- 1Anatomy, Section of Medicine, Faculty of Science and Medicine, University of Fribourg, Fribourg, Switzerland
- 2SIB Data mining, Swiss Institute for Bioinformatics, Geneva, Switzerland
- 3Department of Neurology, Radboud University Medical Center and Donders Center for Medical Neuroscience, Nijmegen, Netherlands
The white matter of the central nervous system (CNS) is difficult to represent in anatomy because it is located predominantly “between” other anatomical entities. In a classic presentation, like a cross section of a brain segment, white matter is present and can be labeled adequately. Several appearances of the same entity are feasible on successive cross section views. The problem is the absence of a global view on long tracts, and more generally, the lack of a comprehensive classification of white matter pathways. Following the recent revision of the Terminologia Anatomica (TA, 1998), in particular the chapter on the nervous system, resulting in the Terminologia Neuroanatomica (TNA, 2017), the authors have developed a new schema for the representation of white matter. In this approach, white matter is directly attached to the CNS, and no longer considered as part of the brain segments. Such a move does not affect the content but redistributes the anatomical entities in a more natural fashion. This paper gives an overall description of this new schema of representation and emphasizes its benefits. The new classification of white matter tracts is developed, selecting the origin as the primary criterion and the type of tract as the secondary criterion.
Introduction
At large, white matter coordinates communication between regions of the brain and the spinal cord. It is described by several nouns such as tract, funiculus, fasciculus, commissure, lemniscus, fibers, decussation, and stria. For convenience, in this article, they will be referred to as tract or alternatively as pathway. However, the above-mentioned specific names will continue to be used in the terminology, because they eventually bring some additional information to the named entity. There is a permanent discussion when building a terminology: how much a term should contribute to the definition of its referred entity. Short terms or longer versatile terms? Both have their advantages.
Any tract has an origin located in a cortical region or in a nucleus (or a group of nuclei). It has terminations in one or more locations of gray matter in the central nervous system (CNS), where it synapses, the locations are not always well-known. A tract is made of several bundles of fibers, possibly not completely identified. Between the origin and the termination, the path may be short between near structures or long between brain segments, either crossing the midline or not.
Our description of tracts is in line with other studies such as the Foundational Model of Connectivity (FMC) developed by Swanson and Bota (2010) which is the basis of the representation of white matter tracts in the Terminologia Neuroanatomica (TNA, 2017). The TNA is a recent revision of the terminology on the CNS, the peripheral nervous system (PNS) and the sensory organs. These were abstracted from the Terminologia Anatomica (TA, 1998) and the Terminologia Histologica (TH, 2008) and were extensively updated by the Neuroanatomy Working Group of the Federative International Programme for Anatomical Terminology (FIPAT) of the International Federation of Anatomical Associations (IFAA), and was merged to form the TNA (ten Donkelaar et al., 2017; TNA, 2017), which currently stands alone. The FMC presentation of the connectome, with its three levels of connections—macroconnections (white matter tracts), mesoconnections (dealing with neuron types), and microconnections (dealing with individual neurons of a neuron type)—is a pertinent schema of representation, totally compatible with our approach. Most of the tract names in the TNA refer to macroconnections, because they reveal enough knowledge for naming a tract, whether the type of cells is reported or not. But there is no doubt that the documentation of mesoconnections in the future will help to differentiate new structures with consequent updates in the terminology.
Today, anatomical terminologies are challenged by the emergence of ontology and its formal approach. There was a time for the atlas of anatomy (and this approach is still valid), and there is a time for computer-based presentations. The basis of modern terminology for the biomedical sciences is the Basic Formal Ontology 2.0 (Smith et al., 2015), on which the FMA and our model are grounded. As seen in Table 2 below, we adopt the taxonomy developed by the FMA (Rosse and Mejino, 2003). The rules governing the taxonomy and the partonomy have been made explicit and they are imperative. An expected benefit is to favor the exchange of data between computer applications. But the overall goal is a precise definition of anatomical entities. This will allow a better understanding among people living in distinct communities, with a specific background, while communicating in different languages. The past terminologies are the initial background for future developments, while modern terminologies are now ready to take the lead. This paper positions itself as a contribution to the advent of a modern terminology.
