- Department of Pharmaceutics, ISF College of Pharmacy, Moga, Punjab, India
Topical drug delivery presents a novel substitute to the conventional drug-distribution routes of oral delivery and injection. Apart from the simplicity and non-invasiveness, the skin also serves as a “reservoir” that sustains administration over a period of days. Nanocarriers provide new potential for the treatment of skin disease. The skin’s barrier function offers a considerable obstacle for the potential nanocarriers to infiltrate into the tissue. However, the barrier is partially weakened in case of damage or inflammation, as in the case of skin cancer. Nanoparticles may promote the penetration of the skin. Extensive research has been done into producing nanoparticles for topical distribution; nevertheless, relatively little progress has been achieved in transferring them to the clinic for treating skin malignancies. The prior art features the critical concepts of skin malignancies and techniques in current clinical care. The present review gives a complete viewpoint of the numerous nanoparticle technologies studied for the topical treatment of skin malignancies and outlines the hurdles that hamper its advancement from the bench to the bedside. The review also intends to give knowledge of the routes that control nanoparticle penetration into the skin and their interactions inside the tissue.
Introduction
Skin cancer is the most common disease in Americans and European countries; one in five Americans is impacted by skin cancer, making it the most frequent disease in the United States and did not account for nonmelanoma skin cancer (NMCS) in the last of 2020 (Katalinic et al., 2003). One million new cases are accounted for in the United State. Although melanoma accounts for only 1% of all skin cancers, it causes the majority of deaths, and it is expected that almost 7,000 people will die from melanoma by the end of the year (Sigmundsdottir, 2010). Over 5.4 million cases of NMSC are treated in the U.S. each year. Basal cell carcinoma (BCC) is the most common form and is usually slow growing and locally invasive (Jain et al., 2020). Squamous cell carcinoma (SCC) is the second most common form of non-melanomatous skin cancer, accounting for approximately 20%–30% of new cases (Leiter-Stöppke et al., 2014). Skin is the largest organ of body it is a potential alternative route of administration for topical and transdermal delivery of many drugs (Rogers et al., 2012). Skin can be used to deliver drugs locally in skin cancer because it offers several advantages like avoidance of the problems associated with stomach emptying, pH, deactivation due to enzymes, and avoidance of first-pass metabolism, which are generally associated with oral administration (Kosmadaki and Gilchrest, 2002; Simões et al., 2015). Another major advantage of topical products is the ease of termination of therapy simply by removing the formulation from the skin surface. Novel topical formulations can be used to manipulate the barrier function of the skin (Lewis and Weinstock, 2007).
Nanocarriers are colloidal drug carrier systems having submicron particle sizes typically <500 nm in the range (Guy et al., 2015). It is a nanomaterial being used as a transport module for another substance, such as drug nanoparticles are promising materials used to deliver biologically active molecules at the desired rate, and the desired time. Nanocarriers can increase solubility, stability, and bioavailability, decrease toxicities, and site-specific drug delivery via different routes and different types of nanomaterial used as a nanocarrier (Lewis and Weinstock, 2004). In the recent decade, topical carriers have provided a unique mode for improving skin penetration across the skin surface. Hence for localized drug delivery, topical carriers favour much more attention to attending desired plasma concentration for a longer period of time and its benefits in chronic conditions. Thus, the prior art features critical appraisal in various nanocarrier along with the literature survey, recent advances and future prospects. The aim of the present review focuses on recent advances in localized topical drug delivery for skin melanoma.
Anatomy of the human skin
The skin comprises three layers, epidermis, dermis, and hypodermis (Andrews et al., 2013). The skin of an average adult covers a surface area of approximately two sq. m. and receives about one-third of the blood circulating through the body and serves as a permeability barrier against the topical absorption of various chemical and biological agents (Bouwstra and Honeywell-Nguyen, 2002). The outermost layer of the epidermis, stratum corneum (SC), is approximately 10–20 μm thick and is non-viable. It is formed by stratified, squamous epithelium composed mainly of keratinocytes, melanocytes, Merkel cells, and Langerhans cells (Jensen et al., 2011). Each cell is approximately 40 μm in diameter and 0.5 μm thick, and the intercellular space between adjacent cells is not more than 0.1 μm. Thus, the drug must pass through the intact SC layer for dermal availability. Once a medication has crossed the SC, it may build up in the nearby fatty tissues, leading to higher tissue concentration for a longer period of time (Prochazka, 2000). To have the edge over alternative delivery methods, tissue localization of medicines applied topically can be investigated. Cancer, vaccines, pain relief, and hormone therapy are just a few of the many possible applications for targeted medication delivery. Systems for delivering drugs via the skin can lower the dosage necessary for a therapeutic effect, minimizing side effects. Drug penetration into specific areas without considerably entering the bloodstream, resulting in improved efficacy due to high local concentration, is one benefit of localized drug delivery utilizing skin (Bogner and Wilkosz, 2005).
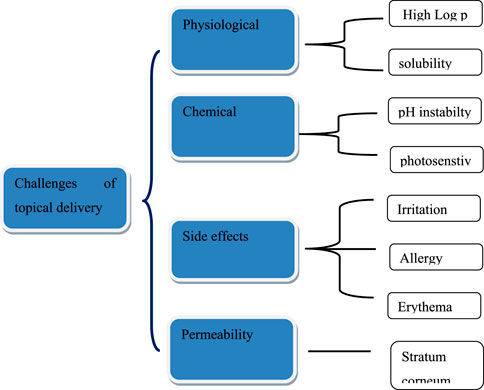
Challenges in transdermal delivery of drugs
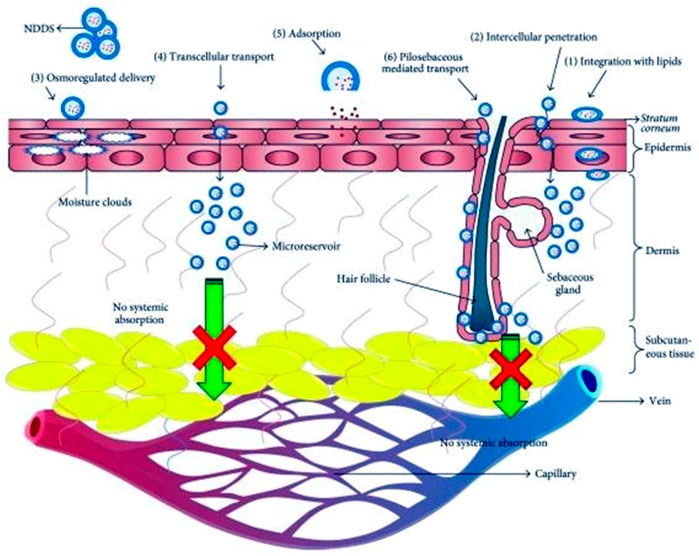
Skin delivery of actives has many benefits, but it also acquired some drawbacks regarding effective permeability and therapeutic effect. The stratum corneum (SC) is impermeable to active pharmacological substances with molecular weights >500 Da, having drugs with very high or low partition coefficients, and high melting temperatures (Bos and Meinardi, 2000). Although, applying a formulation to the skin seems simple, SC is the main barrier that causes the skin impermeability, which is the rate-limiting step for epidermal drug transport and can help achieve therapeutic drug concentration at the target site with low adverse effects (Donnelly et al., 2005; Calin et al., 2013). Passive skin-to-skin diffusion of compounds occurs during percutaneous absorption (Kleinpenning et al., 2006). The process of permeation can entail dispersion through shunts, notably those provided by the very widely dispersed hair follicles and eccrine glands, or transit through the epidermis itself that collectively called transepidermal absorption or transfollicular or shunt pathway (Benson and Watkinson, 2012). Drug molecules may enter the skin through sweat ducts or hair follicles during the initial transitory diffusion stage before being absorbed through the follicular epithelium and sebaceous glands (Kleesz et al., 2012). The predominant channel for topical penetration becomes diffusion via the intact stratum corneum, once a steady state has been established. Another restriction is the potential for allergic reactions to the medications or excipients used in the formulations. Figure 1 illustrates the difficulties in drug administration through cutaneous route. Figure 2 shows the mechanistic pathways for drug delivery using new carriers. New delivery systems interface with skin elements to efficiently distribute the loaded medicine to the different skin layers.
These carriers can deliver the topically applied actives to different skin layers depending on their compositional characteristics (Barua and Mitragotri, 2014). Dissolution within and release from the formulation are generally acquired two steps process by which a medicinal substance is released from a topical formulation and transported to the systemic circulation dividing up into the (SC) diffusion primarily by the lipidic intercellular route through the SC by partitioning into the viable aqueous epidermis.
The viable epidermis gets diffused into the upper dermis, papillary dermal layer and finally uptaken by microcirculation.
Summary on melanoma
Basal cell carcinoma (BCC), cutaneous malignant melanoma, and squamous cell carcinoma (SCC) are the three types of skin cancer (Zhang et al., 2012). In contrast to melanoma skin cancer, an aggressive form and one of the most chemotherapy-resistant malignancies, BCC and SCC are categorized as nonmelanoma skin cancers (Apalla et al., 2017). It results from repeated exposure to sunlight and is made up of pigment-producing cells called melanocytes, found in the deepest epidermis or basal layer (Stockfleth et al., 2008; Calzavara-Pinton et al., 2015). Since most melanocytes are found on the skin, melanoma tends to develop there; however, it can also affect the eyes and, less frequently, the mucous membranes of the nasal passages, mouth, pharyngeal cavity, vagina, and anal cavity. Melanoma, which makes up only 5% of all skin malignancies, is responsible for over 80% of all deaths brought on by skin cancer. Five phases are used to classify melanoma (Schulman and Fisher, 2009). Stage I is in the dermis without ulceration, while Stage 0 is non-invasive and visible in the epidermis. Melanoma in stage II is ulcerative and invades deeper dermal layers. Stages III and IV signify metastases to various bodily locations. Early melanomas can develop slowly and only appear on the skin’s surface for several months or even years.
When melanoma is identified and treated before it spreads to the lymph nodes, the 5-year survival rate is 99 percent (Armstrong and Kricker, 1994; Amini et al., 2010). It can be fatal if it spreads to other body parts unchecked. Malignant melanoma is brought on by melanin-containing melanocytes found in the basal epidermal layer. Most melanomas start in or close to an already present mole or skin-colored area. Normal moles are uniformly colored, have clearly defined edges, and have a round or oval shape, but melanomas look erratic and are typically larger than normal moles. Early melanomas can develop slowly, and only appear on the skin’s surface for several months or even years. Later, lesions thicken and penetrate the skin further. After then, melanoma has a propensity to grow quickly, invade lymph nodes, and spread via the bloodstream to the liver, lungs, bones, and brain. Men can acquire melanomas on their chest and back, while women are more likely to develop them on their legs, face, and neck (MacLennan et al., 1992). There are four different forms of melanoma exists in the biological science. The most prevalent kind, superficial spreading melanoma, affects the trunk and limbs. Lentigo maligna melanoma affects elderly adults. A rare variant of lentiginous melanoma that affects the palms of the hands or soles of the feet and is not related to Sun exposure is acral lentiginous melanoma. The rate-regulating membrane of the skin is called the stratum corneum (SC), composed of dead keratinized cells in a lipid matrix with hair follicles and sweat glands intermingled permeation. Drug penetration is hampered by several major factors, including hyperkeratinization of the stratum corneum (SC) brought on by Sun exposure (Diepgen and Mahler, 2002).
