- 1Department of Pharmacology, Medical School, National and Kapodistrian University of Athens, Athens, Greece
- 2First Department of Psychiatry, Eginition Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Treatment of neuropsychiatric disorders relies on the effective delivery of therapeutic molecules to the target organ, the brain. The blood–brain barrier (BBB) hinders such delivery and proteins acting as transporters actively regulate the influx and importantly the efflux of both endo- and xeno-biotics (including medicines). Neuropsychiatric disorders are also characterized by important sex differences, and accumulating evidence supports sex differences in the pharmacokinetics and pharmacodynamics of many drugs that act on the brain. In this minireview we gather preclinical and clinical findings on how sex and sex hormones can influence the activity of those BBB transporter systems and affect the brain pharmacokinetics of psychotropic medicines. It emerges that it is not well understood which psychotropics are substrates for each of the many and not well-studied brain transporters. Indeed, most evidence originates from studies performed in peripheral tissues, such as the liver and the kidneys. None withstanding, accumulated evidence supports the existence of several sex differences in expression and activity of transport proteins, and a further modulating role of gonadal hormones. It is proposed that a closer study of sex differences in the active influx and efflux of psychotropics from the brain may provide a better understanding of sex-dependent brain pharmacokinetics and pharmacodynamics of psychotropic medicines.
Introduction
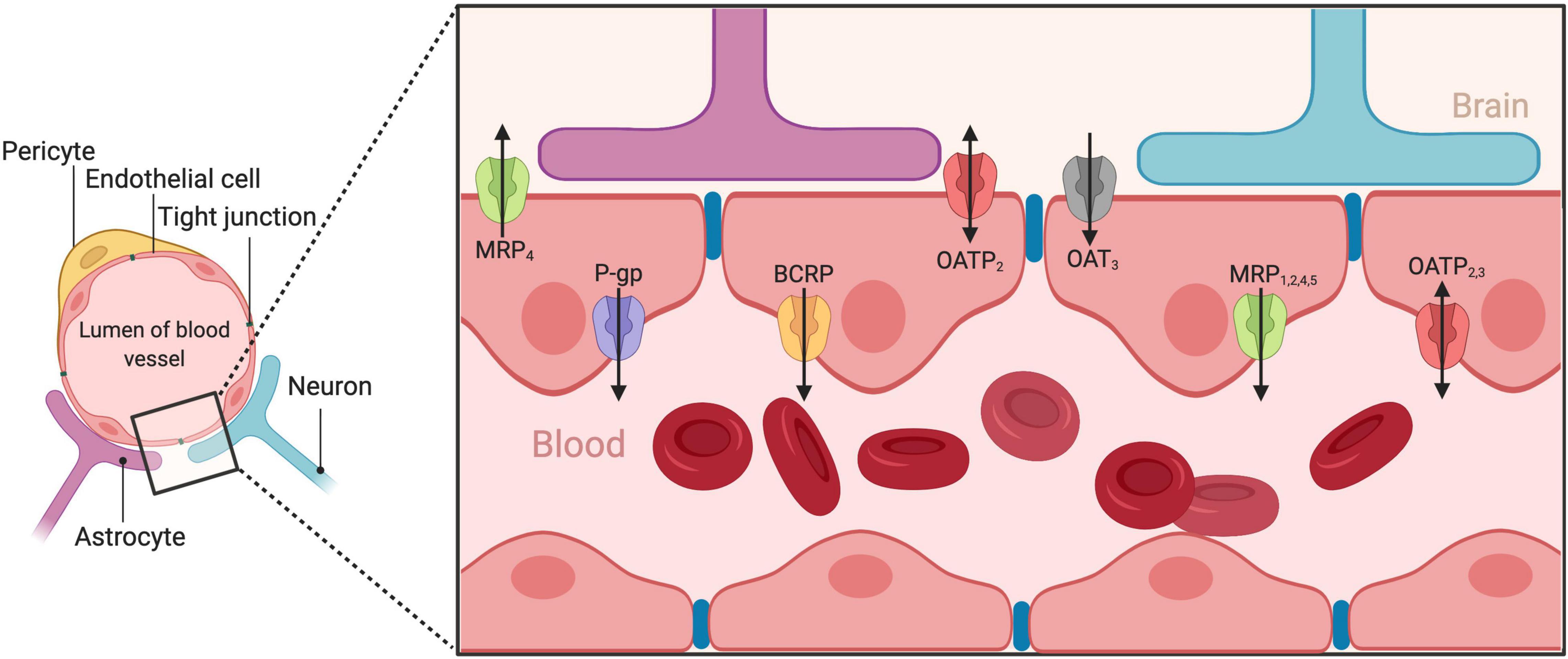
Neuropsychiatric disorders carry a significant burden and disproportionally affect more women than men (Wittchen et al., 2011). Their treatment relies on effective drug delivery to the brain. However, such drug delivery is challenging, as the blood–brain barrier (BBB) allows only endo- and xeno-biotics (including medicines) with specific physicochemical characteristics (lipophilicity, molecular weight, and charge) to enter. This barrier is achieved as brain capillary endothelial cells (BCECs), in very close proximity between them, form complex and tight junctions (Figure 1). The BBB functions within the context of the neurovascular unit (McConnell et al., 2017), a structure consisting of neurons, interneurons, astrocytes, pericytes, basal lamina covered with smooth muscular cells, microglia as well as endothelial cells and extracellular matrix, and regulates the cerebral blood flow (Muoio et al., 2014). Although some substances may diffuse passively though the BBB, the influx and efflux of most substances is actively regulated by a complex system of transporters expressed on the BBB. Emerging evidence suggests that brain pharmacokinetics, and thus psychotropic pharmacodynamics is greatly influenced by these transport systems (O’Brien et al., 2012). However, such knowledge is relatively new and now unfolding for many of those systems, especially with the help of evidence gathered from the presence of those transporters in peripheral barriers, such as in the gastrointestinal tract, the liver, and the kidneys. On the other hand, there is strong evidence that many neuropsychiatric disorders present significant sex differences (Balta et al., 2019) and preclinical research is progressing into incorporating sex as an important biological variable (Butlen-Ducuing et al., 2021). Moreover, psychotropic medication present noteworthy pharmacodynamic and interestingly, pharmacokinetic sex differences (Kokras et al., 2011; Seeman, 2021). Given that psychotropic medication must reach the brain to exert their therapeutic action, it emerges that potential sex differences in the brain’s transport systems might be involved in the action of psychotropic medicines in men and women. Therefore, in this minireview, we gather preclinical and clinical findings on how sex and sex hormones can influence the activity of BBB transporters and, discuss the current state of the art.
