- Department of Occupational Therapy, Faculty of Social Welfare and Health Sciences, University of Haifa, Haifa, Israel
Attention Deficit Hyperactivity Disorder (ADHD) is a common developmental disorder affecting 5-7% of adults and children. We surveyed the literature to examine ADHD through three pillars: developmental characteristics, symptomatology, and treatment strategies. Firstly, in terms of developmental characterstics, early life stress may increase the risk of developing ADHD symptoms according to animal models’ research. Secondly, the current core symptoms of ADHD are comprised of inattention, hyperactivity, and impulsivity. However, the up-to-date literature indicates individuals with ADHD experience emotional and sensory dysregulation as well, which early-life stress may also increase the risk of. Finally, we discuss the therapeutic benefits of methylphenidate on both the current core ADHD symptoms and the sensory and emotional dysregulation found in those with ADHD. In summation, we surveyed the recent literature to analyze (i) the potential role of early-life stress in ADHD development, (ii) the involvement of emotional and sensory dysregulation in ADHD symptomatology and finally, (iii) the therapeutic intervention with methylphenidate, aiming to reduce the potential effect of early life stress in ADHD, and mainly emotional and sensory dysregulation. The apparent but currently less recognized additional symptoms of emotional and sensory dysregulation in ADHD call for further investigation of these possible causes and thus increasing treatments efficacy in individuals with ADHD.
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a common developmental disorder suggested to be caused by a delay in brain maturation (Martine Hoogman, 2017), affecting 5-7% of the population (Polanczyk et al., 2007; Thomas et al., 2015; Xu et al., 2018). Risk factors for ADHD development are continuously being investigated, with childhood stress potentially correlating with the development of ADHD itself (Humphreys et al., 2019) and the emotional difficulties often found in children with ADHD (Kennedy et al., 2016). However, due to the difficult nature of controlling for early-life stress in clinical research, there is no definitive clinical evidence for the role of early-life stress on ADHD development.
The core symptomatology of ADHD comprises of inattention, hyperactivity, and impulsivity, thus forming three subtypes of ADHD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association [APA], 2013). While inattention and/or impulsivity and hyperactivity are the most well-known facets of the disorder, emerging evidence potentially indicates emotional dysregulation (van Stralen, 2016) and sensory processing issues (Sandler, 2001; Panagiotidi et al., 2018) to be potential additional symptoms of ADHD.
Pharmacological strategies for treating ADHD symptoms include methylphenidate (MPH), being one of the most common treatments. This review focuses on MPH specifically since it has a low addictive potential (Robbins, 2002), a shorter half-life than other common ADHD treatments (Manos et al., 1999; Pelham et al., 1999), allowing the comparison of its chronic and acute effects more distinctly, and improving the potential additional symptoms of ADHD: emotional dysregulation (Gamli and Tahiroglu, 2018) and sensory dysregulation (Treister et al., 2015).
Therefore, this review (August 2019 through August 2022) is conducted on three pillars: Developmental characteristics, Symptomatology, and Treatment strategies. Within developmental characteristics, the role of early life stress on the development of ADHD-like symptoms will be discussed. The specific symptoms themselves will then be discussed in the Symptomatology sections, exploring the currently accepted ADHD symptoms (impulsivity, hyperactivity, and inattention), as well as the proposed additional symptoms of ADHD (emotional and sensory dysregulation). Finally, after discussing the way these symptoms develop and the subtleties of the Symptomatology, we will discuss how MPH mitigates ADHD symptoms, including the potential additional ADHD symptoms of sensory and emotional dysregulation. Through this discussion of Developmental Characteristics, Symptomatology, and Treatment strategies, we can follow the line of how early life stress may increase the risk of ADHD symptoms, and how certain ADHD symptoms, particularly emotional and sensory dysregulation, may give rise to the higher order symptoms of impulsivity, hyperactivity, and inattention, and how application of MPH can prevent these symptoms, respectively.
Thus, we aim to understand nuances of the developmental risks, the role of sensory and emotional dysregulation on symptoms, and treatments of ADHD by considering the National Institute of Health’s call to approach a more dimensional diagnostic process for mental health concerns. Furthermore, by understanding the neurophysiological and behavioral symptoms of the disorder and determining the dis-/similarities between ADHD and emotional and sensory dysregulation, we may suggest differential and more accurate treatments of the disorders.
In regards to methodology, we conducted the review between August 2017 through August 2022, as mentioned, through Google Scholar and Pubmed. A limitation worth mentioning is a large subset of these publications were gathered prior to the recent Pubmed website update, meaning some of our citations may not be available through Pubmed currently.
2. Developmental characteristics: The Influence of stress on the development of ADHD
Neglect, deprivation, abuse, and trauma may intensify the risk of ADHD development (Humphreys and Zeanah, 2015). The stress of early-life parental separation induced ADHD-like symptoms of hyperactivity and inattentiveness in a rat model, which is then rescued by MPH application (Bock et al., 2017). This correlation between ADHD and early life stress appears also in humans, as the number of stressful life events as evaluated by the Traumatic Events Screen Inventory for children, both in early and later childhood, was associated with higher levels of ADHD symptoms (Humphreys et al., 2019). Furthermore, the rate of ADHD in the U.S. child welfare agencies is 19%, almost 4 times the rate of the general population (Heneghan et al., 2013). Interestingly, children raised for at least 6 months in “depriving” Romanian orphanages similarly have an ADHD rate of 19%, or 4 times the rate of their counterparts raised in “non-depriving” homes (Kennedy et al., 2016) mirroring the increased rate of early life traumatizing institutional deprivation in these Romanian agencies (Rutter et al., 2010). Traumatizing events in later childhood appear to correlate with higher levels of ADHD symptoms, as ADHD symptoms correlate with K-SADS-PL Post-Traumatic Stress Disorder scores in 14- and 15 years-old (Sibley et al., 2020) and sexual and physical abuse before the age of 16 (Vrijsen et al., 2018) or 17 (Singer et al., 2016). Taken together, these results potentially further emphasize the positive correlation found between high Adverse Childhood Experiences report scores and ADHD risk (Brown et al., 2017).
The potential role of early-life trauma on the development of inattention and hyperactivity, symptoms typically associated with ADHD, may be mitigated through environmental factors. Environmental factors like emotionally supportive parenting in childhood and early adolescence correlated with a reduction of emotional dysregulation symptoms in individuals with ADHD (Breaux et al., 2018). Parents of children with ADHD were assessed by the German Family Climate questionnaire at baseline, one year, and two years then compared to offspring’s ADHD symptoms, which indicated that improvement of the family climate (e.g., “In our family everybody cares about each other’s worries”) was associated with a decrease in overall ADHD symptoms (Wüstner et al., 2019). Based on this data, early-life stress occurring along the developmental trajectory correlates with the development of ADHD, but early environmental intervention in both animal and human models may correlate with reduced emotional dysregulation in children with ADHD and the classical ADHD symptoms. While it appears that early life stress may correlate with increased sensory dysfunction, this needs to be further evaluated in those with ADHD specifically. Overall, these data correlate childhood trauma with the development of ADHD; however, further clinical research to determine whether early-life stress has a causative, rather than correlative, effect on ADHD development is required.
3. Symptomatology: Emotional and sensory dysregulation as potential ADHD symptoms
3.1. Emotional dysregulation in ADHD
Early life stress not only correlates with the typical ADHD symptoms of impulsivity, hyperactivity, and inattention, as above-mentioned, but, according to animal models research, may intensify anxiety-like behaviors in a rat model of “emotional dysregulation” (Ishikawa et al., 2015). Emotional regulation is defined as the “extrinsic and intrinsic processes responsible for monitoring, evaluating, and modifying emotional reactions, especially their intensive and temporal features, to accomplish one’s goals” (Thompson, 1994). Many studies posit emotional dysregulation is a common pathology of ADHD (Retz et al., 2012; Bunford et al., 2018). Indeed, those with ADHD particularly express an elevated anxiety level which subserves as an aspect of emotional dysregulation (Bloch et al., 2017). About 25% of adults with ADHD meet the criteria for generalized anxiety disorder (Piñeiro-Dieguez et al., 2016), and higher anxiety has been associated with greater ADHD symptomatology and even with the most severe form of ADHD (Reimherr et al., 2017).
