
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Behav. Neurosci., 08 September 2022
Sec. Motivation and Reward
Volume 16 - 2022 | https://doi.org/10.3389/fnbeh.2022.977474
This article is part of the Research TopicNeuronal Ensembles and Memory Engrams: Cellular and Molecular MechanismsView all 6 articles
Neuronal ensembles are local, sparsely distributed populations of neurons that are reliably re-activated by a specific stimulus, context or task. Such discrete cell populations can be defined either functionally, by electrophysiological recordings or in vivo calcium imaging, or anatomically, using the expression of markers such as the immediate early gene cFos. A typical example of tasks that involve the formation of neuronal ensembles is reward learning, such as the cue-reward pairing during operant conditioning. These ensembles are re-activated during cue-presentation and increasing evidence suggests that this re-activation is the neurophysiological basis for the execution of reward-seeking behavior. Whilst the pursuit of rewards is a common daily activity, it is also related to the consumption of drugs, such as alcohol, and may result in problematic behaviors including addiction. Recent research has identified neuronal ensembles in several reward-related brain regions that control distinct aspects of a conditioned response, e.g., contextual information about the availability of a specific reward or the actions needed to retrieve this reward under the given circumstances. Here, we review studies using the activity marker cFos to identify and characterize neuronal ensembles related to alcohol and non-drug rewards with a special emphasis on the discrimination between different rewards by meta-ensembles, i.e., by dynamic co-activation of multiple ensembles across different brain areas.
Survival in a changing environment requires experience-dependent or associative learning. This mental process can be empirically studied by the pursuit of consummatory rewards such as food or water. Pleasurable feelings generated by reward consumption act as a positive reinforcement, thereby motivating us to obtain this reward again (McClure et al., 2004). However, powerful rewards can also be obtained from drugs (Kelley and Berridge, 2002). Importantly, the value of any reward depends on the context and the current needs of an individual. A stream’s burbling may serve as a cue for a drink to a thirsty person, for cooling on a hot day or have no reward-association at all when hiking through the rain. Accordingly, the behavior required to obtain a reward, commonly referred to as reward-seeking, depends on the context of stimulus/cue presentation and the recall of a previously established cue-reward memory. Such memories can persist for long times and in the case of drugs, can elicit excessive seeking behavior and relapse even after prolonged periods of abstinence.
The drug with arguably the highest health burden is alcohol (WHO, 2018), which is consumed in moderate quantities by most of the world’s population but excessively by a minority which may develop alcohol use disorder (AUD). A phenomenon often reported by individuals with problematic consumption habits are intrusive thoughts or urges, also called craving. In AUD patients, craving can strongly diminish control over consumption, biased choice and relapse into excessive drinking (Heilig et al., 2019). Thus, gaining insights into the neuronal mechanisms of memory formation, decision making and seeking behavior for drug and natural rewards may open new roads for regaining control in AUD and related diseases (Heinz et al., 2020).
The processing of reward memories and the control over seeking behavior is thought to involve activation of the brain’s reward system, mesolimbic circuits originating in the ventral tegmental area and connecting striatal and prefrontocortical regions as well as the amygdala (Fein and Cardenas, 2015; Lüscher, 2016). These brain regions are broadly activated by reward consumption as well as the presentation of reward-associated cues, leading to the hypothesis that drugs hijack a circuitry that normally serves reward-related learning (Wise, 1987; Nesse and Berridge, 1997).
However, recent studies suggest that presentation of reward cues only activates small subsets of sparsely distributed neurons in a given region (∼3–15%; e.g., Koya et al., 2009; Bossert et al., 2011; Pfarr et al., 2015), which are conceptualized as neuronal ensembles (Hebb, 1949), implying that their activation controls reward-seeking behavior. In this review, we discuss recent progress concerning the importance of neuronal ensembles in reward-seeking. We pay particular attention to recent application of graph theory-based network analysis and the role of co-activation patterns of neuronal ensembles across multiple brain regions with regard to seeking behavior for alcohol and natural, sweet rewards.
Neuronal ensembles are defined as assemblies of neurons that are specifically and reliably activated by a stimulus (Hebb, 1949), although the exact form of co-activation of neurons within an ensemble can range from synchronous action potential firing to synfire chains to activation at some time during a behavioral task (Russo and Durstewitz, 2017). Such a wide definition allows for a variety of methodological approaches to identify the neurons participating in an ensemble and to monitor their activity. High resolution activity patterns in the millisecond range can be obtained by in vivo electrophysiology (e.g., Emberly and Seamans, 2020; Takehara-Nishiuchi et al., 2020; Sachuriga et al., 2021) or calcium imaging (e.g., Shin et al., 2020; Grant et al., 2021) and early studies using these techniques already suggested the existence of different ensembles involved in cocaine and sugar seeking within the nucleus accumbens (Carelli and Wightman, 2004). However, the application of these methods is typically restricted to one brain region and therefore, of limited use for investigating the distribution and interaction of local ensembles across the brain. Furthermore, the real-time detection of the sparsely distributed neurons participating in an ensemble is challenging due to the probabilistic association of environmental stimuli, neuronal responses and behavioral output. Alternatively, ensembles can be defined by the expression of activity markers, such as cFos, NF-κB, or Arc. These markers efficiently label recently activated neurons during a behavioral experiment and provide excellent, single neuron resolution across the entire brain.
