
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neuroanat. , 17 November 2022
Volume 16 - 2022 | https://doi.org/10.3389/fnana.2022.999033
This article is part of the Research Topic Women in Neuroanatomy View all 10 articles
 Lianqing Zhang1,2
Lianqing Zhang1,2 Jinli Meng1,3
Jinli Meng1,3 Hailong Li1,2
Hailong Li1,2 Mengyue Tang1,2
Mengyue Tang1,2 Zan Zhou3
Zan Zhou3 Xingning Zhou3
Xingning Zhou3 Li Feng3
Li Feng3 Xiangwei Li3
Xiangwei Li3 Yongyue Guo3
Yongyue Guo3 Yuanyuan He3
Yuanyuan He3 Wanlin He3*
Wanlin He3* Xiaoqi Huang1,2*
Xiaoqi Huang1,2*The hippocampus is highly plastic and vulnerable to hypoxia. However, it is unknown whether and how it adapts to chronic hypobaric hypoxia in humans. With a unique sample of Tibetans and acclimatized Han Chinese individuals residing on the Tibetan plateau, we aimed to build a neuroanatomic profile of the altitude-adapted hippocampus by measuring the volumetric differences in the whole hippocampus and its subfields. High-resolution T1-weighted magnetic resonance imaging was performed in healthy Tibetans (TH, n = 72) and healthy Han Chinese individuals living at an altitude of more than 3,500 m (HH, n = 27). In addition, healthy Han Chinese individuals living on a plain (HP, n = 72) were recruited as a sea-level reference group. Whereas the total hippocampal volume did not show a significant difference across groups when corrected for age, sex, and total intracranial volume, subfield-level differences within the hippocampus were found. Post hoc analyses revealed that Tibetans had larger core hippocampal subfields (bilateral CA3, right CA4, right dentate gyrus); a larger right hippocampus–amygdala transition area; and smaller bilateral presubiculum, right subiculum, and bilateral fimbria, than Han Chinese subjects (HH and/or HP). The hippocampus and all its subfields were found to be slightly and non-significantly smaller in HH subjects than in HP subjects. As a primary explorational study, our data suggested that while the overall hippocampal volume did not change, the core hippocampus of Tibetans may have an effect of adaptation to chronic hypobaric hypoxia. However, this adaptation may have required generations rather than mere decades to accumulate in the population.
Hypobaric hypoxia, the dramatically decreased availability of oxygen at high altitudes, poses significant challenges to humans residing there. The Tibetan Plateau is one of the highest plateaus in the world, and native Tibetans are adapted to life and reproduction in such a hypoxic environment with their unique protective physiological traits and were studied as an evolution paradigm for the high-altitude adaptation (Petousi and Robbins, 2014). However, newcomers to Tibet from lowland areas suffer from the physical and cognitive influence of high-altitude exposure (Yan, 2014). Studies consistently report noticeable declines in cognitive function, especially impairments in short-term memory, in migrants to a plateau above an altitude of 6,000 m (Virués-Ortega et al., 2004; Chen et al., 2017). Recently, brain structural and functional alterations related to cognitive declines following chronic exposure (2 years) to high altitude in college freshmen were also reported, including decreased gray matter volume in subcortical areas, disrupted white matter integrity, and altered resting-state networks (Chen et al., 2017, 2019, 2021). This evidence raises further questions regarding whether and how the human brain can adapt to high-altitude environments and how long this process requires.
The hippocampus is of particular interest in neuroscience research for its important function in memory and because it is a highly plastic structure that is vulnerable to multiple factors, including hypoxia. Furthermore, the hippocampus consists of several histologically and functionally different subfields, and our previous work found distinctive hippocampal neuroanatomic profiles in psychiatric disorders (Hu et al., 2019; Zhang et al., 2019, 2020). These subfields could be heterogeneously affected by hypoxia. In rats, chronic hypobaric hypoxia induced apoptosis, neuronal pyknosis, cell shrinkage, and consequent intercellular vacuolization in the CA1 and CA3 subfields of the hippocampus (Maiti et al., 2007; Hota et al., 2008a), and therapy such as ceftriaxone, oxygen enrichment or hypothermia could rescue hippocampal neurons from excitotoxicity and memory impairments in chronic hypobaric hypoxia (Hota et al., 2008b; Cai et al., 2021; Ranjan et al., 2022). In human subjects, how the hippocampus reacts to chronic hypobaric hypoxia and whether it can adapt remain largely unknown due to difficulties in finding research samples and the lack of research tools. In patients with obstructive sleep apnea, which causes intermittent hypoxia during sleep, damage to the hippocampus was found with cortical thinning in the molecular layer of the dentate gyrus (DG), CA1, and some layers of the entorhinal cortex (Owen et al., 2019). However, patients with obstructive sleep apnea (OSA) suffer from intermittent hypoxia, which is different from the constant hypoxia that occurs at high altitudes.
There is also evidence showing that permanent residents at high altitudes have adapted to hypoxia via multiple biological processes, including altered hemoglobin levels, regional blood flow, and O2 utilization components of the O2 transport system (Moore, 2017). Among them, the rs1769793 variant reduces EGLN1 expression, a gene that regulates cellular hypoxic responses, in skeletal muscle and the hippocampus (Liu et al., 2020). In new migrants to the plateau, while regional homogeneity (ReHo) as measured by resting-state fMRI was decreased over the brain, increased ReHo was found in the hippocampus, suggesting a special response in the hippocampus compared to other brain regions (Chen et al., 2017). Again, none of this evidence provides information on whether the hippocampus adapts to high altitude.
