- 1Medical School, Kunming University of Science and Technology, Kunming, China
- 2Shenzhen Key Laboratory of Drug Addiction, Brain Cognition and Brain Disease Institute, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
- 3Shenzhen Key Laboratory of Viral Vectors for Biomedicine, Brain Cognition and Brain Disease Institute, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
- 4Shenzhen-Hong Kong Institute of Brain Science, Shenzhen Fundamental Research Institutions, Shenzhen, China
- 5CAS Key Laboratory of Brain Connectome and Manipulation, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Background: The dorsolateral prefrontal cortex (DLPFC) is a key node of the frontal cognitive circuit. It is involved in executive control and many cognitive processes. Abnormal activities of DLPFC are likely associated with many psychiatric diseases. Modulation of DLPFC may have potential beneficial effects in many neural and psychiatric diseases. One of the widely used non-invasive neuromodulation technique is called transcranial direct current stimulation (or tDCS), which is a portable and affordable brain stimulation approach that uses direct electrical currents to modulate brain functions.
Objective: This review aims to discuss the results from the past two decades which have shown that tDCS can relieve clinical symptoms in various neurological and psychiatric diseases.
Methods: Here, we performed searches on PubMed to collect clinical and preclinical studies that using tDCS as neuromodulation technique, DLPFC as the stimulation target in treating neuropsychiatric disorders. We summarized the stimulation sites, stimulation parameters, and the overall effects in these studies.
Results: Overall, tDCS stimulation of DLPFC could alleviate the clinical symptoms of schizophrenia, depression, drug addiction, attention deficit hyperactivity disorder and other mental disorders.
Conclusion: The stimulation parameters used in these studies were different from each other. The lasting effect of stimulation was also not consistent. Nevertheless, DLPFC is a promising target for non-invasive stimulation in many psychiatric disorders. TDCS is a safe and affordable neuromodulation approach that has potential clinical uses. Larger clinical studies will be needed to determine the optimal stimulation parameters in each condition.
Introduction
Neuropsychiatric disorders are combinations of psychiatric and neurologic malfunction that deal with mental disorders, including degenerative diseases, addictions, mood disorders, neurotic disorders, etc. Current treatments of neuropsychiatric diseases mainly include drug therapy, physical therapy and psychotherapy. Common physical therapies included electroconvulsive treatment (ECT), deep brain stimulation (DBS), transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), etc. Among these techniques, tDCS becomes an increasingly employed clinically due to its economical, convenient, non-invasive and mild side effects. However, current dilemma in using tDCS as a option of clinical treatment is that there is no common standard, and the therapeutic effects vary from case to case.
In this review, we discussed: (1) the mechanism of tDCS and the application of tDCS technique in clinical research, focusing on five types of psychiatric disorders; (2) and the potential therapeutic brain target DLPFC.
An Overview of Transcranial Direct Current Stimulation Technique
Accumulating knowledge has supported that transcranial direct current stimulation (tDCS) can relieve symptoms of various diseases, including pain (Wrigley et al., 2013), depression (Sharafi et al., 2019), schizophrenia (Brunelin et al., 2012a), attention deficit disorder (Cosmo et al., 2015), drug addiction (da Silva et al., 2013), and anxiety disorder (Heeren et al., 2017). In recent years, tDCS has been widely used in clinical research due to the advantages mentioned above. tDCS is a non-invasive brain stimulation technique that uses low-intensity direct current (1–2 mA) to modulate cortical activity (Woods et al., 2016). A common tDCS stimulator consists of a controller to generate a constant current, and at least one pair of stimulation electrodes to attach to the surface of the scalp. Although there is no uniform standard for stimulation parameters in clinical studies, electrodes of 20–35 cm2, with application of 1–2 mA currents, 20- or 30-min stimulation duration for one session with one or multiple sessions through a certain period have been employed in a large body of studies.
The activity of the brain is based on the electrical activity of neurons. It is believed that tDCS may modulate the brain activity at different scales. First, from a macro perspective, tDCS likely modulate the brain activity via changing the cortical excitability directly. In general, anodal stimulation depolarizes neurons, whereas cathodal stimulation hyperpolarizes neurons (Purpura and McMurtry, 1965; Bikson et al., 2004). In addition, tDCS may regulate the activity of neural networks by influencing other brain regions associated with the target brain region. It has been suggested that neuronal networks were more sensitive than single neuron in the weak electric field (Francis et al., 2003). By using resting-state functional magnetic resonance imaging (fMRI) technique, it has been found that anode tDCS intensified the functional connection among the thalamus, the temporal lobe and the left caudate nucleus (Dalong et al., 2020). At the neuronal levels, tDCS has been shown to modulate the neural oscillations. McDermott et al. (2019) reported that anode tDCS increased spontaneous activity in the theta (4–7 Hz) and alpha (9–14 Hz) bands in prefrontal and occipital cortices in a flanker task. Finally, from the molecular perspective, tDCS may modulate neurotransmitter release to regulate synaptic plasticity. For example, long-term potentiation (LTP) which was observed after anodal tDCS coupling with synaptic activation (Fritsch et al., 2010). Another study found that the effects of tDCS may be related to the polarity-specific changes in neurotransmitter concentrations. Anodal tDCS caused locally reduced GABA concentrations while cathodal stimulation caused reduced glutamatergic neuronal activity with a highly correlated increase in GABA concentration (Stagg et al., 2009). Liebetanz et al. (2002) showed that, dextromethorphan, an antagonist of N-Methyl-D-Aspartic Acid receptors (NMDAR, receptors that are involved in synaptic plasticity regulation), suppressed the post-stimulation effects of both anode and cathode stimulation.
In order to recommend this convenient technique as a powerful therapeutic strategy, a remarkable effort is still needed to further understand how tDCS modulate the brain activity.
Dorsolateral Prefrontal Cortex Is a Target for Non-Invasive Stimulation in Neuropsychiatric Diseases
One of the most common cortical targets for tDCS is the dorsolateral prefrontal cortex (DLPFC; Figure 1). DLPFC is a structurally and functionally heterogeneous region (Glasser et al., 2016), and is closely related with cognitive functions [attention (Vossel et al., 2014; Bidet-Caulet et al., 2015), decision-making (Philiastides et al., 2011; Rahnev et al., 2016), working memory (Barbey et al., 2013), and emotion regulation (Shahani and Russell, 1969; Buhle et al., 2014; Frank et al., 2014)]. The DLPFC is located in the middle frontal gyrus, and it is a part of the prefrontal cortex (PFC) which regulates the marginal reward area, and involves in higher executive function and impulsive behaviors (Fitzpatrick et al., 2013; Xu et al., 2017). The left DLPFC connects to the primary motor area, primary sensory area, etc. It mainly participates in pain perception and emotional cognitive processing through a top-down neural network (Koenigs and Grafman, 2009; Vaseghi et al., 2015). The right DLPFC is selectively involved in processing pessimistic, negative emotions and mediates vigilance and arousal (Hecht, 2010). DLPFC has become an important target in the treatment for mental disorders.
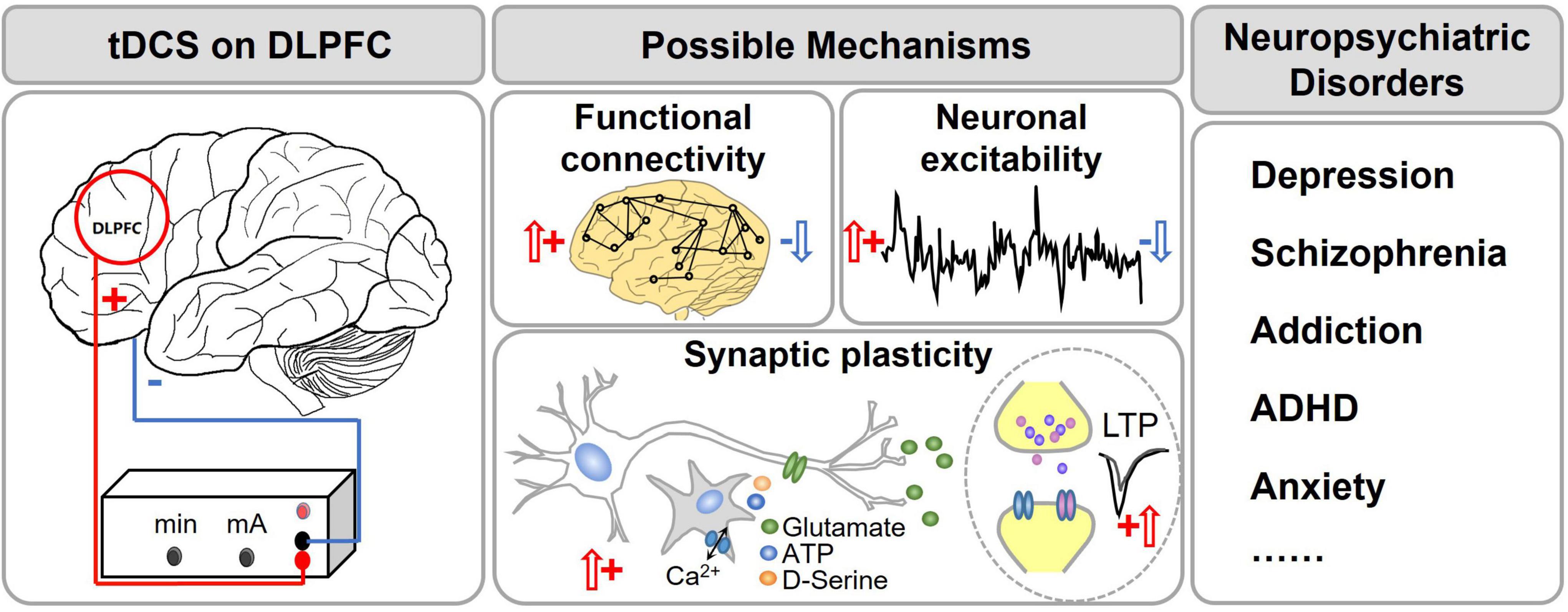
Figure 1. tDCS of the dorsal lateral prefrontal cortex (DLPFC) for treatment of neuropsychiatric disorders. The red circle shows the DLPFC. It is the center for higher brain functions such as working memory, executive function, attention, etc. Dysfunction of DLPFC was found in many psychiatric disorders such as schizophrenia, depression, ADHD, etc. tDCS of DLPFC has become a popular treatment option for these disorders. It has been proposed that tDCS changes the functional connectivity, neuronal excitability and synaptic plasticity of the related brain regions.
A large number of studies have shown that tDCS targeting at DLPFC can alleviate a variety of neuronal and psychiatric diseases symptoms. For example, anode tDCS (2 mA) can reduce the pain caused by multiple sclerosis (Ayache et al., 2016). Anode stimulation of the right DLPFC, and cathode at the left DLPFC improved the risk preference of the subjects (Yang et al., 2017). Studies have shown that anodal tDCS stimulation of left DLPFC could decrease negative emotions and improve cognitive control (Pena-Gomez et al., 2011). Here, we summarize and discuss perspectives of the parameters and effects of tDCS targeting DLPFC in the treatment of different types of neuropsychiatric disorders.