The majority of studies relevant for this review have employed the immediate early gene and transcription factor cFos, an effector linking gene expression to synaptic plasticity in a position- and time-dependent manner (Morgan and Curran, 1989). Because of its rapid response properties and short half-life, it is particularly suited to monitor neuronal activity within a certain time window, i.e., stimulus presentation (Kaczmarek, 1993). cFos expression has been directly linked to distinct cellular, neurochemical and behavioral responses (Sommer et al., 1993; Sommer and Fuxe, 1997), and more recently to cue-induced drug or natural reward-seeking (Cruz et al., 2013; Pfarr et al., 2015). Interference with cued ensemble activation can specifically alter cue-triggered behavior, suggesting that neuronal ensembles underly the formation of memory engrams (Josselyn and Tonegawa, 2020). Hence, a local neuronal ensemble formed during the initial learning of a cue-reward association is reactivated upon re-exposure to the cue, which in turn leads to the recall of the reward memory and reward-seeking behavior (e.g., Bossert et al., 2011; Pfarr et al., 2015).
Neuronal ensembles as the basis for reward-seeking have received increasing attention in the past decade, as classical experiments, using lesions or gross inhibition of whole brain regions, produced conflicting results (Cruz et al., 2013). The indiscriminate inactivation of both, the sparse cue-specific ensemble and the vast majority of unrelated neuronal populations may obscure the function of a region. Thus, a brain region’s function during a task may be dominated by a distinct ensemble, or this ensemble’s role is hidden in a high tonic activity. In recent years, causality between the activation of cue-associated neuronal ensembles reward-seeking has been elegantly demonstrated using the Daun02-inactivation method (Koya et al., 2009; Cruz et al., 2013). This approach relies on transgenic cfos-lacZ rats (Kasof et al., 1995) which express the bacterial enzyme β-galactosidase under the cfos promoter. Neurons strongly activated during a behavioral task express cFos and consequently also β-galactosidase. This enzyme converts the prodrug Daun02 into the neurotoxin daunorubicin. Hence, local administration of Daun02 causes apoptosis of previously activated cells, but spares other neurons (Farquhar et al., 2002; Pfarr et al., 2015). Thereby, cFos-defined ensembles can be directly linked to distinct aspects of reward-seeking. Considering alcohol, seeking-related ensembles have been reported in the infralimbic (IL) cortex (Pfarr et al., 2015; Laque et al., 2019), nucleus accumbens (Leão et al., 2015) and amygdala (de Guglielmo et al., 2016). Another alcohol reward-related ensemble in the accumbens was found using NF-κB (nuclear factor kappa-light chain of activated B cells) as an activity marker and driver of the lacZ transgene (Nennig et al., 2018). Interestingly, in this study only about 60% of the induced NF-κB+ cells were neurons, suggesting glia cells as potential ensemble participants. Activity triggered ablation of a specific cue-associated ensemble in the IL, but not inactivation of the entire region, led to excessive seeking behavior (Pfarr et al., 2015), demonstrating that a small population of IL neurons (∼12%) was responsible for suppressing cue-induced alcohol-seeking. The same ensemble was not involved in stress-induced alcohol-seeking. Thus, specific manipulation of a reward-associated neuronal ensemble strongly implicated a group of neurons in the IL as the cellular correlate of a memory that controls specific aspects of reward-seeking, whereas an ensemble in the neighboring prelimbic region active during the same task had no effect on the behavioral output in this paradigm. Using the Daun02 chemogenetic approach, Suto et al. (2016) demonstrated coexisting neuronal ensembles within the IL that are selectively reactive to different environmental cues and either promoted or suppressed sweet reward-seeking. This extended findings of intermingling cFos ensembles mediating both, food reward and extinction memories in the IL (Warren et al., 2016). Furthermore, context-dependent roles of a sweet reward-seeking related ensemble for distinct aspects of behavioral expression were identified by cFos tagging experiments in mice (Jessen et al., 2022).
Taken together, using neuronal activity markers allows the detection of multiple, context/cue-specific ensembles coexisting within the same mPFC region that mediate different and even opposing aspects of behavior. This conclusion is also supported by in vivo electrophysiology and calcium imaging studies (Moorman and Aston-Jones, 2015; Otis et al., 2017). Importantly, ensembles involved in the control of reward-seeking are neither limited to the mPFC nor alcohol and sweet rewards, but have been found in various regions of the brain and for most known rewards (e.g., Bossert et al., 2011; Cruz et al., 2014; Leão et al., 2015; Rubio et al., 2015; de Guglielmo et al., 2016; Warren et al., 2016, 2019; Laque et al., 2019; Sieburg et al., 2019; Kimbrough et al., 2021). Thus, reward memories are represented in neuronal ensembles dispersed across the brain with ensembles in various regions involved in the control of seeking behavior. Moreover, different aspects of behavioral control (e.g., approach or avoidance) can be supported by ensembles within the same brain region, indicating major influence of experimental conditions on behavioral outcome, rather than clear evidence for functional segregation between brain regions.
