
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci., 28 February 2024
Sec. Cellular and Molecular Mechanisms of Brain-aging
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1368232
This article is part of the Research TopicNeurosensory and Neuro-Cognitive Aging: Molecular and Cellular Mechanisms and Therapeutic Perspectives.View all 4 articles
 He Zhao1,2,3†
He Zhao1,2,3† Yan Wang1,2,3†
Yan Wang1,2,3† Limei Cui2,3†
Limei Cui2,3† Hanjing Wang2,3,4
Hanjing Wang2,3,4 Sha Liu2,3
Sha Liu2,3 Tiantian Liang2,3
Tiantian Liang2,3 Dawei Liu2,3
Dawei Liu2,3 Jingjing Qiu2,3*
Jingjing Qiu2,3* Liang Chen2,3*
Liang Chen2,3* Yan Sun2,3*
Yan Sun2,3*Sensorineural hearing loss (SNHL) is a category of hearing loss that often leads to difficulty in understanding speech and other sounds. Auditory system dysfunction, including deafness and auditory trauma, results in cognitive deficits via neuroplasticity. Cognitive impairment (CI) refers to an abnormality in the brain’s higher intellectual processes related to learning, memory, thinking and judgment that can lead to severe learning and memory deficits. Studies have established a strong correlation between SNHL and CI, but it remains unclear how SNHL contributes to CI. The purpose of this article is to describe three hypotheses regarding this relationship, the mainstream cognitive load hypothesis, the co-morbidity hypothesis, and the sensory deprivation hypothesis, as well as the latest research progress related to each hypothesis.
Hearing loss (HL) is a serious condition that not only diminishes a patient’s quality of life but can also lead to lifelong disability. According to a global survey conducted in 2016, HL ranks as the fourth most prevalent disorder and one of the top five contributors to disability (Vos et al., 2017). Sensorineural hearing loss (SNHL), a type of HL, accounts for 90% of reported cases of HL (Li et al., 2017) and leads to difficulties in directly understanding speech and other sounds. Cross-sectional and longitudinal evidence suggests that dysfunction of the auditory system, including deafness and auditory trauma, leads to cognitive deficits that develop via neuroplasticity (Rutherford et al., 2018; Swords et al., 2018). SNHL is generally categorized as age-related hearing loss (ARHL), noise-related hearing loss, or drug-related hearing loss (DRHL).
Cognitive function (CF) refers to the processes by which the human brain receives external information, processes it, and converts it into internal mental activity in order to acquire knowledge or apply it. CF encompasses memory, language skills, visuospatial abilities, executive functions, computational skills, and comprehension judgments (Liu et al., 2024; Mellow et al., 2024). Cognitive impairment (CI) refers to a pathological process in which abnormalities exist in the higher intellectual processes of the brain related to learning and memory as well as thinking and judgment (Sanford, 2017). Such impairment results in severe deficits in learning and memory accompanied by changes such as aphasia or dysfunction/loss of recognition or behaviors. The pathological changes in the brain associated with CI development primarily involve lesions within the hippocampus.
The aim of this review is to describe the current hypotheses on how CI results from SNHL and to provide an update on research progress in this field. In some experiments involving mice subjected to inner ear hair cell ablation (Liu et al., 2020; Qian and Ricci, 2020) and in animals chronically exposed to noise (Patel et al., 2022), CI is observed after a period of hearing impairment. RNA sequencing analysis has revealed that ARHL shares a common causative gene with Alzheimer’s disease (AD; Xue et al., 2021). Numerous experimental studies have identified multiple mechanisms by which SNHL can lead to CI (Loughrey et al., 2018; Choi et al., 2021; Bikbov et al., 2022). In response to these findings, researchers have proposed three hypotheses on how SNHL brings about CI: the cognitive load (CL) hypothesis, the co-morbidity hypothesis, and the sensory deprivation hypothesis (Slade et al., 2020; Figure 1).
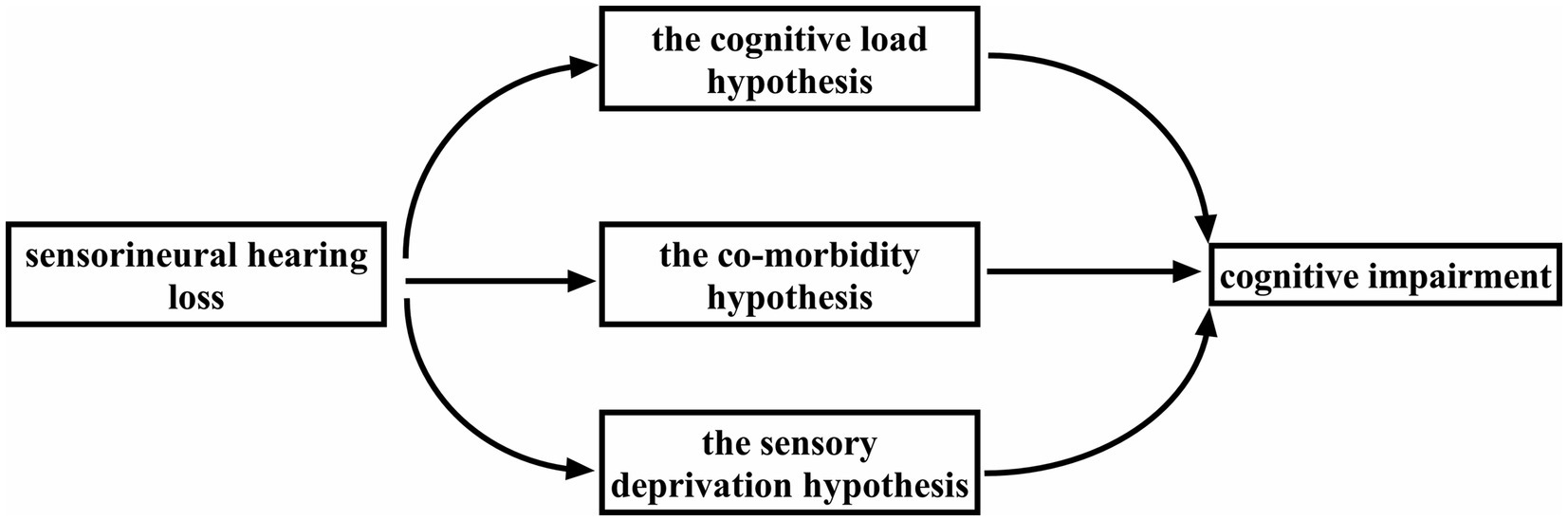
Figure 1. Three hypotheses regarding how SNHL leads to CI: the cognitive load 72 hypothesis, the co-morbidity hypothesis, and the sensory deprivation hypothesis.
The CL hypothesis suggests that the amount of information within the brain that can be processed, held in memory, and accessed at any given time is limited due to the limited availability of processing resources (Wingfield, 2016). ARHL results in patients receiving less information from the outside world. To achieve as much processing and preservation as possible of the limited amount of acoustic information, a higher demand is placed on the limited processing resources of patients with HL. This means that these patients need more cognitive resources for auditory perceptual processing. As a result, cognitive resources are diverted from other cognitive tasks to listening efforts, leading to a depletion of cognitive resources (Wingfield et al., 2005; Tun et al., 2009). This reallocation of resources has a detrimental effect on CF and could theoretically lead to a decrease in cognitive performance (Humes et al., 2013).
Research to date regarding CL has found increases in neurovascular coupling responses as a result of CL (Csipo et al., 2021). One study (Luan et al., 2019) found that patients with SNHL showed a significant increase in distal functional coupling between the dorsolateral prefrontal cortex and the auditory cortex. Additionally, as the hearing status decreases, this coupling response becomes stronger. As is well established, the brain is naturally divided into four regions by the sulcal gyrus: the frontal, parietal, occipital and temporal lobes. The temporal lobe is currently thought to be primarily responsible for language function and auditory perception, as well as involved in long-term memory and emotion (Buchanan et al., 2006). The superior temporal gyrus, where the auditory center is located, is located in the part of the brain between the lateral and superior temporal sulci on the temporal lobe (Tae et al., 2014). In the brains of patients with HL, activities in and loads on the auditory center are increased to allow patients to better recognize acoustic signals, and correspondingly, other areas of cognitive reserve need to be called upon for auditory use, such as memory, emotion, language, and other areas (Hughes et al., 2018). Studies related to the use of hearing aids (Qian et al., 2016; Bucholc et al., 2022) and cochlear implants (Mertens et al., 2021) have demonstrated that as hearing aids and cochlear implants help to restore auditory perception, the onset of CI is delayed. It is hypothesized that hearing aids (Reinten et al., 2021) and cochlear implants (Chatterjee et al., 2023) improve the “effortful process” of sound discrimination in the daily lives of SNHL patients, and that this change reduces the previous over-allocation of cognitive resources, helping to restore balance in the CL.
Research has demonstrated that the balance between excitation and inhibition in the brain is disrupted under different CLs (Bezalel et al., 2019). This balance is critical for the stability of cortical networks. Patients with ARHL have now been found to have reduced levels of gamma- aminobutyricacid (GABA) and glutamate (Glu) in the auditory center (Li et al., 2023). GABA is an important inhibitory neurotransmitter in the brain that is known to regulate inhibitory neurotransmission within the auditory system (Kotak et al., 2008). One study reported a correlation between the mean GABA level in the auditory cortex and mean binaural hearing thresholds, with greater HL associated with lower mean GABA levels. Further research (Dobri and Ross, 2021) found that older adults with ARHL have greater difficulty understanding speech in noisy environments as their GABA level declines. Bezalel et al. (2019) noted that GABA secretion in the dorsal anterior cingulate cortex (ACC), which plays a role in controlling behaviors (Vassena et al., 2020), is increased in high CL situations. GABA, however, is decreased in the auditory center of the temporal lobe in ARHL patients (Zemaitis et al., 2021), which does not contradict the previous decrease in total GABA. Interestingly, it has been suggested that increasing GABA in the auditory cortex or increasing the sensitivity of GABA receptors enhances the response of the auditory center to sound stimuli (Brecht et al., 2017). This reinforces the idea that HL causes a redistribution of CL, resulting in CI.
