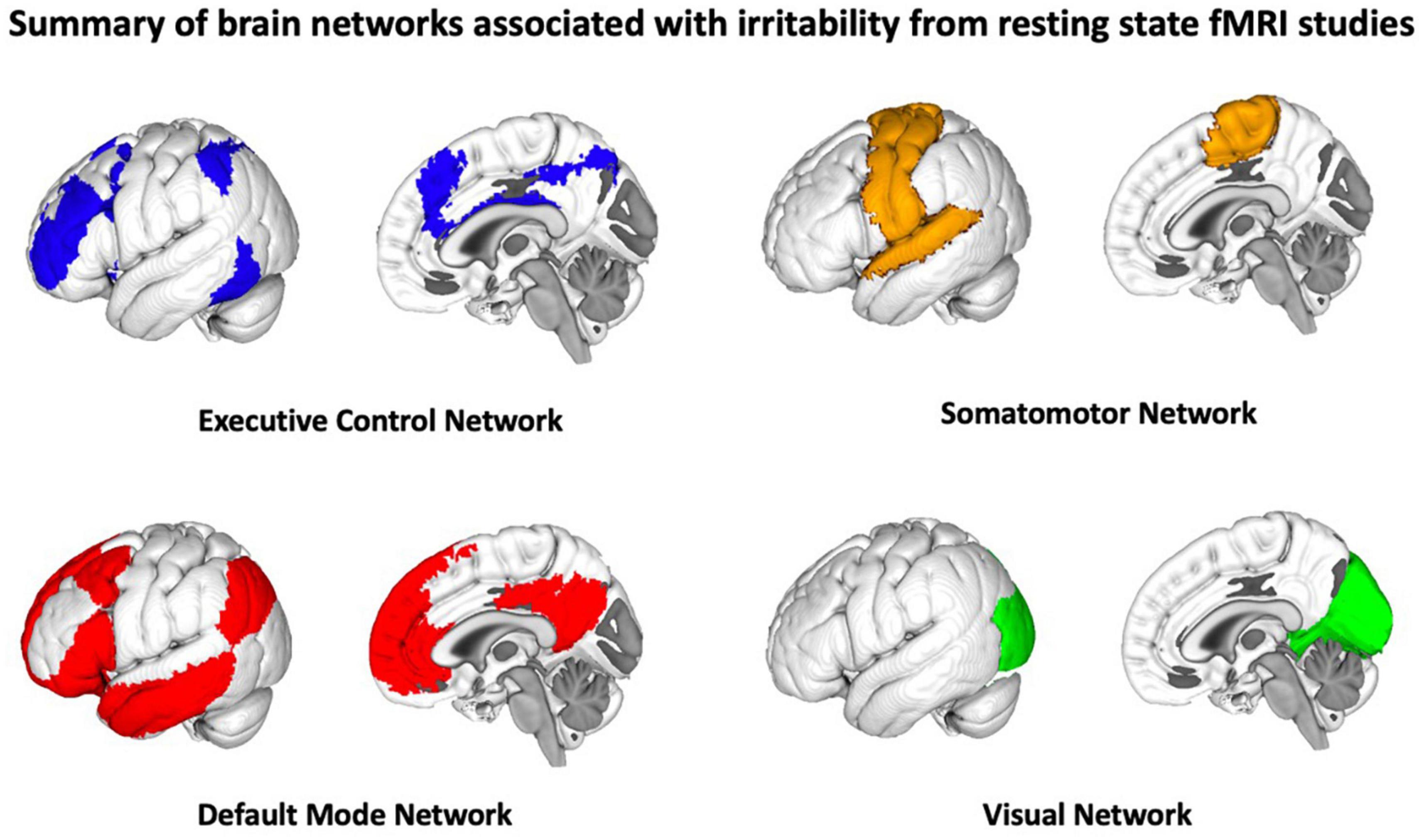
Figure 2. Summary of brain networks associated with irritability from resting state studies, the majority of which focus on the amygdala and its functional connectivity with other brain networks.
