- 1Affiliated Qingdao Third People’s Hospital, Qingdao University, Department of Otorhinolaryngology Head and Neck, Qingdao, China
- 2Affiliated Qingdao Third People’s Hospital, Qingdao University, Department of Clinical Laboratory, Qingdao, China
Background: Hearing loss and tinnitus have been linked to mild cognitive impairment (MCI); however, the evidence is constrained by ethical and temporal constraints, and few prospective studies have definitively established causation. This study aims to utilize Mendelian randomization (MR) and cross-sectional studies to validate and analyze this association.
Methods: This study employs a two-step approach. Initially, the genetic data of the European population from the Genome-wide association studies (GWAS) database is utilized to establish the causal relationship between hearing loss and cognitive impairment through Mendelian randomization using the inverse variance weighted (IVW) method. This is achieved by identifying strongly correlated single nucleotide polymorphisms (SNPs), eliminating linkage disequilibrium, and excluding weak instrumental variables. In the second step, 363 elderly individuals from 10 communities in Qingdao, China are assessed and examined using methods questionnaire survey and pure tone audiology (PTA). Logistic regression and multiple linear regression were used to analyze the risk factors of MCI in the elderly and to calculate the cutoff values.
Results: Mendelian randomization studies have shown that hearing loss is a risk factor for MCI in European populations, with a risk ratio of hearing loss to MCI loss of 1. 23. The findings of this cross-sectional study indicate that age, tinnitus, and hearing loss emerged as significant risk factors for MCI in univariate logistic regression analysis. Furthermore, multivariate logistic regression analysis identified hearing loss and tinnitus as potential risk factors for MCI. Consistent results were observed in multiple linear regression analysis, revealing that hearing loss and age significantly influenced the development of MCI. Additionally, a notable finding was that the likelihood of MCI occurrence increased by 9% when the hearing threshold exceeded 20 decibels.
Conclusion: This study provides evidence from genomic and epidemiological investigations indicating that hearing loss may serve as a risk factor for cognitive impairment. While our epidemiological study has found both hearing loss and tinnitus as potential risk factors for cognitive decline, additional research is required to establish a causal relationship, particularly given that tinnitus can manifest as a symptom of various underlying medical conditions.
Introduction
Mild cognitive impairment (MCI) serves as a transitional phase between typical aging and the onset of clinical dementia, characterized by a mild decline in cognitive abilities, particularly in memory (Cox and Wallace, 2022; Zhao et al., 2023). Individuals with MCI face a heightened risk of developing Alzheimer’s disease (AD), with approximately 32% progressing to dementia within a median timeframe of 2 years (Jafari et al., 2019; Jia et al., 2019; Clark and Swanepoel, 2021; Babulal et al., 2022). Consequently, the identification, assessment, and intervention of MCI warrant further investigation in research endeavors to effectively delay and avert the onset of AD.
Over 1.5 billion individuals globally, constituting approximately 20% of the world’s population, experience varying degrees of hearing loss, with 430 million individuals exhibiting moderate to severe hearing loss (Cunningham and Tucci, 2017; D'Haese et al., 2023). The incidence of hearing loss escalates significantly with advancing age, rising from 12.7% at 60 years to 58.6% at 90 years. Moreover, a substantial portion (58%) of individuals with disabling hearing loss are aged 60 years and above (Lin et al., 2013; Cunningham and Tucci, 2017; Clark and Swanepoel, 2021; McMahon et al., 2021; Zhang et al., 2022; D'Haese et al., 2023).
Hearing loss (HL) is a prevalent disability in today’s increasingly aging society and poses a serious threat to the physical and mental health of older people (Bowl and Dawson, 2019). Hearing loss is the most common chronic sensory impairment in older people, with disabling hearing loss occurring in approximately 50% of people over the age of 70 years, and it is one of the three most common health problems in older people (Ronnberg et al., 2013). The World Health Organization predicts that the global population aged over 65 years will reach 1 billion by 2050 and that hearing loss will become a major public health problem (Lin et al., 2013). In addition to its widespread occurrence, hearing impairment can have a multifaceted effect on elderly individuals and is consequently receiving increasing recognition. Firstly, hearing loss may result in challenges with speech and communication, thereby diminishing older individuals’ verbal communication capabilities (Rutherford et al., 2018). Secondly, hearing loss can precipitate feelings of social isolation, despondency, solitude, and various psychological issues, including depression (Chern and Golub, 2019). Importantly, hearing loss can also lead to cognitive impairment (Livingston et al., 2017). According to Lancet Dementia Commission 2017 and 2020, hearing loss is the greatest potential risk factor for dementia (Livingston et al., 2020), and it is projected that if hearing loss was eliminated, the prevalence of dementia would be reduced by 8%. If the prevalence of each risk factor for hearing loss was reduced by 10 to 20% every 10 years, the number of dementia diagnoses worldwide could be reduced by 8.8 to 16.2 million by 2050 (Czornik et al., 2022).
Tinnitus is a common symptom of the auditory system, which is mainly caused by other diseases, such as noise, hearing loss and mental stress. In Europe and the United States, 10 to 15 percent of people are affected by tinnitus for some time, and the prevalence increases with age (Mazurek et al., 2022). Recent evidence suggests a link between tinnitus and impairment in all aspects of cognitive function. Both hearing loss and tinnitus can affect mental health and contribute to depression, stress, and depression (Jarach et al., 2022; Lau et al., 2022). This study included tinnitus in the community survey project, providing new evidence for the study of tinnitus and cognitive impairment.
Previous observational studies and meta-analyses have identified hearing loss as a possible risk factor for cognitive impairment and dementia, but confounding could not be excluded (Loughrey et al., 2018; Jafari et al., 2019). Recent studies have found that hearing loss is associated with cognitive decline, brain atrophy and tau protein by genetic association analysis using data from different databases, which again confirms that hearing loss is a risk factor for cognitive decline and dementia (Wang et al., 2022). However, there is no direct evidence that hearing loss is causally related to cognitive impairment and dementia. These observational and bioinformatic findings need to be confirmed in randomized, controlled trials and animal studies that are time - and ethically demanding. A 2022 randomized controlled trial at four community study sites in the United States of America in adults aged 70 to 84 years with untreated hearing loss and no severe cognitive impairment. The findings suggest that hearing interventions may reduce cognitive change over 3 years in an older population at increased risk of cognitive decline, but not in a population at reduced risk of cognitive decline (Lin et al., 2023). Therefore, more evidence is needed to confirm the direct relationship between hearing loss and cognitive impairment. Mendelian randomization (MR) uses genetic tools to provide new evidence for confirming the causal relationship between exposure factors and diseases (Emdin et al., 2017; Ji et al., 2024; Levin and Burgess, 2024). Mendelian randomization is widely used in epidemiological etiology research because it can avoid the ethical and time limitations of randomized controlled trials and the interference of confounding factors in case–control trials. Based on large samples of genetic and phenotypic data, MR Screens single nucleotide polymorphisms (SNPS) that are strongly associated with exposure factors but not related to outcomes and confounding factors, and uses them as instrumental variables to assess the causal relationship between exposure factors and outcomes (Larsson et al., 2023).
This study posited that hearing loss, tinnitus, weight, and other variables may serve as potential risk factors for cognitive impairment. Genetic and epidemiological methodologies were employed to investigate these risk factors in European and Chinese populations, with logistic regression and linear regression utilized to analyze the relationship between these factors (Figure 1). The findings of this study aim to contribute new evidence to the understanding of the link between hearing loss, tinnitus, and cognitive impairment.
