
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 06 March 2024
Sec. Neurocognitive Aging and Behavior
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1335878
This article is part of the Research Topic To Know or Not to Know: Causes and Evolution of Lack of Awareness of Cognitive Decline in Neurodegenerative Diseases, Volume II View all 9 articles
 Sharon Wang1
Sharon Wang1 Kayden Mimmack1
Kayden Mimmack1 Federica Cacciamani1,2,3,4
Federica Cacciamani1,2,3,4 Michael Elnemais Fawzy5,6
Michael Elnemais Fawzy5,6 Catherine Munro1,5,6
Catherine Munro1,5,6 Jennifer Gatchel6,7,8
Jennifer Gatchel6,7,8 Gad A. Marshall1,5,6
Gad A. Marshall1,5,6 Geoffroy Gagliardi1,5,6†
Geoffroy Gagliardi1,5,6† Patrizia Vannini1,5,6*† for the Alzheimer's Disease Neuroimaging Initiative
Patrizia Vannini1,5,6*† for the Alzheimer's Disease Neuroimaging InitiativeIntroduction: Both the loss of awareness for cognitive decline (a. k.a anosognosia) and neuropsychiatric symptoms (NPS) are common in patients with Alzheimer's disease (AD) dementia, even in prodromal stages, and may exacerbate functional impairment and negatively impact caregiver burden. Despite the high impact of these symptoms on patients and their caregivers, our knowledge of how they develop across the AD spectrum is limited. Here, we explored the cross-sectional and longitudinal associations between anosognosia and NPS in individuals with mild cognitive impairment (MCI).
Methods: We included 237 participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI) with a baseline clinical diagnosis of MCI. Everyday Cognition (ECog) questionnaire scores were used to measure complaints from participants and study-partners at baseline and annually over a mean of 4.29 years [standard deviation (SD) = 2.72]. Anosognosia was defined as the study-partner having an ECog score ≥2.5/4 and the participant having an ECog score <2.5/4 on their baseline measure and their last observation without more than two consecutive deviating observations during the follow-up period. The 12-item study-partner-rated Neuropsychiatric Inventory determined the presence or absence of specific NPS. Survival analyses were performed to analyze the frequency and temporal onset of NPS over time in individuals with and without anosognosia.
Results: Thirty-eight out of 237 participants displayed anosognosia. Groups had similar lengths of follow-up at baseline (p > 0.9), though participants with anosognosia had lower MMSE scores (p = 0.049) and a higher proportion of amyloid-positivity using PET (p < 0.001. At baseline, the frequencies of agitation (p = 0.029) and disinhibition (p < 0.001) were higher in the anosognosia group compared to the non-anosognosia group. Survival analyses showed earlier onset of seven of the 12 NPS in the anosognosia group (p's < 0.001).
Discussion: Loss of awareness for cognitive decline is associated with greater frequency and earlier onset of NPS over time in participants with MCI. These results support the hypothesis of a potential common underlying neurophysiological process for anosognosia and NPS, a finding that needs to be addressed in future studies.
Alzheimer's Disease (AD) has a progressive evolution that spans several decades. During the preclinical stage, AD biomarkers are present but clinical signs or symptoms of AD are absent (Dubois et al., 2016). In mild cognitive impairment (MCI), impairment in one or more cognitive domains is seen while social or occupational functioning or functional independence are preserved (Albert et al., 2011).
Anosognosia (i.e., decreased awareness of cognitive deficits) is common at the dementia stage, estimated to be as high as 80% (Reed et al., 1993). It has also been shown to occur and develop before dementia onset (Hanseeuw et al., 2020a; Vannini et al., 2020). In addition, low self-awareness toward objective cognitive decline has been shown to predict progression from cognitively normal (CN) to MCI (Hanseeuw et al., 2020a) and from MCI to AD dementia (Gerretsen et al., 2017). Similarly, the presence and severity of anosognosia has been found to be associated with dementia progression, biological and cognitive markers of AD, and neuropsychiatric symptoms (NPS; Galeone et al., 2011; Zhao et al., 2016; Yoon et al., 2017).
Similarly, NPS are also common in the dementia stage (Zhao et al., 2016) and have been shown to be present in the pre-clinical or prodromal stages (MCI; Geda et al., 2008) as well. The most commons NPS in individuals with MCI are depression, apathy, and irritability. Meanwhile, psychotic symptoms are rare in this population (Geda et al., 2008). The presence of affective NPS, such as apathy, agitation, irritability, anxiety, and depression, are diagnostic factors that can help distinguish between CN and MCI groups, and ~50% of individuals with MCI have at least one NPS (Geda et al., 2008). Some NPS such as anxiety, hallucinations, and apathy, at baseline have been found to be associated with AD progression (Wadsworth et al., 2012).
The link between anosognosia and NPS in AD dementia is well-established. For example, a strong relationship has been observed between anosognosia and apathy in several studies (Starkstein et al., 2010; Mak et al., 2015; Azocar et al., 2021). In mild AD dementia patients, impaired awareness has been found to be associated with less depression and anxiety but greater apathy (Azocar et al., 2021). One cross-sectional study found that in mild AD dementia, anosognosia was positively correlated with agitation, aberrant motor behavior, and apathy (Spalletta et al., 2012). Another study looked at the levels of anosognosia in early-onset vs. late-onset AD dementia and its association with the presence of NPS and found that in early-onset dementia there was a strong association between early anosognosia and NPS, particularly apathy (Tondelli et al., 2021). As demonstrated above, many studies have shown that specific NPS, particularly apathy, are more common in unaware AD dementia patients.
This association has, however, not been studied as extensively in MCI. In one study, impaired awareness was found to be linked to greater severity of NPS such as euphoria, irritability, eating disorders, and aberrant motor behavior (Spalletta et al., 2012). In another study, no relationship was found between anosognosia and depression nor apathy (Mak et al., 2015).
