
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Behav. Neurosci. , 03 July 2014
Sec. Emotion Regulation and Processing
Volume 8 - 2014 | https://doi.org/10.3389/fnbeh.2014.00237
This article is part of the Research Topic Decision-making under stress: the importance of cortico-limbic circuits View all 12 articles
Instrumental decision making has long been argued to be vulnerable to emotional responses. Literature on multiple decision making systems suggests that this emotional biasing might reflect effects of a system that regulates innately specified, evolutionarily preprogrammed responses. To test this hypothesis directly, we investigated whether effects of emotional faces on instrumental action can be predicted by effects of emotional faces on bodily freezing, an innately specified response to aversive relative to appetitive cues. We tested 43 women using a novel emotional decision making task combined with posturography, which involves a force platform to detect small oscillations of the body to accurately quantify postural control in upright stance. On the platform, participants learned whole body approach-avoidance actions based on monetary feedback, while being primed by emotional faces (angry/happy). Our data evidence an emotional biasing of instrumental action. Thus, angry relative to happy faces slowed instrumental approach relative to avoidance responses. Critically, individual differences in this emotional biasing effect were predicted by individual differences in bodily freezing. This result suggests that emotional biasing of instrumental action involves interaction with a system that controls innately specified responses. Furthermore, our findings help bridge (animal and human) decision making and emotion research to advance our mechanistic understanding of decision making anomalies in daily encounters as well as in a wide range of psychopathology.
Emotions are well-known to influence decision making (Damasio et al., 1996; Damasio, 1997; Roelofs et al., 2009a,b, 2010b; Von Borries et al., 2012). Such emotional biases may reflect interactions between distinct (e.g., emotional vs. deliberative) systems for behavioral control (Kahneman and Frederick, 2002; Camerer et al., 2005). However, the precise nature of these interactions remains unclear. One possibility is that emotional biasing of action selection reflects an interaction between a Pavlovian and an instrumental control system (Dayan et al., 2006; Seymour and Dolan, 2008). According to this view, emotional biasing of action selection might reflect effects of a Pavlovian system that regulates innately specified, evolutionarily preprogrammed responses to reward- or punishment-predictive stimuli. The power of this Pavlovian system is illustrated, for example, by the inability of chicks to learn to run away from a food cup in order to obtain food (Hershberger, 1986). Thus, the innately specified tendency to approach the food prevented acquisition of the instrumental run-away response. In humans, Pavlovian influences are evidenced by a series of experiments (Crockett et al., 2009; Guitart-Masip et al., 2011, 2012, 2014; Cavanagh et al., 2013) crossing motivational valence (appetitive vs. aversive) and action (behavioral activation vs. behavioral inhibition) showing that reward potentiates behavioral activation, while punishment potentiates behavioral inhibition (see also, Carver and White, 1994; Gray and McNaughton, 2000, where close reward-activation and punishment-inhibition couplings have been suggested). Similar Pavlovian response tendencies have been studied extensively using Pavlovian-to-instrumental transfer paradigms in animals, as well as humans, where reward- or punishment-predictive stimuli modulate instrumental responses (Estes and Skinner, 1941; Estes, 1948; Di Giusto et al., 1974; Lovibond, 1983; Dickinson and Balleine, 1994; Bray et al., 2008; Talmi et al., 2008; Declercq and De Houwer, 2009; Trick et al., 2011; Prévost et al., 2012; Geurts et al., 2013; Lewis et al., 2013; Lovibond et al., 2013). Although it has been shown before that basic learning mechanisms may be relevant to understanding human social interactions (Olsson et al., 2005), the hypothesis that Pavlovian-like response tendencies account for emotional biasing of action selection by emotional faces has never been tested. Elucidating such relationship is important to advance our mechanistic understanding of decision making anomalies that play a critical role in daily encounters as well as in (social) psychopathologies.
The main aim of this study was to test directly the hypothesis that effects of emotional faces on action selection reflect effects of a system that regulates innately specified responses. To this end, we investigated whether effects of emotional faces on approach and avoidance responses can be predicted by effects of emotional faces on bodily freezing, an innately specified response to aversive vs. appetitive cues. Specifically, we predicted that effects of angry vs. happy faces on instrumental avoidance vs. approach responses would be predicted by effects of angry vs. happy faces on bodily freezing.
Bodily freezing, one of the most widely recognized defensive reactions to threat (Blanchard et al., 2001), can be reliably measured in humans using posturography (Carpenter et al., 1999; Azevedo et al., 2005; Stins and Beek, 2007; Roelofs et al., 2010a). Using a force-platform, small bodily oscillations can be accurately detected to quantify postural control in upright stance when watching emotional pictures. Using this method, we have previously shown that bodily freezing to aversive pictures is exacerbated in anxious (Roelofs et al., 2010a) and traumatized (Hagenaars et al., 2014) individuals. Moreover, bodily freezing has been shown to be associated with bradycardia, i.e., reductions in heartrate, which is a well accepted indicator of human freezing (Lang et al., 2000; Marx et al., 2008; Roelofs et al., 2010a; Hermans et al., 2013). Combining posturography with a novel emotional decision making task allowed us to quantify the innately specified response to the emotional face, i.e., bodily freezing, as well as its predictive value for the subsequent emotional biasing effect on the instrumental response.
