
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 12 March 2025
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 17 - 2025 | https://doi.org/10.3389/fnagi.2025.1468895
This article is part of the Research Topic Common pathophysiology underpinning Parkinson's disease and other neurodegenerative diseases View all 14 articles
 Hongjia Xu1
Hongjia Xu1 Xiaolei Zheng1
Xiaolei Zheng1 Xinyue Xing1
Xinyue Xing1 Zhichao Bi1
Zhichao Bi1 Dewei Wang1
Dewei Wang1 Cheng Zhang1
Cheng Zhang1 Lifei Wei1
Lifei Wei1 Yulin Jin2*
Yulin Jin2* Shunliang Xu1,2*
Shunliang Xu1,2*Parkinson’s disease (PD) is a prevalent neurodegenerative disorder, best known for its motor symptoms such as resting tremor, muscle rigidity, and bradykinesia. However, autonomic dysfunction is an important non-motor aspect that often brings considerable discomfort and distress to both patients and their families. In this review, we summarize recent advances in understanding the pathophysiological mechanisms of autonomic dysfunction and explore its relationship with other clinical features. Our aim is to discover novel potential diagnostic and therapeutic strategies, alleviate patient suffering, and pave the way for future clinical and basic research.
Parkinson’s disease (PD) is a common and complex neurodegenerative disease. While PD is most typically observed in the elderly, a growing hypothesis suggests that a prodromal phase, marked by a range of early symptoms, may begin as early as a person’s 20s (Darweesh et al., 2018; Fereshtehnejad et al., 2019). Studied for centuries, the hallmark pathophysiology of PD is involves by a decrease in dopamine in substantia nigra neurons and striatum, and the formation of intracellular inclusion bodies that contain α-synuclein(α-syn) aggregates (Shahmoradian et al., 2019; Tolosa et al., 2021; Chu et al., 2024). The clinical features include motor symptoms represented by bradykinesia, resting tremor, increased muscle tone, and gait abnormalities, as well as non-motor symptoms such as olfactory disturbances, sleep disturbances, cognitive dysfunction, emotional abnormalities, and autonomic dysfunction (Bloem et al., 2021). Autonomic dysfunction (AutD) is an important non-motor symptoms in PD, which includes gastrointestinal dysfunction, cardiovascular dysfunction, urinary dysfunction, thermoregulatory dysfunction, pupillary motor function and sexual dysfunction (Chen et al., 2020; Pfeiffer, 2020). It has been reported that 70–80 per cent of patients may suffer from gastrointestinal autonomic dysfunction (Perez-Pardo et al., 2017). Additionally, about 30–50 per cent of patients experience orthostatic hypotension. Cardiovascular dysfunctions significantly impact patients’ lives by causing extreme blood pressure instability, which not only affects the cognitive functioning but also weakens their ability to perform daily activities (Palma and Cortelli, 2023; Palma et al., 2024). Furthermore, slow gastric emptying and constipation can impair the pharmacodynamics of medications, leading to a deterioration of motor function (Chung and Pfeiffer, 2020; Leta et al., 2023).
Research has revealed that AutD may appear years before than motor symptoms in Parkinson’s disease, and more than half of PD patients experience at least one form of the AutD, which can significantly impact their quality of life (Bloem et al., 2021; Longardner et al., 2022). Moreover, AutD is considered a key feature in the diagnosis of prodromal PD and serves a prognostic biomarker (Schapira et al., 2017; Blesa et al., 2021). Although the current understanding of AutD in PD is well documented, its precise pathogenesis and relationship to other PD symptoms are remain unclear (Chaudhuri, 2021).
Given the significant impact of AutD on the lives of PD patients, and with the International Parkinson and Movement Disorder Society (MDS) introducing various clinical rating scales to assess it, AutD has become a cutting-edge area of PD research (Chen et al., 2020; Chaudhuri, 2021). This review highlights recent understanding of AutD in PD. In contrast to previous reviews, we summarize current research on the correlation between autonomic dysfunction and other related clinical symptoms in patients with PD. Our review describes some of the common pathogenetic mechanisms that have been identified, thus filling the gaps in this area, aiming to explore more diagnostic and therapeutic approaches to autonomic dysfunction and lay the foundation for future high-quality clinical and basic research.
Dopaminergic deficits in nigral neurons and the striatum, along with the accumulation of α-synuclein in intraneuronal inclusion bodies, are recognized as characteristic neuropathological features of Parkinson’s disease (PD). In addition to these well-known mechanisms, other theories suggest that PD pathogenesis may also involve factors such as traumatic injury, genetic mutations, mitochondrial dysfunction, and neuroinflammation. However, the exact pathogenesis of autonomic disorders in PD has not yet been clearly demonstrated. Current research suggests multiple factors may contribute to the pathogenesis of AutD in PD, potentially involving abnormal protein deposition and neuronal destruction, genetic problems, and environmental factors (Chen et al., 2020; Bloem et al., 2021).
The deposition of alpha-synuclein(α-syn) and phosphorylated α-syn in autonomic neurons and their innervated regions, along with the neuronal degeneration, are considered as key factors contributing to autonomic dysfunction in patients with Parkinson’s disease (PD). PD with autonomic impairment is recognized to involve varying degrees of α-syn accumulation and neuronal destruction across both central and peripheral nerves (Pfeiffer, 2020). For example, in the hypothalamus of PD patients with autonomic dysfunction, α-syn deposition was found in the paraventricular, infundibular, and supraoptic nuclei, but the severity of symptoms was not found to be significantly correlated with alpha-syn deposition (De Pablo-Fernandez et al., 2017). Additionally, α-syn deposition has been detected in other central autonomic control centres, such as those in the cortex, brainstem, and spinal cord, which are hypothesized to contribute to autonomic dysfunction in PD (Christopher et al., 2014; De Pablo-Fernandez et al., 2017; Chen et al., 2020).
Neurosonography of PD patients with parasympathetic dysfunction revealed that these patients exhibited a smaller vagal cross-sectional area compared to controls. This reduction was considered to be caused by α-syn deposition, leading to progressive neurodegenerative lesion (Huckemann et al., 2023). Studies in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine(MPTP)-injected mice have shown that phosphorylated α-syn can accumulate in Schwann cells within the vagus nerve, where it interacts with Toll-like receptors to induce apoptosis in these cells. This process is thought to lead to autonomic dysfunction in mice, manifesting primarily as impairments in the gastrointestinal, cardiovascular, and urinary systems (Li et al., 2024). In transgenic mouse model of PD, α-syn deposits were observed to accumulate with age in the colon’s submucosal and myenteric plexus neurons, leading to autonomic dysfunction, predominantly manifesting as constipation. Notably, this pathological change in the gut appeared before the onset of motor symptoms, suggesting that α-syn may spread from the gut to the brain via the vagus nerve (Chen et al., 2018). This finding may explain why some PD patients initially develop autonomic deficits in the gastrointestinal tract. However, a subsequent study found that α-syn deposition in the gastrointestinal tract did not directly affect the autonomic function of its associated segments, suggesting that α-syn may not be the primary driver of gastrointestinal autonomic dysfunction (Lee et al., 2018). Further studies are needed to determine whether gastrointestinal autonomic dysfunction is indeed related to α-syn aggregation.
Research on sympathetic nerves has yet to fully explain the mechanism underlying norepinephrine deficiency in some PD patients. A team has proposed a novel mechanism, which find that α-syn deposits are present in sympathetic nerves within blood vessels, sweat glands, erector spinae, and myocardium. This mechanism suggests that α-syn deposits in sympathetic nerves may contribute to autonomic dysfunction in PD, particularly cardiovascular and thermoregulatory issues, by disrupting norepinephrine release through sympathetic nerve inactivation. However, further studies are required to confirm this hypothesis (Isonaka et al., 2019).
Parkinson’s disease is also a hereditary disorder, with a recent review in The Lancet indicating that the genetic risk of Parkinson’s disease ranges from 22 to 40% (Ben-Shlomo et al., 2024; Morris et al., 2024). Of these, about 10% are due to single-gene mutations, with common mutations occurring in genes like Leucine-rich repeat kinase 2 (LRRK2), Parkin RBR E3 Ubiquitin Protein Ligase (PRKN), and Synuclein Alpha (SNCA) (Wanneveich et al., 2018; Isonaka et al., 2021). In addition, genes such as mutations in the β-glucoside cerebroside gene (GBA) have also been proposed, with patients carrying these mutations exhibiting more severe motor symptoms and greater likelihood of autonomic dysfunction in Parkinson’s disease (Gonçalves et al., 2021).
