- Department of Behavioral Neuroscience, Oregon Health & Science University, Portland, OR, United States
In humans, an acutely traumatic experience can lead to post-traumatic stress disorder (PTSD), which is often characterized by changes in anxiety and motivation months after trauma. There are few demonstrations of the persistent motivational effects of an acute stressor in rodent approaches to PTSD. In two experiments, we evaluated the persistent effects of a battery of footshocks in one context on appetitive Pavlovian conditioning, instrumental learning, and Pavlovian-to-instrumental transfer (PIT) in a different context. In Experiment 1, a battery of footshocks before appetitive training caused deficits in single-outcome PIT (SO-PIT) in male Long Evans rats. The same battery of footshocks after appetitive training, but before testing had little effect on SO-PIT overall, but there were some deficits in within-stimulus expression of SO-PIT. In Experiment 2, the battery of footshocks had no effect on sensory-specific PIT in male or female rats, but two sex differences emerged: males showed more generalized fear from the aversive to the appetitive context compared to females, and females showed less evidence for sensory-specific PIT compared to males. Males showed robust sensory-specific PIT, with clear extinction and spontaneous recovery of the sensory-specific PIT effect across test sessions. These findings show that (a) an acute trauma can have persistent effects on general motivational processes and (b) in sensory-specific PIT, females may show transfer through generalized motivational processes, whereas males may rely on specific features of the cues and outcomes to augment instrumental responding selectively. We discuss implications for current approaches to stress and motivation in preclinical approaches to PTSD.
Introduction
Intensely stressful events can cause long-term aberrations in learning, memory, and motivation. In humans, the experience of either intense acute or chronic stress can lead to post-traumatic stress disorder (PTSD) which is characterized by persistent intrusive memories of the trauma, avoidance of reminders of the trauma, negative changes in cognition and mood, and alterations in arousal/stress reactivity, all which persist 30 days beyond trauma (American Psychiatric Association, 2013). A major difficulty with treating PTSD is that it is highly comorbid with persistent aberrations in reward-related processes. Individuals with PTSD are at greater risk of developing alcohol use, substance use, gambling, and eating disorders, and also show a higher risk of obesity (Kessler et al., 2005; Brewerton, 2007; Swinbourne and Touyz, 2007; Chwastiak et al., 2011; Coughlin et al., 2011; Grubbs et al., 2019). Although there are many preclinical demonstrations of the persistent effects of chronic stressors on reward processing (Willner et al., 1987; Piazza et al., 1990; Ghiglieri et al., 1997), the effects of acute stressors has largely been studied using the stress-induced reinstatement approach, in which a stressor is applied after extinction and the effects on instrumental responding are assessed during that period of acute stress. Remarkably little is known about the effects of acute stress on reward that persist long after that stressful experience.
One approach to evaluating persistent effects of acute stress is stress-enhanced fear learning (SEFL), which is based on years of work on the cellular, molecular, and behavioral mechanisms of fear conditioning. This approach results in a persistent hyperresponsivity to a mild stressor in one context following an exposure to a battery of intense footshocks in a different context (Rau et al., 2005; Rau and Fanselow, 2009). Chronic stressors (such as maternal deprivation or chronic variable stress) often involve exposure to stressors over multiple days in multiple contexts without an easily measured memory component. SEFL consists of a temporally and contextually isolated stressful experience that has a well characterized behavioral response (freezing behavior) associated with it. Thus, the effects of memory for trauma can be evaluated and potentially dissociated from the effects of the stress of the trauma.
Stress-enhanced fear learning has been adapted to study the persistent alterations of trauma on appetitive processes by following the shock battery session with appetitive training and testing in a novel context (Meyer et al., 2013; Pizzimenti et al., 2017). Meyer et al. (2013) found that the battery of footshocks led to a persistent change in alcohol drinking and Pizzimenti et al. (2017) found that the same battery of shocks promoted expression of cocaine-induced conditional place preference in mice and methamphetamine-induced reinstatement in rats. In both of these studies, a single acute stressor resulted in persistent changes in drug-seeking or -taking. However, little is known about how the persistent effects of acute trauma on appetitive learning and motivation in the realm of natural rewards. This is an important line of inquiry because it will provide valuable insights as to unique alterations to the psychological and neurobiological processes imparted by trauma alone without the interference on drug-related plasticity in these same processes.
The Pavlovian-to-instrumental transfer (PIT) procedure allows the influence of Pavlovian stimuli on instrumental behaviors to be assessed. This approach may capture aspects of general affective motivation for reward, as well as sensory-specific memories involving associations among stimuli, responses, and predicted outcomes (Cartoni et al., 2016). In single-outcome PIT (SO-PIT), animals are conditioned to associate a Pavlovian conditional stimulus (CS+) with an unconditional stimulus (US; or outcome, O). Separately animals are trained to associate an instrumental response (R1) with that same outcome. During testing, SO-PIT is captured when presentation of the CS+ augments instrumental responding on R1. SO-PIT is thought to rely on a general affective motivational process and depends on psychological and neurobiological processes that are distinct from another form of PIT, sensory-specific (SS) PIT. With SS-PIT animals are conditioned to form two specific Pavlovian associations (CS1-O1 and CS2-O2) and separately trained to associate two different instrumental responses with these same outcomes (R1-O1 and R2-O2). In testing, both manipulanda are available and the Pavlovian CSs are presented to assess their influence on instrumental responding. SS-PIT occurs when a given CS preferentially invigorates instrumental responding on the manipulandum previously reinforced with the same outcome compared to the manipulandum previously reinforced with the alternate outcome (i.e., S1 increases R1 more than R2 and S2 increases R2 more than R1).
Because PIT separates Pavlovian and instrumental memory processes, it is a powerful tool for investigating how trauma alters specific memory and motivational processes (Colwill et al., 2022). Much of the work on stress and reward (such as the work on cue-induced reinstatement) cannot distinguish between Pavlovian and instrumental processes. In humans, high anxiety levels (not experimentally induced) have been associated with deficits in SO-PIT (Quail et al., 2017), whereas experimentally induced stress just prior to testing has been shown to promote SO-PIT (Pool et al., 2015) and to not alter SS-PIT (Steins-Loeber et al., 2020). In rodents, acute stress just prior to testing has been shown not to affect SO-PIT expression (Pielock et al., 2013), but to transiently block SS-PIT expression (Morgado et al., 2012). In the following experiments we evaluate the persistent (> 21 days) effects of acute trauma (exposure to a battery of 15 intense footshocks in a 90-min session) on SO-PIT in male rats (Experiment 1) and sensory-specific PIT in male and female rats (Experiment 2).
General methods
Subjects
Long Evan rats were used in all the experiments in this study (N = 89); group sizes, and sexes are outlined below in individual methods sections. Rats were pair and triplicate housed and maintained on a reverse light-dark schedule. Experimental procedures were conducted during the dark phase of the cycle. For all experiments rats were food restricted to between 85 and 90% of their ad libitum weights and maintained at this weight for the duration. Weights were maintained by providing daily rations following each training session; for females and males, approximately 10 and 15 g per rat was needed to hold weights steady.
Apparatus
Two distinct contexts housed in separate rooms were used for fear conditioning (Ctx A) and for appetitive training and testing (Ctx B). Chambers differed in dimensions, patterned backdrops, olfactory cues, floor textures, and other internal features. All chambers were standard Med Associates operant chambers housed in sound attenuating cabinets. Ctx A was 29.53 cm × 24.84 cm × 18.67 cm (LxWxH) with metal rod grid floors (19, 4.7 mm rods), a horizontal zig zagged pattern back drop, and a single house light. For olfactory distinctiveness gauze pads infused with clover leaf essential oil (Crafter’s Choice™ Clove Leaf EO- Certified 100% Pure 1050) were placed into the scat collection trays at the base of the chambers. Ctx B was 29.53 cm × 23.5 cm × 27.31 cm with wire mesh inserts (19-Gauge Wire Mesh Fence; 0.5″ mesh size) placed over metal rod grid floors and a backdrop with randomly distributed differently sized squares or circles. For olfactory distinctiveness, citrus cilantro fragrance oil infused gauze pads were placed in the scat trays at the start of each session (Crafter’s Choice™ Citrus Cilantro Fragrance Oil 548). Internal features of Ctx B chambers included two retractable levers flanking a recessed food magazine, a single speaker above the magazine, and two flat lights above the levers on the front wall of the chamber. On the opposing wall, a single hooded houselight was situated at the top center. Food hoppers and syringe pumps were externally housed and connected to the magazine for delivery of pellet and liquid reinforcement.
Shock trauma treatment
Rats were placed in Ctx A, where they received 15, 1 mA footshocks delivered through the metal rods of chamber’s grid floor. The session lasted 90 min and shocks were delivered on variable-time 360 s (VT 360″) schedule. Control rats were exposed to the same context for 90 min without any shocks. All sessions were video recorded for subsequent quantification of freezing behavior.
Single-outcome Pavlovian-to-instrumental transfer (training and testing)
All appetitive training was conducted in Ctx B. First, rats received magazine training to learn to collect pellet reinforcements (Bioserv Dustless Precision Pellets, 45 mg; product# F0021) from the recessed magazine. Each session lasted 20 min, in which 20 pellets were delivered on a VT 60″ schedule. The number of magazine sessions differed across experiments; see individual experimental timelines for more details. Rats then learned to press an Active lever to earn pellets while responses on a simultaneously available Inactive lever had no consequence (lever positions counterbalanced). Initially, rats were trained on a continuous reinforcement schedule (CRF) in which every press earned a single pellet until reaching the acquisition criterion of 50 pellets in a 40 min single session. Next, rats were trained on variable interval (VI) schedules of reinforcement in 20 min sessions where the schedule was thinned across sessions as follows: VI 10″, VI 30″, and VI 60″ (2, 2, and 4 sessions, respectively). At the start of each instrumental session the houselights turned on with simultaneous insertion of the levers and the session terminated with the light turning off and retraction of the levers. After instrumental training, rats received eight 55-min Pavlovian discrimination sessions, in which one conditional stimulus (CS; 2-min 80 dB white noise or 2 kHz tone, counterbalanced) was reinforced (CS+) and one CS was non-reinforced (CS-). On each CS+ trial, four pellets were delivered on a VT 30″ schedule. The first trial began 2 min after the session start and subsequent trials were presented after a variable 5 min intertrial interval (ITI). Each CS was presented four times in a quasi-random order. Each session began with the house light turning on and ended 2 min after the last trial with the house light turning off. Following training, rats were tested for Pavlovian-to-instrumental transfer in 41 min sessions under extinction conditions. Each test began with the house light turning on and insertion of both levers, then 10 min into the session the first CS trial began, and subsequent trials were presented on a fixed 2-min ITI. Each CS was presented four times in a quasi-random order and the session ended 1 min after termination of the last trial. Rats were tested in two separate sessions on two consecutive days.