Our second aim was to test the hypothesis that the interaction between emotion and action selection occurs at the level of motivational valence rather than at the level of motor output. This question parallels a debate in the Pavlovian-to-instrumental transfer literature (Lovibond, 1983), where recent work in humans (Huys et al., 2011) has suggested that such Pavlovian-to-instrumental transfer occurs at the level of motivational valence rather than motor output. Huys et al. (2011) have shown that appetitive Pavlovian cues do not potentiate all “go”-responses, but only those “go”-responses that are labeled as “approach”. In fact, “go”-responses labeled as avoidance were suppressed by appetitive cues. Conversely, aversive Pavlovian cues potentiated “avoid-go”-responses, while suppressing “approach-go”-responses. This action-specificity indicates that Pavlovian-to-instrumental transfer is unlikely to reflect interaction at the level of motor output. Indeed modulation of motor output would have led to potentiation (or suppression) of both types of “go”-responses. Here we build on this observation and extend it to the domain of emotional biasing. In contrast with classic upper-extremity approach-avoidance tasks where forward-backward movement direction is associated with approach-avoidance actions (Rotteveel and Phaf, 2004; Markman and Brendl, 2005), we employed an emotional decision making task, in which, as in Huys et al. (2011), the motor responses are matched for instrumental approach and avoidance. Furthermore, the direction of the emotional response axis (lean forward/freeze/lean backward in response to the emotional faces) and the direction of the instrumental approach/avoidance axis (step left-rightwards) were orthogonalized. Specifically, the instrumental task required participants to learn to step leftwards or rightwards on a balance board, in a manner that corresponded, or was opposite to the location of an instrumental target on the screen. The operationalization of approach-avoidance actions in terms of actual whole-body movements towards/away from a target incidentally also minimized the ambiguity of the experimental definition of approach-avoidance actions, as is the case in classic upper-extremity approach-avoidance tasks (Rotteveel and Phaf, 2004; Markman and Brendl, 2005). In sum, the main purpose of the present study was to test the hypothesis that emotional biasing of action selection reflects effects of a system that controls innately specified responses. Moreover, this system was anticipated to interact with the instrumental action selection system at the level of motivational valence rather than motor output. Thus, we expected the emotional biases to be action-specific, so that angry faces would potentiate instrumental avoidance responses (left- or rightwards steps to the location opposite to that of the instrumental stimulus), and inhibit instrumental approach responses (left- or rightwards steps to the locations corresponding to that of the instrumental stimulus), while the opposite would hold for happy faces. Critically, the strength of this action-specific effect of emotional faces was expected to be related to the strength of the bodily freezing responses.
Given the gender differences in the processing of angry faces (Rotter and Rotter, 1988), and to reduce between-subject variability, this study was restricted to women. Forty-five female students, 18–28 of age (M = 21.8, SD = 2.2), from the Radboud University Nijmegen participated in this study after giving written informed consent. They received payment or course credits as a reimbursement for participation. All participants were healthy with normal or corrected-to-normal visual acuity. Exclusion criteria were regular use of medication (except for contraceptives) or use of psychotropic drugs. The study was performed in accordance with the Declaration of Helsinki and approved by the local ethical committee. Data from two participants were excluded from data analyses. One participant failed to complete the experiment due to nausea. The other participant represented an outlier (>3 standard deviations from the mean) on one of our primary measures: the postural sway difference between angry and happy faces. Accordingly, data are reported from 43 participants.
Figure 1 (bottom panel, left) illustrates the experimental set-up. Participants performed the task on a custom-made strain gauge force plate (dimensions: 1 × 1 m; sampling frequency: 100 Hz; 1 mm accuracy), which consisted of four sensors measuring forces in the (vertical) z-direction. The signals were used to derive time series of the center of pressure (COP), for the anterior-posterior (AP) and the medio-lateral (ML) direction. Approximately 1 m in front of the participant, visual stimuli were presented at eye height on a 22-inch height-adjustable screen.
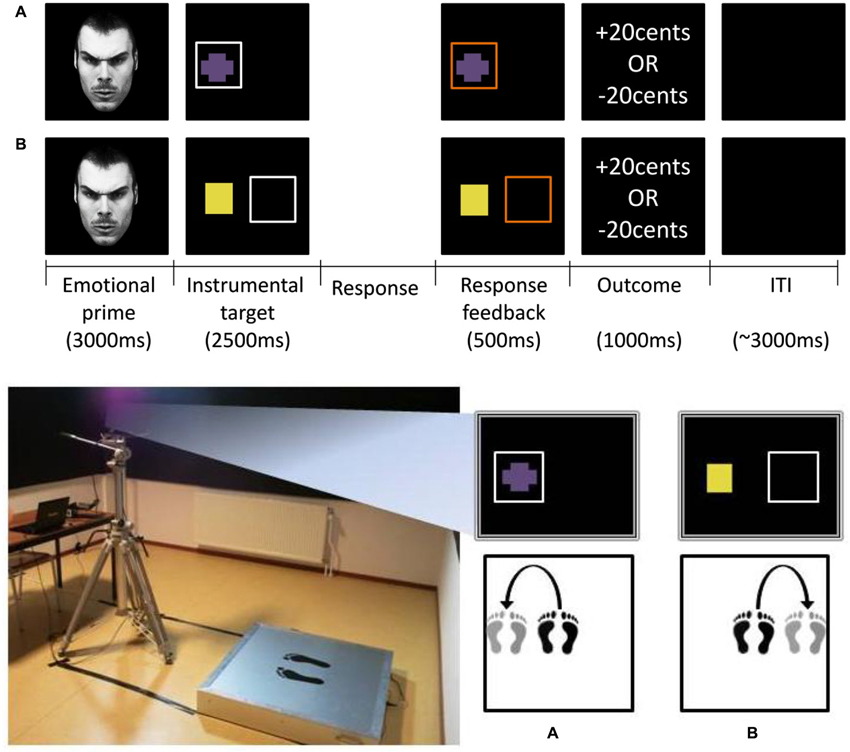
Figure 1. Top panel: emotional decision making task. Trial events from the (A) approach block and (B) avoidance block. After face-prime offset (3000 ms), the instrumental target appeared, to which subjects were required to make a go- or nogo-response within 2500 ms. Response feedback (500 ms) was provided before the monetary outcome (1000 ms). In these examples a go-response had been recorded as indicated by the orange-colored squares during the response feedback phase. The duration of the intertrial interval was 3000 ms on average. Bottom panel: Balance board apparatus (left) and examples of a go-response (right) in (A) the approach block and (B) the avoidance block. Approach-go: sideway step on the balance board towards the side of the instrumental target (A). Avoidance-go: sideway step on the balance board away from the side of the instrumental target (B). Approach-/avoidance- nogo-responses involved remaining stationary at the center of the balance board.