An important question for a better understanding of reward-seeking and associated aspects of AUD is how memories of different rewards are represented in the brain. And along the same lines, whether there are distinct differences in the representation of natural and drug rewards that can explain the different behavioral outcomes, i.e., the bias toward excessive or compulsive responding that is characteristic for addiction. As outlined above, neuronal ensembles associated with seeking behavior for both reward types can be present within the same brain region, a finding that is also supported by in vivo functional magnetic resonance imaging (MRI) studies in humans and animals (Dudek et al., 2015; Noori et al., 2016). Recently, we addressed the identity of neurons comprising neuronal ensembles associated with alcohol (drug) and saccharin (natural reward) by employing a concurrent 2-reward operant self-administration paradigm in which rats learned to associate specific conditioned cues (odors) with the availability of a paired reward (alcohol or saccharin) and to execute the appropriate behavior in order to obtain the reward (press the correct lever). This experimental protocol ensured that each animal had the same training history and consequently had formed neuronal ensembles for both cue-reward pairs. Using event-specific labeling of cFos expression by two-color fluorescence in situ hybridization (FISH), we identified neurons that were active during the presentation of either one of the paired cues or during both cue presentations, in successive sessions under reinstatement conditions. Taking advantage of the rapid splicing of cfos-mRNA (Kasof et al., 1995), the mature mRNA resulting from the first session could be readily distinguished from the nascent cfos-mRNA present directly after the second session. Interestingly, within the IL both ensembles largely overlapped and only about 25% of cfos-mRNA positive neurons could be specifically attributed to either reward with no differences in cortical layer distribution (Pfarr et al., 2018). Thus, each reward seems to recruit a specific subset of neurons in the IL that signal the availability of the specific paired reward, while another subset of neurons may be recruited by rewards in general. The overall size of the alcohol or saccharine associated ensembles appeared to be quite similar in all brain regions examined and seemed to primarily depend on the brain region and not on the specific reward (Figure 1; Pfarr et al., 2018; Wandres et al., 2021). Likewise, neuronal ensembles in the dorsomedial shell of the NAc, activated by two stimuli of the same valence (morphine and cocaine) have been found to be highly overlapping, too, while stimuli of opposing valences (morphine and foot shock) activated ensembles that showed limited overlap (Xiu et al., 2014; Nawarawong and Olsen, 2020). However, studies in the NAc core and the ventromedial PFC using sucrose and cocaine as concurrent rewards (same valence) found less overlapping neuronal ensembles (Bobadilla et al., 2020; Kane et al., 2021).
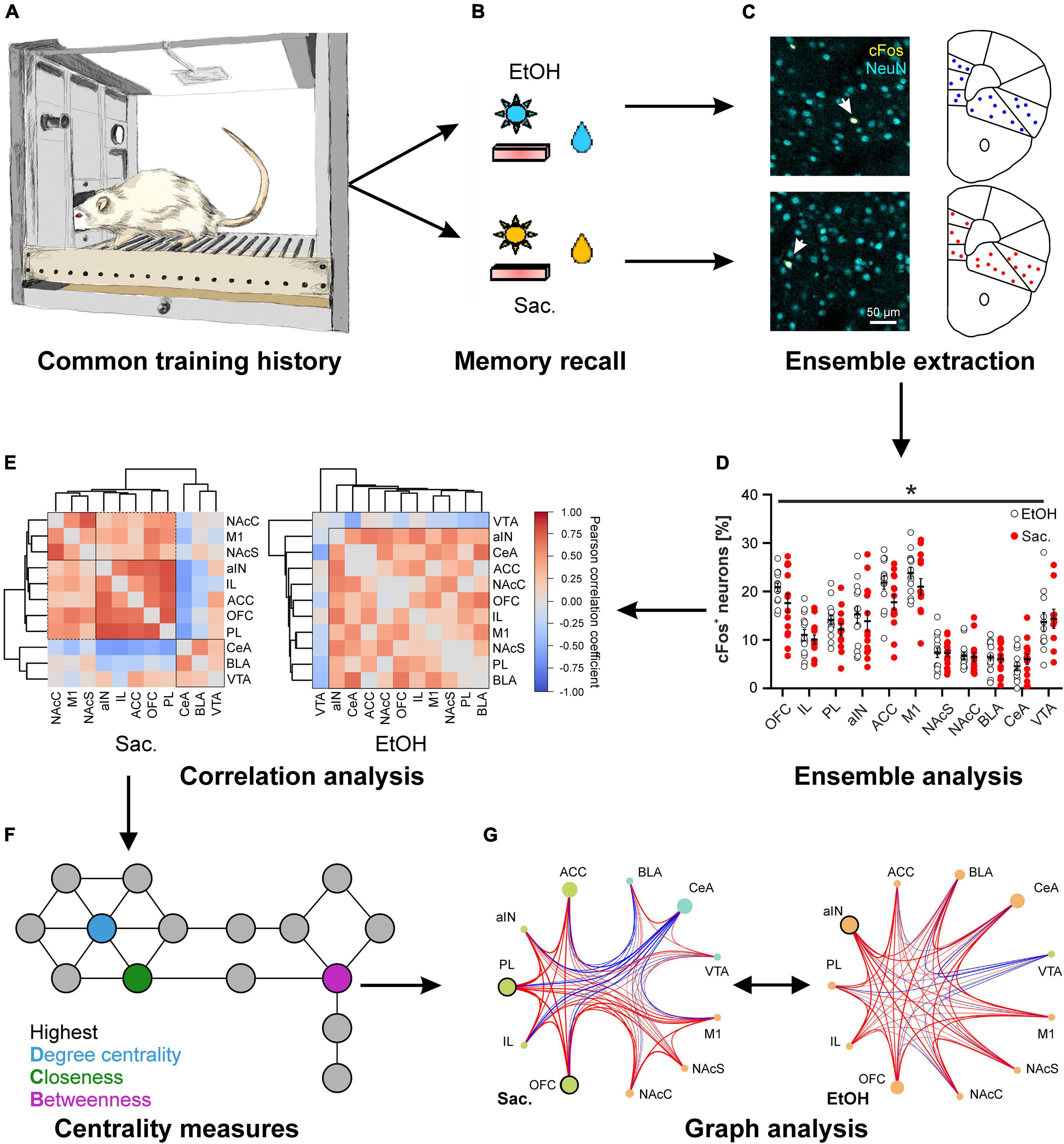
Figure 1. Distinct cue-reward memories are encoded by specific meta-ensembles consisting of co-active neuronal ensembles spread across multiple brain regions. Rats trained on a concurrent 2-reward operant self-administration paradigm establish memories for both rewards (A) that are retrieved under reinstatement conditions, meaning that previously learned reward-specific cues are presented but seeking behavior is not rewarded (B). (C,D) Postmortem cFos expression analysis allows the analysis of neuronal ensembles within distinct brain areas. (C) Representative images of immuno double-labeling of cFos and the pan-neuronal marker NeuN, to determine the fraction of activated neurons, in the central amygdala after reinstatement for alcohol (top) and saccharin (bottom). (D) Quantification of the fraction of cFos labeled, activated neurons in 10 different reward-related brain regions after reinstatement for either alcohol (EtOH, white circles) or saccharin (Sac., red circles) (Data is presented as mean ± s.e.m., *p < 0.05, two-way ANOVA, main effect of group). (E–G) Reward-specific properties of brain activity are only evident after analysis of co-activity across brain regions (E) and subsequent graph theory-based analyses (G) such as analyses of centrality measures (F). (E) Hierarchical clustering of brain regions according to the Pearson correlation coefficients derived from the fraction of cFos labeled neurons after reinstatement for saccharin (Sac., left) or alcohol (EtOH, right). (F) Scheme illustrating three commonly used centrality measures to analyze networks. The degree centrality is defined as the number of nodes directly connected to the node of interest. The closeness and betweenness are measures for the distance from the node of interest to all other nodes and the number of shortest connections between all other nodes that run via the node of interest, respectively. That means, nodes with a high degree