Importantly, very few studies have looked at this relationship longitudinally. In fact, in a systematic review of the association between impaired awareness and NPS, only three of the 27 studies included were longitudinal studies (Azocar et al., 2021). One study by Starkstein et al. (2010) explored the association between anosognosia and apathy longitudinally and found that anosognosia at baseline predicts severity of apathy in the follow-up period. However, the longitudinal relationships between anosognosia and other NPS are more poorly explored (Starkstein et al., 2010). To our knowledge, Tondelli et al. (2021) is the only study that explored the relationship between anosognosia and all 12 NPS in the Neuropsychiatric Inventory-Questionnaire (NPI-Q) longitudinally (Tondelli et al., 2021). However, the association between anosognosia and the onset of NPS has not been explored. In addition, no study has looked at the longitudinal associations between NPS and anosognosia in participants with MCI.
Therefore, the aim of this study was to understand the association between self-awareness of memory decline and NPS at the MCI stage by investigating not only the frequency of NPS but also the onset of NPS. Given previous findings of an association between anosognosia and NPS cross-sectionally, we hypothesized that patients that are unaware of their memory deficits will show earlier onset of NPS over time.
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). ADNI is an ongoing, longitudinal, multicenter study conducted at 59 sites across North America, enrolling CN, amnestic MCI, and AD dementia participants aged 55–94 years. ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and AD dementia. For up-to-date information, see www.adni-info.org.
A total of 237 ADNI participants were included in the current analyses. All participants had a baseline clinical diagnosis of MCI and longitudinal measures available for Mini-Mental State Examination (MMSE; Folstein et al., 1975), Geriatric Depression Scale (GDS; Yesavage et al., 1982) administered to the participant, Everyday Cognition (Ecog; Farias et al., 2008) scores for both participant and study partner, study partner-rated Neuropsychiatric Inventory (NPI; Cummings et al., 1994; Cummings, 1997) score, and Clinical Dementia Rating (CDR; Morris, 1993).
Demographic characteristics are summarized in Table 1.
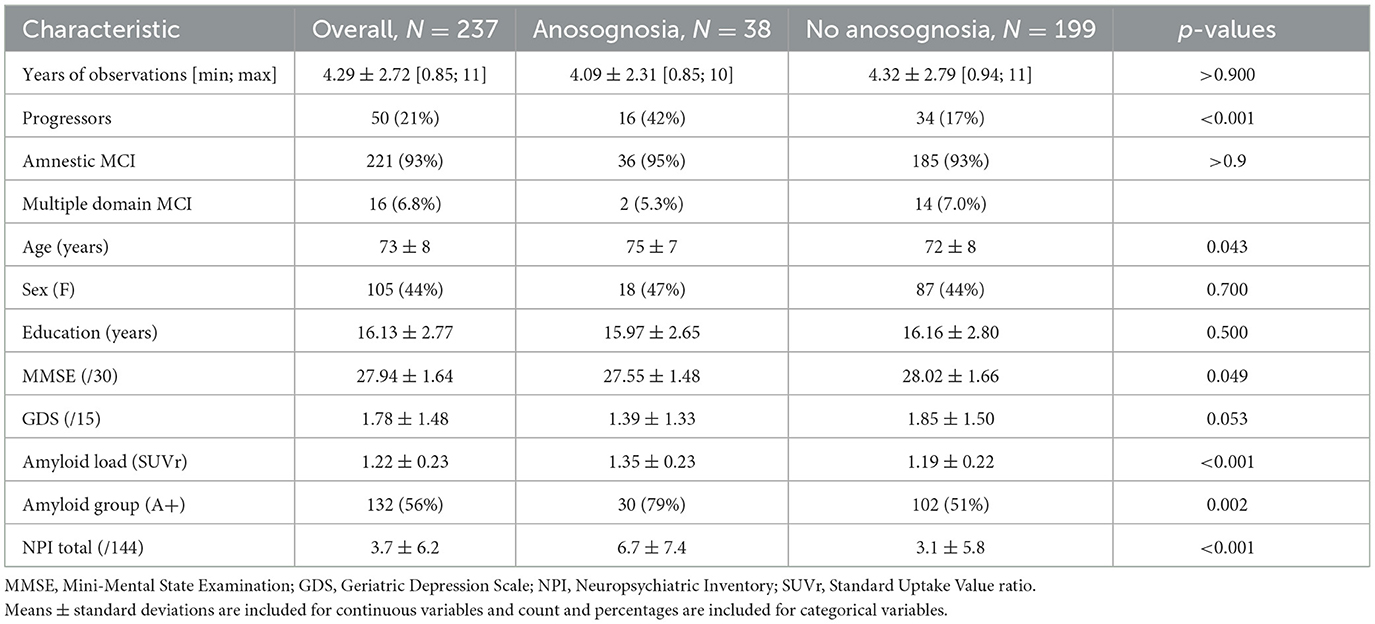
Table 1. Baseline demographic measures and comparisons between participants among the anosognosia and non-anosognosia groups.
ECog (Farias et al., 2008) questionnaire scores were used to measure complaints from participants and study partners over the course of the study. The ECog scale includes six subscales (i.e., Memory, Language, Visual-spatial/Perceptual Abilities, Planning, Organization, and Divided-Attention) exploring cognitive domains over 39 questions. For each question, the respondent is asked to compare the current level of cognitive functioning to 10 years ago using concrete examples (e.g., “Remembering a few shopping items without a list”). The rating is done on a Likert scale from 1 (“Better or no change”) to 4 (“Consistently much worse”), with a higher score describing the perception of a greater cognitive decline. Consistent with prior literature in MCI and AD dementia samples (Hanseeuw et al., 2020b; Gagliardi et al., 2021), we focused on the eight questions of the memory subscale. We computed the participant and study partner average scores and defined the presence or absence of anosognosia depending on these values. Participants were considered to have low awareness of cognitive decline when their average complaint score was < 2.5/4 while their study partner's rating was ≥2.5/4. The rationale for this approach was to create two groups in which the participant and study partner were on opposite sides of the ECog scale. This grouping method was used for the baseline observation and all longitudinal observations and, similarly to the method previously used by Starkstein et al. (2010), we extracted participants showing stable anosognosia as opposed to the consistent absence of anosognosia. Therefore, at least two ECog assessments were needed to determine stable anosognosia. Stability was defined as a similar grouping from baseline to the last observation, without having more than two consecutive “deviating” observations during the follow-up period. For example, a participant who displayed anosognosia at baseline and their second follow-up but not at their first follow-up would be characterized as someone with stable anosognosia.
