- 1Wushu College, Shanghai Sport University, Shanghai, China
- 2Xiamen Medical College, Xiamen, China
- 3School of Psychology, Shanghai Sport University, Shanghai, China
Aim: To identify optimally therapeutic exercise interventions for improving motor ability among patients with Parkinson’s disease (PD), we conducted a network meta-analysis (NMA) of randomized controlled trials comparing different exercise regimens.
Methods: Relevant RCTs were retrieved by searching PubMed, Embase, Cochrane, Web of Science, CINAHL, CBM, China National Knowledge Infrastructure (CNKI), Wan fang, VIP, and other databases from inception to July 9, 2023 is available in English as the primary language. Exercise outcomes as measured by Movement Disorder Society- Unified Parkinson’s Disease Rating Scale Part III (MDS-UPDRS-III) score change were evaluated and ranked using STATA software version 18.0. All included studies were assessed for methodological quality using the Cochrane Risk of Bias tool.
Results: The final NMA included 71 studies involving 3,732 participants, 87 intervention experiments, and 27distinct interventions. Although most exercise interventions showed some efficacy (reducing MDS-UPDRS-III score), cumulative ranking probability surface (SUCRA) values indicated that the best exercise interventions for motor function improvement were archery (95.6%), riding a bicycle (80.9%), and binary rhythm dance (80.8%).
Conclusion: An exercise intervention comprising archery, cycling, and(or) binary rhythm dance may yield superior improvements in motor function among patients with Parkinson’s disease.
1 Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized neuropathologically by the degeneration of dopaminergic neurons and behaviorally by progressive deterioration of motor function and eventually of cognitive capacity. It is the second most common chronic neurodegenerative disease of the central nervous system after Alzheimer’s disease (AD). Like AD, PD onset risk increases with age, so as expected prevalence is rising globally due to population aging, and in particular the number and proportion of individuals aged 65 or older (Hirsch et al., 2016). Current projections suggest that the global prevalence of PD patients will exceed 12 million by the year 2040 (Dorsey and Bloem, 2018). In addition to the aged, a growing number of individuals under the age of 50 are being diagnosed with early-onset PD (Dorsey et al., 2018). The typical clinical manifestations of early- to intermediate-stage PD are bradykinesia, rigidity, reduced range of motion, and diminished automaticity (Mirelman et al., 2019), motor deficits that substantially reduce functional independence and quality of life.
The first-line treatment for PD is dopamine replacement therapy. While dopaminergic medications can improve motor functions such as walking speed and stride length, prolonged therapy often leads to motor fluctuations, dyskinesia (Albani et al., 2014), and non-motor symptoms like hallucinations and impulsive compulsive behaviors (Weintraub et al., 2022). Surgical interventions, such as deep brain stimulation (DBS) may also accelerate the initiation of motor responses and ameliorate functional disturbances, but electrode implantation carries risks of infection, rejection and poor or suboptimal stimulus targeting. Moreover, no current treatment can prevent or alleviate non-motor symptoms and disease progression.
The development of therapies that can augment or replace drug and surgical interventions is a major focus of current PD research. Such studies have reported that exercise, physical rehabilitation, psychological interventions, and caregiving can be cost-effective and feasible adjunct therapies with long-term adherence. In fact, cross-sectional, longitudinal observational, and prospective interventional trials support exercise therapy as more effective for addressing motor symptoms than current pharmacological approaches (Dutta et al., 2022; Foster et al., 2013; Gamborg et al., 2022; Schenkman et al., 2018). However, these improvements are not always observed, potentially due to variations in cognitive engagement, the severity of the disease, and the specific exercises used (Canning et al., 2012; Skidmore et al., 2008). In addition, inconsistencies in outcome may reflect the use of different evaluation metrics. In 2001, the Movement Disorder Society (MDS) commissioned a revision of the Unified Parkinson’s Disease Rating Scale (UPDRS) initially developed in the 1980s. This new version, called the MDS-UPDRS, was further revised in 2008 to enhance its assessment capabilities. It has since become a widely utilized clinical rating scale for comprehensively evaluating various symptoms and complications of PD (Goetz et al., 2007; Ramaker et al., 2002). Among other advantages, this widespread application of the MDS-UPDRS may improve consistency across studies and thereby enhance the feasibility of pooled data analyses (e.g., meta-analyses).
Network meta-analysis (NMA) is a versatile technique for simultaneously comparing multiple interventions (e.g., A vs. B, B vs. C) from individual studies (Lu and Ades, 2004). Moreover, by combining direct and indirect comparisons, NMA techniques can rank the relative efficacies of multiple interventions for selecting the optimal regimen (Garcia-Ruiz et al., 2014). In the current NMA, the MDS-UPDRS was selected as the outcome measure and various exercise interventions evaluated in randomized controlled trials (RCTs) were systematically ranked according to the improvement (decrease) in MDS-UPDRS score post-intervention. The first part of the MDS-UPDRS addresses “non-motor experiences of daily living,” the second “motor experiences of daily living,” the third part remains dedicated to “motor examination,” and the fourth part focuses on “motor complications.” The third part, MDS-UPDRS-III (motor examination) has demonstrated high reliability, validity, and sensitivity to change following treatment, with an assessment time of less than 15 min (Goetz et al., 2008). Therefore, MDS-UPDRS-III is particularly useful for evaluating the efficacies of specific exercise interventions. The exercise interventions examined in the current NMA include treadmill training, stretch training, aerobic exercise, aquatic exercise, balance and gait training, dual-task training, dance (e.g., tango, waltz, Irish dance, Sardinian dance, folk dance, different rhythm-based dance therapies), qigong practices (e.g., Eight Brocades, Five Animal Frolics, Six Healing Sounds), Tai Chi, mindfulness meditation, resistance exercises (e.g., weightlifting, resistance band exercises, progressive resistance exercise), exercise games, rock climbing deficits. While many systematic reviews and meta-analyses have promoted the effectiveness of various physical therapies in PD, most have included non-randomized controlled trials or lacked quantitative analysis. Moreover, these reviews often compared non-pharmacological, physical interventions with placebos, waitlists, or standard treatments, providing insufficient evidence for ranking by efficacy. Therefore, our objective is to systematically review previous RCTs, on diverse exercise interventions for PD, reevaluate the efficacy of each by pooled analysis, and rank exercises by efficacy to provide clinicians and patients with evidence-based selection criteria.
2 Methods
2.1 Eligibility criteria and literature search
PubMed, Embase, Cochrane, Web of Science, CINAHL, CBM, China National Knowledge Infrastructure (CNKI), Wanfang, VIP, and other databases were searched from inception to July 9, 2023, without language restrictions. Search strings included a combination of Medical Subject Headings (MeSH terms or Emtree terms) and free-text terms related to PD (“Parkinson’s disease”, “idiopathic Parkinson’s disease”, “Lewy body dementia”, “tremor paralysis”), exercise intervention (“aerobic exercise”, “strength training”, “balance exercise”, “balance”, “dual-task training”, “stretching exercise”, “Tai Chi”, “Five Animal Frolics”, “Eight Brocades”, “qigong”, “yoga”, “dance”, “boxing”, “resistance training”, “aquatic exercise”) and RCTs (“randomized controlled trial”, “random control”, “placebo”).The MeSH terms and free words were linked by “OR” within each group, and the groups were linked by “AND” for the search. Study selection, data gathering, and reporting were conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement and the Cochrane Collaboration extension statement (Hutton et al., 2015).
2.2 Study selection criteria
Inclusion criteria were based on the PICO (Participant, Intervention, Outcome, Study Design) guideline as follows: (1) Participants were early-to mid-stage Parkinson’s disease classified according to the Hoehn and Yahr (H&Y) scale (Stages I–III); (2) The Intervention was exercise training; (3) Outcome was change in MDS-UPDRS-III score; (4) Study Design was RCT available in English as the primary language.
Studies were excluded if participants had other neurological disorders, the primary outcome measure was not the MDS-UPDRS-III, there was no randomly selected control group, or if only a single acute training event was examined. In addition, feasibility, effectiveness, and pilot studies were excluded, as were study protocols.