centrality have many direct neighbors while those with a high closeness are at short distance to many other nodes. A high betweenness on the other hand indicates that a lot of (short) connections between other nodes, and thus information, run via the node of interest. (G) Example for the analysis of networks derived from the fractions of cFos labeled neurons after reinstatement for saccharin (Sac., left) and alcohol (EtOH, right) showing the recruited meta-ensembles. The size of the circles represents the importance of the brain region in information flow derived from centrality analysis and the strength and color of the connecting lines represents properties of network edges based on the Pearson correlation coefficients (thicker lines = stronger correlation, red: positive correlation, blue: negative correlation). Brain region labels: ACC, anterior cingulate cortex; aIN, anterior insular cortex; BLA, basolateral amygdala; CeA, central amygdala; IL, infralimbic cortex; M1, primary motor cortex; NAcC, nucleus accumbens core; NAcS, nucleus accumbens shell; OFC, orbitofrontal cortex; PL, prelimbic cortex; VTA, ventral tegmental area. [cFos staining in panel (C) as well as panels (D–G) reproduced from Wandres et al. (2021)].
Taken together, the current data suggest that the differences in encoding reward memories for drugs and natural rewards do not necessarily lay in the size or location of the neuronal ensemble, although ensembles in distinct brain regions such as the IL may control certain aspects of reward-seeking behavior and are thus critical to understanding the neurobehavioral processes underlying regaining control over drug taking and seeking.
To gain insight into the differences in memory representation of drug and natural rewards, so far, most studies on the neuronal correlates of reward-seeking focused on one or only a handful of brain regions, ignoring the complexity of the brain’s reward system and possible interactions between neuronal activation patterns in different brain regions. We, therefore, investigated cFos patterns across the reward system in animals that had been trained on the concurrent 2-reward operant self-administration paradigm (Pfarr et al., 2018; Wandres et al., 2021). Since we found no evidence for reward specific encoding by any of the analyzed regions (Figure 1D), we turned to graph theory-based network science, an approach commonly used to analyze the flow of information, e.g., in social media, that has recently been applied to neuroscience (Bullmore and Sporns, 2009). Graph analysis relies on the construction of a network in which brain regions serve as nodes, while the connecting edges are defined by the co-activity between the regions which can be determined by the correlation coefficient of cFos expression (Figure 1E). These networks are described in a formal framework, e.g., in terms of modularity–the presence of functionally segregated modules, centrality–the importance of a region in information flow (Figure 1F), or communication efficiency–a measure of how efficiently information can be exchanged between nodes (Newman, 2010). Thus, graph analysis allows inference on which brain regions form higher order neuronal ensembles or meta-ensembles by high degrees of co-activity and what nodes are most influential to control the network. A major advantage of graph analysis is that the extracted network properties can be tested by proper statistical methods. We and others have recently applied this framework to characterize meta-ensembles involved in reward memories (Kimbrough et al., 2020, 2021; Wandres et al., 2021).
Application of graph analysis to cFos-derived networks revealed significant reward-specific differences in network structure, despite the similar ensemble sizes in individual brain regions (Figure 1; Wandres et al., 2021). Specifically, seeking for saccharin engaged a highly modular network with strong correlations between sub-regions of the network (e.g., mPFC), in which the prelimbic and orbitofrontal cortices were the most important for controlling the flow of information. In contrast, the network recruited during alcohol seeking was not modularly organized and showed a weaker but more broadly connected structure instead. This was also reflected in significantly weaker global and local communication efficiencies in the alcohol condition. Notably, in the alcohol meta-ensemble, the most important region for the control of information flow was the anterior insula while the basolateral amygdala also increased in importance (Figure 1G). Given that alcohol represents a multimodal stimulus–smell, taste, caloric content, and pharmacological effects–the involvement of structures mediating the representation of internal states, such as insula and amygdala, in alcohol-associated memory is highly plausible. Interestingly, the importance of the insula and its connection to the amygdala is one of the most consistent findings across AUD studies (Campbell and Lawrence, 2021; Centanni et al., 2021; Flook et al., 2021; Sommer et al., 2022).