The presence of NPS was assessed using the Neuropsychiatric Inventory (NPI; Cummings et al., 1994; Cummings, 1997). The NPI is a study partner-rated questionnaire assessing the presence and severity/frequency of specific NPS. Twelve domains are explored: delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, sleep, and appetite and eating disorders. For each of these categories, the study partner is first asked whether the described symptom is observed in the participant. If the symptom is observed, study partners are then asked about the severity (i.e., 1 = Mild, 2 = Moderate, and 3 = Severe) and the frequency (1 = rarely, less than once per week; 2 = sometimes, about once per week; 3 = often, several times per week; and 4 = very often, once, or more per day) of the symptom. If the symptom is not observed, these questions are skipped. The NPI total score is computed by summing each of the domain scores, which is calculated by multiplying the severity and frequency scores (a maximum score of 12 per domain), and the NPI total has a maximum score of 144 with higher scores indicating greater symptoms.
All participants underwent Florbetapir (18F−AV45) PET, used to measure brain amyloidosis. We used a whole brain standard uptake value ratio (SUVr) in large areas of the frontal, lateral temporal, and parietal lobes, based on the whole cerebellum as a reference region. For group comparisons, participants were also classified as amyloid positive (A+) or negative (A–) using a 1.11 cut-off as previously described for the ADNI cohort (Landau et al., 2013).
We first compared baseline observations of participants with and without stable anosognosia. Continuous variables, including age and education (in years), MMSE and NPI total scores (/30 and/144, respectively), brain amyloidosis (using the global SUVr at PET) and the number of observations, were compared using Wilcoxon rank sum test. As for categorical variables such as sex (F/M) and amyloid grouping (A+/A-), we used Pearson's Chi-squared test.
Survival analyses were performed to analyze the rate of appearance of NPS over time in individuals with and without anosognosia. We applied Cox proportional hazards regression models, using the presence or absence of a determined NPI as the predicted value, and the awareness grouping (i.e., presence or absence of anosognosia) as the predictor of interest. Demographics (i.e., age, sex and education) and baseline MMSE were added as covariates, and estimate log hazard ratios (HRs) were computed with 95% confidence intervals (CI). Separate models adding amyloidosis and baseline values for baseline phosphorylated tau cerebrospinal fluid as covariates were run as well. Kaplan–Meier curves were computed to visualize the results. P-values were corrected for multiple comparisons using the Bonferroni (1939) method. All statistical analyses were performed using R4.1.0 (https://www.R-project.org/). The survival (Therneau and Grambsch, 2000; Therneau, 2023) and survminer (Kassambara et al., 2021) packages were used to compute the survival analyses and their graphic representations.
Following the longitudinal grouping criteria we described, we split our sample into two groups of participants depending on whether they showed a stable presence or absence of anosognosia. Eighty-four percent of our sample, i.e., 199 participants out of 237, met our criteria for the absence of anosognosia. The remaining 16%, i.e., 38 participants, were considered to have anosognosia.
As reported in Table 1, participants with and without anosognosia showed similar baseline demographic characteristics (i.e., sex and years of education) and years of observations. Participants with anosognosia were older and had lower MMSE scores. Comparing brain amyloidosis levels, the anosognosia group showed greater amyloidosis and a corresponding greater proportion of amyloid-positive participants. Additionally, the anosognosia group was more likely to be progressors, demonstrating a clinical progression from MCI to AD dementia at some point in the follow-up period. Finally, the group with anosognosia showed more NPS at baseline as compared to the participants without anosognosia with a greater NPI Total score. However, the group with anosognosia and the group without anosognosia had similar GDS scores.
We also compared these two groups at baseline on the frequency of NPS. As reported in Table 2, participants with anosognosia showed a greater frequency of NPS on agitation and disinhibition compared to participants without anosognosia.