A recent systematic review and meta-analysis by Lee et al. (2022) sought to determine whether there are convergent neural responses associated with irritability across the domains of threat, reward processing, and cognitive control in task-fMRI (Lee et al., 2022). Although the authors conclude that, across individual task-based studies, the amygdala, putamen and caudate are the brain regions that exhibit the highest number of significant associations with irritable mood, they found little evidence for convergence across the three neurocognitive domains examined. For example, amygdala reactivity (increased activation) was found to be associated with irritability in only 2 out of 10 studies that used a threat-processing/emotional reactivity paradigm, 1 out of 3 studies that involved a cognitive control task, while no association was found in the two reward-processing task studies included in the review. The picture is equally unclear when considering the resting state literature on irritability. Current findings suggest that the neural correlates of irritability comprise amygdala functional connectivity with executive control, default mode and sensorimotor brain networks (Nielsen et al., 2021). However, the direction of these relationships was found to vary across individual studies with some reporting increased activation while others reported decreased or no activation (Nielsen et al., 2021). The inconsistent results across both task-based and resting state irritability studies may be due to a host of factors including, the marked heterogeneity in clinical characteristics, task design, irritability measures and analysis methods, small sample sizes, and a lack of longitudinal research. Moreover, it remains unclear whether the brain mechanisms underpinning irritability vary across disorders (Eshel and Leibenluft, 2020). Some evidence suggests that individual differences in dispositional (i.e., chronic) irritability may be more underpinned by amygdala-DMN connectivity than state (i.e., episodic) irritability, due to more consistent findings in this neural circuitry in resting state (Fulwiler et al., 2012; Gaffrey et al., 2021) compared to task-based studies (Stoddard et al., 2017; Kryza-Lacombe et al., 2020). However, more research, ideally combining resting state and task-based paradigms in larger samples with harmonized protocols is needed to advance our understanding of the neural correlates of irritability.
The heterogeneity present across multiple domains sheds light on several important considerations, especially research involving developmental samples. Probing individual differences in irritability will allow us to better understand its bounds as a normative behavior across development, and what might reflect concerning irritable mood. How this relates to other cognitive processes, such as emotion regulation, and the associated underlying neural circuitry, will help pave the way forward for targeted intervention strategies. Further, there is an overall paucity of research on age-related (and sex-related) changes associated with irritability — longitudinal and sufficiently powered cross-sectional studies that examine age interaction effects are also needed. It may be that the neural underpinnings of irritability vary across development and are related to other typical, as well as divergent, neurodevelopmental changes. Emerging research on brain growth charts for the human lifespan will be helpful in this effort (Bethlehem et al., 2022). Moreover, few studies have examined irritability in later adolescence (only 4/30 studies in the Lee et al. review had a mean age >15 years). Given the varying age of onset for mental health difficulties during adolescence (Solmi et al., 2021) and the distinct neuromaturation that characterizes this life phase (Bethlehem et al., 2022), future studies should be designed in a developmentally sensitive way. Some large, longitudinal youth cohort studies such as IMAGEN and the Adolescent Brain Cognitive Development (ABCD) Study, include variables related to irritable mood alongside neuroimaging data, and have already contributed to our understanding of the neurobiology of youth irritability and psychopathology (e.g., Chaarani et al., 2020, using IMAGEN data).
While the large sample sizes of such cohort studies are well powered to detect more subtle effects (e.g., individual differences in irritability, underlying neural circuitry, and potential contributing factors), the breadth of measures included in such studies comes at the cost of phenotypic depth. For example, ABCD and IMAGEN do not include an irritability-specific questionnaire like the ARI. Instead, a measure of irritability is derived from individual items in broad mental health measures like the Development and Wellbeing Assessment (DAWBA; Goodman et al., 2000) or the Child Behavior Checklist (CBCL; Achenbach, 2011). Thus, rather than a “panacea” to the many unknowns in developmental cognitive neuroscience, cohort studies may be better conceptualized as hypothesis generating tools that can inform directions for future studies (Saragosa-Harris et al., 2022). To develop a finer-grained characterization of irritability, and the functional significance of altered neural circuitry—especially how it relates to psychopathology—we need construct-specific and ecologically valid experimental designs. Ideally, these designs would involve harmonized protocols across studies to minimize sources of error as much as possible. Initiatives like the ENIGMA Irritability Working Group are leading by example in this way.
In sum, the surge of studies on the neurobiology of irritability over the past decade has allowed us to outline the brain areas involved in this transdiagnostic symptom from which myriad directions for future research have emerged. Before embarking upon these new avenues of research, we should reflect on how we plan to move forward to ensure that our journey takes us toward the world of young people rather than away from it.
