- 12nd Department of Psychiatry, School of Medicine, National and Kapodistrian University of Athens, University General Hospital “ATTIKON”, Athens, Greece
- 2Laboratory of Cognitive Neuroscience and Sensorimotor Control, University Mental Health, Neurosciences and Precision Medicine Research Institute “COSTAS STEFANIS”, Athens, Greece
- 3Department of Child and Adolescent Psychiatry, Medical Faculty, University of Freiburg, Freiburg, Germany
- 4Department of Child and Adolescent Psychiatry, Medical Faculty, University of Cologne, Cologne, Germany
In recent years, psychiatric research has focused on the evaluation and implementation of biomarkers in the clinical praxis. Oculomotor function deviances are among the most consistent and replicable cognitive deficits in schizophrenia and have been suggested as viable candidates for biomarkers. In this narrative review, we focus on oculomotor function in first-episode psychosis, recent onset schizophrenia as well as individuals at high risk for developing psychosis. We critically discuss the evidence for the possible utilization of oculomotor function measures as diagnostic, susceptibility, predictive, monitoring, and prognostic biomarkers for these conditions. Based on the current state of research we conclude that there are not sufficient data to unequivocally support the use of oculomotor function measures as biomarkers in schizophrenia.
Introduction
Oculomotor paradigms have been extensively used over the last 50 years in the realm of psychiatric research, in order to assess the integrity of cognitive functions (Gooding and Tallent, 2001; Hutton, 2008; Canu et al., 2021) and evaluate pharmacological intervention (Karpouzian et al., 2019). Performance of these tasks provides a variety of parameters that can be objectively and quantitatively measured (Alexander and Martinez-Conde, 2019; Foulsham, 2019; Lencer et al., 2019; Pierce et al., 2019). Impairments in oculomotor tasks constitute some of the most replicated findings in studies of psychiatric disorders and especially in those of schizophrenia (Levy et al., 1993, 2000; Smyrnis et al., 2019). The expectation behind the use of oculomotor tasks is to associate specific patterns of performance abnormalities with particular clinical syndromes in order to provide tools for the diagnosis, prognosis, and assessment of the response to treatment (Smyrnis, 2008). This research then aspires to come up with reliable oculomotor biomarkers for psychiatric disorders to be used in clinical practice (Smyrnis et al., 2019).
One definition of a biological marker or biomarker in medicine (Califf, 2018) is the following: “a biomarker is a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention”. This broad definition can be derived from molecular, histologic, radiographic, imaging, or physiologic characteristics. A number of different categories of biomarkers have been defined according to their assumed applications (Table 1).
Firstly, diagnostic biomarkers characterize the detection of the presence of a disease or condition of interest, or the identification of an individual with a subtype of the disease or condition (Califf, 2018). Monitoring biomarkers are used to determine the status of a medical condition, for evidence of exposure to a medical product or environmental agent, or to discern an effect of a medical product or biological agent used as treatment (Califf, 2018). A predictive biomarker is defined by the fact that the presence of the biomarker or change in the biomarker levels predicts that an individual or group of individuals is more likely to experience a favorable or unfavorable effect from the subjection to medical intervention or environmental agent (Califf, 2018). A prognostic biomarker is used to identify the likelihood of a clinical event, disease occurrence, or disease progression. It is crucial to distinguish prognostic biomarkers from susceptibility/risk biomarkers, which predict the transition from a healthy state to a disease state (Califf, 2018). Also, susceptibility/risk biomarkers are differentiated from predictive biomarkers, which, as mentioned before, pinpoint factors associated with the effect of intervention or exposure.
At this point, a note should be made on a similar yet distinct concept, that of the endophenotype. Both the terms biomarker and endophenotype have been used interchangeably in the psychiatric research literature (Prata et al., 2014). Endophenotypes reflect a linkage between a clinical phenotype and a specific genotype and a number of criteria have been proposed for their identification (for a review, see Gottesman and Gould, 2003).
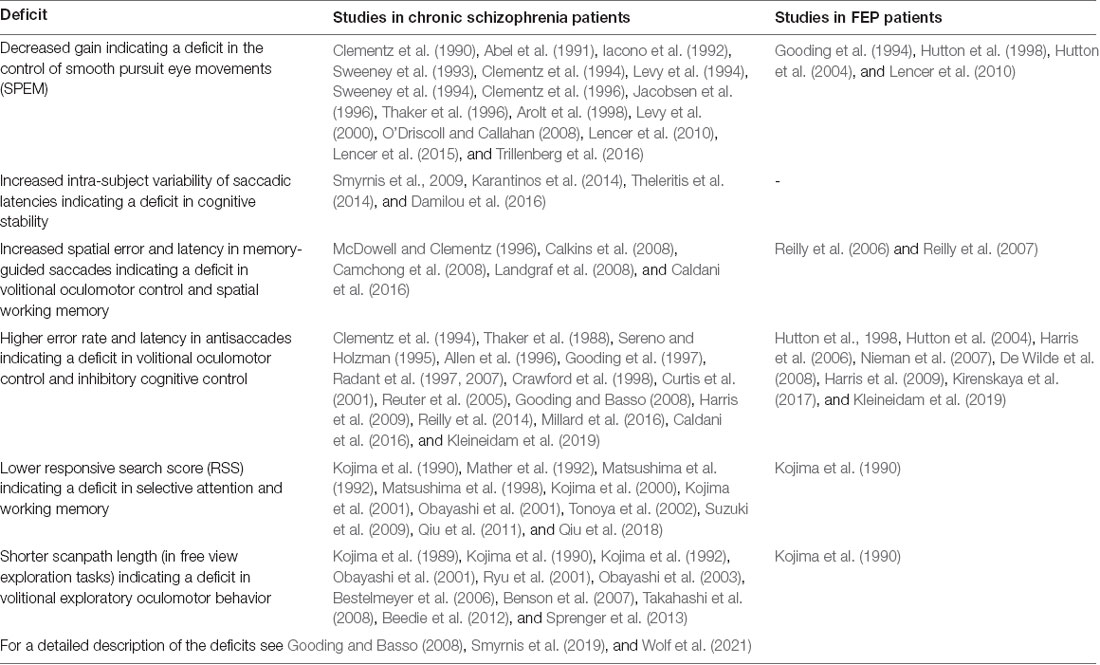
Biomarkers have proven to be valuable tools in the clinical praxis of many medical specialties and are currently the focus of intensive research in medicine. However, for a number of reasons (for a critique, see Venkatasubramanian and Keshavan, 2016) their usage remains absent from the field of psychiatry. One of these reasons is the aetiological “blindness” of the current classification systems of psychiatric disorders, meaning that the underlying pathophysiology of psychiatric syndromes is not taken into account when defining nosological groups. This, in turn, leads to a situation in which one and the same nosological group is likely to be aetiologically heterogeneous while different nosological groups show aetiological overlap (Lee et al., 2019). This leads to a lack of diagnostic sensitivity and specificity of biomarkers, as will be explained below. The research on schizophrenia and oculomotor variables could provide a solution to this conundrum as there is a large body of work indicating robust oculomotor function deviances in schizophrenia (Table 2). The focus, in particular, should be towards the first presentation of the disorder, known as first episode psychosis (FEP), recent-onset schizophrenia, and also individuals at Ultra-High Risk (UHR) of schizophrenia. The ability to diagnose patients better in terms of underlying pathophysiological mechanisms, predict the conversion to psychosis risk for UHR individuals, or anticipate treatment effects in this group would be invaluable as they would set the course for a proper monitoring and therapeutic plan as early as possible in the disease process.