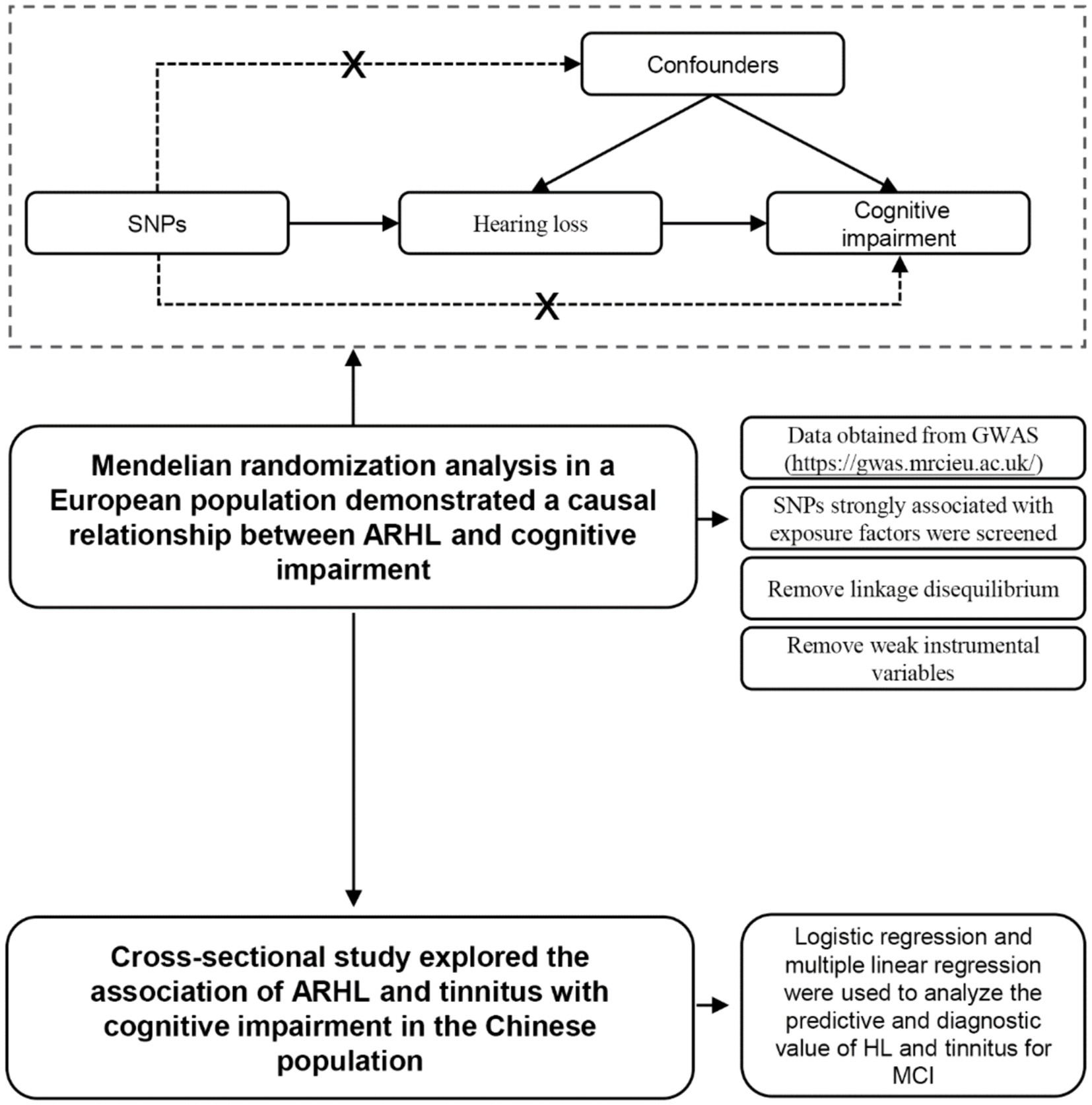
Figure 1. Flow chart of Mendelian randomization and cross-sectional studies and the principle of Mendelian randomization.
Methods
Mendelian randomization analysis between hearing loss and cognitive function
Data of hearing loss (ebi-a-GCST90018857, including 489,493 European adult subjects and consisted of 14,654 cases and 474,839 controls) which obtained from GWAS1 (S1) is defined as exposure data, which was subjected to selection of SNPs for strongly correlated instrumental variables (p < 2 × 10−7), followed by analyses to remove linkage disequilibrium (SNPs within 10,000 kb of the highest SNPs with significance at r2 < 0.001) were removed and filtering of weak instrumental variables (F-value >10) (S2) (Storey and Tibshirani, 2003). Data of cognitive functions (ieu-b-4837, including 9,997 European adult subjects) were defined as outcome data, which was subjected to extraction of instrumental variable SNPs and then merged with exposure data and analyzed by Mendelian randomization.
Two-sample MR Analysis was used in this study. Five methods were used for Mendelian analysis, namely MR Egger method, Weighted median method, Inverse variance weighted method, Simple mode method, and Weighted mode method. Inverse-variance weighting was used as the primary analysis method. Inverse variance weighting methods estimate the causal effects of genes on traits by weighting the causal effects of different genetic variants on traits and then combining the estimated effects after weighting. The advantage of inverse variance weighting method is to reduce the influence of sample size, improve the estimation accuracy and reduce bias, so it is used as the main analysis method (Sanderson, 2021). MR-egger method and weighted median method were used as supplements, and the Beta value was calculated to judge whether there was consistency in the results of MR Analysis to enhance the robustness of causality. MR-Egger method can calculate direct and indirect effects, evaluate the multiple effects of genetic variation on the results, adjust the confounding bias to a certain extent, and improve the accuracy of causality estimation (Burgess and Thompson, 2017). The weighted median method mainly assigns different weights to different genetic variants, thereby reducing the impact of extreme genetic variation on causal inference and improving the stability of the results. In addition, the weighted median method also has the advantages of increasing the estimation accuracy, wide applicability and strong flexibility (Hartley et al., 2022). The results of Mendelian randomization were finally tested for heterogeneity (S3), sensitivity and pleiotropy (S4), and funnel plots, forest plots and scatter plots were created.
Population of the cross-sectional study
All of the subjects were recruited from residents of Liking District, Qingdao, aged 60 years or older. Exclusion criteria were as follows: having dementia, bipolar disorder, schizophrenia, or other developmental or neurodegenerative disorders or that prevented performing cognitive assessment tests, and currently wearing hearing aids such as hearing aids or cochlear implants. Recruitment and testing took place from May to July 2022. The study was approved by the Ethics Committee of the Third People’s Hospital of Qingdao, affiliated with Qingdao University. All the procedures were carried out in accordance with the approval, and the participants provided written informed consent.
Questionnaire survey and pure tone audiometry were conducted among 363 elderly people in 10 communities in Qingdao through recruitment and promotion in community health centers. Gender, age, height, body mass index (BMI), hypertension, diabetes, tinnitus, depression, and hearing loss were used as independent variables, and MCI was used as the dependent variable to conduct univariate logistic regression to preliminarily explore the risk factors of MCI. Univariate logistic regression analysis was used to screen out statistically significant independent variables, and then multivariate logistic regression analysis was used to determine the risk factors of MCI. Finally, the cut-off value of Youden index for the diagnosis of MCI was calculated by logistic regression.
Data collection
Data collection consisted of the following three parts: instrumental measurements, questionnaire, and scale assessment. Height, blood pressure, weight, and hearing test data were obtained from instrumental measurements, depression, and cognitive function data were obtained from scale assessment. Diabetes diagnosis was obtained by asking medical history. The data collection process was completed by clinicians from the Department of Otorhinolaryngology of the Third People’s Hospital of Qingdao affiliated with Qingdao University.