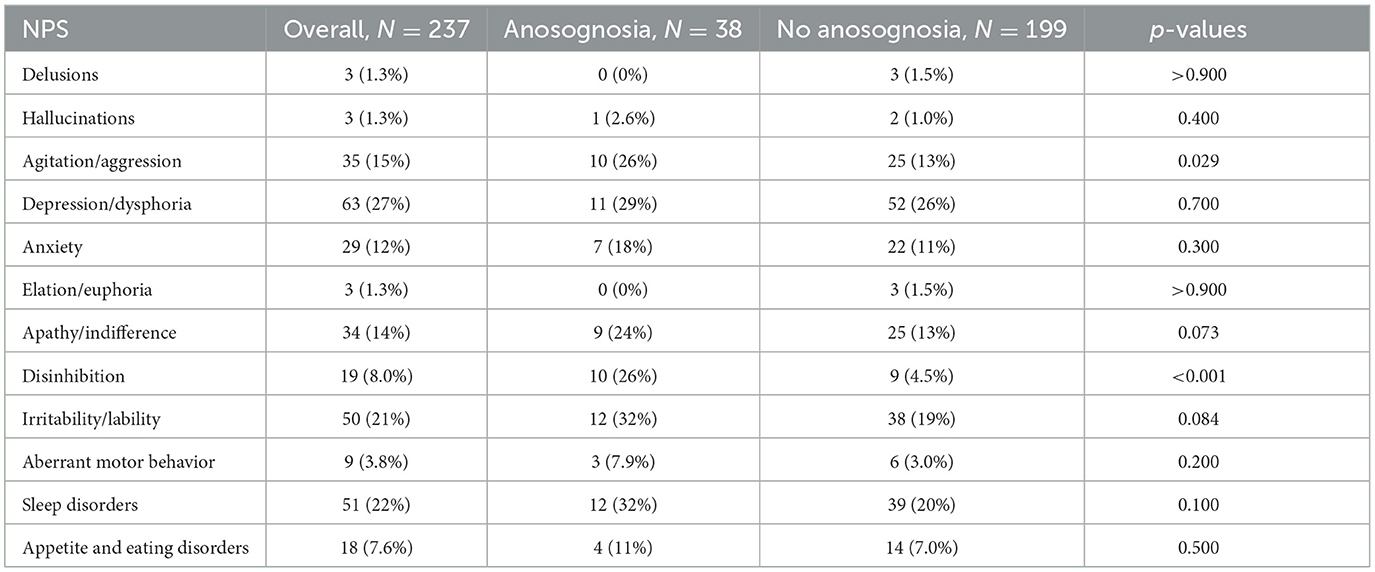
Table 2. Baseline comparisons of frequency of NPS between participants among the anosognosia and non-anosognosia groups.
After Bonferroni correction for multiple comparisons, no significant effects of age nor education were observed within the different models (see Table 3). There was a significant effect of sex on the onset of agitation, elation, and irritability so that males had smaller risks of developing agitation and irritability and had greater risks of developing elation. Additionally, lower baseline MMSE were significantly related to greater risk of progression in anxiety, apathy, and appetite and eating disorders. Regarding the groups with and without anosognosia, a significant difference was observed for seven of the 12 NPI domains (delusions, hallucinations, agitation, apathy, disinhibition, irritability, and aberrant motor behavior) such that participants with anosognosia showed an earlier onset of NPS over time as compared to participants without anosognosia (see Table 3 and Figure 1).
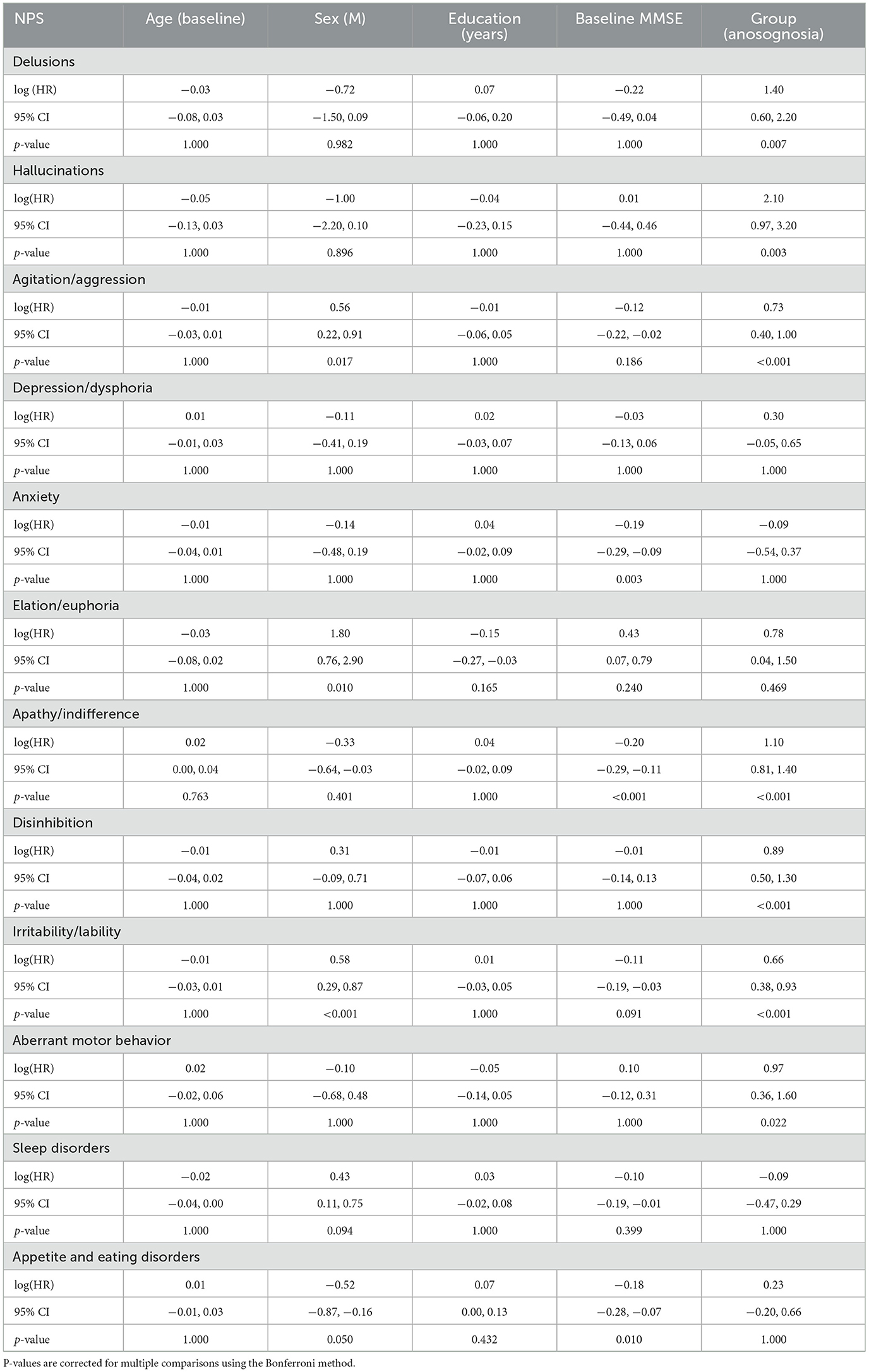
Table 3. Cox regression model outputs comparing the onset of NPS over time for participants with and without stable anosognosia.