Co-produced research, whereby the target population of the study, such as adolescents, are involved in as many steps of the research project as possible, has gained increasing traction in recent times.1 Initiatives like Young Persons’ Advisory Groups (YPAGs) allow young people to be involved in research in an active, meaningful, and mutually beneficial way. As co-researchers, young people and researchers can work together to ensure that the research questions, methods, and dissemination of research findings are relevant to the lives of young people today (MacSweeney et al., 2019). Although co-produced research involves a considerable (and front-loaded) time investment, researchers should approach it like other best practices in research, such as open science (Whitmore and Mills, 2021). Transparent and rigorous research that is attuned to the issues and experiences of today’s young people will be a key tool in our effort to answer complex questions in developmental science. Thankfully, resources are now available to help researchers undertake effective and meaningful co-created research (Whitmore and Mills, 2021).
Although there have been important commentaries on the neuroscience of irritability (Eshel and Leibenluft, 2020), the social context in which irritable mood occurs has been largely overlooked. Notably, irritability occurs in social and interactive contexts between youth and other people in their environment. However, existing fMRI paradigms, like frustrative non-reward tasks and emotional faces tasks, may not appropriately capture the rich social tapestry of adolescence. To enhance both construct and ecological validity, future research on irritability should incorporate social context into the study design. For example, Lee et al. (2022) propose a frustrative social non-reward task that targets behaviors like social rejection. This work would complement existing research on social exclusion in adolescence, which has used socially relevant tasks like Cyberball (Williams et al., 2000; Sebastian et al., 2010). Given that avoidance of social rejection drives adolescent decision-making and behavior (Tomova et al., 2021), this research could provide novel insight into how the nuances of the adolescent social world relate to the emergence and development of irritability and related mental health difficulties. Importantly, this effort to align our research methods with the social world of adolescence could be strengthened even further by undertaking research that is co-produced with young people.
By asking young people questions like, “What situations do you find irritating in your daily life?,” we could design studies that better reflect the experience of irritability as a young person. In turn, this could help disentangle the current heterogenous findings in irritability research. As mentioned by Lee et al. (2022), these insights could be incorporated into task-based fMRI, but there is also opportunity for “hybrid” resting-state paradigms. Recent calls for a “third-wave” of fMRI research propose the use of integrated fMRI paradigms, whereby task-like manipulations are paired with “traditional” resting state approaches (Finn, 2021). Naturalistic stimuli (e.g., movie watching) are some examples of integrated fMRI paradigms (Sonkusare et al., 2019), which allow researchers to regain some degree of experimental control, while acknowledging the dynamic patterns of brain function that arise from self-generated activity. Further, pairing these integrated paradigms with analyses capable of capturing fine grained temporal details, such as dynamic functional connectivity analysis, warrants consideration going forward. It has been argued that progress in our understanding of the human brain and behavior is likely to emerge from these “third-wave” paradigms (Finn, 2021). However, this progress will be hampered if the paradigms are not attuned to the lives of young people today. Co-produced fMRI paradigms will help ensure that the construct of interest is studied in a way that reflects real-world experience. For example, when studying youth irritability, we could ask young people to come up with irritating scenarios based on their own experiences. These scenarios would then form the stimuli for an integrated fMRI paradigm, asking young people to read each irritating scenario and imagine the experience as vividly as possible while in the scanner. This protocol would suit a range of samples (e.g., healthy volunteers, young people with mental health difficulties) but could also be adapted to suit different sample characteristics and research questions.
Importantly, novel paradigms like this would need to be validated against traditional task-based irritability paradigms (e.g., frustrative non-reward and threat response tasks) as well as behavioral measures of irritability (e.g., ARI). Given the lack of convergence in the neural correlates of irritability across neurocognitive domains (Lee et al., 2022), a novel, co-produced integrated fMRI task with improved ecological validity, holds great promise as way to better our understanding of youth irritability, identify tractable intervention targets, and move young people away from illness toward wellbeing.
NM: conceptualization, methodology, formal analysis (literature review), investigation, writing—original draft, and writing—review and editing. PL: conceptualization, formal analysis (literature review), and writing—review and editing. SZ: conceptualization and writing—review and editing. SC: conceptualization, writing—review and editing, and funding acquisition. AK and SL: writing—review and editing. LR and HW: conceptualization, writing—review and editing, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
HW and LR are supported by a Wellcome Trust Institutional Strategic Support Fund Grant. NM was also supported by a Mental Health Research UK Ph.D. studentship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achenbach, T. M. (2011). “Child behavior checklist,” in Encyclopedia of clinical neuropsychology, eds J. S. Kreutzer, J. DeLuca, and B. Caplan (New York, NY: Springer), 546–552. doi: 10.1007/978-0-387-79948-3_1529
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: DSM-5, 5th Edn. Washington, DC: American Psychiatric Association.
