
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 12 June 2024
Sec. Alzheimer's Disease and Related Dementias
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1420290
 Mark A. Dubbelman1,2*
Mark A. Dubbelman1,2* Ibai Diez3
Ibai Diez3 Christopher Gonzalez4
Christopher Gonzalez4 Rebecca E. Amariglio1,2
Rebecca E. Amariglio1,2 J. Alex Becker3
J. Alex Becker3 Jasmeer P. Chhatwal1,2
Jasmeer P. Chhatwal1,2 Jennifer R. Gatchel5,6
Jennifer R. Gatchel5,6 Keith A. Johnson1,2,3
Keith A. Johnson1,2,3 Joseph J. Locascio1
Joseph J. Locascio1 Onyinye J. Udeogu1
Onyinye J. Udeogu1 Sharon Wang1
Sharon Wang1 Kathryn V. Papp1,2
Kathryn V. Papp1,2 Michael J. Properzi1
Michael J. Properzi1 Dorene M. Rentz1,2
Dorene M. Rentz1,2 Aaron P. Schultz1
Aaron P. Schultz1 Reisa A. Sperling1,2
Reisa A. Sperling1,2 Patrizia Vannini1,2
Patrizia Vannini1,2 Gad A. Marshall1,2
Gad A. Marshall1,2Background: Changes in everyday functioning constitute a clinically meaningful outcome, even in the early stages of Alzheimer's disease. Performance-based assessments of everyday functioning might help uncover these early changes. We aimed to investigate how changes over time in everyday functioning relate to tau and amyloid in cognitively unimpaired older adults.
Methods: Seventy-six cognitively unimpaired participants (72 ± 6 years old, 61% female) completed multiple Harvard Automated Phone Task (APT) assessments over 2.0 ± 0.9 years. The Harvard APT consists of three tasks, performed through an automated phone system, in which participants refill a prescription (APT-Script), select a new primary care physician (APT-PCP), and transfer money to pay a bill (APT-Bank). Participants underwent Pittsburgh compound-B and flortaucipir positron emission tomography scans at baseline. We computed distribution volume ratios for a cortical amyloid aggregate and standardized uptake volume ratios for medial temporal and neocortical tau regions. In separate linear mixed models, baseline amyloid by time and tau by time interactions were used to predict longitudinal changes in performance on the Harvard APT tasks. Three-way amyloid by tau by time interactions were also investigated. Lastly, we examined associations between tau and change in Harvard APT scores in exploratory voxel-wise whole-brain analyses. All models were adjusted for age, sex, and education.
Results: Amyloid [unstandardized partial regression coefficient estimate (β) = −0.007, 95% confidence interval (95% CI) = (−0.013, −0.001)], and medial temporal tau [β = −0.013, 95% CI = (−0.022, −0.004)] were associated with change over time in years on APT-PCP only, i.e., higher baseline amyloid and higher baseline tau were associated with steeper rate of decline of APT-PCP. Voxel-wise analyses showed widespread associations between tau and change in APT-PCP scores over time.
Conclusion: Even among cognitively unimpaired older adults, changes over time in the performance of cognitively complex everyday activities relate to cortical amyloid and widespread cerebral tau burden at baseline. These findings support the link between Alzheimer's disease pathology and function and highlight the importance of measuring everyday functioning in preclinical disease stages.
The performance of cognitively complex everyday activities, so-called “instrumental activities of daily living” (IADL), changes gradually throughout the Alzheimer's disease (AD) course, eventually leading to a dependence on caregivers for performing such activities as making (doctor's) appointments, managing finances, and using electronic devices. Impairment in IADL is a source of burden, both for the patient and the caregiver (Sherwood et al., 2005; Feger et al., 2020). Yet even before impairment reaches this point, IADL performance is a clinically meaningful outcome. The earliest, subtle difficulties performing IADL are thought to occur at the end of the preclinical phase of AD (Marshall et al., 2012).
The accumulation of cerebral amyloid and the spreading of tau through the temporal lobe characterize early AD (Banerjee et al., 2017; Maass et al., 2018). Tau distribution in the brain appears more closely associated than amyloid distribution with cognitive performance (Lowe et al., 2019; Ossenkoppele et al., 2019). Similarly, IADL functioning has been related to amyloid and tau, both cross-sectionally and longitudinally (Halawa et al., 2019; Marshall et al., 2019b, 2020; Dubbelman et al., 2020, 2022, 2023; Gonzalez et al., 2023). Consequently, it seems that changes in IADL performance might reflect early biological changes associated with AD. Using sensitive instruments is pivotal to uncovering early changes in IADL performance and how they relate to AD pathology.
