- 1AllExcel, Inc, West Haven, CT, United States
- 2NanoViricides, Inc, Shelton, CT, United States
NV-CoV-2, a nanoviricide composed of covalently attached polyethylene glycol and alkyl pendants that are designed to bind free virion particles of multiple strains of coronaviruses in a broad-spectrum manner at multiple points. The binding interaction is like a nano-velcro-tape and may cause a lipid–lipid fusion between nanoviricide micelle and the lipid envelope of the virus. A nanoviricide can encapsulate the virus and dismantle it without any involvement of the host immune system, ultimately disabling the infectibility of the host cells. Thus, it may be expected to count a stronger and synergistic antiviral effect by combining NV-CoV-2 with other anti-coronavirus regimens like remdesivir. Furthermore, some ligands similar to the SARS-CoV S-protein are designed by molecular modeling and attached to the nanoviricide at the same site as where the cognate cellular receptor, ACE2, binds. As a result, a competitive binding inhibition may occur. A nanoviricide can encapsulate other antiviral compounds and protect them from serum-mediated degradation in vivo. This makes the antiviral compounds available for a longer period of time to interact with RNA polymerase and inhibit it. Altogether, a multipoint antiviral efficacy can be achieved with our nanoviricide, NV-CoV-2.
Introduction
Nanoviricide® is a platform technology-based biopolymer called NV-CoV-2, that is used as a broad-spectrum antiviral compound. The backbone of this flexible biopolymer is composed of polyethylene glycol (PEG) and some alkyl pendants. PEG will form the hydrophilic shell and render non-immunogenicity. The alkyl chains make up the flexible core. The resulting material is different from liposomes, which are dynamic micelles. Polymeric chemical groups in our naonovircide are uniformly distributed allowing them to attach to virus-specific ligands such as peptides, antibody fragments, and various chemical moieties (Diwan et al., 2021a).
Electron photomicrographs can support our aforementioned mechanism in which murine cytomegalovirus (CMV) was incubated with our nanoviricide. Figures 1B and C demonstrate that the binding of CMV to our nanoviricide results in the CMV naked capsids, which are non-infectious.
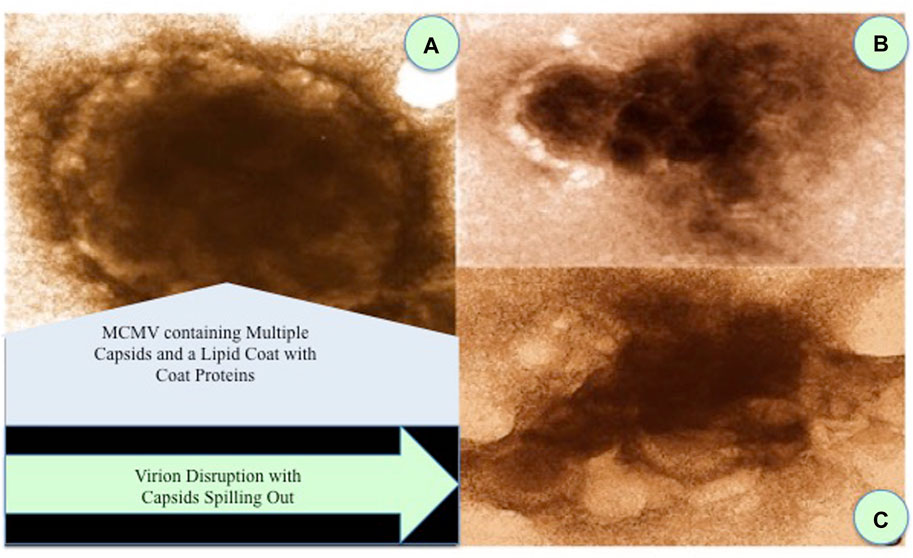
FIGURE 1. Effects of two different nanoviricides binding to murine cytomegalovirus (MCMV). (A) Control treated virion: MCMV containing multiple capsids and a lipid coat with coat proteins. (B,C) MCMV virions treated with two different nanoviricides. Virion disruption with capsids spilling out.
