
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Nanotechnol., 12 August 2022
Sec. Biomedical Nanotechnology
Volume 4 - 2022 | https://doi.org/10.3389/fnano.2022.923634
Nanoplastics are defined as plastic particles broken down to extremely small sizes (1–100 nm) with unknown effects to the human body and immune system. Air and food exposure scenarios involving blood, lungs and intestine are considered in the literature. The fact that plastics also needs to pass the nose, oral cavity, and throat is so far ignored in the literature. The tonsils are immunologically important tissue in the oral cavity in which ingested and inhaled agents are incorporated through crypts with the capacity to capture agents and start early immunologic reactions. We argue that the tonsil is a very important tissue to study in regard to micro and nanoplastic human exposure and immunologic response. Nano-sized particles are known to be able to travel through the natural barriers and have different effects on biology compared to larger particle and the bulk material. It is therefore, although difficult, important to develop experimental methods to detect and identify nanoplastics in the tonsils. In preliminary experiments we have optimized the breakdown of tonsil tissues and tried to retrieve added polystyrene nanoparticles using density-based separation and concentration. The polystyrene was followed by FTIR spectrometry and could be detected in micro- and nano-size, in the tissue breakdown solution but not after density-based separation. When nanoplastics are incorporated in the human body, it is possible that the small plastic pieces can be detected in the tonsil tissue, in the lymph system and it is of importance for future studies to reveal the immunological effects for humans.
Humans are exposed to micro- and nanoplastics through multiple pathways probably mainly through air inhalation (Prata, 2018) and ingestion (Rubio-Armendáriz et al., 2022). Microplastics are commonly defined as plastic pieces with a size <5 mm (Frias and Nash, 2019) and the smallest microplastics are referred to as nanoplastics. While a universal definition currently is lacking, a suggested definition of nanoplastics is: “plastic particles derived from larger plastics with a size below 1 µm” (Gigault et al., 2018). Another definition is in accordance with European Council definitions of nanoparticles stating that at least one dimension should be below 100 nm (Potočnik, 2011). The presence of nanoplastics in organisms are currently difficult to quantify due to the small size of the material and that the chemical composition resembles the surrounding organic matter (Gigault et al., 2018). It is noteworthy that most data describing biological effects and accumulation of nanoplastics derive from studies using commercial polystyrene nanoparticles. This material has been useful to understand size specific effects but may not be representative for environmentally relevant nanoplastics which has been broken down from multiple plastic materials (Gigault et al., 2018). Exposure to, and accumulation of, nanoplastics can be of particular interest in comparison to larger plastic particles because they present a greater surface area that can interact with the organism.
Based on current literature we can conclude that humans are exposed to plastic materials. Inhaled microplastics has been of increasing interest lately due to reports on nanoparticles of plastics in both indoor- and outdoor environments (Prata, 2018). It has also been reported to exist in the lungs of humans in several studies (Pauly et al., 1998; Amato-Lourenço et al., 2021; Jenner et al., 2022). From a food perspective, microplastics have been found in filter feeding aquatic animals as mussels and in fish around the world (Bajt, 2021), as well as in table salt (Kim et al., 2018), and in fresh-, tap-, and bottled water (Koelmans et al., 2019). Oral intake of plastic, by inhalation or ingestion, exposes the oral cavity, oropharynx, and the remainder of the gastrointestinal tract (Ibrahim et al., 2020) to micro- and nanoplastics. These direct exposure scenarios of plastics to humans have, not surprisingly, lead to the detection of microplastics in human colon (Ibrahim et al., 2020), and faeces (Schwabl et al., 2019; Zhang et al., 2021; Yan et al., 2022). A recent study has described the existence of microplastics in human blood (Leslie et al., 2022), however, re-isolation of nanoplastics has not been performed in blood, only chemical identification of polymers, and these studies do not link particle size or presence of nano-sized particles in the samples with the chemical analyses. This is an important aspect as many of the nano specific effects are due to changed interactions with surfaces with high curvature. However, this also add substantial experimental difficulties as isolating the nanoplastics as particles before chemical analyses is further complicated by the risk of recrystallization of polymers as they are isolated and concentrated. Especially if they are characterized after being dried down on for example transmission electron microscope (TEM) grids. One study found microplastics in the placenta (Ragusa et al., 2021) and another study also concluded that there were more microplastics found in faeces of infants compared to adults (Zhang et al., 2021). Transfer from airways to internal organs through the blood has been confirmed in animal studies. In rat, nano-sized polystyrene placed in trachea of the mother could translocate to maternal internal organs as well as in the organs of the fetus (Fournier et al., 2020).