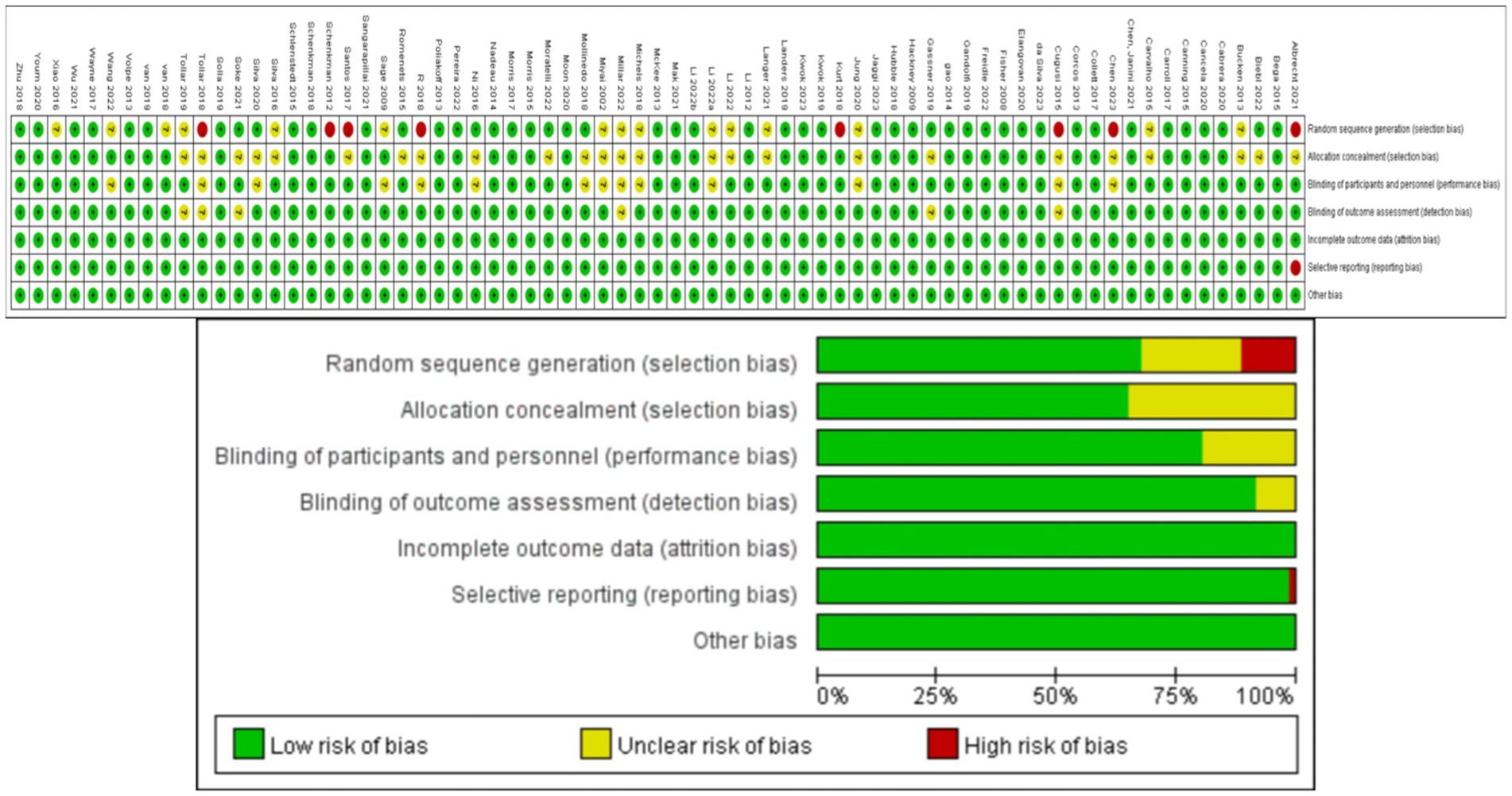
Two authors (ZHF and ZL) independently screened titles, abstracts, and full texts for potential inclusion, and discussed disagreements until reaching a consensus. Data was extracted by the first author (ZHF), and extracted parameters included including participant characteristics (sample size, age, disease duration, Hoehn and Yahr stage, MDS-UPDRS-III scores at baseline and post-intervention), medication status (ON or OFF) during the trial, and type, frequency, and duration, of the exercise intervention. In addition to published RCTs retrieved from the aforementioned literature databases, reports on ongoing or upcoming trials were retrieved from the U.S. National Library of Medicine ClinicalTrials.gov and the Chinese Clinical Trial Registry. Grey literature was also considered. Finally, the reference lists of included articles were searched for eligible studies. Six potential sources of bias (risk of bias, RoB) were assessed for each RCT using the revised Cochrane Collaboration Tool (Abraha and Montedori, 2010): (1) bias from the randomization process, (2) deviation from intended interventions, (3) missing outcome data, (4) bias from the outcome measurement, (5) selective outcome reporting, and (6) overall bias. During this process, the first author (ZHF) independently screened the articles and any discrepancies were resolved by discussion with a third researcher (LL) until consensus was achieved. The risk for each primary source of bias was defined as either “low,” “medium,” or “high” for each trial, and a color-coded risk of bias table was constructed (Figure 1).The risk assessment for each trial was independently entered into Review Manager (RevMan 5.4), generating a summary of bias risk alongside the meta-analysis results.
3 Statistical analysis
The NMA was conducted using STATA 18.0, and the Frequentist framework was employed following the PRISMA NMA guidelines. For all eligible RCTs, the post-intervention mean MDS-UPDRS-III score (with standard deviation) was retrieved for comparison across studies. To depict all available effects for each exercise intervention, a network evidence graph was generated as a concise summary (Figure 2A). In the network graph, nodes represent exercise interventions, node size is proportional to the total number of participants in the studies, the connecting edges between nodes indicate direct pairwise comparisons, and edge is indicative of effect magnitude.
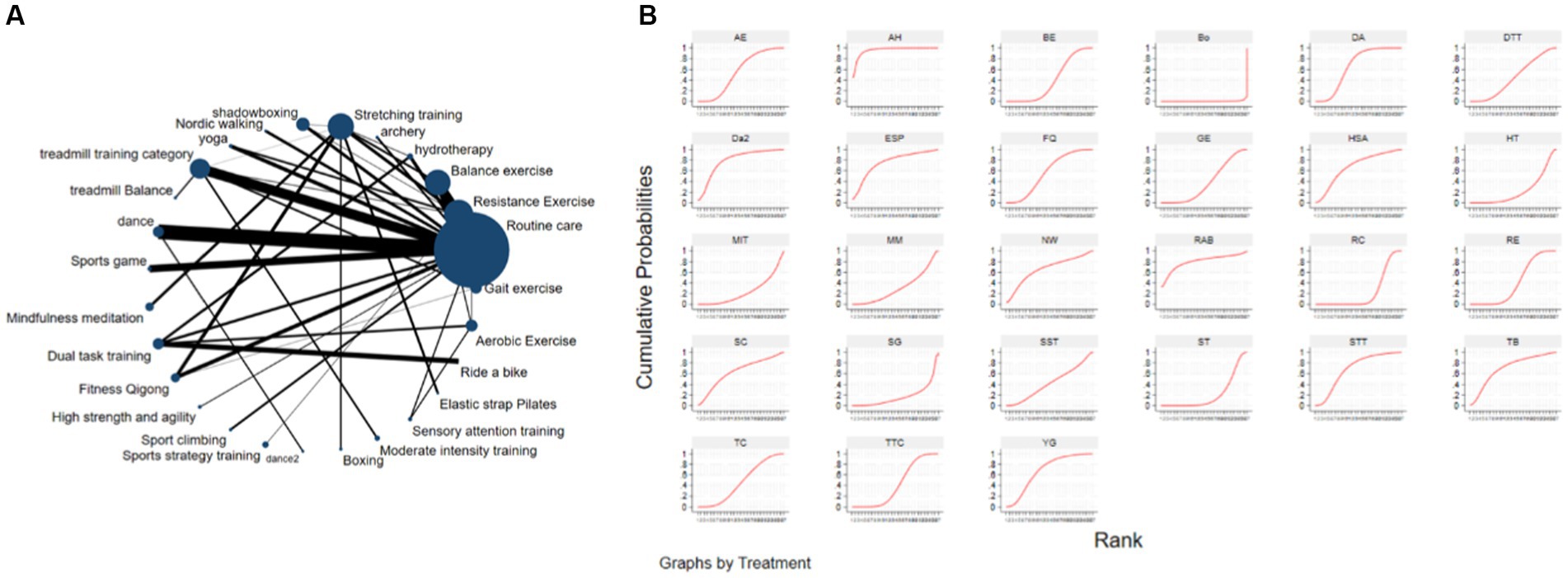
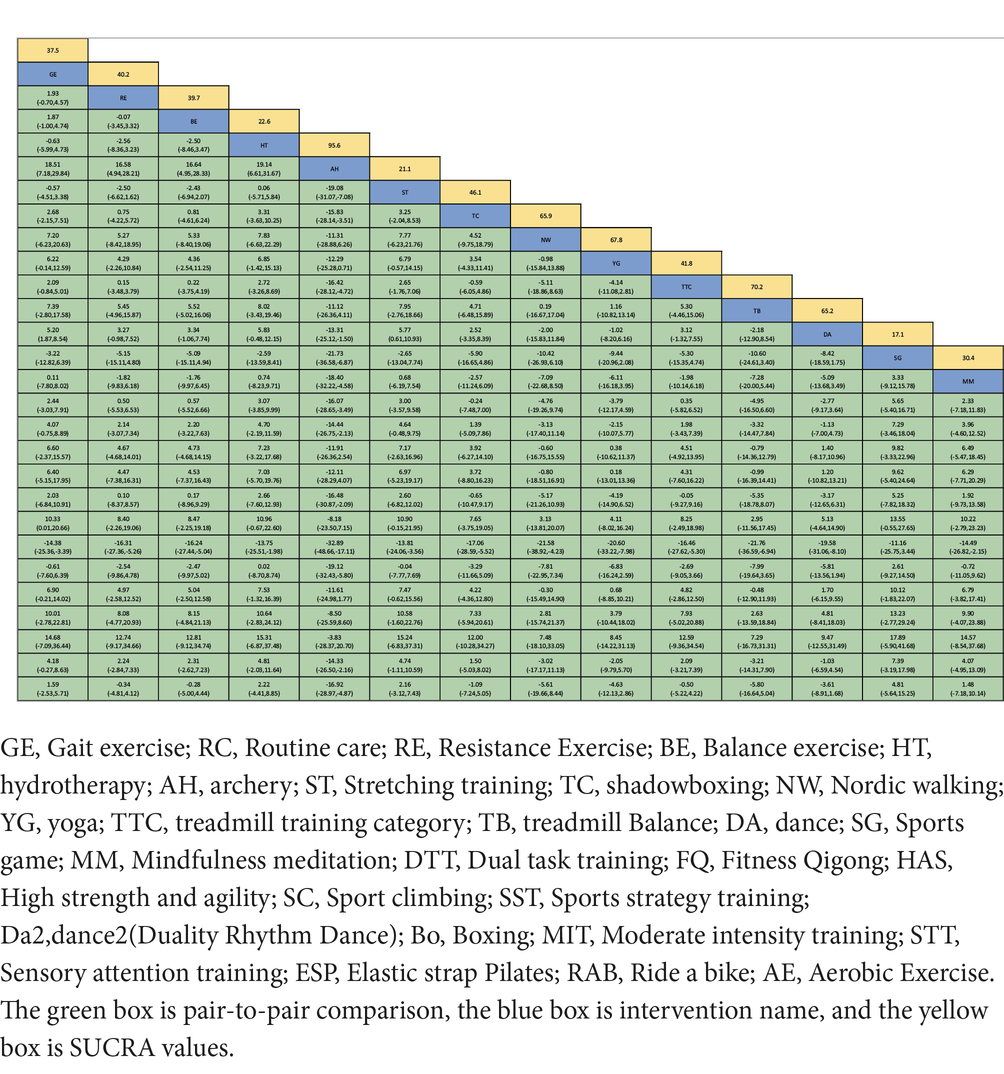
Figure 2. The NMA figure for MDS-UPDRS-III (A). The SUCRA plot for MDS-UPDRS-III (B). GE, Gait exercise; RC, Routine care; RE, Resistance Exercise; BE, Balance exercise; HT, hydrotherapy; AH, archery; ST, Stretching training; TC, shadowboxing; NW, Nordic walking; YG, yoga; TTC, treadmill training category; TB, treadmill Balance; DA, dance; SG, Sports game; MM, Mindfulness meditation; DTT, Dual task training; FQ, Fitness Qigong; HAS, High strength and agility; SC, Sport climbing; SST, Sports strategy training; Da2, dance2(Duality Rhythm Dance); Bo, Boxing; MIT, Moderate intensity training; STT, Sensory attention training; ESP, Elastic strap Pilates; RAB, Ride a bike; AE, Aerobic Exercise.
