
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Aging Neurosci., 21 February 2024
Sec. Neurocognitive Aging and Behavior
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1294681
Introduction: As individuals age, their sleep patterns change, and sleep disturbances can increase the risk of dementia. Poor sleep quality can be a risk factor for mild cognitive impairment (MCI) and dementia. Epidemiological studies show a connection between sleep quality and cognitive changes, with brain imaging revealing grey matter volume reduction and amyloid beta accumulation in Alzheimer’s disease. However, most research has focused on Europeans, with little attention to other ethnic groups.
Methods: This is a cross sectional study comparing effects across countries and ethnicities. Group 1 (n = 193) will be Indians residing in India (new participant recruitment), Group 2 will be South Asians residing in UK and group 3 will be Europeans residing in the UK. For group 2 and 3 (n = 193), data already collected by UK-based Southall and Brent REvisited (SABRE) tri-ethnic study will be used. For group 1, Pittsburgh Sleep Quality Index questionnaire (PSQI) will be used for assessment of sleep quality, Indian Council of Medical Research (Neurocognitive ToolBox) (ICMR-NCTB) for cognition testing and a 3 T MRI cerebral scan for brain morphometry. The data will be compared to sleep, cognitive function and brain MRI parameters from SABRE.
Discussion: Racial and ethnic differences can impact the relationships of cognitive function, sleep quality and brain structure in older adults. Earlier studies have highlighted higher prevalence of poor sleep among black individuals compared to white individuals. Genetic or epigenetic mechanisms may contribute to these variations. Socio-cultural and environmental factors, such as neighbourhood, migration, lifestyle, stress and perceived discrimination may influence sleep patterns. The aim of the study is to examine the ethnogeographic variations in sleep quality, cognitive performance and brain morphometry among Indians living in India, and South Asians and Europeans residing in the UK.
Sleep disturbances may lower the threshold for dementia (Yaffe et al., 2014). Sleep duration pertains to the total hours spent asleep, whereas sleep quality is the subjective sense of feeling refreshed upon waking. Different manifestations of insomnia, including difficulties in falling asleep, maintaining sleep, or experiencing early morning awakenings, along with habitual snoring and other breathing issues during sleep, can lead to fragmented sleep patterns characterized by recurrent brief awakenings. These factors contribute to poor-quality sleep. The quality of sleep of an individual can serve as a risk factor for the development of mild cognitive impairment (MCI) and dementia (Scullin and Bliwise, 2015; Tsapanou et al., 2020; Simmonds et al., 2023). Difficulty in initiation of sleep is related to impaired global cognition (Nebes et al., 2009; Chang-Quan et al., 2012; Auyeung et al., 2013; Blackwell et al., 2014), worse working memory executive functions (Schmutte et al., 2007; Gamaldo et al., 2010; Rana et al., 2018), and verbal fluency. Early morning awakening has been found to be associated with poor performance on executive function tests (Ling et al., 2016), and with cognitive impairment in mid and late life (Jelicic et al., 2002). Excessive daytime sleepiness is also correlated with poor executive function and memory-based tasks in older adults (age 60 and above) (Ohayon, 2002; Okamura et al., 2016).
Although the amount of sleep varies individually, gender has been one of the factors underlying the variation. Some studies have reported that women tend to have longer sleep latency (Silva et al., 2008) and total sleep time (Bixler et al., 2009) as compared to men. Prevalence of insomnia and sleep disturbances is reported to be higher in women as compared to men (Ohayon, 2002; Madrid-Valero et al., 2017; Tang et al., 2017; Zeng et al., 2020).
Epidemiological studies have revealed a significant correlation between sleep quality and brain structure, as visualized by magnetic resonance imaging (MRI) (Tsapanou et al., 2020). Longer sleep latency and midnight awakenings are associated with low grey matter volume in insula and significant Aβ (amyloid beta) accumulation in prefrontal areas which are linked to Alzheimer’s disease (AD), the most common form of dementia (Branger et al., 2016; Rigat et al., 2023). Recent studies on older cohorts have revealed an association between daytime sleepiness and reduced grey matter and cortical volume (Tsapanou et al., 2020). In one study, difficulty in falling asleep was associated with cognitive impairment, and was more pronounced in men (de Souza Medeiros et al., 2022). Correlation of insomnia and atrophy of cortical and subcortical grey matter has also been documented (Grau-Rivera et al., 2020).
A small proportion of studies have reported ethnic differences in these parameters. A previous study using Southall and Brent REvisited (SABRE) tri-ethnic data has reported that Indian Asians, as compared to Europeans, have higher risk of mortality if reporting difficulty falling asleep and snoring problems (Garfield et al., 2019). Another study found that the quality of sleep, specifically snoring during middle age, is linked to the onset of type 2 diabetes in later life, even when accounting for established risk factors for type 2 diabetes. This correlation is evident in South-Asians but is not observed among Europeans and African-Caribbeans (Ong et al., 2021). As the incidence of dementia is increasing worldwide, it is important to understand the geographic, racial and cultural differences in sleep quality, brain health, and cognition.