Assessment of IADL commonly relies on observer and self-reported questionnaires, which attempt to capture self-perceived global everyday functioning. Alternatively, performance-based instruments measure a person's ability to complete one or more specific activities. An example of a performance-based measure is the Harvard Automated Phone Task (APT), which includes several healthcare-related tasks for individuals to perform through a phone menu. Performance on the Harvard APT has been shown to correlate with other measures of IADL (Marshall et al., 2019a), as well as with amyloid and tau cross-sectionally (Gonzalez et al., 2023).
In this study, we aimed to investigate how changes over time in performance on the Harvard APT relate to baseline amyloid and tau. Based on earlier findings with other IADL instruments, we analyzed global cortical amyloid and tau in the medial temporal and neocortical cortices. We hypothesized that, even among those with normal cognition, higher levels of amyloid and tau would be associated with a decreased performance over time on the Harvard APT. Second, in exploratory analyses, we investigated in what (other) areas of the cortex tau deposition related to change over time in IADL performance, to explore regional relationships between tau and everyday functioning.
We selected participants from the Instrumental Activities of Daily Living and Subjective Cognitive Decline studies at Massachusetts General Hospital and Brigham and Women's Hospital in Boston, Massachusetts. Participants were included if they were 55 years or older, had an available study partner, and were fluent in English. Exclusion criteria included history of major psychiatric disorder, neurodegenerative disease, brain tumor, or contraindications for magnetic resonance imaging. For the present study, we selected only participants who completed at least two Harvard Automated Phone Task assessments and underwent both amyloid and tau positron emission tomography (PET) scans.
This study was approved by the institutional review board of Partners Healthcare, Inc. All participants provided written informed consent before starting any study procedures.
The Harvard Automated Phone Task (APT) was collaboratively developed between the Center for Alzheimer's Research and Treatment of Brigham and Women's Hospital, Massachusetts General Hospital, the Connected Health Innovation at Partners Healthcare, and Rip Road. Harvard APT comprises three tasks in which participants navigate an interactive voice response system: APT-Script, APT-PCP, and APT-Bank, as described in detail by Marshall et al. (2015). Briefly, in the APT-Script task, participants are asked to call their pharmacy to refill a prescription; in the APT-PCP task, participants need to contact their health insurance company to select a new primary care physician; and in APT-Bank, participants make a bank account transfer. Each task consisted of multiple successive steps that the participants needed to complete (e.g., for APT-PCP, enter their member ID, date of birth, select the correct menu items, enter the PCP name and the city). Instructions, including expected responses, were provided to the participants on paper. The number of correct responses divided by the total time to complete each task formed the performance metric for the Harvard APT. Higher values indicate better performance. A detailed description of the Harvard APT and its scoring rules can be found in the first validation paper by Marshall et al. (2015). Participants completed the Harvard APT annually.
Positron emission tomography (PET) scans were made for tau, using the [18F]-flortaucipir tracer, and amyloid, using the [11C]-Pittsburgh compound-B tracer. We computed two aggregate regions of interest (ROIs) for tau-PET: (i) the medial temporal lobe, comprising the amygdala, entorhinal, and parahippocampal regions, and (ii) the temporo-parietal neocortex, comprising the inferior temporal, fusiform, middle temporal, and inferior parietal regions. For amyloid-PET, a large cortical aggregate served as the only ROI. We used FreeSurfer version 5.3.0 to compute binding in the ROIs (Fischl, 2012), represented by the distribution value ratio (DVR) for amyloid or standardized uptake value ratio (SUVR) for tau, with cerebellar gray as the reference region, as described in more detail elsewhere (Chien et al., 2013; Johnson et al., 2016).
In addition to the ROIs, we performed voxel-wise whole-brain analyses to analyze tau binding in relation to change over time in Harvard APT performance. Partial volume correction was applied to all ROI and voxel-wise analyses.
Using linear mixed-effects models (LMMs), we investigated change over time in performance on the Harvard APT tasks, with time in years as the primary fixed effect independent variable and each of the Harvard APT tasks as the primary dependent variable, each in a separate analysis. The random model terms were participant intercepts and slopes of time in years. We used an unstructured covariance structure, allowing any correlation between the random slopes and intercepts to be estimated freely. In the first set of LMMs, we analyzed interactions between time and global cortical amyloid and between time and the two tau-PET ROIs. Three-way interactions between time, tau-PET, and amyloid-PET were also investigated. In the second set of LMMs, we ran the same analyses on voxel-wise data. Voxel-wise analyses were adjusted for multiple testing in MATLAB version 2023a using Monte Carlo simulations. All models were adjusted for baseline age, sex, and education. We report estimates and their corresponding 95% confidence intervals (95% CI), as well as t-values (calculated as the estimate divided by the standard error), as an indication of standardized effect size. ROI analyses were run in R version 4.3.2, while voxel-wise analyses were run in R 3.6.3 (R Core Team, 2023).
