
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Nanotechnol. , 04 October 2022
Sec. Biomedical Nanotechnology
Volume 4 - 2022 | https://doi.org/10.3389/fnano.2022.987117
We propose therapy with extracellular vesicles (EVs) for dominant central nervous system aspects of chronic Long COVID Syndromes (LCS). These clinical conditions have a delayed onset of 1–3 months following the cessation of active SARS-CoV-2 virus infections that cause an acute disease called COVID-19. The therapy of LCS will be achieved by direct access to the central nervous system (CNS) by nasal administration of small EVs derived from Mesenchymal Stromal Cells (MSC). When administered nasally, they target CNS microglia and endothelia involved in LCS encephalopathy, as indicated by experimental animal models and human autopsy and spinal fluid studies. Underlying this approach is the discovery that MSC-sEV treatment for healing neuro injury targets, microglia, and macrophages that then likely release secondary trophic EVs that affect the local capillary endothelial cells to restore vascular integrity. It is postulated that the pathways of endothelial and neural pathologies in acute SARS-CoV-2 virus infections may carry over to produce underlying vascular and neurological defects mediating LCS that are susceptible to this proposed nasal therapy with MSC-sEVs.
Long COVID Syndromes (LCS) are debilitating chronic illness that follow acute SARS-CoV-2 virus infections that cause the disease COVID-19 in 10 %–30 % of recovered patients after a gap of time of 1–3 months. Thus, LCS are not a disease continuation but are independent with a delayed onset (Davis et al., 2021; Nasserie et al., 2021). A retrospective cohort study of nearly 250,000 United States patients showed that the incidence of neuro/psychiatric diagnoses in 6 months post COVID-19 was about 33 %, with 12 % of patients diagnosed for the first time with such disorders (Taquet et al., 2021). These neuro/psychiatric aspects of post COVID, when examined by employing high-resolution MRI imaging of intracranial vessel walls and tissue microstructures in 60 COVID-19 3 month-recovered patients vs. age- and sex-matched non-COVID-19 controls, showed disruption and a disturbed functional brain integrity in the recovery stages of COVID-19 (Lu et al., 2020). Therefore, LCS patients constitute a potentially enormous looming health-care crisis, with many demonstrating neuro/psychiatric aspects that will likely represent a major clinical proportion.
In a web-based survey of 3,762 respondents with LCS from 56 countries, the majority had COVID-19 without hospitalization (only 8.4 % were hospitalized) and 96 % reported symptoms beyond 90 days after the initiation of infections. Of 203 symptoms in 10 organ systems the most frequently reported after 6 months were: fatigue (78 %), post-exertional malaise (72 %), and cognitive dysfunction (55 %) (Davis et al., 2021). Respondents with symptoms over 6 months experienced an average of 13.8 symptoms in 7 months and 86 % experienced relapses, with exercise or physical or mental activity and stress as the main triggers (Davis et al., 2021). The trajectory of LCS is unpredictable, episodic and has a relapse-remitting nature with worsening unpredictably or in response to any physical or mental exertions (Twomey et al., 2022).
Many who were infected have had acute severe manifestations, even respiratory failure in some (Evans et al., 2021) and indeed there is a high proportion of LCS in cases surviving more than a year from ICU discharge (Heesakkers et al., 2022). Indeed, early systems biology analysis emphasized that quantitated viral load and the severity of clinical infection predicted subsequent long COVID symptoms (Su et al., 2022). However, and peculiarly, with time, it has been determined that mild and even sub-clinical active COVID-19 infections, even in young previously entirely healthy people infected with the SARS-CoV-2 (Fernández-Castañeda et al., 2022; Tabacof et al., 2022) can also develop significant LCS lasting months and even years (Carfì et al., 2020; Tenforde et al., 2020). People who have had COVID, that subsequently are not complaining of daily long COVID symptoms, nevertheless can demonstrate degraded attention and memory for up to 6–9 months.
Furthermore, LCS can occur no matter which SARS-CoV-2 virus variant has been involved, since there is no evidence yet that there are any differences in the subsequent development of LCS between beta and delta or the present omicron infections (Su et al., 2022). Currently there is no clear unified idea on the pathogenesis of these syndromes (Maltezou et al., 2021; Yoo et al., 2022; Zhao et al., 2022), but the general idea is emerging that these are principally vascular diseases; judging by aspects of the active SARS-CoV-2 viral infection of COVID-19 patients (Kalafatis, 2021; Lei et al., 2021; Ostergaard, 2021; Siddiqi et al., 2021) and elements of the subsequent LCS (Charfeddine et al., 2021; Christensen and Berg, 2021). In fact, brain single-photon emission-computed tomography in fourteen LCS Long COVID patients seen in family practice with severe neurocognitive problems demonstrated vascular encephalopathy that was thought due to persistent coagulation disorders (Jamoulle et al., 2021).
There are as yet no recommendations for specific immune or biological therapies (Greenhalgh et al., 2020; Nalbandian et al., 2021), but here we postulate the ultimate healing effects of MSC-derived sEVs on the abnormal vasculature in LCS patients providing unique properties for beneficial treatment. Thus, this article emphasizes the neurological and neurovascular aspects as the most commonly occurring in LCS and particularly susceptible to the healing effects of MSC-derived sEVs. The idea is proposed of an appropriate protocol for treatment employing the use of particular healing mesenchymal stromal cell (MSC)-derived sEVs administered directly into the brain by direct application via the nose. The use of these MSC-derived sEVs and the nasal route of treatment have considerable histories of positive healing affecting the vascular and neurological abnormalities of neural and psychiatric illnesses that mainly underlie the pathogenesis of LCS.
Long COVID Syndromes consist of complex clinical processes, with literally hundreds of possible symptoms, in which a significant patient subpopulation has neurological dominant manifestations. These may persist for months after infection as a major part of the total constellation of symptoms that are now called Long COVID Syndromes (LCS) (Davis et al., 2021). Such neuro-dominant LCS variably consist mainly of intractable profound fatigue, myalgias, impaired concentration, headache, poor memory, sensory disturbances, dizziness, and the inability to accomplish everyday tasks, as well as depression, and in severe cases, delusions, paranoia, and even psychosis. In some patients, these occur along with respiratory aspects consisting of dry cough, sore throat, palpitations, shortness of breath, and chest tightness.
Individuals can long continue to suffer from cognitive symptoms such as difficult concentration, known as brain fog, as well as forgetfulness and of course, the severe fatigue for many months. There can be a reduced capacity for memory, decreased attention, and cognitive abilities (“walk into a room and forget what you came in for”), expressive aphasias, like forgetting people’s names, how to spell, and having trouble typing or writing. Furthermore, speech can be slower, more measured, and paced, than previously, with having to focus on pronouncing words clearly, or one’s voice becomes “slurry” like after a stroke. Despite debilitating exhaustive fatigue, there are poor fitful sleep disturbances, awakening with soaking diaphoresis, and depression. The chronic over-powering fatigue is a persistent distressing feeling of extreme weariness or exhaustion that is neither alleviated by rest nor proportional to activity and can be increased by attempts at activity; interfering with usual daily functions and negatively impacting the quality of life.
In some patients, there is a characteristic loss of smell and taste (anosmia/hypogeusia) and even smell distortions, (“phantom smells, i.e. perfume can smell like sewage”) (Misra et al., 2021). In more severe and later LCS, evolved neurologic conditions can even include stroke, delirium, brain inflammation, primary psychiatric syndromes, and peripheral nerve abnormalities (Varatharaj et al., 2020; Spudich and Nath, 2022). These can include sensory disturbances, like numbness in the limbs and strange vibrating sensational paresthesias (pins and needles feelings), along with pain or numbness in the hands and feet, weakness, twitching, and burning in the feet and/or hands, and also swallowing difficulties. There frequently is a characteristic post-exercise symptom exacerbation, called post-exertional malaise (Davis et al., 2021) and in a few individuals exhibit extreme vibratory disturbances that can be so severe as to lead to suicide (Massey et al., 2021).
Also, some individuals can have photophobia (sensitivity to light), vertigo, nausea, incredible swings in blood pressure, and severe body and joint pains. Additionally, there can be panic and a feeling of spiraling out of control (Novak et al., 2022). The results can be an inability to return to work because of difficulties performing basic activities of living. Furthermore, there can be a recurrence of similar patterns of symptoms, like a symptom memory when patients attempt over doing an effort. This requires LCS patients to institute careful pacing, since in this case, driving oneself can induce more suffering with repetitive delayed recovery.
We are proposing a therapy of CNS aspects of LCS by nasally administered small extracellular vesicles (sEVs) that are derived from the endoplasmic intracellular pathway of mesenchymal stromal cells (MSC). MSC are harvested as a minor subpopulation of connective tissue cells from a variety of sites like bone marrow, placenta, umbilical, cord amniotic fluid, etc., then cultured in vitro and passaged for enrichment as adherent and self-replenishing stromal cells (Lankford et al., 2018; Nakazaki et al., 2021). Enriched plastic adherent MSC are then detached and changed to serum-free media for a final 2-day culture in order to collect the released sEVs by using differential centrifugation to 10,000 g, or size exclusion chromatography. Then, differential buoyant density centrifugation is advised to harvest floating vesicles and discard pelleted aggregates. The protein content is assessed, and the composition is evaluated for sizes of vesicles by nanoparticle-tracking analysis. This results in great in vitro enrichment to 100 % from generally about only 0.01 % of connective tissue precursors. Tetraspanin protein markers of sEVs are assessed by Western blotting and flow cytometry with appropriate monoclonal antibodies (Lankford et al., 2018; Nakazaki et al., 2021).
In 2014, a remarkable benefit of MSC EV therapy based on its demonstrated in vitro immune suppressive actions was reported in a patient with a severe graft vs. host response that was resistant to all therapies. There was a remarkable reduction of daily diarrhea volume and curative effects on severe skin and necrotic oral mucosal lesions (Kordelas et al., 2014). Prior work had shown that the MSC, when given systemically, had trophic healing actions in a variety of injury models in experimental animals. These included myocardial infarction, acute respiratory distress, renal reperfusion injury, and the rat model of obesity-induced type 2 diabetes (Kotikalapudi et al., 2022).
Very importantly, it was then repeatedly found that the therapeutic effects of systemic MSC can be replicated by the systemic transfer of the sEVS secreted by the MSCs in a variety of experimental injury models, as we reviewed (Askenase, 2020). Recently, we showed that with MSC therapy via intravenous (IV) injection, the cells become entrapped in the lungs for 2–3 days, and in reality, it is the sEVs that release in vivo and then mediate the numerous healing effects (Nakazaki et al., 2021). In fact, our quantitative study showed that multiple IV injections of the sEVs over days can be needed to reach quantitative maximum healing that is produced by single MSC injections (Nakazaki et al., 2021). More clinical studies of similar benefits of MSC and MSC-EVs in patients have been published (Weiss et al., 2013; Nassar et al., 2016; Stolk et al., 2016; Mrahleh et al., 2021) Currently, there are 199 listed NIH clinical trials involving MSC, 52 trials on sEVs, some with MSC-secreted EVs, that are now just beginning to come to favorable published conclusion.
In an important studied example, the nasal delivery of EVs was encapsulated with the anti-inflammatory effectors for delivery non-invasively to the CNS of mice that produced very favorable therapeutic results (Zhuang et al., 2011). Three very different inflammation disease models were tested with similar beneficial results (Zhuang et al., 2011). This favorable result with the Multiple Sclerosis model in 2011 was repeated by others in 2020 (Upadhya et al., 2020a) and in 2021 (Fathollahi et al., 2021). These data demonstrate that nasal administration of sEVs likely will provide a non-invasive and novel therapeutic approach for treating brain inflammatory-related encephalopathy in patients with these aspects in LCS.
Compared to IV administration, the use of EVs nasally has advantages from a therapeutic perspective. It represents a painless, non-invasive entry into the brain that is manageable and easily repeatable. The EVs can immediately cross the blood–brain barrier and thus are able to be delivered into the brain without complex neurosurgical interventions. Therefore, there is a rapid onset of action and a profile of favorable drug delivery and tolerability (Arora et al., 2002). Importantly, nasal administration increases bioavailability by avoiding first-pass loses in non-specific hepatic and splenic uptakes and thus reduce the systemic side effects. Intranasal delivery is of particular interest in the treatment of chronic conditions such as LCS when multiple dosing is likely required and self-dosing becomes possible.