Additionally, some evidence is found in the literature that Glu also accumulates in the brains of animals with ARHL (Tadros et al., 2007; Kirschmann et al., 2019). Glu is the most abundant free amino acid in the brain as well as the main excitatory neurotransmitter in the brain. Glu can be released into the synaptic gap in a vesicular manner (Franco et al., 2021). Accumulation of Glu can increase CL in the auditory center and affect CL distribution. N-Methyl-D-aspartic acid receptors (NMDARs) are central mediators of glutamatergic neurotransmission and widely distributed, mainly regulating the inward flow of Ca2+ ions into neuronal cells and influencing neuronal activity (Joshi et al., 2019). This process is crucial in synaptic plasticity, which underlies activity-dependent learning and memory. Glu was found to be involved in regulating the channel opening of NMDARs (Wang et al., 2021), and overstimulation of NMDARs enhances the release of Glu via calcium channels. Under excitotoxic conditions, Glu leads to synaptic loss and elimination in hippocampal pyramidal cells (Companys-Alemany et al., 2022; Keimasi et al., 2023). It is now widely accepted that over-activation of NMDARs leads to the development of AD (Xu et al., 2021; Abad-Perez et al., 2023). However, recent studies have found that NMDARs are not only expressed in the cranial brain, but also in inner hair cells (IHCs), and that overactivation of NMDARs also results in Glu release in IHCs (Tang et al., 2014; Kaur et al., 2020; Song et al., 2021). Excessive Glu release causes excitatory neurotoxic effects, reducing the number of ribbon synapses in the cochlea and altering synaptic morphology, resulting in impaired signaling of the cochlear nerve and affecting the patient’s hearing (Hong et al., 2018). NMDARs are involved in the regulation of neurogenesis and spatial memory formation, with the NR2A and NR2B subunits playing crucial roles (Hu et al., 2008; Sun et al., 2011). Bone morphogenic protein 4 (BMP4) is a member of the transforming growth factor-β (TGF-β) family, which is involved in the regulation of cell proliferation and survival. Chen et al. (2014) found that BMP4 regulates cochlear epithelial cell survival by altering the expression of the NR2B subunit of NMDARs. Song et al. (2021) and Ralli et al. (2014) found that administration of NMDA channel blockers in their experimental animal model delayed the onset of salicylic acid-induced DRHL and tinnitus. α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMDARs) are Glu-gated ion channels that mediate most of the rapid excitatory synaptic transmission in the brain (Diering and Huganir, 2018). In addition to NMDARs, studies have shown that AMDARs are also modulated by neurotransmitters (Brechet et al., 2017). Previous research has revealed that CI results in a reduction of synaptic AMPAR in the hippocampus (Chang et al., 2006). Specifically, in the cochlea, AMDAR is primarily located in the nerve endings near the base of the IHCs (Hong et al., 2018), Furthermore, in the cochlea, AMDAR plays a crucial role in mediating rapid excitatory transmission at mature spiral ganglion neurons SGNs afferent synapses (Lozier et al., 2023). Interestingly, changes in the subunit composition of synaptic AMDAR occur as a result of HL, leading to long-term effects on synaptic integration (Pilati et al., 2016).
The co-morbidity hypothesis, or common etiology hypothesis, presumes that CI and SNHL are due to a common cause. Clinically, physicians have found by magnetic resonance imaging (MRI) that the volume of temporal gray matter (TGM) is significantly less in patients with AD than in normal older adults (Li et al., 2020), and the same shrinkage of TGM occurs in patients with ARHL (Armstrong et al., 2019; Slade et al., 2022).
The limbic system (LS; Kalus et al., 2006) includes the pear-shaped cortex, internal olfactory area, orbital gyrus, cingulate gyrus (CG), subcallosal gyrus, hippocampal gyrus, insula, temporal pole, amygdala, septum, preoptic area, hypothalamus, hippocampus, papillae, etc. The LS is extensively interconnected with the rest of the nervous system and is closely associated with sensation, regulation of visceral activity, emotion, behavior, learning, and memory (Manchella et al., 2023). The CG consists mainly of the ACC and posterior cingulate cortex (PCC). Adults with ARHL show significant volume atrophy in the ACC, PCC, precentral gyrus, postcentral gyrus, and parahippocampus on MRI in comparison to normal individuals (Belkhiria et al., 2019). The PCC plays an important role in CFs such as episodic memory, spatial attention, and self-evaluation. The precentral gyrus, located in the frontal lobe, is the highest somatic motor center and is responsible for movement of the contralateral limbs. The postcentral gyrus is located in the parietal lobe and is the highest sensory center. The parahippocampus is also part of the LS, and previous studies have shown that they are closely related to emotion regulation (Kleen et al., 2010). In addition to the reduced volume of the TMG and LS, Paciello et al. (2023) found that the auditory cortex (ACx) is damaged in a mouse model of ARHL, and Xu et al. (2023) found that rats given noise stimulation for 6 months exhibited not only binaural HL but also damage to the ACx.
Amyloid plaques formed by β-amyloid (Aβ) and neurofibrillary tangles formed by abnormally modified tau proteins are the hallmarks of AD (Skouras et al., 2020; Ossenkoppele et al., 2022; Tautou et al., 2023). Aβ is a peptide produced by hydrolysis of amyloid precursor protein (APP), and excess Aβ accumulation in mitochondria activates astrocytes and microglia, damages neurons (Huang et al., 2023), and induces mitochondrial autophagy, promoting reactive oxygen species (ROS) production and accelerating neural oxidation (Dou and Tan, 2023). At the same time, over-phosphorylation of tau protein eliminates its ability to form and maintain stable microtubules, reduces the dissociation of microtubule protein molecules, and induces microtubule bundling (Kandimalla et al., 2018; Solas et al., 2023; Zhang et al., 2023). In turn, this affects neuronal cell signaling and the mitosis of other cell types in the brain. Notably, some studies have found that Aβ and tau protein levels are significantly elevated in the brain of HL patients (Xu et al., 2019; Golub et al., 2021; Irace et al., 2022; Wang et al., 2022; Zheng et al., 2022). Consistently, both Aβ and tau were found to be significantly elevated in patients with AD plus HL (Zhang et al., 2022).
Although current clinical studies have found a link between SNHL and CI, no clear pathway for this process has been established. The co-morbidity hypothesis suggests that pathways such as oxidative damage, neuroinflammation and accumulation of harmful substances may be a common cause of both SNHL and CI.
Oxidative damage (Maurya et al., 2022) and neuroinflammation have long been recognized as significant features of neurodegenerative diseases. Previous research has indicated that oxidative damage can impact the activity and expression of DNA methyltransferases. Specifically, an elevated expression of DNA methyltransferase 1 (DNMT1) has been linked to memory impairment in amnesic mice (Srivas and Thakur, 2017). Case reports have also documented that mutations in DNMT1 can lead to hereditary sensory and autonomic neuropathy with CI (Klein et al., 2011).
Adenosine is an endogenous purine nucleoside, and adenosine receptors, including A1R, A2R and A3R, are involved in the regulation of neurotransmitter release, oxidative stress responses, inflammation, blood flow, and a variety of intracellular signaling pathways including apoptosis. Activation of A1R protects inner ear hair cells, reduces hair cell death, and effectively protects against noise damage (Kaur et al., 2016; Chang et al., 2017; Fok et al., 2020). Inhibition of A1R specifically was shown to cause cochlear nerve damage and to increase susceptibility to HL (Vlajkovic et al., 2017). However, intracranially, A1R has a neuroprotective effect (Shi et al., 2021), and disruption of A1R exacerbates long-term potentiation of the hippocampus, which can cause CI (Zhang et al., 2020). A2R represents a class of adenosine receptors that have been extensively studied and found to be associated with pathophysiological conditions such as inflammatory diseases and neurodegenerative disorders. In contrast to the neuroprotective effect of A1R in the ear, inhibition of adenosine A2A receptor (A2AR) increases the resistance of the cochlea to acoustic damage (Vlajkovic et al., 2017; Shin et al., 2021; Oliveros et al., 2022). Within the cranium, activation of A2AR promotes neuroinflammation, reducing synaptic plasticity (Merighi et al., 2022; Chen et al., 2023). However, adenosine A2B receptor was shown to have the opposite effect of A1R (Qiang et al., 2021).
Pattern recognition receptors (PRRs) are important components of the body’s innate immune system, are widely distributed, and are present in a variety of forms. In the brain, glial cells are known to express a variety of PRRs. Toll-like receptors (TLRs) are one type of PRR. Different TLRs determine not only which ligands are recognized but also the nature of the signals generated (Blasius and Beutler, 2010). Among the many TLRs, TLR4 in particular has been studied more in HL (Müller, 2020). TLR4 is activated primarily by lipopolysaccharide from Gram-negative bacteria (Bowyer et al., 2020) and sequentially triggers the immune response of the body, and studies have found that overexpression of TLR4 in the brain activates the nuclear factor kappa B (NF-κB) signaling pathway (Wang et al., 2021; Abd El-Rahman and Fayed, 2022; Kwon and Lee, 2022), exacerbating the inflammatory response, activating microglia, damaging neurons, and causing CI (van Well et al., 2012; Zhang et al., 2018; Campolo et al., 2019; Potter et al., 2019; Tang et al., 2021; Islam et al., 2022). However, other studies have provided evidence that mild activation of TLR4 can enhance the phagocytic capacity of glial cells, clear accumulated Aβ early, and delay the onset of CI (Qin et al., 2016; Wu et al., 2022). In addition to its expression in brain tissue, TLR4 is also expressed in cochlear tissue (Zhang et al., 2019) and, upon lipopolysaccharide stimulation, activates a range of immune responses via the NF-κB signaling pathway (Si et al., 2015; Liu et al., 2020). A recent study found that overexpression of TLR4 causes a significant increase in HL (Zhang et al., 2019). Therefore, we suggest that the process of chronic immune activity induced by the TLR4-mediated NF-κB signaling pathway may be one of the potential mechanisms responsible for HL with CL.