The majority of the studies investigating the association of sleep quality, brain structure and cognitive function have been conducted in Europeans, and little attention has been paid to other ethnic groups. In Asian population, in a cross-sectional, community-based study in older Japanese adults, excessive daytime sleepiness was been found to be associated with subjective memory impairment (Okamura et al., 2016). Few, if any, studies to date have been conducted in the Indian Asian population with similar parameters. It is thus essential to acquire fresh data from the Indian population residing in India. The purpose of this study is to assess the variations in the relationship between sleep quality, cognitive performance, and brain structure among three specific population groups: Indians residing in India, South Asians residing in the UK, and the white European population residing in the UK.
IEC-66/14.01.2022 (AIIMS India) and 14/LO/0108 (UCL UK).
This is a cross sectional study comparing effects across countries and ethnicities. Three groups will be compared in this study. Group 1 will be Indians residing in India (New participant recruitment), Group 2 will be South Asians residing in UK and group 3 will be Europeans residing in the UK. For group 2 and 3, data already collected by Southall and Brent REvisited (SABRE) tri-ethnic study will be used. Figure 1 displays a flowchart depicting the study design.
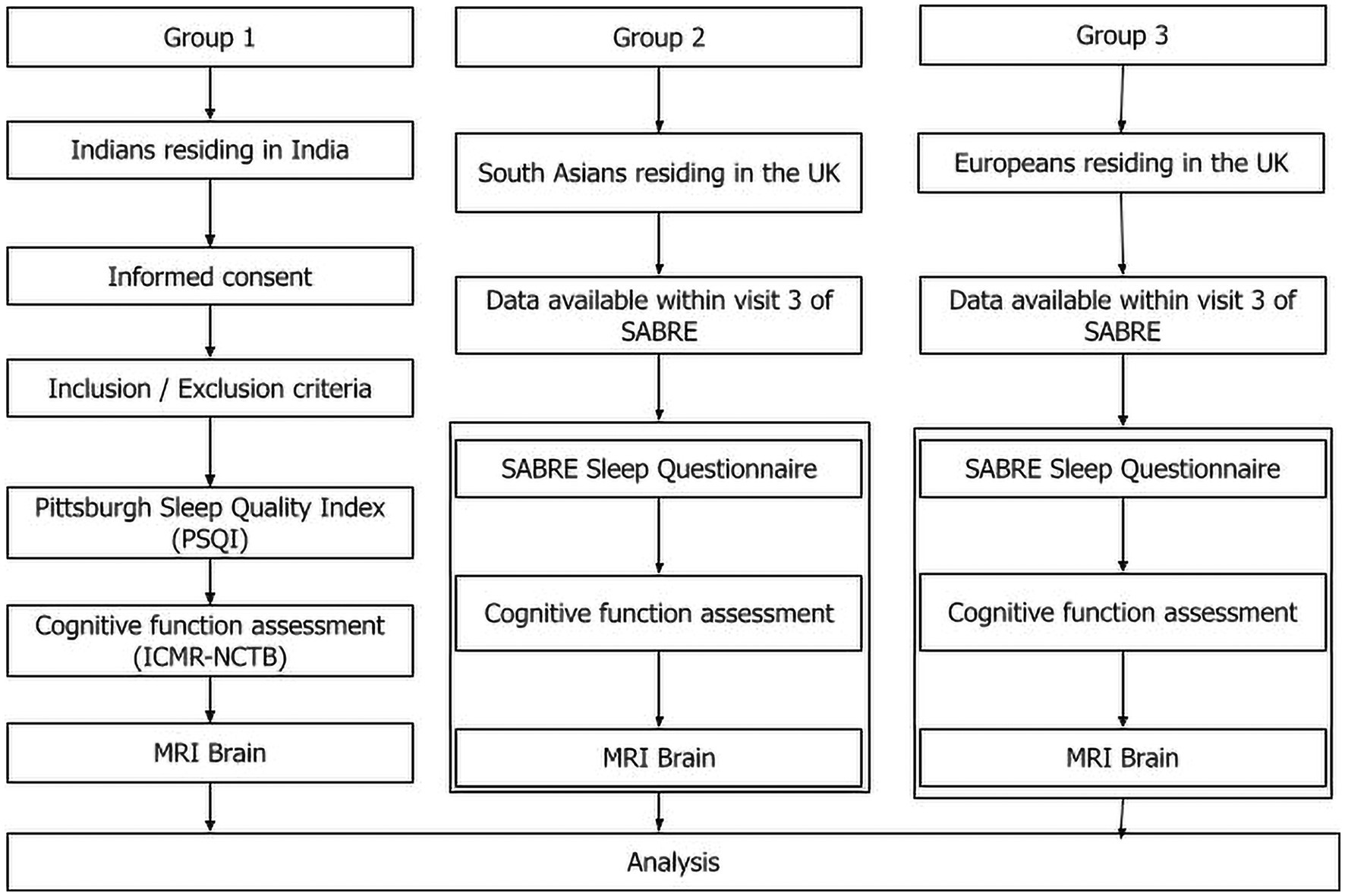
Figure 1. Flow diagram of the study. The study design has three arms: Indians residing in India, South Asians residing in the UK, and Europeans residing in the UK. The data for South Asians residing in UK and European population is already available in the SABRE BioBank. New data on Indian population in India will be collected at AIIMS after informed consent. Sleep quality, cognitive function, and brain MRI of all three groups will be compared.