We have observed that anatomists may be reluctant to ontologies and related formal approaches.
“Terminologies should not be developed by reference to a system of preferred terms, rather they should be developed in such a way that their individual nodes and relations amongst these nodes are modeled on an underlying formal ontology, where the linguistic content of these nodes will be filled in based on a system of terms and synonyms (from many different languages) that is associated with each node based on the intended ontological interpretation of that node.”
(see Baud et al., 2007, where additional references may be found). Indeed, there is no opposition: ontologies are the necessary support of terminologies.
In a classic view of anatomical terminology, white matter is considered as a part of the brain segment where it appears. For example, in the Terminologia Anatomica (TA, 1998), the posterior spinocerebellar tract is described as a part of the myelencephalon. This entity is also present as a part of the spinal cord, but it is absent as part of the cerebellum where it terminates. Many tracts are mentioned several times in other segments, as if they are a part of it. The benefit of this approach was to document cross section views of brain segments and to make the path followed by the different tracks more explicit. This is an important aspect, but, hopefully, it is not lost in our new schema of presentation (see discussion below, see Table 5). The problem with such a presentation is that we depart from the rules of partonomy.
This is a classical error: it is well-known that the relation CONTAINED_IN differs from PART_OF. The blood is contained in the vessels, but not a part of them. The fact that a tract is crossing a brain segment does not makes this tract a part of it. If it did, because it crosses several segments, it would simultaneously be part of several brain segments. That is incompatible with the general statement of single inheritance in the partonomy: no entity can be a part of two entities. Therefore, it is formally incorrect to represent white matter tracts as part of the brain segments. The consequence is an ambiguous representation making it difficult to navigate in the knowledge base, as reported by several authors (Martin et al., 2001; Rosse, 2001; Rosse and Mejino, 2003; Bota and Swanson, 2008; Swanson and Bota, 2010; Swanson, 2014).
This classic view is widespread and shared by the TA (TA, 1998), the Foundational Model of Anatomy (FMA; Rosse and Mejino, 2003) and NeuroNames (Bowden and Martin, 1995; Martin et al., 2001; Bowden et al., 2012). Other initiatives generally claim to be compatible with any of these former studies. In the TA, the pyramidal tract is present as part of the myelencephalon and the mesencephalon with different codes. In the FMA, the pyramidal tract has no partonomic information so far. In NeuroNames, the pyramidal tract is part of the medulla or myelencephalon. Those statements are incomplete, because the pyramidal tract passes from the cerebral cortex to the spinal cord: only some segments of the pyramidal tract could be considered as part of the brain segments as stated above. But is it acceptable to state that myelencephalic segment of pyramidal tract PART_OF myelencephalon instead of myelencephalic segment of pyramidal tract PART_OF pyramidal tract? Certainly not. It is time to adjust our representation of tracts.
New View on Tracts
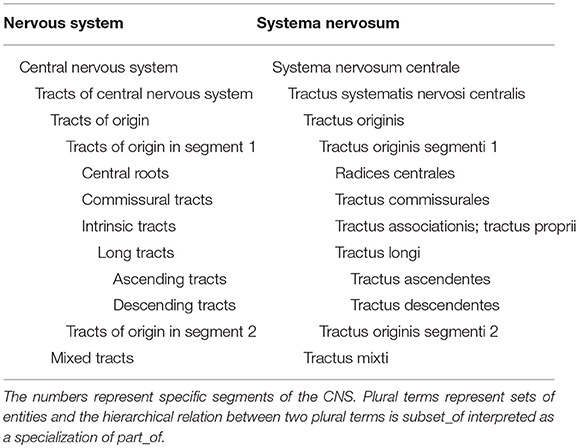
On the contrary, as a general statement, the tracts are not a part of any segment, but should rather be considered in a separate section of the CNS as previously advocated in the Jenaer Nomina Anatomica (JNA, 1936), which contains the collection of all tracts and only those. This places a direct focus on the connections running through the white matter. The current number of tracts is about 100 items, but this number may grow in the coming years due to new discoveries.