Data showing the larger brain accumulation administrating sEVs nasally compared to the usual IV route employed gold-labeled EVs in mice (Betzer et al., 2017). Similar observations were made in a different model (Perets et al., 2018). Furthermore, pertinent recent results indicate rapid clearance of EVs from the circulation after IV administration (Lai et al., 2014) that might be related to the very small amount of EVs able to reach the CNS after IV administration compared to nasal delivery. Finally, DiR-lipid-labeled EVs with their detection by confocal microscopy were used to study the biodistribution of macrophage-derived EVs administered by different routes. On a practical level, non-invasive nasal delivery was judged the best for achieving long duration in the CNS and thus best for chronic dosing (Haney et al., 2020).
These sEVs can be administered directly to the CNS by nasal administration. This is because they naturally pass the blood–brain barrier (BBB) after transit through spaces around the cranial nerves traversing the cribriform plate in the ventral skull (Zhuang et al., 2011). Then, as physiologic, they have the ability to traverse the epithelial and endothelial cells of the BBB by undergoing transcytosis (Banks et al., 2020; Saint-Pol et al., 2020). Once in the brain, these sEVs are subsequently taken up by phagocytic cells like macrophage-related microglia (Zhuang et al., 2011; Kodali et al., 2019; Sabel et al., 2021) to mediate healing effects by restoring the diverse normal functions in a variety of local pathological conditions.
Regarding questions about MSC-sEVs crossing the BBB, in several studies of stroke or traumatic brain injury, intravenous or intranasal administration of MSC-EVs that was labeled with lipophilic dyes or carried the typical EV tetraspanin marker CD63 labeled with GFP, resulted in fluorescent signals in neurons, glia, and cerebral endothelial cells (András and Toborek, 2015; Zhang et al., 2015; Di Rocco et al., 2016; Xiong et al., 2017; Upadhya et al., 2020b).
This has included studies in ischemic and hemorrhagic strokes (Xin et al., 2013a; Xin et al., 2013b; Doeppner et al., 2015a; Chen et al., 2016; Otero-Ortega et al., 2017; Venkat et al., 2019; Mahdavipour et al., 2020; Rohden et al., 2021) and perinatal brain injury (Drommelschmidt et al., 2017; Sisa et al., 2019; Kaminski et al., 2020), some treated with intranasal MSC-EVs (Thomi et al., 2019a; Thomi et al., 2019b). Importantly, for this discussion regarding nasal sEV therapy of the neuro-psychiatric aspects of LCS, this includes the treatment of various neurodegenerative diseases with nasal sEVs, that has succeeded in diminishing pathologies in animal models of Parkinson’s disease (Laso-Garcia et al., 2018; Clark et al., 2019; Narbute et al., 2019; Chen et al., 2020; Jafarinia et al., 2020; Zhu et al., 2021), amyotrophic lateral sclerosis (ALS) (Bonafede et al., 2016; Gugliandolo et al., 2019), and Alzheimer’s disease (Alvarez-Erviti et al., 2011; Ding et al., 2018a; Elia et al., 2019; Reza-Zaldivar et al., 2019; Losurdo et al., 2020a; Ma et al., 2020a; Cone et al., 2021a); as well as psychiatric disorders such as autism (Perets et al., 2018; Alessio et al., 2020) and schizophrenia (Tsivion-Visbord et al., 2020), and further in neurotraumas such as traumatic brain injury (Patel et al., 2018; Ni et al., 2019; Williams et al., 2019; Sun et al., 2020; Williams et al., 2020), spinal cord injury (Guo et al., 2019), penetrating hippocampal injury (León-Moreno et al., 2020), and status epilepticus (Long et al., 2017; Herman et al., 2021), some treated intranasally (Kodali et al., 2019).
These numerous and diverse results support the therapeutic usefulness of nasal administered MSC-derived sEVs in the neuro/psychiatric aspect of LCS. At sites of CNS involvement in these aspects of LCS, such EVs tend to specifically target and be taken up by macrophage family healing cells like microglia, and perhaps trophic M2-type macrophages as well. This can often lead such cells to produce secondary sEVs that usefully target other local cells like endothelial cells and pericytes to mediate the microvascular clearing of pathology and permeability stabilization, as in our work to be reviewed below. This makes the cultured, isolated, and enriched MSC-derived sEVs ideal therapeutic agents for treating the dominant CNS neuro/psychiatric and neuro-vascular effects of clinical LCS by their direct effects in the CNS after nasal administration.
Although there was an early emphasis on respiratory complications with serious pneumonia so dominant, the evidence of neurological manifestations of SARS-CoV-2 during infection contributing to the morbidity and mortality has grown rapidly. SARS-CoV-2 may affect the CNS by neuronal or hematogenous dissemination. Thus, CNS complications with COVID-19 can include encephalitis, acute necrotizing encephalopathy, diffuse leukoencephalopathy, stroke (both ischemic and hemorrhagic), venous sinus thrombosis, meningitis, and neuroleptic malignant syndromes (Zhou et al., 2020; Borah et al., 2021), that could, especially in milder forms, lead to subsequent neurologic syndromes driving the LCS. Also, to be considered as contributing are hypoxic and immune-based neuro injuries (Jha et al., 2021). Brain CTs in intensive care unit patients confirmed the severe vascular pathology (Castellano et al., 2020).
Autopsy studies showed CNS damage caused by thinning and leaky brain blood vessels from patients who died shortly after contracting infectious SARS-CoV-2 disease. However, there were no signs of SARS-CoV-2 in the brain tissue samples; suggesting that the damage was not caused by a direct viral attack on the CNS. Microvascular changes in the olfactory bulb and brainstem showed infiltrating macrophages with activated microglia and astrocytes in the perivascular spaces. High-resolution magnetic resonance imaging showed punctate hyperintensities, representing areas of microvascular injury and fibrinogen leakage indicating vascular permeability (Lee et al., 2021).
The corresponding antibody-staining confirmed the dominant microvascular changes, showing thinned basal lamina of endothelial cells and punctate hypo-intensities corresponding to congested blood vessels with surrounding fibrinogen leakage interpreted as microhemorrhages. The microvascular injury was accompanied by perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes that were frequently adjacent to neurons (Lee et al., 2021). There were CD8-pos T cells in the perivascular spaces adjacent to the endothelial cells that may have contributed to the vascular injury in the endotheliitis of COVID-19 (Varga et al., 2020). Other autopsy studies confirmed hypoxia of brain areas; many hemorrhages were likely caused by vascular insufficiencies, with similarly activated microglia (Thakur et al., 2021).
COVID-19 damage of cerebral small vessels likely causes many neurological symptoms. In the brains of severe acute-infected individuals with increased numbers of empty basement membrane tubes, the so-called “string vessels” represent remnants of lost capillaries of the microvascular brain pathology (Wenzel et al., 2021). Other and similar autopsies in COVID-19 patients confirm these processes demonstrating that megakaryocytes and platelet–fibrin thrombi characterize multi-organ thrombosis (Rapkiewicz et al., 2020). Overall, the autopsy findings confirm that endotheliopathy is a crucial aspect of the CNS pathology of acute SARS-CoV-2 infections. These could explain the confusion and delirium seen in some patients with severe infections and prominent lingering “brain fog” of subsequent LCS in those with mild infections (McAlpine et al., 2021; Ruhl et al., 2021). The fact that injury models show MSC-EVs target brain-healing macrophages that produce secondary sEVS-healing local micro vesicles (Nakazaki et al., 2021) makes this a particularly attractive therapy for patients with LCS.
Examination of cerebrospinal fluid (CSF) samples from infected patients reveals neuroinflammation and aberrant neuroimmune responses during acute COVID-19 infections not seen in the cells of the peripheral blood plasma (Song et al., 2021). Additionally, a CSF-specific clonal expansion of T cells and the presence of antibodies that recognize the epitopes of SARS-CoV-2 spike protein that cross-react with neural antigens suggest compartmentalization of the immune response (Franke et al., 2021a; Song et al., 2021); i.e., the potential of an auto immune role in the subsequent LCS (Bhadelia et al., 2021; Ortona et al., 2021; Rojas et al., 2022). This was emphasized by finding a neurovascular injury with complement activation and inflammation in COVID-19 (Lee et al., 2022) and a high frequency of CSF auto-antibodies in COVID-19 patients with neurological symptoms (Franke et al., 2021b). During this acute phase, with neuronal injury, monocytes with markers of immune activation were detected in CSF (Edén et al., 2021).
In another CSF study, severe COVID-19 patients with neurologic presentations, compared to disease controls with other inflammatory neurologic disorders, had anti-SARS-CoV-2 IgG antibodies in the CSF, but with little upregulation of cytokines and chemokines compared to the controls. However, ICU patients exhibited higher concentrations of chemokines and VEGF in the CSF (Bernard-Valnet et al., 2021). Lumbar punctures in a large cohort of patients with active SARS-CoV-2 infections and neurological symptoms confirmed negative RT-PCR for SARS-CoV-2 in the CSF of all cases, as well as no WBC, as confirmed in other studies (Neumann et al., 2020; Jarius et al., 2022). Thus, the preponderance of evidence from CSF and the brain tissue autopsy examination suggests that immune neuro cell activation with some inflammation within the CNS is the primary driver of neurologic diseases in acute COVID-19 with no evidence of active replication of SARS-CoV-2 to account for subsequent LCS.
Examination of CSF in LCS patients even after mild non-hospitalized COVID infections showed increased proteins of inflammation and unexpected antibodies indicating an activated local immune system, while white blood cells, glucose, and IgG levels typical of neuroinflammation were not found (Matschke J et al., 2020). Consistent with this, abnormal oligoclonal banding immunoglobulin patterns were identified in two-thirds of the samples with cognitive changes compared to none in the cognitive controls (Apple et al., 2022). Some such oligoclonal bands were found in the blood and CSF, implying that these resulted from systemic inflammation.
Humans experiencing LCS with cognitive symptoms had spinal fluid elevation of the chemokine CCL11 (eotaxin), often seen similarly without the resulting eosinophils in diverse neurologic (AmandaHuber et al., 2018) and psychiatric syndromes (Teixeira et al., 2018), and in cancer with analogous brain fog (Gibson and Monje, 2021), compared to those with LCS who lacked cognitive symptoms. Eotaxin/CCL11 is immunologically well-known for its effects on eosinophil chemotaxis. Instead, in neurologic disorders, it has been associated with the inhibition of neurons inducing cognitive changes, such as impaired memory and learning (Villeda et al., 2011; de Miranda et al., 2015), likely by an indirect action on microglia (Parajuli et al., 2015a) that are known to be strongly activated in LCS (Fernández-Castañeda et al., 2022). Finding this unusual chemokine response suggests that LCS patients have immunologically abnormal brains, just like individuals with diverse neurologic diseases and several psychiatric syndromes (Villeda et al., 2011; Parajuli et al., 2015a; de Miranda et al., 2015).
The observations summarized previously strongly suggest that acute COVID-19 is dominantly a vascular endothelial disease, with its endotheliopathy in the CNS microvasculature resulting from inflammation, cytokine storm, oxidative stress, and coagulopathy. Multiple pathways may lead to this dominant endotheliopathy of acute COVID-19 infections that are postulated to carry over to dominate in many CNS aspects of LCS, and are proposed herein to be susceptible to the vascular healing properties of sEVs derived from MSC (Nakazaki et al., 2021) and when given nasally.
As part of the COVID macrophage activation syndrome’s inflammatory cytokines, besides responding to the viral infection with innate and acquired immune responses, there is further stimulation of macrophage/microglial vascular-acting inflammatory cytokines by the RNA of the SARS-CoV-2 pathogen via its pattern recognition receptor TLR-7. Furthermore, there is a release of monocyte-derived microvesicles with the surface tissue factor that triggers the extrinsic coagulation pathway (Cañas et al., 2021), and macrophage-derived EVs carrying microRNAs that drive COVID-19 micro thromboses of vessels (Parajuli et al., 2015b; Sahu et al., 2017).
Additionally, neutrophil extracellular traps (NETs) frequent in severe COVID-19 infections (Veras et al., 2020), releasing DNA with histones that activate platelets and the direct coagulation activating clotting factor XII (Hernández-Huerta et al., 2021). Furthermore, NETs release neutrophil elastase and myeloperoxidase (MPO) that cleave and inactivate natural anticoagulants to promote microvascular bleeding (Goshua et al., 2020; Bonaventura et al., 2021; Fernández et al., 2022). The induction of NETS also comes from SARS-CoV-2 binding to angiotensin-converting enzyme-2 as a viral receptor, increasing the production of reactive oxygen species that decreases vaso protective nitric oxide and prostacyclin, inducing endothelial cell damage, dysfunction, and finally apoptosis (Kuriakose et al., 2021). Furthermore, the release of involved proinflammatory and prothrombotic factors leads to more vascular inflammation, platelet aggregation, and thrombosis (Fodor et al., 2021).