In the following, text we review the evidence for biomarker status for each one of the categories of biomarkers for oculomotor function indices in schizophrenia. We focus this narrative review only to early schizophrenia and in particular FEP and patients up to 2 years after onset (recent-onset). FEP is defined by three alternative definitions: (i) first treatment contact; (ii) duration of antipsychotic medication use; and (iii) duration of psychosis, with methodological limitations for each of these definitions (Breitborde et al., 2009). We also include studies investigating high-risk individuals. The At-Risk Mental State [also termed as the Clinical High-Risk state for psychosis (CHR-P), a synonym to UHR (Fusar-Poli et al., 2013)] is a prodromal phase of schizophrenia characterized by cognitive impairments, mood alterations, anxiety, attenuated psychotic symptoms and a decline in social and occupational functioning (Kahn et al., 2015). The literature search was conducted in the PubMed and Scopus databases. The studies that were taken into consideration were those that included FEP patients, patients with schizophrenia and duration up to 2 years and UHR individuals. Studies that included only chronic schizophrenia patients, patients with illness duration more than 3 years or did not mention illness duration at all were excluded. Key words for our search included the following: schizophrenia and oculomotor, schizophrenia and “eye movement”, schizophrenia and eye and biomarker, “first episode schizophrenia” and “eye movement”, “first episode schizophrenia” and biomarker, “recent onset schizophrenia” and “eye movement”.
Diagnostic Biomarkers
Patients at the time of FEP exhibit a variety of deviances in oculomotor tasks when compared with healthy controls. The most robust and replicable finding is the elevated error rate in the antisaccade task (Hutton et al., 1998, 2004; Nieman et al., 2000, 2007; Harris et al., 2006; De Wilde et al., 2008; Harris et al., 2009; Kirenskaya et al., 2017; Kleineidam et al., 2019). Simultaneously, in the same task, an increased latency of the correct antisaccades has been found (Hutton et al., 2002; Harris et al., 2009; Kleineidam et al., 2019). In Smooth Pursuit Eye Movements (SPEM) patients show higher Root Mean Square Error (RMSE) values (Iacono et al., 1992), lower maintenance gain (both in the open-loop and the closed-loop conditions of pursuit), and more frequent catch-up saccades (Gooding et al., 1994; Hutton et al., 1998, 2004; Lencer et al., 2010). Studies involving memory-guided saccades have shown impairment in accuracy of remembered spatial locations (Reilly et al., 2006, 2007). Finally, prosaccade latencies have been found to be reduced in antipsychotic-naïve FEP patients in some studies (Reilly et al., 2005, 2006, 2007; Hill et al., 2008; Krebs et al., 2010), while unimpaired in others (Harris et al., 2009; see Smyrnis et al., 2019, for a review).
The usage of such deficits as diagnostic biomarkers has been repeatedly proposed (Lencer et al., 2015). However, two very important problems preclude the use of these indices as diagnostic biomarkers (Smyrnis et al., 2019). Firstly, the deficits in patients in such tasks are found on a group level and hardly ever hold for all members of the group, leading to sub-optimal diagnostic sensitivity. A possible compensatory approach could be the usage of scores arising from a combination of multiple variables (Morita et al., 2019). This was demonstrated impressively by Benson et al. (2012) who reached a 98.3% classification accuracy for schizophrenia vs. healthy controls on the basis of a brief assessment of fixation, smooth pursuit, and free viewing eye movements. Secondly, the other important requirement for a diagnostic biomarker, especially for early detection, is a high diagnostic specificity for the disease in question. Initial studies using qualitative measures of SPEM performance showed that deficits, although consistent, were not specific to schizophrenia, but were also detected in patients with major affective disorders such as bipolar disorder and major depression (Levy et al., 1993; Sweeney et al., 1994). Subsequently, studies using quantitative measures of pursuit performance such as closed-loop gain clarified that SPEM deficit is also prominent in patients with affective disorders (Abel et al., 1991; Kathmann et al., 2003; Brakemeier et al., 2019). Other studies employing the step-ramp pursuit task reported closed-loop and initial open-loop pursuit deficits both in schizophrenia and affective disorders (Sweeney et al., 1998, 1999; Lencer et al., 2004, 2010, 2015) while open-loop deficits were simultaneously found in schizophrenia and bipolar disorder patients (Trillenberg et al., 2016). Concerning the antisaccade task, studies with large sample sizes reported that patients with major depression (especially severe depression with psychotic characteristics) as well as patients with bipolar disorder (again, especially those with psychotic characteristics) also have elevated error rates compared to healthy controls (Tien et al., 1996; Sweeney et al., 1998; Gooding and Tallent, 2001; Martin et al., 2007; Harris et al., 2009). In the study of Harris et al. (2009), from a group of depression patients with and without psychotic features, it was shown that those with the psychotic features showed similar deficits as schizophrenic patients and patients with bipolar disorder. Reilly et al. (2014) compared the antisaccade error rate between groups of schizophrenia patients, schizoaffective patients, psychotic bipolar patients, and healthy controls. All patient’ groups presented with elevated error rates, and the schizophrenia patients also differed significantly from both schizoaffective and psychotic bipolar patients. In a study by Grootens et al. (2008), individuals with borderline personality disorder were included. It was shown that they produced more inhibition errors when compared with healthy controls, an increase which was more prominent in the individuals who also presented psychotic symptoms. The aforementioned studies have built a strong argument about increased antisaccade error rate being a trait of psychotic state, in and out of the context of schizophrenia (Gooding and Basso, 2008; Smyrnis et al., 2019). The genetic overlap between schizophrenia and bipolar disorder, but also between these and depression (Lee et al., 2019) may be involved in lowering the diagnostic specificity of the above oculomotor biomarkers.
Many studies have also reported elevated antisaccade error rates with (Lennertz et al., 2012) or without (Tien et al., 1992; Rosenberg et al., 1997) elevated antisaccade latency in patients with Obsessive-Compulsive Disorder (OCD). Damilou et al. (2016) compared the antisaccade task performance between OCD patients and schizophrenic patients and showed that both groups have similar increments in antisaccade error rate and antisaccade latency. Their only difference was in the variability of error prosaccade latencies, which were found increased only for the schizophrenia patients. This evidence indicates that saccadic eye movement abnormalities as measured with different variables are not restricted to patients with schizophrenia (Morita et al., 2019; Smyrnis et al., 2019) but are also observed in patients with other psychotic disorders and other psychiatric conditions such as OCD.