Depression
Depression (also known as depressive disorder) is a mental disease that causes a persistent feeling of sadness and loss of interests, with high recurrence rate, disability rate and suicide rate. In general, it can be classified into major depression, bipolar disorder or treatment-resistant depression. Bipolar disorder, causing extreme mood swings that include emotional highs (mania or hypomania) and lows (depression). Treatment-resistant depression refers to no response to at least two different antidepressant treatments.
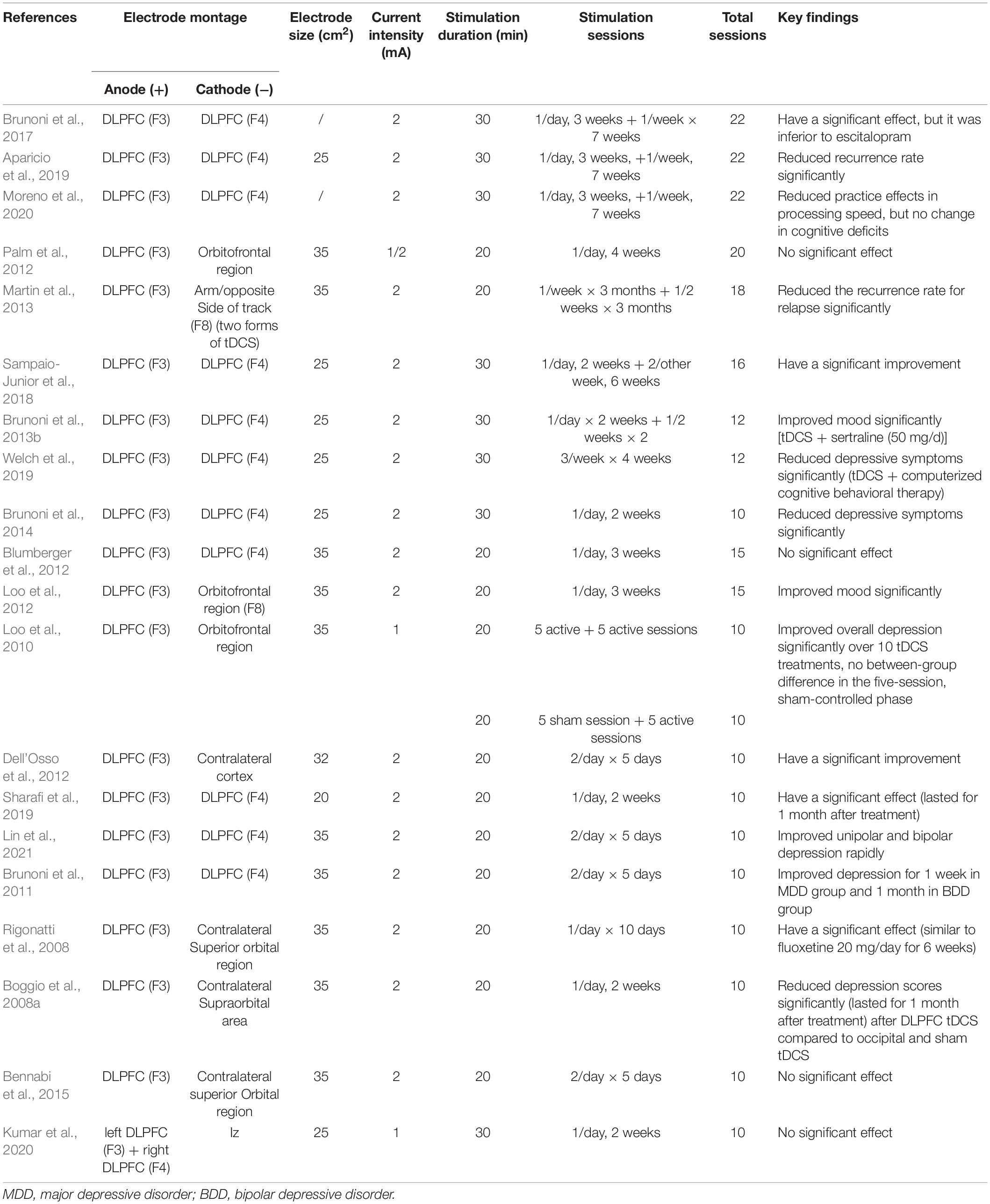
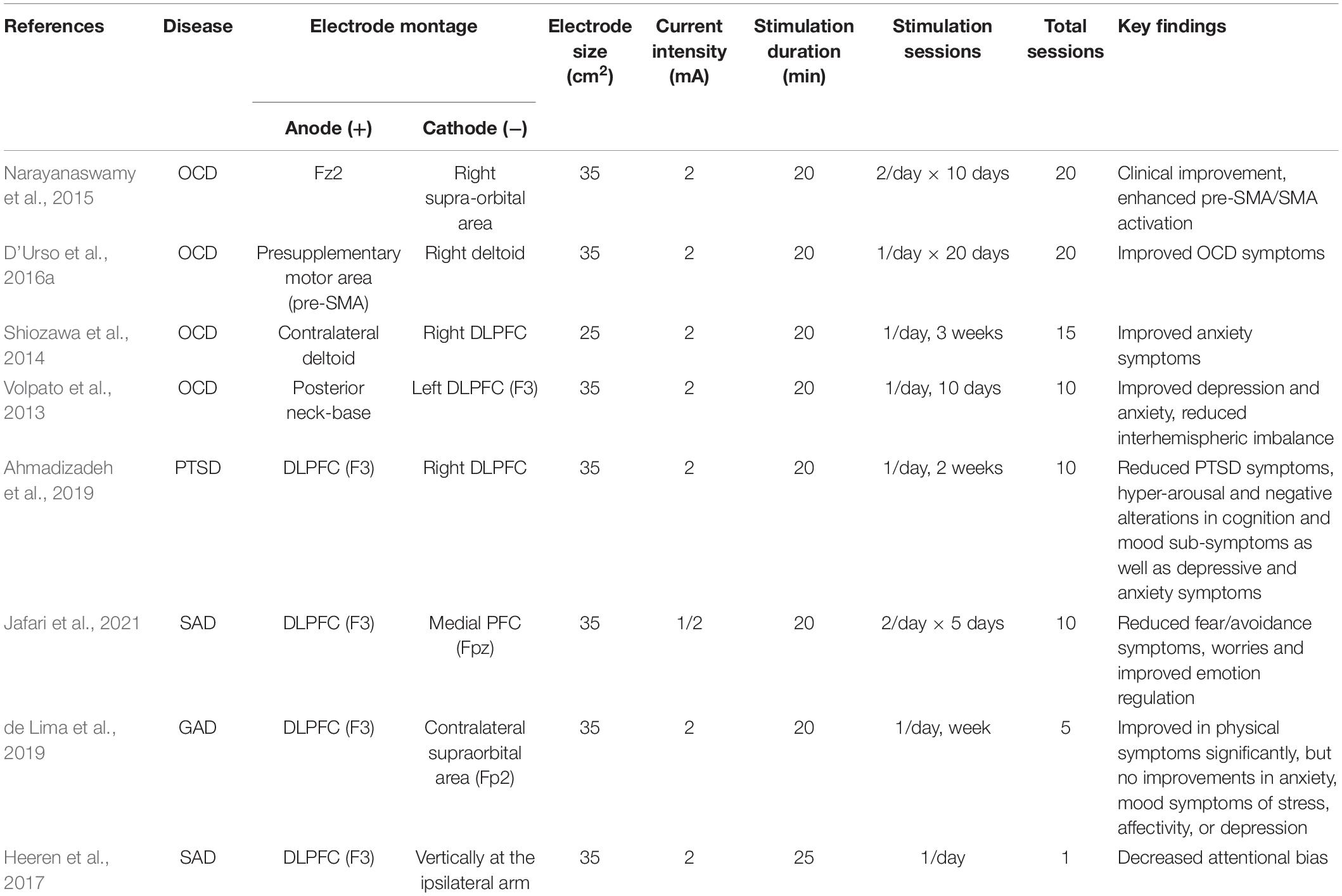
Twenty studies collected from PubMed were shown in Table 1. Majority of these studies have shown that tDCS targeting at DLPFC (mostly the left DLPFC) can significantly improve depression symptoms for a month or longer. All studies placed the anode electrodes on the left DLPFC and the cathode electrodes on the opposite side (right DLPFC or orbitofrontal region). 17 out of 20 studies reported improvement of depressive symptoms. Besides, tDCS also improved working memory and attention (Loo et al., 2012). Importantly, tDCS in combination with other treatments, such as an antidepressant drug (Brunoni et al., 2013b) or with computerized cognitive behavioral therapy (Welch et al., 2019), can reduce depressive symptoms even better than tDCS alone (Brunoni et al., 2013a). It is important to note that tDCS on DLPFC may have some side effects, such as mania, although this is not common (Loo et al., 2012). For the stimulation parameters, most studies have used a current of 2 mA, electrode sizes of 25–35 cm2, and a total of more than five sessions (see details in Table 1). Though various parameters have shown different effects on depression symptoms, most stimulation protocols with longer stimulation duration for one session and repeated sessions were shown to have therapeutic effects.
Schizophrenia
Schizophrenia is a chronic mental disorder. The most typical symptoms of schizophrenia include hallucinations and delusions, which are often referred to as positive symptoms. Schizophrenia may also experience negative symptoms, such as social withdrawal, anhedonia, hyperboulia, affective blunting and alogia (Carpenter et al., 2016). In recent years, clinical studies have shown that tDCS may be effective in reducing auditory hallucination symptoms in patients with schizophrenia. For example, a study reported that anode tDCS showed a significant increase in short- interval intracortical inhibition in the left motor cortex, but no change in intra-cortical facilitation (ICF) compared to sham stimulation (Gordon et al., 2019). Yoon et al. (2019) found that decreased functional network connectivity was negatively correlated with the increase of hallucinogenic behavior at baseline and was significantly enhanced after anode 2 mA tDCS. This may suggest that fronto-temporal tDCS may regulate abnormal hallucination-related functional network connectivity in patients with schizophrenia. Decline in insight is also one of the main symptoms of schizophrenia. Patients with insight deficits often fail to recognize that they are ill and may refuse treatment. Bose et al. (2014) found that 2 mA anode tDCS stimulation over left DLPFC and cathode over the left temporo-parietal junction, could improve the insight and decrease auditory hallucination symptoms in patients. However, no such effect was observed after 1 mA stimulation, which indicates that the current intensity of tDCS is a key factor (Hill et al., 2016). A combination of medication, physical therapy, and psychotherapy usually have a synergic effect. Non-invasive brain stimulation combined with physical therapy has been shown to improve motor performance and language function in stroke patients (Barros Galvao et al., 2014; Rubi-Fessen et al., 2015). Orlov et al. (2017) found that anode tDCS stimulation combined with cognitive behavioral training showed significant improvement in working memory and learning. However, Shiozawa et al. (2016) found that tDCS combined with cognitive training failed to produce a synergic effect in schizophrenia patients. This may due to the small sample size and the use of antipsychotics in patients (Orlov et al., 2017).