ADHD has a high comorbidity with a variety of mental disorders, like the previously mentioned generalized anxiety disorder, leading to the exclusion of emotional dysregulation as a direct feature of ADHD, and consider it a manifestation of a comorbid disorder. However, emotional dysregulation has been found in ADHD with no comorbidities, and emotional dysregulation symptoms respond to common ADHD treatments (Reimherr et al., 2005; Corbisiero et al., 2017). Emotional dysregulation was even a diagnostic criterion of ADHD in the DSM II, only to return as an associated feature in the DSM III (Shaw et al., 2014).
Hypothetically, emotional dysregulation may induce some of the classical symptoms of ADHD, like impulsivity and inattention. The dysregulation of emotion would make paying attention more difficult and would also increase the likelihood of impulsive behavior. This hypothesis is mirrored in data of ADHD individuals, as impulsivity and hyperactivity predict the severity of emotional dysregulation in ADHD individuals (Groves et al., 2020). Some studies show that emotional dysregulation is better defined in adult ADHD individuals than children (Retz et al., 2012; Hirsch et al., 2018), but it is still present in children with ADHD (Nigg et al., 2020). Thus, emotional dysregulation remains prominent over the lifespan of those with ADHD (Cubillo et al., 2012), indicating a need for further research on early detection of emotional dysregulation in children with ADHD. Furthermore, treatment options for emotional dysregulation are not specified, and further research is required on how to mitigate emotional dysregulation found in ADHD (Lenzi et al., 2018) as well.
3.2. Sensory dysregulation in ADHD
Similar to emotional dysregulation, early life stress correlates with increased sensory dysregulation in adolescents as well (Jeon and Bae, 2022). Both children (Dunn and Bennett, 2002; Ghanizadeh, 2011) and adults (Bijlenga et al., 2017) with ADHD express atypical responses to sensory stimuli. The relation between sensory dysregulation and ADHD is found as early as infancy, as infants with reduced habituation of EEG responses to repeated auditory tones is associated with later attentional dysfunction (Hutchison et al., 2017). This association continues into adulthood, when adults with ADHD were administered questionnaires about sensory processing and ADHD symptoms, the number of sensory difficulties was associated with severity of ADHD (Panagiotidi et al., 2018).
This sensory dysregulation, found in those with ADHD, seems to be tied to emotional dysregulation as well, as pain sensitivity in children with ADHD was recently shown to be associated with the severity of emotional dysregulation (Bruton et al., 2022). This result was corroborated by Lane and Reynolds (2019), as they found that children with ADHD that presented sensory over-responsiveness more often, have clinically significant anxiety levels, and that the strength of the children’s response to sensory stimuli mediates the relationship between attention, anxiety, and sympathetic nervous system recovery (Lane and Reynolds, 2019). Both sensory responsivity and poor reactive control aspect of emotional dysregulation are related to the meso-limbic dopamine system, which has already been associated with the typical ADHD symptoms. Others have related the symptoms of hyperactivity and impulsivity to reactive control and stimulus-driven low level neural responses (Nigg, 2006), and sensory responsivity (Nigg et al., 2005). Some have even argued that heightened sensory sensitivity found in children with ADHD strengthens the connection between hyperactive/impulsive symptoms and emotional lability with three or more clinically impairing ADHD symptoms (DeSerisy et al., 2019).
Neurologically, these disruptions in the processing of somatosensation found in ADHD may be related to disruptions in disruptions in central nervous system inhibitory systems (Parush et al., 2007). The sensory dysregulation found in ADHD seems to share neural correlates between sensory symptoms and intrinsic brain functional connectivity in adults. These neural correlates were also associated with the severity of ADHD symptoms (Itahashi et al., 2020). More specifically, the corpus callosum may play a role in this sensory dysregulation as well, as DTI data from adults with ADHD indicates that the degree of sensory symptoms were parallel to white matter alteration in the large areas of the corpus callosum. Specifically, DTI parameter in the corpus callosum was negatively correlated with sensory sensitivity (Ohta et al., 2020).
4. Treatment strategies
4.1. Methylphenidate
Methylphenidate (MPH) is one of most commonly prescribed ADHD medications and is highly efficacious in treating ADHD symptoms (Pliszka et al., 2017). MPH prevents the reuptake of norepinephrine and dopamine within their respective neuroregulation systems (Hannestad et al., 2010; Ihezie et al., 2019). Consequently, MPH reduces resting state functional connectivity (RSFCs) in a large network involving cortical regions, cerebellar regions, and the visual, executive, and default mode networks. In adolescents with ADHD, connectivity in these regions may be heightened; MPH may be “normalizing” these ADHD individuals RSFCs (Silk et al., 2017). Chronic administration of MPH does not appear to increase addictive tendencies (Robbins, 2002) likely because MPH does not promote electrophysiological dopamine signal bursts like with other illicit stimulants (Swanson and Volkow, 2002). MPH does not appear to cause long-term consequences on learning, memory, or social behavior (Martin et al., 2018), and its effects are reversible (Silk et al., 2017).
4.1.1. Acute administration of methylphenidate
Both acute and chronic MPH administration increases activity in the Ventral Tegmental Area (VTA) and Locus Coeruleus (LC) (Karim et al., 2017). Acute MPH application normalizes striatal dopamine levels (Aarts et al., 2015) and has age-dependent effects (Schrantee et al., 2017). Acute MPH appears to have widespread effects on neural connectivity. The acute administration of the drug alters both LC and VTA/Substantia Nigra Pars Compacta connectivity between several brain regions (Kline et al., 2016). However, acute MPH application has a dose-dependent effects on LC firing, with low dose suppressing LC discharge (Devilbiss and Berridge, 2006), while typical dosing increasing LC firing rate in rats (Tang and Dafny, 2012). Overall, acute MPH application may have age- and dose-dependent effects on the dopaminergic and noradrenergic systems, which should be accounted for when determining the most effective therapeutic dosage.