What we propose is that tracts form directly part of the CNS (see Table 1). When concentrating all tracts in a single section, it is necessary to classify these tracts based on natural acceptable criteria. The origin of a tract is finally considered as the primary criterion, because it is always known and relatively well-localized to a single location, in contrast to terminations of tracts that are numerous and not always completely documented. On this basis, the tracts of the CNS are classified by their origin in the following 9 segments: telencephalon (pallium), telencephalon (subpallium), hypothalamus, diencephalon, mesencephalon, cerebellum, rostral rhombencephalon, caudal rhombencephalon, and spinal cord.
In the TNA, a more natural hierarchical classification of brain structures is used for the prosencephalon (forebrain) as implemented in the revised version of the Terminologia Embryologica (TE2, 2017). The forebrain is subdivided into the caudal prosencephalon, giving rise to the diencephalon (pretectum, thalamus with epithalamus, prethalamus, and the prerubral or diencephalic tegmentum), and a rostral or secondary prosencephalon, giving rise to the hypothalamus and the entire telencephalon. The telencephalon is divided into the pallium and the subpallium (striatum, pallidum, basal forebrain, and preoptic area).
The diencephalon in its classic, columnar view (Herrick, 1910) was divided into four dorsoventrally arranged columns separated by ventricular sulci, i.e., the epithalamus, the dorsal thalamus, the ventral thalamus and the hypothalamus. It should be noted that NeuroNames and the FMC still follow this doctrine. Extensive embryological studies (see Puelles et al., 2013; ten Donkelaar et al., 2017, 2018) made it clear that the thalamic “columns” are derived from transversely oriented zones, the prosomeres which, from caudal to rostral, contain, in their alar domains, the pretectum (prosomere 1 or P1), the epithalamus and the thalamus (P2), and the prethalamus and the eminentia thalami (P3). The diencephalic basal plate contains the diencephalic part of the substantia nigra–VTA complex, the interstitial nucleus of Cajal, the nucleus of Darkschewitsch and the fields of Forel, collectively known as the prerubral or diencephalic tegmentum. The entire hypothalamus arises from the alar and basal components of the secondary prosencephalon. The preoptic region arises from the subpallium, but for practical reasons it is listed preceding the hypothalamus. The Kuhlenbeck (1967-1978) terms “interbrain” for diencephalon, and “afterbrain” for myelencephalon, as advocated in the FMC, are not widely recognized.
The term “pons,” as used in colloquial neuroanatomy for the rostral part of the hindbrain, is replaced by the term rostral rhombencephalon, keeping the term pons for the pons proper (the basilar part of the pons in most studies). For the caudal part of the hindbrain, the myelencephalon, the term caudal rhombencephalon is suggested.
Our choice of origin as the primary classification criterion is in accordance with the FMC. The first level or macroconnection is based on the region including the origin of a tract, whereas the second level, mesoconnection, is based on cell types. Therefore, our first criterion corresponds to the FMC first level.
A second criterion was necessary for the classification within a segment. The type of tract was selected for this purpose. The following subdivision was used (see Tables 1, 2):
(1) Central roots, defined as the white matter tracts of the CNS that contribute (a) to cranial nerve roots within the brain stem, including the genu of the facial nerve, the decussation of the trochlear nerve, the spinal and mesencephalic tracts of the trigeminal nerve, and the solitary tract; and (b) the central projections of the dorsal roots of the spinal cord, forming the gracile and cuneate fasciculus. The optic tract may also be viewed as a central root.