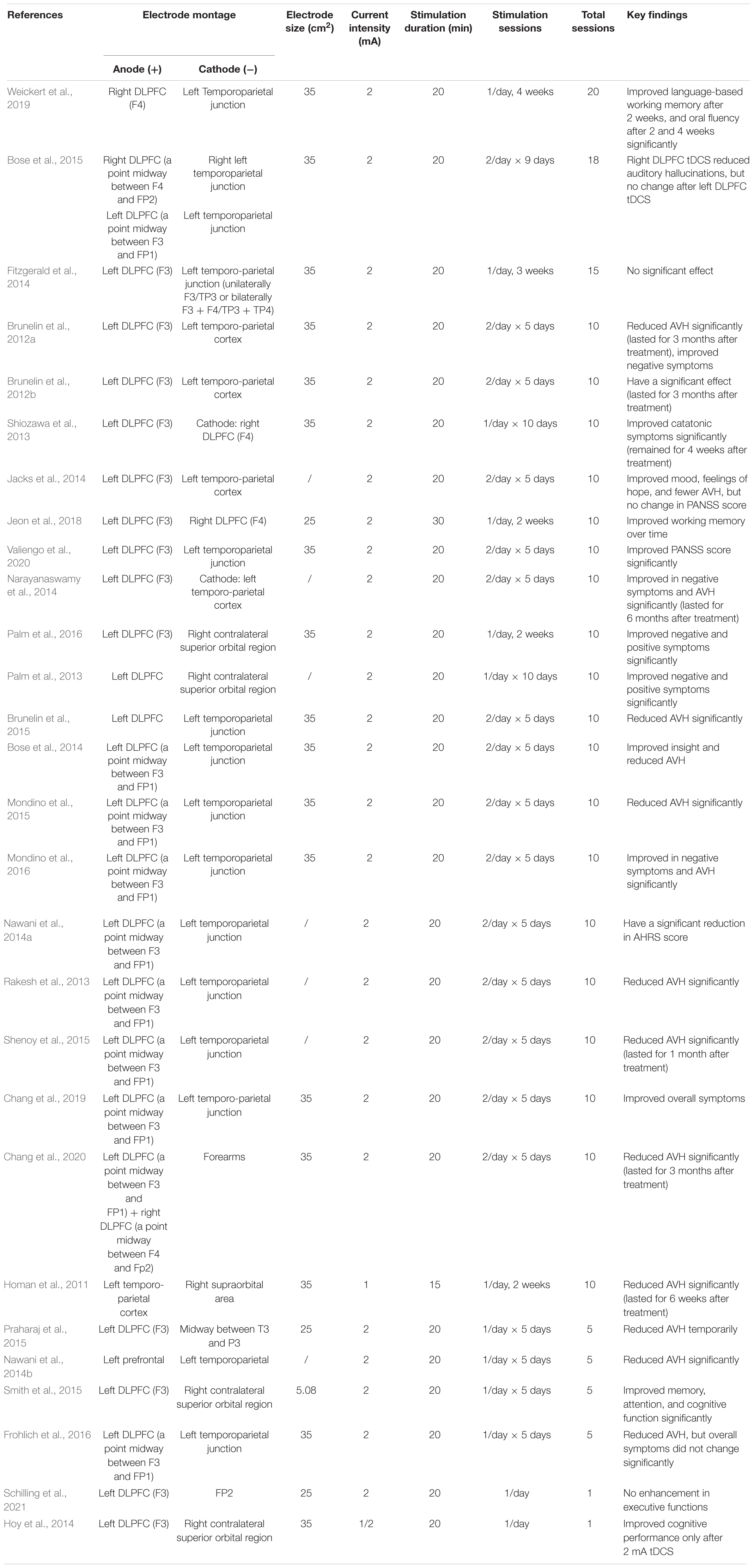
We summarized 28 studies using tDCS as a treatment strategy for schizophrenia in Table 2. Overall, tDCS improved both positive syndromes and negative syndromes in patients with schizophrenia. Only two studies showed no significant improvement after tDCS. For the electrodes positions, in 26 out of 28 studies placed the anode in the left DLPFC (F3) or a point midway between F3 and FP1 and the cathode in the right hemisphere (left temporoparietal junction, FP2, or right contralateral superior orbital region). 20 out of 28 studies used 25–35 cm2 electrodes. For stimulating current intensity, 26 studies used 2 mA current, only 1 study used 1 mA current, and 1 study used both 1 mA and 2 mA current. For stimulation duration, 26 studies used 20 min/session, 1 study used 30 min/session, and 1 study used 15 min/session. All studies adopted multiple stimulation sessions (from 5 to 20 sessions), only two studies used one single session of tDCS. Most multiple sessions of tDCS brought a better curative effect, pointing to a repeated application of tDCS as therapeutic strategy. In studies with one single session of tDCS, 2 mA but not 1 mA was shown to induce a positive effect. Taken together, 2 mA multi-session anodal tDCS of the left DLPFC or left temporoparietal junction area has the most potential to improve symptoms in patients with schizophrenia.
Addiction
Addiction is a chronic brain disease characterized by compulsive use of drugs, with loss of self-control and a high relapse rate (Berke and Hyman, 2000; Preller et al., 2013). Patients may experience negative emotions during withdrawal, such as sadness, restlessness, subdued pleasure. The relapse tendency indicates that a solid memory of drugs, a pathological memory, also called drug memory formed in addiction patients (Boning, 2009; Nestler, 2013). Drug memory is signaled by dynamic neuronal activity patterns in the brain areas such as prefrontal cortex, hippocampus and the ventral tegmental area (VTA; Berke and Hyman, 2000). Drugs increase the activity of VTA dopaminergic neurons as well as the concentration of dopamine in the projection area (Hyman and Malenka, 2001; Pierce and Kumaresan, 2006). The downstream targets of VTA dopaminergic neurons mainly includes ventral striatum, which is responsible for processing reward information, and prefrontal cortex, which is responsible for higher brain functions such as decision making, executive function, etc. (Robbins and Everitt, 2002; Hyman et al., 2006). Reward related perception and executive function can be modulated by the release of dopamine in the frontal lobe (Goldstein and Volkow, 2002).
Many studies have shown that tDCS can significantly relieve the symptoms of addictions (such as craving for cocaine, cigarette, alcohol, etc.). Bilateral DLPFC tDCS stimulation reduced cocaine craving with a linear decrease within 4 weeks, and improved anxiety symptoms and overall quality of life in patients (Batista et al., 2015). In addition to cocaine, tDCS stimulation can also reduce cravings for alcohol and cigarettes. Klauss et al. (2018b) showed that bilateral DLPFC tDCS stimulation significantly reduced alcohol cravings and reduced recurrence rates. Fecteau et al. (2014) found that the number of cigarettes consumed decreased significantly after bilateral DLPFC stimulation, and the effect could last for 4 days after the stimulation. Besides, non-substance addiction, such as food addiction, gambling addiction and internet addiction, shows executive function (such as decision-making and risk- taking processes) and working memory deficits similar to those in drug addiction (Fernandez-Serrano et al., 2010; Marazziti et al., 2014; Potenza, 2014). Studies have shown that anode tDCS stimulation of the right DLPFC decreased craving and negative emotions in addicted internet gaming players (Wu et al., 2020). Fregni et al. (2008b) found that the bilateral tDCS stimulation, left anode/right cathode or right anode/left cathode, reduced the food craving as well.
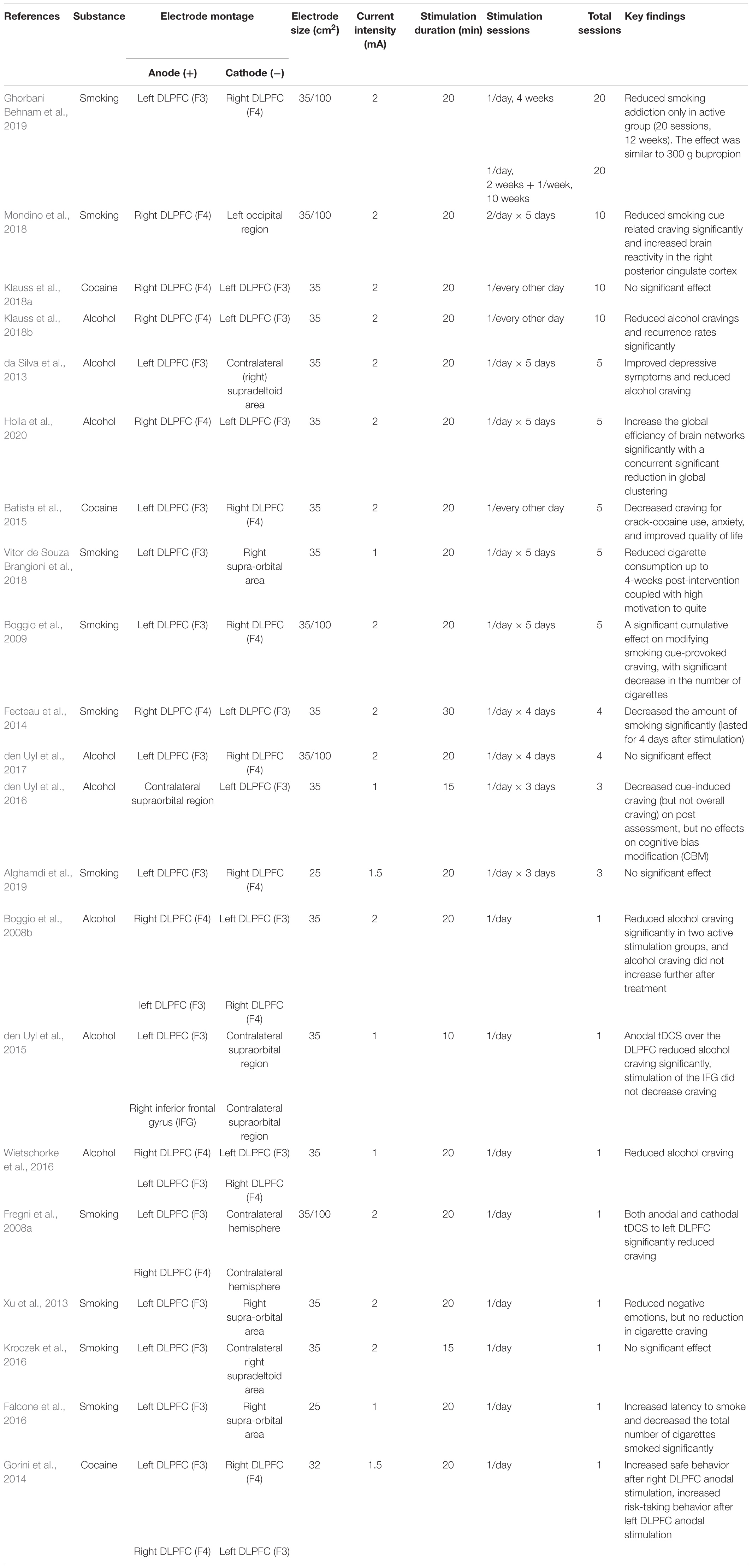
In Table 3, we summarized 21 studies evaluated tDCS treatment in substance addiction. Four studies didn’t observe any improvement after tDCS treatment. All other studies showed tDCS reduced craving, improved behavioral control and reduced likelihood of relapse. Most studies used 25–35 cm2 electrodes. For stimulating current intensity, 14 studies used 2 mA current, and 7 studies used a lower current. For stimulation duration, 4 studies used 10∼15 min/session, other studies used 20 min/session. There are 18 studies applied stimulation sessions from 1 to 4, and three of these studies showed no positive effects the rest studies used stimulation sessions from 5 to 20, which induced significant improvement of addiction symptoms except for one study. Roughly half of the studies placed anodal electrode on the right DLPFC, and the other half on the left. A couple of studies tried both montages. Together, tDCS of the DLPFC (left and/or right) has the potential to improve symptoms and reduce craving in substance addiction.