Besides the fundamentally different structures of the meta-ensembles in control of alcohol and saccharin seeking, the statistical graph analysis implicates additional important insights about the representation of reward memories that would have gone unnoticed by the common region centered view. First, cFos meta-ensembles are dynamically remapped on demand. The different task-states have been entrained by previous learning. Such task-specific representational networks may serve as priors supporting decision-making in complex environments (Niv, 2019). Second, our inability to differentiate between rewards based on the activity levels of local ensembles suggests that the remapping of cFos meta-ensembles is likely to ultimately rely on synaptic plasticity induced by Hebbian mechanisms or changes in neuronal excitability. Furthermore, the meta-ensemble approach described here resembles the study of functional connectivity in the human brain and may therefor support translation of research findings.
To conclude, increasing evidence causally links the activity of diverse neuronal ensembles within the reward system to the representation of reward-associated memories and consequently the control of reward-seeking and taking behavior. Network analysis of cFos-derived co-activation patterns across the reward system revealed distinct, clearly separable connectivity states between local ensembles, termed meta-ensembles, in which information transfer was controlled by different brain regions and which could be dynamically recruited upon demand. However, while the meta-ensembles described here may capture some fundamental properties of brain networks, they cannot constitute the entire representation of a specific memory. A postmortem “snap-shot” of a highly dynamic process has inherent limitations, such as the number of observable states, temporal resolution and stability over time. One problem may arise from the specific marker characteristics, such as different expression levels and induction thresholds of cFos in different brain regions or cell types (see e.g., Figure 1D), which may bias the inclusion of brain regions and observable network states. This could potentially be addressed by the use of multiple marker proteins (e.g., Arc, NF-κB). The principle problem, however, exists for all methods. In fMRI, for example, many subcortical areas are seldom examined due to their deep location and small size.
Nevertheless, future studies of reward memories and addiction will need to focus on animal models that properly represent the pathological condition, including a biased choice of alcohol over natural rewards (Meinhardt and Sommer, 2015; Augier et al., 2018; Russo et al., 2018). These studies will benefit from adopting a network perspective by applying systems level approaches and largely unbiased analyses strategies, as recently demonstrated in a large translational AUD research project (Sommer et al., 2022) and the brain-wide investigation of cFos expression in 123 regions from chronically alcohol-drinking mice using light sheet microscopy that elegantly demonstrated decreased modularity of brain networks (Kimbrough et al., 2020). These results are in line with our findings (Wandres et al., 2021) and supported by recent fMRI results from animals (Degiorgis et al., 2022; Pérez-Ramírez et al., 2022) and humans (Bordier et al., 2022; Camchong et al., 2022), Targeting network modules, rather than individual brain regions non-invasively, holds therapeutic potential as demonstrated by a clinical trial in AUD patients using transcranial magnetic stimulation of the mPFC (Harel et al., 2022).
Finally, the better understanding of the dynamics and fluidity of neuronal ensembles will require monitoring and manipulating ensemble activity over extended periods of time and more specifically during behavioral events, e.g., the lever response or reward consumption. Early applications of such technologies are emerging (Brebner et al., 2020; Visser et al., 2020; Domi et al., 2021) and open exciting new possibilities for studying the representation of reward memories and ultimately how to regain control over addictive behaviors (Heinz et al., 2020).
CK and WS wrote the manuscript. Both authors contributed to the article and approved the submitted version.
This work in the authors labs has been supported by the Deutsche Forschungsgemeinschaft by center grants SFB 1134 and to CK and WS, and TRR 265 to WS. The publication of this article was supported by the DFG and Heidelberg University within the “Open Access Publishing” funding program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Augier, E., Barbier, E., Dulman, R. S., Licheri, V., Augier, G., Domi, E., et al. (2018). A molecular mechanism for choosing alcohol over an alternative reward. Science 360, 1321–1326. doi: 10.1126/science.aao1157
Bobadilla, A. C., Dereschewitz, E., Vaccaro, L., Heinsbroek, J. A., Scofield, M. D., and Kalivas, P. W. (2020). Cocaine and sucrose rewards recruit different seeking ensembles in the nucleus accumbens core. Mol. Psychiatry 25, 3150–3163. doi: 10.1038/s41380-020-00888-z
