
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Behav. Neurosci. , 16 December 2020
Sec. Individual and Social Behaviors
Volume 14 - 2020 | https://doi.org/10.3389/fnbeh.2020.612313
This article is part of the Research Topic Modern Methods in Neuroethology View all 22 articles
The post-embryonal development of arthropod species, including crustaceans and insects, is characterized by ecdysis or molting. This process defines growth stages and is controlled by a conserved neuroendocrine system. Each molting event is divided in several critical time points, such as pre-molt, molt, and post-molt, and leaves the animals in a temporarily highly vulnerable state while their cuticle is re-hardening. The molting events occur in an immediate ecdysis sequence within a specific time window during the development. Each sub-stage takes only a short amount of time, which is generally in the order of minutes. To find these relatively short behavioral events, one needs to follow the entire post-embryonal development over several days. As the manual detection of the ecdysis sequence is time consuming and error prone, we designed a monitoring system to facilitate the continuous observation of the post-embryonal development of the fruit fly Drosophila melanogaster. Under constant environmental conditions we are able to observe the life cycle from the embryonic state to the adult, which takes about 10 days in this species. Specific processing algorithms developed and implemented in Fiji and R allow us to determine unique behavioral events on an individual level—including egg hatching, ecdysis and pupation. In addition, we measured growth rates and activity patterns for individual larvae. Our newly created RPackage PEDtracker can predict critical developmental events and thus offers the possibility to perform automated screens that identify changes in various aspects of larval development. In conclusion, the PEDtracker system presented in this study represents the basis for automated real-time staging and analysis not only for the arthropod development.
Ecdysis or molting is the most important feature of ecdysozoan species including arthropods, nematodes and other relatives (Telford et al., 2008). The whole body surface of these animals is surrounded by a chitinous cuticle which hardened, for example, to an exoskeleton in the whole group of arthropods (Hadley, 1986; Ewer, 2005). Therefore, for a successful growth from juveniles to adults the animals have to shed and renew their body surface throughout their post-embryonal development (Nijhout, 2013). The renewing process is controlled by a highly conserved neuroendocrine system which has been well described in several species, especially in arthropods such as the crab Portunus trituberculatus, the butterfly Manduca sexta and the fly Drosophila melanogaster (Rewitz et al., 2007; Xie et al., 2016, Riddiford et al., 1999). Beside the renewing of the cuticle, some insect groups further evolved a total re-organization (metamorphosis) from the last larval stage to the adult during a pupal stage (holometabolism). Consequently, the post-embryonal development of holometabolous insects is characterized by both, molting and metamorphosis (Weeks and Truman, 1984; Truman, 1996).
Although the molecular pathway and regulation of molting-related molecules are well understood, the question arises whether different factors such as the physiological state of the animals, food choice and environmental stimuli influence the post-embryonal development and directly affect the larval endocrine system and growth (Koyama et al., 2014). While hormones such as 20-hydroxyecdysone, juvenile hormone and insulin are well known to function in molting and growth especially in insects (Chang, 1985; Riddiford et al., 2003; Beckstead et al., 2005; Lin and Smagghe, 2019), the role and importance of the critical weight especially for the initiation of metamorphosis is still not fully understood (Robertson, 1963; Nijhout and Williams, 1974; Davidowitz et al., 2003; Mirth et al., 2005).
To get a better understanding of the physiological state, molecular background and motivation of the individual animal during specific developmental time points, such as molting or metamorphosis, staging of juveniles, or larvae represents an important approach. For example, larvae of holometabolous insects can be manually classified into developmental stages by morphological characteristics such as the differentiation of the mouth hooks or anterior and posterior spiracles (according to, e.g., Okada, 1963; Schubiger et al., 1998; Vaufrey et al., 2018). Another approach for staging juveniles or larvae is to allow females to lay eggs for some hours on a food media and examine larvae after a set time interval, for example every 12 h (according to, e.g., Burmester et al., 1999). However, both manual detection approaches are time-consuming. In addition, specific larval stages and developmental time points are difficult to catch as the main ecdysis behavioral sequence takes only a comparatively short time window of a few minutes. The molting cycle in general comprises four consecutive phases: (1) intermolt; the time between two molting events, (2) pre-molt; the time-window just before the main molt, (3) molt; the time-window where larvae shed their cuticle, and (4) post-molt; the time-window right after the main molt (Locke, 1970). In the fly D. melanogaster the ecdysis behavioral sequence as part of the molting cycle is described to last only 30 min and is divided in four parts—the occurrence of the double mouth hooks and vertical plates, renewing of the tracheal system, pre-ecdysis and ecdysis (Park et al., 2002).
Our long-term goal is the investigation of individual molecular and behavioral changes during the molting cycle of the animal. As the main ecdysis takes about 5 min of a single larval stage of D. melanogaster, the first aim was to find a tool for precisely timing these stages for investigations on development-associated processes such as sensation and food choice. Using D. melanogaster, we established a monitoring system throughout the whole post-embryonal development—from egg to pupa—over 10 days (Dewhurst et al., 1970). Under constant environmental conditions (25°C and 65% humidity) and a yeast-sugar diet, we observed the life cycle from egg to pupa with a camera and a framerate of 3 frames/minute. We subsequently analyzed the videos with the focus on the molting cycle throughout the post-embryonal development such as the main molting sequence on an individual level. Our results reveal insights into larval behavior regarding activity and growth. We clearly observe the specific activity pattern during the molting cycle and the individual growth in size from young to old larval stages. Consisting of a video recording set up and newly developed analysis scripts (in Fiji and R), the PEDtracker (=post-embryonal development tracker) forms the basis for a future real-time tracking system for the prediction of developmental stages which could also be used for various other insects and their relatives.
All experiments were performed with the wild-type Drosophila melanogaster strain Canton-S. Flies were kept on standardized cornmeal medium at 25°C and 65% humidity under a 14:10 light:dark cycle. Adult flies were transferred to new food vials every 72 h.