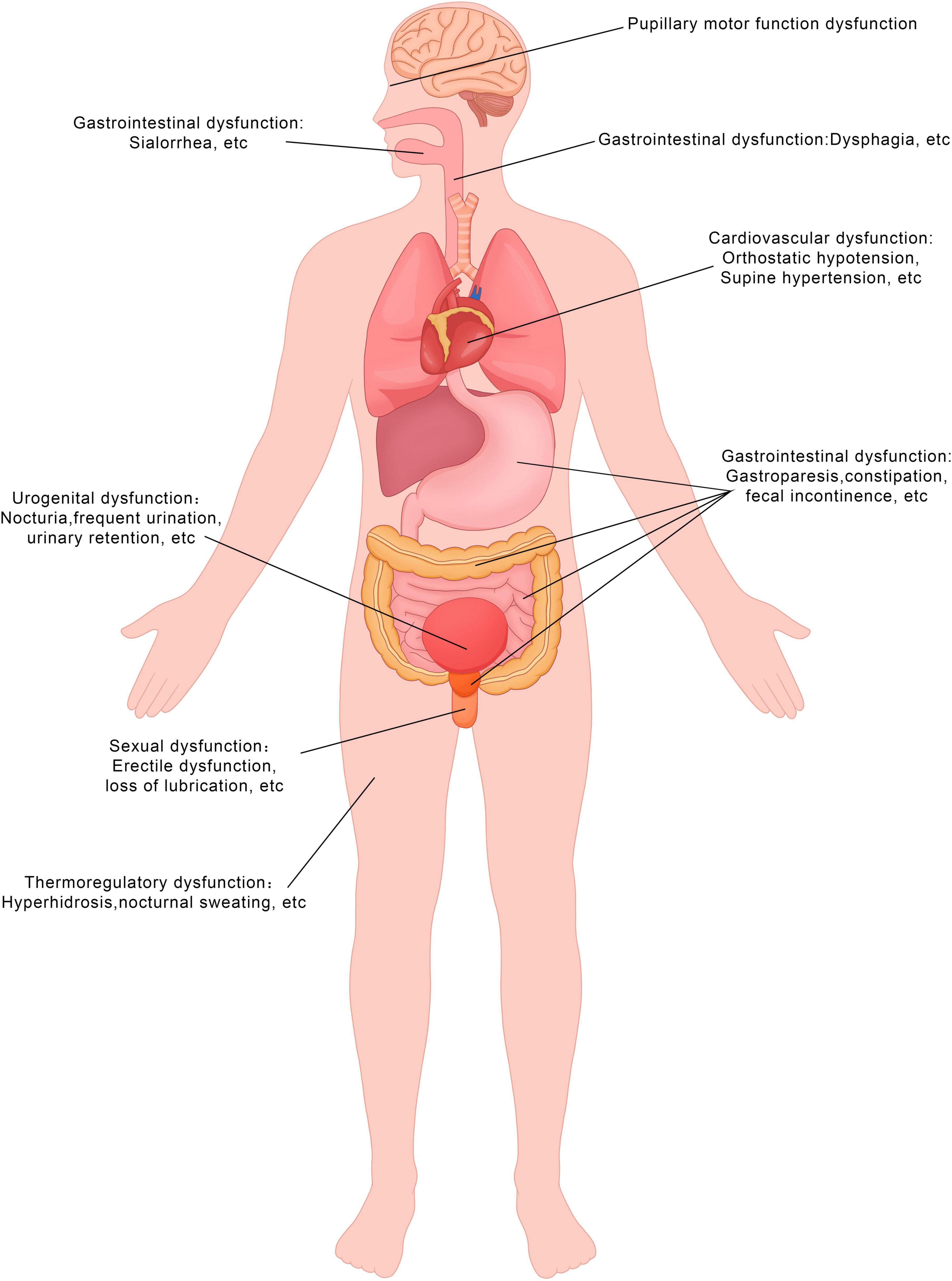
Figure 1. Symptoms of autonomic dysfunction in the PD patients. This figure demonstrates the types of symptoms experienced by patients. The diagram was produced and generated by Adobe Photoshop.
The SNCA gene was the first gene identified as the causative factor of PD. Different point mutations in this gene are associate with different clinical manifestations, with the more recurrent mutations, more likely to be linked to prominent non-motor symptoms, including autonomic dysfunction (Magistrelli et al., 2021). PD patients with E46K-SNCA mutation experience prominent non-motor symptoms. A comparison of skin biopsies from E46K mutation in alpha-synuclein gene (E46K-SNCA) carriers, parkin gene mutation (PARK2) carriers, and healthy controls revealed that E46K-SNCA carriers exhibited more substantial α-syn deposits and a marked reduction in small nerve fiber density. Previous studies have shown pronounced denervation of cardiovascular autonomic nerves in these carriers, supporting the view that E46K-SNCA mutations are associated with significant autonomic dysfunction compared to other genotypes (Carmona-Abellan et al., 2019). Two other common variants, A30P and A53T, also show autonomic dysfunction, significant impairment of the gastrointestinal dysfunction is observed in animal studies (Carmona-Abellan et al., 2019). In addition, rs11931074 mutation in the SNCA gene has been linked to α-syn deposition in the enteric nervous system of PD patients, which impacts gastrointestinal autonomic dysfunction in PD patients (Chung et al., 2019). In contrast, PD patients with the G2385R mutation in LRRK2 exhibit higher prevalence and severity of autonomic dysfunction-primarily in cardiovascular, gastrointestinal, and urinary functions, but the specific pathogenesis of this needs to be further investigated (Yang et al., 2021).
Heart rate and blood pressure are among the earliest and most common indicators of cardiovascular dysfunction in PD patients. Analysis of PD patients using heart rate variability (HRV) has shown that those with GBA mutations tend to have higher resting and upright heart rates, along with greater blood pressure decreases upon standing, compared to patients with idiopathic PD and healthy controls, suggesting parasympathetic impairment. Carandina et al. (2022) propose that the GBA mutation may lead to a deficiency in the lysosomal enzyme glucocerebrosidase (GCase), which impairs the breakdown of lysosomal α-synuclein, resulting in significant cardiovascular autonomic dysfunction. A similar pathogenic process may also underlie gastrointestinal autonomic dysfunction, particularly constipation, in PD patients with GBA mutations. Further analysis has indicated that PD patients with GBA mutations experience impaired sweat gland function, primarily in the distal limbs, with skin biopsies revealing severe autonomic nerve loss and reduced sweat gland density due to α-synuclein deposition (Carandina et al., 2022; Devigili et al., 2023). Recently, Giulia’s team concluded that while patients with GBA mutations are more likely to develop orthostatic hypotension (OH), its severity did not appear to be significantly different between GBA-PD and non-GBA-PD patients after instrumental assessment. This finding suggests that further studies are needed to explore the underlying mechanisms (Giannini et al., 2024).
A recent research has proposed that abnormal functional connectivity between the insula and limbic lobes in patients with early-stage Parkinson’s disease may contribute to severe autonomic dysfunction symptoms (Conti et al., 2024). In addition, secretion, transport and release of central transmitters play crucial roles in autonomic dysfunction observed in PD patients. In particular, Dopamine is essential in regulating autonomic functions. A study of cranial brain imaging and autonomic dysfunction in 310 PD patients found that degeneration of dopaminergic neurons in the striatum is linked to autonomic dysfunction, primarily affecting gastrointestinal and cardiovascular functions (van Deursen et al., 2020). Urinary tract disorders in PD are believed to arise from multiple factors. Given that most PD patients are elderly, it is essential to determine whether urinary symptoms in male patients are due to prostate enlargement or PD-related urinary dysfunction (Valentino et al., 2018). The severity of striatal dopamine transporter deficiency has been correlated with bladder symptoms (Metzger and Emborg, 2019). Additionally, PD pathology affects other anatomical structures involved in bladder function, including the raphe nuclei and the locus coeruleus (VanderHorst et al., 2015). Abnormal pupillary function in PD may also be linked to the loss of dopamine in the central nervous system and retina, which in turn leads to visual hallucinations and ocular dyskinesia. In cases of pupillary constriction disorders, neuron loss in the Edinger-Westphal nucleus is implicated in the pathogenesis of PD (Metzger and Emborg, 2019).
The gut-brain axis has emerged as a hotspot for research into the pathogenesis of autonomic dysfunction in PD. The digestive tract is the main location where interactions occur between the outside environment and the body’s internal environment. Environmental changes in the gastrointestinal tract, especially changes in gastrointestinal flora have been found to have an important role in the pathogenesis of PD. Many studies have found that changes in gastrointestinal flora are correlate with cognitive and motor deficits in PD patients (Barichella et al., 2018; Matheoud et al., 2019; Pietrucci et al., 2019; Chen et al., 2020). Although some studies have pointed out that changes in gastrointestinal flora could trigger gastrointestinal inflammatory, autoimmune reactions, and are associated with α-syn deposition, which can spread to the brain and other nervous systems via the vagus nerve. However, no direct correlation has been found between these changes and gastrointestinal dysfunction or other autonomic dysfunction in patients in either basic experiments or clinical studies (Claudino dos Santos et al., 2023). There have been studies that have found bile acid abnormalities in PD patients with abnormal lipid metabolism, which are caused by an imbalance in the intestinal flora. Therefore, PD patients may also be suffering from an imbalance of intestinal flora that affects the biochemical metabolism of the gastrointestinal tract and thus indirectly affects the autonomic function of the gastrointestinal tract (Hasuike et al., 2020; Claudino dos Santos et al., 2023; Higinbotham and Kilbane, 2024).
Neurodegenerative lesions occur in various brainstem regions of PD patients, including the substantia nigra and locus coeruleus. Most of these affected areas were involved in both motor postural control and autonomic regulation (Benarroch, 1993; Seidel et al., 2014), suggest a potential direct link between motor symptoms and autonomic dysfunction.
PD patients with multiregional damage exhibit significant slowness of movement, lower oxygen pulse, oxygen consumption, systolic blood pressure, and respiratory exchange ratio (RER) at maximal exercise load during the 10-meter walk test. These observation suggested that more severe autonomic dysfunction is associated with poorer exercise capacity (Qin et al., 2024). Additional studies have confirmed higher Scales for Outcomes in Parkinson’s disease - Autonomic (SCOPA-AUT) scores in patients with indeterminate subtypes of PD compared with patients with tremor dominant (TD) and postural instability gait disorder (PIGD) subtypes, suggesting that there may be differences in the severity and progression of autonomic dysfunction across PD subtypes (Jeong et al., 2021). However, the relationship between exercise capacity and autonomic dysfunction in patients with PD is not fully understood and further studies are needed to clarify it. One hypothesis suggests that the co-occurrence of both conditions may be attributed to the presence of Lewy bodies, which are extensively distributed in the hypothalamus, the lateral reticular nucleus of the medulla oblongata, sympathetic ganglia, the dorsal nucleus of the vagus nerve, and sacral parasympathetic nuclei within the spinal cord. This distribution may disrupt autonomic regulatory mechanisms and diminish maximum sympathetic activation during movement, thereby impacting patient mobility (Qin et al., 2024).