Bordier, C., Weil, G., Bach, P., Scuppa, G., Nicolini, C., Forcellini, G., et al. (2022). Increased network centrality of the anterior insula in early abstinence from alcohol. Addict. Biol. 27:e13096. doi: 10.1111/adb.13096
Bossert, J. M., Stern, A. L., Theberge, F. R. M., Cifani, C., Koya, E., Hope, B. T., et al. (2011). Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat. Neurosci. 14, 420–422. doi: 10.1038/nn.2758
Brebner, L. S., Ziminski, J. J., Margetts-Smith, G., Sieburg, M. C., Reeve, H. M., Nowotny, T., et al. (2020). The emergence of a stable neuronal ensemble from a wider pool of activated neurons in the dorsal medial prefrontal cortex during appetitive learning in mice. J. Neurosci. 40, 395–410. doi: 10.1523/JNEUROSCI.1496-19.2019
Bullmore, E., and Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. doi: 10.1038/nrn2575
Camchong, J., Haynos, A. F., Hendrickson, T., Fiecas, M. B., Gilmore, C. S., Mueller, B. A., et al. (2022). Resting hypoconnectivity of theoretically defined addiction networks during early abstinence predicts subsequent relapse in alcohol use disorder. Cereb. Cortex 32, 2688–2702. doi: 10.1093/cercor/bhab374
Campbell, E. J., and Lawrence, A. J. (2021). It’s more than just interoception: The insular cortex involvement in alcohol use disorder. J. Neurochem. 157, 1644–1651. doi: 10.1111/jnc.15310
Carelli, R. M., and Wightman, R. M. (2004). Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr. Opin. Neurobiol. 14, 763–768. doi: 10.1016/j.conb.2004.10.001
Centanni, S. W., Janes, A. C., Haggerty, D. L., Atwood, B., and Hopf, F. W. (2021). Better living through understanding the insula: Why subregions can make all the difference. Neuropharmacology 198, 108765. doi: 10.1016/j.neuropharm.2021.108765
Cruz, F. C., Babin, K. R., Leao, R. M., Goldart, E. M., Bossert, J. M., Shaham, Y., et al. (2014). Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J. Neurosci. 34, 7437–7446. doi: 10.1523/JNEUROSCI.0238-14.2014
Cruz, F. C., Koya, E., Guez-Barber, D. H., Bossert, J. M., Lupica, C. R., Shaham, Y., et al. (2013). New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat. Rev. Neurosci. 14, 743–754. doi: 10.1038/nrn3597
de Guglielmo, G., Crawford, E., Kim, S., Vendruscolo, L. F., Hope, B. T., Brennan, M., et al. (2016). Recruitment of a neuronal ensemble in the central nucleus of the amygdala is required for alcohol dependence. J. Neurosci. 36, 9446–9453. doi: 10.1523/JNEUROSCI.1395-16.2016
Degiorgis, L., Arefin, T. M., Ben-Hamida, S., Noblet, V., Antal, C., Bienert, T., et al. (2022). Translational structural and functional signatures of chronic alcohol effects in mice. Biol. Psychiatry 91, 1039–1050. doi: 10.1016/j.biopsych.2022.02.013
Domi, E., Xu, L., Toivainen, S., Nordeman, A., Gobbo, F., Venniro, M., et al. (2021). A neural substrate of compulsive alcohol use. Sci. Adv. 7:eabg9045. doi: 10.1126/sciadv.abg9045
Dudek, M., Abo-Ramadan, U., Hermann, D., Brown, M., Canals, S., Sommer, W. H., et al. (2015). Brain activation induced by voluntary alcohol and saccharin drinking in rats assessed with manganese-enhanced magnetic resonance imaging. Addict. Biol. 20, 1012–1021. doi: 10.1111/adb.12179
Emberly, E., and Seamans, J. K. (2020). Abrupt, asynchronous changes in action representations by anterior cingulate cortex neurons during trial and error learning. Cereb. Cortex 30, 4336–4345. doi: 10.1093/cercor/bhaa019
Farquhar, D., Pan, B. F., Sakurai, M., Ghosh, A., Mullen, C. A., and Nelson, J. A. (2002). Suicide gene therapy using E. coli beta-galactosidase. Cancer Chemother. Pharmacol. 50, 65–70. doi: 10.1007/s00280-002-0438-2
Fein, G., and Cardenas, V. A. (2015). Neuroplasticity in human alcoholism: studies of extended abstinence with potential treatment implications. Alcohol. Res. 37, 125–141.
Flook, E. A., Luchsinger, J. R., Silveri, M. M., Winder, D. G., Benningfield, M. M., and Blackford, J. U. (2021). Anxiety during abstinence from alcohol: a systematic review of rodent and human evidence for the anterior insula’s role in the abstinence network. Addict. Biol. 26:e12861. doi: 10.1111/adb.12861
Grant, R. I., Doncheck, E. M., Vollmer, K. M., Winston, K. T., Romanova, E. V., Siegler, P. N., et al. (2021). Specialized coding patterns among dorsomedial prefrontal neuronal ensembles predict conditioned reward seeking. eLife 10:e65764. doi: 10.7554/eLife.65764.sa2
Harel, M., Perini, I., Kämpe, R., Alyagon, U., Shalev, H., Besser, I., et al. (2022). Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol. Psychiatry 91, 1061–1069. doi: 10.1016/j.biopsych.2021.11.020
Heilig, M., Augier, E., Pfarr, S., and Sommer, W. H. (2019). Developing neuroscience-based treatments for alcohol addiction: A matter of choice? Transl. Psychiatry 9:255. doi: 10.1038/s41398-019-0591-6
Heinz, A., Kiefer, F., Smolka, M. N., Endrass, T., Beste, C., Beck, A., et al. (2020). Addiction research consortium: losing and regaining control over drug intake (ReCoDe)-From trajectories to mechanisms and interventions. Addict. Biol. 25:e12866. doi: 10.1111/adb.12866