New data of Indians residing in India will be collected afresh by new participant recruitment at a tertiary care center in India. Data acquisition will begin after obtaining the ethical approval from the Institute Ethics Committee. Data from the UK-based Southall and Brent REvisited (SABRE) tri-ethnic study at visit 3 (2014–18) will be analyzed in parallel with the new data collected in India.
New participants in India will be recruited from out-patient departments of neurology, geriatric medicine memory clinic, healthy relatives of patients in the clinics at a tertiary care center, community outreach services, and, via advertisements and social media invitations. Participants will be screened for inclusion and exclusion criteria by history and physical examination. Inclusion criteria will be individuals of both sexes aged between 50 and 80 years, matching the age group of participants in the SABRE study. Exclusion criteria will be non-ambulatory patients, individuals diagnosed with major depression or general anxiety disorder, family history consistent with autosomal dominant Alzheimer’s Disease (AD), a history of any psychiatric condition, individuals currently using psychotropic medication and presence of MRI incompatible implants. Written informed consent will be obtained from all participants.
Will consist of South Asians (Group 2) and Europeans residing in the UK (Group 3) at the 20-year follow-up in the Southall and Brent Revisited (SABRE) study. This study was first conducted between 2008 and 2012. It is a multiethnic community-based prospective cohort comprising older Europeans, South-Asians, and African-Caribbeans from London. SABRE begun in the late 1980s and then they were followed up twice, once at 20 years (this data will not be used in the current study) and then again between 2014-2018 and these are the data which will be analyzed for the study. The primary objective of this study is to investigate ethnic differences in cardiometabolic disorders. The median follow-up period for participants for the current study is 19 years, with an interquartile range (IQR) of 15 to 20 years. Participants’ ethnicity was initially determined by interviewers based on grandparental origin and subsequently confirmed by the participants themselves. Among the South-Asian participants, the majority are Punjabi Sikhs (52%), followed by Gujarati or Punjabi Hindus (20%), Muslims (15%), and other South-Asians (15%). The survivors at the follow-up assessment range from 57 to 90 years old. A comprehensive cohort profile and additional follow-up details are found in previous publications (Tillin et al., 2012, 2013).
Sleep quality will be assessed in the new participants in India using Pittsburg Sleep quality questionnaire. It is a subjective sleep quality assessment scale which includes 19 items that assess perceived sleep disturbance over the preceding 4 weeks. Seven component scores (i.e., sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, daytime dysfunction) are summed to derive a PSQI global score of subjective sleep quality (range 0–21 points). A global score of less than or equal to five is an indicator of good quality sleep. A weighted sleep quality score will be created.
Within the SABRE cohort, participants responded to four questions regarding sleep quality during the baseline assessment. These questions assessed whether individuals experienced difficulty falling asleep, woke up too early, felt tired upon waking, and snored within the past 30 days. The first three questions were adapted from Jenkin’s Sleep Questionnaire (Jenkins et al., 1988), a concise, validated, reliable, and commonly used tool for evaluating sleep disturbances. A weighted sleep quality score will be created.
There are two outcomes- cognitive function and brain morphometry.
The Indian Council of Medical Research-National Cognitive Test Battery (ICMR-NCTB) incorporates tests of the major cognitive domains of attention, executive functions, memory, language, and visuospatial skills adapted and translated into five Indian languages: Hindi, Bengali, Telugu, Kannada, and Malayalam. The ICMR-NCTB consists of a range of tests that evaluate the major cognitive domains: (a) tests of cognition for the various domains of attention-executive functions: Trail Making Test A & B (TMT A & B) and Category Fluency; memory: Verbal Learning Test-Total Learning and Delayed Recall (VLT-TL & DR) and Modified Taylor Complex Figure Test-Delayed Recall (MTCF-DR); and language (Picture Naming Test-PNT) and visuospatial skills (Modified Taylor Complex Figure test); and (b) questionnaires on behavior and functional activities: Geriatric Depression Scale (GDS), Instrumental Activities of Daily Living-Elderly (IADLE), Neuropsychiatric Inventory (NPI), Informant Questionnaire on Cognitive Decline in Elderly (IQCODE), and RAND Short Form Health Survey (RAND SF-36). This test battery was developed to screen and diagnose dementia and mild cognitive impairment in the early stages, across the country in India, and to be suitable for conducting global collaborative research in cognitive disorders (Verma et al., 2021).
Cognitive function as been assessed in the SABRE cohort with a test battery that has been previously validated for cross cultural settings (Stewart et al., 2001). The following domains have been tested: global/overall function (Community Screening Instrument for Dementia [CSID] cognitive assessment); verbal memory (immediate and delayed verbal recall [CERAD 10-word]), visual memory (picture recognition); fluency (animal naming), processing speed (color trail-making A and B); and working memory (forward and backward digit span).
