
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Behav. Neurosci., 05 October 2022
Sec. Emotion Regulation and Processing
Volume 16 - 2022 | https://doi.org/10.3389/fnbeh.2022.995573
This article is part of the Research TopicNoradrenergic Modulation of (Mal)Adaptive BehaviorView all 4 articles
 Samuel J. Duesman1
Samuel J. Duesman1 Sanutha Shetty1
Sanutha Shetty1 Sanil Patel2
Sanil Patel2 Neha Ogale1
Neha Ogale1 Farzanna Mohamed1
Farzanna Mohamed1 Njeri Sparman2
Njeri Sparman2 Prashant Rajbhandari2
Prashant Rajbhandari2 Abha Karki Rajbhandari1*
Abha Karki Rajbhandari1*Severe stress leads to alterations in energy metabolism with sexually dimorphic onset or severity. The locus coeruleus (LC) in the brainstem that mediates fight-or-flight-or-freeze response to stress is sexually dimorphic in morphology, plays a key role in interactions between diet and severe stressors, and has neuronal input to the brown adipose tissue (BAT)—a thermogenic organ important for energy balance. Yet, little is known on how LC coordinates stress-related metabolic adaptations. LC expresses receptors for the neuropeptide PACAP (pituitary adenylate cyclase activating peptide) and PACAP signaling through PAC1 (PACAP receptor) are critical regulators of various types of stressors and energy metabolism. We hypothesized that LC-PAC1 axis is a sex-specific central “gatekeeper” of severe acute stress-driven behavior and energy metabolism. Selective ablation of PAC1 receptors from the LC did not alter stress response in mice of either sex, but enhanced food intake in females and was associated with increased energy expenditure and BAT thermogenesis in male mice. These results show a sexually dimorphic role of the LC-PAC1 in regulating acute stress-related energy metabolism. Thus, by disrupting LC-PAC1 signaling, our studies show a unique and previously unexplored role of LC in adaptive energy metabolism in a sex-dependent manner.
Severe stressors lead to behavior and metabolic dysfunctions with a sexually dimorphic etiology (Dallman et al., 2003; Dedert et al., 2010; Nowotny et al., 2010; Farr et al., 2014; Levine et al., 2014; LeardMann et al., 2015; Michopoulos et al., 2016; Wolf et al., 2016). Understanding neural mechanisms of stress-related energy metabolism can allow development of novel ways of balancing optimal energy metabolism to reduce maladaptive impact on health and well-being (Breslau, 2002; Raikkonen et al., 2002; Dobie et al., 2004; Tolin and Foa, 2006; Heppner et al., 2009; Bell et al., 2011; Roenholt et al., 2012; Kubzansky et al., 2014; Udo et al., 2014; Buta et al., 2018; Pooley et al., 2018; American Psychological Association, 2020; Escarfulleri et al., 2021; Kautzky et al., 2021).
Locus coeruleus (LC) coordinates stress-associated adaptive/maladaptive arousal (alertness via enhanced blood pressure, heart rate, and breathing), “fight-or flight-or freeze” defensive responses, and energy metabolism (Aston-Jones et al., 1999; Aston-Jones and Cohen, 2005; Jovanovic et al., 2010; Fullana et al., 2016; Mothersill and Donohoe, 2016; Borodovitsyna et al., 2018; Kral et al., 2018; Kim et al., 2019). LC is a major source of norepinephrine (NE) to the entire forebrain axis and is a sexually dimorphic structure in morphology, gene expression, and stress responsiveness (Curtis et al., 2006; Samuels and Szabadi, 2008; Bangasser et al., 2016; Mulvey et al., 2018). LC activity and energy metabolism are causally linked, whereby enhanced LC activity decreases feeding, increases body temperature and oxygen consumption, while LC lesion decreases weight gain (Guimaraes et al., 2013). Furthermore, activation of ATP-dependent potassium channel in LC increases epididymal fat, while binge eating, or consumption of palatable foods, decrease LC activity (Guimaraes et al., 2013; Bello et al., 2014). LC also has functional connectivity to the brown adipose tissue (BAT)—a critical determinant of systemic energy balance via sympathetic activation and induction of thermogenic factors such as mitochondrial uncoupling protein 1 (UCP1) (Aston-Jones et al., 1994; Canale et al., 2013; Thorp and Schlaich, 2015; Atzori et al., 2016; Rabasa and Dickson, 2016; Caron et al., 2018; Naegeli et al., 2018; Rabasa et al., 2019; Morris et al., 2020; Yang et al., 2021), which is primarily expressed in brown adipocytes and uncouples oxidative phosphorylation from ATP synthesis to generate heat (Rousset et al., 2004; Fedorenko et al., 2012). LC inhibition reduces BAT thermogenesis and markedly attenuates BAT sympathetic activity independent of cold stress activity (Almeida et al., 2004). Despite clinical or preclinical literature strongly informing that trauma-like stressors contribute to the onset, maintenance, or aggravation of metabolic dysfunctions potentially via LC sympathetic pathways (Pervanidou and Chrousos, 2012), knowledge of combined behavioral and metabolic functions via LC neuromodulation is incomplete due to poor consideration of sex differences in animal models of severe stressors. Given that LC is sexually dimorphic in morphology and regulates stress-associated behavioral changes, we predicted that LC mediates long-lasting metabolic consequences to severe stressors, thereby acting as a switch for arousal and energy mobilization in humans and animals in a sexually dimorphic manner (Cannon, 1932; Southwick et al., 1999; O’Donnell et al., 2004; Borodovitsyna et al., 2018; Li et al., 2018; Grueschow et al., 2021). Therefore, by using a robust model of trauma-like stressor, we tested effects on whole body energy metabolism and BAT thermogenesis via a novel approach of LC neuropeptidergic modulation.
Several neuropeptides like the corticotrophin releasing factor are known to regulate LC functions. However, the receptor PAC1 (gene name ADCYAPR1), which is selective for the neuropeptide PACAP (gene name ADCYAP1), is highly expressed in the LC. Yet, PACAP and PAC1’s role in stress and metabolism has not been previously explored. LC-PAC1 has been shown to be important for regulating somatic symptoms associated with morphine withdrawal (Otto et al., 2001; Martin et al., 2010; Zhang et al., 2021). Human genetic studies have linked PACAP/PAC1 to post-traumatic stress disorder (PTSD) diagnosis and symptom severity. Specifically, mutations to the PAC1 gene are associated with PTSD symptom severity in women (Ressler et al., 2011). PACAP/PAC1 signaling are also sex-specific sympathetic regulators of a variety of stressors, energy homeostasis, mood, feeding, appetite, and metabolism (Beck, 2000; D’Este et al., 2000; Otto et al., 2001; Lein et al., 2007; Vaudry et al., 2009; Boughton and Murphy, 2013; Bangasser et al., 2016; Gastelum et al., 2021; Zhang et al., 2021). PACAP knockout early in development is embryonically lethal due to respiratory or metabolic alterations suggesting its biological importance for survival (Miyata et al., 1990; Hosoya et al., 1992; Gray et al., 2001; Cummings et al., 2004). PAC1 knockout in adult mice causes deficits in lipid metabolism, increase in serum triglycerides, fatty acids, cholesterol, and leptin indicating their role in energy metabolism (Gray et al., 2001; Adams et al., 2008; Hawke et al., 2009; Rudecki and Gray, 2016; Bozadjieva-Kramer et al., 2021; Chang et al., 2021; Filatov et al., 2021; Maunze et al., 2022).
We previously reported that amygdalar PACAP/PAC1 signaling regulates fear behaviors in a sexually dimorphic manner (Rajbhandari et al., 2021). Here, we aimed to unravel the role of LC-PAC1 on severe stress-associated energy homeostasis at the level of: (i) behavioral stress response, (ii) whole-body energy expenditure, and (iii) BAT thermogenesis.
To comprehensibly determine the role of LC-PAC1 in fear, we followed a detailed experimental timeline and setup (Figure 1A). At 12 weeks of age, we conducted stereotaxic surgeries to microinfuse AAV2-hsyn-GFP-Cre or AAV2-hsyn-GFP into LC (coordinates L/M: ±1; A/P: −5.4; D/V: −4.2) for efficient Cre mediated PAC1 knockdown in PAC1loxp/loxp mice. Following a 3-week recovery period to allow for viral transfection, mice received 10 foot shocks on Day 1 as the stressor experience. This protocol produces a long-lasting fear in rodents (Rau and Fanselow, 2009; Rajbhandari et al., 2018). Two way ANOVA did not reveal effect of sex [F(1, 70) = 2.493, p > 0.05] or interaction [F(3,70) = 0.32, p > 0.81). There was a main effect of treatment condition [F(3, 70) = 24.11, p < 0.05] (Figure 1B). Post-hoc analysis revealed that in both males and females freezing was increased (p < 0.05) on day 2 compared to unstressed animals regardless of the level of PAC1 receptors in the LC (Figures 1B,C). However, there was no significant effect of LC-PAC1 deletion on freezing for both males and females (p > 0.05) (Figures 1B,C). The deletion of PAC1 receptors was verified using RNAScope mRNA in situ hybridization (see section “Materials and methods”) for analyzing expression of PAC1 mRNA, Adcyap1r1, in tissue sections. We conducted fluorescence microscopy on mRNA puncta of PAC1 and represented as mean fluorescence (Figure 1D). As shown in Figures 1D–F, mean fluorescence of Adcyap1r1 was measured for each image and showed a significant decrease in Cre injected male and female mice compared to GFP-only control.