Herein, to better understand the reaction and adaptation of the human hippocampus to high altitude, we aim to build a neuroanatomic profile of the hippocampus in a unique sample of healthy Tibetan individuals living at high altitude (HT, adapted population), Han Chinese individuals living at high altitude (HH, acclimatized newcomers), and Han Chinese individuals living on the plain (PH, as a sea-level reference). As in the aforementioned reports showing the toxic effects of hypoxia in pyramidal cells in the hippocampus, we hypothesized that: (1) the hippocampal volumes would be larger in the TH as a result of adaptation and would be smaller in the HH as a result of the toxic effect of hypoxia; and (2) these adaptive/toxic effects of hypoxia would be observed primarily in the core hippocampal subfields (CA and DG), which showed sensitivity to hypoxia.
The study was approved by the local Research Ethics Committee, and written informed consent was obtained prior to study participation. Residents at an altitude of more than 3,500 m were recruited at the Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region (Hospital C.T.) with a poster advertisement. The inclusion criteria were as follows: (1) long-term residence at a high altitude of more than 3,500 m above sea level and residence at such an altitude within the last year; (2) arrival in Chengdu (sea level) for brief travel; (3) age between 18 and 75 years; and (4) right-handedness. The exclusion criteria were as follows: (1) an acute state of stress; (2) head injury, stroke or any other significant medical or neurologic conditions; (3) pregnancy; (4) systemic medical illness thought to interfere with brain function; and (5) contraindications to magnetic resonance imaging. Ultimately, a total of 96 high-altitude residents were recruited and assigned to the Tibetan high-altitude group (TH, n = 72) and the Han Chinese high-altitude group (HH, n = 27) based on their ethnicity. In addition, healthy age- and sex-matched Han Chinese individuals living on the plain (HP, n = 72) was recruited as a sea-level reference group. These HP subjects were screened to confirm the absence of any history of high-altitude exposure, any significant physical conditions or psychiatric disorders. Their demographic data are reported in Table 1; no significant difference was found in age, sex ratio, education level, body mass index (BMI), red blood cell count (RBC), mean corpuscular hemoglobin (MCH) or mean corpuscular hemoglobin concentration (MCHC).
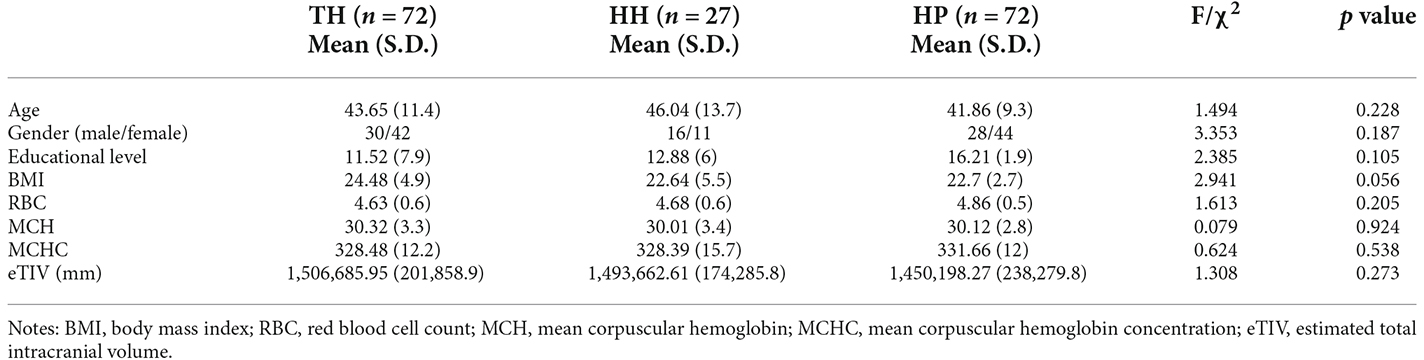
Table 1. Demographic and clinical data in Tibetans (TH) and in Han Chinese individuals living at High Altitude (HH) or on the Plain (HP).
MRI data were acquired using a Philips Achieva 3.0 T MRI system and an eight-channel phase array head coil. A high-resolution T1-weighted 3D spoiled gradient recall (SPGR) sequence was used [repetition time (TR) = 8.1 ms, echo time (TE) = 3.7 ms, flip angle = 12°, slice thickness = 1.0 mm]. The field of view was 256 × 256 mm2 with an acquisition matrix = 256 × 256, which yielded an actual voxel size = 1 × 1 × 1 mm3. Foam padding and earplugs were used to reduce head motion and scanner noise.
The hippocampal subfield volume analysis protocol followed the protocol used in our previous work (Hu et al., 2019; Zhang et al., 2019, 2020). Anatomic images were automatically segmented using FreeSurfer software (V. 7.1.1)1. The recon-all FreeSurfer analysis pipeline was applied. Briefly, T1-weighted images were corrected for head motion and transformed into Talairach space, and normalization and skull-strip procedures were performed (Sled et al., 1998; Fischl et al., 2002, 2004; Reuter et al., 2010). The estimated total intracranial volume (eTIV) of each subject was collected.
Hippocampal subfield segmentation was performed using a module in FreeSurfer software that was designed for the purpose; the module employs a tetrahedral mesh-based probabilistic atlas built from manually delineated hippocampus in both in vivo and ex vivo data (Iglesias et al., 2015). Using this algorithm, the overall volumes of the bilateral hippocampus and their subfields were obtained. Two sets of segmentations with different levels of hierarchy were generated: (1) head, body, and tail (usually referred to as subregions); and (2) CA1, CA3 (which contains CA2), CA4, the molecular and granule cell layers of the dentate gyrus (GC-ML-DG), the molecular layer, subiculum, presubiculum, parasubiculum, fimbria, and hippocampus–amygdala transition area (HATA). An example of the segmentation for a healthy subject is shown in Figure 1. All segmentation was visually verified following a quality control protocol that is similar to the ENIGMA protocol2. In brief, the segmentation of each subject was independently visually checked by two coauthors (LZ and MT), and any subject with segmentation results judged to be incorrect was excluded; no such segmentation failures occurred.