All participants will undergo an MRI scan using a 3 T scanner (Philips, Achieva). MR protocol for T1 weighted images using 32 channel head coil will consist of a slice thickness of 1 mm, echo time (TE) =3.7 ms, repetition time (TR) =8.1 ms, flip angle = 8°, voxel size = 1 × 1 × 1 mm3 (acquired); 1 × 1 × 1 mm3 (reconstructed) and field-of-view (FOV) =240 × 240 × 180 mm3. MR protocol for T2 weighted images will consist of TE = 257 ms, TR = 2,500 ms, voxel size = 1 × 1 × 1 mm3 (reconstructed) and FOV = 250 × 250 × 175 mm3. Any images with motion artifacts will be excluded. Computational Anatomy Toolbox (CAT-12) will be used for cortical and subcortical analysis of T1 weighted images. This toolbox covers diverse morphometric methods such as voxel-based morphometry (VBM), surface-based morphometry (SBM), deformation-based morphometry (DBM), and region- or label-based morphometry (RBM). This will provide the parameters reported in the UK SABRE cohort: hippocampal volume, total brain volume, total intracranial volume, total grey matter volume.
In the SABRE cohort, cerebral MRI has been performed based on the Cardiovascular Health Study protocol (Bryan et al., 1994). Parameters of interest are the total brain volume, total intracranial volume, total grey matter volume and total hippocampal volume. The MRI includes sagittal T1-weighted and axial T1-weighted, proton density, and T2-weighted images of 5-mm thickness with no gaps. For volumetric measures, 3-mm axial fluid-attenuated inversion recovery and coronal 1.5-mm 3-dimensional T1-weighted gradient echo images have been obtained. A third of the scans have been performed on a General Electric Signa HDxt 1.5 T scanner and the rest on a General Electric Discovery MR750 3 T scanner (GE Healthcare, Waukesha, WI). An automated segmentation protocol has been used to quantify total brain and hippocampal volume using FIRST in FSL 4.1 (Patenaude et al., 2011). Total brain volume (TBV) has been computed as the volume after skull stripping of the T1-weighted image using BET (Smith, 2002), in FSL 5.0. Table 1 shows the dependent variables.
These will include demographic factors (age, sex, socioeconomic status), health behaviors (alcohol consumption and smoking) and comorbidities (cardiovascular disease, type-2 diabetes, hypertension, hyperlipidaemia, body mass index). Weight, height, blood pressure, abdominal circumference, neck circumference, waist:hip ratio, and medications will be recorded before the conduct of study in group 1. Records of medications will be maintained to investigate the effect of their medication on their sleep structure. For group 2 and 3, these variables have been obtained at the third visit (2014–18). Table 2 shows the sociodemographic variables for group 1 (age, smoking, alcohol consumption, living circumstances, education, household income, and socioeconomic status).
As the comparison of the study parameters between Indians in the UK and Indians in India is a novel proposition, there is no meta-analysis available for this kind of comparison. We would like the acquisition of new data on the Indian side to approximate the size of the sample already in the SABRE study (population with MRI also), that is 193. Thus, we would like to keep the sample size at 193. SABRE currently possesses the data of 193 Indian Asians and 287 Europeans with all the required measurements.
Exposures will be sleep quality questions from Pittsburgh Sleep Quality Index (PSQI). We will create a weighted sleep quality score as per the analysis in the SABRE Cohort (Ong et al., 2021; Topriceanu et al., 2021). The outcomes will be the following: (a) neuroimaging/morphological outcomes: hippocampal volume, total brain volume, total intracranial volume, total grey matter volume; and other parameters (b) cognitive function: verbal memory, language, reasoning and delayed visual recall, attention, global function (to screen for dementia) and functional literacy. Confounding factors will include demographic factors (age, sex, socioeconomic status, education) and health behaviors (alcohol consumption, smoking) and comorbidities (cardiovascular disease, type-2 diabetes, hypertension, hyperlipidemia, body mass index). To avoid bias in the study, we will use gender as a covariate in one of the sub analyses.
We will fit three sequential models. Model 1 will include adjustment for demographic factors, model 2 will additionally include smoking + alcohol, model 3 will additionally include co-morbidities. We will use linear regression to examine the correlation of sleep quality with neuroimaging outcomes. All three models will be stratified to ethnic groups; hence the SABRE analysis will be done independently in Europeans and Indian Asians. The results which will be obtained from linear regression models for each ethnic group will include standardized beta coefficients with confidence intervals of 95%.
To account for multiple testing within each outcome group (cognition and MRI), we will apply Bonferroni corrections. With seven cognitive outcomes and four MRI outcomes, the adjusted alpha levels will be 0.05/7 = 0.007 for cognition and 0.05/4 = 0.013 for MRI. This helps maintain an overall alpha level of 0.05 while controlling for family-wise error.
For ethnic comparisons, we will perform Cochran’s Q heterogeneity tests. This statistical test will assess whether the effect sizes (represented by standardized beta coefficients in our linear regression models) significantly differ between Indians in India, Indian Asians in the UK, and Europeans in the UK.