New topical formulations required for the treatment of melanoma
Since, melanoma is a progressive condition, chemopreventive therapies are appropriate. This can be accomplished by concentrating on the molecular pathways and processes involved in the disease’s progression (R Khan et al., 2015). Chemopreventive treatments for melanoma fall into three categories: those that prevent the development of the disease, secondly, those that stop premalignant lesions from becoming malignant, and thirdly, those that stop recurrence after primary melanomas have been successfully treated (Safwat et al., 2018). Immunotherapy, targeted therapy, and chemotherapy is given as injectables, such as ipilimumab and oral forms of vemurafenib are all approved treatments for melanoma. Numerous novel anticancer medicines have unfavourable pharmacokinetics, lack stability, have limited water solubility, and are irritating by nature. Conventional nanoformulations have several unfavourable impacts, including sub therapeutic effectiveness, dose-limiting side effects, and low patient quality of life. The inability to identify the protein targets that cause the disease, the absence of successful combination therapy and the inability to get active ingredients into tumor cells specifically are reasons for the ineffective treatment of melanoma. Studies for the chemoprevention of melanoma by medicines that prevent, stop, or reverse its development are being investigated because of the information that is currently known regarding the biology of pigment cells and the neoplastic changes found in melanoma. Sporn et al. first suggested the concept of chemoprevention, which is the use of synthetic or natural medicines to reverse, inhibit, or prevent molecular and histological premalignant lesions that develop with the advancement of invasive cancer (Mukherji et al., 1995). Researchers are looking into melanoma chemoprevention studies using substances that can stop, slow down, or even reverse the disease’s progression. Cancerogenesis can be prevented sensibly and cost-effectively by preventing tumor initiation, development, and advancement. Topical delivery with encapsulated actives can be a promising strategy for melanoma chemoprevention because these formulations can protect anticancer drugs from deterioration, allow for increased tumor penetration, improve drug stability, and lessen skin irritation by preventing the drug from coming into direct contact with the skin’s surface. Tyrosinase activity can be inhibited, which is advantageous because it promotes melanin overproduction in tissues and the serum.
A topical product for the treatment of melanoma has not yet received approval. The approved topical therapies for basal cell and squamous cell carcinoma are creams and solutions containing 2% and 5 % imiquimod, and 2% and 5% 5-fluorouracil (5-FU), respectively. The findings of in vivo testing of topically applied 1% cream of apomine for melanoma chemoprevention showed a significant decrease in tumor incidence (Kuehl et al., 2009). Additionally, apomine has been shown to prevent melanoma growth by 55%, and a topical formulation is currently undergoing clinical testing (Chinembiri et al., 2015). According to reports, actinic keratosis has been linked to melanoma when left untreated (Lebwohl et al., 2013). Precancerous actinic keratosis can be treated with commercially available topical formulations of diclofenac, 5-fluorouracil, ingenol mebutate, and imiquimod (Cornwell and Barry, 1993). Actinic keratosis can also be treated with photodynamic therapy using topical aminolevulinic acid, which works by having a photosensitizer convert a medication or a prodrug intracellularly. The process hurts because aminolevulinic acid makes the skin extremely sensitive (Alvi et al., 2011; Chinembiri et al., 2015). Resiquimod is a more recent medication that is 10–100 times more effective than imiquimod and regulates the immune system when given topically (Alvi et al., 2011). There is an increasing need for additional novel topical formulations that can address the severe side effects of conventional topical products like solutions and creams used to treat actinic keratosis, such as skin irritation, burning, redness, dryness, pain, swelling, and tenderness at the site of application (Kumar and Sinha, 2016). The inability to identify the protein targets that cause the disease, the absence of successful combination therapy and the inability to get active ingredients into tumor cells specifically are the collective reasons for the ineffective treatment of melanoma (Boakye et al., 2016). Additionally, Sun exposure-induced stratum corneum hyperkeratinization is a significant component that prevents drugs from penetrating the skin (Palmer and DeLouise, 2016).
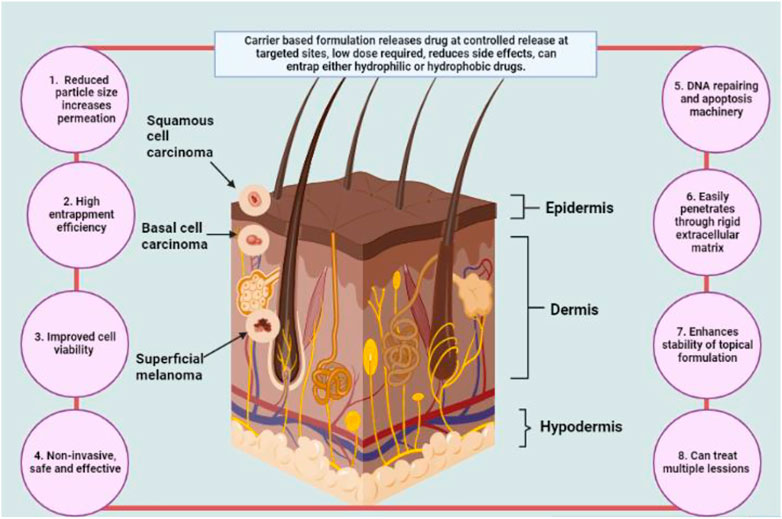
Encapsulated drug-loaded topical formulations to treat melanoma early on and offer chemoprevention are promising strategies (Anselmo and Mitragotri, 2016). Additionally, as high levels of tyrosinase in the serum and tissues promote melanin overproduction reducing tyrosinase activity may help prevent melanoma (Bos and Meinardi, 2000; Fahradyan et al., 2017). Drug-loaded nanosized formulations have several benefits, including the capacity to boost tumor penetration, improve drug stability and lessen skin irritation by preventing direct drug contact with the skin’s surface. Figure 3 describes possibly changes in skin physiochemical properties after incorporating actives into topical nanocarriers.
Numerous biophysical methods have been employed to pinpoint the principal skin penetration route (Ishii, 2017). Using methyl nicotinate as a probe, Albery and Hadgraft reported that, the area around the corneocytes, experienced intercellular penetration (Guy and Hadgraft, 1980; Hadgraft, 2004). Three pathways exist for substances to enter the skin, i.e., first the intercellular pathway (between the corneocyte), second the intracellular pathway (through cells and the lipids separating them) and the third pathway worked through skin appendages like hair follicles and sweat glands (Shah et al., 2007). Iontophoresis, sonophoresis, and electroporation are a few examples of physical enhancers that can be used and chemical enhancers like terpenes, flavonoids are utilized to increase bioavailability. Electricity is used in iontophoresis and electroporation to increase penetration. Using a greater voltage during electroporation, the stratum corneum lipid bilayer might have its structural integrity disturbed. When compared to passive delivery, this method can boost the transport of actives through the skin by 60 times. Sonophoresis, on the other hand, uses ultrasound to create tiny cavitations in the skin to help drugs diffuse. However, due to their high cost, adsorption of these physical penetration procedures uses is restricted. Applying chemical penetration enhancers that temporarily disrupt SC bilayers and restore them to function for enhanced skin penetration is quite simple. As a result of better partitioning between the formulation and (SC), this phenomenon increases actives penetration into tumors cells quite rapidly. Penetration promoters include substances like oleic acid, azone, ethanol, propylene glycol, transcutol, monoolein and dimethylsulfoxide (DMSO). DMSO and monoolein are the most widely used and researched chemical enhancers for topical chemotherapy. Compared to DMSO, monoolein is less harmful and biodegradable. Following are the few instances that support the importance of newer excipients in novel formulations in delivering enhanced efficacy compared to conventional systems.
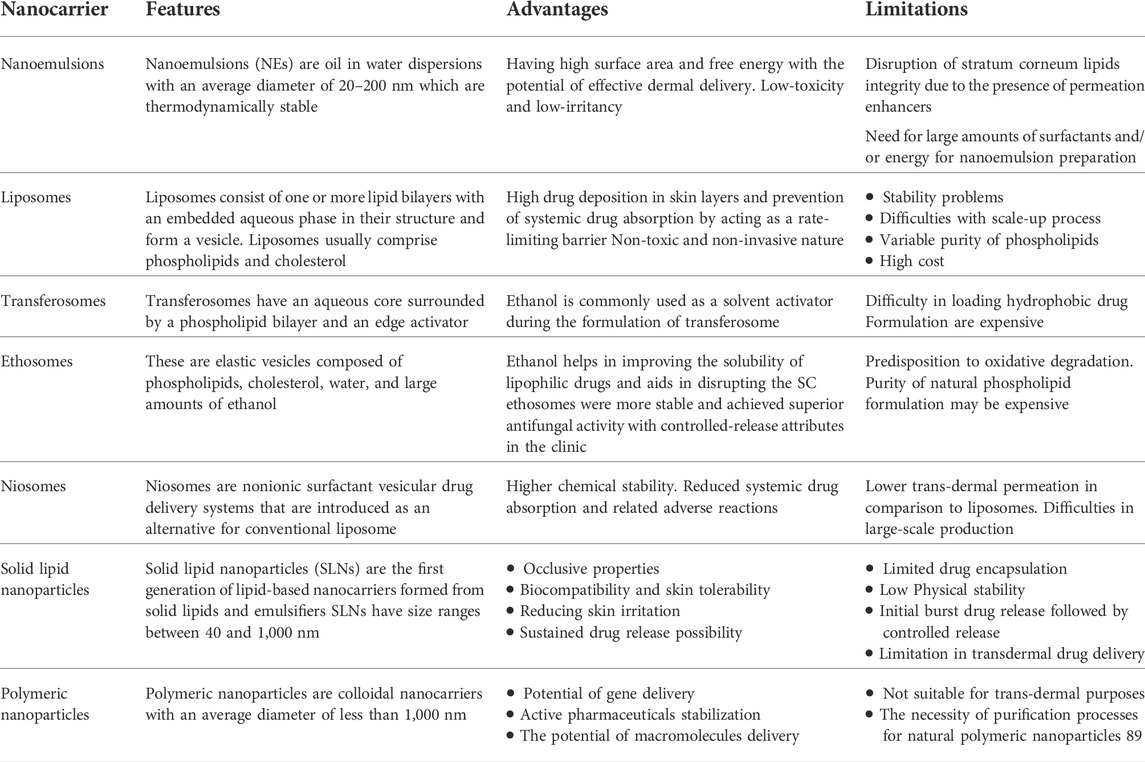
According to published research, ceramides resemble SC lipids they are inactive components in innovative microemulsion formulations that facilitate medication administration into skin compartments and enable deeper penetration than a traditional hydrophilic cream. Similarly, phospholipid-based liposomes containing cholesterol, palmitic acid, ceramides, and cholesterol sulphatesmight show higher drug accumulation in the epidermis and dermis. Additionally, due to the flexibility of the vesicles and the changed membranes, modified liposomes containing oleic acid demonstrate high deposition in the skin and boost the diffusion coefficient of a medication. Published research supports drug delivery methods based on nanotechnologies, such as polymeric and lipidic nanoparticles, nanoemulsions, dendrimers, and liposomes, to improve medication penetration through the skin. Encapsulated 5-fluorouracil and doxorubicin are two examples of the formulations that reduced toxicity, avoided the reticuloendothelial system, and improved tumor uptake. The compiled advantages and disadvantages of topical nanocarriers are enlisted in Table 1.