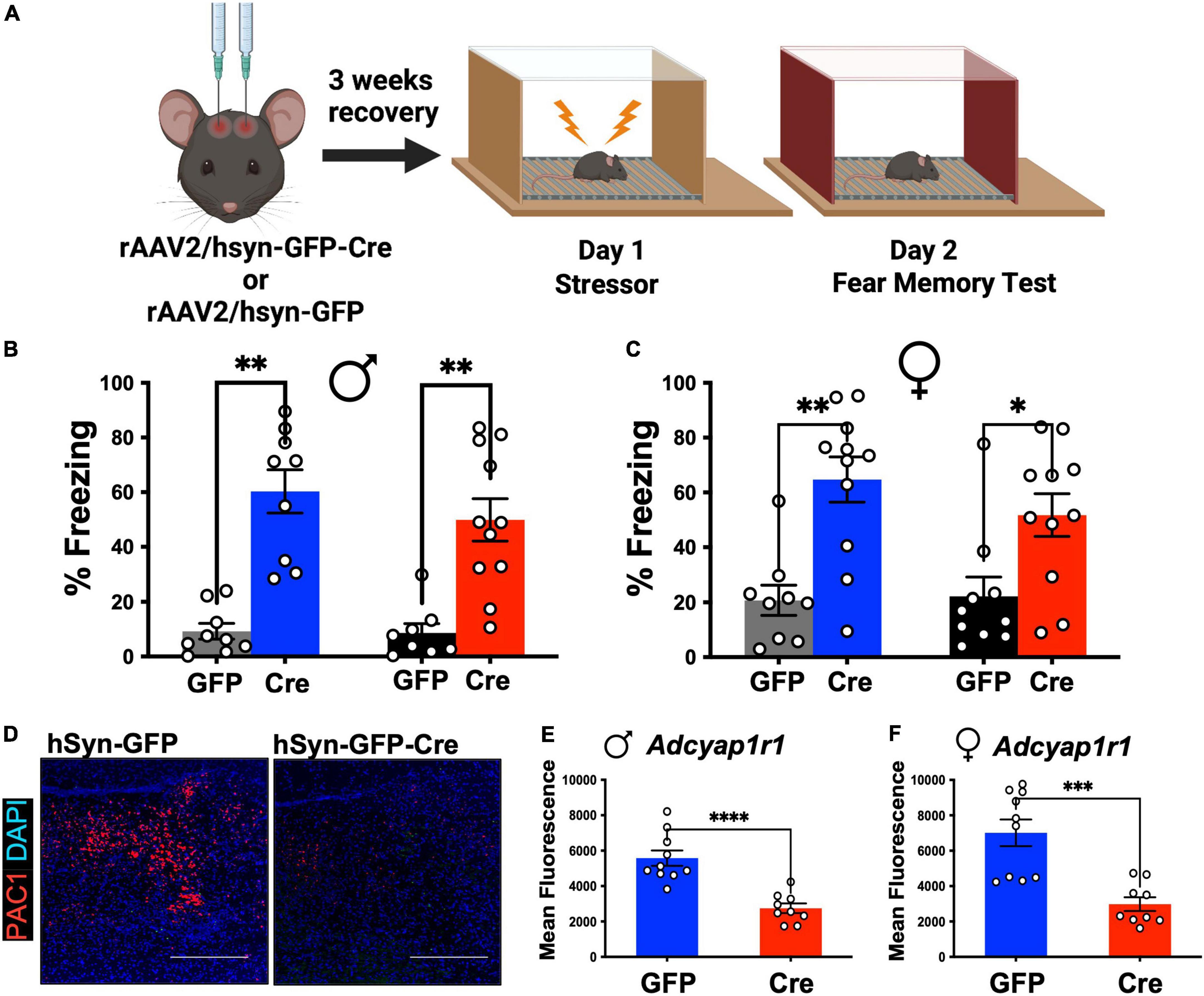
Figure 1. LC-PAC1 expression does not affect memory of the stressor test. (A) Animals were bilaterally injected with either hSyn-GFP or hSyn-GFP-Cre into the LC. After a 3 week recovery period, animals were run through a contextual fear protocol. (B,C) Prior stressors significantly increased freezing in all groups, but no significant effect of LC-PAC1 deletion were observed in memory of the stressor in either males (B) or females (C). N = 8–11/group. (D) Representative RNAScope in situ hybridization image of merged PAC1 mRNA and DAPI levels in rAAV2-hSyn-GFP and rAAV2-hSyn-GFP-Cre mice. (E,F) Mean fluorescence of PAC1 mRNA intensity in LC of indicated mouse groups. Injection of rAAV2-hSyn-GFP-Cre into the LC resulted in a significant reduction of mean fluorescence of PAC1 mRNA in both males and females. N = 9–10/group. *p < 0.05; **p < 0.01. Scale bar = 1 mm.
To determine the role of LC-PAC1 deletion in stress-induced energy metabolism, we performed indirect calorimetry using metabolic chambers in stressed or unstressed mice for 72 h (Figure 2A). Mice were acclimatized to individual metabolic chambers for the first 24 h, during which all the groups showed heightened calorimetric data possibly due to stress in a novel context (Supplementary Figures 1A–L). Therefore, we removed the first 24 h data from our calculation. Results of an ANCOVA and ANOVA test on the rest 48 h showed a main effect of LC-PAC1 deletion in stressed males (Supplementary Table 1). Post-hoc analysis showed that stressed males with LC-PAC1 knockdown (CRE S) had a total respiratory exchange ratio (RER) that was significantly lower, total energy expenditure (EE), oxygen consumption (VO2), and carbon dioxide production (VCO2) was significantly higher than stressed males with intact PAC1 receptors (GFP S) in the LC (hSyn-Cre S versus hSyn-GFP S; p < 0.05) (Figures 2B,C and Supplementary Figures 2A,B). The results of an ANCOVA and ANOVA showed no significant effect of PAC1 receptor depletion in the LC on food consumption or locomotion were observed between stressed males, respectively (hSyn-Cre S versus hSyn-GFP S; p > 0.05) (Supplementary Figures 2C,D and Supplementary Table 1). Interestingly, our results show that GFP S mice showed an overall decrease in EE and increase in RER than other groups (Figures 2B,C), implicating a possible role of LC-PAC1 in attenuating stress-induced increase in energy metabolism. For females, results of an ANCOVA showed that female mice with PAC1 deletion and acute stressor (CRE S) showed no differences in RER, EE, VO2, and VCO2 (Figures 2D,E, Supplementary Figures 2E,G, and Supplementary Table 2) compared to stressed females with intact PAC1 receptors (GFP S). Females showed enhanced total daily food intake compared to mice without deletion or the ones that only received the stress experience (hSyn-Cre S versus hSyn-GFP S; p < 0.05) (Supplementary Figure 2H and Supplementary Table 2). No significant differences in locomotor activity were observed within each group in male or female mice (hSyn-Cre S versus hSyn-GFP S; p > 0.05), or across sexes (Supplementary Figures 2C,G and Supplementary Tables 1, 2).