For egg laying small Petri dishes (4 cm diameter) were filled with a 3% agarose (VWR life science; type number: 97062-250), 3% sucrose (Merck KGaA; type number: 107687), and 30% apple juice (Edeka) mix. To entice female flies to oviposit one drop of yeast was put on top of the plate. Flies were allowed to lay eggs for at least 2 h and then, one egg per chamber was transferred to the larval bed. The bed was prepared as previously described (Szuperak et al., 2018) with the SYLGARD® 184 Silicone Elastomer Kit (type number: 24001673921) and a size of 8.3 cm in length and 5.7 cm in depth in a self-made 3D-printed template (Renkforce 3D-printer RF1000; material PLA). Each bed contains of 24 chambers (4 x 6) with each 1 cm in diameter. For an optimal set-up, with the regard to humidity and the camera resolution, maximal half of the chambers could be filled. Before placing eggs, each chamber was filled with 100 μl food medium containing a mix of 2% agarose (VWR life science; type number: 97062-250), 2% sucrose (Merck KGaA; type number: 107687), and three drops of fresh yeast. To avoid mold a mixture of 0.1% methylparaben-ethanol (methylparaben: Carl Roth GmbH + Co. KG type number: 3646.4; ethanol: CHEMSOLUTE® type number: 2273.1000) was added to the food medium. To avoid an escape of larvae, the bed was covered with clear film and two layers of glass plates. The bed was then placed in a custom-built climate chamber (workshop of the University of Konstanz) on a glass table with constant LED-light (KYG light-table) from below (Figure 1). The climate chamber provided a constant temperature (23–25°C) and humidity (60–65%) for our experiments. Lower humidity (< 50%) led to a stronger dehydration of the food, a higher humidity (>75%) caused condensation on the clear film. Pictures were taken every 20 s over a period of at least 10 days with a 25 mm lens using a Basler camera (acA2040-25gm; type number: 105715) with a resolution of 2048 × 2048.
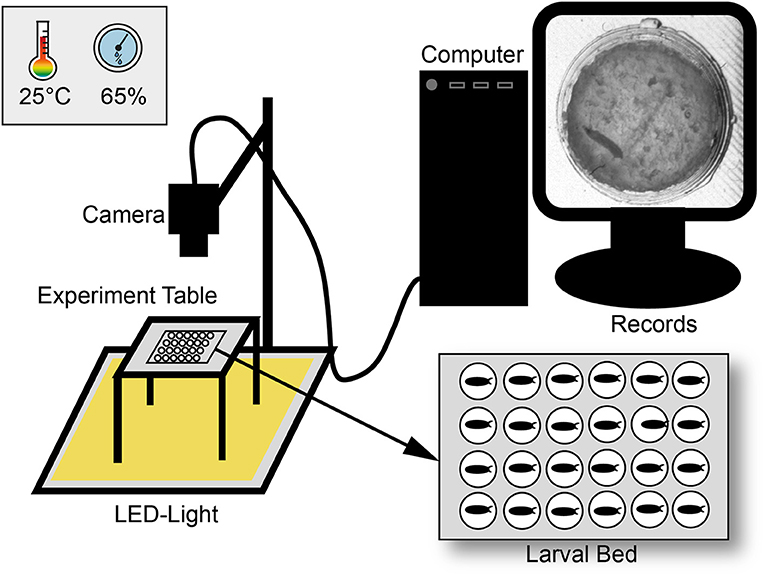
Figure 1. Scheme of the experimental set-up. Larvae were observed over ten days under constant light in a climate chamber with constant abiotic conditions. For observation three pictures per minute were recorded with a camera and saved with a custom-made software. Pictures were then combined to a video sequence and analyzed with custom-made scripts in ImageJ and R.
We recorded 22 experiments (seven runs for finding optimal conditions; 15 runs with optimal conditions) and examined 43 individuals for the hatching timepoint, 36 individuals for the first molting event, 23 individuals for the second molting event such as 33 wandering stages. For data analysis pictures were processed as individual 6-h video sequences (1080 frames) with a custom-made Fiji script (Schindelin et al., 2012; see Supplementary Figure 1). We first defined regions of interest (ROIs) marking the individual chambers. For each ROI a median background image was created and subtracted from the cropped video. The resulting images were thresholded and then analyzed with specific measurement settings (Table 1) in Fiji.
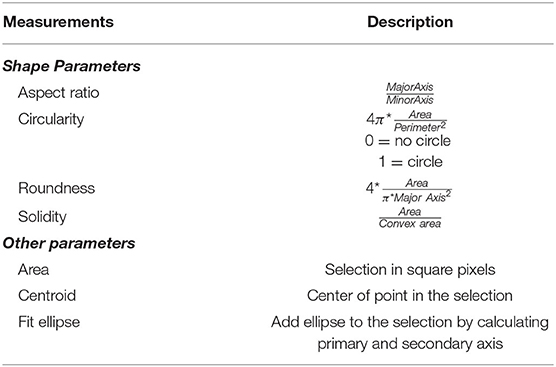
Table 1. Measurements used in ImageJ for video analysis (full description via https://imagej.nih.gov/ij/docs/guide/146-30.html#toc-Subsection-30.7).
After image processing in Fiji (Figure 2), we followed up with error correction and further in-depth analysis in a custom-written R script using R version 3.6.1 (R Core Team, 2019) in RStudio (R Studio Team, 2019; see Supplementary Figure 2). For each larval stage, parameters for size and shape of valid objects were defined (Table 1, Figure 3, Supplementary Figure 3). To determine the length of each larva the major axis of each object was scaled to the diameter of each chamber (~ 300 px = 10 mm). Growth rates were then calculated as follows:
To determine larval movement, the Euclidian distance between the centroids (X1, X2; Y1, Y2) in two successive frames was calculated through Pythagorean function
Next, the relative movement over time was used to get an activity pattern for the individual larvae. Data frames containing analyzed particles were then sorted, merged and plotted. We show examples of the molting behavior of two larval stages in time lapse videos (recorded at 3 fpm; played back at 3 fps; see Supplementary Material 1, 2). Statistical analyses were conducted with R version 3.6.1 (R Core Team, 2019) in RStudio (R Studio Team, 2019) using the R Stats Package stats. For evaluate the difference between manual and computational object detection for place and length we used the paired t-test [t.test(a,b, paired = TRUE)]; to evaluate the variances of different developmental events for timing and size we used the F-test with the greater alternative [var.test(a,b, alternative = “greater”)]. Final figures were designed with R version 3.61 in R Studio using the ggplot2 package and were then edited with Adobe Illustrator CS5 (San Jose, CA, USA).
