
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Behav. Neurosci. , 20 April 2021
Sec. Pathological Conditions
Volume 15 - 2021 | https://doi.org/10.3389/fnbeh.2021.647069
This article is part of the Research Topic Understanding Early Detection Markers in Schizophrenia View all 9 articles
 Tsutomu Takahashi1,2*
Tsutomu Takahashi1,2* Daiki Sasabayashi1,2
Daiki Sasabayashi1,2 Yoichiro Takayanagi1,3
Yoichiro Takayanagi1,3 Yuko Higuchi1,2
Yuko Higuchi1,2 Yuko Mizukami1
Yuko Mizukami1 Shimako Nishiyama1,4
Shimako Nishiyama1,4 Atsushi Furuichi1,2
Atsushi Furuichi1,2 Mikio Kido1,2
Mikio Kido1,2 Tien Viet Pham1,2
Tien Viet Pham1,2 Haruko Kobayashi1,2
Haruko Kobayashi1,2 Kyo Noguchi5
Kyo Noguchi5 Michio Suzuki1,2
Michio Suzuki1,2An increased prevalence of duplicated Heschl’s gyrus (HG), which may reflect an early neurodevelopmental pathology, has been reported in schizophrenia (Sz). However, it currently remains unclear whether individuals at risk of psychosis exhibit similar brain morphological characteristics. This magnetic resonance imaging study investigated the distribution of HG gyrification patterns [i.e., single HG, common stem duplication (CSD), and complete posterior duplication (CPD)] and their relationship with clinical characteristics in 57 individuals with an at-risk mental state (ARMS) [of whom 5 (8.8%) later developed Sz], 63 patients with Sz, and 61 healthy comparisons. The prevalence of duplicated HG patterns (i.e., CSD or CPD) bilaterally was significantly higher in the ARMS and Sz groups than in the controls, whereas no significant differences were observed in HG patterns between these groups. The left CSD pattern, particularly in the Sz group, was associated with a verbal fluency deficit. In the ARMS group, left CSD pattern was related to a more severe general psychopathology. The present results suggest that an altered gyrification pattern on the superior temporal plane reflects vulnerability factors associated with Sz, which may also contribute to the clinical features of high-risk individuals, even without the onset of psychosis.
Heschl’s gyrus (HG), a convolution on the superior temporal plane, hosts the primary auditory cortex (Rademacher et al., 1993; Da Costa et al., 2011) and is also involved in memory (Weinberger, 2015) and emotional (Concina et al., 2019) processing. The morphology of HG markedly varies across individuals, with approximately 30–50% of healthy individuals potentially having complete or partial duplication (Leonard et al., 1998; Rademacher et al., 2001; Abdul-Kareem and Sluming, 2008; Marie et al., 2015). This anatomical variant appears to reflect variations in cytoarchitectonic development during gestation (Chi et al., 1977; Armstrong et al., 1995), and duplicated HG may lead to learning disabilities (Leonard et al., 1993, 2001) and reduced HG activity during auditory processing (Tzourio-Mazoyer et al., 2015) in a non-clinical population. In a recent magnetic resonance imaging (MRI) study, we reported an increased prevalence of HG duplications in first-episode schizophrenia (Sz) (Takahashi et al., in submission), which may reflect the early neurodevelopmental pathology (Weinberger, 1987; Insel, 2010). However, since another MRI study that specifically examined HG duplication patterns in chronic Sz did not find significant results (Hubl et al., 2010), it currently remains unclear whether illness stages affect the HG pattern of Sz. Furthermore, although structural/functional abnormalities in the superior temporal plane may underlie the positive psychotic symptoms (Alderson-Day et al., 2015; Takahashi and Suzuki, 2018) as well as core trait abnormalities [e.g., deficits in social cognition (Mier et al., 2017) and verbal fluency (Antonova et al., 2004)] of Sz, it has not yet been clarified whether the HG gyrification pattern is associated with these clinical features.
MRI studies on individuals at high risk of developing psychosis [i.e., at-risk mental state (ARMS) (Yung et al., 2004, 2005)], who have an increased risk of developing psychosis within a short period of time [approximately 30% at 2 years (Fusar-Poli et al., 2012a)], generally showed similar gross morphological characteristics associated with early neurodevelopment [e.g., an altered sulcogyral pattern in the orbitofrontal region (Nakamura et al., 2019) and widespread cortical hypergyria (Sasabayashi et al., 2017)] to those of overt Sz. Since these brain anomalies are at least partly observed in participants without a later onset of psychosis (Sasabayashi et al., 2017; Nakamura et al., 2019), they may represent biological traits associated with general vulnerability to psychopathology. These gross brain characteristics may contribute to cognitive impairments in the Sz and ARMS groups (Takahashi et al., 2019a), supporting the presentation of cognitive impairments, particularly in social function (Lee et al., 2015) and verbal fluency (Fusar-Poli et al., 2012b), even before the onset of psychosis as a trait vulnerability marker. However, despite evidence of partly shared superior temporal gray matter reductions in the ARMS and Sz groups (Takahashi et al., 2010b), no MRI studies to date have specifically examined the HG duplication pattern and its potential contribution to clinical features (e.g., cognitive deficits) in the ARMS cohort.
Therefore, the present MRI study aimed to examine the HG gyrification pattern (single HG, partial duplication, and complete duplication) in ARMS individuals and Sz patients, compare it with those in healthy controls, and examine its potential contribution to clinical variables (symptoms, social and cognitive functions). Based on our previous MRI findings from an independent sample of Sz (Takahashi et al., in submission) as well as the potential role of brain gyrification as a stable neurodevelopmental marker (Chi et al., 1977; Armstrong et al., 1995), we predicted increased HG duplication in both the ARMS and Sz groups. We also speculated that the HG pattern in these groups may be associated with clinical variables that reflect trait abnormalities, such as cognitive impairments.
