- 1Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom
- 2Department of Psychiatry, Herchel Smith Building for Brain & Mind Sciences, University of Cambridge, Cambridge, United Kingdom
- 3Cambridge Psychosis Centre, Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, United Kingdom
Negative symptoms are a debilitating feature of schizophrenia which are often resistant to pharmacological intervention. The mechanisms underlying them remain poorly understood, and diagnostic methods rely on phenotyping through validated questionnaires. Deeper endo-phenotyping is likely to be necessary in order to improve current understanding. In the last decade, valuable behavioural insights have been gained through the use of effort-based decision making (EBDM) tasks. These have highlighted impairments in reward-related processing in schizophrenia, particularly associated with negative symptom severity. Neuroimaging investigations have related these changes to dysfunction within specific brain networks including the ventral striatum (VS) and frontal brain regions. Here, we review the behavioural and neural evidence associated with negative symptoms, shedding light on potential underlying mechanisms and future therapeutic possibilities. Findings in the literature suggest that schizophrenia is characterised by impaired reward based learning and action selection, despite preserved hedonic responses. Associations between amotivation and reward-processing deficits have not always been clear, and may be mediated by factors including cognitive dysfunction or dysfunctional or self-defeatist beliefs. Successful endo-phenotyping of negative symptoms as a function of objective behavioural and neural measurements is crucial in advancing our understanding of this complex syndrome. Additionally, transdiagnostic research–leveraging findings from other brain disorders, including neurological ones–can shed valuable light on the possible common origins of motivation disorders across diseases and has important implications for future treatment development.
Introduction
Negative symptoms are a core feature of schizophrenia, observed in up to 90% of patients at first-episode psychosis (Kraepelin, 1921; Bleuler, 1950; Mäkinen et al., 2008; An der Heiden et al., 2016). They include a constellation of symptoms unified by the loss of “vital properties” such as speech, motivation and self-expression (Berrios, 1985). Despite being less prominent in presentation than the positive/psychotic features of schizophrenia (e.g., hallucinations and delusions), negative symptoms are independently associated with poor functional outcomes (Milev et al., 2005; Rabinowitz et al., 2012, 2013; Fervaha et al., 2014) and subjective well-being (Strauss et al., 2012), and can predict future psychotic episodes (Piskulic et al., 2012). To date, there are no licensed treatments specifically targeting negative symptoms, in part likely due to a lack of understanding of their underlying mechanisms. A better characterisation of such mechanisms might therefore play a key role in treating their debilitating effects across psychiatric conditions.
Recent development of behavioural paradigms to investigate negative symptoms has significantly improved our understanding of their phenotype (Gold et al., 2015; Culbreth et al., 2018). Importantly, these behavioural measures have been related directly to specific neural regions and networks in the brain (Chase et al., 2018; Culbreth et al., 2018). Here we discuss the history and current mechanistic understanding of motivational aspects of the negative syndrome considering these developments, whilst highlighting current gaps and future therapeutic possibilities.
Negative Symptoms: The State of Play
Our conceptual understanding has progressed considerably since the formalisation of a “negative syndrome” as early as 1974 (Strauss et al., 1974). Current classification of the negative syndrome includes five symptom domains as agreed upon by the National Institute of Mental Health (NIMH) consensus statement (Kirkpatrick et al., 2006). These are: alogia, anhedonia, avolition, asociality, and blunted affect (see Table 1 for definitions). Questionnaire measures, which can help to support clinical diagnosis, have been adapted to reflect the evolving definition of the negative syndrome (Kirkpatrick et al., 2011).
Factor analyses of these questionnaire data have shed further light on possible underlying structures of the negative syndrome. For example, the results of some studies suggest that negative symptom domains aggregate into a two-factor structure where alogia and blunted affect form a “Diminished Expression” (EXP) factor, while the remaining domains form a “Motivation and Pleasure” (MAP) factor, which can be referred to as “apathy” (Figure 1; Kimhy et al., 2006; Nakaya and Ohmori, 2008; Foussias and Remington, 2010; Horan et al., 2011; Kirkpatrick et al., 2011; Kring et al., 2013). This dichotomised view of negative symptoms is reflected in the most recent version of the DSM-5 (Tandon et al., 2013). However, other findings suggest a more complex construct, providing some evidence against a simplistic two factor model of negative symptoms (Strauss et al., 2018, 2019a,b).
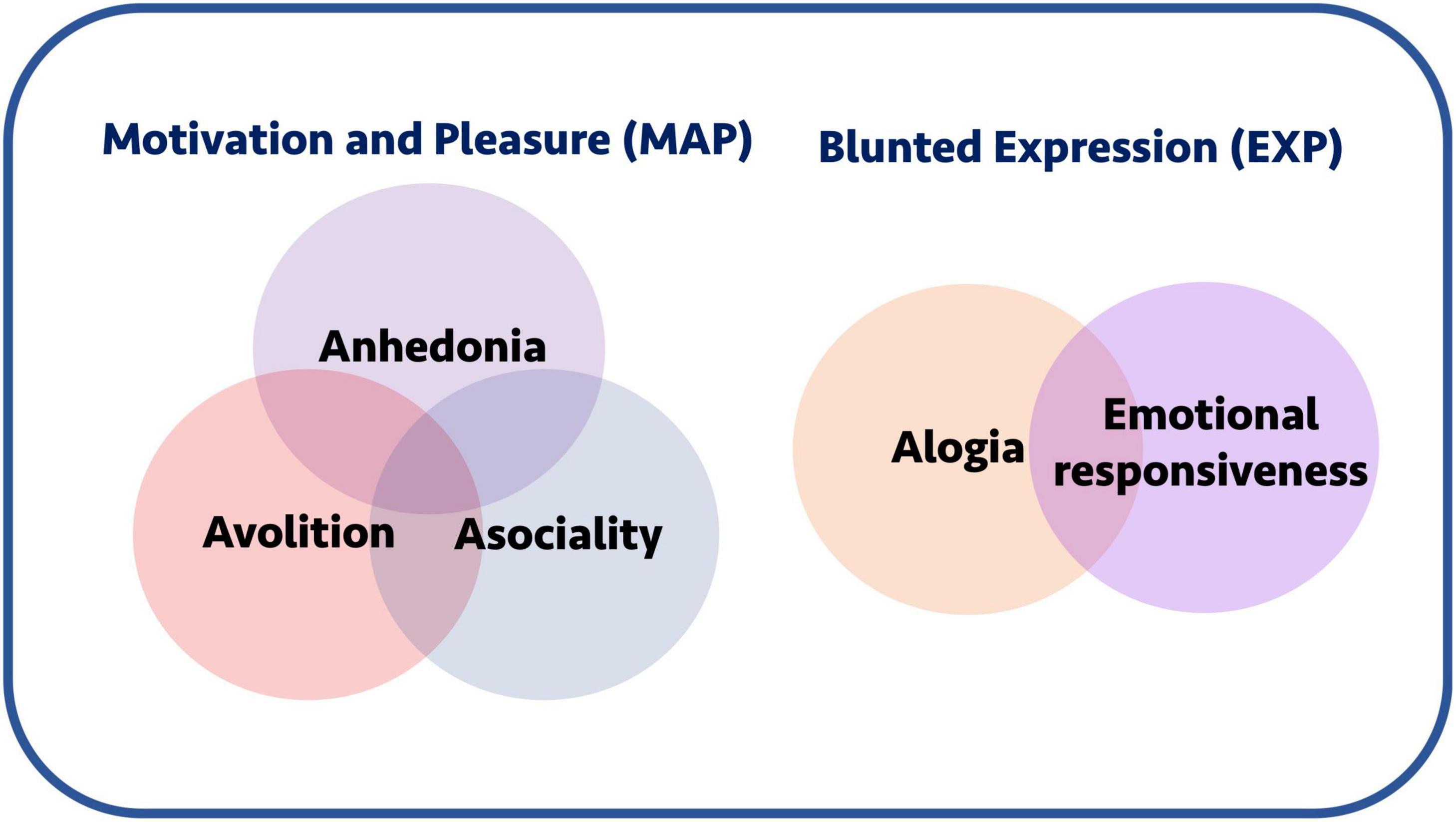
Figure 1. Negative symptoms and conceptual groupings. Negative symptoms are often conceptually grouped into a motivation and pleasure (MAP) and blunted expression (EXP) components. The former includes anhedonia, avolition, and asociality, whereas the latter includes alogia and emotional responsiveness. Collectively, the motivation-pleasure symptoms are also sometimes referred to using the umbrella term “apathy.”
Some challenges with frameworks based on questionnaire analyses may be partly due to the use of different instruments to assess negative symptoms (Garcia-Portilla et al., 2015; Kumari et al., 2017). Previous studies have relied on questionnaires containing symptoms that are no longer considered part of the consensus statement definition of the negative syndrome (Peralta and Cuesta, 1995; Sayers et al., 1996; Kumari et al., 2017). For example, the widely used PANSS questionnaire (Positive And Negative Syndrome Scale) includes “abstract thinking” as a negative symptom, whereas the SANS (Scale for the Assessment of Negative Symptoms) questionnaire includes an “inattention” domain (Andreasen et al., 1991; Mortimer, 2007). Both these components may reflect general cognitive dysfunction, currently considered to arise separate from negative symptom mechanisms in schizophrenia (Kirkpatrick et al., 2006).
It is evident that the complex negative syndrome remains to be fully understood. While questionnaire analyses may advance our understanding at the structural level, they lack the ability to provide deeper mechanistic insight at behavioural and anatomical levels. One potential way to address the current knowledge gap is by application of an endo-phenotyping approach to advance a neurocognitive framework of this syndrome. A key area where this is being applied is in disorders of motivation.
Effort-Based Decision Making Framework for Motivation Deficits
Motivation deficits appear to be central to the negative syndrome (Strauss et al., 2019c, 2021). An emerging framework that has been used to conceptualize the motivational aspects of negative symptoms is that of effort-based decision making for rewards (EBDM). Paradigms that assess EBDM, some translated from animal studies, have the potential to provide important insights into the phenotypes of neuropsychiatric disease by probing different phases of behaviour (Husain and Roiser, 2018). These include the instrumental, consummatory and learning phases of motivated behaviour outlined in Figure 2 and explained below.

Figure 2. Phases of effort-based decision making for rewards. Effort based decisions making can occur across three conceptual phases. Option selection includes the processes of weighing up components of decisions and behaviourally manifests as wanting. Consummatory phase refers to the hedonic response and represents the experience of liking. Learning reflects the process of updating beliefs based on a representation of the environment that is structured (i.e., model based) or otherwise (model free). Framework adapted from Berridge and Robinson (2003) and Husain and Roiser (2018).