P-Glycoprotein
The ABCB1 gene expresses P-glycoprotein (P-gp) (or multi-drug resistance protein 1) in humans and two homologs in rodents, the abcb1a and abcb1b (O’Brien et al., 2012). P-gp has a broad binding site for a wide range of substances, as it is not restricted stereochemically and currently is the most studied transport protein. Regarding psychiatric disorders, P-gp plays an important role in CNS drugs bioavailability (De Klerk et al., 2011). Several antidepressants, like citalopram/escitalopram, paroxetine, imipramine, and venlafaxine are substrates of P-gp (Uhr and Grauer, 2003; Karlsson et al., 2010; O’Brien et al., 2013a,b). Thus, their brain pharmacokinetics are altered by P-gp and response to treatment is affected (Lin et al., 2011). However, other drugs appear not affected by P-gp, like fluoxetine and mirtazapine (Uhr et al., 2000, 2003). Interestingly, some psychotropic medications show a complex interaction with P-gp. For example, sertraline displays a biphasic and time-dependent interaction, fluctuating between inhibition and stimulation of P-gp (Kapoor et al., 2013). Another example is that high doses of nortriptyline saturate the P-gp-dependent transport and thus decrease its clearing effectiveness (Ejsing and Linnet, 2005). Abundant evidence indicates sex differences in the P-gp transport (Baris et al., 2006; Lifschitz et al., 2006; Ueno and Sato, 2012; Tornatore et al., 2013). However, there are also reports showing no significant sex differences (Dagenais et al., 2001; Gottschalk et al., 2011; Long et al., 2016). Such discrepancies, as discussed later, are probably explained by several factors, such as differences in species, the studied substrate, the tissue sampled, etc. Moreover, many P-gp polymorphisms affecting therapeutic drug efficacy are reported (Dizdarevic et al., 2014; Peng et al., 2015; Skalski et al., 2017; Rahikainen et al., 2018). Some are linked with sex-differentiated drug responses and development of specific side effects (Alzoubi et al., 2013; Rahikainen et al., 2018). This highlights the importance of sex segregation in pharmacogenetic research. Lastly, there is evidence that gonadal hormones, such as estrogens, testosterone and progesterone affect the activity of P-gp, and its activity may vary across the menstrual cycle (Axiotis et al., 1991; Peng et al., 2015; Kanado et al., 2019).
Breast Cancer Resistant Protein
Breast cancer resistant protein (BCRP) is an ABC transporter expressed in different tissues, including the brain epithelial cells, and may be responsible for the low bioavailability of several psychotropics. A recent study showed that sertraline is a BCRP substrate along with its P-gp inhibiting properties (Feng et al., 2019). Venlafaxine dose-dependently induces the BCRP expression (Bachmeier et al., 2011). Moreover, BCRP is known to work in synergy with P-gp, cooperatively eliminating xenobiotics from the brain and thus impeding treatment (Kodaira et al., 2010; Agarwal et al., 2011). Several preclinical studies highlight sex differences in BCRP, whose regulation is testosterone-induced and estradiol-inhibited, and point to a higher expression of BCRP in males (Teo et al., 1993; Fu et al., 2012). Hormonal manipulations, such as gonadectomy or hormonal treatment significantly affected its expression, and in general lower BCRP expression in females led to higher drug exposure (Merino et al., 2005; Gulilat et al., 2020). However, most results are obtained from tissues other than the brain. Interestingly a single study showed that specifically in the brain, BCRP expression is higher in female than male mice (Tanaka et al., 2005).
Multidrug Resistance-Associated Proteins
Multidrug resistance-associated protein (MRP) is a family of ABC transporters comprising of currently seven known members which are located at luminal membranes, and also found at the BBB (Ueno et al., 2010). Although considered to be an important drug transport mechanism, there is limited information regarding most psychotropics. One study showed that phenytoin and carbamazepine brain levels were lower following upregulation of MRP1 (Chen et al., 2013). Interestingly, no sex differences were identified regarding Mrp1 and Mrp2 mRNA expression in the choroid plexus. However, after its removal, BBB expression levels of Mrp1, Mrp2, and Mrp4 were twice as higher in female mice than in males (Flores et al., 2017). Studies in tissues such as the liver and the kidneys generally corroborate that females have higher MRP expression (Maher et al., 2005; Lu and Klaassen, 2008) and some evidence points to a progesterone and/or dehydroepiandrosterone regulation of this sex difference (Rost et al., 2005; Evseenko et al., 2007).
Organic Anion Transporters
Organic anion transporter (OAT) is an heterogenous family of negatively charged proteins, mainly located in kidneys and the liver, but OAT1/OAT3 are also found in the brain and are responsible for transporting hydrophobic organic anions. Evidence suggests that valproate, used as a mood stabilizer, is a substrate of OAT1 and homovanillic acid, a metabolite of dopamine, is a substrate of OAT3 (Sekine et al., 2000; Mori et al., 2003). In the kidneys and the liver, OAT expression is affected by androgens, and perhaps different OAT isoforms are stronger expressed in males and females in these tissues. Overall, renal Oat1 expression is androgen-regulated, renal Oat2 expression is modulated by female GH secretion pattern, and hepatic Oat3 expression is influenced by both androgens and female GH secretion pattern (Buist et al., 2003). Although OAT sex differences have been demonstrated in rodents, the direction of sex difference is not consistent and are not confirmed in other species, such as in rabbits (Groves et al., 2006) and in human cells (Breljak et al., 2016). Moreover, regarding specifically the brain, an in vivo BBB preclinical study did not identify a sex difference in OAT3 (Ohtsuki et al., 2005).
