- Department of Psychology, University of Haifa, Haifa, Israel
Cannabinoid agonists generally have a disruptive effect on memory, learning, and operant behavior that is considered to be hippocampus-dependent. Nevertheless, under certain conditions, cannabinoid receptor activation may facilitate neuronal learning processes. For example, CB1 receptors are essential for the extinction of conditioned fear associations, indicating an important role for this receptor in neuronal emotional learning and memory. This review examines the diverse effects of cannabinoids on hippocampal memory and plasticity. It shows how the effects of cannabinoid receptor activation may vary depending on the route of administration, the nature of the task (aversive or not), and whether it involves emotional memory formation (e.g., conditioned fear and extinction learning) or non-emotional memory formation (e.g., spatial learning). It also examines the memory stage under investigation (acquisition, consolidation, retrieval, extinction), and the brain areas involved. Differences between the effects of exogenous and endogenous agonists are also discussed. The apparently biphasic effects of cannabinoids on anxiety is noted as this implies that the effects of cannabinoid receptor agonists on hippocampal learning and memory may be attributable to a general modulation of anxiety or stress levels and not to memory per se. The review concludes that cannabinoids have diverse effects on hippocampal memory and plasticity that cannot be categorized simply into an impairing or an enhancing effect. A better understanding of the involvement of cannabinoids in memory processes will help determine whether the benefits of the clinical use of cannabinoids outweigh the risks of possible memory impairments.
Introduction
Considerable evidence suggests that cannabinoids impair hippocampal-dependent learning and memory processes, such as spatial learning and context-related memory tasks (Sullivan, 2000; Riedel and Davies, 2005). In this review, I will provide evidence that suggests that the effects of cannabinoids on memory and plasticity are complex and depend on several factors, such as the nature of the task (emotional or non-emotional), the memory stage investigated (acquisition, retrieval, and extinction), and the experimental model used. Naturally, the behavioral effects of cannabinoids on memory may vary as a function of dose, route of administration, and the specific drug used.
Cannabinoid Receptors in the Hippocampus
Cannabis has a long history of consumption both for recreational and medicinal uses. The main psychoactive constituent of marijuana, delta-9-tetrahydrocannabinol (THC), was identified in 1964 (Gaoni and Mechoulam, 1964) and this discovery led to the identification of the endogenous endocannabinoid (eCB) system. This system includes cannabinoid receptors (CB1 and CB2), eCBs [anandamide and 2-arachidonoyl-glycerol (2-AG)], enzymes involved in their synthesis and metabolism [fatty acid amide hydrolase (FAAH) for anandamide and the monoacylglycerol lipase (MAGL) for 2-AG], and an eCB transporter (Devane et al., 1992; Freund et al., 2003; Kogan and Mechoulam, 2006). Recent cDNA cloning of the key enzymes such as N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) and diacylglycerol lipase (DAGL) accelerated molecular biological studies on the eCB biosyntheses (Bisogno et al., 2003; Okamoto et al., 2004). eCBs are synthesized “on demand” at the post-synaptic sites of neurons after an increase in neural activity and calcium ion influx, and are then released into the synaptic cleft. Their main function appears to be the suppression of neurotransmitter release from the presynapse. Thus, eCBs act as retrograde neurotransmitters, modulating other neurotransmitter systems.
CB1 and CB2 are metabotropic receptors coupled to G-proteins of the Gi/o type. CB1 receptors are localized mainly in the central nervous system, but are also present in a variety of peripheral tissues; they are among the most abundant and widely distributed G-protein coupled receptors in the brain. CB1 receptors are expressed in multiple brain areas, including the olfactory bulb, neocortex, pyriform cortex, hippocampus, amygdala, basal ganglia, thalamic and hypothalamic nuclei, cerebellar cortex, and brainstem nuclei (Herkenham et al., 1990, 1991; Katona et al., 2001). CB2 receptors are mostly peripherally located on immunological tissues, but they have also been found within the central nervous system on neurons and glial cells with their expression mainly related to conditions of inflammation (Galiegue et al., 1995; Schat et al., 1997; Begg et al., 2005). More recent immunohistochemical analyses have revealed the presence of CB2 receptors in apparently neuronal and glial processes in diverse rat brain areas, including the cerebellum and hippocampus (Van Sickle et al., 2005; Onaivi et al., 2006).
In the hippocampus, CB1 receptors are expressed at an especially high density in the dentate gyrus, CA1, and CA3 regions (Herkenham et al., 1990, 1991; Matsuda et al., 1990; Tsou et al., 1998). CB1 receptors are predominantly localized on the axon terminals and preterminal segments of cholecystokinin (CCK)-expressing GABAergic interneurons (Nyíri et al., 2005); however, they have also been demonstrated to inhibit glutamatergic transmission in cultured hippocampal cells (Shen, et al., 1996). CB1 receptors located on GABAergic axon terminals are activated by lower concentrations of cannabinoid receptor agonists than CB1 receptors located on glutamatergic terminals (Ohno-Shosaku et al., 2001; Hoffman et al., 2007) and CB1 receptor expression is significantly lower on glutamatergic terminals than on GABA axon terminals in the hippocampus (Katona et al., 2006; Kawamura et al., 2006). Specifically, activation of hippocampal CB1 receptors decreases GABA release (Katona et al., 1999; Hajos et al., 2000; Hoffman and Lupica, 2000; Hoffman et al., 2003). The CB1-containing GABergic interneurons are thought to control oscillatory electrical activity in the hippocampus in the theta and gamma frequencies, which plays a role in synchronizing pyramidal cell activity (Hoffman and Lupica, 2000).
Overall, the evidence favors a predominant role for GABAergic pathways in the effects of cannabinoids on hippocampal-dependent memory processes.
Cannabinoid Agonists Impair Hippocampal-Dependent Learning and Memory
In humans, non-human primates, and rodents, cannabinoids impair the performance of a wide variety of memory tasks that share the common feature of requiring the hippocampus for normal performance (Sullivan, 2000; Davies et al., 2002; Riedel and Davies, 2005). In laboratory rodents, activation of cannabinoid receptors via THC or synthetic analogues such as WIN 55,212-2, CP55940, HU-210 or the endogenous agonist anandamide impairs learning (Davies et al., 2002). Administration of THC disrupts hippocampal-dependent learned behavior in operant and spatial maze models of memory (Nakamura et al., 1991; Heyser et al., 1993; Lichtman et al., 1995; Brodkin and Moerschbaecher, 1997; Mallet and Beninger, 1998; Ferrari et al., 1999; Varvel et al., 2001). For example, systemic THC administration (2–6 mg/kg i.p.) impairs working memory tested in the radial-arm spatial task and the cannabinoid antagonist SR141716A (1–10 mg/kg) prevents these deficits in a dose-dependent manner (Lichtman and Martin, 1996). Similarly, THC (8 mg/kg) impairs the acquisition of spatial learning in the water maze and the performance of mice in a working memory task, while consolidation and retrieval of a previously learned task are not affected. Pre-treatment with the antagonist SR 141716A (1 mg/kg i.p.) prevents these learning deficits (Da and Takahashi, 2002). Additionally, systemic administration of THC or the synthetic cannabinoid receptor agonist WIN 55,212-2 reliably impairs performance in delayed-match-to-sample and delayed-non-match-to-sample tasks, and this is accompanied by decreases in hippocampal cell firing during the sample phases of the task (Heyser et al., 1993; Hampson and Deadwyler, 1999, 2000).
Overall, the literature discussed above suggests that activation of cannabinoid receptors impairs learning. However, since the agonists were systemically infused, most of these experiments do not specifically show that cannabinoids impair learning and memory via action on the hippocampus. Rather, the involvement of the hippocampus is assumed because it is an important target for systemically administered cannabinoids and because most of the paradigms described are spatial tasks known to be hippocampus-dependent.