When deciding whether to pursue a course action, an individual may weigh up its potential benefits (e.g., reward/avoiding punishment) against its costs (e.g., effort/risk) vs. other possible actions–a process referred to as “option selection” (Husain and Roiser, 2018). Alternatively, having invested some effort towards a specific goal, one may re-evaluate the merit of sustaining further effort in its pursuit. Paradigms examining these processes in patient groups can manipulate the benefits and costs of actions systematically to highlight potential deficits in effort-based decisions (Treadway et al., 2009; Le Heron et al., 2018b). Some frameworks conceptualise these processes as encompassing the “wanting” or instrumental components of motivated behaviour to seek a particular outcome (Salamone and Correa, 2012; Thomsen et al., 2015).
Whereas the decision making process represents the “wanting” aspect of behaviour, consummatory behaviour encompasses the hedonic response or the “liking” component when interacting with the goal of behaviour (Salamone and Correa, 2012; Thomsen et al., 2015). When an individual engages in pleasurable activity (e.g., watching a comedy), they may respond explicitly (e.g., rating their experience), or implicitly (e.g., smiling) (Berenbaum and Oltmanns, 1992; Berridge and Robinson, 2003). One or more of these responses may be altered in patients with negative symptoms (Berenbaum and Oltmanns, 1992; Cohen and Minor, 2010).
Having engaged in a behaviour with an unexpected positive/negative outcome, expectations of reward may be updated in relation to behaviour. In laboratory settings, this can be achieved by varying the frequency of rewarding different actions. For example, one response may be rewarded 90% of time and another only 60% of the time (Hernaus et al., 2018; Culbreth et al., 2021). Over the course of an experiment, participants learn to favour one action based on the frequency of reward (or reinforcement schedule). Deficits in the learning process may impair individuals from representing the value of reward appropriately to guide future motivated behaviour to influence future decisions.
The last decade has seen a significant rise in EBDM studies in schizophrenia spanning several of these components of decision making (Gold et al., 2013; Barch et al., 2014; Albrecht et al., 2019; Chang et al., 2019, 2020; Cooper et al., 2019; Culbreth et al., 2021). The investigations that have been performed aimed to probe cognitive mechanisms underlying behavioural deficits, including negative symptoms. Phenotypes can be captured using tasks that probe different aspects of EBDM based on behavioural performance, but they can also be derived from computational modelling of behaviour on such tasks and subsequently correlated with symptoms of interest. This approach has been used to investigate apathy and anhedonia across several disorders (Muhammed et al., 2016; Husain and Roiser, 2018; Le Heron et al., 2018a,b; Pessiglione et al., 2018; Berwian et al., 2020). As both these symptoms occur in the negative syndrome, it is possible that analysis of performance on EBDM tasks can also add value to the mechanistic understanding of negative symptoms in schizophrenia.
Instrumental Behaviour: Option Selection
One of the earliest and most commonly used EBDM paradigms is that developed by Treadway et al. (2009, 2015) known as the “Effort Expenditure for Rewards Task” (EEfRT). This paradigm and its variations have been widely used to probe option selection in patients and healthy controls (Fervaha et al., 2013; Barch et al., 2014; Hartmann et al., 2015; Treadway et al., 2015; Chang et al., 2019) and have demonstrated good test-retest reliability (Reddy et al., 2015) and external validity (Horan et al., 2015). In this task participants exert physical effort either by selecting to press on a key using the dominant index finger (low-effort) or the non-dominant little finger (high-effort). While the low effort condition is paired with a single fixed low reward (e.g., $1), the high effort condition can be paired with one of four possible higher reward magnitudes (e.g., $1.3–$4.3). Additionally, the outcome probability is varied across all conditions to incorporate an element of uncertainty (25, 50, or 88%). On a trial by trial basis, participants are offered either a low reward–low effort condition or a high reward–high effort condition with outcome probability varied (Figure 3A).
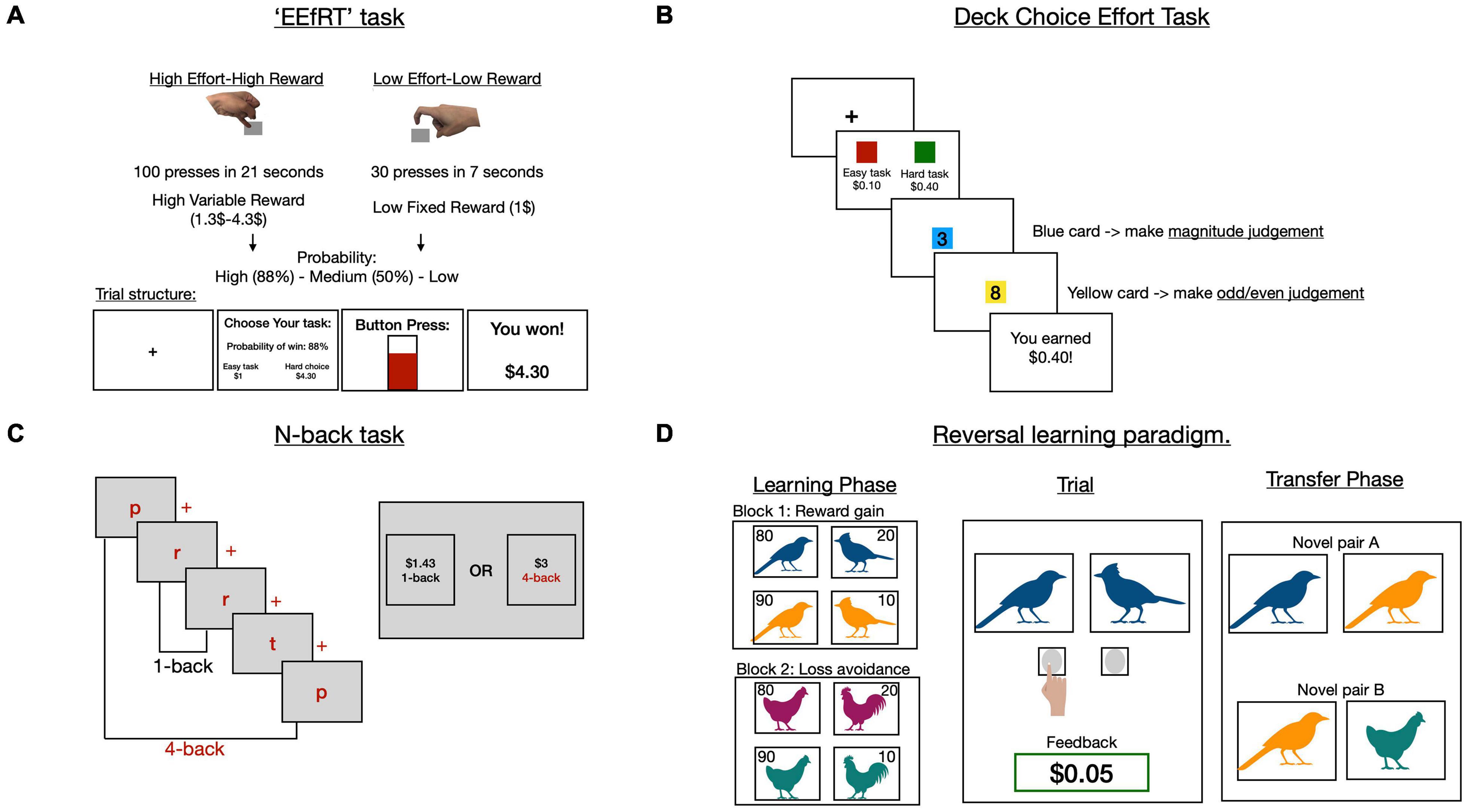
Figure 3. Effort based decision making tasks in schizophrenia. Schematic representation of four paradigms (A–D). The EEfRT task (A) varies physical effort in return for monetary reward. Participants choose between a high effort-high reward condition and low effort-low reward condition. Additionally, probability of winning varies across trial types. Adapted from Treadway et al. (2009). The deck choice effort task and the N-back task (B,C) both use cognitive instead of physical effort in a similar task structure to the EEfRT task, although probability is not varied. (B) In the deck choice task, high effort involves serially alternating between two mental activities, whereas only one mental activity is performed in the low effort option. (C) In the N-back task participants are serially presented with a list of letters and asked if the same letter was presented N steps back. They choose between a 1-back trial for low reward or a higher N-back (e.g., 4-back) for higher reward. (D) Learning paradigms involve choosing between two partially reinforced images to either gain reward, or avoid loss (see subsection “Reinforcement learning of reward value”). In a learning phase, only two images are presented per trial and one type of outcome is varied per block (reward gain vs. loss avoidance). A subsequent transfer phase uses novel combinations of visual stimuli to investigate learning behaviour across trial types.
The most consistent finding reported using this task is that schizophrenia patients paradoxically accept less offers than healthy controls when reward levels and probabilities are the highest (Fervaha et al., 2013; Gold et al., 2013; Barch et al., 2014; Hartmann et al., 2015; Horan et al., 2015; Reddy et al., 2015; Treadway et al., 2015; McCarthy et al., 2016; Moran et al., 2017). Further, some studies demonstrate that this effect is most pronounced in patients with higher negative symptom severity (Gold et al., 2013; Hartmann et al., 2015; Cooper et al., 2019), although this finding is not consistent (Fervaha et al., 2013; Barch et al., 2014; Horan et al., 2015; Moran et al., 2017). These inconsistencies might arise due to a variety of reasons which include use of different analysis approaches (Fervaha et al., 2013; Horan et al., 2015), questionnaire types (Fervaha et al., 2013; Hartmann et al., 2015; Moran et al., 2017) or task variations (Horan et al., 2015). In those investigations where there were significant associations between performance and negative symptoms, correlations tended to bias toward the MAP components (Figure 1) of the negative syndrome (i.e., avolition, asociality, and anhedonia) and not the expressive component (i.e., alogia and blunted affect) (Fervaha et al., 2013; Barch et al., 2014; Hartmann et al., 2015; Cooper et al., 2019).
One critique of the EEfRT task and some of its variations is that it does not vary reward and effort equally. In the version of the task that has been most frequently deployed, there are five reward manipulations and only two effort conditions (Figure 3A; Barch et al., 2014). This makes it difficult to interpret how reward and effort processing individually contribute to altered decision making in schizophrenia. Specifically, it is not clear if the deficits in evaluating reward value across different magnitudes also extend to effort. Future work might benefit from utilising task structures which parametrically and symmetrically co-vary reward and effort. Additionally, it would be important to define negative symptom severity using more modern instruments like the brief negative symptom questionnaire (BNSS) (Kirkpatrick et al., 2011). Consistent use of these measures across studies might improve interpretability of associations between negative symptom severity and behavioural parameters.
While the EEfRT task manipulated physical effort, other studies have investigated the effects of cognitive effort on decision making in schizophrenia (Gold et al., 2014; Reddy et al., 2015, 2018; Culbreth et al., 2016, 2020; Chang et al., 2020). These include paradigms where participants chose to perform either a cognitively simple operation for a fixed-low reward, or a more demanding one for variable high rewards (Reddy et al., 2015, 2018). In one example, the two choices were represented by two decks of cards (Figure 3B; Reddy et al., 2018). In the “difficult” deck asked participants were asked to alternate between one of two mental operations (e.g., is the displayed number odd? Or is this number greater than five?), whereas the “easy” deck repeated a single operation across all trials (e.g., is this number greater than five?) (Reddy et al., 2018). Schizophrenia patients accepted far fewer offers in the high effort-high reward condition when compared to controls. However, there was no direct relationship between offers accepted and negative symptoms, although including mediating factors (e.g., dysfunctional beliefs such as: “Why bother, I’ll just fail again”) in the analyses yielded an indirect significant association (Reddy et al., 2018).