In humans, the tonsils are the first immunologically active tissue that encounters micro- and nanoplastic upon oral exposure. The tonsils are lymphoid tissue in the oral cavity in which agents are incorporated through crypts. The lymphoid draining continues to the submandibular lymph nodes and on to deeper cervical lymph nodes. The location of the tonsils in the oral cavity in the junction between the airway and the alimentary pathway is ideal for optimizing uptake of foreign material like antigens and foreign materials (i.e., possibly also micro- and nanoplastic particles). Hence, it seems likely that if tiny fragments of plastics are ingested or inhaled, the first possible absorbing organs are the tonsils.
The literature is scarce concerning micro- and nanoparticles being incorporated in the tonsils. However, nanoparticles other than plastic, e.g., spherical gold nanoparticles, has been shown to be able to enter the immune system and lymph nodes (Zhang et al., 2019) and smaller particles (5–15 nm) have also been shown to enter the immune system faster than larger (50–100 nm). Despite this, it is currently not known if nanoplastics travel the same path.
Wherever micro- and nanoplastics are deposited in the human body, they may interact with the universally projected immune system. Inferentially, this may produce inflammation, up-regulation of pro-inflammatory cytokines (such as Interleukin-8 (IL-8), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and Tumor Necrosis Factor-α (TNF-α), (Xu et al., 2019), likely a type-1 response (including Interferon gamma IFNγ-production) elicited via the innate immune system, and possibly induce sensitization like described for engineered nanomaterials (Alsaleh and Brown, 2020) but not for nanoplastics. In the oropharynx, the encounter may take place in the tonsils, potentially representing a site where exposure to micro- and nanoplastics, and its effects may be assessed. Furthermore, the tonsils are well-defined and readily available for sampling in connection to routinely performed tonsillectomy.
We here introduce the tonsils as a potentially highly interesting tissue to analyse concerning the existence of micro- and nanoplastic particles in the human body. We also present lessons learned while developing a method to re-isolate and identify the nanoplastics after administration into tonsil tissue which can be of interest for further method developments concerning detection of nanoplastics in the tonsils but also in tissues in general.
Preliminary experiments were performed to explore the breakdown of tonsils and isolation of added polystyrene particles. The experimental procedure is based on established protocols for isolating microplastics from organisms, but with the aim to isolate and describe size of nanoplastics. The entire procedure is described in detail in Supplementary Information and the experimental part is outlined in Figure 1. Shortly, tonsils, obtained from patients who underwent conventional tonsillectomy, were broken down by freeze drying followed by grinding. Polystyrene particles with a nominal diameter of 195 nm and 2.07 µm (Bangs Laboratories Inc.) were added to the grinded tissue and degraded in 2.6 M KOH, 1.36 M NaCl2O at 60 °C for 48 h. After degradation the samples were filtered through a 100 µm mesh filter and neutralized to pH 7. Thereafter 1.5 M NaCl was added to promote flotation of the polystyrene particles after centrifugation (Supplementary Video S1). The resulting pellet was assumed to be organic matter from the tonsils. The supernatant was removed and diluted with water to allow concentration of the presumed polystyrene particles by pelleting. Nanoparticle tracking analysis (195 nm samples), or differential sedimentation centrifugation (2.07 µm samples) showed that there were particles with the size of 195 nm and 2.07 µm, respectively in the pellets, Supplementary Figures S1A, B. The presence of particles was confirmed at different stages of the procedure by Fourier-transformed infrared spectroscopy (FTIR) measurements (Supplementary Figure S1).