In LCS, there may be persistent microclots and hyperactivated platelets perpetuating coagulation and vascular pathology, resulting in cells not getting enough oxygen in the tissues to sustain bodily functions, causing a variety of possible symptoms. Widespread hypoxia may be central to the numerous reported debilitating symptoms of LCS; including those in the CNS. This can be indicated by elevated biomarkers such as persistently elevated D-dimer and C reactive proteins (Mandal et al., 2020) in LCS often due to fibrinolytic-resistant microclots (Grobbelaar et al., 2021; Pretorius et al., 2021).
Additionally, SARS-CoV-2 viral proteins can directly affect vascular permeability. Interestingly, the tight junction microvascular proteins cadherin-5, ZO-1, and β-catenin, that in spinal cord injury are positively affected by the therapeutic healing mediated by MSC-sEV-released M2 macrophage-derived secondary sEVs (Nakazaki et al., 2021), are affected by four different SARS-CoV-2 proteins likely participating in endothelial dysfunction (Hui Shi et al., 2022).
Thus, autopsy studies conducted during active COVID-19 infections repeatedly confirm the importance of endotheliopathy dominance in the absence of active viral infections. This endothelial centric pathogenesis conceptually stimulates several possible pathways whereby vascular injury of the acute infection can cause neurologic defects to possibly carry over to account for the CNS defects of LCS that we propose to treat by the CNS actions of nasal-administered MSC-derived sEVs that likely target macrophages/microglia, and secondarily the local microvasculature (Nakazaki et al., 2021).
Stroke and traumatic brain injuries trigger CNS inflammation that exacerbates brain damage. Several preclinical studies of stroke models of the focal cerebral ischemia in rodents have shown that the systemic treatment with MSC-derived EVs reduces this inflammation (Doeppner et al., 2015b). In further stroke studies, these sEVs ameliorated intense responses of CNS microglia and astrocytes, reduced levels of IL-1β (Kim et al., 2016), and cognitive impairment (Wang et al., 2022). In other studies, like the transient middle cerebral artery occlusion with reperfusion, MSC-derived sEVs significantly decreased the infarct volume and neuronal injury, decreased brain infiltration of neutrophils, monocytes/macrophages, and lymphocytes, and reduced blood–brain barrier permeability (Wang et al., 2020).
Another prominent power of the healing actions of MSC-sEVs in ischemic stroke is the promotion of cerebral angiogenesis (Xin et al., 2017; Zhang et al., 2019). This is especially true when employing sEVs harvested from MSCs cultured under hypoxia (1 % O2) that act via the transfer of distinct sets of upregulated pro-angiogenic miRNAs and via the downregulation of others (Gong et al., 2017; Baruah and Wary, 2020), along with differentially abundant EV proteins (Gregorius et al., 2021). Such pro-angiogenic sEVs are able to act positively on endothelium in vitro (Shabbir et al., 2015). These positive effects of MSC-EVs on vessels in models of stroke encourage our contention that these EVs will be useful in LCS where vascular lesions may dominate.
Of course, MSC are not monoclonal and contain multiple miRNAs in diverse EVs, so such complex possible positive effects in models of stroke are not unexpected (Liu et al., 2021a; Liu et al., 2022). They do show that there may be multiple pathways by which the MSC-EVs can contribute to the healing of neurologic and vascular lesions in the complex entity that is stroke and certainly LCS. Furthermore, these results likely reflect a major seemingly chaotic aspect of this field given that an individual miRNA can affect the function of multiple mRNAs and a given mRNA can be affected by multiple miRNAs (Pillai, 2005; Bartel, 2009; Wu et al., 2010). Thus, similar diverse results should be expected in the MSC EV nasal treatment of the pathological neuro and vascular lesions of the CNS in patients with LCS.
Studies of the MSC-EV treatment of stroke and spinal cord injury offer interesting data about how such a treatment affects severe neuro pathologies. However, it is doubtful that these pathologies relate to LCS. We actually do not yet have detailed data on the anatomic and molecular pathologies of the brains of patients with LCS. Of potential of having great pertinence, there is a long history of treatment of Alzheimer’s animal models with MSC-sEV. Here, following initial dozens of articles demonstrating the value of MSC themselves, likely due to their endogenous production of therapeutic EVs, in the last few years there have been many follow-up studies with MSC-derived sEVs successfully treating Alzheimer’s models, showing that this is highly effective and this is most relevent to our recommendation of this therapy in LCS.
In one such study, human MSC-sEV injection repaired cognitive disfunctions, helped to clear Aβ protein deposits, modulated the activation of microglia to alleviate neuroinflammation, and in vitro alternatively activated microglia from M1 to the M2-type (Ding et al., 2018b). Another systemic treatment with MSC-EVs showed reduced β-amyloid plaque expression and restored expression of neuronal memory/synaptic plasticity-related genes. In a related in vitro study, a human neural cell culture model with familial Alzheimer’s mutations was established and co-cultured with purified MSC-EVs. The uptake of 18F-deoxy-glucose as an objective measure of the brain glucose metabolism, PET imaging, and cognitive function improved significantly in AD transgenic mice (Chen et al., 2021). Yet, in another study of a murine Alzheimer’s model receiving intravenous MSC-EVs, there were improved cognitive impairments and reduced hippocampal Αβ aggregates and neuronal loss, restored brain electrical activities, as well as favorable mitochondrial changes (Wang et al., 2021).
Pertinent to our therapy proposal for LCS, some studies of the MSC-EV treatment of stroke have employed nasal rather than systemic treatment. These successful nasal administrations for stroke models thus provide strong evidence that our protocol of nasal treatment with the MSC-EVs of patients with significant LCS would be an effective approach. Thus, in very similar Alzheimer’s disease models where the MSC-EVs were delivered intranasally, the MSC-EVs exerted powerful neuroprotective effects on the Aβ1-42 oligomer or glutamate-induced neuronal toxicity, effectively ameliorating the neurologic damage in the whole brain, remarkably increasing newborn neurons and powerfully rescuing memory deficits in APP/PS1 transgenic mice. Following this nasal MSC-EV treatment, a proteomic analysis showed that these EVs contained multiple proteins possessing neuroprotective and neurogenesis activities, and neuronal RNA sequencing showed genes enriched in neuroprotection and neurogenesis (Ma et al., 2020b).
Yet in other studies employing the nasal administration of MSC-sEV for Alzheimer’s disease models in mice, there were improvements in cognitive impairments, reduced hippocampal Aβ aggregates, and favorable mitochondrial changes, along with a dampened activation of the microglia and increased density of dendritic spines; structures in the brain that provide cognitive resilience (Losurdo et al., 2020b; Cone et al., 2021b). Observed effects were achieved by only two intranasal injections of MSC-EVs delivered just hours apart and were evident threeweeks later; possibly because the EVs delivered intranasally could reach higher levels than those delivered by other methods, again in Alzheimer’s disease models in mice (Losurdo et al., 2020b; Cone et al., 2021b). These multiple favorable studies of Alzheimer’s disease models in mice treated with nasal MSC-EVs bode well for the prospects of this therapeutic approach for LCS that has many overlaps with the involved pertinent pathogenic mechanisms. Obtaining greater effects by repeated intranasal injections needs to be undertaken for LCS.
Similar to the autopsy and spinal fluid findings described previously, a comprehensive molecular analysis of RNAomes in tissues from the brains of patients who died of COVID-19 acute infections reveals extensive signs of inflammation and neurodegeneration, but there is no sign of the causative virus. Using single-cell RNA sequencing, the activation levels of the transcriptomes of thousands of genes in each of the 65,309 individual cells taken from the brain-tissue samples of the COVID-19 patients and the controls were examined. Activation levels of hundreds of genes in all major cell types differed in the COVID-19 patients’ brains vs. controls. Many of these genes were associated with inflammatory processes and degenerative neuro diseases such as Alzheimer’s and Parkinson’s diseases (Yang et al., 2021; Reiken et al., 2022). The findings may help explain the neurological problems of patients with LCS. Note that acutely, about one-third of individuals hospitalized for COVID-19 reported symptoms of fuzzy thinking, forgetfulness, difficulty in concentrating, and depression and that these become major difficulties of individuals with LCS.
Important related findings were of numerous Alzheimer’s-like focal β-amyloid deposits without the characteristics of amyloid plaques of Alzheimer’s disease in brains of young dead COVID patients’ under 60 years of age, correlating with hypoxia in the neocortex, but the absence of other Alzheimer-type changes that may be precursors of amyloid plaques (Harker Rhodes et al., 2021). A similar examination of brains of patients dying of acute respiratory distress before the COVID era had similar deposits. This suggested that these findings are not unique to COVID-19, but relate to hypoxia (Harker Rhodes et al., 2021). Another study confirmed beta-amyloid aggregation and plaque formation, tauopathy, neuroinflammation, and cell death. Furthermore, SARS-CoV-2 was shown to invade the brain’s cognitive centers, triggering Alzheimer’s-like gene programs in healthy neurons and exacerbating such Alzheimer’s neuropathology in existing patients (Shen et al., 2021). Thus, SARS-CoV-2 can invade the cognitive centers of the brain and induce Alzheimer’s -like neuropathology or enhance Alzheimer’s -like neuropathology. These carried on to LCS may be susceptible to nasal therapy with MSC-derived sEVs as they likely have similar abnormalities in animal models of Alzheimer’s disease.
Microglia are nervous system tissue‐resident macrophages that demonstrate similar and unique features regarding M1/M2-type phenotype polarization. Microglia can be stimulated by LPS or the IFN‐γ to M1- phenotype for expression of pro‐inflammatory cytokines or instead by IL‐4/IL‐13 to an M2 phenotype for resolution of inflammation and tissue repair. In a rat middle cerebral artery occlusion and reperfusion stroke model, treatment with MSC-sEVs significantly improved motor, learning, and memory abilities. The intake of these EVs into microglia was visualized through immunofluorescence staining and MSC-sEV-treatment significantly inhibited M1 microglia polarization and increased M2 microglia cells. The production of pro-inflammatory factors decreased, while the anti-inflammatory cytokines and neurotrophic factors increased, both in the cortex and hippocampus of the ischemic hemisphere as well as in the in vitro culture supernatant of microglia. Furthermore, Western blot analysis demonstrated that M1-polarizing CysLT2R expression and ERK1/2 phosphorylation were downregulated both in vivo and in vitro (Zhao et al., 2020).Therefore, MSC-sEv attenuated brain injury and inhibited microglial inflammation by reversing microglia M1 polarization to healing M2-type that should be a powerful aspect of the benefit of nasal administered MSV-EVs (Nakazaki et al., 2021) that we are recommending for therapy of patients with LCS.
Another study in this system confirmed the neuroprotective effects of MSC-sEVs by similarly modulating microglial polarization, associated with the attenuation of NLRP3 inflammasome-dependent pyroptosis and apoptosis, dose dependently reducing the brain infarct area and water content, and improving the neurological function up to 5 weeks after stroke (Liu et al., 2021b). These results were replicated in a spinal cord injury model confirming that MSC-sEVs promote the functional behavioral recovery by shifting microglial polarization from M1 to M2 in vivo and in vitro (Nakazaki et al., 2021). Furthermore, shuttling of miRNA-216a-5p by these sMSC-EVs was involved and TLR4 was identified as the targeted downstream gene (Liu et al., 2020).
Additionally, in mouse models of stroke such MSC sEV therapy, it also regulated the polarization of microglia, increasing the repair-promoting M2-type and decreasing the pro-inflammatory M1-type via transferring miRNAs acting on STAT1 and PTEN downstream-signaling targets. Furthermore, there was reduced volume of brain atrophy, improved neuromotor and cognitive functions, as well as an attenuated loss of oligodendrocytes, and importantly, there was increased vascular endothelium in the peri-ischemia area (Hu et al., 2022).
Microglia are central players of CNS homeostasis and inflammatory response in COVID-19 that exert their crucial functions in coordination with other CNS cells. This indicates that SARS-CoV-2 likely directly infects and/or immunologically affects human microglia, eliciting M1-like pro-inflammatory responses, leading to other neurological complications featuring the activation of astrocytes and T-lymphocytes, and further disturbing the blood–brain barrier; all together causing neuronal damage and death.
By targeting and prospering M2 healing and trophic macrophages, therapy with MSC-sEVs results in restoring balance by dampening the effects of pathogenic M1-leaning microglia in neurological abnormalities of COVID-19 patients, and likely in LCS as well. Our studies of spinal cord injury in rats showed definitively that MSC sEVs very specifically target M2 macrophages at the site of injury. This stimulates their maturation, proliferation, production of inhibitory TGF-β, and most importantly induces them to produce secondary trophic sEVs that target the local microvasculature. This induces further healing and decline in the altered vascular permeability by activating genes encoding intercellular adhesive molecules (Nakazaki et al., 2021) that together would be quite desirable in improving the neuro function in LCS patients.