Seventy-six participants (aged 72 ± 6 years, 61% female) completed the Harvard APT multiple times. They were either cognitively unimpaired without complaints (CU; n = 32, 42%) or had subjective cognitive decline (SCD; n = 44, 58%) but had no objective cognitive impairment. Table 1 displays all baseline characteristics. There were no differences in baseline demographic variables between those who were CU or had SCD. The mean follow-up duration was 2.0 ± 0.9 years (range 0.9–3.4 years), with 38 participants (50%) having completed the Harvard APT twice, 28 (37%) having completed it three times, and 10 (13%) having completed it four times. The mean time difference between tau and amyloid PET acquisition and baseline Harvard APT completion was 0.29 ± 0.52 years.
In the whole sample, performance on all three Harvard APT tasks was stable over time, i.e., there was no significant change over time. A greater global cortical amyloid burden at baseline was associated with a greater rate of decline in performance over time on APT-PCP [interaction coefficient (β) = −0.003, 95% confidence interval (95% CI) = (−0.006, −0.001), Z value (Z) = −2.40], but not on APT-Script or APT-Bank. Greater medial temporal lobe tau was also associated with decreased performance on APT-PCP over time [β = −0.008, 95% CI = (−0.013, −0.004), Z = −3.56]. Tau in the temporo-parietal neocortex was not associated with a change in performance on any of the APT tasks. A modest three-way interaction between time, amyloid, and medial temporal tau existed for APT-Script, but not for the other tasks [βamyloid × time = −0.055, 95% CI = (−0.103, −0.006), Z = −2.22; βtau × time = −0.042, 95% CI = (−0.082, −0.001), Z = −2.05; βamyloid × tau×time = 0.031, 95% CI = (0.002, 0.059), Z = 2.14]. All results are displayed in Table 2. Figure 1 shows the relationship between amyloid (top row) and medial temporal tau (bottom row) and changes over time on the three APT tasks.
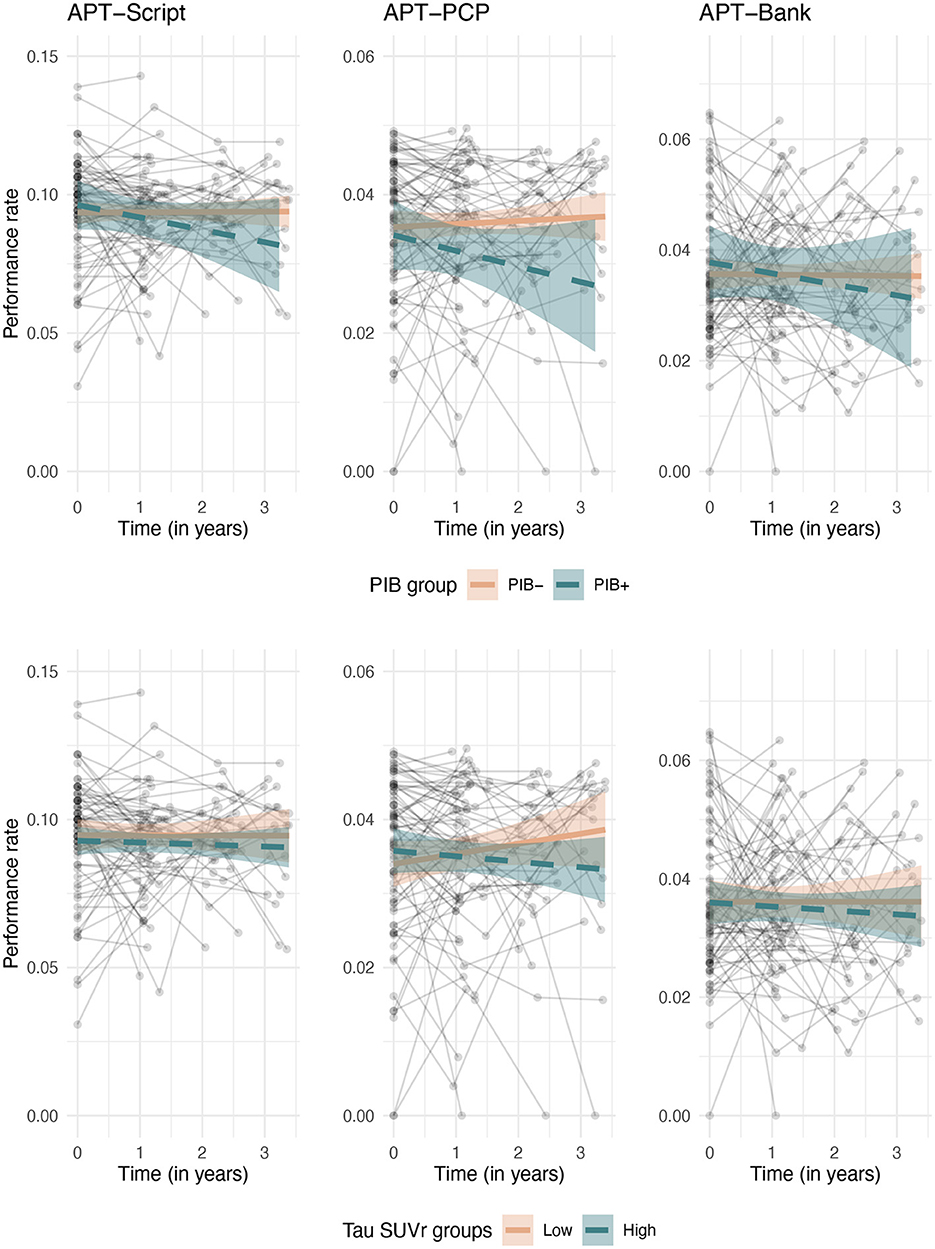
Figure 1. Longitudinal associations between change over time on the Harvard APT tasks and baseline amyloid (top row) and medial temporal tau (bottom row). For visualization purposes, amyloid is binarized into amyloid positive and negative groups based on our center's local cutoff, and tau is split into low and high strata based on median split.