Suggested methods for topical delivery of drugs
Microemulsions and nanoemulsion based systems
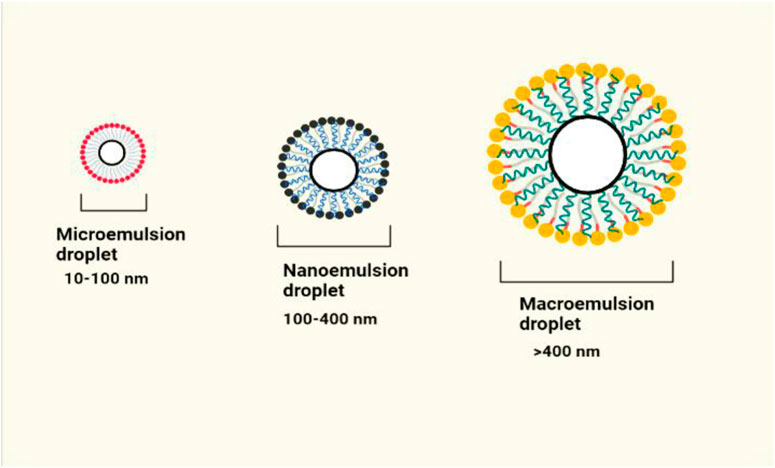
Microemulsions (MEs) are clear, colloidal, isotropic, and thermodynamically stable liquid dispersions of oil and water (Figure 4) (Subramanian et al., 2004). Surfactants and co-surfactants are used to create stable liquid dispersions of water and oil, known as MEs and NEs. Nonionic surfactants are preferred because of their cutaneous tolerability and balanced hydrophilic-lipophilic properties. The most widely used emulsifiers and co-emulsifiers are naturally occurring or modified lecithin, block copolymers that contain polyethylene oxide (PEO), castor oil derivatives with PEG conjugation (Cremophore EL), glycerides, and positively charged lipids for skin delivery. A multiphase system made up of water, oil, a surfactant, and a cosurfactant like alcohol that primarily serves as a cosolvent and demonstrates transparency by supplying globule size below 140 nm was the first to be referred to as a ME by Schulman in 1959 (Grampurohit et al., 2011). MEs provide various benefits for topical distribution, including the capacity to dissolve lipophilic medicines effectively, improved skin permeability, and a longer release of both lipophilic and hydrophilic medications for skin melanoma.
According to reports, oleic acid MEs exhibit a greater capacity for solubilizing drugs and a greater concentration of drug retention in the skin. MEs have a high capacity for drug loading. Because they have a high capacity for solubilizing drugs, they can get through the stratum corneum barrier and partition the medication into the skin. Polyoxyl castor oil derivatives like Cremophor EL and Cremophor RH 40, polysorbates, sorbitan monooleate, and different polyglycolyzed glycerides like Labrafils and Labrasol are nonionic surfactants used in topical formulations (Subramanian et al., 2005). According to conventional insight, surfactants with low HLB values of 3-6 are employed to create w/o ME, whereas those with high HLB values of 8–18 are favoured to create o/w ME for skin application. Co-surfactants are necessary for generating microemulsions when surfactants with HLB larger than 20 are % (Sharma et al., 2021). Conversion of microemulsion to emulgel favors improved topical permeation, better skin retention and deposition and less drug irritation. Moreover, ME based emulgel systems acquires improved drug loading capacity which further increases targeatibility and improved therapeutic effect. There are number of scientific reports published which potentiates the ME based emulgels for skin melanoma.
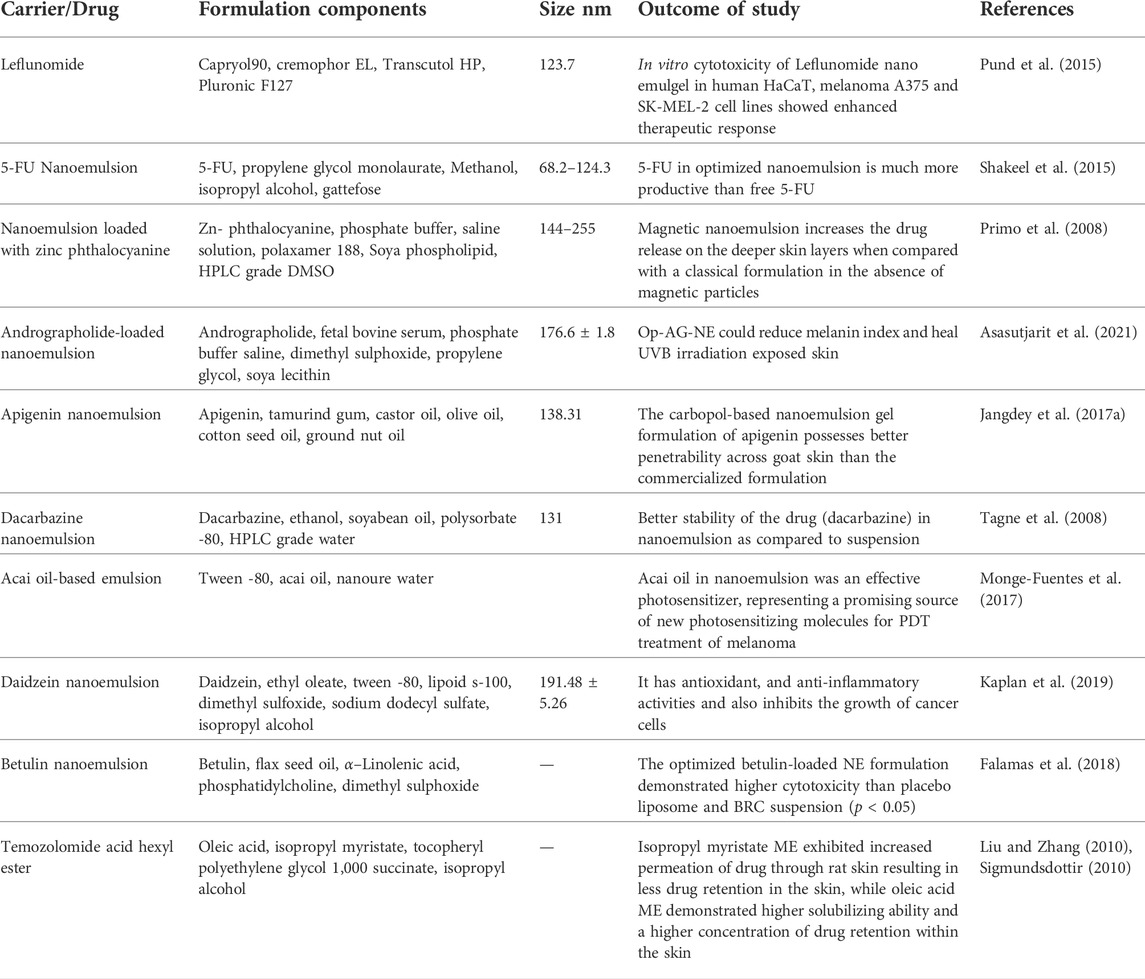
To combat the poor solubility and instability of a prodrug called temozolomide acid hexyl ester (TMZA-HE), created microemulsions of the substance. Oleic acid or isopropyl myristate was used to create microemulsions, and tocopheryl (vitamin E) polyethylene glycol 1,000 succinate and isopropyl alcohol were used as the co-surfactant and surfactant, respectively. In all systems, the microstructures were examined using freeze-fracture electron microscopy. In permeation trials, isopropyl myristate microemulsion showed enhanced drug permeability through rat skin, leading to decreased drug retention in the skin. In contrast, oleic acid microemulsion showed higher solubilizing capacity and higher concentrations of drug retention in the skin. Tagne et al. investigated dacarbazine nanoemulsions and their topical application in a nude mouse xenograft model of a human melanoma cell line. The prepared nanoemulsion’s mean particle size was 131 nm, showing less negative charge, suggesting improved skin permeability. Comparing the formulation to the medication suspension, the Formulation showed a tenfold reduction in tumor growth. This may be explained by (a) the smaller particle size of the nanoemulsion (111 nm) compared to the suspension (6,000 nm) and (b) the smaller zeta potential of the nanoemulsion (−3.2 against −89.1 mV, respectively). Leflunomide (LFD) nanoemulgel’s effectiveness for targeted therapy of skin damaged by melanoma was validated by Pund et al. permeation in ex vivo demonstrated a considerable improvement in flux, apparent permeability coefficient, steady-state diffusion coefficient, and drug deposition through rat abdomen skin. LFD nanoemulgel demonstrated increased therapeutic responses in melanoma A375 and SK-MEL-2 cell lines when tested in vitro. The soy isoflavone genistein, which is useful in treating a variety of cancers, was examined by Chen and Babu concerning several formulation parameters impacting physical *and chemical stability. Different oil phases (Labrafac WL1349, Ethyl Oleate, and Soybean Oil), surfactants (Cremophor EL, Poloxamer188, Lecithin, and Solutol HS-15), and co-surfactants were used to create ternary phase diagrams (ethanol and propanediol). The Formulation showed the maximum cytotoxicity toward the B16BL6 melanoma cell line67 and exceptional physical and chemical stability. A list of documented specialized emulsion systems for cutaneous usage in the treatment of melanoma is presented in Table 2.
Thermosensitive gel spray formulation
At 200°C, thermosensitive gel spray formulations behave Newtonian (like a liquid), and at 37°C, they behave non-Newtonian (like a gel). Poloxamer is the thermo-responsive polymer that is utilized the most frequently. Poloxamers, which come in different grades, are synthetic triblock copolymers consisting of poly (ethylene oxide), poly (propylene oxide), and poly (ethylene oxide). They display great compatibility with other excipients used in topical preparations and are typically considered non-toxic. Reported research has shown that these systems can increase therapeutic efficacy, prolonged drug release, and high drug retention at the site of action. The gel can be administered as a liquid formulation conveniently sprays across larger afflicted areas of skin thanks to its thermo-responsive nature. Due to their ease of administration, aesthetic appeal, optimal drug release characteristics, and superior physicochemical stability, these gels may provide various advantages over conventional gels for skin melanoma. These gels comprises of several polymers like Poloxamer 407, Chitin which showed controllable size and high loading capacity. Thermal gelation properties allow to form a depor at the target skin cancer cells and able to prolong the pharmacological action. Katas et al. developed topical thermosensitive gel formulation containing DsiRNA and showed reduced size of 146 nm with excellent stability. Moreover, the optimized formulation observed with improved cell viability of 88%–93%, as compared to pure gene.