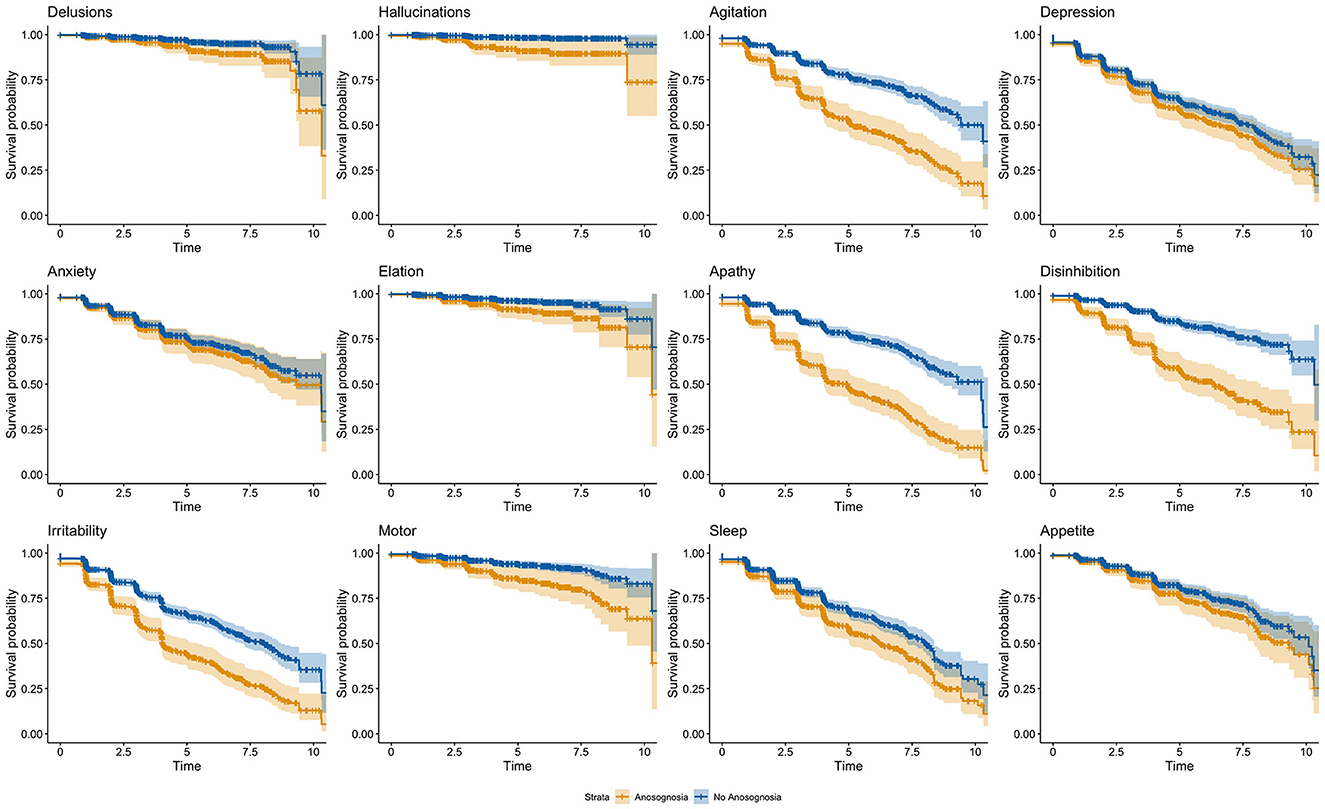
Figure 1. Cox regression model outputs comparing the onset of NPS over time for participants with and without stable anosognosia. The orange line indicates participants with anosognosia, and the blue line indicates participants without anosognosia.
The results of the model adding amyloidosis as a covariate are presented in Supplementary Table 1. The results of the model adding amyloidosis and baseline CSF phosphorylated tau as covariates are presented in Supplementary Table 2.
In this study, we investigated the association between anosognosia and the frequency and development of NPS in individuals with MCI. We observed a significantly earlier onset of seven of the 12 NPS in participants with anosognosia as compared to participants without anosognosia. These seven NPS include delusion, hallucination, agitation, apathy, disinhibition, irritability, and aberrant motor behavior. This study adds to the existing literature by exploring the time to development of NPS using the presence or absence of anosognosia as a predictor. In addition, we assessed baseline comparisons between the two groups on some cognitive, neuropsychiatric, and pathological variables. Our results showed that at baseline, participants classified as having anosognosia demonstrated lower MMSE scores and greater cerebral amyloid levels, in line with previous findings (Hanseeuw et al., 2020b; Gagliardi and Vannini, 2022) indicating that anosognosia is related to AD pathology. Participants with anosognosia had more NPS at baseline than those without, which is consistent with previous results in the dementia population (Spalletta et al., 2012; Conde-Sala et al., 2016; Martyr et al., 2022). Specifically, agitation and disinhibition were more frequent in MCI participants with anosognosia compared to those without (see Table 2). These results are in line with Spalletta et al. (2012) who found that in individuals with amnestic multidomain MCI, impaired awareness was related to greater severity of disinhibition. Yoon et al. (2017) also found that in participants with CDR scores of 0.5, the prevalence of agitation was higher in those with anosognosia compared to those without. Studies that have looked at cross-sectional associations between anosognosia and NPS in dementia have found positive relationships between anosognosia and behavioral disturbances like agitation and disinhibition (Aalten et al., 2005; Kashiwa et al., 2005; Spalletta et al., 2012).