Attention Deficit Hyperactivity Disorder
Attention Deficit Hyperactivity Disorder (ADHD) is a brain disorder that characterized with inattention, impulsivity, hyperactivity and learning disabilities. ADHD mainly occurs in primary and middle schools (6–17 years old), and the prevalence is as high as over 6% (Rowland et al., 2015). The prevalence of ADHD is higher in boys than girls, and the risk for premature infants is also higher (Polanczyk et al., 2015). Neuroimaging studies have shown that the symptoms in ADHD patients may be related to abnormalities in fronto–striato–cerebellar neural circuit, especially the prefrontal lobe (Cubillo et al., 2012; Christakou et al., 2013). Specifically, the activity of bilateral striato-thalamus, left DLPFC and superior parietal cortex was significantly reduced in ADHD patients, and the activity of precuneus was significantly increased (Hart et al., 2013). Adults with childhood ADHD showed reduced activation in bilateral inferior prefrontal cortex, caudate and thalamus compared to controls. Neuro-functional abnormalities in ADHD patients are likely to persist from childhood to adulthood (Cubillo et al., 2010). fMRI studies also showed that striatum activation was abnormal in ADHD children (Durston et al., 2003).
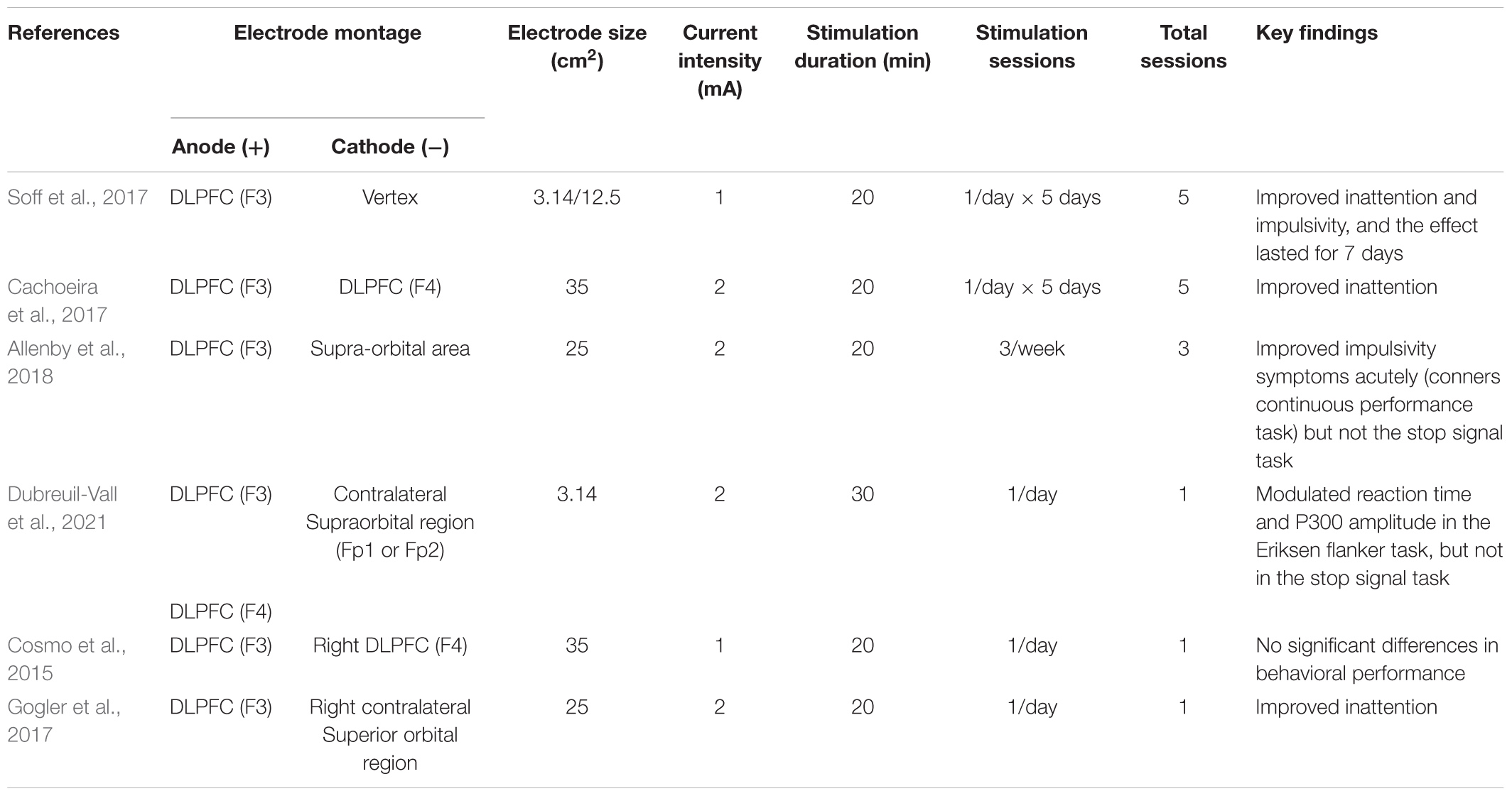
In recent years, tDCS has been considered to have an ameliorative effect on ADHD symptoms. Studies have shown that 1 mA anode tDCS of the left DLPFC improved the executive function in adolescent ADHD patients. After tDCS, they showed better inhibitory control, interference control, working memory and cognitive flexibility (Nejati et al., 2020). Blair’s research showed that inhibitory control is the main executive problem for adolescents with ADHD, and the problems with inhibitory control will lead to dysfunctions in memory, emotion regulation and other executive functions (Blair and Razza, 2007). tDCS improves the symptoms not only in adolescent patients, but also in adult ADHD patients. Left DLPFC tDCS in adult ADHD patients improved the impulsiveness symptoms (Allenby et al., 2018), and bilateral tDCS (anode over right DLPFC, cathode over left DLPFC) improved the inattention symptoms (Cachoeira et al., 2017). Only several studies were collected here which were shown in Table 4. All these studies targeted left DLPFC with anodal stimulation. One out of six studies (used a single session protocol) showed negative results, and all the rest found tDCS improved ADHD related symptoms. The stimulation current was 1 mA or 2 mA, 1 session to 5 sessions in total. While the potential of tDCS of the DLPFC to treat ADHD is promising, the published studies are relatively fewer compared to other diseases.
Anxiety
Anxiety disorders are the most common form of emotional disorder characterized by nervousness, worry and fear. There are several types of anxiety disorders, including generalized anxiety disorder (GAD), Social anxiety disorder (SAD), post-traumatic stress disorder (PTSD), panic disorder (PD), obsessive compulsive disorder (OCD), agoraphobe and specific phobia. Studies have shown that OCD symptoms are related to the cortico-striato-thalamocortical circuitry, including DLPFC, orbital frontal lobe (OFC), medial prefrontal lobe (MPF), and anterior cingulate cortex (ACC; Del Casale et al., 2011; Fineberg et al., 2011). Striatal dysfunction may lead to hypothalamic gating problems and hyperactivity in the orbitofrontal cortex and anterior cingulate cortex in OCD patients (Milad and Rauch, 2012). Sakai et al. (2011) found that functional connections of the orbitofrontal cortex, medial prefrontal cortex, DLPFC and ventral striatum were significantly increased in patients with OCD, but there was no significant correlation between symptom severity and connection strength. D’Urso et al. (2016b) reported that patients received cathode stimulation over the left DLPFC showed significant improvement in OCD symptoms.
Generalized anxiety disorder is characterized by persistent unspecified nervousness, excessive anxiety and worry about everyday life events (Locke et al., 2015; Stein et al., 2017). Previous studies have shown that brain regions related to rumination and introspection in GAD patients were overactivated (Locke et al., 2015). Patients also showed autonomic nervous dysfunction, vagus-mediated decreased heart rate variability, and neurostructural abnormalities in the rostral ACC, left medial orbitofrontal cortex, and right isthmic cingulate gyrus (Etkin and Wager, 2007; Carnevali et al., 2019). Neuroplasticity in prefrontal and limbic regions is also altered in patients with a variety of subtypes of anxiety disorders (Ironside et al., 2019). Vicario et al. (2019) reviewed the using of non-invasive brain stimulation techniques for the treatment of anxiety previously. A study showed that stimulation of the left DLPFC with 2 mA tDCS significantly improved physical stress symptoms in patients, however, there was no significant improvement in major psychological symptoms, such as anxiety, tension, emotion, or depression (de Lima et al., 2019). In another case report, a total of 15 sessions of 2 mA cathode tDCS stimulation improved anxiety symptoms in patients with GAD (Shiozawa et al., 2014).
Social anxiety disorder is an anxiety disorder characterized by extreme fear in getting involved in social interactions. Studies have shown that patients with SAD have attentional bias brought by social threats, and the attentional bias will increase the anxiety of patients with SAD (Klosowska et al., 2015). Anode tDCS of the left DLPFC significantly reduced attentional bias compared to the sham stimulation (Heeren et al., 2017). In addition, a single dose of 1 mA of tDCS reduced pain anxiety caused by burns (Hosseini Amiri et al., 2016), and improved anxiety symptoms caused by major depression (Nishida et al., 2019). Although there are only a few studies on the tDCS treatment of anxiety, these findings indicate that this technique can be an effective therapeutic option. We have summarized some of the published studies in Table 5.
Summary and Outlook
In recent years, tDCS is increasingly being studied for the therapeutic potential in neurological and psychiatric disorders. DLPFC is involved in many higher brain functions such as working memory, decision making, impulsivity, attention, etc. DLPFC also plays an important role in cognition and emotion. These brain functions were often disrupted in neurological and psychiatric diseases. Thus, modulation of the activity of DLPFC is a major strategy in treatment of these diseases. Although the neural mechanisms of tDCS is still not quite clear. It is believed that anodal stimulation increases brain activity while cathodal stimulation inhibits brain activity. One of the major problems of tDCS treatment of neuropsychiatric diseases is that each study used slightly different stimulation parameters. For instance, the current intensities were from 1 to 2 mA, tDCS sessions were from one session to more than 20 sessions. The tDCS frequency varies from twice daily to once every other day. Thus, it’s not appropriate to compare the current results directly side by side. Future studies will need to investigate the effects of tDCS using the different parameters in the same study or the same parameters in different studies. Nevertheless, this review demonstrates clearly that tDCS of DLPFC has a great potential to treat neuropsychiatric disorders.
