- 1Psychology Department and Center for Neural Science, New York University, New York, NY, United States
- 2Emotional Brain Institute, The Nathan S. Kline Institute for Psychiatric Research, Orangeburg, NY, United States
- 3Child and Adolescent Psychiatry, New York University Langone Medical Center, New York, NY, United States
The complex process of regulating physiological functions and homeostasis during external and internal disruptions develops slowly in altricial species, with parental care functioning as a co-regulator of infant physiological and emotional homeostasis. Here, we review our current understanding of the infant’s use of parental behaviors for neurobehavioral regulation and its disruption with harsh parental care. Taking a cross-species view, we briefly review the human developmental literature that highlights the importance of the caregiver in scaffolding the child’s physiological and emotional regulation, especially under threat and stress. We then use emerging corresponding animal literature within the phylogenetically preserved attachment system to help define neural systems supporting caregiver regulation and its supporting causal mechanism to provide translational bridges to inform causation and mechanisms impossible to define in children. Next, we briefly review animal research highlighting the impact of specific sensory stimuli imbedded in parental care as important for infant physiological and emotion regulation. We then highlight the importance of parental sensory stimuli gaining hedonic value to go beyond simple sensory stimuli to further impact neurobehavioral regulation, with poor quality of care compromising the infant’s ability to use these cues for regulation. Clinically, parental regulation of the infant is correlated with later-life neurobehavioral outcome and quality of life. We suggest an understanding of this parental regulation of the infant’s immediate neurobehavioral functioning within the context of attachment quality, that may provide insights into the complex processes during early life, initiating the pathway to pathology.
Introduction
Homeostasis is a complex self-regulating process of maintaining physiological functions at optimal levels and engaging mechanisms to readjust with external and internal challenges (Cannon, 1929). Specifically, in response to internal and external challenges, the body self-regulates its physiological functioning to adjust myriad bodily processes, some of which involve emotional regulation. Homeostasis and self-regulation in response to challenges develop slowly in altricial mammalian species, such as humans, non-human primates, and rats. Sensory stimuli received during parental care are important for the infant to maintain homeostasis across myriad physiological and emotional systems until self-regulation is achieved (Kopp, 1982; Shields et al., 1994; Gunnar and Donzella, 2002; Bridgett et al., 2015; Montroy et al., 2016; Silvers et al., 2017; Li-Grining et al., 2019; Vink et al., 2020; Fotopoulou et al., 2021). The purpose of this review is to present our current understanding of the neurobehavioral response of the infant to parental caregiving and its importance to the infant’s short-term physiological and emotional regulation. We focus on the impact of parental care on infant neurobehavioral functioning, with some emphasis on one specific parental behavior–regulation of infant homeostasis. Next, we review the literature on infant adverse experiences and the disruption of parental regulation, to define an atypical developmental experience that goes beyond the initial adversity.
Self-Regulation Is Immature in Infants of Altricial Species and Parental Care Provides the Sensory Stimulation Regulating Infant Neurobehavioral Function
Children are born with self-regulatory mechanisms in many physiological systems which are necessary for homeostasis and to support myriad processes to sustain life. Other physiological systems use parental co-regulation over the first months to years of life, of varying degrees.
For instance, food intake is heavily dependent on parental regulation, while body temperature can be regulated by the infant but the caregiver optimizes temperature through clothes and the warmth of physical contact (Fotopoulou et al., 2021). Frequently, the impact of maternal care on the infant and the degree of parental co-regulation is subtle, with its impact becoming visible with removal or dramatic changes in parental care. For example, young children can maintain homeostasis of vital functions, such as heart rate and respiration, but the regulatory role of parental care was shown through experiments comparing infants alone v. engaged in parental contact via somatosensory (temperature, touch), olfactory (caregiver odor), visual (face), and auditory (voice) stimulation (Kommers et al., 2019; Suga et al., 2019; Buhler-Wassmann and Hibel, 2021; Ionio et al., 2021). This regulation is also seen in the infant’s co-sleep with the parent, producing improved sleep compared to when the infants sleep alone (Mosko et al., 1997; Richard and Mosko, 2004; Waynforth, 2020; Yoshida and Funato, 2021).
Of course, physiological homeostasis includes neural mechanisms regulating emotional homeostasis. The child’s emotional regulation is enhanced by parents as evidenced by the more effective soothing of a crying child by the parent compared to a stranger engaging in similar comforting behaviors (Bridges et al., 1997; Feinberg et al., 2009; Hazen et al., 2010; Yoshida and Funato, 2021). In children, regular disruption of emotional homeostasis during early life (i.e., through adverse rearings such as deprivation or maltreatment) is highly correlated with later-life compromised functioning (i.e., psychiatric disorders, impaired academics, etc.), although specific causal mechanisms embedded within the infant-caregiver relationship have remained elusive (Raineki et al., 2012; Buhler-Wassmann and Hibel, 2021). The lack of understanding specific infant self-regulatory and co-regulatory mechanisms, such as specific parental behavior or sensory stimuli necessary and causal for infant physiological and emotional homeostasis, has hampered our understanding of the correlational link between infant dysregulation and later-life compromised outcome (Tronick et al., 1977; Feldman et al., 2002; Raposo et al., 2014; Cevasco-Trotter et al., 2019; Palacios-Barrios and Hanson, 2019; Tottenham, 2020; Vink et al., 2020). For example, it hasn’t been determined (1) how exactly an infant regulates its sleep-wake cycle, (2) if sleep-wake cycle dysregulation is a marker for behavioral or emotional difficulties, (3) how much it is self-regulated by the infant or supported by co-regulation from the parent, and (4) what specific parental behavior regulates or dysregulates the sleep-wake cycle of an infant (Scher, 2005; Whittingham and Douglas, 2014).
Early Animal Models: Regulation by Maternal Care With Replacement of Sensory Stimuli
In the 1980s, Myron Hofer and others observed that children separated from the mother exhibited dysregulation of myriad physiological functions and behaviors (Hofer, 1981, 1994). Using rodent infant-mother dyads, Hofer operationalized the role of the parent in regulating infant physiology and behavior. Specifically, Hofer removed maternal care and questioned which sensory stimuli replacement would regain homeostasis. He uncovered specific causal mechanisms hidden in complex maternal behaviors that controlled very specific physiological functions of the infant rat, which he termed “hidden regulators.” For example, the mothers’ tactile stimulation regulates pups’ growth hormones, while the mothers’ phasic provision of milk regulates the pup’s sleep-wake states (Hofer, 1994, 2006). Of course, others also significantly contributed to this research. For example, removal of the mother could be partially reversed by artificial feeding and tactile stimulation (mimic licking) and repaired heightened adrenocorticotrophic hormone and growth hormone levels (Harlow, 1959; Powell et al., 1967; Schanberg et al., 1984; Schanberg and Kuhn, 1985; Pauk et al., 1986; Francis et al., 1999; Oers and Kloet, 1999). Overall, this research went beyond the global “maternal behavior” as causal, to specific links between subcomponents of maternal behavior and homeostasis of specific systems. This was a paradigm shift in our understanding of the importance of maternal care that provided the foundation for a new approach to the care of young infants (Schanberg and Field, 1987; Reite et al., 1989; Field, 2003; Brett et al., 2015; Porges et al., 2019; Fotopoulou et al., 2021; Yoshida and Funato, 2021).