Our recent studies of spinal cord injury in rats showed that MSC-derived sEV therapy leads to secondary suppressive EVs derived at the site of neuro injury from a specifically targeted healing subpopulation of M2-type macrophages, expressing the M2 marker CD206, and not the M1 marker iNOS (Lankford et al., 2018). Furthermore, these targeted M2-macs produce local healing, anti-inflammatory, and immune suppressive TGF-β to positively influence the local endothelium targeted by their locally secreted secondary EVs (Nakazaki et al., 2021) (Figure 1).
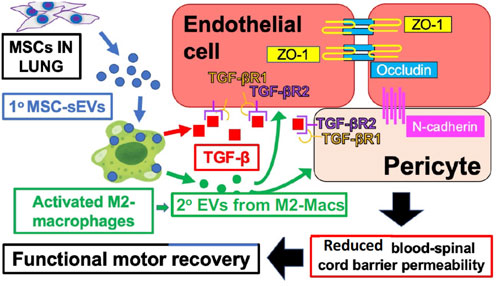
FIGURE 1. IV-injected primary MSC‐sEVs specifically target M2-type macrophages at the site of spinal cord injury, inducing secretion of healing TGF‐β and production of secondary M2-derived sEVs with surface TGF‐β to induce and bind TGF‐βR2 on local vascular cells, and inducing the production of endothelial cell junctional proteins to restore the blood–spinal cord barrier integrity.
Figure 1 shows the proposed mechanisms underlying the therapeutic effects of MSC‐sEVs in spinal cord injury. Circulating MSC‐sEVs [primary (1°)] MSC‐sEVs: blue circles produced either by the release of sEVs from IV-infused MSCs trapped in the lungs or by repeated IV dosing three times with derived MSC‐sEVs, are quite specifically taken up by M2-type macrophages in the spinal cord lesion. sEV uptake by a M2 macrophage subpopulation promotes an increased production of TGFβ (red squares) and sustained M2 polarization. Furthermore, there is induced TGFβ and/or TGFβ surface expressing secondary sEVs (2° sEVs) released by the MSC‐sEV‐stimulated spinal cord infiltrating M2 macrophages (green circles) that then bind to the induced TGF‐βR2 on local vascular endothelial cells and/or pericytes. Complexing with TGF‐βR1 is via enhanced avidity due to a surface array of TGF-β to activate downstream endothelial cell pathways, that lead to the upregulation of three vascular junctional proteins (ZO‐1, occludin, and N‐cadherin) contributing to the restoration of the blood–spinal cord barrier’s integrity. This vascular restoration provides a more favorable environment for neuronal functions and promotes greater recovery of locomotor activities.
There are three unique findings. Firstly, the quantative effects of treatment with MSC alone were compared to the treatment with their released EVs. We considered 2 × 106 MSCs as their IV dose and the sEV output in 24hrs of culture as the sEV dose of 2.5 × 109 nano vesicles (4.6 ± 0.5 µg protein). However, at this dose, the EVs did not significantly improve the locomotion effects attained with the MSC that could be achieved by three doses of the EV over 3 days (Nakazaki et al., 2021).
Secondly, these primary injected MSC-derived sEVs specifically targeted M2-type macrophages in the injured cord (Lankford et al., 2018) to in turn produce M2-Mac-derived endogenous secondary EVs that targeted the loal endothelium. Crucially, these secondary EVs likely transfer miRNAs that favorably alter the genetic programming of their targeted endothelium (Nakazaki et al., 2021).
Thirdly, these 2° Mac-2 EVs induce the synthesis of downstream microvascular proteins to favorably alter the recipient local endothelial cells and pericytes to express not only TGF-β receptors, but also tight adheren junction mRNAs and their corresponding occluden proteins such as zona occludence protein-1 and N-cadherin (Nakazaki et al., 2021).
This results in microvascular stabilization by tightening the occluding cell surfaces of the local endothelial cells, to seal the pathway of intercellular junctional complexes consisting of multiple proteins whose canonical function is to prevent vascular leakage. Thus, there was consequent vascular functional recovery demonstrated by reduced permeability and clearing of pathology (Nakazaki et al., 2021). These secondary sEVs can have a surface array of TGF-β that stimulates the induced neighboring microvasculature endothelial cell TGF-β receptors and pericytes resulting in prolonged targeted cell signaling compared to free-TGFβ-1 (Shelke et al., 2019). These unique local microvascular aspects of spinal cord healing by MSC-sEV therapy might also apply to the treatment of Long COVID neuro/psychiatric patients receiving these nasally applied sEVs.
A variety of studies have demonstrated the benefits of MSC therapy with the MSC in active COVID-19 infections (Kaushal et al., 2020; Yang et al., 2020; Hashemian et al., 2021; Kouroupis et al., 2021; Lanzoni et al., 2021). The next completed level has been a few favorable double-blind, randomized, controlled trials of therapy with MSC alone in active COVID-19 infections (Payares-Herrera et al., 2021; Shi et al., 2021). A recent strictly controlled use of the standard bone marrow-derived MSC therapy in severe COVID-19 pneumonia produced very strong data of efficacy (Grégoire et al., 2022). MSC infusions were tolerated well without adverse effects. Survival was significantly higher at 100 % in the MSC-treated group vs. 71 % in the controls at 60 days (p = 0.0082), as well as equally strongly reduced D-dimer levels. This anti-COVID infectious knowledge was recently added by the demonstration that MSC-EV treatment of SARS-CoV-2-infected human lung epithelial cells suppresses viral replication and mitigates the production/release of infectious virions (Chutipongtanate et al., 2022). All together, these strong data suggest that if residual viruses or viral antigens are driving LCS, which has been reported (Zuo et al., 2021; Cheung et al., 2022; Das et al., 2022; Natarajan et al., 2022; Tejerina et al., 2022), the nasal MSC EVs will be an appropriate efficacious therapy for LCS.
Therefore, there now is the obvious need for further trials employing MSC-secreted sEVs for severe active infections. Recently, there was a prospective non-randomized open-label cohort study of a commercial EV agent derived from 24 SARS-CoV-2 PCR-pos patients at a single hospital center (Sengupta et al., 2020). The patients were injected IV with this agent and monitored daily for 14 days. There were no adverse events and most patients recovered (83 % survival) with improved oxygenation (192 %), decreased blood marker neutrophils (32 %), C Reactive Protein (CRP), ferritin, and fibrin d-dimer (42 %–77 %), with increased lymphocyte counts (46 %); all statistically significant (Sengupta et al., 2020).
Although this study involved an undocumented extracellular vesicle product with no details per the actual composition (Lim et al., 2020), it demonstrated the potential safety and efficacy of BM-MSC-derived sEVs in COVID pneumonia, that may therefore be a promising therapeutic approach for late COVID. This would be after satisfying the cautions of international societies and FDA approval. A subsequent article provided the details about this commercial EV agent derived from allogeneic bone marrow MSC that were requested by experts (Sengupta et al., 2021).
In a similar study, eight subjects experiencing mild-to-moderate COVID-19 infections were treated with another experimental commercial product of sEVs from amniotic fluid-derived MSCs (positive for CD63, CD81, and CD9) (Bellio et al., 2021a). This product was from sterile endotoxin low-cultured sEVs given systemically at 2.63 × 1011/ml. It was administered as a suppressor of cytokine activation to reduce COVID-19 infection severity by targeting TNF-α, IL-6, IL-8, and other associated immune response genes via its carried miRNAs (Bellio et al., 2021a). This is one of a variety of registered patient MSC-derived sEV treatment protocols for COVID-19 lung diseases employing that there are among a variety of 197 current MSC protocols listed at www.clinicaltrials.gov (its identifier: NCT04384445).
Here again, there were no adverse events and all COVID-19-associated symptoms were resolved; including: fatigue, cough, shortness of breath, chest x-rays, inflammatory biomarkers (CRP, IL-6, TNF-α), and absolute lymphocyte counts (Bellio et al., 2021a). Findings from this proof-of-concept, expanded the access trial for treatment of significant COVID-19 infections showing safety and effective prevention of COVID-19 disease progression.
All together, these properties demonstrate the therapeutic potential of MSC-EVs as a suppressor of cytokine activation for the reduction of clinical manifestations of COVID-19 infection, and perhaps the infection itself, with severe respiratory failure, and might indicate one pathway for the treatment of long COVID due to residual viruses or viral antigens.
So far, COVID long haulers receive little to no guidance from physicians with almost no treatment options available. A commercial MSC-derived sEV product of the human amniotic fluid is beginning to be investigated for long COVID therapy. Initially, FDA and IRB approvals were obtained to investigate the therapeutic use of this commercial MSC-derived EV product in a single long hauler patient experiencing prolonged shortness of breath and respiratory impairment in an uncontrolled manner to start.
In careful preparatory testing, this group first used this EV product to successfully treat an experimental model of bronchopulmonary dysplasia (Bellio et al., 2021a). Then, in a first move to LCS patients, they published a case report using these amniotic fluid-derived nanoparticles, said to contain extracellular vesicles and exosomes, in three severely ill COVID-19 patients suffering from severe, multi-organ complications induced by the COVID-19 infection. In this initial uncontrolled clinical trial, all patients had been diagnosed with COVID-19, developed respiratory failure, and were hospitalized for more than 40 days. All the patients showed amelioration in the ICU clinical status with objectively determined respiratory improvements, resolution of acute delirium, and diminution of elevated inflammatory biomarkers (Mitrani et al., 2021a; Bellio et al., 2021b).
Finally, in a subsequent uncontrolled use of this commercial MSC-derived EV product in a single long hauler patient experiencing prolonged shortness of breath and respiratory impairment, intravenous and multi-dose administrations of this agent were safe and well-tolerated without any reported serious adverse events. The patient demonstrated respiratory improvements in chest X-rays and measured oxygen saturation. This long hauler patient had become infected with SARS-CoV-2 two months prior to treatment and suffered from chronic respiratory distress with continuing bilateral pneumonia and shortness of breath. There had been no elevation in the blood laboratory results or in the inflammatory biomarkers linked to these persistent symptoms. The patient began to experience improvements in shortness of breath early in this MSC-EV treatment and continued to improve throughout. By conclusion of the study, the patient returned to normal with no respiratory impairments (Mitrani et al., 2021b).
The advancement represented by this admittedly single patient study of a commercial MSC-derived sEV product derived from human amniotic fluid for the treatment of severe chronic LCS demonstrated the potential therapeutic potential of MSC-EVs in Long COVID, for scientific biologic treatment of patients, beyond use of MSC themselves. Recently, the company announced a U.S. FDA approval of a much needed double-blinded, placebo-controlled, randomized phase I/II trial to investigate the safety and potential efficacy of this MSC-EV product in treating COVID-19 long haulers. It is hoped that this initial success with respiratory dominant long COVID will lead to similar trials of such therapy in neuro/psychiatric-dominant LCS that could eventually lead to trials of nasal treatment with similar MSC-EVs.
LCS frequently are neuro vascular diseases. We recommended appropriate therapy with nasal-administered MSC-derived sEVs when able to meet the recommendations of the ISEV (Witwer et al., 2019) and the FDA, as well as meeting the challenges for their clinical use (Mendt et al., 2019; Gowen et al., 2020). MSC are not stem cells but stromal cells with diverse healing properties in a variety of illnesses and injuries in multiple organs; including the CNS, and importantly in neuro psychiatric syndromes such as Alzheimer’s and Parkinson’s diseases that have parallel aspects with LCS. MSC-sEVs are like broadly effective corticosteroids with diverse polyvalent positive properties when given acutely in hyper physiological doses, but the results of MSC-sEV chronic use are not yet clear. With some times inducing a prominent role of TGF-β in the healing processes, clinical care will be needed to carefully judge the treatment doses and duration of MSC-sEV therapy so that there does not develop excessive fibrosis, along with other unwanted side-effects.
The key to efficient use of MSC-sEV therapy for LCS is the direct approach to the involved CNS by administration via the nasal route enabling the physiologic passage of the blood–brain barrier of numerous EV subgroups to target elements of dominant cerebrovascular vascular diseases involving the micro vessels. Furthermore, MSC-sEV therapy particularly targets macrophage-type cells; especially microglia in the altered nervous system of LCS patients that often subsequently lead to the stimulation of neighboring micro vascular cells via macrophage/microglial release of secondary healing/trophic sEVs that positively affect vascular junctions, reduce vascular permeability, and restore vascular functions.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Yale medical Animal Care Committee.