(2) Intrinsic tracts, restricted to a particular part of the brain, include: (a) association tracts, the association pathways of the telencephalon; (b) intrinsic tracts of other parts of the brain; and (c) the intrinsic, propriospinal tracts of the spinal cord.
(3) Commissural tracts, connecting left and right parts of the brain or spinal cord segment, including the corpus callosum, the anterior, habenular, posterior and supraoptic commissures, and commissural connections in the spinal cord.
(4) Long tracts connect various segments of the CNS and can be divided into (a) ascending tracts; (b) cerebellar efferent tracts; and (c) descending tracts, irrespective of whether they cross the midline or not.
In order to illustrate the use of the two criteria, three examples of partonomy are presented here:
1) long tracts of origin in telencephalon > descending tracts of origin in telencephalon > pyramidal tract > corticorubral fibers.
2) tracts of origin in hypothalamus > efferent tracts of hypothalamus > hypothalamohypophysial tracts > paraventriculohypophysial tract.
3) long tracts of origin in spinal cord > ascending tracts of origin in spinal cord > anterolateral tract > spinoreticular tract.
Once a tract has been classified according to its origin, it is possible to give more information on its path through different brain segments. To do that, it is possible in a partonomic view to present the path of this tract, decomposed in ipsilateral and contralateral segments and decussations as well. These parts of a track may be referenced in the partonomic presentation of the white matter of the brain segments.
An open question remains: how will this model evolve when new discoveries are made? In the domain of TNA, massive data are collected, and new investigation tools are available. There is no doubt that integration of new information will be necessary in the future. There are two open solutions. (1) the origin of tracts may be further defined, and the tract could be split up into subtracts corresponding to detailed origins; (2) different types of cells (mesoconnection) at the origin of a tract may be used to differentiate the tract into several tracts. This paper does not answer this question, however, the new schema formally improves the approach relative to the traditional approach and is consequently better adapted to the evolution of knowledge.
Integration in Taxonomy
Today, there is a single valid taxonomy for the domain of human anatomy, provided by the FMA (Rosse and Mejino, 2003). The emergence of an alternate taxonomy, although theoretically possible, has little chance, because such an enterprise has a workload of several years, currently not available, with no promise of an improved solution. The scientific community must therefore concentrate on the existing FMA taxonomy, and create collaborations to accommodate further improvements. And indeed, the actual FMA taxonomy is of significant value.
The role of the taxonomy is important in a modern ontology. The traditional presentation by the anatomists is the atlas of anatomy, inspired by the partonomic hierarchy. The atlas paradigm is convenient for the inventory of the relevant anatomical entities. Because past terminologies essentially were inventories of the domain, this approach was convenient. But today, a modern terminology must include a classification schema. The taxonomy, based on the genus and differentia principle of Aristotle, is the answer of choice to this need. Consequently, any anatomical entity is positioned in both hierarchies: partonomy and taxonomy. For example: humerus isa long bone and humerus partof free upper limb.
Apparently, the FMA taxonomy was not recently updated to correspond with the many new developments in neuroanatomy. Therefore, a revision of the neuroanatomical subdivision, based largely on NeuroNames, would be welcome. The present study offers a contribution to such a revision. Currently, the white matter that is essentially made of axons is classified (see Table 2) as a cell part cluster of central nervous system and its main child is tract of central nervous system that contains the majority of tracts. There are roughly 100 tracts classified into two groups: tract of brain and tract of spinal cord. Because this figure may be considerably augmented in the future, such a long flat list is no longer acceptable: further subdivisions must be created. Here, the above criterion may be inserted.
Overview of The Tracts
Making an exhaustive inventory of tracts is not the goal of the terminology of today, when our knowledge of the CNS must be permanently updated by new discoveries. Therefore, the presentation of tracts is made of open lists. There are basically two conditions to fulfill before a new tract may enter the tables: (1) it should be recognized as important; and (2) it should have reached a significant level of agreement in the domain. These criteria are obviously subjective: it is the responsibility of the authors of the terminology to decide what is present or not. These two conditions should obviously be updated permanently, making the terminology subject to continual changes.