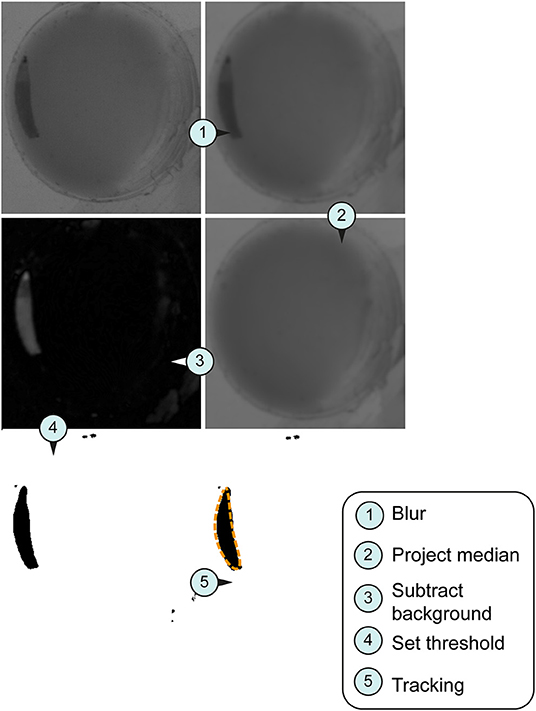
Figure 2. Image processing for tracking system. Larval stage three were used for all imaged pictures. (A) Original section of a video sequence. Note individual larva in the chamber. (B) Section of a video sequence. Picture were smoothed using convolution with Gaussian function. (C) Median calculation of an image shows the median intensity over all images in a stack. (D) Implementation of an arithmetic and logical operation. We used the difference between the source (img1) and destination image (img2)—imgX = | img1—img2 |. (E) Set threshold dependent on the larval stage (L1, 10; L2, 20; L3, 30). (F) Orange dashed line represents the silhouette of the object of interest in the section.
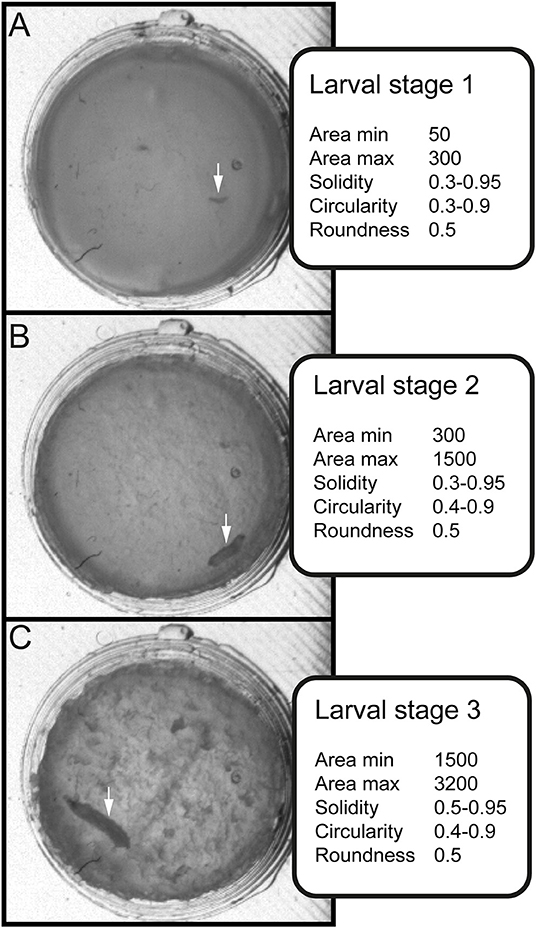
Figure 3. Stage-specific parameters for data sorting and evaluation. Three values for shape description—solidity, circularity and roundness—are used in combination with the area to differentiate between larval stages. (A) Arrow indicates position of larval stage one. Surface of individual larva ranges from 50 to 300 square pixels. (B) Arrow indicates position of larval stage two. Surface of individual larva ranges from 300 to 1500 square pixels. (C) Arrow indicates position of larval stage three. Surface of individual larva ranges from 1500 to 3000 square pixels.
For tracking adult Drosophila melanogaster there exist many different approaches to monitor activity over several days, usually with the focus on circadian activity and sleep research. Some of these approaches make use of infrared light beams (e.g., the Drosophila Activity Monitor; Pfeiffenberger et al., 2010) or video tracking (e.g., Gilestro, 2012) to monitor the flies but these setups are not designed to handle objects of varying size and shape. As a basis for our tracking setup we used the LarvaLodge (Szuperak et al., 2018) which itself is based on earlier work in Caenorhabditis elegans (Churgin et al., 2017). In their experiment up to 20 larvae can be monitored simultaneously for several hours. For our even longer approach—monitoring the whole post-embryonal development of D. melanogaster over the course of several days—we had to overcome multiple challenges. Our first task was to improve the setup to enable proper larval development over several days. For this we had to address humidity issues and prevent the growth of mold. We settled on a frame rate of three frames per minute as a middle ground to still be able to measure activity but also record for a timespan of more than a week without generating a vast amount of data. One typical experiment contains about 60,480 images (14 days; lossless compression as .png-images). The first step of processing was performed in ImageJ where the target objects (i.e., the larvae) are extracted from the video. Here we had to address the problem of the dramatic change in size of the larvae over the course of their development as well as the change in the environment over several days. Difficulties occurred with food drying out or larvae digging into the food which have been solved to some extent in ImageJ with the Gaussian Blur filter and by the application of binary Close and Dilate functions. Further improvement of the quality of our data was then performed in the secondary processing implemented with R in RStudio. A evaluation of the set up regarding the definition of larval parameters, object detection and definition of larval length are shown in Table 2, Supplementary Figures 3–5. Interestingly, during the review process of this paper another system for tracking activity in D. melanogaster has been published (DIAMonDS; Seong et al., 2020). Their setup uses a flatbed scanner to monitor the animals during their whole lifecycle from embryo to death. Their work focuses on transitions between static (embryo, pupa, carcass) and dynamic stages (larva, adult fly), but they do not discriminate between different larval stages.
The characterization of specific developmental time points and larval size are important parameters for the investigation of the body constitution as well as the behavior during the development. Looking at an example of larval activity over a period of about 3 h we can see that the larva crawls through the chamber and stops at frame 213 (video position ~ 1:20 min, see Supplementary Figures 6, 7) and then rests in the same place for almost 30 min. Interestingly, after 14 min the larva turns to the side and rests there for another 15 min. During both resting periods, the record indicates alternating phases of resting, pulsation and contraction (frames 213–300, video position 1:20–1:40 min). After comparing the video to previous studies we suggest that the observed resting phase in the video sequence correspond to the ecdysis behavioral sequence of the D. melanogaster larva (Park et al., 2002). Since the PEDtracker can only detect low activity but not contractions so far, we have examined several detected molting events manually and assume that the first resting phase corresponds to tracheal molt and air filling, and the second one with stronger pulsation and contractions to pre-molt and main molt. The same pattern occurs during the ecdysis behavioral sequence of L2 to L3 (see Supplementary Figures 8, 9). We analyzed both ecdysis behavioral sequences for larval activity (Figure 4) and show that the activity patterns for both larval stages reveal a decrease of movement during molting events. Whereas, larvae move up to 5 mm in the intermolt phases, they slow down to at least 0.5 mm in the pre-molt and main molt stage (Figure 4).