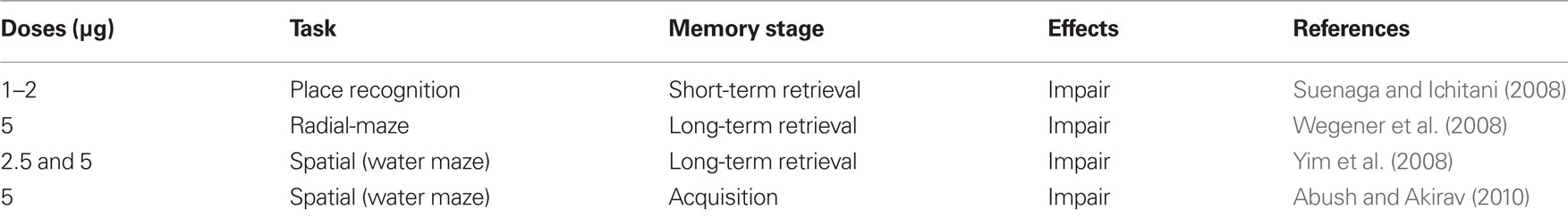
More recent research has directly tested whether specific administration of cannabinoids into the hippocampus would have similar effects (summarized in Table 1). Intrahippocampal infusions of the agonists CP55940, THC, or WIN 55,212-2 were found to disrupt performance in the radial-arm maze, and in T-maze delayed alternation, passive avoidance, spatial learning, and place recognition memory tasks (Lichtman et al., 1995; Mishima et al., 2001; Egashira et al., 2002; Suenaga and Ichitani, 2008; Suenaga et al., 2008; Wegener et al., 2008; Abush and Akirav, 2010). For example, activation of hippocampal cannabinoid receptors by the agonist WIN 55,212-2 (1–2 μg) dose-dependently decreases the exploration of an object in a new place, and this effect is antagonized by pre-treatment with the cannabinoid receptor antagonist AM 281 (2 mg/kg, i.p.; Suenaga and Ichitani, 2008). WIN 55,212-2 (5 μg) injected into the dorsal hippocampus increases the number of reference memory errors in the eight-arm radial-maze task, suggesting impairment of memory retrieval (Wegener et al., 2008). Additionally, post-training intrahippocampal administration of WIN 55,212-2 (2.5 and 5 μg) disrupts long-term spatial memory, but not acquisition or short-term memory, in a rat reference memory task in the water maze (Yim et al., 2008). We have recently found that WIN 55,212-2 administered systemically (0.5 mg/kg) or specifically into the hippocampal CA1 area (5 μg/side) before massed training in the Morris water maze impairs spatial learning (Abush and Akirav, 2010). Thus experiments that specifically targeted the hippocampus confirm the implications of the earlier systemic research as to the impairing effect of cannabinoids on hippocampal-dependent learning and memory.
Cannabinoid Agonists Impair Hippocampal Synaptic Plasticity
In neuronal circuits, memory storage depends on activity-dependent modifications in synaptic efficacy, such as long-term potentiation (LTP) and long-term depression (LTD), which are the two main forms of synaptic plasticity in the brain. A key feature of LTP and LTD is that a short period of synaptic activity (either high- or low-frequency stimulation) can trigger persistent changes in synaptic transmission lasting at least several hours and often longer. This single property initially led investigators to suggest that these forms of plasticity are the cellular correlate of learning (Bliss and Gardner-Medwin, 1973; Bliss and Lomo, 1973). Indeed, efforts to understand synaptic plasticity are driven by the belief that such synaptic modifications might occur during learning and memory. However, it is extremely difficult to demonstrate directly that learning-induced synaptic changes occur following experience.
The mechanisms underlying synaptic plasticity have been studied more intensely in the hippocampus than in any other brain region. Both forms of synaptic plasticity have been studied most intensively at the Schaffer collateral–CA1 synapses of the hippocampus because of the established role of the CA1 area in spatial memory (Behr et al., 2009). LTP and LTD are thought to be involved in memory formation at glutamatergic synapses in the hippocampus. Cannabinoids appear to work by reducing glutamate release below the level needed to activate N-Methyl-D-aspartate (NMDA) receptors that are required for LTP and LTD (Shen et al., 1996; Misner and Sullivan, 1999). CB1 receptors are capable of regulating both inhibitory and excitatory neurotransmitter release in the hippocampus and are thus capable of exerting subtle control over synaptic plasticity.
Most of our knowledge about cannabinoids and activity-dependent changes in synaptic strength comes from studies performed at excitatory synapses, largely using acute hippocampal slices as the experimental model (Chevaleyre et al., 2006). Cannabinoid receptor activation inhibits both LTP and LTD induction in the hippocampal slice. The inhibition of LTP in field potentials in the CA1 region has been demonstrated using THC, HU-210, WIN 55,212-2, 2-AG, and anandamide (Nowicky et al., 1987; Collins et al., 1994, 1995; Terranova et al., 1995; Misner and Sullivan, 1999) and has been found recently to inhibit hippocampal LTD of CA1 field potentials as well (Misner and Sullivan, 1999). The impairment in the induction of LTP in the CA1 is blocked by cannabinoid antagonists such as SR141716A.
We have recently examined cannabinoid modulation of LTP and LTD in a different experimental model: acute anesthetized rats. Using this experimental condition, we found that i.p. administration of WIN 55,212-2 or the CB1 receptor antagonist AM251 at the doses tested impairs LTP in the Schaffer collateral–CA1 projection, with no effect on LTD (Abush and Akirav, 2010; see Figure 1).
de Oliveira Alvares et al. (2006) have also demonstrated impairment of LTP in a CA1 slice preparation following AM251 administration. Sokal et al. (2008) found that the CB1 receptor antagonist SR141716A blocked the potentiation of the fEPSP slope observed following HFS to the perforant path. However, other studies conducted on hippocampal slices of the Schaffer collateral–CA1 synapses have shown that CB1 blockade favors LTP in the hippocampus (Slanina et al., 2005) and that mice lacking CB1 receptors show enhanced LTP (Bohme et al., 2000). However, in the study by Slanina et al. (2005), the drug was present throughout the experiment and LTP was elicited by moderate stimulations (20 or 50 pulses). Thus, the discrepancies with our findings could result from the examination of field potential in an intact rat model versus slices, or from various methodological issues, such as different stimulation protocols, different drug doses, etc.
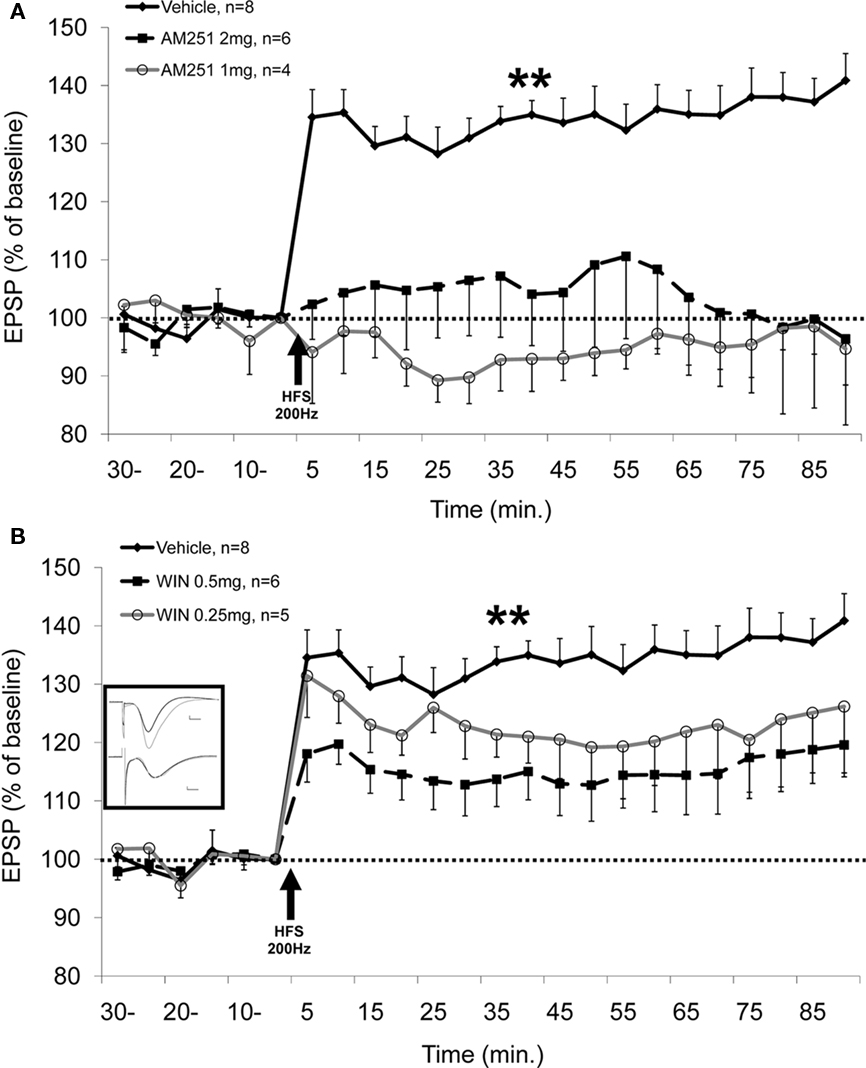
Figure 1. CB1 receptor antagonist and agonist impair the induction of LTP. (A) AM251 injected i.p. (1 or 2 mg/kg) 30 min before application of high frequency stimulation (HFS; 200 Hz) to the Schaffer collateral significantly impairs the induction of LTP in the CA1 compared with the vehicle group (P < 0.01, vehicle differs from all the groups). No significant difference is observed between the groups before HFS. (B) WIN 55,212-2 (0.5 mg/kg) injected i.p. 20 min before application of HFS (200 Hz) to the Schaffer collateral significantly impairs the induction of LTP in the CA1 compared with the vehicle group (P < 0.01). No significant difference is observed between the groups before HFS. Inset: representative traces in the CA1 for vehicle (upper traces) and WIN 0.5 mg (lower traces) groups taken before (black) and 90 min after (gray) HFS to the Schaffer collateral (calibration: 0.2 mV, 10 μs). Data published by Abush and Akirav (2010) in Hippocampus.