Some studies found a strong association between autonomic dysfunction (e.g., OH, gastrointestinal symptoms, etc.) and gait disturbance and falls in patients with advanced PD. Kwon et al. (2021a) proposed that early gastrointestinal and axial symptoms in PD may be interconnected within the pathophysiology of the condition. However, the precise mechanisms remain unclear and necessitate further detailed investigation (Kwon et al., 2021a). PIGD has been found to be significantly and positively correlated with the SCOPA-AUT total score and the score of urinary symptoms in patients with PD. Autonomic dysfunction in these patients can impact gait, particularly in the early to mid-stages of the disease (Kwon et al., 2021b; Zhou et al., 2023). The more severe autonomic dysfunction in patients with new-onset PD is associated with poorer performance in gait speed, stride length, walking rhythm, and more pronounced difficulties with backward movement. Notably, urinary autonomic abnormalities in new-onset PD patients are strongly correlated with gait impairment. The research team posits that severe autonomic dysfunction may signify more extensive brain damage, including regions such as the pontine urination center and the periaqueductal gray matter of the midbrain, thereby indicating a potential for comorbidity (Lee et al., 2023).
Postural instability was first linked to autonomic dysfunction in a study by You and colleagues, who found that postural instability in PD patients was associated with parasympathetic autonomic dysfunction (You et al., 2020). Another prospective study that followed 50 PD patients demonstrated that those with cardiovascular dysautonomia (including but not limited to orthostatic hypotension) were more likely to fall. PD patients with more cardiac sympathetic modulation required more efforts to maintain balance in standing (Romagnolo et al., 2018). In 2020, two research teams led by You and Yoan posits that the autonomic and postural pathways share critical relay points within the brainstem, cerebral cortex, and basal ganglia. Autonomic dysfunction resulting from a loss of dominance in cardiac sympathetic innervation and impairment of parasympathetic nerves contributes to alterations in postural control due to disrupted communication between the cerebral cortex and brainstem (Espinoza-Valdés et al., 2020; You et al., 2020). It can be argued that cardiovascular autonomic deficits may be a strong, independent predictor of falls in patients with PD. Therefore, clinicians should be aware of the possibility of postural instability associated with autonomic dysfunction, even though the patient does not have typical postural instability.
In addition to motor symptoms, cognitive impairment, sleep disorders and emotions changes have been found to strongly correlated with autonomic dysfunction in PD. These symptoms significantly affect the life quality of PD patients and have become a hot topic of research in recent years.
Numerous studies have shown that PD patients with autonomic dysfunction are more likely to experience cognitive impairment. In fact, PD patients may exhibit autonomic symptoms and subtle cognition changes several years before a formal diagnosis, which may affect their differential diagnosis from other α-synucleinopathies (Palermo et al., 2020). Magdalena and coworkers reviewed several studies and found that approximately 25% of these patients showed mild cognitive impairment (MCI) at an early stage, with most also showing blood pressure abnormalities, such as orthostatic hypotension (OH). This may be due to frequent cranial hypoperfusion caused by cardiovascular autonomic dysfunction, leading to unstable blood pressure or neurodegenerative disease affecting central or peripheral noradrenergic pathways (McDonald et al., 2016). However, Magdalena noted that it remains unclear whether the relationship between the cardiovascular system and cognitive impairment is causal or simply correlative, highlighting the need for more rigorous controlled trials to clarify this link (Kwaśniak-Butowska et al., 2021). Recently, Ruiz-Barrio et al. (2023) performed a retrospective analysis and identified that early-stage orthostatic hypotension (OH) is linked to an elevated risk of cognitive impairment. They elucidated that the detrimental effects of OH on cognitive function arise from recurrent episodes of cerebral hypoperfusion, which induce chronic hypoxic changes, thereby activating specific molecular pathways that lead to non-specific neuronal destruction and neurodegeneration. Additionally, they suggested the potential for treating OH as a means to prevent cognitive decline (Ruiz-Barrio et al., 2023).
It has been reported that mild cognitive impairment in patients with new-onset PD is often associated with gastrointestinal symptoms related to autonomic dysfunction, particularly memory and executive function deficits (Jones et al., 2020). One study found that more severe gastrointestinal symptoms predicted a trend toward declining performance on alphabetic fluency, visuospatial, learning, and memory in patients with up to 5-year follow-up period. Notably, these cognitive declines were linked specifically to gastrointestinal autonomic symptoms, rather than to non-autonomic symptoms, suggesting that gastrointestinal symptoms may serve as a predictive marker of cognitive decline in PD patients (Jones et al., 2020). By studying early Parkinson’s patients, a team found that degeneration of the LocusCoeruleus leads to the onset of cognitive deficits and worsening of gastrointestinal symptoms at subsequent follow-up (Kim et al., 2024). Additionally, Camacho and colleagues conducted a cohort study revealing that PD patients exhibiting early symptoms of constipation are at an increased risk of developing dementia. Furthermore, the severity of constipation at disease onset serves as a prognostic indicator for accelerated dementia progression (Camacho et al., 2021). However, the precise mechanisms and causal relationship between cognitive impairment and gastrointestinal symptoms remain unclear. The leading hypothesis suggests that changes in gut microbiology may influence cognitive dysfunction in patients with PD. In the early stages of certain Parkinson’s disease (PD) patients, aggregates of alpha-synuclein protein are observed to accumulate in the gastrointestinal tract. Metabolites resulting from intestinal dysregulation may lead to increased gut permeability, oxidative stress, and localized inflammation, which can influence cerebral function via the gut-brain axis. This cascade ultimately results in damage and deposition of alpha-synuclein, contributing to neurodegeneration within the brain and subsequent cognitive impairment (Nair et al., 2018; Dowling et al., 2022; Warnecke et al., 2022). Additionally, degenerative changes in both the peripheral gastrointestinal system and the central cholinergic system may play a role, potentially impacting both gastrointestinal symptoms and cognitive function. In patients with autonomic dysfunction, early degeneration of cholinergic neurons within the gastrointestinal tract can be observed, contributing to the manifestation of autonomic impairment. Furthermore, cholinergic neurons in the brain primarily function as projection neurons connecting various central nervous system (CNS) regions. Along with motor neurons and certain autonomic neurons, these neurons facilitate interactions between the CNS and peripheral nervous system. The concurrent degeneration of these neuronal populations may adversely impact cognitive functions in Parkinson’s disease (PD) patients with autonomic dysfunction (Titova et al., 2016; Bohnen et al., 2022). Therefore, if PD patients suffer from autonomic dysfunction, clinicians should assess for the signs of mild cognitive impairment (Kwon et al., 2022).
Rapid eye movement sleep behavior disorder (RBD) is an important non-motor symptom of PD. Patients with RBD tend to experience more severe motor and non-motor symptoms, with RBD categorized as either isolated or secondary. The isolated RBD is considered a prodromal symptom of PD as its high rate of progression to the disease (Toft et al., 2021). Sleep disturbances and autonomic dysfunction are both key non-motor symptoms that substantially impact the quality of life of PD patients. Several studies have demonstrated a correlation between RBD and autonomic dysfunction in PD, showing that PD patients with RBD have more severe involuntary dysfunction than those without RBD. Furthermore, the severity of autonomic symptoms may be linked to a faster phenotypic progression in patients with isolated RBD (Kim et al., 2016; Li et al., 2017).
To clarify which autonomic symptoms are associated with RBD in PD, Fujita et al. (2022) conducted a followed up study on 126 PD patients with RBD. They found that the cardiovascular and urinary symptoms were the most severe among autonomic symptoms, with urinary symptoms-particularly “weak urinary stream”-emerging as a key indicator for worsening RBD. This finding suggests that the disease-specific pathology in brainstem nuclei, such as the periaqueductal gray (PAG) and pontine micturition center (PMC), may be more pronounced in PD patients with RBD (Fujita et al., 2022). Kim et al. (2016) reported a strong association between early-stage RBD with OH and cardiac sympathetic denervation, suggesting that cardiovascular symptoms in RBD may be linked to sympathetic denervation.
Another hypothesis suggested that autonomic dysfunction and sleep disorders, particularly RBD, may share overlapping areas of co-morbidity (Cortellia and Lambardi, 2005). A longitudinal cohort study found that all autonomic symptoms-except for pupillary movement-were more severe and deteriorated more rapidly in PD patients with RBD compared to those without RBD (Ashraf-ganjouei et al., 2021; Maggi et al., 2023). Autopsy studies of PD patients with RBD have revealed significant α-synuclein deposits in the subcortical and brainstem nuclei, suggesting that the pathogenesis of RBD may involve regions such as the ventral-lateral gray matter around the aqueduct, the lateral pontine tegmentum, and the nucleus of the pontine pedunculi (Lu et al., 2006; Luppi et al., 2011). Central autonomic network (CAN), which controls autonomic function, is located in brainstem areas such as the periaqueductal gray matter of the midbrain and the parabrachial nucleus of the pons. α-syn deposition in the CAN region is observed in the early stages of PD, which in turn affects autonomic function in PD patients. There is an anatomical overlap between the cranial lesion area of RBD and CAN, which may be the reason for the co-morbidity of the two (Ashraf-ganjouei et al., 2021). Recently, a study by Eckhardt et al. (2023) further demonstrated that PD patients who experiencing autonomic dysfunction share common lesion areas with sleep disorders. Gastrointestinal and cardiovascular dysfunctions in PD are thought to result from degenerative changes in neurons near the brainstem, which also contribute to associated sleep disorders. Additionally, orthostatic hypotension (OH) has been identified as a predictor of REM sleep without atonia (RWA), and the coexistence of these symptoms may indicate a more advanced stage of PD (Eckhardt et al., 2023).