Regulation With the Caregiver Exceeds That Achieved by Sensory Stimulation Alone
More recently, research is suggesting that sensory stimuli infants experience during interactions with the caregiver acquire hedonic value that provides potent capabilities to regulate the infant more robustly (Perry et al., 2016). Indeed, work by Myron Hofer’s team and others hinted that some features of the mother rat could not be duplicated by simple sensory stimulation: for example, the rat pup’s behaviors could not be altered by a neutral novel odor and required the presentation of the mother’s odor (Hofer, 1994). This is supported by observations of a young child interacting (or being hugged) with the parent as opposed to a stranger, which illustrates the infant’s specialized prosocial behaviors to the biological/adoptive (learned) caregiver compared to strangers (Singer et al., 1985; Altenhofen et al., 2013; Tottenham et al., 2019; Yoshida et al., 2020). Overall, this research suggests that young children are using all of their sensory systems to use parental information to maintain homeostasis, as illustrated in Figure 1.
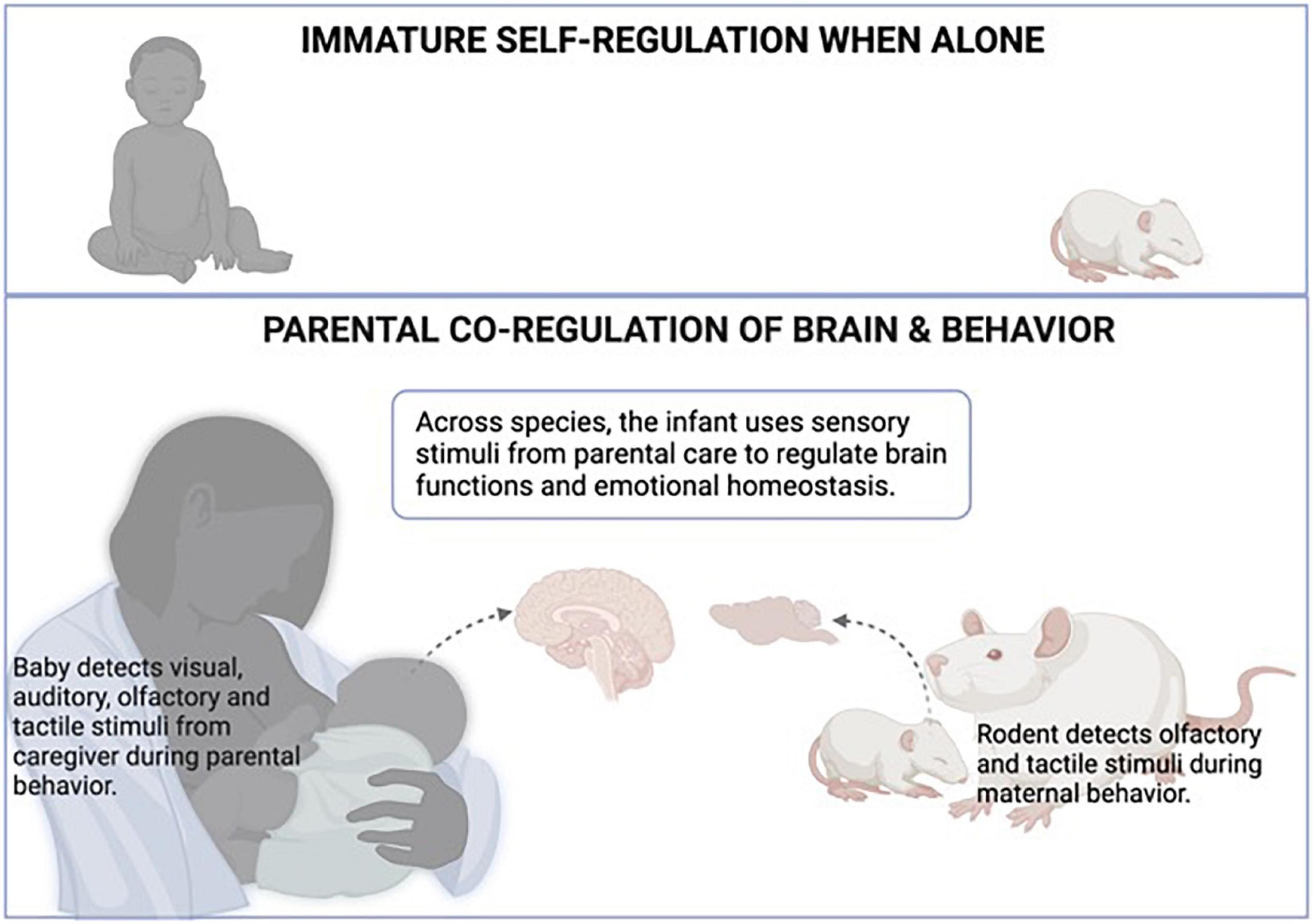
Figure 1. Attachment to the primary caregivers is learned across species. Once the infant learns the attachment figure, the attachment figure acquires special value for the infant, including regulation of homeostasis (Bowlby, 1969; Hofer, 1994). As illustrated in the top panel, when alone, the infant’s ability to maintain physiological and emotional homeostasis is limited. As illustrated in the lower panel, the regulatory role of sensory stimuli from the attachment figure was shown to have a particular impact on the infant’s brain to permit homeostasis and regulation of diverse systems. During maternal care, the infant detects visual, auditory, olfactory, and tactile stimuli from the caregiver during parental behavior, while rodents rely on olfactory and tactile stimuli during maternal behavior. Across species, the infant uses these sensory stimuli from parental care to regulate brain functions, which extends to physiological and emotional homeostasis. Created with BioRender.com.