Susceptibility Biomarkers
Susceptibility biomarkers are used to quantify the risk of transitioning from prodromal state to disease. Studies of UHR subjects could shed some light on the potential use of oculomotor functions as such. However, until now these studies have been very few and their findings are far from conclusive. van Tricht et al. (2010) showed that UHR participants exhibited higher rates of corrective and non-corrective saccades during a SPEM task. Caldani et al. (2017) also reported an increased number of intrusive saccades but only in their UHR subgroup with elevated neurological soft signs score. Nieman et al. (2007) reported a higher antisaccade error rate in UHR subjects as well as a trend towards comparatively higher baseline antisaccade error rates for those UHR participants who eventually transitioned into schizophrenia. Obyedkov et al. (2019) also reported an elevated antisaccade error rate in UHR. In contrast, Caldani et al. (2016) failed to find such a difference. Caldani et al. (2016) also used a memory-guided saccade task and reported an elevated error rate both for UHR and for biological healthy siblings of schizophrenia patients. In the study of Kleineidam et al. (2019), individuals at-risk mental state defined by cognitive basic symptoms as early (E-ARMS) or late at-risk mental state (L-ARMS) and patients with FEP, were studied using the prosaccade and antisaccade paradigm. L-ARMS but not E-ARMS participants exhibited elevated antisaccade latencies compared to controls. It was also reported that none of the variables analyzed (prosaccade and antisaccade latencies, antisaccade error rate, and correction rate) were predictive of conversion to psychosis within 2 years. Clearly, more studies with longitudinal design are needed in this field of research. Furthermore, the possibility that oculomotor variables could be used as predictors not only of conversion to psychosis but also of stability or de-escalation from the UHR status should also be considered.
Monitoring/Predictive Biomarkers
Monitoring and predictive biomarkers can be used for monitoring the effects of treatment and predicting treatment outcomes. A few studies have used oculomotor paradigms in unmedicated or drug-naïve FEP. Hutton et al. (2001) examined whether smooth pursuit deficits in schizophrenia were comparable between first-episode and chronic schizophrenia patients who differed in their lifetime antipsychotic treatment administration and their antipsychotic treatment status at the time of testing. The chronic schizophrenia patients showed an elevated impairment in pursuit velocity gain compared to the FEP patients, and the degree of this effect was interceded by the effects of long–term antipsychotic treatment. Smooth pursuit velocity gain was found to be significantly better in chronic patients who were drug-free from antipsychotics for at least 6 months compared to those who did not stop their administration (Hutton et al., 2001).
The effect of medication in acute psychosis has been investigated by Hill et al. (2008), who found reduced latency of prosaccades in drug-naïve patients with FEP that was no longer present after 6 weeks of risperidone treatment. On the other hand, Keedy et al. (2014) report no effect of medication on prosaccade latency. With regard to the antisaccade paradigm, antipsychotic treatment (Hutton et al., 1998; Müller et al., 1999; Kallimani et al., 2009) and chronicity of the disease (Crawford et al., 1998; Curtis et al., 2001; Gooding et al., 2004) seem to have no impact on the core impairments. Increased frequency of antisaccade errors was present in FEP patients medicated at the time of testing (Hutton et al., 2002, 2004; Kleineidam et al., 2019) and treatment-naïve FEP patients (Harris et al., 2009). In one study, medicated and drug-naive FEP patients showed higher antisaccade error rates, but only drug- naïve patients also had an increased latency in initiating correct antisaccades (Hutton et al., 1998). Harris et al. (2006) reported a decrease in antisaccade error rates and latencies of correct antisaccades after treatment with haloperidol or risperidone. Studies comparing antisaccades early in the course of illness, prior and after treatment with atypical antipsychotics (Harris et al., 2006; Hill et al., 2008), noticed faster initiation of correct antisaccades but reported conflicting results on the error rate.
Some of the studies reported before, propose that selected oculomotor variables could be used as predictive/monitoring biomarkers. However, these studies examine only the medication effects on the oculomotor variables but not the overall relationship between treatment effects, changes in the oculomotor predictor variable, and changes in the symptomatology of the patient. To our knowledge, there are only four studies that have tried to explore this relationship. Gooding et al. (1994) studied FEP patients using a SPEM task and Root Mean Square Error (RMSE) as a measure of their performance. They reported that in their follow-up, after approximately 9.5 months, RMSE remained stable despite medication and clinical improvement. Obayashi et al. (2001) re-evaluated medicated schizophrenia patients (a mix of 24 FEP and 4 s-episode patients) after an average of 8 months in an exploratory eye movement paradigm. Dividing the patients into an improved and an unchanged group (based on their clinical improvement) they reported that exploratory eye movement variables [which included the number of eye fixations, mean eye scanning length (MESL), and RSS] did not change despite the improvement in clinical symptoms of the patients. Finally, Reilly et al. (2007) and Hill et al. (2008) failed to report any relationship between symptom change and oculomotor variables (including measures of visually guided saccades, antisaccades, and memory-guided saccades) change. They raised, however, an interesting point about utilizing those variables as monitoring agents for the cognitive adverse effects of antipsychotic treatment.
Prognostic Biomarkers
It is well known that cognitive function deficits present in FEP and early schizophrenia are the most important predictors of the final outcome of the disorder (Díaz-Caneja et al., 2015; Suvisaari et al., 2018). Thus, the use of oculomotor function indices as prognostic biomarkers predicting the functional outcome of psychosis is probably the most promising area for the application of these measures in clinical practice. Nevertheless, only a few studies have reported such potential effects. RSS, of exploratory eye movement tasks, has been correlated both with negative symptoms (NS) in schizophrenia (blunted affect, emotional withdrawal; Kojima et al., 1990, 1992) as well as with hallucination severity (Qiu et al., 2018). Gaebel et al. (1987) reported a correlation between increased values of fixation parameters (mean duration of a single fixation as well as coefficient of variation) and negative symptoms. Increased RMSE in SPEM tasks was associated with generally impaired functioning (Katsanis et al., 1996). Obayashi et al. (2001) reported that mean eye scanning length (MESL) values were decreased in patients whose symptoms did not improve suggesting that MESL might be a sensitive indicator for monitoring chronicity in schizophrenia. Finally, the antisaccade error rate has been found higher in disorganized patients (DS) compared to patients with negative symptoms (NS) and patients with positive symptoms (PS), indicative of a correlation with specific clinical endophenotypes (Obyedkov et al., 2019).
Reviewing the available evidence it becomes clear that there is a great lack of studies evaluating the various oculomotor function indices as possible prognostic biomarkers. More longitudinal studies are needed to explore the prognostic value of oculomotor function deficits at FEP over the evolution of the disorder, the transition from the first episode to chronic schizophrenia, and the evolution of symptoms from positive symptoms and psychotic episodes to negative symptoms and functional and vocational impairment. Another important missing line of evidence is the investigation of oculomotor function deviances as prognostic biomarkers that would help the classification of patients between subgroups. Such biomarkers could provide critical clinical information for example dissociating the progression of FEP to either a debilitating chronic schizophrenia with prominent cognitive impairment and a poor functional outcome or a psychotic spectrum disorder with minimal cognitive impairment and a favorable functional outcome.