Next, in exploratory analyses where we were not bound by predefined ROIs, we analyzed how a change in performance on the Harvard APT tasks over time related to baseline tau at the voxel level. Change over time in APT-Script scores showed associations with tau deposition in the left posterior cingulate gyrus, angular gyrus, precuneus, occipital pole, middle temporal gyrus, and temporal fusiform cortex, as well as the bilateral frontal orbital cortex. These associations did not remain significant after adjustment for multiple comparisons. Bilateral tau depositions in the middle and inferior temporal gyrus, supramarginal gyrus, frontal pole, and angular gyrus were associated with changes in performance over time on APT-PCP, including after adjustment for multiple comparisons. Furthermore, tau in the right parahippocampal and left superior and middle frontal and left superior temporal gyri, and left occipital pole was associated with a change in APT-PCP, including after adjustment for multiple comparisons. Figure 2 serves as an illustration and displays brain maps of the areas where tau burden was significantly associated with change over time in the APT-PCP task. Finally, changes over time in APT-Bank scores were associated with left middle frontal gyrus, superior parietal lobule, and temporal fusiform cortex tau, right posterior cingulate and precuneus tau, and bilateral parahippocampal gyrus and thalamus tau. None of these areas remained significant after multiple comparison corrections.
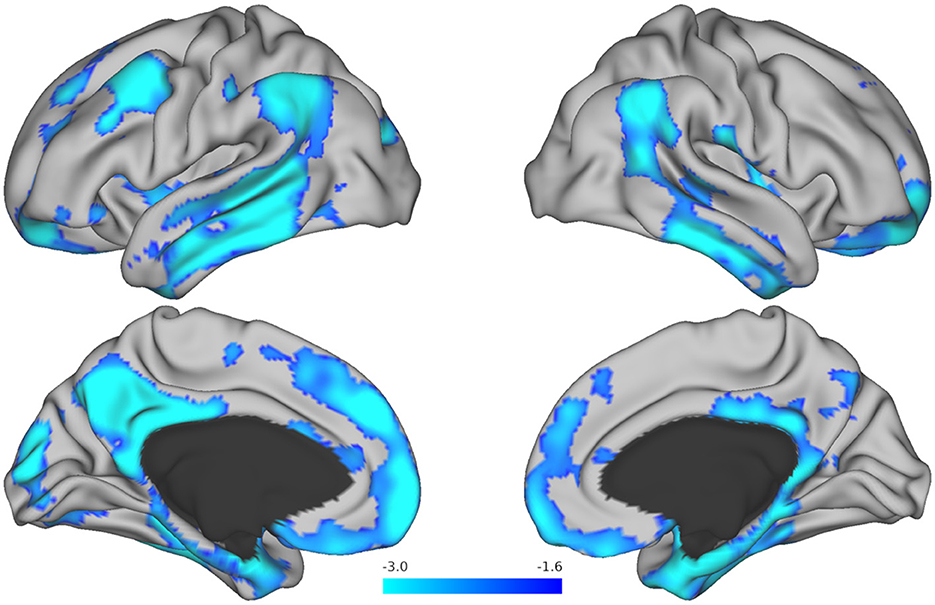
Figure 2. Brain maps resulting from voxel-wise analysis showing tau regions associated with change over time in the Harvard APT-PCP task. The T-values for areas that remained significant after correction for multiple comparisons are displayed in blue. Brighter blue colors represent more robust associations.
In this study, we found that changes in performance over a follow-up span of 2–4 years on a performance-based measure of IADL, the Harvard APT, related to baseline amyloid and tau deposition among cognitively unimpaired older adults. Change over time on one complex task specifically, where participants needed to select a new primary care physician, showed associations with widespread tau throughout the cortex.
