
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 06 January 2025
Sec. Alzheimer's Disease and Related Dementias
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1436171
This article is part of the Research Topic Advances of lipid metabolism in neurological diseases and mental disorders View all 5 articles
 Chengyong Liu1†
Chengyong Liu1† Chi Wang2†
Chi Wang2† Jing Jiang1
Jing Jiang1 Yuyang Bo1
Yuyang Bo1 Lixiu Nan1
Lixiu Nan1 Ying Zhang1
Ying Zhang1 Kongxi Zhu1
Kongxi Zhu1 Xiaoqiu Wang1
Xiaoqiu Wang1 Xinxin Feng3
Xinxin Feng3 Xiaoyang Lian1*
Xiaoyang Lian1* Shan Qin1*
Shan Qin1*Objective: There reportedly exists a significant comorbidity between insomnia and neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), indicative of a potential link to serum metabolic dysregulation.
Method: To elucidate shared pathophysiological mechanisms between insomnia and AD/PD, we performed comprehensive two-sample Mendelian randomization (MR) analyses, investigating 1,400 serum metabolic characteristics for their causal relationships with the risks of insomnia, AD, widely defined AD (WDAD), and PD. We employed publicly available genetic data; the primary estimate was determined using inverse-variance weighting, supplemented by weighted median, simple mode, weighted mode, and the MR-PRESSO and MR-Egger methods to evaluate heterogeneity and pleiotropy.
Results: The ratio of N-palmitoyl-sphingosine to N-palmitoyl-sphinganine is linked to higher risks of insomnia (OR = 1.137, 95% CI = 1.015–1.273, p = 0.026) and AD (OR = 1.090, 95% CI = 1.005–1.183, p = 0.037). The acetylcarnitine to propionylcarnitine ratio is a risk factor for insomnia (OR = 1.190, 95% CI = 1.003–1.370, p = 0.016) but has protective effects against AD (OR = 0.868, 95% CI = 0.784–0.961, p = 0.006) and WDAD (OR = 0.892, 95% CI = 0.817–0.973, p = 0.010). Glutamine conjugate of C7H12O2 levels are associated with reduced risk of insomnia (OR = 0.863, 95% CI = 0.749–0.995, p = 0.042) and PD (OR = 0.856, 95% CI = 0.746–0.981, p = 0.026).
Conclusion: Our findings highlight the crucial role of serum metabolic characteristics in the comorbidity of insomnia with neurodegenerative diseases, providing valuable insights into prospective therapeutic targets and diagnostic markers.
Despite optimal sleep environments and opportunities, individuals suffering from insomnia tend to express dissatisfaction with both sleep duration and quality, often leading to daytime functional impairments (Morin et al., 2015). The prevalence of insomnia is on the rise owing to rapid societal development, with approximately 10% adults meeting the diagnostic criteria for the condition (Hale et al., 2020). Insomnia poses a significant threat to both physiological and psychological health, substantially increasing the risk of comorbid neurodegenerative alterations, thereby imposing a severe healthcare burden on individuals, families, and society. Alzheimer’s disease (AD) and Parkinson’s disease (PD) represent the most common neurodegenerative pathologies, characterized by neuronal structural changes and progressive cognitive decline. An epidemiological study based on national demographic data found a significantly elevated incidence of AD among individuals with insomnia, with a poorer prognosis compared to those without non-insomnia (Baek et al., 2021). Further, a retrospective longitudinal cohort study in Taiwan demonstrated an increased risk of PD associated with insomnia (Hsiao et al., 2017).
Previous investigations have revealed that insomnia are commonly observed early in the progression of dementia, affecting approximately 44% of patients with AD, the highest incidence is found in those with AD and PD, where up to 90% of patients are affected (Ju et al., 2013). Insomnia can lead to various alterations in brain structure and function, including inflammation, degeneration, and changes in glial function. Consequently, they represent a risk factor for dementia and can worsen morbidity in dementia patients (Pillai and Leverenz, 2017; Gottesman et al., 2024). Conversely, alterations in brain structures and functions that are critical for sleep regulation can result in comorbid insomnia in dementia patients, indicating a bidirectional relationship between insomnia and changes in brain structure and function (Mutti et al., 2021). Previous cross-sectional studies have demonstrated that insomnia adversely affects cognitive function, particularly attention and memory, in patients with PD (Jozwiak et al., 2017). Insomnia may be a significant risk factor for cognitive impairment in PD patients. A longitudinal cohort study found that, among PD patients with insomnia, the annual decline in MoCA scores was higher over a 3-year follow-up period (Chahine et al., 2016). Presently, the reasons for the increased comorbidity risk between insomnia and AD/PD remain unclear, we speculate that changes in serum metabolites may be an important contributing factor to the common etiology between insomnia and AD/PD. Serum metabolic characteristics play a crucial role in the pathological processes of insomnia, AD, and PD. A significant increase in the levels of acylcarnitine, glycerophospholipids, and sphingolipids have been reported during acute sleep deprivation as compared to that during the sleep period (Davies et al., 2014). Besides, in comparison with healthy individuals, patients with insomnia exhibit significant alterations in phospholipid metabolites such as 1-palmitoylglycerophosphocholine (Wang et al., 2022). Phospholipids are essential components of biological membranes and play a crucial role in maintaining the structure and function of nerve cells. Untargeted metabolomic studies have revealed metabolic disruptions in carbohydrates, amino acids, and lipids in patients with AD compared to cognitively normal groups (Yu et al., 2023). In patients with PD, significant alterations occur in the levels of acylcarnitine, indolelactic acid, and unsaturated fatty acids, indicating a potential association between these metabolic characteristics and PD pathogenesis (Shao et al., 2021). While these observational studies confirm the correlation between serum metabolic characteristics and insomnia, AD, and PD, in this study, we aimed to explore causality through Mendelian randomization (MR), acknowledging the limitations of observational studies in elucidating causal relationships and accounting for confounding factors and reverse causation.
Previous studies have indicated associations between serum metabolites and various neurodegenerative diseases. However, these findings must be interpreted cautiously due to the potential for confounding factors and reverse causation. Unlike traditional observational studies that are prone to such biases, Mendelian randomization (MR) uses genetic variations as instrumental variables, offering a robust method to establish causal relationships. In this study, we employed MR to investigate the associations between serum metabolites and neuropsychiatric disorders such as insomnia, AD and PD. Through this analysis, we aim to contribute to a broader understanding of the underlying causes for the increased comorbidity risk between insomnia and AD/PD.