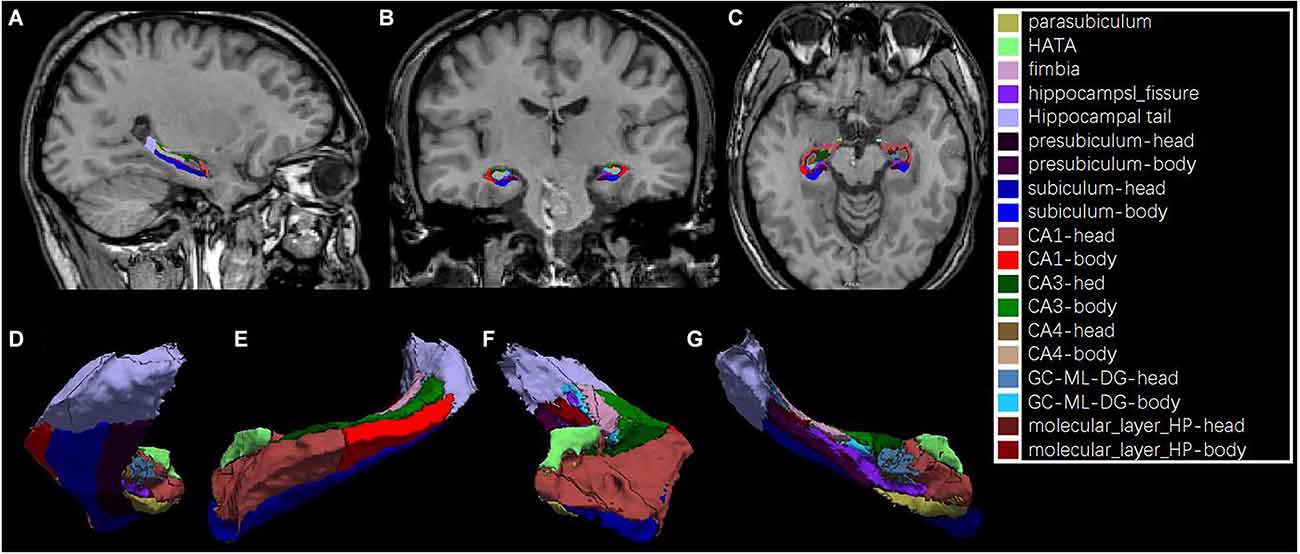
Figure 1. An example of hippocampal subfield segmentation in healthy Han Chinese individuals living on the plain. Upper row: (A) sagittal, (B) coronal and, (C) axial view of the hippocampus in the brain; Lower row: 3D reconstruction of the hippocampal subfields in (D) dorsal, (E) lateral, (F) frontal and, (G) medial view.
A multivariate analysis of covariance (MANCOVA) test was used to test for volume differences in overall hippocampal volume and its subregions/subfields across TH, HH and HP groups, with age, sex and eTIV as covariates, and the false discovery rate (FDR) method was used to correct multiple hypothesis testing issues across subregions/subfields. For subfields that showed significance after FDR correction, post-hoc tests were employed to determine where the difference between groups was. The partial eta squared (η2) was calculated to estimate effect sizes. Partial correlation analyses with age, sex, and eTIV as covariates were conducted between volumes and BMI, RBC, MCH, and MCHC levels to find potential associations.
The whole hippocampal volume or any of the subregions, including the head, body or tail, did not show a significant difference across groups when corrected for age, sex, and ICV (Table 2, Figure 2). However, subfield-level differences within the hippocampus were found. The “core hippocampus,” including the CA subfields and the GC-ML-DG, was largest in the TH group and smallest in the HH group, while the subiculum complex, fimbria, and HATA were smallest in the TH group, except for the right subiculum and right HATA (Table 2, Figure 3). These volume alterations were statistically significant in the subfields of the bilateral CA3, right CA4, right GC-ML-DG, right subiculum, bilateral presubiculum, bilateral fimbria, and right HATA. No associations were found between volumes and BMI, RBC, MCH or MCHC levels.