PA entirely contributed to this manuscript.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ab, antibody; Ag, antigen; APC, antigen presenting cell; BBB, blood brain barrier; CNS, central nervous system; COVID-19, Coronavirus Sars-CoV-2-induced clinical processes; DC, dendritic cell; EV, extracellular vesicle; IV, intravenous; LCS, Long COVID Syndromes; miRNA, micro (small) RNA; mRNA, messenger RNA; MSC, Mesenchymal Stromal Cells; MSC-sEVs, MSC-derived small EVs; sEVs, small EVs that includes exosomes; SARS-CoV-2 virus, induces COVID-19 processes; TGF-β, transforming growth factor-beta; TLR, toll like receptors; TNF-α, tumor necrosis factor-alpha.
Alessio, N., Brigida, A. L., Peluso, G., Antonucci, N., Galderisi, U., and Siniscalco, D. (2020). Stem cell-derived exosomes in autism spectrum disorder. Int. J. Environ. Res. Public Health 17 (3), 944. doi:10.3390/ijerph17030944
Alvarez-Erviti, L., Seow, Y., Yin, H., Betts, C., Lakhal, S., and Wood, M. J. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29 (4), 341–345. doi:10.1038/nbt.1807
AmandaHuber, K. David A. Giles, Segal, Benjamin M., and Irani, David N. (2018). An emerging role for eotaxins in neurodegenerative disease. Clin. Immunol. 189, 29–33. doi:10.1016/j.clim.2016.09.010
András, I. E., and Toborek, M. (2015). Extracellular vesicles of the blood-brain barrier. Tissue Barriers 4 (1), e1131804. doi:10.1080/21688370.2015.1131804
Apple, A. C., Oddi, A., Peluso, M. J., Asken, B. M., Henrich, T. J., Kelly, J. D., et al. (2022). Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. Ann. Clin. Transl. Neurol. 9 (2), 221–226. doi:10.1002/acn3.51498
Arora, P., Sharma, S., and Garg, S. (2002). Permeability issues in nasal drug delivery. Drug Discov. Today 7, 967–975. doi:10.1016/s1359-6446(02)02452-2
Askenase, P. W. (2020). COVID-19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: Do the exosomes in convalescent plasma antagonize the weak immune antibodies? J. Extracell. Vesicles 10 (1), e12004. doi:10.1002/jev2.12004
Banks, W. A., Sharma, P., Bullock, K. M., Hansen, K. M., Ludwig, N., and Whiteside, T. L. (2020). Transport of extracellular vesicles across the blood-brain barrier: Brain pharmacokinetics and effects of inflammation. Int. J. Mol. Sci. 21 (12), 4407. doi:10.3390/ijms21124407
Baruah, J., and Wary, K. K. (2020). Exosomes in the regulation of vascular endothelial cell regeneration. Front. Cell. Dev. Biol. 7, 353. doi:10.3389/fcell.2019.00353
Bellio, M. A., Bennett, C., Arango, A., Khan, A., Xu, X., Barrera, C., et al. (2021). Proof-of-concept trial of an amniotic fluid-derived extracellular vesicle biologic for treating high risk patients with mild-to-moderate acute COVID-19 infection. Biomater. Biosyst. 4, 100031. doi:10.1016/j.bbiosy.2021.100031
Bellio, M. A., Young, K. C., Milberg, J., Santos, I., Abdullah, Z., Stewart, D., et al. (2021). Amniotic fluid-derived extracellular vesicles: Characterization and therapeutic efficacy in an experimental model of bronchopulmonary dysplasia. Cytotherapy 23 (12), 1097–1107. doi:10.1016/j.jcyt.2021.07.011
Bernard-Valnet, R., Perriot, S., Canales, M., Pizzarotti, B., Caranzano, L., Castro-Jiménez, M., et al. (2021). Encephalopathies associated with severe COVID-19 present neurovascular unit alterations without evidence for strong neuroinflammation. Neurol. Neuroimmunol. Neuroinflamm. 8 (5), e1029. doi:10.1212/nxi.0000000000001029
Betzer, O., Perets, N., Angel, A., Motiei, M., Sadan, T., Yadid, G., et al. (2017). In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano 11, 10883–10893. doi:10.1021/acsnano.7b04495
Bhadelia, N., Belkina, A. C., Olson, A., Winters, T., Urick, P., Lin, N., et al. (2021). Distinct autoimmune antibody signatures between hospitalized acute COVID-19 patients, SARS-CoV- 2 convalescent individuals, and unexposed pre-pandemic controls. medRxiv preprint. doi:10.1101/2021.01.21.21249176
Bonafede, R., Scambi, I., Peroni, D., Potrich, V., Boschi, F., Benati, D., et al. (2016). Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp. Cell. Res. 340, 150–158. doi:10.1016/j.yexcr.2015.12.009
Bonaventura, A., Vecchié, A., Dagna, L., Martinod, K., Dixon, D. L., Van Tassell, B. W., et al. (2021). Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 21, 319–329. doi:10.1038/s41577-021-00536-9
Borah, P., Deb, P. K., Chandrasekaran, B., Goyal, M., Bansal, M., Hussain, S., et al. (2021). Neurological consequences of SARS-CoV-2 infection and concurrence of treatment-induced neuropsychiatric adverse events in COVID-19 patients: Navigating the uncharted. Front. Mol. Biosci. 8, 627723. doi:10.3389/fmolb.2021.627723
Cañas, C. A., Cañas, F., Bautista-Vargas, M., and Bonilla-Abadía, F. (2021). Role of tissue factor in the pathogenesis of COVID-19 and the possible ways to inhibit it. Clin. Appl. Thromb. Hemost. 27, 107602962110039. doi:10.1177/10760296211003983
Carfì, A., Bernabei, R., Landi, F., et al. (2020). Persistent symptoms in patients after acute COVID-19. JAMA 324, 603–605. doi:10.1001/jama.2020.12603
Castellano, A., Anzalone, N., Pontesilli, S., Fominskiy, E., and Falini, A. (2020). Pathological brain CT scans in severe COVID-19 ICU patients. Intensive Care Med. 46 (11), 2102–2104. doi:10.1007/s00134-020-06222-z
Charfeddine, S., Ibn Hadj Amor, H., Jdidi, J., Torjmen, S., Kraiem, S., Hammami, R., et al. (2021). Long COVID 19 syndrome: Is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front. Cardiovasc. Med. 8, 745758. doi:10.3389/fcvm.2021.745758
Chen, H. X., Liang, F. C., Gu, P., Xu, B. L., Xu, H. J., Wang, W. T., et al. (2020). Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell. Death Dis. 11, 288. doi:10.1038/s41419-020-2473-5
Chen, K. H., Chen, C. H., Wallace, C. G., Yuen, C. M., Kao, G. S., Chen, Y. L., et al. (2016). Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 7, 74537–74556. doi:10.18632/oncotarget.12902
Chen, Y. A., Lu, C. H., Ke, C. C., Chiu, S. J., Jeng, F. S., Chang, C. W., et al. (2021). Mesenchymal stem cell-derived exosomes ameliorate Alzheimer's disease pathology and improve cognitive deficits. Biomedicines 9 (6), 594. doi:10.3390/biomedicines9060594
Cheung, C. C. L., Goh, D., Lim, X., Tien, T. Z., Lim, J. C. T., Lee, J. N., et al. (2022). Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 71 (1), 226–229. doi:10.1136/gutjnl-2021-324280
Christensen, R. H., and Berg, R. M. G. (2021). Vascular inflammation as a therapeutic target in COVID-19 "long haulers": HIITing the spot? Front. Cardiovasc. Med. 8, 643626. doi:10.3389/fcvm.2021.643626
Chutipongtanate, S., Kongsomros, S., Pongsakul, N., Panachan, J., Khowawisetsut, L., Pattanapanyasat, K., et al. (2022). Anti-SARS-CoV-2 effect of extracellular vesicles released from mesenchymal stem cells. J. Extracell. Vesicles 11 (3), e12201. doi:10.1002/jev2.12201
Cone, A. S., Yuan, X., Sun, L., Duke, L. C., Vreones, M. P., Carrier, A. N., et al. (2021). Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer's disease-like phenotypes in a preclinical mouse model. Theranostics 11 (17), 8129–8142. doi:10.7150/thno.62069
Cone, A. S., Yuan, X., Sun, L., Duke, L. C., Vreones, M. P., Carrier, A. N., et al. (2021). Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer's disease-like phenotypes in a preclinical mouse model. Theranostics 11 (17), 8129–8142. doi:10.7150/thno.62069
Das, A., Vidyarthi, A. J., Khan, S., Singh, S., Bala, K., Wundavalli, L., et al. (2022). Persistence of SARS-CoV-2 in COVID-19 patients during the second wave of the pandemic in India. J. Infect. Dev. Ctries. 16 (6), 959–965. doi:10.3855/jidc.15937
Davis, H. E., Assaf, G. S., McCorkell, L., Wei, H., Low, R. J., Re'em, Y., et al. (2021). Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38, 101019. doi:10.1016/j.eclinm.2021.101019
de Miranda, A. S., Brant, F., Campos, A. C., Vieira, L. B., Rocha, N. P., Cisalpino, D., et al. (2015). Evidence for the contribution of adult neurogenesis and hippocampal cell death in experimental cerebral malaria cognitive outcome. Neuroscience 284, 920–933. doi:10.1016/j.neuroscience.2014.10.062
Di Rocco, G., Baldari, S., and Toietta, G. (2016). Towards therapeutic delivery of extracellular vesicles: Strategies for in vivo tracking and biodistribution analysis. Stem Cells Int., 1–12. doi:10.1155/2016/5029619
Ding, M., Shen, Y., Wang, P., Xie, Z., Xu, S., Zhu, Z., et al. (2018). Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer's disease. Neurochem. Res. 43 (11), 2165–2177. doi:10.1007/s11064-018-2641-5
Ding, M., Shen, Y., Wang, P., Xie, Z., Xu, S., Zhu, Z., et al. (2018). Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in alzheimer’s disease. Neurochem. Res. 43, 2165–2177. doi:10.1007/s11064-018-2641-5
Doeppner, T. R., Herz, J., Görgens, A., Schlechter, J., Ludwig, A.-K., Radtke, S., et al. (2015). Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 4, 1131–1143. doi:10.5966/sctm.2015-0078
Doeppner, T. R., Herz, J., Gorgens, A., Schlechter, J., Ludwig, A. K., Radtke, S., et al. (2015). Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 4, 1131–1143. doi:10.5966/sctm.2015-0078
Drommelschmidt, K., Serdar, M., Bendix, I., Herz, J., Bertling, F., Prager, S., et al. (2017). Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav. Immun. 60, 220–232. doi:10.1016/j.bbi.2016.11.011
Edén, A., Kanberg, N., Gostner, J., Fuchs, D., Hagberg, L., Andersson, L. M., et al. (2021). CSF biomarkers in patients with COVID-19 and neurologic symptoms: A case series. Neurology 96 (2), e294–e300. doi:10.1212/WNL.0000000000010977
Elia, C. A., Tamborini, M., Rasile, M., Desiato, G., Marchetti, S., Swuec, P., et al. (2019)., 8. Cells, 1059. doi:10.3390/cells8091059Intracerebral injection of extracellular vesicles from mesenchymal stem cells exerts reduced Aβ plaque burden in early stages of a preclinical model of alzheimer’s diseaseCells
Evans, R. A., McAuley, H., Harrison, E. M., Shikotra, Aa, Singapuri, A., Sereno, M., et al. (2021). Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet. Respir. Med. 9, 1275–1287. doi:10.1016/S2213-2600(21)00383-0
Fathollahi, A., Hashemi, S. M., Molla Hoseini, HajiM., Tavakoli, S., Farahani, E., and Yeganeh, F. (2021). Intranasal administration of small extracellular vesicles derived from mesenchymal stem cells ameliorated the experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 90, 107207. doi:10.1016/j.intimp.2020.107207
Fernández, S., Moreno-Castaño, A. B., Palomo, M., Martinez-Sanchez, J., Torramade-Moix, S., Tellez, A., et al. (2022). Distinctive biomarker features in the endotheliopathy of COVID-19 and septic syndromes. Shock 57 (1), 95–105. doi:10.1097/shk.0000000000001823
Fernández-Castañeda, A., Lu, P., Geraghty, A. C., Song, E., Lee, M. H., Wood, J., et al. (20222022). etc. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. bioRxiv [Preprint]. doi:10.1101/2022.01.07.475453
Fodor, A., Tiperciuc, B., Login, C., Orasan, O. H., Lazar, A. L., Buchman, C., et al. (2021). Endothelial dysfunction, inflammation, and oxidative stress in COVID-19-mechanisms and therapeutic targets. Oxid. Med. Cell. Longev. 2021, 8671713. doi:10.1155/2021/8671713
Franke, C., Ferse, C., Kreye, J., Reincke, S. M., Sanchez-Sendin, E., Rocco, A., et al. (2021). High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 93, 415–419. doi:10.1016/j.bbi.2020.12.022
Franke, C., Ferse, C., Kreye, J., Reincke, S. M., Sanchez-Sendin, E., Rocco, A., et al. (2021). High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 93, 415–419. doi:10.1016/j.bbi.2020.12.022
Gibson, E. M., and Monje, M. (2021). Microglia in cancer therapy-related cognitive impairment. Trends Neurosci. 44 (6), 441–451. doi:10.1016/j.tins.2021.02.003
Gong, M., Yu, B., Wang, J., Wang, Y., Liu, M., Paul, C., et al. (2017). Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 8 (28), 45200–45212. doi:10.18632/oncotarget.16778
Goshua, G., Pine, A. B., Meizlish, M. L., Chang, C. H., Zhang, H., Bahel, P., et al. (2020). Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet. Haematol. 7 (8), e575–e582. doi:10.1016/S2352-3026(20)30216-7
Gowen, A., Shahjin, F., Chand, S., Odegaard, K. E., and Yelamanchili, S. V. (2020). Mesenchymal stem cell-derived extracellular vesicles: Challenges in clinical applications. Front. Cell. Dev. Biol. 8, 149. doi:10.3389/fcell.2020.00149
Greenhalgh, T., Knight, M., A’Court, C., Buxton, M., and Husain, L. (2020). Management of post-acute COVID-19 in primary care. BMJ 370, m3026. doi:10.1136/bmj.m3026
Grégoire, C., Layios, N., Lambermont, B., Lechanteur, C., Briquet, A., Bettonville, V., et al. (2022). Bone marrow-derived mesenchymal stromal cell therapy in severe COVID-19: Preliminary results of a phase I/II clinical trial. Front. Immunol. 13, 932360. doi:10.3389/fimmu.2022.932360
Gregorius, J., Wang, C., Stambouli, O., Hussner, T., Qi, Y., Tertel, T., et al. (2021). etcSmall extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic Res. Cardiol. 116 (1), 40. doi:10.1007/s00395-021-00881-9
Grobbelaar, L. M., Venter, C., Vlok, M., Ngoepe, M., Laubscher, G. J., Lourens, P. J., et al. (2021). SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19. Biosci. Rep. 41 (8), BSR20210611. doi:10.1042/BSR20210611
Gugliandolo, A., Bramanti, P., and Mazzon, E. (2019). Mesenchymal stem cells: A potential therapeutic approach for amyotrophic lateral sclerosis? Stem Cells Int. 2019, 1–16. doi:10.1155/2019/3675627
Guo, S., Perets, N., Betzer, O., Ben-Shaul, S., Sheinin, A., Michaelevski, I., et al. (2019). Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano 13, 10015–10028. doi:10.1021/acsnano.9b01892
Haney, M. J., Zhao, Y., Jin, Y. S., and Batrakova, E. V. (2020). Extracellular vesicles as drug carriers for enzyme replacement therapy to treat CLN2 batten disease: Optimization of drug administration routes. Cells, 9.