Anxiety and depression are important non-motor symptom of PD. In recent years, many studies have explored the relationship between autonomic dysfunction and mood disorders in PD patients. In Sklerov et al. (2020) conducted a longitudinal study of PD patients with autonomic dysfunction over a period of 60 months. They found that autonomic dysfunction worsened, the patients became progressively more depressed, which further influence their daily lives, particularly in the early stages of PD (Sklerov et al., 2020). In line with other studies, the relationships between autonomic dysfunction and mood disorders such as anxiety and depression in PD may be explained by the overlap of the neural substrates involved in both. Key regions of the central autonomic network (e.g., hypothalamus, anterior cingulate cortex, amygdala, insula) are involved in regulating the balance between sympathetic and parasympathetic activity and also play an important role in mood regulation. Some of these regions are also vulnerable to the accumulation of α-synuclein in PD. Dysregulation of neurotransmitter systems, including norepinephrine and epinephrine, has been linked to both mood and autonomic symptoms (Sklerov et al., 2022). Dysbiosis of gut microbiota in gastrointestinal autonomic dysfunction can lead to the release of lipopolysaccharides, which may ascend along the vagus nerve, circumvent the blood-brain barrier, and excessively activate the hypothalamic-pituitary-adrenal (HPA) axis, resulting in anxiety-like behaviors (Chan et al., 2022). Based on these findings, Sklerov et al. (2020) proposed that treating depression in these individuals may be more effective using drugs that block norepinephrine reuptake. However, they emphasized that further research is needed to determine the correlation between the use of antidepressant medications and the ability to improve autonomic dysfunction and how this might affect daily life for PD.
Although several studies have shown that PD autonomic dysfunction is correlated with anxiety and depression, most do not mention which specific type of autonomic dysfunction is correlated with anxiety or depression. In contrast, Adrianna M and others found that gastrointestinal functioning has a stronger correlation with anxiety and depression than other system dysfunctions through a 5-year follow-up. They hypothesized that the relationship between anxiety and depression and gastrointestinal dysfunction could be related to gut-brain axis interactions. Pathologic processes such as intestinal flora imbalance and inflammation may increase the risk of anxiety and depression in patients with PD by promoting cytokine production, disrupting the blood-brain barrier, and causing inflammation or neuronal dysfunction in the central nervous system. However, it is also suggested that anxiety and depression may themselves lead to changes in the gastrointestinal tract, including alterations in the microbiome composition. In addition, thermoregulatory dysfunction is a unique predictor of anxiety and depression, while urinary and cardiovascular dysfunction are primarily associated with depression in PD patients. The follow-up study also observed a trend of worsening depression and anxiety in patients with new-onset PD, which seemed to correlate with the severity of autonomic symptoms. Therefore, it was hypothesized that interventions and treatments for autonomic symptoms in the early stages of Parkinson’s disease may influence the long-term development of emotional symptoms. They argue that future research should explore how autonomic dysfunction interacts with other PD symptoms to influence the trajectory of mood disorders and whether addressing autonomic dysfunction can improve mood (Ratajska et al., 2023).
Both olfactory dysfunction and AutD are among the earliest pre-motor symptoms of Parkinson’s disease (Yoon et al., 2024). A research team from the United States has discovered that olfactory dysfunction is related to gastrointestinal dysfunction, cardiovascular dysfunction, and pupillary motor function in PD (Kang et al., 2012). Although previous studies have maintained that olfactory dysfunction in PD is associated with both sympathetic and parasympathetic nerve dysfunctions of the heart, the team of this research contends that olfactory dysfunction is primarily positively correlated with parasympathetic nerve dysfunctions, while having a relatively minor connection with sympathetic nerve dysfunctions (Goldstein and Sewell, 2009; Oka et al., 2010; Kang et al., 2012).
Recently, the “Revised Single-Hit Hypothesis” posits that PD might commence in the enteric nervous system or the olfactory bulb and exhibit distinct clinical manifestations based on the different sites of onset (Borghammer et al., 2022). Furthermore, PD can be categorized into two disparate subtypes according to the distinct transmission pathways of α-syn: (1) Body-first PD: α-syn accumulates in the peripheral nervous system or the enteric nervous system, and subsequently diffuses along the vagus nerve before ultimately invading the central nervous system. (2) Brain-first PD: α-syn initiates secondary diffusion from the olfactory bulb or amygdala to the peripheral nervous system (Borghammer et al., 2019; Borghammer et al., 2021; Horsager and Borghammer, 2024). As the enteric nervous system is primarily involved in the onset of body-first PD, patients may exhibit autonomic dysfunction as a prodromal symptom.
Yoon et al. (2024) contend that patients with body-first PD would manifest more severe olfactory dysfunction compared to those with brain-first PD. Furthermore, a 7-year follow-up study verified that a greater AutD at the time of diagnosis typically implies a more significant olfactory dysfunction (Stewart et al., 2023). The most recent autopsy studies have proved that, contrary to the common perception, damage to the olfactory bulb does not herald association with olfactory dysfunction; rather, Lewy pathology in the brain is related to olfactory dysfunction (Nag et al., 2019). Hence, Yoon et al. (2024) put forward that the majority of olfactory tests not only demand intact olfactory bulb function but also higher-order cortical function for the correct naming of odors. Moreover, patients with Body-first PD, represented by AutD, have more extensive Lewy body lesions in the new-onset stage and can give rise to higher-order cortical dysfunction, thereby resulting in olfactory dysfunction (Yoon et al., 2024). Secondly, the current hypothesis suggests that due to the distinct transmission pathways of α-syn, in brain-first PD that has its onset in the olfactory bulb, α-syn spreads unilaterally and does not involve both olfactory bulbs, thereby not giving rise to severe olfactory dysfunction (Borghammer, 2021). Nevertheless, in body-first PD where AutD predominates, when α-syn retrogradely spreads through the bilateral vagus nerves to reach the locus coeruleus, it can be projected from the locus coeruleus to both olfactory bulbs, and the simultaneous impairment of both olfactory bulbs results in more pronounced olfactory dysfunction (Kebschull et al., 2016; Stewart et al., 2023).
A new review from the Movements Disorders Society Evidence-Based Medicine provides a list of drugs that could be effective for each of these systems (Seppi et al., 2019), and the drugs that may be effective for the cardiovascular system are droxidopa, fludrocortisone, midodrine, and domperidone (Joseph et al., 1993; Schoffer et al., 2007; Hauser et al., 2014a; Hauser et al., 2014b; Smith et al., 2016; Schreglmann et al., 2017). Domperidone should be used with caution in PD patients with heart disease. Solifenacin is the only drug thought to be potentially effective for urinary symptoms (Zesiewicz et al., 2015). For patients with constipation, Polyethylene glycol, Lubiprostone, Probiotic strains and prebiotic fibers may have desirable effects (Zangaglia et al., 2007; Ondo et al., 2012; Barichella et al., 2016; Leta et al., 2021b). No ideal drug is given for thermoregulatory disorders. Sildenafil, on the other hand, is recommended for male sexual dysfunction (Bernard et al., 2016; Seppi et al., 2019). Fipamezole, which primarily antagonizes the α2-adrenergic receptor and has a moderate affinity for the 5 -hydroxytryptamine transporter and histamine receptor and a weak affinity for other receptors and transporters, was initially investigated as an antimotor disorder drug (Leta et al., 2019), with one of the adverse effects being an elevation in blood pressure, and was therefore preliminarily investigated in the NCT00758849 clinical trial to see if it could be used to treat OH, but the results have not yet been published (Rukavina et al., 2022). Tomoxetine, a norepinephrine transporter protein blocker that delays postsynaptic reuptake of released norepinephrine, is thought to be applicable and potentially efficacious in PD patients with OH (Shibao et al., 2007; Ramirez et al., 2014; Palma and Kaufmann, 2018). Pyridostigmine can act by increasing cholinergic tone in sympathetic ganglia, and was found to be effective in an open-label study of PD patients treated with Pyridostigmine alone. Patients with OH treated with Pyridostigmine had better results than midodrine alone or a combination of the two (Byun et al., 2017).
Untreated constipation in PD can interferes with the absorption of oral levodopa in the small intestine and can lead to life-threatening complications such as sigmoid colon torsion and bowel perforation. Given that dysregulation of intestinal flora is now believed to be a pathogenetic factor in gastrointestinal dysfunction in PD, approaches targeting intestinal flora modulation—such as probiotics and fecal microbiota transplantation (FMT)—are considered potentially effective (Metta et al., 2021). Linaclotide and Prucalopride have been shown to be effective in small studies, but further randomized controlled trials are needed (Freitas et al., 2018). Elobixibat, which increases bile acid concentrations in the colon, stimulates colonic transit and secretion, and is effective in treating constipation in the general population, is also thought to be beneficial for PD patients (Nakajima et al., 2018; Rukavina et al., 2022). A recent study by a team from China also found that acupuncture can be used as an adjunctive treatment for constipation without causing side effects, but further research is needed to determine its long-term effectiveness and safety (Zhang et al., 2023).
Mirabregon, a selective β3-adrenoceptor agonist that induces relaxation of the urethral muscle, has been shown to be useful in the treatment of patients with PD and has an acceptable incidence of adverse events (Peyronnet et al., 2018; Cho et al., 2021). Additionally, transvesical detrusor injections of botulinum toxin A may be effective in PD patients who have not responded to other treatments (Kulaksizoglu and Parman, 2010; Giannantoni et al., 2011; Quarracino et al., 2020).