The Surface Under the Cumulative RAnking (SUCRA) curve (Figure 2B) is a simple numerical statistic indicating the cumulative ranking probability for each intervention, and serves as an metric for grading the superiority or inferiority of exercise interventions (Page et al., 2016). Specifically, a larger SUCRA value indicates a greater likelihood that a particular exercise intervention is highly ranked (relatively more effective), while a lower value suggests that the intervention is likely less effective. We examined global consistency and employed the node-splitting model to assess local consistency. A p > 0.05 indicated no significant inconsistency between direct and indirect comparisons, and a consistency model was used. Otherwise, an inconsistency model was employed.
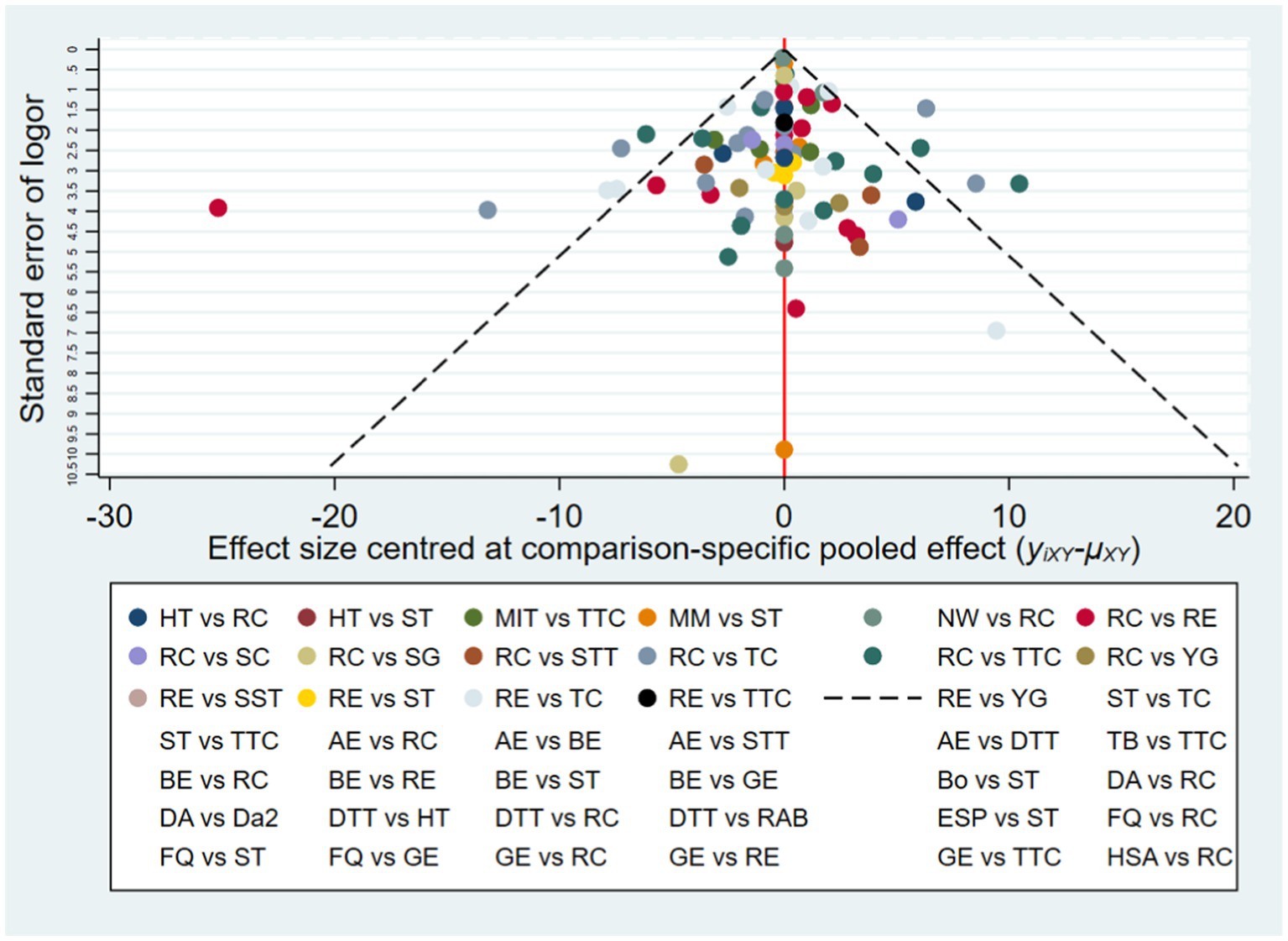
To detect the presence of publication bias, selective reporting, and other biases, we constructed a funnel plot (Figure 3) (Egger et al., 1997). The funnel plot is a simple scatter plot that reflects the estimated intervention effect of a single study with a certain sample size or precision. The distribution width and symmetry are indicative of study heterogeneity and publication bias, respectively. The advantage of the funnel plot is that it is intuitive, as relative differences in effect size can be observed directly.
4 Results
4.1 Study identification
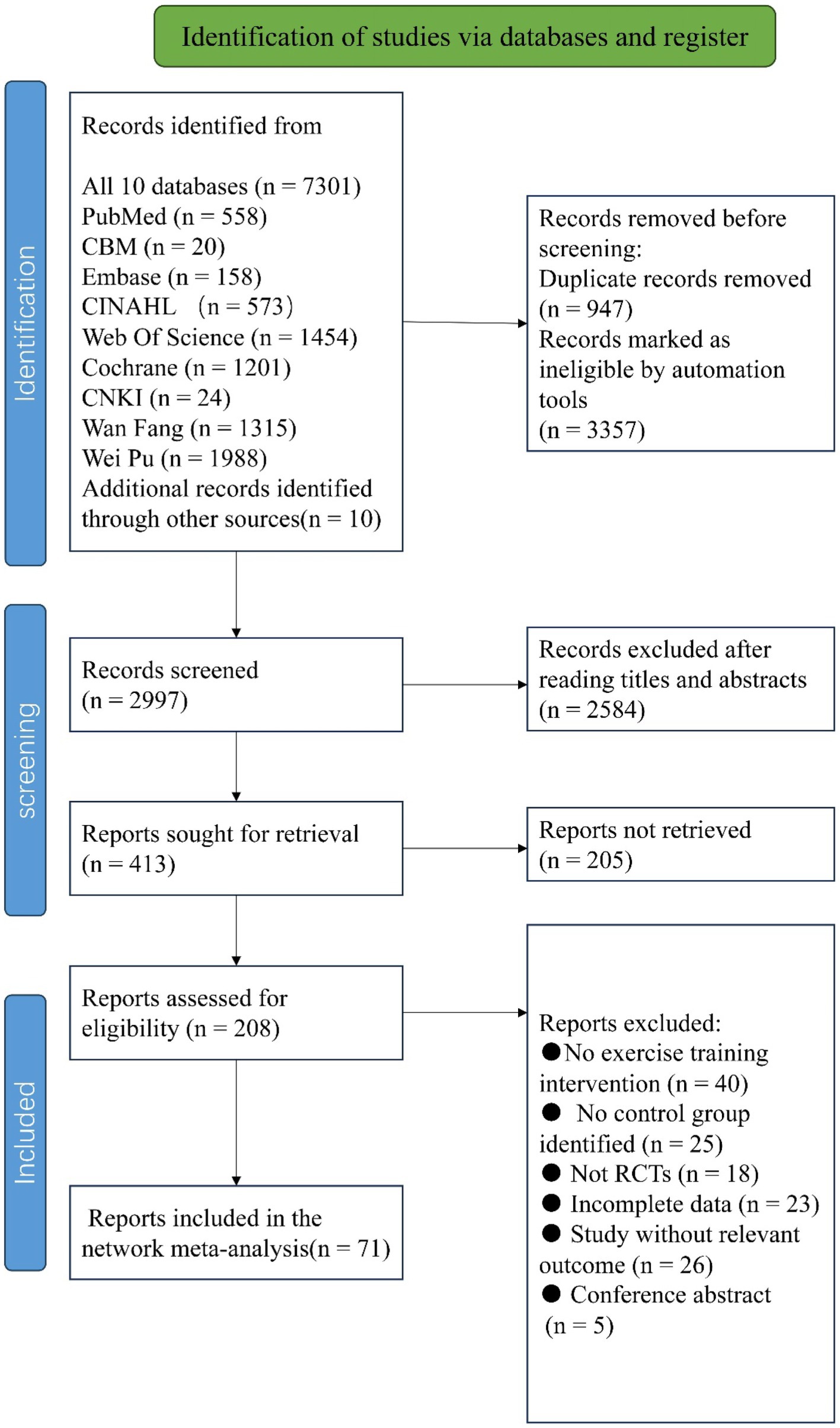
A total of 7,301 articles were retrieved using the pre-established search strategy. After excluding duplicates and for other reasons, the remaining 2,997 articles were screened based on titles and abstracts. Subsequently, 2,584 articles were excluded as irrelevant, and the remaining 413 were subjected to a full-text review. Of these, 342 were excluded as non-randomized controlled trials, for incomplete data, as conference papers, or for non-compliance with intervention measures, among others reasons (Figure 4). Ultimately, 71 articles were included in the NMA (summarized in Tables 1–3).