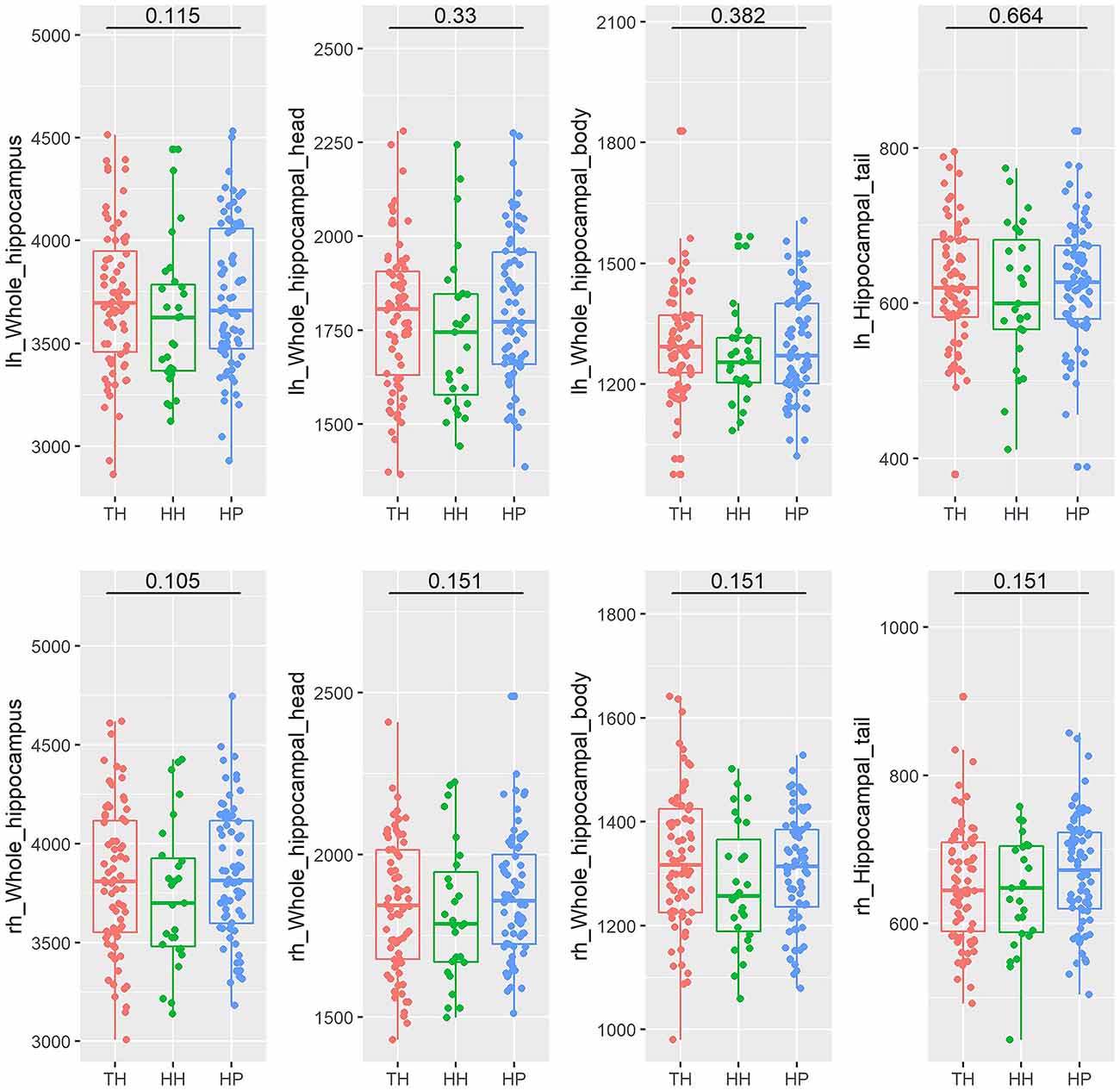
Figure 2. Box plots of the volumes of the entire hippocampus and its subregions (head, body, and tail) in healthy Tibetan individuals living at high altitude (TH, adapted population, shown in red), Han Chinese individuals living at high altitude (HH, acclimatized newcomers, shown in green), and Han Chinese individuals living on the plain (HP, sea-level reference, shown in blue). The annotations show the FDR-corrected p values. lh, left hemisphere; rh, right hemisphere.
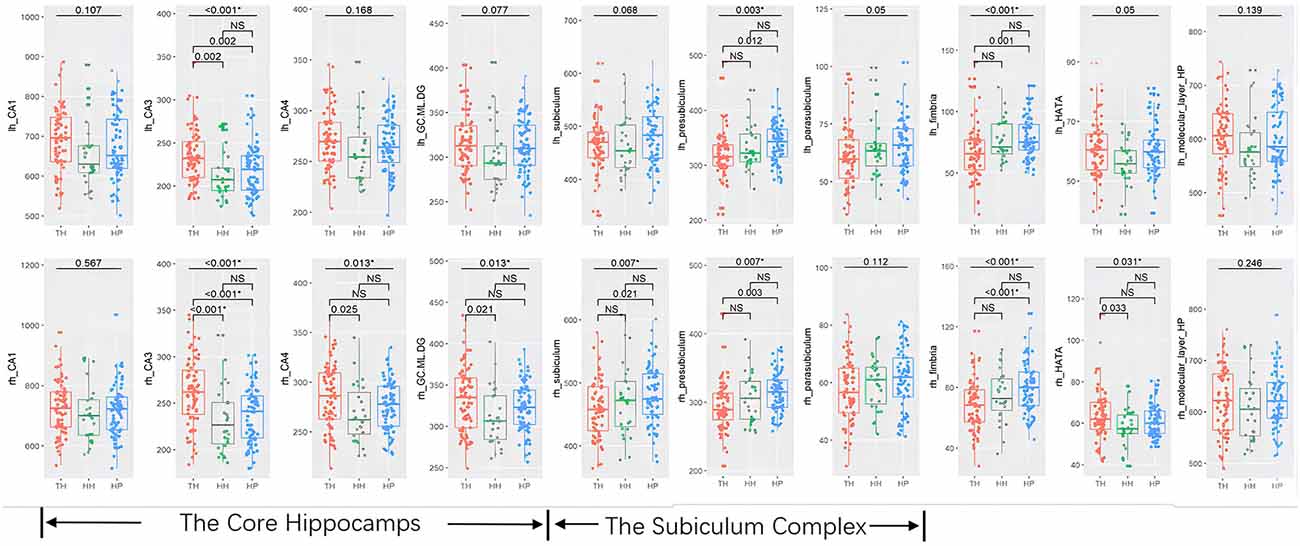
Figure 3. Box-plots of subfields of the hippocampus in healthy Tibetan individuals living at high altitude (TH, adapted population, shown in red), Han Chinese individuals living at high altitude (HH, acclimatized newcomers, shown in green), and Han Chinese individuals living on the plain (HP, sea-level reference, shown in blue). The annotations show the FDR-corrected p values, and * indicates statistical significance. lh, left hemisphere; rh, right hemisphere. NS, not significant.