The interstitial fluid and cerebrospinal fluid are two extracellular fluids present in the cranium. These two extracellular fluids not only provide protective buffering for the brain, but also are involved in the transport of nutrients and waste products, the maintenance of electrolyte homeostasis (Voisin et al., 1999), and signal transduction. Maintenance of this homeostasis in the brain is regulated by the aquaporins (AQPs; Benga and Huber, 2012). The AQPs are cell membrane proteins with the main function of controlling the movement of water in and out of the cell (Voisin et al., 1999). Aquaporin 4 (AQP4) is the major AQP found in the brain where it plays a significant role in water homeostasis. Current research has established that diminished AQP4 expression leads to reduced Aβ clearance and causes Aβ accumulation, leading to deficiencies in memory and learning ability and the development of CI (Hubbard et al., 2018; Chandra et al., 2021; Fang et al., 2021; Liu et al., 2022; Wang et al., 2022; Vasciaveo et al., 2023). In addition to regulating water homeostasis, AQP4 also activates astrocytes (Yang et al., 2022; Liu et al., 2023), and outside of the brain, it is expressed by IHCs (Christensen et al., 2009; Nishio et al., 2013). AQP4 is now widely believed to be involved in maintaining the osmotic balance during the K+ cycle as well as the ionic balance of the endolymphatic fluid (Li and Verkman, 2001; Mhatre et al., 2002). AQP3 and AQP5 are also expressed in the cochlea and, like AQP4, are involved in regulating the ionic balance of endolymphatic fluid. Research studies have proposed that AQPs are closely associated with Ménière’s disease (Eckhard et al., 2014, 2015; Nevoux et al., 2015).
In addition to TLRs, the nucleotide-binding oligomerization domain-like receptor family is the most representative class of PRRs. NLR pyrin domain-containing 3 (NLRP3) is a member of this family that is associated with many diseases. Studies have shown that microglia-mediated activation of NLRP3 is closely related to cognition (Guo et al., 2020; Feng et al., 2021). Mitochondrial damage or production of mitochondrial ROS (mtROS) is an important regulator of NLRP3 activation. mtROS activates NLRP3 in microglia, which in turn promotes activation of caspase-1, a downstream protein of NLRP3, and the secretion of IL-1β and IL-18, which in turn stimulates a neuroinflammatory response (Tian et al., 2021; Barczuk et al., 2022; Liang et al., 2022; Li et al., 2023; Su et al., 2023). Two trials have reported that treatment with NLRP3 inhibitors is effective at alleviating the onset of CI (Zheng et al., 2022; Lin et al., 2023). NLRP3 has also been found to be associated with both AD and HL. In both genetic deafness (Nakanishi et al., 2018; Ma et al., 2022) or SNHL (Kim et al., 2021), NLRP3 is thought to induce inflammatory damage in IHCs and SGNs via ROS activation and to reduce the occurrence of autophagy. The study by Sai et al. (2022) showed that noise exposure activates NLRP3 inflammation in the cochlea and increases the production of IL-18 and IL-1β, inducing inflammation in the cochlea. Tang et al. (2021) found that exogenous application of the chemical BDE-47 activates ROS and NLRP3 inflammatory vesicles in cochlear hair cells as well as the p38 MAPK pathway, causing HL.
The sensory deprivation hypothesis shares some conceptual commonalities with the CL hypothesis, but it places greater emphasis on the long-term reallocation of cognitive resources toward hearing in patients with SNHL due to chronic sensory deprivation, which leads to cognitive decline (Lindenberger and Baltes, 1994; Humes et al., 2013). This hypothesis highlights that prolonged sensory deprivation leads to compensatory cortical reorganization and neural alterations that hinder general cognitive and affective processes. Previous studies have provided evidence supporting cortical alterations in ARHL, including an increased reliance on frontal brain regions during speech perception (Du et al., 2016; Rosemann and Thiel, 2018) and a reduction in gray matter in the auditory cortex caused by diminished hearing ability (Eckert et al., 2019). While inadequate sensory input directly affects cognition through deprivation, it may also indirectly impact cognition through reduced socialization and communication or increased depression (Dawes et al., 2015; Stahl, 2017). This hypothesis suggests that decreased social interaction associated with social isolation and depression may mediate the causal relationship between HL and cognitive decline (Dawes et al., 2015; Whitson et al., 2018). Indeed, significant associations have been found between depressive symptoms, heightened social isolation, and diminished quality of life among patients with SNHL (Panza et al., 2019). According to this perspective, neural changes resulting from SNHL, such as reduced ACC activation, can directly influence mood and emotion regulation (Husain et al., 2014). The anterior ventral location of the ACC within the corpus callosum plays a crucial role in depressive symptoms, and thus, a reduction in ACC volume can lead to impaired emotion processing (Belkhiria et al., 2019).
A significant relationship was identified between HL and emotional loneliness (Jayakody et al., 2022). A study in the United Kingdom reported that the adverse effects of HL are not limited to hearing impairment but may also include negative effects on psychosocial health (Tsimpida et al., 2022). In another study, middle-aged and elderly patients with HL were more likely to have diminished health status, depression, and suicidal ideation compared with participants without HL (Park et al., 2022). Additional studies (Chern et al., 2022; Huang et al., 2022) have suggested that HL can contribute to psychosocial disorders in patients. An MRI study revealed that gray matter volume in the middle cingulate cortex is positively correlated with high-frequency hearing impairment in patients with ARHL (Ma et al., 2022). These results suggest that HL can influence mental health.
An epidemiological investigation estimated that by 2023, 6.7 million Americans aged 65 years and older would have AD and that 73% of Americans 75 years or older would be affected (Alzheimer's and dementia: the journal of the Alzheimer's Association, 2023). In this review, we explore three hypotheses for the co-occurrence of SNHL and CI: the CL hypothesis, the co-morbidity hypothesis, and the sensory deprivation hypothesis. The CL hypothesis emphasizes that when acoustic signals are received, the CL is redistributed in the brain of patients with SNHL, increasing the burden on the auditory center and resulting in a constant high load on the auditory center, leading to a decrease in CL elsewhere and a decline in CF. The co-morbidity hypothesis suggests that SNHL and CI are diseases of the same type and occur due to a common cause. Indeed, SNHL and CI are extremely similar in terms of pathological changes, including volumetric atrophy of functional brain areas and deposition of the toxic and harmful substances Aβ and tau. Chronic neuroinflammation and long-term oxidative damage are now considered to be the common cause of their pathogenesis. In addition, accumulation of toxic substances and alterations in ion channel expression also may play a role in the development of these conditions. Both the general CL hypothesis and the co-morbidity hypothesis address the direct causes of SNHL and CI. In contrast, the sensory deprivation hypothesis proposes that an indirect pathway contributes to CI in patients with SNHL. The sensory deprivation hypothesis suggests that long-term auditory decline affects people’s psycho-spiritual health, increases their sense of isolation, and increases their risk of psycho-spiritual disorders. However, it is currently believed that psychosocial illness and CI are mutually reinforcing. Long-term auditory decline contributes to a high risk of psychosocial illness and increases the risk of CI. We propose that the co-occurrence of SNHL and CI is the result of a combination of direct and indirect causes.
However, there are limitations to the three hypotheses mentioned above. While there is some evidences for all three hypotheses, these evidences are fragmented and still lack a relatively complete basis. The sensory deprivation hypothesis suggests that the absence of sensation causes cortical changes, however, one study found a limited effect of HL on these changes (Parker et al., 2020). CL is thought to be exacerbated by hearing impairment, but this change has also been noted in some cases of visual impairment (Golzan et al., 2017; Vu et al., 2021). Age has long been recognized as one of the main causative factors in the co-morbidity hypothesis; however, aging can lead to a variety of diseases and sensory loss, such as cardiovascular disease (Yang et al., 2023), cerebrovascular disease (Romay et al., 2024), and vision decline (Vanhunsel et al., 2021). All of these diseases can lead to CI and these findings seem to support that age, rather than ARHL, is the underlying cause of CI.
A survey of populations in the UK and France found that HL is significantly and positively associated with an increased risk of AD during an exposure window of 2–10 years prior to AD diagnosis (Nedelec et al., 2022). Despite the limitations of the three hypotheses mentioned in this review, they are still the prevailing viewpoints and have implications for the study of SNHL and CI. As the incidence of SNHL increases each year, we should recognize the dangers of this disabling disease. Hearing aids and cochlear implants have been shown to be effective at improving hearing and delaying the onset of CI. As these devices improve a patient’s hearing, they not only reduce the load on the auditory center but also improve the patient’s ability for interpersonal communication, which can reduce their sense of isolation and risk of psychosocial disorders.
HZ: Formal analysis, Resources, Validation, Writing – original draft. YW: Data curation, Formal analysis, Resources, Writing – original draft. LCu: Formal analysis, Funding acquisition, Investigation, Resources, Writing – review & editing. HW: Data curation, Formal analysis, Resources, Writing – review & editing. SL: Data curation, Resources, Validation, Writing – review & editing. TL: Resources, Supervision, Writing – review & editing. DL: Data curation, Supervision, Writing – review & editing. LCh: Data curation, Funding acquisition, Resources, Validation, Writing – review & editing. JQ: Conceptualization, Formal analysis, Funding acquisition, Validation, Writing – review & editing. YS: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by grants from the National Natural Science Foundation of China (#82371153), the China Postdoctoral Science Foundation (#2023 M731845), the Natural Science Foundation of Shandong Province (#ZR2021MH378 and #ZR2022QH073), and the Yantai Science and Technology Innovation Development Project (#2022YD009, #2023YD050 and #2020MSGY078).