We conducted a two-sample MR investigation to evaluate the causal relationships between 1,400 metabolic characteristics and the risk of insomnia, AD, widely defined AD (WDAD), and PD. The MR framework employs genetic variances as surrogates for risk factors, necessitating robust instruments that adhere to three fundamental assumptions crucial in causal inference: (1) direct association between genetic variances and the exposure, (2) absence of correlation between genetic variances and potential confounding factors bridging the exposure and outcome, and (3) no influence of genetic variances on the outcome via pathways distinct from the exposure (Lawlor, 2016).
We comprehensively analyzed a large-scale genome-wide association study (GWAS) and established instrumental variables (IVs) related to serum metabolic characteristics. The GWAS summary statistics for this study, with accession numbers GCST90199621-90201020 for European GWAS, have been deposited in the GWAS Catalog.1 This comprehensive GWAS delved into the intricate genetic foundations of 1,091 plasma metabolic characteristics and 309 corresponding ratios (Chen et al., 2023).
Of the 1,091 plasma metabolic characteristics, 850 presented unequivocal identities spanning eight super pathways, encompassing lipids, amino acids, xenobiotics, nucleotides, cofactors and vitamins, carbohydrates, peptides, and energy. The remaining 241 were either classified as unknown or only “partially” characterized. To unveil latent biological processes inherent in the study of individual metabolic characteristics, the researchers computed ratios at the metabolite level for pairs sharing an enzyme or transporter (Chen et al., 2023). This meticulous process leveraged associations between metabolic characteristics and proteins meticulously documented in the Human Metabolome Database.
In contrast to an uninformed methodology to derive metabolite ratios, their systematic approach, anchored in empirical evidence, preserves enhanced statistical power by judiciously testing a subset of metabolite ratios displaying heightened biological plausibility (Chen et al., 2023).
The inclusion criteria for this analysis of GWAS data were confined to cohorts of European lineage. The GWAS summary statistics for insomnia were obtained from the FinnGen project (DATA FREEZE 92). The FinnGen Project, a substantial genetic exploration initiative, focuses on elucidating the intricate interplay between genomic data and health attributes within Finnish and broader European populations. The R9 dataset encompasses an extensive reservoir of data, with a total sample size of 377,277 individuals (210,870 females and 166,407 males) actively participating in the investigative undertaking. A meticulous analysis was performed on an extraordinary 20,175,454 genetic variants, probing connections with a diverse array of disease endpoints (phenotypes), totaling 2,272. This substantial repository of genetic revelations was disseminated among collaborative affiliates, with the latest data release occurring in the initial quarter of 2022.
Acquiring GWAS data for insomnia, AD, WDAD, and PD involved the formal submission of a request to the investigators overseeing the FinnGen study for their approval. The dataset includes 13,393 individuals with WDAD, along with 36,384 control individuals. In addition, the dataset includes 9,301 patients with AD and 36,7,976 control individuals, 4,235 patients with PD and 37,3,042 control individuals, and also 4,235 patients with insomnia and 37,3,042 control individuals (Kurki et al., 2023).
In line with contemporary investigations (Sha et al., 2023; Yan et al., 2023), the significance level threshold for IVs related to each metabolite trait was set at 1 × 10−5. The pruning process in PLINK v1.90 was employed to remove single nucleotide polymorphisms (SNPs) (linkage disequilibrium r2 threshold <0.01 within a 10,000 kb span). The computation of F-statistics was performed for each IV to assess the robustness of instrumental strength and prevent the risks of weak instrumental bias. IVs for metabolite characteristics were retained for subsequent analyses, excluding those with suboptimal F-statistics (<10).
All computations were performed using R 4.3.1.3 To examine the causal relationship between 1,400 metabolic characteristics and outcomes, we employed advanced statistical techniques, including inverse-variance weighting (IVW) (Slob and Burgess, 2020), weighted median (Bowden et al., 2016), simple mode, and weighted mode via the MendelianRandomization package v0.4.3 (Yavorska and Burgess, 2017). Cochran’s Q statistic and corresponding p values were utilized to evaluate heterogeneity among the selected IVs (Greco et al., 2015). To address potential horizontal pleiotropy effects, the MR-Egger method was applied, which identifies horizontal multiplicity if the intercept term achieves significance (Bowden et al., 2015; Burgess and Thompson, 2017). Furthermore, the robust MR-PRESSO method was utilized to identify and exclude plausible horizontal pleiotropic outliers that could significantly influence estimation results within the MR-PRESSO package (Verbanck et al., 2018). Our analysis also incorporated visualizations such as scatter, funnel, and leave-one-out plots. Scatter plots confirmed the robustness of outcomes, demonstrating their insensitivity to outliers. Funnel plots graphically represented the resilience of correlation, emphasizing the absence of heterogeneity. Leave-one-out plots helped assess the impact of individual data points on overall analyses, systematically excluding each data point one at a time and revealing resultant changes; moreover, they facilitated a nuanced evaluation of the influence of individual observations on the stability and reliability of the statistical model.
We performed MR analyses with 1,400 metabolic characteristics in relation to insomnia, AD, WDAD, and PD. Based on our preliminary findings, 40, 45, 50, and 50 metabolic characteristics were identified to be potentially causally related to insomnia (Supplementary Table S1), AD (Supplementary Table S2), WDAD (Supplementary Table S3), and PD (Supplementary Table S4), respectively. By curating frequently appearing metabolic characteristics and meticulous examining vertical and horizontal pleiotropy using IVW and the MR-Egger and MR-PRESSO methods (Table 1), we observed that N-palmitoyl-sphingosine/N-palmitoyl-sphinganine concurrently correlated causally with the onset risks of insomnia and AD, while acetylcarnitine (C2)/propionylcarnitine (C3) concurrently influenced the onset risks of insomnia, AD, and WDAD. Further, our findings suggested that N-acetyl-aspartyl-glutamate levels and glutamine conjugate of C7H12O2 levels concurrently influence the onset risks of insomnia and PD. With regard to positive outcomes, the total count of index SNPs chosen for the genetic prediction of metabolic characteristics (Supplementary Tables S5–S12) ranged from 15 to 59 (Figure 1). The F-statistics of these genetic instruments exceeded the commonly adopted threshold value of 10, affirming their robustness and potency (Supplementary Tables S5–S12). SNP effect sizes for the exposure were visualized via scatter plots for the outcome (Figures 2A–G). Leave-one-out sensitivity analysis indicated that no individual SNP significantly influenced the association between the exposure and outcome (Figures 3A–G). Finally, funnel plots revealed no significant heterogeneity among the chosen independent variables (Figures 4A–G).