Also illustrated in Figure 1, other animal species, such as rodents, also use maternal behavior and sensory cues to maintain homeostasis and provide a translational bridge and opportunities to explore neural mechanisms using invasive techniques unsuitable to use on children. For example, rodents can provide a temporally dynamic view of the infant’s brain during mother-infant interactions. Using local field potentials (LFP), we recorded the prefrontal cortex (PFC) of very young pups, targeting a brain region known to be responsive to alteration in maternal care (Opendak et al., 2020). These recordings of neural oscillations illustrated the profound and immediate impact of the maternal presence and maternal behavior on pups’ neural oscillations. For example, when pups are near (not touching) the mother compared to alone, pups’ LFP shows a reduction in high-frequency bands. Contact with the mother produces a further reduction, and maternal behaviors produce transient, rapid increases in LFP power: nipple attachment increased slow-wave activity, while maternal stimulation such as grooming and milk ejection produced very rapid and transient cortical desynchronization (Opendak et al., 2020). The neural controls of these PFC oscillations are complex: Courtiol et al. (2018) have shown that the mother’s presence regulates the activity of cortical oscillations by increasing low-frequency oscillations mediated by the serotonin receptor, 5-hydroxytryptamine-2-receptor, while the transient cortical desynchronization induced by grooming and milk ejection is dependent on norepinephrine (Sarro et al., 2014). Overall, this data illustrated the robust and temporally dynamic impact on pups’ brain rhythmic oscillations, thus impacting a process shown to be critical in guiding brain development (Penn and Shatz, 1999).
Using Parental Reduction of Infant Fear for Understanding Emotional Regulation
Parental reduction of their child’s fear was noted in Bowlby’s Attachment Theory in the 1960s: children in novel or threatening environments showed less fear if their parent was present (Bowlby, 1965, 1969). Expanding on this observation, more recent research showed that the impact of maternal presence on children is particularly salient during the regulation of the threat response: more specifically, maternal presence reduces the child’s fear and is accomplished through suppression of both stress hormone and amygdala activity, as indicated by correlations (Gunnar and Donzella, 2002; Gee et al., 2014; Hostinar et al., 2015; Callaghan et al., 2019). Animal studies showed that this effect is also seen in diverse altricial animal species (Suchecki et al., 1993; Wiedenmayer et al., 2003; Shionoya et al., 2007; Hennessy et al., 2009; Sarro et al., 2014; Sanchez et al., 2015; Sullivan, 2017; Sullivan and Opendak, 2021), which permitted the identification of a causal link between infant fear and maternal presence. Indeed, we now understand maternal presence suppressing infant fear because her presence suppresses the basolateral subarea of the amygdala, a brain area well-documented to support fear in pups and adults (Moriceau et al., 2006; Moriceau and Sullivan, 2006; Shionoya et al., 2007). How the infant detects the mother to then suppress the amygdala is also known: the smell of the mother’s odor enters the pup’s olfactory system and travels to the hypothalamic paraventricular nucleus to suppress activation of the hypothalamic-pituitary-adrenal (Shionoya et al., 2007). The infant amygdala has an age-specific dependence on the stress hormone corticosterone (CORT), and its suppression by the mother is sufficient to block mechanisms (Thompson et al., 2008). Thus, maternal suppression of CORT deprives the infant amygdala of the plasticity mechanisms required for its activation. This plasticity suppression also means the infant amygdala cannot support amygdala-dependent fear learning (Moriceau et al., 2006; Moriceau and Sullivan, 2006; Shionoya et al., 2007), which was recently replicated in children (Tottenham et al., 2019) along with suppression of the amygdala (Gee et al., 2014).
Further capitalizing on our ability to probe the rat brain during interactions with the mother, we focused on the role of the neurotransmitter dopamine (DA) because it’s documented to be altered by maternal care and presence in infant rats (Tamborski et al., 1990; Andersen et al., 1992; Barr et al., 2009; Opendak et al., 2021). We began by using microdialysis, a technique that enables us to measure DA levels in pups during maternal presence, to show that maternal presence blocks DA release into the basolateral amygdala (Barr et al., 2009). Using brain dissection, we showed that the mother also blocked AMPA receptors and plasticity molecules (Opendak et al., 2019) showing the mother was altering the pups global amygdala activity, its connectivity to other brain regions, but also the intracellular machinery within amygdala neurons controlling pups ability to use brain areas to alter behavior and learning (Moriceau and Sullivan, 2006; Barr et al., 2009; Opendak et al., 2019; Robinson-Drummer et al., 2019; Sullivan and Opendak, 2021). For reviews see Kikusui et al. (2006), Gunnar et al. (2015), Sanchez et al. (2015), Al Aïn et al. (2017), Kiyokawa and Hennessy (2018).
While a present and calm parent typically suppress their offspring’s fear, an agitated or fearful parent can enhance their offspring’s fear via social transmission (Chang and Debiec, 2016; Silvers et al., 2021). Rodent and human research have shown that a parent expressing fear to a specific cue will transmit that specific fear to their child, a process associated with heightened amygdala activation. Using rodents to identify causal mechanisms, a learned fearful odor presented to the mother is sufficient to induce an amygdala-dependent, learned odor-specific fear in pups, if the mother had expressed fear in the pups’ presence (Debiec and Sullivan, 2014; Rickenbacher et al., 2017). Specifically, rat mothers were fear-conditioned (peppermint-shock pairing either before mating or during pregnancy) and presented with the odor conditioned stimulus (CS) to express fear in the presence of their pups. Pups immediately showed fear of the peppermint odor and continued to show amygdala-dependent fear of the peppermint odor the next day, whether or not the mother was present. The mechanism for this social transmission is socially communicated: maternal fear expression is accompanied by the release of a fear pheromone (potentially comparable to the child seeing a fearful parental facial expression), which increases pups CORT and amygdala activity to support fear learning (Debiec and Sullivan, 2014; Boulanger-Bertolus et al., 2017). This social transmission of fear occurs throughout the lifespan (Askew and Field, 2008; Chang and Debiec, 2016).
Disrupting Parental Regulation Through Early Life Adversity
To acknowledge how effective co-regulation works, it’s important to understand what happens when parental regulation is disrupted and its effectiveness decreased. For example, even an abused child learns and expresses attachment to their caregiver, which has also been seen in other animal species (Ainsworth, 1969; Perry and Sullivan, 2014), although self-regulation appears compromised with trauma-related attachment (Shields et al., 1994). This abuse-related attachment occurs across species: maltreated chicks following the imprinting figure (Hess, 1962; Salzen, 1970; Rajecki et al., 1978), infant dogs shocked by a human caregiver seek that person (Stanley and Elliot, 1962) and infant monkeys inflicted with pain by a wire surrogate or an abusive mother continue to show attachment (Harlow and Harlow, 1965; Maestripieri et al., 1999; Sanchez et al., 2001; Suomi, 2003; O’Connor and Cameron, 2006). Animal research on rodents suggests the evolutionarily conserved attachment system appears to rely on an attachment learning circuit that is equally responsive to rough and nurturing maternal care to support attachment learning (Sullivan et al., 2000; Sullivan, 2012).