4.2 Study characteristics
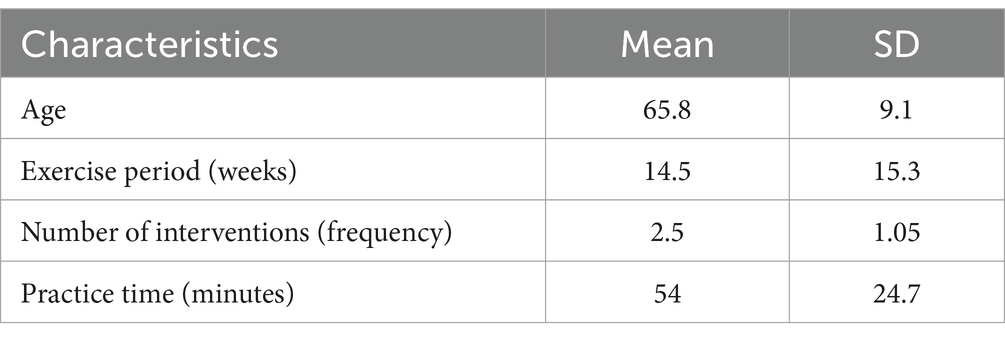
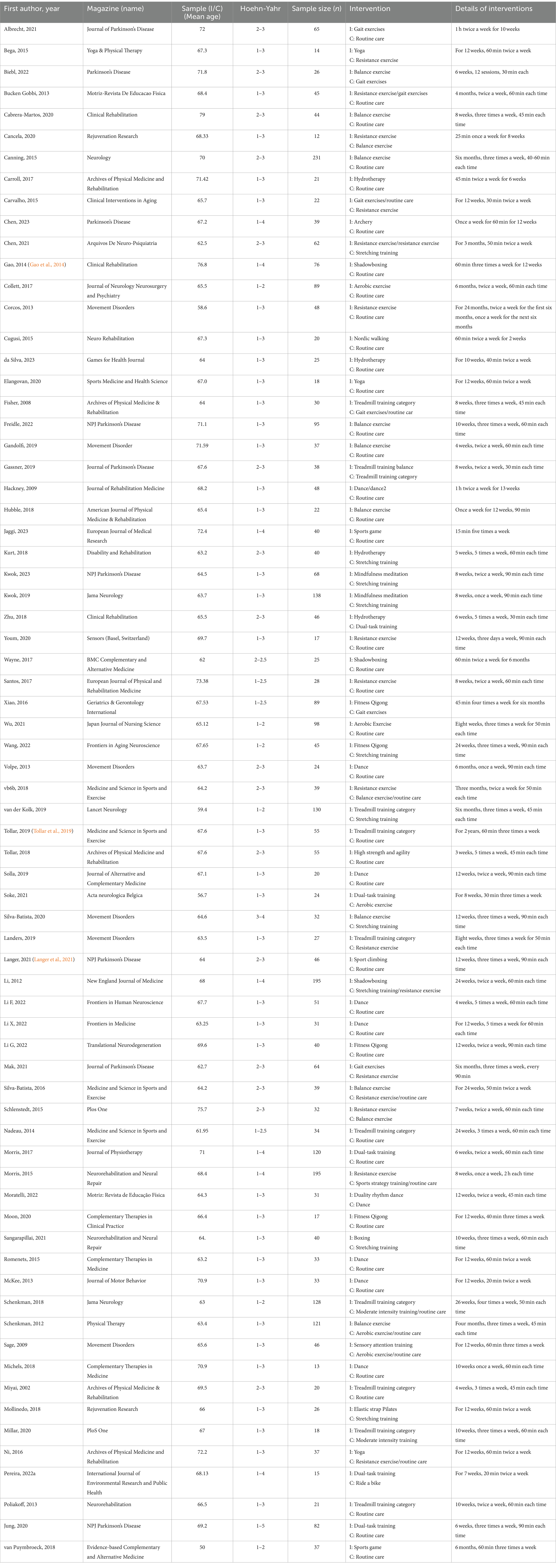
These 71 RCTs were published between 2002 and 2023, and involved a total of 3,732 participants. The NMA included a total of 87 intervention experiments and 27 distinct interventions: gait exercises (GE, such as gait posture interventions and aerobic walking), routine care: (RC), resistance exercises (RE, such as weightlifting, resistance band exercises, and strength training), balance exercises (BE, such as stability exercises and balance training), hydrotherapy (HT, aquatic exercises), archery (AH), stretching exercises (ST, such as limb and joint stretching), Tai Chi (TC), Nordic walking (NW), yoga(YG), treadmill training (TTC, high-intensity, moderate-intensity, or low-intensity), treadmill balance (TB, treadmill walking combined with balance interventions), dance (DA, including tango, Irish dance, improvised dance, waltz), sports games (VR, sports games), mindfulness meditation (MM), dual-task training (DTT), fitness qigong (FQ, Six-character Formula, Five Animal Frolics, Eight Brocades), high-intensity agility training (HAS), sport climbing(SC), sport strategy training(SST), dance 2 (Da2, binary rhythmic dance), boxing (BO), moderate-intensity training (MIT), sensory attention training (STT), elastic band Pilates (ESP), riding a bicycle (RAB), and aerobic exercise (AE). In the included studies, most exercise interventions were compared to routine care, stretching exercises, or aerobic exercises as the control. Among all eligible RCTs, 55 were two-arm (Albrecht et al., 2021; Bega et al., 2015; Biebl et al., 2022; Cabrera-Martos et al., 2020; Cancela et al., 2020; Canning et al., 2015; Carroll et al., 2017; Chen et al., 2023; Collett et al., 2017; Corcos et al., 2013; Cugusi et al., 2015; Da Silva et al., 2023; Elangovan et al., 2020; Freidle et al., 2022; Gandolfi et al., 2019; Gassner et al., 2019; Hubble et al., 2018; Jaggi et al., 2023; Jung et al., 2020; Kurt et al., 2018; Kwok et al., 2023; Kwok et al., 2019; Landers et al., 2016; Landers and Navalta, 2019; Li G. et al., 2022; Mak and Wong-Yu, 2021; McKee and Hackney, 2013; Michels et al., 2018; Millar, 2020; Miyai et al., 2002; Mollinedo-Cardalda et al., 2018; Moon et al., 2020; Moratelli et al., 2022; Morris et al., 2017; Ni et al., 2016; Pereira-Pedro et al., 2022a; Poliakoff et al., 2013; Romenets et al., 2015; Sangarapillai et al., 2021; Santos et al., 2017; Schlenstedt et al., 2015; Silva-Batista et al., 2020; Soke et al., 2021; Solla et al., 2019; van der Kolk et al., 2019; Van Puymbroeck et al., 2018; Volpe et al., 2013; Wang et al., 2022; Wayne et al., 2017; Wu et al., 2021; Xiao and Zhuang, 2016; Youm et al., 2020; Zhu et al., 2018; vb6b, 2018) and 16 were three-arm (Bucken Gobbi et al., 2013; Carvalho et al., 2015; Chen et al., 2021; Fisher et al., 2008; Hackney and Earhart, 2009; Li et al., 2012; Li F. et al., 2022; Li X. et al., 2022; Morris et al., 2015; Nadeau et al., 2014; Sage and Almeida, 2009; Schenkman et al., 2012; Schenkman et al., 2018; Silva-Batista et al., 2016; Tollar et al., 2018). The exercise intervention period for included trials ranged from 4 to 96 weeks (average 14.5 weeks, SD 15.3 weeks), the frequency of exercise intervention from 1 to 5 sessions per week (average 2.5, SD 1.05), and the total time per session from 15 to 120 min (average 54 min, SD 24.7 min).
4.3 Quality assessment
Methodological quality assessment results for the eligible RCTs are depicted in Figure 1. While overall quality was high, 8 trials did not mention random sequence generation or blinding, and one trial reported incomplete results. These trials were classified as “medium risk”. Additionally, 23 trials mentioned randomization and blinding but did not provide specific details. These trials were classified as “low risk”.
4.4 Network meta-analysis for efficacy ranking
Figure 2A depicts the network diagram of different exercise interventions for the MDS-UPDRS-III. The overall network structure indicates numerous comparisons between routine care (control) and dance, stretching training, balance exercise, and resistance exercises, as these interventions are currently popular. Also indicated are numerous pair-wise comparisons between interventions from three-arm trials. Surface Under the Cumulative Ranking (SUCRA) curves for each of the 27 intervention type are shown in Figure 2B (derived from Table 2). In these curves (red lines), a sharp early increase yields a larger area and indicates a greater probability of improving motor ability (higher efficacy rank), whilst a shallow, later increase yields a smaller area and is indicative of lower probability of motor improvement (lower efficacy rank). According to these analyses, archery ranked first (SUCRA = 95.6%), followed by bike riding (SUCRA = 80.9%), duality rhythm dance (SUCRA = 80.8%), elastic strap Pilates (SUCRA = 76.9%), sensory attention training (SUCRA = 70.7%), treadmill balance training (SUCRA = 70.2%), yoga (SUCRA = 67.8%), high-intensity strength and agility training (SUCRA = 67.2%), resistance exercise (SUCRA = 40.2%), and balance exercise (SUCRA = 39.7%). The efficacies of these exercise interventions for reducing MDS-UPDRS-III score were higher than stretching training (SUCRA = 21.1%) and routine care (SUCRA = 22.6%).
Further, archery was significantly superior to routine care (standardized mean difference (SMD = −16.92, 95%CI = −28.97, −4.87), stretch training (SMD = −19.08, 95%CI = −31.07, −7.08), sports games (SMD = −21.73, 95%CI = −36.58, −6.87), and aerobic exercise (SMD = −14.33, 5%CI = −26.50, −2.16) for improving motor abilities in Parkinson’s disease. Overall, boxing was the least effective as MDS-UPDRS-III score was not reduced post-intervention (SUCRA = 0.7%, Sangarapillai et al., 2021). All other interventions were superior to boxing.