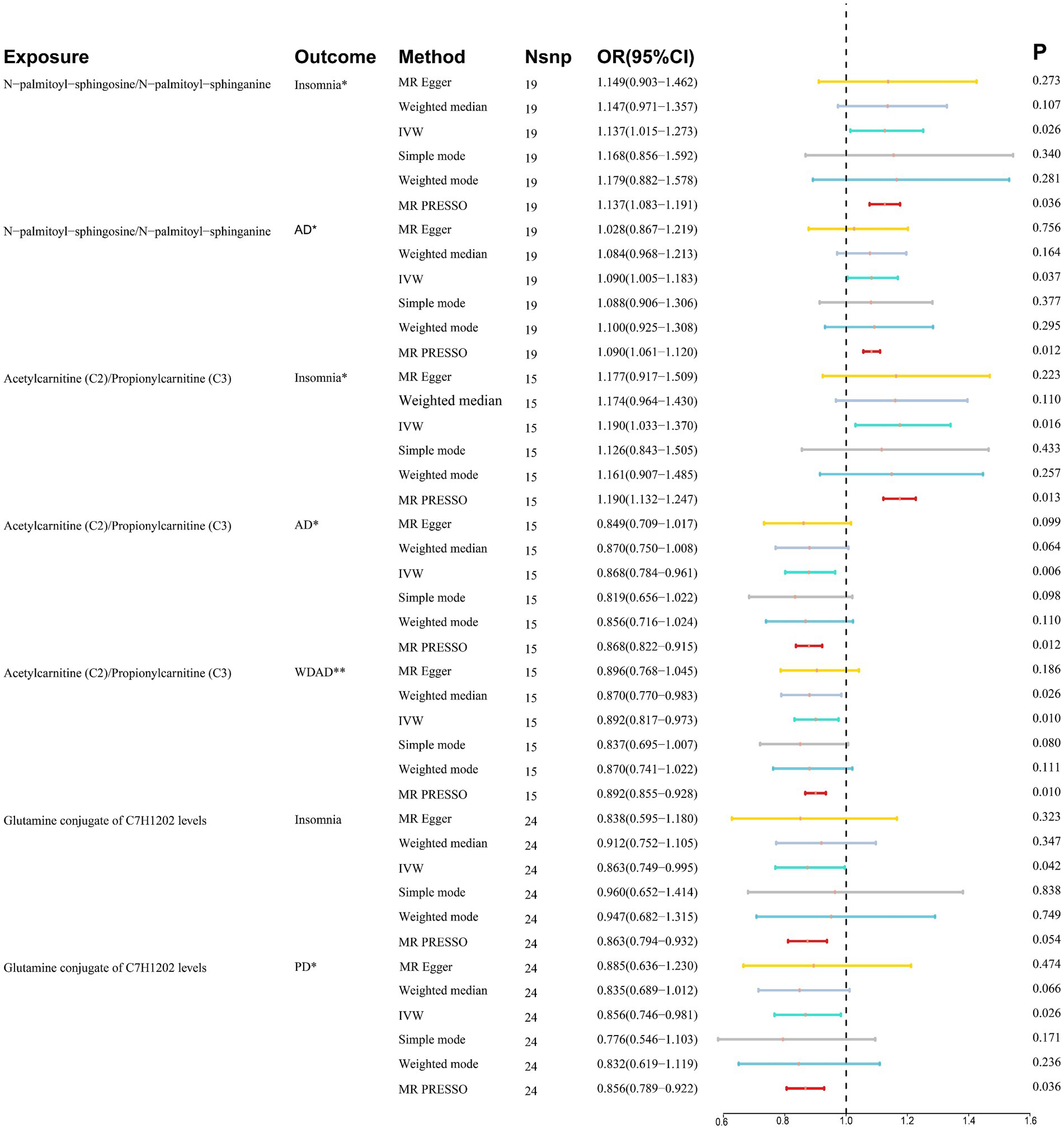
Figure 1. Summary of association between metabolic features and diseases. *Where PIVW < 0.05 and Pweighted median/PMR-PRESSO < 0.05, it means suggestive association. **Where PIVW < 0.05, Pweighted median and PMR-PRESSO < 0.05, it means significant association. CI, confidence interval; IVW, inverse variance weighted; OR, odds ratio; Nsnp, number of single-nucleotide polymorphisms; AD, Alzheimer’s disease; WDAD, widely defined Alzheimer’s disease; PD, Parkinson’s disease.
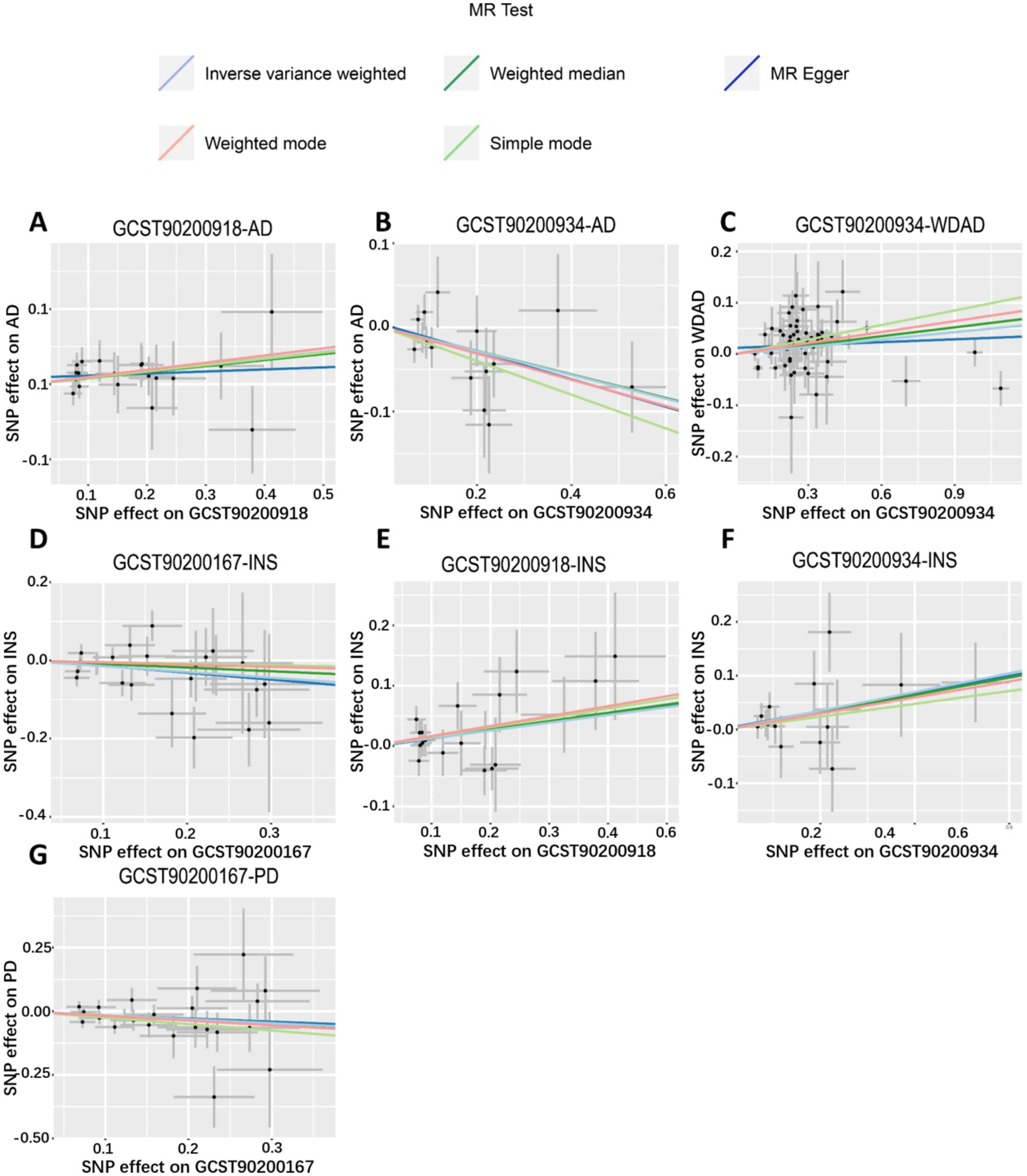
Figure 2. Scatter plots of genetic correlations of metabolic features and insomnia, WDAD, AD, and PD using different MR methods. (A,E) Scatter plot of genetic correlations of N-palmitoyl-sphingosine (d18:1 to 16:0) to N-palmitoyl-sphinganine (d18:0 to 16:0) ratio with AD and insomnia. (B,C,F) Scatter plot of genetic correlations of acetylcarnitine (C2) to