Sensory-specific Pavlovian to instrumental transfer (training and testing)
As with SO-PIT, all training and testing was conducted in Ctx B. Two unconditional stimulus outcomes were used for reinforcement, Bioserv Dustless Precision Pellets (45 mg) and 20% sucrose (0.1 ml). Magazine training was conducted separately for each outcome using the same parameters as described above. Next rats were trained to press one lever for pellets and one for sucrose in separate sessions. Instrumental training proceeded as described for SO PIT starting with CRF, ending with VI 60″ (lever-outcome assignments counterbalanced). Next rats underwent Pavlovian conditioning to associate one CS with pellets and the other with sucrose; a white noise and flashing lights (0.25″ on/off; lights were located directly above both levers and flashed simultaneously) were used as distinct CSs (CS-outcome assignments counterbalanced). Each CS was presented for 2 min per trial during which four US deliveries were made on a VT 30″ schedule. Trials were separated by variable 5-min ITIs. The first trial was presented 2 min into the session and the session ended 2 min following termination of the last trial. Each CS-O pair was trained in separate 27 min sessions (four CSs/Session; eight sessions per CS). Rats were given 20-min instrumental reminder sessions (VI 60″) for each R-O association 1 day before each test. Test sessions were identical to that described in SO-PIT. Rats were tested in three sessions with instrumental reminder sessions between each test. After session 3, rats were given instrumental reminder training on the following day, then food restriction was lifted and rats were tested 1 day later for a final ad libitum SS-PIT test.
Data analysis
For analysis of freezing behavior, videos were scored manually by visually sampling behavior for 2 s every 10 s during the 3-min immediately prior to each shock delivery (or yoked time period for controls). One video in Experiment 2 containing three females was corrupted during the recording and therefore data from shock conditioning were lost for these subjects. For appetitive training and testing, data were collected via automated response measures recorded by the Med Associates software controlling the operant chamber machinery. For statistical analyses, ANOVAs, unpaired t-tests, and Holm-Sidak’s (HS) multiple comparisons were used planned and post hoc comparisons and are specified in more detail in the section “Results.”
Experiment-specific methods
Experiment 1: Effects of massive shock on SO-PIT in males
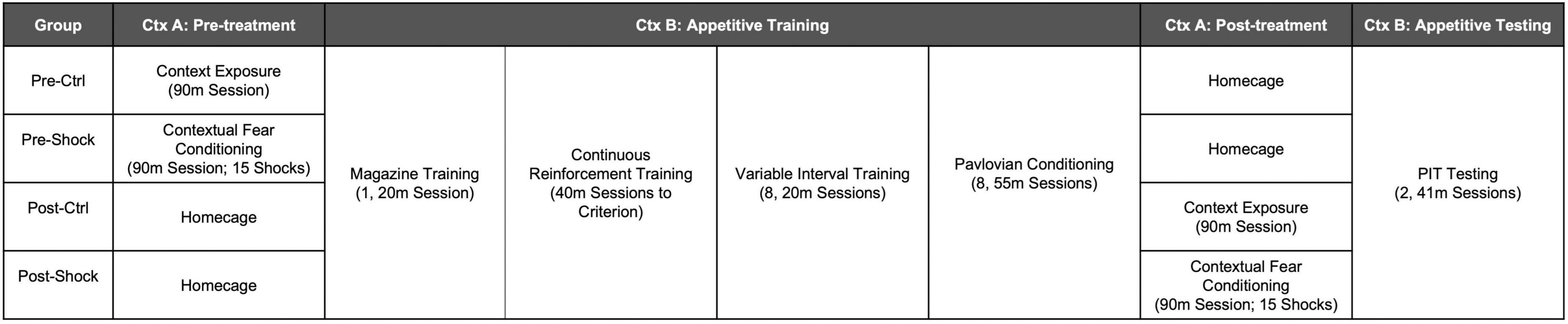
Male Long Evans rats (N = 32) approximately 70 days old at the start of the experiment were purchased from Charles River Laboratories and shipped to the OHSU animal facility 1 week before the start of the experiment. Rats were food restricted to 85–90% of their ad libitum bodyweights for the duration of the experiment. An illustration of the experimental timeline for Experiment 1 is shown in Figure 1. Rats were split into four groups of eight rats. Pre-Ctrl and -Shock groups received either control treatment or massive shock (Ctx A), respectively, 1 day prior to the start of appetitive training (Ctx B). Post-Ctrl and Shock groups received either control treatment or massive shock (Ctx A), respectively, the day after the final Pavlovian conditioning session, 1 day prior to testing. Testing was conducted on the same day for all rats. Rats received two test sessions over two consecutive days. Appetitive training and testing for SO-PIT was conducted as described above.
Experiment 2: Effects of massive shock on SS-PIT in males and females
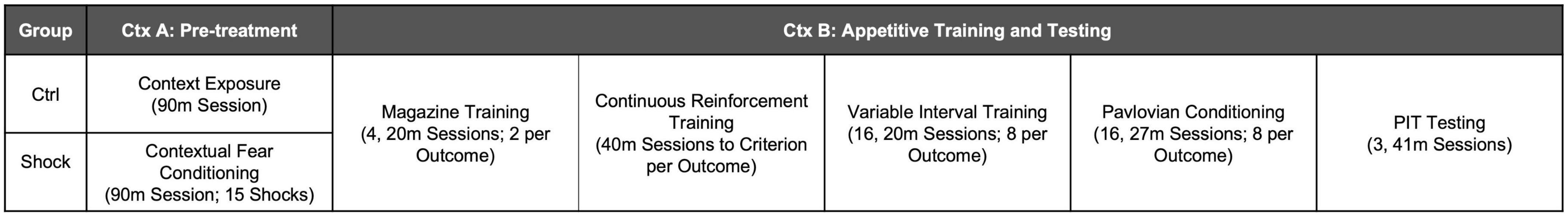
Female (N = 22) and male (N = 35) Long Evans rats were bred at OHSU from breeders purchased from Charles River Laboratories. Rats were weaned at postnatal (PN) day 23. At PN 70 or older, rats were food restricted and the following day given either control treatment or massive shock in Ctx A. The following day appetitive training for SS-PIT began in Ctx B as described above. An illustration of the experimental timeline for Experiment 2 is shown in Figure 2.
Results
Experiment 1: Effect of a battery of footshocks on SO-PIT
Pre-training fear conditioning
Freezing behavior in the Pre-Shock group was evident by the second shock and reached asymptotic levels by the third shock, whereas freezing behavior was expectedly absent in the Pre-Ctrl group (Supplementary Figure 1A). Two-way repeated measures analysis of variance (RM ANOVA), main effect of treatment, F(1, 14) = 361.1, p < 0.01; main effect of time, F(1, 196) = 21.41, p < 0.01; time by treatment interaction, F(14, 196) = 21.41, p < 0.01).
Magazine, continuous reinforcement, and variable interval training
For all training data, when Pre-Ctrl, Post-Ctrl, and Post-Shock groups did not differ (p > 0.05), these groups were collapsed (Collapsed Ctrl; i.e., all of the groups that had not received shock prior to training). Magazine training began the day after fear conditioning. As can be seen in Figure 3, rats that were shocked in Ctx A prior to appetitive training showed less magazine responding compared to the rats that received control context exposure in Ctx A [Figure 3A; Two-way RM ANOVA, significant treatment × time of treatment interaction, F(1, 28) = 4.38, p < 0.05; HS post hoc comparison: Pre-Ctrl vs. Pre-Shock, t(28) = 2.98, p = 0.01]. During CRF training, the time to reach the acquisition criterion was delayed in the Pre-Shock group compared to the Collapsed Ctrl group [Figure 3B); t(30) = 2.31, p = 0.03]. Active lever pressing increased across VI sessions in both groups and despite the ordinal difference in the groups there was no significant difference in Active lever responding between the groups [Figure 3C; Two-way RM ANOVA, main effect of session, F(7, 210) = 16.67, p < 0.01; no effect of group, p = 0.45; no session by group interaction, p = 0.97]. Inactive lever pressing was very low throughout training, but systematically increased without any group differences during the last 4 VI 60″ sessions [Figure 3C; Two-way RM ANOVA, main effect of session, F(7, 210) = 5.12, p < 0.01; no effect of group, p = 0.09; no session by group interaction, p = 0.73]. In both the Collapsed Ctrl and Pre-Shock groups responding on the Active lever was significantly higher than the Inactive lever and this difference increased over sessions [Figure 3C; Two-way RM ANOVA, main effect of session, Collapsed Ctrl, F(7, 161) = 67.94, p < 0.01; Pre-Shock, F(7, 49) = 4.03, p < 0.01; main effect of lever, Collapsed Ctrl, F(1, 23) = 296.00, p < 0.01; Pre-Shock, F(1, 7) = 47.64, p < 0.01; session by lever interaction: Collapsed Ctrl: F(7, 161) = 19.84, p < 0.01; Pre-Shock, F(7, 49) = 2.71, p = 0.02]. As an additional evaluation of the effects of pre-training shock on instrumental responding, we restricted comparison of responding to the Pre-Ctrl and Pre-Shock groups and found no significant difference in response rates, though there was modest ordinal difference with the Pre-Shock group performing lower in some sessions than Pre-Ctrl (Supplementary Figure 2A; Two-way RM ANOVA, no effects of group: Active lever, p = 0.17; Inactive lever, p = 0.15).