In Vitro and In Vivo Anti-SARS-CoV-2 Capacity of Nanoviricide
Physical, Chemical, and Pharmaceutical Properties and Formulation
Chemical Name
The IUPAC name of the polymer NV-CoV-2 is derived using MarvinSketch 6.2.1. Based on polyethylene glycol of 22 repeat units substituted by two hexadecylamine, the IUPAC name of NV-CoV-2 repeat unit is 2-({1-carboxy-3-[(1-{[2-carboxy-1-(hexadecylcarbamoyl)ethyl]sulfanyl}-3-({3-carboxy-3-[(1,2-dicarboxyethyl)sulfanyl]propanoyl}oxy)-4-{[1-(hexadecylcarbamoyl)-3-[(65-hydroxy-3,6,9,12,15,18,21,24,27,30,33,36,39,42,45,48,51,54,57,60,63-henicosaoxapentahexacontan-1-yl)oxy]-3-oxopropyl]sulfanyl}butan-2-yl)oxy]-3-oxopropyl}sulfanyl)butanedioic acid.
Molecular Formula
Based on polyethylene glycol of 22 repeat units (m) substituted by two hexadecylamine (R1), the theoretical molecular formula for the repeat unit of the polymeric drug substance (NV-CoV-2, 6) is C104H188N2O44S4.
Molecular Weight
Nominal calculated formula weight of the polymer repeat unit (RU) is 2298.85 g/mol when the hexadecylamine substitution level (x) per repeat unit is 2, the MMSA substitution level (y) is also 2, and the starting polyethylene glycol (P10, 1) consists of 22 repeat units (m). The actual substitution levels are less than these theoretical limits and are described in the drug substance specifications. The repeat unit (RU) formula weight at hexadecylamine (HDA) substitution level x between 0 and 2 is described by the formula: MWRU = 1853 + 223x. The degree of polymerization, n, in P10M2DT (HDA)x (MMSA)y polymer is 8 ± 2.
Pharmaceutical properties, formulations for injection, physical properties, and chemical properties are all available in bioRxiv preprint doi: https://doi.org/10.1101/2021.11.24.469813. (Diwan et al., 2021a; the full paper was submitted at PLoS One, and it is in press).
Pharmacology
NV-CoV-2 is being developed for the treatment of SARS-CoV-2 infection using the human coronavirus, CoV-NL63. Like SARS-CoV-2, CoV-NL63 binds to angiotensin-converting enzyme 2 (ACE2) for entry into cells. Therefore, CoV-NL63 was used as an appropriate model virus for proof-of-concept studies in vitro and in vivo.
In Vitro Antiviral Proof-of-Concept Studies
The antiviral efficacy, potency, and cytotoxicity of the antiviral compound NV-CoV-2 in cell cultures were studied. A series of experiments were performed to evaluate the inhibitory activity of the human coronavirus, Cov-NL63 infection in both rhesus monkey kidney epithelial (LLC-MK2) cells and human fetal lung fibroblast (MRC-5) cells. NV-CoV-2 and remdesivir were serially diluted in culture medium to desired final concentrations along with equivalent vehicle concentrations. The virus was then diluted to the appropriate infection PFU/well dose: CoV-NL63 and CoV-229E MOI = 0.01. In a separate clear 96-well plate, 60 μL of the compound was added to 60 μL of virus and incubated for 1 h at 34°C. After incubation, 100 μL/well of compound: virus mixture was added to each well of the cell plates and incubated for 5–7 days. This gives the virus time to replicate and cause cell death (cytopathic effect). A viability assay was used to measure cell viability and determine the amount of cell death (CPE) caused by the virus. Relative CPE levels were compared between all groups to identify any reduction in viral replication, as a reduction in CPE compared to untreated infected controls suggests a decrease in virus production, growth, and spread (Diwan et al., 2021a).
In Vivo Antiviral Proof-of-Concept Studies
Dose-Response of NV-CoV-2
Male and female Sprague–Dawley rats that were 8–9 weeks old were infected with CoV-NL63. All untreated rats infected in this manner succumb to the disease in 5–6 days. This set was used as a model for evaluating the efficacy of NV-CoV-2. Both NV-CoV-2 and vehicle were administered by a tail vein I.V. injection (10 ml/kg), once a day (days 0, 1, 3, 5, and 7). Remdesivir (RDV) was administered by tail vein I.V. injection based on the standard administration protocol. On the first day, two doses were given and then once daily for the remaining 7 days. A group of infected, untreated rats was included as an additional control. The rats were observed daily for clinical and behavior changes and body weight; moribund rats were sacrificed. The primary endpoints were survival and body weight loss.