Organic Anion Transporting Polypeptides
These transporters form a superfamily of membrane-solute carriers characterized by significant functional diversity and a widespread role in the transport of endo/xenobiotics (Hagenbuch and Meier, 2004). There is scarce data on whether they are involved in the brain transport of psychotropics, but we know that transport of DHEA-S and opioids occurs via OATP1A2 and a small sex difference favoring women was recently reported (Asaba et al., 2000; Gao et al., 2000; Taniguchi et al., 2020). However, DHEA administration led to a gender-neutral Oatp1a1 and Oatp1b2 decrease and a further decrease in Oatp1a4 expression only in males (Rost et al., 2005). Evidence on sex differences is convoluted because there are many organic anion transporting polypeptide (OATP) transporters with a broad tissue distribution. Most preclinical evidence converges that activity of Oatp1a4, which is also expressed in the BBB, is higher in females, with testosterone probably suppressing it (Zhang et al., 2013; Brzica et al., 2018). However, several preclinical studies showed a tissue-specific variability in the direction or even absence of sex differences regarding various members of the OATP family (Cheng et al., 2005, 2006; Fu et al., 2012; Muzzio et al., 2014; Prasad et al., 2014; Badee et al., 2015).
Organic Cation Transporters
Organic cation transporter (OCT) are responsible for transporting cationic substances, like monoamine neurotransmitters, nicotine, the opioid agonist oxycodone, and antipsychotics like amisulpride and haloperidol (Bostrom et al., 2006; Okura et al., 2008; Sekhar et al., 2019). Interestingly, OCT2 and rOCT are found in the brain, and regulate the concentration of neurotransmitters in the neurons rather than the BBB (Busch et al., 1998). Very few data exist on potential sex differences, mostly on renal OCT2, which is expressed more strongly in males than females and it is upregulated by androgens (Alnouti et al., 2006; Groves et al., 2006; Basit et al., 2019). Plasma membrane monoamine transporter (PMAT/SLC29A4), a known transporter for cationic substances, is implicated in the efflux of amisulpride and haloperidol from the brain and is inhibited by nicotine (Tega et al., 2018; Sekhar et al., 2019). Some evidence on sex differences exist for PMAT, as behavioral changes were noted only in female, but not male, PMAT knockout mice (Gilman et al., 2018).
Monocarboxylate Transporters
Monocarboxylate transporter (MCT) mediate the transport of short chain monocarboxylates such as lactate and pyruvate, indicating their involvement in regulating brain energy substrates. Of 14 MCT members identified, MCT1, MCT2, MCT4, and the sodium-coupled SMCT1 have been described in the brain (Pierre and Pellerin, 2005). They are implicated in the brain transport of several drugs, including notably statins, salicylates and in relation to psychotropics, valproic acid, and γ-hydroxybutyrate (GHB) (Vijay and Morris, 2014). Sex differences have been identified, and are attributed in a tissue-specific regulation by both male and female sex hormones (Felmlee et al., 2020). Hepatic MCT1 and MCT4 regulation appears dependent on both estrogens and androgens (Cao et al., 2017). In muscles testosterone increases MCT1/4 expression but decreases testicular MCT2/4. However, there is a paucity of data regarding sex-dependent patterns of brain MCT regulation, which is important given the tissue-specific profile that emerges.
Multidrug and Toxin Extrusion Proteins
Multidrug and toxin extrusion protein (MATE) family transporters function in concert with OCT, are mostly expressed in the liver and the kidneys, but they are also found in the brain, and are involved in the transport of cationic drugs (Lickteig et al., 2008). Amisulpride and haloperidol, both antipsychotics, as well as nicotine, have been identified as possible substrates of MATE1 (Tsuda et al., 2007; Sekhar et al., 2019). This family of transporters is very recently discovered, and few data exist on potential sex differences. No data is available for the brain, but it appears that hepatic mRNA of MATE1 was notably increased in females in relation to males, but on the contrary, renal mRNA expression was found notably lower in females compared to males (Lickteig et al., 2008; Fu et al., 2012).
Other Transporters
Several other transporters, of which relatively little is known, are located at the BBB. Alanine/serine/cysteine transporter 2 (ASCT2) is located at the abluminal membrane of BACEs and is the only transporter of the Solute Carrier 1A (SLC1A) family to transport glutamine (Albrecht and Zielinska, 2019). BBB also expresses Betaine/GABA transporter-1, which in mice can be found as GAT2 transporter, regulating the efflux of GABA, and is different from GABA transporters, GAT1/3, that mediate transport across neurons and astrocytes (Takanaga et al., 2001). Enkephalins and AVP are effluxed by Peptide Transport System 1 and 2, respectively (Banks, 2006; Ueno et al., 2010). Several sodium-coupled transporters (NHE1, NHES, NBCn1, and NKCC1) are implicated in the active transport of lithium, a mood stabilizer across the BBB (Luo et al., 2018). System A and System L are transport systems of small and large neutral amino acids, respectively. Several drugs are carried by system L into the brain, and there is a strategy to design drugs that resemble the amino acids L-histidine and L-tryptophan for enhanced CNS delivery through LAT1 transporter (Hall et al., 2019). However, for all those transport systems little is known about their potential sex differences.
Discussion
In this minireview we summarized findings about sex differences in brain transport systems. These may affect pharmacokinetics of psychotropic medications in a sex-dependent manner and are important for precision medicine and treatment. In summary, for many transporter systems little is known about their function and the role of sex and gonadal hormones. Some protein transporters are indeed recently discovered, but for many other, evidence accumulates at a slow pace. Moreover, data are more abundant for the peripheral expression and function of these transporters, and less is known about the BBB, with the exception perhaps of the P-gp. This is surprising, as brain-transport systems regulate the influx and massively the efflux (clearance) of psychotropics. Moreover, BBB dysfunction has been implicated in many neuropsychiatric disorders and other diseases which are sex-differentiated (Greene et al., 2020; Profaci et al., 2020; Dion-Albert et al., 2022b). Admittedly, studies on peripheral transporters are methodologically easier, especially in humans where access to the BBB is significantly hindered. However, preclinical studies are also lacking, and more research is needed on which psychotropics are substrates of which BBB transporter system and whether this is sex-differentiated. This research could lead to clinical important findings regarding the treatment of psychiatric disorders in a more precise way.
Despite the paucity of evidence, preclinical studies collectively support the notion of male and female predominant transporters mainly in the periphery (Maher et al., 2005; Klaassen and Aleksunes, 2010; Zhu et al., 2017; Basit et al., 2019). The existence of protein transporter systems in the periphery also adds another layer of complexity in understanding their impact on pharmacokinetics. Most, if not all, of those transporters are heavily expressed in peripheral tissues (intestine, liver, and kidneys) that are crucially implicated in absorption, distribution, and metabolism of drugs. Peripheral transporters play as much an important role in psychotropic pharmacokinetics as do the BBB transporters in delivering to and clearing psychotropics from the brain. Therefore, a psychotropic that is a substrate for a specific transporter may be more extensively absorbed, more broadly distributed and at the same time more readily cleared from the brain and then metabolized and excreted. It remains unknown whether these effects cancel themselves out and, in the context of this review, whether male or female sex affects those transporters equally, in all of their expression sites (brain and periphery) (Cummins et al., 2002; Gottschalk et al., 2011). It is possible that their function is also influenced locally by estrogens – or other steroid – receptors in the BBB. These local interactions represent an interesting new research pathway that could promote our understanding of the BBB and its transporter proteins in the healthy and diseased brain in a sex-dependent way.