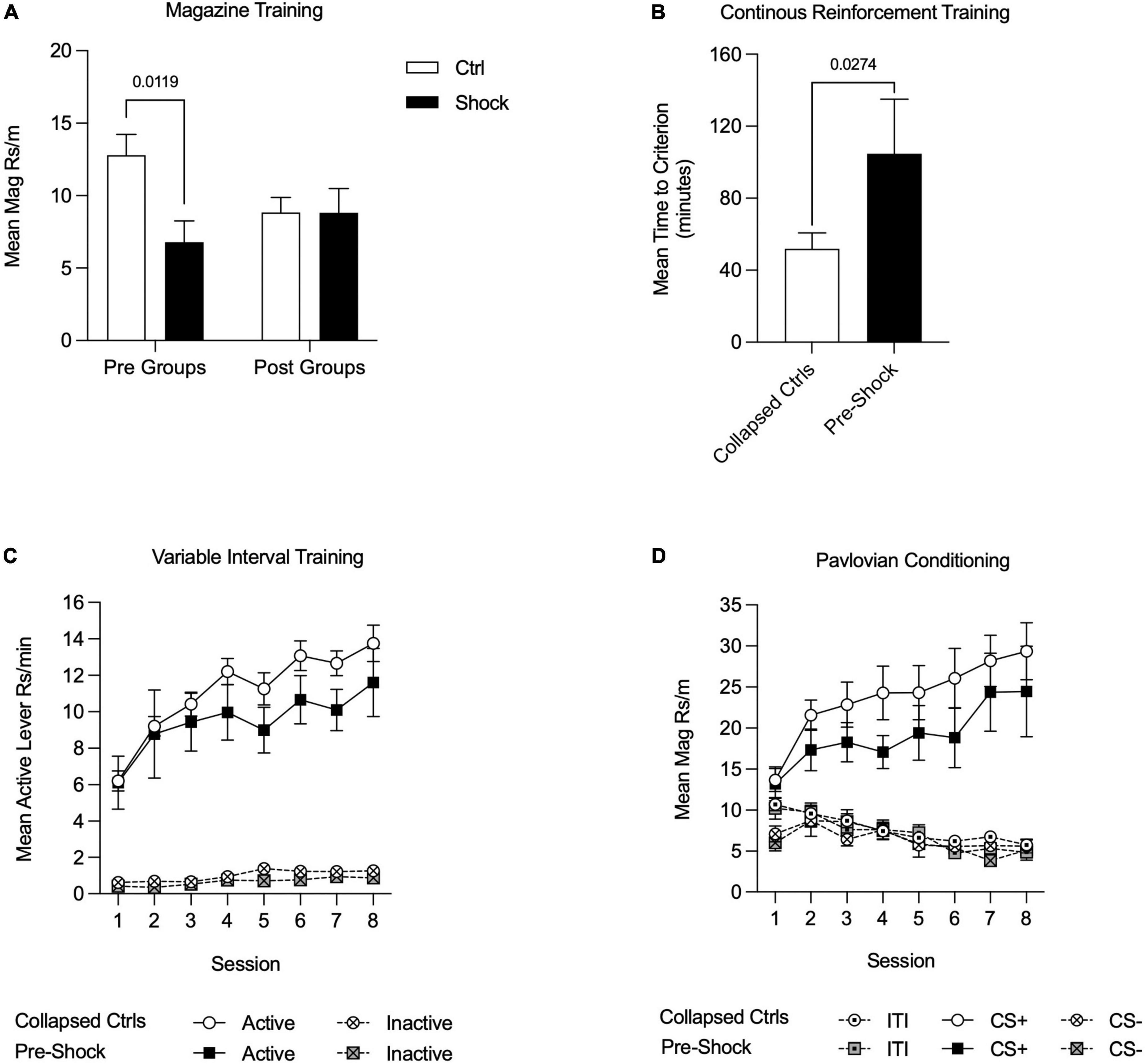
Figure 3. Appetitive training in Context B in Experiment 1. Data are collapsed between Pre-Ctrl, Post-Ctrl, and Post-Shock groups when measures did not differ between these groups. (A) Magazine training was suppressed in the Pre-Shock group relative to the pre-control group and there were no differences between the post groups. (B) Acquisition on a continuous reinforcement schedule was slower in the Pre-Shock group compared to the other groups. (C) During variable interval training, response rates increased across sessions and did not differ between Pre-Shock rats and the Collapsed Ctrl. Inactive lever responding was significantly lower than Active lever responding across sessions. (D) During Pavlovian conditioning, the mean rate of magazine responding during CS+ presentations increased across sessions and there were no group differences between Pre-Shock and Collapsed Ctrl Groups in responding.
Pavlovian conditioning
During the eight sessions of appetitive Pavlovian conditioning, all groups responded more to the CS+ than to the CS- [Figure 3D; Two-way RM ANOVA, main effect of CS: Collapsed Ctrl: F(2, 46) = 52.85, p < 0.01; Pre-Shock: F(2, 14) = 22.51, p < 0.01; CS by session interaction: Collapsed Ctrl: F(14, 322) = 11.90, p < 0.01; Pre Shock: F(14, 98) = 4.59, p < 0.01]. As expected, magazine responding to the CS+ increased systematically across sessions and response rates between the Collapsed Ctrl and Pre-Shock groups did not differ [Figure 3D; Two-way RM ANOVA, main effect of session, F(7, 210) = 6.32, p < 0.01; no effect of group, p = 0.32; no session by group interaction, p = 0.88]. Magazine responding during the ITI and CS- did not differ between the Collapsed Ctrl and Pre-Shock groups and was expectedly low across training [Figure 3D; Two-way RM ANOVA, no effect of group: ITI: p = 0.48; CS-: p = 0.82]. ITI responding significantly decreased across sessions, whereas CS- responding varied across days, but not systematically [Figure 3D; Two-way RM ANOVA, main effect of session: ITI: F(7, 210) = 13.31, p < 0.01; CS-: F(7, 210) = 3.25, p < 0.01]. As an additional evaluation of the effects of pre-training shock on responding during Pavlovian conditioning, we directly compared Pre-Ctrl and Pre-Shock groups, finding no group differences in responding (Supplementary Figure 2B; Two-way RM ANOVA, no effects of group: ITI, p = 0.21; CS+, p = 0.32; CS-, p = 0.30).
Post-training fear conditioning
For the Post-Ctrl and Post-Shock groups, rats were given context exposure or received massive shock in Ctx A the day following the final appetitive Pavlovian conditioning session (the Pre-groups were left undisturbed in their home cages on this day). The Post-Ctrl and Post-Shock were counterbalanced to ensure even distribution of CS-O assignments and to match levels of appetitive Pavlovian conditional responding (data not shown: Two-way RM ANOVA, CS+ responding, no effect of group, p = 0.82, no group × session interaction, p = 0.99). During Ctx A training, as expected the Post-Ctrl group did not freeze significantly across the context exposure session, whereas the freezing in the Post-Shock group emerged rapidly and was significantly increased by the second Pre-Shock period compared to the first [Supplementary Figure 1B; Two-way RM ANOVA, main effect of group, F(1, 14) = 204.2, p < 0.01; main effect of time, F(1, 196) = 7.88, p < 0.01; time by group interaction, F(14, 196) = 7.88, p < 0.01; HS post hoc comparison: Bin 1 v 2: t(196) = 4.45, p < 0.01].
SO-PIT testing
On the following 2 days all groups were tested for SO-PIT in Ctx B (one session per day). Although overall responding was lower during Test 2, there were no interactions with test session, so the two tests were combined for analysis. There also were no differences between Pre- and Post-Ctrl groups, so those data were combined for analysis (data not shown, two-way RM ANOVA, no effects of groups: Pavlovian conditional responding: p = 0.74; Active lever responding: p = 0.53). During testing, we evaluated magazine entries and lever pressing during the Pavlovian CSs. Both groups retained the CS+/CS- discrimination, without notable group differences [Figure 4A; Two-way RM ANOVA, no effect of group, p = 0.81; no group by phase (ITI/CS+/CS-) interaction, p = 0.94]. Each group showed greater magazine responding during the CS+ compared to the ITI [Figure 4A; Two-way RM ANOVA, main effect of phase, F(2, 58) = 30.07, p < 0.01; HS planned comparison: Ctrl: t(58) = 5.18, p < 0.01; Pre-Shock: t(58) = 3.07, p < 0.01; Post-Shock: t(58) = 3.86, p < 0.01]. Furthermore, CS+ magazine responding was significantly greater than CS- magazine responding (Figure 4A; HS planned comparison: Ctrl: t(58) = 5.02, p < 0.01; Pre-Shock: t(58) = 2.95, p < 0.01; Post-Shock: t(58) = 4.14, p < 0.01).