Jessen, K., Slaker Bennett, M. L., Liu, S., and Olsen, C. M. (2022). Comparison of prefrontal cortex sucrose seeking ensembles engaged in multiple seeking sessions: Context is key. J. Neurosci. Res. 100, 1008–1029. doi: 10.1002/jnr.25025
Josselyn, S. A., and Tonegawa, S. (2020). Memory engrams: recalling the past and imagining the future. Science 367:39. doi: 10.1126/science.aaw4325
Kaczmarek, L. (1993). Molecular biology of vertebrate learning: is c-fos a new beginning? J. Neurosci. Res. 34, 377–381. doi: 10.1002/jnr.490340402
Kane, L., Venniro, M., Quintana-Feliciano, R., Madangopal, R., Rubio, F. J., Bossert, J. M., et al. (2021). Fos-expressing neuronal ensemble in rat ventromedial prefrontal cortex encodes cocaine seeking but not food seeking in rats. Addict. Biol. 26:e12943. doi: 10.1111/adb.12943
Kasof, G. M., Mandelzys, A., Maika, S. D., Hammer, R. E., Curran, T., and Morgan, J. I. (1995). Kainic acid-induced neuronal death is associated with DNA damage and a unique immediate-early gene response in c-fos-lacZ transgenic rats. J. Neurosci. 15, 4238–4249. doi: 10.1523/JNEUROSCI.15-06-04238.1995
Kelley, A. E., and Berridge, K. C. (2002). The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 22, 3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002
Kimbrough, A., Kallupi, M., Smith, L. C., Simpson, S., Collazo, A., and George, O. (2021). Characterization of the brain functional architecture of psychostimulant withdrawal using single-cell whole-brain imaging. eNeuro 8:ENEURO.0208-19.2021. doi: 10.1523/ENEURO.0208-19.2021
Kimbrough, A., Lurie, D. J., Collazo, A., Kreifeldt, M., Sidhu, H., Macedo, G. C., et al. (2020). Brain-wide functional architecture remodeling by alcohol dependence and abstinence. Proc. Natl. Acad. Sci. U.S.A. 117, 2149–2159. doi: 10.1073/pnas.1909915117
Koya, E., Golden, S. A., Harvey, B. K., Guez-Barber, D. H., Berkow, A., Simmons, D. E., et al. (2009). Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat. Neurosci. 12, 1069–1073. doi: 10.1038/nn.2364
Laque, A. L., De Ness, G., Wagner, G. E., Nedelescu, H., Carroll, A., Watry, D., et al. (2019). Anti-relapse neurons in the infralimbic cortex of rats drive relapse-suppression by drug omission cues. Nat. Commun. 10:3934. doi: 10.1038/s41467-019-11799-1
Leão, R. M., Cruz, F. C., Vendruscolo, L. F., de Guglielmo, G., Logrip, M. L., Planeta, C. S., et al. (2015). Chronic nicotine activates stress/reward-related brain regions and facilitates the transition to compulsive alcohol drinking. J. Neurosci. 35, 6241–6253. doi: 10.1523/JNEUROSCI.3302-14.2015
Lüscher, C. (2016). The emergence of a circuit model for addiction. Annu. Rev. Neurosci. 39, 257–276. doi: 10.1146/annurev-neuro-070815-013920
McClure, S. M., York, M. K., and Montague, P. R. (2004). The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist 10, 260–268. doi: 10.1177/1073858404263526
Meinhardt, M. W., and Sommer, W. H. (2015). Postdependent state in rats as a model for medication development in alcoholism. Addict. Biol. 20, 1–21. doi: 10.1111/adb.12187
Moorman, D. E., and Aston-Jones, G. (2015). Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc. Natl. Acad. Sci. U.S.A. 112, 9472–9477. doi: 10.1073/pnas.1507611112
Morgan, J. I., and Curran, T. (1989). Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 12, 459–462. doi: 10.1016/0166-2236(89)90096-9
Nawarawong, N. N., and Olsen, C. M. (2020). Within-animal comparisons of novelty and cocaine neuronal ensemble overlap in the nucleus accumbens and prefrontal cortex. Behav. Brain Res. 379:112275. doi: 10.1016/j.bbr.2019.112275
Nennig, S. E., Fulenwider, H. D., Chimberoff, S. H., Smith, B. M., Eskew, J. E., Sequeira, M. K., et al. (2018). Selective lesioning of nuclear factor-κB activated cells in the nucleus accumbens shell attenuates alcohol place preference. Neuropsychopharmacology 43, 1032–1040. doi: 10.1038/npp.2017.214
Nesse, R. M., and Berridge, K. C. (1997). Psychoactive drug use in evolutionary perspective. Science 278, 63–66. doi: 10.1126/science.278.5335.63
Newman, M. E. (2010). Networks: An Introduction. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780199206650.001.0001
Niv, Y. (2019). Learning task-state representations. Nat. Neurosci. 22, 1544–1553. doi: 10.1038/s41593-019-0470-8
Noori, H. R., Cosa Linan, A., and Spanagel, R. (2016). Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: A comprehensive meta-analysis. Eur. Neuropsychopharmacol. 26, 1419–1430. doi: 10.1016/j.euroneuro.2016.06.013
Otis, J. M., Namboodiri, V. M., Matan, A. M., Voets, E. S., Mohorn, E. P., Kosyk, O., et al. (2017). Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature 543, 103–107. doi: 10.1038/nature21376
Pérez-Ramírez, Ú, López-Madrona, V. J., Pérez-Segura, A., Pallarés, V., Moreno, A., Ciccocioppo, R., et al. (2022). Brain network allostasis after chronic alcohol drinking is characterized by functional dedifferentiation and narrowing. J. Neurosci. 42, 4401–4413. doi: 10.1523/JNEUROSCI.0389-21.2022