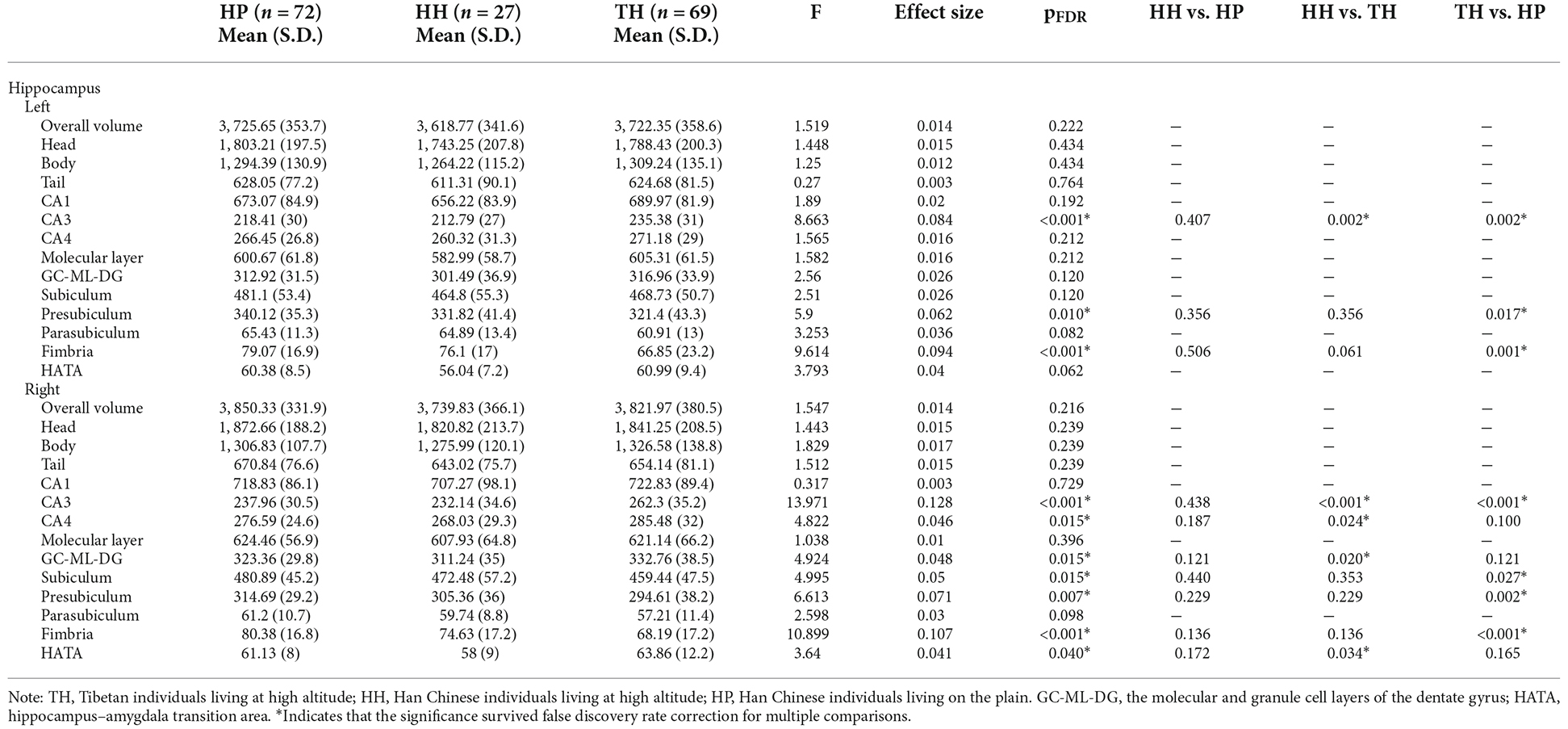
Table 2. Multivariate Analysis of Covariance (MANCOVA) and post hoc analysis of hippocampal volume in Tibetans (TH) and in Han Chinese individuals living at High Altitude (HH) or on the Plain (HP).
Post hoc analyses revealed that Tibetans had a larger core hippocampus and right HATA than Han Chinese individuals. Specifically, the TH group showed larger CA3 bilaterally than the HP (post hoc: left p = 0.002, right p < 0.001) or HH (post hoc: left p = 0.002, right p < 0.001) groups. The right CA4 (post hoc p = 0.024) and right GC-ML-DG (post hoc p = 0.02) were found to be significantly larger in the TH group than in the HH group (Table 2, Figure 3). For the subiculum complex, the bilateral presubiculum (post hoc: left p = 0.017, right p = 0.002) and right subiculum (post hoc: right p = 0.027) were found to be significantly smaller in the TH group than in the HP group (Table 2, Figure 3). Finally, bilateral fimbria were significantly smaller in the TH group than in the HP group (post hoc: left p = 0.001, right p < 0.001). No differences were found to be significant between the HH and HP groups.
To our knowledge, this is the first study to document hippocampal volume in normal residents at high altitudes. Notably, we included Tibetan and Han Chinese individuals living at high altitudes to find possible hippocampal adaptation to chronic hypoxia at different time scales (generational or lifetime) and recruited a group of normal Han Chinese subjects as the sea-level reference. Moreover, with subfield-level analyses, we are able to detect subfield-level hippocampal differences that would not be revealed by the global volume. We found that: (1) the Tibetans (generationally adapted to chronic hypoxia) had larger subfields in the “core hippocampus” (CA3, CA4, and GC-ML-DG) and smaller subfields of subiculum, presubiculum, and fimbria than Han subjects, suggesting an effect of adaptation that could be protective to the “core hippocampus”; and (2) Meanwhile, Han Chinese individuals who are residents on the plateau for decades were observed to have slightly but not significantly smaller hippocampal volume across subfields compared with HP, showing a mild effect of chronic hypobaric hypoxia on hippocampal volume that is not subregional specific.