Performance on the three Harvard APT tasks did not change over time at the group level. People without AD commonly show practice effects after retaking a cognitive test (Goldberg et al., 2015), as repeated exposure to a cognitive test leads to improved performance over time. Practice effects were not evident on any of the Harvard APT tasks in this sample, with the average performance remaining stable over annual assessments at the total group level. We did, however, observe a variety of individual trajectories of change over time, with some individuals showing improvement and others showing decline over time. Potential explanations for the absence of an apparent group-level practice effect on the Harvard APT tasks include the assessment frequency (annually), the possibility that individual improvements and decreases in performance cancel each other out, or the design of the Harvard APT, which is not a standard cognitive test but a measure of cognitively complex everyday tasks. It is also possible that the follow-up duration was insufficiently long for detecting group-level changes in cognition, particularly among older adults with minimal AD biomarkers (Soldan et al., 2016; Dubbelman et al., 2024).
Here, we show that a performance-based assessment, administered unsupervised through an automated phone menu, can detect changes in the performance of everyday tasks that are related to underlying Alzheimer's disease processes in individuals who were initially cognitively unimpaired, even over a relatively short follow-up duration. Specifically, the APT-PCP task showed associations with tau throughout the cortex and even in some subcortical structures. A higher tau burden in the temporal, frontal, and occipital lobes appeared to be related to decreased performance over time on APT-PCP. Notably, higher medial temporal tau was linked to decreased performance over time on APT-PCP. This area is known to accumulate tau in relatively early Alzheimer's disease stages (Vogel et al., 2021), and greater temporal tau has been previously associated with cognitive decline (Chen et al., 2021). It thus seems that early tau tracks with early changes in cognitively complex everyday functioning as measured by the Harvard APT.
In addition to the associations with medial temporal tau, a decline in performance on the APT-PCP subtask over time is also related to global cortical amyloid. It has previously been argued that both amyloid and tau abnormalities are required before cognitive decline occurs in the preclinical stages of Alzheimer's disease (Sperling et al., 2019). Our analyses, however, did not demonstrate any interactions between amyloid and tau and the change over time in APT-PCP scores. We did find such interaction, albeit a weak one, in relation to APT-Script scores, where the effects of amyloid and tau on change both individually suggested a declining performance over time, and together show a positive three-way interaction. As a caveat, only approximately a quarter of the participants in our sample were considered to have an amyloid-positive PET scan, meaning that the amount of amyloid accumulation was relatively low. Still, with these minimal levels of amyloid and tau, we did observe significant associations with the Harvard APT. As the change in performance was only apparent in the presence of increased amyloid and tau, the Harvard APT might be a suitable outcome measure for measuring complex everyday functioning among cognitively unimpaired individuals with limited levels of Alzheimer's disease-related pathology.
These findings provide further evidence for the existence of a relationship between everyday functioning and amyloid and tau. Previous studies have demonstrated that the performance of cognitively complex everyday activities is associated with underlying Alzheimer's disease processes, i.e., accumulation of cerebral amyloid and tau (Okonkwo et al., 2010; Marshall et al., 2011, 2019b; Halawa et al., 2019; Dubbelman et al., 2023). This holds true even among cognitively unimpaired individuals, who show no or only limited changes in the performance of everyday activities (Lilamand et al., 2018; Gonzalez et al., 2021; Dubbelman et al., 2022). Thus, it seems that Alzheimer's disease-related changes in everyday functioning emerge in the early stages of the disease trajectory, before the onset of dementia or even mild cognitive impairment (Marshall et al., 2012, 2020; Dubbelman et al., 2020).
Previous work examining the relationship between AD biomarkers and everyday functioning has primarily employed self- or study partner-reported questionnaires, which reflect either the person's own perception—or that of a spouse, friend, or relative—of the difficulties they experience in everyday life. While these questionnaires usually present information about a wide range of activities, they are subject to reporter bias. It has, for instance, been shown that people with more depressive symptoms report more negatively about their own daily functioning (Verrijp et al., 2021). On the other hand, performance-based measures might reflect actual performance more closely (Wesson et al., 2016), albeit in a highly controlled environment and usually focused on a narrower range of activities. Others have previously shown that performance-based assessments of everyday functioning are associated with neurodegeneration among cognitively unimpaired individuals, thus demonstrating the clinical value of performance-based measures in this early disease stage (Keleman et al., 2022). A comparison of self- or study partner-reported questionnaires and performance-based measures of everyday functioning in cognitively unimpaired individuals at risk for Alzheimer's disease might provide more insight into the utility of these different assessments.