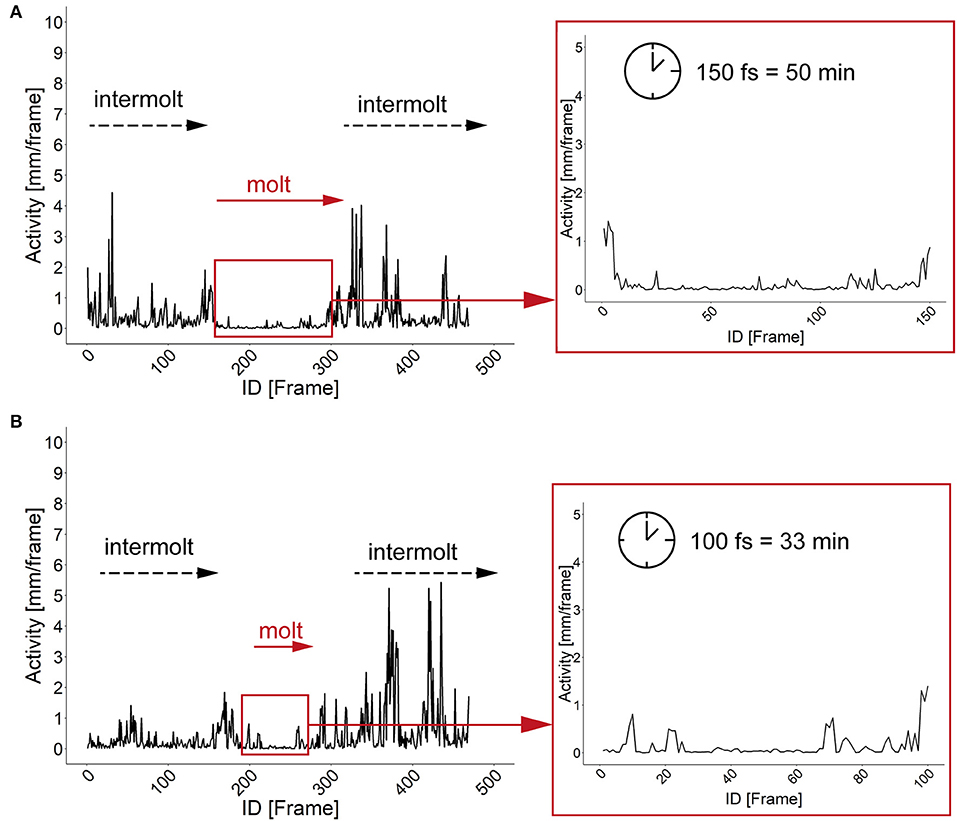
Figure 4. Activity patterns of two developmental stages and related molting events. (A) Activity pattern (in mm) and molting sequence of larval stage one. Insert represents the activity pattern (in mm) of larval stage one during the main molting event. Note a time window of 150 fs of low activity of the larva. (B) Activity pattern (in mm) and molting sequence of larval stage two. Insert represents the activity pattern (in mm) of larval stage two during the main molting event. Note a time window of 100 fs of low activity of the larva. fs, frames; min, minutes.
Our results reveal a mean hatching time point of 18 h after egg laying and a nearly similar size of about 0.5 millimeter for young L1 Canton-S larvae (Figures 5, 6). Interestingly, the later the developmental stage the higher is the variability of the time point of molting events and the respective body sizes of larvae (Figures 5A,C, 6; Table 2). Our results indicate a mean lifespan of D. melanogaster Canton-S larvae of 7.8 days from egg hatching to pupation (Figures 4A, 7). This result is in contrast to previous studies which revealed that D. melanogaster larvae went under pupation five days after egg laying (Table 3, Dewhurst et al., 1970; Ashburner, 1989; Casas-Vila et al., 2017). Our results further indicate less stringency in developmental time points and life spans of single larval stages in comparison with the literature (Figure 7). Whereas the mean hatching time after egg laying is in line with previous studies (about 18 h; 0.7 days; Markow et al., 2009), our results indicate the first molting event 2.6 days after egg laying (=1.9 days after hatching) and the second molting event 4.2 days after egg laying (=3.5 days after hatching). Previous studies indicated that larvae of the same age not pupated at the same time (Casares and Carracedo, 1987). Thus, the life cycle of D. melanogaster might be variable to some degree and dependent on different life-history traits and not only on the expression of molting related molecules such as ecdysone and juvenile hormone.
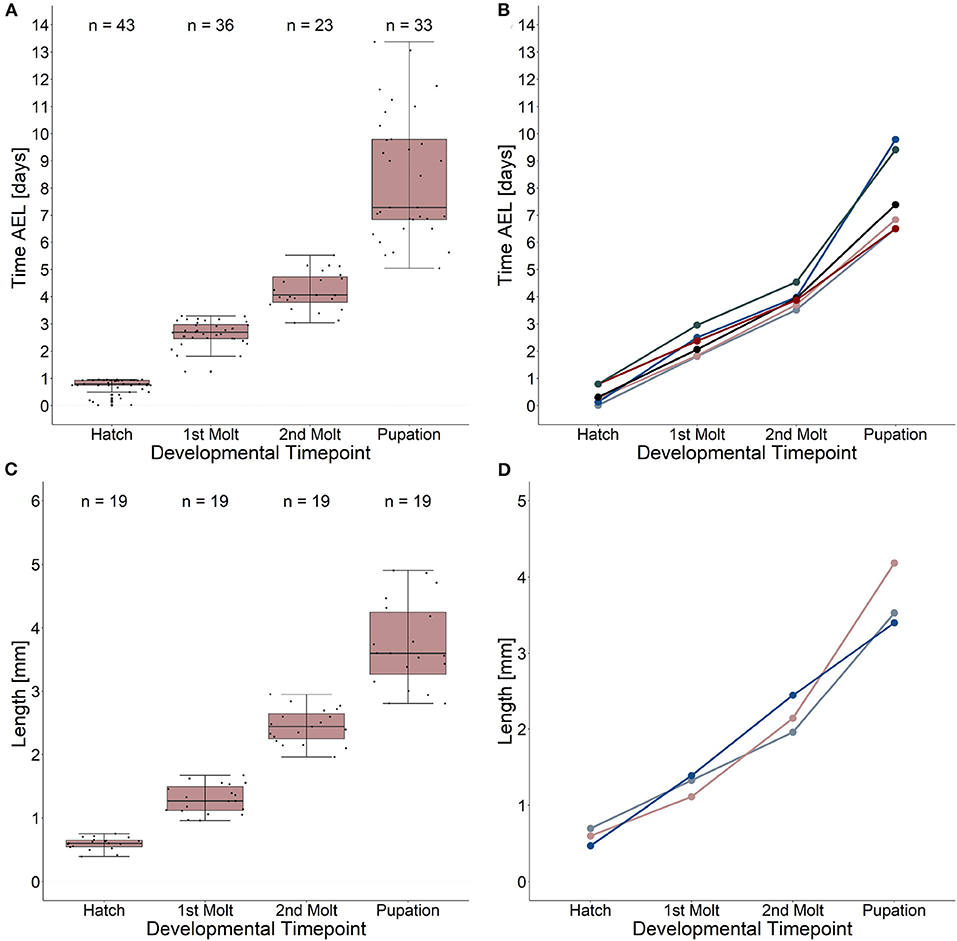
Figure 5. Developmental time points and related growth in length. (A) Time after egg laying and corresponding developmental time points. Note the high variation (five to ten days after egg laying) of the pupation event. (B) Time after egg laying and corresponding developmental time points for five single larvae as an example. (C) Growth in length from egg hatching to pupation and related developmental time points. Note the high variation of larval length at the pupation event. Growth ratemedian = 4.9. (D) Growth in length and related developmental time points for three single larvae as an example. Note the highest growth in length in larval stage three. Growth rate 1(blue line) = 6.2; growth rate 2 (light red line) = 5.9; growth rate 3 (gray line) = 4.06. See table 2 for statistical tests and p-values.