As seen above, tracts are grouped into 9 segments. In this paper, only tracts originating in the diencephalon are presented. An extended version of this paper, including all segments, is available by the authors.
The tables related to each segment are exclusively partonomic: each indentation means a part_of relation between the father entity and the indented entity. Plural terms refer to a set of entities. The link from a plural to a plural term must be understood as a subset_of and from a plural to a singular term as a member_of, both being a specialization of a part_of.
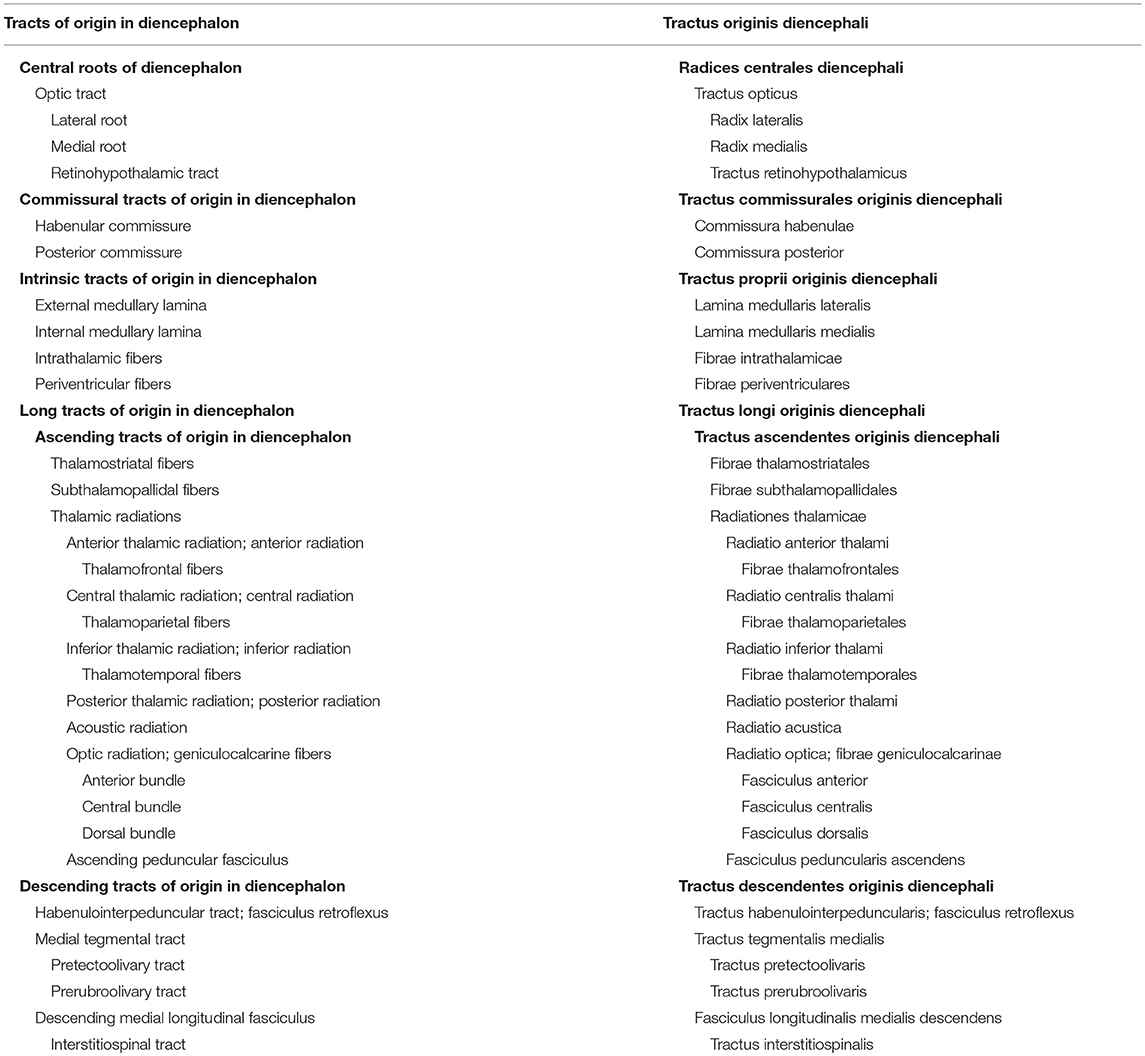
The Tracts of Origin in The Diencephalon
The tracts of origin in diencephalon (tractus originis diencephali) include a central root, commissural, intrinsic and long tracts, ascending as well as descending (Table 3). The unique central root in the diencephalon is the optic tract (tractus opticus) with three distinct parts, the lateral root (radix lateralis), the medial root (radix medialis), and the retinohypothalamic tract (tractus hypothalamicus). Since the optic tract, arising from the retinal ganglion cells, mainly terminates in the lateral geniculate body, it is defined as a diencephalic central root. There are two crossing bundles of fibers, the habenular commissure (commissura habenulae), connecting the epithalami, and the posterior commissure (commissura posterior), a commissure of the pretectum. Intrinsic to the diencephalon are four tracts, the external medullary lamina (lamina medullaris lateralis), the internal medullary lamina (lamina medullaris medialis), intrathalamic fibers (fibrae intrathalamicae), and periventricular fibers (fibrae periventriculares).