Scale assessment
All the subjects were assessed on the Hearing Handicap Inventory for the Elderly-Screening. Higher scores on the Hearing Handicap Inventory for the Elderly-Screening indicated greater hearing loss and a scale score of >8 was defined as the presence of hearing loss (Kim et al., 2016). The Montreal Cognitive Assessment Scale (MoCA) has a high sensitivity and specificity for the rapid screening of patients with MCI and is now widely used internationally (Lee et al., 2020). MoCA is divided into eight cognitive domain subscales, of which the immediate memory subscale is not scored and the other seven cognitive domain subscales are scored as follows: 5 points for visuospatial and executive functions, 3 points for language skills, 6 points for attention, 6 points for orientation, 5 points for delayed memory, 3 points for naming, and 2 points for abstraction. The original English version of the MoCA recommended a score of less than 26 for cognitive impairment. In studies on the Chinese population, a score of 26 is generally considered high. There is some variation in the optimal cutoff values used in different diseases and populations. For example, in the case of non-demented vascular cognitive impairment, some studies have shown that the optimal cutoff score for MoCA is 23.5 (Wang et al., 2018). Based on the results of the study on the Chinese population, the MoCA score of 26 was used as the cut-off value in this study (Jia et al., 2021). Chinese elderly have free physical examination in the community every year, and have chronic disease management files, so that they are very aware of their own chronic disease history, so data on diabetes, hypertension, and tinnitus were obtained through patient self-evaluation (Respondents were asked if they heard a non-pulsating cicada, cricket, or whistle for an extended period of time) (Vasilescu and Weisman, 2023). Depression data were obtained by assessment of the Patient Health Questionnaire-9 (PHQ-9) scale, the scale had 9 items, including mood, sleep, appetite, fatigue, self-identity, attention and self-injury. Each item was composed of four options (0 = not at all, 1 = a few days, 2 = more than half of the days, 3 = almost every day). The total score ranged from 0 to 27, and the total score > 4 was considered positive (Levis et al., 2019).
Hearing test
All the subjects were audiometrically tested by an audiologist for pure tone, with air conduction at 500, 1000, 2000, and 4,000 Hz. The hearing test equipment was Pure audiometer—Clinical diagnostic audiometer (AD226) produced by International Hearing Company, Denmark. If the average of the air conduction thresholds of 500, 1,000, 2000, and 4,000 Hz for the better ear, in accordance with the WHO classification criteria for the degree of hearing loss, was ≤25 decibels hearing level, it was considered normal hearing, while more than 25 decibels hearing level was considered hearing loss.
Statistical methods
Chi-square test and Mann–Whitney U test were used to compare the differences between groups according to the number of samples and the distribution of data. The effect of the independent variables on cognitive impairment was assessed using the odds ratio (OR) in logistic regression, with an OR > 1 and a statistically significant p-value indicating that the variable was a risk factor for cognitive impairment, and an OR < 1 and a statistically significant p-value indicating that the variable was a protective factor for cognitive impairment. Statistical analyses were performed using Stata 12, R 4.2.2 and SPSS (v28.0.1.1) software, and differences were considered statistically significant at p < 0.05. The cut-off value was determined based on the Youden index. The Youden index is the sum of sensitivity and specificity minus 1, and the maximum corresponding value is the Youden index, which is the cut-off value.
Results
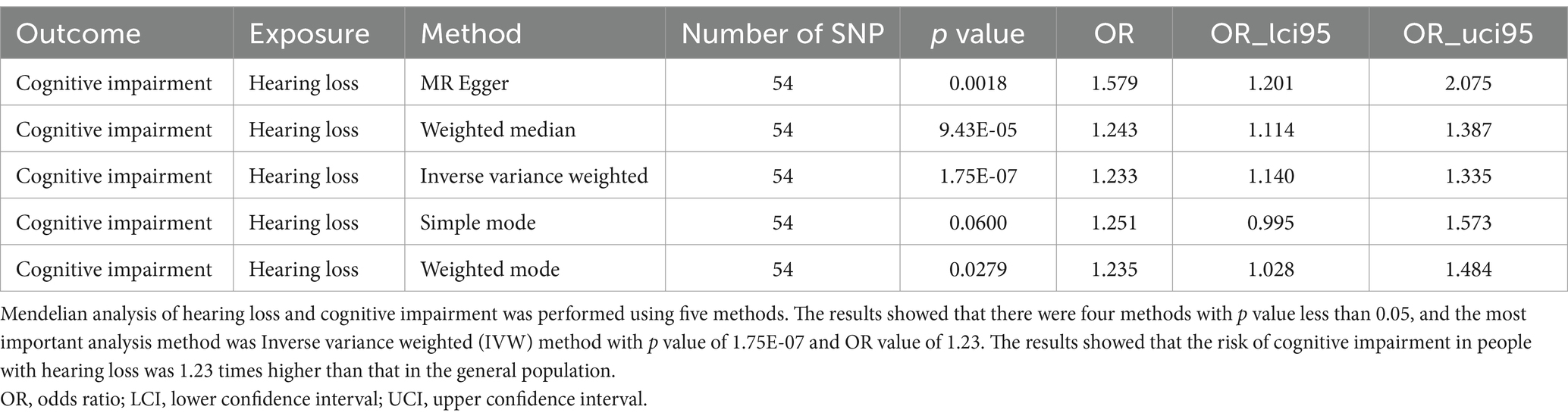
Mendelian randomization
Through Mendelian randomization analysis, we found an instrumental variable consisting of 54 SNPs, thus demonstrating a causal relationship between HL and cognitive impairment. Inverse variance weighted analysis showed an OR of 1.23 with a p-value of 1.75 × 10−7 (Table 1). The p-value for the test of pleiotropy was 0.07 and for the test of heterogeneity was 0.99. Sensitivity analysis and funnel plot of leave-one-out method also showed less bias in Mendelian analysis (Figure 2). Our Mendelian randomization results suggest that HL is a risk factor for cognitive impairment. In order to confirm the results of Mendelian randomization, we conducted a cross-sectional study in a Chinese population for validation.
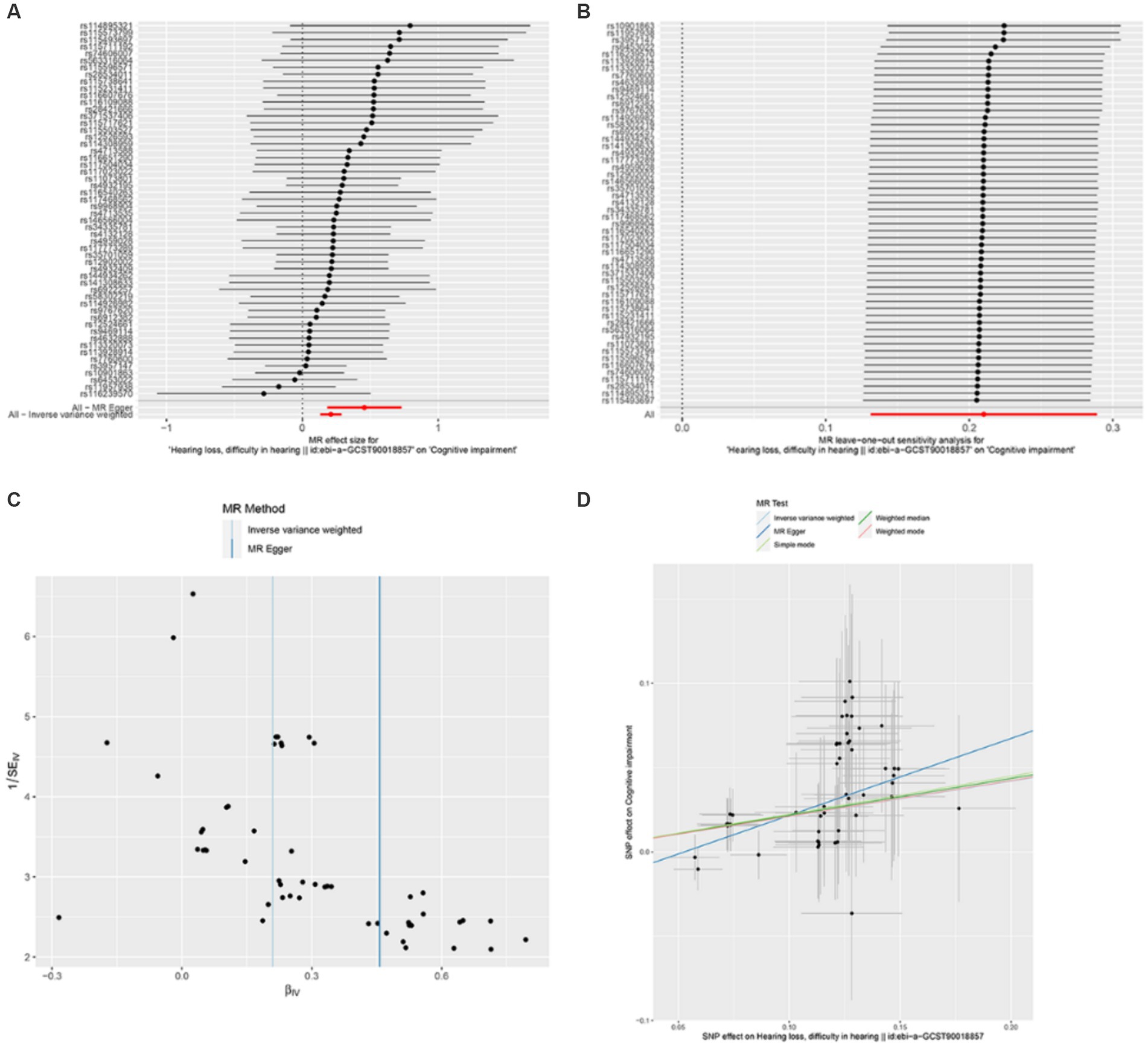
Figure 2. Mendelian randomization analysis between hearing loss and cognitive function. (A) is an effect forest plot of Mendelian randomization showing the effect value of Mendelian randomization for each SNP and the total effect value (beta value), which shows that 50 out of 54 effect SNPs have a hazardous effect, 4 SNPs have a protective effect, and the total effect is a hazardous effect. (B) is a leave-one-out method to analyze the sensitivity of each SNP. After each SNP was taken out, the results of the remaining SNP analysis remained robust. (C) shows the funnel plot to detect bias. The results show that the SNPs are evenly distributed on both sides of the total effect line, demonstrating that the analysis is not significantly biased. (D) shows the scatterplot of the five analytical methods of Mendelian randomization. The horizontal coordinate is the effect of SNP on exposure factors and the vertical coordinate is the effect of SNP on the results. All five methods of analysis showed that hearing loss exerts a detrimental effect on cognitive function.