In our sample, depression was not associated with anosognosia at baseline using the depression item in the NPI-Q nor the self-reported GDS. Some studies have also found no association between depression and anosognosia, which may reflect differences in depressive symptoms from dysthymia or mild depression vs. major depression (Aalten et al., 2005).
Whereas, previous researchers have consistently found apathy and anosognosia to be positively related in the dementia population (Aalten et al., 2005; Starkstein et al., 2010; Tondelli et al., 2021), we did not find that participants with anosognosia had more apathy at baseline. Our results agree with those of Mak et al. (2015) which did not find any association between apathy and anosognosia in MCI but did in their participants with AD dementia. This could be explained by the fact that participants with MCI demonstrate a lower prevalence of NPS than in AD dementia (Geda et al., 2008).
Our longitudinal study aim was to understand the association between anosognosia and the potential development of NPS while adjusting for baseline age, sex, years of education, and MMSE. To our knowledge, this study is the first longitudinal study to investigate the association between anosognosia and the onset of NPS in MCI participants. Our findings are consistent with previous studies in AD dementia that have found anosognosia at baseline to be associated with the development of future NPS, in particular apathy. Starkstein et al. (2010) and Tondelli et al. (2021) found that in the dementia population, anosognosia at baseline predicted more severe apathy at follow-up and that higher levels of anosognosia were associated with subsequent apathy, respectively. However, it should be noted that those studies were done in the dementia population only.
Our longitudinal results, in combination with the cross-sectional findings, suggest that there is an association between anosognosia and NPS. Indeed, previous neuroimaging studies have found that both apathy and anosognosia significantly correlate with right hemisphere dysfunction, specifically the frontal regions, in AD dementia (Ott et al., 1996). In addition, changes to the anterior cingulate cortex is common across almost all NPS (Chen et al., 2021) and are also associated with anosognosia (Valera-Bermejo et al., 2020). Decreased connectivity in the default mode network (DMN), a brain region that supports self-referential processing, has also been linked to both anosognosia (Antoine et al., 2019) and NPS (Lee et al., 2020), specifically hyperactivity symptoms such as agitation, irritability, aberrant motor behavior, euphoria, and disinhibition. While there may not be a direct causal relationship between the two, it is possible that their co-occurrence could be explained by shared neurobiological mechanisms in the frontal lobe, including in the orbitofrontal, prefrontal, and anterior cingulate cortex (Aalten et al., 2005; Starkstein et al., 2010; Spalletta et al., 2012). Therefore, awareness and NPS may both depend on the functioning of the same neural substrates. Interestingly, in our study, participants with anosognosia demonstrated greater global cerebral amyloid levels. This finding is in accordance with previous findings (Vannini et al., 2017; Cacciamani et al., 2020; Hanseeuw et al., 2020b; Gagliardi et al., 2021). Future studies are needed to understand if increased AD pathology might contribute to the joint dysfunction of awareness and NPS.
We acknowledge that this study has some limitations. First, we acknowledge that there are a variety of methods to assess anosognosia and NPS. We assessed the prevalence of NPS with study partner-rated NPI scores and the presence of anosognosia by calculating the discrepancy between study partner and participant E-Cog questionnaire scores. However, there is a possibility that study partners may be hypervigilant and over-report NPS and difficulties with everyday abilities. Similarly, anosognosia was defined as the study-partner having an ECog score ≥2.5/4 and the participant having an ECog score < 2.5/4 on their baseline measure and their last observation without more than two consecutive deviating observations during the follow-up period. We acknowledge that a different definition of stable anosognosia may yield different results. Additionally, study partners may report affective changes (i.e., how the participant is presenting outwardly to others), and their score may not accurately reflect the participant's internalized symptoms. Other alternative assessments that could have been used include clinician-rated evaluations or participant-rated questionnaires (i.e., for awareness, a discrepancy between participant's subjective and objective performances). Future studies are need to replicate our findings using these alternative approaches. Next, this cohort was a highly educated sample that may not be representative of the general population, and future studies should investigate this association using a more heterogeneous sample. Finally, we acknowledge the limited follow-up duration. Future studies should look at this association with longer follow-up to confirm our findings.
In conclusion, our findings provide further support for an association between anosognosia and NPS in individuals diagnosed with MCI at baseline. Participants who were unaware of their memory decline displayed more NPS at baseline and developed NPS earlier than participants who were aware of their cognitive decline. Individuals with anosognosia also had higher frequency of clinical decline over time. Subsequently, these findings suggest that individuals with MCI and anosognosia may be at greater risk for developing NPS and AD dementia. This information is important to relay to clinicians as it may help in the development of clinical interventions that could lessen the combined negative impact of anosognosia and NPS on patients' wellbeing. The findings from this study also support the hypothesis of a potential common underlying neurophysiological process for these manifestations. Future studies should investigate this association using neuroimaging techniques.
Publicly available datasets were analyzed in this study. Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu).