At both dose levels, treatment with NV-CoV-2 markedly extended the survival of the rats infected intratracheally with a lethal dose of human Cov-NL63 virus. More importantly, both treatments were better than RDV treatment alone (Diwan et al., 2021a). The efficacy of NV-CoV-2 on survival was also reflected in the body weight changes. Body weight loss increased significantly as the animals became moribund. Thus, the treatment with NV-CoV-2 at both 160 and 320 mg/kg/injection for days 0, 1, 3, 5, and 7 had a significant effect on survival in rats using a lethal model of infection with the human coronavirus CoV-NL63. Furthermore, NV-CoV-2 treatment was clearly superior to daily treatment with RDV.
Effect of Number of Days of Dosing of NV-CoV-2 in Rats Infected With CoV-NL63
Consistent with the previous study, the increase in survival after 3–5 days of treatment with NV-CoV-2 was also observed in the body weight changes over time post-infection. The first day of administration of NV-CoV-2 did not significantly affect changes in the body weight when compared to vehicle treatment. Thus, the antiviral efficacy of NV-CoV-2 in rats infected with CoV-NL63 was clearly dependent on the duration of the treatment.
Nanoviricide Can Work as a Safe Cargo
It is conjectured that NV-CoV-2 should be capable of delivering other encapsulated antiviral compounds. Furthermore, these nanoviricide-mediated deliveries only happen to the virus-infected cells, sparing healthy cells, because only infected cells, budding virion, or exposed cell membrane structures display the viral antigen S-protein (or S1 and S2 proteins).
Proof-of-concept studies were conducted in CoV-NL63-infected cell in cultures and also in virus-infected rats (Chakraborty and Diwan 2020a; 2020b) as the SARS-CoV-2 and CoV-NL63 bind to the same receptor, angiotensin-converting enzyme 2 (ACE2), for entry into the host cells (Li et al., 2007). With several studies, both in vitro and in vivo, we have shown that encapsulation of RDV in NV-CoV-2 and the stability of RDV as well as its overall antiviral efficacy of the proposed regimen has improved a lot (Chakraborty and Diwan 2020a; 2020b).
Therefore, it is expected that in vivo, our polymer encapsulation can provide RDV for longer periods of time improving its antiviral activity. In our in vivo study, the untreated rats and the vehicle-treated rats infected with the CoV-NL63 virus directly into the lungs only survived for 5 days. The group of rats treated daily with a dose of RDV at 10 mg/kg (NV376, double dose on the first day, mimicking standard Veklury® protocol), survived up to 7.5 days. The group of rats treated with NV-CoV-2, 320 mg/kg (high, given on alternate days for five doses), survived until day 14. The survival rate of the rats administered with NV-CoV-2-R (comprising 160 mg/kg NV-CoV-2/med and 16 mg/kg RDV/low, given on alternate days for five doses) increased to 18 days. The total RDV dose was matched in the RDV and NV-CoV-2-R groups enabling simple comparison of the effect on survival (Chakraborty et al., 2022b).
Safety Issues of Our Generic Biopolymer (NV387) and/or Virus-Specific Biopolymer (NV-CoV-2)
Core battery tests as defined in the ICH S7A guidelines were conducted on the respiratory and central nervous systems in the standardized conscious rat model, and the cardiovascular system in a non-human primate model (cynomolgus monkeys-Macaca fascicularis) upon intravenous administration of NV-CoV-2. No significant adverse effects on respiratory function or neurobehavioral effects were observed at 1 h of post-dose time point in all dose groups of rats. Body temperature in rats was not affected by the intravenous administration of NV-CoV-2.
The intravenous administration of NV-CoV-2 in conscious telemetered cynomolgus monkeys did not induce any significant, biologically relevant effects on heart rate, arterial blood pressure, cardiac rhythm, or ECG parameters. All monkeys maintained sinus rhythm throughout the study. Additionally, NV-387 was found to be non-immunogenic in a rat model. NV-387 was non-allergenic in several animal models given as injection as well as given orally. NV-387 was found to be non-mutagenic in the standardized bacterial reverse mutation test (Ames test) and non-genotoxic in the standardized micronucleus test. The micronucleus test is a genotoxicity test to detect the chromosome damaging potential after exposure to a test chemical in human TK6 cells in vitro.