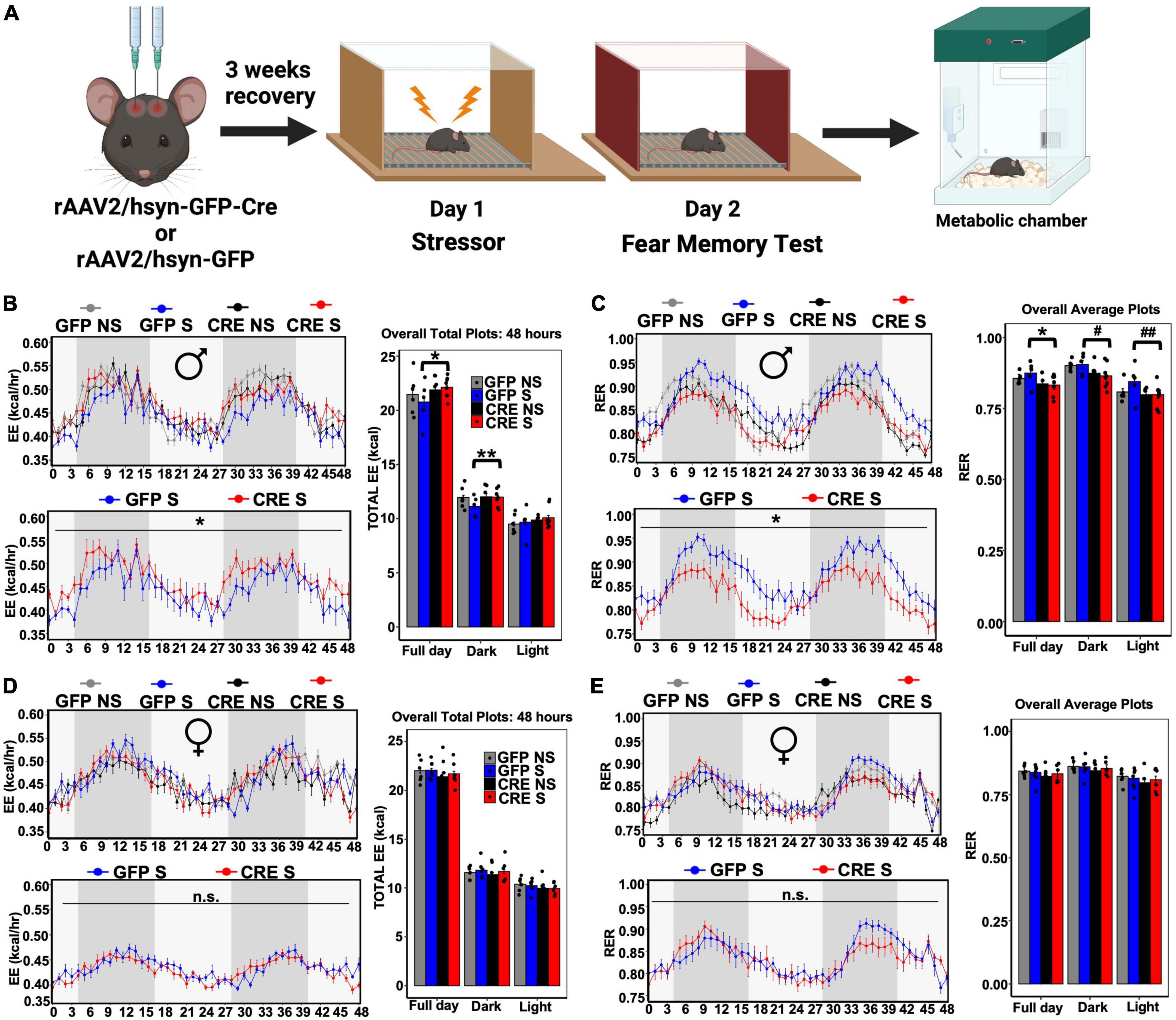
Figure 2. LC-PAC1 deletion results in sexually dimorphic stress-induced metabolic changes. (A) Animals were bilaterally injected with either hSyn-GFP or hSyn-GFP-Cre into the LC. After a 3 week recovery period, animals were run through a contextual fear protocol followed by 72 h in the metabolic chamber. (B,C) In male mice with LC-PAC1 deletion energy expenditure (EE, kcal/h) was significantly increased (B) and respiratory exchange ratio (RER) was significantly reduced (C) as evidenced by analysis in Sable Promethion metabolic chambers (12 h light/dark cycle, 48 h total duration, white bar represent light cycle and grey bar represent night cycle). (D,E) No significant differences in energy expenditure (EE, kcal/h) (D) and respiratory exchange ratio (RER) (E) in females. Analysis was performed in Sable Promethion metabolic chambers (12 h light/dark cycle, 48 h total duration, white bar represent light cycle and gray bar represent night cycle. For each of these variables a line graph and bar graph comparing all four groups (hSyn-Cre S, hSyn-Cre NS, hSyn-GFP S, and hSyn-GFP NS) as well as a line graph comparing stressed groups (hSyn-Cre S and hSyn-GFP S) are displayed. N = 9–10/group. *p < 0.05; **p < 0.01, #p = 0.05, ##p = 0.07. GFP NS, hSyn-GFP No Shock; GFP S, hSyn-GFP Shock; CRE NS, hSyn-Cre No Shock; and CRE S, hSyn-Cre Shock.
Mitochondrial uncoupling by UCP1 is a critical determinant of EE (Aston-Jones et al., 1994; Canale et al., 2013; Thorp and Schlaich, 2015; Atzori et al., 2016; Rabasa and Dickson, 2016; Caron et al., 2018; Naegeli et al., 2018; Rabasa et al., 2019; Morris et al., 2020; Yang et al., 2021). Following indirect calorimetry, stressed and non-stressed mice with and without LC-PAC1 deletion were sacrificed under 3% isoflurane anesthesia, and intrascapular BAT was collected (Figure 3A). RNA purification, cDNA synthesis, and RT-qPCR were performed on BAT samples and expression of genes involved in mitochondrial function, biogenesis, and thermogenesis were measured. Two Way ANOVA revealed no effect of sex and condition [F(1,22) = 1.94, p > 0.05) on the thermogenic gene Ucp1 in non-stressed mice. There was a main effect of sex and condition [F(1,9) = 6.462, p = 0.0316] on Ucp1 in stressed mice. Post-hoc analysis showed that there was a significant increase in thermogenic gene, Ucp1 expression in stressed LC-PAC1 knockout males compared to stressed males with intact LC-PAC1 (hSyn-Cre S versus hSyn-GFP S; p < 0.05) (Figure 3B). Genes encoding proteins important for mitochondrial function such as Ppargc1a and Cox8B were also increased (not significant) in male CRE S mice compared to GFP S mice (Figures 3D–G). Other BAT genes such as PPARa, Elovl3, Dio2, Clstn3 were comparable between CRE S and GFP S male mice (Supplementary Figures 3A–H). No significant differences were observed in BAT expression of Ucp1, PPARa, Cox8b, Elovl3, Dio2, Clstn3, or PPARGC1a in females (Figure 3C and Supplementary Figures 3A–H). Our BAT analysis supports our indirect calorimetric data, suggesting that ablation of PAC1 receptors in LC of male mice increases BAT thermogenic function in stressed mice which could potentially led to increase in EE.
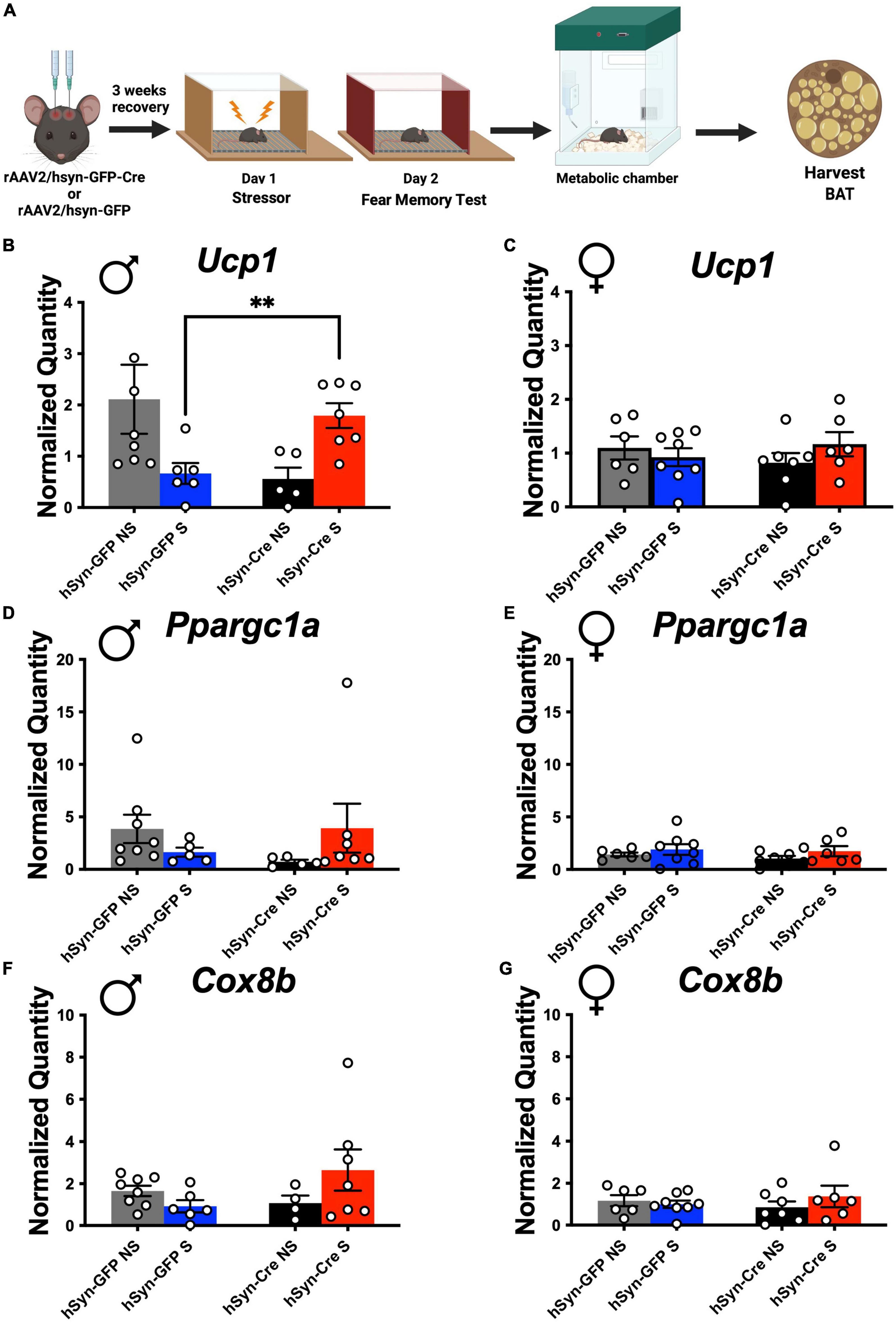
Figure 3. PAC1 deletion in the LC result in a stress-induced thermogenic genetic expression increase in males. (A) Animals were bilaterally injected with either hSyn-GFP or hSyn-GFP-Cre into the LC. After a 3-week recovery period, animals were run through a contextual fear protocol followed by 72 h in the metabolic chamber and BAT harvest. (B–G) Real-time qPCR of indicated genes from male and female BAT of indicated mouse groups. Stressed male mice with LC-PAC1 deletion showed a significant upregulation in Ucp1 compared to stressed males with intact LC-PAC1 (B). No significant differences in Ucp1 expression were observed in females (C). There were no significant differences in expression of PPARGC1a in males (D) or females (E). No significant differences in expression of Cox8b were observed in males (F) or females (G). N = 9–10/group. **p < 0.01. Ucp1, Uncoupling protein 1; PPARa, peroxisome proliferator-activated receptor; Cox8b, cytochrome c oxidase, subunit VIIIb; Elovl3, elongation of very long chain fatty acids-like 3; Clstn3, Calsyntenin 3; PPARGC1a, Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha.