While there are short-term benefits to constructing an attachment system that ensures an infant learns to attach regardless of the quality of care, this system does produce robust detrimental long-term outcomes that primarily emerge around peri-adolescence. Finding biomarkers to early life that can be used to predict later life problems is a major focus of the adversity developmental research. However, subtle immediate behavioral identification of children who have compromised attachment has been shown experimentally by Ainsworth and the stressful Strange Situation Procedure (SSP, i.e., repeated parent-child separations and reunions) (Ainsworth, 1969). Specifically, Ainsworth’s SSP showed that parents can typically calm (regulate emotions) the stressed child, although maltreated children instead show ambivalent, contradictory, and incomplete/undirected behavioral responses, termed Disordered Attachment. Disordered attachment is the only attachment category associated with later life pathology (Main and Solomon, 1990; Mason et al., 2005).
To better understand the role of maternal regulation (or lack of) in disrupted SSP social behavior toward the mother, we developed an SSP test in rodents. This also builds on our previous research showing that pups reared by a maltreating mother have a significantly slower approach response to the mother outside the nest and a blunted neural response to the mother across brain areas that include the amygdala, PFC, and hippocampus (Rincon-Cortes and Sullivan, 2016; Al Aïn et al., 2017; Yan et al., 2017; Perry et al., 2019). We replicated these results in the rodent SSP during the final infant-mother reunion and extended these results to show that the typical dynamic cortical LFP response pups show to the maternal presence and maternal behaviors were significantly blunted. Capitalizing on the power of animal models to define mechanisms, we systemically pharmacologically block pups’ stress hormone release during the SSP, which repaired pups’ disrupted behavior to the mother, and pups regained a significant amount of dynamic LFP in response to the mother (Opendak et al., 2020). The mother was anesthetized (no maternal behaviors) in our SSP test, which was required to mimic the human SSP.
However, to better understand pups’ response to maternal behaviors, we also tested pups during natural interactions with the mother as we recorded cortical LFP. Maltreated pups’ response to maternal care was also blunted, but only to nurturing maternal care. Specifically, the LFP response to harsh maternal care (low occurrence in control pups permits comparison), such as stepping on or dragging pups did not differ between control and maltreated pups, while the LFP response to the maternal nurturing care (i.e., nipple attachment, grooming) was blunted. Importantly, while these LFP measures uncovered differences in pups’ neural response within the nest, even when no behavioral differences were found within the nest–the stress of the SSP was required to uncover pups’ behavioral differences (Opendak et al., 2020).
As illustrated in Figure 2, our integration of these surprising results illustrates that disfunction of maternal regulation of the infant is a sensitive measure of early life pathology and appears to be a robust measure in situations ranging from threat (fear learning) and a stressful novel situation (Ainsworth’s SSP) where both behavior and brain showed atypical responses. Within the nest, where behavioral pathologies are difficult to locate, the LFP response to the mother was atypical and similar to the blunted response found in stressful testing. Thus, infant behavioral effects of maltreatment are subtle, although its disruption of maternal regulation of the infant appears consistent across myriad situations.
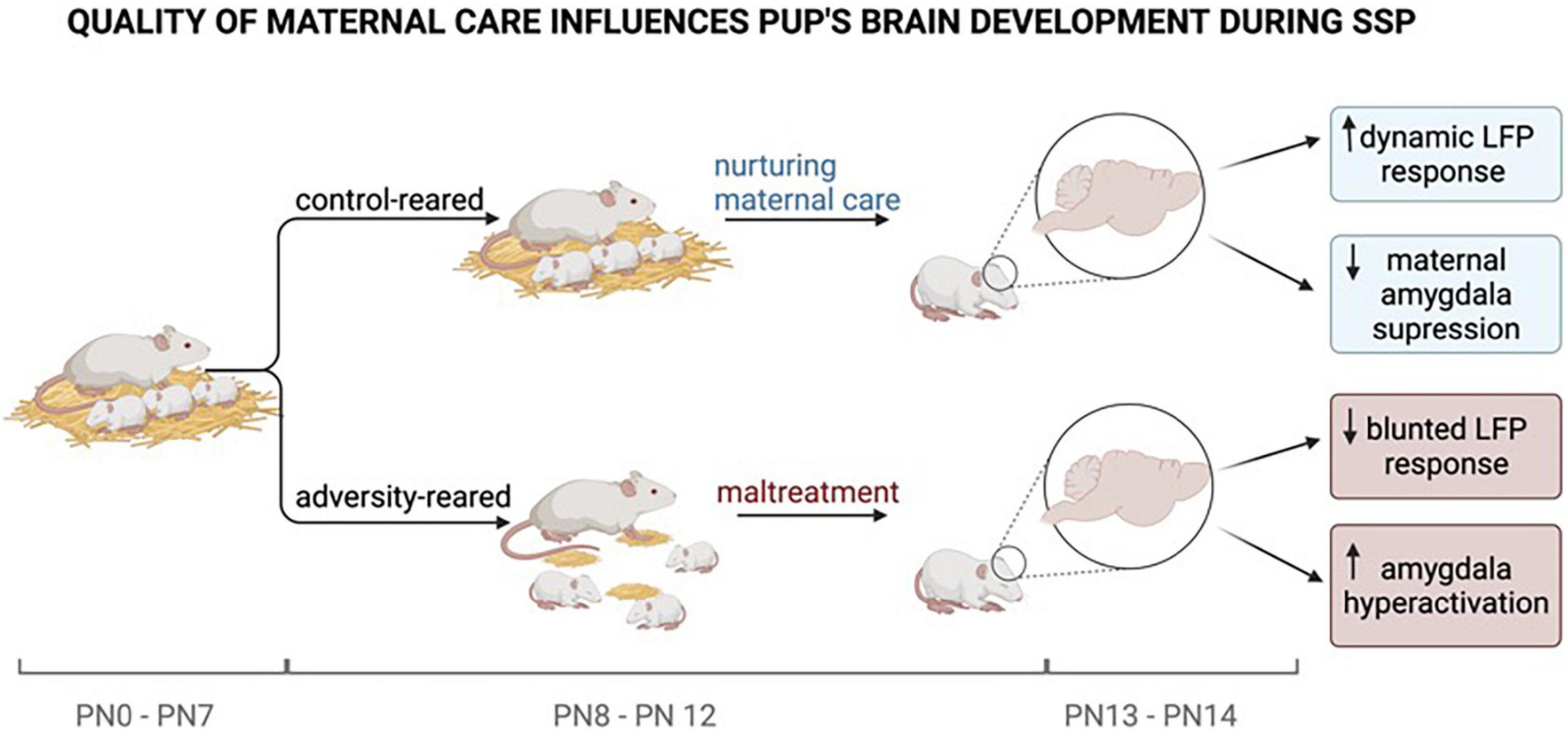
Figure 2. Experiments by Opendak et al. (2020) have shown that pups reared under adverse conditions face repeated maltreatment by their mothers compared to control-reared pups. The quality of maternal care provided is causal for the pup’s brain development. While typically reared pups show a dynamic LFP response (synchronized, low-frequency waves) and a maternally suppressed amygdala response, adversity reared pups to show a blunted LFP response (desynchronized, high-frequency waves) and amygdala hyperactivation. At PN 10–12 the pup’s brain is most sensitive to maternal regulation. The mother’s ability to regulate the pup’s physiological and emotional state diminishes as the pup is getting older and more independent. Neurological changes during brain development caused by maternal maltreatment set the early stage for later-life pathologies. Created with BioRender.com.