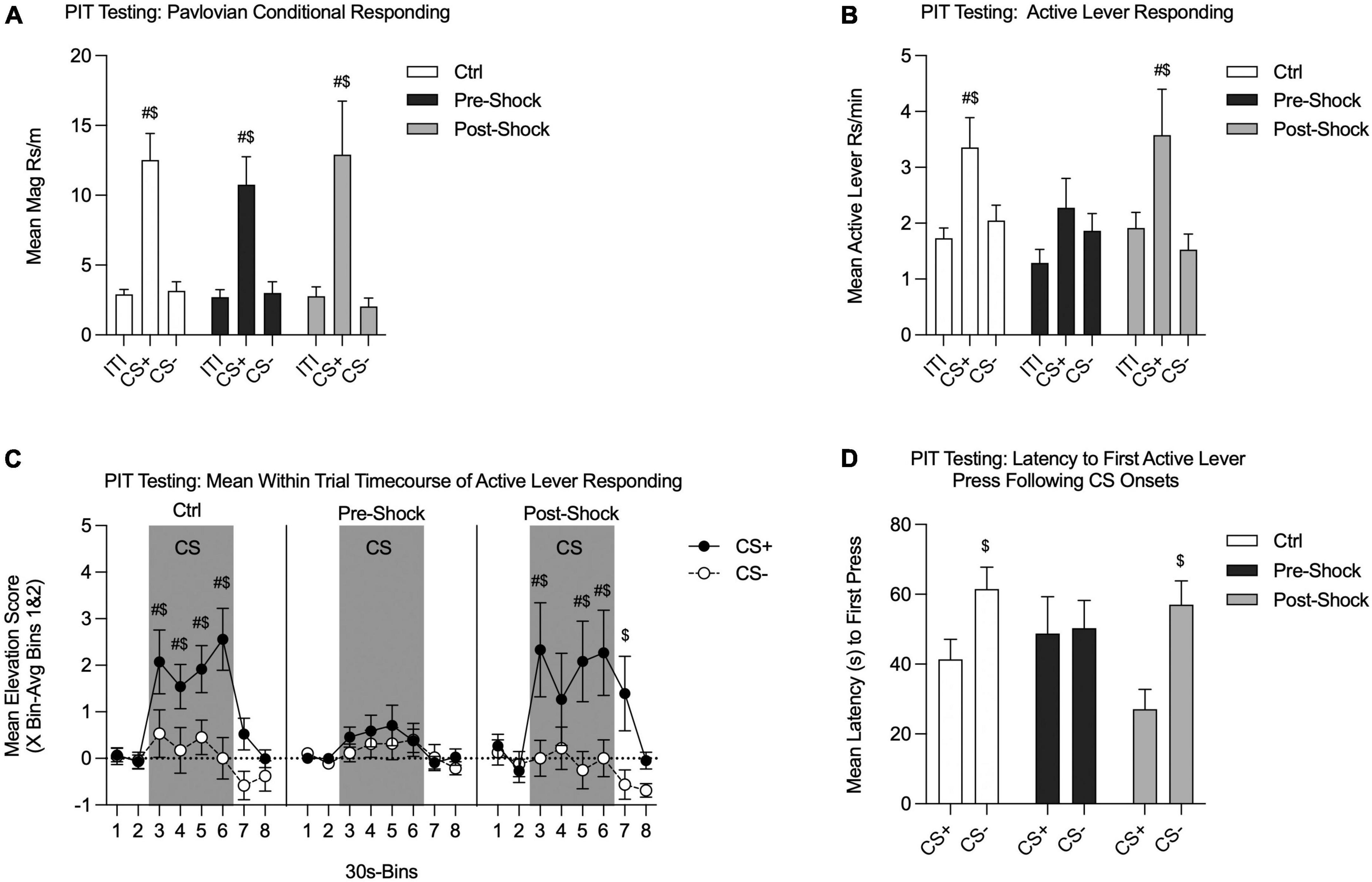
Figure 4. Single-outcome Pavlovian-to-instrumental transfer tests in Context B in Experiment 1. The Pre-Ctrl and Post-Ctrl groups did not differ and were therefore collapsed. Data are presented as means from Tests 1 and 2. (A) Pavlovian conditional responding during PIT testing. All groups showed robust discrimination between CS+ and CS-. (B) Active lever responding measuring the SO-PIT effect. In the Collapsed Ctrl and Post-Shock groups Active lever responding was significantly greater during CS+ presentations than during the ITI and CS-. However, in the Pre-Shock the rate Active lever responding did not differ between the CS+, ITI, and CS- periods. (C) Mean time course of Active lever from 60 s pre- to 60 s post-CS presentations. Data are presented as an elevation score [responding in the pre-CS period (bins 1 and 2) subtracted from each bin]. In the Collapsed Ctrl and Post-Shock groups, Active lever responding was greater during CS+ vs. CS- presentations throughout the 2 m CS window. In the Post-Shock group this effect carried over into the first 30 s after CS offset. In contrast, the Pre-Shock group did not show any significant increase Active lever responding during CS+ vs. CS- presentations at any point within the CS window. (D) Active lever response latency during PIT testing. In the Collapsed Ctrl and the Post-Shock groups, the latency between CS+ onset and the first Active lever response was significantly shorter than the latency to press the Active lever following CS- onset. In contrast, in the Pre-Shock group, there was no difference in the latency to first press following the CS+ vs. the CS- onsets ($CS+ vs. CS-, p < 0.05; #CS vs. ITI, p < 0.05).
Next, we examined Active lever responding to evaluate PIT [Figure 4B; RM ANOVA, main effect of phase, F(2, 58) = 10.07, p < 0.01; no effect of group, p = 0.30; no group by phase interaction, p = 0.51]. The critical determinant of SO-PIT expression is whether Active lever responding is significantly greater during CS+ presentations compared to CS- presentations. Therefore, to determine if each individual group expressed reliable SO-PIT we conducted planned comparisons to evaluate this. Both the Ctrl and the Post-Shock groups showed SO-PIT with CS+ presentations evoking greater Active lever responding compared to CS- presentations, which did not augment responding above ITI levels [Figure 4B; HS planned comparison CS+ vs. CS-: Ctrl: t(58) = 2.81, p = 0.01; Post-Shock, t(58) = 3.11, p = 0.01]. This SO-PIT effect, however, was absent in the Pre-Shock group [Figure 4B; HS planned comparison CS+ vs. CS-: Pre-Shock: p = 0.62]. Furthermore, in the Ctrl and Post-Shock groups Active lever responding was greater during CS+ vs. ITI periods, but this augmentation by the CS+ was absent in the Pre-Shock group [Figure 4B; HS planned comparison: Ctrl, t(58) = 3.49, p < 0.01; Pre-Shock, p = 0.36; Post-Shock, t(58) = 2.53, p = 0.03].
We also evaluated how SO-PIT manifested across the CS presentation window by looking at the mean time course of responding in 30 s bins from 60 s before to 60 s post CS presentations. These data are presented as elevation scores, which were calculated for each individual by subtracting the mean response during the pre-CS window (Bins 1 and 2) from the response rate in a given bin during the CS. In the Ctrl group, SO-PIT was sustained and strengthened across the entire 2-min CS window [Figure 4C (left panel); Two-way RM ANOVA, main effect of time, F(7, 105) = 9.15, p < 0.01; main effect of CS, F(1, 15) = 6.81, p = 0.02; significant time by CS interaction, F(7, 105) = 3.23, p < 0.01]. In the Pre-Shock group, SO-PIT was entirely absent across the CS window and did not change systematically over time [Figure 4C (middle panel); Two-way RM ANOVA, main effect of time, F(7, 49) = 2.80, p = 0.02; no effect of CS, p = 0.60; no time by CS interaction, p = 0.91]. Although SO-PIT was evident across the session in the Post-Shock group when comparing CS+ to CS-, when pre-CS response rates were incorporated, PIT was not evident in every CS time bin and this was enough variability to weaken the overall main effect of CS [Figure 4C (right panel); Two-way RM ANOVA, main effect of time, F(7, 49) = 3.81, p < 0.01; main effect of CS, F(1, 7) = 4.08, p = 0.08; time by CS interaction, F(7, 49) = 2.05, p = 0.07].
As a final measure of PIT, we examined the latency to lever press following CS+ and CS- onsets. Consistent with the rate data, the Ctrl and Post-Shock groups showed a shorter latency to press the Active lever following CS+ vs. CS- presentations, whereas the Pre-Shock group did not show any difference in the latency to press following CS+ vs. CS- presentations [Figure 4D; Two-Way RM ANOVA, main effect of CS, F(1, 29) = 8.32, p < 0.01; no effect of group, p = 0.45; no group by CS interaction, p = 0.21; HS planned CS+ vs. CS- comparison: Ctrl, t(29) = 2.52, p = 0.04; Pre-Shock, p = 0.90; Post-Shock, t(29) = 2.65, p = 0.03].
In Experiment 1, we found that a battery of footshocks in one context led to an impairment in SO-PIT in a different context in male rats. Although there were deficits in acquisition of magazine approach training, there were no effects of shock on acquisition of Pavlovian or instrumental responding. In addition, during testing, which was conducted under extinction conditions, discriminatory magazine responding to CS+ vs. CS- was intact in all groups. Similarly, instrumental response rates were similar across all groups under these extinction conditions. When shock followed appetitive training, there were no profound effects on PIT testing, but when shock preceded training PIT was absent. In Experiment 2, we evaluate the effects of pre-training shock on sensory-specific PIT in male and female rats.
Experiment 2: Effect of a battery of footshocks on SS-PIT in male and female adults
Pre-training fear conditioning
Ctx A treatment was conducted the day before the start of appetitive conditioning. In the Shock groups freezing during the Pre-Shock periods emerged rapidly in both males and females without pronounced sex differences (Supplementary Figure 3; Two-way RM ANOVA, main effect of trial, F(14, 406) = 27.04, p < 0.01; no effect of sex, p = 0.16, trial by sex interaction, F(14, 406) = 1.64, p = 0.07]. To further explore early sex effects on freezing, we focused on freezing behavior in the first four Pre-Shock periods. Here we found that females froze significantly less in these early Pre-Shock periods compared to males [Two-way RM ANOVA, main effect of sex, F(1, 29) = 5.50, p = 0.03]. Freezing did not emerge in Ctrl Female or Male rats (Supplementary Figure 3; No main effect of trial or sex, and no interaction, ps > 0.99).