Avenevoli, S., Blader, J. C., and Leibenluft, E. (2015). Irritability in youth: An update. J. Am. Acad. Child Adolesc. Psychiatry 54, 881–883. doi: 10.1016/j.jaac.2015.08.012
Bennett, R. H., Somandepalli, K., Roy, A. K., and Di Martino, A. (2017). The neural correlates of emotional lability in children with autism spectrum disorder. Brain Connect. 7, 281–288. doi: 10.1089/brain.2016.0472
Bethlehem, R. a. I., Seidlitz, J., White, S. R., Vogel, J. W., Anderson, K. M., Adamson, C., et al. (2022). Brain charts for the human lifespan. Nature 604, 525–533. doi: 10.1038/s41586-022-04554-y
Blakemore, S.-J., and Mills, K. L. (2014). Is adolescence a sensitive period for sociocultural processing? Annu. Rev. Psychol. 65, 187–207. doi: 10.1146/annurev-psych-010213-115202
Brotman, M. A., Kircanski, K., Stringaris, A., Pine, D. S., and Leibenluft, E. (2017). Irritability in youths: A translational model. Am. J. Psychiatry 174, 520–532. doi: 10.1176/appi.ajp.2016.16070839
Chaarani, B., Kan, K.-J., Mackey, S., Spechler, P. A., Potter, A., Banaschewski, T., et al. (2020). Neural correlates of adolescent irritability and its comorbidity with psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry 59, 1371–1379. doi: 10.1016/j.jaac.2019.11.028
Daly, M. (2022). Prevalence of depression among adolescents in the U.S. From 2009 to 2019: Analysis of trends by sex, race/ethnicity, and income. J. Adolesc. Health 70, 496–499. doi: 10.1016/j.jadohealth.2021.08.026
Deveney, C. M., Connolly, M. E., Haring, C. T., Bones, B. L., Reynolds, R. C., Kim, P., et al. (2013). Neural mechanisms of frustration in chronically irritable children. Am. J. Psychiatry 170, 1186–1194. doi: 10.1176/appi.ajp.2013.12070917
Eshel, N., and Leibenluft, E. (2020). New frontiers in irritability research—from cradle to grave and bench to bedside. JAMA Psychiatry 77:227. doi: 10.1001/jamapsychiatry.2019.3686
Finn, E. S. (2021). Is it time to put rest to rest? Trends Cogn. Sci. 25, 1021–1032. doi: 10.1016/j.tics.2021.09.005
Fulwiler, C. E., King, J. A., and Zhang, N. (2012). Amygdala–orbitofrontal resting-state functional connectivity is associated with trait anger. NeuroReport 23, 606–610.