The aim of the study is to examine the ethnogeographic variations in sleep quality, cognitive performance and brain morphometry among Indians living in India, and South Asians and Europeans residing in the UK. We will assess how sleep quality affects cognitive function and brain structure in these populations.
The mechanisms linking sleep and brain structure and cognition encompass a range of crucial processes. During sleep, synaptic pruning and plasticity refine neural connections (Paolicelli et al., 2011), while hormone release, particularly growth hormone and cortisol, influences brain development and maintenance (Kim et al., 2015). In young adults, poor sleep quality may be associated with a significant risk of developing obstructive sleep apnoea (Aggarwal et al., 2021) and frequent arousals (Bhat et al., 2022). Sleep supports neuroplasticity, reorganizing neural networks, and promoting gray matter changes. The hippocampus is closely tied to sleep, aiding memory consolidation, while sleep facilitates the clearance of toxic substances like beta-amyloid (Aggarwal et al., 2021). Proper sleep maintains optimal brain connectivity, supports myelin sheath integrity, and may even promote neurogenesis (Payne and Nadel, 2004; de Vivo and Bellesi, 2019).
Impaired sleep quality has been shown to impact cognitive performance differently in men and women. Some studies suggest that women may be more resilient to the cognitive effects of sleep quality than men (Alhola and Polo-Kantola, 2007; Bixler et al., 2009), whereas others suggest the opposite (Ferrara et al., 2015; Hajali et al., 2019). These studies indicate that gender may impact sleep architecture, and have consequences on cognitive performance. Research on sleep differences between genders holds profound significance for public health, as sleep-related issues are associated with a range of conditions, including cognitive decline, mood disorders, and metabolic disturbances. Gender differences will be analyzed in our study.
The mechanism behind the ethnic differences in sleep quality, brain morphometry and cognition is still under investigation. One review proposed a conceptual model positing that social and environmental factors, including neighborhood characteristics, occupational stress, treatment accessibility, and perceived discrimination, may exert influence over sleep quality, duration, and the prevalence of sleep disorders (Kingsbury et al., 2013; Siow et al., 2022). However, additional long-term studies are necessary to confirm and strengthen the validity of these findings. Other studies have hypothesized that cultural and environmental factors (such as lifestyle, diet, stress, and exposure to different light and noise environments) may influence the relationship between sleep, cognitive performance, and brain morphometry in these distinct populations (Gildner et al., 2014). For Indians residing in the UK, it has been suggested that the differences might be due to the effects of migration and acculturation. This could involve assessing how the adoption of a new cultural and environmental context impacts these factors. Racial and ethnic variations in sleep quality and sleep pattern may affect cognitive function differentially. Inadequate sleep quality may contribute to impaired memory among older adults. Poor sleep tends to be more prevalent among black individuals compared to white individuals, potentially due to variations in health and psychosocial factors (Hokett and Duarte, 2019; Hokett et al., 2022). Moreover, black participants exhibited higher within-person variability in their sleep quality compared to white participants. This increased variability in sleep quality was strongly associated with decreased neural activity related to memory in black participants. The study further revealed that greater variability in sleep quality was linked to poorer memory performance, particularly in older adults. Genetic or epigenetic mechanisms have been reported to contribute to variations in sleep quality and their effects on cognitive function and brain structure in different ethnicities (Zhang et al., 2023). This study will look into some factors that may be contributing to the racial and ethnic differences such as socio-economic status, literacy, sleep quality, etc.
Studying ethnic and racial differences in the relationship between sleep, cognition, and brain structure is important for several reasons. Understanding these disparities can lead to more equitable healthcare and interventions. Insights from this research can inform public health policies (Akhtar and Mallick, 2019) and interventions aimed at improving sleep health and cognitive outcomes for different communities, ultimately enhancing overall well-being. The cross-sectional, cross-country study we are proposing may also help prospectively in the establishment of a cohort in India on the lines of the SABRE cohort in the UK. The cohort can then be used to study the cause-and-effect relationships between current dependent (outcome) and independent (exposure) variables. If a correlation between poor sleep quality and impaired cognition/brain morphometry is found, subsequent studies can target sleep quality as a modifiable risk factor to reduce cognitive decline. Sleep is essential for cognitive function, and impaired sleep quality can lead to a variety of cognitive deficits, including impaired attention, memory, and decision-making (Deak and Stickgold, 2010; Pérez-Carbonell and Iranzo, 2023). Our study might be helpful to determine how disturbed sleep disrupts the normal process of memory consolidation and how sleep quality is correlated with brain function. Our study will be one of the first to examine the association of sleep quality, brain structure, and cognition in a sample of healthy adults from India, a country with a large and diverse population and a high burden of cognitive impairment and dementia. This study will explore the crucial impact of cultural and geographical contexts on sleep patterns, cognitive decline, and brain structure. By assessing differences across ethnicities, races, and nations, it will shed light on how these vital parameters are impacted by diverse societal and environmental factors. This goes beyond identifying sleep quality as simply a correlate of cognitive decline and dementia; it will offer valuable insights into the interplay between these elements in distinct populations. This knowledge will pave the way for developing culturally sensitive interventions that optimize sleep quality, enhance cognitive health, and improve overall well-being in older adults around the world. Ultimately, this study will empower us to move beyond one-size-fits-all healthcare approaches and embrace personalized strategies tailored to the specific needs of each individual within their unique cultural and geographic context.