To test if the behavior and energy metabolism changes were due to sustained PAC1 deletion in LC, after the behavior and metabolic tests were completed (Figure 4A), mice were sacrificed, and their brains extracted and immediately stored in at −80°C. The brains were sliced at 20 microns in a cryostat and slices containing the LC were collected on microscope slides. The deletion of PAC1 receptors was verified using RNAScope for analyzing expression of RNA in tissue sections. We conducted fluorescence microscopy on mRNA puncta of PAC1 and GFP mRNA and represented as mean fluorescence (Figures 4B–D). Mean fluorescence of Adcyap1r1 was measured for each image and showed a significant decrease in Cre injected male and female mice compared to GFP-only control (Figures 4B–D). Our data show that sexually dimorphic metabolic perturbation seen in mice are due to acute stress paired with sustained PAC1 deletion in the LC regions.
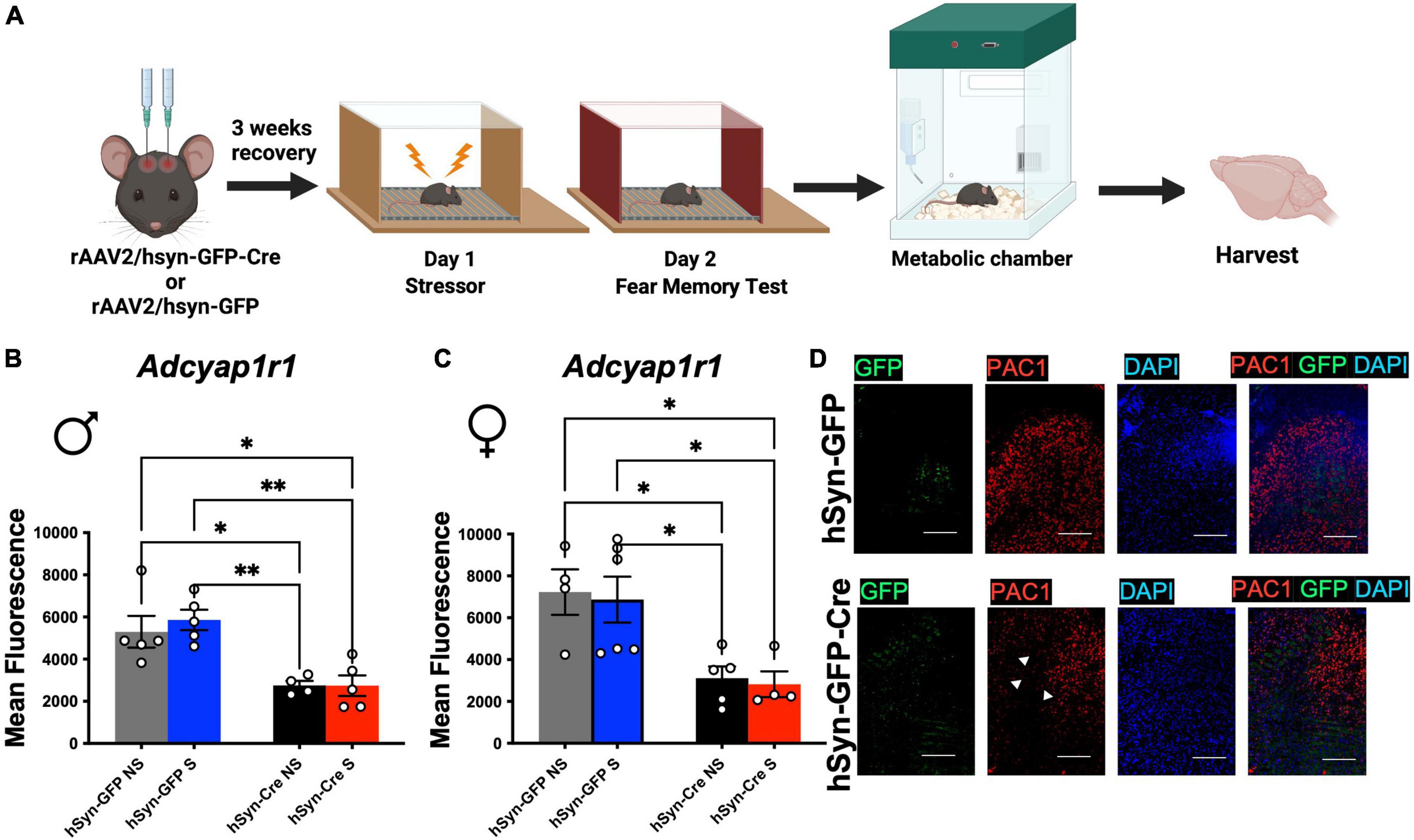
Figure 4. Injection of rAAV2-hSyn-Cre results in a sustained reduction of PAC1 receptors in PAC1loxP/loxP mice. (A) Animals were bilaterally injected with either hSyn-GFP or hSyn-GFP-Cre into the LC. After a 3 week recovery period, animals were run through a contextual fear protocol followed by 72 h in the metabolic chamber and brain harvest. (B,C) Mean fluorescence of PAC1 mRNA intensity in LC of indicated mouse groups. Injection of rAAV2-hSyn-GFP-Cre into the LC resulted in a significant reduction of mean fluorescence of PAC1 mRNA in both males (B) and (C) females. (D) Representative RNAScope in situ hybridization image of PAC1 mRNA, GFP mRNA, DAPI, and merged levels in rAAV2-hSyn-GFP and rAAV2-hSyn-GFP-Cre mice. *p < 0.05; **p < 0.01. Scale bar = 1 mm.
Our results for the first time show that deletion of PAC1 receptors from the LC does not alter fear expression in male or female mice. However, PAC1 knockdown in the LC of male mice led to an increase in EE and decreased RER. PAC1 ablation from LC in female mice significantly enhanced food intake. We also show that PAC1 deletion in the LC in male mice increased the Ucp1 gene, a measure of BAT thermogenic gene program. Overall, these results show a causal role of LC PAC1 receptors in regulating energy metabolism in a sex-specific manner.
Measurement of stressor memory in mice with and without PAC1 deletion in the LC indicates that these receptors in the LC are not involved in fear regulation. Both male and female groups showed similar levels of freezing with and without PAC1 deletion after acute stressor. It is possible that these receptors are involved in other behavioral measures of stress-exposure including fear extinction, grooming, rearing, and other measures including endocrine measurements such as corticosterone and catecholamines, which will require further studies.
Our results on the whole-body energy metabolism showed a sexually dimorphic effect of LC-PAC1 knockout. Male GFP-only control showed reduced VO2, VCO2, EE and increased RER (indicative of decreased fat oxidation and utilization) compared with male mice with PAC1 deletion and acute stressor. These results were not attributable to changes in locomotion. Female mice with PAC1 deletion in the LC and acute stressor showed enhanced food consumption compared to GFP-only control mice. Analysis of BAT tissue also showed that male mice with PAC1 deletion in the LC and acute stress exposure showed enhanced levels of the Ucp1 gene compared to mice without PAC1 deletion that went through acute stressor. This indicates that -LC-PAC1 receptors in male mice regulates energy and fat metabolism but in female mice food intake, which is in line with a recent study has showed that LC inhibition increases food intake in response to acute stressor (Sciolino et al., 2019). These findings indicate that under stressor, PAC1 receptors in the LC are important regulators of metabolic functions, whereby removal of these receptors in female mice increase food intake but in male mice alter the peripheral regulation of energy metabolism.
Based on published reports that show LC neurons innervate the BAT (Morrison and Madden, 2014; Wiedmann et al., 2017) and given that LC is sexually dimorphic in morphology, we predict that LC-PAC1 expressing neuronal innervations to BAT are sexually dimorphic and are altered by severe stressors. One possibility is that LC-PAC1 receptors are highly expressed on BAT projecting neurons in male mice that undergo severe stressors than females or other control groups. Published work show that acute stressors increase BAT Ucp1 activity (Nozu et al., 1992; Lkhagvasuren et al., 2011; Kataoka et al., 2014) and our studies corroborate those findings. We further show nuanced sex-specific differences in LC-PAC1 regulation of BAT activity and thermogenic program. However mechanistic aspects of BAT activity regulation via LC PAC1 remain to be elucidated. First, multiple physiological signals including LC-sympathetic pathways innervate BAT through multiple synapses (Cano et al., 2003; Almeida et al., 2004; Wiedmann et al., 2017). To gain a better understanding of anatomical link between LC-PAC1 and BAT, it will be important to determine how the PAC1 receptor expressing LC neurons project to BAT and whether LC-PAC1 alters BAT sympathetic tone to alter thermogenic program. Second, while LC expresses PAC1 receptors, the source/s of PACAPergic input to LC are unclear. The nucleus of the solitary tract (NTS), a structure known to express PACAP99 is a known major source of preganglionic input to the LC (Van Bockstaele et al., 1999). NTS integrates peripheral autonomic and endocrine signals associated with stressors, and regulates LC functions (Ulrich-Lai and Herman, 2009). The rostral ventrolateral medulla (RVLM) also contains high PACAPergic cells and innervate the LC, but their functions are related to cardiorespiratory functions which could influence metabolic functions indirectly. Thus, it will be important to discern if there are sex differences in NTS to LC projections.