Vesicular delivery systems
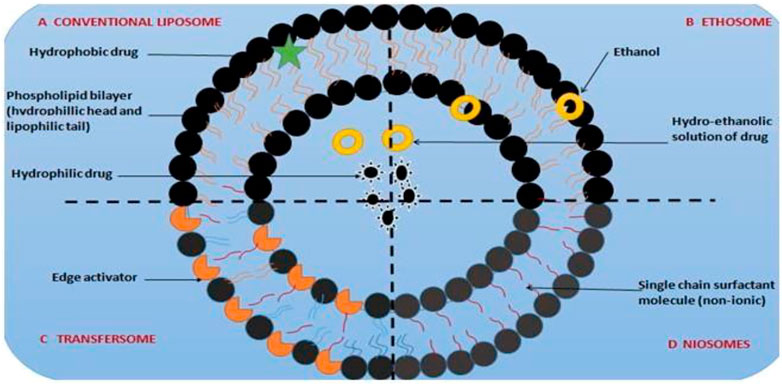
Vesicular drug delivery systems consist of one or more concentric bilayers and are highly organized assemblies. These vesicles are created when amphiphilic building components self-assemble in the presence of water (Tamilvanan et al., 2008; Vanniasinghe et al., 2009). These systems can localize the medication to the site of action, reducing the concentration of the drug at other places in the body, making them effective for targeted drug delivery for skin melanoma (Mezei and Gulasekharam, 1980). Since, conventional topical solution and cream formulations containing 5-FU have been sed to treat several skin cancers, 5-FU has attracted the interest of numerous researchers in developing abundant topical innovative formulations (Kirjavainen et al., 1996). According to studies, vesiculation of 5-FU enhances topical administration by achieving smooth and spherical nanovesicles (Maghraby et al., 2006). Compared to other nonvesiculized dosage forms, the optimized spherical nanovesivles provided decreased cytotoxicty by reducing the expression of biomarkers by achieving improved skin permeability and retention. Some of the most popularly studied vesicular systems include liposomes, transfersomes, ethosomes, and niosomes are examples of vesicular systems for topical delivery (Tabbakhian et al., 2006). A schematic illustration of vesicular systems is shown in Figure 5.
Liposomes
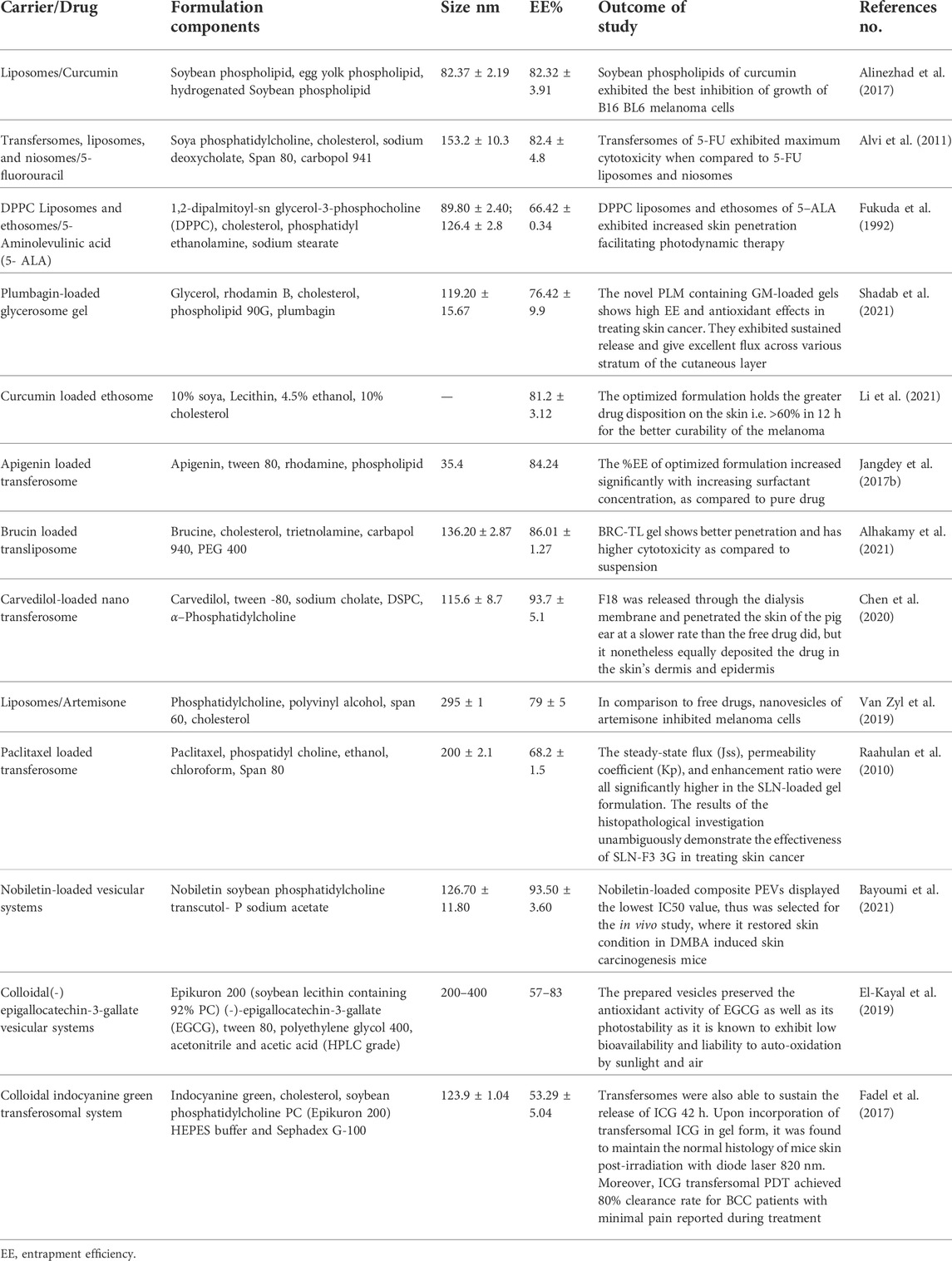
An aqueous core is encircled by a hydrophobic lipid bilayer membrane that contains phospholipids and cholesterol in liposomes, which are biocompatible and biodegradable vesicles (Trapasso et al., 2009; Cui et al., 2016). They allow for better active ingredient absorption through the skin. Although they are simple to prepare, they are prone to structural failure and oxidation (Sinico et al., 2005). The design, content, size, and drug release properties of liposomes are flexible. According to published clinical research, liposomal anticancer medication has less toxicity and better tolerance. For in vitro skin permeation and in vivo antineoplastic effect (Hsueh et al., 1995). Liposomes are versatile lipidic carriers observed with high cellular uptake, escaping reticuloendothelial system and provide enhanced anti-cancer activity, as evident from numerous case studies. In recent decade, the potential of liposomes in improving anti-cancer activity against skin was deeply studied. The potential components of liposomes imparts greater skin retention, selective effect on the potential biomarkers and allows easy pay load against melanoma cells. Recent literature findings displayed curcumin-loaded liposomal formulations comprising soybean phospholipids, egg yolk phospholipids, and hydrogenated soybean phospholipids. Curcumin-loaded soybean phospholipids demonstrated the best penetration and has largest ability to suppress the proliferation of B16BL6 melanoma cells, according to a comparative in vitro skin penetration investigation. Liposomal and 5-ALA formulations demonstrated that the DPPC liposomal formulation had improved protoporphyrin accumulation in tumor tissue, which aided in photodynamic cell killing and better skin penetration ability [Ref].
Transfersomes
Transfersomes are extremely flexible, self-assembled, and ultra deformable vesicles with an aqueous core and a complex lipid bilayer on each side (Cevc, 1996). These vesicles are self-regulating and self-optimizing because of their structure and composition (Honeywell-Nguyen and Bouwstra, 2005). Transfersomes can spontaneously penetrate the SC because they can efficiently cross various transport barriers (Boinpally et al., 2003). They have better efficacy in sustained release applications for topical medication administration and are more elastic than liposomes. A study comparing several vesicular formulations found that 5-FU-loaded transfersomes had a more lethal effect on cancer cell types than liposomes and niosomes (Cevc and Blume, 2001). Characterization of particle size and shape, zeta potential, viscosity, entrapment effectiveness, deformability, in vitro drug release, kinetics, and drug retention (Trotta et al., 2002; Elsayed et al., 2006). The transfersome was created using the solvent evaporation, whereas the reverse-phase evaporation method was employed to create the liposomes and niosomes (Touitou et al., 2000; Maghraby et al., 2001).
Ethosomes
Ethosomes are stretchy phospholipid-based vesicles that contain 20–45 percent ethanol. The polar head group area of the lipid molecules can interact with ethanol, a known permeation enhancer, to lower the melting point of the SC lipid and increase lipid fluidity and cell membrane permeability (Touitou et al., 2000). The additional ethanol makes the vesicular membranes extremely flexible, which enables the elastic vesicles to force their way through the pores (Liu and Hu, 2007). According to published research, ethosomal systems are more effective than traditional liposomes or hydro-alcoholic solutions at delivering chemicals through the skin in quantity and depth. The ability of 5-aminolevulinic acid (ALA)-containing ethosomes to boost skin formation of protoporphyrin IX (PpIX) in contrast to liposome was investigated (Barry, 2001). Confocal laser scanning microscopy was used to detect PpIX in live animal skin (CLSM).
In comparison to ALA in an aqueous solution, the enhancements of all the formulations ranged from 11 to 15 fold in terms of PpIX intensity. Additionally, ethosomes had better penetrating power than liposomes (Godin and Touitou, 2004). A 5-fluorouracil (5-FU) ethosomal formulation that Puri and Jain created was the subject of a comparison investigation (Paolino et al., 2005). It was possible to achieve a six-fold increase in drug deposition, decreased skin irritancy, and improved anti-tumor activity.
Niosomes
Niosomes are composed of nonionic surfactant vesicles and are similar to liposomes in structure (Keservani et al., 2010). These formulations are becoming more and more significant for cutaneous drug administration because they have traits such as improved drug penetration, prolonged drug release, increased drug stability, and the capacity to transport both hydrophilic and lipophilic drugs (Kumar and Rao, 2012; Marianecci et al., 2014; Dwivedi et al., 2018). Cholesterol, Span 80, and Bola-containing 5-FU niosomes were made and tested (Marianecci et al., 2014). In SKMEL-28 (human melanoma) and HaCaT (nonmelanoma skin cancer) cell lines, 5-FU-loaded bola-niosomes were reported to demonstrate favourable cytotoxic activity and four to eight fold increases in drug penetration compared to the free drug (Dwivedi et al., 2018). Furthermore, effectiveness of artemisinin in nanovesicular niosomes and solid lipid nanoparticles against human melanoma A-375 cells and human keratinocytes (HaCaT)as studied. The created formulations showed very low toxicity to healthy skin cells while exhibiting extremely selective cytotoxicity towards melanoma cells (Hamishehkar et al., 2013). Table 3 is a compilation of published anti-melanoma vesicular dermal formulations.
Microparticulate drug delivery systems
Major particulate drug delivery systems for topical application are represented by polymeric microspheres and lipid-based formulations like solid lipid nanoparticles and nano lipid carriers. Microcapsules, microspheres, and micro sponges are microparticulate systems of solid polymeric particles with sizes ranging from 0.1 to 1,000 nm. Numerous formulations of polymeric nanoparticulate materials have been created employing polymers, including polycaprolactone, poly-dl-lactic acid, and poly-lactic-co-glycolic acid (PCL). According to published research, polymeric and solid lipid nanoparticles facilitate prolonged drug release by favoring drug accumulation in the skin for several hours and preventing drug degdradation which prolonging the effect against melanoma. For such formulations to be optimized, it is crucial to characterize elements such as the nanoparticle’s mean diameter, flexibility, and surface charge. Solid lipid nanoparticles are more stable, have a longer drug release time, are simpler to sterilize, and can be produced in larger quantities than liposomes. Solid lipid nanoparticles have a significant advantage over polymeric nanoparticles in that organic solvents are not necessary for their preparation which imparts green synthesis and reduced toxicity towards skin (Desai et al., 1997).