4.5 Efficacy ranking
The cumulative ranking probability according to SUCRA graphs was as follows: Archery > Ride a bike > Duality Rhythm Dance > Elastic strap Pilates > Sensory attention training > Treadmill > Balance > Yoga > High strength and agility > Nordic walking > Dance > Sport climbing > Aerobic Exercise > Fitness Qigong > Shadowboxing > Dual task training > Sports strategy training > Treadmill training category > Resistance Exercise > Balance exercise > Gait exercise > Mindfulness meditation > Moderate intensity training > Routine care > Hydrotherapy > Stretching training > Sports game > Boxing.
4.6 Consistency analysis
The global inconsistency analysis p-value was 0.2170, indicating no significant inconsistency. Additionally, the node-splitting model analysis yielded p-values >0.05, indicating no significant inconsistency between direct and indirect comparisons, supporting adoption of a consistency model.
4.7 Publication bias
Publication bias for the outcome measure (MDS-UPDRS-III) was further evaluated by constructing a funnel plot with relative effect size (odds ratio, OR) on the horizontal axis and standard error of log (OR) on the vertical axis, and then examining plot dispersion and symmetry (Figure 3). This contrast yields narrower, higher plots for studies with larger sample sizes and lower, more dispersed plots for studies with smaller sample sizes. The majority of points falling within the 95% confidence intervals (slash lines) is indicative of little or no heterogeneity, while a symmetrical distribution is indicative of little or no publication bias. The points representing individual comparisons (indicated by color code in the lower panel) fell mainly within the 95%CIs and with high symmetry on each side of the 0 point (no effect), suggesting little publication bias.
5 Discussion
The objective of this NMA was to integrate evidence from 71 RCTs (including 87 interventions and 27 different exercises) to identify those with greatest efficacy for improving the motor abilities of PD patients according to MDS-UPDRS-III score reduction. Surface Under the Cumulative Ranking curve analysis indicated that archery (Chen et al., 2023; Radder et al., 2020) is the most effective intervention for reducing MDS-UPDRS-III scores and improving motor abilities (Chen et al., 2023; Radder et al., 2020), surpassing the efficacy of all other exercises tested (SUCRA = 95.6%), followed by bicycling (Pereira-Pedro et al., 2022b) and duality rhythm dance. This particular dance form, characterized by binary rhythm movements distinct from traditional dance categories such as tango, waltz, Irish dance, and self-created free dance, proved surprisingly effective (SUCRA = 80.8%), providing clues to the precise activity patterns (e.g., muscle groups engaged and contraction–relaxation dynamics) most beneficial for improving motor abilities in PD. Previous studies have reported significant improvements in motor abilities following exercise interventions such as dance, dual-task training, and high-intensity resistance training (Wang et al., 2023; Zhou et al., 2022), and these interventions were also relatively effective according to the current NMA. However, many previous meta-analyses and reviews grouped distinct exercise interventions into a single category, such as “martial arts” for Tai Chi, fitness Qigong, or boxing (Radder et al., 2020). Although this grouping increased statistical power, it did not identify the best specific intervention, and as demonstrated here, there were marked differences in therapeutic efficacy among these interventions. The current analysis thus provides precise information for selecting the most appropriate exercise intervention.
Archery has long been regarded an ideal rehabilitative activity, and was one of the first exercises introduced for the rehabilitation of paralysis and limb palsy patients (Guttmann and Mehra, 1973). Archery involves the activation of trunk latissimus dorsi and serratus anterior muscles, along with the stretching of the palm, finger muscles, and wrist. Participants must mentally focus on specifically ordered steps, from hooking the bowstring with their fingers to releasing the arrow by activating and relaxing various muscle groups in precise sequences, thereby providing opportunities for both strength and coordination enhancement. The practice of archery also provides a definitive metric for success (target hits), thus motivating regular participation (regular upper limb functional exercises) and performance improvement. Indeed, regular archery is reported to improve overall body stability and even non-motor symptoms (Chen et al., 2023). However, there have been a limited number of RCTs applying archery as an intervention for PD, so additional trials are required to confirm these findings. Further, as compliance will improve efficacy, additional studies are needed to compare the effects of exercises matched for weekly frequency, intensity, and total duration.
Riding a bike, the second most effective exercise choice for PD patients (SUCRA = 80.9%), can improve cardiovascular health, motor skills, coping, and cognitive skills as well as provide a sense of independence and promote social inclusion (Alberts et al., 2011; Ridgel et al., 2013; Tiihonen et al., 2021). Low-intensity progressive cycling improved motor dysfunction (Chang et al., 2018) while high-intensity cycling improved motor function, stiffness, and bradykinesia by promoting activity-dependent neuroplasticity (Feng et al., 2020; Oliveira de Carvalho et al., 2018). Forced passive cycling was also reported to enhance functional connectivity between the motor cortex and ipsilateral thalamus and between the subthalamic nucleus and posterior cingulate (Shah et al., 2016), consistent with improvements in motor control via neuroplasticity within sensorimotor pathways. Moreover, the motor improvements conferred by regular cycling may involve enhanced processing of proprioceptive inputs by sensory cortex (Nagano-Saito et al., 2005).
Dance is another exercise intervention widely used as therapy for PD patients, and consistent with previous reports of improved motor abilities, the SUCRA value was among the highest (80.8%). The benefits of dance likely stem from the multifaceted nature of the activity, requiring movement control (fluidity) and appropriate posture, potentially addressing PD-related deficits such as stiffness, bradykinesia, and postural instability (Hashimoto et al., 2015; Sharp and Hewitt, 2014; Šumec et al., 2015). Dance is also highly enjoyable, aiding in compliance (Earhart, 2009). At the neural level, dance stimulates basal ganglia circuits and reward systems to evoke positive emotions (Weintraub et al., 2005).
This NMA has several limitations. First, it included only early- to mid-stage PD patients (average Hoehn-Yahr stage of 1–3), so results may not be applicable to more advanced PD patients. There was also substantial heterogeneity in the frequency and duration of exercise interventions across trials, which could influence efficacy independent of the specific exercise used. Third, despite a comprehensive search, all included studies were in English, which may introduce culture bias against other exercise practices. Many pair-wise comparisons also included only a few individual trials, limiting the statistical power. Although all participants included in the analysis were in the early and middle stages of PD, the MDS-UPDRS-III scores varied markedly, likely reflecting the subjective nature of the assessment and inter-rater variability.
Last, most studies did not report concealed allocation, which may result in selection and performance biases. Large-scale RCTs comparing multiple exercise modalities matched for intensity and duration, and with appropriate safeguards against bias are needed to confirm the rankings presented here.
6 Conclusion
To the best of our knowledge, this study is the first to compare a large number of distinct exercise modalities (n = 27) for efficacy in improving motor function among patients with early- to middle-stage PD. A series of direct and indirect comparisons using NMA and SUCRA methods identified archery, cycling and dual rhythm dance as particularly effective for improving MDS-UPDRS-III scores, while others such as boxing and sports gameplay were largely ineffective. Although larger-sample, multi-arm trials are required for validation, the current findings may serve as a useful guide for healthcare providers when selecting exercise interventions to enhance the motor abilities, quality of life, and cardiovascular health status of individuals with PD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZH: Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. ZL: Investigation, Project administration, Supervision, Writing – original draft. LL: Conceptualization, Data curation, Methodology, Writing – review & editing. GR: Formal analysis, Project administration, Visualization, Writing – original draft. HY: Software, Validation, Writing – review & editing. WZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the “Zhipa is not afraid, join hands in common Martial Arts”—“Parkinson’s Rehabilitation Exercise Camp” popular science activity project of China Martial Arts Museum, the Shanghai Science and Technology Commission (20DZ2300900), and the project “Research on proprioceptive neural mechanism of ankle joint that restricts motor balance function in elderly people over 75 years old” supported by the National Natural Science Foundation (Project No. 31870936).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abraha, I., and Montedori, A. (2010). Modified intention to treat reporting in randomised controlled trials: systematic review. BMJ 340:c2697. doi: 10.1136/bmj.c2697
Albani, G., Cimolin, V., Fasano, A., Trotti, C., Galli, M., and Mauro, A. (2014). "Masters and servants" in parkinsonian gait: a three-dimensional analysis of biomechanical changes sensitive to disease progression. Funct. Neurol. 29, 99–105.
Alberts, J. L., Linder, S. M., Penko, A. L., Lowe, M. J., and Phillips, M. (2011). It is not about the bike, it is about the pedaling: forced exercise and Parkinson's disease. Exerc. Sport Sci. Rev. 39, 177–186. doi: 10.1097/JES.0b013e31822cc71a
Albrecht, F., Pereira, J. B., Mijalkov, M., Freidle, M., Johansson, H., Ekman, U., et al. (2021). Effects of a highly challenging balance training program on motor function and brain structure in Parkinson's disease. J. Parkinsons Dis. 11, 2057–2071. doi: 10.3233/JPD-212801
Bega, D., Corcos, D., Stein, J., Victorson, D., Zadikoff, C., Jovanovic, B., et al. (2015). Yoga versus resistance training in Parkinson's disease: a 12-week pilot feasibility study [journal article; conference proceeding]. Mov. Disord. 30, S69–S70.
Biebl, J. T., Azqueta-Gavaldon, M., Wania, C., Zettl, O., Woiczinski, M., Bauer, L., et al. (2022). Resistance training combined with balance or gait training for patients with Parkinson’s disease: a randomized controlled pilot study. Parkinsons Dis. 2022, 9574516–9574517. doi: 10.1155/2022/9574516
Bucken Gobbi, L. T., Teixeira-Arroyo, C., Lirani-Silva, E., Vitorio, R., Barbieri, F. A., and Pereira, M. P. (2013). Effect of different exercise programs on the psychological and cognitive functions of people with Parkinson's disease [article]. Motriz 19, 597–604. doi: 10.1590/S1980-65742013000300010
Cabrera-Martos, I., Jimenez-Martin, A. T., Lopez-Lopez, L., Rodriguez-Torres, J., Ortiz-Rubio, A., and Valenza, M. C. (2020). Effects of a core stabilization training program on balance ability in persons with Parkinson's disease: a randomized controlled trial [article]. Clin. Rehabil. 34, 764–772. doi: 10.1177/0269215520918631
Cancela, J. M., Mollinedo, I., Montalvo, S., and Vila Suarez, M. E. (2020). Effects of a high-intensity progressive-cycle program on quality of life and motor symptomatology in a Parkinson's disease population: a pilot randomized controlled trial [article]. Rejuvenation Res. 23, 508–515. doi: 10.1089/rej.2019.2267
Canning, C. G., Allen, N. E., Dean, C. M., Goh, L., and Fung, V. S. (2012). Home-based treadmill training for individuals with Parkinson's disease: a randomized controlled pilot trial. Clin. Rehabil. 26, 817–826.