Conclusion
Sensory stimulation in early life has been considered important since the 1950s and further refined in the 1980s by Hofer as sensory stimulation as “Hidden Regulators” of pup physiology. More recently, we have begun to understand that sensory stimuli received from the parent are more than simple sensory stimuli–they’re sensory stimuli that have acquired special value through their learned association with the attachment figures and have significantly more robust strength to alter the infant’s neurobehavioral function. The SSP animal model approach aligns with decades of research on children, highlighting the parent’s special role in guiding the infant’s interaction with the world and as a source of comfort. This review of animal models of human regulation has highlighted that maternal presence regulates the brain on myriad levels, including gene expression, receptors, neurotransmitters, brain regions of interest, circuits, and networks across the brain including data from our lab. This regulatory effect occurs during parent-infant interactions but is more salient during a threat. Further research focusing on understanding what exactly has gone wrong within a parent-infant interaction as well as its neural underpinnings can help in targeted interventions and treatments to repair the relationship and prevent later-life pathologies.
Author Contributions
NG and RMS wrote the manuscript. RMZ contributed specific paragraphs to the manuscript. WS and RR made the figures on BioRender.com. EZ reviewed the manuscript for orthographic mistakes. All authors read and approved the manuscript.
Funding
This work was supported by the National Institutes of Health grant HD083217 (RMS) and Fulbright PS00297326 (NG).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ainsworth, M. D. S. (1969). Object relations, dependency, and attachment: a theoretical review of the infant-mother relationship. Child Dev. 40, 969–1025. doi: 10.2307/1127008
Al Aïn, S., Perry, R. E., Nuñez, B., Kayser, K., Hochman, C., Brehman, E., et al. (2017). Neurobehavioral assessment of maternal odor in developing rat pups: implications for social buffering. Soc. Neurosci. 12, 32–49. doi: 10.1080/17470919.2016.1159605
Altenhofen, S., Clyman, R., Little, C., Baker, M., and Biringen, Z. (2013). Attachment security in three-year-olds who entered substitute care in infancy. Infant Mental Health J. 34, 435–445. doi: 10.1002/imhj.21401
Andersen, S. L., Gazzara, R. A., Robinson, S. R., and Smotherman, W. P. (1992). Effect of milk of dopamine release in the newborn rat: an in vivo microdialysis study. Dev. Brain Res. 68, 286–288. doi: 10.1016/0165-3806(92)90073-6
Askew, C., and Field, A. P. (2008). The vicarious learning pathway to fear 40 years on. Clin. Psychol. Rev. 28, 1249–1265. doi: 10.1016/j.cpr.2008.05.003
Barr, G. A., Moriceau, S., Shionoya, K., Muzny, K., Gao, P., Wang, S., et al. (2009). Transitions in infant learning are modulated by dopamine in the amygdala. Nat. Neurosci. 12, 1367–1369. doi: 10.1038/nn.2403
Boulanger-Bertolus, J., White, A. M., and Debiec, J. (2017). Enduring neural and behavioral effects of early life adversity in infancy: consequences of maternal abuse and neglect, trauma and fear. Curr. Behav. Neurosci. Rep. 4, 107–116. doi: 10.1007/s40473-017-0112-y
Brett, Z. H., Humphreys, K. L., Fleming, A. S., Kraemer, G. W., and Drury, S. S. (2015). Using cross-species comparisons and a neurobiological framework to understand early social deprivation effects on behavioral development. Dev Psychopathol. 27, 347–367. doi: 10.1017/S0954579415000036
Bridges, L. J., Grolnick, W. S., and Connell, J. P. (1997). Infant emotion regulation with mothers and fathers. Infant Behav. Dev. 20, 47–57. doi: 10.1016/S0163-6383(97)90060-6
Bridgett, D. J., Burt, N. M., Edwards, E. S., and Deater-Deckard, K. (2015). Intergenerational transmission of self-regulation: a multidisciplinary review and integrative conceptual framework. Psychol. Bull. 141:602. doi: 10.1037/a0038662
Buhler-Wassmann, A. C., and Hibel, L. C. (2021). Studying caregiver-infant co-regulation in dynamic, diverse cultural contexts: a call to action. Infant Behav. Dev. 64:101586. doi: 10.1016/j.infbeh.2021.101586
Callaghan, B. L., Gee, D. G., Gabard-Durnam, L., Telzer, E. H., Humphreys, K. L., Goff, B., et al. (2019). Decreased amygdala reactivity to parent cues protects against anxiety following early adversity: an examination across 3 years. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 664–671. doi: 10.1016/j.bpsc.2019.02.001
Cannon, W. B. (1929). Organization for physiological homeostasis. Physiol. Rev. 9, 399–431. doi: 10.1152/physrev.1929.9.3.399
Cevasco-Trotter, A. M., Hamm, E. L., Yang, X., and Parton, J. (2019). Multimodal neurological enhancement intervention for self-regulation in premature infants. Adv. Neonatal Care 19:E3–E11. doi: 10.1097/ANC.0000000000000595
Chang, D. J., and Debiec, J. (2016). Neural correlates of the mother-to-infant social transmission of fear. J. Neurosci. Res. 94, 526–534. doi: 10.1002/jnr.23739
Courtiol, E., Wilson, D. A., Shah, R., Sullivan, R. M., and Teixeira, C. M. (2018). Maternal regulation of pups’ cortical activity: role of serotonergic signaling. eNeuro 5:ENEURO.18–ENEURO.93. doi: 10.1523/ENEURO.0093-18.2018