Indeed, transporter function, and thus potential sex differences, are not necessarily identical in peripheral tissues (such as in liver, kidneys, and intestine) and the brain. Although transporters present significant, but not absolute conservation across species, some sex differences observed in one species are not confirmed in another. Therefore, future research should focus on whether findings from one species to another are translatable, regarding both substrates for each transporter, as well as on the significance of potential sex differences of transporters in relation to human disease and treatment. A recent study on P-gp comparing gastro-intestinal tissue from Wistar rats and humans confirmed the translatability of experimental findings on discovered sex differences (Mai et al., 2021). P-gp activity is altered in patients with depression and recent evidence, in post mortem brain, suggest that vascular alterations in the BBB are present in women with depression (de Klerk et al., 2009; Dion-Albert et al., 2022b). Interestingly, BBB dysfunction has been associated with many other diseases, such as dementia, autoimmune disorders, epilepsy, and stroke, that also present sex differences and often co-exist with depression (Greene et al., 2020; Profaci et al., 2020). Therefore, future studies should investigate sex differences in specific transport proteins of the BBB in relation to its dysfunction during depression and other comorbidities. Moreover, transporter activity may be affected by factors such as stress, disease, exercise, or diet in a brain-region specific manner. Indeed, chronic variable stress altered BBB integrity in female, but not in the male mouse prefrontal cortex and this could have contributed to stress vulnerability (Dion-Albert et al., 2022b).
This mini-review focused on sex differences in psychotropic transport across the BBB. As the purpose of such sex differences remains unclear, it is postulated that the mammalian reproductive process exerted a selection pressure that explains those sexual dimorphisms (Gilks et al., 2014; Della Torre and Maggi, 2017). As elegantly reviewed elsewhere, this is reflected to several sex differences at the BBB in health and disease, regarding, but not limited to BBB strength, metabolism, response to stressors and involvement of several pathways, classic and non-classic genomic, as well as non-genomic, involving NO signaling, matrix metalloproteinases, the RhoA/Rho-kinase-2 pathway and other estrogens-mediated pathways (Weber and Clyne, 2021; Dion-Albert et al., 2022a).
In conclusion, accumulated evidence supports the existence of several sex differences in expression and activity of BBB transporters, and a further modulating role of gonadal hormones. A closer study of sex differences in the active influx and efflux of psychotropics from the brain may provide a better understanding of sex-dependent brain pharmacokinetics and pharmacodynamics of psychotropics. This would have a significant impact in precision medicine and treatment. Furthermore, in combination with BBB permeability studies, research on sex differences in BBB transporters will contribute to our understanding of the neurobiology and treatment of psychiatric diseases and their relationship with other disorders, such as autoimmune and neurological.
Author Contributions
D-GS and TG searched the literature and gathered relevant evidence. PP compiled the first draft. NK and CD conceived and coordinated the study, mastered the final and revised manuscripts, and provided guidance. All authors contributed and approved the manuscript.
Funding
This research was co-financed by Greece and the European Union (European Social Fund – ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers – 2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (IKY).
Conflict of Interest
NK and CD have received honoraria and financial support from Janssen-Cilag, Lundbeck, Elpen S.A., and Medochemie S.A.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, S., Hartz, A. M., Elmquist, W. F., and Bauer, B. (2011). Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr. Pharm. Des. 17, 2793–2802. doi: 10.2174/138161211797440186
Albrecht, J., and Zielinska, M. (2019). Exchange-mode glutamine transport across CNS cell membranes. Neuropharmacology 161:107560. doi: 10.1016/j.neuropharm.2019.03.003
Alnouti, Y., Petrick, J. S., and Klaassen, C. D. (2006). Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab. Dispos. 34, 477–482. doi: 10.1124/dmd.105.006932
Alzoubi, K. H., Khabour, O. F., Al-Azzam, S. I., and Mayyas, F. (2013). The role of multidrug resistance-1 (MDR1) variants in response to fexofenadine among Jordanians. Int. J. Clin. Pharmacol. Ther. 51, 880–887. doi: 10.5414/CP201968
Asaba, H., Hosoya, K., Takanaga, H., Ohtsuki, S., Tamura, E., Takizawa, T., et al. (2000). Blood-brain barrier is involved in the efflux transport of a neuroactive steroid, dehydroepiandrosterone sulfate, via organic anion transporting polypeptide 2. J. Neurochem. 75, 1907–1916. doi: 10.1046/j.1471-4159.2000.0751907.x
Axiotis, C. A., Guarch, R., Merino, M. J., Laporte, N., and Neumann, R. D. (1991). P-glycoprotein expression is increased in human secretory and gestational endometrium. Lab. Invest. 65, 577–581.