The authors declare that this research was conducted in the absence of any commercial or financial supports that could be considered a conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abad-Perez, P., Molina-Payá, F. J., Martínez-Otero, L., Borrell, V., Redondo, R. L., and Brotons-Mas, J. R. (2023). Theta/gamma co-modulation disruption after NMDAr blockade by MK-801 is associated with spatial working memory deficits in mice. Neuroscience 519, 162–176. doi: 10.1016/j.neuroscience.2023.03.022
Abd El-Rahman, S. S., and Fayed, H. M. (2022). Improved cognition impairment by activating cannabinoid receptor type 2: modulating CREB/BDNF expression and impeding TLR-4/NFκBp65/M1 microglia signaling pathway in D-galactose-injected ovariectomized rats. PLoS One 17:e0265961. doi: 10.1371/journal.pone.0265961
(2023). Alzheimer's disease facts and figures. Alzheimer's & dementia: J Alzheimer's Assoc 19, 1598–1695. doi: 10.1002/alz.13016
Armstrong, N. M., An, Y., Doshi, J., Erus, G., Ferrucci, L., Davatzikos, C., et al. (2019). Association of Midlife Hearing Impairment with Late-Life Temporal Lobe Volume Loss. JAMA Otolaryngol Head Neck Surg 145, 794–802. doi: 10.1001/jamaoto.2019.1610
Barczuk, J., Siwecka, N., Lusa, W., Rozpędek-Kamińska, W., Kucharska, E., and Majsterek, I. (2022). Targeting NLRP3-mediated Neuroinflammation in Alzheimer's disease treatment. Int. J. Mol. Sci. 23:8979. doi: 10.3390/ijms23168979
Belkhiria, C., Vergara, R. C., San Martín, S., Leiva, A., Marcenaro, B., Martinez, M., et al. (2019). Cingulate cortex atrophy is associated with hearing loss in Presbycusis with Cochlear amplifier dysfunction. Front. Aging Neurosci. 11:97. doi: 10.3389/fnagi.2019.00097
Benga, O., and Huber, V. J. (2012). Brain water channel proteins in health and disease. Mol. Asp. Med. 33, 562–578. doi: 10.1016/j.mam.2012.03.008
Bezalel, V., Paz, R., and Tal, A. (2019). Inhibitory and excitatory mechanisms in the human cingulate-cortex support reinforcement learning: a functional proton magnetic resonance spectroscopy study. NeuroImage 184, 25–35. doi: 10.1016/j.neuroimage.2018.09.016
Bikbov, M. M., Kazakbaeva, G. M., Rakhimova, E. M., Rusakova, I. A., Fakhretdinova, A. A., Tuliakova, A. M., et al. (2022). Concurrent vision and hearing impairment associated with cognitive dysfunction in a population aged 85+ years: the Ural very old study. BMJ Open 12:e058464. doi: 10.1136/bmjopen-2021-058464
Blasius, A. L., and Beutler, B. (2010). Intracellular toll-like receptors. Immunity 32, 305–315. doi: 10.1016/j.immuni.2010.03.012
Bowyer, J. F., Sarkar, S., Burks, S. M., Hess, J. N., Tolani, S., O'Callaghan, J. P., et al. (2020). Microglial activation and responses to vasculature that result from an acute LPS exposure. Neurotoxicology 77, 181–192. doi: 10.1016/j.neuro.2020.01.014
Brechet, A., Buchert, R., Schwenk, J., Boudkkazi, S., Zolles, G., Siquier-Pernet, K., et al. (2017). AMPA-receptor specific biogenesis complexes control synaptic transmission and intellectual ability. Nat. Commun. 8:15910. doi: 10.1038/ncomms15910
Brecht, E. J., Barsz, K., Gross, B., and Walton, J. P. (2017). Increasing GABA reverses age-related alterations in excitatory receptive fields and intensity coding of auditory midbrain neurons in aged mice. Neurobiol. Aging 56, 87–99. doi: 10.1016/j.neurobiolaging.2017.04.003
Buchanan, T. W., Tranel, D., and Adolphs, R. (2006). Memories for emotional autobiographical events following unilateral damage to medial temporal lobe. Brain J. Neurol. 129, 115–127. doi: 10.1093/brain/awh672
Bucholc, M., Bauermeister, S., Kaur, D., McClean, P. L., and Todd, S. (2022). The impact of hearing impairment and hearing aid use on progression to mild cognitive impairment in cognitively healthy adults: An observational cohort study. Alzheimer's & dementia (New York, N Y) 8:e12248. doi: 10.1002/trc2.12248
Campolo, M., Paterniti, I., Siracusa, R., Filippone, A., Esposito, E., and Cuzzocrea, S. (2019). TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson's diseases in vivo model. Brain Behav. Immun. 76, 236–247. doi: 10.1016/j.bbi.2018.12.003
Chandra, A., Farrell, C., Wilson, H., Dervenoulas, G., De Natale, E. R., and Politis, M. (2021). Aquaporin-4 polymorphisms predict amyloid burden and clinical outcome in the Alzheimer's disease spectrum. Neurobiol. Aging 97, 1–9. doi: 10.1016/j.neurobiolaging.2020.06.007
Chang, E. H., Savage, M. J., Flood, D. G., Thomas, J. M., Levy, R. B., Mahadomrongkul, V., et al. (2006). AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knockin mice. Proc. Natl. Acad. Sci. USA 103, 3410–3415. doi: 10.1073/pnas.0507313103
Chang, H., Telang, R. S., Sreebhavan, S., Tingle, M., Thorne, P. R., and Vlajkovic, S. M. (2017). Pharmacokinetic properties of adenosine amine congener in Cochlear perilymph after systemic administration. Biomed. Res. Int. 2017, 1–8. doi: 10.1155/2017/8091462
Chatterjee, M., Gajre, S., Kulkarni, A. M., Barrett, K. C., and Limb, C. J. (2023). Predictors of emotional prosody identification by school-age children with Cochlear implants and their peers with Normal hearing. Ear Hear. 45, 411–424. doi: 10.1097/AUD.0000000000001436
Chen, J. F., Choi, D. S., and Cunha, R. A. (2023). Striatopallidal adenosine a(2A) receptor modulation of goal-directed behavior: homeostatic control with cognitive flexibility. Neuropharmacology 226:109421. doi: 10.1016/j.neuropharm.2023.109421
Chen, J., Zheng, Y., Xiong, H., and Ou, Y. (2014). NMDA receptors are involved in the regulation of BMP4-mediated survival in rat cochlear epithelial cells. Neurosci. Lett. 566, 275–279. doi: 10.1016/j.neulet.2014.02.067
Chern, A., Irace, A. L., and Golub, J. S. (2022). The laterality of age-related hearing loss and depression. Otolog Neurotol: Official Pub American Otolog Society American Neurotol Society European Acad Otolog Neurotol 43, 625–631. doi: 10.1097/MAO.0000000000003531
Choi, J. Y., Lee, S., and Lee, W. (2021). The impact of hearing loss on clinical dementia and preclinical cognitive impairment in later life. J Alzheimer's disease: JAD 81, 963–972. doi: 10.3233/JAD-210074
Christensen, N., D'Souza, M., Zhu, X., and Frisina, R. D. (2009). Age-related hearing loss: aquaporin 4 gene expression changes in the mouse cochlea and auditory midbrain. Brain Res. 1253, 27–34. doi: 10.1016/j.brainres.2008.11.070
Companys-Alemany, J., Turcu, A. L., Schneider, M., Müller, C. E., Vázquez, S., Griñán-Ferré, C., et al. (2022). NMDA receptor antagonists reduce amyloid-β deposition by modulating calpain-1 signaling and autophagy, rescuing cognitive impairment in 5XFAD mice. Cell. Mol. Life Sci. 79:408. doi: 10.1007/s00018-022-04438-4
Csipo, T., Lipecz, A., Mukli, P., Bahadli, D., Abdulhussein, O., Owens, C. D., et al. (2021). Increased cognitive workload evokes greater neurovascular coupling responses in healthy young adults. PLoS One 16:e0250043. doi: 10.1371/journal.pone.0250043
Dawes, P., Emsley, R., Cruickshanks, K. J., Moore, D. R., Fortnum, H., Edmondson-Jones, M., et al. (2015). Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS One 10:e0119616. doi: 10.1371/journal.pone.0119616
Diering, G. H., and Huganir, R. L. (2018). The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329. doi: 10.1016/j.neuron.2018.10.018
Dobri, S. G. J., and Ross, B. (2021). Total GABA level in human auditory cortex is associated with speech-in-noise understanding in older age. NeuroImage 225:117474. doi: 10.1016/j.neuroimage.2020.117474
Dou, Y., and Tan, Y. (2023). Presequence protease reverses mitochondria-specific amyloid-β-induced mitophagy to protect mitochondria. FASEB J: Official Pub Federation of American Societies for Experimental Biol 37:e22890. doi: 10.1096/fj.202200216RRRR
Du, Y., Buchsbaum, B. R., Grady, C. L., and Alain, C. (2016). Increased activity in frontal motor cortex compensates impaired speech perception in older adults. Nat. Commun. 7:12241. doi: 10.1038/ncomms12241
Eckert, M. A., Vaden, K. I. Jr., and Dubno, J. R. (2019). Age-related hearing loss associations with changes in brain morphology. Trends Hear 23:233121651985726. doi: 10.1177/2331216519857267
Eckhard, A., Dos Santos, A., Liu, W., Bassiouni, M., Arnold, H., Gleiser, C., et al. (2015). Regulation of the perilymphatic-endolymphatic water shunt in the cochlea by membrane translocation of aquaporin-5. Arch. Eur. J. Physiol. 467, 2571–2588. doi: 10.1007/s00424-015-1720-6
Eckhard, A., Müller, M., Salt, A., Smolders, J., Rask-Andersen, H., and Löwenheim, H. (2014). Water permeability of the mammalian cochlea: functional features of an aquaporin-facilitated water shunt at the perilymph-endolymph barrier. Arch. Eur. J. Physiol. 466, 1963–1985. doi: 10.1007/s00424-013-1421-y