In our current studies we did not measure the contribution of sex hormones in our sex-specific metabolic phenotype upon LC PAC1 ablation. While sex steroids, specifically estradiol, influence BAT activity (Heine et al., 2000; Pedersen et al., 2001; Karastergiou et al., 2012; Hoene et al., 2014; Valencak et al., 2017), future studies with ovariectomy/hormone replacement studies to determine roles of ovarian hormones (e.g., estradiol vs. progesterone) are needed. Besides estrous cycle, other studies will also be needed to determine if fat mass, lean mass, adult testicular hormones, gonadal hormonal surges in development, or the different complement of genes on the sex chromosomes (McCarthy et al., 2012) influence metabolic functions such as EE and RER and BAT activity between males and females.
Overall, our studies capture a granular detail of the LC in integrating severe stressor and metabolic signals via a genetically defined anatomy of LC via the PAC1 receptors. Our findings that LC-PAC1 neurons regulate metabolic responses under trauma-like stressors are important for further understanding the unique biology of LC via other systematic approach and consideration of sex differences. These studies are also important for growing studies in mapping brain and body interactions under severe stressors.
All experimental procedures were conducted in accordance with guidelines set by the National Institutes of Health and the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai. Mice were provided ad libitum access to food and water in a light- and temperature-controlled vivarium. Mice (3–4 months) were housed with no more than five mice per cage as littermates in a vivarium in a 12 h light:12 h dark cycle. Experiments were performed between 9 AM and 3 PM. The Adcyap1r1loxP/loxP mouse line was utilized for all experiments. These mice were generated in a C57BL/6 background with a conditional knockout (KO) allele (PAC1loxP/loxP mice) through the NIH-funded knockout mouse project (KOMP).
Mice were either injected with AAV2-hsyn-GFP-Cre for Cre-mediated deletion of PAC1 or AAV2-hsyn-GFP for control animals (UNC vector core).
To ablate PAC1 receptors in neurons, mice were secured in a stereotaxic apparatus under 2% isoflurane anesthesia and injected with rAAV2-hsyn-GFP-Cre to achieve neuronal deletion of PAC1 receptors. Control mice were injected with rAAV2-hsyn-eGFP. Mice were injected with 0.3 μl of virus bilaterally into the LC using coordinates (LM: ±1.0; AP: −5.4; DV: 4.2 from Bregma). Virus was microinfused into the LC with a 10 μl Hamilton Syringe fitted with a 1 mm glass pipette with no filament at a rate of 0.2 μl/min. After completion of infusion the glass pipette was left in position for 10 min to allow for diffusion. Using the glass pipettes that are commonly used for electrophysiological recordings with single cell resolution allowed confining the virus infusion to the LC. Using this method, we have previously targeted structures that are smaller than LC (Rajbhandari et al., 2021). Immediately after surgery, mice were given ad libitum access to a 0.5/0.1 mg/kg Sulfamethoxazole/Trimethoprim solution in drinking water for 5 days. The antibiotic regimen is standard procedure to prevent infections after surgeries. Since this is administered for 5 days after surgeries and the experimental procedure do not start until 3 weeks later, we do not expect it to have effects on metabolic functions. Mice also received a subcutaneous injection of the anti-inflammatory drug Rimadyl (5 mg/kg) immediately after, and 1 day following surgery. Mice were allowed 21 days after surgery prior to behavioral testing, which allowed for viral expression sufficient for Cre-mediated deletion of PAC1 receptors in LC neurons.
Mice were run individually in sound and light attenuated conditioning boxes (Med Associates Inc., Georgia, VT, USA) (Figure 1). The boxes were equipped with Near Infra-Red Video Fear Conditioning System and could be configured to represent different contexts by changing the internal structure, illumination, and odor. Context A (28 cm × 21 cm × 21 cm) had a clear Plexiglas back wall, ceiling, and front door with aluminum sidewalls visibly illuminated with a white light. It also had a grid floor with evenly spaced stainless-steel rods. Beneath the grid floor, in Context A, was an aluminum tray with a paper towel scented with 50% Windex. The floor in context A was connected to a scrambled foot shock generator. Context B (28 cm × 21 cm × 21 cm) had a clear plexiglass door with red walls illuminated with red colored LED light emitting from the top of the chamber. Context B also contained a grid floor connected to a scrambled foot shock generator, but beneath the grid floor was a paper towel scented with a 1% acetic acid solution.
Freezing is a complete lack of movement except for respiration (Fanselow, 1980). Freezing was measured using Ethovision software that performed real-time video recordings at 18 frames per second. With this program, adjacent frames are compared to provide the grayscale change for each pixel and the sum of pixels changing from one frame to the next constitutes a momentary activity score. To account for video noise and to approximate scoring by a trained human observer a threshold is set at 0.02 activity units so that an instance of freezing is counted when that the activity score remains below this threshold for 1 s (Anagnostaras et al., 2001). Percentage freezing = Freezing Time/Total Time × 100 for a period of interest. Data are presented as mean percentages (±SEM).
We designed our behavioral tests to capture memory of the stressor. This design was chosen mainly because LC has been shown to be important for fear generalization (Soya et al., 2017).
After mice recovered from surgery an acute stress paradigm was used. On day 1, mice were transported to the behavioral testing area in their clear plastic home cage and placed in the chambers set up in Context A. Mice were then exposed to 10 random foot shocks (1 mA) over the period of 60 min. This stressor has been shown to produce long-lasting effects (Rau and Fanselow, 2009). Mice were transported to the laboratory together in their home cages. For the behavioral experiments male mice were always ran before females and chambers were thoroughly cleaned between mice to avoid effects of pheromones on behavior.
On day 2 mice were transported to the behavioral testing room in a round, opaque plastic container which was distinct from their home cage and placed in chambers set up in Context B. The animals were placed in a different Context (B) for four minutes and thirty seconds. Mice were allowed to explore Context B for 4 min and time spent freezing was measured for this time-period.
Three days after administration of the acute stress paradigm, mice were placed in in indirect calorimetry chambers (Sable Promethion) for 72 h. Data collected from indirect calorimetry included oxygen consumption, carbon dioxide production, energy expenditure (EE), respiratory exchange ratio (RER), energy balance, food and water intake, locomotor activity, and body mass. This information highlights changes in metabolic physiology after severe stressor as a function of presence/absence of LC-PAC1. The first 24 h of indirect calorimetry data was excluded from analysis to allow mice to habituate to the novel environment. Body weight was used as statistical covariates for the analysis of some indirect calorimetry measurements in the metabolic chambers such as oxygen consumption, carbon dioxide production, EE, and food and water intake. RER value and locomotion were analyzed without a covariate as they are known to be measures independent of body weight. The RER value, which is a ratio of the volume carbon dioxide produced over the volume of oxygen consumed, show if the predominant source of energy is fat or carbohydrate after stressor. A higher RER value denotes carbohydrate as the primary source of energy being utilized, while a lower RER value indicates fat as a fuel source. We analyzed the data with CalR (Mina et al., 2018) that considers activity, food intake and other parameters allowing us to derive accurate indirect calorimetry values.
Three days after indirect calorimetry mice were food deprived for 4 hours and anesthetized with isoflurane and rapidly decapitated. Brain and BAT were collected and rapidly frozen and stored in a −80 °C freezer. Brain tissue with LC were used to confirm injection sites and loss of PAC1 using RNAScope in situ hybridization routinely used in our lab58.
The brains were sectioned at 20 μm in a cryostat at −20°C and slices containing the LC were collected on Fisherbrand Superfrost Plus microscope slides (Thermo Fisher Scientific) and stored at −80 °C. Deletion of PAC1 receptors was verified using RNAscope for analyzing expression of RNA tissue sections (ACD Biotechne). Briefly, we performed in situ hybridization steps following RNAscope® 2.5 HD HD Assay - RED protocol for fresh frozen sections. After completion of the labeling, sections were cover-slipped using Prolong Gold (Thermo Fisher Scientific) with 4’,6-diamidino-2- phenylindole (DAPI) and the edges were sealed with clear nail polish. PAC1 mRNA quantification was carried out in sections containing the LC that were captured with a 20X objective on a Zeiss AxioImager Z2M with ApoTome. Analysis of PAC1 mRNA was conducted using FIJI software. For quantification, mean fluorescence of PAC1 mRNA labeled with mCherry inside a standard section inside the LC was assessed.