Tibetans appear to have lived at high altitude the longest over the world among three human populations that have lived at high altitudes for millennia, suggesting that Tibetans have had more time and opportunity for natural selection in response to a hypoxic environment than any other high-altitude human population (Petousi and Robbins, 2014). Our reported neuroanatomic profile of the hippocampal subfield volume could indicate an effect of human hippocampal adaptation to the high-altitude environment. The larger “core hippocampus” found in Tibetans could be a result of their distinctive physiological traits or resistance to certain pathophysiological processes in high-altitude environments. These physiological traits mainly involve cardiovascular, respiratory, and hematopoietic physiology, which affect convective oxygen transport (Petousi and Robbins, 2014).
Multiple genomic loci that underwent natural selection in Tibetans were found, e.g., EGLN1 and EPAS1 encode major components of the hypoxia-inducible factor transcriptional system, which has a central role in oxygen sensing and coordinating an organism’s response to hypoxia (Lorenzo et al., 2014; Petousi and Robbins, 2014). A previous study reported that EGLN1 variants found to be associated with higher VO2max (maximal oxygen consumption, a metric commonly used to evaluate exercise performance) in hypoxia in Peruvian Quechua individuals could significantly reduce EGLN1 expression in the skeletal muscle and hippocampus compared with the other 48 tissues, including other brain tissues from other brain regions (Liu et al., 2020).
This finding could indicate that the hippocampus is more sensitive to increased oxygen transport efficiency than other brain regions. For example, aerobic exercise training could increase the hippocampus volume or effectively reverse age-related loss in hippocampal volume in human subjects, and this effect in the hippocampal volume is significantly correlated with the improvement in VO2max (Erickson et al., 2011; Aghjayan et al., 2021). Strikingly, with higher exercise performance (measured with exercise workload), Tibetans exhibit lower hemoglobin concentration and VO2max than acclimatized newcomers (Ge et al., 1994; Garruto et al., 2003; Petousi and Robbins, 2014). These findings indicate that Tibetans may have adapted in their own way to use oxygen more efficiently. Either way, elevated oxygen transport or consumption could be beneficial to the hippocampus, leading to increased hippocampal volume in its core subfields.
The volume increase in Tibetans was found selectively in the “core hippocampus,” especially the CA3, CA4, and DG. These subfields mainly consist of pyramidal neurons, and neurogenesis was found in these subfields. Meanwhile, a volume decrease was found in subfields of the (pre)subiculum and fimbria, which are widely considered the input/output structure of the hippocampus (Roddy et al., 2019). Such selectivity suggests that there are regionally dependent molecular pathways in Tibetans. Animal studies demonstrated that in adult rodents, aerobic exercise could increase synaptic strength and plasticity in the hippocampus and promote neurogenesis with the DG (van Praag et al., 1999; Rhodes et al., 2003; van Praag, 2008; Hötting and Röder, 2013). This suggests that a higher VO2max may promote neurogenesis in the hippocampus.
Meanwhile, hippocampal neurons are also sensitive to the toxic effect of hypoxia. It was reported that hypobaric hypoxia induced apoptosis in the CA1 region and damaged hippocampal pyramidal neurons in rats (Maiti et al., 2007; Hota et al., 2008a). We hypothesize that the protective physiological mechanism to hypoxia adapted in Tibetans is more sensitive in neuron types in the subfields of the “core hippocampus” but less sensitive in other subfields, which resulted in a unique neuroanatomic profile of the hippocampus in Tibetans found in the current study.
Although the difference was not statistically significant, the hippocampal volume was slightly smaller across all its subfields in acclimatized Han Chinese lowlanders who migrated to a high altitude compared with their sea-level counterparts. This is partly in line with our hypothesis that hypoxia is toxic to the hippocampus and may lead to volume decreases in highland newcomers. The observed hippocampal neuroanatomic profile indicates an effect of long-term acclimatization in lowlanders who migrate to high altitudes. A previous study showed significantly lowered accuracy in memory tests and longer reaction times after exposure, together with markedly decreased volumes or gray matter volumes in other subcortical nuclei but not the hippocampus in lowlanders who relocated to the Tibetan Plateau for 2 years (Chen et al., 2017, 2019). These results suggest that even if hypoxia is toxic to the hippocampus (as discussed above), the effect may not be substantial enough for its volume decrease to reach statistical significance.
As we recruited a sample of lowlanders who migrated to high altitude for longer than 2 years, the effect of their acclimatization to hypoxia could further normalize hippocampal alterations under hypoxia. For example, in rats, accumulation was found to promote neuronal regeneration together with memory/cognitive function recovery in the hippocampus during exposure to a chronic high-altitude hypoxic environment (Chen et al., 2022). Another possible explanation is that the effect of hypoxia on hippocampal neurons could be complex. While the hippocampus is more susceptible to acute hypoxic injury (Zhang et al., 2022), chronic intermittent hypobaric hypoxia could restore hippocampal function and may serve as a potential treatment for epilepsy-induced cognitive impairments (Sun et al., 2020). Taken together, the outcome of brain hypoxia may depend on the type, severity, exposure duration, and frequency of hypoxia (Burtscher et al., 2021). However, hippocampal neuron changes across time under chronic continuous hypobaric hypoxia on the plateau remain to be clarified.
Our study has some limitations. First, while it is interesting that we documented a unique neuroanatomic profile of the hippocampus in Tibetans, our result may not be generalizable to other highland populations (Andeans on the Andean Altiplano and Ethiopians on the Simien Plateau) because Tibetan populations may have followed different evolutionary pathways with their unique culture and daily habits (Beall, 2000). Second, the HH group, the Han Chinese individuals who migrated to a high altitude, had a relatively small sample size compared with the TH and HP groups and thus reduce the statistical power. Potential confounds related to migration (e.g., smoking history, stress from migrating, etc.) could contribute to hippocampal volume differences, hence, the results should be interpreted with caution. Finally, we did not report cognitive assessments for Tibetans which could be informative. We had tried to collect such data by translating these tests into local dialog. However, we found that more than 50% of Tibetan subjects could not finish the test and we believe that finding validated cognitive tests for Tibetan is a topic worth further study.