propionylcarnitine (C3) ratio with AD, WDAD, and insomnia. (D,G) Scatter plot of genetic correlations of glutamine conjugate of C7H12O2 levels with insomnia and PD. AD, Alzheimer’s disease; WDAD, widely defined Alzheimer’s disease; PD, Parkinson’s disease.
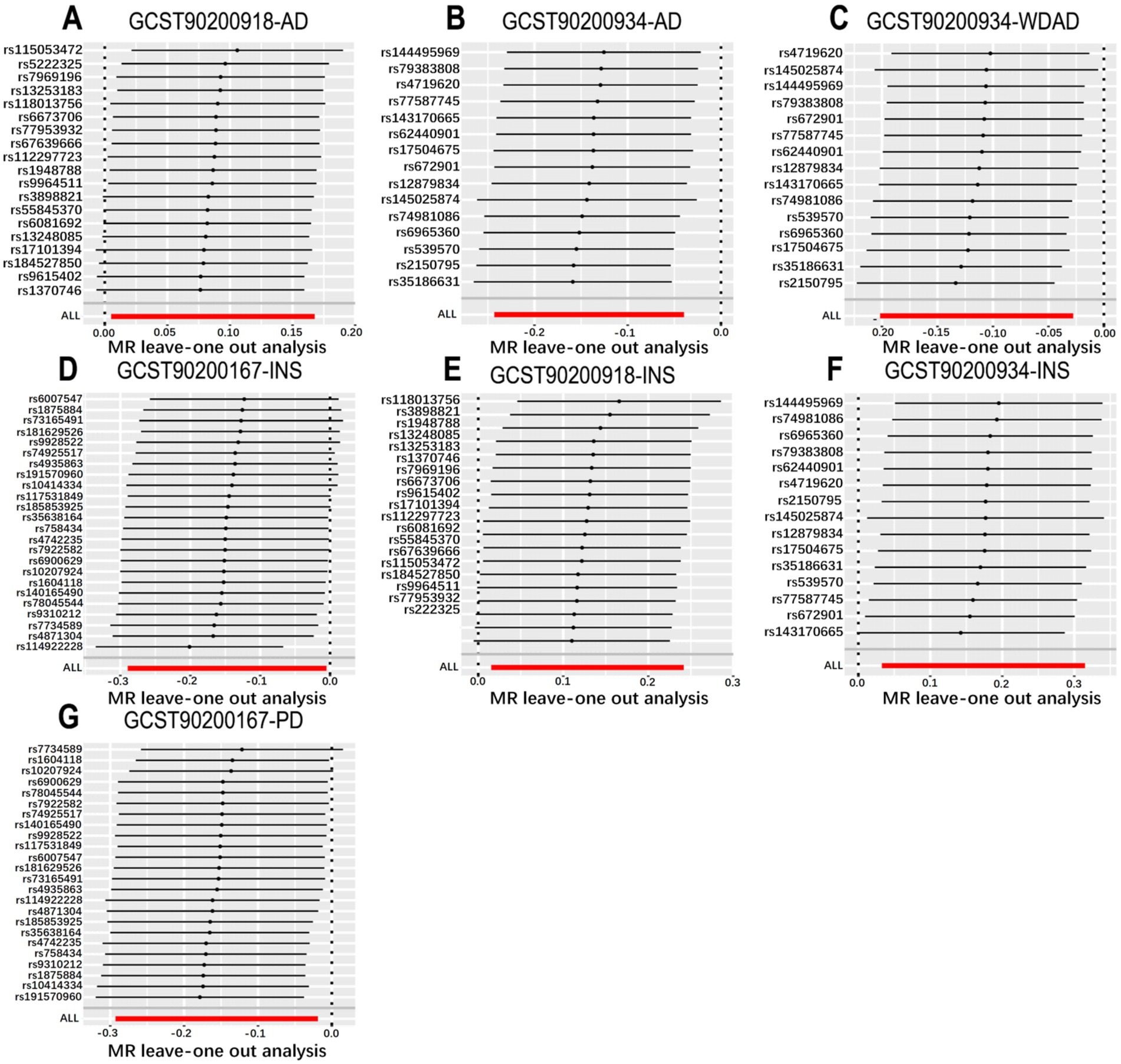
Figure 3. Leave-one-out plot from genetically predicted metabolic features on insomnia, WDAD, AD, and PD. (A,E) Leave-one-out plot of genetic correlations of N-palmitoyl-sphingosine (d18:1 to 16:0) to N-palmitoyl-sphinganine (d18:0 to 16:0) ratio with AD and insomnia. (B,C,F) The leave-one-out plot of genetic correlations of acetylcarnitine (C2) to propionylcarnitine (C3) ratio with AD, WDAD and insomnia. (D,G) Leave-one-out plot of genetic correlations of glutamine conjugate of C7H12O2 levels with insomnia and PD. AD, Alzheimer’s disease; WDAD, widely defined Alzheimer’s disease; PD, Parkinson’s disease.