Author Contributions
CL and ZM discussed and initiated the review topic and edited the manuscript substantially. QL drafted the manuscript. All authors interpreted the results together, revised the manuscript critically, and contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Innovation 2030-Major Project (2021ZD0202103), National Natural Science Foundation of China (U20A2017, U20A6005, and 32071009), Science, Technology and Innovation Commission of Shenzhen Municipality (ZDSYS20190902093601675, NYKFKT20190020, and ZDSYS20200811142401005), Key Laboratory of Brain Connectome and Manipulation, Chinese Academy of Sciences (2019DP173024), and Natural Science Foundation of Guangdong Province (2020A1515011055).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmadizadeh, M. J., Rezaei, M., and Fitzgerald, P. B. (2019). Transcranial direct current stimulation (tDCS) for post-traumatic stress disorder (PTSD): a randomized, double-blinded, controlled trial. Brain Res. Bull. 153, 273–278. doi: 10.1016/j.brainresbull.2019.09.011
Alghamdi, F., Alhussien, A., Alohali, M., Alatawi, A., Almusned, T., Fecteau, S., et al. (2019). Effect of transcranial direct current stimulation on the number of smoked cigarettes in tobacco smokers. PLoS One 14:e0212312. doi: 10.1371/journal.pone.0212312
Allenby, C., Falcone, M., Bernardo, L., Wileyto, E. P., Rostain, A., Ramsay, J. R., et al. (2018). Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul. 11, 974–981. doi: 10.1016/j.brs.2018.04.016
Aparicio, L. V. M., Rosa, V., Razza, L. M., Sampaio-Junior, B., Borrione, L., Valiengo, L., et al. (2019). Transcranial direct current stimulation (tDCS) for preventing major depressive disorder relapse: results of a 6-month follow-up. Depress. Anxiety 36, 262–268. doi: 10.1002/da.22878
Ayache, S. S., Palm, U., Chalah, M. A., Al-Ani, T., Brignol, A., Abdellaoui, M., et al. (2016). Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front. Neurosci. 10:147. doi: 10.3389/fnins.2016.00147
Barbey, A. K., Koenigs, M., and Grafman, J. (2013). Dorsolateral prefrontal contributions to human working memory. Cortex 49, 1195–1205. doi: 10.1016/j.cortex.2012.05.022
Barros Galvao, S. C., Borba Costa dos Santos, R., Borba dos Santos, P., Cabral, M. E., and Monte-Silva, K. (2014). Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil. 95, 222–229. doi: 10.1016/j.apmr.2013.10.023
Batista, E. K., Klauss, J., Fregni, F., Nitsche, M. A., and Nakamura-Palacios, E. M. (2015). A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int. J. Neuropsychopharmacol. 18:pyv066. doi: 10.1093/ijnp/pyv066
Bennabi, D., Nicolier, M., Monnin, J., Tio, G., Pazart, L., Vandel, P., et al. (2015). Pilot study of feasibility of the effect of treatment with tDCS in patients suffering from treatment-resistant depression treated with escitalopram. Clin. Neurophysiol. 126, 1185–1189. doi: 10.1016/j.clinph.2014.09.026
Berke, J. D., and Hyman, S. E. (2000). Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25, 515–532. doi: 10.1016/s0896-6273(00)81056-9
Bidet-Caulet, A., Buchanan, K. G., Viswanath, H., Black, J., Scabini, D., Bonnet-Brilhault, F., et al. (2015). Impaired facilitatory mechanisms of auditory attention after damage of the lateral prefrontal cortex. Cereb. Cortex 25, 4126–4134. doi: 10.1093/cercor/bhu131
Bikson, M., Inoue, M., Akiyama, H., Deans, J. K., Fox, J. E., Miyakawa, H., et al. (2004). Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. 557(Pt 1), 175–190. doi: 10.1113/jphysiol.2003.055772
Blair, C., and Razza, R. P. (2007). Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child. Dev. 78, 647–663. doi: 10.1111/j.1467-8624.2007.01019.x
Blumberger, D. M., Tran, L. C., Fitzgerald, P. B., Hoy, K. E., and Daskalakis, Z. J. (2012). A randomized double-blind sham-controlled study of transcranial direct current stimulation for treatment-resistant major depression. Front. Psychiatry 3:74. doi: 10.3389/fpsyt.2012.00074
Boggio, P. S., Liguori, P., Sultani, N., Rezende, L., Fecteau, S., and Fregni, F. (2009). Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci. Lett. 463, 82–86. doi: 10.1016/j.neulet.2009.07.041
Boggio, P. S., Rigonatti, S. P., Ribeiro, R. B., Myczkowski, M. L., Nitsche, M. A., Pascual-Leone, A., et al. (2008a). A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int. J. Neuropsychopharmacol. 11, 249–254. doi: 10.1017/S1461145707007833
Boggio, P. S., Sultani, N., Fecteau, S., Merabet, L., Mecca, T., Pascual-Leone, A., et al. (2008b). Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 92, 55–60. doi: 10.1016/j.drugalcdep.2007.06.011
Boning, J. (2009). Addiction memory as a specific, individually learned memory imprint. Pharmacopsychiatry 42(Suppl. 1), S66–S68. doi: 10.1055/s-0029-1216357
Bose, A., Shivakumar, V., Narayanaswamy, J. C., Nawani, H., Subramaniam, A., Agarwal, S. M., et al. (2014). Insight facilitation with add-on tDCS in schizophrenia. Schizophr. Res. 156, 63–65. doi: 10.1016/j.schres.2014.03.029
Bose, A., Sowmya, S., Shenoy, S., Agarwal, S. M., Chhabra, H., Narayanaswamy, J. C., et al. (2015). Clinical utility of attentional salience in treatment of auditory verbal hallucinations in schizophrenia using transcranial direct current stimulation (tDCS). Schizophr. Res. 164, 279–280. doi: 10.1016/j.schres.2015.01.040
Brunelin, J., Hasan, A., Haesebaert, F., Nitsche, M. A., and Poulet, E. (2015). Nicotine smoking prevents the effects of frontotemporal transcranial direct current stimulation (tDCS) in hallucinating patients with schizophrenia. Brain Stimul. 8, 1225–1227. doi: 10.1016/j.brs.2015.08.002
Brunelin, J., Mondino, M., Gassab, L., Haesebaert, F., Gaha, L., Suaud-Chagny, M. F., et al. (2012a). Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am. J. Psychiatry 169, 719–724. doi: 10.1176/appi.ajp.2012.11071091
Brunelin, J., Mondino, M., Haesebaert, F., Saoud, M., Suaud-Chagny, M. F., and Poulet, E. (2012b). Efficacy and safety of bifocal tDCS as an interventional treatment for refractory schizophrenia. Brain Stimul. 5, 431–432. doi: 10.1016/j.brs.2011.03.010
Brunoni, A. R., Boggio, P. S., De Raedt, R., Bensenor, I. M., Lotufo, P. A., Namur, V., et al. (2014). Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. J. Affect. Disord. 162, 43–49. doi: 10.1016/j.jad.2014.03.026
Brunoni, A. R., Ferrucci, R., Bortolomasi, M., Scelzo, E., Boggio, P. S., Fregni, F., et al. (2013a). Interactions between transcranial direct current stimulation (tDCS) and pharmacological interventions in the major depressive episode: findings from a naturalistic study. Eur. Psychiatry 28, 356–361. doi: 10.1016/j.eurpsy.2012.09.001
Brunoni, A. R., Valiengo, L., Baccaro, A., Zanao, T. A., de Oliveira, J. F., Goulart, A., et al. (2013b). The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 70, 383–391. doi: 10.1001/2013.jamapsychiatry.32
Brunoni, A. R., Ferrucci, R., Bortolomasi, M., Vergari, M., Tadini, L., Boggio, P. S., et al. (2011). Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 96–101. doi: 10.1016/j.pnpbp.2010.09.010
Brunoni, A. R., Moffa, A. H., Sampaio-Junior, B., Borrione, L., Moreno, M. L., Fernandes, R. A., et al. (2017). Trial of electrical direct-current therapy versus escitalopram for depression. N. Engl. J. Med. 376, 2523–2533. doi: 10.1056/NEJMoa1612999
Buhle, J. T., Silvers, J. A., Wager, T. D., Lopez, R., Onyemekwu, C., Kober, H., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex 24, 2981–2990. doi: 10.1093/cercor/bht154
Cachoeira, C. T., Leffa, D. T., Mittelstadt, S. D., Mendes, L. S. T., Brunoni, A. R., Pinto, J. V., et al. (2017). Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder – a pilot randomized controlled study. Psychiatry Res. 247, 28–32. doi: 10.1016/j.psychres.2016.11.009
Carnevali, L., Mancini, M., Koenig, J., Makovac, E., Watson, D. R., Meeten, F., et al. (2019). Cortical morphometric predictors of autonomic dysfunction in generalized anxiety disorder. Auton. Neurosci. 217, 41–48. doi: 10.1016/j.autneu.2019.01.001
Carpenter, W. T., Blanchard, J. J., and Kirkpatrick, B. (2016). New standards for negative symptom assessment. Schizophr. Bull. 42, 1–3. doi: 10.1093/schbul/sbv160
Chang, C. C., Kao, Y. C., Chao, C. Y., and Chang, H. A. (2019). Enhancement of cognitive insight and higher-order neurocognitive function by fronto-temporal transcranial direct current stimulation (tDCS) in patients with schizophrenia. Schizophr. Res. 208, 430–438. doi: 10.1016/j.schres.2018.12.052
Chang, C. C., Kao, Y. C., Chao, C. Y., Tzeng, N. S., and Chang, H. A. (2020). Examining bi-anodal transcranial direct current stimulation (tDCS) over bilateral dorsolateral prefrontal cortex coupled with bilateral extracephalic references as a treatment for negative symptoms in non-acute schizophrenia patients: a randomized, double-blind, sham-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry. 96:109715. doi: 10.1016/j.pnpbp.2019.109715
Christakou, A., Murphy, C. M., Chantiluke, K., Cubillo, A. I., Smith, A. B., Giampietro, V., et al. (2013). Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with autism. Mol. Psychiatry 18, 236–244. doi: 10.1038/mp.2011.185
Cosmo, C., Baptista, A. F., de Araujo, A. N., do Rosario, R. S., Miranda, J. G., Montoya, P., et al. (2015). A randomized, double-blind, sham-controlled trial of transcranial direct current stimulation in attention-deficit/hyperactivity disorder. PLoS One 10:e0135371. doi: 10.1371/journal.pone.0135371
Cubillo, A., Halari, R., Ecker, C., Giampietro, V., Taylor, E., and Rubia, K. (2010). Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J. Psychiatr. Res. 44, 629–639. doi: 10.1016/j.jpsychires.2009.11.016
Cubillo, A., Halari, R., Smith, A., Taylor, E., and Rubia, K. (2012). A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex 48, 194–215. doi: 10.1016/j.cortex.2011.04.007
da Silva, M. C., Conti, C. L., Klauss, J., Alves, L. G., do Nascimento Cavalcante, H. M., Fregni, F., et al. (2013). Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J. Physiol. Paris 107, 493–502. doi: 10.1016/j.jphysparis.2013.07.003
Dalong, G., Jiyuan, L., Ying, Z., Lei, Z., Yanhong, H., and Yongcong, S. (2020). Transcranial direct current stimulation reconstructs diminished thalamocortical connectivity during prolonged resting wakefulness: a resting-state fMRI pilot study. Brain Imaging Behav. 14, 278–288. doi: 10.1007/s11682-018-9979-9
de Lima, A. L., Braga, F. M. A., da Costa, R. M. M., Gomes, E. P., Brunoni, A. R., and Pegado, R. (2019). Transcranial direct current stimulation for the treatment of generalized anxiety disorder: a randomized clinical trial. J. Affect. Disord. 259, 31–37. doi: 10.1016/j.jad.2019.08.020
Del Casale, A., Kotzalidis, G. D., Rapinesi, C., Serata, D., Ambrosi, E., Simonetti, A., et al. (2011). Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology 64, 61–85. doi: 10.1159/000325223
Dell’Osso, B., Zanoni, S., Ferrucci, R., Vergari, M., Castellano, F., D’Urso, N., et al. (2012). Transcranial direct current stimulation for the outpatient treatment of poor-responder depressed patients. Eur. Psychiatry 27, 513–517. doi: 10.1016/j.eurpsy.2011.02.008
den Uyl, T. E., Gladwin, T. E., Rinck, M., Lindenmeyer, J., and Wiers, R. W. (2017). A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict. Biol. 22, 1632–1640. doi: 10.1111/adb.12463
den Uyl, T. E., Gladwin, T. E., and Wiers, R. W. (2015). Transcranial direct current stimulation, implicit alcohol associations and craving. Biol. Psychol. 105, 37–42. doi: 10.1016/j.biopsycho.2014.12.004