Canning, C. G., Sherrington, C., Lord, S. R., Close, J. C. T., Heritier, S., Heller, G. Z., et al. (2015). Exercise for falls prevention in Parkinson disease a randomized controlled trial [article]. Neurology 84, 304–312. doi: 10.1212/WNL.00000000000001155
Carroll, L. M., Volpe, D., Morris, M. E., Saunders, J., and Clifford, A. M. (2017). Aquatic exercise therapy for people with Parkinson disease: a randomized controlled trial [article]. Arch. Phys. Med. Rehabil. 98, 631–638. doi: 10.1016/j.apmr.2016.12.006
Carvalho, A., Barbirato, D., Araujo, N., Martins, J. V., Sa Cavalcanti, J. L., Santos, T. M., et al. (2015). Comparison of strength training, aerobic training, and additional physical therapy as supplementary treatments for Parkinson's disease: pilot study [article]. Clin. Interv. Aging 10, 183–191. doi: 10.2147/CIA.S68779
Chang, H. C., Lu, C. S., Chiou, W. D., Chen, C. C., Weng, Y. H., and Chang, Y. J. (2018). An 8-week low-intensity progressive cycling training improves motor functions in patients with early-stage Parkinson's disease. J. Clin. Neurol. 14, 225–233. doi: 10.3988/jcn.2018.14.2.225
Chen, J., Chien, H. F., Valente Francato, D. C., Barbosa, A. F., Souza, C. D. O., Voos, M. C., et al. (2021). Effects of resistance training on postural control in Parkinson's disease: a randomized controlled trial [article]. Arq. Neuropsiquiatr. 79, 511–520. doi: 10.1590/0004-282x-anp-2020-0285
Chen, C.-Y., Wang, W.-N., Lu, M.-K., Yang, Y.-W., Yu, T., Wu, T.-N., et al. (2023). The rehabilitative effect of archery exercise intervention in patients with Parkinson's disease [article]. Parkinsons Dis. 2023:9175129. doi: 10.1155/2023/9175129
Collett, J., Franssen, M., Meaney, A., Wade, D., Izadi, H., Tims, M., et al. (2017). Phase II randomised controlled trial of a 6-month self-managed community exercise programme for people with Parkinson's disease [article]. J. Neurol. Neurosurg. Psychiatry 88, 204–211. doi: 10.1136/jnnp-2016-314508
Corcos, D. M., Robichaud, J. A., David, F. J., Leurgans, S. E., Vaillancourt, D. E., Poon, C., et al. (2013). A two-year randomized controlled trial of progressive resistance exercise for Parkinson's disease [article]. Mov. Disord. 28, 1230–1240. doi: 10.1002/mds.25380
Cugusi, L., Solla, P., Serpe, R., Carzedda, T., Piras, L., Oggianu, M., et al. (2015). Effects of a Nordic walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson's disease [journal article]. NeuroRehabilitation 37, 245–254. doi: 10.3233/NRE-151257
Da Silva, K. G., Nuvolini, R. A., Bacha, J. M. R., De Freitas, T. B., Dona, F., Torriani-Pasin, C., et al. (2023). Comparison of the effects of an Exergame-based program with conventional physiotherapy protocol based on Core areas of the European guideline on postural control, functional mobility, and quality of life in patients with Parkinson's disease: randomized clinical trial [article]. Games Health J. 12, 228–241. doi: 10.1089/g4h.2022.0039
Dorsey, E. R., and Bloem, B. R. (2018). The Parkinson pandemic-a call to action. JAMA Neurol. 75, 9–10. doi: 10.1001/jamaneurol.2017.3299
Dorsey, E. R., Sherer, T., Okun, M. S., and Bloem, B. R. (2018). The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 8, S3–s8. doi: 10.3233/JPD-181474
Dutta, D., Paidi, R. K., Raha, S., Roy, A., Chandra, S., and Pahan, K. (2022). Treadmill exercise reduces α-synuclein spreading via PPARα. Cell Rep. 40:111058. doi: 10.1016/j.celrep.2022.111058
Earhart, G. M. (2009). Dance as therapy for individuals with Parkinson disease. Eur. J. Phys. Rehabil. Med. 45, 231–238
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Elangovan, N., Cheung, C., Mahnan, A., Wyman, J. F., Tuite, P., and Konczak, J. (2020). Hatha yoga training improves standing balance but not gait in Parkinson's disease. Sports Med. Health Sci. 2, 80–88. doi: 10.1016/j.smhs.2020.05.005
Feng, Y. S., Yang, S. D., Tan, Z. X., Wang, M. M., Xing, Y., Dong, F., et al. (2020). The benefits and mechanisms of exercise training for Parkinson's disease. Life Sci. 245:117345.
Fisher, B. E., Wu, A. D., Salem, G. J., Song, J., Lin, C.-H., Yip, J., et al. (2008). The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch. Phys. Med. Rehabil. 89, 1221–1229. doi: 10.1016/j.apmr.2008.01.013
Foster, E. R., Golden, L., Duncan, R. P., and Earhart, G. M. (2013). Community-based argentine tango dance program is associated with increased activity participation among individuals with Parkinson's disease [article]. Arch. Phys. Med. Rehabil. 94, 240–249. doi: 10.1016/j.apmr.2012.07.028
Freidle, M., Johansson, H., Ekman, U., Lebedev, A. V., Schalling, E., Thompson, W. H., et al. (2022). Behavioural and neuroplastic effects of a double-blind randomised controlled balance exercise trial in people with Parkinson's disease. NPJ Parkinson's Dis. 8:12. doi: 10.1038/s41531-021-00269-5
Gamborg, M., Hvid, L. G., Dalgas, U., and Langeskov-Christensen, M. (2022). Parkinson's disease and intensive exercise therapy – an updated systematic review and meta-analysis. Acta Neurol. Scand. 145, 504–528. doi: 10.1111/ane.13579
Gandolfi, M., Tinazzi, M., Magrinelli, F., Buselli, G., Dimitrova, E., Polo, N., et al. (2019). Four-week trunk-specific exercise program decreases forward trunk flexion in Parkinson's disease: a single-blinded, randomized controlled trial [journal article; conference proceeding]. Mov. Disord. 34:S263. doi: 10.1016/j.parkreldis.2019.05.006
Gao, Q., Leung, A., Yang, Y., Wei, Q., Guan, M., Jia, C., et al. (2014). Effects of Tai Chi on balance and fall prevention in Parkinson\u0027s disease: a randomized controlled trial [Article]. Clin Rehabil. 28, 748–753. Doi: doi: 10.1177/0269215514521044
Garcia-Ruiz, P. J., Chaudhuri, K. R., and Martinez-Martin, P. (2014). Non-motor symptoms of Parkinson's disease a review…from the past. J. Neurol. Sci. 338, 30–33. doi: 10.1016/j.jns.2014.01.002
Gassner, H., Steib, S., Klamroth, S., Pasluosta, C. F., Adler, W., Eskofier, B. M., et al. (2019). Perturbation treadmill training improves clinical characteristics of gait and balance in Parkinson's disease [article]. J. Parkinsons Dis. 9, 413–426. doi: 10.3233/JPD-181534
Goetz, C. G., Fahn, S., Martinez-Martin, P., Poewe, W., Sampaio, C., Stebbins, G. T., et al. (2007). Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov. Disord. 22, 41–47.
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., et al. (2008). Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170.
Guttmann, L., and Mehra, N. C. (1973). Experimental studies on the value of archery in paraplegia. Paraplegia 11, 159–165.