SW: Formal analysis, Investigation, Visualization, Writing – original draft. KM: Writing – review & editing. FC: Writing – review & editing. ME: Writing – review & editing. CM: Writing – review & editing. JG: Writing – review & editing. GM: Writing – review & editing. GG: Formal analysis, Investigation, Supervision, Visualization, Writing – original draft. PV: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. PV reported receiving grants from the National Institute on Aging (NIA) and National Institutes of Health (NIH) R01 AG061083 during the conduct of the study. Data collection and sharing for this project was funded by the ADNI (NIH U01 AG024904 (NIANIA) and Department of Defense W81XWH-12-2-0012). The ADNI was funded by the NIH National Institute on Aging and the NIH National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association, Alzheimer's Drug Discovery Foundation, Araclon Biotech, BioClinica, Biogen, Bristol-Myers Squibb Company, CereSpir, Cogstate, Eisai, Elan Pharmaceuticals, Eli Lilly and Company, EuroImmun, F. Hoffmann-La Roche and its affiliated company Genentech, Fujirebio, GE Healthcare, IXICO, Janssen Alzheimer Immunotherapy Research and Development, Johnson & Johnson Pharmaceutical Research and Development, Lumosity, Lundbeck, Merck & Co, Meso Scale Diagnostics, NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer, Piramal Imaging, Servier, Takeda Pharmaceutical Company, and Transition Therapeutics. The Canadian Institutes of Health Research provides funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
The investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1335878/full#supplementary-material
Aalten, P., Van Valen, E., Clare, L., Kenny, G., and Verhey, F. (2005). Awareness in dementia: a review of clinical correlates. Aging Ment. Health 9, 414–422. doi: 10.1080/13607860500143075
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer. Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Antoine, N., Bahri, M. A., Bastin, C., Collette, F., Phillips, C., Balteau, E., et al. (2019). Anosognosia and default mode subnetwork dysfunction in Alzheimer's disease. Hum. Brain Mapp. 40, 5330–5340. doi: 10.1002/hbm.24775
Azocar, I., Livingston, G., and Huntley, J. (2021). The association between impaired awareness and depression, anxiety, and apathy in mild to moderate Alzheimer's disease: a systematic review. Front. Psychiatry 12:633081. doi: 10.3389/fpsyt.2021.633081
Bonferroni, C. E. (1939). Teoria statistica delle classi e calcolo delle probabilità. Paris: Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze.
Cacciamani, F., Sambati, L., Houot, M., Habert, M. O., Dubois, B., Epelbaum, S., et al. (2020). Awareness of cognitive decline trajectories in asymptomatic individuals at risk for AD. Alzheimer. Res. Ther. 12:129. doi: 10.1186/s13195-020-00700-8
Chen, Y., Dang, M., and Zhang, Z. (2021). Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer's disease: a systematic review of symptom-general and -specific lesion patterns. Mol. Neurodegener. 16:38. doi: 10.1186/s13024-021-00456-1
Conde-Sala, J. L., Turró-Garriga, O., Piñán-Hernández, S., Portellano-Ortiz, C., Viñas-Diez, V., Gascón-Bayarri, J., et al. (2016). Effects of anosognosia and neuropsychiatric symptoms on the quality of life of patients with Alzheimer's disease: a 24-month follow-up study: anosognosia and neuropsychiatric symptoms in AD. Int. J. Geriatr. Psychiatry 31, 109–119. doi: 10.1002/gps.4298
Cummings, J. L. (1997). The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology 48:10S. doi: 10.1212/WNL.48.5_Suppl_6.10S
Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., and Gornbein, J. (1994). The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2308. doi: 10.1212/WNL.44.12.2308
Dubois, B., Hampel, H., Feldman, H. H., Scheltens, P., Aisen, P., Andrieu, S., et al. (2016). Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimer. Dement. 12, 292–323. doi: 10.1016/j.jalz.2016.02.002
Farias, S. T., Mungas, D., Reed, B. R., Cahn-Weiner, D., Jagust, W., Baynes, K., et al. (2008). The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology 22, 531–544. doi: 10.1037/0894-4105.22.4.531
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). Mini-mental state. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Gagliardi, G., Kuppe, M., Lois, C., Hanseeuw, B., and Vannini, P. (2021). Pathological correlates of impaired self-awareness of memory function in Alzheimer's disease. Alzheimer. Res. Ther. 13:118. doi: 10.1186/s13195-021-00856-x
Gagliardi, G., and Vannini, P. (2022). Episodic memory impairment mediates the loss of awareness in mild cognitive impairment. Front. Aging Neurosci. 13:802501. doi: 10.3389/fnagi.2021.802501
Galeone, F., Pappalardo, S., Chieffi, S., Iavarone, A., and Carlomagno, S. (2011). Anosognosia for memory deficit in amnestic mild cognitive impairment and Alzheimer's disease. Int. J. Geriatr. Psychiatry 26, 695–701. doi: 10.1002/gps.2583
Geda, Y. E., Roberts, R. O., Knopman, D. S., Petersen, R. C., Christianson, T. J. H., Pankratz, V. S., et al. (2008). Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch. Gen. Psychiatry 65:1193. doi: 10.1001/archpsyc.65.10.1193
Gerretsen, P., Chung, J. K., Shah, P., Plitman, E., Iwata, Y., Caravaggio, F., et al. (2017). Anosognosia is an independent predictor of conversion from mild cognitive impairment to Alzheimer's disease and is associated with reduced brain metabolism. J. Clin. Psychiatry 78, e1187–e1196. doi: 10.4088/JCP.16m11367
Hanseeuw, B. J., Scott, M. R., Sikkes, S. A. M., Properzi, M., Gatchel, J. R., Salmon, E., et al. (2020a). Evolution of anosognosia in alzheimer's disease and its relationship to amyloid. Ann. Neurol. 87, 267–280. doi: 10.1002/ana.25649
Hanseeuw, B. J., Scott, M. R., Sikkes, S. A. M., Properzi, M., Gatchel, J. R., Salmon, E., et al. (2020b). Evolution of anosognosia in alzheimer's disease and its relationship to amyloid. Ann. Neurol. 87, 267–280.