Another paradigm that has been used to examine cognitive effort is the “N-back” working memory task, where participants are asked if a displayed letter in a rapid, serial sequence is identical to the one presented “N” steps previously (Figure 3C; Westbrook et al., 2013). For example, the “easy” 1-back condition involves comparing each letter to the preceding one in the sequence and reporting if it is the same or different. On the other hand, the more challenging 4-back condition requires a comparison of each letter with the one shown four steps previously. Typically, participants were given a choice between a 1-back trial at low reward ($1) or a 2–4 back trial for high reward ($2) (Culbreth et al., 2016, 2020; Chang et al., 2020). Critically, the value of the low-reward condition was adjusted based on the previous trial’s response. For example, if the low-reward trial was rejected, in subsequent trials its value was increased stepwise up to an “indifference point” where participants were equally likely to choose either offer. The indifference point is a measure of how effort “discounts” reward across trial types, and was used to compute a per condition subjective value as well as an overall summary measure of value known as the area under the curve (AUC).
Schizophrenia patients discounted rewards more steeply than controls as a function of cognitive effort on the N-back task (Culbreth et al., 2016, 2020; Chang et al., 2020). This effect, as measured by the subjective value (Chang et al., 2020; Culbreth et al., 2020) or AUC (Culbreth et al., 2016), was specifically associated with negative symptom severity (Culbreth et al., 2016, 2020; Chang et al., 2020). However, the nature of these associations is not very clear. For example, in a recent study by Chang et al. (2020) the relationship between behaviour and negative symptoms varied depending on the outcome measure of interest. After taking the sum of anhedonia, asociality, and avolition scores on the BNSS questionnaire, schizophrenia patients were split down the median into amotivation+ and amotivation– sub-groups. When comparing the proportion of high reward-high effort responses, both amotivation+ and amotivation– sub-groups accepted fewer offers compared to healthy volunteers. On the other hand, a comparison of subjective value between groups revealed a significant decrease in this parameter the amotivation+ sub-group compared to controls, but there was no difference between the amotivation+ and amotivation– patients. Finally, there was no association between the AUC measure and negative symptoms in this particular study, which contrasts with findings by other groups using very similar paradigms (Culbreth et al., 2016).
Taken together, these findings suggest that schizophrenia patients are impaired in the “option selection” process of motivated behaviour (Figures 3A–C). Specifically, they forego high reward opportunities when they are coupled with high effort costs. This effect is present regardless of whether the cost is physical (EEfRT) or cognitive (N-back). Investigations utilising the EEfRT task suggest these deficits might arise due to impairments in constructing reward value (Gold et al., 2013; Barch et al., 2014; Reddy et al., 2015; Treadway et al., 2015) while N-back experiments support an additional role for cognitive effort avoidance (Culbreth et al., 2016, 2020; Chang et al., 2020). Both behavioural deficits are associated with negative symptom severity, however, these relationships may be more nuanced than initially proposed. If the value of reward is discounted more easily in patients with negative symptoms, is this because they cannot experience pleasure?
Interaction With Behavioural Goals: “Liking” but Not “Wanting”
It might be argued that schizophrenia patients are less motivated by reward because they experience less pleasure. However, the evidence points to the contrary (Berenbaum and Oltmanns, 1992; Horan et al., 2006; Burbridge and Barch, 2007; Herbener et al., 2007; Barch et al., 2016). In fact, schizophrenia patients frequently report similar levels of pleasure to healthy volunteers in response to positive experiences like consuming a sugary drink or watching a funny video clip (Berenbaum and Oltmanns, 1992; Horan et al., 2006). This phenomenon of preserved hedonic responses has been replicated throughout the literature, and is summarised in a meta-analysis by Cohen and Minor (2010). So why do patients continue to display poor motivation for rewards while enjoying pleasurable activities? One view is that it is not the hedonic response per se that is affected in schizophrenia, but the ability to anticipate future pleasure from self-guided action. In other words patients may not “want” to engage in pleasurable activities despite reporting that they “like” those activities (Gard et al., 2007; Herbener et al., 2007; Edwards et al., 2015; Moran and Kring, 2018).
Early research supporting this proposal involved serial collection of self-ratings from schizophrenia patients and healthy individuals over the course of a week (Gard et al., 2007). Participants reported how much pleasure they derived from current and future activities (e.g., eating dinner). Despite showing similar pleasure ratings while engaged in current activities, they reported significantly less anticipatory pleasure for future actions. Forthcoming, but not current, activity ratings were associated with familial and social functional outcome measures. Other paradigms relied on the presentation of serial images with positive (cupcake), neutral (chair), or negative (snake) associations (Herbener et al., 2007; Edwards et al., 2015; Moran and Kring, 2018).
Subtle methodological variations permitted separate measurements of both consummatory (i.e., current) and anticipatory (i.e., future) pleasure assessments. For example, in one setting a preceding symbol denoted the image category (e.g., +sign before positive image) and ratings were collected at this stage as well as during image presentation (Edwards et al., 2015). In another, images rated as highly pleasant or unpleasant at presentation were then associated with a neutral cue and subsequent ratings were made in response to the neutral cue presentation (Moran and Kring, 2018). In both studies, patients reported similar levels of consummatory but reduced anticipatory pleasure (Edwards et al., 2015; Moran and Kring, 2018). Further, they not only undervalued the pleasantness of future reward (Moran and Kring, 2018), but rated unpleasant images less severely in comparison to healthy participants (Edwards et al., 2015; Moran and Kring, 2018). This dampening of anticipatory pleasure was correlated with negative symptom severity (Moran and Kring, 2018).
Taken together, this evidence suggests that schizophrenia patients are less able to represent reward value when considering future actions–the “wanting” or instrumental aspects of motivated behaviour. Moreover, this is both associated with functional outcomes, as well as negative symptom severity. So why does a patient with negative symptoms lose the ability to represent future reward appropriately? Do they suffer from a general inability to learn about reward? Or might there be specific learning deficits that give rise to this?
Reinforcement Learning of Reward Value
Several investigations have employed reinforcement learning paradigms to further explore the hypothesis that the representation of reward value is impaired in schizophrenia (Figure 3D; Gold et al., 2008, 2012; Strauss et al., 2011; Yilmaz et al., 2012; Moran et al., 2017; Hernaus et al., 2018; Culbreth et al., 2020). In one experiment conducted by Gold et al. (2012), participants were presented with image pairs of different landscapes and asked to choose the “correct” option. In half the trials, choosing the correct answer resulted in monetary gain (vs. no gain if incorrect), and in the other half it prevented monetary loss (vs. a loss if incorrect). Correct responses were reinforced at varying probabilities for each pair (i.e., they were rewarded on 90 or 80% of trials). After learning all possible associations, participants were asked to choose the best option between novel combinations of the original images in a “transfer” phase (Gold et al., 2012). Investigators were particularly interested in comparing the most frequently rewarded option (FR) with the most frequently loss avoidant one (FLA) (Figure 3D).
In the learning phase, schizophrenia patients with high negative symptom severity demonstrated impaired learning in the conditions rewarded with high probability (90%) when compared to controls. However, loss-avoidance learning was intact, suggesting that reward-based learning was specifically affected. When faced with a direct comparison between FR and FLA choices, healthy participants selected the FR option significantly more often than patients with high negative symptoms. In other words, healthy participants and patients without negative symptoms incorporated the expected value of responses in their decisions more frequently than patients with high negative symptoms.
In computational terms, updating decisions based on future expected value was considered to be encoded in a “model-based” process termed Q-learning (Hernaus et al., 2018). One hypothesis is that patients with high negative symptom severity have lower Q-learning rates. Instead, they select options more frequently based on the accumulation of unexpected positive outcomes, or reward prediction errors, regardless of the future expected value. This process is encoded in a “model-free” process known as the actor-critic model (Hernaus et al., 2018). In this scenario, avoiding loss and gaining reward both classify as positive outcomes when compared to their paired alternatives (no gain and loss, respectively). As a result, when directly compared, they are selected indiscriminately despite only one of these outcomes (gaining reward) increasing expected value. In summary, this study proposed that reward processing deficits in schizophrenia are characterised by a reduction in Q-learning and an increase in actor-critic learning.
Subsequently, the same group proposed that Q-learning deficits should be most noticeable when comparing options with the largest (i.e., easiest) discriminations in expected value (Hernaus et al., 2018). To address this possibility, Hernaus et al. (2018) used a similar paradigm to compare responses to image pairs with three different probabilities (90:10, 75:25, and 60:40). As expected, schizophrenia patients accepted less offers than controls at the highest (90:10 and 75:25) probabilities, but not lower probabilities (60:40). Using a logistic regression, the authors were able to confirm that the group differences could be detected as a function of the expected value difference (Hernaus et al., 2018).
Next, they attempted to capture these findings using a computational model that incorporated model-based (Q-learning) and model free (actor-critic) learning. The hypothesis was that the expected value difference could be modelled using a mixing parameter (m) representing the extent of model-based/model-free learning employed by each subject. Further, this parameter should relate to both group differences and possibly negative symptom severity (Hernaus et al., 2018). At the group level, the investigators were able to demonstrate that the m parameter captured the difference in expected value and was lower in schizophrenia patients compared to healthy volunteers. However, there was no association between this parameter and negative symptoms severity. This finding that reward based learning is most impaired at the highest probabilities has been reported across different learning paradigms (Yilmaz et al., 2012; Chang et al., 2016; Moran et al., 2017), but not accounted for with a single model previously.
In view of these results, one surprising finding across studies is that schizophrenia patients demonstrate intact general learning ability (Gold et al., 2012; Moran et al., 2017; Hernaus et al., 2018; Culbreth et al., 2020). That is, they can learn about rewards in general. For example, schizophrenia patients display similar overall accuracy in choices to controls in the transfer phase (Moran et al., 2017; Hernaus et al., 2018; Culbreth et al., 2020) and accurately favour frequently rewarding options over frequent loss ones (Gold et al., 2012). When faced with two relatively positive choices, however, they are less able to determine which response leads the best outcomes.
To sum up, schizophrenia patients appear to suffer impairments in reward-based learning due to a specific deficit in representing expected value. This impairment is specific to reward learning and not loss avoidance (Gold et al., 2012). Nevertheless, there still seems to be a lack of clarity about how exactly these behavioural deficits specifically relate to negative symptoms severity. One possible explanation is that the behavioural deficits related to negative symptoms are mediated by factors including cognitive impairment, dopaminergic treatment, or dysfunctional attitudes. We explore these possibilities briefly in the following section.