The hydrophobicity, or more specifically, the hydrophilic/hydrophobic balance in the polymer, is expected to affect its aggregation properties as well as interactions with proteins and cell membranes. Nevertheless, the design principles that we used, of incorporating PEG in the backbone to enable “stealth mode,” and pendant alkyl chains to enable mimicking cellular membranes, and enabling self-assembly that presents an unstructured hydrophilic PEG surface for the micelle, are expected to enable similar non-mutagenic, non-immunogenic, non-allergenic, and non-genotoxic characteristics to the TheraCour polymer platform.
Discussion
Viruses bind to specific cell surface ligands in order to attach to cells and internalize. A nanoviricide is designed to act like a decoy of a human cell. When the virus sees the appropriate ligand displayed on a nanoviricide micelle, the virus is believed to bind to it. This may cause the flexible nanoviricide to lead to a chain binding to an additional viral coat protein, analogous to the “Velcro” effect from cooperative interactions. This property enables the ligands which individually may have low affinity but achieve very high avidity when their polyvalency result. This concept was shown earlier convincingly with polymeric vs. monomeric inhibitors in the scientific literature (Barton et al., 2011, 2016).
Unlike antiviral antibodies, nanoviricide on binding with a virus particle develops van der Waals interactions which promote encapsulation of the virus particle by conformational re-arrangement of the polymeric chains of the nanoviricide. The lipid tails of the nanoviricide would integrate with the lipid membrane coating of the virus. This is similar to a coordinated surfactant attack on the virus particle which leads to dismantling of the virus envelope as well as stripping off the glycoproteins necessary for viral adsorption and cellular entry.
The antiviral efficacy and potency and the cytotoxicity of the antiviral compound NV-CoV-2 in cell cultures were studied (Li et al., 2007; Barton et al., 2011, 2016; Chakraborty and Diwan 2020a; Chakraborty and Diwan 2020b; Diwan et al., 2021b). Relative CPE levels were compared between all groups to identify any reduction in viral replication, as a reduction in CPE compared to untreated infected controls suggests a decrease in virus production/growth/spread.
NV-CoV-2, in addition to its possessing intrinsic antiviral activity, the polymer encapsulation renders RDV highly effective against SARS-CoV-2 by protecting the drug from plasma-mediated degradation (Chakraborty et al., 2021b) (Figure 2).
Treatment with NV-CoV-2 and NV-CoV-2-R, extended dose dependently the survival of rats infected intratracheally with a lethal dose of human CoV-NL-63 virus when NV-CoV-2-R showed a much better effect than NV-CoV-2. Importantly, both treatments were also markedly better than RDV treatment alone. Thus, NV-CoV-2 containing encapsulated RDV, NV-CoV-2-R, clearly demonstrates antiviral efficacy in this lethal infection model with CoV-NL-63 virus.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The authors declare that this study received funding from Nanoviricides, Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
AC, JT, RP, PH, NH, and BP are employed by the company AllExcel, Inc. AC, AD, RB, VA, YT, VC, JT, RP, PH, NH, and BP are employed by the company Nanoviricides, Inc.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All our colleagues and secretaries are hereby acknowledged for their help during the preparation of this manuscript.
Abbreviations
NV, nanoviricides; NV-387, nanoviricides-polymer 387; NV-387-R, nanoviricides-polymer 387-remdesivir conjugate; RDV, remdesivir; SBECD, commercial encapsulating agent SBECD (GILEAD); and SBECD-R, commercial remdesivir conjugated with SBECD.
References
Barton, R. W., Tatake, J. G., and Diwan, A. R. (2016). in Nanoviricides: Targeted Anti-viral Nanomaterials Handbook of Clinical Nanomedicine, Nanoparticles, Imaging, Therapy, and Clinical Applications. Editors B Raj, G F. Audette, and I Rubinstein (Singapore: Jenny Stanford Publishing), 1039–1046. ISBN 9789814669207. Published February 24.
Barton, R. W., Tatake, J. G., and Diwan, A. R. (2011). “Nanoviricides-A Novel Approach to Antiviral Therapeutics,” in Bionanotechnology II. Editor E. R David (Boca Raton, FL: CRC Press. Taylor and Francis Group), 141–154. Available at: www.crcpress.com.