Fifty-seven ARMS individuals, 63 Sz patients, and 61 healthy controls participated in the present study (Table 1); they were physically healthy and had no history of severe obstetric complications, serious head trauma, neurological illness, substance abuse, or serious medical disease (e.g., diabetes, thyroid disease, hypertension, or steroid use). Handedness (Okada et al., 2014a), IQ scores measured using the Japanese version of the National Adult Reading Test (JART) (Matsuoka et al., 2006), and the personal and parental socioeconomic status (SES) (Okada et al., 2014b) were also evaluated. We recently detected an altered HG gyrification pattern in first-episode Sz (Takahashi et al., in submission); however, there was no sample overlap between these findings and the present results.
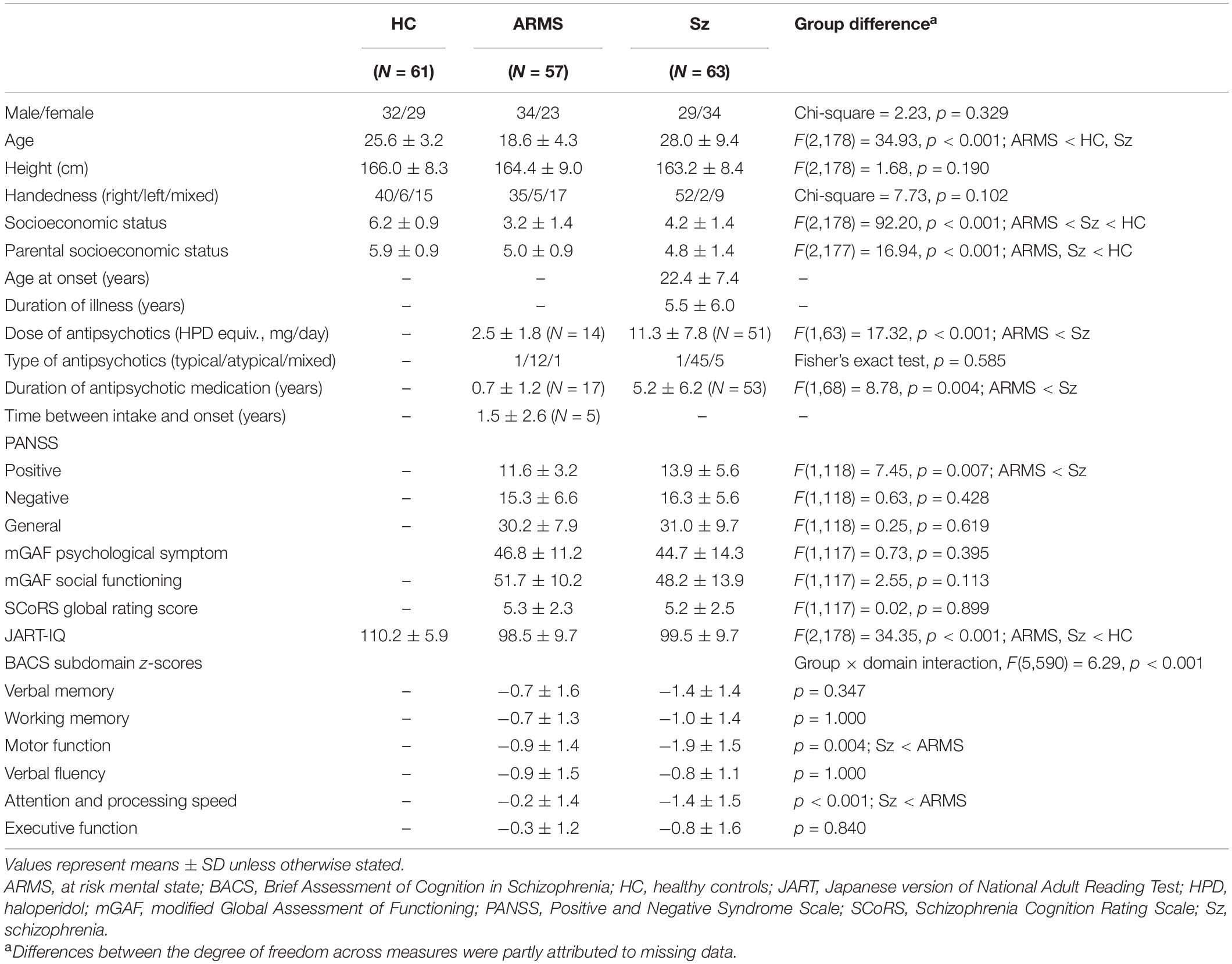
Table 1. Demographic/clinical characteristics and sociocognitive functions in ARMS, schizophrenia, and control subjects.
As described previously (Takahashi et al., 2017, 2018), individuals with ARMS were enrolled from the Consultation Support Service in Toyama (CAST), which is a regional clinical setting that specializes in early interventions (Mizuno et al., 2009). All individuals met the criteria for attenuated psychotic symptoms (APS) based on the Comprehensive Assessment of At-Risk Mental States (CAARMS) (Yung et al., 2005), while 6 also fulfilled brief and limited intermittent psychotic symptoms (BLIPS) (N = 1) or genetic risk and deterioration syndrome (GRD) (N = 5) criteria. Major comorbid DSM Axis I disorder (American Psychiatric Association, 2000) comprised anxiety disorders (N = 13), adjustment disorders (N = 11), schizotypal personality disorders (N = 10), pervasive developmental disorders (N = 9), or depressive disorders (N = 8). Five participants (8.8%) developed Sz during the clinical follow-up at Toyama University Hospital (mean = 3.2 ± 2.9 years, median = 2.4). Medication and other clinical data are summarized in Table 1. Eleven participants were also being treated with antidepressants (N = 5) and/or benzodiazepines (N = 8) when scans were performed.