According to Levy et al., the flux and percutaneous absorption of H-labelled 5-fluorouracil from formulations containing 0.5% fluorouracil porous microspheres were 20–40 times lower than those from commercial formulation (Jorizzo et al., 2002). The study also showed that, compared to 54% of the marketed 5% product, 86%–92% of the absorbed fluorouracil from the 0.5 %t microsphere formulation was still present in the skin after 24 h. The prolonged period of activity at a concentration one-tenth that of the standard formulation is made possible by the innovative microsphere formulation’s which observed with better skin retention (Zafrani et al., 2012).
Drug delivery methods utilizing lipid nanoparticles
Solid lipid particles with surfactants display the best in vivo tolerability, good physico-chemical stability, and prolonged drug release. Solid lipid particles are made up of isotropic lipids having FDA regulatory status which prepared in a lowe energy thermodynamically state and acquires Smicrometer size range (Gupta et al., 2013). Compared to liposomes, they have been described as lipidic drug carrier systems for topical applications that can replace polymers and enable large-scale manufacture at a relatively lower cost (Mandawgade and Patravale, 2008). Solid lipid microparticles (SLMs) have the potential for topical and transdermal medication delivery, although having received less research attention than solid lipid nanoparticles (SLNs) for skin applications (Fang et al., 2008). Several techniques, including solvent evaporation, melt dispersion, hot and cold homogenization, spray drying, and spray congealing, can be used to create SLMs (Štecová et al., 2007).
Since of their small size and intimate contact with the stratum corneum, lipid nanoparticles are ideal for dermal administration because they increase the amount of medication absorbed through the skin. An effective and safe substitute for colloidal emulsions, liposomes, and polymeric nanoparticles is the family of lipophilic particle-based colloidal drug delivery systems that include solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). Solid lipid nanoparticles are more stable, have a longer drug release time, are simpler to sterilize, and can be produced in larger quantities than liposomes.
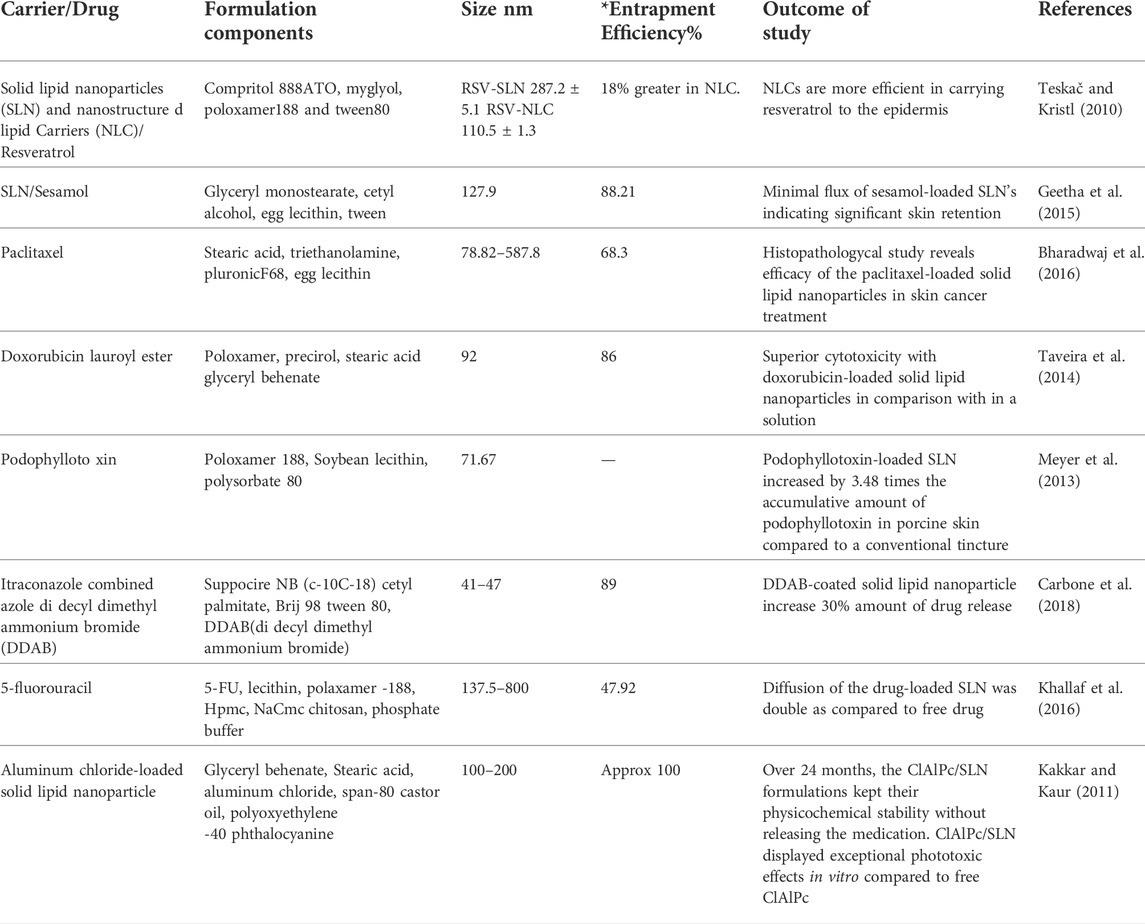
NLCs are made by combining solid and liquid lipids, whereas SLNs use solid lipids in place of the oils in an oil/water emulsion. Because solid lipid has reduced drug mobility. Thus, sustained medication release can enhanced by using nanoparticles. Since NLCs have a matrix with lower water content, they can improve drug loading, stability, bioavailability, and targeted administration in addition to reducing side effects, which is the main disadvantage of SLNs. Alkylating agent dacarbazine is utilized as a preferred medication for treating melanoma skin cancer. Cream with dacarbazine-loaded stearic acid nanostructured nanoparticles was examined by Hafeez and Kazmi (2017) According to the study, encapsulation efficiency is around 70%, and the spherical particles can penetrate cancer cells because they are between 10 and 20 nm in size. A higher rate of drug release than drug suspension was discovered, demonstrating the possibility of efficient melanoma treatment. Lecithin and poloxamer-188 were used to create highly penetrating shell-enriched solid lipid nanoparticles (SLN) that contained the hydrophilic medication 5-Fluorouracil (5-FU). The creation of inverted micelles within SLN was demonstrated by using 5-FU nanogold particles as a tracer. In comparison to the free drug the SLN formulation quadrupled the mobility of 5-FU via a hydrophobic membrane. Resveratrol-loaded SLN and NLC particles were created by Gokce et al. (2012). Utilizing high shear homogenization with ingredients such as compritol 888ATO, miglyol, poloxamer188, and tween 80. Particle size, polydispersity index, drug entrapment effectiveness, yield, and zeta potential were the metrics used to assess the drug-loaded solid lipid nanoparticles and nano lipid carriers. Nanostructured lipid carriers were more effective at transporting resveratrol through the epidermis, according to ex vivo skin studies.
Geetha et al. demonstrated the requirement for sesamol-loaded solid lipid nanoparticles to treat skin cancer by observing a sizable flow through mice’s skin after topical administration of sesamol. The in vivo skin retention and ex vivo skin permeation investigations supported the considerable retention in the skin with low flux across the skin after topical application of sesamol-loaded solid lipid nanoparticles in a cream base. The in vitro antiproliferative MTT assay and DNA fragmentation investigations in HL 60 cell lines supported the apoptotic nature of sesamol. Bharadwaj et al. used high-speed homogenization and ultrasonication techniques to generate paclitaxel solid lipid nanoparticles, which were then injected into a carbopol gel. It was discovered that the drug’s in vitro release from solid lipid nanoparticle dispersion was biphasic, with an early burst effect and then a gradual release.
Chen et al. have investigated solid lipid nanoparticles containing podophyllotoxin for topical use. Podophyllotoxin-loaded solid lipid nanoparticles enhanced the cumulative amount of podophyllotoxin in pig skin, i.e., 3.48 times over a standard tincture, according to an in vitro penetration investigation. Images of the formulated formulation showed robust Podophyllotoxin’s regulated release and localization in the epidermis can result in epidermal targeting. Using the solvent evaporation method, Chen et al. (2006) produced cationic, anionic, and neutral tripterine-loaded NLC. They assessed how the surface charge of nano lipid carriers affected in vitro skin permeability. Compared to pure tripterine, cationic tripterine-loaded NLC may have improved percutaneous penetration and anti-melanoma efficacy. Taveira et al. created cationic SLNs carrying doxorubicin and investigated how these particles affected the drug’s cytotoxicity and cellular absorption in B16F10 murine melanoma cells using a 32 factorial design-based model (Gratieri et al., 2017). Higher concentrations of stearic acid resulted in 97 percent entrapment efficiency. This demonstrated the interaction between the cationic charges on the molecules of doxorubicin and the negative charges in stearic acid. Doxorubicin’s cytotoxicity was considerably boosted by doxorubicin encapsulation in melanoma culture cell experiments. The effectiveness of doxorubicin-loaded solid lipid nanoparticles for topical administration against melanoma was examined (Tupal et al., 2016). An MTT assay and mice induced melanoma were used to test the in vitro and in vivo cytotoxicity of the optimized formulation on murine melanoma cells (B16F10), respectively. The results showed that doxorubicin-loaded solid lipid nanoparticles performed more cytotoxically than a doxorubicin solution. According to Peira et al., doxorubicin amide and lauroyl ester are lipophilic derivatives that can be trapped in spherical nanosized particles with drug entrapment efficiencies of 80%–94% w/w. MTT and colony-forming assays were used to examine the effect on cell proliferation in four different tumor cell lines (Ribeiro et al., 2017). The development of nanoparticulate drug delivery systems using a variety of biodegradable polymers is currently the research subject. In this regard, PLGA deserves special notice for its use in drug encapsulation since it is readily biodegradable, non-toxic, and exhibits a controlled release profile of its medication. Examples of reported particle topical administration systems for treating skin cancer are shown in Table 4.
Future prospects on topical formulations for skin cancer
Numerous studies on encapsulated formulations for enhanced cutaneous medication delivery for the treatment of melanoma have been published in the past 10 years. Chinembiri et al. looked at the impact of Pheroid TM technology, a different colloidal drug delivery system, on the skin permeability and anti-melanoma effectiveness of 5-fluorouracil. With Pheroid TM formulations, statistically significant amounts of 5-fluorouracil diffused into and through the skin, resulting in improved in vitro skin permeation and, as a result, medication getting to the intended place. Topical delivery of therapeutic peptides, proteins, and nucleic acids utilising such delivery vehicles has advanced alongside the use of small molecule medications, even though nanoparticulate systems produced employing lipids, polymers, and surfactants have grown considerably. In addition, more recent biological techniques that use biologicals as drug carriers, such as proteins and peptides, antibodies, and nucleic acids, have developed. Framework nucleic acid (FNA) and protein- or peptide-drug conjugates are two examples of these uncommon systems. Spherical nucleic acids are a novel nucleic acid-based nanoparticulate delivery technology that has demonstrated significant promise for topical application. The core of SNAs, which can be siRNA, mRNA, or oligonucleotides, is a gold nanoparticulate core that is densely covered in nucleic acid molecules. Similar ideas of using nucleic acids for topical drug administration as well as nucleic acids acting as a carrier for other pharmaceuticals were stimulated by advancements in the field of SNAs. FNAs, which represent a 3D network of single-stranded deoxyribonucleic acid molecules to produce nanoparticulate structural characteristics that can be used for drug administration, serve as an excellent illustration. The use of cell-penetrating peptides (CPPs), which provide safer choices for drug administration over skin, is another area that has experienced tremendous expansion (so-called SPPs, i.e., skin penetrating peptides). These peptides typically share the characteristics of being cationic and having an arginine-rich structure.