There are three groups of ascending fibers: the thalamostriatal fibers (fibrae thalamostriatales), the subthalamopallidal fibers (fibrae subthalamopallidales), and the extensive connections of the thalamus to the cerebrum, known by the generic name thalamic radiations (radiationes thalamicae). These radiations, anterior, central, inferior and posterior (see Table 3) reach each lobe of the cerebrum. The acoustic radiation (radiatio acustica) projects to the temporal lobe, the optic radiation (radiatio optica) to the occipital lobe, whereas the ascending peduncular fasciculus (fasciculus peduncularis ascendens) connects the thalamus to the claustrum. This mixed tract (see Table 4) also contains fibers from the claustrum to the thalamus (the descending peduncular fasciculus).
The descending fibers arising in the diencephalon include: (1) the habenulointerpeduncular tract or fasciculus retroflexus (tractus habenulointerpeduncularis or fasciculus retroflexus) from the habenular nuclei to the mesencephalic interpeduncular nucleus; (2) the medial tegmental tract (tractus tegmentalis medialis), arising in the pretectum and the prerubral tegmentum, projecting to the inferior olivary complex, and composed of the pretectoolivary tract (tractus pretectoolivaris) from the pretectum, and the prerubroolivary tract (tractus prerubrolivaris) from the elliptic nucleus (the nucleus of Darkschewitsch) in the prerubral tegmentum; (3) the descending medial longitudinal fasciculus (fasciculus longitudinalis medialis descendens), containing the interstitiospinal tract (tractus interstitiospinalis), arising in the interstitial nucleus of Cajal.