Sz patients fulfilling the DSM-IV-TR criteria (American Psychiatric Association, 2000) were enrolled from the in- and outpatient clinics of the Department of Neuropsychiatry of Toyama University Hospital. They were diagnosed based on the Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition (SCID-I/P) (First et al., 1997) and a detailed chart review. The Sz group was divided into first-episode [illness duration ≤1 year (N = 17)] and chronic [illness duration ≥3 years (N = 38)] subgroups to examine the effects of illness chronicity.
Healthy controls with no personal or family history (among first-degree relatives) of neuropsychiatric disorders were enrolled from both the community and hospital staff and screened using the SCID-I Non-patient Edition (First et al., 1997). The present study was approved by the Committee on Medical Ethics of Toyama University (No. I2013006). Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. When participants were <20 years old, written consent was also obtained from a parent/guardian.
The clinical symptoms of ARMS and Sz participants were rated by experienced psychiatrists using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). The Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al., 2004), the Schizophrenia Cognition Rating Scale (SCoRS) (Keefe et al., 2006), and the modified Global Assessment of Functioning (mGAF) scale (Eguchi et al., 2015) were used to evaluate social and cognitive functions.
Magnetic resonance imaging was performed using the 3-T Magnetom Verio (Siemens, Erlangen, Germany). A three-dimensional magnetization-prepared rapid gradient echo (MPRAGE) sequence provided 176 contiguous 1.2-mm-thick T1-weighted slices in the sagittal plane. The following imaging parameters were used: repetition time = 2,300 ms; echo time = 2.9 ms; flip angle = 9°; field of view = 256 mm; and matrix size = 256 pixels × 256 pixels, with a voxel size of 1.0 mm × 1.0 mm × 1.2 mm.
Brain images were coded randomly and analyzed blind to participants’ information (e.g., diagnosis and gender). The images were then realigned using Dr. View software (Infocom, Tokyo, Japan) into three dimensions to account for differences in head tilting during the acquisition of images. They were reconstructed into entire contiguous 1-mm-thick coronal images that were perpendicular to the anterior commissure-posterior commissure line.
As reported previously (Leonard et al., 1998; Rademacher et al., 2001; Abdul-Kareem and Sluming, 2008; Marie et al., 2015), the HG gyrification pattern on each hemisphere was classified into single HG, common stem duplication (CSD), and complete posterior duplication (CPD) (Figure 1). Among duplicated HG patterns, the CSD pattern was characterized by the gyrus being partially split by the sulcus intermedius (SI), which forms a ‘heart-shaped’ HG. The hemisphere with fully separate gyri [two (N = 80) or three (N = 4) gyri per hemisphere in the present study] was defined as the CPD pattern. Fourteen hemispheres (3.9%), which had a separate HG posterior to the HG with partial duplication, were categorized as the CSD pattern.

Figure 1. Sample MR images of Heschl’s gyrus (HG; colored in blue) in participants with different gyrification patterns. Schematic drawings of the superior temporal surface on an axial view are also shown (right). A, anterior; CPD, complete posterior duplication; CSD, common stem duplication; FTS, first transverse sulcus; HS, Heschl’s sulcus; L, lateral; Lt, left; P, posterior; M, medial; PP, planum polare; PT, planum temporale; Rt, right; sHG, second Heschl’s gyrus; sHS, second Heschl’s sulcus; SI, sulcus intermedius.
In the present study, one rater (TT) classified HG gyrification patterns without knowledge of subject identities. Intra- (TT) and inter-rater (TT and DS) reliabilities in 15 randomly selected brains (30 hemispheres) were ≥0.83 (Cronbach’s α).
Demographic and clinical data were compared between groups using a one-way analysis of variance (ANOVA) or the χ2 test.
Group differences in the HG pattern distribution were compared on each hemisphere by the χ2 test. Potential relationship between the HG pattern and age, IQ, or medication (dose, duration) was assessed using ANOVA with the HG pattern as an independent variable. For assessing the potential contribution of the HG pattern to clinical variables (PANSS, BACS, SCoRS, and mGAF scores) in the ARMS and Sz groups, analysis of covariance (ANCOVA) was used with age and medication (dose, duration) as covariates. The relationship between the HG pattern and clinical variables with non-normal distribution (SCoRS, mGAF, and BACS executive function scores for both groups and BACS verbal/working memory scores for Sz group; tested by Kolmogorov–Smirnov tests) was also assessed by non-parametric Kruskal–Wallis tests. PANSS and other BACS scores were normally distributed. A post hoc Newman–Keuls test was used to follow-up these analyses. A p-value of <0.05 was considered to be significant.
No significant differences were observed in sex, height, or handedness between groups, whereas age, IQ, and parental/personal SES significantly differed.
Lower doses of antipsychotics, less severe positive symptoms, and higher BACS scores for motor function and attention subdomains were observed in the ARMS group than in the Sz group.
Both the ARMS (left, χ2 = 9.08, p = 0.003; right, χ2 = 6.93, p = 0.008) and Sz (left, χ2 = 10.51, p = 0.001; right, χ2 = 11.63, p < 0.001) groups had a significantly higher prevalence of duplicated HG patterns (i.e., CSD or CPD) bilaterally than the controls, whereas the HG pattern did not significantly differ between these groups (left, χ2 = 0.02, p = 0.880; right, χ2 = 0.53, p = 0.465) (Table 2 and Figure 2). When we examined participants with HG duplication only, no group difference was noted in HG patterns (CSD vs. CPD; all χ2 < 1.82, p > 0.177). We also compared the first-episode and chronic subgroups of Sz, but found no significant differences in the HG patterns (left, χ2 = 0.60, p = 0.741; right, χ2 = 0.06, p = 0.969).
