- 1 Departments of Psychiatry and Neuroscience, Mt. Sinai School of Medicine, New York NY, USA
- 2 Department of Psychology and Center for Neural science, New York University, New York, NY, USA
Evidence for reconsolidation in non-human animals has accumulated rapidly in the last decade, providing compelling` demonstration for this phenomenon across species and memory paradigms. In vast contrast, scant evidence exists for human reconsolidation to date. A major reason for this discrepancy is the invasive nature of current techniques used to investigate reconsolidation, which are difficult to apply in humans. Pharmacological blockade of reconsolidation, for example, has been typically used in animals as a proof of concept. However, most compounds used in these studies are toxic for humans, and those compounds that are safe target related, but not direct mechanisms of reconsolidation. Thus, although human reconsolidation has been hypothesized, there is limited evidence it actually exists. The best evidence for human reconsolidation emerges from non-invasive techniques that “update” memory during reconsolidation rather than block it, a technique only rarely used in animal research. Here we discuss the current state of human reconsolidation and the challenges ahead. We review findings on reconsolidation of emotional associative, episodic, and procedural memories, using invasive and non-invasive techniques. We discuss the possible interpretation of these results, attempt to reconcile some inconsistencies, and suggest a conceptual framework for future research.
A Brief History of Human Reconsolidation
Attempts to Alter Reconsolidation in Humans
The origin of today’s reconsolidation hypothesis is in the studies reported by Donald Lewis and colleagues during the late 1960s (Misanin et al., 1968; Lewis, 1969). These studies established the criteria to which an experimental protocol of reconsolidation should obey: (1) Reactivate a consolidated memory by means of a reminder cue; (2) Administer the treatment aimed at altering reconsolidation post reactivation and not prior to it; (3) Test for retention after the effects of the treatment have dissipated and the window of reconsolidation has closed. Because reconsolidation is a process affecting long-term memory storage (or re-storage), short-term memory immediately after treatment should be intact. If the memory is no longer expressed following this protocol (compared to control groups with no treatment after reactivation, or treatment without reactivation) it is possible to claim that there is a consolidation process occurring with retrieval, and the treatment was effective in blocking it. This phenomenon was initially termed “cue-dependent amnesia” (Misanin et al., 1968; Lewis, 1969) and only later was referred to as “reconsolidation” (Spear, 1973; Przybyslawski and Sara, 1997).
The initial studies supporting the reconsolidation hypothesis were exclusively based on motivationally driven learning, such as aversive Pavlovian conditioning (Misanin et al., 1968; Lewis, 1969; DeVietti and Holliday, 1972; DeVietti and Kirkpatrick, 1976), passive avoidance (Gordon and Spear, 1973), hypothermia (Mactutus et al., 1979), or complex maze tasks with food reward (Lewis et al., 1972; Lewis and Bregman, 1973). This triggered the criticism that cue-dependent amnesia could be attributed to the heightened state of arousal induced by the reminder cue rather than a general memory process (Schneider and Sherman, 1968; Squire et al., 1976). In subsequent years, Lewis and colleagues went on to address this criticism and developed a cognitive approach to cue-dependent amnesia (Lewis, 1976). On the basis of this work, Lewis (1979) proposed a novel theory of memory. Accordingly, initially new memories are in an unstable active state and stabilize over time into an inactive state. Active and inactive states are akin to the short- and long-term memory stages (respectively) of the consolidation hypothesis of memory (McGaugh, 1966). The novelty of Lewis’s model was his proposal that the act of remembering returns inactive memories into an active state, whereas the original consolidation theory suggested that the instability period happens only once when the memory is formed.
During the time the reconsolidation hypothesis evolved, there were two attempts to examine these ideas in humans albeit in ways fundamentally different from the animal research. The first was a translational study trying to make clinical use of the phenomenon of cue-dependent amnesia. Rubin (1976) and Rubin et al. (1969) adapted Lewis’s animal protocol to human patients suffering from obsessive-compulsive disorder (OCD) and hallucinations. The equivalent of the animal retrieval cue was to prompt the patients to focus on the subject matter of their psychopathology (e.g., fear of contamination). Rubin assumed that this would return their maladaptive memory into an active state vulnerable to disruption. He then administered his patients with electroconvulsive shock (ECS), just as in the animal protocol. In contrast to the animal protocol, however, where a simple memory was created in the laboratory, here the patients brought their own real life memories. The results were consistent with the reconsolidation hypothesis in that the patients’ OCD symptoms were altered. The patients reached levels of improvement that were not observed when ECS was previously given under anesthesia.
The second attempt created new memories in a laboratory setting using more traditional tasks examining non-emotional, episodic memory. In this study, Squire et al. (1976) used ECS on depressed psychiatrist patients. Their goal was to examine performance on a battery of memory tests including object recognition, paired-associate learning, as well as remote memories. The results showed that reactivating memories just before ECS did not produce amnesia. In other words, they failed to find evidence for reconsolidation in humans, as did a few other studies in animals around that time (Banker et al., 1969; Dawson and McGaugh, 1969; Weaver and Magnus, 1969; Jamieson and Albert, 1970; Gold and King, 1972). Squire and colleagues speculated that their results had to do with the fact that their human subjects were not under a state of arousal or heightened motivation, as were the animals in previous studies. As mentioned above, the initial animal studies of this phenomenon typically used footshocks or hunger to motivate learning. Squire and colleagues claimed that cues associated with such experimental settings are bound to highly arouse the animal when presented. What these studies measured, they claimed, was the interactive effects of ECS and arousal on performance and not a direct effect of ECS on memory. Because Squire and colleagues examined neutral episodic memories instead of memories linked to emotional consequences they could avoid such interaction. They therefore concluded that “previously learned material is not easily disrupted by simply calling it to mind before treatment” (p. 342).
Why Squire and colleagues failed to find evidence for the disruption of reconsolidation is unclear. The arousal explanation was refuted by other studies in non-human animals (Lewis, 1976). It could be that the ECS treatment was insufficient as its effects on initial consolidation were anyway mild. Or perhaps an important factor was that Squire and colleagues examined neutral episodic memories, which have a different neural representation than emotional associative memories. As we describe below, inconsistent findings are the hallmark of extant human reconsolidation research. Indeed, one of the major challenges to human reconsolidation research is to reconcile findings from studies examining different memory systems and identifying the potential interactions between them.
The Malleability of Human Episodic Memory
Around the same time these first few human reconsolidation studies appeared, a similar idea emerged within the cognitive psychology literature, suggesting memory is not a snapshot of the original event, but rather an ongoing process that incorporates new information available at the time of retrieval. The roots of this idea began with James (1892) who argued that memory is constantly changing due to being retrieved in different cognitive environments. Bartlett (1932) provided experimental support for this hypothesis by showing that the more subjects were to retrieve information, the more it was biased toward their cultural expectations. But perhaps the most compelling evidence for the dynamic nature of memory comes from a large body of work in cognitive psychology on the malleability of human memory. Research over the past 30 years has shown that information, and even more so, misinformation, could profoundly influence old memories if provided at the time of retrieval (Tulving and Thomson, 1973; Loftus, 1979, 1981, 2005a,b; Loftus and Yuille, 1984; Lindsay and Johnson, 1989; Johnson et al., 1993; Schacter, 1999; Roediger et al., 2007).
In a classic study that helped re-energize the debate of the nature of memory malleability, Loftus et al., (1978) presented subjects with a series of slides depicting an automobile accident involving either a “stop” sign or a “yield” sign. At a later time, subjects were given a questionnaire that presented misinformation about the nature of the sign (e.g., a “stop” sign was referred to as a “yield” sign). For a large proportion of the subjects, this misinformation was incorporated into subsequent memory for the accident. The mechanisms underlying the nature of this misinformation effect were hotly debated. In their initial research, Loftus and colleagues suggested that new information is integrated into the old memory, resulting in the reconstruction of a memory that was never experienced. Others argued against this integration or re-writing hypothesis suggesting that the original memory is not altered; rather the misinformation effect occurs because subjects forget the original event and are lured into responding with the incorrect information (McCloskey and Zaragoza, 1985). It was also suggested the misinformation effect could be due to misattributing the source of the inconsistent information, with or without an intact original memory trace (Lindsay and Johnson, 1989).