The studies involving humans were approved by Institute Ethics Committee of Postgraduate Research (IECPG), AIIMS, New Delhi. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
RS: Writing – original draft, Writing – review & editing. CD: Writing – review & editing. VG: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. NA: Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by University College of London-All India Institute of Medical Sciences, New Delhi Collaborative Research Grant.
We would like to acknowledge the funding agencies- University College of London and All India Institute of Medical Sciences, New Delhi.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aggarwal, K., Akhtar, N., and Mallick, H. N. (2021). Sleep quality mediates the relationship between risk of obstructive sleep apnea and acute stress in young adults. J. Physiol. Pharmacol. 72:11. doi: 10.26402/jpp.2021.1.11
Akhtar, N., and Mallick, H. (2019). Recommendations for a National Sleep Policy in India. Natl. Med. J. India 32, 59–60. doi: 10.4103/0970-258X.272131
Alhola, P., and Polo-Kantola, P. (2007). Sleep deprivation: impact on cognitive performance. Neuropsychiatr. Dis. Treat. 3, 553–567.
Auyeung, T. W., Lee, J. S. W., Leung, J., Kwok, T., Leung, P. C., Woo, J., et al. (2013). Cognitive deficit is associated with phase advance of sleep-wake rhythm, daily napping, and prolonged sleep duration--a cross-sectional study in 2,947 community-dwelling older adults. Age 35, 479–486. doi: 10.1007/s11357-011-9366-6
Bhat, S. Y., Akhtar, N., Sengupta, T., Netam, R., Kumar, V. M., and Mallick, H. N. (2022). Electroencephalographic and electromyographic events during spontaneous and final arousal from sleep: study of the sequence of appearance and significance. Sleep Vigil. 6, 153–163. doi: 10.1007/s41782-021-00185-x
Bixler, E. O., Papaliaga, M. N., Vgontzas, A. N., et al. (2009). Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J. Sleep Res. 18, 221–228. doi: 10.1111/j.1365-2869.2008.00713.x
Blackwell, T., Yaffe, K., Laffan, A., Ancoli-Israel, S., Redline, S., Ensrud, K. E., et al. (2014). Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep 37, 655–663. doi: 10.5665/sleep.3562
Branger, P., Arenaza-Urquijo, E. M., Tomadesso, C., Mézenge, F., André, C., de Flores, R., et al. (2016). Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol. Aging 41, 107–114. doi: 10.1016/j.neurobiolaging.2016.02.009
Bryan, R. N., Manolio, T. A., Schertz, L. D., Jungreis, C., Poirier, V. C., Elster, A. D., et al. (1994). A method for using MR to evaluate the effects of cardiovascular disease on the brain: the cardiovascular health study. AJNR Am. J. Neuroradiol. 15, 1625–1633.
Chang-Quan, H., Bi-Rong, D., and Yan, Z. (2012). Association between sleep quality and cognitive impairment among Chinese nonagenarians/centenarians. J. Clin. Neurophysiol. 29, 250–255. doi: 10.1097/WNP.0b013e3182570f2e
de Souza Medeiros, L., Santos, F. H., Almeida, A. P., Alves, D. M., Rocca, R. R., Tufik, S., et al. (2022). Sex differences in the cognitive performance in adults: role of impaired sleep. Sleep Sci 15, 17–25. doi: 10.5935/1984-0063.20210022
de Vivo, L., and Bellesi, M. (2019). The role of sleep and wakefulness in myelin plasticity. Glia 67, 2142–2152. doi: 10.1002/glia.23667
Deak, M. C., and Stickgold, R. (2010). Sleep and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 1, 491–500. doi: 10.1002/wcs.52
Ferrara, M., Bottasso, A., Tempesta, D., Carrieri, M., De Gennaro, L., and Ponti, G. (2015). Gender differences in sleep deprivation effects on risk and inequality aversion: evidence from an economic experiment. PLoS One 10:e0120029. doi: 10.1371/journal.pone.0120029
Gamaldo, A. A., Allaire, J. C., and Whitfield, K. E. (2010). Exploring the within-person coupling of sleep and cognition in older African Americans. Psychol. Aging 25, 851–857. doi: 10.1037/a0021378
Garfield, V., Joshi, R., Garcia-Hernandez, J., Tillin, T., and Chaturvedi, N. (2019). The relationship between sleep quality and all-cause, CVD and cancer mortality: the Southall and Brent REvisited study (SABRE). Sleep Med. 60, 230–235. doi: 10.1016/j.sleep.2019.03.012
Gildner, T. E., Liebert, M. A., Kowal, P., Chatterji, S., and Snodgrass, J. J. (2014). Associations between sleep duration, sleep quality, and cognitive test performance among older adults from six middle income countries: results from the study on global ageing and adult health (SAGE). J. Clin. Sleep Med. 10, 613–621. doi: 10.5664/jcsm.3782