Bachmeier, C. J., Beaulieu-Abdelahad, D., Ganey, N. J., Mullan, M. J., and Levin, G. M. (2011). Induction of drug efflux protein expression by venlafaxine but not desvenlafaxine. Biopharm. Drug Dispos. 32, 233–244. doi: 10.1002/bdd.753
Badee, J., Achour, B., Rostami-Hodjegan, A., and Galetin, A. (2015). Meta-analysis of expression of hepatic organic anion-transporting polypeptide (OATP) transporters in cellular systems relative to human liver tissue. Drug Metab. Dispos. 43, 424–432. doi: 10.1124/dmd.114.062034
Balta, G., Dalla, C., and Kokras, N. (2019). Women’s Psychiatry. Adv. Exp. Med. Biol. 1192, 225–249. doi: 10.1007/978-981-32-9721-0_11
Banks, W. A. (2006). The CNS as a target for peptides and peptide-based drugs. Expert Opin. Drug Deliv. 3, 707–712. doi: 10.1517/17425247.3.6.707
Baris, N., Kalkan, S., Guneri, S., Bozdemir, V., and Guven, H. (2006). Influence of carvedilol on serum digoxin levels in heart failure: Is there any gender difference? Eur. J. Clin. Pharmacol. 62, 535–538. doi: 10.1007/s00228-006-0138-7
Basit, A., Radi, Z., Vaidya, V. S., Karasu, M., and Prasad, B. (2019). Kidney cortical transporter expression across species using quantitative proteomics. Drug Metab. Dispos. 47, 802–808. doi: 10.1124/dmd.119.086579
Bostrom, E., Simonsson, U. S., and Hammarlund-Udenaes, M. (2006). In vivo blood-brain barrier transport of oxycodone in the rat: indications for active influx and implications for pharmacokinetics/pharmacodynamics. Drug Metab. Dispos. 34, 1624–1631. doi: 10.1124/dmd.106.009746
Breljak, D., Ljubojevic, M., Hagos, Y., Micek, V., Balen Eror, D., Vrhovac Madunic, I., et al. (2016). Distribution of organic anion transporters NaDC3 and OAT1-3 along the human nephron. Am. J. Physiol. Renal Physiol. 311, F227–F238. doi: 10.1152/ajprenal.00113.2016
Brzica, H., Abdullahi, W., Reilly, B. G., and Ronaldson, P. T. (2018). Sex-specific differences in organic anion transporting polypeptide 1a4 (Oatp1a4) functional expression at the blood-brain barrier in Sprague-Dawley rats. Fluids Barriers CNS 15:25. doi: 10.1186/s12987-018-0110-9
Buist, S. C., Cherrington, N. J., and Klaassen, C. D. (2003). Endocrine regulation of rat organic anion transporters. Drug Metab. Dispos. 31, 559–564. doi: 10.1124/dmd.31.5.559
Busch, A. E., Karbach, U., Miska, D., Gorboulev, V., Akhoundova, A., Volk, C., et al. (1998). Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol. Pharmacol. 54, 342–352. doi: 10.1124/mol.54.2.342
Butlen-Ducuing, F., Balkowiec-Iskra, E., Dalla, C., Slattery, D. A., Ferretti, M. T., Kokras, N., et al. (2021). Implications of sex-related differences in central nervous system disorders for drug research and development. Nat. Rev. Drug Discov. 20, 881–882. doi: 10.1038/d41573-021-00115-6
Cao, J., Ng, M., and Felmlee, M. A. (2017). Sex hormones regulate rat hepatic monocarboxylate transporter expression and membrane trafficking. J. Pharm. Pharm. Sci. 20, 435–444. doi: 10.18433/J3CH29
Chen, Y. H., Wang, C. C., Xiao, X., Wei, L., and Xu, G. (2013). Multidrug resistance-associated protein 1 decreases the concentrations of antiepileptic drugs in cortical extracellular fluid in amygdale kindling rats. Acta Pharmacol. Sin. 34, 473–479. doi: 10.1038/aps.2012.183
Cheng, X., Maher, J., Chen, C., and Klaassen, C. D. (2005). Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps). Drug Metab. Dispos. 33, 1062–1073. doi: 10.1124/dmd.105.003640
Cheng, X., Maher, J., Lu, H., and Klaassen, C. D. (2006). Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol. Pharmacol. 70, 1291–1297. doi: 10.1124/mol.106.025122
Cummins, C. L., Wu, C. Y., and Benet, L. Z. (2002). Sex-related differences in the clearance of cytochrome P450 3A4 substrates may be caused by P-glycoprotein. Clin. Pharmacol. Ther. 72, 474–489. doi: 10.1067/mcp.2002.128388
Dagenais, C., Zong, J., Ducharme, J., and Pollack, G. M. (2001). Effect of mdr1a P-glycoprotein gene disruption, gender, and substrate concentration on brain uptake of selected compounds. Pharm. Res. 18, 957–963. doi: 10.1023/a:1010984110732
De Klerk, O. L., Bosker, F. J., Luurtsema, G., Nolte, I. M., Dierckx, R., Den Boer, J. A., et al. (2011). The role of p-glycoprotein in psychiatric disorders: A reliable guard of the brain? Cent. Nerv. Syst. Agents Med. Chem. 11, 197–209. doi: 10.2174/187152411798047744
de Klerk, O. L., Willemsen, A. T., Roosink, M., Bartels, A. L., Hendrikse, N. H., Bosker, F. J., et al. (2009). Locally increased P-glycoprotein function in major depression: a PET study with [11C]verapamil as a probe for P-glycoprotein function in the blood-brain barrier. Int. J. Neuropsychopharmacol. 12, 895–904. doi: 10.1017/S1461145709009894
Della Torre, S., and Maggi, A. (2017). Sex differences: A resultant of an evolutionary pressure? Cell Metab. 25, 499–505. doi: 10.1016/j.cmet.2017.01.006
Dion-Albert, L., Bandeira Binder, L., Daigle, B., Hong-Minh, A., Lebel, M., and Menard, C. (2022a). Sex differences in the blood-brain barrier: implications for mental health. Front. Neuroendocrinol. 65:100989. doi: 10.1016/j.yfrne.2022.100989
Dion-Albert, L., Cadoret, A., Doney, E., Kaufmann, F. N., Dudek, K. A., Daigle, B., et al. (2022b). Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat. Commun. 13:164. doi: 10.1038/s41467-021-27604-x
Dizdarevic, S., Aplin, M., Newport, M. J., Ryan, N., Holt, S., Goubet, S., et al. (2014). Old tracer for a new purpose: potential role for 99mTc-2-Methoxyisobutylisonitrile (99mTc-MIBI) in renal transplant care. Nucl. Med. Commun. 35, 1058–1066. doi: 10.1097/MNM.0000000000000165
Ejsing, T. B., and Linnet, K. (2005). Influence of P-glycoprotein inhibition on the distribution of the tricyclic antidepressant nortriptyline over the blood-brain barrier. Hum. Psychopharmacol. 20, 149–153. doi: 10.1002/hup.667
Evseenko, D. A., Paxton, J. W., and Keelan, J. A. (2007). Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab. Dispos. 35, 595–601. doi: 10.1124/dmd.106.011478
Felmlee, M. A., Jones, R. S., Rodriguez-Cruz, V., Follman, K. E., and Morris, M. E. (2020). Monocarboxylate transporters (SLC16): function, regulation, and role in health and disease. Pharmacol. Rev. 72, 466–485. doi: 10.1124/pr.119.018762
Feng, S., Zheng, L., Tang, S., Gu, J., Jiang, X., and Wang, L. (2019). In-vitro and in situ assessment of the efflux of five antidepressants by breast cancer resistance protein. J. Pharm. Pharmacol. 71, 1133–1141. doi: 10.1111/jphp.13100
Flores, K., Manautou, J. E., and Renfro, J. L. (2017). Gender-specific expression of ATP-binding cassette (Abc) transporters and cytoprotective genes in mouse choroid plexus. Toxicology 386, 84–92. doi: 10.1016/j.tox.2017.05.019
Fu, Z. D., Csanaky, I. L., and Klaassen, C. D. (2012). Effects of aging on mRNA profiles for drug-metabolizing enzymes and transporters in livers of male and female mice. Drug Metab. Dispos. 40, 1216–1225. doi: 10.1124/dmd.111.044461
Gao, B., Hagenbuch, B., Kullak-Ublick, G. A., Benke, D., Aguzzi, A., and Meier, P. J. (2000). Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 294, 73–79.