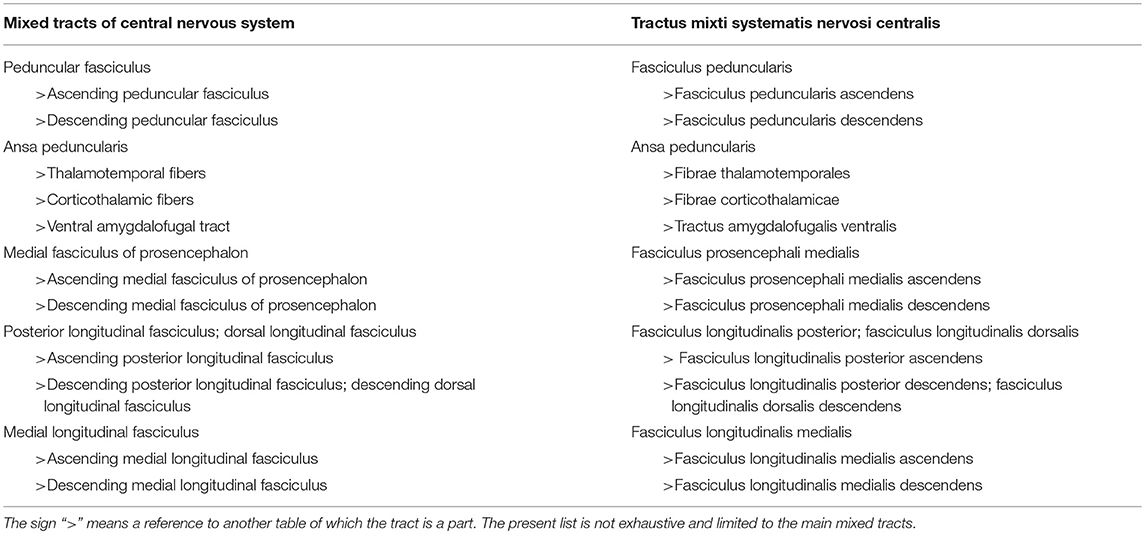
Mixed Tracts
Mixed tracts have ascending and descending components, using the same tract, exemplified by the medial fasciculus of the prosencephalon, also known as the medial forebrain bundle. See Table 4 for a list of mixed tracts.
Rostrocaudal Sequencing
A general rule has been adopted for the sequence of entities, when there are several children from a father entity. In general, in a partonomic hierarchy, the order of children is open. It was decided to always sequence the tracts from the most rostral to the most caudal, when this sequence is significant. In other situations, like the spinal laminae, the sequence is determined by the current usage, here from posterior to anterior position.
Brain Segment White Matter
It was also necessary to make explicit the links between the white matter representation in a segment and the tracts originating, terminating and traversing this segment. Because the representation is done by a partonomy, and the tracts are no more a part of the segment, we used a convention, named a reference. In a brain segment, we were concerned with tracts of several origins. We had to point to all of them in a specific order, proper to the segment. A reference is a pointer to an anatomical entity located elsewhere in the partonomic hierarchy. It is here represented by the sign “>.”
Typically, in the representation of the white matter of the cerebellum, we would point to the tracts running in the brachium conjunctivum using references. The long tracts using this pathway to enter or exit the cerebellum are not part of it and can only be revealed by a reference. See Table 5 about the white matter of the cerebellum.
Conclusion
The new schema for white matter gives a direct focus on the several tracts of the central nervous system. It brings a formally correct representation that is necessary for further developments of a highly developing domain.
The need to improve the formal aspect of a modern terminology has been underlined. The dual approach with two facets–the partonomy and the taxonomy–becomes evidence. Multiple computer applications are developed today, and they will exchange their data only on the condition that they have a common background: the taxonomy plays this role. The terminological aspect remains a predominant source of problems and a universal consensus has not yet been reached. However, sound principles as exemplified in this paper contribute to the solutions of tomorrow.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RR and handling Editor declared their shared affiliation at the time of review.
Acknowledgments
Thanks to Jose Leonardo V. Mejino Jr., M.D., for reviewing the manuscript of this paper.
References
Baud, R., Ceusters, W., Ruch, P., Rassinoux, A. M., Lovis, C., and Geissbühler, A. (2007). Reconciliation of ontology and terminology to cope with linguistics. Stud. Health Technol. Inform. 29(Pt. 1):796–801.