Grau-Rivera, O., Operto, G., Falcón, C., et al. (2020). Association between insomnia and cognitive performance, gray matter volume, and white matter microstructure in cognitively unimpaired adults. Alzheimers Res. Ther. 12:4. doi: 10.1186/s13195-019-0547-3
Hajali, V., Andersen, M. L., Negah, S. S., and Sheibani, V. (2019). Sex differences in sleep and sleep loss-induced cognitive deficits: the influence of gonadal hormones. Horm. Behav. 108, 50–61. doi: 10.1016/j.yhbeh.2018.12.013
Hokett, E., Arunmozhi, A., Campbell, J., and Duarte, A. (2022). Factors that protect against poor sleep quality in an adult lifespan sample of non-Hispanic black and non-Hispanic white adults during COVID-19: a cross-sectional study. Front. Psychol. 13:949364. doi: 10.3389/fpsyg.2022.949364
Hokett, E., and Duarte, A. (2019). Age and race-related differences in sleep discontinuity linked to associative memory performance and its neural underpinnings. Front. Hum. Neurosci. 13:176. doi: 10.3389/fnhum.2019.00176
Jelicic, M., Bosma, H., Ponds, R. W. H. M., Van Boxtel, M. P. J., Houx, P. J., and Jolles, J. (2002). Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht ageing study (MAAS). Int. J. Geriatr. Psychiatry 17, 73–77. doi: 10.1002/gps.529
Jenkins, C. D., Stanton, B. A., Niemcryk, S. J., and Rose, R. M. (1988). A scale for the estimation of sleep problems in clinical research. J. Clin. Epidemiol. 41, 313–321. doi: 10.1016/0895-4356(88)90138-2
Kim, T. W., Jeong, J. H., and Hong, S. C. (2015). The impact of sleep and circadian disturbance on hormones and metabolism. Int. J. Endocrinol. 2015:591729, 1–9. doi: 10.1155/2015/591729
Kingsbury, J. H., Buxton, O. M., and Emmons, K. M. (2013). Sleep and its relationship to racial and ethnic disparities in cardiovascular disease. Curr. Cardiovasc. Risk Rep. 7, 387–394. doi: 10.1007/s12170-013-0330-0
Ling, A., Lim, M. L., Gwee, X., Ho, R. C. M., Collinson, S. L., and Ng, T. P. (2016). Insomnia and daytime neuropsychological test performance in older adults. Sleep Med. 17, 7–12. doi: 10.1016/j.sleep.2015.07.037
Madrid-Valero, J. J., Martínez-Selva, J. M., Ribeiro do Couto, B., Sánchez-Romera, J. F., and Ordoñana, J. R. (2017). Age and gender effects on the prevalence of poor sleep quality in the adult population. Gac. Sanit. 31, 18–22. doi: 10.1016/j.gaceta.2016.05.013
Nebes, R. D., Buysse, D. J., Halligan, E. M., Houck, P. R., and Monk, T. H. (2009). Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 64, 180–187. doi: 10.1093/geronb/gbn037
Ohayon, M. M. (2002). Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med. Rev. 6, 97–111. doi: 10.1053/smrv.2002.0186
Okamura, T., Ura, C., Miyamae, F., Sugiyama, M., Niikawa, H., Ito, K., et al. (2016). Excessive daytime sleepiness is related to subjective memory impairment in late life: a cross-sectional community-based study. Psychogeriatrics 16, 196–201. doi: 10.1111/psyg.12139
Ong, Z. L., Chaturvedi, N., Tillin, T., Dale, C., and Garfield, V. (2021). Association between sleep quality and type 2 diabetes at 20-year follow-up in the Southall and Brent REvisited (SABRE) cohort: a triethnic analysis. J. Epidemiol. Community Health 75, 1117–1122. doi: 10.1136/jech-2020-215796
Paolicelli, R. C., Bolasco, G., Pagani, F., Maggi, L., Scianni, M., Panzanelli, P., et al. (2011). Synaptic pruning by microglia is necessary for Normal brain development. Science 333, 1456–1458. doi: 10.1126/science.1202529
Patenaude, B., Smith, S. M., Kennedy, D. N., and Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 56, 907–922. doi: 10.1016/j.neuroimage.2011.02.046
Payne, J. D., and Nadel, L. (2004). Sleep, dreams, and memory consolidation: the role of the stress hormone cortisol. Learn. Mem. 11, 671–678. doi: 10.1101/lm.77104
Pérez-Carbonell, L., and Iranzo, A. (2023). Sleep-related changes prior to cognitive dysfunction. Curr. Neurol. Neurosci. Rep. 23, 177–183. doi: 10.1007/s11910-023-01258-2
Rana, B. K., Panizzon, M. S., Franz, C. E., Spoon, K. M., Jacobson, K. C., Xian, H., et al. (2018). Association of Sleep Quality on memory-related executive functions in middle age. J. Int. Neuropsychol. 24, 67–76. doi: 10.1017/S1355617717000637
Rigat, L., Ouk, K., Kramer, A., and Priller, J. (2023). Dysfunction of circadian and sleep rhythms in the early stages of Alzheimer’s disease. Acta Physiol. 238:e13970. doi: 10.1111/apha.13970