Fang, Y., Dai, S., Jin, C., Si, X., Gu, L., Song, Z., et al. (2021). Aquaporin-4 polymorphisms are associated with cognitive performance in Parkinson's disease. Front. Aging Neurosci. 13:740491. doi: 10.3389/fnagi.2021.740491
Feng, X., Zhan, F., Luo, D., Hu, J., Wei, G., Hua, F., et al. (2021). LncRNA 4344 promotes NLRP3-related neuroinflammation and cognitive impairment by targeting miR-138-5p. Brain Behav. Immun. 98, 283–298. doi: 10.1016/j.bbi.2021.08.230
Fok, C., Bogosanovic, M., Pandya, M., Telang, R., Thorne, P. R., and Vlajkovic, S. M. (2020). Regulator of G protein Signalling 4 (RGS4) as a novel target for the treatment of sensorineural hearing loss. Int. J. Mol. Sci. 22:3. doi: 10.3390/ijms22010003
Franco, R., Rivas-Santisteban, R., Lillo, J., Camps, J., Navarro, G., and Reyes-Resina, I. (2021). 5-Hydroxytryptamine, glutamate, and ATP: much more than neurotransmitters. Front Cell Dev Biol 9:667815. doi: 10.3389/fcell.2021.667815
Golub, J. S., Sharma, R. K., Rippon, B. Q., Brickman, A. M., and Luchsinger, J. A. (2021). The association between early age-related hearing loss and brain β-amyloid. Laryngoscope 131, 633–638. doi: 10.1002/lary.28859
Golzan, S. M., Goozee, K., Georgevsky, D., Avolio, A., Chatterjee, P., Shen, K., et al. (2017). Retinal vascular and structural changes are associated with amyloid burden in the elderly: ophthalmic biomarkers of preclinical Alzheimer's disease. Alzheimers Res. Ther. 9:13. doi: 10.1186/s13195-017-0239-9
Guo, D. H., Yamamoto, M., Hernandez, C. M., Khodadadi, H., Baban, B., and Stranahan, A. M. (2020). Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J. Clin. Invest. 130, 1961–1976. doi: 10.1172/JCI126078
Hong, J., Chen, Y., Zhang, Y., Li, J., Ren, L., Yang, L., et al. (2018). N-methyl-D-aspartate receptors involvement in the gentamicin-induced hearing loss and pathological changes of ribbon synapse in the mouse Cochlear inner hair cells. Neural Plast 2018, 1–16. doi: 10.1155/2018/3989201
Hu, M., Sun, Y. J., Zhou, Q. G., Chen, L., Hu, Y., Luo, C. X., et al. (2008). Negative regulation of neurogenesis and spatial memory by NR2B-containing NMDA receptors. J. Neurochem. 106, 1900–1913. doi: 10.1111/j.1471-4159.2008.05554.x
Huang, H., Wang, J., Jiang, C. Q., Zhu, F., Jin, Y. L., Zhu, T., et al. (2022). Hearing loss and depressive symptoms in older Chinese: whether social isolation plays a role. BMC Geriatr. 22:620. doi: 10.1186/s12877-022-03311-0
Huang, Y., Zhang, W., Guo, X., Zhang, Y., Wu, J., and Zu, H. (2023). Cellular cholesterol loss by DHCR24 knockdown leads to Aβ production by changing APP intracellular localization. J. Lipid Res. 64:100367. doi: 10.1016/j.jlr.2023.100367
Hubbard, J. A., Szu, J. I., and Binder, D. K. (2018). The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res. Bull. 136, 118–129. doi: 10.1016/j.brainresbull.2017.02.011
Hughes, S. E., Hutchings, H. A., Rapport, F. L., McMahon, C. M., and Boisvert, I. (2018). Social connectedness and perceived listening effort in adult Cochlear implant users: a grounded theory to establish content validity for a new patient-reported outcome measure. Ear Hear. 39, 922–934. doi: 10.1097/AUD.0000000000000553
Humes, L. E., Busey, T. A., Craig, J., and Kewley-Port, D. (2013). Are age-related changes in cognitive function driven by age-related changes in sensory processing? Atten. Percept. Psychophysiol. 75, 508–524. doi: 10.3758/s13414-012-0406-9
Husain, F. T., Carpenter-Thompson, J. R., and Schmidt, S. A. (2014). The effect of mild-to-moderate hearing loss on auditory and emotion processing networks. Front. Syst. Neurosci. 8:10. doi: 10.3389/fnsys.2014.00010
Irace, A. L., Rippon, B. Q., Brickman, A. M., Luchsinger, J. A., and Golub, J. S. (2022). The laterality of early age-related hearing loss and brain β-amyloid. Otolog Neurotol: Official Pub American Otolog Society, American Neurotol Society European Acad Otolog Neurotol 43, e382–e390. doi: 10.1097/MAO.0000000000003454
Islam, R., Vrionis, F., and Hanafy, K. A. (2022). Microglial TLR4 is critical for neuronal injury and cognitive dysfunction in subarachnoid hemorrhage. Neurocrit. Care. 37, 761–769. doi: 10.1007/s12028-022-01552-w
Jayakody, D. M. P., Wishart, J., Stegeman, I., Eikelboom, R., Moyle, T. C., Yiannos, J. M., et al. (2022). Is there an association between untreated hearing loss and psychosocial outcomes? Front. Aging Neurosci. 14:868673. doi: 10.3389/fnagi.2022.868673
Joshi, D. C., Zhang, C. L., Babujee, L., Vevea, J. D., August, B. K., Sheng, Z. H., et al. (2019). Inappropriate intrusion of an axonal mitochondrial anchor into dendrites causes neurodegeneration. Cell Rep. 29, 685–96.e5. doi: 10.1016/j.celrep.2019.09.012
Kalus, P., Slotboom, J., Gallinat, J., Mahlberg, R., Cattapan-Ludewig, K., Wiest, R., et al. (2006). Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia. NeuroImage 30, 713–720. doi: 10.1016/j.neuroimage.2005.10.035
Kandimalla, R., Manczak, M., Yin, X., Wang, R., and Reddy, P. H. (2018). Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer's disease. Hum. Mol. Genet. 27, 30–40. doi: 10.1093/hmg/ddx381
Kaur, T., Borse, V., Sheth, S., Sheehan, K., Ghosh, S., Tupal, S., et al. (2016). Adenosine A1 receptor protects against cisplatin ototoxicity by suppressing the NOX3/STAT1 inflammatory pathway in the cochlea. J. Neurosci. 36, 3962–3977. doi: 10.1523/JNEUROSCI.3111-15.2016
Kaur, C., Saini, S., Pal, I., Kumar, P., Chandra Sati, H., Jacob, T. G., et al. (2020). Age-related changes in the number of cresyl-violet-stained, parvalbumin and NMDAR 2B expressing neurons in the human spiral ganglion. Hear. Res. 388:107883. doi: 10.1016/j.heares.2020.107883
Keimasi, M., Salehifard, K., Keimasi, M., Amirsadri, M., Esfahani, N. M. J., Moradmand, M., et al. (2023). Alleviation of cognitive deficits in a rat model of glutamate-induced excitotoxicity, using an N-type voltage-gated calcium channel ligand, extracted from Agelena labyrinthica crude venom. Front. Mol. Neurosci. 16:1123343. doi: 10.3389/fnmol.2023.1123343
Kim, B. J., Kim, Y. H., Lee, S., Han, J. H., Lee, S. Y., Seong, J., et al. (2021). Otological aspects of NLRP3-related autoinflammatory disorder focusing on the responsiveness to anakinra. Rheumatology (Oxford) 60, 1523–1532. doi: 10.1093/rheumatology/keaa511
Kirschmann, E. K., Pollock, M. W., Nagarajan, V., and Torregrossa, M. M. (2019). Development of working memory in the male adolescent rat. Dev Cogn Neurosci 37:100601. doi: 10.1016/j.dcn.2018.11.003
Kleen, J. K., Scott, R. C., Holmes, G. L., and Lenck-Santini, P. P. (2010). Hippocampal interictal spikes disrupt cognition in rats. Ann. Neurol. 67, 250–257. doi: 10.1002/ana.21896
Klein, C. J., Botuyan, M. V., Wu, Y., Ward, C. J., Nicholson, G. A., Hammans, S., et al. (2011). Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat. Genet. 43, 595–600. doi: 10.1038/ng.830
Kotak, VC, Takesian, AE, and Sanes, DH. (2008). Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cerebral cortex (New York, NY). 18:2098–2108. doi: 10.1093/cercor/bhm233
Kwon, O. Y., and Lee, S. H. (2022). Ishige okamurae attenuates Neuroinflammation and cognitive deficits in mice Intracerebroventricularly injected with LPS via regulating TLR-4/MyD88-dependent pathways. Antioxidants (Basel, Switzerland) 12:78. doi: 10.3390/antiox12010078
Li, L., Chao, T., Brant, J., O'Malley, B. Jr., Tsourkas, A., and Li, D. (2017). Advances in nano-based inner ear delivery systems for the treatment of sensorineural hearing loss. Adv. Drug Deliv. Rev. 108, 2–12. doi: 10.1016/j.addr.2016.01.004
Li, P., Li, S., Wang, L., Li, H., Wang, Y., Liu, H., et al. (2023). Mitochondrial dysfunction in hearing loss: oxidative stress, autophagy and NLRP3 inflammasome. Front Cell Dev Biol 11:1119773. doi: 10.3389/fcell.2023.1119773
Li, N., Ma, W., Ren, F., Li, X., Li, F., Zong, W., et al. (2023). Neurochemical and functional reorganization of the cognitive-ear link underlies cognitive impairment in presbycusis. NeuroImage 268:119861. doi: 10.1016/j.neuroimage.2023.119861
Li, J., and Verkman, A. S. (2001). Impaired hearing in mice lacking aquaporin-4 water channels. J. Biol. Chem. 276, 31233–31237. doi: 10.1074/jbc.M104368200
Li, X., Xia, J., Ma, C., Chen, K., Xu, K., Zhang, J., et al. (2020). “Accelerating structural degeneration in temporal regions and their effects on cognition in aging of MCI patients” in Cerebral cortex, vol. 30 (New York, NY), 326–338.