The significant associations in the primary analyses using ROIs, as well as the exploratory voxel-wise analyses, were noted with the APT-PCP task but not the APT-Script or APT-Bank tasks. This aligns with prior cross-sectional analyses showing an association between cortical thinning and APT-PCP (Marshall et al., 2015) and between the interaction of amyloid and tau and APT-PCP (Gonzalez et al., 2023). The latter study also showed associations with APT-Bank. APT-Script is a more straightforward task that primarily relates to processing speed, while APT-PCP and APT-Bank are more complex tasks that primarily relate to executive functioning (Marshall et al., 2015). Therefore, the more complex tasks may be more sensitive to early changes in Alzheimer's disease.
We should note that the radioactive tracers used to obtain both amyloid and tau PET scans may bind to proteins or tissues that they are not supposed to bind to; this is referred to as off-target binding, and it is undesirable. Off-target binding is a well-described issue with the flortaucipir tracer, particularly in the basal ganglia, choroid plexus, and other areas (Leuzy et al., 2019). This might explain why we observed a relationship between striatal tau and performance on APT-Script and between the putamen and APT-PCP, which do not appear to bear apparent clinical significance. The findings in the medial temporal cortex are more credible, as they emerged from different analyses as well and were less affected by off-target binding.
Our study had a few limitations. The sample was predominantly non-Hispanic White and highly educated, limiting the generalizability of these results to other populations. Further, the sample was relatively small, and the follow-up duration was relatively short. The results from this study should be interpreted with these limitations in mind. While a larger sample and more extensive follow-up might have shown more or stronger relationships, even with these data we show changes over time in performance in relation to amyloid and tau. Important strengths of this study include the availability of baseline amyloid and tau PET scans, which allow for localization of Alzheimer's disease-related pathology with a high spatial resolution.
In conclusion, our study demonstrated that changes in everyday functioning over time, as measured using a performance-based assessment, are related to baseline amyloid and tau among cognitively unimpaired individuals. As we unravel the early disease processes, self-completed performance-based measures of everyday functioning, such as the Harvard APT, might allow for the detection of early decline in the face of Alzheimer's disease pathology and should be considered as clinically meaningful outcome measures in prevention trials.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Partners Healthcare, Inc./Mass General Brigham. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MD: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. ID: Data curation, Formal analysis, Visualization, Writing – review & editing. CG: Writing – review & editing. RA: Funding acquisition, Writing – review & editing. JB: Resources, Writing – review & editing. JC: Resources, Writing – review & editing. JG: Writing – review & editing. KJ: Writing – review & editing. JL: Writing – review & editing. OU: Data curation, Writing – review & editing. SW: Data curation, Writing – review & editing. KP: Writing – review & editing. MP: Data curation, Writing – review & editing. DR: Writing – review & editing. AS: Writing – review & editing. RS: Writing – review & editing. PV: Resources, Writing – review & editing. GM: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The National Institutes of Health–National Institute on Aging provided funding for the collection of data for this study, with grant numbers R01 AG053184, R01 AG067021 (PI: GM), and R01 AG058825 (PI: RA). MD received salary support from Alzheimer's Nederland (WE.06-2023-02), paid to his institution. GM has received research salary support for serving as site principal investigator for clinical trials funded by Eisai Inc., Eli Lilly and Company, and Genentech. The sponsors of these studies played no role in the design, methods, subject recruitment, data collection, analysis, or preparation of this article.
We thank the participants and all study personnel for making this study possible.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Banerjee, G., Carare, R., Cordonnier, C., Greenberg, S. M., Schneider, J. A., Smith, E. E., et al. (2017). The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice. J. Neurol. Neurosurg. Psychiatr. 88, 982–994. doi: 10.1136/jnnp-2016-314697
