
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 04 March 2025
Sec. Alzheimer's Disease and Related Dementias
Volume 17 - 2025 | https://doi.org/10.3389/fnagi.2025.1522434
We reviewed the literature on sex differences in genetically determined Alzheimer’s disease (AD), focusing on autosomal dominant AD (ADAD), Down syndrome-associated AD (DSAD), and APOE4 homozygosity, particularly regarding disease penetrance, symptom onset and clinical progression, and trajectories for markers of amyloidosis (A), tau pathology (T) and neurodegeneration (N). Data suggests that sex differences in disease penetrance, symptom onset, and AT(N) biomarker trajectories are typically subtle for genetically determined AD populations. Noteworthy exceptions, such as increased neurodegeneration in later stages of the disease in females while similar cognitive outcomes, suggest a potential differential cognitive reserve that warrants further investigation. Additionally, the interaction between APOE genotype and sex reveals complex and multifaceted effects in DSAD, with potential implications for ADAD that remain underexplored. The smaller sex differences observed compared to sporadic AD offer insights into the different underlying disease mechanisms in genetically determined AD populations. Future research should prioritize sex-specific investigations in genetically determined AD, focusing on refining methodologies. This includes prioritizing longitudinal designs, adjustment for key confounders, and adherence to sex-specific guidelines.
Alzheimer’s disease (AD) is the most common cause of dementia worldwide (Gustavsson et al., 2023), and its prevalence reveals notable differences between sexes (Alzheimer’s Disease Facts and Figures, 2024). Females account for about two-thirds of AD cases globally (Huque et al., 2023; Zhu et al., 2021), a disparity often attributed to their longer lifespans (Mielke et al., 2014). Selective survival may also play a role, as males who reach older ages often represent a biologically and genetically advantaged subset, potentially reducing their observed AD risk compared to females (Shaw et al., 2021). In addition, emerging research suggest that factors beyond life expectancy and selective survival may also play a role in shaping sex-specific risks for AD (Chêne et al., 2015; Shaw et al., 2021). For instance, hormonal changes, particularly the rapid decline in estrogen during menopause, may increase AD vulnerability in females by impairing glucose metabolism, increasing amyloid-beta deposition, and exacerbating neuroinflammation (Silva et al., 2024). Cardiovascular health disparities (Ferretti et al., 2018) and immune differences further contribute to sex-specific AD risks (Deming et al., 2018).
Research indicates not only a higher prevalence of AD in females but also a faster progression from mild cognitive impairment (MCI) to AD dementia compared to males, with females eventually losing their verbal memory advantage as the disease advances (Arenaza-Urquijo et al., 2024; Castro-Aldrete et al., 2023; Lin et al., 2015; Sohn et al., 2018). Sexual dimorphism in cognitive skills—where males typically excel in visuospatial tasks, reaction times, and mathematical reasoning, while females outperform in verbal abilities, executive functioning, attention, and episodic memory (Levine et al., 2016; Lauer et al., 2019; Sang et al., 2024; Hirnstein et al., 2023; DeCasien et al., 2022) —is particularly relevant in this context. Current cognitive measures designed to detect AD-related decline may not fully account for these sex-specific differences, potentially leading to biased diagnoses and assessments. Specifically, the female verbal memory advantage may mask early cognitive deficits, delaying AD diagnosis when standard verbal-based memory tests are used. This could contribute to the perception of faster cognitive decline in later stages for females, as deficits become more apparent when the verbal advantage diminishes.
Sexual dimorphism also extends to brain structure. Males generally have larger overall brain sizes, including absolute gray and white matter volumes, while females exhibit proportionally larger gray and white matter volumes and thicker cortices when adjusted for total brain volume (TBV) or intracranial volume (ICV) (Eliot et al., 2021). Females also show relatively larger hippocampal volumes—an area critical for memory and AD pathology—while males display larger amygdala and putamen volumes when normalized for TBV or ICV (Eliot et al., 2021). Although these structural and functional differences often show small and inconsistent effects across studies, they underscore the importance of considering sex as a critical factor in understanding AD. Thus, sexual dimorphism across various levels—including genetics, hormones, brain structure, and cognition—along with cultural influences (Reilly, 2012; Springer et al., 2012) likely contributes to sex-related disparities in AD prevalence.
Given the potential role of sexual dimorphism in AD pathology, understanding sex-specific trajectories in AD biomarkers has become a critical focus in research. Efforts to elucidate these differences often rely on the AT(N) biomarker framework, which characterizes AD pathology through amyloid plaques (A), tau tangles (T), and neurodegeneration (N). Amyloid-beta (A) biomarkers, such as CSF amyloid-β (Aβ)42 levels, Aβ42/Aβ40 ratios, and amyloid-PET scans, consistently show no significant sex differences (Mielke, 2020). Conversely, tau biomarkers (T) present a divergent picture; while plasma and CSF phosphorylated tau (p-tau181) levels are comparable between sexes, several studies have shown a more pronounced tau accumulation in tau-PET scans in key brain regions in females, even after adjusting for overall disease severity (Buckley et al., 2019). This observation is supported by autopsy studies indicating higher neurofibrillary tangle densities in females (Liesinger et al., 2018). Regarding neurodegeneration (N), some studies suggest males have higher concentrations of neurofilament light chain (NfL) in CSF (Bridel et al., 2019), a marker of axonal damage, but other studies have not found differences in plasma. Additionally, MRI biomarkers present mixed results across sexes (Ferretti et al., 2018). Importantly, a recent study adds a layer of complexity in the interpretation of sex differences in AD biomarkers: While plasma p-tau181 levels were found to be similar between sexes, females showed greater neurodegeneration, faster cognitive decline, and a higher risk of developing AD dementia associated with elevated p-tau181 compared to males (Tsiknia et al., 2022). These findings suggest that sex differences may affect the clinical interpretation of plasma p-tau181 as an AD biomarker, paralleling previously reported challenges in assessing cognitive changes related to verbal memory.
In addition, recent multi-omics studies have provided valuable insights into the molecular mechanisms underlying sex-differences in AD (Guo et al., 2021). Transcriptomic analyses link gene downregulation in female neurons and transcriptional activation in male oligodendrocytes to disease progression (Mathys et al., 2019). Females show more immune-related gene expression changes, while males exhibit differences in synaptic signaling and autophagy (Paranjpe et al., 2021). Females also show greater immune and neuronal pathway alterations, with stronger associations to amyloid and tau pathologies (Deming et al., 2018), and metabolomic findings indicate dysregulated lipid metabolism and energy pathways, particularly in female APOE4 carriers (Shang et al., 2020) with distinct methylation and RNA profiles (Cao et al., 2019; Mano et al., 2017). The APOE ε4 allele is the strongest genetic risk factor for late-onset AD. Research has consistently shown a more pronounced impact on females, who show worse memory performance, global cognition, and higher cerebrospinal tau levels compared to males (Walters et al., 2023; Yan et al., 2021).
Genetically determined AD offers unique opportunities to understanding sex effects on AD pathophysiology. Late onset sporadic AD dementia cases, even when confirmed by biomarkers, often involves additional co-pathologies, complicating the disease landscape. At autopsy, AD pathology is frequently accompanied by other neurodegenerative conditions such as vascular dementia, Lewy body dementia, or other forms of pathology. These additional conditions, for which biomarkers are less robust, make it more challenging to disentangle and analyze sex-related differences in sporadic AD (Mortimer, 2013). This complex landscape can significantly influence the observed differences between sexes in late-onset sporadic AD adding further complexity to the study of sex-related differences in this population. In contrast, genetic forms of AD might offer a clearer perspective on disease development and sex-related variations, as co-pathologies are less frequent in these cases. However, it is important to note that Cerebral Amyloid Angiopathy (CAA) is more prevalent and severe in these genetic forms than in sporadic AD (Carmona-Iragui et al., 2019). Three genetic forms of AD have been proposed; autosomal dominant AD (ADAD) (Bateman et al., 2012), Down syndrome associated AD (DSAD) (Fortea et al., 2020, 2021), and more recently, apolipoprotein E epsilon 4 (APOE4) homozygosity (Fortea et al., 2024).
In ADAD, mutations in the amyloid precursor protein (APP), presenilin 1 (PSEN-1), or presenilin 2 (PSEN-2) gene lead to altered processing of the amyloid precursor protein, resulting in an increased ratio of longer amyloid-β (Aβ) fragments, particularly Aβ42. These longer Aβ fragments are more prone to aggregation and are associated with early and extensive deposition of amyloid plaques in the brain. This amyloid deposition triggers a cascade of neurodegenerative processes that lead to neuronal death the early onset of clinical symptoms (i.e., before 65 years of age), and eventual dementia. Mutations in APP, PSEN1, and PSEN2 have an estimated prevalence of 5.3 per 100,000 persons (i.e., 5.3e-05% worldwide) (Lanoiselée et al., 2017). Among these, PSEN1 mutations are the most common, followed by APP and PSEN2 mutations.
DS is the most frequent genetic cause of intellectual disability (ID), affecting approximately 0.14% of the general population. Due to the triplication of the APP gene, which is encoded on chromosome 21, individuals have near full penetrance of AD dementia (Fortea et al., 2021) and AD now represents the main cause of death in adults of this population (Iulita et al., 2022). Notably, ADAD and DSAD have been recognized as genetic forms in the new Alzheimer’s Association criteria (Dubois et al., 2014).
We have recently proposed APOE4 homozygotes as another form of genetic AD as it also fulfills the key features of genetically determined AD: (1) near-full penetrance of the disease (defined biologically), (2) predictability of the age at symptom onset and of clinical changes, and (3) a consistent sequence of biomarker and pathological alterations. The three forms of the disease show striking similarities in these features (Bateman et al., 2012; Fortea et al., 2020, 2021, 2024), suggesting that APOE4 homozygotes could be reclassified as a distinct form of genetically determined AD. Notably, approximately 2% of the global population is homozygous for APOE4, accounting for 15–20% of AD cases.
Our review will examine sex differences in three genetically determined AD populations: ADAD, DSAD, and APOE4 homozygotes. For each, we will summarize studies on sex differences in biomarkers, cognition, and neural dimorphism unrelated to AD that may influence disease risk and progression. We will then explore sex effects on disease penetrance, symptom onset, clinical progression, and AT(N) biomarker changes, including interactions between sex and APOE status in ADAD and DSAD. To deepen understanding, we will review multi-omics studies to provide a comprehensive overview of sex effects in genetically determined AD.