Harker Rhodes, C., Priemer, D. S., Karlovich, E., Daniel, P. P., and Goldman, J. E. (2021). β-Amyloid deposits in young COVID patients. SSRN J. doi:10.2139/ssrn.4003213
Hashemian, S. R., Aliannejad, R., Zarrabi, M., Soleimani, M., Vosough, M., Hosseini, S. E., et al. (2021). Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: A case series. Stem Cell. Res. Ther. 12 (1), 91. doi:10.1186/s13287-021-02165-4
Heesakkers, H., van der Hoeven, J. G., Corsten, S., Janssen, I., Ewalds, E., Simons, K. S., et al. (2022). Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA 327 (6), 559–565. doi:10.1001/jama.2022.0040
Herman, S., Fishel, I., and Offen, D. (2021). Intranasal delivery of mesenchymal stem cells-derived extracellular vesicles for the treatment of neurological diseases. Stem Cells 39 (12), 1589–1600. doi:10.1002/stem.3456
Hernández-Huerta, M. T., Pérez-Santiago, A. D., Pérez-Campos Mayoral, L., Sánchez Navarro, L. M., Rodal Canales, F. J., Majluf-Cruz, A., et al. (2021). Mechanisms of immunothrombosis by SARS-CoV-2. Biomolecules 11 (11), 1550. doi:10.3390/biom11111550
Hu, X., Pan, J., Li, Y., Jiang, Y., Zheng, H., Shi, R., et al. (2022). Extracellular vesicles from adipose-derived stem cells promote microglia M2 polarization and neurological recovery in a mouse model of transient middle cerebral artery occlusion. Stem Cell. Res. Ther. 13 (1), 21. doi:10.1186/s13287-021-02668-0
Hui Shi, M. D., PhDYu Zuo, M. D., Sherwin Navaz, B. S., Alyssa Harbaugh, B. S., Claire, K., Hoy, B. S., et al. (2022). Endothelial cell-activating antibodies in COVID-19. doi:10.1002/art.42094
Jafarinia, M., Alsahebfosoul, F., Salehi, H., Eskandari, N., Azimzadeh, M., Mahmoodi, M., et al. (2020). Therapeutic effects of extracellular vesicles from human adipose‐derived mesenchymal stem cells on chronic experimental autoimmune encephalomyelitis. J. Cell. Physiol. 235, 8779–8790. doi:10.1002/jcp.29721
Jamoulle, M., Kazeneza-Mugisha, G., and Ayoub, Z. (2021). Descriptive and narrative study of long Covid cases in general practice and diagnostic value of single photon emission computed tomography (SPECT scan). doi:10.1101/2022.03.01.22270897
Jarius, S., Pache, F., Körtvelyessy, P., Jelčić, I., Stettner, M., Franciotta, D., et al. (2022). Cerebrospinal fluid findings in COVID-19: A multicenter study of 150 lumbar punctures in 127 patients. J. Neuroinflammation 19 (1), 19. doi:10.1186/s12974-021-02339-0
Jha, N. K., Ojha, S., Jha, S. K., Dureja, H., Singh, S. K., Shukla, S. D., et al. (2021). Evidence of Coronavirus (CoV) pathogenesis and emerging pathogen SARS-CoV-2 in the nervous system: A review on neurological impairments and manifestations. J. Mol. Neurosci. 71 (11), 2192–2209. doi:10.1007/s12031-020-01767-6
Kalafatis, M. (2021). COVID-19: A serious vascular disease with primary symptoms of a respiratory ailment. J. Appl. Laboratory Med. 6 (5), 1099–1104. doi:10.1093/jalm/jfab084
Kaminski, N., Köster, C., Mouloud, Y., Börger, V., Felderhoff-Müser, U., Bendix, I., et al. (2020). Mesenchymal stromal cell-derived extracellular vesicles reduce neuroinflammation, promote neural cell proliferation and improve oligodendrocyte maturation in neonatal hypoxic-ischemic brain injury. Front. Cell. Neurosci. 14, 601176. doi:10.3389/fncel.2020.601176
Kaushal, S., Khan, A., Deatrick, K., Ng, D. K., Snyder, A., Shah, A., et al. (2020). Intravenous mesenchymal stem cells in extracorporeal oxygenation patients with severe COVID-19 acute respiratory distress syndrome. medRxiv. doi:10.1101/2020.10.15.20122523etc
Kim, D. K., Nishida, H., An, S. Y., Shetty, A. K., Bartosh, T. J., and Prockop, D. J. (2016). Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. U. S. A. 113 (1), 170–175. doi:10.1073/pnas.1522297113
Kodali, M., Castro, O. W., Kim, D. K., Thomas, A., Shuai, B., Attaluri, S., et al. (2019). Intranasally administered human MSC-derived extracellular vesicles pervasively incorporate into neurons and microglia in both intact and status epilepticus injured forebrain. Int. J. Mol. Sci. 21 (1), 181. doi:10.3390/ijms21010181
Kordelas, L., Rebmann, V., Ludwig, A. K., Radtke, S., Ruesing, J., Doeppner, T. R., et al. (2014). MSC-Derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28, 970–973. doi:10.1038/leu.2014.41
Kotikalapudi, N., Sampath, S. J. P., Sinha, S. N., Bhonde, R., Mungamuri, S. K., and Venkatesan, V. (2022). Human placental mesenchymal stromal cell therapy restores the cytokine efflux and insulin signaling in the skeletal muscle of obesity-induced type 2 diabetes rat model. Hum. Cell. 35 (2), 557–571. doi:10.1007/s13577-021-00664-3
Kouroupis, D., Lanzoni, G., Linetsky, E., Messinger Cayetano, S., Wishnek Metalonis, S., Leñero, C., et al. (2021). Umbilical cord-derived mesenchymal stem cells modulate TNF and soluble TNF receptor 2 (sTNFR2) in COVID-19 ARDS patients. Eur. Rev. Med. Pharmacol. Sci. 25 (12), 4435–4438. doi:10.26355/eurrev_202106_26156
Kuriakose, J., Montezano, A. C., and Touyz, R. M. (2021). ACE2/Ang-(1-7)/Mas1 axis and the vascular system: Vasoprotection to COVID-19-associated vascular disease. Clin. Sci. 135 (2), 387–407. doi:10.1042/CS20200480
Lai, C. P., Mardini, O., Ericsson, M., Prabhakar, S., Maguire, C., Chen, J. W., et al. (2014). Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8, 483–494. doi:10.1021/nn404945r
Lankford, Karen L., Arroyo, E. J., Nazimek, K., Bryniarski, K., Askenase, P. W., and Kocsis, J. D. (2018). Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One 13 (1), e0190358. doi:10.1371/journal.pone.0190358
Lanzoni, G., Linetsky, E., Correa, D., Messinger Cayetano, S., Alvarez, R. A., Kouroupis, D., et al. (2021). Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 10, 660–673. doi:10.1002/sctm.20-0472
Laso-Garcia, F., Ramos-Cejudo, J., Carrillo-Salinas, F. J., Otero-Ortega, L., Feliu, A., Gomez-de Frutos, M., et al. (2018). Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS One 13, e0202590. doi:10.1371/journal.pone.0202590
Lee, M. H., Perl, D. P., Nair, G., Li, W., Maric, D., Murray, H., et al. (2021). Microvascular injury in the brains of patients with covid-19. N. Engl. J. Med. Overseas. Ed. 384 (5), 481–483. doi:10.1056/nejmc2033369
Lee, M. H., Perl, D. P., Steiner, J., Pasternack, N., Li, W., Maric, D., et al. (2022). Neurovascular injury with complement activation and inflammation in COVID-19. Brain 145 (7), 2555–2568. doi:10.1093/brain/awac151
Lei, Y., Zhang, J., Schiavon, C. R., He, M., Chen, L., Shen, H., et al. (2021). SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE2. Circ. Res. 128, 1323–1326. doi:10.1161/circresaha.121.318902
León-Moreno, L. C., Castañeda-Arellano, R., Aguilar-García, I. G., Desentis-Desentis, M. F., Torres-Anguiano, E., Gutierrez-Almeida, C. E., et al. (2020). Kinematic changes in a mouse model of penetrating hippocampal injury and their recovery after intranasal administration of endometrial mesenchymal stem cell-derived extracellular vesicles. Front. Cell. Neurosci. 14, 579162. doi:10.3389/fncel.2020.579162
Lim, S. K., Giebel, B., Weiss, D. J., Witwer, K. W., and Rhode, E. (2020). Re: “exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19” by sengupta et al. Stem Cells Dev. 29, 877–878. doi:10.1089/scd.2020.0089
Liu, W., Rong, Y., Wang, J., Zhou, Z., Ge, X., Ji, C., et al. (2020). Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflammation 17, 47. doi:10.1186/s12974-020-1726-7
Liu, X., Zhang, M., Liu, H., Zhu, R., He, H., Zhou, Y., et al. (2021). Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp. Neurol. 341, 113700. doi:10.1016/j.expneurol.2021.113700
Liu, Z., Li, X., Ye, Z., and Lin, H. (2022). Neuroprotective effect of exosomes derived from bone marrow mesenchymal stem cells via activating TGR5 and suppressing apoptosis. Biochem. Biophys. Res. Commun. 593, 13–19. doi:10.1016/j.bbrc.2022.01.039
Liu, Z., Ye, H., Ye, Z., and Lin, H. (2021). Neuroprotective effect of exosomes derived from bone marrow mesenchymal stem cells via activating TGR5 and suppressing apoptosis. Biochem. Biophys. Res. Commun. 593 (2022), 13–19. doi:10.1016/j.bbrc.2022.01.039
Long, Q., Upadhya, D., Hattiangady, B., Kim, D. K., An, S. Y., Shuai, B., et al. (2017). Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. U. S. A. 114 (17), E3536-E3545–E3545. doi:10.1073/pnas.1703920114
Losurdo, M., Pedrazzoli, M., D'Agostino, C., Elia, C. A., Massenzio, F., et al. (2020). Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl. Med. 9 (9), 1068–1084. doi:10.1002/sctm.19-0327
Losurdo, M., Pedrazzoli, M., D'Agostino, C., Elia, C. A., Massenzio, F., Lonati, E., et al. (2020). Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl. Med. 9 (9), 1068–1084. doi:10.1002/sctm.19-0327
Lu, Y., Li, X., Geng, D., Mei, N., Wu, P. Y., Huang, C. C., et al. (2020). Cerebral micro-structural changes in COVID-19 patients; an MRI-based 3-month follow-up study. EClinicalMedicine 25, 100484. doi:10.1016/j.eclinm.2020.100484
Ma, X., Huang, M., Zheng, M., Dai, C., Song, Q., Zhang, Q., et al. (2020). ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer's disease. J. Control. Release 327, 688–702. doi:10.1016/j.jconrel.2020.09.019
Ma, X., Huang, M., Zheng, M., Dai, C., Song, Q., Zhang, Q., et al. (2020). ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer's disease. J. Control. Release 327, 688–702. doi:10.1016/j.jconrel.2020.09.019