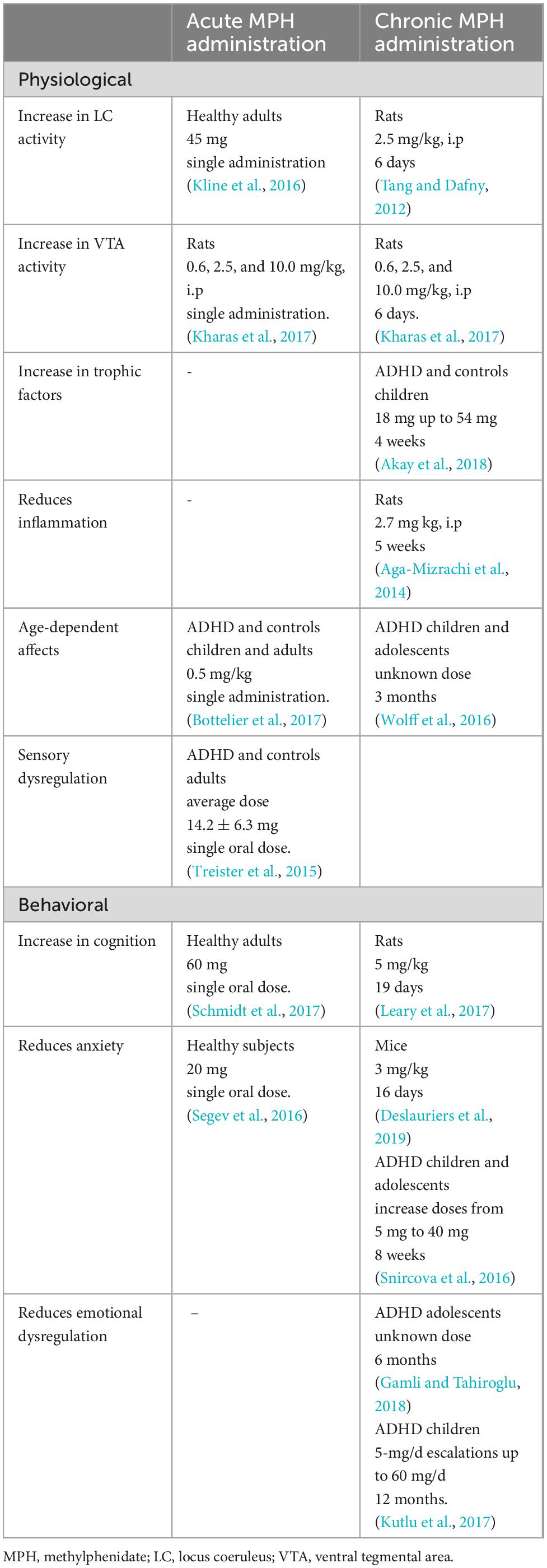
Acute MPH has a higher efficacy in treating attentional symptoms than impulsive symptoms (Dougherty et al., 2016). Through the improvement of attention, acute MPH improves cognitive performance even in healthy individuals (Schmidt et al., 2017), perhaps by reducing noradrenergic and dopaminergic dysfunction (Howlett et al., 2017). Acute MPH may rescue this noradrenergic and dopaminergic dysfunction through enhancing the transport of amino acids involved in NE and DA metabolism (Quansah et al., 2017a). Acute MPH application normalizes pupil size, a correlate of the LC-NE system, during attentional performance in children with ADHD (Wainstein et al., 2017). During reward sustained attention tasks, acute administration of MPH regulated the increased activation in the orbitofrontal cortex in children with ADHD (Rubia et al., 2009). Furthermore, acute administration of MPH has been found to reduce right amygdala reactivity in both children and adults with ADHD, but reduces left amygdala reactivity only in adults. These age-dependent effects of acute MPH on amygdala reactivity are important to note, as amygdala reactivity is one of the functional neural mechanisms underlying emotional processing (Bottelier et al., 2017). As noted above, the amygdala changes significantly throughout development, which may cause age-dependent effects of the medication on amygdalar functioning. Acute doses of MPH appear to be anxiolytic (Segev et al., 2016; Lelieveld et al., 2019), and alter reactions to fear stimuli (Ritov and Richter-Levin, 2017). Many studies find MPH induces locomotion, which suggest the drug is anxiogenic, but this heightened locomotion is likely independent of anxiety-like behaviors (Boyette-Davis et al., 2018); in humans, enhanced somatomotor functional connectivity to the thalamus and striatum, is increasing motor function (Farr et al., 2014), offering a potential mechanism in which MPH increases motor function without an increase in anxiety. Nonetheless, the anxiolytic mechanism of MPH is up for debate. MPH improves cognition, which may allow for anxiety to be processed cognitively, rather than the drug being directly anxiolytic (Ernst et al., 2016). Interestingly, acute MPH application may exert anti-nociceptive properties on adults with ADHD, who had increased sensitivity to pain compared to controls, indicating a potential therapeutic relation between MPH and sensory dysregulation found in ADHD as well (Treister et al., 2015). Ultimately, even acute doses of MPH reduce anxiety, a component of emotional dysregulation, and appear to stabilize some aspects of sensory dysregulation, potentially relating to acute MPH application as a therapeutic treatment of some aspects of sensory and emotional dysregulation found in ADHD. A summarization comparing the effects of acute and chronic MPH application has been supplied in Table 1.
4.1.2. Chronic administration of methylphenidate
Chronic MPH administration increases neuroplasticity in a variety of brain regions (Simchon-Tenenbaum et al., 2015; Quansah et al., 2017b) and upregulates expression of genes associated with plasticity and dendritic spine formation (Quansah et al., 2017b). MPH has been shown to increase trophic factors, specifically BDNF serum levels in both ADHD individuals and healthy controls (Akay et al., 2018), which may be responsible for the increase in neuroplasticity and cognition. Chronic MPH application at the typical dose increases LC firing in rats (Tang and Dafny, 2012), which is to be expected, an MPH increases NE release. Dopaminergically, chronic application of MPH increases D1 receptor expression in the striatum in rats, and increases D2 receptor expression in female rats (Izquierdo et al., 2016). More generally, MPH also reverses high levels of inflammation (Aga-Mizrachi et al., 2014), which may indirectly reduce overall ADHD symptoms, as high levels of inflammation are associated with ADHD (Dunn et al., 2019). In an age-dependent manner, chronic MPH application has been shown to increase blood flow within the thalamus and striatum of older children, but not adults (Schrantee et al., 2016). Additionally, the adolescent pFC is more susceptible to neuromodulation from chronic MPH use than adults (Venkataraman et al., 2019) and affects boy’s white matter more than men’s (Bouziane et al., 2019). These age-dependent, neurological effects of MPH mirror the developmental trajectory of ADHD.
After MPH treatment, adolescents with ADHD report improved quality of life (Karci et al., 2018). Regarding sensory dysregulation, pain perception in children and adolescents with ADHD is regulated with chronic MPH application (Wolff et al., 2016). Furthermore, the stimulant lessens anxiety (Snircova et al., 2016; Deslauriers et al., 2019; Jager et al., 2019), and has been shown to decrease emotional dysregulation in children with ADHD (Kutlu et al., 2017; Gamli and Tahiroglu, 2018; Ventura et al., 2022) potentially through reducing impulsivity (Gamli and Tahiroglu, 2018). MPH may lessen anxiety through the reduction of dopaminergic functional connectivity between the amygdala and the rostral anterior cingulate cortex (Berry et al., 2019). Chronic administration of MPH also reported to decrease blood flow to the orbitofrontal cortex in children with ADHD, providing an additional mean through which MPH may reduce anxiety (Lee et al., 2005). While the potential efficacy of MPH on emotional and sensory dysregulation warrants additional investigation, the role of ADHD medications with different mechanisms of action than MPH may elucidate on differing neurobiological mechanisms of these dysregulations as well.
5. Conclusion
The current core ADHD symptoms are comprised of inattention, hyperactivity, and impulsivity (American Psychiatric Association [APA], 2013). Despite not being current diagnostic criterion, emotional and sensory dysregulation appear to be key components of ADHD as well (Dunn and Bennett, 2002; Ghanizadeh, 2011; Retz et al., 2012; Bijlenga et al., 2017; Bunford et al., 2018). Table 2 describes potential objective, physiological diagnostic tests for emotional dysregulation, sensory dysregulation, and attentional dysregulation found in ADHD. The pupil reflex test, correlates pupil constriction and subsequent dilation in response to light to attentional dysregulation (Wainstein et al., 2017) and autonomic dysfunction (Hamrakova et al., 2019, 2020; Bellato et al., 2021) in ADHD. Additionally, if we further explore the relation between emotional and sensory dysregulation and the classical ADHD symptoms, more potential diagnostic practices may arise.