To our knowledge, six studies have investigated sex-related differences in ADAD outcomes, with five of them being cross-sectional. Three studies were conducted as part of The Colombian Alzheimer’s Prevention Initiative (API) Registry (Vila-Castelar et al., 2020, 2022, 2023), focusing on members of the PSEN1 E280A family from Antioquia, Colombia. Another Latin-American cohort from Jalisco, Mexico, involving PSEN1 mutation carriers, has published few studies on the clinical phenotype and progression of AD, some of which included sex-related analyses (Dumois-Petersen et al., 2020). The final two studies investigating sex-related differences come from the international Dominantly Inherited Alzheimer Network (DIAN) cohort (Kommaddi et al., 2023; Wagemann et al., 2024) which recruited family members with APP, PSEN1, and PSEN2 mutations from Asia, Australia, Europe, and the Americas. Notably, only one of these studies included longitudinal data (Kommaddi et al., 2023).
Regarding sexual dimorphism, female asymptomatic mutation carriers exhibit significantly higher episodic verbal memory scores compared to their male counterparts, a trend observed in both the Colombian cohort and in DIAN (Vila-Castelar et al., 2022; Wagemann et al., 2024). Interestingly, this sex difference is evident since childhood (Fox-Fuller et al., 2021). The study of 1,354 children aged 6 to 16 years from the Colombian cohort, including 265 with the PSEN1 variant, showed that girls outperformed boys in working memory, perceptual reasoning, and verbal comprehension, regardless of genetic status. The disparity in working memory, however, was especially pronounced in the PSEN1 carriers. No differences in other cognitive outcome shave been explored. In addition, the DIAN cohort which included 436 participants (257 mutation carriers and 179 non-carriers) of approximately 7 years before expected symptom onset, revealed that there was a Brain Age Gap (calculated by predicting the brain’s “age” based on imaging features such as cortical thickness, gray matter volume, and white matter integrity, and then subtracting the individual’s chronological age) of approximately 3 years greater in males than females regardless of mutations status (Millar et al., 2023). Such findings underscore that nuanced differences in cognitive resilience and brain aging trajectories may exist in this population. No further sex differences have been found in other brain structures [e.g., hippocampus and amygdala (Vila-Castelar et al., 2022; Wagemann et al., 2024)] or in cortical thickness (Wagemann et al., 2024) in pre-symptomatic ADAD.
Regarding sex effects on AD penetrance, both the Colombian (Aguirre-Acevedo et al., 2016) and Mexican kindred (Dumois-Petersen et al., 2020) as well the cross-sectional DIAN cohort studies (Wagemann et al., 2024) demonstrated full disease penetrance without significant sex differences in symptom onset or clinical progression. While the female advantage in verbal memory generally diminishes with age, these studies indicate that this does not significantly affect the clinical onset or progression of AD. However, the longitudinal study revealed some interesting sex differences in clinical progression (Kommaddi et al., 2023). Specifically, females demonstrated slower cognitive decline in tasks involving logical verbal memory. Conversely, in tasks requiring executive function—symptomatic female mutation carriers declined faster than their male counterparts. No significant sex differences were found in other cognitive outcomes, including episodic verbal memory tasks or in the Mini-Mental State Examination (MMSE). These findings suggest that while females may show greater resilience in specific verbal memory tasks, they may experience more rapid decline in areas like executive function, which are not typically emphasized in AD-related examinations.
Regarding the sequence of AD biomarker changes, most studies do not show significant sex differences in amyloid (CSF Aβ42/40 ratio), tau (CSF p-tau181, t-tau levels), or markers of neurodegeneration, with two exceptions: A study from the Colombian Kindred found that female carriers exhibited a greater increase in plasma NfL levels compared to male carriers, but no significant differences in p-tau217 levels. Interestingly, despite the increased plasma NfL levels in females, this did not correlate with differences in cognitive performance (Vila-Castelar et al., 2023). Furthermore, the cross-sectional study from the DIAN cohort noted that with disease progression, symptomatic female carriers showed more increased cortical thinning and decreased volumes of the hippocampus and amygdala compared to male carriers, highlighting a greater degree of neurodegeneration in advanced stages for females (Wagemann et al., 2024). These findings suggest that while core biomarker changes are mostly similar between sexes, distinct differences in neurodegeneration and cognitive resilience may become evident, particularly in the later stages of the disease.
The role of APOE in ADAD, particularly among PSEN1 mutation carriers, has shown mixed findings. Several studies in the Colombian kindred suggested that carriers of the APOE ε4 allele developed earlier dementia onset compared to non-carriers (Pastor et al., 2003; Langella et al., 2023). In contrast, a study conducted in the Mexican kindred found the opposite effect, suggesting a later onset for APOE ε4 carriers (Valdez-Gaxiola et al., 2023). Adding to the complexity, other studies in the same cohort (Langella et al., 2024; Vélez et al., 2016) found no significant effect of APOE4 on symptom onset and trajectories, while noted that the APOE ε2 allele delayed the onset of clinical symptoms by approximately 8 years. Similarly, the APOE3 Christchurch variant has been associated with delayed cognitive impairment in PSEN1 carriers (Quiroz et al., 2024). Broader studies involving various ADAD families have indicated differences between APP and PSEN1 mutations, which may obscure the effects of APOE in pooled analyses (Lanoiselée et al., 2017). Of note, the role of APOE4 in ADAD has shown to be variable across the lifespan and it is possible that APOE4 may provide some biological or cognitive benefit in younger PSEN1 carriers. Regarding biomarker progression, a recent study in the Colombian kindred found that APOE4 accelerates age-related plasma NfL increases and APOE ε2 attenuates the relationship between higher plasma NfL levels and cognitive decline in ADAD (Langella et al., 2024).
Regarding the interaction between APOE ε4 and sex, studies in the Colombian cohort found no significant relationship between APOE, memory, amyloid burden, or cerebral hypometabolism, though the sample size was small, with only 18 APOE4 carriers per sex (Vila-Castelar et al., 2022). The DIAN study reported no significant differences between sexes for APOE4 status but did not examine the interactive effects between APOE4 status and sex on AD outcomes. However, a 2014 metanalysis (Ryman et al., 2014) that examined combined dataset—including the DIAN database and two large kindreds of Colombian (PSEN1 E280A) and Volga German (PSEN2 N141I) ancestry—found no interaction between sex and APOE4 status on symptom onset or disease progression. Neither sex nor APOE4 status independently influenced AD outcomes. Further research is required to validate the absence of interactive effects in this population.
Finally, only two studies from the DIAN cohort have investigated CSF proteomics in ADAD. The first reported no significant impact of sex or APOE4 status on proteomic changes or their progression relative to estimated years to disease onset (Johnson et al., 2023). Similarly, the second study compared proteomic profiles between mutation carriers and controls, with no explicit sex-related differences observed in CSF proteomics or associated biomarker pathways (Van Der Ende et al., 2023).
In conclusion, current research on sex-related differences in ADAD indicates that female asymptomatic mutation carriers consistently demonstrate higher verbal memory scores from childhood, highlighting the influence of sexual dimorphism rather than a specific sex effect on AD progression. Despite this verbal advantage, most studies have found no significant sex differences in AD penetrance, symptom onset and progression, or biomarker changes. However, in advanced disease stages, females may exhibit more pronounced brain atrophy, particularly in regions such as the hippocampus and amygdala, although this does not consistently correlate with greater cognitive decline. See Table 1 for an overview of the published studies investigating the role of sex in ADAD.
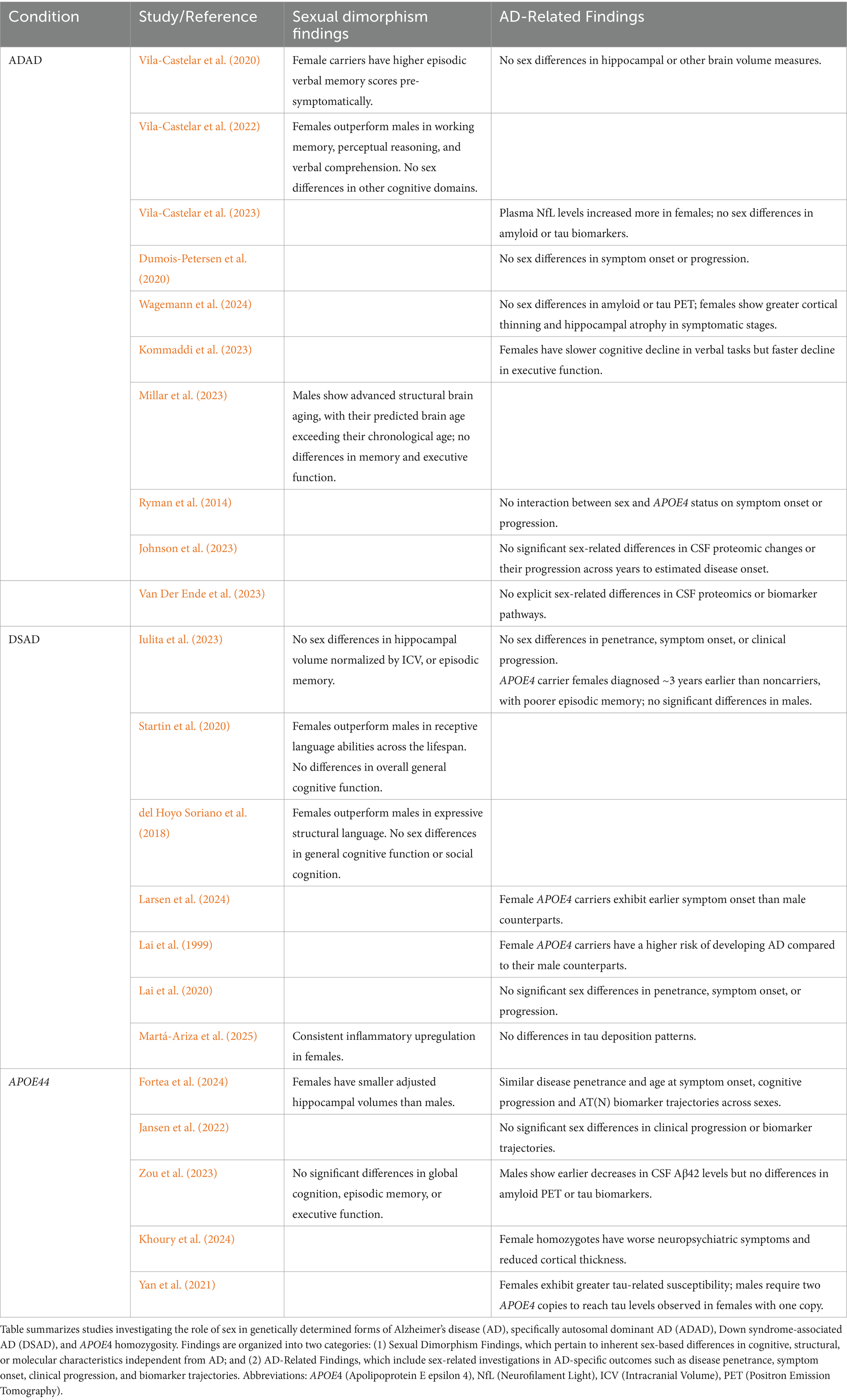
Table 1. Overview of studies investigating the role of sex in genetically determined Alzheimer’s disease.