Conclusion
Melanoma is a progressive carcinoma disorder and devastates livinghood worldwide. A limited number of medications and delivery vehicles are available to combat this disease. Currently, topical drug delivery is a developing and challenging field in procuring skin cancer. There is a number of carriers available, like microemulsions, solid lipid nanoparticles, and nanostructured lipid carrier that produces marked therapeutic efficacy on the skin cancer. Hence, we highlighted the important properties of carriers along with mechanistic insignts and applications in the developing field. The outcome of this article produces insights on the skin cancer mediated drug delivery system.
Author contributions
DS conceptualized the idea; DS, NG, and GG, approved the final draft and submitted to the journal.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alhakamy, N. A., Aldawsari, H. M., Ali, J., Gupta, D. K., Warsi, M. H., Bilgrami, A. L., et al. (2021). Brucine-loaded transliposomes nanogel for topical delivery in skin cancer: Statistical optimization, in vitro and dermatokinetic evaluation. 3 Biotech. 11 (6), 288–313. doi:10.1007/s13205-021-02841-5
Alinezhad, V., Alinezhad, H., Ataee, R., and Ataie, A. (2017). Utilization of curcumine and nanocurcumine compounds in cancer therapy. Mazums-pbr. 3 (3), 1–11. doi:10.29252/pbr.3.3.1
Alvi, I. A., Madan, J., Kaushik, D., Sardana, S., Pandey, R. S., and Ali, A. (2011). Comparative study of transfersomes, liposomes, and niosomes for topical delivery of 5-fluorouracil to skin cancer cells: Preparation, characterization, in-vitro release, and cytotoxicity analysis. Anti-cancer drugs 22 (8), 774–782. doi:10.1097/cad.0b013e328346c7d6
Amini, S., Viera, M. H., Valins, W., and Berman, B. (2010). Nonsurgical innovations in the treatment of nonmelanoma skin cancer. J. Clin. Aesthet. Dermatol. 3 (6), 20–34.
Andrews, S. N., Jeong, E., and Prausnitz, M. R. (2013). Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 30 (4), 1099–1109. doi:10.1007/s11095-012-0946-7
Anselmo, A. C., and Mitragotri, S. (2016). Nanoparticles in the clinic. Bioeng. Transl. Med. 1 (1), 10–29. doi:10.1002/btm2.10003
Apalla, Z., Nashan, D., Weller, R. B., and Castellsagué, X. (2017). Skin cancer: Epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol. Ther. (Heidelb). 7 (1), 5–19. doi:10.1007/s13555-016-0165-y
Asasutjarit, R., Sooksai, N., Fristiohady, A., Lairungruang, K., Ng, S-F., and Fuongfuchat, A. (2021). Optimization of production parameters for andrographolide-loaded nanoemulsion preparation by microfluidization and evaluations of its bioactivities in skin cancer cells and uvb radiation-exposed skin. Pharmaceutics 13 (8), 1290. doi:10.3390/pharmaceutics13081290
Barry, B. W. (2001). Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 14 (2), 101–114. doi:10.1016/s0928-0987(01)00167-1
Barua, S., and Mitragotri, S. (2014). Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano today 9 (2), 223–243. doi:10.1016/j.nantod.2014.04.008
Bayoumi, M., Arafa, M. G., Nasr, M., and Sammour, O. A. (2021). Nobiletin-loaded composite penetration enhancer vesicles restore the normal miRNA expression and the chief defence antioxidant levels in skin cancer. Sci. Rep. 11 (1), 20197–20218. doi:10.1038/s41598-021-99756-1
Benson, H. A., and Watkinson, A. C. (2012). Topical and transdermal drug delivery: Principles and practice. John Wiley & Sons.
Bharadwaj, R., Das, P. J., Pal, P., and Mazumder, B. (2016). Topical delivery of paclitaxel for treatment of skin cancer. Drug Dev. industrial Pharm. 42 (9), 1482–1494. doi:10.3109/03639045.2016.1151028
Boakye, C. H., Patel, K., Doddapaneni, R., Bagde, A., Behl, G., Chowdhury, N., et al. (2016). Ultra-flexible nanocarriers for enhanced topical delivery of a highly lipophilic antioxidative molecule for skin cancer chemoprevention. Colloids Surfaces B Biointerfaces 143, 156–167. doi:10.1016/j.colsurfb.2016.03.036
Bogner, R., and Wilkosz, M. (2005). Transdermal drug delivery part 1: Current Status. Available at: www. uspharmacist. comindex. asp? show= article&page= 8_1061htm.
Boinpally, R. R., Zhou, S-L., Poondru, S., Devraj, G., and Jasti, B. R. (2003). Lecithin vesicles for topical delivery of diclofenac. Eur. J. Pharm. Biopharm. 56 (3), 389–392. doi:10.1016/s0939-6411(03)00143-7
Bos, J. D., and Meinardi, M. M. (2000). The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 9 (3), 165–169. doi:10.1034/j.1600-0625.2000.009003165.x
Bouwstra, J., and Honeywell-Nguyen, P. (2002). Skin structure and mode of action of vesicles. Adv. drug Deliv. Rev. 54, S41–S55. doi:10.1016/s0169-409x(02)00114-x
Calin, M. A., Parasca, S. V., Savastru, R., Calin, M. R., and Dontu, S. (2013). Optical techniques for the noninvasive diagnosis of skin cancer. J. Cancer Res. Clin. Oncol. 139 (7), 1083–1104. doi:10.1007/s00432-013-1423-3
Calzavara-Pinton, P., Ortel, B., Venturini, M., Pan, R., Egberts, M. R., Nascimento, L. C., et al. (2015). Non-melanoma skin cancer, sun exposure and sun protection. J. Burn Care & Res. (LWW) 36 (4).
Carbone, C., Martins-Gomes, C., Pepe, V., Silva, A., Musumeci, T., Puglisi, G., et al. (2018). Repurposing itraconazole to the benefit of skin cancer treatment: A combined azole-DDAB nanoencapsulation strategy. Colloids Surfaces B Biointerfaces 167, 337–344. doi:10.1016/j.colsurfb.2018.04.031
Cevc, G., and Blume, G. (2001). New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers, Transfersomes. Biochimica Biophysica Acta - Biomembr. 1514 (2), 191–205. doi:10.1016/s0005-2736(01)00369-8
Cevc, G. (1996). Transfersomes, liposomes and other lipid suspensions on the skin: Permeation enhancement, vesicle penetration, and transdermal drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 13 (3-4), 257–388. doi:10.1615/critrevtherdrugcarriersyst.v13.i3-4.30
Chen, H., Chang, X., Du, D., Liu, W., Liu, J., Weng, T., et al. (2006). Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J. Control. release 110 (2), 296–306. doi:10.1016/j.jconrel.2005.09.052
Chen, M., Shamim, M. A., Shahid, A., Yeung, S., Andresen, B. T., Wang, J., et al. (2020). Topical delivery of carvedilol loaded nano-transfersomes for skin cancer chemoprevention. Pharmaceutics 12 (12), 1151. doi:10.3390/pharmaceutics12121151
Chinembiri, T. N., Gerber, M., Du Plessis, L., Du Preez, J., and Du Plessis, J. (2015). Topical delivery of 5-fluorouracil from Pheroid™ formulations and the in vitro efficacy against human melanoma. AAPS PharmSciTech 16 (6), 1390–1399. doi:10.1208/s12249-015-0328-7
Cornwell, P., and Barry, B. (1993). The routes of penetration of ions and 5-fluorouracil across human skin and the mechanisms of action of terpene skin penetration enhancers. Int. J. Pharm. 94 (1-3), 189–194. doi:10.1016/0378-5173(93)90023-9
Cui, H., Li, W., Li, C., Vittayapadung, S., and Lin, L. (2016). Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 32 (2), 215–225. doi:10.1080/08927014.2015.1134516
Desai, M. P., Labhasetwar, V., Walter, E., Levy, R. J., and Amidon, G. L. (1997). The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 14 (11), 1568–1573. doi:10.1023/a:1012126301290
Diepgen, T. L., and Mahler, V. (2002). The epidemiology of skin cancer. Br. J. Dermatol. 146, 1–6. doi:10.1046/j.1365-2133.146.s61.2.x
Donnelly, R. F., McCarron, P. A., and Woolfson, A. D. (2005). Drug delivery of aminolevulinic acid from topical formulations intended for photodynamic therapy. Photochem. Photobiol. 81 (4), 750–767. doi:10.1562/2004-08-23-ir-283
Dwivedi, A., Mazumder, A., and Nasongkla, N. (2018). Layer-by-layer nanocoating of antibacterial niosome on orthopedic implant. Int. J. Pharm. 547 (1-2), 235–243. doi:10.1016/j.ijpharm.2018.05.075
El-Kayal, M., Nasr, M., Elkheshen, S., and Mortada, N. (2019). Colloidal (-)-epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: A comprehensive experimental study with preclinical investigation. Eur. J. Pharm. Sci. 137, 104972. doi:10.1016/j.ejps.2019.104972
Elsayed, M. M., Abdallah, O. Y., Naggar, V. F., and Khalafallah, N. M. (2006). Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. Int. J. Pharm. 322 (1-2), 60–66. doi:10.1016/j.ijpharm.2006.05.027
Fadel, M., Samy, N., Nasr, M., and Alyoussef, A. A. (2017). Topical colloidal indocyanine green-mediated photodynamic therapy for treatment of basal cell carcinoma. Pharm. Dev. Technol. 22 (4), 545–550. doi:10.3109/10837450.2016.1146294
Fahradyan, A., Howell, A. C., Wolfswinkel, E. M., Tsuha, M., Sheth, P., and Wong, A. K. (Editors) (2017). Updates on the management of non-melanoma skin cancer (NMSC) (HealthcareMDPI).