A recent study of PD patients after deep brain stimulation (DBS) found some improvement in temperature perception, as well as reductions in hyperhidrosis and heat intolerance. However, due to the small sample size and short follow-up period, further research is needed to validate these findings (Leta et al., 2021a; Zhang et al., 2022).
Autonomic dysfunction is a common non-motor symptom of Parkinson’s disease. It seriously affects patients’ quality of life and has the potential to exacerbate their motor deficits, thereby both the care and financial burden on PD patients (Pfeiffer, 2020; Bloem et al., 2021). While research into autonomic dysfunction is gaining traction, its exact pathogenesis remains unclear and requires further investigation. Autonomic dysfunction is likely to co-exist with motor and other non-motor symptoms of PD, and some may share common pathogenetic mechanisms. Therefore, it is crucial to determine whether there is a causal relationship or merely a correlation, whether multiple symptoms can be treated simultaneously, and whether improving autonomic dysfunction can alleviate other comorbidities. The treatment and management of autonomic dysfunction in patients with PD is very challenging. It may be overlooked by the patient in the early stages or interfered with by other conditions present in the patient. Therefore, clinicians should carefully identify and select potentially useful medications. Future research should focus on exploring potential new treatments for autonomic dysfunction as they are developed (Quarracino et al., 2020).
HX: Conceptualization, Investigation, Writing – original draft, Writing – review and editing. XZ: Investigation, Supervision, Writing – review and editing. XX: Investigation, Writing – review and editing. ZB: Supervision, Writing – review and editing. DW: Investigation, Writing – review and editing. CZ: Investigation, Writing – review and editing. LW: Supervision, Writing – review and editing. YJ: Conceptualization, Supervision, Writing – review and editing. SX: Conceptualization, Supervision, Writing – review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the Shandong Provincial Natural Science Foundation #ZR2024MH332 and Shandong Provincial Natural Science Foundation Youth Project #ZR2022QH100.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ashraf-ganjouei, A., Moradi, K., Aarabi, M., Abdolalizadeh, A., Kazemi, S. Z., Kasaeian, A., et al. (2021). The association between REM sleep behavior disorder and autonomic dysfunction in Parkinson’s disease. J. Parkinson’s Dis. 11, 747–755. doi: 10.3233/jpd-202134
Barichella, M. P., Pacchetti, C., Bolliri, C., Cassani, E., Iorio, L., and Pusani, C. (2016). Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology 17, 928–929.
Barichella, M., Severgnini, M., Cilia, R., Cassani, E., Bolliri, C., Caronni, S., et al. (2018). Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 34, 396–405. doi: 10.1002/mds.27581
Benarroch, E. E. (1993). The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proc. 68, 988–1001. doi: 10.1016/s0025-6196(12)62272-1
Ben-Shlomo, Y., Darweesh, S., Llibre-Guerra, J., Marras, C., San Luciano, M., and Tanner, C. (2024). The epidemiology of Parkinson’s disease. Lancet 403, 283–292. doi: 10.1016/s0140-6736(23)01419-8
Bernard, B. A., Metman, L. V., Levine, L., Ouyang, B., Leurgans, S., and Goetz, C. G. (2016). Sildenafil in the treatment of erectile dysfunction in Parkinson’s disease. Mov. Disord. Clin. Pract. 4, 412–415. doi: 10.1002/mdc3.12456
Blesa, J., Foffani, G., Dehay, B., Bezard, E., and Obeso, J. A. (2021). Motor and non-motor circuit disturbances in early Parkinson disease: which happens first? Nat. Rev. Neurosci. 23, 115–128. doi: 10.1038/s41583-021-00542-9
Bloem, B. R., Okun, M. S., and Klein, C. (2021). Parkinson’s disease. Lancet 397, 2284–2303. doi: 10.1016/s0140-6736(21)00218-x
Bohnen, N. I., Yarnall, A. J., Weil, R. S., Moro, E., Moehle, M. S., Borghammer, P., et al. (2022). Cholinergic system changes in Parkinson’s disease: Emerging therapeutic approaches. Lancet Neurol. 21, 381–392. doi: 10.1016/s1474-4422(21)00377-x
Borghammer, P. (2021). The α-synuclein origin and connectome model (SOC Model) of Parkinson’s disease: Explaining motor asymmetry, non-motor phenotypes, and cognitive decline. J. Parkinson’s Dis. 11, 455–474. doi: 10.3233/jpd-202481
Borghammer, P., Horsager, J., Andersen, K., Van Den Berge, N., Raunio, A., Murayama, S., et al. (2021). Neuropathological evidence of body-first vs. brain-first Lewy body disease. Neurobiol. Dis. 161:105557. doi: 10.1016/j.nbd.2021.105557
Borghammer, P., Just, M. K., Horsager, J., Skjærbæk, C., Raunio, A., Kok, E. H., et al. (2022). A postmortem study suggests a revision of the dual-hit hypothesis of Parkinson’s disease. npj Parkinson’s Dis. 8, 166. doi: 10.1038/s41531-022-00436-2
Borghammer, P., Van Den Berge, N., and van Laar, T. (2019). Brain-first versus gut-first Parkinson’s disease: A hypothesis. J. Parkinson’s Dis. 9, S281–S295. doi: 10.3233/jpd-191721
Byun, J., Moon, J., Kim, D., Shin, H., Sunwoo, J., Lim, J., et al. (2017). Efficacy of single or combined midodrine and pyridostigmine in orthostatic hypotension. Neurology 89, 1078–1086.
Camacho, M., Macleod, A. D., Maple-Grødem, J., Evans, J. R., Breen, D. P., Cummins, G., et al. (2021). Early constipation predicts faster dementia onset in Parkinson’s disease. npj Parkinson’s Dis. 7:45. doi: 10.1038/s41531-021-00191-w
Carandina, A., Lazzeri, G., Rodrigues, G. D., Franco, G., Monfrini, E., Arienti, F., et al. (2022). Dysautonomia in Parkinson’s disease: Impact of glucocerebrosidase gene mutations on cardiovascular autonomic control. Front. Neurosci. 16:842498. doi: 10.3389/fnins.2022.842498
Carmona-Abellan, M., Gabilondo, I., Murueta-Goyena, A., Khurana, V., Tijero, B., Luquin, M. R., et al. (2019). Small fiber neuropathy and phosphorylated alpha-synuclein in the skin of E46K-SNCA mutation carriers. Parkinsonism Relat. Disord. 65, 139–145. doi: 10.1016/j.parkreldis.2019.05.038
Chan, D. G., Ventura, K., Villeneuve, A., Du Bois, P., and Holahan, M. R. (2022). Exploring the connection between the gut microbiome and Parkinson’s disease symptom progression and pathology: Implications for supplementary treatment options. J. Parkinson’s Dis. 12, 2339–2352. doi: 10.3233/jpd-223461
Chaudhuri, K. R. (2021). Thirty years of research on autonomic dysfunction, non-motor features, and endophenotypes in Parkinson disease. Clin. Autonomic Res. 31, 37–39. doi: 10.1007/s10286-021-00771-z
Chen, Q.-Q., Haikal, C., Li, W., Li, M.-T., Wang, Z.-Y., and Li, J.-Y. (2018). Age-dependent alpha-synuclein accumulation and aggregation in the colon of a transgenic mouse model of Parkinson’s disease. Transl. Neurodegeneration 7:13. doi: 10.1186/s40035-018-0118-8
Chen, Z., Li, G., and Liu, J. (2020). Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 134:104700. doi: 10.1016/j.nbd.2019.104700
Cho, S. Y., Jeong, S. J., Lee, S., Kim, J., Lee, S. H., Choo, M. S., et al. (2021). Mirabegron for treatment of overactive bladder symptoms in patients with Parkinson’s disease: A double-blind, randomized placebo-controlled trial (Parkinson’s disease overactive bladder mirabegron. PaDoMi study). Neurourol. Urodyn. 40, 286–294. doi: 10.1002/nau.24552
Christopher, L., Koshimori, Y., Lang, A. E., Criaud, M., and Strafella, A. P. (2014). Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain 137, 2143–2154. doi: 10.1093/brain/awu084
Chu, Y., Hirst, W. D., Federoff, H. J., Harms, A. S., Stoessl, A. J., and Kordower, J. H. (2024). Nigrostriatal tau pathology in parkinsonism and Parkinson’s disease. Brain 147, 444–457. doi: 10.1093/brain/awad388
Chung, K. A., and Pfeiffer, R. F. (2020). Gastrointestinal dysfunction in the synucleinopathies. Clin. Autonomic Res. 31, 77–99. doi: 10.1007/s10286-020-00745-7
Chung, S. J., König, I. R., Lohmann, K., Hinrichs, F., Kim, J., Ryu, H.-S., et al. (2019). Association of SNCA variants with α-synuclein of gastric and colonic mucosa in Parkinson’s disease. Parkinsonism Relat. Disord. 61, 151–155. doi: 10.1016/j.parkreldis.2018.10.028
Claudino dos Santos, J. C., Lima, M. P. P., Brito, G. A. D. C., and Viana, G. S. D. B. (2023). Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res. Rev. 84:101812. doi: 10.1016/j.arr.2022.101812
Conti, M., Garasto, E., Bovenzi, R., Ferrari, V., Mercuri, N. B., Di Giuliano, F., et al. (2024). Insular and limbic abnormal functional connectivity in early-stage Parkinson’s disease patients with autonomic dysfunction. Cereb. Cortex 34:bhae270. doi: 10.1093/cercor/bhae270
Cortellia, P., and Lambardi, C. (2005). Sleep and autonomic nervous system dysfunction. Handb. Clin. Neurophysiol. 6, 343–353.