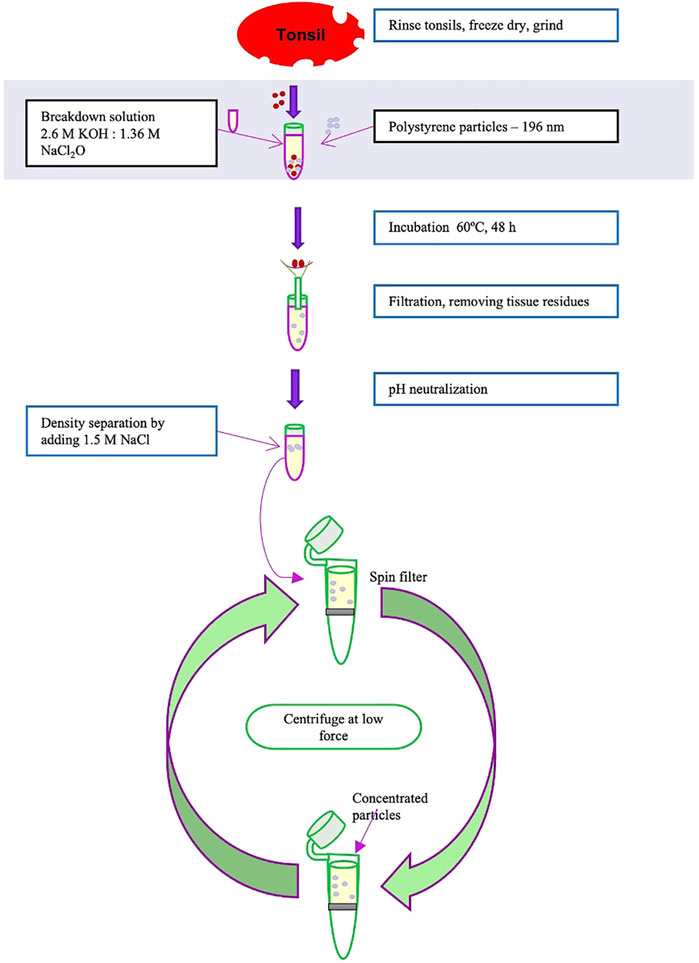
FIGURE 1. Overview of the breakdown of tonsil through freeze dry, grinding, breakdown with KOH, filtration, and separation of particles.
In summary, polystyrene particles in homogenizing solution are present in pellets after centrifugation, in homogenizing solution and floating solution (Supplementary Figures S1C, D). The same is true for polystyrene particles together with tissue in homogenizing solution after pelleting (Supplementary Figure S1E). In all cases, repeated washing of the pellets was needed to remove interference from the high concentrations of salt and bases. However, surprisingly, we could not recognize a polystyrene signal in the spectra from polystyrene particles first in homogenizing and then floating solution when tonsil tissue was present (Supplementary Figure S1F). The challenges with identifying the particles could be due to interference with e.g. fats in the tissue floating and/or interference with the polystyrene particles emphasizing the difficulties with isolation and identification of nanoplastics from different tissues.
In a real scenario there will be different types of plastics which make isolating them difficult. For example, many plastics have a density below one which makes pelleting in water impossible. Therefore, we tried another approach mimicking low density by adding NaCl to the polystyrene particles solution and concentrate the floating particles by low-speed centrifugation in spin filters (Supplementary Video S1). We believe it is a possible way forward to concentrate all types of nanoplastics. Characterization of microplastics after isolation is traditionally done by capturing the particles on filters and subsequent analyses of single particles by FTIR or Raman spectrometry, alternatively by pyrolysis. Gas chromatography (GC) and GC (in conjunction with MS (mass spectrometry) is also used frequently for analysing micro/nano plastics (Leslie et al., 2022).
We here introduce the theory of tonsils as a possible entry port for nanoplastics to the human body and suggest that future focus is warranted on possible immunologic effects induced by nanoplastics. When developing the field further it is of importance to detect the actual particles and describe sizes of nanoparticles in human tissue.