Falamas, A., Dehelean, C. A., and Pinzaru, S. C. (2018). Monitoring of betulin nanoemulsion treatment and molecular changes in mouse skin cancer using surface enhanced Raman spectroscopy. Vib. Spectrosc. 95, 44–50. doi:10.1016/j.vibspec.2018.01.004
Fang, J-Y., Fang, C-L., Liu, C-H., and Su, Y-H. (2008). Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 70 (2), 633–640. doi:10.1016/j.ejpb.2008.05.008
Fukuda, H., Paredes, S., and Battle, A. MdC. (1992). Tumour-localizing properties of porphyrins in vivo studies using free and liposome encapsulated aminolevulinic acid. Comp. Biochem. Physiology Part B Comp. Biochem. 102 (2), 433–436. doi:10.1016/0305-0491(92)90147-j
Geetha, T., Kapila, M., Prakash, O., Deol, P. K., Kakkar, V., and Kaur, I. P. (2015). Sesamol-loaded solid lipid nanoparticles for treatment of skin cancer. J. drug Target. 23 (2), 159–169. doi:10.3109/1061186x.2014.965717
Godin, B., and Touitou, E. (2004). Mechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrier. J. Control. Release 94 (2-3), 365–379. doi:10.1016/j.jconrel.2003.10.014
Gokce, E. H., Korkmaz, E., Dellera, E., Sandri, G., Bonferoni, M. C., and Ozer, O. (2012). Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomedicine 7, 1841–1850. doi:10.2147/ijn.s29710
Grampurohit, N., Ravikumar, P., and Mallya, R. (2011). Microemulsions for topical use–a review. Ind. J. Pharm. Edu Res. 45 (1), 100–107.
Gratieri, T., Krawczyk-Santos, A. P., da Rocha, P. B., Gelfuso, G. M., Marreto, R. N., Taveira, S. F., et al. (2017). SLN-And NLC-encapsulating antifungal agents: Skin drug delivery and their unexplored potential for treating onychomycosis. Curr. Pharm. Des. 23 (43), 6684–6695. doi:10.2174/1381612823666171115112745
Gupta, M., Tiwari, S., and Vyas, S. P. (2013). Influence of various lipid core on characteristics of SLNs designed for topical delivery of fluconazole against cutaneous candidiasis. Pharm. Dev. Technol. 18 (3), 550–559. doi:10.3109/10837450.2011.598161
Guy, G. P., Machlin, S. R., Ekwueme, D. U., and Yabroff, K. R. (2015). Prevalence and costs of skin cancer treatment in the US, 2002− 2006 and 2007− 2011. Am. J. Prev. Med. 48 (2), 183–187. doi:10.1016/j.amepre.2014.08.036
Guy, R. H., and Hadgraft, J. (1980). A theoretical description relating skin penetration to the thickness of the applied medicament. Int. J. Pharm. 6 (3-4), 321–332. doi:10.1016/0378-5173(80)90115-5
Hadgraft, J. (2004). Skin deep. Eur. J. Pharm. Biopharm. 58 (2), 291–299. doi:10.1016/j.ejpb.2004.03.002
Hafeez, A., and Kazmi, I. (2017). Dacarbazine nanoparticle topical delivery system for the treatment of melanoma. Sci. Rep. 7 (1), 16517–16610. doi:10.1038/s41598-017-16878-1
Hamishehkar, H., Rahimpour, Y., and Kouhsoltani, M. (2013). Niosomes as a propitious carrier for topical drug delivery. Expert Opin. drug Deliv. 10 (2), 261–272. doi:10.1517/17425247.2013.746310
Honeywell-Nguyen, P. L., and Bouwstra, J. A. (2005). Vesicles as a tool for transdermal and dermal delivery. Drug Discov. today Technol. 2 (1), 67–74. doi:10.1016/j.ddtec.2005.05.003
Hsueh, Y-M., Cheng, G., Wu, M., Yu, H., Kuo, T., and Chen, C. J. (1995). Multiple risk factors associated with arsenic-induced skin cancer: Effects of chronic liver disease and malnutritional status. Br. J. Cancer 71 (1), 109–114. doi:10.1038/bjc.1995.22
Ishii, H. (2017). Skin permeation and disposition of therapeutic and cosmeceutical compounds. Springer, 263–271.Drugs in topical formulations
Jain, R., Sarode, I., Singhvi, G., and Dubey, S. K. (2020). Nanocarrier based topical drug delivery-A promising strategy for treatment of skin cancer. Curr. Pharm. Des. 26 (36), 4615–4623. doi:10.2174/1381612826666200826140448
Jangdey, M. S., Gupta, A., and Saraf, S. (2017). Fabrication, in-vitro characterization, and enhanced in-vivo evaluation of carbopol-based nanoemulsion gel of apigenin for UV-induced skin carcinoma. Drug Deliv. 24 (1), 1026–1036. doi:10.1080/10717544.2017.1344333
Jangdey, M. S., Gupta, A., Saraf, S., and Saraf, S. (2017). Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: In vitro evaluation. Artif. Cells, Nanomedicine, Biotechnol. 45 (7), 1452–1462. doi:10.1080/21691401.2016.1247850
Jensen, L., Petersson, K., and Nielsen, H. (2011). In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur. J. Pharm. Biopharm. 79 (1), 68–75. doi:10.1016/j.ejpb.2011.05.012
Jorizzo, J., Stewart, D., Bucko, A., Davis, S. A., Espy, P., Hino, P., et al. (2002). Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-2-or 4-week treatment in patients with actinic keratosis. Cutis 70 (6), 335–339.
Kakkar, V., and Kaur, I. P. (2011). Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 49 (11), 2906–2913. doi:10.1016/j.fct.2011.08.006
Kaplan, A. B. U., Cetin, M., Orgul, D., Taghizadehghalehjoughi, A., Hacımuftuoglu, A., and Hekimoglu, S. (2019). Formulation and in vitro evaluation of topical nanoemulsion and nanoemulsion-based gels containing daidzein. J. Drug Deliv. Sci. Technol. 52, 189–203. doi:10.1016/j.jddst.2019.04.027
Katalinic, A., Kunze, U., and Schäfer, T. (2003). Epidemiology of cutaneous melanoma and non‐melanoma skin cancer in schleswig‐holstein, Germany: Incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer). Br. J. Dermatol. 149 (6), 1200–1206. doi:10.1111/j.1365-2133.2003.05554.x
Keservani, R. K., Sharma, A. K., and Ramteke, S. (2010). Novel vesicular approach for topical delivery of baclofen via niosomes. Lat. Am. J. Pharm. 29 (8), 1364–1370.
Khallaf, R. A., Salem, H. F., and Abdelbary, A. (2016). 5-Fluorouracil shell-enriched solid lipid nanoparticles (SLN) for effective skin carcinoma treatment. Drug Deliv. 23 (9), 3452–3460. doi:10.1080/10717544.2016.1194498
Kirjavainen, M., Urtti, A., Jääskeläinen, I., Suhonen, T. M., Paronen, P., Valjakka-Koskela, R., et al. (1996). Interaction of liposomes with human skin in vitro—The influence of lipid composition and structure. Biochimica Biophysica Acta - Lipids Lipid Metabolism 1304 (3), 179–189. doi:10.1016/s0005-2760(96)00126-9
Kleesz, P., Darlenski, R., and Fluhr, J. (2012). Full-body skin mapping for six biophysical parameters: Baseline values at 16 anatomical sites in 125 human subjects. Skin. Pharmacol. Physiol. 25 (1), 25–33. doi:10.1159/000330721
Kleinpenning, M., Smits, T., Ewalds, E., Van Erp, P., Van De Kerkhof, P., and Gerritsen, M. (2006). Heterogeneity of fluorescence in psoriasis after application of 5‐aminolaevulinic acid: An immunohistochemical study. Br. J. Dermatology 155 (3), 539–545. doi:10.1111/j.1365-2133.2006.07341.x
Kosmadaki, M. G., and Gilchrest, B. A. (2002). The demographics of aging in the United States: Implications for dermatology. Archives dermatology 138 (11), 1427–1428. doi:10.1001/archderm.138.11.1427-a
Kuehl, P. J., Stratton, S. P., Powell, M. B., and Myrdal, P. B. (2009). Preformulation, formulation, and in vivo efficacy of topically applied apomine. Int. J. Pharm. 382 (1-2), 104–110. doi:10.1016/j.ijpharm.2009.08.016
Kumar, G. P., and Rao, P. R. (2012). Ultra deformable niosomes for improved transdermal drug delivery: The future scenario. Asian J. Pharm. Sci. 7 (1).
Kumar, S., and Sinha, V. R. (2016). Design, development and characterization of topical microemulsions of 5-fluorouracil for the treatment of non melanoma skin cancer and its precursor lesions. Anticancer. Agents Med. Chem. 16 (2), 259–268. doi:10.2174/1871520615666150907093551
Lebwohl, M., Shumack, S., Gold, L. S., Melgaard, A., Larsson, T., and Tyring, S. K. (2013). Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 149 (6), 666–670. doi:10.1001/jamadermatol.2013.2766
Leiter-Stöppke, U. M. B., Eigentler, T. K., and Garbe, C. (2014). Epidemiology of skin cancer. Adv. Exp. Med. Biol. 810, 120–140. doi:10.1007/978-1-4939-0437-2_7
Lewis, K. G., and Weinstock, M. A. (2004). Nonmelanoma skin cancer mortality (1988-2000): The Rhode Island follow-back study. Arch. Dermatol. 140 (7), 837–842. doi:10.1001/archderm.140.7.837
Lewis, K. G., and Weinstock, M. A. (2007). Trends in nonmelanoma skin cancer mortality rates in the United States, 1969 through 2000. J. Investigative Dermatology 127 (10), 2323–2327. doi:10.1038/sj.jid.5700897
Li, Y., Xu, F., Li, X., Chen, S-Y., Huang, L-Y., Bian, Y-Y., et al. (2021). Development of curcumin-loaded composite phospholipid ethosomes for enhanced skin permeability and vesicle stability. Int. J. Pharm. 592, 119936. doi:10.1016/j.ijpharm.2020.119936
Liu, D., and Zhang, N. (2010). Cancer chemotherapy with lipid-based nanocarriers. Crit. Rev. Ther. Drug Carr. Syst. 27 (5), 371–417. doi:10.1615/critrevtherdrugcarriersyst.v27.i5.10
Liu, J., and Hu, G. (2007). Advances in studies of phospholipids as carriers in skin topical application. J. Nanjing Med. Univ. 21 (6), 349–353. doi:10.1016/s1007-4376(07)60076-8
MacLennan, R., Green, A., McLeod, G., and Martin, N. (1992). Increasing incidence of cutaneous melanoma in Queensland, Australia. JNCI J. Natl. Cancer Inst. 84 (18), 1427–1432. doi:10.1093/jnci/84.18.1427
Maghraby, G. M. E., Williams, A. C., and Barry, B. W. (2006). Can drug‐bearing liposomes penetrate intact skin? J. Pharm. Pharmacol. 58 (4), 415–429. doi:10.1211/jpp.58.4.0001
Maghraby, G. M. E., Williams, A. C., and Barry, B. W. (2001). Skin delivery of 5‐fluorouracil from ultradeformable and standard liposomes in-vitro. J. Pharm. Pharmacol. 53 (8), 1069–1077.