Debiec, J., and Sullivan, R. M. (2014). Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc. Natl. Acad. Sci. U.S.A 111, 12222–12227. doi: 10.1073/pnas.1316740111
Feinberg, M. E., Kan, M. L., and Goslin, M. C. (2009). Enhancing co-parenting, parenting, and child self-regulation: effects of family foundations 1 year after birth. Prev. Sci. 10, 276–285. doi: 10.1007/s11121-009-0130-4
Feldman, R., Weller, A., Sirota, L., and Eidelman, A. I. (2002). Skin-to-Skin contact (Kangaroo care) promotes self-regulation in premature infants: sleep-wake cyclicity, arousal modulation, and sustained exploration. Dev. Psychol. 38, 194–207. doi: 10.1037//0012-1649.38.2.194
Field, T. M. (2003). Stimulation of preterm infants. Pediatr. Rev. 24, 4–11. doi: 10.1542/pir.24-1-4
Fotopoulou, A., Von Mohr, M., and Krahé, C. (2021). Affective regulation through touch: homeostatic and allostatic mechanisms. Curr. Opin. Behav. Sci. 43, 80–87. doi: 10.31234/osf.io/ksj3x
Francis, D., Diorio, J., Liu, D., and Meaney, M. J. (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286, 1155–1158. doi: 10.1126/science.286.5442.1155
Gee, D. G., Gabard-Durnam, L., Telzer, E. H., Humphreys, K. L., Goff, B., Shapiro, M., et al. (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 25, 2067–2078. doi: 10.1177/0956797614550878
Gunnar, M. R., and Donzella, B. (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology 27, 199–220. doi: 10.1016/S0306-4530(01)00045-2
Gunnar, M. R., Hostinar, C. E., Sanchez, M. M., Tottenham, N., and Sullivan, R. M. (2015). Parental buffering of fear and stress neurobiology: reviewing parallels across rodent, monkey, and human models. Soc. Neurosci. 10, 474–478. doi: 10.1080/17470919.2015.1070198
Harlow, H., and Harlow, M. (1965). “The affectional systems,” in Behavior of Nonhuman Primates, Vol. 2, eds A. Schrier, H. Harlow, and F. Stollnitz (Cambridge, MA: Academic Press). 287–334. doi: 10.1016/b978-1-4832-2821-1.50008-2
Harlow, H. F. (1959). Love in infant monkeys. Sci. Am. 200, 68–75. doi: 10.1038/scientificamerican0659-68
Hazen, N. L., McFarland, L., Jacobvitz, D., and Boyd-Soisson, E. (2010). Fathers’ frightening behaviors and sensitivity with infants: relations with fathers’ attachment representations, father–infant attachment, and children’s later outcomes. Early Child Dev. Care 180, 51–69. doi: 10.1080/03004430903414703
Hennessy, M. B., Kaiser, S., and Sachser, N. (2009). Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 30:470–482. doi: 10.1016/j.yfrne.2009.06.001
Hess, E. (1962). “Ethology: an approach to the complete analysis of behavior,” in New Directions in Psychology, eds R. Brown, E. Galanter, E. Hess, and G. Mendler (New York, NY: Holt, Rinehart, and Winston). 159–199.
Hofer, M. (1981). Toward a developmental basis for disease predisposition: the effects of early maternal separation on brain, behavior, and cardiovascular system. Res. Pub. Assoc. Res. Nerv. Mental Dis. 59, 209–228.
Hofer, M. A. (1994). Hidden Regulators in Attachment, Separation, and Loss. Monogr. Soc. Res. Child Dev. 59, 192–207. doi: 10.2307/1166146
Hofer, M. A. (2006). Psychobiological roots of early attachment. Curr. Dir. Psychol. Sci. 15, 84–88. doi: 10.1111/j.0963-7214.2006.00412.x
Hostinar, C. E., Johnson, A. E., and Gunnar, M. R. (2015). Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev. Sci. 18, 281–297. doi: 10.1111/desc.12195
Ionio, C., Ciuffo, G., and Landoni, M. (2021). Parent-Infant Skin-to-Skin Contact and Stress Regulation: a systematic review of the literature. Int. J. Environ. Res. Public Health 18:4695. doi: 10.3390/ijerph18094695
Kikusui, T., Winslow, J. T., and Mori, Y. (2006). Social buffering: relief from stress and anxiety. Philos. Trans. R. Soc. B Biol. Sci. 361, 2215–2228. doi: 10.1098/rstb.2006.1941
Kiyokawa, Y., and Hennessy, M. B. (2018). Comparative studies of social buffering: a consideration of approaches, terminology, and pitfalls. Neurosci. Biobehav. Rev. 86, 131–141. doi: 10.1016/j.neubiorev.2017.12.005
Kommers, D. R., Joshi, R., van Pul, C., Feijs, L., Bambang Oetomo, S., and Andriessen, P. (2019). Changes in autonomic regulation due to kangaroo care remain unaffected by using a swaddling device. Acta Paediatr. 108, 258–265. doi: 10.1111/apa.14484
Kopp, C. B. (1982). Antecedents of self-regulation: a developmental perspective. Dev. Psychol. 18:199. doi: 10.1037/0012-1649.18.2.199
Li-Grining, C. P., McKinnon, R. D., and Raver, C. C. (2019). Self-Regulation in early and middle childhood as a precursor to social adjustment among low-income, ethnic minority children. Merrill Palmer Q. (Wayne State Univ Press) 65, 265–293. doi: 10.13110/merrpalmquar1982.65.3.0265
Maestripieri, D., Tomaszycki, M., and Carroll, K. A. (1999). Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Dev. Psychobiol. 34, 29–35. doi: 10.1002/(sci)1098-2302(199901)34:1<29:aid-dev5<3.0.co;2-u
Main, M., and Solomon, J. (1990). Procedures for identifying infants as disorganized/disoriented during the ainsworth strange situation. In M. T. Greenberg, D. Cicchetti, & E. M. Cummings (Eds.) Attachment In The Preschool Years: Theory, Research, And Intervention 1, 121–160. Chicago, IL: The University of Chicago Press.