As this classic research in cognitive psychology suggests, viewing memory as fundamentally dynamic is in fact an old tradition in cognitive psychology without using the term “reconsolidation.” However, the debate about the nature of the storage (or re-storage) mechanism underlying the misinformation effect and other findings demonstrating the malleability of human memory has not been conclusively resolved based on the psychological literature alone. As we suggest below, the reconsolidation hypothesis, and our increasing understanding of its neurobiological underpinnings, may provide an alternative framework for interpreting this literature and provide novel insights into this old debate (see Hardt et al., 2009 for an interesting review bridging animal reconsolidation with cognitive psychology).
The Current State of Human Reconsolidation
What Happened in the Last Decade?
Research of human reconsolidation has evolved only in recent years (Table 1). This is perhaps not surprising given that the reconsolidation field as a whole was silent for the most part during the last five decades. The early studies of the 60s and 70s, and those of the last decade, represent the birth and the rebirth of the field, respectively. Human reconsolidation research shares the same dynamics albeit in drastically smaller numbers. While research with non-human animals has produced over 300 papers just in the last 10 years, human research provided about 13. Below we discuss the reasons for these disproportionate numbers, the current state of research on reconsolidation in humans, and the substantial challenges ahead.
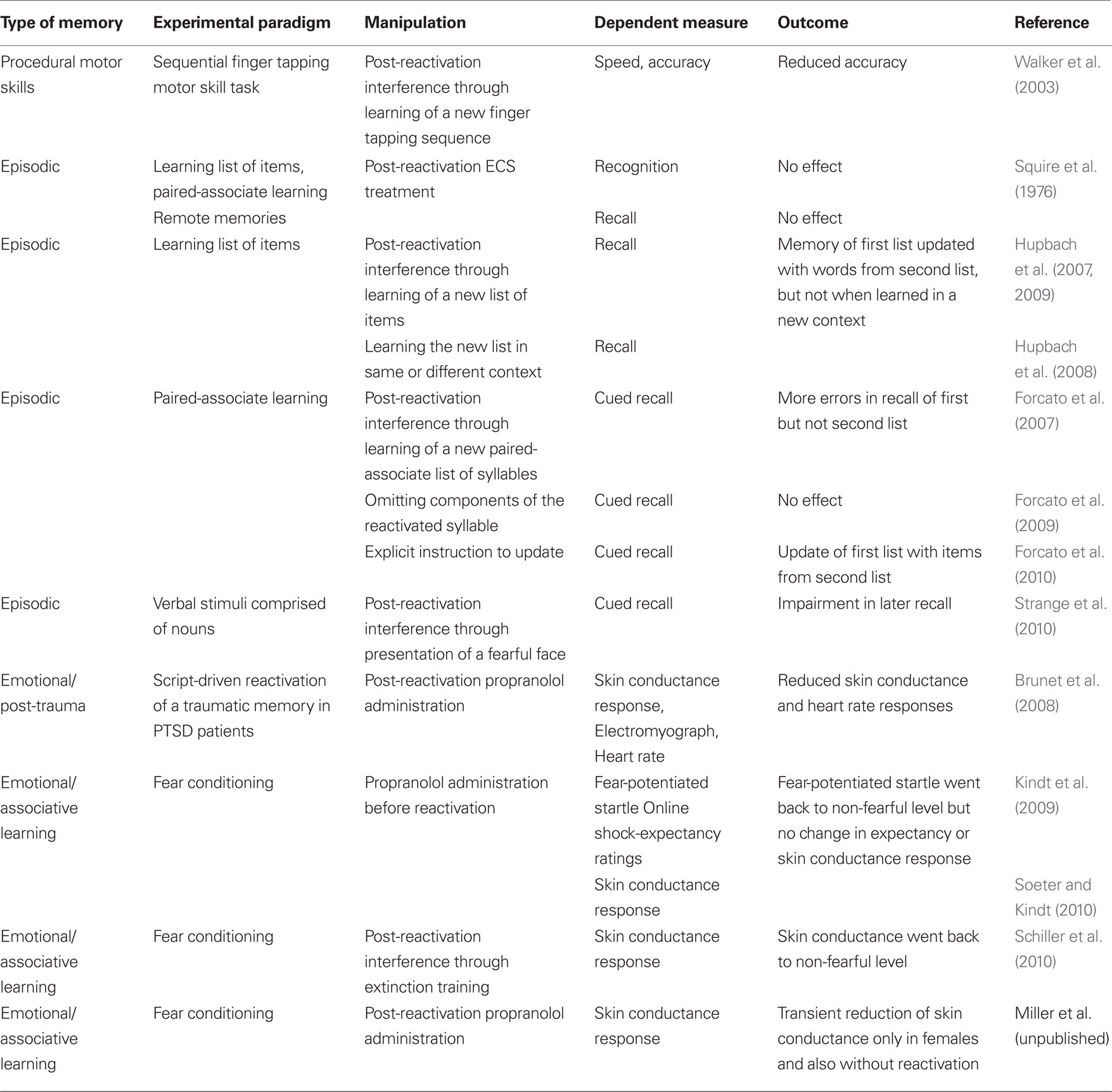
Table 1. Memory systems and experimental paradigms in which reconsolidation has been examined in humans.
Amygdala-dependent memories
The vast majority of animal studies on reconsolidation have used aversive Pavlovian conditioning as the paradigm of choice (Sara, 2008; see for example Nader et al., 2000). In this procedure, a neutral stimulus, such as a tone, is paired with an aversive outcome, such as a shock. After a few pairings, a fear response is triggered by the tone itself because of its association with the shock. A day later, after allowing the memory to be fully consolidated into long-term storage, the tone alone is presented only once, serving as a reminder cue. This reactivation cue is what presumably triggers the reconoslidation process. Evidence for the timing of reconsolidation, or the reconsolidation “window,” is still emerging, but it is thought to require somewhere between 3 and 10 min after reactivation for the reconsolidation process to begin and it lasts at least an hour or more (Monfils et al., 2009). By 6 h the reconsolidation process is complete (Nader et al., 2000; Duvarci and Nader, 2004). Interfering with reconsolidation by pharmacological or behavioral means during this window blocks or alters the re-storage of the memory. This is apparent when examining the animal’s response to the tone when it is presented again 24 h later. Importantly, there is no interference when examining short-term memory before the reconsolidation process is complete.
A long line of research in non-human animals suggests that the acquisition, storage, and expression of conditioned fear, require an intact amygdala. The lateral nucleus of the amygdala is thought to be the site of storage for conditioned fear memories (see LeDoux, 2000 for a review). Because of this, studies examining consolidation (LaBar et al., 1998; Fanselow and LeDoux, 1999; Davis, 2000; LeDoux, 2000; Maren, 2001; Phelps and LeDoux, 2005; Schafe et al., 2005) and reconsolidation (Nader et al., 2000; Duvarci et al., 2005; Jin et al., 2007) have focused on this brain region. For example, Nader et al. (2000) injected a protein synthesis inhibitor directly into the lateral amygdala during reconsolidation of conditioned fear. It was already known that inhibition of protein synthesis blocks reconsolidation (Judge and Quartermain, 1982) but the particular locus within the neural circuitry of fear conditioning was unknown. Nader and colleagues then tested the rats a day later, in the absence of the drug. These rats no longer showed fear of the tone, compared to rats that received placebo injection following reactivation, or drug without reactivation. The protein synthesis inhibitor had no effect when testing the rats 4 h after reactivation. This suggests that the short-term memory was intact, and only the long-term memory was impaired through effects of the drug on reconsolidation.
In humans, only four published studies have examined reconsolidation of amygdala-dependent memories. It is impossible to use protein synthesis inhibitors in humans because they are highly toxic. Alternatively, it is safe to use beta-adrenergic receptor blockers, such as the drug propranolol, which may modulate protein synthesis (Gelinas and Nguyen, 2005) and thereby may regulate long-term memory storage. Indeed, propranolol has similar effects as protein synthesis inhibition when injected systematically or directly into the rat amygdala (Debiec and LeDoux, 2004). Below we describe the details of three published studies using this drug, one unpublished study, and one proposing a non-invasive technique.