Effects of Cannabinoid Agonists on Emotional and Non-Emotional Memory
Although considerable evidence suggests that activation of CB1 receptors can induce learning and memory impairments (Sullivan, 2000; Robinson et al., 2003; O’Shea et al., 2004; Varvel et al., 2005), CB1 receptors are essential for the extinction of conditioned fear associations (Marsicano et al., 2002), indicating an important role for this receptor in neuronal emotional learning and memory.
Role of the Cannabinoid System in Extinction
Extinction was established as a tool to treat conditioned fear by Freud in the 1920s. It has become widely accepted that a deficit in the capacity to extinguish memories of fear is at the root of fear disorders as a result of the distinction between those who do and do not develop serious symptoms after fearsome experiences, and the fact that fear disorders are treated with therapy based on extinction procedures. Moreover, panic attacks, phobias, and particularly post-traumatic stress disorder (PTSD) are viewed by many as a deficit of extinction that should therefore be treated by an intensification of extinction (Charney et al., 1993; Wessa and Flor, 2007; Milad et al., 2008).
Conditioned fear is induced by pairing a neutral, conditioned stimulus (CS; e.g., a light, a tone, or a context) with an aversive stimulus (unconditioned stimulus, US; e.g., a mild footshock) that evokes a measurable fear response. Experimental extinction learning occurs when a CS that previously predicted a US no longer does so, and over time, the conditioned response (e.g., freezing or elevated skin conductance responses) decreases. Extinction learning involves the ventromedial prefrontal cortex (PFC), amygdala, and hippocampus (Milad and Quirk, 2002; Phelps et al., 2004; Bouton et al., 2006). PTSD patients continue to re-experience the traumatic event over a long timeframe and avoid trauma-related stimuli, even though they recognize that the traumatic event is no longer occurring. It has been suggested that dysfunctional fear extinction plays an important role in the development of clinical symptoms, such as reexperiencing trauma in PTSD (Rothbaum and Davis, 2003; Milad et al., 2006; Quirk et al., 2006; Rauch et al., 2006). PTSD patients also demonstrate impaired extinction in the aftermath of new trauma. For example, Milad et al. (2008) have shown deficient extinction recall as measured in skin conductance response in a 2-day fear conditioning and extinction procedure in PTSD patients.
Clearly, animal models do not entirely mimic the complex features of psychiatric disorders. However, they can predict the clinical effects of substances and provide insights into the biological mechanisms of these diseases. Marsicano et al. (2002) found that CB1 receptor-deficient mice show normal acquisition and consolidation in a fear conditioning task, but fear extinction is strongly impaired. Impaired extinction is also observed when the antagonist SR141716 is injected systemically into wild-type mice before the extinction trial, indicating that CB1 receptors are required at the moment of the extinction training. The findings that CB1 knockout mice exhibit impaired short- and long-term extinction of cue-induced conditioned fear responses have been replicated by other groups for the extinction of both cue- and context-induced fear responses (Finn et al., 2004; Suzuki et al., 2004; Chhatwal et al., 2005; Lafenêtre et al., 2007; Lutz, 2007; Niyuhire et al., 2007). We have recently shown that microinjecting the antagonist AM251 (6 ng) into the BLA or the CA1 significantly impairs extinction of inhibitory avoidance (Ganon-Elazar and Akirav, 2009; Abush and Akirav, 2010). Several studies suggest that the eCB system is not involved in the extinction of non-aversive memories (Hölter et al., 2005; Niyuhire et al., 2007).
On the other hand, studies have demonstrated that pharmacological activation of eCB signaling promotes extinction of fear memories. For example, Chhatwal et al. (2005) found that systemic administration of the eCB transporter AM404 (10 mg/kg) promotes extinction of fear that was conditioned using fear-potentiated startle. This was replicated using systemic (Pamplona et al., 2008) and intracerebroventricular (Bitencourt et al., 2008) injections. In another study (Varvel et al., 2007), OL-135 (30 mg/kg), an inhibitor of FAAH, enhanced the rate of extinction in a water maze task. Pamplona et al. (2006) showed that WIN 55,212-2 (0.25 mg/kg) facilitates the extinction of contextual fear in the fear conditioning task and of spatial memory in the water maze reversal task. We have used the light–dark inhibitory avoidance procedure to demonstrate the effects of WIN 55,212-2 administered into the CA1 or the BLA on extinction. This procedure is dependent on both the amygdala and hippocampus as a single CS–US (context–footshock) pairing establishes a robust long-term memory, expressed as an increase in latency to enter the dark chamber at testing. Repeated retrieval of the avoidance response in the absence of the US induces extinction of inhibitory avoidance memory, meaning that the animal learns that the context no longer predicts the footshock. We found that WIN 55,212-2 administered into the CA1 facilitates the extinction of inhibitory avoidance, with no effect on extinction kinetics when microinjected into the BLA (Ganon-Elazar and Akirav, 2009; Abush and Akirav, 2010).
Hence, the results of Marsicano et al. (2002) and subsequent investigations demonstrate that inhibition of eCB transmission robustly inhibits (or prolongs) fear extinction (Suzuki et al., 2004; Pamplona et al., 2006; Ganon-Elazar and Akirav, 2009; Abush and Akirav, 2010). Conversely, stimulation of eCB transmission accelerates fear extinction (Suzuki et al., 2004; Chhatwal et al., 2005; Barad et al., 2006; Abush and Akirav, 2010).
Comparing the Effects of Cannabinoid Agonists on Aversive and Non-Aversive Tasks
It has been suggested that the neural processes underlying emotional memory formation (such as extinction learning) and non-emotional memories (such as spatial learning) are differentially sensitive to cannabinoid receptor activation (Chhatwal and Ressler, 2007). An intriguing question is whether cannabinoids have a similar effect on other types of emotional memories that do not involve fear and extinction learning.
We have recent findings suggesting that cannabinoid receptor activation has differential effects on learning and memory that are task-, brain region-, and memory stage-dependent (Segev and Akirav, 2011). We examined the effects of WIN 55,212-2 microinjected into the amygdala and the subiculum on the acquisition and retrieval of a neutral learning task (i.e., social discrimination) and an aversive learning task (i.e., contextual fear conditioning). The subiculum is the principal target of CA1 pyramidal cells. It functions as a mediator of hippocampal–cortical interaction and has been proposed to play an important role in the encoding and retrieval of long-term memory. In fear conditioning paradigms, the BLA plays a central role in the formation and consolidation of fear-related memory traces (LeDoux, 2003; Maren and Quirk, 2004), whereas the hippocampus’s role is to integrate the features of the context and not to form a context–shock association (Fanselow, 1998). Unlike the aversive fear conditioning task, social discrimination is considered neutral or even rewarding. This finding was established using both conditioned place preference paradigms and T-maze learning rewarded by social interaction (Van den Berg et al., 1999). Social recognition processes depend on brain regions such as the medial amygdala, which modulates the initial social encounter and formation of social memory (Ferguson et al., 2001; Bielsky and Young, 2004) and the ventral hippocampus (Van Wimersma Greidanus and Maigret, 1996; Kogan et al., 2000).
We found that in the aversive contextual fear task, WIN 55,212-2 administered into the BLA impairs fear acquisition/consolidation, but not retrieval, whereas in the ventral subiculum (vSub), WIN 55,212-2 impairs fear retrieval. In the non-aversive or rewarding social discrimination task, WIN 55,212-2 into the vSub impairs acquisition/consolidation and retrieval, whereas in the medial amygdala, WIN 55,212-2 impairs acquisition (Segev and Akirav, 2011). These findings suggest that cannabinoid agonists can impair emotional (or aversive) as well as neutral (or rewarding) memory-related processes in a task-, region-, and memory stage-dependent manner. This is consistent with other studies suggesting that exogenous acute cannabinoid treatment may have different outcomes depending on task aversiveness and the brain region involved (Suzuki et al., 2004; de Oliveira Alvares et al., 2005; Varvel et al., 2005; Ganon-Elazar and Akirav, 2009; Abush and Akirav, 2010).
Effects of Cannabinoids on Stress and Anxiety
Considerable evidence suggests that cannabinoids are anxiolytics and modulate the behavioral and physiological response to stressful events (Viveros et al., 2007; Hill et al., 2010). Consequently, the effects of CB1 agonists on learning and memory may be attributable to a general modulation of anxiety or stress levels and not to memory per se.
Stress is most readily defined as any stimulus that presents a challenge to homeostasis including any actual or potential disturbance of an individual’s environment. The stress response enables the animal to adapt to the changing environment (Joëls and Baram, 2009). Fear is an adaptive component of the acute stress response to potentially dangerous stimuli that threaten the integrity of the individual. However, when disproportionate in its intensity, chronic, irreversible, and/or not associated with any actual risk, it constitutes a maladaptive response and may be symptomatic of anxiety-related neuropsychiatric disorders (Taber and Hurley, 2009).