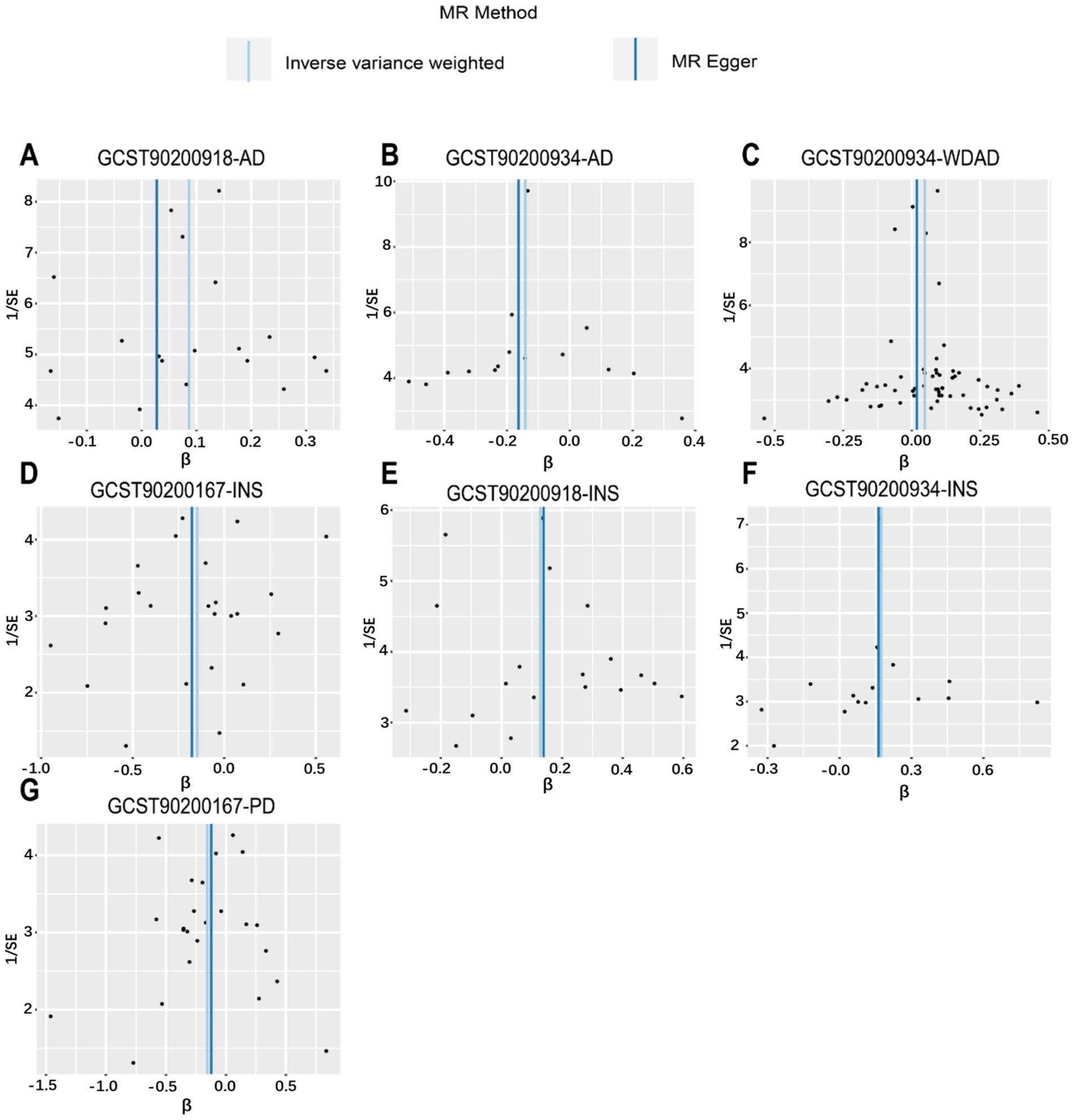
Figure 4. Funnel plot from genetically predicted metabolic features on insomnia, WDAD, AD, and PD. (A,E) Funnel plot of genetic correlations of N-palmitoyl-sphingosine (d18:1 to 16:0) to N-palmitoyl-sphinganine (d18:0 to 16:0) ratio with AD and insomnia. (B,C,F) Funnel plot of genetic correlations of acetylcarnitine (C2) to propionylcarnitine (C3) ratio with AD, WDAD, and insomnia. (D,G) Funnel plot of genetic correlations of glutamine conjugate of C7H12O2 levels with insomnia and PD. AD, Alzheimer’s disease; WDAD, widely defined Alzheimer’s disease; PD, Parkinson’s disease.
The ratio of N-palmitoyl-sphingosine to N-palmitoyl-sphinganine was associated with increased risk of insomnia (MR IVW method: OR = 1.137, 95% CI = 1.015–1.273, p = 0.026) and AD (MR IVW method: OR = 1.090, 95% CI = 1.005–1.183, p = 0.037).
The ratio of acetylcarnitine to propionylcarnitine emerged as a risk factor for insomnia (MR IVW method: OR = 1.190, 95% CI = 1.003–1.370, p = 0.016) while exhibiting a protective effect against AD (MR IVW method: OR = 0.868, 95% CI = 0.784–0.961, p = 0.006) and WDAD (MR IVW method: OR = 0.892, 95% CI = 0.817–0.973, p = 0.010). The consistency of the effects of the ratio of acetylcarnitine to propionylcarnitine on WDAD was validated in the subsequent sensitivity analyses (weighted median: OR = 0.870, 95% CI = 0.770–0.983, p = 0.026).