Conclusion
In summary research of oculomotor deficits in early schizophrenia does not provide sufficient evidence for biomarker candidates. Individual variables lack both sensitivity and specificity as diagnostic biomarkers while there are not enough data for the use of oculomotor variables as susceptibility, predictive or prognostic biomarkers. Most of the studies do not set out with the question of whether an oculomotor variable can be used as a biomarker. They rather try to answer that a posteriori, an issue which seems to be common among the biomarker research in psychiatry (Prata et al., 2014). Longitudinal studies designed to investigate the effectiveness of oculomotor function measures as biomarkers combined with rigorous methodology are needed. Moreover, it is our belief that such studies should move away from the effort of detecting diagnostic oculomotor biomarkers in early schizophrenia and should focus on other types of biomarkers. Studies should be designed to explore the possibility that oculomotor function measures or combinations of them could be used as monitoring biomarkers for the effects of treatment and treatment outcome in early schizophrenia and FEP. As new therapeutic agents are being introduced in the treatment of cognitive deficits in schizophrenia oculomotor function measures could be target biomarkers assessing the effectiveness of such new treatments. Studies should be conducted addressing the relation of oculomotor function to patients’ symptomatology over the course of the disorder with the aim of predicting symptom progression especially in the realm of negative and cognitive symptoms and the transition to chronic schizophrenia with a poor functional outcome. Also, longitudinal studies will provide definite information for oculomotor function indices use as possible susceptibility/risk biomarkers in high-risk populations. The investigation and possible clinical implementation of oculomotor biomarkers is an intriguing prospect that should be introduced as an independent objective in oculomotor function research.
Author Contributions
FA, MM, and O-VS did the literature search and wrote the original manuscript. NS and CK edited the final mansuscipt and conceived the idea for this review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abel, L., Friedman, L., Jesberger, J., Malki, A., and Meltzer, H. (1991). Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biol. Psychiatry 29, 1063–1072. doi: 10.1016/0006-3223(91)90248-k
Alexander, R. G., and Martinez-Conde, S. (2019). “Fixational eye movements,” in Eye Movement Research. Studies in Neuroscience, Psychology and Behavioral Economics, eds C. Klein and U. Ettinger (Cham: Springer), 73–115.
Allen, J. S., Lambert, A. J., Johnson, F. Y., Schmidt, K., and Nero, K. L. (1996). Antisaccadic eye movements and attentional asymmetry in schizophrenia in three Pacific populations. Acta Psychiatr. Scand. 94, 258–265. doi: 10.1111/j.1600-0447.1996.tb09858.x
Arolt, V., Teichert, H. M., Steege, D., Lencer, R., and Heide, W. (1998). Distinguishing schizophrenic patients from healthy controls by quantitative measurement of eye movement parameters. Biol. Psychiatry 44, 448–458. doi: 10.1016/s0006-3223(97)00479-4
Beedie, S. A., Benson, P. J., Giegling, I., Rujescu, D., and St Clair, D. M. (2012). Smooth pursuit and visual scanpaths: independence of two candidate oculomotor risk markers for schizophrenia. World J. Biol. Psychiatry 13, 200–210. doi: 10.3109/15622975.2011.566628
Benson, P. J., Beedie, S. A., Shephard, E., Giegling, I., Rujescu, D., and St. Clair, D. (2012). Simple viewing tests can detect eye movement abnormalities that distinguish schizophrenia cases from controls with exceptional accuracy. Biol. Psychiatry 72, 716–724. doi: 10.1016/j.biopsych.2012.04.019
Benson, P. J., Leonards, U., Lothian, R. M., St Clair, D. M., and Merlo, M. C. G. (2007). Visual scan paths in first-episode schizophrenia and cannabis-induced psychosis. J. Psychiatry Neurosci. 32, 267–274. Available online at: https://pubmed.ncbi.nlm.nih.gov/17653295/.
Bestelmeyer, P. E., Tatler, B. W., Phillips, L. H., Fraser, G., Benson, P. J., and St. Clair, D. (2006). Global visual scanning abnormalities in schizophrenia and bipolar disorder. Schizophr. Res. 87, 212–222. doi: 10.1016/j.schres.2006.06.015
Brakemeier, S., Sprenger, A., Meyhöfer, I., McDowell, J. E., Rubin, L. H., Hill, S. K., et al. (2019). Smooth pursuit eye movement deficits as a biomarker for psychotic features in bipolar disorder—Findings from the PARDIP study. Bipolar Disord. 22, 602–611. doi: 10.1111/bdi.12865
Breitborde, N. J., Srihari, V. H., and Woods, S. W. (2009). Review of the operational definition for First-episode psychosis. Early Interv. Psychiatry 3, 259–265. doi: 10.1111/j.1751-7893.2009.00148.x
Caldani, S., Amado, I., Bendjemaa, N., Vialatte, F., Mam-La-Fook, C., Gaillard, R., et al. (2017). Oculomotricity and neurological soft signs: can we refine the endophenotype? A study in subjects belonging to the spectrum of schizophrenia. Psychiatry Res. 256, 490–497. doi: 10.1016/j.psychres.2017.06.013
Caldani, S., Bucci, M. P., Lamy, J.-C., Seassau, M., Bendjemaa, N., Gadel, R., et al. (2016). Saccadic eye movements as markers of schizophrenia spektrum: exploration in at-risk mental states. Schizophr. Res. 181, 30–37. doi: 10.1016/j.schres.2016.09.003
Califf, R. M. (2018). Biomarker definitions and their applications. Exp. Biol. Med. 243, 213–221. doi: 10.1177/1535370217750088
Calkins, M. E., Iacono, W. G., and Ones, D. S. (2008). Eye movement dysfunction in first-degree relatives of patients with schizophrenia: a meta-analytic evaluation of candidate endophenotypes. Brain Cogn. 68, 436–461. doi: 10.1016/j.bandc.2008.09.001
Camchong, J., Dyckman, K. A., Austin, B. P., Clementz, B. A., and McDowell, J. E. (2008). Common neural circuitry supporting volitional saccades and its disruption in schizophrenia patients and relatives. Biol. Psychiatry 64, 1042–1050. doi: 10.1016/j.biopsych.2008.06.015
Canu, D., Ioannou, C., Müller, K., Martin, B., Fleischhaker, C., Biscaldi, M., et al. (2021). Visual search in neurodevelopmental disorders: evidence towards a continuum of impairment. Eur. Child Adolesc. Psychiatry doi: 10.1007/s00787-021-01756-z [Epub ahead of print].