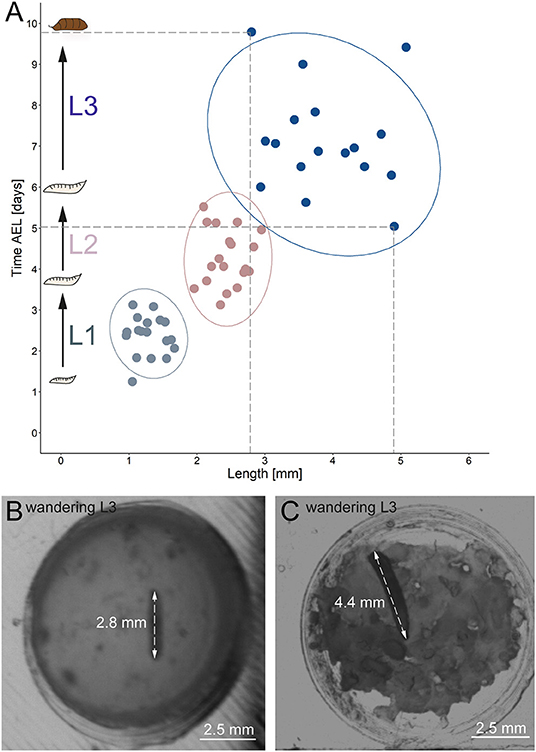
Figure 6. Correlation between developmental time and size. (A) Y-axis indicates time after egg laying in days; x-axis represents larval length in mm. Light gray dots indicate larval stage one, light red dots indicate larval stage two and blue dots indicate larval stage three. Ellipses represent 95% confidence level. (B) Arrows indicate the length of a small late stage three larvae, approximately one hour before pupation. (C) Arrows indicate the length of a large late stage three larvae approximately one hour before pupation. AEL, after egg laying; mm, millimeter.
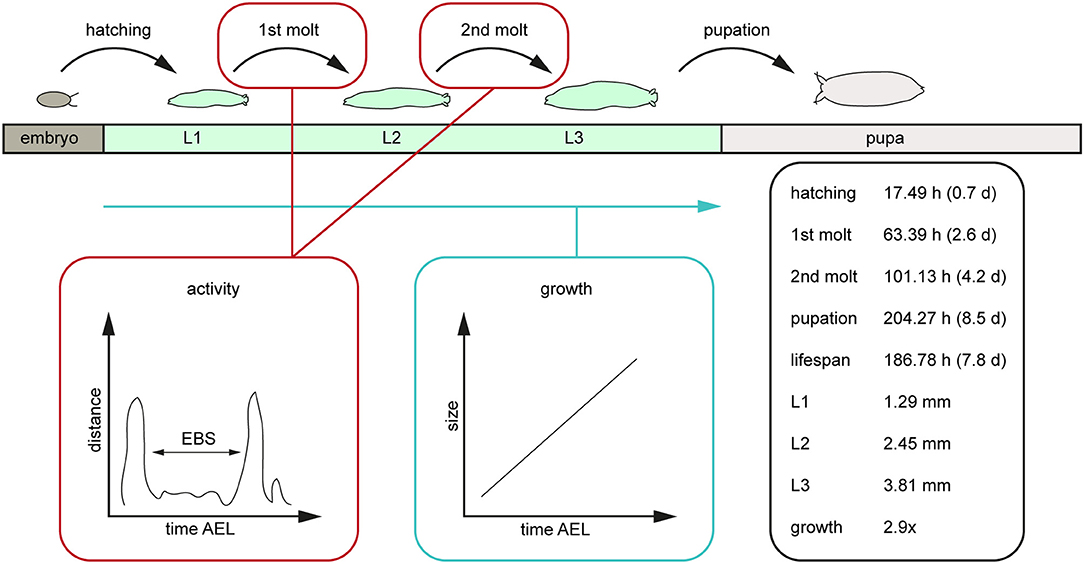
Figure 7. Scheme of the post-embryonal development of D. melanogaster and related values generated by the PEDtracker. The given values represent the average values of relevant developmental time points after egg laying such as the larval lifespan, the average length of all larval stages in length and the related growth rate. AEL, after egg laying; d, days; EBS, ecdysis behavioral sequence; h, hours; L1, larval stage one; L2, larval stage two; L3, larval stage three; mm, millimeter.
Previous studies showed that the developmental period of drosophilid species depends on environmental stimuli such as temperature, humidity or light. The developmental period successively decreases to seven days depending on the increase of the temperature up to 28 degree (Ashburner and Thompson, 1978; Al-Saffar et al., 1996; Tochen et al., 2014). Moreover, the highest survival rate (88–97%) for pupae of D. melanogaster is described for 60 to 100% relative humidity (Elwyn et al., 1917; Al-Saffar et al., 1996). But then, the sensitivity of larvae to temperature and humidity differ between drosophilid species (Geisler, 1942; McKenzie and Parsons, 1974). In contrast to the effect of temperature and humidity, the role of light on the developmental period and larval circadian clock is still under debate. Some studies indicated that permanent night has no effect on the animals, but in other cases the culture in constant darkness revealed a reduced longevity (Payne, 1910, 1911; Erk and Samis, 1970). Similar effects have been shown for culture in constant light (Allemand et al., 1973).