Magazine, continuous reinforcement schedule, and variable interval training
Appetitive training in Ctx B began with magazine training; data were analyzed as the mean rate of responding across all sessions (Figure 5A). There was an overall main effect of shock treatment [F(1,57) = 7.15, p < 0.01] with no reliable main effect of sex or interaction, ps = 0.19 (Figure 5A). Planned comparisons to evaluate the effect of shock within each sex found no differences in magazine entries in females (p = 0.40), but shocked males made fewer entries than did control males [Figure 5A; HS planned comparison, t(57) = 3.33, p < 0.01]. Next rats went through CRF training and data were analyzed by taking the mean time to acquire both instrumental responses. The time to reach the acquisition criteria did not differ between treatment groups or sex [Figure 5B; HS planned comparison Ctrl vs. Shock: females and males, ps > 0.20]. In the next phase, rats were trained on VI reinforcement schedules for eight sessions. Data were analyzed as the mean rate of responding across both levers. In both sexes, response rates increased across sessions, were similar between Ctrl and Shock groups, and this did not interact with session [Figure 5C; Two-way RM ANOVA, main effect of session: Females: F(7, 140) = 2.61, p = 0.01; Males: F(7, 259) = 5.64, p < 0.01; no effects of treatment groups, and no interactions, ps > 0.41]. We also compared responding between sexes for each treatment group. In Ctrl, responding did not differ between sexes, but in the Shock groups, response rates in Females were lower than in Males overall [Two-way RM ANOVA, main effects of sex: Ctrl: p = 0.27; Shock: F(1, 32) = 4.35, p < 0.05].
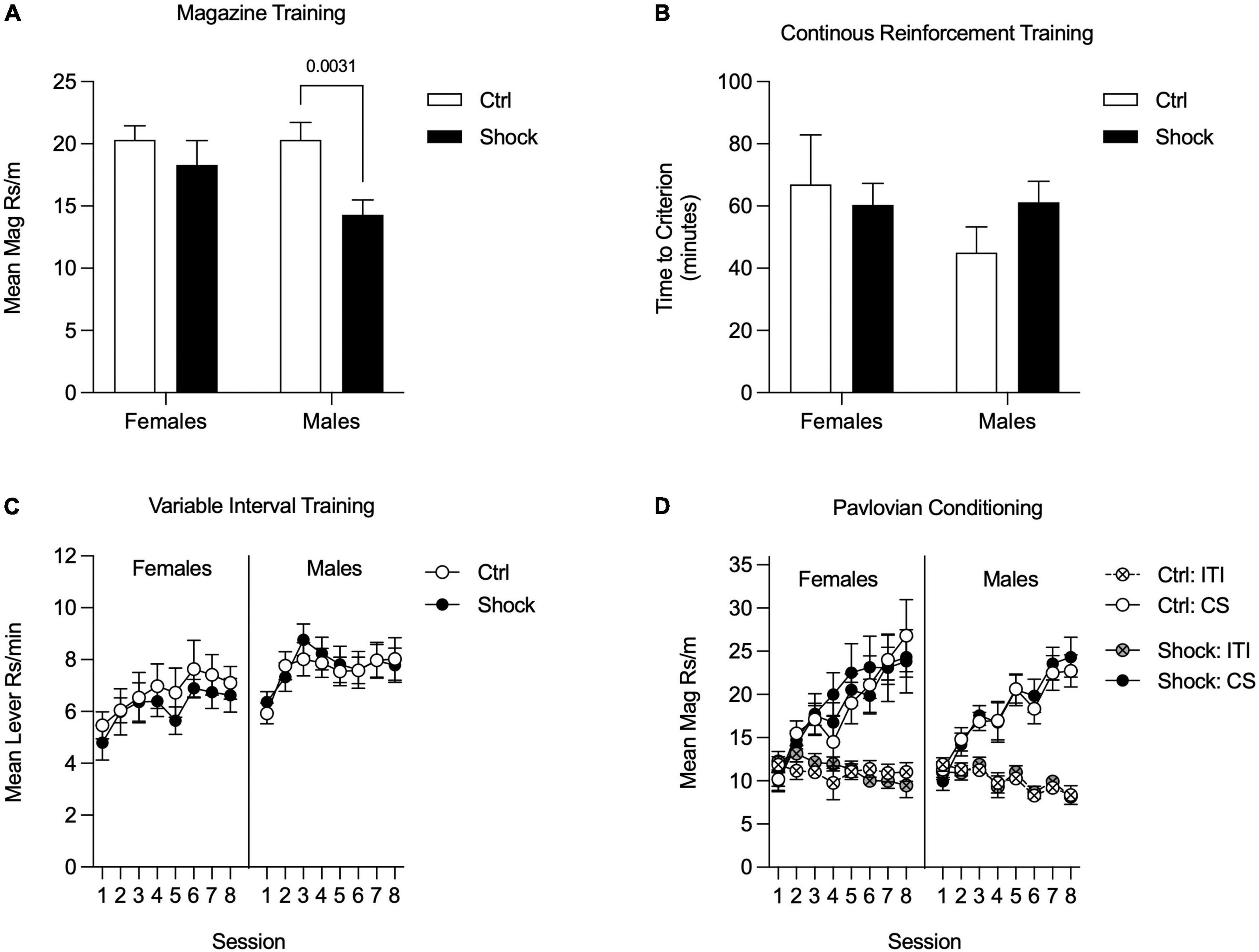
Figure 5. Appetitive training in Context B in Experiment 2. (A) During magazine training, responding was similar between female Ctrl and Shock groups, whereas in males responding was suppressed in the Shock group relative to the Ctrl group. (B) During CRF training, the time to reach the acquisition criteria did not differ between the Ctrl and Shock groups in either sex and there was no indication of a sex difference in acquisition times. (C) During VI training, instrumental responding increased across sessions, but was not significantly different between the Ctrl and Shock groups in females or in males (Though not shown directly here, responding in Shock groups was lower in females than males throughout training; there were no sex differences in responding in the Ctrl groups). (D) During Pavlovian conditioning, magazine responding during CS+ presentations increased across sessions without any differences between the Ctrl and Shock groups and no differences or interactions with sex. Magazine responding during the ITI and CS- did not increase across session and there were not differences across groups.
Pavlovian conditioning
In the last appetitive training phase rats underwent sensory-specific Pavlovian conditioning. These data were analyzed by averaging response rates across both CSs. In both sexes, magazine entries during the CSs increased across training sessions and did not differ between treatment groups [Figure 5D; Two-way RM ANOVA, main effect of session: Females: F(7, 140) = 17.10, p < 0.01; Males: F(7, 259) = 29.46, p < 0.01; no effects of treatment groups, Females, p = 0.80; Males: p = 0.87]. In both sexes, response rates during the ITI were low and did not differ between treatment groups [Figure 5D; Two-way RM ANOVA, no effects of treatment group: Females, p = 0.81; Males: p = 0.94]. Finally, we compared response rates between sexes for each treatment group and found no sex differences in either Ctrl or Shock groups (Two-way RM ANOVA, main effects of sex: Ctrl: p = 0.83; Shock: p = 0.71).
SS-PIT testing
Rats were tested in three separate tests with instrumental reminder sessions on the days between testing. Initial analysis was conducted by averaging performance in these tests. First, to evaluate the possibility of any notable sex differences or interactions between sex and trauma on the magnitude of SS-PIT, we calculated a mean score for SS-PIT magnitude by subtracting the Diff lever response rate from the Same lever rate on each test and then averaging these discrimination scores. We found no effect of shock treatment, but we did find a sex difference with overall lower SS-PIT in females compared to males and this did not interact with shock treatment [Figure 6A; Two-way ANOVA, main effect of sex F(1,55) = 34.97, p = 0.03; no effect of treatment, p = 0.92; no sex by treatment interaction, p = 0.28].

Figure 6. SS-PIT testing in Ctx B in Experiment 2. (A) SS-PIT magnitudes did not differ between Ctrl and Shock groups, but overall SS-PIT magnitudes were lower in females than males independent of shock treatment. (B) In females, SS-PIT was apparent in that lever responses on the Same lever were greater than responding on the Different lever in both Ctrl and Shock groups. General PIT was also evident in that Different lever responding was also significantly elevated above ITI lever response rates in both groups. (C) In males, SS-PIT was also evident in both the Ctrl and Shock groups with Same lever responses being significantly higher than Different lever responses and as in the females, there was a notable General PIT effect with Different lever response rates being greater than ITI response rates. (D) In females the trial-by-trial SS-PIT effect was not strong and focusing on the first trial of each test it was only evident on 1/3 tests in Ctrl and Shock groups. Overall, response rates began high and extinguished across the trials within each test and SS-PIT was absent in Trial 4 of all 3 tests. (E) In males in both the Ctrl and Shock groups the SS-PIT effect was apparent early in each test and disappeared with repeated trials. This pattern repeated across testing in a spontaneous recovery like effect. In males, SS-PIT was evident on the first trial of every test, but was absent on Trial 4 of every test demonstrating the within-session extinction effect and between-session recovery effect ($Same vs. Diff, p < 0.05; #significantly greater than ITI, p < 0.05).
Next, to directly examine SS-PIT we looked at the means lever response rates for ITI, Same lever, and Different lever responding averaged across all tests. In females, both Ctrl and Shock groups exhibited sensory-specific PIT with a given CS, preferentially increasing responding on the lever associated with the same outcome as the CS and there were no differences between the Ctrl and Shock groups [Figure 6B; Two-way RM ANOVA, main effect of lever response type (ITI, Same CS, Diff CS): F(2,40) = 29.70, p < 0.01; HS planned comparison: Same vs. Diff: Ctrl, t(40) = 3.07, p < 0.01; Shock, t(40) = 3.37, p < 0.01; no effect of treatment, p = 0.58]. In addition to sensory-specific transfer (Same > Diff), both Ctrl and Shock females exhibited significant General transfer on the Different lever such that CS presentations significantly elevated responding on the Different lever over ITI response rates, though this effect was marginal in the Ctrl group [Figure 6B; HS planned comparison: ITI vs. Diff CS: Ctrl, t(40) = 2.00, p = 0.05; Shock, t(40) = 2.72, p < 0.01]. The pattern was the same in males, such that both Ctrl and Shock groups showed sensory specific transfer with greater Same lever responding than Different with no differences between the Ctrl and Shock groups [Figure 6C; Two-way RM ANOVA, main effect of lever response type: F(2,74) = 114.00, p < 0.01; HS planned comparison: Same vs. Diff: Ctrl, t(74) = 6.37, p < 0.01; Shock, t(74) = 9.50, p < 0.01; no effect of treatment, p = 0.34]. As in the females, both the Ctrl and Shock groups showed significant General transfer with presentation of CS evoking a significant increase in responses on the Different lever from ITI responding [Figure 6C; HS planned comparison: ITI vs. Diff CS: Ctrl, t(74) = 2.58, p = 0.01; Shock, t(42) = 2.18, p = 0.03]. In summary, Shock did not disrupt SS-PIT in either female or male rats.