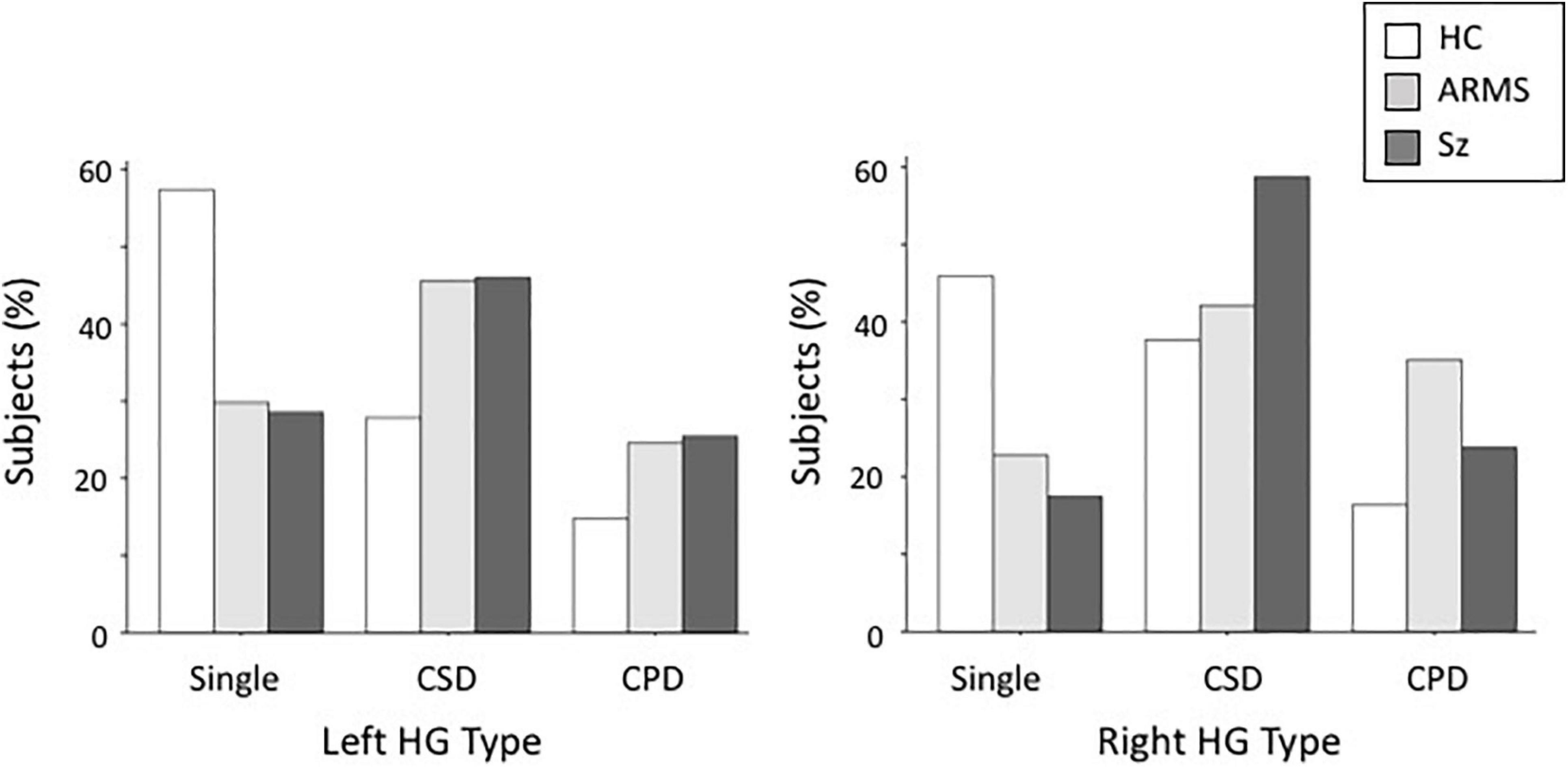
Figure 2. Distribution of Heschl’s gyrus (HG) gyrification patterns in schizophrenia (SZ), at-risk mental state (ARMS), and healthy comparison (HC) groups. CPD, complete posterior duplication; CSD, common stem duplication.
Furthermore, HG patterns did not significantly differ between male and female participants (left, χ2 = 0.87, p = 0.648; right, χ2 = 1.03, p = 0.596), while HG duplication (i.e., CSD or CPD) was more frequent in the right hemisphere (χ2 = 4.01, p = 0.045) when all diagnostic groups were combined.
Medication (for the ARMS and Sz groups), age, and IQ were not associated with the HG pattern for all diagnostic groups (Supplementary Table).
In the combined sample of ARMS and Sz participants, there was a significant effect of the left HG pattern on the BACS verbal fluency score [F(2,114) = 3.89, p = 0.023]; participants with CSD had a lower score than those with CPD (p = 0.040). This effect was significant also for the Sz group only [F(2,57) = 3.69, p = 0.031; post hoc test, p = 0.044].
At-risk mental state individuals with the left CSD pattern had a higher PANSS general psychopathology score than those with the CPD pattern [F(2,51) = 4.97, p = 0.011; post hoc test, p = 0.016].
No association was observed between the HG pattern and other clinical variables (e.g., SCoRS and mGAF scores; Supplementary Table).Kruskal–Wallis tests for the clinical variables with non-normal distribution also showed no significant association.
To the best of our knowledge, this is the first MRI study to examine the HG duplication pattern in clinical high-risk individuals for developing psychosis. We demonstrated that ARMS individuals and patients with established Sz both exhibited a significantly higher prevalence of duplicated HG patterns than healthy controls. Furthermore, the HG pattern was associated with global symptom ratings and verbal fluency ability in these participants. The present results suggest that the gross morphological characteristics of the superior temporal plane represent vulnerability factors associated with psychosis, which may be associated with clinical trait abnormalities.