Hackney, M. E., and Earhart, G. M. (2009). Effects of dance on movement control in Parkinson\u0027s disease: a comparison of Argentine tango and American ballroom [Journal article]. J Rehabil Med. 41, 475‐481. doi: 10.2340/16501977-0362
Hashimoto, H., Takabatake, S., Miyaguchi, H., Nakanishi, H., and Naitou, Y. (2015). Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson's disease: a quasi-randomized pilot trial [article]. Complement. Ther. Med. 23, 210–219. doi: 10.1016/j.ctim.2015.01.010
Hirsch, M. A., Iyer, S. S., and Sanjak, M. (2016). Exercise-induced neuroplasticity in human Parkinson's disease: what is the evidence telling us? [Journal article]. Parkinsonism Relat. Disord. 22, S78–S81. doi: 10.1016/j.parkreldis.2015.09.030
Hubble, R. P., Naughton, G., Silburn, P. A., and Cole, M. H. (2018). Trunk exercises improve gait symmetry in Parkinson disease: a blind phase II randomized controlled trial [article]. Am. J. Phys. Med. Rehabil. 97, 151–159. doi: 10.1097/PHM.0000000000000858
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162, 777–784. doi: 10.7326/M14-2385
Jaggi, S., Wachter, A., Adcock, M., de Bruin, E. D., Moeller, J. C., Marks, D., et al. (2023). Feasibility and effects of cognitive-motor exergames on fall risk factors in typical and atypical Parkinson's inpatients: a randomized controlled pilot study [article]. Eur. J. Med. Res. 28, 2–3. doi: 10.1186/s40001-022-00963-x
Jung, S. H., Hasegawa, N., Mancini, M., King, L. A., Carlson-Kuhta, P., Smulders, K., et al. (2020). Effects of the agility boot camp with cognitive challenge (ABC-C) exercise program for Parkinson's disease. NPJ Parkinson's Dis. 6:31. doi: 10.1038/s41531-020-00132-z
Kurt, E. E., Buyukturan, B., Buyukturan, O., Erdem, H. R., and Tuncay, F. (2018). Effects of Ai chi on balance, quality of life, functional mobility, and motor impairment in patients with Parkinson's disease [article]. Disabil. Rehabil. 40, 791–797. doi: 10.1080/09638288.2016.1276972
Kwok, J. Y. Y., Choi, E. P. H., Wong, J. Y. H., Lok, K. Y. W., Ho, M. H., Fong, D. Y. T., et al. (2023). A randomized clinical trial of mindfulness meditation versus exercise in Parkinson’s disease during social unrest [journal article]. NPJ Parkinson's Dis. 9:7. doi: 10.1038/s41531-023-00452-w
Kwok, J. Y. Y., Kwan, J. C. Y., Auyeung, M., Mok, V. C. T., Lau, C. K. Y., Choi, K. C., et al. (2019). Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease a randomized clinical trial [article]. JAMA Neurol. 76, 755–763. doi: 10.1001/jamaneurol.2019.0534
Landers, M. R., Hatlevig, R. M., Davis, A. D., Richards, A. R., and Rosenlof, L. E. (2016). Does attentional focus during balance training in people with Parkinson's disease affect outcome? A randomised controlled clinical trial [article]. Clin. Rehabil. 30, 53–63. doi: 10.1177/0269215515570377
Landers, M. R., and Navalta, J. W. (2019). A high-intensity exercise boot camp for persons with Parkinson disease: a phase II, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms [journal article; conference proceeding]. Mov. Disord. 43, 12–25. doi: 10.1097/NPT.0000000000000249
Langer, A., Hasenauer, S., Flotz, A., Gassner, L., Pokan, R., Dabnichki, P., et al. (2021). A randomised controlled trial on effectiveness and feasibility of sport climbing in Parkinson’s disease [Journal article]. NPJ Parkinson\u0027s dis. 7, 1-7. Doi: doi: 10.1038/s41531-021-00193-8
Li, F., Harmer, P., Fitzgerald, K., Eckstrom, E., Stock, R., Galver, J., et al. (2012). Tai chi and postural stability in patients with Parkinson's disease. N. Engl. J. Med. 366, 511–519. doi: 10.1056/NEJMoa1107911
Li, G., Huang, P., Cui, S. S., Tan, Y. Y., He, Y. C., Shen, X., et al. (2022). Mechanisms of motor symptom improvement by long-term tai chi training in Parkinson's disease patients. Transl. Neurodegener. 11:6. doi: 10.1186/s40035-022-00280-7
Li, X., Lv, C., Liu, X., and Qin, X. (2022). Effects of health qigong exercise on lower limb motor function in Parkinson's disease [Journal article]. Front. Med. 16:809134. doi: 10.3389/fmed.2021.809134
Li, F., Wang, D., Ba, X., Liu, Z., and Zhang, M. (2022). The comparative effects of exercise type on motor function of patients with Parkinson's disease: a three-arm randomized trial [article]. Front. Hum. Neurosci. 16::1033289. doi: 10.3389/fnhum.2022.1033289
Lu, G., and Ades, A. E. (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 23, 3105–3124. doi: 10.1002/sim.1875
Mak, M. K. Y., and Wong-Yu, I. S. K. (2021). Six-month community-based brisk walking and balance exercise alleviates motor symptoms and promotes functions in people with Parkinson's disease: a randomized controlled trial [article]. J. Parkinsons Dis. 11, 1431–1441. doi: 10.3233/JPD-202503
McKee, K. E., and Hackney, M. E. (2013). The effects of adapted tango on spatial cognition and disease severity in Parkinson's disease [article]. J. Mot. Behav. 45, 519–529. doi: 10.1080/00222895.2013.834288
Michels, K., Dubaz, O., Hornthal, E., and Bega, D. (2018). "Dance therapy" as a psychotherapeutic movement intervention in Parkinson's disease [article]. Complement. Ther. Med. 40, 248–252. doi: 10.1016/j.ctim.2018.07.005
Millar, P. (2020). Physiological benefits of high-intensity interval training for individuals with Parkinson's disease [trial registry record].
Mirelman, A., Bonato, P., Camicioli, R., Ellis, T. D., Giladi, N., Hamilton, J. L., et al. (2019). Gait impairments in Parkinson's disease. Lancet Neurol. 18, 697–708. doi: 10.1016/S1474-4422(19)30044-4
Miyai, I., Fujimoto, Y., Yamamoto, H., Ueda, Y., Saito, T., Nozaki, S., et al. (2002). Long-term effect of body weight-supported treadmill training in Parkinson's disease: a randomized controlled trial. Arch. Phys. Med. Rehabil. 83, 1370–1373. doi: 10.1053/apmr.2002.34603
Mollinedo-Cardalda, I., Maria Cancela-Carral, J., and Helena Vila-Suarez, M. (2018). Effect of a mat Pilates program with TheraBand on dynamic balance in patients with Parkinson's disease: feasibility study and randomized controlled trial [article; early access]. Rejuvenation Res. 21, 423–430. doi: 10.1089/rej.2017.2007
Moon, S., Sarmento, C. V. M., Steinbacher, M., Smirnova, I. V., Colgrove, Y., Lai, S. M., et al. (2020). Can qigong improve non-motor symptoms in people with Parkinson's disease – a pilot randomized controlled trial? [journal article]. Complement. Ther. Clin. Pract. 39:101169. doi: 10.1016/j.ctcp.2020.101169
Moratelli, J. A., Alexandre, K. H., Boing, L., De Carvalho Souza Vieira, M., and De Azevedo Guimaraes, A. C. (2022). Functional training versus mat Pilates in motor and non-motor symptoms of individuals with Parkinson's disease: study protocol for a randomized controlled trial [journal article]. Motriz 28:28. doi: 10.1590/s1980-657420220019321
Morris, M. E., Menz, H. B., McGinley, J. L., Watts, J. J., Huxham, F. E., Murphy, A. T., et al. (2015). A randomized controlled trial to reduce falls in people with Parkinson's disease [article]. Neurorehabil. Neural Repair 29, 777–785. doi: 10.1177/1545968314565511
Morris, M. E., Taylor, N. F., Watts, J. J., Evans, A., Horne, M., Kempster, P., et al. (2017). A home program of strength training, movement strategy training and education did not prevent falls in people with Parkinson's disease: a randomised trial [article]. J. Physiother. 63, 94–100. doi: 10.1016/j.jphys.2017.02.015
Nadeau, A., Pourcher, E., and Corbeil, P. (2014). Effects of 24 wk of treadmill training on gait performance in Parkinson's disease [article]. Med. Sci. Sports Exerc. 46, 645–655. doi: 10.1249/MSS.0000000000000144
Nagano-Saito, A., Washimi, Y., Arahata, Y., Kachi, T., Lerch, J. P., Evans, A. C., et al. (2005). Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64, 224–229. doi: 10.1212/01.WNL.0000149510.41793.50
Ni, M., Signorile, J. F., Mooney, K., Balachandran, A., Potiaumpai, M., Luca, C., et al. (2016). Comparative effect of power training and high-speed yoga on motor function in older patients with Parkinson disease [article]. Arch. Phys. Med. Rehabil. 97, 345–354.e15. doi: 10.1016/j.apmr.2015.10.095
Oliveira de Carvalho, A., Filho, A. S. S., Murillo-Rodriguez, E., Rocha, N. B., Carta, M. G., and Machado, S. (2018). Physical exercise for Parkinson's disease: clinical and experimental evidence. Clin. Pract. Epidemiol. Ment. Health 14, 89–98. doi: 10.2174/1745017901814010089
Page, M. J., Shamseer, L., Altman, D. G., Tetzlaff, J., Sampson, M., Tricco, A. C., et al. (2016). Epidemiology and reporting characteristics of systematic reviews of biomedical research: a cross-sectional study. PLoS Med. 13:e1002028. doi: 10.1371/journal.pbio.3001177
Pereira-Pedro, K. P., de Oliveira, I. M., Mollinedo Cardalda, I., and Cancela-Carral, J. M. (2022b). Effects of a forced cycling program with cognitive stimulation on symptomatology, physical condition, and cognition in people diagnosed with Parkinson disease. Medicine 101:e31920. doi: 10.1097/MD.00000000000031920
Pereira-Pedro, K. P., de Oliveira, I. M., Mollinedo-Cardalda, I., and Cancela-Carral, J. M. (2022a). Effects of cycling dual-task on cognitive and physical function in Parkinson's disease: a randomized double-blind pilot study [article]. Int. J. Environ. Res. Public Health 19:7847. doi: 10.3390/ijerph19137847
Poliakoff, E., Galpin, A. J., McDonald, K., Kellett, M., Dick, J. P. R., Hayes, S., et al. (2013). The effect of gym training on multiple outcomes in Parkinson's disease: a pilot randomised waiting-list controlled trial [article]. NeuroRehabilitation 32, 125–134. doi: 10.3233/NRE-130829
Radder, D. L. M., Silva, L., de Lima, A., Domingos, J., Keus, S. H. J., van Nimwegen, M., et al. (2020). Physiotherapy in Parkinson's disease: a Meta-analysis of present treatment modalities. Neurorehabil. Neural Repair 34, 871–880. doi: 10.1177/1545968320952799