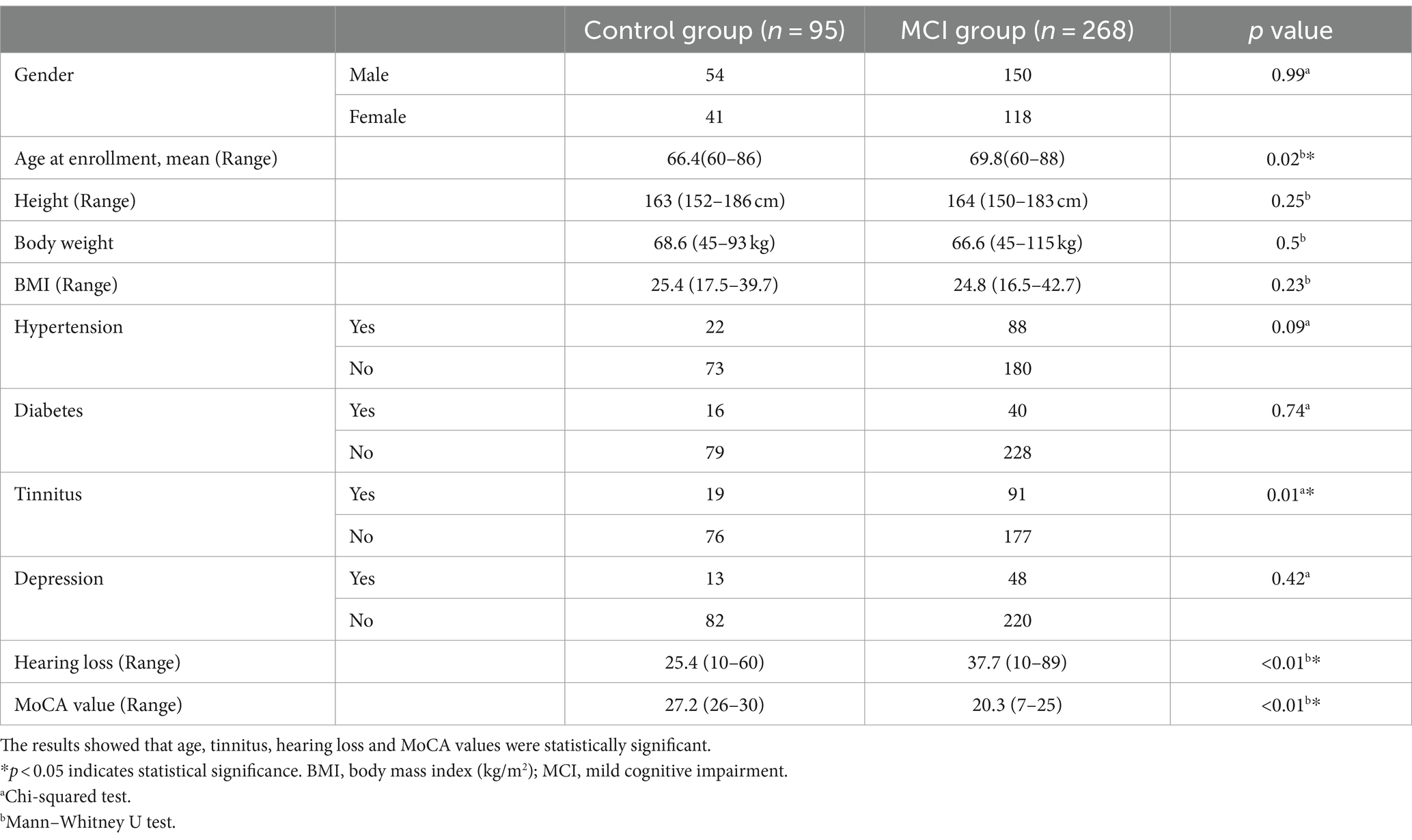
Characteristics of the survey population
As shown in Table 2, a total of 363 subjects were included in this study. They were aged 60 to 88 years, with 204 men and 159 women. According to the MoCA scale, 95 of the 363 study subjects had a normal cognitive function and 268 had a cognitive decline. Age, hearing loss, and tinnitus were significantly different between the two groups, while all the other variables were not significantly different.