Gilks, W. P., Abbott, J. K., and Morrow, E. H. (2014). Sex differences in disease genetics: evidence, evolution, and detection. Trends Genet. 30, 453–463. doi: 10.1016/j.tig.2014.08.006
Gilman, T. L., George, C. M., Vitela, M., Herrera-Rosales, M., Basiouny, M. S., Koek, W., et al. (2018). Constitutive plasma membrane monoamine transporter (PMAT, Slc29a4) deficiency subtly affects anxiety-like and coping behaviours. Eur. J. Neurosci. [Epub ahead of print]. doi: 10.1111/ejn.13968
Gottschalk, S., Cummins, C. L., Leibfritz, D., Christians, U., Benet, L. Z., and Serkova, N. J. (2011). Age and sex differences in the effects of the immunosuppressants cyclosporine, sirolimus and everolimus on rat brain metabolism. Neurotoxicology 32, 50–57. doi: 10.1016/j.neuro.2010.10.006
Greene, C., Hanley, N., and Campbell, M. (2020). Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl. Psychiatry 10:373. doi: 10.1038/s41398-020-01054-3
Groves, C. E., Suhre, W. B., Cherrington, N. J., and Wright, S. H. (2006). Sex differences in the mRNA, protein, and functional expression of organic anion transporter (Oat) 1, Oat3, and organic cation transporter (Oct) 2 in rabbit renal proximal tubules. J. Pharmacol. Exp. Ther. 316, 743–752. doi: 10.1124/jpet.105.094979
Gulilat, M., Keller, D., Linton, B., Pananos, A. D., Lizotte, D., Dresser, G. K., et al. (2020). Drug interactions and pharmacogenetic factors contribute to variation in apixaban concentration in atrial fibrillation patients in routine care. J. Thromb. Thrombolysis 49, 294–303. doi: 10.1007/s11239-019-01962-2
Hagenbuch, B., and Meier, P. J. (2004). Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflügers Arch. 447, 653–665. doi: 10.1007/s00424-003-1168-y
Hall, C., Wolfe, H., Wells, A., Chien, H. C., Colas, C., Schlessinger, A., et al. (2019). l-Type amino acid transporter 1 activity of 1,2,3-triazolyl analogs of l-histidine and l-tryptophan. Bioorg. Med. Chem. Lett. 29, 2254–2258. doi: 10.1016/j.bmcl.2019.06.033
Kanado, Y., Tsurudome, Y., Omata, Y., Yasukochi, S., Kusunose, N., Akamine, T., et al. (2019). Estradiol regulation of P-glycoprotein expression in mouse kidney and human tubular epithelial cells, implication for renal clearance of drugs. Biochem. Biophys. Res. Commun. 519, 613–619. doi: 10.1016/j.bbrc.2019.09.021
Kapoor, A., Iqbal, M., Petropoulos, S., Ho, H. L., Gibb, W., and Matthews, S. G. (2013). Effects of sertraline and fluoxetine on p-glycoprotein at barrier sites: in vivo and in vitro approaches. PLoS One 8:e56525. doi: 10.1371/journal.pone.0056525
Karlsson, L., Schmitt, U., Josefsson, M., Carlsson, B., Ahlner, J., Bengtsson, F., et al. (2010). Blood-brain barrier penetration of the enantiomers of venlafaxine and its metabolites in mice lacking P-glycoprotein. Eur. Neuropsychopharmacol. 20, 632–640. doi: 10.1016/j.euroneuro.2010.04.004
Klaassen, C. D., and Aleksunes, L. M. (2010). Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol. Rev. 62, 1–96. doi: 10.1124/pr.109.002014
Kodaira, H., Kusuhara, H., Ushiki, J., Fuse, E., and Sugiyama, Y. (2010). Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J. Pharmacol. Exp. Ther. 333, 788–796. doi: 10.1124/jpet.109.162321
Kokras, N., Dalla, C., and Papadopoulou-Daifoti, Z. (2011). Sex differences in pharmacokinetics of antidepressants. Expert Opin. Drug Metab. Toxicol. 7, 213–226. doi: 10.1517/17425255.2011.544250
Lickteig, A. J., Cheng, X., Augustine, L. M., Klaassen, C. D., and Cherrington, N. J. (2008). Tissue distribution, ontogeny and induction of the transporters Multidrug and toxin extrusion (MATE) 1 and MATE2 mRNA expression levels in mice. Life Sci. 83, 59–64. doi: 10.1016/j.lfs.2008.05.004
Lifschitz, A., Ballent, M., Virkel, G., Sallovitz, J., and Lanusse, C. (2006). Sex-related differences in the gastrointestinal disposition of ivermectin in the rat: P-glycoprotein involvement and itraconazole modulation. J. Pharm. Pharmacol. 58, 1055–1062. doi: 10.1211/jpp.58.8.0005
Lin, K. M., Chiu, Y. F., Tsai, I. J., Chen, C. H., Shen, W. W., Liu, S. C., et al. (2011). ABCB1 gene polymorphisms are associated with the severity of major depressive disorder and its response to escitalopram treatment. Pharmacogenet. Genomics 21, 163–170. doi: 10.1097/FPC.0b013e32833db216
Long, B., Su, Y. Q., Xia, Y., Zou, Y. Y., Tang, B., Chen, Z. J., et al. (2016). No sex difference in overall P-glycoprotein activity as assessed by talinolol disposition in humans. Int. J. Clin. Pharmacol. Ther. 54, 157–162. doi: 10.5414/CP202477
Lu, H., and Klaassen, C. (2008). Gender differences in mRNA expression of ATP-binding cassette efflux and bile acid transporters in kidney, liver, and intestine of 5/6 nephrectomized rats. Drug Metab. Dispos. 36, 16–23. doi: 10.1124/dmd.107.014845
Luo, H., Gauthier, M., Tan, X., Landry, C., Poupon, J., Dehouck, M. P., et al. (2018). Sodium transporters are involved in lithium influx in brain endothelial cells. Mol. Pharm. 15, 2528–2538. doi: 10.1021/acs.molpharmaceut.8b00018