Investigation of dietary influence on the developmental period in larvae further implicated that the food composition has a prevalent effect on the life span and body parameters of drosophilids (Anagnostou et al., 2010; Ormerod et al., 2017). The developmental period increases with the linear availability of carbohydrates (negative correlation) and decreases (positive correlation) with the linear availability of proteins. Consequently, larvae are capable to differentiate their needs of nutrients and regulate their dietary intake toward a minimal developmental period (Rodrigues et al., 2015). In two drosophilid species D. melanogaster and D. subobscura a correlation between the metabolic rate, the developmental period and social parameters was observed (Marinkovié et al., 1986; Cluster et al., 1987; Hoffmann and Parsons, 1989; Sevenster and Alphen, 1993). Additional investigation on larval life, mortality, and pupal viability in D. melanogaster and D. simulans revealed a correlation between the density of larval numbers and the developmental period—high density and crowding of larvae in culture leads to a longer developmental period in both species (Powsner, 1935; Miller, 1964). To conclude, a decrease or increase of the developmental period of D. melanogaster is caused by different environmental stimuli and sociality. However, due to our results and experimental set-up, the examination of a single larva in one chamber on basic food medium with optimal temperature (25°C) and humidity (65%), we assume that the metabolic rate might influences the developmental time rather than social interactions or competition in their environment.
Our results further indicate a high variability of the size, especially for L3 Canton-S larvae. Whereas, young L1 Canton-S larvae showed a similar size (about one millimeter) until the first molting event, late wandering L3 Canton-S larvae (shortly before pupation) differ in size from about three to five millimeters (Figure 5C, Table 4). Therefore, we plotted larvae on an individual level to observe the time span and size between egg hatching and pupation on an individual level. Our results indicate that the pupation time point and the size of late L3 Canton-S larvae are independent of the egg hatching time and size (Figures 5, 6). We further expect that a specific size plays a key role for the initiation of molting and is therefore more relevant for younger larvae than for older (Figure 6). Our correlation between the time after egg laying and larval size showed that also small L3 larvae of the Canton-S strain went under pupation after a relatively long developmental time (Figure 6A). Previous studies inferred that larvae have to grow to a minimum size before entering the next growth stage and that growth phases before pupation can divided into two distinct phases which are independently genetically regulated (Robertson, 1963). Our results reproduce this finding that even smaller L3 larvae are able to pupate but due to individual variations we assume that the size might not be the only initiator for pupation and metamorphosis (Figures 6B,C). In comparison with other wild-type strains the variability of size in Canton-S larvae is also higher (Vaufrey et al., 2018).
In this study we followed D. melanogaster larva during several stages of development from egg hatching to pupation over up to 14 days (Figure 7). We defined tracking parameters to identify the larva most consistently in our environment and over a large range of body sizes. We found and analyzed critical developmental events like molting while looking at the activity and growth of the larva (Figure 7). With the combination of a video-sequence and a particle analyzer we are able to manually detect important developmental stages which will be the basis for a future real-time tracking system. For future improvement of the PEDtracker system, the analyzed particles regarding larval size and activity we presented in this methodology illustrate the basis for a custom-made software program for the analysis of insect larvae and prediction of behavioral events. Our focus was on getting data for the future implementation of such a real-time tracking system with an integrated molting detector for the prediction of important development time points such as molting or metamorphosis.
Taken together, our PEDtracker system provides a novelty in tracking systems for the observation of the whole post-embryonal development on an individual level which is not only suitable for insects but also for other molting animals such as chelicerates, nematodes, and other ecdysozoans. Besides the usage in the observation of developmental time points, the PEDtracker represents a useful tool for further molecular and behavioral experiment such as the culture of different genotypes under different food regimes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
IS established the experimental procedure. TT developed the Fiji script. IS and TT developed the Rpackage, designed the figures, and wrote the manuscript.
This research was supported by the University of Leipzig. Article fees were supported by the Leipzig University Open Access Fund.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are thankful to Andreas S. Thum who supported the whole project and made helpful comments on the manuscript. We are furthermore grateful to the whole Department of Genetics for support and comments on the establishment of the experimental procedure. We are also thankful to Ingo Kannetzky for providing technical material during the establishment of the experimental procedure.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2020.612313/full#supplementary-material
Supplementary Video 1. Molting event larval stage one to larval stage two; recorded at 3 fpm, played back at 3 fps.
Supplementary Video 2. Molting event larval stage two to larval stage three; recorded at 3 fpm, played back at 3 fps.
Supplementary Figure 1. Scheme of the Fiji macro script. Frames were distributed in packages of 1080 frames (equivalent to 6 h at 3 fpm) and then cropped in regions of interest (ROIs). ROIs were analyzed with specific parameters. Parameters for analyzed particles were saved in a csv-file. Batches at the border of two developmental stages were analyzed with parameters of both respective stages.
Supplementary Figure 2. Scheme of the R script. Csv-files were loaded in R and combined into a newly created list. Data were evaluated in two steps. First, fitting settings where selected and then objects where analyzed with these settings. After final evaluation of the data, new csv-files were saved and results were plotted using ggplot2.
Supplementary Figure 3. Definition of larval parameters for analyzing larval objects in Fiji and R. Parameters (Area, Circularity, Aspect Ratio, Roundness, Solidity) were defined for each larval stage with the objective to reliably sort out non-larval objects. To distinguish one or more objects from a larval object we have examined 60 cases (orange bar plot) for different parameters. In 56 cases (93%) the parameter “solidity” was higher for larval objects. For this reason, we used the value for solidity to distinguish larval from other objects in one frame.
Supplementary Figure 4. Evaluation of the centroid of an object in a frame. (A) Point cloud represents 51 cases of objects. X- and Y-Axis represents the width of the larval bed in pixel. Red dots indicate manually detected objects every 10 frames, black triangles represent the analyzed objects with Fiji and R, respectively. Note that the two-paired points are close together, except for non-detected objects which shows that PEDtracker detects larval objects and avoids false detections. (B) Bar plot indicates the detection probability for larval objects. Note that the detection probability is highest for L2 and lowest for L3. (C) Bar plot indicates the difference of the manual and computational detected object position on the X-axis. The difference of the detection between manual and computational detected object positions on the X-axis is below 5 pixels (lower than one larval length). (D) Bar plot indicates the difference of the manual and computational marked object position at the Y-Axis. The difference of the detection between manual and computational marked object positions on the Y-axis is below 5 pixels (lower than one larval length).
Supplementary Figure 5. Comparison of larval length between manual and computational detection. Note that the lengths of the computational detected larvae are slightly higher than the manual values due to the more precise area determination and resulting longitudinal axis.