The goal of the paradigm was to assess whether emotional biasing of instrumental action selection would be predicted by innately specified responses, elicited by negative relative to positive emotional stimuli. To this end we employed a probabilistic learning paradigm combined with a balance board apparatus (Figure 1; bottom panel [left]). In this paradigm participants had to learn whole body go- and nogo-responses by trial and error on the basis of monetary outcome (wins/losses of €0.20), while being primed by emotional (angry/happy) faces. The rational for using a probabilistic learning paradigm was to promote recruitment of a model-free habit system, which is thought to be more vulnerable to influences of emotion than is a model- (rule-)based system (Dickinson et al., 1995; Holland, 2004).
The task was framed in terms of a gems collecting and sorting task and consisted of two blocks with different action-contexts (approach/avoidance). These action-contexts determined whether a go-response upon an instrumental target (colored shape, representing a gem) was an active approach- or an active avoidance-action. Thus, there were four types of instrumental responses in total: approach-go, approach-nogo, avoidance-go, avoidance-nogo. We rewarded go- and nogo-responses equally by designating the go-response as the optimal response to half of the instrumental targets. In the approach block, good gems (targets 1–3) were to be approached, whereas bad gems (targets 4–6) were not to be approached. Similarly, bad gems in the avoidance block (targets 7–9) were to be avoided, whereas good gems (targets 10–12) were not to be avoided. As such the value of the four types of instrumental responses was matched. This resulted in a multi-factorial design, in which we manipulated emotional prime (angry/happy), action-context (approach/avoidance), and optimal response (go/nogo) independently (Table 1). Participants were informed that correct choices were reinforced probabilistically. However, they were not informed about the nature of the probabilistic associations.
Figure 1 (top panel) illustrates trial events for (a) the approach block; and (b) the avoidance block respectively. At the beginning of each trial, a task-irrelevant emotional face-prime (angry/happy) was presented centrally on the screen for 3000 ms. Participants were instructed to maintain their view on these stimuli, while remaining stationary. After face-prime offset, the instrumental target appeared either on the left or the right side of the screen, upon which the participant had to make a go- or a nogo-response. If a go-response had not been made within 2500 ms, a nogo-response was recorded. Response feedback in terms of a square (1) turning orange when a go-response was recorded; and (2) remaining white when a nogo-response was recorded, was provided during 500 ms before the monetary outcome (1000 ms). The intertrial interval was jittered (3000 ± 1000 ms). Participants were required to return to their starting position (i.e., the center of the balance board) during the monetary outcome and intertrial interval, if go-response had been made.
Participants were instructed to remain stationary in the center of the balance board for a nogo-response (approach-nogo/avoid-nogo). Importantly, for the go-responses (approach-go/avoidance-go), participants were instructed to make sideway (not forward/backward) steps towards (approach-go) or away from (avoid-go) the side where the gem was presented to approach or avoid the gem respectively (Figure 1, bottom panel [right]). Thus, in contrast with traditional (upper-extremity) approach-avoidance tasks, we ensured that instrumental approach and avoidance required the same motoric “go”-response. Furthermore, the direction of the emotional response axis and the direction of the instrumental approach/avoidance were orthogonalized; that is, the direction of the instrumental response (left-rightward) was orthogonal to the forward-backward movement direction, which has traditionally been associated with approach-avoidance. This more abstract representation of approach-avoidance actions enabled us to address our secondary aim, to disentangle whether Pavlovian-instrumental interaction occurs at the level of motivational valence rather than at the level of motor output: action-specificity (e.g., angry-induced speeding of avoidance, but slowing of approach) would indicate that the interaction occurred at the level of motivational valence rather than motor output. In total, the task consisted of 240 trials. The order of blocks was randomized across participants.
Facial emotional expressions are able to communicate behavioral intentions or action demands to the perceiver (Horstmann, 2003). We have used happy and angry faces, because these emotional faces have been suggested to activate the appetitive (approach) and aversive (avoidance) motivational systems respectively (Roelofs et al., 2009a,b, 2010b; Volman et al., 2011; Von Borries et al., 2012). In contrast with emotional faces such as fear, disgust, sadness, for which the elicited behavioral tendencies may be complex (Marsh et al., 2005; Seidel et al., 2010), happy and angry faces have been shown to induce approach and avoidance behavioral tendencies respectively (Marsh et al., 2005; Roelofs et al., 2009a,b, 2010b; Seidel et al., 2010; Volman et al., 2011; Von Borries et al., 2012).
The face-primes consisted of adult Caucasian faces from 36 models (18 male) from several databases (Ekman and Friesen, 1976; Matsumoto and Ekman, 1988; Lundqvist et al., 1998; Martinez and Benavente, 1998). Model identity was counterbalanced, such that the model occurred equally often for each instrumental target. Each model showed two emotions (angry/happy), matched for brightness and contrast values, displayed against a black background. Faces were trimmed to exclude influence from hair and nonfacial contours (Van Peer et al., 2007). The instrumental targets (gems) consisted of 12 different colored shapes. The emotional primes and the instrumental targets were randomly assigned to each of the two blocks, such that different emotional stimuli and different instrumental stimuli occurred in the two blocks. Stimulus presentation and response acquisition were controlled by a laptop running Presentation software 14.8 12.30.10 (Neurobehavioral Systems, inc.).
Upon arrival, the participants were reminded of the experimental procedure. Subsequently, the participants practiced sideway stepping on the balance board until they felt comfortable with stepping while maintaining their view on the screen. Participants received instructions of the task before each block. To increase ecological validity and participants’ motivation during the task, we told participants that they would receive the total amount of monetary gain as a bonus (on top of the reimbursement).
Data-analyses were performed in MATLAB R2009b (The MathWorks, Natick, MA). Statistical analyses were performed using IBM SPSS Statistics 19. We calculated the following parameters: (1) postural mobility (mm/s) for angry vs. happy faces; and (2) reaction time (RT) of instrumental go-responses, and the proportion of instrumental go-responses (Pgo) in the emotional decision making task. Repeated measures analysis of variance (ANOVA) were conducted with postural mobility as dependent variable and emotion (angry/happy) as a within-subject variable to assess effects of emotion on bodily freezing. Additionally, two repeated measures ANOVAs with RT and Pgo as dependent variables were performed with emotion (angry/happy) and action-context (avoidance/approach) as within-subject variables, and postural mobility difference score (Dpostural mobility = postural mobility for angry minus happy) as a covariate of interest to assess whether performance changes on the emotional decision making task were a function of bodily freezing. Significant interaction effects were followed up by simple effects analyses and Pearson correlational analyses. Alpha was set at 0.05.