Glutamine conjugate of C7H12O2 levels were associated with decreased risk of insomnia (MR IVW method: OR = 0.863, 95% CI = 0.749–0.995, p = 0.042) and PD (MR IVW method: OR = 0.856, 95% CI = 0.746–0.981, p = 0.026).
Previous observational studies have indicated an increased comorbidity risk of AD and PD in individuals with insomnia (Baek et al., 2021; Hsiao et al., 2017). Considering the limitations of observational studies and the significant role of serum metabolite-mediated oxidative stress reactions in patients with insomnia with comorbid AD and PD, herein we performed MR analyses to mitigate confounding factors and reverse causation. Our core objective was to elucidate the causal relationship between serum metabolic characteristics and insomnia, AD, and PD. We found that increased N-palmitoyl-sphingosine to N-palmitoyl-sphinganine ratio simultaneously increased the risk of insomnia and AD, while glutamine conjugate of C7H12O2 levels decreased the risk of insomnia and PD, indicative of shared etiology between insomnia and AD/PD. In addition, increased ratio of acetylcarnitine to propionylcarnitine was observed to be positively correlated with the risk of insomnia and negatively correlated with the risk of AD and ADWD.
There is a significant correlation between oxidative stress and insomnia. During wakefulness, neurons produce reactive oxygen species (ROS), while sleep facilitates the clearance of these ROS, thereby mitigating oxidative damage. Sleep deprivation leads to the accumulation of ROS and subsequent oxidative stress (Vaccaro et al., 2020). Our research reveals a causal relationship between insomnia and specific serum metabolite levels, including the N-palmitoyl sphingosine/dipalmitoyl sphingosine ratio, the acetylcarnitine/propionylcarnitine ratio, and the C7H12O2-glutamine conjugate. These metabolic alterations suggest that insomnia may not only be a symptom but also a potential pathophysiological contributor to neurodegenerative diseases such as AD and PD.
AD is the most prevalent clinical neurodegenerative disorder, characterized by the accumulation of abnormal proteins—β-amyloid plaques and tau tangles—within the brain, leading to neuronal dysfunction or death (Atlante and Valenti, 2023). Mitochondrial dysfunction and oxidative stress are critically implicated in the onset and progression of AD (Song et al., 2021). Mitochondrial impairment induces oxidative stress, reduces ATP synthesis, disrupts normal neuronal metabolism, and promotes the accumulation of β-amyloid and tau by enhancing β-secretase activity through elevated ROS levels. This ultimately affects the anatomy and physiology of neurons, resulting in cell death (Bell et al., 2021). We have observed that an elevated N-palmitoyl sphingosine/dipalmitoyl sphingosine ratio is associated with increased risk of insomnia and AD. Elevated levels of N-palmitoyl sphingosine may promote inflammation and apoptosis, potentially compromising the integrity of the blood–brain barrier (BBB) and exacerbating neurodegenerative processes (Huang et al., 2020). Similarly, an imbalance in the acetylcarnitine/propionylcarnitine ratio may indicate mitochondrial dysfunction, a critical factor in the development and progression of AD (Ke et al., 2021; McCann et al., 2021). Acetylcarnitine and propionylcarnitine are intermediates in fatty acid oxidation with potent antioxidant properties (Moskaleva et al., 2022). Their ratio can indirectly reflect oxidative stress levels (Solevåg et al., 2021), and alterations in this ratio may lead to increased deposition of abnormal proteins such as β-amyloid and tau (Islam, 2017), hallmark features of AD. Inhibiting the synthesis of these substances may offer new therapeutic strategies for neurodegenerative diseases like AD.
PD is the second most common neurodegenerative disorder after AD, characterized by a reduction in the number of dopaminergic neurons in the substantia nigra, leading to decreased dopamine levels in the basal ganglia (Korczowska-Łącka et al., 2023). Mitochondrial dysfunction and oxidative stress play pivotal roles in PD pathology. Research has shown that oxidative stress promotes the synthesis of cytotoxic compounds, which accumulate in dopaminergic neurons, leading to protein misfolding, enzyme inactivation, lipid peroxidation, and cell death. These processes ultimately contribute to the degenerative changes in dopaminergic neurons (Chakrabarti and Bisaglia, 2023; Dong-Chen et al., 2023). In this study, we found that elevated levels of the C7H12O2-glutamine conjugate are associated with a reduced risk of PD, suggesting that mitigating oxidative stress may be one of its underlying mechanisms. Glutamine, the most abundant free amino acid in the body, reduces oxidative stress by inhibiting the activation of the PI3K/Akt signaling pathway, thereby minimizing damage to dopaminergic neurons (Cruzat et al., 2018; Yan et al., 2020). This finding supports the hypothesis that reducing oxidative stress may serve as a protective mechanism in PD; however, further research is needed to determine whether the C7H12O2-glutamine conjugate exerts its antioxidant effects through this pathway.
The increased risk of comorbid insomnia with PD and AD imposes a significant healthcare burden on individuals, families, and society. However, our understanding of the shared pathophysiological mechanisms between insomnia and AD/PD remains incomplete. In this study, we employed serum metabolic profiling to identify risk and protective factors associated with insomnia, AD, and PD. Our findings confirm the existence of common pathophysiological mechanisms linking insomnia with AD/PD. We believe that serum metabolic profiling can aid in predicting neurodegenerative comorbidities in patients with insomnia, offering new avenues for the prevention and treatment of comorbid insomnia and AD/PD.
Although metabolites such as N-palmitoyl sphingosine and dipalmitoyl sphingosine have limited ability to cross the blood–brain barrier, thereby restricting their direct impact on insomnia and neurodegenerative diseases, changes in their levels in the peripheral system may reflect pathological alterations in the brain (Quinville et al., 2021). We propose that future research should investigate whether these metabolites are synthesized within the brain, as well as their local effects and potential connections between the brain and peripheral systems, to achieve a more comprehensive understanding of their roles in neurodegenerative diseases and insomnia.
This study has several merits. For example, while observational studies are limited to correlational analyses, MR studies facilitate causal inference, mitigating the impact of confounding factors and reverse causation. The inclusion of a large sample size enabled thorough MR analyses across various metabolic characteristics. Furthermore, we utilized the most recent genomic data for metabolic characteristics, which ensured comprehensive metabolite coverage. Open-access datasets and open-source software were employed, enhancing the transparency and reproducibility of our findings; moreover, rigorous testing for heterogeneity (Cochran’s Q) and pleiotropy (MR-PRESSO global and MR-Egger regression interval trial tests) was performed, demonstrating the reliability of our key results.