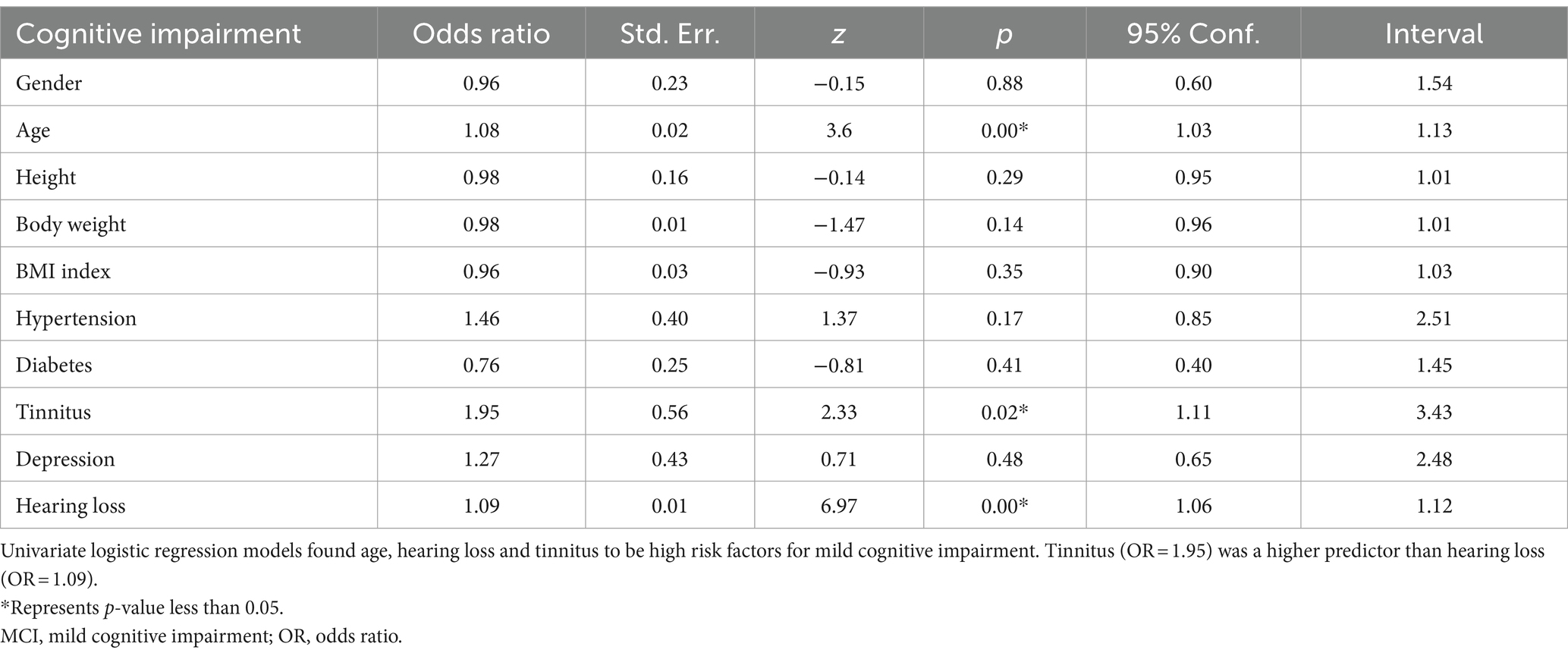
Univariate logistic regression screening for high risk factors
To verify the accuracy of risk factors, we applied univariate logistic regression to continue screening high risk factors for cognitive impairment. As shown in Table 3, the p values for age, hearing loss and tinnitus were statistically significant, and the OR values were both higher than 1. These results suggest that age, hearing loss and tinnitus are high risk factors for cognitive impairment. The results of our analysis suggest that older individuals with tinnitus have a 1.95 times higher risk of developing cognitive impairment compared with normal older people, while the risk of developing cognitive impairment increases by 1.08 times for every year of age. In contrast, in older patients with hearing loss, the risk of developing cognitive impairment increases by 1.09 times for each decibel of hearing loss.
Multi-factor logistic regression to develop a diagnostic model to evaluate the early diagnostic value of hearing loss and tinnitus on MCI
Univariate logistic regression screened variables including age, tinnitus and hearing loss were subjected to multifactorial logistic regression to ultimately screen for high-risk factors that may lead to cognitive impairment. Through multifactorial logistic regression analysis, we found that hearing loss and tinnitus were high risk factors for cognitive impairment as shown in Table 4. Our results identified hearing loss and tinnitus as potential risk factors for cognitive impairment, while age factors were excluded due to p-values greater than 0.05.
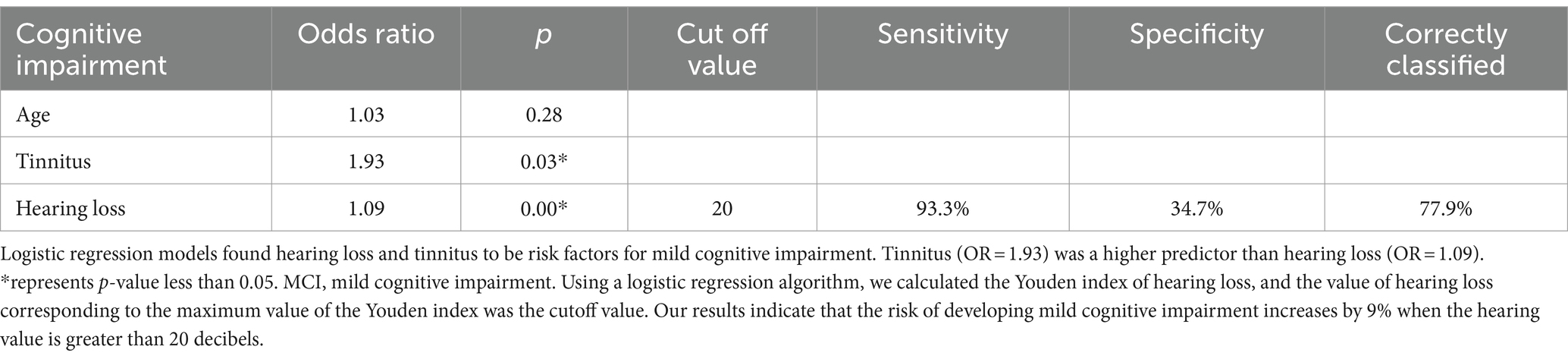
Table 4. Multi-factor logistic regression analysis of the predictive effect of each variable on MCI.
In Figure 3 the ROC diagnostic model was constructed using logistic regression with cognitive impairment as the dependent variable and hearing loss and tinnitus as the independent variables. The results showed that the AUC for the diagnostic value of tinnitus on cognitive impairment was 0.56; the AUC for the diagnostic value of hearing loss on cognitive impairment was 0.77; and the AUC for the diagnostic value of the combined hearing loss and tinnitus on cognitive impairment was 0.78.
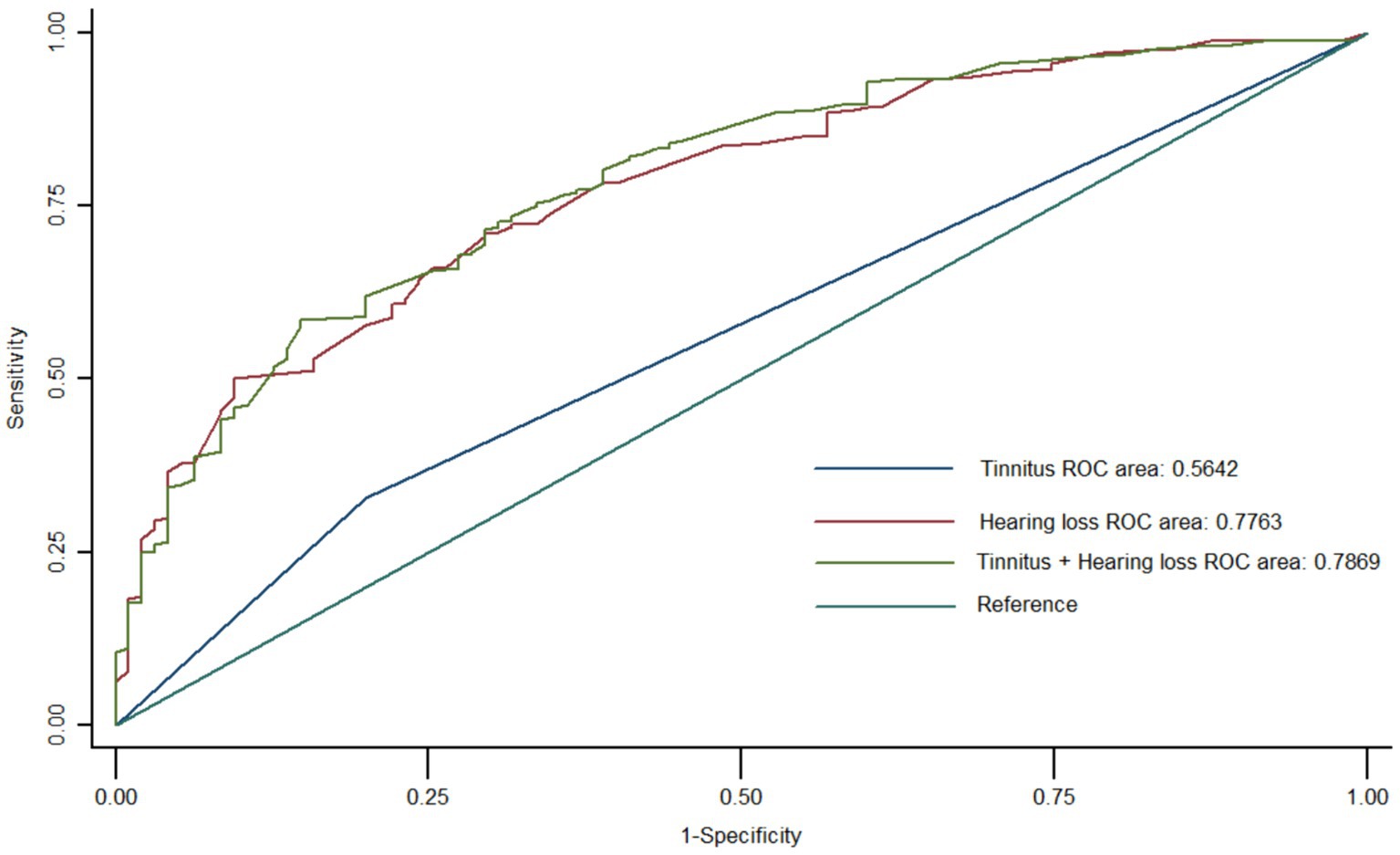
Figure 3. Multifactorial logistic regression model to evaluate the diagnostic efficacy of hearing loss and tinnitus on mild cognitive impairment. Hearing loss has a higher diagnostic efficacy than tinnitus for mild cognitive impairment. Hearing loss and tinnitus have the highest combined diagnostic effect on mild cognitive impairment.