Cognitive Dysfunction and Reward Processing
Cognitive dysfunction is a well-documented core phenomenon in schizophrenia (Heinrichs and Zakzanis, 1998; Kahn and Keefe, 2013; Schaefer et al., 2013), but the association with negative symptom questionnaires is complex (Harvey et al., 2006). While questionnaire analyses reveal correlations between negative symptoms and cognitive impairment (O’Leary et al., 2000; Good et al., 2004), these associations are often modest and weakened when adjusting for confounding factors such as functional outcomes (Heydebrand et al., 2004; Bowie et al., 2006; Couture et al., 2011). In the case of EBDM, impaired cognitive function has been shown to be associated with altered decision making for rewards (Heerey et al., 2007, 2008). Notably, a recent re-analysis of several studies using the EEfRT task demonstrated that schizophrenia patients with difficulties in constructing value representations were characterised by greater impairments across measures of cognitive function (Cooper et al., 2019). Hence, reward sensitivity is not only altered by negative symptoms but also by cognitive impairment.
These findings suggest negative symptoms and cognitive dysfunction are, at the very least, highly interdependent and might operate in tandem to regulate motivated behaviour (Robison et al., 2020). A better characterisation of this interaction at the behavioural level would be imperative in uncovering the relationship between negative symptoms, cognitive impairment and EBDM. One emerging hypothesis suggests that altered behaviour associated with negative symptoms and cognitive impairment is mediated by dysfunctional attitudes and/or defeatist beliefs (Grant and Beck, 2009; Horan et al., 2010; Quinlan et al., 2014). So far, two investigations have additionally associated these beliefs to performance on EBDM tasks (Granholm et al., 2016; Reddy et al., 2018).
In one study, patients with high scores on self-defeatist beliefs demonstrated reduced pupillary dilation while recalling increasingly complex numeric sequences (Granholm et al., 2016). Additionally, negative symptom severity positively correlated with self-defeatist beliefs. More recently, Reddy et al. (2018) showed that schizophrenia patients accepted less offers at high reward-high cognitive effort compared to healthy volunteers. While there was no direct association between responses and negative symptoms, when the interaction term between negative symptoms and self-defeatist beliefs was included as a covariate, there was a significant positive association between the number of offers accepted and this interaction term (Reddy et al., 2018). Additionally, cognitive impairment was the strongest predictor of task performance across analyses, but the authors did not explore the interactions between cognitive function, negative symptoms, and dysfunctional beliefs. These preliminary findings suggest that mediating factors like cognition and/or dysfunctional beliefs may play a role in linking EBDM deficits and negative symptoms in schizophrenia. Hypothesis driven mediation analyses in future behavioural work may further clarify these complex associations.
Dopamine and Effort-Based Decision Making
Regulation of the neurotransmitter dopamine is heavily implicated both in EBDM and the pathophysiological changes in schizophrenia (Davis et al., 1991; Salamone et al., 2007; Maia and Frank, 2017). Additionally, most antipsychotic medications used in schizophrenia target dopaminergic D2 receptors (Miyamoto et al., 2005). Several studies have found no associations between antipsychotic dose equivalents and negative symptoms (Gold et al., 2013; Chang et al., 2020). Crucially, they also failed to find a relationship between EBDM deficits and negative symptom severity (Fervaha et al., 2013; Gold et al., 2013; Barch et al., 2014; Chang et al., 2020).
One interpretation of this lack of association is that most investigations do not dissociate between antipsychotic medication based on D2 receptor affinity (Gold et al., 2015). Specifically, Gold et al. (2015) showed in a small sample that patients on first generation antipsychotics were significantly less likely to expend effort for rewards in comparison to patients on second generation medications like clozapine. However, this was based on a small sample size (16 cases on clozapine) and a more sizeable replication of this work is necessary.
A recent analysis of 196 patients demonstrated an inverse association between negative symptom severity and medication dose that was specific for atypical antipsychotic therapy, but this study did not investigate effort-based decisions (Fujimaki et al., 2018). There would be considerable potential value in a conducting well-powered EBDM tasks in patients on different antipsychotic medications. Demonstrating distinct behavioural and questionnaire responses on first vs. second generation anti-dopaminergic drugs might influence both current clinical practice as well as future therapeutic development.
Neural Correlates of Reward Processing in Schizophrenia
The results of neuroimaging studies in healthy people have revealed that reward related processing occurs in distinct areas of the brain (Figure 4; Pessiglione et al., 2018). Typically, these are regions in the medial frontal cortex and basal ganglia: the orbitofrontal cortex (OFC) sometimes referred to as the ventromedial pre-frontal cortex (vmPFC), anterior cingulate cortex (ACC), ventral striatum (VS) including the nucleus accumbens, and the ventral tegmental area (VTA) of the midbrain (Croxson et al., 2009; Bartra et al., 2013; Bonnelle et al., 2016). Tracer studies in animals and tractography analyses in humans have revealed that these areas are interconnected in an extensive network (Haber and Knutson, 2010). Functional neuroimaging studies demonstrate that both the OFC and VS are activated in anticipation of rewards (Croxson et al., 2009; Kroemer et al., 2014), while the ACC encodes subjective value by integrating reward and effort signals (Croxson et al., 2009; Bonnelle et al., 2016). Lesions studies offer additional support for these findings. Specifically, OFC and VS lesions are associated with altered sensitivity to rewards as measured by pupillary responses (Manohar and Husain, 2016). Recently, Manohar and Husain (2016) showed that patients with vmPFC lesion completing a gambling task were less reliant on the expected value of rejected options when compared to controls. Additionally, lesions within the ACC are associated with severe forms of apathy known as akinetic mutism (Barris and Schuman, 1953; Németh et al., 1988), whereas lesions in the basal ganglia, and thalamus can also lead to significant decreases in motivation, although this may improve with dopaminergic therapy (Adam et al., 2013; Blundo and Gerace, 2015). Preclinical studies lend further support to the role of these brain regions in facilitating motivated behaviour, namely through dopaminergic transmission (reviewed in Floresco et al., 2008; Salamone et al., 2018). In food deprived rodents, dopamine depletion within the nucleus accumbens leads to significant reductions in effort exertion during feeding behaviour (Salamone, 1988; Salamone et al., 1991; Koch et al., 2000). Together, this evidence implicates a distinct neural network in the regulation of motivated behaviour, possibly through dopaminergic transmission.
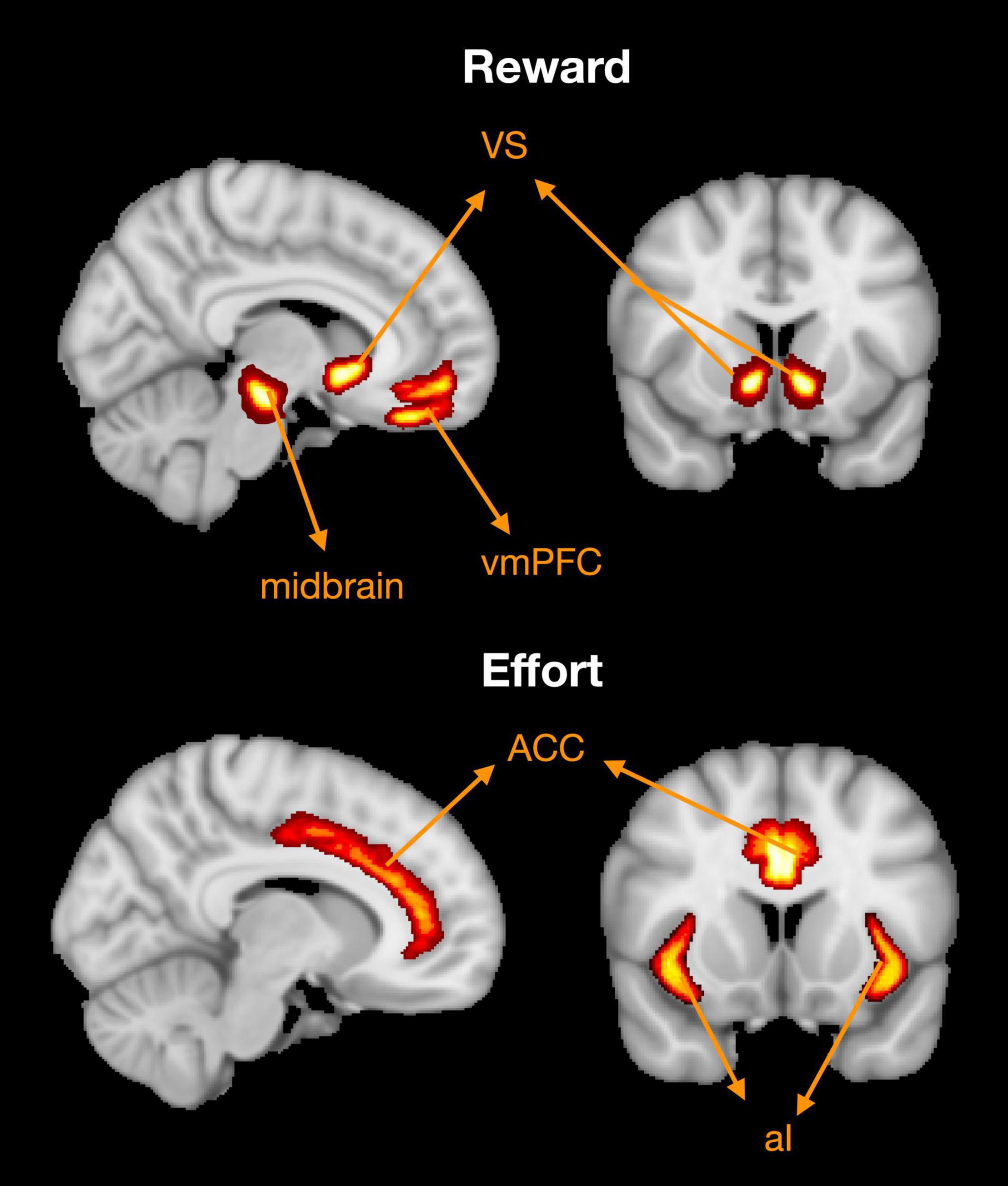
Figure 4. Brain regions associated with reward and effort processing. Based on a meta-analysis of functional MRI studies (Pessiglione et al., 2018). Reward processing associated with activation in the ventromedial pre-frontal cortex (vmPFC), ventral striatum (VS), and ventral tegmental area within the midbrain. Effort processing clusters are principally found in the dorsal anterior cingulate cortex (ACC) and anterior insula (AI). Adapted from Figure 5 in Pessiglione et al. (2018).