Mahdavipour, M., Hassanzadeh, G., Seifali, E., Mortezaee, K., Aligholi, H., Shekari, F., et al. (2020). Effects of neural stem cell‐derived extracellular vesicles on neuronal protection and functional recovery in the rat model of middle cerebral artery occlusion. Cell. biochem. Funct. 38, 373–383. doi:10.1002/cbf.3484
Maltezou, H. C., Pavli, A., and Tsakris, A. (2021). Post-COVID syndrome: An insight on its pathogenesis. Vaccines (Basel) 9 (5), 497. doi:10.3390/vaccines9050497
Mandal, S., Barnett, J., Brill, S. E., Brown, J. S., Denneny, E. K., Hare, S. S., et al. (2020). Long-COVID”: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 76, 396–398. doi:10.1136/thoraxjnl-2020-215818
Massey, D., Baker, A. D., Berrent, D. Z., Güthe, N., Shidlovsky, S. P., et al. (2021). Internal tremors and vibration symptoms among people with post-acute sequelae of sars- CoV-2: A narrative review of patient reports. medRxiv preprint. doi:10.1101/2021.12.03.21267146
Matschke J, J., Lütgehetmann, M., Hagel, C., Sperhake, J. P., Schröder, A. S., Edler, C., et al. (2020). Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet. Neurol. 19 (11), 919–929. doi:10.1016/S1474-4422(20)30308-2
McAlpine, L. S., Zubair, A. S., Maran, I., Chojecka, P., Lleva, P., Jasne, A. S., et al. (2021). Ischemic stroke, inflammation, and endotheliopathy in COVID-19 patients. Stroke 52 (6), e233–e238. doi:10.1161/strokeaha.120.031971
Mendt, M., Rezvani, K., and Shpall, E. (2019). Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transpl. 54 (2), 789–792. doi:10.1038/s41409-019-0616-z
Misra, S., Kolappa, K., Prasad, M., Radhakrishnan, D., Thakur, K. T., Solomon, T., et al. (2021). Frequency of neurologic manifestations in COVID-19: A systematic review and meta-analysis. Neurology 97 (23), e2269–e2281. doi:10.1212/wnl.0000000000012930
Mitrani, M. I., Bellio, M. A., Meglin, A., Khan, A., Xu, X., Haskell, G., et al. (2021). Treatment of a COVID-19 long hauler with an amniotic fluid-derived extracellular vesicle biologic. Respir. Med. Case Rep. 34, 101502. doi:10.1016/j.rmcr.2021.101502
Mitrani, M. I., Bellio, M. A., Sagel, A., Saylor, M., Kapp, W., VanOsdol, K., et al. (2021). Case report: Administration of amniotic fluid-derived nanoparticles in three severely ill COVID-19 patients. Front. Med. 8, 583842. doi:10.3389/fmed.2021.583842
Mrahleh, M. A., Matar, S., Jafar, H., Wehaibi, S., Aslam, N., and Awidi, A. (2021). Human wharton's jelly-derived mesenchymal stromal cells primed by tumor necrosis factor-α and interferon-γ modulate the innate and adaptive immune cells of type 1 diabetic patients. Front. Immunol. 12, 732549. doi:10.3389/fimmu.2021.732549
Nakazaki, M., Morita, T., Lankford, K. L., Askenase, P. W., and Kocsis, J. D. (2021). Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J. Extracell. Vesicles 10 (11), e12137. doi:10.1002/jev2.12137
Nalbandian, A., Sehgal, K., Gupta, A., Madhavan, M. V., McGroder, C., Stevens, J. S., et al. (2021). Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615. doi:10.1038/s41591-021-01283-z
Narbute, K., Piļipenko, V., Pupure, J., Dzirkale, Z., Jonavičė, U., et al. (2019). Intranasal administration of extracellular vesicles derived from human teeth stem cells improves motor symptoms and normalizes tyrosine hydroxylase expression in the substantia nigra and striatum of the 6-hydroxydopamine-treated rats. Stem Cells Transl. Med. 8 (5), 490–499. doi:10.1002/sctm.18-0162
Nassar, W., El‐Ansary, M., Sabry, D., Mostafa, M. A., Fayad, T., Kotb, E., et al. (2016). Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 20, 21. doi:10.1186/s40824-016-0068-0
Nasserie, T., Hittle, M., and Goodman, S. N. (2021). Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review. JAMA Netw. Open 4, e2111417.,
Natarajan, A., Zlitni, S., Brooks, E. F., Vance, S. E., Dahlen, A., Hedlin, H., et al. (2022). Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 3 (6), 371–387.e9. doi:10.1016/j.medj.2022.04.001
Neumann, B., Schmidbauer, M. L., Dimitriadis, K., Otto, S., Knier, B., Niesen, W. D., et al. (2020). Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J. Neurol. Sci. 418, 117090. doi:10.1016/j.jns.2020.117090
Ni, H., Yang, S., Siaw Debrah, F., Hu, J., Wu, K., He, Z., et al. (2019). Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front. Neurosci. 13, 14. doi:10.3389/fnins.2019.00014
Novak, P., Mukerji, S. S., Alabsi, H. S., Systrom, D., Marciano, S. P., Felsenstein, D., et al. (2022). Multisystem involvement in post-acute sequelae of Coronavirus disease 19. Ann. Neurol. 91 (3), 367–379. doi:10.1002/ana.26286
Ortona, E., Buonsenso, D., Carfi, A., and Malorni, W. (2021). Long COVID: An estrogen-associated autoimmune disease? Cell. Death Discov. 7, 77. doi:10.1038/s41420-021-00464-6
Ostergaard, L. (2021). SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 9, 14726. doi:10.14814/phy2.14726
Otero-Ortega, L., Laso-Garcia, F., Gomez-de Frutos, M. D., Rodriguez-Frutos, B., Pascual-Guerra, J., Fuentes, B., et al. (2017). White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci. Rep. 7, 44433. doi:10.1038/srep44433
Parajuli, B., Horiuchi, H., Mizuno, T., Takeuchi, H., and Suzumura, A. (2015). CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia 63 (12), 2274–2284. doi:10.1002/glia.22892
Parajuli, B., Horiuchi, H., Mizuno, T., Takeuchi, H., and Suzumura, A. (2015). CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia 63 (12), 2274–2284. doi:10.1002/glia.22892
Patel, N. A., Moss, L. D., Lee, J. Y., Tajiri, N., Acosta, S., Hudson, C., et al. (2018). Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflammation 15, 204. doi:10.1186/s12974-018-1240-3
Payares-Herrera, C., Martínez-Muñoz, M. E., Vallhonrat, I. L., de Molina, R. M., Torres, M. P., Trisan, A., et al. (2021). Double-blind, randomized, controlled, trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 (COVID-at): A structured summary of a study protocol for a randomised controlled trial. Trials 22 (1), 9. doi:10.1186/s13063-020-04964-1
Perets, N., Hertz, S., London, M., and Offen, D. (2018). Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Mol. Autism 9, 57. doi:10.1186/s13229-018-0240-6
Pillai, R. S. (2005). MicroRNA function: Multiple mechanisms for a tiny RNA? RNA 11 (12), 1753–1761. doi:10.1261/rna.2248605
Pretorius, E., Vlok, M., Venter, C., Bezuidenhout, J. A., Laubscher, G. J., Steenkamp, J., et al. (2021). Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 20 (1), 172. doi:10.1186/s12933-021-01359-7
Rapkiewicz, A. V., Mai, X., Carsons, S. E., Pittaluga, S., Kleiner, D. E., Berger, J. S., et al. (2020). etc. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 24, 100434. doi:10.1016/j.eclinm.2020.100434
Reiken, S., Sittenfeld, L., Dridi, H., Liu, Y., Liu, X., and Marks, A. R. (2022). Alzheimer's-like signaling in brains of COVID-19 patients. Alzheimers Dement. 18 (5), 955–965. doi:10.1002/alz.12558
Reza-Zaldivar, E. E., Hernandez-Sapiens, M. A., Gutierrez-Mercado, Y. K., Sandoval-Avila, S., Gomez-Pinedo, U., Marquez-Aguirre, A. L., et al. (2019). Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regen. Res. 14, 1626–1634. doi:10.4103/1673-5374.255978
Rohden, F., Teixeira, L. V., Bernardi, L. P., Ferreira, P. C. L., Colombo, M., Teixeira, G. R., et al. (2021). Functional recovery caused by human adipose tissue mesenchymal stem cell-derived extracellular vesicles administered 24 h after stroke in rats. Int. J. Mol. Sci. 22 (23), 12860. doi:10.3390/ijms222312860
Rojas, M., Rodríguez, Y., Acosta-Ampudia, Y., Monsalve, D. M., Zhu, C., Li, Q. Z., et al. (2022). Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 20, 129. doi:10.1186/s12967-022-03328-4
Ruhl, L., Pink, I., Kühne, J. F., Beushausen, K., Keil, J., Christoph, S., et al. (2021). Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct. Target. Ther. 6, 418. doi:10.1038/s41392-021-00819-6
Sabel, B. A., Zhou, W., Huber, F., Schmidt, F., Sabel, K., Gonschorek, A., et al. (2021). Non-invasive brain microcurrent stimulation therapy of long-COVID-19 reduces vascular dysregulation and improves visual and cognitive impairment. Restor. Neurol. Neurosci. 39 (6), 393–408. doi:10.3233/rnn-211249
Sahu, A., Jha, P. K., Prabhakar, A., Singh, H. D., Gupta, N., Chatterjee, T., et al. (2017). MicroRNA-145 impedes thrombus formation via targeting tissue factor in venous thrombosis. EBioMedicine 26, 175–186. doi:10.1016/j.ebiom.2017.11.022
Saint-Pol, J., Gosselet, F., Duban-Deweer, S., Pottiez, G., and Karamanos, Y. (2020). Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells 9, 851. doi:10.3390/cells9040851
Sengupta, V., Sengupta, S., Lazo, A., Woods, P., Nolan, A., and Bremer, N. (2020). Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 29, 747–754. doi:10.1089/scd.2020.0080
Sengupta, V., SenguptaLazo, S. A., Hicok, K., and Moseley, T. A. (2021). Response to Lim et al. re: “Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19”. Stem Cells Dev. 29 (14), 879–881. doi:10.1089/scd.2020.0095
Shabbir, A., Cox, A., Rodriguez-Menocal, L., Salgado, M., and Van Badiavas, E. (2015). Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 24 (14), 1635–1647. doi:10.1089/scd.2014.0316
Shelke, G. V., Yin, Y., Jang, S. C., Lasser, C., Wennmalm, S., Hoffmann, H. J., et al. (2019). Endosomal signalling via exosome surface TGF-β-1. J. Extracell. Vesicles 8, 1650458.