Chen, S.-D., Lu, J.-Y., Li, H.-Q., Yang, Y.-X., Jiang, J.-H., Cui, M., et al. (2021). Staging tau pathology with tau PET in Alzheimer's disease: a longitudinal study. Transl. Psychiatry 11, 483. doi: 10.1038/s41398-021-01602-5
Chien, D. T., Bahri, S., Szardenings, A. K., Walsh, J. C., Mu, F., Su, M.-Y., et al. (2013). Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J. Alzheimers Dis. 34, 457–468. doi: 10.3233/JAD-122059
Dubbelman, M. A., Hendriksen, H. M. A., Harrison, J. E., Vijverberg, E. G. B., Prins, N. D., Kroeze, L. A., et al. (2024). Cognitive and functional change over time in cognitively healthy individuals according to Alzheimer disease biomarker-defined subgroups. Neurology 102:e207978. doi: 10.1212/WNL.0000000000207978
Dubbelman, M. A., Jutten, R. J., Tomaszewski Farias, S. E., Amariglio, R. E., Buckley, R. F., Visser, P. J., et al. (2020). Decline in cognitively complex everyday activities accelerates along the Alzheimer's disease continuum. Alzheimers Res. Ther. 12:138. doi: 10.1186/s13195-020-00706-2
Dubbelman, M. A., Mimmack, K. J., Sprague, E. H., Amariglio, R. E., Vannini, P., Marshall, G. A., et al. (2023). Regional cerebral tau predicts decline in everyday functioning across the Alzheimer's disease spectrum. Alzheimers Res. Ther. 15:120. doi: 10.1186/s13195-023-01267-w
Dubbelman, M. A., Sanchez, J., Schultz, A. P., Rentz, D. M., Amariglio, R. E., Sikkes, S. A. M., et al. (2022). Everyday functioning and entorhinal and inferior temporal tau burden in cognitively normal older adults. J. Prev. Alzheimers Dis. 9, 801–808. doi: 10.14283/jpad.2022.58
Feger, D. M., Willis, S. L., Thomas, K. R., Marsiske, M., Rebok, G. W., Felix, C., et al. (2020). Incident instrumental activities of daily living difficulty in older adults: which comes first? findings from the advanced cognitive training for independent and vital elderly study. Front. Neurol. 11:550577. doi: 10.3389/fneur.2020.550577
Goldberg, T. E., Harvey, P. D., Wesnes, K. A., Snyder, P. J., and Schneider, L. S. (2015). Practice effects due to serial cognitive assessment: implications for preclinical Alzheimer's disease randomized controlled trials. Alzheimers Dement. 1, 103–111. doi: 10.1016/j.dadm.2014.11.003
Gonzalez, C., Mimmack, K. J., Amariglio, R. E., Becker, J. A., Chhatwal, J. P., Fitzpatrick, C. D., et al. (2023). Associations of the harvard automated phone task and alzheimer's disease pathology in cognitively normal older adults: preliminary findings. J. Alzheimers. Dis. 94, 217–226. doi: 10.3233/JAD-220885
Gonzalez, C., Tommasi, N. S., Briggs, D., Properzi, M. J., Amariglio, R. E., Marshall, G. A., et al. (2021). Financial capacity and regional cerebral tau in cognitively normal older adults, mild cognitive impairment, and alzheimer's disease dementia. J. Alzheimers Dis. 79, 1133–1142. doi: 10.3233/JAD-201122
Halawa, O. A., Gatchel, J. R., Amariglio, R. E., Rentz, D. M., Sperling, R. A., Johnson, K. A., et al. (2019). Inferior and medial temporal tau and cortical amyloid are associated with daily functional impairment in Alzheimer's disease. Alzheimers Res. Ther. 11:14. doi: 10.1186/s13195-019-0471-6
Johnson, K. A., Schultz, A., Betensky, R. A., Becker, J. A., Sepulcre, J., Rentz, D., et al. (2016). Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 79, 110–119. doi: 10.1002/ana.24546
Keleman, A. A., Bollinger, R. M., Wisch, J. K., Grant, E. A., Benzinger, T. L., Ances, B. M., et al. (2022). Assessment of instrumental activities of daily living in preclinical alzheimer disease. OTJR 42, 277–285. doi: 10.1177/15394492221100701
Leuzy, A., Chiotis, K., Lemoine, L., Gillberg, P.-G., Almkvist, O., Rodriguez-Vieitez, E., et al. (2019). Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol. Psychiatry 24, 1112–1134. doi: 10.1038/s41380-018-0342-8
Lilamand, M., Cesari, M., Cantet, C., Payoux, P., Andrieu, S., Vellas, B., et al. (2018). Relationship between brain amyloid deposition and instrumental activities of daily living in older adults: a longitudinal study from the multidomain Alzheimer prevention trial. J. Am. Geriatr. Soc. 66, 1940–1947. doi: 10.1111/jgs.15497
Lowe, V. J., Bruinsma, T. J., Wiste, H. J., Min, H.-K., Weigand, S. D., Fang, P., et al. (2019). Cross-sectional associations of tau-PET signal with cognition in cognitively unimpaired adults. Neurology 93, e29–e39. doi: 10.1212/WNL.0000000000007728
Maass, A., Lockhart, S. N., Harrison, T. M., Bell, R. K., Mellinger, T., Swinnerton, K., et al. (2018). Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. J. Neurosci. 38, 530–543. doi: 10.1523/JNEUROSCI.2028-17.2017