Gaffrey, M. S., Barch, D. M., Luby, J. L., and Petersen, S. E. (2021). Amygdala functional connectivity is associated with emotion regulation and amygdala reactivity in 4- to 6-year-olds. J. Am. Acad. Child Adolesc. Psychiatry 60, 176–185. doi: 10.1016/j.jaac.2020.01.024
Goodman, R., Ford, T., Richards, H., Gatward, R., and Meltzer, H. (2000). The development and well-being assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. J. Child Psychol. Psychiatry 41, 645–655. doi: 10.1111/j.1469-7610.2000.tb02345.x
James, S. L., Abate, D., Abate, K. H., Abay, S. M., Abbafati, C., Abbasi, N., et al. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. doi: 10.1016/S0140-6736(18)32279-7
Kryza-Lacombe, M., Kiefer, C., Schwartz, K. T. G., Strickland, K., and Wiggins, J. L. (2020). Attention shifting in the context of emotional faces: Disentangling neural mechanisms of irritability from anxiety. Depress. Anxiety 37, 645–656. doi: 10.1002/da.23010
Lee, K. S., Hagan, C. N., Hughes, M., Cotter, G., McAdam Freud, E., Kircanski, K., et al. (2022). Systematic review and meta-analysis: Task-based fMRI studies in youths with irritability. J. Am. Acad. Child Adolesc. Psychiatry 62, 208–229. doi: 10.1016/j.jaac.2022.05.014
Leibenluft, E., and Stoddard, J. (2013). The developmental psychopathology of irritability. Dev. Psychopathol. 25, 1473–1487. doi: 10.1017/S0954579413000722
Leibenluft, E., Cohen, P., Gorrindo, T., Brook, J. S., and Pine, D. S. (2006). Chronic versus episodic irritability in youth: A community-based, longitudinal study of clinical and diagnostic associations. J. Child Adolesc. Psychopharmacol. 16, 456–466. doi: 10.1089/cap.2006.16.456
Liuzzi, M. T., Kryza-Lacombe, M., Christian, I. R., Palumbo, D. E., Amir, N., and Wiggins, J. L. (2020). Neural and behavioral correlates of inhibitory control in youths with varying levels of irritability. J. Affect. Disord. 273, 567–575. doi: 10.1016/j.jad.2020.04.049
MacSweeney, N., Bowman, S., and Kelly, C. (2019). More than just characters in a story: Effective and meaningful involvement of young people in mental health research. J. Public Ment. Health 18, 14–16. doi: 10.1108/JPMH-07-2018-0053
Malhi, G. S., and Mann, J. J. (2018). Depression. Lancet (Lond. Engl.) 392, 2299–2312. doi: 10.1016/S0140-6736(18)31948-2
Nielsen, A. N., Wakschlag, L. S., and Norton, E. S. (2021). Linking irritability and functional brain networks: A transdiagnostic case for expanding consideration of development and environment in RDoC. Neurosci. Biobehav. Rev. 129, 231–244. doi: 10.1016/j.neubiorev.2021.07.022
Perlman, S. B., Jones, B. M., Wakschlag, L. S., Axelson, D., Birmaher, B., and Phillips, M. L. (2015). Neural substrates of child irritability in typically developing and psychiatric populations. Dev. Cogn. Neurosci. 14, 71–80. doi: 10.1016/j.dcn.2015.07.003
Roy, A. K., Bennett, R., Posner, J., Hulvershorn, L., Castellanos, F. X., and Klein, R. G. (2018). Altered intrinsic functional connectivity of the cingulate cortex in children with severe temper outbursts. Dev. Psychopathol. 30, 571–579. doi: 10.1017/S0954579417001080
Saragosa-Harris, N. M., Chaku, N., MacSweeney, N., Guazzelli Williamson, V., Scheuplein, M., Feola, B., et al. (2022). A practical guide for researchers and reviewers using the ABCD Study and other large longitudinal datasets. Dev. Cogn. Neurosci. 55:101115. doi: 10.1016/j.dcn.2022.101115
Sawyer, S. M., Azzopardi, P. S., Wickremarathne, D., and Patton, G. C. (2018). The age of adolescence. Lancet Child Adolesc. Health 2, 223–228. doi: 10.1016/S2352-4642(18)30022-1
Scheinost, D., Dadashkarimi, J., Finn, E. S., Wambach, C. G., MacGillivray, C., Roule, A. L., et al. (2021). Functional connectivity during frustration: A preliminary study of predictive modeling of irritability in youth. Neuropsychopharmacology 46, 1300–1306. doi: 10.1038/s41386-020-00954-8
Sebastian, C., Viding, E., Williams, K. D., and Blakemore, S.-J. (2010). Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 72, 134–145. doi: 10.1016/j.bandc.2009.06.008
Solmi, M., Radua, J., Olivola, M., Croce, E., Soardo, L., Salazar de Pablo, G., et al. (2021). Age at onset of mental disorders worldwide: Large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatry 27, 281–295. doi: 10.1038/s41380-021-01161-7