den Uyl, T. E., Gladwin, T. E., and Wiers, R. W. (2016). Electrophysiological and behavioral effects of combined transcranial direct current stimulation and alcohol approach bias retraining in hazardous drinkers. Alcohol Clin. Exp. Res. 40, 2124–2133. doi: 10.1111/acer.13171
Dubreuil-Vall, L., Gomez-Bernal, F., Villegas, A. C., Cirillo, P., Surman, C., Ruffini, G., et al. (2021). Transcranial direct current stimulation to the left dorsolateral prefrontal cortex improves cognitive control in patients with attention-deficit/hyperactivity disorder: a randomized behavioral and neurophysiological study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 439–448. doi: 10.1016/j.bpsc.2020.11.006
D’Urso, G., Brunoni, A. R., Anastasia, A., Micillo, M., de Bartolomeis, A., and Mantovani, A. (2016a). Polarity-dependent effects of transcranial direct current stimulation in obsessive-compulsive disorder. Neurocase 22, 60–64. doi: 10.1080/13554794.2015.1045522
D’Urso, G., Brunoni, A. R., Mazzaferro, M. P., Anastasia, A., de Bartolomeis, A., and Mantovani, A. (2016b). Transcranial direct current stimulation for obsessive-compulsive disorder: a randomized, controlled, partial crossover trial. Depress. Anxiety 33, 1132–1140. doi: 10.1002/da.22578
Durston, S., Tottenham, N. T., Thomas, K. M., Davidson, M. C., Eigsti, I. M., Yang, Y., et al. (2003). Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry 53, 871–878. doi: 10.1016/s0006-3223(02)01904-2
Etkin, A., and Wager, T. D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488. doi: 10.1176/appi.ajp.2007.07030504
Falcone, M., Bernardo, L., Ashare, R. L., Hamilton, R., Faseyitan, O., McKee, S. A., et al. (2016). Transcranial direct current brain stimulation increases ability to resist smoking. Brain Stimul. 9, 191–196. doi: 10.1016/j.brs.2015.10.004
Fecteau, S., Agosta, S., Hone-Blanchet, A., Fregni, F., Boggio, P., Ciraulo, D., et al. (2014). Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend. 140, 78–84. doi: 10.1016/j.drugalcdep.2014.03.036
Fernandez-Serrano, M. J., Perez-Garcia, M., Perales, J. C., and Verdejo-Garcia, A. (2010). Prevalence of executive dysfunction in cocaine, heroin and alcohol users enrolled in therapeutic communities. Eur. J. Pharmacol. 626, 104–112. doi: 10.1016/j.ejphar.2009.10.019
Fineberg, N. A., Chamberlain, S. R., Hollander, E., Boulougouris, V., and Robbins, T. W. (2011). Translational approaches to obsessive-compulsive disorder: from animal models to clinical treatment. Br. J. Pharmacol. 164, 1044–1061. doi: 10.1111/j.1476-5381.2011.01422.x
Fitzgerald, P. B., McQueen, S., Daskalakis, Z. J., and Hoy, K. E. (2014). A negative pilot study of daily bimodal transcranial direct current stimulation in schizophrenia. Brain Stimul. 7, 813–816. doi: 10.1016/j.brs.2014.08.002
Fitzpatrick, S., Gilbert, S., and Serpell, L. (2013). Systematic review: are overweight and obese individuals impaired on behavioural tasks of executive functioning? Neuropsychol. Rev. 23, 138–156. doi: 10.1007/s11065-013-9224-7
Francis, J. T., Gluckman, B. J., and Schiff, S. J. (2003). Sensitivity of neurons to weak electric fields. J. Neurosci. 23, 7255–7261. doi: 10.1523/JNEUROSCI.23-19-07255.2003
Frank, D. W., Dewitt, M., Hudgens-Haney, M., Schaeffer, D. J., Ball, B. H., Schwarz, N. F., et al. (2014). Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 45, 202–211. doi: 10.1016/j.neubiorev.2014.06.010
Fregni, F., Liguori, P., Fecteau, S., Nitsche, M. A., Pascual-Leone, A., and Boggio, P. S. (2008a). Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J. Clin. Psychiatry 69, 32–40. doi: 10.4088/jcp.v69n0105
Fregni, F., Orsati, F., Pedrosa, W., Fecteau, S., Tome, F. A., Nitsche, M. A., et al. (2008b). Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 51, 34–41. doi: 10.1016/j.appet.2007.09.016
Fritsch, B., Reis, J., Martinowich, K., Schambra, H. M., Ji, Y., Cohen, L. G., et al. (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66, 198–204. doi: 10.1016/j.neuron.2010.03.035
Frohlich, F., Burrello, T. N., Mellin, J. M., Cordle, A. L., Lustenberger, C. M., Gilmore, J. H., et al. (2016). Exploratory study of once-daily transcranial direct current stimulation (tDCS) as a treatment for auditory hallucinations in schizophrenia. Eur. Psychiatry 33, 54–60. doi: 10.1016/j.eurpsy.2015.11.005
Ghorbani Behnam, S., Mousavi, S. A., and Emamian, M. H. (2019). The effects of transcranial direct current stimulation compared to standard bupropion for the treatment of tobacco dependence: a randomized sham-controlled trial. Eur. Psychiatry 60, 41–48. doi: 10.1016/j.eurpsy.2019.04.010
Glasser, M. F., Coalson, T. S., Robinson, E. C., Hacker, C. D., Harwell, J., Yacoub, E., et al. (2016). A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. doi: 10.1038/nature18933
Gogler, N., Willacker, L., Funk, J., Strube, W., Langgartner, S., Napiorkowski, N., et al. (2017). Single-session transcranial direct current stimulation induces enduring enhancement of visual processing speed in patients with major depression. Eur. Arch. Psychiatry Clin. Neurosci. 267, 671–686. doi: 10.1007/s00406-016-0761-y
Goldstein, R. Z., and Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159, 1642–1652. doi: 10.1176/appi.ajp.159.10.1642
Gordon, P. C., Valiengo, L., de Paula, V. J. R., Galhardoni, R., Ziemann, U., de Andrade, D. C., et al. (2019). Changes in motor cortical excitability in schizophrenia following transcranial direct current stimulation. Prog. Neuropsychopharmacol. Biol. Psychiatry 90, 43–48. doi: 10.1016/j.pnpbp.2018.11.004
Gorini, A., Lucchiari, C., Russell-Edu, W., and Pravettoni, G. (2014). Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front. Hum. Neurosci. 8:661. doi: 10.3389/fnhum.2014.00661
Hart, H., Radua, J., Nakao, T., Mataix-Cols, D., and Rubia, K. (2013). Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 70, 185–198. doi: 10.1001/jamapsychiatry.2013.277
Hecht, D. (2010). Depression and the hyperactive right-hemisphere. Neurosci. Res. 68, 77–87. doi: 10.1016/j.neures.2010.06.013
Heeren, A., Billieux, J., Philippot, P., De Raedt, R., Baeken, C., de Timary, P., et al. (2017). Impact of transcranial direct current stimulation on attentional bias for threat: a proof-of-concept study among individuals with social anxiety disorder. Soc. Cogn. Affect. Neurosci. 12, 251–260. doi: 10.1093/scan/nsw119
Hill, A. T., Fitzgerald, P. B., and Hoy, K. E. (2016). Effects of anodal transcranial direct current stimulation on working memory: a systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stimul. 9, 197–208. doi: 10.1016/j.brs.2015.10.006
Holla, B., Biswal, J., Ramesh, V., Shivakumar, V., Bharath, R. D., Benegal, V., et al. (2020). Effect of prefrontal tDCS on resting brain fMRI graph measures in alcohol use disorders: a randomized, double-blind, sham-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry 102:109950. doi: 10.1016/j.pnpbp.2020.109950
Homan, P., Kindler, J., Federspiel, A., Flury, R., Hubl, D., Hauf, M., et al. (2011). Muting the voice: a case of arterial spin labeling-monitored transcranial direct current stimulation treatment of auditory verbal hallucinations. Am. J. Psychiatry 168, 853–854. doi: 10.1176/appi.ajp.2011.11030496
Hosseini Amiri, M., Tavousi, S. H., Mazlom, S. R., and Manzari, Z. S. (2016). Effect of transcranial direct current stimulation on pain anxiety during burn wound care. Burns 42, 872–876. doi: 10.1016/j.burns.2016.01.006
Hoy, K. E., Arnold, S. L., Emonson, M. R., Daskalakis, Z. J., and Fitzgerald, P. B. (2014). An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophr. Res. 155, 96–100. doi: 10.1016/j.schres.2014.03.006
Hyman, S. E., and Malenka, R. C. (2001). Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2, 695–703. doi: 10.1038/35094560
Hyman, S. E., Malenka, R. C., and Nestler, E. J. (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 29, 565–598. doi: 10.1146/annurev.neuro.29.051605.113009
Ironside, M., Browning, M., Ansari, T. L., Harvey, C. J., Sekyi-Djan, M. N., Bishop, S. J., et al. (2019). Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: a randomized clinical trial. JAMA Psychiatry 76, 71–78. doi: 10.1001/jamapsychiatry.2018.2172
Jacks, S., Kalivas, B., Mittendorf, A., Kindt, C., and Short, E. B. (2014). Transcranial direct-current stimulation as an adjunct to electroconvulsive therapy and clozapine for refractory psychosis. Prim. Care Companion CNS Disord. 16:PCC.14l01635. doi: 10.4088/PCC.14l01635
Jafari, E., Alizadehgoradel, J., Pourmohseni Koluri, F., Nikoozadehkordmirza, E., Refahi, M., Taherifard, M., et al. (2021). Intensified electrical stimulation targeting lateral and medial prefrontal cortices for the treatment of social anxiety disorder: a randomized, double-blind, parallel-group, dose-comparison study. Brain Stimul. 14, 974–986. doi: 10.1016/j.brs.2021.06.005
Jeon, D. W., Jung, D. U., Kim, S. J., Shim, J. C., Moon, J. J., Seo, Y. S., et al. (2018). Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: a double-blind 12-week study. Schizophr. Res. 197, 378–385. doi: 10.1016/j.schres.2017.12.009
Klauss, J., Anders, Q. S., Felippe, L. V., Ferreira, L. V. B., Cruz, M. A., Nitsche, M. A., et al. (2018a). Lack of effects of extended sessions of transcranial direct current stimulation (tDCS) over dorsolateral prefrontal cortex on craving and relapses in crack-cocaine users. Front. Pharmacol. 9:1198. doi: 10.3389/fphar.2018.01198
Klauss, J., Anders, Q. S., Felippe, L. V., Nitsche, M. A., and Nakamura-Palacios, E. M. (2018b). Multiple sessions of transcranial direct current stimulation (tDCS) reduced craving and relapses for alcohol use: a randomized placebo-controlled trial in alcohol use disorder. Front. Pharmacol. 9:716. doi: 10.3389/fphar.2018.00716
Klosowska, J., Blaut, A., and Paulewicz, B. (2015). [Attentional bias training in reducing symptoms of anxiety]. Psychiatr. Pol. 49, 57–66. doi: 10.12740/PP/27628
Koenigs, M., and Grafman, J. (2009). The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 201, 239–243. doi: 10.1016/j.bbr.2009.03.004
Kroczek, A. M., Haussinger, F. B., Rohe, T., Schneider, S., Plewnia, C., Batra, A., et al. (2016). Effects of transcranial direct current stimulation on craving, heart-rate variability and prefrontal hemodynamics during smoking cue exposure. Drug Alcohol Depend. 168, 123–127. doi: 10.1016/j.drugalcdep.2016.09.006
Kumar, S., Batist, J., Ghazala, Z., Zomorrodi, R. M., Brooks, H., Goodman, M., et al. (2020). Effects of bilateral transcranial direct current stimulation on working memory and global cognition in older patients with remitted major depression: a pilot randomized clinical trial. Int. J. Geriatr. Psychiatry 35, 1233–1242. doi: 10.1002/gps.5361
Liebetanz, D., Nitsche, M. A., Tergau, F., and Paulus, W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125(Pt 10), 2238–2247. doi: 10.1093/brain/awf238
Lin, Y. Y., Chang, C. C., Huang, C. C., Tzeng, N. S., Kao, Y. C., and Chang, H. A. (2021). Efficacy and neurophysiological predictors of treatment response of adjunct bifrontal transcranial direct current stimulation (tDCS) in treating unipolar and bipolar depression. J. Affect. Disord. 280(Pt A), 295–304. doi: 10.1016/j.jad.2020.11.030
Locke, A. B., Kirst, N., and Shultz, C. G. (2015). Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am. Fam. Phys. 91, 617–624.