Anxiety disorders are marked by excessive fear (and avoidance), often in response to specific objects or situations, in the absence of true danger, and they are common in the general population (Shin and Liberzon, 2010). As excessive fear is a key component of anxiety disorders, the search for the neurocircuitry of anxiety disorders has focused extensively on studies of fear circuits in animal models. These studies examined the neurocircuitry associated with fear responses in rats and mice using fear conditioning paradigms, inhibitory avoidance, and fear-potentiated startle models. The amygdala, PFC, and hippocampus have arisen as clear regions of interest in studies of anxiety disorders and are implicated in PTSD (Shin and Liberzon, 2010).
The hippocampus is often implicated in the neurobiology of stress. Mineralocorticoid and glucocorticoid receptors are expressed in high numbers within the hippocampus. Although stress-induced corticosteroid signaling in the hippocampus has a beneficial role in regulating the time course of the hypothalamic–pituitary–adrenal (HPA) axis stress response (De Kloet et al., 2005), prolonged glucocorticoid signaling can damage the hippocampus as measured by dendritic atrophy, decreased neurogenesis, and deficits in synaptic plasticity (McEwen and Gould, 1990; Sapolsky, 1996; McEwen, 1999; Meaney, 2001). In PTSD and major depression patients, hippocampus volumes are reduced (Bremner et al., 1995; Sheline et al., 1999; Woon and Hedges, 2008), and smaller hippocampal volumes are predictive of vulnerability to developing stress-related disorders (Pitman et al., 2006).
Role of the Endocannabinoid System in Unconditioned Stress and Anxiety
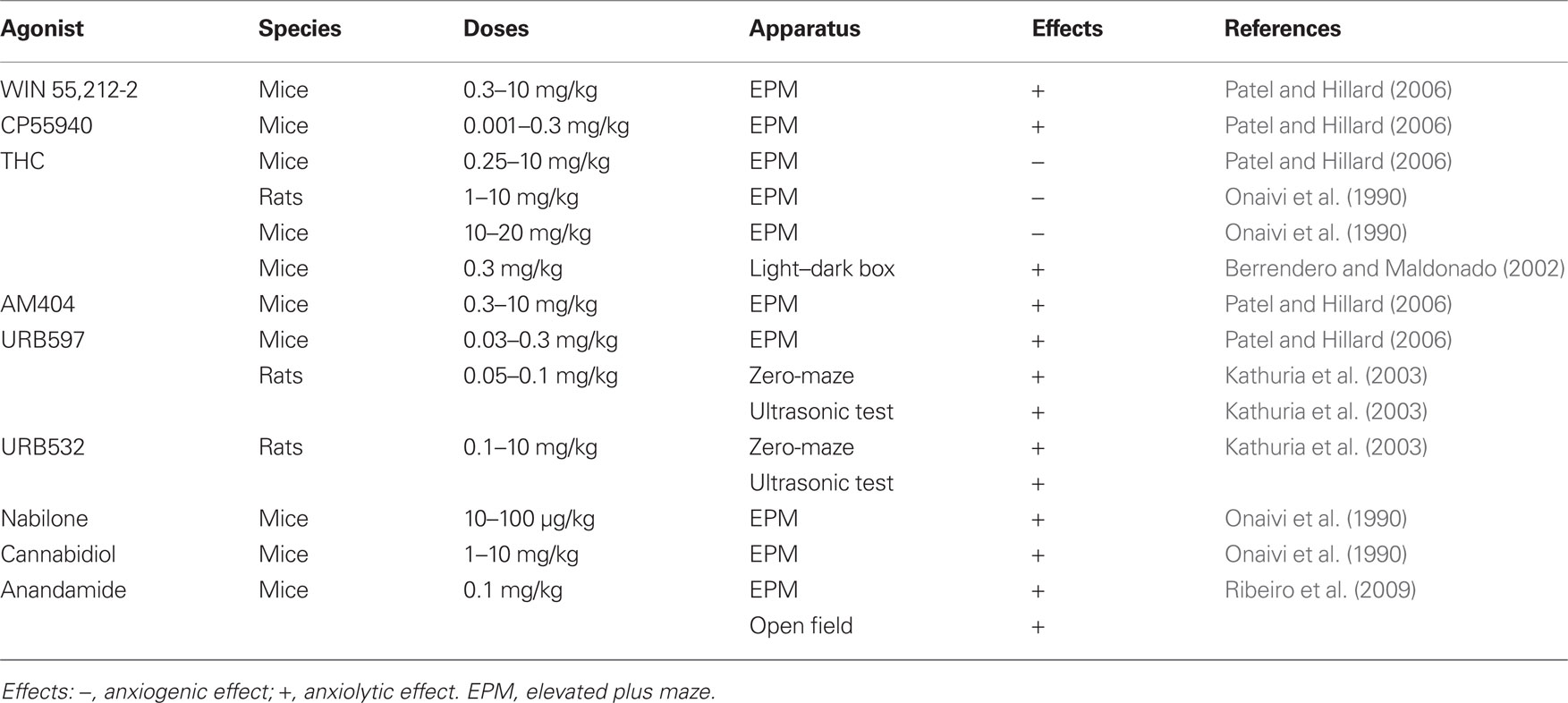
Results from many studies indicate that the eCB system modulates unconditioned stress- and anxiety-like responses (Viveros et al., 2005; Gorzalka et al., 2008; Lutz, 2009). A general conclusion that can be tentatively derived from the complicated and often contradictory literature is that inhibition of eCB signaling increases stress and anxiety, while moderate increases in eCB signaling decrease stress and anxiety (Lutz, 2009; summarized in Table 2). The term “moderate” is used because strong stimulation of eCB signaling by high doses of CB1 receptor agonists potentiates stress- and anxiety-like responses (Rodriguez de Fonseca et al., 1996; Scherma et al., 2008; Lutz, 2009). This biphasic effect has been demonstrated in animal models of anxiety (Lafenêtre et al., 2007; Hill and Gorzalka, 2009), and also in humans. Cannabis may induce aversive states in some smokers, precipitating anxiety and panic attacks (Hall and Solowij, 1998). Furthermore, THC administration may result in psychotic-like states (Linszen and van Amelsvoort, 2007). These bidirectional effects of cannabinoids observed in humans can be mimicked in laboratory animals. Hence, in models predictive of anxiolytic-like activity, low doses of CB1 agonists tend to be anxiolytic and high doses tend to increase aversion and anxiety-related behaviors (Viveros et al., 2005).
Procedures used in studies on the role of eCBs in stress and anxiety evaluate the anxiolytic/anxiogenic effects of drugs by using standard tasks such as the elevated plus maze (EPM), social interaction, and defensive burying (Viveros et al., 2005; Lutz, 2009). Using the EPM, Patel and Hillard (2006) found that cannabinoid receptor agonists WIN 55212-2 (0.3–10 mg/kg) and CP55940 (0.001–0.3 mg/kg) administered systemically increase the time mice spend on the open arms (i.e., elicit an anxiolytic response) only at low doses. At the highest doses, both compounds alter overall locomotor activity. In contrast, THC (0.25–10 mg/kg) produces a dose-dependent reduction in time spent on open arms. The eCB uptake/catabolism inhibitor AM404 (0.3–10 mg/kg) produces an increase in time spent on the open arms at low doses and has no effect at the highest dose tested. The FAAH inhibitor URB597 (0.03–0.3 mg/kg) produces a monophasic, dose-dependent increase in time spent on the open arms. Systemic administration of the CB1 receptor antagonists SR141716 (1–10 mg/kg) and AM251 (1–10 mg/kg) produce dose-related decreases in time spent on open arms. Onaivi et al. (1990) have shown that THC induces increased aversion to the open arms of the EPM in both rats and mice that is similar to the aversion produced by anxiogenic agents. In contrast, mice treated with the agonists cannabidiol and nabilone spend a greater amount of time in the open arms of the maze, an effect similar to that produced by diazepam, the reference anxiolytic agent.
In the light–dark box, Berrendero and Maldonado (2002) have shown that the systemic administration of a low dose of THC (0.3 mg/kg) produces clear anxiolytic-like responses. The CB1 cannabinoid receptor antagonist SR 141716A (0.5 mg/kg) completely blocks the anxiolytic-like response induced by THC, suggesting that this effect is mediated by CB1 cannabinoid receptors. In another study, systemic administration of the FAAH inhibitors URB597 and URB532 reduces anxiety-related behavior in the rat elevated zero-maze and in isolation-induced ultrasonic vocalization tests (Kathuria et al., 2003). These effects are dose-dependent and blocked by the antagonist rimonabant. The FAAH inhibitor and eCB re-uptake inhibitor AM404 also exhibit a dose-dependent anxiolytic profile in the EPM, defensive withdrawal test, and ultrasonic vocalization test (Bortolato et al., 2006). URB597 has also been shown to be anxiolytic in the rat EPM and open-field tests (Hill et al., 2007) and has recently been shown to reduce anxiety-related behavior in the EPM in Syrian hamsters (Moise et al., 2008).