The present study replicated our previous findings from an independent cohort of first-episode Sz (Takahashi et al., in submission) showing increased HG duplication in Sz patients and also demonstrated that illness stages (i.e., first-episode vs. chronic stages) did not significantly influence HG patterns. On the other hand, a previous study by Hubl et al. (2010) only found a slightly higher prevalence of duplicated HG in chronic Sz patients. However, their negative finding may be partly due to the small sample size examined (13 Sz and 13 control participants) as well as their definition of HG duplication, which classified the CSD pattern as a variant of single HG. Since we demonstrated increased HG duplication in Sz regardless of the subtype (i.e., CSD or CPD), the Sz group examined by Hubl et al. (2010) must have had a higher prevalence of the duplicated HG pattern according to the traditional HG pattern definition [single vs. duplicated (CSD or CPD) (Leonard et al., 1998; Rademacher et al., 2001; Abdul-Kareem and Sluming, 2008; Marie et al., 2015)]. While the mechanisms regulating the development of cortical gyrification remain unclear, the secondary gyri of HG, which form variations in the HG gyrification pattern, predominantly develop during the late gestation period (i.e., after 36 weeks of gestation) (Chi et al., 1977) along with local neuronal connectivity and synaptic development (Van Essen, 1997), but remain stable after birth (Armstrong et al., 1995). Therefore, HG gyrification studies in Sz generally support the notion that the gyrification pattern in Sz represents a stable trait marker associated with early neurodevelopmental pathology (Matsuda and Ohi, 2018).
One of the primary results of the present study was that ARMS individuals, who may be vulnerable to psychopathology but will not necessarily develop overt psychosis (Yung et al., 2004; Fusar-Poli et al., 2012a), exhibited an increased HG duplication pattern similar to that in Sz. Based on the potential relationship between brain gyrification and local neuronal connectivity (Van Essen, 1997), the present results appear to be consistent with previous functional neuroimaging findings showing that the ARMS and Sz groups share local connectivity disruption involved in HG (Yoon et al., 2015; Du et al., 2018). A few MRI studies on cortical surface features in clinical high-risk individuals also showed similar gross morphological characteristics, such as altered sulcogyral patterns (Sasabayashi et al., 2017; Nakamura et al., 2019) and sulcal-depth abnormalities (Takahashi et al., 2019b), with patients with established Sz. In contrast to the evidence of active gray matter reductions in the superior temporal plane (e.g., HG and planum temporale) during the early illness stages of psychosis (Takahashi and Suzuki, 2018), a recent longitudinal study demonstrated the stability of gyrification features during the clinical high-risk period as a marker of early neurodevelopmental insults (Damme et al., 2019). Nevertheless, high-risk individuals with the later onset of psychosis may exhibit greater gyrification abnormalities before illness onset (Sasabayashi et al., 2017; Das et al., 2018) because greater and/or more prolonged neurodevelopmental deviations during gestation and consequent anomalous post-pubertal brain changes may lead to overt and sustained psychosis (Pantelis et al., 2005). Since the present ARMS group with a short follow-up period (median = 2.4 years) only examined a small number of participants with a later onset of psychosis (N = 5), the potential of the HG gyrification pattern as a predictive marker of the later onset of psychosis remains unclear.
The present results suggested that the partial duplication of HG (i.e., CSD) was associated with a more severe general psychopathology in ARMS individuals, supporting aberrant connectivity in the superior temporal region potentially contributing to prodromal-like symptoms (Yoon et al., 2015). However, the present Sz cohort (predominantly chronic cases) did not replicate the relationship between the CPD pattern and mild positive symptom severity observed in first-episode Sz (N = 62) (Takahashi et al., in submission), implicating that neurodevelopmental pathology may be associated with susceptibility to positive psychotic symptoms of Sz but this relationship may be influenced by various factors including illness stages and treatment. On the other hand, as also suggested in our sample (Table 1), cognitive deficits, particularly in verbal fluency and memory functioning, may exist even before the onset of psychosis as markers of increased vulnerability (Fusar-Poli et al., 2012b; Lee et al., 2015). In the present study, we found that participants with the left CSD pattern had a greater deficit in verbal fluency, but not in other domains or social functioning, than those with the left CPD pattern in the Sz (N = 63) or combined Sz and ARMS (N = 120) groups. This result appears to be consistent with the notion that candidate neural circuits for verbal fluency deficits include the superior temporal region for both the Sz (Frith et al., 1995; Antonova et al., 2004) and ARMS (Meijer et al., 2011) groups. While the functional role of the HG duplication type (i.e., CPD vs. CSD) remains largely unknown, participants with the CSD pattern may have a significantly smaller planum temporale gray matter than those with the CPD pattern bilaterally for both the Sz and control groups (Takahashi et al., in submission), which may lead to deficits in verbal ability (Shapleske et al., 1999). However, the potential contribution of different HG patterns to the pathophysiology of psychotic disorders warrants further study at various illness stages, particularly using functional neuroimaging.
Several potential limitations in the present study need to be addressed. First, as described above, it was not possible to examine whether the HG gyrification pattern was associated with the future onset of psychosis because only 5 participants (8.8%) in the ARMS group developed psychosis in the clinical follow-up period. Furthermore, the ARMS group was younger than the other groups in the present study. Second, the majority of Sz and 14 ARMS participants were being treated with antipsychotics during the present study. These factors were not expected to significantly affect gross sulcogyral patterns; however, antipsychotic medication may be a confounding factor for the morphology of the superior temporal plane (Takahashi and Suzuki, 2018) and cognitive functioning (Keefe, 2014). Therefore, future studies using a larger antipsychotic naïve ARMS cohort (particularly participants with a later onset of psychosis) and well-matched comparison groups are needed to examine the HG gyrification pattern and its potential contribution to clinical features (including the later onset of psychosis). Third, we did not correct our results of ANOVA/ANCOVA for multiple comparisons due to exploratory nature of our study. We predicted that the HG pattern would be associated with cognitive impairments, but we had no clear hypothesis and comprehensively assessed the potential contribution of HG pattern to all available cognitive subdomains, which might lead to potential Type I error. Finally, since superior temporal gray matter reductions (Takahashi et al., 2010a, c) and altered brain gyrification patterns (Yang et al., 2016; Maggioni et al., 2019) have been reported in other neuropsychiatric disorders (e.g., mood and anxiety disorders and autism), the disease specificity of the present results warrant further study.