The first study directly implemented the reconsolidation hypothesis in a clinical population. Brunet et al. (2008) examined 19 individuals with post-traumatic stress disorder (PTSD). PTSD is a chronic syndrome marked by intrusive and distressing memories of intensely emotional events. The symptoms and susceptibility to PTSD have been linked with an over-reactive amygdala (Rauch et al., 2006; Admon et al., 2009; Brohawn et al., 2010; Brunetti et al., 2010). To reactivate the traumatic memory in this study, the patients were prompted to describe in writing the event that caused their PTSD using a standard script preparation form that takes about 20 min. Immediately after this script-driven retrieval, half the patients received propranolol (a short acting 40 mg pill and a long acting 60 mg pill 2 h later). The other half received matching placebos, and the administration was randomized and double-blind. One week later, the patients were reminded again of their traumatic memory by listening to a recording describing the traumatic event. The recording was prepared in advance by one of the investigators based on the scripts, and they were asked to imagine the events while listening. At that time, their physiological responses were recorded using measures of autonomic nervous system arousal (heart rate and skin conductance response, or SCR) and electromyogram (EMG) of the facial frowning muscle (the left corrugator). The investigators compared the level of these physiological measures to normative cut-offs for PTSD based on prior research. They found that heart rate and SCR levels were above normative PTSD cut-offs in the placebo group, but below in the propranolol group, although not significantly below the PTSD cut-off for SCR. The EMG responses were below the normative cut-offs in both groups and therefore were not indicative of drug-induced reduction of the expression of negative affect.
These results suggest that propranolol given after reactivation of a traumatic memory might be effective in reducing some of the maladaptive physiological responses triggered by the memory. As of yet, it is unclear if this is a long-lasting effect, and if the administration of propranolol during the reconsolidation period is effective in reducing PTSD symptomatology as well, which is the ultimate goal of the treatment. Directly linking the effects of propranolol to reconsolidation from these results, however, should be done with caution. In the absence of a control group that receives propranolol without memory reactivation it is impossible to rule out the possibility that propranolol had more general effects that are not necessarily related to reconsolidation (Nader, 2003).
Shortly after the publication of these results, another study reported using propranolol but this time in healthy volunteers that underwent fear conditioning (Kindt et al., 2009). The use of a normal population and experimental procedures that closely mimic animal research allows for appropriate controls and better interpretation of the results (Rasmusson and Charney, 1997; Myslobodsky and Weiner, 2000). This is naturally harder to achieve in clinical populations, which are less available and introduce significant variability across participants. Kindt et al. (2009) fear-conditioned their subjects by presenting fear-relevant images (spiders) on a computer screen and pairing them with a mild electric shock to the wrist. There were two spider images – one was designated as the conditioned stimulus (CS) and was paired with the shock (the unconditioned stimulus, or US) and the other was never paired. A day later they reactivated the memory using a single presentation of the CS. Propranolol was administered 1.5 h before memory reactivation. On the following day, the stimuli were presented again 10 times each without the US (extinction session). After extinction, a few non-signaled shocks were given in order to reinstate the memory (Bouton, 2002), and this was followed by another extinction session. The investigators had two measures of fear: (1) fear-potentiated startle, where the indication of conditioned fear is the potentiation of the eyeblink startle reflex to a loud noise (the startle reflex is initially habituated) during the presentation of the CS; (2) explicit knowledge of the contingency between the CS and the US. This was measured through online shock-expectancy ratings that the subjects indicated at the beginning of each trial (the shock itself co-terminated with stimulus presentation). The experimental groups were: propranolol with reactivation, placebo with reactivation, and importantly, propranolol without reactivation. The investigators found that only the group that received memory reactivation in conjunction with propranolol failed to show conditioned fear at extinction and also after reinstatement. In contrast, subjects who were reminded of the conditioned fear but got placebo, or got only propranolol without reactivation, continued to show conditioned fear. These results, however, were obtained only with the fear-potentiated startle measure. According to the explicit knowledge ratings, all subjects remembered the image-shock contingency, and appropriately expected the shocks throughout.
Although this initial study was encouraging, there are several issues with the Kindt et al. (2009) study that suggest a mechanism other than the blocking of the reconsolidation of conditioned fear. The primary issue is that propranolol was given 1.5 h prior to reactivation and reconsolidation. This detail creates a major caveat in linking the effects of the drug with reconsolidation. The reason the authors chose to do so is that it takes about 90 min for propranolol to reach peak plasma concentration in the blood (Gilman and Goodman, 1996). The authors coordinated the peak level with memory reactivation, not memory reconsolidation. Because of this, they cannot rule out effects of the drug on retrieval itself. It could be that retrieval of the fear memory in the presence of the drug had a lasting effect on the expression of the fear potentiated startle measure, rather than blocking the reconsolidation of the fear memory itself.
Consistent with this hypothesis, in a follow up study using a similar procedure with another measure of conditioned fear this same group failed to find evidence that reactivation of the fear memory after the administration of propranolol disrupts the later expression of fear conditioning (Soeter and Kindt, 2010). These inconsistent results suggest that their procedure is only partially effective at altering the expression of conditioned fear. Importantly, their second measure was autonomic nervous system arousal as assessed with SCR. Clinically, autonomic nervous system arousal is a primary symptom of fear related disorders, such as PTSD. SCR is also the most frequently assessed measure of amygdala-dependent conditioned fear in humans (see Phelps and LeDoux, 2005 for a review), and the only one that has been linked to focal amygdala damage (Bechara et al., 1995). This lack of replication provides further support for the suggestion that the administration of propranolol prior to reactivation may have altered the later expression of potentiated startle, rather than disrupting the reconsolidation of the conditioned fear memory.
Interestingly, the latter finding is consistent with results obtained in our laboratory (Miller Altemus, Debiec, LeDoux, and Phelps, unpublished). Our study had a similar design as Kindt et al. (2009) with the three experimental groups undergoing fear conditioning, with Day 1: acquisition, Day 2: reactivation followed by propranolol or placebo (or no reactivation followed by drug), and Day 3: test of conditioned fear. The primary difference was that we administered propranolol immediately after reactivation to assure that we were testing the effect of propranolol on reconsolidation, rather than reactivation. Our measure of conditioned fear was SCR. Similar to Soeter and Kindt (2010), we found evidence of conditioned fear on the Day 3 test in all three groups. However, a detailed analysis of our data suggested a transient effect of propranolol. That is, subjects who received propranolol on Day 2 showed no evidence of conditioned fear on the first trial of the Day 3 test. By the second trial (12 s later) conditioned fear returned. Although our temporary disruption of fear memory with administration of propranolol was suggestive of a partial disruption of conditioned fear, this paradigm was ultimately unsuccessful. However, the pattern of results we obtained provides some hints as to factors that may be important to consider in future efforts to disrupt the reconsolidation of conditioned fear in humans using propranolol.
Specifically, the temporary disruption of fear suggests that something must be driving the return of the fear response. We hypothesize that this return of fear may be the result of subjects having intact explicit knowledge or episodic memory of the relationship between with the CS and the US. There is abundant evidence that knowledge of the CS–US contingency alone, in the absence of pairing of the CS and US and fear conditioning, can result in a physiological fear response that is almost identical to conditioned fear (see Olsson and Phelps, 2007, for a review). This episodic memory top-down driven fear response has been most frequently observed as measured with potentiated startle (Grillon et al., 1991, 1994; Funayama et al., 2001), but has also been observed with SCR (Phelps et al., 2001). Interestingly, although the acquisition and storage of explicit knowledge of the CS–US contingency does not depend on the amygdala (Bechara et al., 1995; LaBar et al., 1995), the physiological expression of this fear representation is amygdala-dependent (Funayama et al., 2001). In our unsuccessful study described above (Miller et al., unpublished), it is possible that the first trial of the Day 3 test served to remind the subjects of their episodic memory of the CS–US contingency, which led to a return of the fear response. Critically, these findings highlight the importance of understanding the relationship between different memory systems and fear representations when developing protocols to disrupt fears by influencing reconsolidation mechanisms.
In addition to the temporary fear disruption in this study, there were three other unexpected results. First, this disruption of fear was only observed in female participants. There is evidence that females metabolize propranolol differently than males (Walle et al., 1994a,b), which could impact the success of this treatment in influencing reconsolidation. Second we observed both a temporary decrease in SCR to the CS, and an increase in SCR to a second stimulus that was explicitly not paired with shock (i.e., a CS- or safety stimulus). The amygdala may code both fear and safety memories (Phelps et al., 2001; Rogan et al., 2005; Schiller et al., 2008; Ostroff et al., 2010) and it is possible that our temporary memory disruption may have inadvertently influenced both. Finally, we found an effect for propranolol on the expression of fear, regardless of whether the fear memory was reactivated. Our subjects who received propranolol with no reactivation cue may have generated the cue themselves by simply being placed back in the hospital context. This finding suggests that either propranolol had a more general fear dampening effect, or alternatively, it may be difficult to precisely control memory reactivation in human subjects.