Ribeiro et al. (2009) examined the dose-response effects of exogenous anandamide at doses of 0.01, 0.1, and 1.0 mg/kg in mice sequentially submitted to the open field and EPM. Systemically administered at 0.1 mg/kg (but not at 0.01 or 1 mg/kg), anandamide increases the time spent and the distance covered in the central zone of the open field, as well as exploration of the open arms of the EPM. Recently, Rubino et al. (2008b) demonstrated that the anxiolytic-like effect of a low anandamide dose is reversed by administration of the antagonist AM251, whereas the anxiogenic-like effect is inhibited by pre-treatment with capsazepine, a transient receptor potential vanilloid type 1 (TRPV1) receptor antagonist. The authors suggested that the anxiolytic effect evoked by anandamide might be due to the interaction with the CB1 cannabinoid receptor, whereas vanilloid receptors seem to be involved in the anxiogenic action of anandamide (Rubino et al., 2008b). Marsch et al. (2007) reported that TRPV1 “null” mice exhibit a significantly reduced response to anxiogenic stimuli. Therefore, the anandamide-induced inverted U-shape pattern might be based on the fact that the intrinsic efficacy of anandamide on TRPV1 is relatively low compared to that observed on the CB1 receptor (Ross, 2003).
Transgenic mice deficient for FAAH, the enzyme that degrades anandamide, demonstrate reduced anxiety-like behavior in the EPM and light–dark box compared with wild-type mice and these effects are prevented by systemic administration of the antagonist rimonabant (Moreira et al., 2008). By contrast, transgenic mice lacking expression of the CB1 receptor demonstrate an anxiogenic profile in the EPM, the light–dark box, open-field arena, and social interaction test (Haller et al., 2002, 2004; Maccarrone et al., 2002; Martin et al., 2002; Urigüen et al., 2004) and demonstrate impaired stress coping behavior in the forced swim test (Steiner et al., 2008). Similarly, CB1 receptor antagonists increase anxiety-related behaviors in the EPM (Patel and Hillard, 2006). Taken together, these studies suggest that eCBs act at CB1 receptors to reduce anxiety.
Role of the Endocannabinoid System in Conditioned Fear and Anxiety
Understanding the role of the eCB system in conditioned fear and aversive memories is important because a number of anxiety disorders, including PTSD and phobias, are thought to result from dysregulated fear neurocircuitry (Rauch et al., 2006). Investigators have examined the effect of CB1 receptor agonists and antagonists on contextual and cue fear conditioning. Results from these studies were somewhat mixed. In rats, systemic injections of the CB1 receptor antagonist AM251 enhance both the acquisition and expression of cue fear conditioning (Arenos et al., 2006; Reich et al., 2008). Administering AM251 (5 mg/kg, i.p) during tone–footshock conditioning enhances acquisition of freezing behavior for both trace fear conditioning (hippocampal-dependent) and delay fear conditioning (amygdala-dependent; Reich et al., 2008). Recently, we used an inhibitory avoidance task and found that microinjecting AM251 (6 ng) into the BLA significantly enhances conditioned avoidance but has no effect on conditioning when microinjected into the hippocampal CA1 area (Ganon-Elazar and Akirav, 2009; Abush and Akirav, 2010). However, others have shown that mice lacking the CB1 receptor or systemically administered with the CB1 receptor antagonist AM251 (0.3–3 mg/kg) 30 min before behavioral testing show no contextually induced fear response (Mikics et al., 2006). Furthermore, the CB1 receptor antagonist rimonabant or genetic deletion of the CB1 receptor has no effect on the acquisition of cue and context fear conditioning in mice (Marsicano et al., 2002; Suzuki et al., 2004). On the other hand, cue-fear-potentiated startle is decreased by medial PFC injections of the CB1 receptor agonist WIN 55212-2 or the FAAH inhibitor URB597 (Lin et al., 2008, 2009) and contextual fear conditioning is decreased by dorsolateral periaqueductal gray injections of either anandamide or the anandamide transport inhibitor AM404 (Resstel et al., 2008). Overall it appears that, as in the case of unconditioned fear, inhibition of eCB transmission increases fear while moderate stimulation of eCB transmission decreases fear.
The Involvement of the Hippocampus in Endocannabinoid Modulation of Stress and Anxiety
Techniques based on intracranial injections of cannabinoids in rats revealed that activation of CB1 receptors is involved in inducing anxiolytic- or antidepressant-like effects (Bambico et al., 2007; Moreira et al., 2007; Rubino et al., 2008a,b). For example, Rubino et al. (2008a) found that low doses of THC microinjected into the PFC (10 μg) or ventral hippocampus (5 μg) in rats induces an anxiolytic-like response during tests in the EPM, while higher doses do not show an anxiolytic effect and even seem to switch into an anxiogenic profile. Nevertheless, other studies demonstrated that eCB activation in the amygdala and dorsal hippocampus results in an anxiogenic-like response. Low THC doses (1 μg) in the BLA produce an anxiogenic-like response whereas higher doses are ineffective (Rubino et al., 2008a). WIN-55212-2 in the dorsal hippocampus (2.5 and 5 μg) produces a significant anxiogenic-like effect in rats that is reversed by AM251 (Roohbakhsh et al., 2007).
Local infusion of cannabinoid compounds into specific brain areas might be instrumental in identifying neural pathways and neuroanatomically separated CB1 receptor subpopulations that may play distinct roles in and mediate the opposing actions of cannabinoids, notably, anxiolytic versus anxiogenic effects (Moreira et al., 2007; Viveros et al., 2007). We examined the role of cannabinoids in modulating aversive and non-aversive learning paradigms in the hippocampus and amygdala (Ganon-Elazar and Akirav, 2009; Abush and Akirav, 2010; Segev and Akirav, 2011). Microinjecting the antagonist AM251 (6 ng) or the agonist WIN-55212-2 (5 μg) into the BLA, CA1, or vSub had no effect on anxiety levels as measured in the open-field, pain sensitivity (Ganon-Elazar and Akirav, 2009; Abush and Akirav, 2010; Segev and Akirav, 2011), or EPM tests (Abush and Akirav, 2010). However, both agonist and antagonist had profound effects on aversive and non-aversive learning tasks. These findings suggest that in these studies the impairing and facilitating effects of local infusions of WIN-55212-2 on learning and memory are probably not attributable to a general modulation of anxiety. Nevertheless, the effects of cannabinoids on the interplay between anxiety and memory processes are difficult to separate and further examination of the effects of different cannabinoids is required.
To summarize the role of the eCB system in stress, anxiety, and conditioned fear, there is a general consensus that the effects of cannabinoid agonists on anxiety seem to be biphasic, with low doses being anxiolytic and high doses being ineffective or possibly anxiogenic. There are several important characteristics of the eCB system that might explain these different effects of eCB modulation. First, in a physiological situation, eCB synthesis, and thus CB1 receptor activation, occurs in particular activated neuronal circuits. This is a notable difference from the situation following pharmacological treatment with receptor agonists, when the agent activates all CB1 receptors in the brain regardless of their specific involvement in a particular physiological process. Second, the CB1 receptor is expressed in diverse brain structures of relevance to psychiatric disorders and is mainly located presynaptically where it can suppress the release of other neurotransmitters (Marsicano and Lutz, 1999, 2006; Mackie, 2005). These neurotransmitters include the main inhibitory neurotransmitter GABA, the main excitatory neurotransmitter glutamate, as well as acetylcholine, noradrenaline, and serotonin (Katona et al., 1999; Harkany et al., 2005; Monory et al., 2006; Häring et al., 2007; Oropeza et al., 2007). Thus, synthetic compounds delivered systemically lack both the spatial and temporal specificity of endogenous compounds (Lafenêtre et al., 2007; Viveros et al., 2007; Moreira and Lutz, 2008). This may explain not only the bell-shaped relationship between dose and effect that some studies have observed, but also why elevation of eCB levels sometimes has effects that are different from those observed with exogenous cannabinoids. Finally, the diversity of eCB ligands with their multiple synthetic and degradation pathways adds a further level of complexity to the eCB system (Di Marzo, 2008).
Summary
The findings demonstrate that the cannabinoid system has diverse effects on hippocampal memory and plasticity that cannot be categorized simply into an impairing or an enhancing effect, but are rather dependent on important variables such as the nature of the task (i.e., aversive, emotional or not), the memory stage under investigation (acquisition, consolidation, retrieval, extinction), and the brain areas involved.
The involvement of the eCB system in multiple aspects of brain function provides new targets for the development of novel therapeutic agents for a wide range of psychiatric disorders, including the treatment of anxiety disorders. Studies examining the involvement of cannabinoids in memory processes advance our understanding of the potential harmful consequences of cannabis use and the mechanisms underlying the close relationship between cannabinoids and cognition. This will help in determining whether the clinical benefits of using cannabinoids outweigh the risks, and to better cope with the deficits induced by cannabinoids.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abush, H., and Akirav, I. (2010). Cannabinoids modulate hippocampal memory and plasticity. Hippocampus 20, 1126–1138.
Arenos, J. D., Musty, R. E., and Bucci, D. J. (2006). Blockade of cannabinoid CB1 receptors alters contextual learning and memory. Eur. J. Pharmacol. 539, 177–183.