Marshall, G. A., Aghjayan, S. L., Dekhtyar, M., Locascio, J. J., Jethwani, K., Amariglio, R. E., et al. (2019a). Measuring instrumental activities of daily living in non-demented elderly: a comparison of the new performance-based Harvard Automated Phone Task with other functional assessments. Alzheimers Res. Ther. 11:4. doi: 10.1186/s13195-018-0464-x
Marshall, G. A., Amariglio, R. E., Sperling, R. A., and Rentz, D. M. (2012). Activities of daily living: where do they fit in the diagnosis of Alzheimer's disease? Neurodegener. Dis. Manag. 2, 483–491. doi: 10.2217/nmt.12.55
Marshall, G. A., Dekhtyar, M., Bruno, J. M., Jethwani, K., Amariglio, R. E., Johnson, K. A., et al. (2015). The Harvard automated phone task: new performance-based activities of daily living tests for early Alzheimer's disease. J. Prev. Alzheimers Dis. 2, 242–253. doi: 10.14283/jpad.2015.72
Marshall, G. A., Gatchel, J. R., Donovan, N. J., Muniz, M. C., Schultz, A. P., Becker, J. A., et al. (2019b). Regional tau correlates of instrumental activities of daily living and apathy in mild cognitive impairment and alzheimer's disease dementia. J. Alzheimers Dis. 67, 757–768. doi: 10.3233/JAD-170578
Marshall, G. A., Olson, L. E., Frey, M. T., Maye, J., Becker, J. A., Rentz, D. M., et al. (2011). Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement. Geriatr. Cogn. Disord. 31, 443–450. doi: 10.1159/000329543
Marshall, G. A., Sikkes, S. A. M., Amariglio, R. E., Gatchel, J. R., Rentz, D. M., Johnson, K. A., et al. (2020). Instrumental activities of daily living, amyloid, and cognition in cognitively normal older adults screening for the A4 Study. Alzheimers Dement. 12:e12118. doi: 10.1002/dad2.12118
Okonkwo, O. C., Alosco, M. L., Griffith, H. R., Mielke, M. M., Shaw, L. M., Trojanowski, J. Q., et al. (2010). Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch. Neurol. 67, 688–696. doi: 10.1001/archneurol.2010.118
Ossenkoppele, R., Smith, R., Ohlsson, T., Strandberg, O., Mattsson, N., Insel, P. S., et al. (2019). Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology 92, e601–e612. doi: 10.1212/WNL.0000000000006875
R Core Team (2023). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Sherwood, P. R., Given, C. W., Given, B. A., and von Eye, A. (2005). Caregiver burden and depressive symptoms: analysis of common outcomes in caregivers of elderly patients. J. Aging Health 17, 125–147. doi: 10.1177/0898264304274179
Soldan, A., Pettigrew, C., Cai, Q., Wang, M.-C., Moghekar, A. R., O'Brien, R. J., et al. (2016). Hypothetical preclinical alzheimer disease groups and longitudinal cognitive change. JAMA Neurol. 73, 698–705. doi: 10.1001/jamaneurol.2016.0194
Sperling, R. A., Mormino, E. C., Schultz, A. P., Betensky, R. A., Papp, K. V., Amariglio, R. E., et al. (2019). The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann. Neurol. 85, 181–193. doi: 10.1002/ana.25395
Verrijp, M., Dubbelman, M. A., Visser, L. N. C., Jutten, R. J., Nijhuis, E. W., Zwan, M. D., et al. (2021). Everyday functioning in a community-based volunteer population: differences between participant- and study partner-report. Front. Aging Neurosci. 13:761932. doi: 10.3389/fnagi.2021.761932
Vogel, J. W., Young, A. L., Oxtoby, N. P., Smith, R., Ossenkoppele, R., Strandberg, O. T., et al. (2021). Four distinct trajectories of tau deposition identified in Alzheimer's disease. Nat. Med. 27, 871–881. doi: 10.1038/s41591-021-01309-6
Wesson, J., Clemson, L., Brodaty, H., and Reppermund, S. (2016). Estimating functional cognition in older adults using observational assessments of task performance in complex everyday activities: a systematic review and evaluation of measurement properties. Neurosci. Biobehav. Rev. 68, 335–360. doi: 10.1016/j.neubiorev.2016.05.024
Keywords: Alzheimer's disease, function, instrumental activities of daily living, amyloid, tau, longitudinal
Citation: Dubbelman MA, Diez I, Gonzalez C, Amariglio RE, Becker JA, Chhatwal JP, Gatchel JR, Johnson KA, Locascio JJ, Udeogu OJ, Wang S, Papp KV, Properzi MJ, Rentz DM, Schultz AP, Sperling RA, Vannini P and Marshall GA (2024) Amyloid and tau burden relate to longitudinal changes in the performance of complex everyday activities among cognitively unimpaired older adults: results from the performance-based Harvard Automated Phone Task. Front. Aging Neurosci. 16:1420290. doi: 10.3389/fnagi.2024.1420290
Received: 19 April 2024; Accepted: 29 May 2024;
Published: 12 June 2024.
Edited by:
Umesh K. Jinwal, University of South Florida, United StatesReviewed by:
Minhong Neenah Huang, Mayo Clinic, United StatesCopyright © 2024 Dubbelman, Diez, Gonzalez, Amariglio, Becker, Chhatwal, Gatchel, Johnson, Locascio, Udeogu, Wang, Papp, Properzi, Rentz, Schultz, Sperling, Vannini and Marshall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark A. Dubbelman, bWR1YmJlbG1hbkBid2guaGFydmFyZC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.