In spite of the initial encouraging but incomplete results using propranolol to disrupt fear memory reconsolidation in a clinical population (Brunet et al., 2008), the findings from controlled laboratory studies using a pharmacological manipulation to alter reconsolidation in humans have been problematic. The paradigms used have not targeted the reconsolidation mechanism (Kindt et al., 2009), have been inconsistent across measures of fear (Soeter and Kindt, 2010), and when a post-reactivation manipulation was used, a long-lasting effect was not observed (Miller et al., unpublished). Nevertheless, these largely unsuccessful attempts represent an important step in this research agenda. Each study provides insights into how future paradigms might be more successful. By understanding the problems and complexity of the pharmacological manipulation of reactivation and reconsolidation in humans, we may eventually be able to develop successful techniques that can be more precisely and effectively translated to the treatment of clinical disorders.
The last and most recent study to examine the reconsolidation of amygdala-dependent memories took a different approach. We (Schiller et al., 2010) again used a fear conditioning paradigm in healthy volunteers, but did not use propranolol to interfere with reconsolidation. Instead we used behaviorally induced interference by introducing new information about the value of the CS during the reconsolidation window. This approach capitalizes on reconsolidation as an update mechanism. Instead of blocking reconsolidation and the memory, this is an attempt to update the memory by allowing the incorporation of new information through the reconsolidation process. The advantage of this protocol is that it is non-invasive and thus relatively safe and easy to use in humans.
The experimental design included three groups and was conducted over 3 days. The measure of fear was SCR. On Day 1, all subjects acquired conditioned fear to a colored square (the CS) paired with an electric shock (the US). Another colored square was also presented but never paired with the shock. On Day 2, two-thirds of the subjects reactivated the fear memory by being exposed to a single presentation of the CS without the US. One-third did not receive the reactivation cue. Next, all the subjects underwent extinction training in which they were repeatedly exposed to the two colored squares without the shocks. For half of the subjects who received the reactivation cue, extinction training occurred after a delay of 10 min (allowing time for the reconsolidation process to start), and for the other half of the reactivated subjects, extinction occurred after 6 h (after the reconsolidation window was closed). We used extinction training in this paradigm to teach subjects that the previously aversive CS is now safe. During fear acquisition, the subjects form a CS–US memory trace. Standard extinction training (without prior reactivation) is thought to result in a second CS–noUS memory trace. After standard extinction training, these two memory representations about the value of the CS compete for expression. Because the initial CS–US trace is still available, fear can return with the passage of time (spontaneous recovery), stress (reinstatement) or in different contexts (renewal; see Bouton, 2002 for a review). In our study, we hypothesized that if extinction training occurs during the reconsolidation process, while the original memory is being re-stored, this safety information may be incorporated into the original memory trace, rather than resulting in a second, alternative memory about the value of the CS, thus preventing the return of fear.
The results showed spontaneous recovery in the group that had regular extinction without reactivation, which was expected (Bouton, 2002). As for the two groups exposed to the reactivation cue, only the one that underwent extinction 6 h later, outside the reconsolidation window, showed fear recovery. The group that underwent extinction training 10 min after reactivation, within the reconsolidation window, showed no recovery of fear. Interestingly, this effect persisted at a follow up test a year later. In a second study, we also showed that this manipulation was effective in preventing the reinstatement of one CS but not another within the same individual. To this aim we presented subjects with three colored squares. Two of them were paired with a shock and the third was not. We subsequently reactivated only one of the CSs. Extinction training with all three stimuli followed this. When tested a day later after reinstatement, only the non-reactivated CS elicited a conditioned fear response. There was no evidence of a return of fear to the CS that was reactivated 10 min prior to extinction training.
These results are consistent with a study in rats that used a similar protocol whereby extinction interfered with reconsolidation of conditioned fear and demonstrated comparable effects on rats’ freezing behavior (Monfils et al., 2009). Together, these studies show that introducing new safety information during reconsolidation of a conditioned fear memory might lead to the re-storage of this memory as safe, and permanently change its fearful properties. An animal study of hippocampal-dependent memories reported in this issue (Lee, 2010) supports the same idea, that the “purpose” of reconsolidation is updating, but demonstrates it in the opposite direction – a neutral contextual representation changed into a contextual fear memory. As we mentioned above, the idea that memories could be modified by the incorporation of new information available at the time of retrieval is an old tradition in cognitive psychology (Loftus, 1979). These ideas have now infiltrated the study of human reconsolidation of amygdala-dependent memories. In the next section we discuss how these ideas are being systematically examined in the reconsolidation of declarative or episodic memories in humans as well.
Revisiting episodic memory
As mentioned above, Squire et al. (1976) observed a discrepancy between their findings in humans and previous results in animals when they failed to find evidence for memory disruption after reactivating declarative or episodic memories prior to ECS. They attributed this inconsistency to the different memory systems investigated, namely episodic versus emotional associative memories, respectively. Squire and colleagues argued that episodic memory simply provides a better model that is not confounded by arousal and motivational state. The use of this model system, they claimed, revealed that the cue-dependent amnesia (i.e., reconsolidation blockade) is not a general characteristic of memory, but rather limited to very specific experimental conditions.
More recently, however, research with non-human animals have provided evidence that reconsolidation blockade is not unique to motivationally driven learning (see Nader and Hardt, 2009 for review). Animal studies successfully demonstrate this in various non-emotional tasks, such as spatial learning (Przybyslawski et al., 1999; Suzuki et al., 2004; Morris et al., 2006) and object recognition (Bozon et al., 2003; Kelly et al., 2003). Moreover, there is clear evidence for reconsolidation blockade when specifically targeting the hippocampus. For example, Debiec et al. (2002) showed that intra-hippocampal injection of a protein synthesis inhibitor blocks contextual fear conditioning, which is known to be hippocampal-dependent (Fanselow, 2000). Other studies showed effects of various pharmacological agents injected into the hippocampus on reconsolidation of inhibitory avoidance (Milekic and Alberini, 2002; Boccia et al., 2004, 2007, 2010; Inda et al., 2011).
These findings suggest that reconsolidation is a general property of memory and is common to different memory systems. As in the emotional memory studies, Squire et al. (1976) were searching for “amnesia,” that is, impaired or a complete lack of memory, just as one would observe when interfering with consolidation of the initial learning. Indeed, they observed impaired recognition of a 32-item list and lower retention of paired-associate learning when subjects learned less than 10 min before ECS. In contrast, ECS 10 min after a reminder of this learning had no effect. It should be noted, however, that the effects of ECS on initial memory consolidation were rather mild and in some cases marginally significant. Given this, it is questionable whether their ESC treatment could cause amnesia at all.
The studies conducted in the last decade on human reconsolidation of declarative or episodic memories searched for something other than amnesia. Much like the Schiller et al. (2010) study described above, they examined if the original memory was “updated” with the introduction of new information. For example, in a recent series of studies aimed at investigating episodic memory reconsolidation, Hupbach et al. (2007, 2009) examined how the reconsolidation of a list of random objects was affected by learning a second list. In their paradigm, the experimenter pulled out the items (e.g., balloon, envelope, tennis ball etc.) one at a time from a bag and put them in a distinct blue basket. Subjects were instructed to name the objects and to memorize them. One day later, the experimenter reminded half the subjects of the list by showing them the blue basket and prompted them to remember what happened with it (but not to recall the items). The other half went into another room with a different experimenter. All subjects at this point learned a new list. However they learned this list using a different procedure to avoid being reminded of the previous list. For the second list, the items were spread on a table and the subjects had to name and memorize them. On Day 3, the experimenter asked the subjects to remember as many items as possible from the Day 1 list.