To assess the expression of PIT across time and to evaluate within session extinction of this effect, we examined lever responding on the first and last trial of each test using planned comparisons. In females this trial analysis revealed that SS-PIT was only evident on the first trial of one of the tests and was absent in the final trial of every test [Figure 6D; Trial 1: Test 1: Ctrl, p = 0.96; Shock, t(1,108) = 4.38, p < 0.01; Test 2: Ctrl, t(1,99) = 3.31, p = 0.02; Shock, p = 0.92; Test 3: all ps > 0.20; Trial 4 Tests 1–3, all ps > 0.78]. In contrast in males, the trial analysis revealed robust SS-PIT on the first trial of all three tests in both Ctrl and Shock groups; SS-PIT was absent on the final trials of each test (Figure 6E; Trial 1: Tests 1–3: all p’s < 0.03; Trial 4: Tests 1–3, all p’s > 0.40]. Thus in males, SS-PIT was strong at the beginning of each test session, but extinguished over the course of test trials. This spontaneous recovery of SS-PIT effect occurred in each test and the pattern of effects was parallel in the male Ctrl and Shock groups. The main effect of sex in the SS-PIT magnitude (Figure 5A) and the subgroup trial-by-trial data point to a sex difference in the propensity of SS-PIT to manifest at a trial level independent of shock treatment, with males showing more consistent SS-PIT across testing. Trial-by-trial analysis of General PIT (Diff vs. ITI) was generally weak and did not show a consistent pattern across trials as we observed with SS-PIT.
Given the mild deficits in SS-PIT in females, we conducted an additional SS-PIT test, but under ad libitum conditions. The purpose of relieving food restriction for this test is based on the finding that SS-PIT is insensitive to alterations in satiation, whereas General PIT is weakened or entirely abolished by satiety (Balleine, 1994; Corbit et al., 2007; Lingawi et al., 2022). Thus, the goal here was to drive down any General PIT process that might be masking effects in females. During this final test, SS-PIT was absent in both Ctrl and Shock females; lever responding on the Same and Different levers to the CS in presentation did not differ in these groups [Figure 7A; Two-way RM ANOVA, main effect of lever response type (ITI, Same CS, Diff CS): F(2,26) = 10.24, p < 0.01; HS planned comparison: Same vs. Diff: Ctrl, p = 0.28; Shock, p = 0.17; no effect of treatment, p = 0.95]. In contrast, in both male Ctrl and Shock groups SS-PIT was evident and with CS presentations evoking greater Same lever responses than Different lever responses [Figure 7B; Two-way RM ANOVA, main effect of lever response type (ITI, Same CS, Diff CS): F(2,58) = 24.84, p < 0.01; HS planned comparison: Same vs. Diff: Ctrl, t(52) = 2.82, p = 0.01; Shock, t(58) = 4.34, p < 0.01; no effect of treatment, p = 0.24].
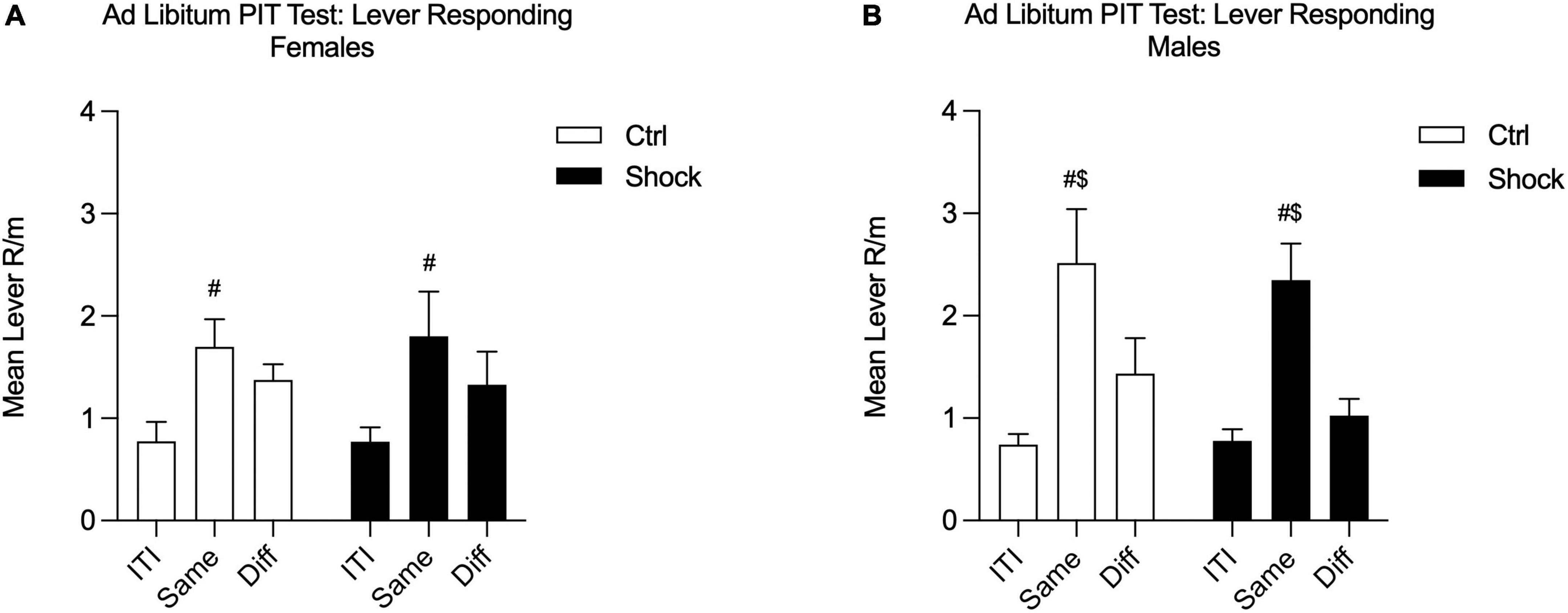
Figure 7. Ad libitum SS-PIT test in Ctx B in Experiment 2. (A) In females, the sensory-specificity of transfer was abolished in this ad libitum testing; Same and Different lever responses did not differ in either the Ctrl or Shock female groups. Same lever responses were greater than ITI in both groups. (B) In males, SS-PIT was maintained under ad libitum test conditions and Same responding was significantly greater than Different lever responding ($Same vs. Diff, p < 0.05; #significantly greater than ITI, p < 0.05).
Discussion
General overview
In this series of experiments, we show that an acute intensely stressful experience prior to appetitive training persistently reduces single-outcome Pavlovian-to-instrumental transfer (SO-PIT) in male rats, but this same experience does not alter the expression of Sensory-Specific PIT (SS-PIT) in male or female rats. These data show an acute highly stressful experience produces long lasting changes in general affective motivation for natural reward, as captured here with SO-PIT without overtly altering sensory-specific memory processes.
The effects of stress on learning, memory, and motivation are well documented in the literature. These studies have used acute stressors (such as footshock, yohimbine, or restraint stress) to evaluate the effects of stress on appetitive responding during or soon after a stressful experience (Mantsch et al., 2016). Typically, these acute stressors are thought to cause relatively short-term increases in stress. Other studies using chronic stressors (such as chronic variable stress, maternal separation, or long-term social isolation) have been shown to cause more persistent effects on consumption of and operant responding for natural rewards (Willner et al., 1987; Ghiglieri et al., 1997; Yan et al., 2010).
Our approach showing persistent changes of stress in appetitive responding is derived from the stress-enhanced fear learning (SEFL) procedure, in which a battery of footshocks in one context causes increased reactivity to a single footshock in a different context. SEFL occurs in contexts that have a long history of drug self-administration (Pizzimenti et al., 2017) and the battery of footshocks used to produce SEFL causes persistent changes in cue-induced reinstatement of methamphetamine-seeking (Pizzimenti et al., 2017) and alcohol drinking (Meyer et al., 2013).
These findings parallel two important aspects of PTSD as it often develops in humans: (1) a triggering traumatic event and (2) behavioral aberrations that persist long after the trauma. The results of work on drug-seeking in the context of SEFL parallel an important comorbidity seen in humans where PTSD is a major risk factor for substance use disorder. However, drugs of abuse alone trigger profound and persistent changes in appetitive motivation and the underlying circuitry, which makes it difficult to isolate the persistent impact of the acute trauma alone on appetitive processes. Furthermore, while many individuals suffering from PTSD experience substance use problems many do not, and understanding the impact of trauma on motivation for natural reward is important to understanding how PTSD manifests in such individuals and how to treat these comorbid symptoms.
Shock trauma prior to appetitive training blocks SO-PIT
In Experiment 1 we demonstrated that in male rats a battery of footshocks before, but not after appetitive training blocks expression of SO-PIT. This effect was specific to the test phase; there were no differences in acquisition of the Pavlovian CS+ vs. CS- discrimination or in acquisition of instrumental responding. During the PIT test, animals shocked prior to appetitive training did not show augmentation of instrumental responding during the CS+. The absence of SO-PIT was also apparent in the average time course of responding per trial and in the latency to press the levers following CS+ vs. CS- presentations. Despite the failure for the excitatory properties of the CS+ to transfer to instrumental responding, these rats still showed reliable Pavlovian conditional responding, preferentially entering the food cup during CS+ vs. CS- presentations. Thus, the failure for SO-PIT to manifest could not be explained by a failure to encode the Pavlovian discrimination. With all these considerations in mind, our data suggest that trauma can induce lasting deficits in the ability for Pavlovian cues associated with natural reward to acquire general motivational properties in males.