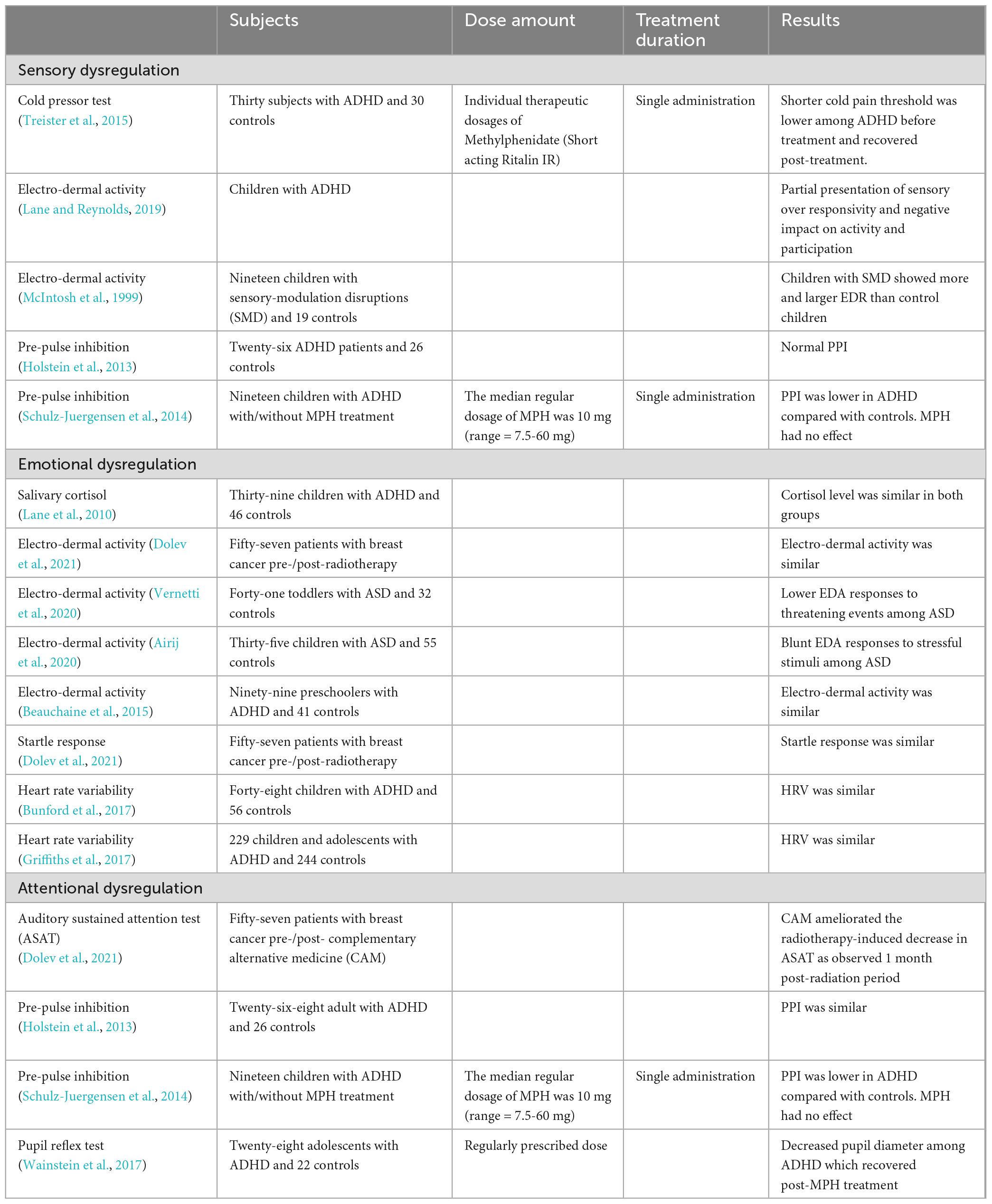
Table 2. Potential physiological diagnostic tests for attention deficit hyperactivity disorder: Studies mentioned evaluated sensory, emotional and attentional dysregulation.
Emotional and sensory dysregulation may give rise to the current core symptoms of ADHD, which in turn induce higher order, classical symptoms. For instance, dysregulated emotions and improperly processed sensory input is quite distracting of itself. Sensorimotor gating is a preconscious regulator of attention, and is why the pre-pulse inhibition of startle is often impaired in disorders with attentional abnormalities (Feifel et al., 2009), and visuospatial working memory may underlie choice-impulsivity in ADHD (Patros et al., 2015). Emotional and sensory dysregulation can induce higher emotionality which in turn can increase impulsivity. Research supports this hypothesis, as higher levels of sensory issues in ADHD can predict aggression levels (Mangeot et al., 2001), impulsivity and hyperactivity can predict the severity of emotional dysregulation in ADHD (Groves et al., 2020), and heightened sensory sensitivity strengthens the relationship between hyperactive/impulsive symptoms and emotional dysregulation in children with three or more clinically impairing ADHD symptoms (DeSerisy et al., 2019). Therefore, we suggest that emotional and sensory dysregulation give rise to the core classical symptoms of ADHD of impulsivity, inattention, and hyperactivity.
On the treatment end, we conclude that MPH mitigates ADHD symptoms once they have developed. Acute and chronic MPH administration has beneficial effects on ADHD symptomatology (Dougherty et al., 2016; Pliszka et al., 2017; Karci et al., 2018). Acute and chronic administration of MPH in those with ADHD reduces sensory dysregulation (Durukan et al., 2011; Treister et al., 2015; Shang et al., 2020), emotional dysregulation (Segev et al., 2016; Kutlu et al., 2017; Lelieveld et al., 2019; Ventura et al., 2020). Hypothetically, as above-discussed, MPH treatment effectiveness on the ‘classical’ ADHD symptoms may be due to its’ additional role in lessening sensory and emotional dysregulation, preventing the development of higher-order symptoms of impulsivity, hyperactivity, and inattention. Overall, MPH proves to be an appropriate, relatively safe therapy for ADHD, normalizing the neurological and behavioral deficits found in the disorder. Further clinical measures may be taken in both the evaluation and treatment of ADHD, with an integration of sensory and emotional dysregulation into the diagnostic process and the application of psychotherapies targeting these dysregulations a well in populations with ADHD.
These results lead us to conclude that: (i) developmental stress may correlate with an increased risk of ADHD development, potentially mediated by the increase of sensory and emotional dysregulation; (ii) This increased sensory and emotional dysregulation give rise to the ‘classical’ ADHD symptoms of inattention, impulsivity, and hyperactivity. These symptoms may be diagnosed through (iii) objective, physiological means, and finally (iv) mitigated with MPH application, and, following further research, possibly also by treatments aimed at these dysregulation processes. To properly further aid ADHD individuals, further research is required to better define causes, symptoms and preventative techniques. Further research is also needed to determine the role of early-life stress effects on the development of ADHD, particularly as there is limited clinical evidence for the causative role of early-life stress on ADHD development. This creates a limitation within our review paper, as the lack of research limits the potential number of references. A deeper investigation into the additional suggested symptoms of ADHD (emotional and sensory dysregulation) beyond the current core symptoms of impulsivity, hyperactivity, and inattention, bares an important potential in improving both ADHD diagnosis (and differential diagnosis) and treatment.
In sum, better ADHD treatment strategies may be achieved by the inclusion of further research on (i) developmental stress on ADHD development, (ii) emotional and sensory dysregulation as potential ADHD additional symptoms, and finally (iii) the effects MPH on emotional and sensory dysregulation in ADHD populations. This new and comprehensive prism could lead to new tools for diagnosing and treating individuals with ADHD.
Author contributions
AG surveyed the literature, drafted the manuscript, and co-wrote the conclusion. AA conceptualized the manuscript, co-wrote the conclusion, and edited previous versions into the final one. Both authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Dr. Janne L. Punski-Hoogervorst for her important feedback on the manuscript.