Bota, M., and Swanson, L. W. (2008). BAMS neuroanatomical ontology: design and implementation. Front. Neuroinform. 2:8. doi: 10.3389/neuro.11.002.2008
Bowden, D. M., and Martin, R. F. (1995). NeuroNames brain hierarchy. Neuroimage 2, 63–83. doi: 10.1006/nimg.1995.1009
Bowden, D. M., Song, E., Kosheleva, J., and Dubach, M. F. (2012). neuronames: an ontology for the braininfo portal to neuroscience on the web. Neuroinformatics 10, 97–114. doi: 10.1007/s12021-011-9128-8
Herrick, C. J. (1910). The morphology of the forebrain in amphibia and reptilia. J. Comp. Neurol. 20, 413–547 doi: 10.1002/cne.920200502
JNA Jenaer Nomina Anatomica. (1936). Approved June 1935 by the Anatomische Gesellschaft in Jena, Published Early 1936 by H.Stieve. Jena: Fischer
Martin, R. F., Mejino, J. L. V. Jr, Bowden, D. M., Brinkley, J. F., and Rosse, C. (2001). Foundational model of neuroanatomy: implications for the human brain project. Proc. AMIA Symp. 2001, 438–442.
Puelles, L., Harrison, M., Paxinos, G., and Watson, C. (2013). A developmental ontology for the mammalian brain based on the prosomeric model. Trends Neurosci. 36, 570–578. doi: 10.1016/j.tins.2013.06.004
Rosse, C. (2001). Terminologia Anatomica: considered from the perspective of next-generation knowledge sources. Clin. Anat. 14, 120–133. doi: 10.1002/1098-2353(200103)14:2<120::AID-CA1020>3.0.CO;2-V
Rosse, C., and Mejino, J. L. V Jr. (2003). A reference ontology for biomedical informatics: the foundational model of anatomy. J. Biomed. Inform. 36, 478–500. doi: 10.1016/j.jbi.2003.11.007
Smith, B., Almeida, M., Bona, J., Brochhausen, M., Ceusters, W., Courtot, M., et al. (2015). BFO 2.0 Specification and User's Guide. Available online at: https://raw.githubusercontent.com/BFO-ontology/BFO/v2.0/BFO2-Reference.docx
Swanson, L. W. (2014). Neuroanatomical Terminology. A Lexicon of Classical Origins and Historical Foundations. New York, NY: Oxford University Press.
Swanson, L. W., and Bota, M. (2010). Foundational model of structural connectivity in the nervous system with a schema for wiring diagrams, connectome, and basic plan architecture. Proc. Natl. Acad. Sci. U. S. A. 107, 20610–20617. doi: 10.1073/pnas.1015128107
TA (1998) Terminologia Anatomica. Federative Committee on Anatomical Terminology. Stuttgart: Thieme.
TE2 (2017) Terminologia Embryologica. FIPAT.Library.dal.ca. Federative International Programme for Anatomical Terminology.
ten Donkelaar, H. J., Broman, J., Neumann, P. E., Puelles, L., Riva, A., Tubbs, R. S., et al. (2017). Towards a terminologia neuroanatomica. Clin. Anat. 30, 145–155. doi: 10.1002/ca.22809
ten Donkelaar, H. J., Kachlík, D., and Tubbs, S. T. (2018). An Illustrated Terminologia Neuroanatomica: A Concise Encyclopedia of Human Neuroanatomy. Heidelberg: Springer.
Keywords: neuroanatomy, terminology, white matter, ontology, knowledge representation
Citation: Baud R, Sprumont P and ten Donkelaar HJ (2018) The Representation of White Matter in the Central Nervous System. Front. Neuroanat. 12:102. doi: 10.3389/fnana.2018.00102
Received: 30 July 2018; Accepted: 15 November 2018;
Published: 13 December 2018.
Edited by:
Kathleen S. Rockland, Boston University, United StatesReviewed by:
Trygve B. Leergaard, University of Oslo, NorwayRichard Jarrett Rushmore, Boston University, United States
Copyright © 2018 Baud, Sprumont and ten Donkelaar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert Baud, cm9iZXJ0LmJhdWRAdW5pZnIuY2g=
 Robert Baud
Robert Baud Pierre Sprumont
Pierre Sprumont Hans J. ten Donkelaar
Hans J. ten Donkelaar