Our finding here that shock prior to training induces deficits in SO-PIT are consistent with a study in humans showing that participants scoring high on anxiety and stress metrics did not show general PIT, which is thought to rely on the same mechanisms as SO-PIT, whereas those with low stress and anxiety did (Quail et al., 2017). Although our study did not examine anxiety and stress directly, the SEFL treatment itself is associated with long lasting elevations in stress and anxiety in rats and in humans a major feature of PTSD is abnormal elevations in stress and anxiety.
Although SO-PIT was disrupted by shock trauma administered prior to training, this same treatment after training, but before testing, had less impact on SO-PIT, with no effect overall but some subtle within-trial deficits. This result is consistent with a study in rats showing that both single and multiple stressors administer either in the light or the dark phase of the circadian cycle did not block SO-PIT in Lister Hooded male rats (Pielock et al., 2013). It is notable that our procedure uses a very intense stress protocol which is distinct from that used in this prior study. Similarly, in humans, experimentally induced stress enhanced SO-PIT expression (Pool et al., 2015), thus it would seem that perhaps the immediate effects of stress on SO-PIT are variable and this warrants further investigation.
Shock prior to training does not alter SS-PIT
SO-PIT taps into basic motivational properties of Pavlovian cues, however it does not provide a clean read out of sensory-specific learning, memory, and motivation. In Experiment 1, all groups showed reliable conditional stimulus discrimination (CS+ vs. CS-) regardless of shock history, arguing against trauma-induced problems with sensory memory encoding; however, the possibility remained that sensory-specific learning may be altered by a history of acute trauma. We therefore tested whether shock before training would alter the expression of SS-PIT, a clean measure of sensory-specific encoding. Here we used both female and male rats. Shock prior to training did not prevent the expression of SS-PIT in either male or female rats. Specifically, CS presentation selectively invigorated responding on the lever that previously shared the same outcome as the CS in presentation vs. the lever that previously delivered a different outcome. This finding confirms that a history of trauma does not prevent the formation of distinct sensory-specific memories in either males or females. This finding is consistent with a previous study that used chronic unpredictable stress to examine the effects of stress on SS-PIT in male Wistar rats (Morgado et al., 2012). In the Morgado study, SS-PIT deficits were observed when testing was conducted shortly after the stress treatment period, but this deficit disappeared when rats were given a period of time off from stress treatment. In our design we used an acute stress and SS-PIT testing occurred more than 20 days after that stressful experience. Although we did not observe a short-term effect with SO-PIT in Experiment 1, it is possible that a test closer in time to the shock trauma would produce short-term deficits in SS-PIT.
Sex differences in training and testing
In Experiment 2, there were several sex differences during the course of fear conditioning and PIT training and testing. First, during fear conditioning, females showed delayed acquisition relative to males early in the conditioning session. This delay may reflect early differences in expression of different behaviors (freezing in males, increased activity/escape in females; Gruene et al., 2015). Despite some differences in rate of acquisition, asymptotic levels of freezing were identical in males and females (Poulos et al., 2015).
Second, during magazine training in both experiments, which is the first appetitive training phase after shock, shocked males showed lower rates of magazine approach compared to control males, but in females the rates of magazine responding were similar in shocked and control subjects. Unfortunately, we had no video record of the magazine training session, but we and others have found that males show more generalized freezing to a novel context than do females (Colon et al., 2018). This effect in Experiment 1 persisted into the first session of CRF training, but in Experiment 2, where there was more magazine training and thus more opportunity for generalized fear to extinguish prior to CRF training, there was no difference in CRF training. To the best of our knowledge, differences in freezing between males and females to a non-threatening novel context following intense fear conditioning has not been documented. During VI training, we did not see any sex differences between Ctrl groups, but in the Shock groups, females showed significantly lower rates of lever responding. This suggests that trauma may have differing effects on instrumental responding between sexes. It is possible that this effect is unique to responding under VI schedules or reinforcement or may reflect broader female specific trauma-induced deficits in instrumental responding. We did not see any indication of sex differences or interactions during Pavlovian conditioning.
Third, although females and males showed SS-PIT, this effect (i.e., the difference in Same vs. Diff) was more consistent across testing in males, with females showing SS-PIT on only one of the 12 test trials. Moreover, when tested in ad libitum state, females failed to show SS-PIT such that responding to the Same vs. Different lever did not significantly differ. Given that SS-PIT has been shown to be insensitive to shifts in motivational states, particularly from hunger to satiety, we utilized this ad libitum test to determine to what extent females were relying on a sensory-specific process to solve the task (Balleine, 1994; Corbit et al., 2007; Lingawi et al., 2022). The loss of sensory-specificity in transfer suggests that females, in our study, were relying more heavily on a generalized motivational process. It is possible these data represent a broad sex differences in reliance on or preferential engagement of sensory-specific vs. general motivational information to guide cue triggered behavior. Together, these findings suggest that sex differences in fear conditioning, generalization, and SS-PIT are present, even if perhaps sometimes subtle. These effects warrant future study because differences in sex hormones may be enough to cause subtle changes in behavioral performance (Bangasser and Wicks, 2017).
The novel observation of the return of extinguished SS-PIT
A noteworthy finding in Experiment 2 was within-session extinction and robust between-session spontaneous recovery of SS-PIT in males. On each test the SS-PIT (Same > Diff) effect was strongest in early trials growing weaker across trials and then re-emerging without any notable degradation upon retesting. Given that PIT testing is conducted under extinction conditions, the Pavlovian CSs are essentially given extinction training and while rats are retrained on the instrumental associations between tests, they never received additional CS-US pairings. Thus, the robust revival of the PIT effect on each subsequent test is quite similar to classic spontaneous recovery. It is also consistent with the intervening instrumental retraining establishing a reinstatement of selective associations via exposure to the outcomes (Delamater, 1997). The resistance of SS-PIT to Pavlovian extinction has previously been documented (Delamater, 1996; Delamater et al., 2017). However, the vast majority of SS-PIT studies that conduct multiple testing present the data in summary format potentially obscuring observation of this spontaneous recovery effect (Panayi and Killcross, 2018; Alarcon and Delamater, 2019; Lingawi et al., 2022; Sommer et al., 2022). To our knowledge, this is the first documentation of this effect.
Shock trauma and motivation for drug vs. natural reward
Pizzimenti et al. (2017) found that shock trauma prior to acquisition of methamphetamine self-administration resulted in enhanced cue-associated reinstatement of methamphetamine-seeking weeks after the initial trauma. These data are most parallel in design to our current study, but our current findings found a deficit rather than an enhancement in Pavlovian control of reward-seeking. The procedural differences between cue-associated reinstatement and PIT might explain some of the disparity between Pizzimenti’s finding and ours, however, there are alternative possibilities worth considering. Drugs of abuse exploit and engage the same circuitry that mediates appetitive motivation for natural rewards, but drugs of abuse themselves induce dramatic changes in plasticity and cues associated with drugs are more difficult to extinguish compared to cues associated with natural rewards (Jacobs et al., 2022). Drugs of abuse also carry with them distinctively stressful properties. Thus, the effects observed by Pizzimenti may specifically reflect the unique plasticity induced by the interaction of acute trauma with drug abuse and drug withdrawal. Whereas in our study, the effects observed on PIT are more readily attributable to the acute stress alone, rather than stress or plasticity induced by the natural reward.
Another possibility is that our data reflect a stress-induced insensitivity to the motivational aspects of natural reward, which may render individuals more vulnerable to the motivational properties of drug reward. The suppressive effects of stress and depression on consumption and instrumental motivation for natural rewards has been well documented (Willner et al., 1987; Ghiglieri et al., 1997; Willner, 2005; Yan et al., 2010). Thus our effects here on SO-PIT may represent an extension of these anhedonic effects to include the ability for Pavlovian CSs to acquire standard motivational properties. Given this possibility, perhaps acute trauma imparts a persistent anhedonia which we capture here in the form of blunted general affective motivation, which may render individuals more sensitive to the effects hedonic perturbation experienced by drugs resulting in a more salient experience thereby enhancing the incentive salience of drug associated stimuli. Ideally a direct comparison of drug versus natural reward using the same methodology (either PIT or cue-associated reinstatement) would be able to address his interesting discrepancy.
Neuronal implications of the persistent effect of trauma on SO-, but not SS-PIT
SO- and SS-PIT are dissociable both as psychological constructs and also rely on different brain circuits (Blundell et al., 2001; Corbit et al., 2001; Hall et al., 2001; Holland and Gallagher, 2003; Corbit and Balleine, 2005; Corbit et al., 2007; Corbit and Balleine, 2011; Laurent et al., 2012; Cartoni et al., 2016; Lingawi et al., 2022). Lesion and inactivation studies have shown that SO-PIT relies on the Nucleus Accumbens Core (NAc Core), and the Central Nucleus of the Amygdala (CeA) (Holland and Gallagher, 2003; Corbit et al., 2007). In contrast, SS-PIT depends on the NAc Shell, and the Basolateral Amygdala (BLA), and the connection between these structures. Given this distinction at the neuronal level, it would seem likely that the persistent effects of trauma on SO-PIT, but not SS-PIT point to long-term trauma-induced plasticity in either the NAc Core and or the CeA. The absence of effects on SS-PIT does not rule out long term trauma induced changes in the NAc Shell and BLA, but suggest that whatever changes may be taking place they are not impacting the sub circuitry of these nuclei that are dedicated to the expression of this appetitive behavior (SS-PIT).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the OHSU IACUC.
Author contributions
RD and KL designed the experiments and wrote the manuscript. RD conducted the experimental work and performed the statistical analyses. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants R01 DA047981 to KL and T32 DA007262 to RD.