It is well known that nanoparticles, other than plastics, can activate the innate immune system in vivo: administration (onto the middle ear mucosa) of poly (γ-glutamic acid) conjugated with l-phenylalanine ethylester (γ-PGA-Phe) produces innate immune actions in rats: pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, MIP-1α, and TNF-α) and associated histopathological changes (Nilsson et al., 2014). Furthermore, these nanoparticles affect antigen-presentation (by monocyte-derived dendritic cells) and subsequent T-cell responses (cytokine release and proliferation) in vitro (Broos et al., 2010). There are immune reactions linked with nanoparticles of plastics as well. Polystyrene nanoplastics induces signifcant up-regulation of pro-inflammatory cytokines such as IL-8, NF-κB, and TNF-α (Xu et al., 2019). Yang et al. (2022) have investigated effects of 40 nm polystyrene nanoplastics on human lung epithelial cells and found a proinflammatory effect with increased levels of IL-6, MCP-1 and TNF-alpha (Yang et al., 2021).
Microplastic particles have been found in internal organs the human body; blood (Leslie et al., 2022), lung (Amato-Lourenço et al., 2021), colon (Ibrahim et al., 2020), and placenta (Ragusa et al., 2021). The study with results on microplastics in blood has somewhat changed the perspectives on small plastic materials in the human body. If in blood, there might be microplastics in the entire body. However, the lymphoid system is a separate system, and it is not known if and how the particles influence the immune system.
Little is known about the effects of plastic nanoparticles on humans, but it is possible that those particles have a greater impact than larger plastic particles (Yan et al., 2022). The tonsils have not been studied to detect if nanoparticles enter the lymphoid system as far as we are aware of.
To develop our method further, we aim to be able detect polylactic acid nanoparticles and we also plan to use breakdown nanoplastics of polyethylene (PE), polyethylene terephthalate (PET), and polylactide (PLA), origin. These will be generated by mechanical breakdown of common polymers used in everyday life of humans described in (Ekvall et al., 2018) and (Ekvall et al., 2022) and size fractionated as described in (Ekvall et al., 2022). In this way the project can further investigate the possible presence and further effects of micro-/nanoplastics on tonsils, lymph system and immune responses.
The presented approach is a stepping stone towards investigating if there is an uptake of nanoplastic particles in the tonsils and if transportation occurs in the body and possible immunological and other responses. This is an issue of major importance to focus on in future environmental medicine with unknown and possibly large effects on human health (Yong et al., 2020). We argue that, in order to claim that nanoplastics are present in organic samples, it is necessary to link isolated particles with a confirmed size in the nano range with a chemical identification. Nevertheless, these results indicate that this may not be possible due to polymer modification. It may be that we need, for now, to settle for a less direct solution. One approach could then be to filter the breakdown tissue using filters with a cut off at 1 µm or lower and then extract the plastics using solvents and identify the dissolved polymers. This approach has been explored for detecting the presence of plastics in for example placenta (Ragusa et al., 2021).
• Humans are, based on the current literature, exposed to micro- and nanoplastics through food and inhalation.
• Exposure scenarios involving lungs and intestine are considered in the literature. However, the fact that plastics needs to pass the nose, oral cavity and throat is so far ignored.
• Tonsils are immunologically important tissues with the capacity to capture agents and activate early immunologic reactions.
• The isolation and identification of small micro- and nanoplastics may be even harder than expected due to massive chemical modifications of the polymers during standard tissue breakdown protocol.
If nanoplastics are incorporated in the human body, it is possible that the small plastic materials can be detected in the tonsil tissue and future studies will hopefully reveal the immunological and over all effects for humans.
The manuscript includes no use of copyrighted material from other sources (including re-published/adapted/modified/partial figures and images from the Internet).