Compared to ADAD, there are more studies investigating the sex-differences in DSAD outcomes (Iulita et al., 2023; Lai et al., 2020; Larsen et al., 2024; Mhatre et al., 2021; Rubenstein et al., 2024; Rubenstein et al., 2020). However, only one has also looked at the sequence of AT(N) biomarker changes across age and sex in 628 adults with DS from the Down Alzheimer Barcelona Neuroimaging Initiative (DABNI), Spain and the University of Cambridge, UK (Iulita et al., 2023).
Regarding sexual dimorphism, a recent study on cognitive abilities in DS (Startin et al., 2020) examined 602 individuals aged 3 months to 73 years and found that males consistently scored lower than females in receptive language abilities across the lifespan. Similarly, another study reported that during adolescence, females outperformed males in expressive structural language using speech samples (del Hoyo Soriano et al., 2018). Additionally, research on asymptomatic young adults with DS targeting multiple cognitive domains, found that females excelled males only in episodic memory and executive functioning (de Sola et al., 2015). Regarding functional and structural brain differences, DS male brains are generally larger than female brains, with greater cortical thickness (Romano et al., 2015) and larger unadjusted hippocampal volumes (Romano et al., 2015). However, most studies show no significant sex-related differences in adjusted hippocampal volumes or glucose metabolism for asymptomatic adults with DS (Iulita et al., 2023; Koenig et al., 2021). Nonetheless, a study–based on 54 MRI scans- found smaller hippocampal volumes in asymptomatic females (controlling for intracranial volume) (Peven et al., 2022).
Mixed results have been found regarding disease penetrance across sexes in the DS population, possibly due to methodological differences between studies. Some studies report a greater risk of dementia in males for older age ranges (Mhatre et al., 2021; Schupf et al., 1998), while others find increased risk in females aged 40 to 54 but no sex differences in those younger than 40 or older than 55 (Rubenstein et al., 2020). Some research shows a mildly greater risk for females across all ages (Lai et al., 1999), while other studies find no sex differences at any age range (Iulita et al., 2023; Lai et al., 2020; Rubenstein et al., 2024). Of such, a recent epidemiological study in adults with DS enrolled in Medicaid or Medicare between 2011 and 2019 (N = 132,720) identified via ICD codes, found no sex-related differences in prevalence and incidence of AD by age (Rubenstein et al., 2024). The inconsistencies across studies may be attributed to variations in study design, such as longitudinal vs. cross-sectional, prospective vs. retrospective studies and research cohorts vs. epidemiological studies based medical records or healthcare reimbursement requests. The retrospective and epidemiological approaches may be subject to biases such as referral differences, coding errors, variations in provider documentation, or disparities in healthcare access and utilization. Additionally, age ranges in the studies varied widely (20 to 70 years), and sample sizes ranged from as few as 21 to over 100,000 participants. Overall, larger and more recent prospective studies, such as those from the DABNI cohort and the large epidemiological study in adults with DS enrolled in Medicaid or Medicare in the U.S have not found significant sex differences in disease risk in the DS population (Iulita et al., 2023; Rubenstein et al., 2024). These findings suggest that, despite earlier conflicting reports, there appears to be no substantial sex difference in AD penetrance among individuals with DS.
Regarding sex-differences in symptom onset, studies have shown mixed results as well. In the 1990s, some studies indicated an earlier onset in males (Schupf et al., 1998), while others found this trend in females (Lai et al., 1999). However, studies from 2020 onwards have found no significant differences in symptom onset across sexes (Iulita et al., 2023; Lai et al., 2020). Regarding clinical progression, longitudinal research shows that rate of cognitive decline does not differ by sex when transitioning from the preclinical stage to prodromal AD (Hartley et al., 2020). Additionally, a recent a meta-analysis with the assessment of mortality data from US death certificates (n = 77,347 case records [49%] female) and from a longitudinal cohort study (DANBI, n = 889 individuals; 46% female; 3.2 [2.1] years of follow-up) found no sex differences in the age of death among the older participants, for whom AD is the predominant cause of mortality (Iulita et al., 2022). The large epidemiological study involving adults with DS enrolled in Medicaid or Medicare in the U.S. found no differences in the incidence of AD dementia between sexes (Rubenstein et al., 2024; Rubenstein et al., 2020).
Regarding the sequence of biomarkers by sex, the study conducted on the DABNI and UK cohorts (Iulita et al., 2023) found that males and females exhibit similar trajectories with age for markers of amyloidosis (CSF amyloid-β 42/40 and amyloid-PET), tau pathology (CSF and plasma phosphorylated-tau181), and neurodegeneration (CSF and plasma neurofilament light, total-tau, fluorodeoxyglucose-PET, and MRI). However, it is important to note that tau-PET data was not available in this study, leaving a gap in the assessment of sex differences in tau deposition. Overall, the findings suggest that there is no significant sex effect on the trajectory of AD biomarkers in the DS population, though further research is needed to confirm these results, particularly with the inclusion of tau-PET data.
Several studies show that DS adults carrying the APOE ε4 allele face a higher risk (Prasher et al., 2008) of developing AD dementia and tend to have an earlier age of onset (Bejanin et al., 2021; Silverman et al., 2013) and a higher degree of cognitive decline (Gorijala et al., 2024) and earlier changes in amyloid (cerebrospinal fluid Aβ1-42/1-40 and amyloid positron emission tomography), tau (plasma phosphorylated tau 181), and neurodegeneration (cerebral glucose hypometabolism and hippocampal atrophy) biomarkers (Bejanin et al., 2021). In addition, APOE ε2 carriers have shown an increased protection against AD pathology (Lai et al., 1999). These trends are in line with observations in sporadic AD, though they tend to be less pronounced in DSAD. Regarding the interaction between APOE and sex on DSAD outcomes, conflicting results have been found. Some studies have reported that females ε4 carriers tend to be diagnosed at an earlier age than their male counterparts (Larsen et al., 2024), while others have found no such association (Iulita et al., 2023; Lai et al., 1999). However, the study in the Spanish and British (Iulita et al., 2023) cohort found that females with an APOE ε4 allele had poorer episodic verbal memory and were diagnosed with Alzheimer’s disease an average of 3 years earlier than non-carriers, while no such differences were observed between male ε4 carriers and non-carriers. At the biomarker level, female ε4 carriers showed a lower CSF Aβ42/Aβ40 ratio and reduced hippocampal volume compared to non-carriers, a pattern not seen in males. Findings from this study underscore the complexity of the interaction between sex and APOE in DSAD, highlighting the need for further research to clarify these relationships and their implications for clinical outcomes.
Significant transcriptomic sex differences between DSAD and sporadic AD have been identified using spatial and single-nucleus transcriptomics (Miyoshi et al., 2024). This recent study revealed that the role of sex differed between DSAD and sporadic AD. In DSAD, transcriptomic differences between sexes were more pronounced, with females showing consistent upregulation of inflammatory and glial genes across brain regions and stronger neuroinflammatory signatures, particularly in late stages of the disease. This contrasts with sporadic AD, where sex differences were more region- and stage-specific. Another recent study (Martá-Ariza et al., 2025) evaluated the Aβ plaque proteome in four cohorts—ADAD, late-onset AD, DSAD, and controls—finding significantly increased amyloid-β and tau pathologies in DS, but no significant sex effects on proteomic profiles. However, this study did not examine the relationship between sex and proteomic profiles specific to each AD population. Similarly, a study of plasma metabolomic profiles in individuals with DS (ages 10–63) found no significant effects of age or sex on metabolite concentrations or patterns, nor any sex-specific differences within age groups (Antonaros et al., 2020).
In conclusion, females with DS exhibit higher verbal scores from childhood, and smaller hippocampal areas, mirroring the sexual dimorphism observed in the general population. When examining AD outcomes, recent prospective studies with larger samples have found no significant sex differences in disease penetrance, symptom onset and clinical progression and biomarker trajectories. However, females with DS and the APOE4 allele tend to be diagnosed earlier and exhibit lower CSF Aβ42/Aβ40 ratios and reduced hippocampal volumes than males’ counterparts, indicating an interaction between sex and APOE4 in the DS population. Furthermore, multi-omics findings reveal pronounced transcriptomic sex differences in DSAD compared to sporadic AD, with DSAD females showing consistent upregulation of inflammatory and glial genes, particularly in late stages of the disease. See Table 1 for an overview of the published studies investigating the role of sex in DSAD.
Most studies tend to group APOE4 heterozygotes and homozygotes into a single ‘APOE4 carriers’ category, largely due to sample size constraints. However, there is still a body research focused on examining the distinct effects of APOE4 dosage, particularly the differences between homozygosity heterozygosity, and noncarriers on penetrance, clinical progression, and biomarker changes by sex (Bocancea et al., 2023). Although this section will primarily address sex differences in APOE4 homozygotes as a genetically determined form of AD, we will also discuss literature on the broader implications of APOE4 dosage effects at the end of this section.
Due to sample size limitations, few studies have specifically analyzed sex differences in AD penetrance, symptom onset, clinical progression, and the biomarker sequence in APOE4 homozygotes (Fortea et al., 2024; Jansen et al., 2022; Zou et al., 2023). Among the research available, the two most extensive studies to date are cross-sectional. One study analyzed data from 3,297 brain donors from the National Alzheimer’s Coordinating Center (NACC) and10,039 individuals from 5 large prospective cohorts (Fortea et al., 2024). The second study utilized data from the 85 cohorts of the Amyloid Biomarker Study (Jansen et al., 2015), an ongoing global data-pooling initiative that began in 2013, and conducted a pooled analysis on 19,097 participants, including 783 APOE4 homozygotes (Jansen et al., 2022).
Regarding sexual dimorphism among individuals with APOE4 homozygosity, studies have shown that asymptomatic males exhibit worse global cognition, episodic memory, executive and visuospatial function compared to females, while similar attentional levels and expressive language outcomes (Zou et al., 2023). Additionally, adult females with APOE4 homozygosity have significantly smaller hippocampal volumes (adjusted for ICV) than their male counterparts across all age ranges and AD statuses (Fortea et al., 2024).