Mason, O., Platts, H., and Tyson, M. (2005). Early maladaptive schemas and adult attachment in a UK clinical population. Psychol. Psychother. Theory Res. Pract. 78, 549–564. doi: 10.1348/147608305X41371
Montroy, J. J., Bowles, R. P., Skibbe, L. E., McClelland, M. M., and Morrison, F. J. (2016). The development of self-regulation across early childhood. Dev. Psychol. 52, 1744–1762. doi: 10.1037/dev0000159
Moriceau, S., and Sullivan, R. M. (2006). Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 9, 1004–1006. doi: 10.1038/nn1733
Moriceau, S., Wilson, D. A., Levine, S., and Sullivan, R. M. (2006). Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 26, 6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006
Mosko, S., Richard, C., and McKenna, J. (1997). Infant arousals during mother-infant bed sharing: implications for infant sleep and sudden infant death syndrome research. Pediatrics 100, 841–849. doi: 10.1542/peds.100.5.841
O’Connor, T. G., and Cameron, J. L. (2006). Translating research findings on early experience to prevention: animal and human evidence on early attachment relationships. Am. J. Prev. Med. 31:S175–S181. doi: 10.1016/j.amepre.2006.07.005
Oers, V., and Kloet, D. (1999). Persistent effects of maternal deprivation on HPA regulation can be reversed by feeding and stroking, but not by dexamethasone. J. Neuroendocrinol. 11, 581–588. doi: 10.1046/j.1365-2826.1999.00329.x
Opendak, M., Robinson-Drummer, P., Blomkvist, A., Zanca, R. M., Wood, K., Jacobs, L., et al. (2019). Neurobiology of maternal regulation of infant fear: the role of mesolimbic dopamine and its disruption by maltreatment. Neuropsychopharmacology 44, 1247–1257. doi: 10.1038/s41386-019-0340-9
Opendak, M., Theisen, E., Blomkvist, A., Hollis, K., Lind, T., Sarro, E., et al. (2020). Adverse caregiving in infancy blunts neural processing of the mother. Nat. Commun. 11:1119. doi: 10.1038/s41467-020-14801-3
Opendak, M., Raineki, C., Perry, R., Wood, E., Packard, K., Woo, J., et al. (2021). Bidirectional control of infant social behavior by dopaminergic innervation of the basolateral amygdala. Neuron 109:4018.e–4035.e. doi: 10.1016/j.neuron.2021.09.041
Palacios-Barrios, E. E., and Hanson, J. L. (2019). Poverty and self-regulation: connecting psychosocial processes, neurobiology, and the risk for psychopathology. Compre. Psychiatry 90, 52–64. doi: 10.1016/j.comppsych.2018.12.012
Pauk, J., Kuhn, C. M., Field, T. M., and Schanberg, S. M. (1986). Positive effects of tactile versus kinesthetic or vestibular stimulation on neuroendocrine and ODC activity in maternally-deprived rat pups. Life Sci. 39, 2081–2087. doi: 10.1016/0024-3205(86)90359-0
Penn, A. A., and Shatz, C. J. (1999). Brain waves and brain wiring: the role of endogenous and sensory-driven neural activity in development. Pediatr. Res. 45, 447–458. doi: 10.1203/00006450-199904010-00001
Perry, R., and Sullivan, R. M. (2014). Neurobiology of attachment to an abusive caregiver: short-term benefits and long-term costs. Dev. Psychobiol. 56, 1626–1634. doi: 10.1002/dev.21219
Perry, R. E., Al Aïn, S., Raineki, C., Sullivan, R. M., and Wilson, D. A. (2016). Development of odor hedonics: experience-dependent ontogeny of circuits supporting maternal and predator odor responses in rats. J. Neurosci. 36, 6634–6650. doi: 10.1523/JNEUROSCI.0632-16.2016
Perry, R. E., Finegood, E. D., Braren, S. H., Dejoseph, M. L., Putrino, D. F., Wilson, D. A., et al. (2019). Developing a neurobehavioral animal model of poverty: drawing cross-species connections between environments of scarcity-adversity, parenting quality, and infant outcome. Dev. Psychopathol. 31, 399–418. doi: 10.1017/S095457941800007X
Porges, S. W., Davila, M. I., Lewis, G. F., Kolacz, J., Okonmah-Obazee, S., Hane, A. A., et al. (2019). Autonomic regulation of preterm infants is enhanced by family nurture intervention. Dev. Psychobiol. 61, 942–952. doi: 10.1002/dev.21841
Powell, G. F., Brasel, J., and Blizzard, R. (1967). Emotional deprivation and growth retardation simulating idiopathic hypopituitarism: clinical evaluation of the syndrome. New Engl. J. Med. 276, 1271–1278. doi: 10.1056/NEJM196706082762301
Raineki, C., Cortés, M. R., Belnoue, L., and Sullivan, R. M. (2012). Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J. Neurosci. 32, 7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012
Rajecki, D., Lamb, M., and Obmascher, P. (1978). Towards a general theory of infantile attachment: a comparative review of aspects of the social bond. Behav. Brain Sci. 3, 417–464. doi: 10.1037/0090-5550.40.2.111
Raposo, S. M., Mackenzie, C. S., Henriksen, C. A., and Afifi, T. O. (2014). Time does not heal all wounds: older adults who experienced childhood adversities have higher odds of mood, anxiety, and personality disorders. Am. J. Geriatr. Psychiatry 22, 1241–1250. doi: 10.1016/j.jagp.2013.04.009
Reite, M., Kaemingk, K., and Boccia, M. L. (1989). Maternal separation in bonnet monkey infants: altered attachment and social support. Child Dev. 60, 473–480. doi: 10.2307/1130991
Richard, C. A., and Mosko, S. S. (2004). Mother-infant bedsharing is associated with an increase in infant heart rate. Sleep 27, 507–511. doi: 10.1093/sleep/27.3.507
Rickenbacher, E., Perry, R. E., Sullivan, R. M., and Moita, M. A. (2017). Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviors and mother-pup interactions. Elife 6:e24080. doi: 10.7554/eLife.24080
Rincon-Cortes, M., and Sullivan, R. M. (2016). Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Transl. Psychiatry 6:e930. doi: 10.1038/tp.2016.205
Robinson-Drummer, P. A., Opendak, M., Blomkvist, A., Chan, S., Tan, S., Delmer, C., et al. (2019). Infant trauma alters social buffering of threat learning: emerging role of prefrontal cortex in preadolescence. Front. Behav. Neurosci. 13:132. doi: 10.3389/fnbeh.2019.00132
Salzen, E. (1970). “Imprinting and environmental learning,” in Development and Evolution of Behavior, eds L. Aronson, E. Tobach, D. Lehrman, and J. Rosenblatt (New York, NY: W.H. Freeman). doi: 10.1037/h0034885
Sanchez, M., Ladd, C., and Plotsky, P. (2001). Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 13, 419–449. doi: 10.1017/s0954579401003029
Sanchez, M. M., McCormack, K. M., and Howell, B. R. (2015). Social buffering of stress responses in nonhuman primates: maternal regulation of the development of emotional regulatory brain circuits. Soci. Neurosci. 10, 512–526. doi: 10.1080/17470919.2015.1087426
Sarro, E. C., Wilson, D. A., and Sullivan, R. M. (2014). Maternal regulation of infant brain state. Curr. Biol. 24, 1664–1669. doi: 10.1016/j.cub.2014.06.017