Might these brain regions be disrupted in schizophrenia, and what is their association with negative symptom severity? Early evidence from Functional MRI studies suggests both schizophrenia and negative symptom severity are associated with impairments within the fronto-striatal network (Walter et al., 2009; Dowd and Barch, 2012; Arrondo et al., 2015; Subramaniam et al., 2015; Moran et al., 2019; Shukla et al., 2019; Xu et al., 2019). Neural activation, inferred from blood oxygen level dependent (BOLD) signals, was compared between patients and healthy controls while performing various reinforcement learning paradigms (Walter et al., 2009; Dowd and Barch, 2012; Arrondo et al., 2015; Subramaniam et al., 2015). One example involved responding with a key press to either a positive cue (e.g., upwards arrow) or neutral cue (e.g., horizontal arrow). Positive cues were followed by one of two monetary rewards (a pound vs. a penny), whereas neutral cues preceded one of two colours (yellow vs. brown circle). When anticipating rewards, schizophrenia patients showed less activation in several brain regions in comparison to healthy participants (Walter et al., 2009; Dowd and Barch, 2012; Arrondo et al., 2015; Moran et al., 2019). These included the VS (Walter et al., 2009; Arrondo et al., 2015), ACC (Walter et al., 2009; Arrondo et al., 2015; Moran et al., 2019), and OFC (Arrondo et al., 2015). Blood oxygen level dependent signal strength in these regions was found to be negatively associated with MAP symptoms in patients (Moran et al., 2019), although this finding is not consistent (Dowd and Barch, 2012; Arrondo et al., 2015). For example, two earlier experiments demonstrated that reduced VS activation during reward anticipation was only associated with anhedonia, and not other components of the negative syndrome (Dowd and Barch, 2012; Arrondo et al., 2015). Functional connectivity has also been assessed in schizophrenia (Shukla et al., 2019; Xu et al., 2019). This involves voxel-wise correlation of BOLD signal time series between spatially distributed brain areas (Shukla et al., 2019; Xu et al., 2019). The assumption is that regions that show high similarity in BOLD signal are likely to be functionally connected (Woodward and Cascio, 2015). Compared to 139 healthy volunteers, 95 schizophrenia patients showed significant reductions in functional connectivity within the striatum-OFC and striatum-ACC (Shukla et al., 2019). Additionally, the extent of the impairment within the striatum-OFC was dependent on negative symptom severity. This finding was replicated in a cohort study of 84 schizophrenia patients, where VTA-OFC connectivity was negatively associated with social amotivation (Xu et al., 2019). Together, this evidence suggests that negative symptoms in schizophrenia patients are associated with reduced functional activation during reward anticipation as well as altered functional connectivity within the fronto-striatal network.
Clinical apathy in neurological conditions like Parkinson’s disease (Baggio et al., 2015) and cerebrovascular small vessel disease (Hollocks et al., 2015; Saleh et al., 2021) is also associated with structural and functional deficits within the medial forebrain regions and the basal ganglia, including the VS (Hollocks et al., 2015; Le Heron et al., 2018a; Saleh et al., 2021). This suggests that some negative symptoms, like apathy/motivation may have similar underlying neural origins, across different diseases (Husain and Roiser, 2018). Such a view has been recently proposed by Strauss and Cohen (2017) in a transdiagnostic view of negative symptoms.
Summary and Conclusion
Negative symptoms are an important feature of schizophrenia. Several lines of evidence suggest that they have a deleterious impact on functional outcomes, so finding treatments that could ameliorate them are considered an important goal of current research. Our understanding of the “negative syndrome” might be advanced by using well-designed EBDM tasks that probe the different phases of behaviour (Figure 2). To date, these paradigms reveal consistent deficits in motivated decision making when evaluating rewards against physical (EEfRT) or cognitive (N-back) effort (Gold et al., 2013; Barch et al., 2014; Chang et al., 2020; Culbreth et al., 2020). This may be due to one or a combination of reduced reward sensitivity and increased effort avoidance. Deeper exploration of schizophrenia patients’ hedonic responses demonstrates that negative symptoms do not reduce the experience of “liking” pleasure (Barch et al., 2016). Instead, they anticipate less enjoyment from future activities, and might therefore “want” to do less than healthy people.
Assessments using reinforcement learning paradigms suggest that reward processing deficits arise in patients because they put less emphasis on the expected value of decisions (Hernaus et al., 2018). Instead, they are guided by the basic stimulus-response relationships between action and outcome. Computational models which attempt to frame behavioural deficits this way are very appealing, but while they capture overall behaviour they do not currently consistently relate it to negative symptoms (Hernaus et al., 2018). Perhaps such models may overfit the data, and subsequently affect associations with questionnaires. Alternatively, it might be that conventional negative symptom questionnaires do not provide the most robust link to the behavioural observations of interest. One factor complicating interpretation is that different studies are inconsistent in which questionnaires they use.
Another is that the relationship between negative symptoms and reward representation might be mediated by other factors. There is increasing interest in how cognitive impairment, or accompanying dysfunctional beliefs, might bridge the association between negative symptoms and altered effort-based decisions (Reddy et al., 2018; Cooper et al., 2019; Robison et al., 2020). This area may prove valuable in explaining some of the inconsistencies between studies when relating deficits in reward based learning and option selection to negative symptoms. Similarly, there is a gap in the current understanding of how treatment with different dopamine receptor antagonists differentially impacts negative symptoms and EBDM.
Negative symptoms are associated with specific changes within a fronto-striatal network that is closely associated with EBDM (Moran et al., 2019; Shukla et al., 2019; Xu et al., 2019). This is particularly interesting as this network is also associated with clinical apathy in neurological diseases (Hollocks et al., 2015; Saleh et al., 2021). Exploring the neural and behavioural differences between negative symptoms across diseases is an exciting future area of research. An important factor in pursuing transdiagnostic enquiries of negative symptoms is to account for variations in terminology across clinical specialties. For example, negative symptoms include avolition, anhedonia, and asociality under a single rubric (MAP). On the other hand, neurologists and mood disorder specialists often refer to clinical apathy as an independent syndrome which may overlap with anhedonia (Husain and Roiser, 2018). Further, clinical apathy as defined in this manner contains several symptom dimensions (e.g., behavioural, social, and emotional) which overlap with MAP symptoms (Marin et al., 1991; Kirkpatrick et al., 2006; Sockeel et al., 2006; Ang et al., 2017). Behaviourally, apathetic patients with cerebrovascular (Le Heron et al., 2018a; Saleh et al., 2021), and Parkinson’s disease (Le Heron et al., 2018b) display reward processing deficits when performing an EBDM task. Specifically, they accept less monetary offers at low reward compared to their motivated counterparts (Le Heron et al., 2018a,b; Saleh et al., 2021). This is a slightly different behavioural pattern to that demonstrated in schizophrenia patients with negative symptoms, where less offers are accepted at the highest reward levels (Gold et al., 2013, 2015). These subtle differences imply that while both syndromes alter reward processing, their behavioural phenotypes are not identical. Differences may be due to diverging pathological processes at the neural level, specific co-morbidities (e.g., cognitive impairment), or pharmacological interactions (e.g., with anti-dopaminergic medication). Transdiagnostic investigations across motivation disorders will ensure that these differences are better defined and accounted for. Additionally, using questionnaires designed specifically for transdiagnostic purposes can be valuable when comparing findings across patient groups.
One limitation of the conceptual framework discussed here is that it does not address components and contributors of negative symptoms outside of EBDM. Specifically, while we explore negative symptoms at the individual level we do not discuss the environmental contributions to negative symptom severity, which can be significant (van Os et al., 2002; Oshima et al., 2005; Van Os et al., 2010). A recent review poses a theoretical framework of how this can be integrated within individual factors (Strauss, 2021). While our current concepts of negative symptoms remains relatively modest, future approaches might explore the interactions between environmental factors and EBDM at the individual level. A related consideration is whether the expressive symptoms (alogia/blunted affect) of schizophrenia can be similarly quantified using behavioural tasks. While effort-based decisions seem to be more influenced by motivational deficits (anhedonia/avolition/asociality), there is an increasing body of literature using different approaches to quantify both emotional (Trémeau, 2006; Carter et al., 2009; Prochwicz and Rózycka, 2012) and verbal (Cohen et al., 2014) deficits in schizophrenia. These include facial recognition tasks (Carter et al., 2009), as well as speech analysis techniques (Cohen et al., 2020), both of which may offer valuable contributions to our future understanding of negative symptoms (Jeganathan and Breakspear, 2021).
In summary, motivational deficits are an important facet of schizophrenia and its underlying mechanisms has the potential to be advanced through well-designed behavioural and neuroimaging research. A better understanding of how amotivation alters reward-processing and which brain regions are implicated can provide the foundations for future treatment strategies. The transdiagnostic nature of these deficits poses the distinct possibility that future treatments for motivation deficits in schizophrenia might benefit patients with other brain disorders.
Author Contributions
YS conducted the literature review, critically analysed the literature, and drafted the manuscript and figures. IJ-B contributed to the reading and writing of the manuscript. EF-E performed the critical revision and approval of the current version. MH performed the overall design and planning of review, critical revision, and approval of the current version. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Wellcome Trust and the NIHR Oxford Biomedical Research Centre (Grant Code 206330/Z/17/Z).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NIMH, National Institute of Mental Health; EXP, diminished expression factor within the negative syndrome; MAP, motivation and pleasure factor within the negative syndrome; PANSS, positive and negative syndrome scale; SANS, scale for the assessment of negative symptoms; EBDM, effort based decision making; EEfRT, effort expenditure for rewards task; BNSS, brief negative symptom questionnaire; AUC, area under the curve; FR, frequently rewarded; FLA, frequently loss avoidant; OFC/vmPFC, orbitofrontal cortex/ventromedial pre-frontal cortex; ACC, anterior cingulate cortex; VS, ventral striatum; VTA, ventral tegmental area; MRI, magnetic resonance imaging; BOLD, blood level oxygen dependent.