Darweesh, S. K. L., Ikram, M. K., Faber, M. J., de Vries, N. M., Haaxma, C. A., Hofman, A., et al. (2018). Professional occupation and the risk of Parkinson’s disease. Eur. J. Neurol. 25, 1470–1476. doi: 10.1111/ene.13752
De Pablo-Fernandez, E., Courtney, R., Holton, J. L., and Warner, T. T. (2017). Hypothalamic α-synuclein and its relation to weight loss and autonomic symptoms in Parkinson’s disease. Mov. Disord. 32, 296–298. doi: 10.1002/mds.26868
Devigili, G., Straccia, G., Cereda, E., Garavaglia, B., Fedeli, A., Elia, A. E., et al. (2023). Unraveling autonomic dysfunction in GBA-related Parkinson’s disease. Mov. Disord. Clin. Pract. 10, 1620–1638. doi: 10.1002/mdc3.13892
Dowling, L. R., Strazzari, M. R., Keely, S., and Kaiko, G. E. (2022). Enteric nervous system and intestinal epithelial regulation of the gut-brain axis. J. Allergy Clin. Immunol. 150, 513–522. doi: 10.1016/j.jaci.2022.07.015
Eckhardt, C., Fanciulli, A., Högl, B., Heidbreder, A., Eschlböck, S., Raccagni, C., et al. (2023). Analysis of sleep, daytime sleepiness, and autonomic function in multiple system atrophy and Parkinson disease: A prospective study. J. Clin. Sleep Med. 19, 63–71. doi: 10.5664/jcsm.10268
Espinoza-Valdés, Y., Córdova-Arellano, R., Espinoza-Espinoza, M., Méndez-Alfaro, D., Bustamante-Aguirre, J. P., Maureira-Pareja, H. A., et al. (2020). Association between cardiac autonomic control and postural control in patients with Parkinson’s disease. Int. J. Environ. Res. Public Health 18:249. doi: 10.3390/ijerph18010249
Fereshtehnejad, S.-M., Yao, C., Pelletier, A., Montplaisir, J. Y., Gagnon, J.-F., and Postuma, R. B. (2019). Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 142, 2051–2067. doi: 10.1093/brain/awz111
Freitas, M. E., Alqaraawi, A., Lang, A. E., and Liu, L. W. C. (2018). Linaclotide and prucalopride for management of constipation in patients with Parkinsonism. Mov. Disord. Clin. Pract. 5, 218–220. doi: 10.1002/mdc3.12577
Fujita, H., Shiina, T., Sakuramoto, H., Nozawa, N., Ogaki, K., and Suzuki, K. (2022). Sleep and autonomic manifestations in Parkinson’s disease complicated with probable rapid eye movement sleep behavior disorder. Front. Neurosci. 16:874349. doi: 10.3389/fnins.2022.874349
Giannantoni, A., Conte, A., Proietti, S., Giovannozzi, S., Rossi, A., Fabbrini, G., et al. (2011). Botulinum toxin type A in patients with Parkinson’s disease and refractory overactive bladder. J. Urol. 186, 960–964. doi: 10.1016/j.juro.2011.04.071
Giannini, G., Minardi, R., Barletta, G., Cani, I., Cecere, A., Baldelli, L., et al. (2024). The degree of cardiovascular autonomic dysfunction is not different in GBA-related and idiopathic Parkinson’s disease patients: A case-control instrumental evaluation. J. Parkinson’s Dis. 14, 335–346. doi: 10.3233/jpd-230334
Goldstein, D. S., and Sewell, L. (2009). Olfactory dysfunction in pure autonomic failure: Implications for the pathogenesis of Lewy body diseases. Parkinsonism Relat. Disord. 15, 516–520. doi: 10.1016/j.parkreldis.2008.12.009
Gonçalves, V. C., Cuenca-Bermejo, L., Fernandez-Villalba, E., Martin-Balbuena, S., and da Silva Fernandes, M. J. (2021). Heart matters: Cardiac dysfunction and other autonomic changes in Parkinson’s disease. Neuroscientist 28, 530–542. doi: 10.1177/1073858421990000
Hasuike, Y., Endo, T., Koroyasu, M., Matsui, M., Mori, C., Yamadera, M., et al. (2020). Bile acid abnormality induced by intestinal dysbiosis might explain lipid metabolism in Parkinson’s disease. Med. Hypotheses 134:109436. doi: 10.1016/j.mehy.2019.109436
Hauser, R. A., Hewitt, L. A., and Isaacson, S. (2014a). Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson’s disease (NOH306A). J. Parkinson’s Dis. 4, 57–65. doi: 10.3233/jpd-130259
Hauser, R. A., Isaacson, S., Lisk, J. P., Hewitt, L. A., and Rowse, G. (2014b). Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson’s disease (nOH306B). Mov. Disord. 30, 646–654. doi: 10.1002/mds.26086
Higinbotham, A. S., and Kilbane, C. W. (2024). The gastrointestinal tract and Parkinson’s disease. Front. Cell. Infection Microbiol. 13:1158986. doi: 10.3389/fcimb.2023.1158986
Horsager, J., and Borghammer, P. (2024). Brain-first vs. body-first Parkinson’s disease: An update on recent evidence. Parkinsonism Relat. Disord. 122:106101. doi: 10.1016/j.parkreldis.2024.106101
Huckemann, S., Mueller, K., Averdunk, P., Kühn, E., Hilker, L., Kools, S., et al. (2023). Vagal cross-sectional area correlates with parasympathetic dysfunction in Parkinson’s disease. Brain Commun. 5:fcad006. doi: 10.1093/braincomms/fcad006
Isonaka, R., Goldstein, D. S., Zhu, W., Yoon, E., Ehrlich, D., Schindler, A. B., et al. (2021). α−Synuclein deposition in sympathetic nerve fibers in genetic forms of Parkinson’s disease. Mov. Disord. 36, 2346–2357. doi: 10.1002/mds.28667
Isonaka, R., Rosenberg, A. Z., Sullivan, P., Corrales, A., Holmes, C., Sharabi, Y., et al. (2019). Alpha-synuclein deposition within sympathetic noradrenergic neurons is associated with myocardial noradrenergic deficiency in neurogenic orthostatic hypotension. Hypertension 73, 910–918. doi: 10.1161/hypertensionaha.118.12642
Jeong, E. H., Sunwoo, M. K., Hyung, S. W., Han, S.-K., Lee, J. Y., and Colosimo, C. (2021). Correlation of dopaminergic denervation and the progression of autonomic dysfunctions in different clinical subtypes of Parkinson’s disease. Parkinson’s Dis. 2021, 1–5. doi: 10.1155/2021/2268651
Jones, J. D., Rahmani, E., Garcia, E., and Jacobs, J. P. (2020). Gastrointestinal symptoms are predictive of trajectories of cognitive functioning in de novo Parkinson’s disease. Parkinsonism Relat. Disord. 72, 7–12. doi: 10.1016/j.parkreldis.2020.01.009
Joseph, J., Gilden, J., Hiner, B., Kaufmann, H., Brown, D., and Coghlan, C. (1993). Neurogenic orthostatic hypotension: A double-blind, placebo-controlled study with midodrine. Am. J. Med. 95, 38–48.
Kang, P., Kloke, J., and Jain, S. (2012). Olfactory dysfunction and parasympathetic dysautonomia in Parkinson’s disease. Clin. Autonomic Res. 22, 161–166. doi: 10.1007/s10286-012-0158-6
Kebschull, J. M., Garcia da Silva, P., Reid, A. P., Peikon, I. D., and Albeanu, D. F. (2016). High-throughput mapping of single-neuron projections by sequencing of barcoded RNA. Neuron 91, 975–987. doi: 10.1016/j.neuron.2016.07.036
Kim, J.-S., Park, H.-E., Oh, Y.-S., Lee, S.-H., Park, J.-W., Son, B.-C., et al. (2016). Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder. J. Neurol. Sci. 362, 59–63. doi: 10.1016/j.jns.2016.01.020
Kim, S., Woo, K. A., Choi, H., Shin, J. H., and Kim, H.-J. (2024). Monoaminergic degeneration, cognition, and autonomic symptom trajectory in early Parkinson’s disease. Parkinsonism Relat. Disord. 127:107086. doi: 10.1016/j.parkreldis.2024.107086
Kulaksizoglu, H., and Parman, Y. (2010). Use of botulinim toxin-A for the treatment of overactive bladder symptoms in patients with Parkinsons’s disease. Parkinsonism Relat. Disord. 16, 531–534. doi: 10.1016/j.parkreldis.2010.06.006
Kwaśniak-Butowska, M., Dulski, J., Pierzchliñska, A., Białecka, M., Wieczorek, D., and Sławek, J. (2021). Cardiovascular dysautonomia and cognition in Parkinson’s disease — A possible relationship. Neurol. Neurochirurgia Polska 55, 525–535. doi: 10.5603/PJNNS.a2021.0040
Kwon, K. Y., Lee, E. J., Lee, M., Ju, H., and Im, K. (2021b). Impact of motor subtype on non-motor symptoms and fall-related features in patients with early Parkinson’s disease. Geriatrics Gerontol. Int. 21, 416–420. doi: 10.1111/ggi.14156
Kwon, K.-Y., Park, S., Kim, R. O., Lee, E. J., and Lee, M. (2022). Associations of cognitive dysfunction with motor and non-motor symptoms in patients with de novo Parkinson’s disease. Sci. Rep. 12:11461. doi: 10.1038/s41598-022-15630-8
Kwon, K.-Y., Park, S., Lee, E. J., Lee, M., and Ju, H. (2021a). Association of fall risk factors and non-motor symptoms in patients with early Parkinson’s disease. Sci. Rep. 11:5171. doi: 10.1038/s41598-021-84720-w
Lee, H. J., Jung, K. W., Chung, S. J., Hong, S.-M., Kim, J., Lee, J. H., et al. (2018). Relation of enteric α-Synuclein to gastrointestinal dysfunction in patients with Parkinson’s disease and in neurologically intact subjects. J. Neurogastroenterol. Motility 24, 469–478. doi: 10.5056/jnm17141
Lee, S.-M., Lee, M., Lee, E. J., Kim, R. O., Kim, Y., and Kwon, K.-Y. (2023). Association between gait and dysautonomia in patients with de novo Parkinson’s Disease: Forward gait versus backward gait. J. Mov. Disord. 16, 59–67. doi: 10.14802/jmd.22045
Leta, V., Dafsari, H. S., Sauerbier, A., Metta, V., Titova, N., Timmermann, L., et al. (2021a). Personalised advanced therapies in Parkinson’s disease: The role of non-motor symptoms profile. J. Pers. Med. 11:773. doi: 10.3390/jpm11080773
Leta, V., Jenner, P., Chaudhuri, K. R., and Antonini, A. (2019). Can therapeutic strategies prevent and manage dyskinesia in Parkinson’s disease? An update. Exp. Opin. Drug Safety 18, 1203–1218. doi: 10.1080/14740338.2019.1681966
Leta, V., Klingelhoefer, L., Longardner, K., Campagnolo, M., Levent, H. Ç, Aureli, F., et al. (2023). Gastrointestinal barriers to levodopa transport and absorption in Parkinson’s disease. Eur. J. Neurol. 30, 1465–1480. doi: 10.1111/ene.15734
Leta, V., Ray Chaudhuri, K., Milner, O., Chung-Faye, G., Metta, V., Pariante, C. M., et al. (2021b). Neurogenic and anti-inflammatory effects of probiotics in Parkinson’s disease: A systematic review of preclinical and clinical evidence. Brain Behav. Immunity 98, 59–73. doi: 10.1016/j.bbi.2021.07.026
Li, Y., Kang, W., Yang, Q., Zhang, L., Zhang, L., Dong, F., et al. (2017). Predictive markers for early conversion of iRBD to neurodegenerative synucleinopathy diseases. Neurology 88, 1493–1500.