Bambico, F. R., Katz, N., Debonnel, G., and Gobbi, G. (2007). Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J. Neurosci. 27, 11700–11711.
Barad, M., Gean, P. W., and Lutz, B. (2006). The role of the amygdala in the extinction of conditioned fear. Biol. Psychiatry 60, 322–328.
Begg, M., Pacher, P., Bátkai, S., Osei-Hyiaman, D., Offertáler, L., Mo, F. M., Liu, J., and Kunos, G. (2005). Evidence for novel cannabinoid receptors. Pharmacol. Ther. 106, 133–145.
Behr, J., Wozny, C., Fidzinski, P., and Schmitz, D. (2009). Synaptic plasticity in the subiculum. Prog. Neurobiol. 89, 334–342.
Berrendero, F., and Maldonado, R. (2002). Involvement of the opioid system in the anxiolytic-like effects induced by G9-tetrahydrocannabinol. Psychopharmacology (Berl.) 163, 111–117.
Bielsky, I. F., and Young, L. J. (2004). Oxytocin, vasopressin, and social recognition in mammals. Peptides 25, 1565–1574.
Bisogno, T., Howell, F., Williams, G., Minassi, A., Cascio, M. G., Ligresti, A., Matias, I., Schiano-Moriello, A., Paul, P., Williams, E. J., Gangadharan, U., Hobbs, C., Di Marzo, V., and Doherty, P. (2003). Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 163, 463–468.
Bitencourt, R. M., Pamplona, F. A., and Takahashi, R. N. (2008). Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur. Neuropsychopharmacol. 18, 849–859.
Bliss, T. V., and Gardner-Medwin, A. R. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the unanaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 357–374.
Bliss, T. V., and Lomo, T. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356.
Bohme, G. A., Laville, M., Ledent, C., Parmentier, M., and Imperato, A. (2000). Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience 95, 5–7.
Bortolato, M., Campolongo, P., Mangieri, R. A., Scattoni, M. L., Frau, R., Trezza, V., La Rana, G., Russo, R., Calignano, A., Gessa, G. L., Cuomo, V., and Piomelli, D. (2006). Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology 31, 2652–2659.
Bouton, M. E., Westbrook, R. F., Corcoran, K. A., and Maren, S. (2006). Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol. Psychiatry 60, 352–360.
Bremner, J. D., Randall, P., Scott, T. M., Bronen, R. A., Seibyl, J. P., Southwick, S. M., Delaney, R. C., McCarthy, G., Charney, D. S., and Innis, R. B. (1995). MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. Psychiatry 152, 973–981.
Brodkin, J., and Moerschbaecher, J. M. (1997). SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J. Pharmacol. Exp. Ther. 282, 1526–1532.
Charney, D. S., Deutch, A. Y., Krystal, J. H., Southwick, S. M., and Davis, M. (1993). Psychobiologic mechanisms of posttraumatic stress disorder. Arch. Gen. Psychiatry 50, 294–305.
Chevaleyre, V., Takahashi, K. A., and Castillo, P. E. (2006). Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 29, 37–76.
Chhatwal, J. P., Myers, K. M., Ressler, K. J., and Davis, M. (2005). Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J. Neurosci. 25, 502–506.
Chhatwal, J. P., and Ressler, K. J. (2007). Modulation of fear and anxiety by the endogenous cannabinoid system. CNS Spectr. 12, 211–220.
Collins, D. R., Pertwee, R. G., and Davies, S. N. (1994). The action of synthetic cannabinoids on the induction of long-term potentiation in the rat hippocampal slice. Eur. J. Pharmacol. 11, R7–R8.
Collins, D. R., Pertwee, R. G., and Davies, S. N. (1995). Prevention by the cannabinoid antagonist, SR141716A, of cannabinoid-mediated blockade of long-term potentiation in the rat hippocampal slice. Br. J. Pharmacol. 115, 869–870.
Da, S., and Takahashi, R. N. (2002). SR 141716A prevents delta 9-tetrahydrocannabinol-induced spatial learning deficit in a Morris-type water maze in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 26, 321–325.
Davies, S. N., Pertwee, R. G., and Riedel, G. (2002). Functions of cannabinoid receptors in the hippocampus. Neuropharmacology 42, 993–1007.
De Kloet, E. R., Joëls, M., and Holsboer, F. (2005). Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475.
de Oliveira Alvares, L., de Oliveira, L. F., Camboim, C., Diehl, F., Genro, B. P., Lanziotti, V. B., and Quillfeldt, J. A. (2005). Amnestic effect of intrahippocampal AM251, a CB1-selective blocker, in the inhibitory avoidance, but not in the open field habituation task, in rats. Neurobiol. Learn. Mem. 83, 119–124.
de Oliveira Alvares, L., Genro, B. P., Vaz Breda, R., Pedroso, M. F., Da Costa, J. C., and Quillfeldt, J. A. (2006). AM251, a selective antagonist of the CB1 receptor, inhibits the induction of long-term potentiation and induces retrograde amnesia in rats. Brain Res. 1075, 60–67.
Devane, W. A., Hanus, L., Breuer, A., Pertwee, R. G., Stevenson, L. A., Griffin, G., Gibson, D., Mandelbaum, A., Etinger, A., and Mechoulam, R. (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949.
Di Marzo, V. (2008). Endocannabinoids: synthesis and degradation. Rev. Physiol. Biochem. Pharmacol. 160, 1–24.
Egashira, N., Mishima, K., Iwasaki, K., and Fujiwara, M. (2002). Intracerebral microinjections of delta 9-tetrahydrocannabinol: search for the impairment of spatial memory in the eight-arm radial maze in rats. Brain Res. 952, 239–245.
Fanselow, M. S. (1998). Pavlovian conditioning, negative feedback, and blocking: mechanisms that regulate association formation. Neuron 20, 625–627.
Ferguson, J. N., Aldag, J. M., Insel, T. R., and Young, L. J. (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 21, 278–8285.
Ferrari, F., Ottani, A., Vivoli, R., and Giuliani, D. (1999). Learning impairment produced in rats by the cannabinoid agonist HU 210 in a water-maze task. Pharmacol. Biochem. Behav. 64, 555–561.
Finn, D., Beckett, S., Richardson, D., Kendall, D., Marsden, C., and Chapman, V. (2004). Evidence for differential modulation of conditioned aversion and fear-conditioned analgesia by CB1 receptors. Eur. J. Neurosci. 20, 848–852.
Freund, T. F., Katona, I., and Piomelli, D. (2003). Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 83, 1017–1066.
Galiegue, S., Mary, S., Marchand, J., Dussossoy, D., Carrière, D., Carayon, P., Bouaboula, M., Shire, D., Le Fur, G., and Casellas, P. (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232, 54–61.
Ganon-Elazar, E., and Akirav, I. (2009). Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J. Neurosci. 29, 11078–11088.
Gaoni, Y., and Mechoulam, R. (1964). Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 86, 1646–1647.
Gorzalka, B. B., Hill, M. N., and Hillard, C. J. (2008). Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci. Biobehav. Rev. 32, 1152–1160.
Hajos, N., Katona, I., Naiem, S., MacKie, K., Ledent, C., Mody, I., and Freund, T. (2000). Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur. J. Neurosci. 12, 3239–1349.
Haller, J., Bakos, N., Szirmay, M., Ledent, C., and Freund, T. (2002). The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci. 16, 1395–1398.
Haller, J., Varga, B., Ledent, C., Barna, I., and Freund, T. (2004). Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur. J. Neurosci. 19, 1906–1912.
Hampson, R. E., and Deadwyler, S. A. (1999). Cannabinoids, hippocampal function and memory. Life Sci. 65, 715–723.
Hampson, R. E., and Deadwyler, S. A. (2000). Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J. Neurosci. 20, 8932–8942.
Häring, M., Marsicano, G., Lutz, B., and Monory, K. (2007). Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience 146, 1212–1219.
Harkany, T., Dobszay, M., Cayetanot, F., Härtig, W., Siegemund, T., Aujard, F., and Mackie, K. (2005). Redistribution of CB1 cannabinoid receptors during evolution of cholinergic basal forebrain territories and their cortical projection areas: A comparison between the gray mouse lemur (microcebus murinus, primates) and rat. Neuroscience 135, 595–609.
Herkenham, M., Lynn, A. B., Johnson, M. R., Melvin, L. S., de Costa, B. R., and Rice, K. C. (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 11, 563–583.
Herkenham, M., Lynn, A. B., Little, M. D., Johnson, M. R., Melvin, L. S., de Costa, B. R., and Rice, K. C. (1990). Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. U.S.A. 87, 1932–1936.
Heyser, C. J., Hampson, R. E., and Deadwyler, S. A. (1993). Effects of Δ9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J. Pharmacol. Exp. Ther. 264, 294–307.
Hill, M. N., and Gorzalka, B. B. (2009). The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol. Disord. Drug Targets 8, 451–458.
Hill, M. N., Karacabeyli, E. S., and Gorzalka, B. B. (2007). Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology 32, 350–357.