References
Adam, R., Leff, A., Sinha, N., Turner, C., Bays, P., Draganski, B., et al. (2013). Dopamine reverses reward insensitivity in apathy following globus pallidus lesions. Cortex 49, 1292–1303. doi: 10.1016/j.cortex.2012.04.013
Albrecht, M. A., Waltz, J. A., Cavanagh, J. F., Frank, M. J., and Gold, J. M. (2019). Increased conflict-induced slowing, but no differences in conflict-induced positive or negative prediction error learning in patients with schizophrenia. Neuropsychologia 123, 131–140. doi: 10.1016/j.neuropsychologia.2018.04.031
An der Heiden, W., Leber, A., and Häfner, H. (2016). Negative symptoms and their association with depressive symptoms in the long-term course of schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 266, 387–396. doi: 10.1007/s00406-016-0697-2
Andreasen, N. C., Flaum, M., Arndt, S., Alliger, R., and Swayze, V. W. (1991). “Positive and negative symptoms: assessment and validity,” in Negative Versus Positive Schizophrenia, eds A. Marneros, N. C. Andreasen, and M. T. Tsuang (Berlin: Springer), 28–51. doi: 10.1007/978-3-642-76841-5_3
Ang, Y. S., Lockwood, P., Apps, M. A. J., Muhammed, K., and Husain, M. (2017). Distinct subtypes of apathy revealed by the apathy motivation index. PLoS One 12:e0169938. doi: 10.1371/journal.pone.0169938
Arrondo, G., Segarra, N., Metastasio, A., Ziauddeen, H., Spencer, J., Reinders, N. R., et al. (2015). Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front. Psychol. 6:1280. doi: 10.3389/fpsyg.2015.01280
Baggio, H. C., Segura, B., Garrido-Millan, J. L., Marti, M. J., Compta, Y., Valldeoriola, F., et al. (2015). Resting-state frontostriatal functional connectivity in Parkinson’s disease-related apathy. Mov. Disord. 30, 671–679. doi: 10.1002/mds.26137
Barch, D. M., Pagliaccio, D., and Luking, K. (2016). Mechanisms underlying motivational deficits in psychopathology: similarities and differences in depression and schizophrenia. Curr. Top. Behav. Neurosci. 27, 411–449. doi: 10.1007/7854_2015_376
Barch, D. M., Treadway, M. T., and Schoen, N. (2014). Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J. Abnorm. Psychol. 123, 387–397. doi: 10.1037/a0036299
Barris, R. W., and Schuman, H. R. (1953). Bilateral anterior cingulate gyrus lesions: syndrome of the anterior cingulate gyri. Neurology 3, 44–52. doi: 10.1212/wnl.3.1.44
Bartra, O., McGuire, J. T., and Kable, J. W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76, 412–427. doi: 10.1016/j.neuroimage.2013.02.063
Berenbaum, H., and Oltmanns, T. F. (1992). Emotional experience and expression in schizophrenia and depression. J. Abnorm. Psychol. 101, 37–44. doi: 10.1037/0021-843X.101.1.37
Berridge, K. C., and Robinson, T. E. (2003). Parsing reward. Trends Neurosci. 26, 507–513. doi: 10.1016/S0166-2236(03)00233-9
Berrios, G. E. (1985). Positive and negative symptoms and jackson: a conceptual history. Arch. Gen. Psychiatry 42. doi: 10.1001/archpsyc.1985.01790240097011
Berwian, I. M., Wenzel, J. G., Collins, A. G. E., Seifritz, E., Stephan, K. E., Walter, H., et al. (2020). Computational mechanisms of effort and reward decisions in patients with depression and their association with relapse after antidepressant discontinuation. JAMA Psychiatry 77, 513–522. doi: 10.1001/jamapsychiatry.2019.4971
Bleuler, E. (1950). Dementia Praecox or the Group of Schizophrenias, ed. J. Zinkin (New York, NY: International Universities Press).
Blundo, C., and Gerace, C. (2015). Dopamine agonists can improve pure apathy associated with lesions of the prefrontal-basal ganglia functional system. Neurol. Sci. 36, 1197–1201. doi: 10.1007/s10072-014-2061-5
Bonnelle, V., Manohar, S., Behrens, T., and Husain, M. (2016). Individual differences in premotor brain systems underlie behavioral apathy. Cereb. Cortex 26, 807–819. doi: 10.1093/cercor/bhv247
Bowie, C. R., Reichenberg, A., Patterson, T. L., Heaton, R. K., and Harvey, P. D. (2006). Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am. J. Psychiatry 163, 418–425. doi: 10.1176/appi.ajp.163.3.418
Burbridge, J. A., and Barch, D. M. (2007). Anhedonia and the experience of emotion in individuals with schizophrenia. J. Abnorm. Psychol. 116, 30–42. doi: 10.1037/0021-843X.116.1.30
Carter, C. S., Barch, D. M., Gur, R., Gur, R., Pinkham, A., and Ochsner, K. (2009). CNTRICS final task selection: social cognitive and affective neuroscience-based measures. Schizophr. Bull. 35, 153–162. doi: 10.1093/schbul/sbn157
Chang, W. C., Chu, A. O. K., Treadway, M. T., Strauss, G. P., Chan, S. K. W., Lee, E. H. M., et al. (2019). Effort-based decision-making impairment in patients with clinically-stabilized first-episode psychosis and its relationship with amotivation and psychosocial functioning. Eur. Neuropsychopharmacol. 29, 629–642. doi: 10.1016/j.euroneuro.2019.03.006
Chang, W. C., Waltz, J. A., Gold, J. M., Chan, T. C. W., and Chen, E. Y. H. (2016). Mild reinforcement learning deficits in patients with first-episode psychosis. Schizophr. Bull. 42, 1476–1485. doi: 10.1093/schbul/sbw060
Chang, W. C., Westbrook, A., Strauss, G. P., Chu, A. O. K., Chong, C. S. Y., Siu, C. M. W., et al. (2020). Abnormal cognitive effort allocation and its association with amotivation in first-episode psychosis. Psychol. Med. 50, 2599–2609. doi: 10.1017/S0033291719002769
Chase, H. W., Loriemi, P., Wensing, T., Eickhoff, S. B., and Nickl-Jockschat, T. (2018). Meta-analytic evidence for altered mesolimbic responses to reward in schizophrenia. Hum. Brain Mapp. 39, 2917–2928. doi: 10.1002/hbm.24049
Cohen, A. S., Cox, C. R., Le, T. P., Cowan, T., Masucci, M. D., Strauss, G. P., et al. (2020). Using machine learning of computerized vocal expression to measure blunted vocal affect and alogia. NPJ Schizophr. 6:26. doi: 10.1038/s41537-020-00115-2
Cohen, A. S., McGovern, J. E., Dinzeo, T. J., and Covington, M. A. (2014). Speech deficits in serious mental illness: a cognitive resource issue? Schizophr. Res. 160, 173–179. doi: 10.1016/j.schres.2014.10.032
Cohen, A. S., and Minor, K. S. (2010). Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull. 36, 143–150. doi: 10.1093/schbul/sbn061
Cooper, J. A., Barch, D. M., Reddy, L. F., Horan, W. P., Green, M. F., and Treadway, M. T. (2019). Effortful goal-directed behavior in schizophrenia: computational subtypes and associations with cognition. J. Abnorm. Psychol. 128, 710–722. doi: 10.1037/abn0000443
Couture, S. M., Granholm, E. L., and Fish, S. C. (2011). A path model investigation of neurocognition, theory of mind, social competence, negative symptoms and real-world functioning in schizophrenia. Schizophr. Res. 125, 152–160. doi: 10.1016/j.schres.2010.09.020
Croxson, P. L., Walton, M. E., O’Reilly, J. X., Behrens, T. E. J., and Rushworth, M. F. S. (2009). Effort-based Cost-benefit valuation and the human brain. J. Neurosci. 29, 4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009
Culbreth, A., Westbrook, A., and Barch, D. (2016). Negative symptoms are associated with an increased subjective cost of cognitive effort. J. Abnorm. Psychol. 125, 528–536. doi: 10.1037/abn0000153
Culbreth, A. J., Moran, E. K., and Barch, D. M. (2018). Effort-based decision-making in schizophrenia. Curr. Opin. Behav. Sci. 22, 1–6. doi: 10.1016/j.cobeha.2017.12.003
Culbreth, A. J., Moran, E. K., Kandala, S., Westbrook, A., and Barch, D. M. (2020). Effort, avolition, and motivational experience in schizophrenia: analysis of behavioral and neuroimaging data with relationships to daily motivational experience. Clin. Psychol. Sci. 8, 555–568. doi: 10.1177/2167702620901558
Culbreth, A. J., Waltz, J. A., Frank, M. J., and Gold, J. M. (2021). Retention of value representations across time in people with schizophrenia and healthy control subjects. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 6, 420–428. doi: 10.1016/j.bpsc.2020.05.009
Davis, K. L., Kahn, R. S., Ko, G., and Davidson, M. (1991). Dopamine in schizophrenia: a review and reconceptualization. Am. J. Psychiatry 148, 1474–1486. doi: 10.1176/ajp.148.11.1474
Dowd, E. C., and Barch, D. M. (2012). Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS One 7:e0035622. doi: 10.1371/journal.pone.0035622
Edwards, C. J., Cella, M., Tarrier, N., and Wykes, T. (2015). Predicting the future in schizophrenia: the discrepancy between anticipatory and consummatory pleasure. Psychiatry Res. 229, 462–469. doi: 10.1016/j.psychres.2015.05.091
Fervaha, G., Foussias, G., Agid, O., and Remington, G. (2014). Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur. Psychiatry 29, 449–455. doi: 10.1016/j.eurpsy.2014.01.007
Fervaha, G., Graff-Guerrero, A., Zakzanis, K. K., Foussias, G., Agid, O., and Remington, G. (2013). Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J. Psychiatr. Res. 47, 1590–1596. doi: 10.1016/j.jpsychires.2013.08.003
Floresco, S. B., St. Onge, J. R., Ghods-Sharifi, S., and Winstanley, C. A. (2008). Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn. Affect. Behav. Neurosci. 8, 375–389. doi: 10.3758/CABN.8.4.375
Foussias, G., and Remington, G. (2010). Negative symptoms in schizophrenia: avolition and occam’s razor. Schizophr. Bull. 36, 359–369. doi: 10.1093/schbul/sbn094
Fujimaki, K., Toki, S., Yamashita, H., Oyamada, T., and Yamawaki, S. (2018). Predictors of negative symptoms in the chronic phase of schizophrenia: a cross-sectional study. Psychiatry Res. 262, 600–608. doi: 10.1016/j.psychres.2017.09.051
Garcia-Portilla, M. P., Garcia-Alvarez, L., Saiz, P. A., Al-Halabi, S., Bobes-Bascaran, M. T., Bascaran, M. T., et al. (2015). Psychometric evaluation of the negative syndrome of schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 265, 559–566. doi: 10.1007/s00406-015-0595-z
Gard, D. E., Kring, A. M., Gard, M. G., Horan, W. P., and Green, M. F. (2007). Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res. 93, 253–260. doi: 10.1016/j.schres.2007.03.008
Gold, J. M., Kool, W., Botvinick, M. M., Hubzin, L., August, S., and Waltz, J. A. (2014). Cognitive effort avoidance and detection in people with schizophrenia. Cogn. Affect. Behav. Neurosci. 15, 145–154. doi: 10.3758/s13415-014-0308-5
Gold, J. M., Strauss, G. P., Waltz, J. A., Robinson, B. M., Brown, J. K., and Frank, M. J. (2013). Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol. Psychiatry 74, 130–136. doi: 10.1016/j.biopsych.2012.12.022
Gold, J. M., Waltz, J. A., and Frank, M. J. (2015). Effort cost computation in schizophrenia: a commentary on the recent literature. Biol. Psychiatry 78, 747–753. doi: 10.1016/j.biopsych.2015.05.005