Li, Y., Tong, Q., Wang, Y., Cheng, Y., Geng, Y., Tian, T., et al. (2024). Phosphorylated α-synuclein deposited in Schwann cells interacting with TLR2 mediates cell damage and induces Parkinson’s disease autonomic dysfunction. Cell Death Discov. 10:52. doi: 10.1038/s41420-024-01824-8
Longardner, K., Merola, A., Litvan, I., De Stefano, A. M., Maule, S., Vallelonga, F., et al. (2022). Differential impact of individual autonomic domains on clinical outcomes in Parkinson’s disease. J. Neurol. 269, 5510–5520. doi: 10.1007/s00415-022-11221-9
Lu, J., Sherman, D., Devor, M., and Saper, C. B. (2006). A putative flip–flop switch for control of REM sleep. Nature 441, 589–594. doi: 10.1038/nature04767
Luppi, P.-H., Clement, O., Sapin, E., Peyron, C., Gervasoni, D., Léger, L., et al. (2011). Brainstem mechanisms of paradoxical (REM) sleep generation. Pflügers Archiv Eur. J. Physiol. 463, 43–52. doi: 10.1007/s00424-011-1054-y
Maggi, G., Vitale, C., Cerciello, F., and Santangelo, G. (2023). Sleep and wakefulness disturbances in Parkinson’s disease: A meta-analysis on prevalence and clinical aspects of REM sleep behavior disorder, excessive daytime sleepiness and insomnia. Sleep Med. Rev. 68:101759. doi: 10.1016/j.smrv.2023.101759
Magistrelli, L., Contaldi, E., and Comi, C. (2021). The impact of SNCA variations and its product alpha-synuclein on non-motor features of Parkinson’s disease. Life 11:804. doi: 10.3390/life11080804
Matheoud, D., Cannon, T., Voisin, A., Penttinen, A.-M., Ramet, L., Fahmy, A. M., et al. (2019). Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1-/- mice. Nature 571, 565–569. doi: 10.1038/s41586-019-1405-y
McDonald, C., Newton, J. L., and Burn, D. J. (2016). Orthostatic hypotension and cognitive impairment in Parkinson’s disease: Causation or association? Mov. Disord. 31, 937–946. doi: 10.1002/mds.26632
Metta, V., Leta, V., Mrudula, K. R., Prashanth, L. K., Goyal, V., Borgohain, R., et al. (2021). Gastrointestinal dysfunction in Parkinson’s disease: molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation. J. Neurol. 269, 1154–1163. doi: 10.1007/s00415-021-10567-w
Metzger, J. M., and Emborg, M. E. (2019). Autonomic dysfunction in Parkinson disease and animal models. Clin. Auton. Res. 29, 397–414. doi: 10.1007/s10286-018-00584-7
Morris, H. R., Spillantini, M. G., Sue, C. M., and Williams-Gray, C. H. (2024). The pathogenesis of Parkinson’s disease. Lancet 403, 293–304. doi: 10.1016/s0140-6736(23)01478-2
Nag, S., Yu, L., VanderHorst, V. G., Schneider, J. A., Bennett, D. A., Buchman, A. S., et al. (2019). Neocortical Lewy bodies are associated with impaired odor identification in community-dwelling elders without clinical PD. J. Neurol. 266, 3108–3118. doi: 10.1007/s00415-019-09540-5
Nair, A. T., Ramachandran, V., Joghee, N. M., Antony, S., and Ramalingam, G. (2018). Gut microbiota dysfunction as reliable non-invasive early diagnostic biomarkers in the pathophysiology of Parkinson’s disease: A critical review. J. Neurogastroenterol. Motility 24, 30–42. doi: 10.5056/jnm17105
Nakajima, A., Seki, M., Taniguchi, S., Ohta, A., Gillberg, P.-G., Mattsson, J. P., et al. (2018). Safety and efficacy of elobixibat for chronic constipation: results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial. Lancet Gastroenterol. Hepatol. 3, 537–547. doi: 10.1016/s2468-1253(18)30123-7
Oka, H., Toyoda, C., Yogo, M., and Mochio, S. (2010). Olfactory dysfunction and cardiovascular dysautonomia in Parkinson’s disease. J. Neurol. 257, 969–976. doi: 10.1007/s00415-009-5447-1
Ondo, W. G., Kenney, C., Sullivan, K., Davidson, A., Hunter, C., Jahan, I., et al. (2012). Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology 78, 1650–1654. doi: 10.1212/WNL.0b013e3182574f28
Palermo, G., Del Prete, E., Bonuccelli, U., and Ceravolo, R. (2020). Early autonomic and cognitive dysfunction in PD, DLB and MSA: Blurring the boundaries between α-synucleinopathies. J. Neurol. 267, 3444–3456. doi: 10.1007/s00415-020-09985-z
Palma, J. A., and Kaufmann, H. (2018). Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov. Disord. 33, 372–390. doi: 10.1002/mds.27344
Palma, J.-A., and Cortelli, P. (2023). Blood pressure and risk of dementia in Parkinson disease and multiple system atrophy. Neurology 100, 451–453. doi: 10.1212/wnl.0000000000206803
Palma, J.-A., Thijs, R. D., Kalbe, E., Bloem, B. R., Kalia, L. V., and Nieuwboer, A. (2024). Non-pharmacological treatment of autonomic dysfunction in Parkinson’s disease and other synucleinopathies. J. Parkinson’s Dis. 14, S81–S92. doi: 10.3233/jpd-230173
Perez-Pardo, P., Kliest, T., Dodiya, H. B., Broersen, L. M., Garssen, J., Keshavarzian, A., et al. (2017). The gut-brain axis in Parkinson’s disease: Possibilities for food-based therapies. Eur. J. Pharmacol. 817, 86–95. doi: 10.1016/j.ejphar.2017.05.042
Peyronnet, B., Vurture, G., Palma, J.-A., Malacarne, D. R., Feigin, A., Sussman, R. D., et al. (2018). Mirabegron in patients with Parkinson disease and overactive bladder symptoms: A retrospective cohort. Parkinsonism Relat. Disord. 57, 22–26. doi: 10.1016/j.parkreldis.2018.07.005
Pfeiffer, R. F. (2020). Autonomic dysfunction in Parkinson’s disease. Neurotherapeutics 17, 1464–1479. doi: 10.1007/s13311-020-00897-4
Pietrucci, D., Cerroni, R., Unida, V., Farcomeni, A., Pierantozzi, M., Mercuri, N. B., et al. (2019). Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Parkinsonism Relat. Disord. 65, 124–130. doi: 10.1016/j.parkreldis.2019.06.003
Qin, Y., Meng, D.-T., Jin, Z.-H., Du, W.-J., and Fang, B.-Y. (2024). Association between autonomic dysfunction with motor and non-motor symptoms in patients with Parkinson’s disease. J. Neural Transmission 131, 323–334. doi: 10.1007/s00702-024-02745-7
Quarracino, C., Otero-Losada, M., Capani, F., and Pérez-Lloret, S. (2020). State-of-the-art pharmacotherapy for autonomic dysfunction in Parkinson’s disease. Exp. Opin. Pharmacother. 21, 445–457. doi: 10.1080/14656566.2020.1713097
Ramirez, C. E., Okamoto, L. E., Arnold, A. C., Gamboa, A., Diedrich, A., Choi, L., et al. (2014). Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension 64, 1235–1240. doi: 10.1161/hypertensionaha.114.04225
Ratajska, A. M., Etheridge, C. B., Lopez, F. V., Kenney, L. E., Rodriguez, K., Schade, R. N., et al. (2023). The relationship between autonomic dysfunction and mood symptoms in De Novo Parkinson’s disease patients over time. J. Geriatric Psychiatry Neurol. 37, 242–252. doi: 10.1177/08919887231204542
Romagnolo, A., Zibetti, M., Merola, A., Canova, D., Sarchioto, M., Montanaro, E., et al. (2018). Cardiovascular autonomic neuropathy and falls in Parkinson disease: A prospective cohort study. J. Neurol. 266, 85–91. doi: 10.1007/s00415-018-9104-4
Ruiz-Barrio, I., Miki, Y., Jaunmuktane, Z. T., Warner, T., and De Pablo-Fernandez, E. (2023). Association between orthostatic hypotension and dementia in patients with Parkinson disease and multiple system atrophy. Neurology 100, e998–e1008. doi: 10.1212/wnl.0000000000201659