We operationalized bodily freezing as a reduction in postural mobility for aversive (angry) vs. appetitive (happy) stimuli (Dpostural mobility). This operationalization was based on previous studies using posturography to objectively assess human freezing (Carpenter et al., 1999; Azevedo et al., 2005; Stins and Beek, 2007; Roelofs et al., 2010a; Hagenaars et al., 2012, 2014). We quantified postural mobility as the sway path length or the sum of the COP displacements in the AP-ML plane over 1 s in time.
Reaction time on go-responses. Based on previous work (Stins et al., 2011), we calculated the time interval between instrumental stimulus onset and the first overt movement into the intended direction (RT). The left and right panels in Figure 2 show the COP profile of a representative leftward step and the velocity profile of the same step respectively. The step was initiated by lifting the left limb resulting in a brief rightward displacement of the COP (Figure 2, left), which was then followed by a movement of the COP to the left. Directional changes in the COP profile coincide with drops in the velocity profile of the COP (Figure 2, right). The moment, at which the velocity between the first and second peak reaches its minimum, was defined as the RT. Incorrect trials were excluded from RT analyses. Incorrect trials were defined as trials on which participants made a suboptimal or an incorrect instrumental action (approach-go in an avoidance action-context, or avoidance-go in an approach action-context).
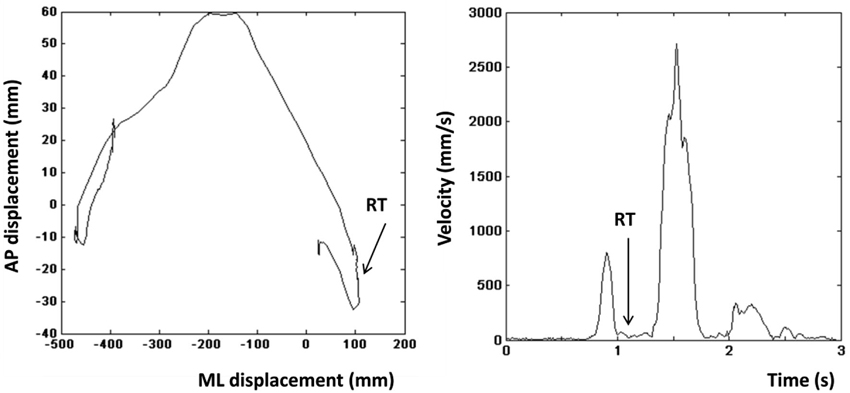
Figure 2. Left: Center of pressure (COP) displacement of a representative leftward step. Right: Velocity profile of the step shown in the left panel. Arrow indicates the moment that was defined as the reaction time.
Proportion go-responses.The proportion of go-responses was calculated by: go/(go+nogo).
ANOVA of the sway path length did not show a main effect of emotion (F(1,42) = 0.02, p = 0.883, η2 < 0.001), indicating that on average there was no difference in postural mobility between angry and happy faces.
Mean RTs are shown in Table 2. Repeated measures ANOVA of RTs showed a significant main effect of action (avoidance/approach) indicating faster approach compared with avoidance actions (F(1,41) = 19.7, p < 0.001, η2 = 0.32). Moreover, we found a significant emotion (angry/happy) × action-context (avoidance/approach) interaction effect (F(1,41) = 8.0, p = 0.007, η2 = 0.16), indicating slower approach compared with avoidance after angry vs. happy face-primes (Figure 3, left). Post-hoc analyses suggested that this interaction effect was mainly driven by slower RT after angry vs. happy face-primes in the approach condition (F(1,41) = 5.4, p = 0.025, η2 = 0.12), but not in the avoidance condition (F(1,41) = 0.52, p = 0.477, η2 = 0.01).
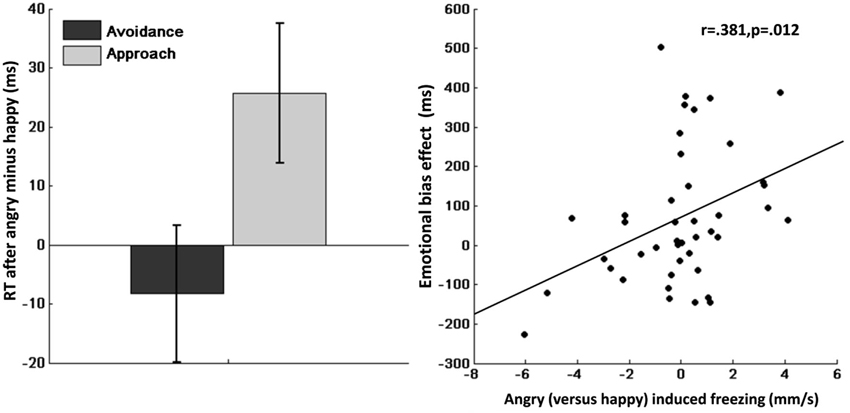
Figure 3. Left: Emotional bias effect on instrumental approach-avoidance. Slower approach (vs. avoidance) after angry (vs. happy) faces, showing an action-specific effect of emotional primes. Error bars represent standard error of the mean. Right: Correlation between bodily freezing and the emotional bias effect. Angry (vs. happy) induced freezing corresponds to sway (mm/s) during happy minus angry (x-axis). Emotional bias effect corresponds to angry (vs. happy) induced slowing of approach (vs. avoidance) (y-axis).