Schmutte, T., Harris, S., Levin, R., Zweig, R., Katz, M., and Lipton, R. (2007). The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav. Sleep Med. 5, 39–56. doi: 10.1207/s15402010bsm0501_3
Scullin, M. K., and Bliwise, D. L. (2015). Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect. Psychol. Sci. 10, 97–137. doi: 10.1177/1745691614556680
Silva, A., Andersen, M. L., De Mello, M. T., Bittencourt, L. R. A., Peruzzo, D., and Tufik, S. (2008). Gender and age differences in polysomnography findings and sleep complaints of patients referred to a sleep laboratory. Braz. J. Med. Biol. Res. 41, 1067–1075. doi: 10.1590/s0100-879x2008001200005
Simmonds, E., Levine, K. S., Han, J., et al. Sleep disturbances as risk factors for neurodegeneration later in life. MedRxiv [Epub ahead of preprint] (2023). doi: 10.1101/2023.11.08.23298037
Siow, T. Y., Toh, C. H., Hsu, J. L., Liu, G. H., Lee, S. H., Chen, N. H., et al. (2022). Association of Sleep, neuropsychological performance, and gray matter volume with Glymphatic function in community-dwelling older adults. Neurology 98, e829–e838. doi: 10.1212/WNL.0000000000013215
Smith, S. M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155. doi: 10.1002/hbm.10062
Stewart, R., Richards, M., Brayne, C., and Mann, A. (2001). Cognitive function in UK community-dwelling African Caribbean elders: normative data for a test battery. Int. J. Geriatr. Psychiatry 16, 518–527. doi: 10.1002/gps.384
Tang, J., Liao, Y., Kelly, B. C., Xie, L., Xiang, Y. T., Qi, C., et al. (2017). Gender and regional differences in sleep quality and insomnia: a general population-based study in Hunan Province of China. Sci. Rep. 7:43690. doi: 10.1038/srep43690
Tillin, T., Forouhi, N. G., PM, M. K., and Chaturvedi, N.SABRE Study Group (2012). Southall and Brent REvisited: cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int. J. Epidemiol. 41, 33–42. doi: 10.1093/ije/dyq175
Tillin, T., Hughes, A. D., Godsland, I. F., Whincup, P., Forouhi, N. G., Welsh, P., et al. (2013). Insulin resistance and truncal obesity as important determinants of the greater incidence of diabetes in Indian Asians and African Caribbeans compared with Europeans: the Southall and Brent REvisited (SABRE) cohort. Diabetes Care 36, 383–393. doi: 10.2337/dc12-0544
Topriceanu, C. C., Tillin, T., Chaturvedi, N., Joshi, R., and Garfield, V. (2021). The association between plasma metabolites and sleep quality in the Southall and Brent Revisited (SABRE) study: a cross-sectional analysis. J. Sleep Res. 30:e13245. doi: 10.1111/jsr.13245
Tsapanou, A., Scarmeas, N., and Stern, Y. (2020). Sleep and the aging brain. A multifaceted approach. Sleep Sci. 13, 152–156. doi: 10.5935/1984-0063.20190128
Verma, M., Tripathi, M., Nehra, A., Paplikar, A., Varghese, F., Alladi, S., et al. (2021). Validation of ICMR neurocognitive toolbox for dementia in the linguistically diverse context of India. Front. Neurol. 12:661269. doi: 10.3389/fneur.2021.661269
Yaffe, K., Falvey, C. M., and Hoang, T. (2014). Connections between sleep and cognition in older adults. Lancet Neurol. 13, 1017–1028. doi: 10.1016/S1474-4422(14)70172-3
Zeng, L. N., Zong, Q. Q., Yang, Y., Zhang, L., Xiang, Y. F., Ng, C. H., et al. (2020). Gender difference in the prevalence of insomnia: a Meta-analysis of observational studies. Front. Psych. 11:577429. doi: 10.3389/fpsyt.2020.577429
Keywords: sleep, MRI, brain, cognition, sleep quality, SABRE, brain morphometry
Citation: Soni R, Dale C, Garfield V and Akhtar N (2024) A cross-sectional observational study for ethno-geographical disparities in sleep quality, brain morphometry and cognition (a SOLACE study) in Indians residing in India, and South Asians and Europeans residing in the UK – a study protocol. Front. Aging Neurosci. 16:1294681. doi: 10.3389/fnagi.2024.1294681
Received: 15 September 2023; Accepted: 12 February 2024;
Published: 21 February 2024.
Edited by:
Nadine Correia Santos, University of Minho, PortugalReviewed by:
Richa Nigam, Thapar Institute of Engineering and Technology, IndiaCopyright © 2024 Soni, Dale, Garfield and Akhtar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victoria Garfield, di5nYXJmaWVsZEB1Y2wuYWMudWs=; Nasreen Akhtar, ZHJuYXNyZWVuYWtodGFyQGFpaW1zLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.