Hupbach and colleagues found that the reminder in fact did not reduce the number of items recalled from the Day 1. Rather it resulted in subjects incorporating items from the Day 2 list into the Day 1 list. Complying with the reconsolidation protocol criteria outlined earlier, the authors also confirmed that (1) the intermixing of the items was unidirectional – no items from the Day 1 infiltrated the Day 2 list, and (2) the effect did not occur immediately after learning the Day 2 list, but only 24 h later. In a follow up study these same investigators demonstrated that exposure to the context of the first list was a necessary and sufficient reminder in triggering reconsolidation of episodic memory, and a reminder outside of the spatial context was ineffective (Hupbach et al., 2008). The authors suggested that space plays a superior role in triggering memory reconsolidation by providing a “scaffold” to which concomitant events are bound. Context reactivation renders these events labile such that they could be modified and updated. Lee (2010) provided empirical evidence that hippocampal memory modification indeed selectively recruits reconsolidation mechanisms. He demonstrated in animals, with a cellular approach, that reactivation of a neutral contextual representation destabilized it, allowing to incorporate emotionally salient information introduced within the same context.
Similar to the studies by Hupbach and colleagues, Forcato et al. (2007) also examined the reconsolidation of episodic memories using a paired-associate learning task. The subjects in this study learned to associate a list of cue syllables with their respective response syllables (a cue syllable, for example, would be “FLI” and the response syllable “AIO”). The reminder was a cue from this list, after which subjects learned a second list of paired associates. They found that introducing the reminder 5 min before training on the second list induced errors in the retention of the first list when tested 1 day later. The same group later demonstrated that the cue reminder ceased to be efficient when removing one of its components (Forcato et al., 2009). Lastly, they showed that the interference effect could turn into an update when subjects were explicitly instructed to do so (Forcato et al., 2010). Without direct instruction to update the first list, they simply made more errors while retrieving it after learning the second list post-reactivation. But when explicitly instructed to incorporate the new information, they retrieved the first list correctly in addition to items from the new paired-associate list.
In an effort to show selective impairment of a specific target episodic memory, Strange et al. (2010) examined reconsolidation of verbal memory interfered with an aversive facial expression. Their participants encoded various nouns on Day 1. The experimenter reactivated the memory for these nouns by presenting the word stems. The participants were asked to complete the stems out loud to make a word from Day 1. In this way, the authors made sure that reactivation of specific words from Day 1 was successful. They then examined the proportion of remembered words a Day later and a week later out of the words successfully reactivated on Day 2. They found impaired recall for those words preceding the presentation of a face with a fearful expression. The authors took these findings to suggest that reconsolidation of specific verbal memories in humans could be impaired using emotionally aversive stimuli.
Unlike the human reconsolidation studies on amygdala-dependent memories described above, the recent research on reconsolidation of hippocampal-dependent memories has relied on behavioral interference techniques. This may be due in part to the lack of an identified pharmacological agent that is safe for human use and has been shown to disrupt hippocampal reconsolidation in non-human animals. Without such a drug, it is not possible to pharmacologically disrupt hippocampal reconsolidation in humans. Nevertheless, the pharmacological research in non-human animals has inspired a revival in behavioral studies on this topic in humans. Interestingly, even though the behavioral interference paradigms used in recent human reconsolidation studies of episodic memory are similar to the one we used (Schiller et al., 2010) examining fear conditioning, the outcome is not. Our findings on amygdala-dependent memory suggest the fear memory was updated and was no longer expressed. In contrast, studies of behavioral interference during reconsolidation in episodic memory tasks find the original memory is still expressed, but it is confused or merged with new information presented during the reconsolidation window. As described in the next section, using a similar behavioral interference paradigm while examining a third type of memory (procedural memory), also yields a slightly different outcome.
Procedural or skill memory
By definition, procedural memories are non-declarative and generally do not require conscious awareness (White and McDonald, 2002; Squire, 2004). The learning is incremental and requires the step-by-step execution of sensory or motor procedures (e.g., learning to ride bicycles or playing an instrument). Although less is known about the neural basis of procedural memories than either fear memories or episodic memories, there is evidence that some types of procedural memories depend on the striatum (Knowlton et al., 1996; Poldrack et al., 2005), and motor skill learning in particular is thought to involve changes in the motor cortex and cerebellum (Molinari et al., 1997; Middleton and Strick, 2000; Poldrack et al., 2005; Kantak et al., 2010).
In what is believed to be the first study to conclusively demonstrate evidence for reconsolidation in humans, Walker et al. (2003) examined the reconsolidation of a motor skill memory using a finger-tapping task. On Day 1, participants learned a five-element sequence comprised of four numeric keys (for example, “4-1-3-2-4”) in a 12-trial training session. On Day 2, they had a brief reactivation of that memory using a three-trial retention session, after which they learned a novel five-element sequence. On the next day they were tested on both sequences. The measures of learning were speed and accuracy when performing the practiced sequences, in contrast to a random sequence.
Walker et al. (2003) found that the initial improvement achieved when learning the first sequence on Day 1 was diminished when tested on Day 3 (accuracy decreased by 50% and speed was non-significantly worse). This was due to reminding subjects of the first sequence on Day 2 before training on the second sequence. The investigators did not observe a decrease in performance on a short-term memory test of performance on the first sequence immediately after learning the second sequence on Day 2. They also did not observe a change in performance of the second sequence, which in fact got better from Day 2 (training) to Day 3 (test). A similar improvement was observed for the first sequence when tested on Day 2 (initial retention/reactivation).
The Walker et al. (2003) paradigm satisfies the three criteria of tests of reconsolidation outlined by Lewis and colleagues in the 1960s (Misanin et al., 1968, Lewis, 1969): (1) the memory is reactivated; (2) the intervention occurs during reconsolidation and not before; (3) the test for retention occurs after the reconsolidation window has closed. In addition, a test of performance on the first sequence immediately after interference by the second sequence shows intact short-term memory. For this motor skill task, the behavioral interference paradigm resulted in impaired performance, although there was still some evidence of expression of the original skill memory. Below, we speculate as to why behavioral interference paradigms examining human reconsolidation may result in different patterns of performance when investigating different memory systems.
Behavioral Interference of Reconsolidation Across Different Memory Systems
One of the most important findings to emerge from memory research in the last century is that there are multiple forms of memory that are independent and have distinct neural representations (see White and McDonald, 2002; Squire, 2004 for reviews). Although these memory systems may have unique neural signatures, they also interact. For example, as mentioned above, experiencing a fear conditioning paradigm engages at least two of these memory systems. The amygdala is critical for the simple CS–US association and the physiological expression of this learning, whereas the hippocampus is necessary for episodic, explicit knowledge of the CS–US relationship (Bechara et al., 1995; LaBar et al., 1995), as well as the contextual modulation of physiological fear expression (Fanselow, 2000, LaBar and Phelps, 2005). We may be able differentiate the engagement of different memory systems by their means of expression, the task, or the qualities of learning (e.g., incremental or immediate), but at times their interaction may make this differentiation difficult (Cohen et al., 1997; Willingham, 1998; Foerde et al., 2006; Olsson and Phelps, 2007).
In our review of the current literature on reconsolidation in humans, it is clear that the behavioral interference paradigms thus far have been the most successful at demonstrating evidence for reconsolidation in humans. However, across different memory systems, the consequence of presenting interfering information during the reconsolidation window seems to differ. For amygdala-dependent expressions of fear learning, presenting safety information during reconsolidation appears to re-write or over-write the original fear memory (Schiller et al., 2010)because there is no evidence for the expression of the original memory as assessed by SCR. When examining hippocampal-dependent episodic memory, the primary content of this original episodic memory appears to be relatively intact following interference during reconsolidation, but the memory is now confused or merged with the interfering information. Finally, presenting an interfering motor skill during reconsolidation results in impaired expression of the original skill memory, but there still evidence that it exists, albeit in a degraded form.
There are at least two possible reasons why these different reconsolidation/interference paradigms yield different patterns of results. The first obvious reason is the method of memory assessment. For example, if the episodic memory test used in the Hupbach et al. (2007, 2009) studies described earlier was an assessment of source memory, rather than memory for list items, their subjects would have tested as impaired in recounting the original memory. Similarly, in our study of fear conditioning (Schiller et al., 2010), explicit knowledge of the CS–US contingency throughout the study might have been unaffected. However, perhaps more important is the fact that the different memory systems examined in these human reconsolidation/interference studies have quite distinct patterns of neural representation. It may primarily be these differences in the organization of the neural systems mediating these different types of memories that yield the diverse effects of interference during reconsolidation on the behavioral outcome.