Kashiwa, Y., Kitabayashi, Y., Narumoto, J., Nakamura, K., Ueda, H., and Fukui, K. (2005). Anosognosia in Alzheimer's disease: association with patient characteristics, psychiatric symptoms and cognitive deficits. Psychiatry Clin. Neurosci. 59, 697–704. doi: 10.1111/j.1440-1819.2005.01439.x
Kassambara, A., Kosinski, M., and Biecek, P. (2021). Survminer: Drawing Survival Curves Using “ggplot2”. R Package Version 0. 4.9. CRAN.R. Available online at: https://cran.r-project.org/package=survminer
Landau, S. M., Breault, C., Joshi, A. D., Pontecorvo, M., Mathis, C. A., Jagust, W. J., et al. (2013). Amyloid-imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J. Nucl. Med. 54, 70–77. doi: 10.2967/jnumed.112.109009
Lee, J. S., Kim, J. H., and Lee, S. K. (2020). The relationship between neuropsychiatric symptoms and default-mode network connectivity in Alzheimer's disease. Psychiatry Investig. 17, 662–666. doi: 10.30773/pi.2020.0009
Mak, E., Chin, R., Ng, L. T., Yeo, D., and Hameed, S. (2015). Clinical associations of anosognosia in mild cognitive impairment and Alzheimer's disease: anosognosia and dementia. Int. J. Geriatr. Psychiatry 30, 1207–1214. doi: 10.1002/gps.4275
Martyr, A., Gamble, L. D., Nelis, S. M., Collins, R., Alexander, C. M., Morris, R. G., et al. (2022). Predictors of awareness of functional ability in people with dementia: the contribution of personality, cognition, and neuropsychiatric symptoms—findings from the IDEAL Program. Dement. Geriatr. Cogn. Disord. 51, 221–232. doi: 10.1159/000524607
Morris, J. C. (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. doi: 10.1212/WNL.43.11.2412-a
Ott, B. R., Noto, R. B., and Fogel, B. S. (1996). Apathy and loss of insight in Alzheimer's disease: a SPECT imaging study. J. Neuropsychiatry Clin. Neurosci. 8, 41–46. doi: 10.1176/jnp.8.1.41
Reed, B. R., Jagust, W. J., and Coulter, L. (1993). Anosognosia in Alzheimer's disease: relationships to depression, cognitive function, and cerebral perfusion. J. Clin. Exp. Neuropsychol. 15, 231–244. doi: 10.1080/01688639308402560
Spalletta, G., Girardi, P., Caltagirone, C., and Orfei, M. D. (2012). Anosognosia and neuropsychiatric symptoms and disorders in mild Alzheimer's disease and mild cognitive impairment. J. Alzheimer. Dis. 29, 761–772. doi: 10.3233/JAD-2012-111886
Starkstein, S. E., Brockman, S., Bruce, D., and Petracca, G. (2010). Anosognosia is a significant predictor of apathy in Alzheimer's disease. J. Neuropsychiatry Clin. Neurosci. 22, 378–383. doi: 10.1176/jnp.2010.22.4.378
Therneau, T. M. (2023). A Package for Survival Analysis in R. R package. Version 3, 5-0. CRAN.R. Available online at: https://cran.r-project.org/package=survival
Therneau, T. M., and Grambsch, P. M. (2000). Modeling Survival Data: Extending the Cox Model. Berlin: Springer.
Tondelli, M., Galli, C., Vinceti, G., Fiondella, L., Salemme, S., Carbone, C., et al. (2021). Anosognosia in early- and late-onset dementia and its association with neuropsychiatric symptoms. Front. Psychiatry 12:658934. doi: 10.3389/fpsyt.2021.658934
Valera-Bermejo, J. M., Marco De Mitolo, M., McGeown, M. W. J., and Venneri, A. (2020). Neuroanatomical and cognitive correlates of domain-specific anosognosia in early Alzheimer's disease. Cortex 129, 236–246. doi: 10.1016/j.cortex.2020.04.026
Vannini, P., Amariglio, R., Hanseeuw, B., Johnson, K. A., McLaren, D. G., Chhatwal, J., et al. (2017). Memory self-awareness in the preclinical and prodromal stages of Alzheimer's disease. Neuropsychologia 99, 343–349. doi: 10.1016/j.neuropsychologia.2017.04.002
Vannini, P., Hanseeuw, B. J., Gatchel, J. R., Sikkes, S. A. M., Alzate, D., Zuluaga, Y., et al. (2020). Trajectory of unawareness of memory decline in individuals with autosomal dominant Alzheimer's disease. J. Am. Med. Assoc. Netw. Open 3:e2027472. doi: 10.1001/jamanetworkopen.2020.27472
Wadsworth, L. P., Lorius, N., Donovan, N. J., Locascio, J. J., Rentz, D. M., Johnson, K. A., et al. (2012). Neuropsychiatric symptoms and global functional impairment along the Alzheimer's continuum. Dement. Geriatr. Cogn. Disord. 34, 96–111. doi: 10.1159/000342119
Yesavage, J. A., Brink, T. L., Rose, T. L., Lum, O., Huang, V., Adey, M., et al. (1982). Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 17, 37–49. doi: 10.1016/0022-3956(82)90033-4
Yoon, B., Shim, Y. S., Hong, Y. J., Choi, S. H., Park, H. K., Park, S. A., et al. (2017). Anosognosia and its relation to psychiatric symptoms in early-onset Alzheimer's disease. J. Geriatr. Psychiatry Neurol. 30, 170–177. doi: 10.1177/0891988717700508
Keywords: awareness, Alzheimer's disease, mild cognitive impairment, neuropsychiatric symptoms, anosognosia
Citation: Wang S, Mimmack K, Cacciamani F, Elnemais Fawzy M, Munro C, Gatchel J, Marshall GA, Gagliardi G and Vannini P (2024) Anosognosia is associated with increased prevalence and faster development of neuropsychiatric symptoms in mild cognitive impairment. Front. Aging Neurosci. 16:1335878. doi: 10.3389/fnagi.2024.1335878
Received: 09 November 2023; Accepted: 05 February 2024;
Published: 06 March 2024.
Edited by:
Julie Suhr, Ohio University, United StatesReviewed by:
Riccardo Manca, Brunel University London, United KingdomCopyright © 2024 Wang, Mimmack, Cacciamani, Elnemais Fawzy, Munro, Gatchel, Marshall, Gagliardi and Vannini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrizia Vannini, cGF0cml6aWFAYndoLmhhcnZhcmQuZWR1
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.