Crucially, we found a significant emotion (angry/happy) × action-context (avoidance/approach) × Dpostural mobility interaction (F(1,41) = 7.0, p = 0.012, η2 = 0.15). Correlational analyses indicated that this interaction was explained by an association between reduced postural mobility to angry vs. happy faces and slower RT for approach compared with avoidance after angry vs. happy faces (r = 0.381, p = 0.012; Figure 3, right). This suggests that individual differences in postural mobility to angry vs. happy faces predict the extent to which angry faces (vs. happy faces) slowed instrumental approach (relative to instrumental avoidance).
Post-hoc analysis suggested that this interaction effect was mainly driven by an association between Dpostural mobility and the emotional effects on instrumental approach (Emotion × Dpostural mobility; F(1,41) = 5.2, p = 0.029, η2 = 0.11). This was reflected by an association between reduced postural mobility to angry vs. happy faces and slower RT for approach after angry vs. happy face-primes (r = 0.333, p = 0.029), There was no emotion (angry/happy) × Dpostural mobility interaction in the avoidance action-contect (F(1,41) = 0.33, p = 0.566, η2 = 0.008).
There were no significant main effects and interaction effects for the proportion go-responses (all F(1,42) < 2.3, p > 0.131, η2 = 0.05).
In the current study, we combined a novel emotional decision making task with posturography and showed that emotional biasing of action selection reflects effects of a system that controls innately specified responses. We demonstrated that angry vs. happy faces slowed instrumental approach relative to avoidance. Critically, individual differences in this emotional biasing effect were predicted by individual differences in bodily freezing, one of the major innately specified defensive responses to threat (Blanchard et al., 2001). Unlike previous studies on individual differences in motivated behavior, which have often relied on subjective self-report questionnaires (e.g., Carver and White, 1994), we have adopted a mechanistic approach to obtain objective indices of motivated behavior.
The finding that angry vs. happy faces slowed instrumental approach relative to avoidance, demonstrates that the effects of the emotional faces are action-specific. Thus, we extend previous findings by Huys et al. (2011) to the emotional domain by showing that angry vs. happy faces slowed “go”-responses vs. “nogo”-responses related to an approach-action, while having no effect, or, if anything, the opposite effect on an avoidance-action.
A critical finding of the current study is that individual differences in this emotional biasing effect was predicted by individual differences in bodily freezing, one of the major innately specified defensive responses to threat (Blanchard et al., 2001). This finding suggests that emotional biasing of instrumental action involves interaction with a system that controls innately specified responses. This finding is generally in line with contemporary literature stating that behavior depends on multiple systems for decision making (Rangel et al., 2008; Dolan and Dayan, 2013), and more specifically with the idea that emotional anomalies reflect interactions between a Pavlovian and an instrumental control system (Dayan et al., 2006; Seymour and Dolan, 2008). According to this literature, a system that regulates innately specified responses to reward- and punishment-predictive stimuli can bias the instrumental action selection system. Our finding supports this literature, and extends this literature to the emotional domain. Moreover, this finding generally concurs with recent ideas about the survival circuit concept (LeDoux, 2012), which is a concept that integrates ideas about emotion, motivation, reinforcement, and arousal to understand how organisms survive and thrive in daily life. According to this concept, mechanisms that instantiate survival functions, such as a defensive mechanism, are of critical importance in the study of emotional processes.
Research using upper-extremity emotional approach-avoidance tasks has previously shown similar emotion-action interaction effects (also in terms of reaction time; Roelofs et al., 2009a). However, this prior work did not demonstrate that such emotion-action interaction reflects interaction with a system that controls innately specified responses. Moreover, this work did not speak to the degree to which these interaction effects occurred at the level of motivational valence or another level (e.g., motor output). Specifically, in these emotional approach-avoidance tasks, participants are instructed to “approach” and “avoid” visually presented emotional faces by pulling and pushing a joystick. Therefore, the directional axes (forward-backward) of the emotional response and the instructed (instrumental) response overlap. Any modulation of approach/avoidance might thus reflect a competition at the level of motor output rather than a bias at the level of motivational valence. In our current study, the interaction between emotional faces and instrumental action selection was demonstrated when we matched the motor responses for instrumental approach and avoidance, and orthogonalized the directional axes of the emotional approach/avoidance (forward-backward) and instrumental approach/avoidance (left-rightward). Therefore, the present results suggest that the emotional biasing effect occurred at the level of motivational valence rather than at the level of motor output. These findings speak to a traditional debate in the Pavlovian-to-instrumental transfer literature (Lovibond, 1983) about the role of Pavlovian influences, and support the idea that Pavlovian stimuli influence motivational systems that underlie instrumental responding posed by traditional theories on incentive-motivation and two-process theories (Mowrer, 1960; Rescorla and Solomon, 1967; Bindra, 1968; Gray, 1975).
The current study is the first to employ posturography in the investigation of emotional biasing in instrumental approach-avoidance (although see, Gélat et al., 2011; Naugle et al., 2011; Stins et al., 2011, for relevant whole-body movement research on emotional bias in gait initiation). Posturography allowed us to derive more reliable and ecologically valid measures of instrumental approach-avoidance by asking subjects to make actual whole-body movements towards/away from a target incidentally, in contrast with ambiguous experimental categorizations of approach-avoidance in classic upper-extremity approach-avoidance tasks (Rotteveel and Phaf, 2004; Markman and Brendl, 2005). Importantly, this design allowed us to measure bodily freezing (Roelofs et al., 2010a). This implicit and objective measure of an innately specified defensive response to threat allowed us to investigate directly and objectively the degree to which emotional biases on action selection reflect effects of a system that controls innately specified responses, rather than explicit awareness of the experimenters’ demands. Our findings underscore the relevance of integrative emotion, decision making and movement research.
We did not observe a main effect of emotion on postural mobility, i.e., bodily freezing across the whole group. Instead, there was large individual variability, possibly reflecting individual differences in the degree to which angry vs. happy faces elicit defensive behavior; for example in aggressive vs. anxious individuals (Roelofs et al., 2009b, 2010b; Von Borries et al., 2012; see also for a review on the relation between anger and approach motivation, Carver and Harmon-Jones, 2009). Here, we exploit this individual variability in bodily freezing and demonstrate that it predicts the effects of emotional stimuli on instrumental action.