For example, as outlined earlier, fear conditioning results in a CS–US association whose neural representation is localized in a relatively discrete manner in a small region of the amygdala (i.e., the lateral amygdala – see LeDoux, 2000 for a review). In contrast, hippocampal-dependent episodic memory is not believed to be stored solely in the hippocampus. Instead, it is suggested that the hippocampus acts to pull together or associate a cortical network of discrete representations that make up the components of the episode or event. The episodic memory representation itself is the cortical network that is tied together by the hippocampus. In contrast to conditioned fear memories, the episodic memory trace is the opposite of localized. It is widespread and distributed throughout the brain, although the binding of this network relies on the hippocampus (see Davachi, 2006; Dickerson and Eichenbaum, 2010 for reviews). Finally, although less is known about the neural representation of procedural or skill memories, the existing evidence suggests that it is neither as localized as amygdala-dependent conditioned fear, nor as distributed as hippocampal-dependent episodic memory (Knowlton et al., 1996; Poldrack et al., 2005; Kantak et al., 2010). Rather, is depends on a restricted number of regions including the motor cortex, the striatum and the cerebellum.
If we view the behavioral interference results for conditioned fear (Schiller et al., 2010), episodic memory (e.g., Hupbach et al., 2007), and motor skill memory (Walker et al., 2003) in light of their unique underlying neural representations, the different behavioral outcomes of presenting interfering information during reconsolidation is understandable. Given the neurally localized and relatively simple representation of the CS–US association that drives the expression of conditioned fear, one might expect that updating the original memory with safety information could so fundamentally alter the representation of the value of the CS as to eliminate the expression of the conditioned fear response. In contrast, episodic memory for a list of items is thought to be represented as a distributed, but bound, cortical network. Introducing a new list of items, or a new mnemonic network, while the original memory is undergoing reconsolidation might simply serve to merge or bind the two memory networks together, as opposed to re-writing the original memory. If this is the case, one might expect the primary consequence not to be reflected as impaired memory for the original list items, but rather impaired memory for the source of the items, as Hupbach et al. (2009) observed. The effect of interference on skill memories does not appear to eliminate the expression of the original memory (as in fear conditioning), nor leave it relatively intact (as in episodic memory). Instead, the effect of behavioral interference on skill memories is an impairment with some expression of the original memory, consistent with a neural representation of this memory that is neither discretely localized, nor widely distributed.
The Future of Human Reconsolidation Research
In contrast to research with non-human animals, research examining reconsolidation in humans has been slow to emerge and the data is not nearly as compelling. Although there appears to be good evidence for a reconsolidation mechanism across memory systems in humans using behavioral interference techniques (e.g., Walker et al., 2003; Hupbach et al., 2007: Schiller et al., 2010), these techniques are subject to many of the same concerns that arose when trying to understand the nature of memory representation in cognitive studies of memory malleability in the 1980s. That is, behavioral data alone cannot provide conclusive evidence of a permanently altered memory representation following reconsolidation. Since that time, however, new techniques for examining human brain function have been developed. The conjunction of human brain function with behavioral interference during reconsolidation may provide support for an altered mnemonic representation. Although changes in the underlying neural signature should be observed across memory systems, the best possibility for clear evidence of influencing a reconsolidation mechanism through interference may come from studies of fear conditioning. The successful reconsolidation/interference paradigm in humans (Schiller et al., 2010) combined with extensive knowledge of the localized neural representation of this simple type of learning that has been investigated across species (Phelps and LeDoux, 2005) leads to specific hypothesis that can be confirmed. For example, the ventral medial prefrontal cortex is known to be critical in the expression of extinction learning (Phelps et al., 2004; Quirk and Mueller, 2008), but this region should not be equally involved if the amygdala-dependent fear representation is altered during reconsolidation.
A critical factor that has enabled the proliferation of research on reconsolidation in non-human animals is a detailed understanding of the synaptic processes needed for memory storage and the availability of drugs that block this process. This same factor may explain why human reconsolidation research is so slow to emerge. It is simply not possible to use these same powerful pharmacological manipulations in humans. As outlined above, even when a drug safe for human use was shown to block the reconsolidation of fear memories in rats (Debiec and LeDoux, 2004), using this drug in humans was not as effective (Brunet et al., 2008; Soeter and Kindt, 2010; Miller et al., unpublished).
Why propranolol appears to be less effective in disrupting the reconsolidation of fear memories in humans is unclear, but it is possible to identify some factors that can be assessed in future studies. First, the animal research used a much higher dose than the equivalent dose in humans. Future studies in humans might increase the dose and future research in rats could determine if a systemically delivered lower dose equivalent to one that humans can safely use is effective. Second, there is reason to believe that human conditioned fear responses may be driven by both simple CS–US associations and top-down knowledge of the CS–US contingency (Olsson and Phelps, 2007). The interaction of these different memory representations likely play an important role in fear related disorders as well. The human pharmacological studies to date have not intentionally and systematically manipulated these independent memory representations. It is possible that future interference and pharmacological studies examining reconsolidation could independently manipulate these different memory representations to understand and clarify how their interaction may be linked to the effectiveness of pharmacological interventions. Finally, propranolol has only been shown to be effective in altering reconsolidation in amygdala-dependent cued fear conditioning (Debiec and LeDoux, 2004; Muravieva and Alberini, 2010). The development of safe biological interventions that may impact the reconsolidation of other types of memory and memory systems, or additional ones that impact amygdala-dependent memory, would greatly enhance our understanding of the details of human reconsolidation processes.
Because most of the recent research on reconsolidation in non-human animals has focused on fear conditioning, human research on this type of memory has been able benefit. To date, this has been less true for human research examining reconsolidation of other memory systems. This is apparent not only in the lack of potential pharmacological manipulations, but also in factors that may be relevant to behavioral interference paradigms. For example, the research on fear memory consolidation in rats provides some information about the timing of when the reconsolidation window “opens” and when it “closes.” A similar understanding of the reconsolidation timing for hippocampal-dependent memories might allow researcher to develop more nuanced interference paradigms that may help differentiate between memories that are integrated and bound through the reconsolidation process verses those that are simply forgotten or blocked from expression. Integrating insights from neurobiological models of hippocampal reconsolidation (Milekic and Alberini, 2002; Inda et al., 2011) with human reconsolidation/interference paradigms may also help address some of the old debates that emerged concerning the malleability of memory in the cognitive psychology literature.
Finally, although reconsolidation is known to be a memory updating mechanism, this view of reconsolidation has been more apparent in research on humans, perhaps because of the lack of safe pharmacological manipulations. If reconsolidation serves to update memories, rather than block them, then we might extend our current reconsolidation/interference paradigms, in both humans and other animals, to more precisely manipulate this process. For example, the type of interference, interference (blockade or update) following reactivation, following reactivation should result in different, but predictable patterns of memory outcomes. Similarly, re-learning following the behavioral interference of reconsolidation should differ in predictable ways from re-learning after pharmacological blockade of reconsolidation. If, for instance, safety information is introduced during reconsolidation of a fear-eliciting cue, re-learning should be slower because this cue now represents safety. But in the case of pharmacological blockade the memory trace is altered so that the memory is no longer expressed. In this case, re-learning should be similar to learning anew. In short, by understanding the principles of memory reconsolidation in humans and other animals, we should be much more effective at specifically altering memories, both in the laboratory and the clinic.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge support from the James S. McDonnell Foundation and National Institutes of Health (R21 MH072279 to Elizabeth A. Phelps).
References
Admon, R., Lubin, G., Stern, O., Rosenberg, K., Sela, L., Ben-Ami, H., and Hendler, T. (2009). Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc. Natl. Acad. Sci. U.S.A. 106, 14120–14125.
Banker, G., Hunt, E., and Pagano, R. (1969). Evidence supporting the memory disruption hypothesis of electroconvulsive shock action. Physiol. Behav. 4, 895–899.
Bartlett, F. C. (1932). Remembering: A Study in Experimental and Social Psychology. Cambridge: Cambridge University Press.
Bechara, A., Tranel, D., Damasio, H., Adolphs, R., Rockland, C., and Damasio, A. R. (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269, 1115–1118.
Boccia, M. M., Acosta, G. B., Blake, M. G., and Baratti, C. M. (2004). Memory consolidation and reconsolidation of an inhibitory avoidance response in mice: effects of i.c.v. injections of hemicholinium-3. Neuroscience 124, 735–741.
Boccia, M. M., Blake, M. G., Krawczyk, M. C., and Baratti, C. M. (2010). Hippocampal α7 nicotinic receptors modulate memory reconsolidation of an inhibitory avoidance task in mice. Neuroscience 171, 531–543.