Maher, J. M., Slitt, A. L., Cherrington, N. J., Cheng, X., and Klaassen, C. D. (2005). Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab. Dispos. 33, 947–955. doi: 10.1124/dmd.105.003780
Mai, Y., Dou, L., Yao, Z., Madla, C. M., Gavins, F. K. H., Taherali, F., et al. (2021). Quantification of P-Glycoprotein in the gastrointestinal tract of humans and rodents: methodology, gut region, sex, and species matter. Mol. Pharm. 18, 1895–1904. doi: 10.1021/acs.molpharmaceut.0c00574
McConnell, H. L., Kersch, C. N., Woltjer, R. L., and Neuwelt, E. A. (2017). The translational significance of the neurovascular unit. J. Biol. Chem. 292, 762–770. doi: 10.1074/jbc.R116.760215
Merino, G., van Herwaarden, A. E., Wagenaar, E., Jonker, J. W., and Schinkel, A. H. (2005). Sex-dependent expression and activity of the ATP-binding cassette transporter breast cancer resistance protein (BCRP/ABCG2) in liver. Mol. Pharmacol. 67, 1765–1771. doi: 10.1124/mol.105.011080
Mori, S., Takanaga, H., Ohtsuki, S., Deguchi, T., Kang, Y. S., Hosoya, K., et al. (2003). Rat organic anion transporter 3 (rOAT3) is responsible for brain-to-blood efflux of homovanillic acid at the abluminal membrane of brain capillary endothelial cells. J. Cereb. Blood Flow Metab. 23, 432–440. doi: 10.1097/01.WCB.0000050062.57184.75
Muoio, V., Persson, P. B., and Sendeski, M. M. (2014). The neurovascular unit - concept review. Acta Physiol. 210, 790–798. doi: 10.1111/apha.12250
Muzzio, A. M., Noyes, P. D., Stapleton, H. M., and Lema, S. C. (2014). Tissue distribution and thyroid hormone effects on mRNA abundance for membrane transporters Mct8, Mct10, and organic anion-transporting polypeptides (Oatps) in a teleost fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 167, 77–89. doi: 10.1016/j.cbpa.2013.09.019
O’Brien, F. E., Clarke, G., Dinan, T. G., Cryan, J. F., and Griffin, B. T. (2013a). Human P-glycoprotein differentially affects antidepressant drug transport: relevance to blood-brain barrier permeability. Int. J. Neuropsychopharmacol. 16, 2259–2272. doi: 10.1017/S1461145713000692
O’Brien, F. E., O’Connor, R. M., Clarke, G., Dinan, T. G., Griffin, B. T., and Cryan, J. F. (2013b). P-glycoprotein inhibition increases the brain distribution and antidepressant-like activity of escitalopram in rodents. Neuropsychopharmacology 38, 2209–2219. doi: 10.1038/npp.2013.120
O’Brien, F. E., Dinan, T. G., Griffin, B. T., and Cryan, J. F. (2012). Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: clinical significance of in vitro and in vivo findings. Br. J. Pharmacol. 165, 289–312. doi: 10.1111/j.1476-5381.2011.01557.x
Ohtsuki, S., Tomi, M., Hata, T., Nagai, Y., Hori, S., Mori, S., et al. (2005). Dominant expression of androgen receptors and their functional regulation of organic anion transporter 3 in rat brain capillary endothelial cells; comparison of gene expression between the blood-brain and -retinal barriers. J. Cell Physiol. 204, 896–900. doi: 10.1002/jcp.20352
Okura, T., Hattori, A., Takano, Y., Sato, T., Hammarlund-Udenaes, M., Terasaki, T., et al. (2008). Involvement of the pyrilamine transporter, a putative organic cation transporter, in blood-brain barrier transport of oxycodone. Drug Metab. Dispos. 36, 2005–2013. doi: 10.1124/dmd.108.022087
Peng, R., Zhang, H., Zhang, Y., and Wei, D. Y. (2015). Effects of the ABCB1 (1199G > A) polymorphism on steroid sex hormone-induced P-Glycoprotein expression, ATPase activity, and hormone efflux. Med. Sci. 3, 124–137. doi: 10.3390/medsci3040124
Pierre, K., and Pellerin, L. (2005). Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 94, 1–14. doi: 10.1111/j.1471-4159.2005.03168.x
Prasad, B., Evers, R., Gupta, A., Hop, C. E., Salphati, L., Shukla, S., et al. (2014). Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab. Dispos. 42, 78–88. doi: 10.1124/dmd.113.053819
Profaci, C. P., Munji, R. N., Pulido, R. S., and Daneman, R. (2020). The blood-brain barrier in health and disease: important unanswered questions. J. Exp. Med. 217:e20190062. doi: 10.1084/jem.20190062
Rahikainen, A. L., Palo, J. U., Haukka, J., and Sajantila, A. (2018). Post-mortem analysis of suicide victims shows ABCB1 haplotype 1236T-2677T-3435T as a candidate predisposing factor behind adverse drug reactions in females. Pharmacogenet. Genomics 28, 99–106. doi: 10.1097/FPC.0000000000000328
Rost, D., Kopplow, K., Gehrke, S., Mueller, S., Friess, H., Ittrich, C., et al. (2005). Gender-specific expression of liver organic anion transporters in rat. Eur. J. Clin. Invest. 35, 635–643. doi: 10.1111/j.1365-2362.2005.01556.x
Seeman, M. V. (2021). The pharmacodynamics of antipsychotic drugs in women and men. Front. Psychiatry 12:468. doi: 10.3389/fpsyt.2021.650904
Sekhar, G. N., Fleckney, A. L., Boyanova, S. T., Rupawala, H., Lo, R., Wang, H., et al. (2019). Region-specific blood-brain barrier transporter changes leads to increased sensitivity to amisulpride in Alzheimer’s disease. Fluids Barriers CNS 16:38. doi: 10.1186/s12987-019-0158-1