Conflict of interest
AA is a scientific consultant of Mindtension, Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarts, E., Van Holstein, M., Hoogman, M., Onnink, M., Kan, C., Franke, B., et al. (2015). Reward modulation of cognitive function in adult attention-deficit/hyperactivity disorder: a pilot study on the role of striatal dopamine. Behav. Pharmacol. 26, 227–240. doi: 10.1097/FBP.0000000000000116
Aga-Mizrachi, S., Cymerblit-Sabba, A., Gurman, O., Balan, A., Shwam, G., Deshe, R., et al. (2014). Methylphenidate and desipramine combined treatment improves PTSD symptomatology in a rat model. Transl. Psychiatry 4, e447–e449. doi: 10.1038/tp.2014.82
Airij, A. G., Sudirman, R., Sheikh, U. U., Khuan, L. Y., and Zakaria, N. A. (2020). Significance of electrodermal activity response in children with autism spectrum disorder. Indonesian J. Electr. Eng. Comput. Sci. 19, 1113–1120. doi: 10.11591/ijeecs.v19.i2.pp1113-1120
Akay, A. P., Resmi, H., Güney, S. A., Erkuran, H. Ö, Özyurt, G., Sargin, E., et al. (2018). Serum brain-derived neurotrophic factor levels in treatment-naïve boys with attention-deficit/hyperactivity disorder treated with methylphenidate: an 8-week, observational pretest–posttest study. Eur. Child Adolesc. Psychiatry 27, 127–135. doi: 10.1007/s00787-017-1022-y
American Psychiatric Association [APA]. (2013). Diagnostic and statistical manual of mental disorders. Virginia: American Psychiatric Association. doi: 10.1176/appi.books.9780890425596
Beauchaine, T. P., Neuhaus, E., Gatzke-Kopp, L. M., Reid, M. J., Chipman, J., Brekke, A., et al. (2015). Electrodermal responding predicts responses to, and may be altered by, preschool intervention for ADHD. J. Consult. Clin. Psychol. 83, 293–303. doi: 10.1037/a0038405
Bellato, A., Arora, I., Kochhar, P., Ropar, D., Hollis, C., and Groom, M. J. (2021). Heart rate variability in children and adolescents with autism, ADHD and co-occurring Autism and ADHD, during passive and active experimental conditions. J. Autism Dev. Disord. 52, 4679–4691. doi: 10.1007/s10803-021-05244-w
Berry, A. S., White, R. L., Furman, D. J., Naskolnakorn, J. R., Shah, V. D., D’Esposito, M., et al. (2019). Dopaminergic mechanisms underlying normal variation in trait anxiety. J. Neurosci. 39, 2735–2744. doi: 10.1523/JNEUROSCI.2382-18.2019
Bijlenga, D., Tjon-Ka-Jie, J. Y. M., Schuijers, F., and Kooij, J. J. S. (2017). Atypical sensory profiles as core features of adult ADHD, irrespective of autistic symptoms. Eur. Psychiatry 43, 51–57. doi: 10.1016/j.eurpsy.2017.02.481
Bloch, Y., Aviram, S., Segev, A., Nitzan, U., Levkovitz, Y., Braw, Y., et al. (2017). Methylphenidate reduces state anxiety during a continuous performance test that distinguishes adult ADHD patients from controls. J. Atten. Disord. 21, 46–51. doi: 10.1177/1087054712474949
Bock, J., Breuer, S., Poeggel, G., and Braun, K. (2017). Early life stress induces attention-deficit hyperactivity disorder (ADHD)-like behavioral and brain metabolic dysfunctions: functional imaging of methylphenidate treatment in a novel rodent model. Brain Struct. Funct. 222, 765–780. doi: 10.1007/s00429-016-1244-7
Bottelier, M. A., Schrantee, A., Ferguson, B., Tamminga, H. G. H., Bouziane, C., Kooij, J. J. S., et al. (2017). Age-dependent effects of acute methylphenidate on amygdala reactivity in stimulant treatment-naive patients with Attention Deficit/Hyperactivity Disorder. Psychiatry Res. Neuroimaging 269, 36–42. doi: 10.1016/j.pscychresns.2017.09.009
Bouziane, C., Filatova, O. G., Schrantee, A., Caan, M. W. A., Vos, F. M., and Reneman, L. (2019). White matter by diffusion MRI following methylphenidate treatment: a randomized control trial in males with attention-deficit/hyperactivity disorder. Radiology 293, 186–192. doi: 10.1148/radiol.2019182528
Boyette-Davis, J. A., Rice, H. R., Shoubaki, R. I., Gonzalez, C. M. F., Kunkel, M. N., Lucero, D. A., et al. (2018). A recreational dose of methylphenidate, but not methamphetamine, decreases anxiety-like behavior in female rats. Neurosci. Lett. 682, 21–26. doi: 10.1016/j.neulet.2018.06.005
Breaux, R. P., McQuade, J. D., Harvey, E. A., and Zakarian, R. J. (2018). Longitudinal associations of parental emotion socialization and children’s emotion regulation: the moderating role of ADHD symptomatology. J. Abnorm. Child Psychol. 46, 671–683. doi: 10.1007/s10802-017-0327-0
Brown, N. M., Brown, S. N., Briggs, R. D., Germán, M., Belamarich, P. F., and Oyeku, S. O. (2017). Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad. Pediatr. 17, 349–355. doi: 10.1016/j.acap.2016.08.013
Bruton, A. M., Senders, A., Tost, G., Ast, H., Robinette, L. M., Leung, B., et al. (2022). Pain sensitivity and perceptual sensitivity are associated with severity of emotional dysregulation in children with ADHD: a cross-sectional analysis using the temperament in middle childhood questionnaire. Disabil. Rehabil. 45, 848–856. doi: 10.1080/09638288.2022.2043946
Bunford, N., Evans, S. W., and Langberg, J. M. (2018). Emotion dysregulation is associated with social impairment among young adolescents with ADHD. J. Atten. Disord. 22, 66–82. doi: 10.1177/1087054714527793
Bunford, N., Evans, S. W., Zoccola, P. M., Owens, J. S., Flory, K., and Spiel, C. F. (2017). Correspondence between heart rate variability and emotion dysregulation in children, including children with ADHD. J. Abnorm. Child Psychol. 45, 1325–1337. doi: 10.1007/s10802-016-0257-2
Corbisiero, S., Mörstedt, B., Bitto, H., and Stieglitz, R. D. (2017). Emotional dysregulation in adults with attention-deficit/hyperactivity disorder–validity, predictability, severity, and comorbidity. J. Clin. Psychol. 73, 99–112. doi: 10.1002/jclp.22317
Cubillo, A., Halari, R., Smith, A., Taylor, E., and Rubia, K. (2012). A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex 48, 194–215. doi: 10.1016/j.cortex.2011.04.007
DeSerisy, M., Hirsch, E., and Roy, A. K. (2019). The contribution of sensory sensitivity to emotional lability in children with ADHD symptoms. Evid. Based Pract. Child Adolesc. Ment. Health 4, 319–327. doi: 10.1080/23794925.2019.1647122
Deslauriers, J., Toth, M., Zhou, X., and Risbrough, V. B. (2019). Heritable differences in catecholamine signaling modulate susceptibility to trauma and response to methylphenidate treatment: relevance for PTSD. Front. Behav. Neurosci. 13:111. doi: 10.3389/fnbeh.2019.00111
Devilbiss, D. M., and Berridge, C. W. (2006). Low-dose methylphenidate actions on tonic and phasic locus coeruleus discharge. J. Pharmacol. Exp. Ther. 319, 1327–1335. doi: 10.1124/jpet.106.110015
Dolev, T., Ben-David, M., Shahadi, I., Freed, Y., Zubedat, S., Aga-Mizrachi, S., et al. (2021). Attention dysregulation in breast cancer patients following a complementary alternative treatment routine: a double-blind randomized trial. Integr. Cancer Ther. 20:15347354211019470. doi: 10.1177/15347354211019470
Dougherty, D. M., Olvera, R. L., Acheson, A., Hill-Kapturczak, N., Ryan, S. R., and Mathias, C. W. (2016). Acute effects of methylphenidate on impulsivity and attentional behavior among adolescents comorbid for ADHD and conduct disorder. J. Adolesc. 53, 222–230. doi: 10.1016/j.adolescence.2016.10.013
Dunn, G. A., Nigg, J. T., and Sullivan, E. L. (2019). Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 182, 22–34. doi: 10.1016/j.pbb.2019.05.005
Dunn, W., and Bennett, D. (2002). Patterns of sensory processing in children with attention deficit hyperactivity disorder. Occup. Ther. J. Res. 22, 4–15.