Using a logistic regression algorithm in Table 4, we calculated the Youden index of hearing loss, and the value of hearing loss corresponding to the maximum value of the Youden index was 20 decibels. Our results indicate that the risk of developing mild cognitive impairment increases by 9% when the hearing value is greater than 20 decibels.
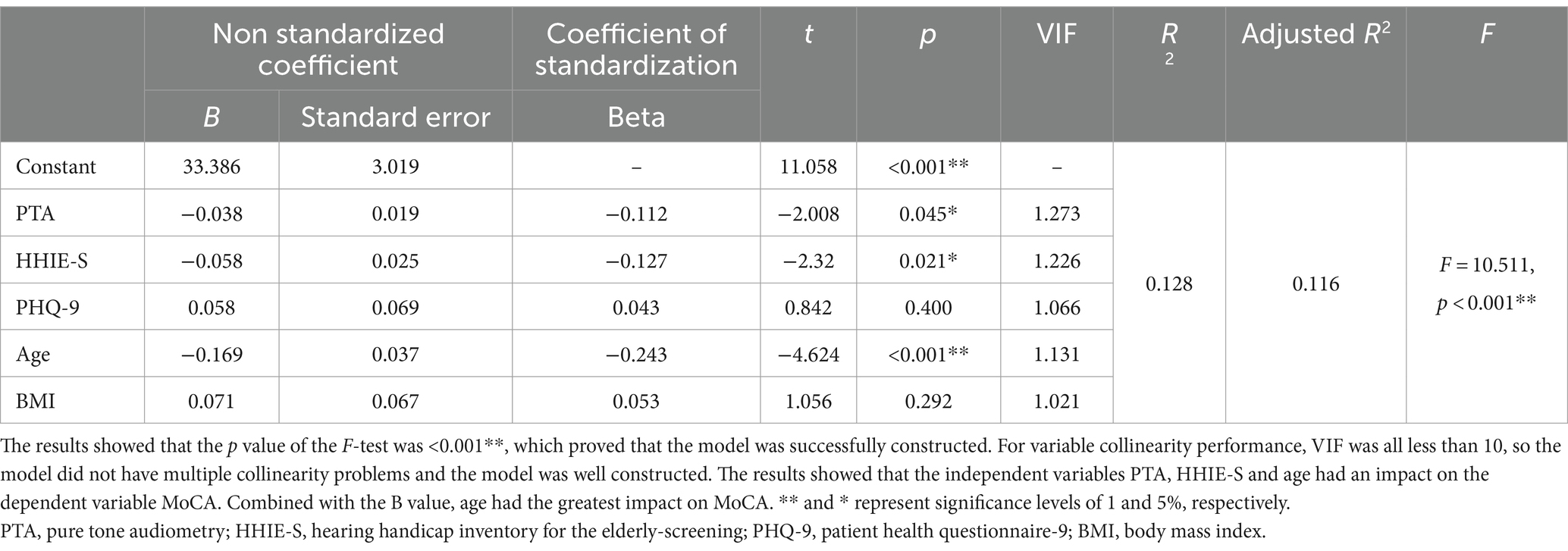
Multiple linear regression was used to explore the influencing factors of MCI
To verify the logistic regression results, multiple linear regression analysis was performed with MoCA as the dependent variable and pure-tone audiometry, HHIE-S, PHQ-9, age, and body mass index as independent variables (Table 5). The model results showed that the p value <0.001**, which proved that the model was successfully constructed. For variable collinearity performance, VIF was all less than 10, so the model did not have multiple collinearity problems and the model was well constructed. The results found that the independent variables PTA, HHIE-S and Age had an impact on the dependent variable MoCA. Combined with the B value, age had the greatest impact on MoCA.
Discussion
Previous studies (Livingston et al., 2017; Chern and Golub, 2019; Livingston et al., 2020) have shown that MCI is a cause of AD, while both hearing loss and tinnitus are strongly associated with cognitive impairment. The cause of tinnitus is complex, may be emotional or fatigue, or tumor, or infection, or chronic diseases like diabetes or high blood pressure, or auditory nerve damage and so on. There are many confounding factors between tinnitus and hearing loss, as well as between tinnitus and cognitive function. Patients with tinnitus do not necessarily have hearing loss, while some patients with hearing loss are accompanied by tinnitus. Therefore, it is difficult to study the pathological relationship among tinnitus, hearing loss and cognitive impairment, and epidemiological investigation is one of the commonly used research methods. This study further validated the association of hearing loss and tinnitus with cognitive impairment through genetic and epidemiological studies across ethnic populations.
Mendelian Randomization is a data analysis technique for assessing etiological inferences in epidemiological studies that uses genetic variants with strong correlations with exposure factors as instrumental variables to assess causal relationships between exposure factors and outcomes (Emdin et al., 2017). In this study, the first Mendelian randomization method using SNPs as an instrumental variable was used to prove the causal relationship between HL and MCI, and it was found that the risk ratio of HL leading to MCI was 1.23. For the first time, we used Mendelian randomization to analyze the causal relationship between hearing loss and cognitive impairment. We identified 54 SNPs that were genetically strongly associated with hearing loss but not cognitive impairment, but whose expression changed when cognitive impairment developed. In the follow-up study, we can use these 54 SNPs as a combination to detect the patients with hearing loss by targeted sequencing, confirm and screen out the most significant SNPs to calculate the diagnostic cutoff value. If the patient’s SNPs value exceeds the cutoff value, it means that the risk of cognitive impairment is very high. At present, there is no biomarker for prediction and diagnosis of cognitive impairment in clinical practice. With the maturity of sequencing technology, the above SNPs may provide new ideas for early prevention and diagnosis of cognitive impairment.
On the basis of MR results, the correlation between HL and MCI were verified with the data from the Chinese checkups. This study initially explored an elderly population in the eastern coastal region of China. By analyzing factors such as pure tone hearing threshold, hearing loss, depression status, cognitive function scales, and chronic illness. Our study demonstrated a high prevalence of early cognitive impairment (73.8%) among Chinese older adults with suspected HL and tinnitus. In addition, this study confirmed the correlation between HL and tinnitus and early cognitive impairment, and explored the predictive and diagnostic ability of HL and tinnitus for early cognitive impairment. This study provides new ideas for the prevention of MCI.