The original contributions presented in the study are included in the article/Supplementary Material further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Regional Ethical Committee (Dnr 2018/611) and the Regional Bioethical Committee (136/BD16). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
ME was part of the study setup, supervised and performed experiments, wrote part of the manuscript, and was part of the review in all versions of the manuscript. SN did experiments, took part in writing the manuscript and reviewed the final version of the manuscript. ML took part in experiments, took part in writing the manuscript and reviewed the final version of the manuscript. TC was part of the study setup, supervised experiments, wrote part of the manuscript, and was part of the review in all versions of the manuscript. MV was the initiator of the project, coordinated the different cites of the project, wrote parts of the manuscript, and was part of the review in all versions of the manuscript.
This was received from Stiftelsen Acta Oto-Laryngologica and Kungl. Fysiografiska Sällskapet in Lund.
Morgan Andersson (deceased), Department of Otorhinolaryngology (ORL), Head and Neck Surgery, Skåne University Hospital, Lund, Sweden, for being part of study start-up. Bengt Olsson, Anna Elfvik, and Natalia Ioukhnenko, and the doctors and the department of ORL surgery, Department of ORL, Ystad Hospital, Ystad, Sweden, for tonsil collection. Lennart Greiff, Department of Otorhinolaryngology (ORL), Head and Neck Surgery, Skåne University Hospital, Lund, Sweden, for valuable comments on the manuscript. Lena Glantz-Larsson, Department of ORL, Head and Neck Surgery, Skåne University Hospital, Lund, Sweden, for logistics support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnano.2022.923634/full#supplementary-material
Alsaleh, N. B., and Brown, J. M. (2020). Engineered nanomaterials and type I allergic hypersensitivity reactions. Front. Immunol. 11, 222. doi:10.3389/fimmu.2020.00222
Amato-Lourenço, L. F., Carvalho-Oliveira, R., Júnior, G. R., Galvãodos, L. S., Ando, R. A., and Mauad, T. (2021). Presence of airborne microplastics in human lung tissue. J. Hazard Mater 416, 126124. doi:10.1016/j.jhazmat.2021.126124
Bajt, O. (2021). From plastics to microplastics and organisms. Febs Open Bio 11, 954–966. doi:10.1002/2211-5463.13120
Broos, S., Lundberg, K., Akagi, T., Kadowaki, K., Akashi, M., Greiff, L., et al. (2010). Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: Implications for specific immunotherapy. Vaccine 28, 5075–5085. doi:10.1016/j.vaccine.2010.05.004
Ekvall, M. T., Gimskog, I., Hua, J., Kelpsiene, E., Lundqvist, M., and Cedervall, T. (2022). Size fractionation of high-density polyethylene breakdown nanoplastics reveals different toxic response in Daphnia magna. Sci. Rep. 12, 3109. doi:10.1038/s41598-022-06991-1
Ekvall, M. T., Lundqvist, M., Kelpsiene, E., Šileikis, E., Gunnarsson, S. B., and Cedervall, T. (2018). Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 1, 1055–1061. doi:10.1039/c8na00210j
Fournier, S. B., D’Errico, J. N., Adler, D. S., Kollontzi, S., Goedken, M. J., Fabris, L., et al. (2020). Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 17, 55. doi:10.1186/s12989-020-00385-9
Frias, J. P. G. L., and Nash, R. (2019). Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 138, 145–147. doi:10.1016/j.marpolbul.2018.11.022
Gigault, J., Halle, A., Baudrimont, M., Pascal, P.-Y., Gauffre, F., Phi, T.-L., et al. (2018). Current opinion: What is a nanoplastic? Environ. Pollut. 235, 1030–1034. doi:10.1016/j.envpol.2018.01.024
Ibrahim, Y. S., Anuar, S. T., Azmi, A. A., Khalik, W. M. A. W. M., Lehata, S., Hamzah, S. R., et al. (2020). Detection of microplastics in human colectomy specimens. JGH Open 5, 116–121. doi:10.1002/jgh3.12457