Liang, T., Zhang, Y., Wu, S., Chen, Q., and Wang, L. (2022). The role of NLRP3 Inflammasome in Alzheimer's disease and potential therapeutic targets. Front. Pharmacol. 13:845185. doi: 10.3389/fphar.2022.845185
Lin, W., Li, Z., Liang, G., Zhou, R., Zheng, X., Tao, R., et al. (2023). TNEA therapy promotes the autophagic degradation of NLRP3 inflammasome in a transgenic mouse model of Alzheimer's disease via TFEB/TFE3 activation. J. Neuroinflammation 20:21. doi: 10.1186/s12974-023-02698-w
Lindenberger, U., and Baltes, P. B. (1994). Sensory functioning and intelligence in old age: a strong connection. Psychol. Aging 9, 339–355. doi: 10.1037/0882-7974.9.3.339
Liu, Y., Fang, S., Liu, L. M., Zhu, Y., Li, C. R., Chen, K., et al. (2020). Hearing loss is an early biomarker in APP/PS1 Alzheimer's disease mice. Neurosci. Lett. 717:134705. doi: 10.1016/j.neulet.2019.134705
Liu, Y., Hu, P. P., Zhai, S., Feng, W. X., Zhang, R., Li, Q., et al. (2022). Aquaporin 4 deficiency eliminates the beneficial effects of voluntary exercise in a mouse model of Alzheimer's disease. Neural Regen. Res. 17, 2079–2088. doi: 10.4103/1673-5374.335169
Liu, S., Li, H., Shen, Y., Zhu, W., Wang, Y., Wang, J., et al. (2023). Moxibustion improves hypothalamus Aqp4 polarization in APP/PS1 mice: evidence from spatial transcriptomics. Front. Aging Neurosci. 15:1069155. doi: 10.3389/fnagi.2023.1069155
Liu, X. H., Liang, F., Jia, X. Y., Zhao, L., Zhou, Y., and Yang, J. (2020). Hyperbaric oxygen treatment improves hearing level via attenuating TLR4/NF-κB mediated inflammation in sudden sensorineural hearing loss patients. Biomed Environ Sci: BES 33, 331–337. doi: 10.3967/bes2020.045
Liu, J., Pan, M., Sun, M., Shi, H., and Feng, R. (2024). Nutritional status and physical exercise are associated with cognitive function in Chinese community-dwelling older adults: the role of happiness. Nutrients. 16:203. doi: 10.3390/nu16020203
Loughrey, D. G., Kelly, M. E., Kelley, G. A., Brennan, S., and Lawlor, B. A. (2018). Association of age-Related Hearing Loss with Cognitive Function, cognitive impairment, and dementia: a systematic review and Meta-analysis. JAMA Otolaryngol Head Neck Surg 144, 115–126. doi: 10.1001/jamaoto.2017.2513
Lozier, N. R., Muscio, S., Pal, I., Cai, H. M., and Rubio, M. E. (2023). Sex differences in glutamate AMPA receptor subunits mRNA with fast gating kinetics in the mouse cochlea. Front. Syst. Neurosci. 17:1100505. doi: 10.3389/fnsys.2023.1100505
Luan, Y., Wang, C., Jiao, Y., Tang, T., Zhang, J., and Teng, G. J. (2019). Prefrontal-temporal pathway mediates the cross-modal and cognitive reorganization in sensorineural hearing loss with or without tinnitus: a multimodal MRI study. Front. Neurosci. 13:222. doi: 10.3389/fnins.2019.00222
Ma, J. H., Lee, E., Yoon, S. H., Min, H., Oh, J. H., Hwang, I., et al. (2022). Therapeutic effect of NLRP3 inhibition on hearing loss induced by systemic inflammation in a CAPS-associated mouse model. EBioMedicine 82:104184. doi: 10.1016/j.ebiom.2022.104184
Ma, W., Zhang, Y., Li, X., Liu, S., Gao, Y., Yang, J., et al. (2022). High-frequency hearing loss is associated with anxiety and brain structural plasticity in older adults. Front. Aging Neurosci. 14:821537. doi: 10.3389/fnagi.2022.821537
Manchella, M. K., Logan, P. E., Perry, B. L., Peng, S., Risacher, S. L., Saykin, A. J., et al. (2023). Associations between social network characteristics and brain structure among older adults. Alzheimers Dement. 20, 1406–1420. doi: 10.1002/alz.13534
Maurya, S. K., Gupta, S., Bakshi, A., Kaur, H., Jain, A., Senapati, S., et al. (2022). Targeting mitochondria in the regulation of neurodegenerative diseases: a comprehensive review. J. Neurosci. Res. 100, 1845–1861. doi: 10.1002/jnr.25110
Mellow, M. L., Dumuid, D., Olds, T., Stanford, T., Dorrian, J., Wade, A. T., et al. (2024). Cross-sectional associations between 24-hour time-use composition, grey matter volume and cognitive function in healthy older adults. Int. J. Behav. Nutr. Phys. Act. 21:11. doi: 10.1186/s12966-023-01557-4
Merighi, S., Borea, P. A., Varani, K., Vincenzi, F., Jacobson, K. A., and Gessi, S. (2022). A(2A) adenosine receptor antagonists in neurodegenerative diseases. Curr. Med. Chem. 29, 4138–4151. doi: 10.2174/0929867328666211129122550
Mertens, G., Andries, E., Claes, A. J., Topsakal, V., Van de Heyning, P., Van Rompaey, V., et al. (2021). Cognitive improvement after Cochlear implantation in older adults with severe or profound hearing impairment: a prospective, longitudinal, controlled, multicenter study. Ear Hear. 42, 606–614. doi: 10.1097/AUD.0000000000000962
Mhatre, A. N., Stern, R. E., Li, J., and Lalwani, A. K. (2002). Aquaporin 4 expression in the mammalian inner ear and its role in hearing. Biochem. Biophys. Res. Commun. 297, 987–996. doi: 10.1016/S0006-291X(02)02296-9
Müller, U. (2020). Exosome-mediated protection of auditory hair cells from ototoxic insults. J. Clin. Invest. 130, 2206–2208. doi: 10.1172/JCI135710
Nakanishi, H., Kawashima, Y., Kurima, K., Muskett, J. A., Kim, H. J., Brewer, C. C., et al. (2018). Gradual symmetric progression of DFNA34 hearing loss caused by an NLRP3 mutation and Cochlear autoinflammation. Otolog Neurotol: Official Pub American Otological Society, American Neurotol Society European Acad Otolog Neurotol 39, e181–e185. doi: 10.1097/MAO.0000000000001715
Nedelec, T., Couvy-Duchesne, B., Monnet, F., Daly, T., Ansart, M., Gantzer, L., et al. (2022). Identifying health conditions associated with Alzheimer's disease up to 15 years before diagnosis: an agnostic study of French and British health records. Lancet Digital health 4, e169–e178. doi: 10.1016/S2589-7500(21)00275-2
Nevoux, J., Viengchareun, S., Lema, I., Lecoq, A. L., Ferrary, E., and Lombès, M. (2015). Glucocorticoids stimulate endolymphatic water reabsorption in inner ear through aquaporin 3 regulation. Arch. Eur. J. Physiol. 467, 1931–1943. doi: 10.1007/s00424-014-1629-5
Nishio, N., Teranishi, M., Uchida, Y., Sugiura, S., Ando, F., Shimokata, H., et al. (2013). Polymorphisms in genes encoding aquaporins 4 and 5 and estrogen receptor α in patients with Ménière's disease and sudden sensorineural hearing loss. Life Sci. 92, 541–546. doi: 10.1016/j.lfs.2013.01.019
Oliveros, A., Yoo, K. H., Rashid, M. A., Corujo-Ramirez, A., Hur, B., Sung, J., et al. (2022). Adenosine a(2A) receptor blockade prevents cisplatin-induced impairments in neurogenesis and cognitive function. Proc. Natl. Acad. Sci. USA 119:e2206415119. doi: 10.1073/pnas.2206415119
Ossenkoppele, R., Pichet Binette, A., Groot, C., Smith, R., Strandberg, O., Palmqvist, S., et al. (2022). Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat. Med. 28, 2381–2387. doi: 10.1038/s41591-022-02049-x
Paciello, F., Pisani, A., Rinaudo, M., Cocco, S., Paludetti, G., Fetoni, A. R., et al. (2023). Noise-induced auditory damage affects hippocampus causing memory deficits in a model of early age-related hearing loss. Neurobiol. Dis. 178:106024. doi: 10.1016/j.nbd.2023.106024
Panza, F., Lozupone, M., Sardone, R., Battista, P., Piccininni, M., Dibello, V., et al. (2019). Sensorial frailty: age-related hearing loss and the risk of cognitive impairment and dementia in later life. Therapeutic Advan Chronic disease 10:204062231881100. doi: 10.1177/2040622318811000
Park, J., Lee, O., and McKee, M. (2022). Association between hearing loss and suicidal ideation among middle-aged and older adults. Aging Ment. Health 26, 1287–1294. doi: 10.1080/13607863.2021.1919991
Parker, T., Cash, D. M., Lane, C., Lu, K., Malone, I. B., Nicholas, J. M., et al. (2020). Pure tone audiometry and cerebral pathology in healthy older adults. J. Neurol. Neurosurg. Psychiatry 91, 172–176. doi: 10.1136/jnnp-2019-321897
Patel, S. V., DeCarlo, C. M., Book, S. A., Schormans, A. L., Whitehead, S. N., Allman, B. L., et al. (2022). Noise exposure in early adulthood causes age-dependent and brain region-specific impairments in cognitive function. Front. Neurosci. 16:1001686. doi: 10.3389/fnins.2022.1001686
Pilati, N., Linley, D. M., Selvaskandan, H., Uchitel, O., Hennig, M. H., Kopp-Scheinpflug, C., et al. (2016). Acoustic trauma slows AMPA receptor-mediated EPSCs in the auditory brainstem, reducing GluA4 subunit expression as a mechanism to rescue binaural function. J. Physiol. 594, 3683–3703. doi: 10.1113/JP271929
Potter, O. V., Giedraitis, M. E., Johnson, C. D., Cox, M. N., and Kohman, R. A. (2019). Young and aged TLR4 deficient mice show sex-dependent enhancements in spatial memory and alterations in interleukin-1 related genes. Brain Behav. Immun. 76, 37–47. doi: 10.1016/j.bbi.2018.10.010
Qian, Z. J., and Ricci, A. J. (2020). Effects of cochlear hair cell ablation on spatial learning/memory. Sci. Rep. 10:20687. doi: 10.1038/s41598-020-77803-7
Qian, Z. J., Wattamwar, K., Caruana, F. F., Otter, J., Leskowitz, M. J., Siedlecki, B., et al. (2016). Hearing aid use is associated with better Mini-mental state exam performance. American J Geriatric Psychiatry: Official J American Association for Geriatric Psychiatry 24, 694–702. doi: 10.1016/j.jagp.2016.03.005
Qiang, Q., Manalo, J. M., Sun, H., Zhang, Y., Song, A., Wen, A. Q., et al. (2021). Erythrocyte adenosine A2B receptor prevents cognitive and auditory dysfunction by promoting hypoxic and metabolic reprogramming. PLoS Biol. 19:e3001239. doi: 10.1371/journal.pbio.3001239
Qin, Y., Liu, Y., Hao, W., Decker, Y., Tomic, I., Menger, M. D., et al. (2016). Stimulation of TLR4 attenuates Alzheimer's disease-related symptoms and pathology in tau-transgenic mice. J. Immunol. 197, 3281–3292. doi: 10.4049/jimmunol.1600873
Ralli, M., Troiani, D., Podda, M. V., Paciello, F., Eramo, S. L., de Corso, E., et al. (2014). The effect of the NMDA channel blocker memantine on salicylate-induced tinnitus in rats. Acta otorhinolaryngologica Italica: organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale 34, 198–204.