A Brazilian census in 2023 evaluated data on 1,335 older adults which results found the prevalence of cognitive impairment was 20.5%. Older adults with hearing loss were 2.66 times more likely to have cognitive impairment than those without hearing loss (Paiva et al., 2023). A Japanese cross-sectional study using multivariate logistic regression analysis showed that HL was associated with MCI (odds ratio 1.60), independent of age, living alone, memory impairment, and impaired self-care (Saji et al., 2021). Correspondingly, our community survey was not a census, it was a specialized focus on a group of older adults suspected of having hearing loss or tinnitus, and our results demonstrate the higher prevalence of cognitive impairment in older adults suspected of having HL. Our results showed that an independent correlation between hearing loss and cognitive decline with an OR of 1.09. A recent meta-analysis of 34 studies reporting data on 48,017 participants found that people with hearing loss had an overall risk ratio of 1.44 for developing MCI (Mannarelli et al., 2017). Our findings also showed that older people with tinnitus had a 1.95-fold increased risk of MCI compared with normal older people, which is consistent with previous research findings (Brinkmann et al., 2021). In addition, our study did not find that factors such as weight and diabetes were associated with cognitive impairment, and the results are inconsistent with previous studies (Cox and Wallace, 2022). In this study, we calculated the diagnostic efficacy of hearing loss and tinnitus for early cognitive impairment by logistic regression and found that the diagnostic efficacy of hearing loss combined with tinnitus was consistent with the diagnostic efficacy of hearing loss alone for the diagnosis of disease. Subsequently, we calculated the cutoff value of hearing loss and found that the risk of developing mild cognitive impairment increased by 9% when the hearing value was greater than 20 decibels. While our study found that PTA, HHIE-S and age were the influencing factors of MoCA using multiple linear regression analysis, which was consistent with the results of our logistic regression.
The predictive analysis of MCI is complex, with social and clinical factors containing many interactions and covariates (Jang et al., 2017; Hu et al., 2021), and the analysis without proving causality is subject to many confounding factors. In this study, the genetic data of Europeans were first used to demonstrate the causal relationship between hearing loss as exposure factor and cognitive impairment as outcome through Mendelian randomization analysis (Yoneda et al., 2021). Subsequently, a cross-sectional study of the Chinese population was conducted to explore the risk factors for cognitive impairment. In this study, genome-wide association analysis combined with epidemiological investigation was used to explore and verify the risk factors of cognitive impairment in different ethnic groups, and the results have high reliability, providing a new idea for the prevention and early diagnosis of MCI patients.
However, this study has some limitations. First of all, the cognitive function assessment used in this study is an audio-based, oral oriented tool, and hearing loss may affect subjects’ performance during the cognitive assessment process, leading to an overestimation of cognitive decline in patients with hearing loss. Future in-depth research could be conducted by designing longitudinal cohort studies using cognitive function tools appropriate for patients with hearing loss. Second, results may be biased due to design flaws such as small sample sizes or different survey scale choices and different screening models. Third, hearing loss above 4000 Hz was not taken into account, which may cause some bias to the study results. Fourth, the etiology of tinnitus is very complex, and further clinical examination is needed to clarify the etiology of tinnitus. However, the tinnitus information in this study was obtained through the self-report of the respondents, and the results based on this analysis have limitations. In addition, future studies should provide a more specific description of tinnitus examinations in order to better understand the relationship between tinnitus and hearing loss and cognitive function. Finally, free community screening is not a census, which will lead to bias in the data. We hope that the results based on this study can attract the attention of the government, so as to conduct accurate research with the help of the government.
Conclusion
Our Mendelian randomization and cross-sectional studies have showed that HL is a risk factor for MCI. The role of genetic, environmental, and lifestyle factors in the development and progression of hearing loss remains a major area of research in this field. More large-scale population-based epidemiologic surveys and clinical cohort studies should be conducted to explore early diagnosis and intervention strategies for HL based on lifelong hearing health, for reducing the incidence of cognitive impairment and improve the quality of life of the elderly population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Third People’s Hospital of Qingdao, affiliated with Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TX: Formal analysis, Investigation, Software, Writing – original draft. TZ: Investigation, Software, Writing – original draft. JL: Investigation, Methodology, Software, Writing – original draft. LZ: Investigation, Methodology, Supervision, Validation, Writing – review & editing. HG: Investigation, Methodology, Supervision, Writing – review & editing. RY: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. ZL: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the General Project of Shandong Provincial Natural Science Foundation (ZR2020MH058).
Acknowledgments
We would like to thank Prof. Yu Zhou for his help in proofreading the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1380145/full#supplementary-material
Abbreviations
HL, hearing loss; MCI, mild cognitive impairment; AD, Alzheimer’s disease; MoCA, Montreal Cognitive Assessment Scale; OR, odds ratio; BMI, body mass index
Footnotes
References
Babulal, G. M., Zhu, Y., Roe, C. M., Hudson, D. L., Williams, M. M., Murphy, S. A., et al. (2022). The complex relationship between depression and progression to incident cognitive impairment across race and ethnicity. Alzheimers Dement. 18, 2593–2602. doi: 10.1002/alz.12631
Bowl, M. R., and Dawson, S. J. (2019). Age-Related Hearing Loss. Cold Spring Harb. Perspect. Med. 9:33217. doi: 10.1101/cshperspect.a033217
Brinkmann, P., Kotz, S. A., Smit, J. V., Janssen, M. L. F., and Schwartze, M. (2021). Auditory thalamus dysfunction and pathophysiology in tinnitus: a predictive network hypothesis. Brain Struct. Funct. 226, 1659–1676. doi: 10.1007/s00429-021-02284-x
Burgess, S., and Thompson, S. G. (2017). Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32, 377–389. doi: 10.1007/s10654-017-0255-x
Chern, A., and Golub, J. S. (2019). Age-related Hearing Loss and Dementia. Alzheimer Dis. Assoc. Disord. 33, 285–290. doi: 10.1097/WAD.0000000000000325
Clark, J. L., and Swanepoel, W. (2021). The World Report on Hearing - a new era for global hearing care. Int. J. Audiol. 60:161. doi: 10.1080/14992027.2021.1881318
Cox, R., and Wallace, R. B. (2022). The Role of Incarceration as a Risk Factor for Cognitive Impairment. J. Gerontol. B Psychol. Sci. Soc. Sci. 77, e247–e262. doi: 10.1093/geronb/gbac138
Cunningham, L. L., and Tucci, D. L. (2017). Hearing Loss in Adults. N. Engl. J. Med. 377, 2465–2473. doi: 10.1056/NEJMra1616601
Czornik, M., Malekshahi, A., Mahmoud, W., Wolpert, S., and Birbaumer, N. (2022). Psychophysiological treatment of chronic tinnitus: A review. Clin. Psychol. Psychother. 29, 1236–1253. doi: 10.1002/cpp.2708