Supplementary Figure 6. Activity pattern from a period of about 1,000 frames (~ 5.5 h) of a molting event and a non-molting event of first instar larvae. Insert compares the level of low activity of a molting event and the non-molting event. Note the phases no activity (light blue and red line).
Supplementary Figure 7. Molting events of individual first instar larvae. Note the phases of low activity in all images. Low activity indicates ecdysis behavioral sequence of D. melanogaster larvae.
Supplementary Figure 8. Activity pattern from a period of about 1,000 frames (~ 5.5 h) of a molting and a non-molting event of second instar larvae.
Supplementary Figure 9. Molting events of individual second instar larvae. Note the phases of low activity in all images. Low activity indicates ecdysis behavioral sequence of D. melanogaster larvae.
Allemand, R., Cohet, Y., and David, J. (1973). Increase in the longevity of adult Drosophila melanogaster kept in permanent darkness. Exp. Gerontol. 8, 279–283. doi: 10.1016/0531-5565(73)90040-5
Al-Saffar, Z. Y., Grainger, J. N. R., and Aldrich, J. (1996). Temperature and humidity affecting development, survival and weight loss of the pupal stage of Drosophila melanogaster, and the influence of alternating temperature on the larvae. J. Therm. Biol. 21, 389–396. doi: 10.1016/S0306-4565(96)00025-3
Anagnostou, C., Dorsch, M., and Rohlfs, M. (2010). Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomol. Exp. Appl. 136, 1–11. doi: 10.1111/j.1570-7458.2010.00997.x
Ashburner, M. (1989). Drosophila. A Laboratory Handbook. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Ashburner, M., and Thompson, Jr. J. N. (1978). Laboratory culture of Drosophila. Genet. Biol. Drosoph. 2a.
Beckstead, R. B., Lam, G., and Thummel, C. S. (2005). The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 6:R99. doi: 10.1186/gb-2005-6-12-r99
Burmester, T., Antoniewski, C., and Lepesant, J. (1999). Ecdysone regulation of synthesis and processing of fat body protein 1, the larval serum protein receptor of Drosophila melanogaster. Eur. J. Biochem. 262, 49–55. doi: 10.1046/j.1432-1327.1999.00315.x
Casares, P., and Carracedo, M. C. (1987). Pupation height in Drosophila: Sex differences and influence of larval developmental time. Behav. Genet. 17, 523–535. doi: 10.1007/BF01073119
Casas-Vila, N., Bluhm, A., Sayols, S., Dinges, N., Dejung, M., Altenhein, T., et al. (2017). The developmental proteome of Drosophila melanogaster. Genome Res. 27, 1273–1285. doi: 10.1101/gr.213694.116
Chang, E. S. (1985). Hormonal control of molting in decapod Crustacea. Am. Zool. 25, 179–185. doi: 10.1093/icb/25.1.179
Churgin, M. A., Jung, S.-K., Yu, C.-C., Chen, X., Raizen, D. M., and Fang-Yen, C. (2017). Longitudinal imaging of Caenorhabditis elegans in a microfabricated device reveals variation in behavioral decline during aging. Elife 6:e26652. doi: 10.7554/eLife.26652
Cluster, P. D., Marinkovi,ć, D., Allard, R. W., and Ayala, F. J. (1987). Correlations between development rates, enzyme activities, ribosomal DNA spacer-length phenotypes, and adaptation in Drosophila melanogaster. Proc. Natl. Acad. Sci.U.S.A. 84, 610–614. doi: 10.1073/pnas.84.2.610
Davidowitz, G., D'Amico, L. J., and Nijhout, H. F. (2003). Critical weight in the development of insect body size. Evol. Dev. 5, 188–197. doi: 10.1046/j.1525-142X.2003.03026.x
Dewhurst, S. A., McCaman, R. E., and Kaplan, W. D. (1970). The time course of development of acetylcholinesterase and choline acetyltransferase in Drosophila melanogaster. Biochem. Genet. 4, 499–508. doi: 10.1007/BF00486600
Elwyn, A., Lutz, F. E., and Tower, R. W. (1917). Effect of humidity on pupal duration and on pupal mortality of Drosophila ampelophila Loew. Bull. AMNH 37:15.
Ewer, J. (2005). How the ecdysozoan changed its coat. PLoS Biol. 3:e30349. doi: 10.1371/journal.pbio.0030349
Geisler, F. S. (1942). Effects of humidity on Drosophila melanogaster pupae. Am. Nat. 76, 223–224. doi: 10.1086/281039
Gilestro, G. F. (2012). Video tracking and analysis of sleep in Drosophila melanogaster. Nat Protoc. 7, 995–1007. doi: 10.1038/nprot.2012.041
Hoffmann, A. R. Y., and Parsons, P. A. (1989). An integrated approach to environmental stress tolerance and life-history variation: desiccation tolerance in Drosophila. Biol. J. Linn. Soc. 37, 117–136. doi: 10.1111/j.1095-8312.1989.tb02098.x
Koyama, T., Rodrigues, M. A., Athanasiadis, A., Shingleton, A. W., and Mirth, C. K. (2014). Nutritional control of body size through FoxO-Ultraspiracle mediated ecdysone biosynthesis. Elife 3:e03091. doi: 10.7554/eLife.03091
Lin, X., and Smagghe, G. (2019). Roles of the insulin signaling pathway in insect development and organ growth. Peptides 122:169923. doi: 10.1016/j.peptides.2018.02.001
Locke, M. (1970). The molt/intermolt cycle in the epidermis and other tissues of an insect Calpodes ethlius (Lepidoptera, hesperiidae). Tissue Cell 2, 197–223. doi: 10.1016/S0040-8166(70)80016-7
Marinkovié, D., Milošević, M., and Milanović, M. (1986). Enzyme activity and dynamics of Drosophila development. Genetica 70, 43–52.
Markow, T. A., Beall, S., and Matzkin, L. M. (2009). Egg size, embryonic development time and ovoviviparity in Drosophila species. J. Evol. Biol. 22, 430–434. doi: 10.1111/j.1420-9101.2008.01649.x
McKenzie, A., and Parsons, P. A. (1974). The genetic architecture of resistance to desiccation in populations of Drosophila melanogaster and D. simulans. Aust. J. Biol. Sci. 27, 441–456. doi: 10.1071/bi9740441
Miller, R. S. (1964). Larval competition in Drosophila melanogaster and D. simulans. Ecology 45, 132–148. doi: 10.2307/1937114
Mirth, C., Truman, J. W., and Riddiford, L. M. (2005). The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr. Biol. 15, 1796–1807. doi: 10.1016/j.cub.2005.09.017
Nijhout, H. F. (2013). “Arthropod developmental endocrinology,” in Arthropod Biology and Evolution, ed. A. B. Minelli, G. Fusco (Heidelberg: Springer), 123–148.