In terms of disease penetrance, research consistently shows that males and females with APOE4 homozygosity have a similar risk and predictability of the age at AD diagnosis (Fortea et al., 2024; Jansen et al., 2022; Therriault et al., 2021; Zou et al., 2023). Importantly, despite smaller adjusted hippocampal volumes in females (Fortea et al., 2024), this did not lead to greater cognitive decline over time. The sequence of biomarker changes with age in APOE4 homozygotes is strikingly similar to that described in ADAD and DSAD and largely sex-independent. Among the biomarkers examined, one of the few significant findings in one of the cross-sectional studies was an earlier decrease in Aβ42 levels in males (Fortea et al., 2024), which should be interpreted cautiously due to the limited number of younger participants. Additionally, amyloid PET scans did not reveal any sex differences, suggesting that the Aβ42 trend may not reflect broader amyloid pathology differences between sexes. Finally, a recent study (Khoury et al., 2024) involving 752 patients with an AD diagnosis reported that female APOE4 homozygote carriers had worse neuropsychiatric symptoms and reduced cortical thickness, particularly in the medial-lateral temporal regions, compared to APOE4 homozygous males. However, these findings are based on cross-sectional data in symptomatic patients only, which limits the ability to determine quantify reductions or increases in cortical thickness as true changes in gray matter related to AD pathology.
To conclude, research in APOE4 homozygotes targeting sex-related differences indicates that asymptomatic females have better cognitive performance than their male counterparts but exhibit smaller hippocampal volumes across all age ranges possibly reflecting the sexual dimorphism rather than AD risk. Indeed, regarding AD outcomes, no significant sex differences in disease penetrance, symptom onset, progression, or biomarker trajectories for APOE4 homozygotes, suggesting similar risks for males and females in this subgroup.
A substantial body of research explores the effects of APOE4 dosage on penetrance, clinical progression, and biomarker changes by sex, with mixed findings (Belloy et al., 2023; Bocancea et al., 2023; Farrer et al., 1997; Liu et al., 2022). For instance, Bocancea et al. (2023) conducted a multicenter longitudinal study with 366 participants with MCI or dementia, including 71 APOE4 homozygotes, and found no significant cross-sectional or longitudinal effects of APOE4 status on cognition and cortical thickness when sex was considered. Additionally, APOE4 status did not significantly moderate the association between tau pathology and cognitive decline or cortical thinning when stratified by sex. In contrast, another study with 117 participants with AD, including 21 APOE4 homozygotes, found a sex-specific response to APOE4 dosage: males required two copies of the allele to reach tau levels that females achieved with just one, suggesting higher susceptibility in females to the effects of APOE4 on tau pathology (Yan et al., 2021). This finding aligns with earlier studies (Farrer et al., 1997) which showed that APOE4 heterozygosity is sufficient to increase AD risk in females, while APOE4 homozygosity is necessary in males. The inconsistencies between Bocancea et al. (2023) and the remaining studies may be attributed to different methodologies and statistical approaches (e.g., use of linear mixed-effects models with sex and APOE4 status as interactive variables versus sex-stratified analyses).
Metabolomic and transcriptomic studies highlight sex-specific differences among APOE4 carriers. Females show metabolic vulnerabilities, such as acylcarnitine C10’s association with increased CSF p-tau and higher proline levels linked to reduced brain glucose uptake (Arnold et al., 2020). Single-cell transcriptomic reveal that female APOE4 carriers display unique transcriptional changes in excitatory neurons and astrocytes and a distinct neutrophil phenotype characterized by IL-17/IL-1 gene expression linked to cognitive impairment (Yu et al., 2024). These molecular differences suggest heightened female susceptibility to APOE4-driven neuroinflammation and neurodegeneration.
To add another layer of complexity, the APOE4-AD association varies by race and ethnicity, with a stepwise decrease in effect estimates across East Asian, non-Hispanic White, non-Hispanic Black, and Hispanic individuals (Belloy et al., 2023). While APOE4 dosage generally increases AD risk in women across races, the magnitude and manifestations of this effect differ by racial and ethnic background (Belloy et al., 2023; Tang et al., 1998) suggesting that findings from research focused predominantly on Caucasian populations may not fully capture sex-specific risks in diverse racial and ethnic groups. These observations highlight the intricate interplay between genetic, racial, and sex-specific factors in AD and underscore the critical need for racial and ethnic stratification in studies of APOE4 effects to improve our understanding of these mechanisms in both men and women.
In summary, although no significant sex differences are observed in AD outcomes among APOE4 homozygotes, the impact of APOE dosage on AD risk might differ between males and females. This discrepancy could be due to several factors. For example, in heterozygotes, the interaction between APOE4 and other genes might trigger sex-specific biological mechanisms, whereas in homozygotes, the strong impact of two APOE4 alleles could overshadow these differences. Additionally, genetic resilience and compensatory mechanisms may vary by sex in heterozygotes but may be insufficient to counterbalance the effects in homozygotes. See Table 1 for an overview of the published studies investigating the role of sex in APOE4 homozygotes.
Genetically determined AD has been critical in our understanding of the pathophysiology of AD and offers the unique opportunity of studying the role of sex in disease penetrance, predictability of the age at symptom onset and of clinical changes, and biomarker trajectories within the AT(N) framework in these more predictable forms of the disease.
First, there is a consistent sexual dimorphism in cognitive function across genetically determined AD, with females showing a cognitive advantage before AD onset. Structurally, females generally have smaller brain and hippocampal volumes compared to males; however, when normalized by ICV, no significant sex differences in hippocampal volume are observed in DS (with one study as an exception) or in ADAD. However, asymptomatic APOE4 homozygous females tend to have smaller adjusted hippocampal volumes compared to their male counterparts, which contrasts with findings in the general population. Similarly, lower cortical thickness has been observed in asymptomatic DS females compared to males, again opposing trends typically seen in the general population. This difference in cortical thickness has not been observed in asymptomatic ADAD and remains unexplored in asymptomatic APOE4 homozygotes. Significant gaps in our understanding of these differences highlight the need for further research on sexual dimorphism in cognitive and structural outcomes in these populations (Figure 1).

Figure 1. Illustration of sexual dimorphism in cognitive performance and brain structures across asymptomatic genetically determined Alzheimer’s disease (AD) populations (including Down syndrome [DS], autosomal dominant AD [ADAD], and APOE44 carriers) compared to the general population. The figure highlights differences in total brain volume, cortical thickness, and hippocampal volume (normalized by total intracranial volume), with females generally exhibiting smaller brain structures compared to males. However, after adjustment for intracranial volume, sex differences in hippocampal volume largely disappear (except for APOE44 and the general population). Cognitive differences between sexes are mild, with females showing an advantage in episodic memory across population. Notably, research gaps persist in understanding sex differences in some cognitive areas and brain structures.
Second, while the limited research necessitates cautious interpretation, and there are mixed results in the literature, the more recent studies with larger sample sizes show minimal sex differences in disease penetrance, symptom onset and both clinical and biomarker changes in these populations, with some nuances. For example, females in advanced stages of ADAD and APOE4 homozygotes may show increased neurodegeneration compared to males with similar cognitive outcomes, possibly reflecting greater cognitive reserve. See Figure 2 for an illustration on sex differences in penetrance, symptom onset, and biomarker progression across genetically determined AD populations. Additionally, an interaction between APOE4 and sex has been observed in DSAD, aligning with findings in sporadic AD (Figure 3).
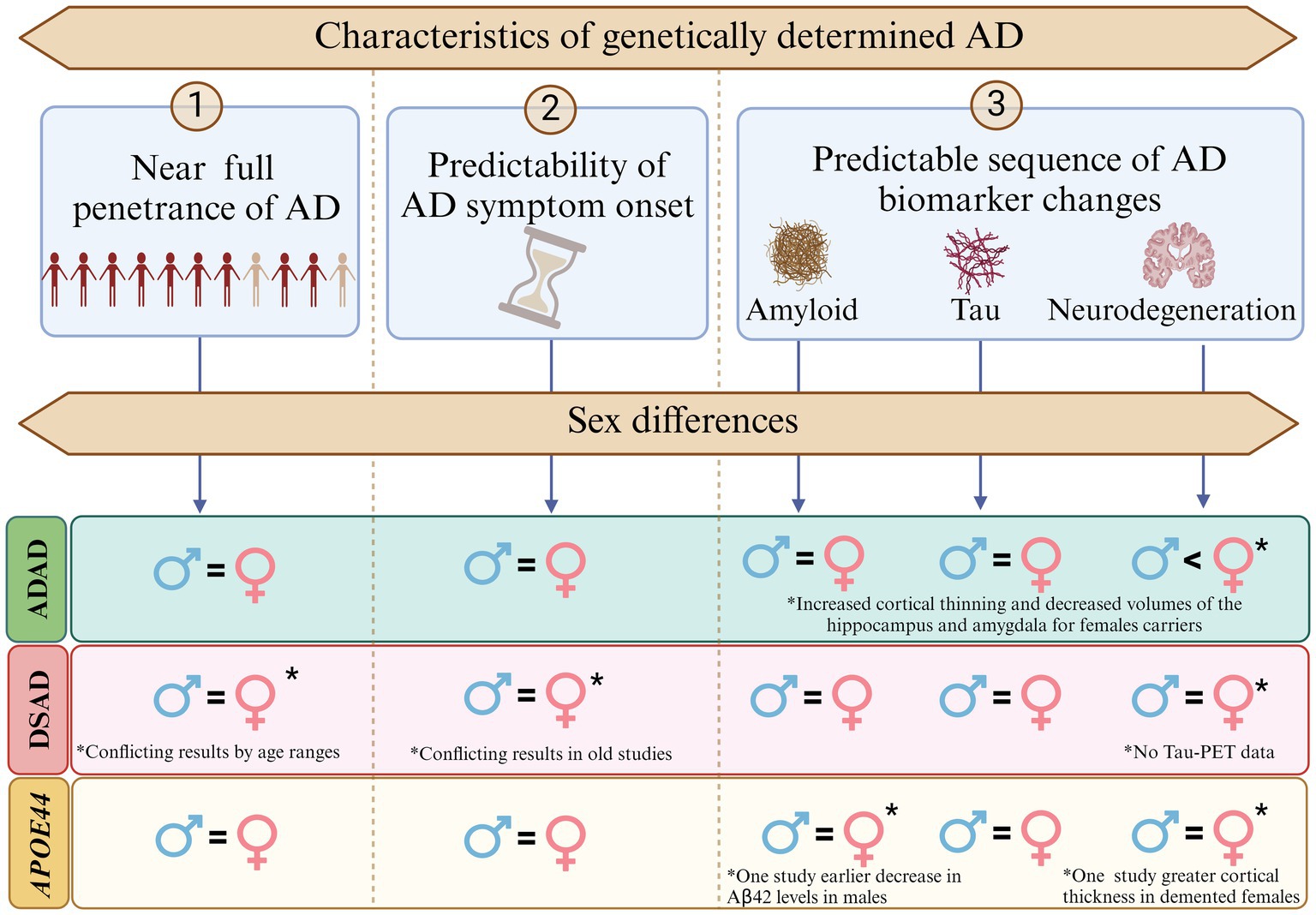
Figure 2. Sex differences in penetrance, symptom onset, and biomarker progression in Alzheimer’s disease (AD) across genetically determined AD populations. This figure illustrates the near-full penetrance of AD in autosomal dominant Alzheimer’s disease (ADAD), Down syndrome-associated AD (DSAD), and APOE44 carriers, alongside the predictable sequence of AD biomarker changes (amyloid deposition, tau accumulation, and neurodegeneration). No significant sex differences were observed in most aspects of penetrance, symptom onset, or biomarker progression. However, notable exceptions include increased cortical thinning and reduced hippocampal and amygdala volumes in female ADAD carriers compared to males. Additionally, earlier decreases in Aβ42 levels were reported in APOE44 males, while greater cortical thickness was observed in APOE44 females. Conflicting findings were noted in DSAD for penetrance and symptom onset, with larger recent studies reporting no significant sex differences.