This study also has few limitations. While MR analysis serves as a robust tool to assess causal relationships between metabolic characteristics and diseases, validation through experimental data is imperative. Herein we only established the presence of shared etiology between insomnia and AD/PD, postulating that aberrations in metabolic characteristics potentially contribute to comorbidity. Further research needs to be conducted to explore specific pathophysiological mechanisms. It is notable that this study exclusively involved participants of European descent, potentially limiting the generalizability of our results to other ethnicities. We believe that future studies should employ a combination of observational and genetic methods to further explore the impact of serum metabolic characteristics on insomnia and AD/PD. This exploration should involve careful consideration of stratification factors, including age and gender.
To summarize, we performed MR analyses and identified several metabolic characteristics that were potentially causally related to insomnia, AD, WDAD, and PD. In particular, we identified a causal relationship between N-palmitoyl-sphingosine/N-palmitoyl-sphinganine and insomnia and AD and also that between acetylcarnitine/propionylcarnitine and insomnia, AD, and WDAD. Further, glutamine conjugate of C7H12O2 levels were causally associated with insomnia and PD. Our results provide novel insights into the pathological mechanisms underlying the comorbidity of insomnia and AD/PD and should contribute to the development of new therapeutic approaches. Further investigations are warranted to validate our findings and elucidate potential molecular mechanisms linking these metabolites to insomnia and neurodegenerative conditions (Abad et al., 2018).
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
CL: Writing – review & editing. CW: Writing – original draft. JJ: Writing – original draft. YB: Writing – original draft. LN: Writing – original draft. YZ: Writing – original draft. KZ: Writing – original draft. XW: Writing – review & editing. XF: Writing – review & editing. XL: Writing – review & editing. SQ: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China grant number 82274631 and 82205252, the Jiangsu Provincial Department of Science and Technology grant Number BE2021751 and BE2023793, the TCM science and technology program of Zhejiang Province grant number 2024ZL86, the General project of Health Commission of Jiangsu Province grant number Ym2023105, and the Jiangsu Basic Research Program Youth Fund Project grant number BK20210986.
We gratefully acknowledge the authors and participants of all GWAS from which we used summary statistics data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1436171/full#supplementary-material
1. ^https://www.ebi.ac.uk/gwas/
2. ^https://www.finngen.fi/en, accessed on November 28, 2023.
Abad, V. C., and Guilleminault, C. (2018). Insomnia in elderly patients: recommendations for pharmacological management. Drugs Aging 35, 791–817. doi: 10.1007/s40266-018-0569-8
Atlante, A., and Valenti, D. (2023). Mitochondrial complex I and β-amyloid peptide interplay in Alzheimer’s disease: a critical review of new and old little regarded findings. Int. J. Mol. Sci. 24:15951. doi: 10.3390/ijms242115951
Baek, M. S., Han, K., Kwon, H. S., Lee, Y. H., Cho, H., and Lyoo, C. H. (2021). Risks and prognoses of Alzheimer’s disease and vascular dementia in patients with insomnia: a Nationwide population-based study. Front. Neurol. 12:611446. doi: 10.3389/fneur.2021.611446
Bell, S. M., Barnes, K., de Marco, M., Shaw, P. J., Ferraiuolo, L., Blackburn, D. J., et al. (2021). Mitochondrial dysfunction in Alzheimer’s disease: a biomarker of the future? Biomedicines 9:63. doi: 10.3390/biomedicines9010063
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. doi: 10.1002/gepi.21965
Burgess, S., and Thompson, S. G. (2017). Interpreting findings from Mendelian randomization using the MR-egger method. Eur. J. Epidemiol. 32, 377–389. doi: 10.1007/s10654-017-0255-x
Chahine, L. M., Xie, S. X., Simuni, T., Tran, B., Postuma, R., Amara, A., et al. (2016). Longitudinal changes in cognition in early Parkinson’s disease patients with REM sleep behavior disorder. Parkinsonism Relat. Disord. 27, 102–106. doi: 10.1016/j.parkreldis.2016.03.006
Chakrabarti, S., and Bisaglia, M. (2023). Oxidative stress and Neuroinflammation in Parkinson’s disease: the role of dopamine oxidation products. Antioxidants 12:955. doi: 10.3390/antiox12040955
Chen, Y., Lu, T., Pettersson-Kymmer, U., Stewart, I. D., Butler-Laporte, G., Nakanishi, T., et al. (2023). Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 55, 44–53. doi: 10.1038/s41588-022-01270-1
Cruzat, V., Macedo Rogero, M., Noel Keane, K., Curi, R., and Newsholme, P. (2018). Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients 10:1564. doi: 10.3390/nu10111564
Davies, S. K., Ang, J. E., Revell, V. L., Holmes, B., Mann, A., Robertson, F. P., et al. (2014). Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 111, 10761–10766. doi: 10.1073/pnas.1402663111
Dong-Chen, X., Yong, C., Yang, X., Chen-Yu, S., and Li-Hua, P. (2023). Signaling pathways in Parkinson’s disease: molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 8:73. doi: 10.1038/s41392-023-01353-3
Gottesman, R. F., Lutsey, P. L., Benveniste, H., Brown, D. L., Full, K. M., Lee, J. M., et al. (2024). Impact of sleep disorders and disturbed sleep on brain health: a scientific statement from the American Heart Association. Stroke 55, e61–e76. doi: 10.1161/STR.0000000000000453
Greco, M. F. D., Minelli, C., Sheehan, N. A., and Thompson, J. R. (2015). Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 34, 2926–2940. doi: 10.1002/sim.6522
Hale, L., Troxel, W., and Buysse, D. J. (2020). Sleep health: an opportunity for public health to address health equity. Annu. Rev. Public Health 41, 81–99. doi: 10.1146/annurev-publhealth-040119-094412