Nijhout, H. F., and Williams, C. M. (1974). Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): cessation of juvenile hormone secretion as a trigger for pupation. J. Exp. Biol. 61, 493–501.
Okada, T. (1963). Caenogenetic differentiation of mouth hooks in drosophilid larvae. Evolution 17, 84–98.
Ormerod, K. G., LePine, O. K., Abbineni, P. S., Bridgeman, J. M., Coorssen, J. R., Mercier, A. J., et al. (2017). Drosophila development, physiology, behavior, and lifespan are influenced by altered dietary composition. Fly. 11, 153–170. doi: 10.1080/19336934.2017.1304331
Park, Y., Filippov, V., Gill, S. S., and Adams, M. E. (2002). Deletion of the ecdysis-triggering hormone gene leads to lethal ecdysis deficiency. Development 129, 493–503.
Parkin, C. A., and Burnet, B. (1986). Growth arrest of Drosophila melanogaster on erg-2 and erg-6 sterol mutant strains of Saccharomyces cerevisiae. J. Insect Physiol. 32, 463–471. doi: 10.1016/0022-1910(86)90007-7
Payne, F. (1911). Drosophila ampelophila Loew bred in the dark for sixty-nine generations. Biol. Bull. 21, 297–301.
Pfeiffenberger, C., Lear, B. C., Keegan, K. P., and Allada, R. (2010). Locomotor activity level monitoring using the Drosophila activity monitoring (DAM) system. Cold Spring Harb Protoc. 2010:pdb.prot5518. doi: 10.1101/pdb.prot5518
Powsner, L. (1935). The effects of temperature on the durations of the developmental stages of Drosophila melanogaster. Physiol. Zool. 8, 474–520. doi: 10.1086/physzool.8.4.30151263
Rewitz, K. F., O'Connor, M. B., and Gilbert, L. I. (2007). Molecular evolution of the insect Halloween family of cytochrome P450s: phylogeny, gene organization and functional conservation. Insect Biochem. Mol. Biol. Mol Biol 37:12. doi: 10.1016/j.ibmb.2007.02.012
Riddiford, L. M., Hiruma, K., Que, L., and Baohua, Z. (1999). Regulation and role of nuclear receptors during larval molting and metamorphosis of Lepidoptera. Am. Zool. 39, 736–746. doi: 10.1093/icb/39.4.736
Riddiford, L. M., Hiruma, K., Zhou, X., and Nelson, C. A. (2003). Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochem. Mol. Biol. 33, 1327–1338. doi: 10.1016/j.ibmb.2003.06.001
Robertson, F. W. (1963). The ecological genetics of growth in Drosophila 6. The genetic correlation between the duration of the larval period and body size in relation to larval diet. Genet. Res. 4, 74–92. doi: 10.1017/S001667230000344X
Rodrigues, M. A., Martins, N. E., Balanc,é, L. F., Broom, L. N., Dias, A. J. S., Fernandes, A. S. D., et al. (2015). Drosophila melanogaster larvae make nutritional choices that minimize developmental time. J. Insect Physiol. 81, 69–80. doi: 10.1016/j.jinsphys.2015.07.002
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. doi: 10.1038/nmeth.2019
Schubiger, M., Wade, A. A., Carney, G. E., Truman, J. W., and Bender, M. (1998). Drosophila EcR-B ecdysone receptor isoforms are required for larval molting and for neuron remodeling during metamorphosis. Development 125, 2053–2062.
Seong, K. H., Matsumura, T., Shimada-Niwa, Y., Niwa, R., and Kang, S. (2020). The Drosophila individual activity monitoring and detection system (DIAMonDS). Elife 9:e58630. doi: 10.7554/eLife.58630
Sevenster, J. G., and Alphen, J. J. M. (1993). A life history trade-off in Drosophila species and community structure in variable environments. J. Anim. Ecol. 62, 720–736. doi: 10.2307/5392
Szuperak, M., Churgin, M. A., Borja, A. J., Raizen, D. M., Fang-Yen, C., and Kayser, M. S. (2018). A sleep state in Drosophila larvae required for neural stem cell proliferation. Elife 7:e33220. doi: 10.7554/eLife.33220
Telford, M. J., Bourlat, S. J., Economou, A., Papillon, D., and Rota-Stabelli, O. (2008). The evolution of the Ecdysozoa. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 363, 1529–1537. doi: 10.1098/rstb.2007.2243
Tochen, S., Dalton, D. T., Wiman, N., Hamm, C., Shearer, P. W., and Walton, V. M. (2014). Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 43, 501–510. doi: 10.1603/EN13200
Truman, J. W. (1996). Steroid receptors and nervous system metamorphosis in insects. Dev. Neurosci. 18, 87–101. doi: 10.1159/000111398
Vaufrey, L., Balducci, C., Lafont, R., Prigent, C., and Le Bras, S. (2018). Size matters! Aurora A controls Drosophila larval development. Dev. Biol. 440, 88–98. doi: 10.1016/j.ydbio.2018.05.005
Weeks, J. C., and Truman, J. W. (1984). Neural organization of peptide-activated ecdysis behaviors during the metamorphosis of Manduca sexta. J. Comp. Physiol. A 155, 407–422. doi: 10.1007/BF00610594
Xie, X., Liu, Z., Liu, M., Tao, T., Shen, X., and Zhu, D. (2016). Role of Halloween genes in ecdysteroids biosynthesis of the swimming crab (Portunus trituberculatus): Implications from RNA interference and eyestalk ablation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 199, 105–110. doi: 10.1016/j.cbpa.2016.06.001
Keywords: monitoring, development, drosophila, larva, ecdysis behavior, tracking
Citation: Schumann I and Triphan T (2020) The PEDtracker: An Automatic Staging Approach for Drosophila melanogaster Larvae. Front. Behav. Neurosci. 14:612313. doi: 10.3389/fnbeh.2020.612313
Received: 30 September 2020; Accepted: 25 November 2020;
Published: 16 December 2020.
Edited by:
Bart R. H. Geurten, University of Göttingen, GermanyReviewed by:
Benjamin Risse, Universität Münster, GermanyCopyright © 2020 Schumann and Triphan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabell Schumann, aXNhYmVsbC5zY2h1bWFubkB1bmktbGVpcHppZy5kZQ==; Tilman Triphan, dGlsbWFuLnRyaXBoYW5AdW5pLWxlaXB6aWcuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.