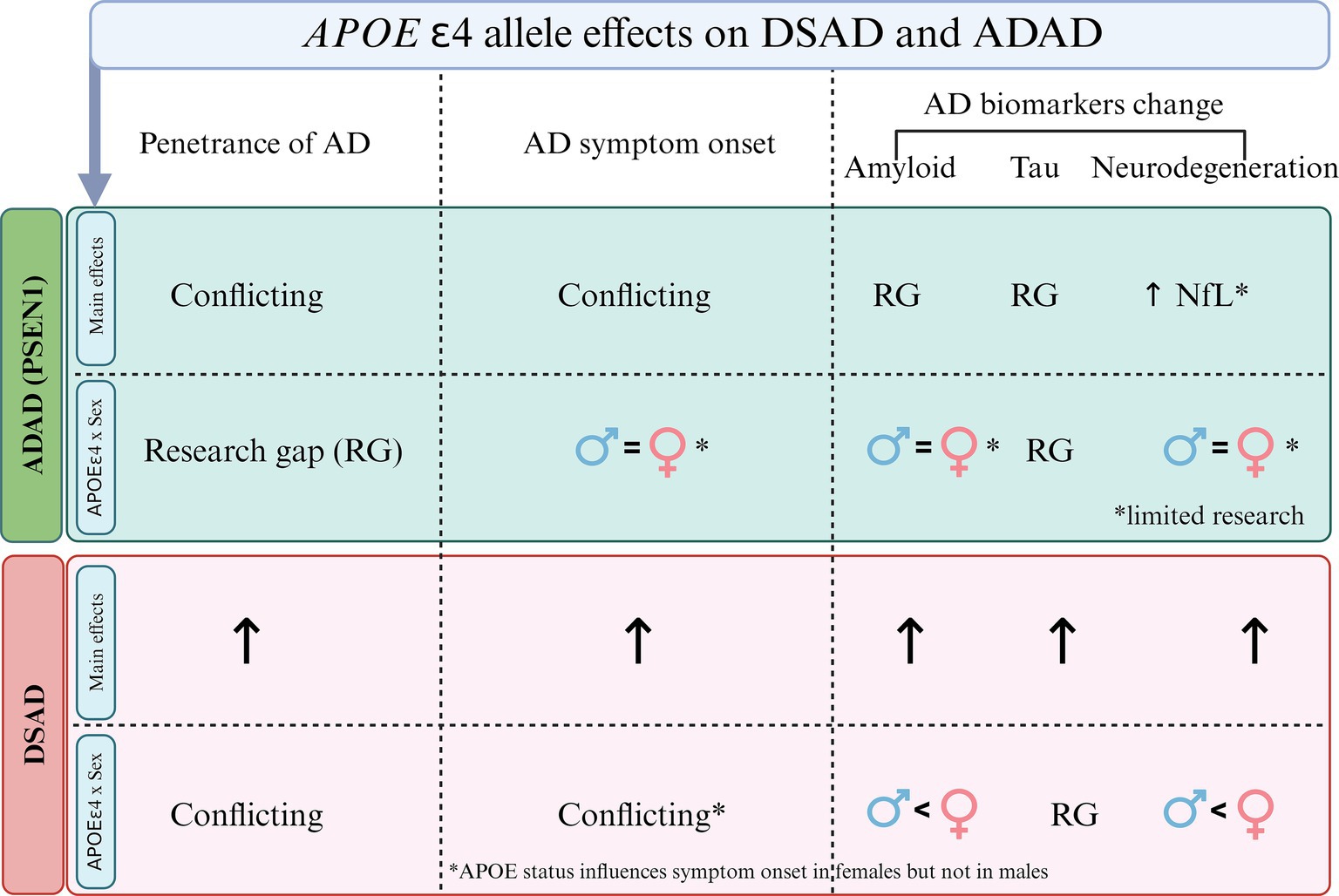
Figure 3. Overview of the main effects of APOE4 and its interaction with sex in Alzheimer’s disease (AD) penetrance, symptom onset and biomarker changes in autosomal dominant AD (ADAD) and Down syndrome-associated AD (DSAD). In ADAD, there are conflicting findings regarding the role of APOE4 in penetrance and symptom onset, with notable research gaps in amyloid and tau changes. However elevated neurofilament light chain (NfL) concentrations have been observed in PSEN1 and APOE4 carriers. The figure also highlights limited evidence for sex-APOE interactions in symptom onset and amyloid changes in ADAD, with many research gaps (RG). In DSAD, APOE4 shows an effect on penetrance, symptom onset, and biomarker changes. Regarding sex, APOE4 appears to influence symptom onset more in females than males, with a stronger impact on amyloid and neurodegeneration, though there are RG in tau biomarker changes.
Emerging multi-omics studies have revealed sex-specific molecular patterns in ADAD, DSAD and APOE4 carriers, with a notable absence of research specifically focused on APOE4 homozygotes. Transcriptomic analyses in DSAD highlighted pronounced neuroinflammatory and glial gene expression differences in females, while proteomic studies in ADAD showed largely sex-independent biomarker trajectories but raised questions about differential neurodegenerative processes. In APOE4 carriers, metabolomic analyses revealed distinct vulnerabilities in energy production pathways, particularly among females, with links to increased p-tau levels and reduced brain glucose uptake. These findings contrast with those in sporadic AD, where multi-omics analyses consistently report more widespread sex differences across immune, synaptic, and metabolic pathways. However, it remains unclear whether these differences are truly less pronounced or simply understudied compared to sporadic AD. Integrating transcriptomics, proteomics, and metabolomics is critical to understanding the interplay between sex and genetic risk factors, advancing sex-specific biomarkers and therapeutic strategies and further research is warranted in these genetic populations.
The smaller sex differences observed in genetically determined AD compared to sporadic AD suggest a lesser influence from sex-specific factors in genetically determined AD. Cognitive resilience and brain reserve may offer less protection in genetic AD, where lifestyle and environmental factors, more influential in sporadic AD, could also play a role in observed differences. Additionally, sporadic AD often involves other pathologies, such as vascular dysfunction, that play a limited role in genetically determined AD. These distinctions highlight the importance of tailoring research and interventions to the specific mechanisms and timelines of genetic and sporadic forms of AD while considering the role of sex in disease pathogenesis.
To comprehensively understand the role of sex in genetically determined AD populations and develop personalized diagnostic, preventive, and therapeutic strategies, future research efforts address the following areas (Table 2):
In summary, while current evidence suggests that sex-specific differences in genetically determined AD are small regarding overall disease penetrance and progression, certain variations may exist. These findings highlight the need for further research into both sex and genetic factors in AD outcomes to enhance our understanding and management of the disease in genetically determined populations. Longitudinal studies that are robustly powered and adhere to sex-specific guidelines (Castro-Aldrete et al., 2023) are critical. It is essential that these studies do not merely control for sex as a confounding variable but instead perform stratified analyses by sex. This approach enables a deeper understanding of how sex-specific factors influence key outcomes, such as disease penetrance, symptom onset, and biomarker trajectories, which may otherwise be obscured in aggregate data. Similarly, race-specific analyses should be conducted to elucidate how the interaction of sex and race impacts AD outcomes in genetically determined forms such as DSAD, ADAD, and APOE44. In this regard, research efforts should prioritize including underrepresented groups, such as Black, Hispanic, and Asian individuals, to better understand how genetic, environmental, and sex-specific factors interact in diverse populations. Current studies largely focus on non-Hispanic White populations, limiting the generalizability of findings. Addressing this gap is critical for ensuring equitable and comprehensive insights into AD risk and progression across different populations. Studies should also include variables unique to each sex, such as hormonal status, to ensure a comprehensive assessment of their impact on AD outcomes. Hormonal influences, such as those occurring during menopause or andropause, likely interact with genetic predispositions like APOE4, influencing disease risk and progression which could be specific to DSAD or ADAD. Cognitive assessment tools should be developed and validated to capture early declines specific to each sex. Current assessments, which often focus on verbal episodic memory, may overlook early declines in females due to their inherent strengths in this domain. Tools that assess cognitive areas where females lack a preexisting advantage, such as visuospatial or executive functions, may provide earlier and more accurate detection of cognitive decline. In this same line, individuals with DS require specialized cognitive tools capable of distinguishing early decline from variations in premorbid intellectual disability. Sex-related differences in cognitive resilience also warrant further study, particularly in DSAD, ADAD, and APOE44 populations, where females often exhibit preserved verbal memory despite underlying neurodegeneration. Understanding these differences has significant implications for precision medicine strategies aimed at enhancing cognitive resilience in genetically determined AD populations. Finally, research findings should always be communicated clearly and transparently, adhering to the MAGIC principles [Magnitude, Accuracy, Generalizability, Inflation, Credibility (Rippon et al., 2024)]. This ensures that findings are impactful and credible while avoiding overstatement of results or misinterpretation. By addressing these gaps and prioritizing the outlined areas, future research can advance understanding of the role of sex in genetically determined AD populations. This will enable the development of more effective, personalized interventions and therapies to improve outcomes for both males and females.
LDHS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. OW: Writing – review & editing. AB: Writing – review & editing. JL: Writing – review & editing. JF: Conceptualization, Data curation, Investigation, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. We thank the institutions that funded this study, the Fondo de Investigaciones Sanitario, Carlos III Health Institute, the Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas and the Generalitat de Catalunya and La Caixa Foundation, the Jérôme Lejeune Foundation as well as the NIH, Horizon 2020 and the Alzheimer’s Association. Funding: National Institute on Aging. This study was supported by the Fondo de Investigaciones Sanitario, Carlos III Health Institute (INT21/00073, PI20/01473 and PI23/01786 to JF, through the Miguel Servet program co-funded by the European Union CP24/00112 to LDHS, and CP20/00038 to AB) and the Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas Program 1, partly jointly funded by Fondo Europeo de Desarrollo Regional, Unión Europea, Una Manera de Hacer Europa. This work was also supported by the National Institutes of Health grants (R01 AG056850; R21 AG056974, R01 AG061566, R01 AG081394 and R61AG066543 to JF) and ADNI (U01 AG024904), the Department de Salut de la Generalitat de Catalunya, Pla Estrategic de Recerca I Innovació en Salut (SLT006/17/00119 to JF). It was also supported by Fundación Tatiana Pérez de Guzmán el Bueno (IIBSP-DOW-2020-151 to JF) and Horizon 2020-Research and Innovation Framework Program from the European Union (H2020-SC1-BHC-2018-2020 to JF), the Jérôme Lejeune Foundation (2326 -GRT-2024A to LDHS) and the Alzheimer’s Association (AARG-22-923680 to AB).