Future studies could benefit from including subjects from different populations, such as patient populations. Our findings may be not only be relevant for understanding the basis of decision making anomalies seen in a wide range of social psychopathology, but also in healthy states, such as fatigue and stress. Particularly, our findings may be interesting in light of recent findings showing that stress may affect systems controlling instrumental action (e.g., Schwabe and Wolf, 2009, 2011). It would be particularly interesting for future studies to assess the interactive effects of stress on the current paradigm. Finally, future studies should test whether these findings can be generalized to men.
In sum, we show that the effects of emotional faces on instrumental responses are action-specific and can occur at the level of motivational valence. Importantly, we found that individual differences in this emotional biasing effect were predicted by individual differences in bodily freezing. This finding suggests that the emotional biasing of action selection by emotional faces reflects effects of a system that controls innately specified responses. Although, this finding is based on correlational analyses, and we have to be careful with causal interpretations, the finding is in support of literature suggesting that behavior depends on multiple decision making systems, an account that may help us understand the mechanisms underlying emotional biasing in decision making. Furthermore, our findings help bridge (animal and human) decision making and emotion research to advance our mechanistic understanding of decision making anomalies in daily encounters as well as in a wide range of psychopathology.
All authors contributed to the study concept and design. Verena Ly was responsible for data-acquisition. Verena Ly analyzed and interpreted the data under the supervision of Karin Roelofs and Roshan Cools, with input by Quentin J. M. Huys and John F. Stins. Verena Ly drafted the manuscript under supervision of Roshan Cools and Karin Roelofs and all others provided critical revisions. All authors approved the final version of the paper prior to submission.
This study was supported by the Mosaic grant 017.007.043 from the Netherlands Organization for Scientific Research (NWO) awarded to Verena Ly and VIDI grants from NWO awarded to Karin Roelofs and Roshan Cools, a Human Frontiers Research Grant to Roshan Cools and a James McDonnell Scholar Award to Roshan Cools. Quentin J. M. Huys was supported by the German Research Foundation RA1047/2-1 (Deutsche Forschungsgemeinschaft, DFG).
The authors reported no biomedical financial interests or potential conflicts of interest. Roshan Cools is consultant for Abbott laboratories and Pfizer, but not an employee or stock shareholder.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank P. de Water en M. van de Hei for their technical support.
Azevedo, T. M., Volchan, E., Imbiriba, L. A., Rodrigues, E. C., Oliveira, J. M., Oliveira, L. F., et al. (2005). A freezing-like posture to pictures of mutilation. Psychophysiology 42, 255–260. doi: 10.1111/j.1469-8986.2005.00287.x
Bindra, D. (1968). Neuropsychological interpretation of the effects of drive and incentive-motivation on general activity and instrumental behavior. Psychol. Rev. 75, 1–22. doi: 10.1037/h0025306
Blanchard, D. C., Griebel, G., and Blanchard, R. J. (2001). Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci. Biobehav. Rev. 25, 205–218. doi: 10.1016/s0149-7634(01)00009-4
Bray, S., Rangel, A., Shimojo, S., Balleine, B., and O’Doherty, J. P. (2008). The neural mechanisms underlying the influence of pavlovian cues on human decision making. J. Neurosci. 28, 5861–5866. doi: 10.1523/jneurosci.0897-08.2008
Camerer, C., Loewenstein, G., and Prelec, D. (2005). Neuroeconomics: how neuroscience can inform economics. J. Econ. Lit. 43, 9–64. doi: 10.1257/0022051053737843
Carpenter, M. C., Frank, J. S., and Silcher, C. P. (1999). Surface height effects on postural control: a hypothesis for a stiffness strategy for stance. J. Vestib. Res. 9, 277–286.
Carver, C. S., and Harmon-Jones, E. (2009). Anger is an approach-related affect: evidence and implications. Psychol. Bull. 135, 183–204. doi: 10.1037/a0013965
Carver, C. S., and White, T. L. (1994). Behavioral inhibition, behavioral activation and affective responses to impending reward and punishment: the BIS/BAS Scales. J. Pers. Soc. Psychol. 67, 319–333. doi: 10.1037/0022-3514.67.2.319
Cavanagh, J. F., Eisenberg, I., Guitart-Masip, M., Huys, Q., and Frank, M. J. (2013). Frontal theta overrides Pavlovian learning biases. J. Neurosci. 33, 8541–8548. doi: 10.1523/jneurosci.5754-12.2013
Crockett, M. J., Clark, L., and Robbins, T. W. (2009). Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J. Neurosci. 29, 11993–11999. doi: 10.1523/jneurosci.2513-09.2009
Damasio, A. R. (1997). Towards a neuropathology of emotion and mood. Nature 386, 769–770. doi: 10.1038/386769a0
Damasio, A. R., Everitt, B. J., and Bishop, D. (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex [ and Discussion ]. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 1413–1420. doi: 10.1098/rstb.1996.0125
Dayan, P., Niv, Y., Seymour, B., and Daw, N. D. (2006). The misbehavior of value and the discipline of the will. Neural Netw. 19, 1153–1160. doi: 10.1016/j.neunet.2006.03.002
Declercq, M., and De Houwer, J. (2009). Transfer of avoidance responding to a sensory preconditioned cue: evidence for the role of S-S and R-S knowledge in avoidance learning. Learn. Motiv. 40, 197–208. doi: 10.1016/j.lmot.2008.11.003
Dickinson, A., and Balleine, B. (1994). Motivational control of goal-directed action. Anim. Learn. Behav. 22, 1–18. doi: 10.3758/bf03199951
Dickinson, A., Balleine, B., Watt, A., Gonzalez, F., and Boakes, R. A. (1995). Motivational control after extended instrumental training. Anim. Learn. Behav. 23, 197–206. doi: 10.3758/bf03199935
Di Giusto, J. A., Di Giusto, E. L., and King, M. G. (1974). Heart rate and muscle tension correlates of conditioned suppression in humans. J. Exp. Psychol. 103, 515–521. doi: 10.1037/h0037207
Dolan, R. J., and Dayan, P. (2013). Goals and habits in the brain. Neuron 80, 312–325. doi: 10.1016/j.neuron.2013.09.007
Ekman, P., and Friesen, W. V. (1976). Pictures of Facial Affect. Palo Alto (CA): Consulting Psychologist Press.