Acknowledgments
We thank Kamlyn Yosick for assistance with data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2022.1028262/full#supplementary-material
References
Alarcon, D. E., and Delamater, A. R. (2019). Outcome-specific Pavlovian-to-instrumental transfer (PIT) with alcohol cues and its extinction. Alcohol 76, 131–146. doi: 10.1016/j.alcohol.2018.09.003
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders : DSM-5. Washington, DC: American Psychiatric Association.
Balleine, B. (1994). Asymmetrical interactions between thirst and hunger in Pavlovian-instrumental transfer. Q. J. Exp. Psychol. B 47, 211–231.
Bangasser, D. A., and Wicks, B. (2017). Sex-specific mechanisms for responding to stress. J. Neurosci. Res. 95, 75–82. doi: 10.1002/jnr.23812
Blundell, P., Hall, G., and Killcross, S. (2001). Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J. Neurosci. 21, 9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001
Brewerton, T. D. (2007). Eating disorders, trauma, and comorbidity: focus on PTSD. Eat. Disord. 15, 285–304. doi: 10.1080/10640260701454311
Cartoni, E., Balleine, B., and Baldassarre, G. (2016). Appetitive Pavlovian-instrumental transfer: A review. Neurosci. Biobehav. Rev. 71, 829–848. doi: 10.1016/j.neubiorev.2016.09.020
Chwastiak, L. A., Rosenheck, R. A., and Kazis, L. E. (2011). Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics 52, 230–236. doi: 10.1016/j.psym.2010.12.009
Colon, L., Odynocki, N., Santarelli, A., and Poulos, A. M. (2018). Sexual differentiation of contextual fear responses. Learn. Mem. 25, 230–240. doi: 10.1101/lm.047159.117
Colwill, R. M., Delamater, A. R., and Lattal, K. M. (2022). Developments in associative theory: A tribute to the contributions of Robert A. Rescorla. J. Exp. Psychol. 48, 245–264.
Corbit, L. H., and Balleine, B. W. (2005). Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J. Neurosci. 25, 962–970. doi: 10.1523/JNEUROSCI.4507-04.2005
Corbit, L. H., and Balleine, B. W. (2011). The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J. Neurosci. 31, 11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011
Corbit, L. H., Janak, P. H., and Balleine, B. W. (2007). General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur. J. Neurosci. 26, 3141–3149. doi: 10.1111/j.1460-9568.2007.05934.x
Corbit, L. H., Muir, J. L., and Balleine, B. W. (2001). The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J. Neurosci. 21, 3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001
Coughlin, S. S., Kang, H. K., and Mahan, C. M. (2011). Selected health conditions among overweight, obese, and non-obese veterans of the 1991 Gulf War: results from a survey conducted in 2003-2005. Open Epidemiol. J. 4, 140–146. doi: 10.2174/1874297101104010140
Delamater, A. (1996). Effects of several extinction treatments upon the integrity of Pavlovian stimulus-outcome associations. Anim. Learn. Behav. 24:13. doi: 10.3758/BF03199015
Delamater, A. R. (1997). Selective reinstatement of stimulus-outcome associations. Anim. Learn. Behav. 25, 400–412. doi: 10.3758/BF03209847
Delamater, A. R., Schneider, K., and Derman, R. C. (2017). Extinction of specific stimulus-outcome (S-O) Associations in pavlovian learning with an EXTENDED CS procedure. J. Exp. Psychol. Anim. Learn. Cogn. 4, 243–261. doi: 10.1037/xan0000138
Ghiglieri, O., Gambarana, C., Scheggi, S., Tagliamonte, A., Willner, P., and Montis, M. G. D. (1997). Palatable food induces an appetitive behaviour in satiated rats which can be inhibited by chronic stress. Behav. Pharmacol. 8, 619–628. doi: 10.1097/00008877-199711000-00018
Grubbs, J. B., Chapman, H., and Shepherd, K. A. (2019). Post-traumatic stress and gambling related cognitions: Analyses in inpatient and online samples. Addict. Behav. 89, 128–135. doi: 10.1016/j.addbeh.2018.09.035
Gruene, T. M., Flick, K., Stefano, A., Shea, S. D., and Shansky, R. M. (2015). Sexually divergent expression of active and passive conditioned fear responses in rats. eLife 4:e11352. doi: 10.7554/eLife.11352.013
Hall, J., Parkinson, J. A., Connor, T. M., Dickinson, A., and Everitt, B. J. (2001). Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur. J. Neurosci. 13, 1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x
Holland, P. C., and Gallagher, M. (2003). Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 17, 1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x
Jacobs, D. S., Hitchcock, L. N., Williams, R. G., and Lattal, K. M. (2022). Effects of a cue associated with cocaine or food reinforcers on extinction and postextinction return of behavior. Behav. Neurosci. 136, 307–317. doi: 10.1037/bne0000519
Kessler, R. C., Chiu, W. T., Demler, O., Merikangas, K. R., and Walters, E. E. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 617–627. doi: 10.1001/archpsyc.62.6.617
Laurent, V., Leung, B., Maidment, N., and Balleine, B. W. (2012). Mu- and delta-opioid-related processes in the accumbens core and shell differentially mediate the influence of reward-guided and stimulus-guided decisions on choice. J. Neurosci. 32, 1875–1883. doi: 10.1523/JNEUROSCI.4688-11.2012
Lingawi, N. W., Berman, T., Bounds, J., and Laurent, V. (2022). Sensory-specific satiety dissociates general and specific pavlovian-instrumental transfer. Front. Behav. Neurosci. 16:877720. doi: 10.3389/fnbeh.2022.877720
Mantsch, J. R., Baker, D. A., Funk, D., Lê, A. D., and Shaham, Y. (2016). Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology 41, 335–356. doi: 10.1038/npp.2015.142
Meyer, E. M., Long, V., Fanselow, M. S., and Spigelman, I. (2013). Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol. Clin. Exp. Res. 37, 566–574. doi: 10.1111/acer.12012
Morgado, P., Silva, M., Sousa, N., and Cerqueira, J. J. (2012). Stress transiently affects Pavlovian-to-instrumental transfer. Front. Neurosci. 6:93. doi: 10.3389/fnins.2012.00093
Panayi, M. C., and Killcross, S. (2018). Functional heterogeneity within the rodent lateral orbitofrontal cortex dissociates outcome devaluation and reversal learning deficits. eLife 7:e37357. doi: 10.7554/eLife.37357.014
Piazza, P. V., Deminiere, J. M., Le Moal, M., and Simon, H. (1990). Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 514, 22–26. doi: 10.1016/0006-8993(90)90431-A
Pielock, S. M., Braun, S., and Hauber, W. (2013). The effects of acute stress on Pavlovian-instrumental transfer in rats. Cogn. Affect. Behav. Neurosci. 13, 174–185. doi: 10.3758/s13415-012-0129-3
Pizzimenti, C. L., Navis, T. M., and Lattal, K. M. (2017). Persistent effects of acute stress on fear and drug-seeking in a novel model of the comorbidity between post-traumatic stress disorder and addiction. Learn. Mem. 24, 422–431. doi: 10.1101/lm.044164.116
Pool, E., Brosch, T., Delplanque, S., and Sander, D. (2015). Stress increases cue-triggered “wanting” for sweet reward in humans. J. Exp. Psychol. Anim. Learn. Cogn. 41, 128–136. doi: 10.1037/xan0000052
Poulos, A. M., Zhuravka, I., Long, V., Gannam, C., and Fanselow, M. (2015). Sensitization of fear learning to mild unconditional stimuli in male and female rats. Behavioral neuroscience, 129, 62–67. doi: 10.1037/bne0000033
Quail, S. L., Morris, R. W., and Balleine, B. W. (2017). Stress associated changes in Pavlovian-instrumental transfer in humans. Q. J. Exp. Psychol. 70, 675–685. doi: 10.1080/17470218.2016.1149198
Rau, V., and Fanselow, M. S. (2009). Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress 12, 125–133. doi: 10.1080/10253890802137320
Rau, V., Decola, J. P., and Fanselow, M. S. (2005). Stress-induced enhancement of fear learning: An animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 29, 1207–1223. doi: 10.1016/j.neubiorev.2005.04.010
Sommer, S., Münster, A., Fehrentz, J.-A., and Hauber, W. (2022). Effects of motivational downshifts on specific Pavlovian-instrumental transfer in rats. Int. J. Neuropsychopharmacol. 25, 173–184. doi: 10.1093/ijnp/pyab075
Steins-Loeber, S., Lörsch, F., Van Der Velde, C., Müller, A., Brand, M., Duka, T., et al. (2020). Does acute stress influence the Pavlovian-to-instrumental transfer effect? Implications for substance use disorders. Psychopharmacology 237, 2305–2316. doi: 10.1007/s00213-020-05534-8
Swinbourne, J. M., and Touyz, S. W. (2007). The co-morbidity of eating disorders and anxiety disorders: a review. Eur. Eat. Disord. Rev. 15, 253–274. doi: 10.1002/erv.784
Willner, P. (2005). Chronic Mild Stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52, 90–110. doi: 10.1159/000087097
Willner, P., Towell, A., Sampson, D., Sophokleous, S., and Muscat, R. (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93, 358–364. doi: 10.1007/BF00187257
Keywords: post-traumatic stress disorder (PTSD), aversive-appetitive interactions, trauma, affective motivation, natural reward, animal models, stress-enhanced fear learning
Citation: Derman RC and Lattal KM (2022) Persistent effects of acute trauma on Pavlovian-to-instrumental transfer. Front. Behav. Neurosci. 16:1028262. doi: 10.3389/fnbeh.2022.1028262
Received: 25 August 2022; Accepted: 10 October 2022;
Published: 31 October 2022.
Edited by:
Johannes Passecker, Medical University of Innsbruck, AustriaReviewed by:
Briac Halbout, University of California, Irvine, United StatesVincent D. Campese, University of Evansville, United States
Copyright © 2022 Derman and Lattal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: K. Matthew Lattal, bGF0dGFsbUBvaHN1LmVkdQ==
 Rifka C. Derman
Rifka C. Derman K. Matthew Lattal
K. Matthew Lattal