Chakraborty, A., and Diwan, A. (2020a). Pharmacodynamics of Remdesivir: How to Improve for COVID-19 Treatment. J. Biomed. Res. Environ. Sci. 1 (8), 431–438. doi:10.37871/jbres1175
Chakraborty, A., and Diwan, A. (2020b). NL-63: A Better Surrogate Virus for Studying SARS-CoV-2. Integr. Mol. Med. 7, 1–9. doi:10.15761/IMM.1000408
Chakraborty, A., Diwan, A., Arora, V., Thakur, Y., Holkar, P., and Chinige, V. (2021a). Nanoviricides Platform Technology Based NV-387 Polymer Protects Remdesivir from Plasma-Mediated Catabolism in Vitro: Importance of its Increased Lifetime for in Vivo Action. bioRxiv preprint, Cold Spring Harbor. this version posted October 23, 2021. doi:10.1101/2021.10.22.465399
Chakraborty, A., Diwan, A., Arora, V., Thakur, Y., Chiniga, V., Tatake, J., et al. (2021b). Nanoviricide’s Platform Technology Based NV-CoV-2 Polymer Increases the Half-Life of Remdesivir in Vivo. bioRxiv preprint, Cold Spring Harbor. 11.17, 468980. doi:10.1101/2021.11.17.468980
Chakraborty, A., Diwan, A., Arora, V., Thakur, Y., Holkar, P., and Chiniga, V. (2022a). Nanoviricides Platform Technology Based NV-387 Polymer Protects Remdesivir from Plasma-Mediated Catabolism In Vitro: Importance of its Increased Lifetime for In Vivo Action. Recent Adv. Clin. Trials 1 (1), 1–8. https://www.scivisionpub.com/pdfs/nanoviricides-platform-technology-based-nv387-polymer-protects-remdesivir-from-plasmamediated-catabolism-in-vitro-importance-of-it-2039.pdf
Chakraborty, A., Diwan, A., Arora, V., Thakur, Y., Chiniga, V., Tatake, J., et al. (2022b). Encapsulation of Remdesivir in Nanoviricide’s Platform Technology Based NV-CoV-2 Polymer Protects the Drug and Improves its Pharmacokinetics (In Vivo). EC Pharmacol. Toxicol. 10 (2), 108–118. https://www.ecronicon.com/ecpt/ECPT-10-00707.php
Diwan, A., Chakraborty, A., Tatake, J., Barton, R., Chiniga, V., Pandey, R., et al. (2021b). Broad Spectrum Antiviral Therapy Can Supersede Vaccination Strategy for Combating COVID-19 Pandemic. Med. J. Clin. Trials Case Stud. 5 (4), 000298. doi:10.23880/mjccs-16000298
Diwan, A., Chakraborty, A., Chiniga, V., Arora, V., Holkar, P., Thakur, Y., et al. (2021a). Dual Effects of Nanoviricides Platform Technology Based NV-CoV-2 Biomimetic Polymer against COVID-19. bioRxiv preprint, Cold Spring Harbor. this version posted November 26, 2021. doi:10.1101/2021.11.24.469813
Keywords: biopolymer active center, NV-CoV-2, SARS-CoV-2, COVID-19, antiviral mechanism
Citation: Chakraborty A, Diwan A, Barton R, Arora V, Thakur Y, Chiniga V, Tatake J, Pandey R, Holkar P, Holkar N and Pond B (2022) A New Antiviral Regimen Against SARS-CoV-2 Based on Nanoviricide’s Biopolymer (NV-CoV-2). Front. Nanotechnol. 4:891605. doi: 10.3389/fnano.2022.891605
Received: 07 March 2022; Accepted: 30 May 2022;
Published: 13 July 2022.
Edited by:
Aliasgar Shahiwala, Dubai Pharmacy College, United Arab EmiratesReviewed by:
Stergios Pispas, National Hellenic Research Foundation, GreeceBiswajit Sarkar, Intel, United States
Copyright © 2022 Chakraborty, Diwan, Barton, Arora, Thakur, Chiniga, Tatake, Pandey, Holkar, Holkar and Pond. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashok Chakraborty, YXNob2suY2hha3JhYm9ydHlAYWxsZXhjZWwuY29t
 Ashok Chakraborty
Ashok Chakraborty Anil Diwan2
Anil Diwan2