Estes, W. K. (1948). Discriminative conditioning. II. Effects of a Pavlovian conditioned stimulus upon a subsequently established operant response. J. Exp. Psychol. 38, 173–177. doi: 10.1037/h0057525
Estes, W., and Skinner, B. (1941). Some quantitative aspects of anxiety. J. Exp. Psychol. 29, 390–400. doi: 10.1037/h0062283
Gélat, T., Coudrat, L., and Le Pellec, A. (2011). Gait initiation is affected during emotional conflict. Neurosci. Lett. 497, 64–67. doi: 10.1016/j.neulet.2011.04.030
Geurts, D. E. M., Huys, Q. J. M., Den Ouden, H. E. M., and Cools, R. (2013). Aversive Pavlovian control of instrumental behaviour in humans. J. Cogn. Neurosci. 25, 1428–1441. doi: 10.1162/jocn_a_00425
Gray, J. A., and McNaughton, M. (2000). The Neuropsychology of Anxiety: An Inquiry into the Function of the Septohippocampal System. Edn. 2. Oxford: Oxford UP.
Guitart-Masip, M., Duzel, E., Dolan, R., and Dayan, P. (2014). Action versus valence in decision making. Trends Cogn. Sci. 18, 194–202. doi: 10.1016/j.tics.2014.01.003
Guitart-Masip, M., Fuentemilla, L., Bach, D. R., Huys, Q. J. M., Dayan, P., Dolan, R. J., et al. (2011). Action dominates valence in anticipatory representations in the human striatum and dopaminergic midbrain. J. Neurosci. 31, 7867–7875. doi: 10.1523/jneurosci.6376-10.2011
Guitart-Masip, M., Huys, Q. J. M., Fuentemilla, L., Dayan, P., Duzel, E., and Dolan, R. J. (2012). Go and no-go learning in reward and punishment: Interactions between affect and effect. NeuroImage 62, 154–166. doi: 10.1016/j.neuroimage.2012.04.024
Hagenaars, M. A., Roelofs, K., and Stins, J. (2014). Human freezing in response to affective films. Anxiety Stress Coping 27, 27–37. doi: 10.1080/10615806.2013.809420
Hagenaars, M. A., Stins, J., and Roelofs, K. (2012). Aversive life events enhance human freezing. J. Exp. Psychol. Gen. 141, 98–105. doi: 10.1037/a0024211
Hermans, E. J., Henckens, M. J. A. G., Roelofs, K., and Fernández, G. (2013). Fear bradycardia and activation of the human periaqueductal grey. Neuroimage 66, 278–287. doi: 10.1016/j.neuroimage.2012.10.063
Hershberger, W. A. (1986). An approach through the looking-glass. Anim. Learn. Behav. 14, 443–451. doi: 10.3758/bf03200092
Holland, P. C. (2004). Relations between pavlovian-instrumental transfer and reinforcer devaluation. J. Exp. Psychol. Anim. Behav. Process 30, 104–117. doi: 10.1037/0097-7403.30.2.104
Horstmann, G. (2003). What do facial expressions convey: feeling states, behavioral intentions, or action requests? Emotion 3, 150–166. doi: 10.1037/1528-3542.3.2.150
Huys, Q. J. M., Cools, R., Gölzer, M., Friedel, E., Heinz, A., Dolan, R. J., et al. (2011). Disentangling the roles of approach, activation and valence in instrumental and pavlovian responding. PLoS Comput. Biol. 7:e1002028. doi: 10.1371/journal.pcbi.1002028
Kahneman, D., and Frederick, S. (2002). “Representativeness revisited: attribute substitution in intuitive judgment,” in Heuristics and Biases: The Psychology of Intuitive Judgment, eds T. Gilovich, D. Griffin and D. Kahneman (New York: Cambridge University Press), 49–81.
Lang, P. J., Davis, M., and Öhman, A. (2000). Fear and anxiety: animal models and human cognitive psychophysiology. J. Affect. Disord. 61, 137–159. doi: 10.1016/s0165-0327(00)00343-8
LeDoux, J. (2012). Rehinking the emotional brain. Neuron 73, 653–676. doi: 10.1016/j.neuron.2012.02.004
Lewis, A. H., Niznikiewicz, M. A., Delamater, A. R., and Delgado, M. R. (2013). Avoidance-based human Pavlovian-to-instrumental transfer. Eur. J. Neurosci. 38, 3740–3748. doi: 10.1111/ejn.12377
Lovibond, P. F. (1983). Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process. 9, 225–247. doi: 10.1037//0097-7403.9.3.225
Lovibond, P. F., Chen, S. X., Mitchell, C. J., and Weidemann, G. (2013). Competition between an avoidance response and a safety signal: evidence for a single learning system. Biol. Psychol. 92, 9–16. doi: 10.1016/j.biopsycho.2011.09.007
Lundqvist, D., Flykt, A., and Ohman, A. (1998). The Karolinska Direct Emotional Faces (KDEF) [CD ROM]. Sweden: Department of Clinical Neuroscience, Section of Psychology, Karolinska Institute.
Markman, A. B., and Brendl, C. M. (2005). Constraining theories of embodied cognition. Psychol. Sci. 16, 6–10. doi: 10.1111/j.0956-7976.2005.00772.x
Marsh, A. A., Ambady, N., and Kleck, R. E. (2005). The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion 5, 119–124. doi: 10.1037/1528-3542.5.1.119
Marx, B. P., Forsyth, J. P., Gallup, G. G., Fusé, T., and Lexington, J. M. (2008). Tonic immobility as an evolved predator defense: implications for sexual assault survivors. Clin. Psychol. Sci. Practice 15, 74–90. doi: 10.1111/j.1468-2850.2008.00112.x
Matsumoto, D., and Ekman, P. (1988). Japanese and Caucasian Facial Expressions of Emotion (JACFEE) [slides]. San Francisco (CA): University of California, Human Interaction Laboratory.