The data presented in the current study are available from the corresponding authors upon reasonable request.
The studies involving human participants were reviewed and approved by Ethics committee of Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region (Hospital C.T.). The patients/participants provided their written informed consent to participate in this study.
HL, YG, WH, and XH conceived and designed the study. ZZ, XZ, and LF collected MRI data and performed the quality control. LZ, HL, and MT analyzed the data and performed statistical analyses. LZ, JM, and XL interpreted the statistical outcomes and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Project of Sichuan Province (Grant No. 2021YJ0161), the Medical Research Project of Sichuan Province (Fund No. Q20042), the Science and Technology Project of Tibet Autonomous Region: (“The central government guides local projects,” Grant No. XZ202102YD0032C), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21041), The Research Project of Shanghai Science and Technology Commission (20dz2260300), and The Fundamental Research Funds for the Central Universities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aghjayan, S. L., Lesnovskaya, A., Esteban-Cornejo, I., Peven, J. C., Stillman, C. M., and Erickson, K. I. (2021). Aerobic exercise, cardiorespiratory fitness and the human hippocampus. Hippocampus 31, 817–844. doi: 10.1002/hipo.23337
Beall, C. M. (2000). Tibetan andean contrasts in adaptation to high-altitude hypoxia. Adv. Exp. Med. Biol. 475, 63–74. doi: 10.1007/0-306-46825-5_7
Burtscher, J., Mallet, R. T., Burtscher, M., and Millet, G. P. (2021). Hypoxia and brain aging: neurodegeneration or neuroprotection? Ageing Res. Rev. 68:101343. doi: 10.1016/j.arr.2021.101343
Cai, J., Ruan, J., Shao, X., Ding, Y., Xie, K., Tang, C., et al. (2021). Oxygen enrichment mitigates high-altitude hypoxia-induced hippocampal neurodegeneration and memory dysfunction associated with attenuated tau phosphorylation. High. Alt. Med. Biol. 22, 274–284. doi: 10.1089/ham.2020.0218
Chen, C., Li, B., Chen, H., Qin, Y., Cheng, J., He, B., et al. (2022). Epigallocatechin-3-Gallate ameliorated iron accumulation and apoptosis and promoted neuronal regeneration and memory/cognitive functions in the hippocampus induced by exposure to a chronic high-altitude hypoxia environment. Neurochem. Res. 47, 2254–2262. doi: 10.1007/s11064-022-03611-2
Chen, X., Li, H., Zhang, Q., Wang, J., Zhang, W., Liu, J., et al. (2019). Combined fractional anisotropy and subcortical volumetric abnormalities in healthy immigrants to high altitude: a longitudinal study. Hum. Brain Mapp. 40, 4202–4212. doi: 10.1002/hbm.24696
Chen, X., Liu, J., Wang, J., Xin, Z., Zhang, Q., Zhang, W., et al. (2021). Altered resting-state networks may explain the executive impairment in young health immigrants into high-altitude area. Brain Imaging Behav. 15, 147–156. doi: 10.1007/s11682-019-00241-1
Chen, X., Zhang, Q., Wang, J., Liu, J., Zhang, W., Qi, S., et al. (2017). Cognitive and neuroimaging changes in healthy immigrants upon relocation to a high altitude: a panel study. Hum. Brain Mapp. 38, 3865–3877. doi: 10.1002/hbm.23635
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U S A 108, 3017–3022. doi: 10.1073/pnas.1015950108
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. doi: 10.1016/s0896-6273(02)00569-x
Fischl, B., Salat, D. H., van der Kouwe, A. J., Makris, N., Segonne, F., Quinn, B. T., et al. (2004). Sequence-independent segmentation of magnetic resonance images. Neuroimage 23, S69–S84. doi: 10.1016/j.neuroimage.2004.07.016
Garruto, R. M., Chin, C. T., Weitz, C. A., Liu, J. C., Liu, R. L., and He, X. (2003). Hematological differences during growth among Tibetans and Han Chinese born and raised at high altitude in Qinghai, China. Am. J. Phys. Anthropol. 122, 171–183. doi: 10.1002/ajpa.10283
Ge, R. L., Chen, Q. H., Wang, L. H., Gen, D., Yang, P., Kubo, K., et al. (1994). Higher exercise performance and lower VO2max in Tibetan than Han residents at 4,700 m altitude. J. Appl. Physiol. (1985) 77, 684–691. doi: 10.1152/jappl.1994.77.2.684
Hota, S. K., Barhwal, K., Singh, S. B., and Ilavazhagan, G. (2008a). Chronic hypobaric hypoxia induced apoptosis in CA1 region of hippocampus: a possible role of NMDAR mediated p75NTR upregulation. Exp. Neurol. 212, 5–13. doi: 10.1016/j.expneurol.2008.01.030
Hota, S. K., Barhwal, K., Ray, K., Singh, S. B., and Ilavazhagan, G. (2008b). Ceftriaxone rescues hippocampal neurons from excitotoxicity and enhances memory retrieval in chronic hypobaric hypoxia. Neurobiol. Learn. Mem. 89, 522–532. doi: 10.1016/j.nlm.2008.01.003
Hötting, K., and Röder, B. (2013). Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 37, 2243–2257. doi: 10.1016/j.neubiorev.2013.04.005