Clementz, B. A., Iacono, W. G., and Grove, W. M. (1996). The construct validity of root-mean-square error for quantifying smooth-pursuit eye tracking abnormalities in schizophrenia. Biol. Psychiatry 39, 448–450. doi: 10.1016/0006-3223(95)00549-8
Clementz, B. A., McDowell, J. E., and Zisook, S. (1994). Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J. Abnorm. Psychol. 103, 277–287. doi: 10.1037/0021-843x.103.2.277
Clementz, B. A., Sweeney, J. A., Hirt, M., and Haas, G. (1990). Pursuit gain and saccadic intrusions in first-degree relatives of probands with schizophrenia. J. Abnorm. Psychol. 99, 327–335. doi: 10.1037//0021-843x.99.4.327
Crawford, T. J., Sharma, T., Puri, B. K., Murray, R. M., Berridge, D. M., and Lewis, S. W. (1998). Saccadic eye movements in families multiply affected with schizophrenia: the Maudsley family study. Am. J. Psychiatry 155, 1703–1710. doi: 10.1176/ajp.155.12.1703
Curtis, C. E., Calkins, M. E., Grove, W. M., Feil, K. J., and Iacono, W. G. (2001). Saccadic disinhibition in acute and remitted schizophrenia and their first-degree biological relatives. Am. J. Psychiatry 158, 100–106. doi: 10.1176/appi.ajp.158.1.100
Damilou, A., Apostolakis, S., Thrapsanioti, E., Theleritis, C., and Smyrnis, N. (2016). Shared and distinct oculomotor function deficits in schizophrenia and obsessive compulsive disorder. Psychophysiology 53, 796–805. doi: 10.1111/psyp.12630
De Wilde, O., Bour, L., Dingemans, P., Boerée, T., and Linszen, D. (2008). Antisaccade deficit is present in young first-episode patients with schizophrenia but not in their healthy young siblings. Psychol. Med. 38, 871–875. doi: 10.1017/S0033291707001894
Díaz-Caneja, C. M., Pina-Camacho, L., Rodríguez-Quiroga, A., Fraguas, D., Parellada, M., and Arango, C. (2015). Predictors of outcome in early-onset psychosis: a systematic review. NPJ Schizophrenia 1:14005. doi: 10.1038/npjschz.2014.5
Foulsham, T. (2019). “Scenes, saliency maps and scanpaths,” in Eye Movement Research. Studies in Neuroscience, Psychology and Behavioral Economics, eds C. Klein and U. Ettinger (Cham: Springer), 197–238.
Fusar-Poli, P., Borgwardt, S., Bechdolf, A., Addington, J., Riecher-Rössler, A., Schultze-Lutter, F., et al. (2013). The psychosis high-risk state. JAMA Psychiatry 70, 107–120. doi: 10.1001/jamapsychiatry.2013.269
Gaebel, W., Ulrich, G., and Frick, K. (1987). Visuomotor performance of schizophrenic patients and normal controls in a picture viewing task. Biol. Psychiatry 22, 1227–1237. doi: 10.1016/0006-3223(87)90030-8
Gooding, D. C., and Basso, M. A. (2008). The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain Cogn. 68, 371–390. doi: 10.1016/j.bandc.2008.08.024
Gooding, D. C., Iacono, W. G., and Beiser, M. (1994). Temporal stability of smooth-pursuit eye tracking in First-episode psychosis. Psychophysiology 31, 62–67. doi: 10.1111/j.1469-8986.1994.tb01025.x
Gooding, D. C., Iacono, W. G., and Grove, W. M. (1997). Ocular motor performance in schizophrenic patients and neurological patients. Schizophr. Res. 24, 242–243. doi: 10.1016/s0920-9964(97)82697-7
Gooding, D. C., Mohapatra, L., and Shea, H. B. (2004). Temporal stability of saccadic task performance in schizophrenia and bipolar patients. Psychol. Med. 34, 921–932. doi: 10.1017/s003329170300165x
Gooding, D. C., and Tallent, K. A. (2001). The association between antisaccade task and working memory task performance in schizophrenia and bipolar disorder. J. Nerv. Ment. Dis. 189, 8–16. doi: 10.1097/00005053-200101000-00003
Gottesman, I. I., and Gould, T. D. (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645. doi: 10.1176/appi.ajp.160.4.636
Grootens, K. P., van Luijtelaar, G., Buitelaar, J. K., van der Laan, A., Hummelen, J. W., and Verkes, R. J. (2008). Inhibition errors in borderline personality disorder with psychotic-like symptoms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 32, 267–273. doi: 10.1016/j.pnpbp.2007.08.020
Harris, M. S., Reilly, J. L., Keshavan, M. S., and Sweeney, J. A. (2006). Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychol. Med. 36, 485–494. doi: 10.1017/S0033291705006756
Harris, M. S., Reilly, J. L., Thase, M. E., Keshavan, M. S., and Sweeney, J. A. (2009). Response suppression deficits in treatment-Naïve first-episode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Res. 170, 150–156. doi: 10.1016/j.psychres.2008.10.031
Hill, S. K., Reilly, J. L., Harris, M. S., Khine, T., and Sweeney, J. A. (2008). Oculomotor and neuropsychological effects of antipsychotic treatment for schizophrenia. Schizophr. Bull. 34, 494–506. doi: 10.1093/schbul/sbm112
Hutton, S. (2008). Cognitive control of saccadic eye movements. Brain Cogn. 68, 327–340. doi: 10.1016/j.bandc.2008.08.021
Hutton, S., Huddy, V., Barnes, T. R., Robbins, T. W., Crawford, T. J., Kennard, C., et al. (2004). The relationship between Antisaccades, smooth pursuit, and executive dysfunction in first-episode schizophrenia. Biol. Psychiatry 56, 553–559. doi: 10.1016/j.biopsych.2004.07.002
Hutton, S., Crawford, T. J., Puri, B. K., Duncan, L., Chapman, M., Kennard, C., et al. (1998). Smooth pursuit and Saccadic abnormalities in first-episode schizophrenia. Psychol. Med. 28, 685–692. doi: 10.1017/s0033291798006722
Hutton, S., Crawford, T., Gibbins, H., Cuthbert, I., Barnes, T., Kennard, C., et al. (2001). Short- and long-term effects of antipsychotic medication on smooth pursuit eye tracking in schizophrenia. Psychopharmacology 157, 284–291. doi: 10.1007/s002130100803
Hutton, S., Joyce, E., Barnes, T., and Kennard, C. (2002). Saccadic distractibility in first-episode schizophrenia. Neuropsychologia 40, 1729–1736. doi: 10.1016/s0028-3932(01)00145-2
Iacono, W. G., Moreau, M., Beiser, M., Fleming, J. A., and Lin, T. (1992). Smooth-pursuit eye tracking in first-episode psychotic patients and their relatives. J. Abnorm. Psychol. 101, 104–116. doi: 10.1037/0021-843x.101.1.104
Jacobsen, L. K., Hong, W. L., Hommer, D. W., Hamburger, S. D., Castellanos, F. X., Frazier, J. A., et al. (1996). Smooth pursuit eye movements in childhood-onset schizophrenia: comparison with attention-deficit hyperactivity disorder and normal controls. Biol. Psychiatry 40, 1144–1154. doi: 10.1016/S0006-3223(95)00630-3
Kahn, R. S., Sommer, I. E., Murray, R. M., Meyer-Lindenberg, A., Weinberger, D. R., Cannon, T. D., et al. (2015). Schizophrenia. Nat. Rev. Dis. Primers 1:15067. doi: 10.1038/nrdp.2015.67
Kallimani, D., Theleritis, C., Evdokimidis, I., Stefanis, N. C., Chatzimanolis, I., and Smyrnis, N. (2009). The effect of change in clinical state on eye movement dysfunction in schizophrenia. Eur. Psychiatry 24, 17–26. doi: 10.1016/j.eurpsy.2008.08.003
Karantinos, T., Tsoukas, E., Mantas, A., Kattoulas, E., Stefanis, N. C., Evdokimidis, I., et al. (2014). Increased intra-subject reaction time variability in the volitional control of movement in schizophrenia. Psychiatry Res. 215, 26–32. doi: 10.1016/j.psychres.2013.10.031
Karpouzian, T., Petrovsky, N., Ettinger, U., and Reilly, J. (2019). “Eye movements as biomarkers to evaluate pharmacological effects on brain systems,” in Eye Movement Research. Studies in Neuroscience, Psychology and Behavioral Economics, eds C. Klein and U. Ettinger (Cham: Springer), 775–816.