RNA was isolated from BAT using phenol-chloroform extraction. After isolated, the RNA pellet was washed and resuspended in diethyl pyrocarbonate (DEPC) water at a concentration of 200 ng/μL. RNA samples were reversely transcribed to cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Real Time qPCR was performed by using a real time PCR SYBR green master mix (Diagenode) and primers for Ucp1, peroxisome proliferator-activated receptor (PPARa), cytochrome c oxidase, subunit VIIIb (Cox8b), elongation of very long chain fatty acids-like 3 (Elovl3), Calsyntenin 3 (Clstn3), peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PPARGC1a). Samples were run and analyzed on a Quantstudio 5 (Applied Biosystems). The qPCR targets were normalized to the expression of the housekeeping gene 36B4.
The tissue sections were analyzed using a Zeiss AxioImager Z2M with ApoTome microscope. Images were analyzed with Fiji image processing software (NIH, Bethesda, MD, USA; RRID:SCR_002285). Mean fluorescent intensity of PAC1 RNA was measured on a section of LC tagged with GFP.
We measured freezing for the first 4 min of the session on day 2 of the acute stress paradigm. For the behavioral and qPCR experiments, a two-way analysis of variance (ANOVA) was used to measure differences in means with two between sex and condition (virus and stress/control) factors. Significant effects indicated by the ANOVA were further analyzed with a post-hoc Holm-Sida’s post-hoc analysis. For metabolic experiments, a two-way analysis of variance (ANOVA) was performed on measurements not associated with mass (i.e., respiratory exchange ratio (RER) and locomotor activity) and analysis of covariance (ANCOVA) with total mass as a covariate for measurements that are associated with mass [i.e., oxygen consumption, carbon dioxide production, food and water consumption, and energy expenditure (EE)]. The level of significance used for all analyses was p < 0.05.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
This animal study was reviewed and approved by IACUC.
SD, NO, SS, SP, and FM ran the experiments. SD, PR, and AR wrote the manuscript. All authors contributed to the article and approved the submitted version.
This research in the AKR lab were supported by NARSAD-AKR (29227), Whitehall Foundation-AKR, Akira Arimura Foundation-AKR, and Friedman Brain Institute. PR was supported by R00DK114571, NIDDK-supported Einstein-Sinai Diabetes Research Center (DRC) Pilot & Feasibility Award, and Diabetes Research and Education Foundation (DREF) Grant #501.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2022.995573/full#supplementary-material
Supplementary Figure 1 | (A–H) Oxygen consumption (VO2, ml/h) (A,B), carbon dioxide production (VCO2, ml/h) (C,D), respiratory exchange ratio (RER) (E,F), energy expenditure (EE) (G,H), food consumption (kcal/h) (I,J), and locomoter activity (beam breaks/h) (K,L), (C) of male and female mice analyzed in Sable Promethion metabolic chambers (12 h light/dark cycle, first 24 h total duration, white bar represent light cycle and grey bar represent night cycle). For each of these variables a line graph comparing all four groups (hSyn-Cre S, hSyn-Cre NS, hSyn-GFP S, and hSyn-GFP NS) are displayed. N = 9–10/group. GFP NS (hSyn-GFP No Shock), GFP S (hSyn-GFP Shock), CRE NS (hSyn-Cre No Shock), and CRE S (hSyn-Cre Shock). No significant differences were observed in these variables.
Supplementary Figure 2 | (A–D) No significant differences were observed in oxygen consumption (VO2, ml/h) (A), carbon dioxide production (VCO2, ml/h) (B), locomotor activity (beam breaks/h), locomotor activity (beam breaks/h) (C), and food consumption (kcal/h) (D) of male mice. (E–G) Similarly, no significant differences in oxygen consumption (VO2, ml/h) (E), carbon dioxide production (VCO2, ml/h) (F), and locomotor activity (beam breaks/h) (G) were observed in females. However, stressed females with LC-PAC1 deletion showed a significant increase in food consumption (kcal/h) compared to stressed females with intact LC-PAC1 (H). All data were collected using Sable Promethion metabolic chambers (12 h light/dark cycle, 48 h total duration, white bar represent light cycle and gray bar represent night cycle). For each of these variables a line graph and bar graph comparing all four groups (hSyn-Cre S, hSyn-Cre NS, hSyn-GFP S, and hSyn-GFP NS) as well as a line graph comparing stressed groups (hSyn-Cre S and hSyn-GFP S) are displayed. N = 9–10/group. *p < 0.05; **p < 0.01. GFP NS, hSyn-GFP No Shock; GFP S, hSyn-GFP Shock; CRE NS, hSyn-Cre No Shock; CRE S, hSyn-Cre Shock.
Supplementary Figure 3 | (A–H) Real-time qPCR of indicated genes from male and female BATs of indicated mouse groups. No significant differences were observed in these variables. N = 9-10/group. PPARa, peroxisome proliferator-activated receptor; Elovl3, elongation of very long chain fatty acids-like 3; Dio, idothyronine deiodinase 2; Clstn3, Calsyntenin 3.
Supplementary Table 1 | Generalized linear model (GLM) ANCOVA and ANOVA tests for the listed parameters from male mice housed in metabolic chambers.
Supplementary Table 2 | Generalized linear model (GLM) ANCOVA and ANOVA tests for the listed parameters from female mice housed in metabolic chambers.
Adams, B. A., Gray, S. L., Isaac, E. R., Bianco, A. C., Vidal-Puig, A. J., and Sherwood, N. M. (2008). Feeding and metabolism in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology 149, 1571–1580.
Almeida, M. C., Steiner, A. A., Coimbra, N. C., and Branco, L. G. (2004). Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus. J. Physiol. 558, 283–294. doi: 10.1113/jphysiol.2004.066654
Anagnostaras, S. G., Gale, G. D., and Fanselow, M. S. (2001). Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus 11, 8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7
American Psychological Association (2020). Stress in America, United States, 2007-2019. Inter-university Consortium for Political and Social Research. Washington, DC: American Psychological Association
Aston-Jones, G., and Cohen, J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450.
Aston-Jones, G., Rajkowski, J., and Cohen, J. (1999). Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 46, 1309–1320.
Aston-Jones, G., Valentino, R. J., Van Bockstaele, E., and Meyerson, A. (1994). Locus Coeruleus, Stress and PTSD:Neurobiological and Clinical Parallels. Washington, DC: American Psychiatric Press.
Atzori, M., Cuevas-Olguin, R., Esquivel-Rendon, E., Garcia-Oscos, F., Salgado-Delgado, R. C., Saderi, N., et al. (2016). Locus Ceruleus Norepinephrine Release: A Central Regulator of CNS Spatio-Temporal Activation? Front. Synaptic. Neurosci. 8:25. doi: 10.3389/fnsyn.2016.00025
Bangasser, D. A., Wiersielis, K. R., and Khantsis, S. (2016). Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 1641, 177–188.
Bell, J. F., Zimmerman, F. J., Arterburn, D. E., and Maciejewski, M. L. (2011). Health-care expenditures of overweight and obese males and females in the medical expenditures panel survey by age cohort. Obesity 19, 228–232.
Bello, N. T., Yeh, C. Y., Verpeut, J. L., and Walters, A. L. (2014). Binge-like eating attenuates nisoxetine feeding suppression, stress activation, and brain norepinephrine activity. PLoS One 9:e93610. doi: 10.1371/journal.pone.0093610
Borodovitsyna, O., Flamini, M. D., and Chandler, D. J. (2018). Acute Stress Persistently Alters Locus Coeruleus Function and Anxiety-like Behavior in Adolescent Rats. Neuroscience 373, 7–19. doi: 10.1016/j.neuroscience.2018.01.020
Boughton, C. K., and Murphy, K. G. (2013). Can neuropeptides treat obesity? A review of neuropeptides and their potential role in the treatment of obesity. Br. J. Pharmacol. 170, 1333–1348.
Bozadjieva-Kramer, N., Ross, R. A., Johnson, D. Q., Fenselau, H., Haggerty, D. L., Atwood, B., et al. (2021). The Role of Mediobasal Hypothalamic PACAP in the Control of Body Weight and Metabolism. Endocrinology 162:bqab012. doi: 10.1210/endocr/bqab012
Breslau, N. (2002). Gender differences in trauma and posttraumatic stress disorder. J. Gend. Specif. Med. 5, 34–40.
Buta, E., Masheb, R., Gueorguieva, R., Bathulapalli, H., Brandt, C. A., and Goulet, J. L. (2018). Posttraumatic stress disorder diagnosis and gender are associated with accelerated weight gain trajectories in veterans during the post-deployment period. Eat Behav. 29, 8–13. doi: 10.1016/j.eatbeh.2018.01.002
Canale, M. P., Manca Di Villahermosa, S., Martino, G., Rovella, V., Noce, A., De Lorenzo, A., et al. (2013). Obesity-related metabolic syndrome: mechanisms of sympathetic overactivity. Int. J. Endocrinol. 2013:865965. doi: 10.1155/2013/865965
Cano, G., Passerin, A. M., Schiltz, J. C., Card, J. P., Morrison, S. F., and Sved, A. F. (2003). Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 460, 303–326. doi: 10.1002/cne.10643
Caron, A., Lee, S., Elmquist, J. K., and Gautron, L. (2018). Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 19, 153–165. doi: 10.1038/nrn.2018.7
Chang, R., Hernandez, J., Gastelum, C., Guadagno, K., Perez, L., and Wagner, E. J. (2021). Pituitary Adenylate Cyclase-Activating Polypeptide Excites Proopiomelanocortin Neurons: Implications for the Regulation of Energy Homeostasis. Neuroendocrinology 111, 45–69. doi: 10.1159/000506367
Cummings, K. J., Pendlebury, J. D., Sherwood, N. M., and Wilson, R. J. (2004). Sudden neonatal death in PACAP-deficient mice is associated with reduced respiratory chemoresponse and susceptibility to apnoea. J. Physiol. 555, 15–26. doi: 10.1113/jphysiol.2003.052514
Curtis, A. L., Bethea, T., and Valentino, R. J. (2006). Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31, 544–554.