Gold, J. M., Waltz, J. A., Matveeva, T. M., Kasanova, Z., Strauss, G. P., Herbener, E. S., et al. (2012). Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch. Gen. Psychiatry 69, 129–138. doi: 10.1001/archgenpsychiatry.2011.1269
Gold, J. M., Waltz, J. A., Prentice, K. J., Morris, S. E., and Heerey, E. A. (2008). Reward processing in schizophrenia: a deficit in the representation of value. Schizophr. Bull. 34, 835–847. doi: 10.1093/schbul/sbn068
Good, K. P., Rabinowitz, J., Whitehorn, D., Harvey, P. D., DeSmedt, G., and Kopala, L. C. (2004). The relationship of neuropsychological test performance with the PANSS in antipsychotic naïve, first-episode psychosis patients. Schizophr. Res. 68, 11–19. doi: 10.1016/j.schres.2003.07.001
Granholm, E., Ruiz, I., Gallegos-Rodriguez, Y., Holden, J., and Link, P. C. (2016). Pupillary responses as a biomarker of diminished effort associated with defeatist attitudes and negative symptoms in schizophrenia. Biol. Psychiatry 80, 581–588. doi: 10.1016/j.biopsych.2015.08.037
Grant, P. M., and Beck, A. T. (2009). Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr. Bull. 35, 798–806. doi: 10.1093/schbul/sbn008
Haber, S. N., and Knutson, B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26. doi: 10.1038/npp.2009.129
Hartmann, M. N., Hager, O. M., Reimann, A. V., Chumbley, J. R., Kirschner, M., Seifritz, E., et al. (2015). Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr. Bull. 41, 503–512. doi: 10.1093/schbul/sbu102
Harvey, P. D., Koren, D., Reichenberg, A., and Bowie, C. R. (2006). Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr. Bull. 32, 250–258. doi: 10.1093/schbul/sbj011
Heerey, E. A., Bell-Warren, K. R., and Gold, J. M. (2008). Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol. Psychiatry 64, 62–69. doi: 10.1016/j.biopsych.2008.02.015
Heerey, E. A., Robinson, B. M., McMahon, R. P., and Gold, J. M. (2007). Delay discounting in schizophrenia. Cogn. Neuropsych. 12, 213–221. doi: 10.1080/13546800601005900
Heinrichs, R. W., and Zakzanis, K. K. (1998). Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12, 426–445. doi: 10.1037/0894-4105.12.3.426
Herbener, E. S., Rosen, C., Khine, T., and Sweeney, J. A. (2007). Failure of positive but not negative emotional valence to enhance memory in schizophrenia. J. Abnorm. Psychol. 116, 43–55. doi: 10.1037/0021-843X.116.1.43
Hernaus, D., Gold, J. M., Waltz, J. A., and Frank, M. J. (2018). Impaired expected value computations coupled with overreliance on stimulus-response learning in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 3, 916–926. doi: 10.1016/j.bpsc.2018.03.014
Heydebrand, G., Weiser, M., Rabinowitz, J., Hoff, A. L., DeLisi, L. E., and Csernansky, J. G. (2004). Correlates of cognitive deficits in first episode schizophrenia. Schizophr. Res. 68, 1–9. doi: 10.1016/S0920-9964(03)00097-5
Hollocks, M. J., Lawrence, A. J., Brookes, R. L., Barrick, T. R., Morris, R. G., Husain, M., et al. (2015). Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain 138, 3803–3815. doi: 10.1093/brain/awv304
Horan, W. P., Felice Reddy, L., Barch, D. M., Buchanan, R. W., Dunayevich, E., Gold, J. M., et al. (2015). Effort-based decision-making paradigms for clinical trials in schizophrenia: Part 2 - external validity and correlates. Schizophr. Bull. 41, 1055–1065. doi: 10.1093/schbul/sbv090
Horan, W. P., Green, M. F., Kring, A. M., and Nuechterlein, K. H. (2006). Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J. Abnorm. Psychol. 115, 496–508. doi: 10.1037/0021-843X.115.3.496
Horan, W. P., Kring, A. M., Gur, R. E., Reise, S. P., and Blanchard, J. J. (2011). Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr. Res. 132, 140–145. doi: 10.1016/j.schres.2011.06.030
Horan, W. P., Rassovsky, Y., Kern, R. S., Lee, J., Wynn, J. K., and Green, M. F. (2010). Further support for the role of dysfunctional attitudes in models of real-world functioning in schizophrenia. J. Psychiatr. Res. 44, 499–505. doi: 10.1016/j.jpsychires.2009.11.001
Husain, M., and Roiser, J. P. (2018). Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat. Rev. Neurosci. 19, 470–484. doi: 10.1038/s41583-018-0029-9
Jeganathan, J., and Breakspear, M. (2021). An active inference perspective on the negative symptoms of schizophrenia. Lancet Psychiatry 366, 732–738. doi: 10.1016/s2215-0366(20)30527-7
Kahn, R. S., and Keefe, R. S. E. (2013). Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry 70, 1107–1112. doi: 10.1001/jamapsychiatry.2013.155
Kimhy, D., Yale, S., Goetz, R. R., McFarr, L. M., and Malaspina, D. (2006). The factorial structure of the schedule for the deficit syndrome in schizophrenia. Schizophr. Bull. 32, 274–278. doi: 10.1093/schbul/sbi064
Kirkpatrick, B., Fenton, W. S., Carpenter, W. T., and Marder, S. R. (2006). The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 32, 214–219. doi: 10.1093/schbul/sbj053
Kirkpatrick, B., Strauss, G. P., Nguyen, L., Fischer, B. A., Daniel, D. G., Cienfuegos, A., et al. (2011). The brief negative symptom scale: psychometric properties. Schizophr. Bull. 37, 300–305. doi: 10.1093/schbul/sbq059
Koch, M., Schmid, A., and Schnitzler, H. U. (2000). Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and pavlovian paradigms of conditioned reward. Psychopharmacology 152, 67–73. doi: 10.1007/s002130000505
Kraepelin, E. (1921). Dementia praecox and paraphrenia. J. Nerv. Ment. Dis. 54:384. doi: 10.1097/00005053-192110000-00104
Kring, A. M., Gur, R. E., Blanchard, J. J., Horan, W. P., and Reise, S. P. (2013). The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am. J. Psychiatry 170, 165–172. doi: 10.1176/appi.ajp.2012.12010109
Kroemer, N. B., Guevara, A., Ciocanea Teodorescu, I., Wuttig, F., Kobiella, A., and Smolka, M. N. (2014). Balancing reward and work: anticipatory brain activation in NAcc and VTA predict effort differentially. Neuroimage 102, 510–519. doi: 10.1016/j.neuroimage.2014.07.060
Kumari, S., Malik, M., Florival, M. C., Manalai, M. P., and Sonje, S. (2017). An assessment of five (PANSS, SAPS, SANS, NSA-16, CGI-SCH) commonly used symptoms rating scales in schizophrenia and comparison to newer scales (CAINS, BNSS). J. Addict. Res. Ther. 08:324. doi: 10.4172/2155-6105.1000324
Le Heron, C., Manohar, S., Plant, O., Muhammed, K., Griffanti, L., Nemeth, A., et al. (2018a). Dysfunctional effort-based decision-making underlies apathy in genetic cerebral small vessel disease. Brain 141, 3193–3210. doi: 10.1093/brain/awy257
Le Heron, C., Plant, O., Manohar, S., Ang, Y. S., Jackson, M., Lennox, G., et al. (2018b). Distinct effects of apathy and dopamine on effort-based decision-making in Parkinson’s disease. Brain 141, 1455–1469. doi: 10.1093/brain/awy110
Maia, T. V., and Frank, M. J. (2017). An Integrative perspective on the role of dopamine in Schizophrenia. Biol. Psychiatry 81, 52–66. doi: 10.1016/j.biopsych.2016.05.021
Mäkinen, J., Miettunen, J., Isohanni, M., and Koponen, H. (2008). Negative symptoms in schizophrenia - a review. Nord. J. Psychiatry 62, 334–341. doi: 10.1080/08039480801959307
Manohar, S. G., and Husain, M. (2016). Human ventromedial prefrontal lesions alter incentivisation by reward. Cortex 76, 104–120. doi: 10.1016/j.cortex.2016.01.005
Marin, R. S., Biedrzycki, R. C., and Firinciogullari, S. (1991). Reliability and validity of the apathy evaluation scale. Psychiatry Res. 38, 143–162. doi: 10.1016/0165-1781(91)90040-V
McCarthy, J. M., Treadway, M. T., Bennett, M. E., and Blanchard, J. J. (2016). Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr. Res. 170, 278–284. doi: 10.1016/j.schres.2015.12.017
Milev, P., Ho, B. C., Arndt, S., and Andreasen, N. C. (2005). Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry 162, 495–506. doi: 10.1176/appi.ajp.162.3.495
Miyamoto, S., Duncan, G. E., Marx, C. E., and Lieberman, J. A. (2005). Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 10, 79–104. doi: 10.1038/sj.mp.4001556
Moran, E. K., Culbreth, A. J., and Barch, D. M. (2017). Ecological momentary assessment of negative symptoms in schizophrenia: relationships to effort-based decision making and reinforcement learning. J. Abnorm. Psychol. 126, 96–105. doi: 10.1037/abn0000240
Moran, E. K., Culbreth, A. J., Kandala, S., and Barch, D. M. (2019). From neuroimaging to daily functioning: a multimethod analysis of reward anticipation in people with schizophrenia. J. Abnorm. Psychol. 128, 723–734. doi: 10.1037/abn0000461
Moran, E. K., and Kring, A. M. (2018). Anticipatory emotion in schizophrenia. Clin. Psychol. Sci. 6, 63–75. doi: 10.1177/2167702617730877
Mortimer, A. M. (2007). Symptom rating scales and outcome in schizophrenia. Br. J. Psychiatry 191, S7–S14. doi: 10.1192/bjp.191.50.s7
Muhammed, K., Manohar, S., Ben Yehuda, M., Chong, T. T. J., Tofaris, G., Lennox, G., et al. (2016). Reward sensitivity deficits modulated by dopamine are associated with apathy in Parkinson’s disease. Brain 139, 2706–2721. doi: 10.1093/brain/aww188
Nakaya, M., and Ohmori, K. (2008). A two-factor structure for the schedule for the deficit syndrome in schizophrenia. Psychiatry Res. 158, 256–259. doi: 10.1016/j.psychres.2007.10.008
Németh, G., Hegedüs, K., and Molnâr, L. (1988). Akinetic mutism associated with bicingular lesions: clinicopathological and functional anatomical correlates. Eur. Arch. Psychiatry Neurol. Sci. 237, 218–222. doi: 10.1007/BF00449910
O’Leary, D. S., Flaum, M., Kesler, M. L., Flashman, L. A., Arndt, S., and Andreasen, N. C. (2000). Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. J. Neuropsychiatry Clin. Neurosci. 12, 4–15. doi: 10.1176/jnp.12.1.4
Oshima, I., Mino, Y., and Inomata, Y. (2005). Effects of environmental deprivation on negative symptoms of schizophrenia: a nationwide survey in Japan’s psychiatric hospitals. Psychiatry Res. 136, 163–171. doi: 10.1016/j.psychres.2005.06.001
Peralta, V., and Cuesta, M. J. (1995). Negative symptoms in schizophrenia: a confirmatory factor analysis of competing models. Am. J. Psychiatry 152, 1450–1457. doi: 10.1176/ajp.152.10.1450
Pessiglione, M., Vinckier, F., Bouret, S., Daunizeau, J., and Le Bouc, R. (2018). Why not try harder? Computational approach to motivation deficits in neuro-psychiatric diseases. Brain 141, 629–650. doi: 10.1093/brain/awx278
Piskulic, D., Addington, J., Cadenhead, K. S., Cannon, T. D., Cornblatt, B. A., Heinssen, R., et al. (2012). Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 196, 220–224. doi: 10.1016/j.psychres.2012.02.018
Prochwicz, K., and Rózycka, J. (2012). Rozpoznawanie emocjonalnej ekspresji mimicznej przez osoby chore na schizofreniȩ. Psychiatr. Pol. 46, 1073–1088.