Hsiao, Y. H., Chen, Y. T., Tseng, C. M., Wu, L. A., Perng, D. W., Chen, Y. M., et al. (2017). Sleep disorders and an increased risk of Parkinson’s disease in individuals with non-apnea sleep disorders: a population-based cohort study. J. Sleep Res. 26, 623–628. doi: 10.1111/jsr.12545
Huang, X., Hussain, B., and Chang, J. (2020). Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms. CNS Neurosci. Ther. 27, 36–47. doi: 10.1111/cns.13569
Islam, M. T. (2017). Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 39, 73–82. doi: 10.1080/01616412.2016.1251711
Jozwiak, N., Postuma, R. B., Montplaisir, J., Latreille, V., Panisset, M., Chouinard, S., et al. (2017). REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep 40:zsx101. doi: 10.1093/sleep/zsx101
Ju, Y. E. S., McLeland, J. S., Toedebusch, C. D., Xiong, C., Fagan, A. M., Duntley, S. P., et al. (2013). Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 70, 587–593. doi: 10.1001/jamaneurol.2013.2334
Ke, J., Tian, Q., Xu, Q., Fu, Z., and Fu, Q. (2021). Mitochondrial dysfunction: a potential target for Alzheimer’s disease intervention and treatment. Drug Discov. Today 26, 1991–2002. doi: 10.1016/j.drudis.2021.04.025
Korczowska-Łącka, I., Hurła, M., Banaszek, N., Kobylarek, D., Szymanowicz, O., Kozubski, W., et al. (2023). Selected biomarkers of oxidative stress and energy metabolism disorders in neurological diseases. Mol. Neurobiol. 60, 4132–4149. doi: 10.1007/s12035-023-03329-4
Kurki, M. I., Karjalainen, J., Palta, P., Sipilä, T. P., Kristiansson, K., Donner, K. M., et al. (2023). FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518. doi: 10.1038/s41586-022-05473-8
Lawlor, D. A. (2016). Commentary: two-sample Mendelian randomization: opportunities and challenges. Int. J. Epidemiol. 45, 908–915. doi: 10.1093/ije/dyw127
McCann, M. R., George De la Rosa, M. V., Rosania, G. R., and Stringer, K. A. (2021). L-carnitine and Acylcarnitines: mitochondrial biomarkers for precision medicine. Meta 11:51. doi: 10.3390/metabo11010051
Morin, C. M., Drake, C. L., Harvey, A. G., Krystal, A. D., Manber, R., Riemann, D., et al. (2015). Insomnia disorder. Nat. Rev. Dis. Primers 1:15026. doi: 10.1038/nrdp.2015.26
Moskaleva, N. E., Shestakova, K. M., Kukharenko, A. V., Markin, P. A., Kozhevnikova, M. V., Korobkova, E. O., et al. (2022). Target metabolome profiling-based machine learning as a diagnostic approach for cardiovascular diseases in adults. Meta 12:1185. doi: 10.3390/metabo12121185
Mutti, C., Rausa, F., and Parrino, L. (2021). Sleep in Neurologic Disorders. Sleep Med. Clin. 16, 499–512. doi: 10.1016/j.jsmc.2021.05.002
Pillai, J. A., and Leverenz, J. B. (2017). Sleep and neurodegeneration: a critical appraisal. Chest 151, 1375–1386. doi: 10.1016/j.chest.2017.01.002
Quinville, B. M., Deschenes, N. M., Ryckman, A. E., and Walia, J. S. (2021). A comprehensive review: sphingolipid metabolism and implications of disruption in sphingolipid homeostasis. Int. J. Mol. Sci. 22:5793. doi: 10.3390/ijms22115793
Sha, T., Wang, N., Wei, J., He, H., Wang, Y., Zeng, C., et al. (2023). Genetically predicted levels of serum metabolites and risk of sarcopenia: a Mendelian randomization study. Nutrients 15:3964. doi: 10.3390/nu15183964
Shao, Y., Li, T., Liu, Z., Wang, X., Xu, X., Li, S., et al. (2021). Comprehensive metabolic profiling of Parkinson’s disease by liquid chromatography-mass spectrometry. Mol. Neurodegener. 16:4. doi: 10.1186/s13024-021-00425-8
Slob, E. A. W., and Burgess, S. (2020). A comparison of robust Mendelian randomization methods using summary data. Genet. Epidemiol. 44, 313–329. doi: 10.1002/gepi.22295
Solevåg, A. L., Zykova, S. N., Thorsby, P. M., and Schmölzer, G. M. (2021). Metabolomics to diagnose oxidative stress in perinatal asphyxia: towards a non-invasive approach. Antioxidants 10:1753. doi: 10.3390/antiox10111753
Song, T., Song, X., Zhu, C., Patrick, R., Skurla, M., Santangelo, I., et al. (2021). Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: a meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 72:101503. doi: 10.1016/j.arr.2021.101503
Vaccaro, A., Kaplan Dor, Y., Nambara, K., Pollina, E. A., Lin, C., Greenberg, M. E., et al. (2020). Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell 181, 1307–1328.e15. doi: 10.1016/j.cell.2020.04.049
Verbanck, M., Chen, C. Y., Neale, B., and Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698. doi: 10.1038/s41588-018-0099-7
Wang, Q., Chen, B., Sheng, D., Yang, J., Fu, S., Wang, J., et al. (2022). Multiomics analysis reveals aberrant metabolism and immunity linked gut microbiota with insomnia. Microbiol Spectr. 10:e0099822. doi: 10.1128/spectrum.00998-22
Yan, S., Hui, Y., Li, J., Xu, X., Li, Q., and Wei, H. (2020). Glutamine relieves oxidative stress through PI3K/Akt signaling pathway in DSS-induced ulcerative colitis mice. Iran. J. Basic Med. Sci. 23, 1124–1129. doi: 10.22038/ijbms.2020.39815.9436
Yan, W., Jiang, M., Hu, W., Zhan, X., Liu, Y., Zhou, J., et al. (2023). Causality investigation between gut microbiota, derived metabolites, and obstructive sleep apnea: a bidirectional Mendelian randomization study. Nutrients 15:4544. doi: 10.3390/nu15214544
Yavorska, O. O., and Burgess, S. (2017). MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46, 1734–1739. doi: 10.1093/ije/dyx034
Keywords: insomnia, neurodegenerative diseases, Alzheimer’s disease, Parkinson’s disease, Mendelian randomization
Citation: Liu C, Wang C, Jiang J, Bo Y, Nan L, Zhang Y, Zhu K, Wang X, Feng X, Lian X and Qin S (2025) Role of metabolic characteristics in the co-occurrence of insomnia, Alzheimer’s disease, and Parkinson’s disease: a Mendelian randomization study. Front. Aging Neurosci. 16:1436171. doi: 10.3389/fnagi.2024.1436171
Received: 21 May 2024; Accepted: 25 November 2024;
Published: 06 January 2025.
Edited by:
Chao Luo, Shanghai Mental Health Center, ChinaCopyright © 2025 Liu, Wang, Jiang, Bo, Nan, Zhang, Zhu, Wang, Feng, Lian and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyang Lian, bGlhbnhpYW95YW5nNjY2NkAxNjMuY29t; Shan Qin, cWluc2hhbm5vMUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.