Hill, M. N., Patel, S., Campolongo, P., Tasker, J. G., Wotjak, C. T., and Bains, J. S. (2010). Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J. Neurosci. 10, 14980–14986.
Hoffman, A. F., and Lupica, C. R. (2000). Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J. Neurosci. 20, 2470–2479.
Hoffman, A. F., Oz, M., Caulder, T., and Lupica, C. R. (2003). Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J. Neurosci. 23, 4815–4820.
Hoffman, A. F., Oz, M., Yang, R., Lichtman, A. H., and Lupica, C. R. (2007). Opposing actions of chronic {Delta} 9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn. Mem. 14, 63–74.
Hölter, S. M., Kallnik, M., Wurst, W., Marsicano, G., Lutz, B., and Wotjak, C. T. (2005). Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur. J. Pharmacol. 510, 69–74.
Kathuria, S., Gaetani, S., Fegley, D., Valiño, F., Duranti, A., Tontini, A., Mor, M., Tarzia, G., La Rana, G., Calignano, A., Giustino, A., Tattoli, M., Palmery, M., Cuomo, V., and Piomelli, D. (2003). Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 9, 76–81.
Katona, I., Rancz, E. A., Acsády, L., Ledent, C., Mackie, K., Hajos, N., and Freund, T. F. (2001). Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neurosci. 21, 9506–9518.
Katona, I., Sperlagh, B., Sik, A., Kafalvi, A., Vizi, E. S., Mackie, K., and Freund, T. F. (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 19, 4544–4558.
Katona, I., Urban, G. M., Wallace, M., Ledent, C., Jung, K. M., Piomelli, D., Mackie, K., and Freund, T. F. (2006). Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 26, 5628–5637.
Kawamura, Y., Fukaya, M., Maejima, T., Yoshida, T., Miura, E., Watanabe, M., Ohno-Shosaku, T., and Kano, M. (2006). The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 26, 2991–3001.
Kogan, J. H., Frankland, P. W., and Silva, A. J. (2000). Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10, 47–56.
Kogan, N., and Mechoulam, R. (2006). The chemistry of endocannabinoids. J. Endocrinol. Invest. 29, 3–14.
Lafenêtre, P., Chaouloff, F., and Marsicano, G. (2007). The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol. Res. 56, 367–381.
Lichtman, A. H., Dimen, K. R., and Martin, B. R. (1995). Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology 119, 282–290.
Lichtman, A. H., and Martin, B. R. (1996). Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology (Berl.) 126, 125–131.
Lin, H. C., Mao, S. C., Chen, P. S., and Gean, P. (2008). Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learn. Mem. 15, 876–884.
Lin, H. C., Mao, S. C., Su, C. L., and Gean, P. W. (2009). The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb. Cortex 19, 165–175.
Linszen, D., and van Amelsvoort, T. (2007). Cannabis and psychosis: an update on course and biological plausible mechanisms. Curr. Opin. Psychiatry 20, 116–120.
Lutz, B. (2009). Endocannabinoid signals in the control of emotion. Curr. Opin. Pharmacol. 9, 46–52.
Maccarrone, M., Valverde, O., Barbaccia, M. L., Castañé, A., Maldonado, R., Ledent, C., Parmentier, M., and Finazzi-Agrò, A. (2002). Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur. J. Neurosci. 15, 1178–1186.
Mackie, K. (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp Pharmacol. 168, 299–325.
Mallet, P. E., and Beninger, R. J. (1998). The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta-9-tetrahydrocannabinol or anandamide. Psychopharmacology 140, 11–19.
Maren, S., and Quirk, G. J. (2004). Neuronal signalling of fear memory. Nat. Rev. Neurosci. 5, 844–852.
Marsch, R., Foeller, E., Rammes, G., Bunck, M., Kossl, M., Holsboer, F., Zieglgänsberger, W., Landgraf, R., Lutz, B., and Wotjak, C. T. (2007). Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J. Neurosci. 27, 832–839.
Marsicano, G., and Lutz, B. (1999). Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 11, 4213–4225.
Marsicano, G., and Lutz, B. (2006). Neuromodulatory functions of the endocannabinoid system. J. Endocrinol. Invest. 29, 27–46.
Marsicano, G., Wotjak, C. T., Azad, S. C., Bisogno, T., Rammes, G., Cascio, M. G., Hermann, H., Tang, J., Hofmann, G., Zieglansberger, W., Di Marzo, V., and Lutz, B. (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature 418, 530–534.
Martin, M., Ledent, C., Parmentier, M., Maldonado, R., and Valverde, O. (2002). Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl.) 159, 379–387.
Matsuda, L. A., Lolait, S. J., Brownstein, M. J., Young, A. C., and Bonner, T. I. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564.
McEwen, B. C., and Gould, E. (1990). Adrenal steroid influences on the survival of hippocampal neurons. Biochem. Pharmacol. 40, 2393–2402.
Meaney, M. J. (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Neuroscience 24, 1161–1192.
Mikics, é., Dombi, T., Barsvári, B., Varga, B., Ledent, C., Freund, T. F., and Haller, J. (2006). The effects of cannabinoids on contextual conditioned fear in CB1 knockout and CD1 mice. Behav. Pharmacol. 17, 223–230.
Milad, M. R., Orr, S. P., Lasko, N. B., Chang, Y., Rauch, S. L., and Pitman, R. K. (2008). Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J. Psychiatr. Res. 42, 515–520.
Milad, M. R., and Quirk, G. J. (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74.
Milad, M. R., Rauch, S. L., Pitman, R. K., and Quirk, G. J. (2006). Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol. Psychol. 73, 61–71.
Mishima, K., Egashira, N., Hirosawa, N., Fujii, M., Matsumoto, Y., Iwasaki, K., and Fujiwara, M. (2001). Characteristics of learning and memory impairment induced by delta9-tetrahydrocannabinol in rats. Jpn. J. Pharmacol. 87, 297–308.
Misner, D. L., and Sullivan, J. M. (1999). Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J. Neurosci. 19, 6795–6805.
Moise, A. M., Eisenstein, S. A., Astarita, G., Piomelli, D., and Hohmann, A. G. (2008). An endocannabinoid signaling system modulates anxiety-like behavior in male syrian hamsters. Psychopharmacology (Berl.) 200, 333–346.
Monory, K., Massa, F., Egertová, M., Eder, M., Blaudzun, H., Westenbroek, R., Kelsch, W., Jacob, W., Marsch, R., Ekker, M., Long, J., Rubenstein, J. L., Goebbels, S., Nave, K. A., During, M., Klugmann, M., Wölfel, B., Dodt, H. U., Zieglgänsberger, W., Wotjak, C. T., Mackie, K., Elphick, M. R., Marsicano, G., and Lutz, B. (2006). The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51, 455–466.
Moreira, F. A., Aguiar, D. C., and Guimarães, F. S. (2007). Anxiolytic-like effect of cannabinoids injected into the rat dorsolateral periaqueductal gray. Neuropharmacology 52, 958–965.
Moreira, F. A., Kaiser, N., Monory, K., and Lutz, B. (2008). Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology 54, 141–150.
Moreira, F. A., and Lutz, B. (2008). The endocannabinoid system: emotion, learning and addiction. Addict. Biol. 13, 196–212.
Nakamura, E. M., da Silva, E. A., Concilio, G. V., Wilkinson, D. A., and Masur, J. (1991). Reversible effects of acute and long-term administration of Δ9-tetrahydrocannabinol (THC) on memory in the rat. Drug Alcohol Depend. 28, 167–175.
Niyuhire, F., Varvel, S. A., Thorpe, A. J., Stokes, R. J., Wiley, J. L., and Lichtman, A. H. (2007). The disruptive effects of the CB 1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berl.) 191, 223–231.
Nowicky, A. V., Teyler, T. J., and Vardaris, R. M. (1987). The modulation of long term potentiation by delta-9-tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Res. Bull. 19, 663–672.
Nyíri, G., Cserép, C., Szabadits, E., Mackie, K., and Freund, T. F. (2005). CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience 136, 811–822.
Ohno-Shosaku, T., Maejima, T., and Kano, M. (2001). Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29, 729–738.
Okamoto, Y., Morishita, J., Tsuboi, K., Tonai, T., and Ueda, N. (2004). Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 279, 5298–5305.
Onaivi, E., Green, M., and Martin, B. (1990). Pharmacological characterization of cannabinoids in the elevated plus maze. J. Pharmacol. Exp. Ther. 253, 1002–1009.
Onaivi, E. S., Ishiguro, H., Gong, J.-P., Patel, S., Perchuk, A., Meozzi, P. A., Myers, L., Mora, Z., Tagliaferro, P., Gardner, E., Brusco, A., Akinshola, B. E., Liu, Q. R., Hope, B., Iwasaki, S., Arinami, T., Teasenfitz, L., and Uhl, G. R. (2006). Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N. Y. Acad. Sci. 1074, 514–536.
Oropeza, V. C., Mackie, K., and Van Bockstaele, E. J. (2007). Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 1127, 36–44.
O’Shea, M., Singh, M. E., McGregor, I. S., and Mallet, P. E. (2004). Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J. Psychopharmacol. 18, 502–508.