Jenner, L. C., Rotchell, J. M., Bennett, R. T., Cowen, M., Tentzeris, V., and Sadofsky, L. R. (2022). Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 831, 154907. doi:10.1016/j.scitotenv.2022.154907
Kim, J.-S., Lee, H.-J., Kim, S.-K., and Kim, H.-J. (2018). Global pattern of microplastics (MPs) in commercial food-grade salts: Sea salt as an indicator of seawater MP pollution. Environ. Sci. Technol. 52, 12819–12828. doi:10.1021/acs.est.8b04180
Koelmans, A. A., Nor, N. H. M., Hermsen, E., Kooi, M., Mintenig, S. M., and France, J. D. (2019). Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 155, 410–422. doi:10.1016/j.watres.2019.02.054
Leslie, H. A., Velzen, M. J. M. van, Brandsma, S. H., Vethaak, D., Garcia-Vallejo, J. J., and Lamoree, M. H. (2022). Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 163, 107199. doi:10.1016/j.envint.2022.107199
Nilsson, J. S., Broos, S., Akagi, T., Akashi, M., Hermansson, A., Cayé-Thomasen, P., et al. (2014). Amphiphilic γ-PGA nanoparticles administered on rat middle ear mucosa produce adjuvant-like immunostimulation in vivo. Acta Otolaryngol. 134, 1034–1041. doi:10.3109/00016489.2014.918278
Pauly, J. L., Stegmeier, S. J., Allaart, H. A., Cheney, R. T., Zhang, P. J., Mayer, A. G., et al. (1998). Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol. Biomarkers Prev. 7, 419–428.
Potočnik, J. (2011). Commission recommendation of 18 october 2011 on the definition of nanomaterial text with EEA relevance. Europe: Official Journal of the European Union.
Prata, J. C. (2018). Airborne microplastics: Consequences to human health? Environ. Pollut. 234, 115–126. doi:10.1016/j.envpol.2017.11.043
Ragusa, A., Svelato, A., Santacroce, C., Catalano, P., Notarstefano, V., Carnevali, O., et al. (2021). Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 146, 106274. doi:10.1016/j.envint.2020.106274
Rubio-Armendáriz, C., Alejandro-Vega, S., Paz-Montelongo, S., Gutiérrez-Fernández, Á. J., Carrascosa-Iruzubieta, C. J., and Torre, A. H. (2022). Microplastics as emerging food contaminants: A challenge for food safety. Int. J. Environ. Res. Public Health 19, 1174. doi:10.3390/ijerph19031174
Schwabl, P., Köppel, S., Königshofer, P., Bucsics, T., Trauner, M., Reiberger, T., et al. (2019). Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 171, 453–457. doi:10.7326/m19-0618
Xu, M., Halimu, G., Zhang, Q., Song, Y., Fu, X., Li, Y., et al. (2019). Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 694, 133794. doi:10.1016/j.scitotenv.2019.133794
Yan, Z., Liu, Y., Zhang, T., Zhang, F., Ren, H., and Zhang, Y. (2022). Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 56, 414–421. doi:10.1021/acs.est.1c03924
Yang, S., Cheng, Y., Chen, Z., Liu, T., Yin, L., Pu, Y., et al. (2021). In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol. Environ. Saf. 226, 112837. doi:10.1016/j.ecoenv.2021.112837
Yong, C. Q. Y., Valiyaveetill, S., and Tang, B. L. (2020). Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health 17, 1509. doi:10.3390/ijerph17051509
Zhang, J., Wang, L., Trasande, L., and Kannan, K. (2021). Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ. Sci. Technol. Lett. 8, 989–994. doi:10.1021/acs.estlett.1c00559
Keywords: nano, micro, plastic, tonsil, polystyrene
Citation: Ekvall MT, Naidu S, Lundqvist M, Cedervall T and Värendh M (2022) The forgotten tonsils—does the immune active organ absorb nanoplastics?. Front. Nanotechnol. 4:923634. doi: 10.3389/fnano.2022.923634
Received: 19 April 2022; Accepted: 07 July 2022;
Published: 12 August 2022.
Edited by:
Chandra Dixit, NanoDx Inc., United StatesReviewed by:
Pandiaraj Manickam, Central Electrochemical Research Institute (CSIR), IndiaCopyright © 2022 Ekvall, Naidu, Lundqvist, Cedervall and Värendh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Värendh, bWFyaWEudmFyZW5kaEBtZWQubHUuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.