Shen, W-B., James, L., Yang, P., Baracco, L., Elahi, Mr, Albert Reece, E., et al. (2021). SARS-CoV-2 invades cognitive centers of the brain and induces Alzheimer's-like neuropathology, doi:10.1101/2022.01.31.478476
Shi, L., Huang, H., Lu, X., Yan, X., Jiang, X., Xu, R., et al. (2021). Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 6, 58. doi:10.1038/s41392-021-00488-5
Siddiqi, H. K., Libby, P., and Ridker, P. M. (2021). COVID-19 – a vascular disease. Trends cardiovasc. Med. 31, 1–5. doi:10.1016/j.tcm.2020.10.005
Sisa, C., Kholia, S., Naylor, J., Herrera Sanchez, M. B., Bruno, S., Deregibus, M. C., et al. (2019). Mesenchymal stromal cell derived extracellular vesicles reduce hypoxia-ischaemia induced perinatal brain injury. Front. Physiol. 10, 282. doi:10.3389/fphys.2019.00282
Song, Eric, Bartley, Christopher M., Chow, Ryan D., Pleasure, Samuel J., Wilson, Michael R., Farhadian, Shelli F., et al. (2021). Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell. Rep. Med. 2 (5), 100288. doi:10.1016/j.xcrm.2021.100288
Spudich, Serena, and Nath, Avindra (2022). Nervous system consequences of COVID-19. SCIENCE 375 (6578), 267–269. doi:10.1126/science.abm2052
Stolk, J., Broekman, W., Mauad, T., Zwaginga, J., Roelofs, H., Fibbe, W., et al. (2016). A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM 109 (5), 331–336. doi:10.1093/qjmed/hcw001
Su, Yapeng, Yuan, Dan, DanielChen, G., Choi, J., Davis, Mark M., Goldman, Jason D., et al. (2022). Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 185, 881–895.e20. doi:10.1016/j.cell.2022.01.014
Sun, M. K., Passaro, A. P., Latchoumane, C. F., Spellicy, S. E., Bowler, M., Goeden, M., et al. (2020). Extracellular vesicles mediate neuroprotection and functional recovery after traumatic brain injury. J. Neurotrauma 37 (11), 1358–1369. doi:10.1089/neu.2019.6443
Tabacof, L., Tosto-Mancuso, J., Wood, J., Cortes, M., Kontorovich, A., McCarthy, D., et al. (2022). Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am. J. Phys. Med. Rehabil. 101 (1), 48–52. doi:10.1097/phm.0000000000001910
Taquet, M., Geddes, J. R., Husain, M., Luciano, S., and Harrison, P. J. (2021). 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 8, 416–427. doi:10.1016/s2215-0366(21)00084-5
Teixeira, Antonio L., Gama, Clarissa S., Rocha, Natalia P., and Teixeira, Mauro M. (2018). Revisiting the role of eotaxin-1/CCL11 in psychiatric disorders. Front. Psychiatry 9, 241. doi:10.3389/fpsyt.2018.00241
Tejerina, F., Catalan, P., Rodriguez-Grande, C., Adan, J., Rodriguez-Gonzalez, C., Muñoz, P., Aldamiz, T., Diez, C., Perez, L., Fanciulli, C., Garcia de Viedma, D., et al. Gregorio Marañon MicrobiologyID COVID 19 Study Group (2022). Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 22 (1), 211. doi:10.1186/s12879-022-07153-4
Tenforde, M. W., Kim, S. S., Lindsell, C. J., Billig Rose, E., Shapiro, N. I., Files, D. C., et al. (2020). Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, march–june 2020. MMWR. Morb. Mortal. Wkly. Rep. 69, 993–998. doi:10.15585/mmwr.mm6930e1
Thakur, K. T., Miller, E. H., Glendinning, M. D., Al-Dalahmah, O., Banu, M. A., Boehme, A. K., et al. (2021). COVID-19 neuropathology at columbia university irving medical center/New York presbyterian hospital. Brain 144 (9), 2696–2708. doi:10.1093/brain/awab148
Thomi, G., Joerger-Messerli, M., Haesler, V., Muri, L., Surbek, D., and Schoeberlein, A. (2019). Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells 8 (8), 855. doi:10.3390/cells8080855
Thomi, G., Surbek, D., Haesler, V., Joerger-Messerli, M., and Schoeberlein, A. (2019). Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell. Res. Ther. 10 (1), 105. doi:10.1186/s13287-019-1207-z
Tsivion-Visbord, H., Perets, N., Sofer, T., Bikovski, L., Goldshmit, Y., Ruban, A., et al. (2020). Mesenchymal stem cells derived extracellular vesicles improve behavioral and biochemical deficits in a phencyclidine model of schizophrenia. Transl. Psychiatry 10, 305. doi:10.1038/s41398-020-00988-y
Twomey, R., DeMars, J., Franklin, K., Culos-Reed, S. N., Weatherald, J., and Wrightson, J. G. (2022). Chronic fatigue and postexertional malaise in people living with long COVID: An observational study. Phys. Ther. 102 (4), pzac005. doi:10.1093/ptj/pzac005
Upadhya, R., Madhu, L. N., Attaluri, S., Góes Gitaí, D. L., Pinson, M. R., Kodali, M., et al. (2020). Extracellular vesicles from human iPSC-derived neural stem cells: miRNA and protein signatures, and anti-inflammatory and neurogenic properties. J. Extracell. Vesicles 9, 1809064. doi:10.1080/20013078.2020.1809064
Upadhya, R., Madhu, L. N., Attaluri, S., Gitaí, D. L. G., Pinson, M. R., Kodali, M., et al. (2020). Extracellular vesicles from human iPSC‐derived neural stem cells: miRNA and protein signatures, and anti‐inflammatory and neurogenic properties. J. Extracell. Vesicles 9 (1), 1809064. doi:10.1080/20013078.2020.1809064
Varatharaj, A., Thomas, N., Ellul, M. A., Davies, N. W. S., Pollak, T. A., Tenorio, E. L., et al. (20201420). Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet. Psychiatry 7 (10), 875–882. doi:10.1016/S2215-0366(20)30287-X
Varga, Z., Flammer, A. J., Steiger, P., Haberecker, M., Andermatt, R., Zinkernagel, A. S., et al. (2020). Endothelial cell infection and endotheliitis in COVID-19. Lancet 395 (10234), 1417–1418. doi:10.1016/s0140-6736(20)30937-5
Venkat, P., Cui, C., Chopp, M., Zacharek, A., Wang, F., Landschoot-Ward, J., et al. (2019). MiR-126 mediates brain endothelial cell exosome treatment–induced neurorestorative effects after stroke in type 2 diabetes mellitus mice. Stroke 50, 2865–2874. doi:10.1161/strokeaha.119.025371
Veras, F. P., Pontelli, M. C., Silva, C. M., Toller-Kawahisa, J. E., de Lima, M., Nascimento, D. C., et al. (2020). SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 217 (12), e20201129. doi:10.1084/jem.20201129
Villeda, S. A., Luo, J., Mosher, K. I., Zou, B., Britschgi, M., Bieri, G., et al. (2011). The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477 (7362), 90–94. doi:10.1038/nature10357
Wang, C., Börger, V., Mohamud Yusuf, A., Tertel, T., Stambouli, O., Murke, F., et al. (2022). Postischemic neuroprotection associated with anti-inflammatory effects by mesenchymal stromal cell-derived small extracellular vesicles in aged mice. Stroke 53 (1), e14–e18. doi:10.1161/strokeaha.121.035821
Wang, C., Börger, V., Sardari, M., Murke, F., Skuljec, J., Pul, R., et al. (2020). Mesenchymal stromal cell-derived small extracellular vesicles induce ischemic neuroprotection by modulating leukocytes and specifically neutrophils. Stroke Vasc. Interv. Neurol. 51 (6), 1825–1834. doi:10.1161/strokeaha.119.028012
Wang, H., Liu, Y., Li, J., Wang, T., Hei, Y., Li, H., et al. (2021). Tail-vein injection of MSC-derived small extracellular vesicles facilitates the restoration of hippocampal neuronal morphology and function in APP /PS1 mice. Cell. Death Discov. 7, 230. doi:10.1038/s41420-021-00620-y
Weiss, D. J., Casaburi, R., Flannery, R., LeRoux-Williams, M., and Tashkin, D. P. (2013). A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 143 (6), 1590–1598. doi:10.1378/chest.12-2094
Wenzel, J., Lampe, J., Müller-Fielitz, H., Schuster, R., Zille, M., Müller, K., et al. (2021). The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat. Neurosci. 24 (11), 1522–1533. doi:10.1038/s41593-021-00926-1
Williams, A. M., Bhatti, U. F., Brown, J. F., Biesterveld, B. E., Kathawate, R. G., Graham, N. J., et al. (2020). Early single-dose treatment with exosomes provides neuroprotection and improves blood-brain barrier integrity in swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 88, 207–218. doi:10.1097/ta.0000000000002563
Williams, A. M., Dennahy, I. S., Bhatti, U. F., Halaweish, I., Xiong, Y., Chang, P., et al. (2019). Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J. Neurotrauma 36, 54–60. doi:10.1089/neu.2018.5711
Witwer, K. W., Van Balkom, B. W. M., Bruno, S., Choo, A., Dominici, M., Gimona, M., et al. (2019). Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 8 (1), 1609206. doi:10.1080/20013078.2019.1609206
Wu, S., Huang, S., Ding, J., Zhao, Y., Liang, L., Liu, T., et al. (2010). Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3' untranslated region. Oncogene 29 (15), 2302–2308. doi:10.1038/onc.2010.34
Xin, H., Wang, F., Li, Y., Lu, Q. E., Cheung, W. L., Zhang, Y., et al. (2017). Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from MicroRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell. Transpl. 26 (2), 243–257. doi:10.3727/096368916x693031
Xin, H., Li, Y., Cui, Y., Yang, J. J., Zhang, Z. G., and Chopp, M. (2013). Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow. Metab. 33, 1711–1715. doi:10.1038/jcbfm.2013.152
Xin, H., Li, Y., Liu, Z., Wang, X., Shang, X., Cui, Y., et al. (2013). MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31, 2737–2746. doi:10.1002/stem.1409
Xiong, Y., Mahmood, A., and Chopp, M. (2017). Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen. Res. 12 (1), 19–22. doi:10.4103/1673-5374.198966
Yang, A. C., Kern, F., Losada, P. M., Agam, M. R., Maat, C. A., Schmartz, G. P., et al. (2021). Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 595, 565–571. doi:10.1038/s41586-021-03710-0
Yang, L., Zhai, Y., Hao, Y., Zhu, Z., and Cheng, G. (2020). The regulatory functionality of exosomes derived from hUMSCs in 3D culture for alzheimer’s disease therapy. Small 16 (3), 1906273. doi:10.1002/smll.201906273
Yoo, S. M., Liu, T. C., Motwani, Y., Sim, M. S., Viswanathan, N., Samras, N., et al. (2022). Factors associated with post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J. Gen. Intern Med. 37, 1988–1995. doi:10.1007/s11606-022-07523-3
Zhang, H., Lu, J., Wu, Q., Wu, B., Xu, C., Fan, Y., et al. (2019). A perioperative small dose of dexamethasone enhances postoperative recovery by reducing volume and inflammatory contents in wound drainage after thyroid surgery: A double-blinded, randomized, prospective study. World J. Surg. 43 (7), 1721–1727. doi:10.1007/s00268-019-04986-0
Zhang, Y., Chopp, M., Meng, Y., Katakowski, M., Xin, H., Mahmood, A., et al. (2015). Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 122 (4), 856–867. doi:10.3171/2014.11.jns14770
Zhao, S., Shibata, K., Hellyer, P. J., Trender, W., Manohar, S., Hampshire, A., et al. (2022). Rapid vigilance and episodic memory decrements in COVID-19 survivors. Brain Commun. 4 (1), fcab295. fcab295. doi:10.1093/braincomms/fcab295
Zhao, Y., Gan, Y., Xu, G., Yin, G., and Liu, D. (2020). MSCs-derived exosomes attenuate acute brain injury and inhibit microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglia M1 polarization. Neurochem. Res. 45 (5), 1180–1190. doi:10.1007/s11064-020-02998-0
Zhou, Z., Kang, H., Li, S., and Zhao, X. (2020). Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 267 (8), 2179–2184. doi:10.1007/s00415-020-09929-7
Zhu, C., Bilousova, T., Focht, S., Jun, M., Elias, C. J., Melnik, M., et al. (2021). Pharmacological inhibition of nSMase2 reduces brain exosome release and alpha-synuclein pathology in a Parkinson’s disease model. Mol. Brain 14, 70. doi:10.1186/s13041-021-00776-9
Zhuang, X., Xiang, X., Grizzle, W., Sun, D., Zhang, S., Axtell, R. C., et al. (2011). Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 19 (10), 1769–1779. doi:10.1038/mt.2011.164
Keywords: long COVID syndromes, COVID-19, exosomes, extracellular vesicles, mesenchymal stromal cell-small EVs
Citation: Askenase PW (2022) Recommendation: Treatment of clinical long COVID encephalopathies with nasal administered mesenchymal stromal cell extracellular vesicles. Front. Nanotechnol. 4:987117. doi: 10.3389/fnano.2022.987117
Received: 05 July 2022; Accepted: 31 August 2022;
Published: 04 October 2022.
Edited by:
Di Huang, Massachusetts Eye & Ear Infirmary andHarvard Medical School, United StatesReviewed by:
Dinesh Devadoss, Florida International University, United StatesCopyright © 2022 Askenase. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip W. Askenase, cGhpbGlwLmFza2VuYXNlQHlhbGUuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.