Kathmann, N., Hochrein, A., Uwer, R., and Bondy, B. (2003). Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. Am. J. Psychiatry 160, 696–702. doi: 10.1176/appi.ajp.160.4.696
Katsanis, J., Iacono, W., and Beiser, M. (1996). Eye-tracking performance and adaptive functioning over the short-term course of first-episode psychosis. Psychiatry Res. 64, 19–26. doi: 10.1016/0165-1781(96)02889-2
Keedy, S. K., Reilly, J. L., Bishop, J. R., Weiden, P. J., and Sweeney, J. A. (2014). Impact of antipsychotic treatment on attention and motor learning systems in first-episode schizophrenia. Schizophr. Bull. 41, 355–365. doi: 10.1093/schbul/sbu071
Kirenskaya, A. V., Tkachenco, A. A., and Novototsky-Vlasov, V. Y. (2017). The study of the Antisaccade performance and contingent Negative Variation characteristics in First-episode and chronic schizophrenia patients. Span. J. Psychol. 20:E55. doi: 10.1017/sjp.2017.40
Kleineidam, L., Frommann, I., Ruhrmann, S., Klosterkötter, J., Brockhaus-Dumke, A., Wölwer, W., et al. (2019). Antisaccade and prosaccade eye movements in individuals clinically at risk for psychosis: comparison with first-episode schizophrenia and prediction of conversion. Eur. Arch. Psychiatry Clin. Neurosci. 269, 921–930. doi: 10.1007/s00406-018-0973-4
Kojima, T., Matsushima, E., and Ando, K. (2000). Eyes and the Mind: Psychophysiological Approach to Psychiatric Disorders Through Visual and Ocular Functions. Tokyo: Japan Scientific Societies Press and Karger.
Kojima, T., Matsushima, E., Ando, K., Ando, H., Sakurada, M., Ohta, K., et al. (1992). Exploratory eye movements and neuropsychological tests in schizophrenic patients. Schizophr. Bull. 18, 85–94. doi: 10.1093/schbul/18.1.85
Kojima, T., Matsushima, E., Nakajima, K., Shiraishi, H., Ando, K., Ando, H., et al. (1990). Eye movements in acute, chronic, and remitted schizophrenics. Biol. Psychiatry 27, 975–989. doi: 10.1016/0006-3223(90)90035-z
Kojima, T., Matsushima, E., Ohta, K., Toru, M., Han, Y. H., Shen, Y. C., et al. (2001). Stability of exploratory eye movements as a marker of schizophrenia—a who multi-center study. World Health Organization. Schizophr. Res. 52, 203–213. doi: 10.1016/s0920-9964(00)00181-x
Kojima, T., Potkin, S. G., Kharazmi, M., Matsushima, E., Herrera, J., and Shimazono, Y. (1989). Limited eye movement patterns in chronic schizophrenic patients. Psychiatry Res. 28, 307–314. doi: 10.1016/0165-1781(89)90211-4
Krebs, M. O., Bourdel, M., Cherif, Z. R., Bouhours, P., Lôo, H., Poirier, M. F., et al. (2010). Deficit of inhibition motor control in untreated patients with schizophrenia: further support from visually guided saccade paradigms. Psychiatry Res. 179, 279–284. doi: 10.1016/j.psychres.2009.07.008
Landgraf, S., Amado, I., Bourdel, M.-C., Leonardi, S., and Krebs, M.-O. (2008). Memory-guided saccade abnormalities in schizophrenic patients and their healthy, full biological siblings. Psychol. Med. 38, 861–870. doi: 10.1017/S0033291707001912
Lee, P. H., Anttila, V., Won, H., Feng, Y. A., Rosenthal, J., Zhu, Z., et al. (2019). Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469.e11–1482.e11. doi: 10.1016/j.cell.2019.11.020
Lencer, R., Reilly, J. L., Harris, M. S., Sprenger, A., Keshavan, M. S., and Sweeney, J. A. (2010). Sensorimotor transformation deficits for smooth pursuit in First-Episode Affective psychoses and schizophrenia. Biol. Psychiatry 67, 217–223. doi: 10.1016/j.biopsych.2009.08.005
Lencer, R., Sprenger, A., and Trillenberg, P. (2019). “Smooth eye movements in humans: smooth pursuit, optokinetic nystagmus and vestibular ocular reflex,” in Eye Movement Research. Studies in Neuroscience, Psychology and Behavioral Economics, eds C. Klein and U. Ettinger (Cham: Springer), 117–163.
Lencer, R., Sprenger, A., Reilly, J. L., McDowell, J. E., Rubin, L. H., Badner, J. A., et al. (2015). Pursuit eye movements as an intermediate phenotype across psychotic disorders: evidence from the b-snip study. Schizophr. Res. 169, 326–333. doi: 10.1016/j.schres.2015.09.032
Lencer, R., Trillenberg, P., Trillenberg-Krecker, K., Junghanns, K., Kordon, A., Broocks, A., et al. (2004). Smooth pursuit deficits in schizophrenia, affective disorder and obsessive-compulsive disorder. Psychol. Med. 34, 451–460. doi: 10.1017/s0033291703001314
Lennertz, L., Rampacher, F., Vogeley, A., Schulze-Rauschenbach, S., Pukrop, R., Ruhrmann, S., et al. (2012). Antisaccade performance in patients with obsessive-compulsive disorder and unaffected relatives: further evidence for impaired response inhibition as a candidate endophenotype. Eur. Arch. Psychiatry Clin. Neurosci. 262, 625–634. doi: 10.1007/s00406-012-0311-1
Levy, D. L., Holzman, P. S., Matthysse, S., and Mendell, N. R. (1993). Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr. Bull. 19, 461–536. doi: 10.1093/schbul/19.3.461
Levy, D. L., Holzman, P. S., Matthysse, S., and Mendell, N. R. (1994). Eye tracking and schizophrenia: a selective review. Schizophr. Bull. 20, 47–62. doi: 10.1093/schbul/20.1.47
Levy, D. L., Lajonchere, C. M., Dorogusker, B., Min, D., Lee, S., Tartaglini, A., et al. (2000). Quantitative characterization of eye tracking dysfunction in schizophrenia. Schizophr. Res. 42, 171–185. doi: 10.1016/s0920-9964(99)00122-x
Martin, L. F., Hall, M.-H., Ross, R. G., Zerbe, G., Freedman, R., and Olincy, A. (2007). Physiology of schizophrenia, bipolar disorder and schizoaffective disorder. Am. J. Psychiatry 164, 1900–1906. doi: 10.1176/appi.ajp.2007.06010017
Mather, J. A., Neufeld, R. W., Merskey, H., and Russell, N. C. (1992). Disruption of saccade production during oculomotor tracking in schizophrenia and the use of its changes across target velocity as a discriminator of the disorder. Psychiatry Res. 43, 93–109. doi: 10.1016/0165-1781(92)90145-s
Matsushima, E., Kojima, T., Obayashi, S., Ando, H., Ando, K., and Shimazono, Y. (1992). Exploratory eye movements in schizophrenic patients and patients with frontal lobe lesions. Eur. Arch. Psychiatry Clin. Neurosci. 241, 210–214. doi: 10.1007/BF02190255