Schanberg, S. M., Evoniuk, G., and Kuhn, C. M. (1984). Tactile and nutritional aspects of maternal care: specific regulators of neuroendocrine function and cellular development [Review]. Proc. Soc. Exp. Biol. Med. 175, 135–146. doi: 10.3181/00379727-175-41779
Schanberg, S. M., and Field, T. M. (1987). Sensory deprivation stress and supplemental stimulation in the rat pup and preterm human neonate. Child Dev. 58, 1431–1447. doi: 10.2307/1130683
Schanberg, S. M., and Kuhn, C. M. (1985). “The biochemical effects of tactile deprivation in neonatal rats,” in Perspectives on Behavioral Medicine, ed. R. B. Williams (Amsterdam: Elsevier). 133–148. doi: 10.1016/b978-0-12-532102-0.50011-5
Scher, A. (2005). Infant sleep at 10 months of age as a window to cognitive development. Early Hum. Dev. 81, 289–292. doi: 10.1016/j.earlhumdev.2004.07.005
Shields, A. M., Cicchetti, D., and Ryan, R. M. (1994). The development of emotional and behavioral self-regulation and social competence among maltreated school-age children. Dev. Psychopathol. 6, 57–75. doi: 10.1017/S0954579400005885
Shionoya, K., Moriceau, S., Bradstock, P., and Sullivan, R. M. (2007). Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweaning rat pups. Horm. Behav. 52, 391–400. doi: 10.1016/j.yhbeh.2007.06.004
Silvers, J. A., Callaghan, B. L., VanTieghem, M., Choy, T., O’Sullivan, K., and Tottenham, N. (2021). An exploration of amygdala-prefrontal mechanisms in the intergenerational transmission of learned fear. Dev. Sci. 24, e13056. doi: 10.1111/desc.13056
Silvers, J. A., Insel, C., Powers, A., Franz, P., Helion, C., Martin, R. E., et al. (2017). vlPFC-vmPFC-Amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb. Cortex 27, 3502–3514. doi: 10.1093/cercor/bhw073
Singer, L. M., Brodzinsky, D. M., Ramsay, D., Steir, M., and Waters, E. (1985). Mother-infant attachment in adoptive families. Child Dev. 56, 1543–1551. doi: 10.2307/1130473
Stanley, W. C., and Elliot, O. (1962). Differential human handling as reinforcing events and as treatments influencing later social behavior in basenji puppies. Psychol. Rep. 10, 775–788. doi: 10.2466/pr0.1962.10.3.775
Suchecki, D., Rosenfeld, P., and Levine, S. (1993). Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Dev. Brain Res. 75, 185–192. doi: 10.1016/0165-3806(93)90022-3
Suga, A., Uraguchi, M., Tange, A., Ishikawa, H., and Ohira, H. (2019). Cardiac interaction between mother and infant: enhancement of heart rate variability. Sci. Rep. 9:20019. doi: 10.1038/s41598-019-56204-5
Sullivan, R. M. (2012). The Neurobiology of attachment to nurturing and abusive caregivers. Hastings Law J. 63, 1553–1570.
Sullivan, R. M. (2017). Attachment figure’s regulation of infant brain and behavior. Psychodyn. Psychiatry 45, 475–498. doi: 10.1521/pdps.2017.45.4.475
Sullivan, R. M., Landers, M., Yeaman, B., and Wilson, D. A. (2000). Good memories of bad events in infancy. Nature 407, 38–39. doi: 10.1038/35024156
Sullivan, R. M., and Opendak, M. (2021). Neurobiology of infant fear and anxiety: impacts of delayed amygdala development and attachment figure quality. Biol. Psychiatry 89, 641–650. doi: 10.1016/j.biopsych.2020.08.020
Suomi, S. J. (2003). Gene-environment interactions and the neurobiology of social conflict. Ann. N. Y. Acad. Sci. 1008, 132–139. doi: 10.1196/annals.1301.014
Tamborski, A., Lucot, J. B., and Hennessy, M. B. (1990). Central dopamine turnover in guinea pig pups during separation from their mothers in a novel environment. Behav. Neurosci. 104:607. doi: 10.1037/0735-7044.104.4.607
Thompson, J. V., Sullivan, R. M., and Wilson, D. A. (2008). Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res. 1200, 58–65. doi: 10.1016/j.brainres.2008.01.057
Tottenham, N. (2020). Early adversity and the neotenous human brain. Biol. Psychiatry 87, 350–358. doi: 10.1016/j.biopsych.2019.06.018
Tottenham, N., Shapiro, M., Flannery, J., Caldera, C., and Sullivan, R. M. (2019). Parental presence switches avoidance to attraction learning in children. Nat. Hum. Behav. 3, 1070–1077. doi: 10.1038/s41562-019-0656-9
Tronick, E. D., Als, H., and Brazelton, T. B. (1977). Mutuality in mother-infant interaction. J. Commun. 27, 74–79. doi: 10.1111/j.1460-2466.1977.tb01829.x
Vink, M., Gladwin, T. E., Geeraerts, S., Pas, P., Bos, D., Hofstee, M., et al. (2020). Towards an integrated account of the development of self-regulation from a neurocognitive perspective: a framework for current and future longitudinal multi-modal investigations. Dev. Cogn. Neurosci. 45:100829. doi: 10.1016/j.dcn.2020.100829
Waynforth, D. (2020). Mother-Infant co-sleeping and maternally reported infant breathing distress in the Uk millennium cohort. Int. J. Environ. Res. Public Health 17:2985. doi: 10.3390/ijerph17092985
Whittingham, K., and Douglas, P. (2014). Optimizing parent-infant sleep from birth to 6 months: a new paradigm. Infant Mental Health J. 35, 614–623. doi: 10.1002/imhj.21455
Wiedenmayer, C. P., Magarinos, A. M., McEwen, B. S., and Barr, G. A. (2003). Mother lowers glucocorticoid levels of preweaning rats after acute threat. Ann. N. Y. Acad. Sci. 1008, 304–307. doi: 10.1196/annals.1301.038
Yan, C. G., Rincon-Cortes, M., Raineki, C., Sarro, E., Colcombe, S., Guilfoyle, D. N., et al. (2017). Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Transl. Psychiatry 7:e1005. doi: 10.1038/tp.2016.276
Yoshida, S., and Funato, H. (2021). Physical contact in parent-infant relationship and its effect on fostering a feeling of safety. iScience 24:102721. doi: 10.1016/j.isci.2021.102721
Keywords: caregiver regulation, trauma bonding, attachment, homeostasis, social buffering, stress, mother, mother-infant dyad
Citation: Graf N, Zanca RM, Song W, Zeldin E, Raj R and Sullivan RM (2022) Neurobiology of Parental Regulation of the Infant and Its Disruption by Trauma Within Attachment. Front. Behav. Neurosci. 16:806323. doi: 10.3389/fnbeh.2022.806323
Received: 31 October 2021; Accepted: 10 March 2022;
Published: 07 April 2022.
Edited by:
Sophie Tronel, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Brittany Rollins Howell, Fralin Biomedical Research Institute, Virginia Tech Carilion, United StatesFrederic Levy, Institut National de la Recherche Agronomique (INRA), France
Copyright © 2022 Graf, Zanca, Song, Zeldin, Raj and Sullivan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nina Graf, bmcyNDM4QG55dS5lZHU=; Regina M. Sullivan, cmVnaW5hLnN1bGxpdmFuQG55dW1jLm9yZw==
 Nina Graf
Nina Graf Roseanna M. Zanca
Roseanna M. Zanca Wei Song
Wei Song Elizabeth Zeldin
Elizabeth Zeldin Roshni Raj
Roshni Raj Regina M. Sullivan
Regina M. Sullivan