The results of this MRI study demonstrated that clinical high-risk individuals for psychosis exhibited an increased HG duplication similar to that in patients with Sz, which may reflect common vulnerability factors. These groups partly shared cognitive impairments, which were associated with HG gyrification patterns. We also found a relationship between the HG pattern and severity of general symptoms observed in high-risk individuals. Therefore, the gross morphology of the superior temporal plane may represent the biological trait abnormalities of Sz that exist prior to illness onset; however, our findings should be replicated in an independent and larger cohort especially for high-risk individuals with and without the later onset of psychosis in order to investigate potential role of HG pattern as a predictive marker of Sz.
The data analyzed in this study is subject to the following licenses/restrictions: the datasets generated during the current study will not be available for public use, since we do not have permission to share the data. Requests to access these datasets should be directed to TT, dHN1dG9tdUBtZWQudS10b3lhbWEuYWM=.
The studies involving human participants were reviewed and approved by the Committee on Medical Ethics of Toyama University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
MS, YH, and TT conceived the idea and methodology of the study. TT conducted the statistical analyses and wrote the manuscript. DS, YH, MK, and HK recruited participants and were involved in clinical and diagnostic assessments. TT, DS, and TP analyzed MRI data. YM and SN assessed the sociocognitive functions of the study participants. KN provided technical support for MRI scanning and data processing. AF managed the MRI and clinical data. MS and YT contributed to the writing and editing of the manuscript. All authors contributed to and approved the final manuscript.
This work was supported by JSPS KAKENHI Grant Numbers JP18K07550 to TT, JP18K15509 to DS, and JP20H03598 to MS, and by the Health and Labour Sciences Research Grants for Comprehensive Research on Persons with Disabilities from the Japan Agency for Medical Research and Development (AMED) Grant Number JP19dk0307029 to MS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2021.647069/full#supplementary-material
Abdul-Kareem, I. A., and Sluming, V. (2008). Heschl gyrus and its included primary auditory cortex: structural MRI studies in healthy and diseased subjects. J. Magn. Reson. Imaging 28, 287–299. doi: 10.1002/jmri.21445
Alderson-Day, B., McCarthy-Jones, S., and Fernyhough, C. (2015). Hearing voices in the resting brain: A review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci. Biobehav. Rev. 55, 78–87. doi: 10.1016/j.neubiorev.2015.04.016
American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders, 4rd Edn. Washington, DC: American Psychiatric Association. Text Revised.
Antonova, E., Sharma, T., Morris, R., and Kumari, V. (2004). The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr. Res. 70, 117–145. doi: 10.1016/j.schres.2003.12.002
Armstrong, E., Schleicher, A., Omran, H., Curtis, M., and Zilles, K. (1995). The ontogeny of human gyrification. Cereb. Cortex 5, 56–63. doi: 10.1093/cercor/5.1.56
Chi, J. G., Dooling, E. C., and Gilles, F. H. (1977). Gyral development of the human brain. Ann. Neurol. 1, 86–93. doi: 10.1002/ana.410010109
Concina, G., Renna, A., Grosso, A., and Sacchetti, B. (2019). The auditory cortex and the emotional valence of sounds. Neurosci. Biobehav. Rev. 98, 256–264. doi: 10.1016/j.neubiorev.2019.01.018
Da Costa, S., van der Zwaag, W., Marques, J. P., Frackowiak, R. S., Clarke, S., and Saenz, M. (2011). Human primary auditory cortex follows the shape of Heschl’s gyrus. J. Neurosci. 31, 14067–14075. doi: 10.1523/JNEUROSCI.2000-11.2011
Damme, K. S. F., Gupta, T., Nusslock, R., Bernard, J. A., Orr, J. M., and Mittal, V. A. (2019). Cortical morphometry in the psychosis risk period: a comprehensive perspective of surface features. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 434–443. doi: 10.1016/j.bpsc.2018.01.003
Das, T., Borgwardt, S., Hauke, D. J., Harrisberger, F., Lang, U. E., Riecher-Rössler, A., et al. (2018). Disorganized gyrification network properties during the transition to psychosis. JAMA Psychiatry 75, 613–622. doi: 10.1001/jamapsychiatry.2018.0391
Du, Y., Fryer, S. L., Fu, Z., Lin, D., Sui, J., Chen, J., et al. (2018). Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. Neuroimage 180(Pt B), 632–645. doi: 10.1016/j.neuroimage.2017.10.022
Eguchi, S., Koike, S., Suga, M., Takizawa, R., and Kasai, K. (2015). Psychological symptom and social functioning subscales of the modified global assessment of functioning scale: reliability and validity of the Japanese version. Psychiatry Clin. Neurosci. 69, 126–127. doi: 10.1111/pcn.12250
First, M. B., Gibbon, M., Spitzer, R. L., and Williams, J. B. W. (1997). Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press.
Frith, C. D., Friston, K. J., Herold, S., Silbersweig, D., Fletcher, P., Cahill, C., et al. (1995). Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br. J. Psychiatry 167, 343–349. doi: 10.1192/bjp.167.3.343
Fusar-Poli, P., Bonoldi, I., Yung, A. R., Borgwardt, S., Kempton, M. J., Valmaggia, L., et al. (2012a). Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry 69, 220–229. doi: 10.1001/archgenpsychiatry.2011.1472
Fusar-Poli, P., Deste, G., Smieskova, R., Barlati, S., Yung, A. R., Howes, O., et al. (2012b). Cognitive functioning in prodromal psychosis: a meta-analysis. Arch. Gen. Psychiatry 69, 562–571. doi: 10.1001/archgenpsychiatry.2011.1592
Hubl, D., Dougoud-Chauvin, V., Zeller, M., Federspiel, A., Boesch, C., Strik, W., et al. (2010). Structural analysis of Heschl’s gyrus in schizophrenia patients with auditory hallucinations. Neuropsychobiology 61, 1–9. doi: 10.1159/000258637
Kay, S. R., Fiszbein, A., and Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276.