We would like to thank the Center for Advanced Studies (CAS) at Ludwig-Maximilians University Munich (CAS LMU) for supporting the CAS Research Group “Tools for Transnational Neuropsychiatric Research.” LDHS and JF were part of the group, of which JL was the spokesperson. All the figures of this manuscript have been created in BioRender.
JF has received personal fees for service on advisory boards, adjudication committees, or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Eisai, Esteve, Fujirebio, Ionis, Laboratorios Carnot, Life Molecular Imaging, Lilly, Lundbeck, Perha, and Roche, outside the submitted work. JF also holds a patent for markers of synaptopathy in neurodegenerative disease (licensed to Adx, EPI8382175.0). JL reports speaker fees from Bayer Vital, Biogen, EISAI, TEVA, Zambon, Esteve, Merck and Roche, consulting fees from Axon Neuroscience, EISAI and Biogen, author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers and is inventor in a patent “Oral Phenylbutyrate for Treatment of Human 4-Repeat Tauopathies” (PCT/EP2024/053388) filed by LMU Munich. In addition, JL reports compensation for serving as chief medical officer for MODAG GmbH is beneficiary of the phantom share program of MODAG GmbH and is inventor in a patent “Pharmaceutical Composition and Methods of Use” (EP 22 159 408.8) filed by MODAG GmbH, all activities outside the submitted work. The other authors report no relevant disclosures.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SG declared a past collaboration with the author JF.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aguirre-Acevedo, D. C., Lopera, F., Henao, E., Tirado, V., Muñoz, C., Giraldo, M., et al. (2016). Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease a retrospective cohort study. JAMA Neurol. 73, 431–438. doi: 10.1001/jamaneurol.2015.4851
Alzheimer’s Disease Facts and Figures (2024). Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 20, 3708–3821. doi: 10.1002/ALZ.13809
Antonaros, F., Ghini, V., Pulina, F., Ramacieri, G., Cicchini, E., Mannini, E., et al. (2020). Plasma metabolome and cognitive skills in down syndrome. Sci. Rep. 10, 10491–10412. doi: 10.1038/s41598-020-67195-z
Arenaza-Urquijo, E. M., Boyle, R., Casaletto, K., Anstey, K. J., Vila-Castelar, C., Colverson, A., et al. (2024). Sex and gender differences in cognitive resilience to aging and Alzheimer’s disease. Alzheimers Dement. 20, 5695–5719. doi: 10.1002/ALZ.13844
Arnold, M., Nho, K., Kueider-Paisley, A., Massaro, T., Huynh, K., Brauner, B., et al. (2020). Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat Commun. 11:1148. doi: 10.1038/S41467-020-14959-W
Bateman, R. J., Xiong, C., Benzinger, T. L. S., Fagan, A. M., Goate, A., Fox, N. C., et al. (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804. doi: 10.1056/NEJMoa1202753
Bejanin, A., Iulita, M. F., Eduard, V., Carmona-Iragui, M., Benejam, B., Videla, L., et al. (2021). Association of Apolipoprotein E ε4 allele with clinical and multimodal biomarker changes of Alzheimer disease in adults with down syndrome supplemental content. JAMA Neurol. 78, 937–947. doi: 10.1001/jamaneurol.2021.1893
Belloy, M. E., Andrews, S. J., Le Guen, Y., Cuccaro, M., Farrer, L. A., Napolioni, V., et al. (2023). APOE genotype and Alzheimer disease risk across age, sex, and population ancestry. JAMA Neurol. 80, 1284–1294. doi: 10.1001/JAMANEUROL.2023.3599
Bocancea, D. I., Svenningsson, A. L., Van Loenhoud, A. C., Groot, C., Barkhof, F., Strandberg, O., et al. (2023). Determinants of cognitive and brain resilience to tau pathology: a longitudinal analysis. Brain 146, 3719–3734. doi: 10.1093/BRAIN/AWAD100
Bridel, C., Van Wieringen, W. N., Zetterberg, H., Tijms, B. M., Teunissen, C. E., Alvarez-Cermeño, J. C., et al. (2019). Diagnostic value of cerebrospinal fluid Neurofilament light protein in neurology: A systematic review and Meta-analysis. JAMA Neurol. 76, 1035–1048. doi: 10.1001/JAMANEUROL.2019.1534
Buckley, R. F., Mormino, E. C., Rabin, J. S., Hohman, T. J., Landau, S., Hanseeuw, B. J., et al. (2019). Sex differences in the Association of Global Amyloid and Regional tau Deposition Measured by positron emission tomography in clinically Normal older adults. JAMA Neurol. 76, 542–551. doi: 10.1001/JAMANEUROL.2018.4693
Cao, M., Li, H., Zhao, J., Cui, J., and Hu, G. (2019). Identification of age- and gender-associated long noncoding RNAs in the human brain with Alzheimer’s disease. Neurobiol. Aging 81, 116–126. doi: 10.1016/J.NEUROBIOLAGING.2019.05.023
Carmona-Iragui, M., Videla, L., Lleó, A., and Fortea, J. (2019). Down syndrome, Alzheimer disease, and cerebral amyloid angiopathy: the complex triangle of brain amyloidosis. Dev. Neurobiol. 79, 716–737. doi: 10.1002/DNEU.22709
Castro-Aldrete, L., Moser, M. V., Putignano, G., Ferretti, M. T., Schumacher Dimech, A., and Santuccione Chadha, A. (2023). Sex and gender considerations in Alzheimer’s disease: the Women’s brain project contribution. Front. Aging Neurosci. 15:1105620. doi: 10.3389/fnagi.2023.1105620
Chêne, G., Beiser, A., Au, R., Preis, S. R., Wolf, P. A., Dufouil, C., et al. (2015). Gender and incidence of dementia in the Framingham heart study from mid-adult life. Alzheimers Dement. 11, 310–320. doi: 10.1016/J.JALZ.2013.10.005
de Sola, S., de la Torre, R., Sánchez-Benavides, G., Benejam, B., Cuenca-Royo, A., Del Hoyo, L., et al. (2015). A new cognitive evaluation battery for down syndrome and its relevance for clinical trials. Front. Psychol. 6:708. doi: 10.3389/fpsyg.2015.00708
DeCasien, A. R., Guma, E., Liu, S., and Raznahan, A. (2022). Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biol. Sex Differ. 13:43. doi: 10.1186/s13293-022-00448-w
del Hoyo Soriano, L., Thurman, A. J., and Abbeduto, L. (2018). Specificity: A phenotypic comparison of communication-relevant domains between youth with down syndrome and fragile X syndrome. Front. Genet. 9:424. doi: 10.3389/fgene.2018.00424
Deming, Y., Dumitrescu, L., Barnes, L. L., Thambisetty, M., Kunkle, B., Gifford, K. A., et al. (2018). Sex-specific genetic predictors of Alzheimer’s disease biomarkers. Acta Neuropathol. 136, 857–872. doi: 10.1007/S00401-018-1881-4
Dubois, B., Feldman, H. H., Jacova, C., Hampel, H., Molinuevo, J. L., Blennow, K., et al. (2014). “Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria” Lancet Neurol. 2014 Jun;13(6):614-29. doi: 10.1016/S1474-4422(14)70090-0. Erratum in: Lancet Neurol. 13:757.