Sekine, T., Cha, S. H., and Endou, H. (2000). The multispecific organic anion transporter (OAT) family. Pflugers Arch. 440, 337–350. doi: 10.1007/s004240000297
Skalski, D., Wendorff, J., Romanowicz, H., Rysz, A., Marchel, A., Stasiolek, M., et al. (2017). Associations between MDR1 C3435T polymorphism and drug-resistant epilepsy in the Polish population. Acta Neurol. Belg. 117, 153–158. doi: 10.1007/s13760-016-0690-6
Takanaga, H., Ohtsuki, S., Hosoya, K., and Terasaki, T. (2001). GAT2/BGT-1 as a system responsible for the transport of gamma-aminobutyric acid at the mouse blood-brain barrier. J. Cereb. Blood Flow Metab. 21, 1232–1239. doi: 10.1097/00004647-200110000-00012
Tanaka, Y., Slitt, A. L., Leazer, T. M., Maher, J. M., and Klaassen, C. D. (2005). Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem. Biophys. Res. Commun. 326, 181–187. doi: 10.1016/j.bbrc.2004.11.012
Taniguchi, T., Zanetti-Yabur, A., Wang, P., Usyk, M., Burk, R. D., and Wolkoff, A. W. (2020). Interindividual diversity in expression of organic anion uptake transporters in normal and cirrhotic human liver. Hepatol. Commun. 4, 739–752. doi: 10.1002/hep4.1489
Tega, Y., Yamazaki, Y., Akanuma, S. I., Kubo, Y., and Hosoya, K. I. (2018). Impact of nicotine transport across the blood-brain barrier: carrier-mediated transport of nicotine and interaction with central nervous system drugs. Biol. Pharm. Bull. 41, 1330–1336. doi: 10.1248/bpb.b18-00134
Teo, W. S., Tan, A., Ng, A., and Wong, J. (1993). New directions in cardiovascular mapping and therapy. Ann. Acad. Med. Singap. 22, 197–204.
Tornatore, K. M., Brazeau, D., Dole, K., Danison, R., Wilding, G., Leca, N., et al. (2013). Sex differences in cyclosporine pharmacokinetics and ABCB1 gene expression in mononuclear blood cells in African American and Caucasian renal transplant recipients. J. Clin. Pharmacol. 53, 1039–1047. doi: 10.1002/jcph.123
Tsuda, M., Terada, T., Asaka, J., Ueba, M., Katsura, T., and Inui, K. (2007). Oppositely directed H+ gradient functions as a driving force of rat H+/organic cation antiporter MATE1. Am. J. Physiol. Renal Physiol. 292, F593–F598. doi: 10.1152/ajprenal.00312.2006
Ueno, K., and Sato, H. (2012). Sex-related differences in pharmacokinetics and pharmacodynamics of anti-hypertensive drugs. Hypertens. Res. 35, 245–250. doi: 10.1038/hr.2011.189
Ueno, M., Nakagawa, T., Wu, B., Onodera, M., Huang, C. L., Kusaka, T., et al. (2010). Transporters in the brain endothelial barrier. Curr. Med. Chem. 17, 1125–1138. doi: 10.2174/092986710790827816
Uhr, M., and Grauer, M. T. (2003). abcb1ab P-glycoprotein is involved in the uptake of citalopram and trimipramine into the brain of mice. J. Psychiatr. Res. 37, 179–185. doi: 10.1016/s0022-3956(03)00022-0
Uhr, M., Grauer, M. T., and Holsboer, F. (2003). Differential enhancement of antidepressant penetration into the brain in mice with abcb1ab (mdr1ab) P-glycoprotein gene disruption. Biol. Psychiatry 54, 840–846. doi: 10.1016/s0006-3223(03)00074-x
Uhr, M., Steckler, T., Yassouridis, A., and Holsboer, F. (2000). Penetration of amitriptyline, but not of fluoxetine, into brain is enhanced in mice with blood-brain barrier deficiency due to mdr1a P-glycoprotein gene disruption. Neuropsychopharmacology 22, 380–387. doi: 10.1016/S0893-133X(99)00095-0
Vijay, N., and Morris, M. E. (2014). Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 20, 1487–1498. doi: 10.2174/13816128113199990462
Weber, C. M., and Clyne, A. M. (2021). Sex differences in the blood-brain barrier and neurodegenerative diseases. APL Bioeng. 5:011509. doi: 10.1063/5.0035610
Wittchen, H. U., Jacobi, F., Rehm, J., Gustavsson, A., Svensson, M., Jonsson, B., et al. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 21, 655–679. doi: 10.1016/j.euroneuro.2011.07.018
Zhang, Y., Csanaky, I. L., Selwyn, F. P., Lehman-McKeeman, L. D., and Klaassen, C. D. (2013). Organic anion-transporting polypeptide 1a4 (Oatp1a4) is important for secondary bile acid metabolism. Biochem. Pharmacol. 86, 437–445. doi: 10.1016/j.bcp.2013.05.020
Keywords: sex differences, blood–brain barrier, psychotropics drugs, transporters and channels, brain, females, transporters, mental disorders
Citation: Dalla C, Pavlidi P, Sakelliadou D-G, Grammatikopoulou T and Kokras N (2022) Sex Differences in Blood–Brain Barrier Transport of Psychotropic Drugs. Front. Behav. Neurosci. 16:844916. doi: 10.3389/fnbeh.2022.844916
Received: 29 December 2021; Accepted: 27 April 2022;
Published: 23 May 2022.
Edited by:
Matthew J. Robson, University of Cincinnati, United StatesReviewed by:
Patrick T. Ronaldson, University of Arizona, United StatesThomas Paul Davis, University of Arizona, United States
Copyright © 2022 Dalla, Pavlidi, Sakelliadou, Grammatikopoulou and Kokras. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikolaos Kokras, bmtva3Jhc0BtZWQudW9hLmdy
 Christina Dalla
Christina Dalla Pavlina Pavlidi
Pavlina Pavlidi Danai-Georgia Sakelliadou
Danai-Georgia Sakelliadou Tatiana Grammatikopoulou
Tatiana Grammatikopoulou Nikolaos Kokras
Nikolaos Kokras