D’Este, L., Casini, A., Wimalawansa, S. J., and Renda, T. G. (2000). Immunohistochemical localization of amylin in rat brainstem. Peptides 21, 1743–1749.
Dallman, M. F., Pecoraro, N., Akana, S. F., La Fleur, S. E., Gomez, F., Houshyar, H., et al. (2003). Chronic stress and obesity: a new view of “comfort food”. Proc. Natl. Acad. Sci. U.S.A. 100, 11696–11701. doi: 10.1073/pnas.1934666100
Dedert, E. A., Calhoun, P. S., Watkins, L. L., Sherwood, A., and Beckham, J. C. (2010). Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann. Behav. Med. 39, 61–78.
Dobie, D. J., Kivlahan, D. R., Maynard, C., Bush, K. R., Davis, T. M., and Bradley, K. A. (2004). Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch. Intern. Med. 164, 394–400.
Escarfulleri, S., Ellickson-Larew, S., Fein-Schaffer, D., Mitchell, K. S., and Wolf, E. J. (2021). Emotion regulation and the association between PTSD, diet, and exercise: a longitudinal evaluation among US military veterans. Eur. J. Psychotraumatol. 12:1895515. doi: 10.1080/20008198.2021.1895515
Fanselow, M. S. (1980). Conditioned and unconditional components of post-shock freezing. Pavlov. J. Biol. Sci. 15, 177–182. doi: 10.1007/BF03001163
Farr, O. M., Sloan, D. M., Keane, T. M., and Mantzoros, C. S. (2014). Stress- and PTSD-associated obesity and metabolic dysfunction: a growing problem requiring further research and novel treatments. Metabolism 63, 1463–1468. doi: 10.1016/j.metabol.2014.08.009
Fedorenko, A., Lishko, P. V., and Kirichok, Y. (2012). Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413.
Filatov, E., Short, L. I., Forster, M. A. M., Harris, S. S., Schien, E. N., Hughes, M. C., et al. (2021). Contribution of thermogenic mechanisms by male and female mice lacking pituitary adenylate cyclase-activating polypeptide in response to cold acclimation. Am. J. Physiol. Endocrinol. Metab. 320:E475–E487. doi: 10.1152/ajpendo.00205.2020
Fullana, M. A., Harrison, B. J., Soriano-Mas, C., Vervliet, B., Cardoner, N., Àvila-Parcet, A., et al. (2016). Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol. Psychiatry 21, 500–508. doi: 10.1038/mp.2015.88
Gastelum, C., Perez, L., Hernandez, J., Le, N., Vahrson, I., Sayers, S., et al. (2021). Adaptive Changes in the Central Control of Energy Homeostasis Occur in Response to Variations in Energy Status. Int. J. Mol. Sci. 22:2728. doi: 10.3390/ijms22052728
Gray, S. L., Cummings, K. J., Jirik, F. R., and Sherwood, N. M. (2001). Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol. Endocrinol. 15, 1739–1747. doi: 10.1210/mend.15.10.0705
Grueschow, M., Stenz, N., Thorn, H., Ehlert, U., Breckwoldt, J., Brodmann Maeder, M., et al. (2021). Real-world stress resilience is associated with the responsivity of the locus coeruleus. Nat. Commun. 12, 2275. doi: 10.1038/s41467-021-22509-1
Guimaraes, J., Moura, E., Silva, E., Aguiar, P., Garrett, C., and Vieira-Coelho, M. A. (2013). Locus coeruleus is involved in weight loss in a rat model of Parkinson’s disease: an effect reversed by deep brain stimulation. Brain Stimul. 6, 845–855. doi: 10.1016/j.brs.2013.06.002
Hawke, Z., Ivanov, T. R., Bechtold, D. A., Dhillon, H., Lowell, B. B., and Luckman, S. M. (2009). PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J. Neurosci. 29, 14828–14835. doi: 10.1523/JNEUROSCI.1526-09.2009
Heine, P. A., Taylor, J. A., Iwamoto, G. A., Lubahn, D. B., and Cooke, P. S. (2000). Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U.S.A. 97, 12729–12734.
Heppner, P. S., Crawford, E. F., Haji, U. A., Afari, N., Hauger, R. L., Dashevsky, B. A., et al. (2009). The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Med. 7:1. doi: 10.1186/1741-7015-7-1
Hoene, M., Li, J., Haring, H. U., Weigert, C., Xu, G., and Lehmann, R. (2014). The lipid profile of brown adipose tissue is sex-specific in mice. Biochim. Biophys. Acta. 1842, 1563–1570.
Hosoya, M., Kimura, C., Ogi, K., Ohkubo, S., Miyamoto, Y., Kugoh, H., et al. (1992). Structure of the human pituitary adenylate cyclase activating polypeptide (PACAP) gene. Biochim. Biophys. Acta. 1129, 199–206.
Jovanovic, T., Norrholm, S. D., Blanding, N. Q., Davis, M., Duncan, E., Bradley, B., et al. (2010). Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety 27, 244–251.
Karastergiou, K., Smith, S. R., Greenberg, A. S., and Fried, S. K. (2012). Sex differences in human adipose tissues - the biology of pear shape. Biol. Sex Differ. 3:13. doi: 10.1186/2042-6410-3-13
Kataoka, N., Hioki, H., Kaneko, T., and Nakamura, K. (2014). Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 20, 346–358. doi: 10.1016/j.cmet.2014.05.018
Kautzky, A., Heneis, K., Stengg, K., Frohlich, S., and Kautzky-Willer, A. (2021). Biological and psychological stress correlates are linked to glucose metabolism, obesity and gender roles in women. Neuroendocrinology 112, 130–142. doi: 10.1159/000514484
Kim, J., Lee, S., Fang, Y. Y., Shin, A., Park, S., Hashikawa, K., et al. (2019). Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat. Neurosci. 22, 576–585. doi: 10.1038/s41593-019-0342-2
Kral, T. R. A., Schuyler, B. S., Mumford, J. A., Rosenkranz, M. A., Lutz, A., and Davidson, R. J. (2018). Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. Neuroimage 181, 301–313. doi: 10.1016/j.neuroimage.2018.07.013
Kubzansky, L. D., Bordelois, P., Jun, H. J., Roberts, A. L., Cerda, M., Bluestone, N., et al. (2014). The weight of traumatic stress: a prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry 71, 44–51. doi: 10.1001/jamapsychiatry.2013.2798
LeardMann, C. A., Woodall, K. A., Littman, A. J., Jacobson, I. G., Boyko, E. J., Smith, B., et al. (2015). Post-traumatic stress disorder predicts future weight change in the Millennium Cohort Study. Obesity 23, 886–892. doi: 10.1002/oby.21025
Lein, E. S., Hawrylycz, M. J., Ao, N., Ayres, M., Bensinger, A., Bernard, A., et al. (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176.
Levine, A. B., Levine, L. M., and Levine, T. B. (2014). Posttraumatic stress disorder and cardiometabolic disease. Cardiology 127, 1–19.
Li, L., Feng, X., Zhou, Z., Zhang, H., Shi, Q., and Lei, Z. (2018). Stress Accelerates Defensive Responses to Looming in Mice and Involves a Locus Coeruleus-Superior Colliculus Projection. Curr. Biol. 28:859–871.e5. doi: 10.1016/j.cub.2018.02.005
Lkhagvasuren, B., Nakamura, Y., Oka, T., Sudo, N., and Nakamura, K. (2011). Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur. J. Neurosci. 34, 1442–1452. doi: 10.1111/j.1460-9568.2011.07863.x
Martin, E. I., Ressler, K. J., Jasnow, A. M., Dabrowska, J., Hazra, R., Rainnie, D. G., et al. (2010). A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol. Psychiatry 67, 1212–1216.
Maunze, B., Bruckner, K. W., Desai, N. N., Chen, C., Chen, F., Baker, D., et al. (2022). Pituitary adenylate cyclase-activating polypeptide receptor activation in the hypothalamus recruits unique signaling pathways involved in energy homeostasis. Am. J. Physiol. Endocrinol. Metab. 322:E199–E210. doi: 10.1152/ajpendo.00320.2021
McCarthy, M. M., Arnold, A. P., Ball, G. F., Blaustein, J. D., and Vries, G. J. (2012). Sex differences in the brain: the not so inconvenient truth. J. Neurosci. 32, 2241–2247.