Reinten, I., De Ronde-Brons, I., Houben, R., and Dreschler, W. (2021). Measuring the influence of noise reduction on listening effort in hearing-impaired listeners using response times to an arithmetic task in noise. Trends in hearing 25:233121652110144. doi: 10.1177/23312165211014437
Romay, M. C., Knutsen, R. H., Ma, F., Mompeón, A., Hernandez, G. E., Salvador, J., et al. (2024). Age-related loss of Notch3 underlies brain vascular contractility deficiencies, glymphatic dysfunction, and neurodegeneration in mice. J. Clin. Invest. 134:e166134. doi: 10.1172/JCI166134
Rosemann, S., and Thiel, C. M. (2018). Audio-visual speech processing in age-related hearing loss: stronger integration and increased frontal lobe recruitment. NeuroImage 175, 425–437. doi: 10.1016/j.neuroimage.2018.04.023
Rutherford, B. R., Brewster, K., Golub, J. S., Kim, A. H., and Roose, S. P. (2018). Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am. J. Psychiatry 175, 215–224. doi: 10.1176/appi.ajp.2017.17040423
Sai, N., Yang, Y. Y., Ma, L., Liu, D., Jiang, Q. Q., Guo, W. W., et al. (2022). Involvement of NLRP3-inflammasome pathway in noise-induced hearing loss. Neural Regen. Res. 17, 2750–2754. doi: 10.4103/1673-5374.339499
Sanford, A. M. (2017). Mild cognitive impairment. Clin. Geriatr. Med. 33, 325–337. doi: 10.1016/j.cger.2017.02.005
Shi, Y., Dai, Q., Ji, B., Huang, L., Zhuang, X., Mo, Y., et al. (2021). Electroacupuncture pretreatment prevents cognitive impairment induced by cerebral ischemia-reperfusion via adenosine A1 receptors in rats. Front. Aging Neurosci. 13:680706. doi: 10.3389/fnagi.2021.680706
Shin, M., Pandya, M., Espinosa, K., Telang, R., Boix, J., Thorne, P. R., et al. (2021). Istradefylline mitigates age-related hearing loss in C57BL/6J mice. Int. J. Mol. Sci. 22:8000. doi: 10.3390/ijms22158000
Si, Y., Chen, Y. B., Chen, S. J., Zheng, Y. Q., Liu, X., Liu, Y., et al. (2015). TLR4 drives the pathogenesis of acquired cholesteatoma by promoting local inflammation and bone destruction. Sci. Rep. 5:16683. doi: 10.1038/srep16683
Skouras, S., Torner, J., Andersson, P., Koush, Y., Falcon, C., Minguillon, C., et al. (2020). Earliest amyloid and tau deposition modulate the influence of limbic networks during closed-loop hippocampal downregulation. Brain J. Neurol. 143, 976–992. doi: 10.1093/brain/awaa011
Slade, K., Plack, C. J., and Nuttall, H. E. (2020). The effects of age-related hearing loss on the brain and cognitive function. Trends Neurosci. 43, 810–821. doi: 10.1016/j.tins.2020.07.005
Slade, K., Reilly, J. H., Jablonska, K., Smith, E., Hayes, L. D., Plack, C. J., et al. (2022). The impact of age-related hearing loss on structural neuroanatomy: a meta-analysis. Front. Neurol. 13:950997. doi: 10.3389/fneur.2022.950997
Solas, M., Vela, S., Smerdou, C., Martisova, E., Martínez-Valbuena, I., Luquin, M. R., et al. (2023). JNK activation in Alzheimer's disease is driven by amyloid β and is associated with tau pathology. ACS Chem. Neurosci. 14, 1524–1534. doi: 10.1021/acschemneuro.3c00093
Song, A., Cho, G. W., Vijayakumar, K. A., Moon, C., Ang, M. J., Kim, J., et al. (2021). Neuroprotective effect of Valproic acid on salicylate-induced tinnitus. Int. J. Mol. Sci. 23:23. doi: 10.3390/ijms23010023
Srivas, S., and Thakur, M. K. (2017). Epigenetic regulation of neuronal immediate early genes is associated with decline in their expression and memory consolidation in scopolamine-induced amnesic mice. Mol. Neurobiol. 54, 5107–5119. doi: 10.1007/s12035-016-0047-4
Stahl, S. M. (2017). Does treating hearing loss prevent or slow the progress of dementia? Hearing is not all in the ears, but who's listening? CNS Spectr 22, 247–250. doi: 10.1017/S1092852917000268
Su, S., Chen, G., Gao, M., Zhong, G., Zhang, Z., Wei, D., et al. (2023). Kai-Xin-San protects against mitochondrial dysfunction in Alzheimer's disease through SIRT3/NLRP3 pathway. Chin. Med. 18:26. doi: 10.1186/s13020-023-00722-y
Sun, W., Tang, L., and Allman, B. L. (2011). Environmental noise affects auditory temporal processing development and NMDA-2B receptor expression in auditory cortex. Behav. Brain Res. 218, 15–20. doi: 10.1016/j.bbr.2010.11.028
Swords, G. M., Nguyen, L. T., Mudar, R. A., and Llano, D. A. (2018). Auditory system dysfunction in Alzheimer disease and its prodromal states: a review. Ageing Res. Rev. 44, 49–59. doi: 10.1016/j.arr.2018.04.001
Tadros, S. F., D'Souza, M., Zettel, M. L., Zhu, X., Waxmonsky, N. C., and Frisina, R. D. (2007). Glutamate-related gene expression changes with age in the mouse auditory midbrain. Brain Res. 1127, 1–9. doi: 10.1016/j.brainres.2006.09.081
Tae, W. S., Yakunina, N., Kim, T. S., Kim, S. S., and Nam, E. C. (2014). Activation of auditory white matter tracts as revealed by functional magnetic resonance imaging. Neuroradiology 56, 597–605. doi: 10.1007/s00234-014-1362-y
Tang, T., Guo, Y., Xu, X., Zhao, L., Shen, X., Sun, L., et al. (2021). BoDV-1 infection induces neuroinflammation by activating the TLR4/MyD88/IRF5 signaling pathway, leading to learning and memory impairment in rats. J. Med. Virol. 93, 6163–6171. doi: 10.1002/jmv.27212
Tang, J., Hu, B., Zheng, H., Qian, X., Zhang, Y., Zhu, J., et al. (2021). 2,2′,4,4'-Tetrabromodiphenyl ether (BDE-47) activates aryl hydrocarbon receptor (AhR) mediated ROS and NLRP3 inflammasome/p38 MAPK pathway inducing necrosis in cochlear hair cells. Ecotoxicol. Environ. Saf. 221:112423. doi: 10.1016/j.ecoenv.2021.112423
Tang, X., Zhu, X., Ding, B., Walton, J. P., Frisina, R. D., and Su, J. (2014). Age-related hearing loss: GABA, nicotinic acetylcholine and NMDA receptor expression changes in spiral ganglion neurons of the mouse. Neuroscience 259, 184–193. doi: 10.1016/j.neuroscience.2013.11.058
Tautou, M., Descamps, F., Larchanché, P. E., Buée, L., El Bakali, J., Melnyk, P., et al. (2023). A Polyaminobiaryl-based β-secretase modulator alleviates cognitive impairments, amyloid load, Astrogliosis, and Neuroinflammation in APP(Swe)/PSEN1(ΔE9) mice model of amyloid pathology. Int. J. Mol. Sci. 24:5285. doi: 10.3390/ijms24065285
Tian, D., Xing, Y., Gao, W., Zhang, H., Song, Y., Tian, Y., et al. (2021). Sevoflurane aggravates the Progress of Alzheimer's disease through NLRP3/Caspase-1/Gasdermin D pathway. Front Cell Dev Biol 9:801422. doi: 10.3389/fcell.2021.801422
Tsimpida, D., Kontopantelis, E., Ashcroft, D. M., and Panagioti, M. (2022). The dynamic relationship between hearing loss, quality of life, socioeconomic position and depression and the impact of hearing aids: answers from the English longitudinal study of ageing (ELSA). Soc. Psychiatry Psychiatr. Epidemiol. 57, 353–362. doi: 10.1007/s00127-021-02155-0
Tun, P. A., McCoy, S., and Wingfield, A. (2009). Aging, hearing acuity, and the attentional costs of effortful listening. Psychol. Aging 24, 761–766. doi: 10.1037/a0014802
van Well, G. T., Sanders, M. S., Ouburg, S., van Furth, A. M., and Morré, S. A. (2012). Polymorphisms in toll-like receptors 2, 4, and 9 are highly associated with hearing loss in survivors of bacterial meningitis. PLoS One 7:e35837. doi: 10.1371/journal.pone.0035837
Vanhunsel, S., Bergmans, S., Beckers, A., Etienne, I., Van Houcke, J., Seuntjens, E., et al. (2021). The killifish visual system as an in vivo model to study brain aging and rejuvenation. NPJ Aging Mech Dis 7:22. doi: 10.1038/s41514-021-00077-4