Matsushima, E., Kojima, T., Ohta, K., Obayashi, S., Nakajima, K., Kakuma, T., et al. (1998). Exploratory eye movement dysfunctions in patients with schizophrenia: possibility as a discriminator for schizophrenia. J. Psychiatr. Res. 32, 289–295. doi: 10.1016/S0022-3956(98)00019-3
McDowell, J. E., and Clementz, B. A. (1996). Ocular-motor delayed-response task performance among schizophrenia patients. Neuropsychobiology 34, 67–71. doi: 10.1159/000119294
Millard, S. P., Shofer, J., Braff, D., Calkins, M., Cadenhead, K., Freedman, R., et al. (2016). Prioritizing schizophrenia endophenotypes for future genetic studies: an example using data from the COGS-1 family study. Schizophr. Res. 174, 1–9. doi: 10.1016/j.schres.2016.04.011
Morita, K., Miura, K., Kasai, K., and Hashimoto, R. (2019). Eye movement characteristics in schizophrenia: a recent update with clinical implications. Neuropsychopharmacol. Rep. 40, 2–9. doi: 10.1002/npr2.12087
Müller, N., Riedel, M., Eggert, T., and Straube, A. (1999). Internally and externally guided voluntary saccades in unmedicated and medicated schizophrenic patients. Part II. Saccadic latency, gain, and fixation suppression errors. Eur. Arch. Psychiatry Clin. Neurosci. 249, 7–14. doi: 10.1007/s004060050059
Nieman, D. H., Bour, L. J., Linszen, D. H., Goede, J., Koelman, J. H., Gersons, B. P., et al. (2000). Neuropsychological and clinical correlates of antisaccade task performance in schizophrenia. Neurology 54, 866–871. doi: 10.1212/wnl.54.4.866
Nieman, D., Becker, H., Van de Fliert, R., Plat, N., Bour, L., Koelman, H., et al. (2007). Antisaccade task performance in patients at ultra-high risk for developing psychosis. Schizophr. Res. 95, 54–60. doi: 10.1016/j.schres.2007.06.022
O’Driscoll, G. A., and Callahan, B. L. (2008). Smooth pursuit in schizophrenia: a meta-analytic review of research since 1993. Brain Cogn. 68, 359–370. doi: 10.1016/j.bandc.2008.08.023
Obayashi, S., Msatsushima, E., Ando, H., Ando, K., and Kojima, T. (2003). Exploratory eye movements during the Benton visual retention test: characteristics of visual behavior in schizophrenia. Psychiatry Clin. Neurosci. 57, 409–415. doi: 10.1046/j.1440-1819.2003.01140.x
Obayashi, S., Matsushima, E., Okubo, Y., Ohkura, T., Kojima, T., and Kakuma, T. (2001). Relationship between exploratory eye movements and clinical course in schizophrenic patients. Eur. Arch. Psychiatry Clin. Neurosci. 251, 211–216. doi: 10.1007/s004060170029
Obyedkov, I., Skuhareuskaya, M., Skugarevsky, O., Obyedkov, V., Buslauski, P., Skuhareuskaya, T., et al. (2019). Saccadic eye movements in different dimensions of schizophrenia and in clinical high-risk state for psychosis. BMC Psychiatry 19:110. doi: 10.1186/s12888-019-2093-8
Pierce, J. E., Clementz, B. A., and McDowell, J. E. (2019). “Saccades: fundamentals and neural mechanisms,” in Eye Movement Research. Studies in Neuroscience, Psychology and Behavioral Economics, eds C. Klein and U. Ettinger (Cham: Springer), 11–71.
Prata, D., Mechelli, A., and Kapur, S. (2014). Clinically meaningful biomarkers for psychosis: a systematic and quantitative review. Neurosci. Biobehav. Rev. 45, 134–141. doi: 10.1016/j.neubiorev.2014.05.010
Qiu, L., Tian, L., Pan, C., Zhu, R., Liu, Q., Yan, J., et al. (2011). Neuroanatomical circuitry associated with exploratory eye movement in schizophrenia: a voxel-based morphometric study. PLoS One 6:e25805. doi: 10.1371/journal.pone.0025805
Qiu, L., Yan, H., Zhu, R., Yan, J., Yuan, H., Han, Y., et al. (2018). Correlations between exploratory eye movement, hallucination, and cortical gray matter volume in people with schizophrenia. BMC Psychiatry 18:226. doi: 10.1186/s12888-018-1806-8
Radant, A. D., Claypoole, K., Wingerson, D. K., Cowley, D. S., and Roy-Byrne, P. P. (1997). Relationships between neuropsychological and oculomotor measures in schizophrenia patients and normal controls. Biol. Psychiatry 42, 797–805. doi: 10.1016/s0006-3223(96)00464-7
Radant, A. D., Dobie, D. J., Calkins, M. E., Olincy, A., Braff, D. L., Cadenhead, K. S., et al. (2007). Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophr. Res. 89, 320–329. doi: 10.1016/j.schres.2006.08.010
Reilly, J. L., Frankovich, K., Hill, S., Gershon, E. S., Keefe, R. S., Keshavan, M. S., et al. (2014). Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr. Bull. 40, 1011–1021. doi: 10.1093/schbul/sbt132
Reilly, J. L., Harris, M. S., Keshavan, M. S., and Sweeney, J. A. (2005). Abnormalities in visually guided saccades suggest corticofugal dysregulation in never-treated schizophrenia. Biol. Psychiatry 57, 145–154. doi: 10.1016/j.biopsych.2004.10.024
Reilly, J. L., Harris, M. S., Keshavan, M. S., and Sweeney, J. A. (2006). Adverse effects of Risperidone on spatial working memory in first-episode schizophrenia. Arch. Gen. Psychiatry 63, 1189–1197. doi: 10.1001/archpsyc.63.11.1189
Reilly, J. L., Harris, M. S., Khine, T. T., Keshavan, M. S., and Sweeney, J. A. (2007). Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biol. Psychiatry 62, 818–821. doi: 10.1016/j.biopsych.2006.10.031
Reuter, B., Rakusan, L., and Kathmanna, N. (2005). Poor antisaccade performance in schizophrenia: an inhibition deficit? Psychiatry Res. 135, 1–10. doi: 10.1016/j.psychres.2004.12.006
Rosenberg, D. R., Averbach, D. H., O’Hearn, K. M., Seymour, A. B., Birmaher, B., and Sweeney, J. A. (1997). Oculomotor response inhibition abnormalities in pediatric obsessive-compulsive disorder. Arch. Gen. Psychiatry 54, 831–838. doi: 10.1001/archpsyc.1997.01830210075008
Ryu, H., Morita, K., Yamaguchi, H., Waseda, Y., and Maeda, H. (2001). Abnormal exploratory eye movements in schizophrenic patients vs. healthy subjects. Acta Neurol. Scand. 104, 369–376. doi: 10.1034/j.1600-0404.2001.00279.x
Sereno, A. B., and Holzman, P. S. (1995). Antisaccades and smooth pursuit eye movements in schizophrenia. Biol. Psychiatry 37, 394–401. doi: 10.1016/0006-3223(94)00127-O
Smyrnis, N. (2008). Metric issues in the study of eye movements in psychiatry. Brain Cogn. 68, 341–358. doi: 10.1016/j.bandc.2008.08.022
Smyrnis, N., Amado, I., Krebs, M. O., and Sweeney, J. A. (2019). “Eye movements in psychiatry,” in Eye Movement Research. Studies in Neuroscience, Psychology and Behavioral Economics, eds C. Klein and U. Ettinger (Cham: Springer), 703–748.