Dumois-Petersen, S., Gallegos-Arreola, M. P., Magaña-Torres, M. T., Perea-Díaz, F. J., Ringman, J. M., and Figuera, L. E. (2020). Autosomal dominant early onset Alzheimer’s disease in the Mexican state of Jalisco: high frequency of the mutation PSEN1 c.1292C>A and phenotypic profile of patients. Am. J. Med. Genet. C: Semin. Med. Genet. 184, 1023–1029. doi: 10.1002/AJMG.C.31865
Eliot, L., Ahmed, A., Khan, H., and Patel, J. (2021). Dump the “dimorphism”: comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci. Biobehav. Rev. 125, 667–697. doi: 10.1016/J.NEUBIOREV.2021.02.026
Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., et al. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A Meta-analysis. JAMA 278, 1349–1356. doi: 10.1001/JAMA.1997.03550160069041
Ferretti, M. T., Iulita, M. F., Cavedo, E., Chiesa, P. A., Dimech, A. S., Chadha, A. S., et al. (2018). Sex differences in Alzheimer disease — the gateway to precision medicine. Nat. Rev. Neurol. 14, 457–469. doi: 10.1038/s41582-018-0032-9
Fortea, J., Pegueroles, J., Alcolea, D., Belbin, O., Dols-Icardo, O., Vaqué-Alcázar, L., et al. (2024). APOE4 homozygosity represents a distinct genetic form of Alzheimer’s disease. Nat. Med. 30, 1284–1291. doi: 10.1038/s41591-024-02931-w
Fortea, J., Vilaplana, E., Carmona-Iragui, M., Benejam, B., Videla, L., Barroeta, I., et al. (2020). Clinical and biomarker changes of Alzheimer’s disease in adults with down syndrome: a cross-sectional study. Lancet 395, 1988–1997. doi: 10.1016/S0140-6736(20)30689-9
Fortea, J., Zaman, S. H., Hartley, S., Rafii, M. S., Head, E., and Carmona-Iragui, M. (2021). Alzheimer’s disease associated with down syndrome: a genetic form of dementia. Lancet Neurol. 20, 930–942. doi: 10.1016/S1474-4422(21)00245-3
Fox-Fuller, J. T., Artola, A., Chen, K., Pulsifer, M., Ramirez, D., Londono, N., et al. (2021). Sex differences in cognitive abilities among children with the autosomal dominant Alzheimer disease Presenilin 1 E280A variant from a Colombian cohort. JAMA Netw. Open 4:E2121697. doi: 10.1001/JAMANETWORKOPEN.2021.21697
Gorijala, P., Aslam, M. M., Dang, L. H. T., Xicota, L., Fernandez, M. V., Sung, Y. J., et al. (2024). Alzheimer’s polygenic risk scores are associated with cognitive phenotypes in down syndrome. Alzheimers Dementia 20, 1038–1049. doi: 10.1002/ALZ.13506
Guo, L., Zhong, M. B., Zhang, L., Zhang, B., and Cai, D. (2021). Sex differences in Alzheimer’s disease: insights from the multiomics landscape. Biol. Psychiatry 91, 61–71. doi: 10.1016/J.BIOPSYCH.2021.02.968
Gustavsson, A., Norton, N., Fast, T., Frölich, L., Georges, J., Holzapfel, D., et al. (2023). Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 19, 658–670. doi: 10.1002/ALZ.12694
Hartley, S. L., Handen, B. L., Devenny, D., Tudorascu, D., Piro-Gambetti, B., Zammit, M. D., et al. (2020). Cognitive indicators of transition to preclinical and prodromal stages of Alzheimer’s disease in down syndrome. Alzheimers Dementia 12:e12096. doi: 10.1002/DAD2.12096
Hirnstein, M., Stuebs, J., Moè, A., and Hausmann, M. (2023). Sex/gender differences in verbal fluency and verbal-episodic memory: A Meta-analysis. Perspect. Psychol. Sci. 18, 67–90. doi: 10.1177/17456916221082116
Huque, H., Eramudugolla, R., Chidiac, B., Ee, N., Ehrenfeld, L., Matthews, F. E., et al. (2023). Could country-level factors explain sex differences in dementia incidence and prevalence? A systematic review and Meta-analysis. J. Alzheimers Disease 91, 1231–1241. doi: 10.3233/JAD-220724
Iulita, M. F., Bejanin, A., Vilaplana, E., Carmona-Iragui, M., Benejam, B., Videla, L., et al. (2023). Association of biological sex with clinical outcomes and biomarkers of Alzheimer’s disease in adults with down syndrome. Brain Commun. 5:fcad074. doi: 10.1093/BRAINCOMMS/FCAD074
Iulita, M. F., Garzón Chavez, D., Klitgaard Christensen, M., Valle Tamayo, N., Plana-Ripoll, O., Rasmussen, S. A., et al. (2022). Association of Alzheimer Disease with Life Expectancy in people with down syndrome. JAMA Netw. Open 5:e2212910. doi: 10.1001/JAMANETWORKOPEN.2022.12910
Jansen, W. J., Janssen, O., Tijms, B. M., Vos, S. J. B., Ossenkoppele, R., Visser, P. J., et al. (2022). Prevalence estimates of amyloid abnormality across the Alzheimer disease clinical Spectrum. JAMA Neurol. 79, 228–243. doi: 10.1001/JAMANEUROL.2021.5216
Jansen, W. J., Ossenkoppele, R., Knol, D. L., Tijms, B. M., Scheltens, P., Verhey, F. R. J., et al. (2015). Prevalence of cerebral amyloid pathology in persons without dementia: A Meta-analysis. JAMA 313, 1924–1938. doi: 10.1001/JAMA.2015.4668
Johnson, E. C. B., Bian, S., Haque, R. U., Carter, E. K., Watson, C. M., Gordon, B. A., et al. (2023). Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer’s disease. Nat. Med. 29, 1979–1988. doi: 10.1038/s41591-023-02476-4
Khoury, M. A., Valcic, M., Churchill, N. W., Di Battista, A., De Luca, V., Fornazzari, L. R., et al. (2024). Sex differences in cortical thickness and neuropsychiatric symptom burden based on APOE4 homozygosity in Alzheimer’s disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 198:e33008. doi: 10.1002/AJMG.B.33008
Koenig, K. A., Oh, S. H., Stasko, M. R., Roth, E. C., Taylor, H. G., Ruedrich, S., et al. (2021). High resolution structural and functional MRI of the hippocampus in young adults with down syndrome. Brain Commun. 3:fcab088. doi: 10.1093/BRAINCOMMS/FCAB088
Kommaddi, R. P., Verma, A., Muniz-Terrera, G., Tiwari, V., Chithanathan, K., Diwakar, L., et al. (2023). Sex difference in evolution of cognitive decline: studies on mouse model and the dominantly inherited Alzheimer network cohort. Transl. Psychiatry. 13, 123–112. doi: 10.1038/s41398-023-02411-8
Lai, F., Kammann, E., Rebeck, G. W., Anderson, A., Chen, Y., and Nixon, R. A. (1999). APOE genotype and gender effects on Alzheimer disease in 100 adults with down syndrome. Neurology 53, 331–336. doi: 10.1212/WNL.53.2.331
Lai, F., Mhatre, P. G., Yang, Y., Wang, M. C., Schupf, N., and Rosas, H. D. (2020). Sex differences in risk of Alzheimer’s disease in adults with down syndrome. Alzheimers Dementia 12:e12084. doi: 10.1002/DAD2.12084
Langella, S., Bonta, K., Chen, Y., Su, Y., Vasquez, D., Aguillon, D., et al. (2024). Impact of APOE ε4 and ε2 on plasma neurofilament light chain and cognition in autosomal dominant Alzheimer’s disease. Alzheimers Res. Ther. 16:208. doi: 10.1186/S13195-024-01572-Y
Langella, S., Gil Barksdale, N., Vasquez, D., Aguillon, D., Chen, Y., Su, Y., et al. (2023). Effect of apolipoprotein genotype and educational attainment on cognitive function in autosomal dominant Alzheimer’s disease. Nat. Commun. 14:5120. doi: 10.1038/s41467-023-40775-z
Lanoiselée, H. M., Nicolas, G., Wallon, D., Rovelet-Lecrux, A., Lacour, M., Rousseau, S., et al. (2017). APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 14:e1002270. doi: 10.1371/JOURNAL.PMED.1002270
Larsen, F. K., Baksh, R. A., McGlinchey, E., Langballe, E. M., Benejam, B., Beresford-Webb, J., et al. (2024). Age of Alzheimer’s disease diagnosis in people with down syndrome and associated factors: results from the horizon 21 European down syndrome consortium. Alzheimers Dementia 20, 3270–3280. doi: 10.1002/ALZ.13779
Lauer, J. E., Yhang, E., and Lourenco, S. F. (2019). The development of gender differences in spatial reasoning: a meta-analytic review. Psychol. Bull. 145, 537–565. doi: 10.1037/BUL0000191
Levine, S. C., Foley, A., Lourenco, S., Ehrlich, S., and Ratliff, K. (2016). Sex differences in spatial cognition: advancing the conversation. Wiley Interdiscip. Rev. Cogn. Sci. 7, 127–155. doi: 10.1002/wcs.1380
Liesinger, A. M., Graff-Radford, N. R., Duara, R., Carter, R. E., Hanna Al-Shaikh, F. S., Koga, S., et al. (2018). Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol. 136, 873–885. doi: 10.1007/S00401-018-1908-X
Lin, K. A., Choudhury, K. R., Rathakrishnan, B. G., Marks, D. M., Petrella, J. R., and Doraiswamy, P. M. (2015). Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dementia 1, 103–110. doi: 10.1016/J.TRCI.2015.07.001
Liu, M., Paranjpe, M. D., Zhou, X., Duy, P. Q., Goyal, M. S., Benzinger, T. L. S., et al. (2022). Sex modulates the ApoE ε4 effect on brain tau deposition measured by 18F-AV-1451 PET in individuals with mild cognitive impairment. Theranostics 9, 4959–4970. doi: 10.7150/thno.35366
Mano, T., Nagata, K., Nonaka, T., Tarutani, A., Imamura, T., Hashimoto, T., et al. (2017). Neuron-specific methylome analysis reveals epigenetic regulation and tau-related dysfunction of BRCA1 in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 114, E9645–E9654. doi: 10.1073/PNAS.1707151114
Martá-Ariza, M., Leitner, D. F., Kanshin, E., Suazo, J., Pedrosa, A. G., Thierry, M., et al. (2025). Comparison of the amyloid plaque proteome in Down syndrome, early-onset Alzheimer’s disease, and late-onset Alzheimer’s disease. Acta Neuropathol. 149:9. doi: 10.1007/s00401-025-02844-z
Mathys, H., Davila-Velderrain, J., Peng, Z., Gao, F., Mohammadi, S., Young, J. Z., et al. (2019). Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337. doi: 10.1038/S41586-019-1195-2
Mhatre, P. G., Lee, J. H., Pang, D., Zigman, W. B., Tycko, B., Krinsky-Mchale, S. J., et al. (2021). The association between sex and risk of Alzheimer’s disease in adults with down syndrome. J. Clin. Med. 10:2966. doi: 10.3390/JCM10132966
Mielke, M. M. (2020). Consideration of sex differences in the measurement and interpretation of Alzheimer disease-related biofluid-based biomarkers. J. Appl. Lab. Med. 5, 158–169. doi: 10.1373/JALM.2019.030023
Mielke, M. M., Vemuri, P., and Rocca, W. A. (2014). Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin. Epidemiol. 6, 37–48. doi: 10.2147/CLEP.S37929
Millar, P. R., Gordon, B. A., Wisch, J. K., Schultz, S. A., Benzinger, T. L., Cruchaga, C., et al. (2023). Advanced structural brain aging in preclinical autosomal dominant Alzheimer disease. Mol. Neurodegener. 18, 1–17. doi: 10.1186/S13024-023-00688-3/FIGURES/5
Miyoshi, E., Morabito, S., Henningfield, C. M., Das, S., Rahimzadeh, N., Shabestari, S. K., et al. (2024). Spatial and single-nucleus transcriptomic analysis of genetic and sporadic forms of Alzheimer’s disease. Nat. Genet. 56, 2704–2717. doi: 10.1038/S41588-024-01961-X
Mortimer, J. A. (2013). The Nun study: risk factors for pathology and clinical-pathologic correlations. Curr. Alzheimer Res. 9, 621–627. doi: 10.2174/156720512801322546
Paranjpe, M. D., Belonwu, S., Wang, J. K., Oskotsky, T., Gupta, A., Taubes, A., et al. (2021). Sex-specific cross tissue Meta-analysis identifies immune dysregulation in women with Alzheimer’s disease. Front. Aging Neurosci. 13:735611. doi: 10.3389/fnagi.2021.735611
Pastor, P., Roe, C. M., Villegas, A., Bedoya, G., Chakraverty, S., García, G., et al. (2003). Apolipoprotein Eepsilon4 modifies Alzheimer’s disease onset in an E280A PS1 kindred. Ann. Neurol. 54, 163–169. doi: 10.1002/ANA.10636
Peven, J. C., Handen, B. L., Laymon, C. M., Fleming, V., Piro-Gambetti, B., Christian, B. T., et al. (2022). Physical activity, memory function, and hippocampal volume in adults with down syndrome. Front. Integr. Neurosci. 16:919711. doi: 10.3389/FNINT.2022.919711
Prasher, V. P., Sajith, S. G., Rees, S. D., Patel, A., Tewari, S., Schupf, N., et al. (2008). Significant effect of APOE epsilon 4 genotype on the risk of dementia in Alzheimer’s disease and mortality in persons with down syndrome. Int. J. Geriatr. Psychiatry 23, 1134–1140. doi: 10.1002/GPS.2039
Quiroz, Y. T., Aguillon, D., Aguirre-Acevedo, D. C., Vasquez, D., Zuluaga, Y., Baena, A. Y., et al. (2024). APOE3 Christchurch heterozygosity and autosomal dominant Alzheimer’s disease. N. Engl. J. Med. 390, 2156–2164. doi: 10.1056/NEJMOA2308583
Reilly, D. (2012). Gender, culture, and sex-typed cognitive abilities. PLoS One 7:e39904. doi: 10.1371/JOURNAL.PONE.0039904
Rippon, G., Losse, K., and White, S. (2024). Impression management in sex and gender neuroscience research reporting: the MAGIC guidelines. Nat. Commun. 15, 1–3. doi: 10.1038/s41467-024-47261-0
Romano, A., Moraschi, M., Cornia, R., Bozzao, A., Gagliardo, O., Chiacchiararelli, L., et al. (2015). Age effects on cortical thickness in young Down’s syndrome subjects: a cross-sectional gender study. Neuroradiology 57, 401–411. doi: 10.1007/S00234-014-1482-4
Rubenstein, E., Hartley, S., and Bishop, L. (2020). Epidemiology of dementia and Alzheimer disease in individuals with down syndrome. JAMA Neurol. 77, 262–264. doi: 10.1001/JAMANEUROL.2019.3666
Rubenstein, E., Tewolde, S., Scott, A., Michals, A., Fox, M., Weuve, J., et al. (2024). “Alzheimer’s disease in a full Medicaid and Medicare sample of US adults with down syndrome” in Poster presented at the 5th international conference of the trisomy 21 research society (Rome: Italy).