Rukavina, K., Batzu, L., Leta, V., and Chaudhuri, K. R. (2022). New approaches to treatments for sleep, pain and autonomic failure in Parkinson’s disease - Pharmacological therapies. Neuropharmacology 208:108959. doi: 10.1016/j.neuropharm.2022.108959
Schapira, A. H. V., Chaudhuri, K. R., and Jenner, P. (2017). Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450. doi: 10.1038/nrn.2017.62
Schoffer, K. L., Henderson, R. D., O’Maley, K., and O’Sullivan, J. D. (2007). Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson’s disease. Mov. Disord. 22, 1543–1549. doi: 10.1002/mds.21428
Schreglmann, S. R., Büchele, F., Sommerauer, M., Epprecht, L., Kägi, G., Hägele-Link, S., et al. (2017). Pyridostigmine bromide versus fludrocortisone in the treatment of orthostatic hypotension in Parkinson’s disease – A randomized controlled trial. Eur. J. Neurol. 24, 545–551. doi: 10.1111/ene.13260
Seidel, K., Mahlke, J., Siswanto, S., Krüger, R., Heinsen, H., Auburger, G., et al. (2014). The brainstem pathologies of Parkinson’s disease and dementia with lewy bodies. Brain Pathol. 25, 121–135. doi: 10.1111/bpa.12168
Seppi, K., Ray Chaudhuri, K., Coelho, M., Fox, S. H., Katzenschlager, R., Perez Lloret, S., et al. (2019). Update on treatments for nonmotor symptoms of Parkinson’s disease—An evidence-based medicine review. Mov. Disord. 34, 180–198. doi: 10.1002/mds.27602
Shahmoradian, S. H., Lewis, A. J., Genoud, C., Hench, J., Moors, T. E., Navarro, P. P., et al. (2019). Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 22, 1099–1109. doi: 10.1038/s41593-019-0423-2
Shibao, C., Raj, S. R., Gamboa, A., Diedrich, A., Choi, L., Black, B. K., et al. (2007). Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension 50, 47–53. doi: 10.1161/hypertensionaha.107.089961
Sklerov, M., Browner, N., Dayan, E., Rubinow, D., and Frohlich, F. (2022). Autonomic and depression symptoms in Parkinson’s disease: Clinical evidence for overlapping physiology. J. Parkinson’s Dis. 12, 1059–1067. doi: 10.3233/jpd-213075
Sklerov, M., Shih, C.-H., Browner, N., Palma, J.-A., Styner, M., and Dayan, E. (2020). Longitudinal change in autonomic symptoms predicts activities of daily living and depression in Parkinson’s disease. Clin. Autonomic Res. 30, 223–230. doi: 10.1007/s10286-020-00672-7
Smith, W., Wan, H., Much, D., Robinson, A. G., and Martin, P. (2016). Clinical benefit of midodrine hydrochloride in symptomatic orthostatic hypotension: A phase 4, double-blind, placebo-controlled, randomized, tilt-table study. Clin. Autonomic Res. 26, 269–277. doi: 10.1007/s10286-016-0363-9
Stewart, C. B., Ledingham, D., Foster, V. K., Anderson, K. N., Sathyanarayana, S., Galley, D., et al. (2023). The longitudinal progression of autonomic dysfunction in Parkinson’s disease: A 7-year study. Front. Neurol. 14:1155669. doi: 10.3389/fneur.2023.1155669
Titova, N., Padmakumar, C., Lewis, S. J. G., and Chaudhuri, K. R. (2016). Parkinson’s: A syndrome rather than a disease? J. Neural Transmission 124, 907–914. doi: 10.1007/s00702-016-1667-6
Toft, M., Iijima, M., Okuma, Y., Suzuki, K., Yoshii, F., Nogawa, S., et al. (2021). Associations between probable REM sleep behavior disorder, olfactory disturbance, and clinical symptoms in Parkinson’s disease: A multicenter cross-sectional study. PLoS One 16:e0247443. doi: 10.1371/journal.pone.0247443
Tolosa, E., Garrido, A., Scholz, S. W., and Poewe, W. (2021). Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 20, 385–397. doi: 10.1016/s1474-4422(21)00030-2
Valentino, F., Bartolotta, T. V., Cosentino, G., Mastrilli, S., Arnao, V., Aridon, P., et al. (2018). Urological dysfunctions in patients with Parkinson’s disease: Clues from clinical and non-invasive urological assessment. BMC Neurol. 18:148. doi: 10.1186/s12883-018-1151-z
van Deursen, D. N., van den Heuvel, O. A., Booij, J., Berendse, H. W., and Vriend, C. (2020). Autonomic failure in Parkinson’s disease is associated with striatal dopamine deficiencies. J. Neurol. 267, 1922–1930. doi: 10.1007/s00415-020-09785-5
VanderHorst, V. G., Samardzic, T., Saper, C. B., Anderson, M. P., Nag, S., Schneider, J. A., et al. (2015). α−synuclein pathology accumulates in sacral spinal visceral sensory pathways. Ann. Neurol. 78, 142–149. doi: 10.1002/ana.24430
Wanneveich, M., Moisan, F., Jacqmin-Gadda, H., Elbaz, A., and Joly, P. (2018). Projections of prevalence, lifetime risk, and life expectancy of Parkinson’s disease (2010-2030) in France. Mov. Disord. 33, 1449–1455. doi: 10.1002/mds.27447
Warnecke, T., Schäfer, K. H., Claus, I., Del Tredici, K., and Jost, W. H. (2022). Gastrointestinal involvement in Parkinson’s disease: Pathophysiology, diagnosis, and management. npj Parkinson’s Dis. 8, 31. doi: 10.1038/s41531-022-00295-x
Yang, J., Wang, H., Yuan, Y., Fan, S., Li, L., Jiang, C., et al. (2021). Peripheral synucleinopathy in Parkinson disease with LRRK2 G2385R variants. Ann. Clin. Transl. Neurol. 8, 592–602. doi: 10.1002/acn3.51301
Yoon, S. H., You, D. H., Na, H. K., Kang, S., Baik, K., Park, M., et al. (2024). Parkinson’s disease with hyposmia and dysautonomia: Does it represent a distinct subtype? J. Neurol. 271, 5064–5073. doi: 10.1007/s00415-024-12332-1
You, S., Kim, H. A., and Lee, H. (2020). Association of postural instability with autonomic dysfunction in early Parkinson’s disease. J. Clin. Med. 9:3786. doi: 10.3390/jcm9113786
Zangaglia, R., Martignoni, E., Glorioso, M., Ossola, M., Riboldazzi, G., Calandrella, D., et al. (2007). Macrogol for the treatment of constipation in Parkinson’s disease. A randomized placebo-controlled study. Mov. Disord. 22, 1239–1244. doi: 10.1002/mds.21243
Zesiewicz, T. A., Evatt, M., Vaughan, C. P., Jahan, I., Singer, C., Ordorica, R., et al. (2015). Randomized, controlled pilot trial of solifenacin succinate for overactive bladder in Parkinson’s disease. Parkinsonism Relat. Disord. 21, 514–520. doi: 10.1016/j.parkreldis.2015.02.025
Zhang, F., Wang, F., Li, C.-H., Wang, J.-W., Han, C.-L., Fan, S.-Y., et al. (2022). Subthalamic nucleus-deep brain stimulation improves autonomic dysfunctions in Parkinson’s disease. BMC Neurol. 22:124. doi: 10.1186/s12883-022-02651-z
Zhang, J., Ge, X., Zhang, K., Qi, Y., Ren, S., and Zhai, X. (2023). Acupuncture for Parkinson’s disease-related constipation: Current evidence and perspectives. Front. Neurol. 14:1253874. doi: 10.3389/fneur.2023.1253874
Keywords: Parkinson’s disease, autonomic dysfunction, review, motor symptoms, non-motor symptoms
Citation: Xu H, Zheng X, Xing X, Bi Z, Wang D, Zhang C, Wei L, Jin Y and Xu S (2025) Advances in autonomic dysfunction research in Parkinson’s disease. Front. Aging Neurosci. 17:1468895. doi: 10.3389/fnagi.2025.1468895
Received: 22 July 2024; Accepted: 28 February 2025;
Published: 12 March 2025.
Edited by:
Sheila Pirooznia, National Institutes of Health (NIH), United StatesReviewed by:
Steven Gunzler, Case Western Reserve University, United StatesCopyright © 2025 Xu, Zheng, Xing, Bi, Wang, Zhang, Wei, Jin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunliang Xu, c2x4dUBsaXZlLmNvbQ==; Yulin Jin, eXVsaW4uamluQGVtb3J5LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.