Michopoulos, V., Vester, A., and Neigh, G. (2016). Posttraumatic stress disorder: A metabolic disorder in disguise? Exp. Neurol. 284, 220–229. doi: 10.1016/j.expneurol.2016.05.038
Mina, A. I., Leclair, R. A., Leclair, K. B., Cohen, D. E., Lantier, L., and Banks, A. S. (2018). CalR: A Web-Based Analysis Tool for Indirect Calorimetry Experiments. Cell Metab. 28, 656–666.e2. doi: 10.1016/j.cmet.2018.06.019
Miyata, A., Jiang, L., Dahl, R. D., Kitada, C., Kubo, K., Fujino, M., et al. (1990). Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem. Biophys. Res. Commun. 170, 643–648. doi: 10.1016/0006-291x(90)92140-u
Morris, L. S., Mccall, J. G., Charney, D. S., and Murrough, J. W. (2020). The role of the locus coeruleus in the generation of pathological anxiety. Brain Neurosci. Adv. 4:2398212820930321.
Morrison, S. F., and Madden, C. J. (2014). Central nervous system regulation of brown adipose tissue. Compr. Physiol. 4, 1677–1713.
Mothersill, O., and Donohoe, G. (2016). Neural effects of social environmental stress - an activation likelihood estimation meta-analysis. Psychol. Med. 46, 2015–2023.
Mulvey, B., Bhatti, D. L., Gyawali, S., Lake, A. M., Kriaucionis, S., Ford, C. P., et al. (2018). Molecular and Functional Sex Differences of Noradrenergic Neurons in the Mouse Locus Coeruleus. Cell. Rep. 23, 2225–2235.
Naegeli, C., Zeffiro, T., Piccirelli, M., Jaillard, A., Weilenmann, A., Hassanpour, K., et al. (2018). Locus Coeruleus Activity Mediates Hyperresponsiveness in Posttraumatic Stress Disorder. Biol. Psychiatry 83, 254–262. doi: 10.1016/j.biopsych.2017.08.021
Nowotny, B., Cavka, M., Herder, C., Loffler, H., Poschen, U., Joksimovic, L., et al. (2010). Effects of acute psychological stress on glucose metabolism and subclinical inflammation in patients with post-traumatic stress disorder. Horm. Metab. Res. 42, 746–753. doi: 10.1055/s-0030-1261924
Nozu, T., Okano, S., Kikuchi, K., Yahata, T., and Kuroshima, A. (1992). Effect of immobilization stress on in vitro and in vivo thermogenesis of brown adipose tissue. JPN J. Physiol. 42, 299–308.
O’Donnell, T., Hegadoren, K. M., and Coupland, N. C. (2004). Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology 50, 273–283.
Otto, C., Martin, M., Wolfer, D. P., Lipp, H. P., Maldonado, R., and Schutz, G. (2001). Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res. Mol. Brain Res. 92, 78–84. doi: 10.1016/s0169-328x(01)00153-x
Pedersen, S. B., Bruun, J. M., Kristensen, K., and Richelsen, B. (2001). Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem. Biophys. Res. Commun. 288, 191–197. doi: 10.1006/bbrc.2001.5763
Pervanidou, P., and Chrousos, G. P. (2012). Metabolic consequences of stress during childhood and adolescence. Metabolism 61, 611–619.
Pooley, A. E., Benjamin, R. C., Sreedhar, S., Eagle, A. L., Robison, A. J., Mazei-Robison, M. S., et al. (2018). Sex differences in the traumatic stress response: PTSD symptoms in women recapitulated in female rats. Biol. Sex Differ. 9:31.
Rabasa, C., Askevik, K., Schele, E., Hu, M., Vogel, H., and Dickson, S. L. (2019). Divergent Metabolic Effects of Acute Versus Chronic Repeated Forced Swim Stress in the Rat. Obesity 27, 427–433. doi: 10.1002/oby.22390
Rabasa, C., and Dickson, S. L. (2016). Impact of stress on metabolism and energy balance. Curr. Opin. Bhevaioral. Sci. 9, 71–77.
Raikkonen, K., Matthews, K. A., and Kuller, L. H. (2002). The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism 51, 1573–1577.
Rajbhandari, A. K., Gonzalez, S. T., and Fanselow, M. S. (2018). Stress-Enhanced Fear Learning, a Robust Rodent Model of Post-Traumatic Stress Disorder. J. Vis. Exp. 58306.
Rajbhandari, A. K., Octeau, J. C., Gonzalez, S., Pennington, Z. T., Mohamed, F., and Trott, J. (2021). A basomedial amygdala to intercalated cells microcircuit expressing PACAP and its receptor PAC1 regulates contextual fear. J. Neurosci. 41, 3446–3461. doi: 10.1523/JNEUROSCI.2564-20.2021
Rau, V., and Fanselow, M. S. (2009). Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress 12, 125–133.
Ressler, K. J., Mercer, K. B., Bradley, B., Jovanovic, T., Mahan, A., Kerley, K., et al. (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470, 492–497.
Roenholt, S., Beck, N. N., Karsberg, S. H., and Elklit, A. (2012). Post-traumatic stress symptoms and childhood abuse categories in a national representative sample for a specific age group: associations to body mass index. Eur. J. Psychotraumatol. 3. doi: 10.3402/ejpt.v3i0.17188
Rousset, S., Alves-Guerra, M. C., Mozo, J., Miroux, B., Cassard-Doulcier, A. M., Bouillaud, F., et al. (2004). The biology of mitochondrial uncoupling proteins. Diabetes 53:S130–S135.
Rudecki, A. P., and Gray, S. L. (2016). PACAP in the Defense of Energy Homeostasis. Trends Endocrinol. Metab. 27, 620–632. doi: 10.1016/j.tem.2016.04.008
Samuels, E. R., and Szabadi, E. (2008). Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr. Neuropharmacol. 6, 235–253. doi: 10.2174/157015908785777229
Sciolino, N. R., Mazzone, C. R., Plummer, N. W., Evsyukova, I., Amin, J., Smith, K. G., et al. (2019). A role for the locus coeruleus in the modulation of feeding. Biorxiv [Preprint]. doi: 10.1101/2019.12.18.881599
Southwick, S. M., Bremner, J. D., Rasmusson, A., Morgan, C. A. 3RD, Arnsten, A., and Charney, D. S. (1999). Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol. Psychiatry 46, 1192–1204.
Soya, S., Takahashi, T. M., Mchugh, T. J., Maejima, T., Herlitze, S., Abe, M., et al. (2017). Orexin modulates behavioral fear expression through the locus coeruleus. Nat. Commun. 8:1606.
Thorp, A. A., and Schlaich, M. P. (2015). Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015:341583.
Tolin, D. F., and Foa, E. B. (2006). Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol. Bull. 132, 959–992. doi: 10.1037/0033-2909.132.6.959
Udo, T., Grilo, C. M., and Mckee, S. A. (2014). Gender differences in the impact of stressful life events on changes in body mass index. Prev. Med. 69, 49–53.
Ulrich-Lai, Y. M., and Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409.
Valencak, T. G., Osterrieder, A., and Schulz, T. J. (2017). Sex matters: The effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 12, 806–813. doi: 10.1016/j.redox.2017.04.012
Van Bockstaele, E. J., Peoples, J., and Telegan, P. (1999). Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J. Comp. Neurol. 412, 410–428. doi: 10.1002/(sici)1096-9861(19990927)412:3<410::aid-cne3>3.0.co;2-f
Vaudry, D., Falluel-Morel, A., Bourgault, S., Basille, M., Burel, D., Wurtz, O., et al. (2009). Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 61, 283–357. doi: 10.1124/pr.109.001370
Wiedmann, N. M., Stefanidis, A., and Oldfield, B. J. (2017). Characterization of the central neural projections to brown, white, and beige adipose tissue. FASEB J. 31, 4879–4890. doi: 10.1096/fj.201700433R
Wolf, E. J., Sadeh, N., Leritz, E. C., Logue, M. W., Stoop, T. B., Mcglinchey, R., et al. (2016). Posttraumatic Stress Disorder as a Catalyst for the Association Between Metabolic Syndrome and Reduced Cortical Thickness. Biol. Psychiatry 80, 363–371. doi: 10.1016/j.biopsych.2015.11.023
Yang, B., Sanches-Padilla, J., Kondapalli, J., Morison, S. L., Delpire, E., Awatramani, R., et al. (2021). Locus coeruleus anchors a trisynaptic circuit controlling fear-induced suppression of feeding. Neuron 109, 823–838.e6. doi: 10.1016/j.neuron.2020.12.023
Keywords: locus coeruleus, energy expenditure, brown adipose fat tissue, stress, PACAP, PAC1, metabolism
Citation: Duesman SJ, Shetty S, Patel S, Ogale N, Mohamed F, Sparman N, Rajbhandari P and Rajbhandari AK (2022) Sexually dimorphic role of the locus coeruleus PAC1 receptors in regulating acute stress-associated energy metabolism. Front. Behav. Neurosci. 16:995573. doi: 10.3389/fnbeh.2022.995573
Received: 16 July 2022; Accepted: 24 August 2022;
Published: 05 October 2022.
Edited by:
Danai Riga, University Medical Center Utrecht, NetherlandsReviewed by:
Ananya Chowdhury, University of California, Los Angeles, United StatesCopyright © 2022 Duesman, Shetty, Patel, Ogale, Mohamed, Sparman, Rajbhandari and Rajbhandari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abha Karki Rajbhandari, YWJoYS5yYWpiaGFuZGFyaUBtc3NtLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.