Durukan, I., Yucel, M., Erdem, M., Kara, K., Oz, O., Karaman, D., et al. (2011). P50 sensory gating in children and adolescents with ADHD and effects of methylphenidate administration on P50 sensory gating. Klinik Psikofarmakoloji Bulteni 21, 42–48. doi: 10.5350/kpb-bcp201121107
Ernst, M., Lago, T., Davis, A., and Grillon, C. (2016). The effects of methylphenidate and propranolol on the interplay between induced-anxiety and working memory. Psychopharmacology 233, 3565–3574. doi: 10.1007/s00213-016-4390-y
Farr, O. M., Zhang, S., Hu, S., Matuskey, D., Abdelghany, O., Malison, R. T., et al. (2014). The effects of methylphenidate on resting-state striatal, thalamic and global functional connectivity in healthy adults. Int. J. Neuropsychopharmacol. 17, 1177–1191. doi: 10.1017/S1461145714000674
Feifel, D., Minassian, A., and Perry, W. (2009). Prepulse inhibition of startle in adults with ADHD. J. Psychiatr. Res. 43, 484–489. doi: 10.1016/j.jpsychires.2008.06.004
Gamli, I. S., and Tahiroglu, A. Y. (2018). Six months methylphenidate treatment improves emotion dysregulation in adolescents with attention deficit/hyperactivity disorder: a prospective study. Neuropsychiatr. Dis. Treat. 14, 1329–1337. doi: 10.2147/NDT.S164807
Ghanizadeh, A. (2011). Sensory processing problems in children with ADHD, a systematic review. Psychiatry Investig. 8, 89–94. doi: 10.4306/pi.2011.8.2.89
Griffiths, K. R., Quintana, D. S., Hermens, D. F., Spooner, C., Tsang, T. W., Clarke, S., et al. (2017). Sustained attention and heart rate variability in children and adolescents with ADHD. Biol. Psychol. 124, 11–20. doi: 10.1016/j.biopsycho.2017.01.004
Groves, N. B., Kofler, M. J., Wells, E. L., Day, T. N., and Chan, E. S. M. (2020). An examination of relations among working memory, ADHD symptoms, and emotion regulation. J. Abnorm. Child Psychol. 48, 525–537. doi: 10.1007/s10802-019-00612-8
Hamrakova, A., Ondrejka, I., Sekaninova, N., Bona Olexova, L., Visnovcova, Z., Cesnekova, D., et al. (2020). Central autonomic regulation assessed by pupillary light reflex is impaired in children with attention deficit hyperactivity disorder. Physiol. Res. 69, S513–S521. doi: 10.33549/PHYSIOLRES.934589
Hamrakova, A., Ondrejka, I., Sekaninova, N., Peregrim, L., and Tonhajzerova, I. (2019). Pupillary light reflex in children with ADHD. Acta Med. Martiniana 19, 30–37. doi: 10.2478/acm-2019-0004
Hannestad, J., Gallezot, J. D., Planeta-Wilson, B., Lin, S. F., Williams, W. A., Van Dyck, C. H., et al. (2010). Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol. Psychiatry 68, 854–860. doi: 10.1016/j.biopsych.2010.06.017
Heneghan, A., Stein, R. E. K., Hurlburt, M. S., Zhang, J., Rolls-Reutz, J., Fisher, E., et al. (2013). Mental health problems in teens investigated by U.S. child welfare agencies. J. Adolesc. Health 52, 634–640. doi: 10.1016/j.jadohealth.2012.10.269
Hirsch, O., Chavanon, M. L., Riechmann, E., and Christiansen, H. (2018). Emotional dysregulation is a primary symptom in adult attention-deficit/hyperactivity disorder (ADHD). J. Affect. Disord. 232, 41–47. doi: 10.1016/j.jad.2018.02.007
Holstein, D. H., Vollenweider, F. X., Geyer, M. A., Csomor, P. A., Belser, N., and Eich, D. (2013). Sensory and sensorimotor gating in adult attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 205, 117–126. doi: 10.1016/j.psychres.2012.08.013
Howlett, J. R., Huang, H., Hysek, C. M., and Paulus, M. P. (2017). The effect of single-dose methylphenidate on the rate of error-driven learning in healthy males: a randomized controlled trial. Psychopharmacology 234, 3353–3360. doi: 10.1007/s00213-017-4723-5
Humphreys, K. L., and Zeanah, C. H. (2015). Deviations from the expectable environment in early childhood and emerging psychopathology. Neuropsychopharmacology 40, 154–170. doi: 10.1038/npp.2014.165
Humphreys, K. L., Watts, E. L., Dennis, E. L., King, L. S., Thompson, P. M., and Gotlib, I. H. (2019). Stressful life events, ADHD symptoms, and brain structure in early adolescence. J. Abnorm. Child Psychol. 47, 421–432. doi: 10.1007/s10802-018-0443-5
Hutchison, A. K., Hunter, S. K., Wagner, B. D., Calvin, E. A., Zerbe, G. O., and Ross, R. G. (2017). Diminished infant P50 sensory gating predicts increased 40-month-old attention, anxiety/depression, and externalizing symptoms. J. Atten. Disord. 21, 209–218. doi: 10.1177/1087054713488824
Ihezie, S. A., Thomas, M. M., and Dafny, N. (2019). Acute and chronic methylphenidate administration in intact and VTA-specific and nonspecific lesioned rats. J. Neural Transm. 126, 173–182. doi: 10.1007/s00702-018-1963-4
Ishikawa, J., Nishimura, R., and Ishikawa, A. (2015). Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. Eur. J. Neurosci. 41, 442–453. doi: 10.1111/ejn.12825
Itahashi, T., Fujino, J., Sato, T., Ohta, H., Nakamura, M., Kato, N., et al. (2020). Neural correlates of shared sensory symptoms in autism and attention-deficit/hyperactivity disorder. Brain Commun. 2:fcaa186. doi: 10.1093/braincomms/fcaa186
Izquierdo, A., Pozos, H., De La Torre, A., DeShields, S., Cevallos, J., Rodriguez, J., et al. (2016). Sex differences, learning flexibility, and striatal dopamine D1 and D2 following adolescent drug exposure in rats. Behav. Brain Res. 308, 104–114. doi: 10.1016/j.bbr.2016.04.028
Jager, A., Kanters, D., Geers, F., Buitelaar, J. K., Kozicz, T., and Glennon, J. C. (2019). Methylphenidate dose-dependently affects aggression and improves fear extinction and anxiety in BALB/cJ mice. Front. Psychiatry 10:768. doi: 10.3389/fpsyt.2019.00768
Jeon, M. S., and Bae, E. B. (2022). Emotions and sensory processing in adolescents: the effect of childhood traumatic experiences. J. Psychiatr. Res. 151, 136–143. doi: 10.1016/j.jpsychires.2022.03.054
Karci, C. K., Toros, F., Tahiroglu, A. Y., and Metin, O. (2018). Effects of methylphenidate treatment on quality of life in adolescents. Dusunen Adam 31, 11–20. doi: 10.5350/DAJPN2018310101
Karim, T. J., Reyes-Vazquez, C., and Dafny, N. (2017). Comparison of the VTA and LC response to methylphenidate: a concomitant behavioral and neuronal study of adolescent male rats. J. Neurophysiol. 118, 1501–1514. doi: 10.1152/jn.00145.2017
Kennedy, M., Kreppner, J., Knights, N., Kumsta, R., Maughan, B., Golm, D., et al. (2016). Early severe institutional deprivation is associated with a persistent variant of adult attention-deficit/hyperactivity disorder: clinical presentation, developmental continuities and life circumstances in the English and Romanian Adoptees study. J. Child Psychol. Psychiatry 57, 1113–1125. doi: 10.1111/jcpp.12576
Kharas, N., Reyes-Vazquez, C., and Dafny, N. (2017). Locus coeruleus neuronal activity correlates with behavioral response to acute and chronic doses of methylphenidate (Ritalin) in adolescent rats. J. Neural Transm. 124, 1239–1250. doi: 10.1007/s00702-017-1760-5
Kline, R. L., Zhang, S., Farr, O. M., Hu, S., Zaborszky, L., Samanez-Larkin, G. R., et al. (2016). The effects of methylphenidate on resting-state functional connectivity of the basal nucleus of meynert, locus coeruleus, and ventral tegmental area in healthy adults. Front. Hum. Neurosci. 10:149. doi: 10.3389/fnhum.2016.00149
Kutlu, A., Akyol Ardic, U., and Ercan, E. S. (2017). Effect of methylphenidate on emotional dysregulation in children with attention-deficit/hyperactivity disorder + oppositional defiant disorder/conduct disorder. J. Clin. Psychopharmacol. 37, 220–225. doi: 10.1097/JCP.0000000000000668
Lane, S. J., and Reynolds, S. (2019). Sensory over-responsivity as an added dimension in ADHD. Front. Integr. Neurosci. 13:40. doi: 10.3389/fnint.2019.00040
Lane, S. J., Reynolds, S., and Thacker, L. (2010). Sensory over-responsivity and ADHD: differentiating using electrodermal responses, cortisol, and anxiety. Front. Integr. Neurosci. 4:8. doi: 10.3389/fnint.2010.00008
Leary, J. B., Bondi, C. O., Laporte, M. J., Carlson, L. J., Radabaugh, H. L., Cheng, J. P., et al. (2017). The therapeutic efficacy of environmental enrichment and methylphenidate alone and in combination after controlled cortical impact injury. J. Neurotrauma 34, 444–450. doi: 10.1089/neu.2016.4438
Lee, J. S., Kim, B. N., Kang, E., Lee, D. S., Kim, Y. K., Chung, J. K., et al. (2005). Regional cerebral blood flow in children with attention deficit hyperactivity disorder: comparison before and after methylphenidate treatment. Hum. Brain Mapp. 24, 157–164. doi: 10.1002/hbm.20067
Lelieveld, I., Storre, G., and Ziabari, S. S. M. (2019). “A temporal cognitive model of the influence of methylphenidate (Ritalin) on test anxiety,” in Proceedings of the 4th International congress on information and communication, (London: Springer).