Boccia, M. M., Freudenthal, R., Blake, M. G., de la Fuente, V., Acosta, G., Baratti, C. M., and Romano, A. (2007). Activation of hippocampal nuclear factor-kappa B by retrieval is required for memory reconsolidation. J. Neurosci. 27, 13436–13455.
Bouton, M. E. (2002). Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry 52, 976–986.
Bozon, B., Davis, S., and Laroche, S. (2003). A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 40, 695–701.
Brohawn, K. H., Offringa, R., Pfaff, D. L., Hughes, K. C., and Shin, L. M. (2010). The neural correlates of emotional memory in posttraumatic stress disorder. Biol. Psychiatry 68, 1023–1030.
Brunet, A., Orr, S. P., Tremblay, J., Robertson, K., Nader, K., and Pitman, R. K. (2008). Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J. Psychiatr. Res. 42, 503–506.
Brunetti, M., Sepede, G., Mingoia, G., Catani, C., Ferretti, A., Merla, A., Del Gratta, C., Romani, G. L., and Babiloni, C. (2010). Elevated response of human amygdala to neutral stimuli in mild post traumatic stress disorder: neural correlates of generalized emotional response. Neuroscience 168, 670–679.
Cohen, N. J., Poldrack, R. A., and Eichenbaum, H. (1997). Memory for items and memory for relations in the procedural/declarative memory framework. Memory 5, 131–178.
Davachi, L. (2006). Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 16, 693–700.
Davis, M. (2000). “The role of the amygdala in conditioned and unconditioned fear and anxiety,” in The Amygdala: A Functional Analysis, ed. J. P. Aggleton (Oxford: Oxford University Press), 213–288.
Dawson, R. G., and McGaugh, J. L. (1969). Electroconvulsive shock effects on a reactivated memory trace: further examination. Science 166, 525–527.
Debiec, J., and LeDoux, J. E. (2004). Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129, 267–272.
Debiec, J., LeDoux, J. E., and Nader, K. (2002). Cellular and systems reconsolidation in the hippocampus. Neuron 36, 527–538.
DeVietti, T. L., and Holliday, J. H. (1972). Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace: a replication. Psychonom. Sci. 29, 137–138.
DeVietti, T. L., and Kirkpatrick, B. R. (1976). The amnesia gradient: inadequate as evidence for a memory consolidation process. Science 194, 438–440.
Dickerson, B. C., and Eichenbaum, H. (2010). The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 35, 86–104.
Duvarci, S., and Nader, K. (2004). Characterization of fear memory reconsolidation. J. Neurosci. 24, 9269–9275.
Duvarci, S., Nader, K., and Ledoux, J. E. (2005). Activation of extracellular signal-regulated kinase-mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur. J. Neurosci. 21, 283–289.
Fanselow, M. S. (2000). Contextual fear, gestalt memories, and the hippocampus. Behav. Brain Res. 110, 73–81.
Fanselow, M. S., and LeDoux, J. E. (1999). Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23, 229–232.
Foerde, K., Knowlton, B. J., and Poldrack, R. A. (2006). Modulation of competing memory systems by distraction. Proc. Natl. Acad. Sci. U.S.A. 103, 11778–11783.
Forcato, C., Argibay, P. F., Pedreira, M. E., and Maldonado, H. (2009). Human reconsolidation does not always occur when a memory is retrieved: the relevance of the reminder structure. Neurobiol. Learn. Mem. 91, 50–57.
Forcato, C., Burgos, V. L., Argibay, P. F., Molina, V. A., Pedreira, M. E., and Maldonado, H. (2007). Reconsolidation of declarative memory in humans. Learn Mem. 14, 295–303.
Forcato, C., Rodrìguez, M. L., Pedreira, M. E., and Maldonado, H. (2010). Reconsolidation in humans opens up declarative memory to the entrance of new information. Neurobiol. Learn. Mem. 93, 77–84.
Funayama, E. S., Grillon, C., Davis, M., and Phelps, E. A. (2001). A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. J. Cogn. Neurosci. 13, 721–729.
Gelinas, J. N., and Nguyen, P. V. (2005). Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J. Neurosci. 25, 3294–3303.
Gilman, A. G., and Goodman, L. S. (1996). Goodman and Gilman’s the Pharmacological Basis of Therapeutics. New York:McGraw-Hill.
Gold, P. E., and King, R. A. (1972). Tests of the effect of delayed footshock-electroconvulsive shock pairings. Physiol. Behav. 8, 797–800.
Gordon, W. C., and Spear, N. E. (1973). The effects of strychnine on recently acquired and reactivated passive avoidance memories. Physiol. Behav. 10, 1071–1075.
Grillon, C., Ameli, R., Woods, S. W., Merikangas, K., and Davis, M. (1991). Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 28, 588–595.
Grillon, C., Falls, W. A., Ameli, R., and Davis, M. (1994). Safety signals and human anxiety: a fear-potentiated startle study. Anxiety 1, 13–21.
Hardt, O., Einarsson, E. O., and Nader, K. (2009). A bridge over troubled water: reconsolidation as a link between cognitive and neuroscientific memory research traditions. Ann. Rev. Psychol. 61, 141–167.
Hupbach, A., Gomez, R., Hardt, O., and Nadel, L. (2007). Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn. Mem. 14, 47–53.
Hupbach, A., Gomez, R., and Nadel, L. (2009). Episodic memory reconsolidation: updating or source confusion? Memory 17, 502–510.
Hupbach, A., Hardt, O., Gomez, R., and Nadel, L. (2008). The dynamics of memory: context-dependent updating. Learn. Mem. 15, 574–579.
Inda, M. C., Muravieva, E. V., and Alberini, C. M. (2011). Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J. Neurosci. 31, 1635–1643.
Jamieson, J. L., and Albert, D. J. (1970). Amnesia from ECS: the effect of pairing ECS and footshock. Psychonom. Sci. 18, 14–15.
Jin, X. C., Lu, Y. F., Yang, X. F., Ma, L., and Li, B. M. (2007). Glucocorticoid receptors in the basolateral nucleus of amygdala are required for postreactivation reconsolidation of auditory fear memory. Eur. J. Neurosci. 25, 3702–3712.
Johnson, M. K., Hashtroudi, S., and Lindsay, D. S. (1993). Source monitoring. Psychol. Bull. 114, 3–28.
Judge, M. E., and Quartermain, D. (1982). Characteristics of retrograde amnesia following reactivation of memory in mice. Physiol. Behav. 28, 585–590.
Kantak, S. S., Sullivan, K. J., Fisher, B. E., Knowlton, B. J., and Winstein, C. J. (2010). Neural substrates of motor memory consolidation depend on practice structure. Nat. Neurosci. 13, 923–925.
Kelly, A., Laroche, S., and Davis, S. (2003). Activation of mitogenactivated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 23, 5354–5360.
Kindt, M., Soeter, M., and Vervliet, B. (2009). Beyond extinction: erasing human fear responses and preventing the return of fear. Nat. Neurosci. 12, 256–258.
Knowlton, B. J., Mangels, J. A., and Squire, L. R. (1996). A neostriatal habit learning system in humans. Science 273, 1399–1402.
LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E., and Phelps, E. A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20, 937–945.
LaBar, K. S., LeDoux, J. E., Spencer, D. D., and Phelps, E. A. (1995). Impaired fear conditioning following unilateral temporal lobectomy in humans. J. Neurosci. 15, 6846–6855.
LaBar, K. S., and Phelps, E. A. (2005). Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav. Neurosci. 119, 677–686.
Lee, J. L. C. (2010). Sources of experimental amnesia. Front. Behav. Neurosci. 4:168. doi: 10.3389/fnbeh.2010.00168
Lewis, D. J., and Bregman, N. J. (1973). The source of the cues for cue-dependent amnesia. J. Comp. Physiol. Psychol. 85, 421–426.
Lewis, D. J., Bergman, N. J., and Mahan, J. J. Jr. (1972). Cue-dependent amnesia in the K-maze. J. Comp. Physiol. Psychol. 81, 243–247.
Lindsay, D. S., and Johnson, M. K. (1989). The eyewitness suggestibility effect and memory for source. Mem. Cognit. 17, 349–358.
Loftus, E. F., Miller, D. G., and Burns, H. J. (1978). Semantic integration of verbal information into a visual memory. J. Exp. Psychol. Hum. Learn. 4, 19–31.