Pfarr, S., Meinhardt, M. W., Klee, M. L., Hansson, A. C., Vengeliene, V., Schönig, K., et al. (2015). Losing control: excessive alcohol seeking after selective inactivation of cue-responsive neurons in the infralimbic cortex. J. Neurosci. 35, 10750–10761. doi: 10.1523/JNEUROSCI.0684-15.2015
Pfarr, S., Schaaf, L., Reinert, J. K., Paul, E., Herrmannsdörfer, F., Roßmanith, M., et al. (2018). Choice for drug or natural reward engages largely overlapping neuronal ensembles in the infralimbic prefrontal cortex. J. Neurosci. 38, 3507–3519. doi: 10.1523/JNEUROSCI.0026-18.2018
Rubio, F. J., Liu, Q.-R., Li, X., Cruz, F. C., Leão, R. M., Warren, B. L., et al. (2015). Context-induced reinstatement of methamphetamine seeking is associated with unique molecular alterations in Fos-expressing dorsolateral striatum neurons. J. Neurosci. 35, 5625–5639. doi: 10.1523/JNEUROSCI.4997-14.2015
Russo, E., and Durstewitz, D. (2017). Cell assemblies at multiple time scales with arbitrary lag constellations. eLife 6:e19428. doi: 10.7554/eLife.19428
Russo, M., Funk, D., Loughlin, A., Coen, K., and Lê, A. D. (2018). Effects of alcohol dependence on discrete choice between alcohol and saccharin. Neuropsychopharmacology 43, 1859–1866. doi: 10.1038/s41386-018-0101-1
Sachuriga, Nishimaru, H., Takamura, Y., Matsumoto, J., Ferreira Pereira de Araújo, M., Ono, T., et al. (2021). Neuronal representation of locomotion during motivated behavior in the mouse anterior cingulate cortex. Front. Syst. Neurosci. 15:655110. doi: 10.3389/fnsys.2021.655110
Shin, J. H., Song, M., Paik, S. B., and Jung, M. W. (2020). Spatial organization of functional clusters representing reward and movement information in the striatal direct and indirect pathways. Proc. Natl. Acad. Sci. U.S.A. 117, 27004–27015. doi: 10.1073/pnas.2010361117
Sieburg, M. C., Ziminski, J. J., Margetts-Smith, G., Reeve, H. M., Brebner, L. S., Crombag, H. S., et al. (2019). Reward devaluation attenuates cue-evoked sucrose seeking and is associated with the elimination of excitability differences between ensemble and non-ensemble neurons in the nucleus accumbens. eNeuro 6:ENEURO.0338-19.2019. doi: 10.1523/ENEURO.0338-19.2019
Sommer, W., Bjelke, B., Ganten, D., and Fuxe, K. (1993). Antisense oligonucleotide to c-fos induces ipsilateral rotational behaviour to d-amphetamine. Neuroreport 5, 277–280. doi: 10.1097/00001756-199312000-00024
Sommer, W., and Fuxe, K. (1997). On the role of c-fos expression in striatal transmission, the antisense oligonucleotide approach. Neurochem. Int. 31, 425–436. doi: 10.1016/S0197-0186(96)00112-X
Sommer, W. H., Canals, S., Bifone, A., Heilig, M., and Hyytiä, P. (2022). From a systems view to spotting a hidden island: a narrative review implicating insula function in alcoholism. Neuropharmacology 209:108989. doi: 10.1016/j.neuropharm.2022.108989
Suto, N., Laque, A., De Ness, G. L., Wagner, G. E., Watry, D., Kerr, T., et al. (2016). Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. eLife 5:e21920. doi: 10.7554/eLife.21920.007
Takehara-Nishiuchi, K., Morrissey, M. D., and Pilkiw, M. (2020). Prefrontal neural ensembles develop selective code for stimulus associations within minutes of novel experiences. J. Neurosci. 40, 8355–8366. doi: 10.1523/JNEUROSCI.1503-20.2020
Visser, E., Matos, M. R., van der Loo, R. J., Marchant, N. J., de Vries, T. J., Smit, A. B., et al. (2020). A persistent alcohol cue memory trace drives relapse to alcohol seeking after prolonged abstinence. Sci. Adv. 6:eaax7060. doi: 10.1126/sciadv.aax7060
Wandres, M., Pfarr, S., Molnár, B., Schöllkopf, U., Ercsey-Ravasz, M., Sommer, W. H., et al. (2021). Alcohol and sweet reward are encoded by distinct meta-ensembles. Neuropharmacology 195:108496. doi: 10.1016/j.neuropharm.2021.108496
Warren, B. L., Kane, L., Venniro, M., Selvam, P., Quintana-Feliciano, R., Mendoza, M. P., et al. (2019). Separate vmPFC ensembles control cocaine self-administration versus extinction in rats. J. Neurosci. 39, 7394–7407. doi: 10.1523/JNEUROSCI.0918-19.2019
Warren, B. L., Mendoza, M. P., Cruz, F. C., Leao, R. M., Caprioli, D., Rubio, F. J., et al. (2016). Distinct fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J. Neurosci. 36, 6691–6703. doi: 10.1523/JNEUROSCI.0140-16.2016
Wise, R. A. (1987). The role of reward pathways in the development of drug dependence. Pharmacol. Ther. 35, 227–263. doi: 10.1016/0163-7258(87)90108-2
Keywords: alcohol, substance abuse, reward-seeking, neuronal ensemble, graph theory, infralimbic, amygdala, insula
Citation: Körber C and Sommer WH (2022) From ensembles to meta-ensembles: Specific reward encoding by correlated network activity. Front. Behav. Neurosci. 16:977474. doi: 10.3389/fnbeh.2022.977474
Received: 24 June 2022; Accepted: 15 August 2022;
Published: 08 September 2022.
Edited by:
Sean B. Ostlund, University of California, Irvine, United StatesReviewed by:
Christopher Olsen, Medical College of Wisconsin, United StatesCopyright © 2022 Körber and Sommer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christoph Körber, a29lcmJlckB1bmktaGVpZGVsYmVyZy5kZQ==; Wolfgang H. Sommer, d29sZmdhbmcuc29tbWVyQHppLW1hbm5oZWltLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.