Lenzi, F., Cortese, S., Harris, J., and Masi, G. (2018). Pharmacotherapy of emotional dysregulation in adults with ADHD: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 84, 359–367. doi: 10.1016/j.neubiorev.2017.08.010
Mangeot, S. D., Miller, L. J., Mclntosh, D. N., McGrath-Clarke, J., Simon, J., Hagerman, R. J., et al. (2001). Sensory modulation dysfunction in children with attention-deficit-hyperactivity disorder. Dev. Med. Child Neurol. 43, 399–406. doi: 10.1017/s0012162201000743
Manos, M. J., Short, E. J., and Findling, R. L. (1999). Differential effectiveness of methylphenidate and Adderall® in school- age youths with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 38, 813–819. doi: 10.1097/00004583-199907000-00010
Martin, C., Fricke, D., Vijayashanthar, A., Lowinger, C., Koutsomitis, D., Popoola, D., et al. (2018). Recovery from behavior and developmental effects of chronic oral methylphenidate following an abstinence period. Pharmacol. Biochem. Behav. 172, 22–32. doi: 10.1016/j.pbb.2018.07.001
Martine Hoogman, P. (2017). Subcortical brain volume differences of participants with ADHD across the lifespan: an ENIGMA collaboration. Lancet Psychiatry 4, 310–319. doi: 10.1016/S2215-0366(17)30049-4.Subcortical
McIntosh, D. N., Miller, L. J., Shyu, V., and Hagerman, R. J. (1999). Sensory-modulation disruption, electrodermal responses, and functional behaviors. Dev. Med. Child Neurol. 41, 608–615. doi: 10.1017/S0012162299001267
Nigg, J. T. (2006). Temperament and developmental psychopathology. J. Child Psychol. Psychiatry 47, 395–422. doi: 10.1111/j.1469-7610.2006.01612.x
Nigg, J. T., Karalunas, S. L., Gustafsson, H. C., Bhatt, P., Ryabinin, P., Mooney, M. A., et al. (2020). Evaluating chronic emotional dysregulation and irritability in relation to ADHD and depression genetic risk in children with ADHD. J. Child Psychol. Psychiatry 61, 205–214. doi: 10.1111/jcpp.13132
Nigg, J. T., Willcutt, E. G., Doyle, A. E., and Sonuga-Barke, E. J. S. (2005). Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol. Psychiatry 57, 1224–1230. doi: 10.1016/j.biopsych.2004.08.025
Ohta, H., Aoki, Y. Y., Itahashi, T., Kanai, C., Fujino, J., Nakamura, M., et al. (2020). White matter alterations in autism spectrum disorder and attention-deficit/hyperactivity disorder in relation to sensory profile. Mol. Autism 11:77. doi: 10.1186/s13229-020-00379-6
Panagiotidi, M., Overton, P. G., and Stafford, T. (2018). The relationship between ADHD traits and sensory sensitivity in the general population. Compr. Psychiatry 80, 179–185. doi: 10.1016/j.comppsych.2017.10.008
Parush, S., Sohmer, H., Steinberg, A., and Kaitz, M. (2007). Somatosensory function in boys with ADHD and tactile defensiveness. Physiol. Behav. 90, 553–558. doi: 10.1016/j.physbeh.2006.11.004
Patros, C. H. G., Alderson, R. M., Lea, S. E., Tarle, S. J., Kasper, L. J., and Hudec, K. L. (2015). Visuospatial working memory underlies choice-impulsivity in boys with attention-deficit/hyperactivity disorder. Res. Dev. Disabil. 38, 134–144. doi: 10.1016/j.ridd.2014.12.016
Pelham, W. E., Gnagy, E. M., Chronis, A. M., Burrows-MacLean, L., Fabiano, G. A., Onyango, A. N., et al. (1999). A comparison of morning-only and morning/late afternoon Adderall to morning-only, twice-daily, and three times-daily methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 104, 1300–11. doi: 10.1542/peds.104.6.1300
Piñeiro-Dieguez, B., Balanzá-Martínez, V., García-García, P., Soler-López, B., Domingo, M. A., Labarra, J. D. A., et al. (2016). Psychiatric comorbidity at the time of diagnosis in adults with ADHD: the CAT study. J. Atten. Disord. 20, 1066–1075. doi: 10.1177/1087054713518240
Pliszka, S. R., Wilens, T. E., Bostrom, S., Arnold, V. K., Marraffino, A., Cutler, A. J., et al. (2017). Efficacy and safety of HLD200, delayed-release and extended-release methylphenidate, in children with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 27, 474–482. doi: 10.1089/cap.2017.0084
Polanczyk, G., De Lima, M. S., Horta, B. L., Biederman, J., and Rohde, L. A. (2007). The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry 164, 942–948. doi: 10.1176/ajp.2007.164.6.942
Quansah, E., Ruiz-Rodado, V., Grootveld, M., Probert, F., and Zetterström, T. S. C. (2017a). 1H NMR-based metabolomics reveals neurochemical alterations in the brain of adolescent rats following acute methylphenidate administration. Neurochem. Int. 108, 109–120. doi: 10.1016/j.neuint.2017.03.003
Quansah, E., Sgamma, T., Jaddoa, E., and Zetterström, T. S. C. (2017b). Chronic methylphenidate regulates genes and proteins mediating neuroplasticity in the juvenile rat brain. Neurosci. Lett. 654, 93–98. doi: 10.1016/j.neulet.2017.06.012
Reimherr, F. W., Marchant, B. K., Gift, T. E., and Steans, T. A. (2017). ADHD and anxiety: clinical significance and treatment implications. Curr. Psychiatry Rep. 19:109. doi: 10.1007/s11920-017-0859-6
Reimherr, F. W., Marchant, B. K., Strong, R. E., Hedges, D. W., Adler, L., Spencer, T. J., et al. (2005). Emotional dysregulation in adult ADHD and response to atomoxetine. Biol. Psychiatry 58, 125–131. doi: 10.1016/j.biopsych.2005.04.040
Retz, W., Stieglitz, R. D., Corbisiero, S., Retz-Junginger, P., and Rösler, M. (2012). Emotional dysregulation in adult ADHD: what is the empirical evidence? Expert Rev. Neurother. 12, 1241–1251. doi: 10.1586/ern.12.109
Ritov, G., and Richter-Levin, G. (2017). Pre-trauma methylphenidate in rats reduces PTSD-like reactions one month later. Transl. Psychiatry 7:e1000. doi: 10.1038/tp.2016.277
Rubia, K., Halari, R., Cubillo, A., Mohammad, A. M., Brammer, M., and Taylor, E. (2009). Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology 57, 640–652. doi: 10.1016/j.neuropharm.2009.08.013
Rutter, M., Sonuga-Barke, E. J., and Castle, J. I. (2010). Investigating the impact of early institutional deprivation on development: background and research strategy of the English and Romanian adoptees (ERA) study. Monogr. Soc. Res. Child Dev. 75, 1–20. doi: 10.1111/j.1540-5834.2010.00548.x
Sandler, A. D. (2001). Sensory modulation dysfunction in children with attention-deficit hyperactivity disorder. J. Dev. Behav. Pediatr. 22:449. doi: 10.1097/00004703-200112000-00021
Schmidt, A., Müller, F., Dolder, P. C., Schmid, Y., Zanchi, D., Liechti, M. E., et al. (2017). Comparative effects of methylphenidate, modafinil, and mdma on response inhibition neural networks in healthy subjects. Int. J. Neuropsychopharmacol. 20, 712–720. doi: 10.1093/ijnp/pyx037
Schrantee, A., Mutsaerts, H. J. M. M., Bouziane, C., Tamminga, H. G. H., Bottelier, M. A., and Reneman, L. (2017). The age-dependent effects of a single-dose methylphenidate challenge on cerebral perfusion in patients with attention-deficit/hyperactivity disorder. Neuroimage Clin. 13, 123–129. doi: 10.1016/j.nicl.2016.11.021