Ramaker, C., Marinus, J., Stiggelbout, A. M., and Van Hilten, B. J. (2002). Systematic evaluation of rating scales for impairment and disability in Parkinson's disease. Mov. Disord. 17, 867–876. doi: 10.1002/mds.10248
Ridgel, A. L., Abdar, H. M., Alberts, J. L., Discenzo, F. M., and Loparo, K. A. (2013). Variability in cadence during forced cycling predicts motor improvement in individuals with Parkinson's disease. IEEE Trans. Neural Syst. Rehabil. Eng. 21, 481–489. doi: 10.1109/tnsre.2012.2225448
Romenets, S. R., Anang, J., Fereshtehnejad, S.-M., Pelletier, A., and Postuma, R. (2015). Tango for treatment of motor and non-motor manifestations in Parkinson's disease: a randomized control study [article]. Complement. Ther. Med. 23, 175–184. doi: 10.1016/j.ctim.2015.01.015
Sage, M. D., and Almeida, Q. J. (2009). Symptom and gait changes after sensory attention focused exercise vs aerobic training in Parkinson's disease [journal article]. Mov. Disord. 24, 1132–1138. doi: 10.1002/mds.22469
Sangarapillai, K., Norman, B. M., and Almeida, Q. J. (2021). Boxing vs sensory exercise for Parkinson's disease: a double-blinded randomized controlled trial [article]. Neurorehabil. Neural Repair 35, 769–777. doi: 10.1177/15459683211023197
Santos, S. M., da Silva, R. A., Terra, M. B., Almeida, I. A., de Melo, L. B., and Ferraz, H. B. (2017). Balance versus resistance training on postural control in patients with Parkinson's disease: a randomized controlled trial [article]. Eur. J. Phys. Rehabil. Med. 53:173-+. doi: 10.23736/S1973-9087.16.04313-6
Schenkman, M., Hall, D. A., Baron, A. E., Schwartz, R. S., Mettler, P., and Kohrt, W. M. (2012). Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial [article]. Phys. Ther. 92, 1395–1410. doi: 10.2522/ptj.20110472
Schenkman, M., Moore, C. G., Kohrt, W. M., Hall, D. A., Delitto, A., Comella, C. L., et al. (2018). Effect of high-intensity treadmill exercise on motor symptoms in patients with De novo Parkinson disease a phase 2 randomized clinical trial [article]. JAMA Neurol. 75, 219–226. doi: 10.1001/jamaneurol.2017.3517
Schlenstedt, C., Paschen, S., Kruse, A., Raethjen, J., Weisser, B., and Deuschl, G. (2015). Resistance versus balance training to improve postural control in Parkinson's disease: a randomized rater blinded controlled study [article]. PLoS One 10:e0140584. doi: 10.1371/journal.pone.0140584
Shah, C., Beall, E. B., Frankemolle, A. M., Penko, A., Phillips, M. D., Lowe, M. J., et al. (2016). Exercise therapy for Parkinson's disease: pedaling rate is related to changes in motor connectivity. Brain Connect. 6, 25–36. doi: 10.1089/brain.2014.0328
Sharp, K., and Hewitt, J. (2014). Dance as an intervention for people with Parkinson's disease: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 47, 445–456. doi: 10.1016/j.neubiorev.2014.09.009
Silva-Batista, C., Corcos, D. M., Roschel, H., Kanegusuku, H., Gobbi, L. T. B., Piemonte, M. E. P., et al. (2016). Resistance training with instability for patients with Parkinson's disease. Med. Sci. Sports Exerc. 48, 1678–1687. doi: 10.1249/MSS.0000000000000945
Silva-Batista, C., de Lima-Pardini, A. C., Nucci, M. P., Coelho, D. B., Batista, A., Piemonte, M. E. P., et al. (2020). A randomized, controlled trial of exercise for parkinsonian individuals with freezing of gait [journal article]. Mov. Disord. 35, 1607–1617. doi: 10.1002/mds.28128
Skidmore, F. M., Patterson, S. L., Shulman, L. M., Sorkin, J. D., and Macko, R. F. (2008). Pilot safety and feasibility study of treadmill aerobic exercise in Parkinson disease with gait impairment. J. Rehabil. Res. Dev. 45, 117–124. doi: 10.1682/jrrd.2006.10.0130
Soke, F., Guclu-Gunduz, A., Kocer, B., Fidan, I., and Keskinoglu, P. (2021). Task-oriented circuit training combined with aerobic training improves motor performance and balance in people with Parkinson's disease [journal article]. Acta Neurol. Belg. 121, 535–543. doi: 10.1007/s13760-019-01247-8
Solla, P., Cugusi, L., Bertoli, M., Cereatti, A., Della Croce, U., Pani, D., et al. (2019). Sardinian folk dance for individuals with Parkinson's disease: a randomized controlled pilot trial [article]. J. Altern. Complement. Med. 25, 305–316. doi: 10.1089/acm.2018.0413
Šumec, R., Filip, P., Sheardová, K., and Bareš, M. (2015). Psychological benefits of nonpharmacological methods aimed for improving balance in Parkinson's disease: a systematic review. Behav. Neurol. 2015:620674, 1–16. doi: 10.1155/2015/620674
Tiihonen, M., Westner, B. U., Butz, M., and Dalal, S. S. (2021). Parkinson's disease patients benefit from bicycling – a systematic review and meta-analysis. NPJ Parkinsons Dis. 7:86. doi: 10.1038/s41531-021-00222-6
Tollar, J., Nagy, F., Kovacs, N., and Hortobagyi, T. (2018). A high-intensity multicomponent agility intervention improves Parkinson Patients' clinical and motor symptoms [article]. Arch. Phys. Med. Rehabil. 99, 2478–2484.e1. doi: 10.1016/j.apmr.2018.05.007
Tollar, J., Nagy, F., Kovacs, N., and Hortobagyi, T. (2019). Two-Year Agility Maintenance Training Slows the Progression of Parkinsonian Symptoms [Article]. Med Sci Sports Exerc. 51, 237–245. Doi: doi: 10.1249/mss.0000000000001793
van der Kolk, N. M., de Vries, N. M., Kessels, R. P. C., Joosten, H., Zwinderman, A. H., Post, B., et al. (2019). Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson's disease: a double-blind, randomised controlled trial [article]. Lancet Neurol. 18, 998–1008. doi: 10.1016/S1474-4422(19)30285-6
Van Puymbroeck, M., Walter, A. A., Hawkins, B. L., Sharp, J. L., Woschkolup, K., Urrea-Mendoza, E., et al. (2018). Functional improvements in Parkinson's disease following a randomized trial of yoga [journal article]. Evid. Based Complement. Alternat. Med. 2018:8516351. doi: 10.1155/2018/8516351
vb6b, R. B. R. (2018). The effect of resistance exercise with instability in the patients with Parkinson's disease [Trial registry record]. Available at: https://trialsearch.who.int/Trial2.aspx?TrialID=RBR-83vb6b (Accessed July 13, 2023).
Volpe, D., Zanin, A., Clifford, A., Shahannan, J., and Morris, M. E. (2013). A randomized controlled feasibility trial to determine the effectiveness of Irish set dancing for people with Parkinson's disease [journal article; conference proceeding]. Mov. Disord. 28, S169–S170.
Wang, D., Cui, W. J., Hou, Z. H., and Gao, Y. (2023). Effectiveness of different exercises in improving postural balance among Parkinson's disease patients: a systematic review and network meta-analysis. Front. Aging Neurosci. 15:1215495. doi: 10.3389/fnagi.2023.1215495
Wang, Z., Pi, Y., Tan, X., Chen, R., Liu, Y., Guo, W., et al. (2022). Effects of Wu Qin xi exercise on reactive inhibition in Parkinson's disease: a randomized controlled clinical trial [article]. Front. Aging Neurosci. 14, 5–9. doi: 10.3389/fnagi.2022.961938
Wayne, P., Osypiuk, K., Vergara-Diaz, G., Bonato, P., Gow, B., Hausdorff, J., et al. (2017). Tai chi for reducing dual task gait variability, a potential mediator of fall risk, in individuals with Parkinson's disease: a pilot randomized controlled trial [conference abstract]. BMC Complement. Altern. Med. 17, 2–3. doi: 10.1177/2164956118775385
Weintraub, D., Aarsland, D., Chaudhuri, K. R., Dobkin, R. D., Leentjens, A. F., Rodriguez-Violante, M., et al. (2022). The neuropsychiatry of Parkinson's disease: advances and challenges. Lancet Neurol. 21, 89–102.
Weintraub, D., Newberg, A. B., Cary, M. S., Siderowf, A. D., Moberg, P. J., Kleiner-Fisman, G., et al. (2005). Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson's disease. J. Nucl. Med. 46, 227–232.
Wu, P.-L., Lee, M., Wu, S.-L., Ho, H.-H., Chang, M.-H., Lin, H.-S., et al. (2021). Effects of home-based exercise on motor, non-motor symptoms and health-related quality of life in Parkinson's disease patients: a randomized controlled trial [article]. Japan J. Nurs. Sci. 18:e12418. doi: 10.1111/jjns.12418
Xiao, C. M., and Zhuang, Y. C. (2016). Effect of health Baduanjin qigong for mild to moderate Parkinson's disease. Geriatr Gerontol Int 16, 911–919. doi: 10.1111/ggi.12571
Youm, C., Kim, Y., Noh, B., Lee, M., Kim, J., and Cheon, S. M. (2020). Impact of trunk resistance and stretching exercise on fall-related factors in patients with Parkinson's disease: a randomized controlled pilot study [journal article]. Sensors (Basel, Switzerland) 20, 2–3. doi: 10.3390/s20154106
Zhou, X., Zhao, P., Guo, X., Wang, J., and Wang, R. (2022). Effectiveness of aerobic and resistance training on the motor symptoms in Parkinson's disease: systematic review and network meta-analysis. Front. Aging Neurosci. 14:935176.
Zhu, Z., Yin, M., Cui, L., Zhang, Y., Hou, W., Li, Y., et al. (2018). Aquatic obstacle training improves freezing of gait in Parkinson’s disease patients: a randomized controlled trial [article]. Clin. Rehabil. 32, 29–36. doi: 10.1177/0269215517715763
Glossary
Keywords: network meta-analysis, Parkinson’s disease, exercise intervention, motor function, randomized controlled trial
Citation: HongFei Z, Li Z, Liang L, Ru GW, Yi HL and Zhen W (2024) Current interventional model for movement in Parkinson’s disease: network meta-analysis based on the improvement of motor ability. Front. Aging Neurosci. 16:1431277. doi: 10.3389/fnagi.2024.1431277
Edited by:
Yildiz Degirmenci, Istanbul Health and Technology University, TürkiyeReviewed by:
Anupa A. Vijayakumari, Cleveland Clinic, United StatesNaoya Hasegawa, Hokkaido University, Japan
Copyright © 2024 HongFei, Li, Liang, Ru, Yi and Zhen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Zhen, d2FuZ3poZW5Ac3VzLmVkdS5jbg==
 Zhao HongFei
Zhao HongFei Zhang Li2
Zhang Li2 Li Liang
Li Liang Wang Zhen
Wang Zhen