Loftus, E. F. (2005a). Planting misinformation in the human mind: a 30-year investigation of the malleability of memory. Learn. Mem. 12, 361–366.
Loftus, E. F. (2005b). Searching for the neurobiology of the misinformation effect. Learn. Mem. 12, 1–2.
Loftus, E. F., and Yuille, J. C. (1984). “Departures from reality in human perception and memory,” in Memory Consolidation: Psychobiology of Cognition, eds H. Weingartner and E. S. Parker (Hillsdale, NJ: Lawrence Erlbaum Associates), 163–184.
Mactutus, C. F., Riccio, D. C., and Ferek, J. M. (1979). Retrograde amnesia for old reactivated memory, some anomalous characteristics. Science 204, 1319–1320.
McCloskey, M., and Zaragoza, M. (1985). Misleading postevent information and memory for events: arguments and evidence against memory impairment hypotheses. J. Exp. Psychol. Gen. 114, 1–16.
Middleton, F. A., and Strick, P. L. (2000). Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Rev. 31, 236–250.
Milekic, M. H., and Alberini, C. M. (2002). Temporally graded requirement for protein synthesis following memory reactivation. Neuron 36, 521–524.
Misanin, J. R., Miller, R. R., and Lewis, D. J. (1968). Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160, 203–204.
Molinari, M., Leggio, M. G., Solida, A., Ciorra, R., Misciagna, S., Silveri, M. C., and Petrosini, L. (1997). Cerebellum and procedural learning: evidence from focal cerebellar lesions. Brain 120, 1753–1762.
Monfils, M.-H., Cowansage, K. K., Klann, E., and LeDoux, J. E. (2009). Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324, 951–955.
Morris, R. G. M., Inglis, J., Ainge, J. A., Olverman, H. J., Tulloch, J., Dudai, Y., and Kelly, P. A. T. (2006). Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron 50, 479–489.
Muravieva, E. V., and Alberini, C. M. (2010). Limited efficacy of proproanolol on the reconsolidation of fear memories. Learn. Mem. 17, 306–319.
Myslobodsky, S. M., and Weiner, I. (2000). Contemporary Issues in Modeling of Psychopathology. Boston: Kluwer Academic Publishers.
Nader, K., and Hardt, O. (2009). A single standard for memory: the case for reconsolidation. Nat. Rev. Neurosci. 10, 224–234.
Nader, K., Schafe, G. E., and LeDoux, J. E. (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726
Ostroff, L. E., Cain, C. K., Bedont, J., Monfils, M. H., and Ledoux, J. E. (2010). Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 107, 9418–9423.
Phelps, E. A., Delgado, M. R., Nearing, K. I., and LeDoux, J. E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905.
Phelps, E. A., and LeDoux, J. E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48, 175–187.
Phelps, E. A., O’Connor, K. J., Gatenby, J. C., Gore, J. C., Grillon, C., and Davis, M. (2001). Activation of the left amygdala to a cognitive representation of fear. Nat. Neurosci. 4, 437–441.
Poldrack, R. A., Sabb, F. W., Foerde, K., Tom, S. M., Asarnow, R. F., Bookheimer, S. Y., and Knowlton, B. J. (2005). The neural correlates of motor skill automaticity. J. Neurosci. 25, 5356–5364.
Przybyslawski, J., Roullet, P., and Sara, S. J. (1999). Attenuation of emotional and nonemotional memories after their reactivation, role of beta adrenergic receptors. J. Neurosci. 19, 6623–6628.
Przybyslawski, J., and Sara, S. J. (1997). Reconsolidation of memory after its reactivation. Behav. Brain Res. 84, 241–246.
Quirk, G. J., and Mueller, D. (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72.
Rasmusson, A. M., and Charney, D. S. (1997). Animal models of relevance to PTSD. Ann. N. Y. Acad. Sci. 821, 332–351.
Rauch, S. L., Shin, L. M., and Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol. Psychiatry 60, 376–382.
Roediger, H. L., Dudai, Y., and Fitzpatrick, S. M. (eds.). (2007). Science of Memory Concepts. Oxford: Oxford University Press.
Rogan, M. T., Leon, K. S., Perez, D. L., and Kandel, E. R. (2005). Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron 46, 309–320.
Rubin, R. D. (1976). Clinical use of retrograde amnesia produced by electroconvulsive shock: a conditioning hypothesis. Can. Psychiatr. Assoc. J. 21, 87–90.
Rubin, R. D., Fried, R., and Franks, C. M. (1969). “New application of ECT,” in Advances in Behavior Therapy, eds R. D. Rubin and C. Franks (New York: Academic Press), 37–44.
Sara, S. (2008). “Reconsolidation: historical perspective and theoretical aspects,” in Learning Theory and Behavior. Vol. [1] of Learning and Memory: A Comprehensive Reference (4 Vols), eds R. Menzel and J. Byrne (Oxford: Elsevier), 461–476.
Schacter, D. L. (1999). The seven sins of memory. Insights from psychology and cognitive neuroscience. Am. Psychol. 54, 182–203.
Schafe, G. E., Doyère, V., and LeDoux, J. E. (2005). Tracking the fear engram: the lateral amygdala is an essential locus of fear memory storage. J Neurosci. 25, 10010–10014.
Schiller, D., Levy, I., Niv, Y., LeDoux, J. E., and Phelps, E. A. (2008). From fear to safety and back: reversal of fear in the human brain. J. Neurosci. 28, 11517–11525.
Schiller, D., Monfils, M. H., Raio, C. M., Johnson, D. C., Ledoux, J. E., and Phelps, E. A. (2010). Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463, 49–53.
Schneider, A. M., and Sherman, W. (1968). Amnesia: a function of the temporal relation of footshock to electroconvulsive shock. Science 159, 219–221.
Soeter, M., and Kindt, M. (2010). Dissociating response systems: erasing fear from memory. Neurobiol. Learn. Mem. 94, 30–41.
Squire, L. R. (2004). Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem. 82, 171–177.
Squire, L. R., Slater, P. C., and Chace, P. M. (1976). Reactivation of recent or remote memory before electroconvulsive therapy does not produce retrograde amnesia. Behav. Biol. 18, 335–343.
Strange, B. A., Kroes, M. C. W., Fan, J. E., and Dolan, R. J. (2010). Emotion causes targeted forgetting of established memories. Front. Behav. Neurosci. 4:175. doi: 10.3389/fnbeh.2010.00175
Suzuki, A., Josselyn, S. A., Frankland, P. W., Masushige, S., Silva, A. J., and Kida, S. (2004). Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24, 4787–4795.
Tulving, E., and Thomson, D. (1973). Encoding specificity and retrieval processes in episodic memory. Psychol. Rev. 80, 352–372.
Walker, M. P., Brakefield, T., Hobson, J. A., and Stickgold, R. (2003). Dissociable stages of human memory consolidation and reconsolidation. Nature 425, 616–620.
Walle, U. K., Fagan, T. C., Topmiller, M. J., Conradi, E. C., and Walle, T. (1994a). The influence of gender and sex steroid hormones on the plasma binding of propranolol enantiomers. Br. J. Clin. Pharmacol. 37, 21–25.
Walle, T., Walle, K., Mathur, R. S., Palesch, Y. Y., and Conradi, E. C. (1994b). Propranolol metabolism in normal subjects: association with sex steroid hormones. Clin. Pharmacol. Ther. 56, 127–132.
Weaver, T. A., and Magnus, J. G. (1969). Effects of unconditioned stimulus-linked subconvulsive current in chicks. Psychonom. Sci. 16, 265–266.
White, N. M., and McDonald, R. J. (2002). Multiple parallel memory systems in the brain of the rat. Neurobiol. Learn. Mem. 77, 125–184.
Keywords: fear conditioning, reconsolidation, emotion, episodic memory, procedural memory, amygdala, hippocampus, prorpanolol
Citation: Schiller D and Phelps EA (2011) Does reconsolidation occur in humans?. Front. Behav. Neurosci. 5:24. doi: 10.3389/fnbeh.2011.00024
Received: 07 January 2011;
Paper pending published: 24 January 2011;
Accepted: 22 April 2011;
Published online: 17 May 2011.
Edited by:
Jacek Debiec, New York University, USACopyright: © 2011 Schiller and Phelps. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Elizabeth A. Phelps, Department of Psychology and Center for Neural science, New York University, 6 Washington Place, New York, NY 10003, USA. e-mail:bGl6LnBoZWxwc0BueXUuZWR1