Schrantee, A., Tamminga, H. G. H., Bouziane, C., Bottelier, M. A., Bron, E. E., Mutsaerts, H. J. M. M., et al. (2016). Age-dependent effects of methylphenidate on the human dopaminergic system in young vs adult patients with attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry 73, 955–962. doi: 10.1001/jamapsychiatry.2016.1572
Schulz-Juergensen, S., Thiemann, A., Gebhardt, J., Baumgarten-Walczak, A., and Eggert, P. (2014). Prepulse inhibition of acoustic startle and the influence of methylphenidate in children with ADHD. J. Atten. Disord. 18, 117–122. doi: 10.1177/1087054712448960
Segev, A., Gvirts, H. Z., Strouse, K., Mayseless, N., Gelbard, H., Lewis, Y. D., et al. (2016). A possible effect of methylphenidate on state anxiety: a single dose, placebo controlled, crossover study in a control group. Psychiatry Res. 241, 232–235. doi: 10.1016/j.psychres.2016.05.009
Shang, C. Y., Shih, H. H., Pan, Y. L., Lin, H. Y., and Gau, S. S. F. (2020). Comparative efficacy of methylphenidate and atomoxetine on social adjustment in youths with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 30, 148–158. doi: 10.1089/cap.2019.0139
Shaw, P., Stringaris, A., Nigg, J., and Leibenluft, E. (2014). Emotion dysregulation in attention deficit hyperactivity disorder. Am. J. Psychiatry 171, 276–293. doi: 10.1176/appi.ajp.2013.13070966
Sibley, M. H., Ortiz, M., Graziano, P., Dick, A., and Estrada, E. (2020). Metacognitive and motivation deficits, exposure to trauma, and high parental demands characterize adolescents with late-onset ADHD. Eur. Child. Adolesc. Psychiatry [Internet]. 29:537–548. doi: 10.1007/s00787-019-01382-w
Silk, T. J., Malpas, C., Vance, A., and Bellgrove, M. A. (2017). The effect of single-dose methylphenidate on resting-state network functional connectivity in ADHD. Brain Imaging Behav. 11, 1422–1431. doi: 10.1007/s11682-016-9620-8
Simchon-Tenenbaum, Y., Weizman, A., and Rehavi, M. (2015). Alterations in brain neurotrophic and glial factors following early age chronic methylphenidate and cocaine administration. Behavioural Brain Research 282, 125–132. doi: 10.1016/j.bbr.2014.12.058
Singer, M. J., Humphreys, K. L., and Lee, S. S. (2016). Coping self-efficacy mediates the association between child abuse and ADHD in adulthood. J. Atten. Disord. 20, 695–703. doi: 10.1177/1087054712465337
Snircova, E., Marcincakova-Husarova, V., Hrtanek, I., Kulhan, T., Ondrejka, I., and Nosalova, G. (2016). Anxiety reduction on atomoxetine and methylphenidate medication in children with ADHD. Pediatr. Int. 58, 476–481. doi: 10.1111/ped.12847
Swanson, J. M., and Volkow, N. D. (2002). Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav. Brain Res. 130, 73–78. doi: 10.1016/S0166-4328(01)00433-8
Tang, B., and Dafny, N. (2012). Methylphenidate modulates the locus ceruleus neuronal activity in freely behaving rat. Eur. J. Pharmacol. 695, 48–56. doi: 10.1016/j.ejphar.2012.08.016
Thomas, R., Sanders, S., Doust, J., Beller, E., and Glasziou, P. (2015). Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 135, e994–e1001. doi: 10.1542/peds.2014-3482
Thompson, R. A. (1994). Emotional regulation: a theme in search of definition. Monogr. Soc. Res. Child Dev. 59, 25–52. doi: 10.1111/j.1540-5834.1994.tb01276.x
Treister, R., Eisenberg, E., Demeter, N., and Pud, D. (2015). Alterations in pain response are partially reversed by methylphenidate (Ritalin) in adults with attention deficit hyperactivity disorder (ADHD). Pain Pract. 15, 4–11. doi: 10.1111/papr.12129
van Stralen, J. (2016). Emotional dysregulation in children with attention-deficit/hyperactivity disorder. Attent. Defic. Hyperact. Disord. 8, 175–187. doi: 10.1007/s12402-016-0199-0
Venkataraman, S. S., Joseph, M., and Dafny, N. (2019). Concomitant behavioral and prefrontal cortex neuronal responses following acute and chronic methylphenidate exposure in adolescent and adult rats. Brain Res. Bull. 144, 200–212. doi: 10.1016/j.brainresbull.2018.11.004
Ventura, P., de Giambattista, C., Spagnoletta, L., Trerotoli, P., Cavone, M., di Gioia, A., et al. (2020). Methylphenidate in autism spectrum disorder: a long-term follow up naturalistic study. J. Clin. Med. 9:2566. doi: 10.3390/jcm9082566
Ventura, P., de Giambattista, C., Trerotoli, P., Cavone, M., di Gioia, A., and Margari, L. (2022). Methylphenidate use for emotional dysregulation in children and adolescents with ADHD and ADHD and ASD: a naturalistic study. J. Clin. Med. 11:2922. doi: 10.3390/jcm11102922
Vernetti, A., Shic, F., Boccanfuso, L., Macari, S., Kane-Grade, F., Milgramm, A., et al. (2020). Atypical emotional electrodermal activity in toddlers with autism spectrum disorder. Autism Res. 13, 1476–1488. doi: 10.1002/aur.2374
Vrijsen, J. N., Tendolkar, I., Onnink, M., Hoogman, M., Schene, A. H., Fernández, G., et al. (2018). ADHD symptoms in healthy adults are associated with stressful life events and negative memory bias. Attent. Defic. Hyperact. Disord. 10, 151–160. doi: 10.1007/s12402-017-0241-x
Wainstein, G., Rojas-Líbano, D., Crossley, N. A., Carrasco, X., Aboitiz, F., and Ossandón, T. (2017). Pupil size tracks attentional performance in attention-deficit/hyperactivity disorder. Sci. Rep. 7:8228. doi: 10.1038/s41598-017-08246-w
Wolff, N., Rubia, K., Knopf, H., Hölling, H., Martini, J., Ehrlich, S., et al. (2016). Reduced pain perception in children and adolescents with ADHD is normalized by methylphenidate. Child Adolesc. Psychiatry Ment. Health 10:24. doi: 10.1186/s13034-016-0112-9
Wüstner, A., Otto, C., Schlack, R., Hölling, H., Klasen, F., and Ravens-Sieberer, U. (2019). Risk and protective factors for the development of ADHD symptoms in children and adolescents: results of the longitudinal BELLA study. PLoS One 14:e0214412. doi: 10.1371/journal.pone.0214412
Keywords: attention deficit hyperactivity disorder (ADHD), emotional dysregulation, sensory dysregulation, developmental stress, methylphenidate (MPH)
Citation: Grossman A and Avital A (2023) Emotional and sensory dysregulation as a possible missing link in attention deficit hyperactivity disorder: A review. Front. Behav. Neurosci. 17:1118937. doi: 10.3389/fnbeh.2023.1118937
Received: 08 December 2022; Accepted: 16 February 2023;
Published: 02 March 2023.
Edited by:
Nuno Sousa, University of Minho, PortugalReviewed by:
Xenia Gonda, Semmelweis University, HungaryEvgeniia Y. Chibikova, Samara Regional Clinical Psychiatric Hospital, Russia
Copyright © 2023 Grossman and Avital. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Avi Avital, YXZpdGFsYXZpQGhvdG1haWwuY29t
 Anna Grossman
Anna Grossman Avi Avital
Avi Avital