Vasciaveo, V., Iadarola, A., Casile, A., Dante, D., Morello, G., Minotta, L., et al. (2023). Sleep fragmentation affects glymphatic system through the different expression of AQP4 in wild type and 5xFAD mouse models. Acta Neuropathol Commun 11:16. doi: 10.1186/s40478-022-01498-2
Vassena, E., Deraeve, J., and Alexander, W. H. (2020). Surprise, value and control in anterior cingulate cortex during speeded decision-making. Nat. Hum. Behav. 4, 412–422. doi: 10.1038/s41562-019-0801-5
Vlajkovic, S. M., Ambepitiya, K., Barclay, M., Boison, D., Housley, G. D., and Thorne, P. R. (2017). Adenosine receptors regulate susceptibility to noise-induced neural injury in the mouse cochlea and hearing loss. Hear. Res. 345, 43–51. doi: 10.1016/j.heares.2016.12.015
Voisin, D. L., Chakfe, Y., and Bourque, C. W. (1999). Coincident detection of CSF Na+ and osmotic pressure in osmoregulatory neurons of the supraoptic nucleus. Neuron 24, 453–460. doi: 10.1016/S0896-6273(00)80858-2
Vos, T., Abajobir, A. A., Abate, K. H., Abbafati, C., Abbas, K. M., Abd-Allah, F., et al. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet (London, England) 390, 1211–1259. doi: 10.1016/S0140-6736(17)32154-2
Vu, T. A., Fenwick, E. K., Gan, A. T. L., Man, R. E. K., Tan, B. K. J., Gupta, P., et al. (2021). The bidirectional relationship between vision and cognition: a systematic review and Meta-analysis. Ophthalmology 128, 981–992. doi: 10.1016/j.ophtha.2020.12.010
Wang, T., Chen, Y., Zou, Y., Pang, Y., He, X., Chen, Y., et al. (2022). Locomotor hyperactivity in the early-stage Alzheimer's disease-like pathology of APP/PS1 mice: associated with impaired polarization of astrocyte aquaporin 4. Aging Dis. 13, 1504–1522. doi: 10.14336/AD.2022.0219
Wang, H., Lv, S., Stroebel, D., Zhang, J., Pan, Y., Huang, X., et al. (2021). Gating mechanism and a modulatory niche of human GluN1-GluN2A NMDA receptors. Neuron 109, 2443–56.e5. doi: 10.1016/j.neuron.2021.05.031
Wang, Q., Shen, Y., Pan, Y., Chen, K., Ding, R., Zou, T., et al. (2021). Tlr2/4 double knockout attenuates the degeneration of primary auditory neurons: potential mechanisms from transcriptomic perspectives. Front Cell Dev Biol 9:750271. doi: 10.3389/fcell.2021.750271
Wang, H. F., Zhang, W., Rolls, E. T., Li, Y., Wang, L., Ma, Y. H., et al. (2022). Hearing impairment is associated with cognitive decline, brain atrophy and tau pathology. EBioMedicine 86:104336. doi: 10.1016/j.ebiom.2022.104336
Whitson, H. E., Cronin-Golomb, A., Cruickshanks, K. J., Gilmore, G. C., Owsley, C., Peelle, J. E., et al. (2018). American Geriatrics Society and National Institute on Aging bench-to-bedside conference: sensory impairment and cognitive decline in older adults. J. Am. Geriatr. Soc. 66, 2052–2058. doi: 10.1111/jgs.15506
Wingfield, A. (2016). Evolution of models of working memory and cognitive resources. Ear Hear. 37, 35s–43s. doi: 10.1097/AUD.0000000000000310
Wingfield, A., Tun, P. A., and McCoy, S. L. (2005). Hearing loss in older adulthood: what it is and how it interacts with cognitive performance. Curr. Dir. Psychol. Sci. 14, 144–148. doi: 10.1111/j.0963-7214.2005.00356.x
Wu, L., Xian, X., Xu, G., Tan, Z., Dong, F., Zhang, M., et al. (2022). Toll-like receptor 4: a promising therapeutic target for Alzheimer's disease. Mediat. Inflamm. 2022, 1–20. doi: 10.1155/2022/7924199
Xu, X. M., Feng, Y., Wang, J., Salvi, R., Yin, X., Gao, J., et al. (2023). Auditory-limbic-cerebellum interactions and cognitive impairments in noise-induced hearing loss. CNS Neurosci Ther 29, 932–940. doi: 10.1111/cns.14028
Xu, W., Zhang, C., Li, J. Q., Tan, C. C., Cao, X. P., Tan, L., et al. (2019). Age-related hearing loss accelerates cerebrospinal fluid tau levels and brain atrophy: a longitudinal study. Aging 11, 3156–3169. doi: 10.18632/aging.101971
Xu, L., Zhou, Y., Hu, L., Jiang, H., Dong, Y., Shen, H., et al. (2021). Deficits in N-methyl-D-aspartate receptor function and synaptic plasticity in hippocampal CA1 in APP/PS1 mouse model of Alzheimer's disease. Front. Aging Neurosci. 13:772980. doi: 10.3389/fnagi.2021.772980
Xue, N., Song, L., Song, Q., Santos-Sacchi, J., Wu, H., and Navaratnam, D. (2021). Genes related to SNPs identified by genome-wide association studies of age-related hearing loss show restriction to specific cell types in the adult mouse cochlea. Hear. Res. 410:108347. doi: 10.1016/j.heares.2021.108347
Yang, K., Hou, R., Zhao, J., Wang, X., Wei, J., Pan, X., et al. (2023). Lifestyle effects on aging and CVD: a spotlight on the nutrient-sensing network. Ageing Res. Rev. 92:102121. doi: 10.1016/j.arr.2023.102121
Yang, S., Kong, X. Y., Hu, T., Ge, Y. J., Li, X. Y., Chen, J. T., et al. (2022). Aquaporin-4, Connexin-30, and Connexin-43 as biomarkers for decreased objective sleep quality and/or cognition dysfunction in patients with chronic insomnia disorder. Front. Psychol. 13:856867. doi: 10.3389/fpsyt.2022.856867
Zemaitis, K., Kaliyappan, K., Frerichs, V., Friedman, A., and Krishnan Muthaiah, V. P. (2021). Mass spectrometry imaging of blast overpressure induced modulation of GABA/glutamate levels in the central auditory neuraxis of Chinchilla. Exp. Mol. Pathol. 119:104605. doi: 10.1016/j.yexmp.2021.104605
Zhang, Y., Cao, H., Qiu, X., Xu, D., Chen, Y., Barnes, G. N., et al. (2020). Neuroprotective effects of adenosine A1 receptor signaling on cognitive impairment induced by chronic intermittent hypoxia in mice. Front. Cell. Neurosci. 14:202. doi: 10.3389/fncel.2020.00202
Zhang, W. J., Li, D. N., Lian, T. H., Guo, P., Zhang, Y. N., Li, J. H., et al. (2022). Clinical features and potential mechanisms relating neuropathological biomarkers and blood-brain barrier in patients with Alzheimer's disease and hearing loss. Front. Aging Neurosci. 14:911028. doi: 10.3389/fnagi.2022.911028
Zhang, Z. H., Peng, J. Y., Chen, Y. B., Wang, C., Chen, C., and Song, G. L. (2023). Different effects and mechanisms of selenium compounds in improving pathology in Alzheimer's disease. Antioxidants (Basel, Switzerland) 12:702. doi: 10.3390/antiox12030702
Zhang, J., Yu, C., Zhang, X., Chen, H., Dong, J., Lu, W., et al. (2018). Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J. Neuroinflammation 15:37. doi: 10.1186/s12974-017-1052-x
Zhang, G., Zheng, H., Pyykko, I., and Zou, J. (2019). The TLR-4/NF-κB signaling pathway activation in cochlear inflammation of rats with noise-induced hearing loss. Hear. Res. 379, 59–68. doi: 10.1016/j.heares.2019.04.012
Zheng, X., Gong, T., Tang, C., Zhong, Y., Shi, L., Fang, X., et al. (2022). Gastrodin improves neuroinflammation-induced cognitive dysfunction in rats by regulating NLRP3 inflammasome. BMC Anesthesiol. 22:371. doi: 10.1186/s12871-022-01915-y
Keywords: sensorineural hearing loss, cognitive impairment, cognitive load hypothesis, co-morbidity hypothesis, sensory deprivation hypothesis
Citation: Zhao H, Wang Y, Cui L, Wang H, Liu S, Liang T, Liu D, Qiu J, Chen L and Sun Y (2024) Sensorineural hearing loss and cognitive impairment: three hypotheses. Front. Aging Neurosci. 16:1368232. doi: 10.3389/fnagi.2024.1368232
Received: 10 January 2024; Accepted: 20 February 2024;
Published: 28 February 2024.
Edited by:
Norshamsiah Md Din, National University of Malaysia, MalaysiaReviewed by:
Meghraj Singh Baghel, Johns Hopkins University, United StatesCopyright © 2024 Zhao, Wang, Cui, Wang, Liu, Liang, Liu, Qiu, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Sun, ZW50c3VueWFuQDEyNi5jb20=; Liang Chen, ZW50Y2hlbmxpYW5nQGhvdG1haWwuY29t; Jingjing Qiu, ZW50amluZ2ppbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.