Loo, C. K., Alonzo, A., Martin, D., Mitchell, P. B., Galvez, V., and Sachdev, P. (2012). Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br. J. Psychiatry 200, 52–59. doi: 10.1192/bjp.bp.111.097634
Loo, C. K., Sachdev, P., Martin, D., Pigot, M., Alonzo, A., Malhi, G. S., et al. (2010). A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Int. J. Neuropsychopharmacol. 13, 61–69. doi: 10.1017/S1461145709990411
Marazziti, D., Presta, S., Baroni, S., Silvestri, S., and Dell’Osso, L. (2014). Behavioral addictions: a novel challenge for psychopharmacology. CNS Spectr. 19, 486–495. doi: 10.1017/S1092852913001041
Martin, D. M., Alonzo, A., Ho, K. A., Player, M., Mitchell, P. B., Sachdev, P., et al. (2013). Continuation transcranial direct current stimulation for the prevention of relapse in major depression. J. Affect. Disord. 144, 274–278. doi: 10.1016/j.jad.2012.10.012
McDermott, T. J., Wiesman, A. I., Mills, M. S., Spooner, R. K., Coolidge, N. M., Proskovec, A. L., et al. (2019). tDCS modulates behavioral performance and the neural oscillatory dynamics serving visual selective attention. Hum. Brain Mapp. 40, 729–740. doi: 10.1002/hbm.24405
Milad, M. R., and Rauch, S. L. (2012). Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn. Sci. 16, 43–51. doi: 10.1016/j.tics.2011.11.003
Mondino, M., Haesebaert, F., Poulet, E., Suaud-Chagny, M. F., and Brunelin, J. (2015). Fronto-temporal transcranial direct current stimulation (tDCS) reduces source-monitoring deficits and auditory hallucinations in patients with schizophrenia. Schizophr. Res. 161, 515–516. doi: 10.1016/j.schres.2014.10.054
Mondino, M., Jardri, R., Suaud-Chagny, M. F., Saoud, M., Poulet, E., and Brunelin, J. (2016). Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr. Bull. 42, 318–326. doi: 10.1093/schbul/sbv114
Mondino, M., Luck, D., Grot, S., Januel, D., Suaud-Chagny, M. F., Poulet, E., et al. (2018). Effects of repeated transcranial direct current stimulation on smoking, craving and brain reactivity to smoking cues. Sci. Rep. 8:8724. doi: 10.1038/s41598-018-27057-1
Moreno, M. L., Goerigk, S. A., Bertola, L., Suemoto, C. K., Razza, L. B., Moffa, A. H., et al. (2020). Cognitive changes after tDCS and escitalopram treatment in major depressive disorder: results from the placebo-controlled ELECT-TDCS trial. J. Affect. Disord. 263, 344–352. doi: 10.1016/j.jad.2019.12.009
Narayanaswamy, J. C., Jose, D., Chhabra, H., Agarwal, S. M., Shrinivasa, B., Hegde, A., et al. (2015). Successful application of add-on transcranial direct current stimulation (tDCS) for treatment of SSRI resistant OCD. Brain Stimul. 8, 655–657. doi: 10.1016/j.brs.2014.12.003
Narayanaswamy, J. C., Shivakumar, V., Bose, A., Agarwal, S. M., Venkatasubramanian, G., and Gangadhar, B. N. (2014). Sustained improvement of negative symptoms in schizophrenia with add-on tDCS: a case report. Clin. Schizophr. Relat. Psychoses 8, 135–136. doi: 10.3371/CSRP.JNVS.061314
Nawani, H., Bose, A., Agarwal, S. M., Shivakumar, V., Chhabra, H., Subramaniam, A., et al. (2014a). Modulation of corollary discharge dysfunction in schizophrenia by tDCS: preliminary evidence. Brain Stimul. 7, 486–488. doi: 10.1016/j.brs.2014.01.003
Nawani, H., Kalmady, S. V., Bose, A., Shivakumar, V., Rakesh, G., Subramaniam, A., et al. (2014b). Neural basis of tDCS effects on auditory verbal hallucinations in schizophrenia: a case report evidence for cortical neuroplasticity modulation. J. ECT 30, e2–e4. doi: 10.1097/YCT.0b013e3182a35492
Nejati, V., Salehinejad, M. A., Nitsche, M. A., Najian, A., and Javadi, A. H. (2020). Transcranial direct current stimulation improves executive dysfunctions in ADHD: implications for inhibitory control, interference control, working memory, and cognitive flexibility. J. Atten. Disord. 24, 1928–1943. doi: 10.1177/1087054717730611
Nestler, E. J. (2013). Cellular basis of memory for addiction. Dialog. Clin. Neurosci. 15, 431–443. doi: 10.31887/DCNS.2013.15.4/enestler
Nishida, K., Koshikawa, Y., Morishima, Y., Yoshimura, M., Katsura, K., Ueda, S., et al. (2019). Pre-stimulus brain activity is associated with state-anxiety changes during single-session transcranial direct current stimulation. Front. Hum. Neurosci. 13:266. doi: 10.3389/fnhum.2019.00266
Orlov, N. D., Tracy, D. K., Joyce, D., Patel, S., Rodzinka-Pasko, J., Dolan, H., et al. (2017). Stimulating cognition in schizophrenia: a controlled pilot study of the effects of prefrontal transcranial direct current stimulation upon memory and learning. Brain Stimul. 10, 560–566. doi: 10.1016/j.brs.2016.12.013
Palm, U., Keeser, D., Blautzik, J., Pogarell, O., Ertl-Wagner, B., Kupka, M. J., et al. (2013). Prefrontal transcranial direct current stimulation (tDCS) changes negative symptoms and functional connectivity MRI (fcMRI) in a single case of treatment-resistant schizophrenia. Schizophr. Res. 150, 583–585. doi: 10.1016/j.schres.2013.08.043
Palm, U., Keeser, D., Hasan, A., Kupka, M. J., Blautzik, J., Sarubin, N., et al. (2016). Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr. Bull. 42, 1253–1261. doi: 10.1093/schbul/sbw041
Palm, U., Schiller, C., Fintescu, Z., Obermeier, M., Keeser, D., Reisinger, E., et al. (2012). Transcranial direct current stimulation in treatment resistant depression: a randomized double-blind, placebo-controlled study. Brain Stimul. 5, 242–251. doi: 10.1016/j.brs.2011.08.005
Pena-Gomez, C., Vidal-Pineiro, D., Clemente, I. C., Pascual-Leone, A., and Bartres-Faz, D. (2011). Down-regulation of negative emotional processing by transcranial direct current stimulation: effects of personality characteristics. PLoS One 6:e22812. doi: 10.1371/journal.pone.0022812
Philiastides, M. G., Auksztulewicz, R., Heekeren, H. R., and Blankenburg, F. (2011). Causal role of dorsolateral prefrontal cortex in human perceptual decision making. Curr. Biol. 21, 980–983. doi: 10.1016/j.cub.2011.04.034
Pierce, R. C., and Kumaresan, V. (2006). The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev. 30, 215–238. doi: 10.1016/j.neubiorev.2005.04.016
Polanczyk, G. V., Salum, G. A., Sugaya, L. S., Caye, A., and Rohde, L. A. (2015). Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 56, 345–365. doi: 10.1111/jcpp.12381
Potenza, M. N. (2014). Non-substance addictive behaviors in the context of DSM-5. Addict. Behav. 39, 1–2. doi: 10.1016/j.addbeh.2013.09.004
Praharaj, S. K., Behere, R. V., and Sharma, P. S. (2015). Cathodal transcranial direct current stimulation over left temporoparietal area for treatment-refractory delusions and auditory hallucinations in schizophrenia: a case study. J. ECT 31, 277–278. doi: 10.1097/YCT.0000000000000237
Preller, K. H., Wagner, M., Sulzbach, C., Hoenig, K., Neubauer, J., Franke, P. E., et al. (2013). Sustained incentive value of heroin-related cues in short- and long-term abstinent heroin users. Eur. Neuropsychopharmacol. 23, 1270–1279. doi: 10.1016/j.euroneuro.2012.11.007
Purpura, D. P., and McMurtry, J. G. (1965). Intracellular activities and evoked potential changes during polarization of motor cortex. J. Neurophysiol. 28, 166–185. doi: 10.1152/jn.1965.28.1.166
Rahnev, D., Nee, D. E., Riddle, J., Larson, A. S., and D’Esposito, M. (2016). Causal evidence for frontal cortex organization for perceptual decision making. Proc. Natl. Acad. Sci. U.S.A. 113, 6059–6064. doi: 10.1073/pnas.1522551113
Rakesh, G., Shivakumar, V., Subramaniam, A., Nawani, H., Amaresha, A. C., Narayanaswamy, J. C., et al. (2013). Monotherapy with tDCS for schizophrenia: a case report. Brain Stimul. 6, 708–709. doi: 10.1016/j.brs.2013.01.007
Rigonatti, S. P., Boggio, P. S., Myczkowski, M. L., Otta, E., Fiquer, J. T., Ribeiro, R. B., et al. (2008). Transcranial direct stimulation and fluoxetine for the treatment of depression. Eur. Psychiatry. 23, 74–76. doi: 10.1016/j.eurpsy.2007.09.006
Robbins, T. W., and Everitt, B. J. (2002). Limbic-striatal memory systems and drug addiction. Neurobiol. Learn. Mem. 78, 625–636. doi: 10.1006/nlme.2002.4103
Rowland, A. S., Skipper, B. J., Umbach, D. M., Rabiner, D. L., Campbell, R. A., Naftel, A. J., et al. (2015). The prevalence of ADHD in a population-based sample. J. Atten. Disord. 19, 741–754. doi: 10.1177/1087054713513799