Hu, X., Zhang, L., Hu, X., Lu, L., Tang, S., Li, H., et al. (2019). Abnormal hippocampal subfields may be potential predictors of worse early response to antidepressant treatment in Drug-Naïve patients with major depressive disorder. J. Magn. Reson. Imaging 49, 1760–1768. doi: 10.1002/jmri.26520
Iglesias, J. E., Augustinack, J. C., Nguyen, K., Player, C. M., Player, A., Wright, M., et al. (2015). A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115, 117–137. doi: 10.1016/j.neuroimage.2015.04.042
Liu, G., Zhao, W., Zhang, H., Wang, T., Han, Z., and Ji, X. (2020). rs1769793 variant reduces EGLN1 expression in skeletal muscle and hippocampus and contributes to high aerobic capacity in hypoxia. Proc. Natl. Acad. Sci. U S A 117, 29283–29285. doi: 10.1073/pnas.2010073117
Lorenzo, F. R., Huff, C., Myllymäki, M., Olenchock, B., Swierczek, S., Tashi, T., et al. (2014). A genetic mechanism for Tibetan high-altitude adaptation. Nat. Genet. 46, 951–956. doi: 10.1038/ng.3067
Maiti, P., Singh, S. B., Muthuraju, S., Veleri, S., and Ilavazhagan, G. (2007). Hypobaric hypoxia damages the hippocampal pyramidal neurons in the rat brain. Brain Res. 1175, 1–9. doi: 10.1016/j.brainres.2007.06.106
Moore, L. G. (2017). Measuring high-altitude adaptation. J. Appl. Physiol. (1985) 123, 1371–1385. doi: 10.1152/japplphysiol.00321.2017
Owen, J. E., BenediktsdOttir, B., Gislason, T., and Robinson, S. R. (2019). Neuropathological investigation of cell layer thickness and myelination in the hippocampus of people with obstructive sleep apnea. Sleep 42:zsy199. doi: 10.1093/sleep/zsy199
Petousi, N., and Robbins, P. A. (2014). Human adaptation to the hypoxia of high altitude: the Tibetan paradigm from the pregenomic to the postgenomic era. J. Appl. Physiol. (1985) 116, 875–884. doi: 10.1152/japplphysiol.00605.2013
Ranjan, R., Amitabh, Prasad, D. N., and Kohli, E. (2022). Hypothermic preconditioning attenuates hypobaric hypoxia induced spatial memory impairment in rats. Behav. Brain Res. 416:113568. doi: 10.1016/j.bbr.2021.113568
Reuter, M., Rosas, H. D., and Fischl, B. (2010). Highly accurate inverse consistent registration: a robust approach. Neuroimage 53, 1181–1196. doi: 10.1016/j.neuroimage.2010.07.020
Rhodes, J. S., van Praag, H., Jeffrey, S., Girard, I., Mitchell, G. S., Garland, T., et al. (2003). Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav. Neurosci. 117, 1006–1016. doi: 10.1037/0735-7044.117.5.1006
Roddy, D. W., Farrell, C., Doolin, K., Roman, E., Tozzi, L., and Frodl, T. (2019). The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol Psychiatry 85, 487–497. doi: 10.1016/j.biopsych.2018.08.021
Sled, J. G., Zijdenbos, A. P., and Evans, A. C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 17, 87–97. doi: 10.1109/42.668698
Sun, C., Fu, J., Qu, Z., Jia, L., Li, D., Zhen, J., et al. (2020). Chronic intermittent hypobaric hypoxia restores hippocampus function and rescues cognitive impairments in chronic epileptic rats via Wnt/β-catenin signaling. Front. Mol. Neurosci. 13:617143. doi: 10.3389/fnmol.2020.617143
van Praag, H. (2008). Neurogenesis and exercise: past and future directions. Neuromolecular Med. 10, 128–140. doi: 10.1007/s12017-008-8028-z
van Praag, H., Kempermann, G., and Gage, F. H. (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270. doi: 10.1038/6368
Virués-Ortega, J., Buela-Casal, G., Garrido, E., and Alcázar, B. (2004). Neuropsychological functioning associated with high-altitude exposure. Neuropsychol. Rev. 14, 197–224. doi: 10.1007/s11065-004-8159-4
Yan, X. (2014). Cognitive impairments at high altitudes and adaptation. High Alt. Med. Biol. 15, 141–145. doi: 10.1089/ham.2014.1009
Zhang, L., Hu, X., Lu, L., Li, B., Hu, X., Bu, X., et al. (2019). Abnormalities of hippocampal shape and subfield volumes in medication-free patients with obsessive-compulsive disorder. Hum. Brain Mapp. 40, 4105–4113. doi: 10.1002/hbm.24688
Zhang, L., Hu, X., Lu, L., Li, B., Hu, X., Bu, X., et al. (2020). Anatomic alterations across amygdala subnuclei in medication-free patients with obsessive-compulsive disorder. J. Psychiatry Neurosci. 45:190114. doi: 10.1503/jpn.190114
Keywords: hippocampus, hypoxia, adaptation, high-altitude, Tibetan
Citation: Zhang L, Meng J, Li H, Tang M, Zhou Z, Zhou X, Feng L, Li X, Guo Y, He Y, He W and Huang X (2022) Hippocampal adaptation to high altitude: a neuroanatomic profile of hippocampal subfields in Tibetans and acclimatized Han Chinese residents. Front. Neuroanat. 16:999033. doi: 10.3389/fnana.2022.999033
Received: 20 July 2022; Accepted: 25 October 2022;
Published: 17 November 2022.
Edited by:
Lidia Alonso-Nanclares, Spanish National Research Council (CSIC), SpainReviewed by:
Diego Iacono, Neuroscience - Uniformed Services University of the Health Sciences (USU), United StatesCopyright © 2022 Zhang, Meng, Li, Tang, Zhou, Zhou, Feng, Li, Guo, He, He and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqi Huang, anVsaWFuYWh1YW5nQDE2My5jb20=; Wanlin He, MTI5NzQzMTU2NkBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.