Smyrnis, N., Karantinos, T., Malogiannis, I., Theleritis, C., Mantas, A., Stefanis, N. C., et al. (2009). Larger variability of saccadic reaction times in schizophrenia patients. Psychiatry Res. 168, 129–136. doi: 10.1016/j.psychres.2008.04.015
Sprenger, A., Friedrich, M., Nagel, M., Schmidt, C. S., Moritz, S., and Lencer, R. (2013). Advanced analysis of free visual exploration patterns in schizophrenia. Front. Psychol. 4:737. doi: 10.3389/fpsyg.2013.00737
Suvisaari, J., Mantere, O., Keinänen, J., Mäntylä, T., Rikandi, E., Lindgren, M., et al. (2018). Is it possible to predict the future in first-episode psychosis? Front. Psychiatry 9:580. doi: 10.3389/fpsyt.2018.00580
Suzuki, M., Takahashi, S., Matsushima, E., Tsunoda, M., Kurachi, M., Okada, T., et al. (2009). Exploratory eye movement dysfunction as a discriminator for schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 259, 186–194. doi: 10.1007/s00406-008-0850-7
Sweeney, J. A., Clementz, B. A., Escobar, M. D., Li, S., Pauler, D. K., and Haas, G. L. (1993). Mixture analysis of pursuit eye-tracking dysfunction in schizophrenia. Biol. Psychiatry 34, 331–340. doi: 10.1016/0006-3223(93)90090-z
Sweeney, J. A., Clementz, B. A., Haas, G. L., Escobar, M. D., Drake, K., and Frances, A. J. (1994). Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity, and the role of attention. J. Abnorm. Psychol. 103, 222–230. doi: 10.1037//0021-843x.103.2.222
Sweeney, J. A., Luna, B., Haas, G. L., Keshavan, M. S., Mann, J., and Thase, M. E. (1999). Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol. Psychiatry 46, 671–680. doi: 10.1016/s0006-3223(99)00132-8
Sweeney, J. A., Luna, B., Srinivasagam, N. M., Keshavan, M. S., Schooler, N. R., Haas, G. L., et al. (1998). Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biol. Psychiatry 44, 698–708. doi: 10.1016/s0006-3223(98)00035-3
Takahashi, S., Tanabe, E., Yara, K., Matsuura, M., Matsushima, E., and Kojima, T. (2008). Impairment of exploratory eye movement in schizophrenia patients and their siblings. Psychiatry Clin. Neurosci. 62, 487–493. doi: 10.1111/j.1440-1819.2008.01840.x
Thaker, G. K., Cassady, S., Adami, H., Moran, M., and Ross, D. E. (1996). Eyemovements in spectrum personality disorders: comparison of community subjects and relatives of schizophrenic patients. Am. J. Psychiatry 153, 362–368. doi: 10.1176/ajp.153.3.362
Thaker, G., Kirkpatrick, B., Buchanan, R. W., Ellsberry, R., Lahti, A., and Tamminga, C. (1988). Oculomotor abnormalities and their clinical correlates in schizophrenia. Psychopharmacol. Bull. 25, 491–497.
Theleritis, C., Evdokimidis, I., and Smyrnis, N. (2014). Variability in the decision process leading to saccades: a specific marker for schizophrenia? Psychophysiology 51, 327–336. doi: 10.1111/psyp.12178
Tien, A. Y., Pearlson, G. D., Machlin, S. R., Bylsma, F. W., and Hoehn-Saric, R. (1992). Oculomotor performance in obsessive-compulsive disorder. Am. J. Psychiatry 149, 641–646. doi: 10.1176/ajp.149.5.641
Tien, A. Y., Ross, D. E., Pearlson, G., and Strauss, M. E. (1996). Eye movements and psychopathology in schizophrenia and bipolar disorder. J. Nerv. Ment. Dis. 184, 331–338. doi: 10.1097/00005053-199606000-00001
Tonoya, Y., Matsui, M., Kurachi, M., Kurokawa, K., and Sumiyoshi, T. (2002). Exploratory eye movements in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 252, 255–261. doi: 10.1007/s00406-002-0390-5
Trillenberg, P., Sprenger, A., Talamo, S., Herold, K., Helmchen, C., Verleger, R., et al. (2016). Visual and non-visual motion information processing during pursuit eye tracking in schizophrenia and bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 267, 225–235. doi: 10.1007/s00406-016-0671-z
van Tricht, M. J., Nieman, D. H., Bour, L. J., Boerée, T., Koelman, J. H. T. M., and de Haan, L. (2010). Increased saccadic rate during smooth pursuit eye movements in patients at Ultra High Risk for developing a psychosis. Brain Cogn. 73, 215–221. doi: 10.1016/j.bandc.2010.05.005
Venkatasubramanian, G., and Keshavan, M. (2016). Biomarkers in psychiatry—a critique. Ann. Neurosci. 23, 3–5. doi: 10.1159/000443549
Keywords: oculomotor, biomarker, schizophrenia, first episode psychosis, recent onset, ultra-high risk
Citation: Athanasopoulos F, Saprikis O-V, Margeli M, Klein C and Smyrnis N (2021) Towards Clinically Relevant Oculomotor Biomarkers in Early Schizophrenia. Front. Behav. Neurosci. 15:688683. doi: 10.3389/fnbeh.2021.688683
Received: 31 March 2021; Accepted: 11 May 2021;
Published: 10 June 2021.
Edited by:
Kyriaki Sidiropoulou, University of Crete, GreeceReviewed by:
Sina Hafizi, University of Manitoba, CanadaDiane Carol Gooding, University of Wisconsin-Madison, United States
Copyright © 2021 Athanasopoulos, Saprikis, Margeli, Klein and Smyrnis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikolaos Smyrnis, smyrnis@med.uoa.gr
† These authors have contributed equally to this work and share first authorship
 Fotios Athanasopoulos
Fotios Athanasopoulos Orionas-Vasilis Saprikis
Orionas-Vasilis Saprikis Myrto Margeli
Myrto Margeli Christoph Klein1,3,4
Christoph Klein1,3,4 Nikolaos Smyrnis
Nikolaos Smyrnis