Rubi-Fessen, I., Hartmann, A., Huber, W., Fimm, B., Rommel, T., Thiel, A., et al. (2015). Add-on effects of repetitive transcranial magnetic stimulation on subacute aphasia therapy: enhanced improvement of functional communication and basic linguistic skills. A randomized controlled study. Arch. Phys. Med. Rehabil. 96, 1935–1944.e1932. doi: 10.1016/j.apmr.2015.06.017
Sakai, Y., Narumoto, J., Nishida, S., Nakamae, T., Yamada, K., Nishimura, T., et al. (2011). Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur. Psychiatry 26, 463–469. doi: 10.1016/j.eurpsy.2010.09.005
Sampaio-Junior, B., Tortella, G., Borrione, L., Moffa, A. H., Machado-Vieira, R., Cretaz, E., et al. (2018). Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression: a randomized clinical trial. JAMA Psychiatry 75, 158–166. doi: 10.1001/jamapsychiatry.2017.4040
Schilling, T. M., Bossert, M., Konig, M., Wirtz, G., Weisbrod, M., and Aschenbrenner, S. (2021). Acute effects of a single dose of 2 mA of anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex on executive functions in patients with schizophrenia-a randomized controlled trial. PLoS One 16:e0254695. doi: 10.1371/journal.pone.0254695
Shahani, B., and Russell, W. R. (1969). Motor neurone disease. An abnormality of nerve metabolism. J. Neurol. Neurosurg. Psychiatry 32, 1–5. doi: 10.1136/jnnp.32.1.1
Sharafi, E., Taghva, A., Arbabi, M., Dadarkhah, A., and Ghaderi, J. (2019). Transcranial direct current stimulation for treatment-resistant major depression: a double-blind randomized sham-controlled trial. Clin. EEG Neurosci. 50, 375–382. doi: 10.1177/1550059419863209
Shenoy, S., Bose, A., Chhabra, H., Dinakaran, D., Agarwal, S. M., Shivakumar, V., et al. (2015). Transcranial direct current stimulation (tDCS) for auditory verbal hallucinations in schizophrenia during pregnancy: a case report. Brain Stimul. 8, 163–164. doi: 10.1016/j.brs.2014.10.013
Shiozawa, P., da Silva, M. E., Cordeiro, Q., Fregni, F., and Brunoni, A. R. (2013). Transcranial direct current stimulation (tDCS) for catatonic schizophrenia: a case study. Schizophr. Res. 146, 374–375. doi: 10.1016/j.schres.2013.01.030
Shiozawa, P., Gomes, J. S., Ducos, D. V., Akiba, H. T., Dias, A. M., Trevizol, A. P., et al. (2016). Effect of transcranial direct current stimulation (tDCS) over the prefrontal cortex combined with cognitive training for treating schizophrenia: a sham-controlled randomized clinical trial. Trends Psychiatry Psychother. 38, 175–177. doi: 10.1590/2237-6089-2015-0043
Shiozawa, P., Leiva, A. P., Castro, C. D., da Silva, M. E., Cordeiro, Q., Fregni, F., et al. (2014). Transcranial direct current stimulation for generalized anxiety disorder: a case study. Biol. Psychiatry 75, e17–e18. doi: 10.1016/j.biopsych.2013.07.014
Smith, R. C., Boules, S., Mattiuz, S., Youssef, M., Tobe, R. H., Sershen, H., et al. (2015). Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. Schizophr. Res. 168, 260–266. doi: 10.1016/j.schres.2015.06.011
Soff, C., Sotnikova, A., Christiansen, H., Becker, K., and Siniatchkin, M. (2017). Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J. Neural. Transm (Vienna). 124, 133–144. doi: 10.1007/s00702-016-1646-y
Stagg, C. J., Best, J. G., Stephenson, M. C., O’Shea, J., Wylezinska, M., Kincses, Z. T., et al. (2009). Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 29, 5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009
Stein, D. J., Scott, K. M., de Jonge, P., and Kessler, R. C. (2017). Epidemiology of anxiety disorders: from surveys to nosology and back. Dialog. Clin. Neurosci. 19, 127–136. doi: 10.31887/DCNS.2017.19.2/dstein
Valiengo, L., Goerigk, S., Gordon, P. C., Padberg, F., Serpa, M. H., Koebe, S., et al. (2020). Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatry 77, 121–129. doi: 10.1001/jamapsychiatry.2019.3199
Vaseghi, B., Zoghi, M., and Jaberzadeh, S. (2015). A meta-analysis of site-specific effects of cathodal transcranial direct current stimulation on sensory perception and pain. PLoS One 10:e0123873. doi: 10.1371/journal.pone.0123873
Vicario, C. M., Salehinejad, M. A., Felmingham, K., Martino, G., and Nitsche, M. A. (2019). A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neurosci. Biobehav. Rev. 96, 219–231. doi: 10.1016/j.neubiorev.2018.12.012
Vitor de Souza Brangioni, M. C., Pereira, D. A., Thibaut, A., Fregni, F., Brasil-Neto, J. P., and Boechat-Barros, R. (2018). Effects of prefrontal transcranial direct current stimulation and motivation to quit in tobacco smokers: a randomized, sham controlled, double-blind trial. Front. Pharmacol. 9:14. doi: 10.3389/fphar.2018.00014
Volpato, C., Piccione, F., Cavinato, M., Duzzi, D., Schiff, S., Foscolo, L., et al. (2013). Modulation of affective symptoms and resting state activity by brain stimulation in a treatment-resistant case of obsessive-compulsive disorder. Neurocase 19, 360–370. doi: 10.1080/13554794.2012.667131
Vossel, S., Geng, J. J., and Fink, G. R. (2014). Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 20, 150–159. doi: 10.1177/1073858413494269
Weickert, T. W., Salimuddin, H., Lenroot, R. K., Bruggemann, J., Loo, C., Vercammen, A., et al. (2019). Preliminary findings of four-week, task-based anodal prefrontal cortex transcranial direct current stimulation transferring to other cognitive improvements in schizophrenia. Psychiatry Res. 280:112487. doi: 10.1016/j.psychres.2019.112487
Welch, E. S., Weigand, A., Hooker, J. E., Philip, N. S., Tyrka, A. R., Press, D. Z., et al. (2019). Feasibility of computerized cognitive-behavioral therapy combined with bifrontal transcranial direct current stimulation for treatment of major depression. Neuromodulation 22, 898–903. doi: 10.1111/ner.12807
Wietschorke, K., Lippold, J., Jacob, C., Polak, T., and Herrmann, M. J. (2016). Transcranial direct current stimulation of the prefrontal cortex reduces cue-reactivity in alcohol-dependent patients. J. Neural. Transm (Vienna). 123, 1173–1178. doi: 10.1007/s00702-016-1541-6
Woods, A. J., Antal, A., Bikson, M., Boggio, P. S., Brunoni, A. R., Celnik, P., et al. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 127, 1031–1048. doi: 10.1016/j.clinph.2015.11.012
Wrigley, P. J., Gustin, S. M., McIndoe, L. N., Chakiath, R. J., Henderson, L. A., and Siddall, P. J. (2013). Longstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trial. Pain 154, 2178–2184. doi: 10.1016/j.pain.2013.06.045
Wu, L. L., Potenza, M. N., Zhou, N., Kober, H., Shi, X. H., Yip, S. W., et al. (2020). A role for the right dorsolateral prefrontal cortex in enhancing regulation of both craving and negative emotions in internet gaming disorder: a randomized trial. Eur. Neuropsychopharmacol. 36, 29–37. doi: 10.1016/j.euroneuro.2020.04.003
Xu, J., Fregni, F., Brody, A. L., and Rahman, A. S. (2013). Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front. Psychiatry 4:112. doi: 10.3389/fpsyt.2013.00112
Xu, X., Deng, Z. Y., Huang, Q., Zhang, W. X., Qi, C. Z., and Huang, J. A. (2017). Prefrontal cortex-mediated executive function as assessed by stroop task performance associates with weight loss among overweight and obese adolescents and young adults. Behav. Brain Res. 321, 240–248. doi: 10.1016/j.bbr.2016.12.040
Yang, X., Gao, M., Shi, J., Ye, H., and Chen, S. (2017). Modulating the activity of the DLPFC and OFC has distinct effects on risk and ambiguity decision-making: a tDCS study. Front. Psychol. 8:1417. doi: 10.3389/fpsyg.2017.01417
Keywords: non-invasive neuromodulation, dorsolateral prefrontal cortex (DLPFC), schizophrenia, addiction, depression, psychiatric disease
Citation: Li Q, Fu Y, Liu C and Meng Z (2022) Transcranial Direct Current Stimulation of the Dorsolateral Prefrontal Cortex for Treatment of Neuropsychiatric Disorders. Front. Behav. Neurosci. 16:893955. doi: 10.3389/fnbeh.2022.893955
Received: 11 March 2022; Accepted: 04 April 2022;
Published: 25 May 2022.
Edited by:
Dongdong Qin, Yunnan University of Traditional Chinese Medicine, ChinaReviewed by:
Yunpeng Zhang, Brandeis University, United StatesTi-Fei Yuan, Shanghai Jiao Tong University, China
Copyright © 2022 Li, Fu, Liu and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang Liu, Y2hhbmcubGl1M0BzaWF0LmFjLmNu; Zhiqiang Meng, emhpcWlhbmctbWVuZ0AxNjMuY29t
 Qing Li
Qing Li Yu Fu1
Yu Fu1 Chang Liu
Chang Liu Zhiqiang Meng
Zhiqiang Meng