Ryman, D. C., Acosta-Baena, N., Aisen, P. S., Bird, T., Danek, A., Fox, N. C., et al. (2014). Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 83, 253–260. doi: 10.1212/WNL.0000000000000596
Sang, F., Zhao, S., Li, Z., Yang, Y., Chen, Y., and Zhang, Z. (2024). Cortical thickness reveals sex differences in verbal and visuospatial memory. Cerebral Cortex 34:bhae067. doi: 10.1093/CERCOR/BHAE067
Schupf, N., Kapell, D., Nightingale, B., Rodriguez, A., Tycko, B., and Mayeux, R. (1998). Earlier onset of Alzheimer’s disease in men with down syndrome. Neurology 50, 991–995. doi: 10.1212/WNL.50.4.991
Shang, Y., Mishra, A., Wang, T., Wang, Y., Desai, M., Chen, S., et al. (2020). Evidence in support of chromosomal sex influencing plasma based metabolome vs APOE genotype influencing brain metabolome profile in humanized APOE male and female mice. PLoS One 15:e0225392. doi: 10.1371/JOURNAL.PONE.0225392
Shaw, C., Hayes-Larson, E., Glymour, M. M., Dufouil, C., Hohman, T. J., Whitmer, R. A., et al. (2021). Evaluation of selective survival and sex/gender differences in dementia incidence using a simulation model. JAMA Netw. Open 4:e211001. doi: 10.1001/JAMANETWORKOPEN.2021.1001
Silva, G. B., Pascucci, J. A., Karim, H., Kaur, G., Lerma, R. O., Mann, A. K., et al. (2024). Influence of the onset of menopause on the risk of developing Alzheimer’s disease. Cureus 16:e69124. doi: 10.7759/CUREUS.69124
Silverman, W. P., Zigman, W. B., Krinsky-Mchale, S. J., Ryan, R., and Schupf, N. (2013). Intellectual disability, mild cognitive impairment, and risk for dementia. J. Policy Pract. Intellect. Disabil. 10, 245–251. doi: 10.1111/JPPI.12042
Sohn, D., Shpanskaya, K., Lucas, J. E., Petrella, J. R., Saykin, A. J., Tanzi, R. E., et al. (2018). Sex differences in cognitive decline in subjects with high likelihood of mild cognitive impairment due to Alzheimer’s disease. Sci. Rep. 8:7490. doi: 10.1038/S41598-018-25377-W
Springer, K. W., Hankivsky, O., and Bates, L. M. (2012). Gender and health: relational, intersectional, and biosocial approaches. Soc. Sci. Med. 74, 1661–1666. doi: 10.1016/J.SOCSCIMED.2012.03.001
Startin, C. M., D’Souza, H., Ball, G., Hamburg, S., Hithersay, R., Hughes, K. M. O., et al. (2020). Health comorbidities and cognitive abilities across the lifespan in down syndrome. J. Neurodev. Disord. 12:4. doi: 10.1186/s11689-019-9306-9
Tang, M. X., Stern, Y., Marder, K., Bell, K., Gurland, B., Lantigua, R., et al. (1998). The APOE-∊4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 279, 751–755. doi: 10.1001/JAMA.279.10.751
Therriault, J., Pascoal, T. A., Benedet, A. L., Tissot, C., Savard, M., Chamoun, M., et al. (2021). Frequency of biologically defined Alzheimer disease in relation to age, sex, APOE ϵ4, and cognitive impairment. Neurology 96, E975–E985. doi: 10.1212/WNL.0000000000011416/ASSET/425395AF-DD29-4E3B-B419-D54D4A386573/ASSETS/GRAPHIC/5TTU1.GIF
Tsiknia, A. A., Edland, S. D., Sundermann, E. E., Reas, E. T., Brewer, J. B., Galasko, D., et al. (2022). Sex differences in plasma p-tau181 associations with Alzheimer’s disease biomarkers, cognitive decline, and clinical progression. Mol. Psychiatry 27, 4314–4322. doi: 10.1038/S41380-022-01675-8
Valdez-Gaxiola, C. A., Maciel-Cruz, E. J., Hernández-Peña, R., Dumois-Petersen, S., Rosales-Leycegui, F., Gallegos-Arreola, M. P., et al. (2023). Potential modifying effect of the APOEε4 allele on age of onset and clinical manifestations in patients with early-onset Alzheimer’s disease with and without a pathogenic variant in PSEN1 in a sample of the Mexican population. Int. J. Mol. Sci. 24, 24:15687. doi: 10.3390/IJMS242115687
Van Der Ende, E. L., Int Veld, S. G. J. G., Hanskamp, I., Van Der Lee, S., Dijkstra, J. I. R., Hok-A-Hin, Y. S., et al. (2023). CSF proteomics in autosomal dominant Alzheimer’s disease highlights parallels with sporadic disease. Brain 146, 4495–4507. doi: 10.1093/BRAIN/AWAD213
Vélez, J. I., Lopera, F., Sepulveda-Falla, D., Patel, H. R., Johar, A. S., Chuah, A., et al. (2016). APOE*E2 allele delays age of onset in PSEN1 E280A Alzheimer’s disease. Mol. Psychiatry 21, 916–924. doi: 10.1038/MP.2015.177
Vila-Castelar, C., Chen, Y., Langella, S., Lopera, F., Zetterberg, H., Hansson, O., et al. (2023). Sex differences in blood biomarkers and cognitive performance in individuals with autosomal dominant Alzheimer’s disease. Alzheimers Dement. 19, 4127–4138. doi: 10.1002/ALZ.13314
Vila-Castelar, C., Guzmán-Vélez, E., Pardilla-Delgado, E., Buckley, R. F., Bocanegra, Y., Baena, A., et al. (2020). Examining sex differences in markers of cognition and neurodegeneration in autosomal dominant Alzheimer’s disease: preliminary findings from the Colombian Alzheimer’s prevention initiative biomarker study. J. Alzheimers Disease 77, 1743–1753. doi: 10.3233/JAD-200723
Vila-Castelar, C., Tariot, P. N., Sink, K. M., Clayton, D., Langbaum, J. B., Thomas, R. G., et al. (2022). Sex differences in cognitive resilience in preclinical autosomal-dominant Alzheimer’s disease carriers and non-carriers: baseline findings from the API ADAD Colombia trial. Alzheimers Dement. 18, 2272–2282. doi: 10.1002/ALZ.12552
Wagemann, O., Li, Y., Hassenstab, J., Aschenbrenner, A. J., McKay, N. S., Gordon, B. A., et al. (2024). Investigation of sex differences in mutation carriers of the dominantly inherited Alzheimer network. Alzheimers Dement. 20, 47–62. doi: 10.1002/ALZ.13460
Walters, S., Contreras, A. G., Eissman, J. M., Mukherjee, S., Lee, M. L., Choi, S.-E., et al. (2023). Associations of sex, race, and apolipoprotein E alleles with multiple domains of cognition among older adults. JAMA Neurol. 80, 929–939. doi: 10.1001/JAMANEUROL.2023.2169
Yan, S., Zheng, C., Paranjpe, M. D., Li, Y., Li, W., and Wang, X. (2021). Sex modifies APOE ε4 dose effect on brain tau deposition in cognitively impaired individuals. Brain 144, 3201–3211. doi: 10.1093/BRAIN/AWAB160
Yu, G., Thorpe, A., Zeng, Q., Wang, E., Cai, D., Wang, M., et al. (2024). The landscape of sex- and APOE genotype-specific transcriptional changes in Alzheimer’s disease at the single cell level. BioRxiv 2024:01.626234. doi: 10.1101/2024.12.01.626234
Zhu, D., Montagne, A., and Zhao, Z. (2021). Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell. Mol. Life Sci. 78, 4907–4920. doi: 10.1007/S00018-021-03830-W
Keywords: genetically determined Alzheimer’s disease, autosomal dominant Alzheimer’s disease (ADAD), down syndrome-associated Alzheimer’s disease (DS-AD), apolipoprotein E epsilon 4 (APOE4) homozygosity, sex differences in Alzheimer’s disease, disease penetrance in Alzheimer’s disease, symptom onset in Alzheimer’s disease, AT(N) biomarker trajectories in Alzheimer’s disease (amyloid, tau and neurodegeneration biomarkers)
Citation: Del Hoyo Soriano L, Wagemann O, Bejanin A, Levin J and Fortea J (2025) Sex-related differences in genetically determined Alzheimer’s disease. Front. Aging Neurosci. 17:1522434. doi: 10.3389/fnagi.2025.1522434
Received: 04 November 2024; Accepted: 19 February 2025;
Published: 04 March 2025.
Edited by:
Stephen D. Ginsberg, Nathan S. Kline Institute for Psychiatric Research, United StatesReviewed by:
Jaclyn Eissman, Vanderbilt University, United StatesCopyright © 2025 Del Hoyo Soriano, Wagemann, Bejanin, Levin and Fortea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Del Hoyo Soriano, bGhveW9Ac2FudHBhdS5jYXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.