Pamplona, F. A., Bitencourt, R. M., and Takahashi, R. N. (2008). Short-and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol. Learn. Mem. 90, 290–293.
Pamplona, F. A., Prediger, R. D. S., Pandolfo, P., and Takahashi, R. N. (2006). The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl.) 188, 641–649.
Patel, S., and Hillard, C. J. (2006). Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 318, 304–311.
Phelps, E. A., Delgado, M. R., Nearing, K. I., and LeDoux, J. E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905.
Pitman, R. K., Gilbertson, M. W., Gurvits, T. V., May, F. S., Lasko, N. B., Metzger, L. J., Shenton, M. E., Yehuda, R., Orr, S. P., and Harvard/VA PTSD Twin Study Investigators. (2006). Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann. N. Y. Acad. Sci. 1071, 242–254.
Quirk, G. J., Garcia, R., and González-Lima, F. (2006). Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry 60, 337–343.
Rauch, S. L., Shin, L. M., and Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research – past, present, and future. Biol. Psychiatry 60, 376–382.
Reich, C. G., Mohammadi, M. H., and Alger, B. E. (2008). Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. J. Psychopharmacol. 22, 769–777.
Resstel, L. B. M., Lisboa, S., Aguiar, D., Corrêa, F. M. A., and Guimaraes, F. (2008). Activation of CB1 cannabinoid receptors in the dorsolateral periaqueductal gray reduces the expression of contextual fear conditioning in rats. Psychopharmacology (Berl.) 198, 405–411.
Ribeiro, A., Ferraz-de-Paula, V., Pinheiro, M., and Palermo-Neto, J. (2009). Dose-response effects of systemic anandamide administration in mice sequentially submitted to the open field and elevated plus-maze tests. Braz. J. Med. Biol. Res. 42, 556–560.
Riedel, G., and Davies, S. N. (2005). Cannabinoid function in learning, memory and plasticity. Handb. Exp. Pharmacol. 168, 445–477.
Robinson, L., Hinder, L., Pertwee, R. G., and Riedel, G. (2003). Effects of delta-9-THC and WIN 55,212-2 on place preference in the water maze in rats. Psychopharmacology (Berl.) 166, 40–50.
Rodriguez de Fonseca, F., Rubio, P., Menzaghi, F., Merlo-Pich, E., Rivier, J., Koob, G. F., and Navarro, M. (1996). Corticotropin-releasing factor (CRF) antagonist [D-Phe12, Nle21, 38, C alpha MeLeu37] CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J. Pharmacol. Exp. Ther. 276, 56–64.
Roohbakhsh, A., Moghaddam, A. H., Massoudi, R., and Zarrindast, M. R. (2007). Role of dorsal hippocampal cannabinoid receptors and nitric oxide in anxiety like behaviours in rats using the elevated plus-maze test. Clin. Exp. Pharmacol. Physiol. 34, 223–229.
Rothbaum, B. O., and Davis, M. (2003). Applying learning principles to the treatment of post-trauma reactions. Ann. N. Y. Acad. Sci. 1008, 112–121.
Rubino, T., Guidali, C., Vigano, D., Realini, N., Valenti, M., Massi, P., and Parolaro, D. (2008a). CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology 54, 151–160.
Rubino, T., Realini, N., Castiglioni, C., Guidali, C., Viganó, D., Marras, E., Petrosino, S., Perletti, G., Maccarrone, M., Di Marzo, V., and Parolaro, D. (2008b). Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb. Cortex 18, 1292–1301.
Schat, A. R., Lee, M., Condie, R. B., Pulaski, J. T., and Kaminski, N. E. (1997). Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol. Appl. Pharmacol. 142, 278–287.
Scherma, M., Medalie, J., Fratta, W., Vadivel, S. K., Makriyannis, A., Piomelli, D., Mikics, E., Haller, J., Yasar, S., Tanda, G., and Goldberg, S. R. (2008). The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology 54, 129–140.
Segev, A., and Akirav, I. (2011). Differential effects of cannabinoid receptor agonist on social discrimination and contextual fear in amygdala and hippocampus. Learn. Mem. 18, 254–259.
Sheline, Y. I., Sanghavi, M., Mintun, M. A., and Gado, M. H. (1999). Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. 19, 5034–5043.
Shen, M., Piser, T. M., Seybold, V. S., and Thayer, S. A. (1996). Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J. Neurosci. 16, 4322–4334.
Shin, L. M., and Liberzon, I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191.
Slanina, K. A., Roberto, M., and Schweitzer, P. (2005). Endocannabinoids restrict hippocampal long term potentiation via CB1. Neuropharmacology 49, 660–668.
Sokal, D. M., Benetti, C., Girlanda, E., and Large, C. H. (2008). The CB1 receptor antagonist, SR141716A, prevents high-frequency stimulation-induced reduction of feedback inhibition in the rat dentate gyrus following perforant path stimulation in vivo. Brain Res. 1223, 50–58.
Steiner, M. A., Marsicano, G., Nestler, E. J., Holsboer, F., Lutz, B., and Wotjak, C. T. (2008). Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology 33, 54–67.
Suenaga, T., and Ichitani, Y. (2008). Effects of hippocampal administration of a cannabinoid receptor agonist WIN 55,212-2 on spontaneous object and place recognition in rats. Behav. Brain Res. 190, 248–252.
Suenaga, T., Kaku, M., and Ichitani, Y. (2008). Effects of intrahippocampal cannabinoid receptor agonist and antagonist on radial maze and T-maze delayed alternation performance in rats. Pharmacol. Biochem. Behav. 91, 91–96.
Sullivan, J. M. (2000). Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn. Mem. 7, 132–139.
Suzuki, A., Josselyn, S. A., Frankland, P., Masushige, S., Silva, A. J., and Kida, S. (2004). Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24, 4787–4795.
Taber, K. H., and Hurley, R. A. (2009). Endocannabinoids: stress, anxiety, and fear. J. Neuropsychiatry Clin. Neurosci. 21, 108–113.
Terranova, J. P., Michaud, J. C., Le Fur, G., and Soubrie, P. (1995). Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212–2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch. Pharmacol. 352, 576–579.
Tsou, K., Brown, S., Sanudo-Pena, M. C., Mackie, K., and Walker, J. M. (1998). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411.
Urigüen, L., Pérez-Rial, S., Ledent, C., Palomo, T., and Manzanares, J. (2004). Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology 46, 966–973.
Van den Berg, C. L., Hol, T., Van Ree, J. M., Spruijt, B. M., Everts, H., and Koolhaas, J. M. (1999). Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 34, 129–138.
Van Sickle, M. D., Duncan, M., Kingsley, P. J., Mouihate, A., Urbani, P., Mackie, K., Stella, N., Makriyannis, A., Piomelli, D., Davison, J. S., Marnett, L. J., Di Marzo, V., Pittman, Q. J., Patel, K. D., and Sharkey, K. A. (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332.
Van Wimersma Greidanus, T. B., and Maigret, C. (1996). The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 713, 153–159.
Varvel, S. A., Anum, E. A., and Lichtman, A. H. (2005). Disruption of CB1 receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl.) 179, 863–872.
Varvel, S. A., Hamm, R. J., Martin, B. R., and Lichtman, A. H. (2001). Differential effects of delta9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl.) 157, 142–150.
Varvel, S. A., Wise, L. E., Niyuhire, F., Cravatt, B. F., and Lichtman, A. H. (2007). Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology 32, 1032–1041.
Viveros, M., Marco, E., Llorente, R., and Lopez-Gallardo, M. (2007). Endocannabinoid system and synaptic plasticity: implications for emotional responses. Neural Plast. 2007, 52908.
Viveros, M., Marco, E. M., and File, S. E. (2005). Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 81, 331–342.
Wegener, N., Kuhnert, S., Thuns, A., Roese, R., and Koch, M. (2008). Effects of acute systemic and intra-cerebral stimulation of cannabinoid receptors on sensorimotor gating, locomotion and spatial memory in rats. Psychopharmacology (Berl.) 198, 375–385.
Wessa, M., and Flor, H. (2007). Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am. J. Psychiatry 164, 1684–1692.
Woon, F. L., and Hedges, D. W. (2008). Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus 18, 729–736.
Keywords: cannabinoids, CB1 receptors, hippocampus, LTP, stress, emotional memory, anxiety, extinction
Citation: Akirav I (2011) The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front. Behav. Neurosci. 5:34. doi: 10.3389/fnbeh.2011.00034
Received: 26 May 2011;
Paper pending published: 07 June 2011;
Accepted: 14 June 2011;
Published online: 23 June 2011.
Edited by:
Patrizia Campolongo, Università degli Studi di Roma La Sapienza, ItalyReviewed by:
Antonella Gasbarri, University of L’Aquila, ItalyCathy Fernandes, King’s College London, UK
Copyright: © 2011 Akirav. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Irit Akirav, Department of Psychology, University of Haifa, Haifa 31905, Israel. e-mail:aXJpdC5ha2lyYXZAZ21haWwuY29t