Mandawgade, S. D., and Patravale, V. B. (2008). Development of SLNs from natural lipids: Application to topical delivery of tretinoin. Int. J. Pharm. 363 (1-2), 132–138. doi:10.1016/j.ijpharm.2008.06.028
Marianecci, C., Di Marzio, L., Rinaldi, F., Celia, C., Paolino, D., Alhaique, F., et al. (2014). Niosomes from 80s to present: The state of the art. Adv. colloid interface Sci. 205, 187–206. doi:10.1016/j.cis.2013.11.018
Meyer, T., Surber, C., French, L. E., and Stockfleth, E. (2013). Resiquimod, a topical drug for viral skin lesions and skin cancer. Expert Opin. investigational drugs 22 (1), 149–159. doi:10.1517/13543784.2013.749236
Mezei, M., and Gulasekharam, V. (1980). Liposomes-a selective drug delivery system for the topical route of administration I. Lotion dosage form. Life Sci. 26 (18), 1473–1477. doi:10.1016/0024-3205(80)90268-4
Monge-Fuentes, V., Muehlmann, L. A., Longo, J. P. F., Silva, J. R., Fascineli, M. L., de Souza, P., et al. (2017). Photodynamic therapy mediated by acai oil (euterpe oleracea martius) in nanoemulsion: A potential treatment for melanoma. J. Photochem. Photobiol. B Biol. 166, 301–310. doi:10.1016/j.jphotobiol.2016.12.002
Mukherji, B., Chakraborty, N. G., Yamasaki, S., Okino, T., Yamase, H., Sporn, J. R., et al. (1995). Induction of antigen-specific cytolytic T cells in situ in human melanoma by immunization with synthetic peptide-pulsed autologous antigen presenting cells. Proc. Natl. Acad. Sci. U. S. A. 92 (17), 8078–8082. doi:10.1073/pnas.92.17.8078
Palmer, B. C., and DeLouise, L. A. (2016). Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 21 (12), 1719. doi:10.3390/molecules21121719
Paolino, D., Lucania, G., Mardente, D., Alhaique, F., and Fresta, M. (2005). Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Release 106 (1-2), 99–110. doi:10.1016/j.jconrel.2005.04.007
Primo, F. L., Rodrigues, M. M., Simioni, A. R., Bentley, M. V., Morais, P. C., and Tedesco, A. C. (2008). In vitro studies of cutaneous retention of magnetic nanoemulsion loaded with zinc phthalocyanine for synergic use in skin cancer treatment. J. Magnetism Magnetic Mater. 320 (14), e211–e214. doi:10.1016/j.jmmm.2008.02.050
Prochazka, A. V. (2000). New developments in smoking cessation. Chest 117 (4), 169S–75S. doi:10.1378/chest.117.4_suppl_1.169s
Pund, S., Pawar, S., Gangurde, S., and Divate, D. (2015). Transcutaneous delivery of leflunomide nanoemulgel: Mechanistic investigation into physicomechanical characteristics, in vitro anti-psoriatic and anti-melanoma activity. Int. J. Pharm. 487 (1-2), 148–156. doi:10.1016/j.ijpharm.2015.04.015
R Khan, N., Harun, S. M., Nawaz, A., Harjoh, N., and Wong, W. T. (2015). Nanocarriers and their actions to improve skin permeability and transdermal drug delivery. Curr. Pharm. Des. 21 (20), 2848–2866. doi:10.2174/1381612821666150428145216
Raahulan, S., Sanapalli, B. K. R., and Karri, V. V. S. R. (2010). Paclitaxel loaded transfersomal vesicular drug delivery for the treatment of melanoma skin cancers.
Ribeiro, S. MdF., Braga, C. B. M., Peria, F. M., Martinez, E. Z., Rocha, J. J. Rd, and Cunha, S. F. C. (2017). Effects of zinc supplementation on fatigue and quality of life in patients with colorectal cancer. Einstein Sao. Paulo. 15, 24–28. doi:10.1590/s1679-45082017ao3830
Rogers, H. W., Weinstock, M. A., Feldman, S. R., and Coldiron, B. M. (2012). Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population. JAMA Dermatol. 151 (10), 1081–1086. doi:10.1001/jamadermatol.2015.1187
Safwat, M. A., Soliman, G. M., Sayed, D., and Attia, M. A. (2018). Fluorouracil-loaded gold nanoparticles for the treatment of skin cancer: Development, in vitro characterization, and in vivo evaluation in a mouse skin cancer xenograft model. Mol. Pharm. 15 (6), 2194–2205. doi:10.1021/acs.molpharmaceut.8b00047
Schulman, J. M., and Fisher, D. E. (2009). Indoor ultraviolet tanning and skin cancer: Health risks and opportunities. Curr. Opin. Oncol. 21 (2), 144–149. doi:10.1097/cco.0b013e3283252fc5
Shadab, Md, Alhakamy, N. A., Aldawsari, H. M., Husain, M., Khan, N., Alfaleh, M. A., et al. (2021). Plumbagin-loaded glycerosome gel as topical delivery system for skin cancer therapy. Polymers 13 (6), 923. doi:10.3390/polym13060923
Shah, K. A., Date, A. A., Joshi, M. D., and Patravale, V. B. (2007). Solid lipid nanoparticles (SLN) of tretinoin: Potential in topical delivery. Int. J. Pharm. 345 (1-2), 163–171. doi:10.1016/j.ijpharm.2007.05.061
Shakeel, F., Haq, N., Al-Dhfyan, A., Alanazi, F. K., and Alsarra, I. A. (2015). Chemoprevention of skin cancer using low HLB surfactant nanoemulsion of 5-fluorouracil: A preliminary study. Drug Deliv. 22 (4), 573–580. doi:10.3109/10717544.2013.868557
Sharma, H., Kumar Sahu, G., and Kaur, C. D. (2021). Development of ionic liquid microemulsion for transdermal delivery of a chemotherapeutic agent. SN Appl. Sci. 3 (2), 215–310. doi:10.1007/s42452-021-04235-x
Sigmundsdottir, H. (2010). Improving topical treatments for skin diseases. Trends Pharmacol. Sci. 31 (6), 239–245. doi:10.1016/j.tips.2010.03.004
Simões, M. F., Sousa, J. S., and Pais, A. C. (2015). Skin cancer and new treatment perspectives: A review. Cancer Lett. 357 (1), 8–42. doi:10.1016/j.canlet.2014.11.001
Sinico, C., Manconi, M., Peppi, M., Lai, F., Valenti, D., and Fadda, A. M. (2005). Liposomes as carriers for dermal delivery of tretinoin: In vitro evaluation of drug permeation and vesicle-skin interaction. J. Control. Release 103 (1), 123–136. doi:10.1016/j.jconrel.2004.11.020
Štecová, J., Mehnert, W., Blaschke, T., Kleuser, B., Sivaramakrishnan, R., Zouboulis, C. C., et al. (2007). Cyproterone acetate loading to lipid nanoparticles for topical acne treatment: Particle characterisation and skin uptake. Pharm. Res. 24 (5), 991–1000. doi:10.1007/s11095-006-9225-9
Stockfleth, E., Ferrandiz, C., Grob, J. J., Leigh, I., Pehamberger, H., Kerl, H., et al. (2008). Development of a treatment algorithm for actinic keratoses: A European consensus. Eur. J. Dermatol. 18 (6), 651–659. doi:10.1684/ejd.2008.0514
Subramanian, N., Ghosal, S. K., and Moulik, S. (2005). Enhanced in vitro percutaneous absorption and in vivo anti-inflammatory effect of a selective cyclooxygenase inhibitor using microemulsion. Drug Dev. industrial Pharm. 31 (4-5), 405–416. doi:10.1080/03639040500214605
Subramanian, N., Ghosal, S. K., and Moulik, S. P. (2004). Topical delivery of celecoxib using microemulsion. Acta Pol. Pharm. 61 (5), 335–341.
Tabbakhian, M., Tavakoli, N., Jaafari, M. R., and Daneshamouz, S. (2006). Enhancement of follicular delivery of finasteride by liposomes and niosomes: 1. in vitro permeation and in vivo deposition studies using hamster flank and ear models. Int. J. Pharm. 323 (1-2), 1–10. doi:10.1016/j.ijpharm.2006.05.041
Tagne, J-B., Kakumanu, S., and Nicolosi, R. J. (2008). Nanoemulsion preparations of the anticancer drug dacarbazine significantly increase its efficacy in a xenograft mouse melanoma model. Mol. Pharm. 5 (6), 1055–1063. doi:10.1021/mp8000556
Tamilvanan, S., Venkateshan, N., and Ludwig, A. (2008). The potential of lipid-and polymer-based drug delivery carriers for eradicating biofilm consortia on device-related nosocomial infections. J. Control. Release 128 (1), 2–22. doi:10.1016/j.jconrel.2008.01.006
Taveira, S. F., De Santana, D. C., Araújo, L. M., Marquele-Oliveira, F., Nomizo, A., and Lopez, R. F. (2014). Effect of iontophoresis on topical delivery of doxorubicin-loaded solid lipid nanoparticles. J. Biomed. Nanotechnol. 10 (7), 1382–1390. doi:10.1166/jbn.2014.1834
Teskač, K., and Kristl, J. (2010). The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 390 (1), 61–69. doi:10.1016/j.ijpharm.2009.10.011
Touitou, E., Dayan, N., Bergelson, L., Godin, B., and Eliaz, M. (2000). Ethosomes—novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. release 65 (3), 403–418. doi:10.1016/s0168-3659(99)00222-9
Trapasso, E., Cosco, D., Celia, C., Fresta, M., and Paolino, D. (2009). Retinoids: New use by innovative drug-delivery systems. Expert Opin. drug Deliv. 6 (5), 465–483. doi:10.1517/17425240902832827
Trotta, M., Peira, E., Debernardi, F., and Gallarate, M. (2002). Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int. J. Pharm. 241 (2), 319–327. doi:10.1016/s0378-5173(02)00266-1
Tupal, A., Sabzichi, M., Ramezani, F., Kouhsoltani, M., and Hamishehkar, H. (2016). Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 33 (4), 372–380. doi:10.1080/02652048.2016.1200150
Van Zyl, L., Viljoen, J. M., Haynes, R. K., Aucamp, M., Ngwane, A. H., and du Plessis, J. (2019). Topical delivery of artemisone, clofazimine and decoquinate encapsulated in vesicles and their in vitro efficacy against Mycobacterium tuberculosis. AAPS PharmSciTech 20 (1), 33–11. doi:10.1208/s12249-018-1251-5
Vanniasinghe, A. S., Bender, V., and Manolios, N. (Editors) (2009). “The potential of liposomal drug delivery for the treatment of inflammatory arthritis,” Seminars in arthritis and rheumatism (Elsevier).
Zafrani, L., Gerotziafas, G., Byrnes, C., Hu, X., Perez, J., Lévi, C., et al. (2012). Calpastatin controls polymicrobial sepsis by limiting procoagulant microparticle release. Am. J. Respir. Crit. Care Med. 185 (7), 744–755. doi:10.1164/rccm.201109-1686oc
Keywords: melanoma, nanocarriers, stratum corneum, nanoparticles, noninvasiveness
Citation: Gupta N, Gupta GD and Singh D (2022) Localized topical drug delivery systems for skin cancer: Current approaches and future prospects. Front. Nanotechnol. 4:1006628. doi: 10.3389/fnano.2022.1006628
Received: 29 July 2022; Accepted: 10 October 2022;
Published: 21 October 2022.
Edited by:
Bhupendra Gopalbhai Prajapati, Ganpat University, IndiaReviewed by:
Maha Nasr, Ain Shams University, EgyptShalin Parikh, Senores Pharmaceuticals Pvt. Ltd., India
Copyright © 2022 Gupta, Gupta and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dilpreet Singh, ZGlscHJlZXQuZGFtYW5AZ21haWwuY29t
 Nimish Gupta
Nimish Gupta Dilpreet Singh
Dilpreet Singh