Keefe, R. S. (2014). The longitudinal course of cognitive impairment in schizophrenia: an examination of data from premorbid through posttreatment phases of illness. J. Clin. Psychiatry 75(Suppl. 2), 8–13. doi: 10.4088/JCP.13065su1.02
Keefe, R. S., Goldberg, T. E., Harvey, P. D., Gold, J. M., Poe, M. P., and Coughenour, L. (2004). The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 68, 283–297.
Keefe, R. S., Poe, M., Walker, T. M., Kang, J. W., and Harvey, P. D. (2006). The schizophrenia cognition rating scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am. J. Psychiatry 163, 426–432.
Lee, T. Y., Hong, S. B., Shin, N. Y., and Kwon, J. S. (2015). Social cognitive functioning in prodromal psychosis: a meta-analysis. Schizophr. Res. 164, 28–34. doi: 10.1016/j.schres.2015.02.008
Leonard, C. M., Eckert, M. A., Lombardino, L. J., Oakland, T., Kranzler, J., Mohr, C. M., et al. (2001). Anatomical risk factors for phonological dyslexia. Cereb. Cortex 11, 148–157. doi: 10.1093/cercor/11.2.148
Leonard, C. M., Puranik, C., Kuldau, J. M., and Lombardino, L. J. (1998). Normal variation in the frequency and location of human auditory cortex landmarks. Heschl’s gyrus: where is it? Cereb. Cortex 8, 397–406. doi: 10.1093/cercor/8.5.397
Leonard, C. M., Voeller, K. K., Lombardino, L. J., Morris, M. K., Hynd, G. W., Alexander, A. W., et al. (1993). Anomalous cerebral structure in dyslexia revealed with magnetic resonance imaging. Arch. Neurol. 50, 461–469. doi: 10.1001/archneur.1993.00540050013008
Maggioni, E., Delvecchio, G., Grottaroli, M., Garzitto, M., Piccin, S., Bonivento, C., et al. (2019). Common and different neural markers in major depression and anxiety disorders: A pilot structural magnetic resonance imaging study. Psychiatry Res. Neuroimaging 290, 42–50. doi: 10.1016/j.pscychresns.2019.06.006
Marie, D., Jobard, G., Crivello, F., Perchey, G., Petit, L., Mellet, E., et al. (2015). Descriptive anatomy of Heschl’s gyri in 430 healthy volunteers, including 198 left-handers. Brain Struct. Funct. 220, 729–743. doi: 10.1007/s00429-013-0680-x
Matsuda, Y., and Ohi, K. (2018). Cortical gyrification in schizophrenia: current perspectives. Neuropsychiatr. Dis. Treat. 14, 1861–1869. doi: 10.2147/NDT.S145273
Matsuoka, K., Uno, M., Kasai, K., Koyama, K., and Kim, Y. (2006). Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words: Japanese version of national adult reading test. Psychiatry Clin. Neurosci. 60, 332–339.
Meijer, J. H., Schmitz, N., Nieman, D. H., Becker, H. E., van Amelsvoort, T. A., Dingemans, P. M., et al. (2011). Semantic fluency deficits and reduced grey matter before transition to psychosis: a voxelwise correlational analysis. Psychiatry Res. 194, 1–6. doi: 10.1016/j.pscychresns.2011.01.004
Mier, D., Eisenacher, S., Rausch, F., Englisch, S., Gerchen, M. F., Zamoscik, V., et al. (2017). Aberrant activity and connectivity of the posterior superior temporal sulcus during social cognition in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 267, 597–610. doi: 10.1007/s00406-016-0737-y
Mizuno, M., Suzuki, M., Matsumoto, K., Murakami, M., Takeshi, K., Miyakoshi, T., et al. (2009). Clinical practice and research activities for early psychiatric intervention at Japanese leading centres. Early Interv. Psychiatry 3, 5–9. doi: 10.1111/j.1751-7893.2008.00104.x
Nakamura, M., Takahashi, T., Takayanagi, Y., Sasabayashi, D., Katagiri, N., Sakuma, A., et al. (2019). Surface morphology of the orbitofrontal cortex in individuals at risk of psychosis: a multicenter study. Eur. Arch. Psychiatry Clin. Neurosci. 269, 397–406. doi: 10.1007/s00406-018-0890-6
Okada, N., Kasai, K., Takahashi, T., Suzuki, M., Hashimoto, R., Kameyama, T., et al. (2014a). Rating scale of handedness for biological psychiatry research among Japanese people. Japanese J. Biol. Psychiatry 25, 118–119.
Okada, N., Kasai, K., Takahashi, T., Suzuki, M., Hashimoto, R., and Kawakami, N. (2014b). Brief rating scale of socioeconomic status for biological psychiatry research among Japanese people: a scaling based on an educational history. Japanese J. Biol. Psychiatry 25, 115–117.