Naugle, K. M., Hass, C. J., Joyner, J., Coombes, S. A., and Janelle, C. M. (2011). Emotional state affects the initiation of forward gait. Emotion 11, 267–277. doi: 10.1037/a0022577
Olsson, A., Ebert, J. P., Banaji, M. R., and Phelps, E. A. (2005). The role of social groups in the persistence of learned fear. Science 309, 785–787. doi: 10.1126/science.1113551
Prévost, C., Liljeholm, M., Tyszka, J. M., and O’Doherty, J. P. (2012). Neural correlates of specific and general Pavlovian-to-instrumental transfer within human amygdalar subregions: a high-resolution fMRI study. J. Neurosci. 32, 8383–8390. doi: 10.1523/jneurosci.6237-11.2012
Rangel, A., Camerer, C., and Montage, P. R. (2008). A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 9, 545–556. doi: 10.1038/nrn2357
Rescorla, R. A., and Solomon, R. L. (1967). Two-process learning theory: relationships between Pavlovian conditioning and instrumental learning. Psychol. Rev. 74, 151–182. doi: 10.1037/h0024475
Roelofs, K., Hagenaars, M. A., and Stins, J. (2010a). Facing freeze: social threat induces bodily freeze in humans. Psychol. Sci. 21, 1575–1581. doi: 10.1177/0956797610384746
Roelofs, K., Minelli, A., Mars, R. B., Van Peer, J., and Toni, I. (2009a). On the neural control of social emotional behavior. Soc. Cogn. Affect. Neurosci. 4, 50–58. doi: 10.1093/scan/nsn036
Roelofs, K., Putman, P., Schouten, S., Lange, W.-G., Volman, I., and Rinck, M. (2010b). Gaze direction differentially affects avoidance tendencies to happy and angry faces in socially anxious individuals. Behav. Res. Ther. 48, 290–294. Elsevier Ltd. doi: 10.1016/j.brat.2009.11.008
Roelofs, K., Van Peer, J., Berretty, E., Jong, P. D., Spinhoven, P., and Elzinga, B. M. (2009b). Hypothalamus-pituitary-adrenal axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. Biol. Psychiatry 65, 336–343. doi: 10.1016/j.biopsych.2008.08.022
Rotter, N. G., and Rotter, G. S. (1988). Sex differences in the encoding and decoding of negative facial emotions. J. Nonverbal Behav. 12, 139–148. doi: 10.1007/bf00986931
Rotteveel, M., and Phaf, R. H. (2004). Automatic affective evaluation does not automatically predispose for arm flexion and extension. Emotion 4, 156–172. doi: 10.1037/1528-3542.4.2.156
Schwabe, L., and Wolf, O. T. (2009). Stress prompts habit behavior in humans. J. Neurosci. 29, 7191–7198. doi: 10.1523/jneurosci.0979-09.2009
Schwabe, L., and Wolf, O. T. (2011). Stress-induced modulation of instrumental behavior: from goal-directed to habitual control of action. Behav. Brain Res. 219, 321–328. doi: 10.1016/j.bbr.2010.12.038
Seidel, E.-M., Habel, U., Kirschner, M., Gur, R. C., and Derntl, B. (2010). The impact of facial emotional expressions on behavioral tendencies in females and males. J. Exp. Psychol. Hum. Percept. Perform. 36, 500–507. doi: 10.1037/a0018169
Seymour, B., and Dolan, R. (2008). Emotion, decision making and the amygdala. Neuron 58, 662–671. doi: 10.1016/j.neuron.2008.05.020
Stins, J. F., and Beek, P. J. (2007). Effects of affective picture viewing on postural control. BMC Neurosci. 8:83. doi: 10.1186/1471-2202-8-83
Stins, J. F., Roelofs, K., Villan, J., and Beek, P. J. (2011). Walk to me when I smile, step back when I ’m angry: emotional faces modulate whole-body approach-avoidance behaviors. Exp. Brain Res. 212, 603–611. doi: 10.1007/s00221-011-2767-z
Talmi, D., Seymour, B., Dayan, P., and Dolan, R. J. (2008). Human pavlovian-instrumental transfer. J. Neurosci. 28, 360–368. doi: 10.1523/JNEUROSCI.4028-07.2008
Trick, L., Hogarth, L., and Duka, T. (2011). Prediction and uncertainty in human Pavlovian to instrumental transfer. J. Exp. Psychol. Learn. Mem. Cogn. 37, 757–765. doi: 10.1037/a0022310
Van Peer, J. M. V., Roelofs, K., Rotteveel, M., Dijk, J. G. V., Spinhoven, P., and Ridderinkhof, K. R. (2007). The effects of cortisol administration on approach – avoidance behavior: an event-related potential study. Biol. Psychology 76, 135–146. doi: 10.1016/j.biopsycho.2007.07.003
Volman, I., Roelofs, R., Koch, S., Verhagen, L., and Toni, I. (2011). Anterior prefrontal cortex inhibition impairs control over social emotional actions. Curr. Biol. 21, 1766–1770. doi: 10.1016/j.cub.2011.08.050
Keywords: emotion, freezing, decision making, learning, approach-avoidance
Citation: Ly V, Huys QJM, Stins JF, Roelofs K and Cools R (2014) Individual differences in bodily freezing predict emotional biases in decision making. Front. Behav. Neurosci. 8:237. doi: 10.3389/fnbeh.2014.00237
Received: 09 April 2014; Accepted: 16 June 2014;
Published online: 03 July 2014.
Edited by:
Ruud Van Den Bos, Radboud University Nijmegen, NetherlandsReviewed by:
Tony W. Buchanan, Saint Louis University, USACopyright © 2014 Ly, Huys, Stins, Roelofs and Cools. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Verena Ly, Donders Institute for Brain, Cognition, and Behaviour, Centre for Cognitive Neuroimaging, Radboud University Nijmegen, Kapittelweg 29, 6500 HB, Nijmegen, Netherlands e-mail:di5seUBkb25kZXJzLnJ1Lm5s
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.