Quinlan, T., Roesch, S., and Granholm, E. (2014). The role of dysfunctional attitudes in models of negative symptoms and functioning in schizophrenia. Schizophr. Res. 157, 182–189. doi: 10.1016/j.schres.2014.05.025
Rabinowitz, J., Berardo, C. G., Bugarski-Kirola, D., and Marder, S. (2013). Association of prominent positive and prominent negative symptoms and functional health, well-being, healthcare-related quality of life and family burden: a CATIE analysis. Schizophr. Res. 150, 339–342. doi: 10.1016/j.schres.2013.07.014
Rabinowitz, J., Levine, S. Z., Garibaldi, G., Bugarski-Kirola, D., Berardo, C. G., and Kapur, S. (2012). Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr. Res. 137, 147–150. doi: 10.1016/j.schres.2012.01.015
Reddy, L. F., Horan, W. P., Barch, D. M., Buchanan, R. W., Dunayevich, E., Gold, J. M., et al. (2015). Effort-based decision-making paradigms for clinical trials in schizophrenia: Part 1 - psychometric characteristics of 5 paradigms. Schizophr. Bull. 41, 1045–1054. doi: 10.1093/schbul/sbv089
Reddy, L. F., Horan, W. P., Barch, D. M., Buchanan, R. W., Gold, J. M., Marder, S. R., et al. (2018). Understanding the association between negative symptoms and performance on effort-based decision-making tasks: the importance of defeatist performance beliefs. Schizophr. Bull. 44, 1217–1226. doi: 10.1093/schbul/sbx156
Robison, A. J., Thakkar, K. N., and Diwadkar, V. A. (2020). Cognition and reward circuits in schizophrenia: synergistic, not separate. Biol. Psychiatry 87, 204–214. doi: 10.1016/j.biopsych.2019.09.021
Salamone, J. D. (1988). Dopaminergic involvement in activational aspects of motivation: effects of haloperidol on schedule-induced activity, feeding, and foraging in rats. Psychobiology 16, 196–206. doi: 10.3758/BF03327307
Salamone, J. D., and Correa, M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76, 470–485. doi: 10.1016/j.neuron.2012.10.021
Salamone, J. D., Correa, M., Farrar, A., and Mingote, S. M. (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191, 461–482. doi: 10.1007/s00213-006-0668-9
Salamone, J. D., Correa, M., Yang, J. H., Rotolo, R., and Presby, R. (2018). Dopamine, effort-based choice, and behavioral economics: basic and translational research. Front. Behav. Neurosci. 12:52. doi: 10.3389/fnbeh.2018.00052
Salamone, J. D., Steinpreis, R. E., McCullough, L. D., Smith, P., Grebel, D., and Mahan, K. (1991). Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology 104, 515–521. doi: 10.1007/BF02245659
Saleh, Y., Le Heron, C., Petitet, P., Veldsman, M., Drew, D., Plant, O., et al. (2021). Apathy in small vessel cerebrovascular disease is associated with deficits in effort-based decision making. Brain 144, 1247–1262. doi: 10.1093/brain/awab013
Sayers, S. L., Curran, P. J., and Mueser, K. T. (1996). Factor structure and construct validity of the scale for the assessment of negative symptoms. Psychol. Assess. 8, 269–280. doi: 10.1037/1040-3590.8.3.269
Schaefer, J., Giangrande, E., Weinberger, D. R., and Dickinson, D. (2013). The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr. Res. 150, 42–50. doi: 10.1016/j.schres.2013.07.009
Shukla, D. K., Chiappelli, J. J., Sampath, H., Kochunov, P., Hare, S. M., Wisner, K., et al. (2019). Aberrant frontostriatal connectivity in negative symptoms of schizophrenia. Schizophr. Bull. 45, 1051–1059. doi: 10.1093/schbul/sby165
Sockeel, P., Dujardin, K., Devos, D., Denève, C., Destée, A., and Defebvre, L. (2006). The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: validation in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 77, 579–584. doi: 10.1136/jnnp.2005.075929
Strauss, G. P. (2021). A bioecosystem theory of negative symptoms in schizophrenia. Front. Psychiatry 12:655471. doi: 10.3389/fpsyt.2021.655471
Strauss, G. P., Ahmed, A. O., Young, J. W., and Kirkpatrick, B. (2019a). Reconsidering the latent structure of negative symptoms in schizophrenia: a review of evidence supporting the 5 consensus domains. Schizophr. Bull. 45, 725–729. doi: 10.1093/schbul/sby169
Strauss, G. P., Esfahlani, F. Z., Galderisi, S., Mucci, A., Rossi, A., Bucci, P., et al. (2019b). Network analysis reveals the latent structure of negative symptoms in schizophrenia. Schizophr. Bull. 45, 1033–1041. doi: 10.1093/schbul/sby133
Strauss, G. P., Esfahlani, F. Z., Kirkpatrick, B., Allen, D. N., Gold, J. M., Visser, K. F., et al. (2019c). Network analysis reveals which negative symptom domains are most central in schizophrenia vs bipolar disorder. Schizophr. Bull. 45, 1319–1330. doi: 10.1093/schbul/sby168
Strauss, G. P., Bartolomeo, L. A., and Harvey, P. D. (2021). Avolition as the core negative symptom in schizophrenia: relevance to pharmacological treatment development. NPJ Schizophr. 7:16. doi: 10.1038/s41537-021-00145-4
Strauss, G. P., and Cohen, A. S. (2017). A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr. Bull. 43, 712–729. doi: 10.1093/schbul/sbx066
Strauss, G. P., Frank, M. J., Waltz, J. A., Kasanova, Z., Herbener, E. S., and Gold, J. M. (2011). Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol. Psychiatry 69, 424–431. doi: 10.1016/j.biopsych.2010.10.015
Strauss, G. P., Nuñez, A., Ahmed, A. O., Barchard, K. A., Granholm, E., Kirkpatrick, B., et al. (2018). The latent structure of negative symptoms in schizophrenia. JAMA Psychiatry 75:1303. doi: 10.1001/jamapsychiatry.2018.2475
Strauss, G. P., Sandt, A. R., Catalano, L. T., and Allen, D. N. (2012). Negative symptoms and depression predict lower psychological well-being in individuals with schizophrenia. Compr. Psychiatry 53, 1137–1144. doi: 10.1016/j.comppsych.2012.05.009
Strauss, J. S., Carpenter, W. T., and Bartko, J. J. (1974). The diagnosis and understanding of schizophrenia. Part III. speculations on the processes that underlie schizophrenic symptoms and signs. Schizophr. Bull. 1, 61–69. doi: 10.1093/schbul/1.11.61
Subramaniam, K., Hooker, C. I., Biagianti, B., Fisher, M., Nagarajan, S., and Vinogradov, S. (2015). Neural signal during immediate reward anticipation in schizophrenia: relationship to real-world motivation and function. Neuroimage Clin. 9, 153–163. doi: 10.1016/j.nicl.2015.08.001
Tandon, R., Gaebel, W., Barch, D. M., Bustillo, J., Gur, R. E., Heckers, S., et al. (2013). Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 150, 3–10. doi: 10.1016/j.schres.2013.05.028
Thomsen, K. R., Whybrow, P. C., and Kringelbach, M. L. (2015). Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Front. Behav. Neurosci. 9:49. doi: 10.3389/fnbeh.2015.00049
Treadway, M. T., Buckholtz, J. W., Schwartzman, A. N., Lambert, W. E., and Zald, D. H. (2009). Worth the “EEfRT”? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One 4:e6598. doi: 10.1371/journal.pone.0006598
Treadway, M. T., Peterman, J. S., Zald, D. H., and Park, S. (2015). Impaired effort allocation in patients with schizophrenia. Schizophr. Res. 161, 382–385. doi: 10.1016/j.schres.2014.11.024
Trémeau, F. (2006). A review of emotion deficits in schizophrenia. Dialog. Clin. Neurosci. 8, 59–70. doi: 10.31887/dcns.2006.8.1/ftremeau
van Os, J., Hanssen, M., de Graaf, R., and Vollebergh, W. (2002). Does the urban environment independently increase the risk for both negative and positive features of psychosis? Soc. Psychiatry Psychiatr. Epidemiol. 37, 460–464. doi: 10.1007/s00127-002-0588-x
Van Os, J., Kenis, G., and Rutten, B. P. F. (2010). The environment and schizophrenia. Nature 468, 203–212. doi: 10.1038/nature09563
Walter, H., Kammerer, H., Frasch, K., Spitzer, M., and Abler, B. (2009). Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology 206, 121–132. doi: 10.1007/s00213-009-1586-4
Westbrook, A., Kester, D., and Braver, T. S. (2013). What is the subjective cost of cognitive effort? load, trait, and aging effects revealed by economic preference. PLoS One 8:e68210. doi: 10.1371/journal.pone.0068210
Woodward, N. D., and Cascio, C. J. (2015). Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry 72, 743–744. doi: 10.1001/jamapsychiatry.2015.0484
Xu, P., Klaasen, N. G., Opmeer, E. M., Pijnenborg, G. H. M., van Tol, M. J., Liemburg, E. J., et al. (2019). Intrinsic mesocorticolimbic connectivity is negatively associated with social amotivation in people with schizophrenia. Schizophr. Res. 208, 353–359. doi: 10.1016/j.schres.2019.01.023
Keywords: effort-based decision making, motivated action selection, apathy, schizophrenia, negative symptom, reward based learning
Citation: Saleh Y, Jarratt-Barnham I, Fernandez-Egea E and Husain M (2021) Mechanisms Underlying Motivational Dysfunction in Schizophrenia. Front. Behav. Neurosci. 15:709753. doi: 10.3389/fnbeh.2021.709753
Received: 14 May 2021; Accepted: 20 August 2021;
Published: 10 September 2021.
Edited by:
David Zald, Rutgers, The State University of New Jersey - Busch Campus, United StatesReviewed by:
Styliani (Stella) Vlachou, Dublin City University, IrelandYvan M. Vachez, Washington University School of Medicine in St. Louis, United States
Copyright © 2021 Saleh, Jarratt-Barnham, Fernandez-Egea and Husain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youssuf Saleh, eW91c3N1Zi5zYWxlaEBuZGNuLm94LmFjLnVr
 Youssuf Saleh
Youssuf Saleh Isaac Jarratt-Barnham
Isaac Jarratt-Barnham Emilio Fernandez-Egea
Emilio Fernandez-Egea Masud Husain1
Masud Husain1