Sonkusare, S., Breakspear, M., and Guo, C. (2019). Naturalistic stimuli in neuroscience: Critically acclaimed. Trends Cogn. Sci. 23, 699–714. doi: 10.1016/j.tics.2019.05.004
Stoddard, J., Hsu, D., Reynolds, R. C., Brotman, M. A., Ernst, M., Pine, D. S., et al. (2015). Aberrant amygdala intrinsic functional connectivity distinguishes youths with bipolar disorder from those with severe mood dysregulation. Psychiatry Res. Neuroimaging 231, 120–125. doi: 10.1016/j.pscychresns.2014.11.006
Stoddard, J., Tseng, W.-L., Kim, P., Chen, G., Yi, J., Donahue, L., et al. (2017). Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiatry 74:95. doi: 10.1001/jamapsychiatry.2016.3282
Stringaris, A., Goodman, R., Ferdinando, S., Razdan, V., Muhrer, E., Leibenluft, E., et al. (2012). The affective reactivity index: A concise irritability scale for clinical and research settings. J. Child Psychol. Psychiatry 53, 1109–1117. doi: 10.1111/j.1469-7610.2012.02561.x
Stringaris, A., Maughan, B., Copeland, W. S., Costello, E. J., and Angold, A. (2013). Irritable mood as a symptom of depression in youth: Prevalence, developmental, and clinical correlates in the Great Smoky Mountains study. J. Am. Acad. Child Adolesc. Psychiatry 52, 831–840. doi: 10.1016/j.jaac.2013.05.017
Thapar, A., Collishaw, S., Pine, D. S., and Thapar, A. K. (2012). Depression in adolescence. Lancet 379, 1056–1067. doi: 10.1016/S0140-6736(11)60871-4
Tomova, L., Andrews, J. L., and Blakemore, S.-J. (2021). The importance of belonging and the avoidance of social risk taking in adolescence. Dev. Rev. 61:100981. doi: 10.1016/j.dr.2021.100981
Tseng, W.-L., Deveney, C. M., Stoddard, J., Kircanski, K., Frackman, A. E., Yi, J. Y., et al. (2019). Brain mechanisms of attention orienting following frustration: Associations with irritability and age in youths. Am. J. Psychiatry 176, 67–76. doi: 10.1176/appi.ajp.2018.18040491
Tseng, W.-L., Thomas, L. A., Harkins, E., Pine, D. S., Leibenluft, E., and Brotman, M. A. (2016). Neural correlates of masked and unmasked face emotion processing in youth with severe mood dysregulation. Soc. Cogn. Affect. Neurosci. 11, 78–88. doi: 10.1093/scan/nsv087
Vidal-Ribas, P., and Stringaris, A. (2021). How and why are irritability and depression linked? Child Adolesc. Psychiatr. Clin. North Am. 30, 401–414. doi: 10.1016/j.chc.2020.10.009
Weathersby, F. L., King, J. B., Fox, J. C., Loret, A., and Anderson, J. S. (2019). Functional connectivity of emotional well-being: Overconnectivity between default and attentional networks is associated with attitudes of anger and aggression. Psychiatry Res. Neuroimaging 291, 52–62. doi: 10.1016/j.pscychresns.2019.08.001
Whitmore, L. B., and Mills, K. L. (2021). Co-creating developmental science. Infant Child Dev. 31:e2273. doi: 10.1002/icd.2273
Wiggins, J. L., Brotman, M. A., Adleman, N. E., Kim, P., Oakes, A. H., Reynolds, R. C., et al. (2016). Neural correlates of irritability in disruptive mood dysregulation and bipolar disorders. Am. J. Psychiatry 173, 722–730. doi: 10.1176/appi.ajp.2015.15060833
Williams, K. D., Cheung, C. K. T., and Choi, W. (2000). Cyberostracism: Effects of being ignored over the Internet. J. Pers. Soc. Psychol. 79, 748–762. doi: 10.1037/0022-3514.79.5.748
Keywords: irritability, adolescent depression, co-production, fMRI, adolescence
Citation: MacSweeney N, Louvet P, Zafar S, Chan SWY, Kwong ASF, Lawrie SM, Romaniuk L and Whalley HC (2023) Keeping up with the kids: the value of co-production in the study of irritability in youth depression and its underlying neural circuitry. Front. Behav. Neurosci. 17:1124940. doi: 10.3389/fnbeh.2023.1124940
Received: 15 December 2022; Accepted: 30 May 2023;
Published: 15 June 2023.
Edited by:
Lingling Kong, Goldbelt Frontier, United StatesReviewed by:
Ahmed A. Karim, University Hospital Tübingen, GermanyCopyright © 2023 MacSweeney, Louvet, Zafar, Chan, Kwong, Lawrie, Romaniuk and Whalley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niamh MacSweeney, bmlhbWgubWFjc3dlZW5leUBwc3lrb2xvZ2kudWlvLm5v
†Youth-researchers
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.