D'Haese, P., Topsakal, V., and Greenham, P. (2023). The World Report on Hearing, what does it mean for me and how can it improve access to hearing devices? Ear Nose Throat J. :014556132311579. doi: 10.1177/01455613231157937
Emdin, C. A., Khera, A. V., and Kathiresan, S. (2017). Mendelian Randomization. JAMA 318, 1925–1926. doi: 10.1001/jama.2017.17219
Hartley, A. E., Power, G. M., Sanderson, E., and Smith, G. D. (2022). A Guide for Understanding and Designing Mendelian Randomization Studies in the Musculoskeletal Field. JBMR Plus 6:e10675. doi: 10.1002/jbm4.10675
Hu, M., Shu, X., Yu, G., Wu, X., Välimäki, M., and Feng, H. (2021). A Risk Prediction Model Based on Machine Learning for Cognitive Impairment Among Chinese Community-Dwelling Elderly People With Normal Cognition: Development and Validation Study. J. Med. Internet Res. 23:e20298. doi: 10.2196/20298
Jafari, Z., Kolb, B. E., and Mohajerani, M. H. (2019). Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res. Rev. 56:100963. doi: 10.1016/j.arr.2019.100963
Jang, H., Ye, B. S., Woo, S., Kim, S. W., Chin, J., Choi, S. H., et al. (2017). Prediction Model of Conversion to Dementia Risk in Subjects with Amnestic Mild Cognitive Impairment: A Longitudinal, Multi-Center Clinic-Based Study. J. Alzheimers Dis. 60, 1579–1587. doi: 10.3233/JAD-170507
Jarach, C. M., Lugo, A., Scala, M., van den Brandt, P. A., Cederroth, C. R., Odone, A., et al. (2022). Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-analysis. JAMA Neurol. 79, 888–900. doi: 10.1001/jamaneurol.2022.2189
Ji, D., Chen, W. Z., Zhang, L., Zhang, Z. H., and Chen, L. J. (2024). Gut microbiota, circulating cytokines and dementia: a Mendelian randomization study. J. Neuroinflammation 21:2. doi: 10.1186/s12974-023-02999-0
Jia, R. X., Liang, J. H., Xu, Y., and Wang, Y. Q. (2019). Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. 19:181. doi: 10.1186/s12877-019-1175-2
Jia, X., Wang, Z., Huang, F., Su, C., du, W., Jiang, H., et al. (2021). A comparison of the Mini-Mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: a cross-sectional study. BMC Psychiatry 21:485. doi: 10.1186/s12888-021-03495-6
Kim, G., Na, W., Kim, G., Han, W., and Kim, J. (2016). The development and standardization of Self-assessment for Hearing Screening of the Elderly. Clin. Interv. Aging 11, 787–795. doi: 10.2147/CIA.S107102
Larsson, S. C., Butterworth, A. S., and Burgess, S. (2023). Mendelian randomization for cardiovascular diseases: principles and applications. Eur. Heart J. 44, 4913–4924. doi: 10.1093/eurheartj/ehad736
Lau, K., Dimitriadis, P. A., Mitchell, C., Martyn-St-James, M., Hind, D., and Ray, J. (2022). Age-related hearing loss and mild cognitive impairment: a meta-analysis and systematic review of population-based studies. J. Laryngol. Otol. 136, 103–118. doi: 10.1017/S0022215121004114
Lee, S. Y., Lee, J. Y., Han, ·. S. Y., Seo, Y., Shim, Y. J., and Kim, Y. H. (2020). Neurocognition of Aged Patients With Chronic Tinnitus: Focus on Mild Cognitive Impairment. Clin Exp Otorhinolaryngol 13, 8–14. doi: 10.21053/ceo.2018.01914
Levin, M. G., and Burgess, S. (2024). Mendelian Randomization as a Tool for Cardiovascular Research: A Review. JAMA Cardiol. 9, 79–89. doi: 10.1001/jamacardio.2023.4115
Levis, B., Benedetti, A., and Thombs, B. D. (2019). Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ 365:l1476. doi: 10.1136/bmj.l1476
Lin, F. R., Pike, J. R., Albert, M. S., Arnold, M., Burgard, S., Chisolm, T., et al. (2023). Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet 402, 786–797. doi: 10.1016/S0140-6736(23)01406-X
Lin, F. R., Yaffe, K., Xia, J., Xue, Q. L., Harris, T. B., Purchase-Helzner, E., et al. (2013). Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 173, 293–299. doi: 10.1001/jamainternmed.2013.1868
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734. doi: 10.1016/S0140-6736(17)31363-6
Loughrey, D. G., Kelly, M. E., Kelley, G. A., Brennan, S., and Lawlor, B. A. (2018). Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 144, 115–126. doi: 10.1001/jamaoto.2017.2513
Mannarelli, D., Pauletti, C., Mancini, P., Fioretti, A., Greco, A., de Vincentiis, M., et al. (2017). Selective attentional impairment in chronic tinnitus: Evidence from an event-related potentials study. Clin. Neurophysiol. 128, 411–417. doi: 10.1016/j.clinph.2016.12.028
Mazurek, B., Hesse, G., Dobel, C., Kratzsch, V., Lahmann, C., Sattel, H., et al. (2022). Chronic Tinnitus. Dtsch. Arztebl. Int. 119, 219–225. doi: 10.3238/arztebl.m2022.0135
McMahon, C. M., Nieman, C. L., Thorne, P. R., Emmett, S. D., and Bhutta, M. F. (2021). The inaugural World Report on Hearing: From barriers to a platform for change. Clin. Otolaryngol. 46, 459–463. doi: 10.1111/coa.13756
Paiva, K. M., Böell, A. L., Haas, P., Samelli, A. G., Hillesheim, D., Figueiró, T. H., et al. (2023). Self-reported hearing loss and cognitive impairment: a cross-sectional analysis of the EpiFloripa Aging study. Cad. Saude Publica 39:e00127622. doi: 10.1590/0102-311xen127622
Ronnberg, J., Lunner, T., Zekveld, A., Sörqvist, P., Danielsson, H., Lyxell, B., et al. (2013). The Ease of Language Understanding (ELU) model: theoretical, empirical, and clinical advances. Front. Syst. Neurosci. 7:31. doi: 10.3389/fnsys.2013.00031
Rutherford, B. R., Brewster, K., Golub, J. S., Kim, A. H., and Roose, S. P. (2018). Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. Am. J. Psychiatry 175, 215–224. doi: 10.1176/appi.ajp.2017.17040423
Saji, N., Makizako, H., Suzuki, H., Nakai, Y., Tabira, T., Obuchi, S., et al. (2021). Hearing impairment is associated with cognitive function in community-dwelling older adults: A cross-sectional study. Arch. Gerontol. Geriatr. 93:104302. doi: 10.1016/j.archger.2020.104302
Sanderson, E. (2021). Multivariable Mendelian Randomization and Mediation. Cold Spring Harb. Perspect. Med. 11:38984. doi: 10.1101/cshperspect.a038984
Storey, J. D., and Tibshirani, R. (2003). Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 100, 9440–9445. doi: 10.1073/pnas.1530509100
Vasilescu, L., and Weisman, A. E. (2023). Diagnostic Value of Different Types of Tinnitus. Int. Tinnitus J. 27, 47–53. doi: 10.5935/0946-5448.20230008
Wang, Y., Zhang, J. N., Hu, W., Li, J. J., Zhou, J. X., Zhang, J. P., et al. (2018). The characteristics of cognitive impairment in subjective chronic tinnitus. Brain Behav. 8:e00918. doi: 10.1002/brb3.918
Wang, H. F., Zhang, W., Rolls, E. T., Li, Y., Wang, L., Ma, Y. H., et al. (2022). Hearing impairment is associated with cognitive decline, brain atrophy and tau pathology. EBioMedicine 86:104336. doi: 10.1016/j.ebiom.2022.104336
Yoneda, K., Hababeh, M., Kitamura, A., Seita, A., and Kamiya, Y. (2021). Prevalence and characteristics of Palestine refugee mothers at risk of postpartum depression in Amman, Jordan: a cross-sectional study. Lancet 398:S28. doi: 10.1016/S0140-6736(21)01514-2
Zhang, Y., Chen, J., Zhang, Y., Sun, B., and Liu, Y. (2022). Using Auditory Characteristics to Select Hearing Aid Compression Speeds for Presbycusic Patients. Front. Aging Neurosci. 14:869338. doi: 10.3389/fnagi.2022.869338
Keywords: Mendelian randomization, mild cognitive impairment, hearing loss, tinnitus, risk
Citation: Xu T, Zong T, Liu J, Zhang L, Ge H, Yang R and Liu Z (2024) Correlation between hearing loss and mild cognitive impairment in the elderly population: Mendelian randomization and cross-sectional study. Front. Aging Neurosci. 16:1380145. doi: 10.3389/fnagi.2024.1380145
Edited by:
Chinedu Udeh-Momoh, Wake Forest University, United StatesReviewed by:
Anusha Yasoda-Mohan, Trinity College Dublin, IrelandDarina Bassil, Harvard University, United States
Copyright © 2024 Xu, Zong, Liu, Zhang, Ge, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Yang, jhtyr@sina.com; Zongtao Liu, liutao009@163.com
†These authors have contributed equally to this work
 Tong Xu1†
Tong Xu1†