Pantelis, C., Yücel, M., Wood, S. J., Velakoulis, D., Sun, D., Berger, G., et al. (2005). Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr. Bull. 31, 672–696. doi: 10.1093/schbul/sbi034
Rademacher, J., Caviness, V. S. Jr., Steinmetz, H., and Galaburda, A. M. (1993). Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb. Cortex 3, 313–329. doi: 10.1093/cercor/3.4.313
Rademacher, J., Morosan, P., Schormann, T., Schleicher, A., Werner, C., Freund, H. J., et al. (2001). Probabilistic mapping and volume measurement of human primary auditory cortex. Neuroimage 13, 669–683. doi: 10.1006/nimg.2000.0714
Sasabayashi, D., Takayanagi, Y., Takahashi, T., Koike, S., Yamasue, H., Katagiri, N., et al. (2017). Increased occipital gyrification and development of psychotic disorders in individuals with an at-risk mental state: a multicenter study. Biol. Psychiatry 82, 737–745. doi: 10.1016/j.biopsych.2017.05.018
Shapleske, J., Rossell, S. L., Woodruff, P. W., and David, A. S. (1999). The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res. Brain Res. Rev. 29, 26–49. doi: 10.1016/s0165-0173(98)00047-2
Takahashi, T., Higuchi, Y., Komori, Y., Nishiyama, S., Nakamura, M., Sasabayashi, D., et al. (2017). Quality of life in individuals with attenuated psychotic symptoms: possible role of anxiety, depressive symptoms, and socio-cognitive impairments. Psychiatry Res. 257, 431–437. doi: 10.1016/j.psychres.2017.08.024
Takahashi, T., Malhi, G. S., Wood, S. J., Yücel, M., Walterfang, M., Kawasaki, Y., et al. (2010a). Gray matter reduction of the superior temporal gyrus in patients with established bipolar I disorder. J. Affect. Disord. 123, 276–282. doi: 10.1016/j.jad.2009.08.022
Takahashi, T., Nakamura, M., Nishikawa, Y., Komori, Y., Nishiyama, S., Takayanagi, Y., et al. (2019a). Potential role of orbitofrontal surface morphology on social and cognitive functions in high-risk subjects for psychosis and schizophrenia patients. Psychiatry Res. Neuroimaging 283, 92–95. doi: 10.1016/j.pscychresns.2018.12.002
Takahashi, T., Nakamura, M., Sasabayashi, D., Komori, Y., Higuchi, Y., Nishikawa, Y., et al. (2018). Olfactory deficits in individuals at risk for psychosis and patients with schizophrenia: relationship with socio-cognitive functions and symptom severity. Eur. Arch. Psychiatry Clin. Neurosci. 268, 689–698. doi: 10.1007/s00406-017-0845-3
Takahashi, T., Nakamura, M., Sasabayashi, D., Nishikawa, Y., Takayanagi, Y., Furuichi, A., et al. (2019b). Association between olfactory sulcus morphology and olfactory functioning in schizophrenia and psychosis high-risk status. Heliyon 5:e02642. doi: 10.1016/j.heliyon.2019.e02642
Takahashi, T., and Suzuki, M. (2018). Brain morphologic changes in early stages of psychosis: implications for clinical application and early intervention. Psychiatry Clin. Neurosci. 72, 556–571. doi: 10.1111/pcn.12670
Takahashi, T., Wood, S. J., Yung, A. R., Walterfang, M., Phillips, L. J., Soulsby, B., et al. (2010b). Superior temporal gyrus volume in antipsychotic-naive people at risk of psychosis. Br. J. Psychiatry 196, 206–211. doi: 10.1192/bjp.bp.109.069732
Takahashi, T., Yücel, M., Lorenzetti, V., Walterfang, M., Kawasaki, Y., Whittle, S., et al. (2010c). An MRI study of the superior temporal subregions in patients with current and past major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 98–103. doi: 10.1016/j.pnpbp.2009.10.005
Tzourio-Mazoyer, N., Marie, D., Zago, L., Jobard, G., Perchey, G., Leroux, G., et al. (2015). Heschl’s gyrification pattern is related to speech-listening hemispheric lateralization: fMRI investigation in 281 healthy volunteers. Brain Struct. Funct. 220, 1585–1599. doi: 10.1007/s00429-014-0746-4
Van Essen, D. C. (1997). A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313–318. doi: 10.1038/385313a0
Weinberger, D. R. (1987). Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44, 660–669. doi: 10.1001/archpsyc.1987.01800190080012
Weinberger, N. M. (2015). New perspectives on the auditory cortex: learning and memory. Handb. Clin. Neurol. 129, 117–147. doi: 10.1016/B978-0-444-62630-1.00007-X
Yang, D. Y., Beam, D., Pelphrey, K. A., Abdullahi, S., and Jou, R. J. (2016). Cortical morphological markers in children with autism: a structural magnetic resonance imaging study of thickness, area, volume, and gyrification. Mol. Autism 7:11. doi: 10.1186/s13229-016-0076-x
Yoon, Y. B., Yun, J. Y., Jung, W. H., Cho, K. I., Kim, S. N., Lee, T. Y., et al. (2015). Altered fronto-temporal functional connectivity in individuals at ultra-high-risk of developing psychosis. PLoS One 10:e0135347. doi: 10.1371/journal.pone.0135347
Yung, A. R., Phillips, L. J., and McGorry, P. D. (2004). Treating Schizophrenia in the Prodromal Phase. London: Taylor & Francis.
Keywords: at-risk mental state, schizophrenia, Heschl’s gyrus, gyrification, early neurodevelopment
Citation: Takahashi T, Sasabayashi D, Takayanagi Y, Higuchi Y, Mizukami Y, Nishiyama S, Furuichi A, Kido M, Pham TV, Kobayashi H, Noguchi K and Suzuki M (2021) Heschl’s Gyrus Duplication Pattern in Individuals at Risk of Developing Psychosis and Patients With Schizophrenia. Front. Behav. Neurosci. 15:647069. doi: 10.3389/fnbeh.2021.647069
Received: 29 December 2020; Accepted: 29 March 2021;
Published: 20 April 2021.
Edited by:
Stella G. Giakoumaki, University of Crete, GreeceReviewed by:
Hideo Hagihara, Fujita Health University, JapanCopyright © 2021 Takahashi, Sasabayashi, Takayanagi, Higuchi, Mizukami, Nishiyama, Furuichi, Kido, Pham, Kobayashi, Noguchi and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tsutomu Takahashi, dHN1dG9tdUBtZWQudS10b3lhbWEuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.