
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci., 13 December 2023
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1320240
This article is part of the Research TopicEffectiveness of Exercise and Diet on Movement DisordersView all 9 articles
Background: Hydrotherapy can improve the motor and non-motor symptoms of Parkinson’s disease (PD), but the long-term effects of hydrotherapy on PD are still unclear.
Objective: The purpose of this systematic evaluation and meta-analysis was to explore the long-term effects of hydrotherapy on balance function in PD patients.
Methods: A systematic search of five databases was conducted to identify appropriate randomized controlled trials (RCTs) according to the established inclusion and exclusion criteria. The general characteristics and outcome data (balance, exercise, mobility, quality of life, etc.) of the included studies were extracted, and the quality of the included studies was evaluated using the Cochrane risk of bias assessment tool. Finally, the outcome data were integrated for meta-analysis.
Results: A total of 149 articles were screened, and 5 high-quality RCTs involving 135 PD patients were included. The results of the meta-analysis showed positive long-term effects of hydrotherapy on balance function compared to the control group (SMD = 0.69; 95% CI = 0.21, 1.17; p = 0.005; I2 = 44%), However, there were no significant long-term effects of hydrotherapy on motor function (SMD = 0.06; 95% CI = −0.33, 0.44; p = 0.77; I2 = 0%), mobility and quality of life (SMD = −0.21; 95% CI = −0.98, 0.57; p = 0.6; I2 = 71%). Interestingly, the results of the sensitivity analysis performed on mobility showed a clear continuation effect of hydrotherapy on mobility compared to the control group (SMD = −0.80; 95% CI = −1.23, −0.37; p < 0.001; I2 = 0%).
Conclusion: The long-term effects of hydrotherapy on PD patients mainly focus on balance function, and the continuous effects on motor function, mobility, and quality of life are not obvious.
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by progressive worsening of motor and non-motor dysfunction (Sveinbjornsdottir, 2016). Epidemiologic studies show a global incidence of PD of 200/100,000 people, with one person diagnosed approximately every hour (Titova and Chaudhuri, 2018). Additionally, the incidence increases 5 to 10 times from the age of 60 to 90 years, resulting in an increase of 5/100,000 to more than 35/100,000 new cases per year (Simon et al., 2020). Disease progression is associated with limited mobility, increased risk of falls, and decreased quality of life (Hariz and Forsgren, 2011). Impaired balance is a common symptom that increases the risk of falls in people with PD (Simpkins and Yang, 2023). As PD progresses, the processing of vestibular, visual, and proprioceptive signals that maintain body balance changes (Samoudi et al., 2015). PD patients tend to move their center of gravity forward, making it difficult for them to perform compensatory movements and adjustments of such body balance, which leads to more frequent falls (Samoudi et al., 2015).
The treatment of PD includes drug therapy and physical therapy (Poewe and Seppi, 2001; Reinoso et al., 2015). Medication can relieve PD symptoms, but it does not change the progression of the disease and has some side effects (Reinoso et al., 2015). For example, levodopa may cause side effects such as nausea, delusions, drowsiness, and dystonia (Hayes, 2019). Physical therapy is used by an increasing number of scholars and physicians as an adjunct to PD treatment (Gage and Storey, 2004). Physical therapy for PD focuses on transfers, posture, upper extremity function, balance (and falls), gait, and physical ability and (in) mobility (Tomlinson et al., 2012). Hydrotherapy as a physical therapy for the treatment of motor and non-motor symptoms in patients with early-stage PD has been shown to have favorable results in terms of slowed movement, dystonia, balance, pain, quality of life, and physical function (Alves Da Rocha et al., 2015; Carroll et al., 2020, 2022). The aquatic environment has specific mechanical advantages due to hydrostatic and hydrodynamic principles of buoyancy, viscosity, and resistance (Denning et al., 2012). Specific properties of the aquatic environment (density, specific gravity, hydrostatic pressure, buoyancy, viscosity, and thermodynamics) may help to modulate sensory feedback of motor output (Cugusi et al., 2019). Buoyancy reduces weight and allows the patient to transfer safely at a lower speed (Carregaro and AMd, 2008). Due to its ability to enhance functional mobility, the effect on PD patients at the same time is pleasant. Hydrotherapy has become a popular treatment (Cugusi et al., 2015). The current meta-analysis reports that hydrotherapy has positive outcomes in gait, balance, and mobility, but only analyzes efficacy during treatment and does not explore the long-term effects of hydrotherapy on PD patients or (how long these improvements can be sustained?) (Carroll et al., 2020). Therefore, the purpose of this systematic evaluation and meta-analysis was to explore the long-term effects of hydrotherapy on balance function in PD patients.
This systematic review was designed and implemented by the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Page et al., 2021). The study was registered in PROSPERO (CRD42023465857).
Searches were conducted in five databases (PubMed, Cochrane Library, Embase, Web of Science, and Ovid) by the 2020 PRISMA statement (Page et al., 2021). The search was limited to September 24, 2023. The search strategy in the Pubmed database uses subject terms paired with keywords for the search. For example: [(“Hydrotherapy”[Mesh]) OR (Whirlpool Baths) OR (Bath, Whirlpool)] AND [(“Parkinson Disease, Secondary”[Mesh]) OR (Parkinson Disease) OR (Secondary Parkinson Disease) OR (Parkinsonism, Symptomatic)].
The criteria for inclusion in the study were: (1) The study population were PD patients (Hoehn and Yahr I–IV); (2) Intervention was hydrotherapy (or physical therapy or exercise was done in a pool setting); (3) The interventions in the control group was land-based treatments or routine exercises; (4) The outcome indicators included in the study must contain the Berg Balance Scale (BBS). In addition, the patient’s motor function, mobility, or quality of life will be evaluated (meeting one of these is sufficient); (5) Follow-up assessment was mandatory for the included studies; (6) The type of study was limited to randomized controlled trials (RCTs). (6) Exclusion Criteria: (1) Follow-up time from the end of the intervention was too short, less than 15 days; (2) Data could not be extracted from the included studies.
Two researchers (ZCL and MH) assessed the studies separately. First, they eliminate duplicates and then evaluate the titles and abstracts of the remaining literature. Finally, studies that met the criteria were read in full text. All differences were discussed and resolved by two researchers.
Two researchers (ZL and MH) independently evaluated the studies based on the above inclusion/exclusion criteria. They extract data as well as information in two parts. The first part is the basic information about the included studies, including authors, year of publication, type of study design, intervention program, and assessment indicators. The second part is the extracted data such as the number of people, age, duration of follow-up, and assessment data. All differences were discussed and resolved by two researchers.
Two researchers (XX and CT) independently evaluated the quality of the method and the risk of bias included in the study through the Cochrane Bias Risk Tool and checked seven items including potential selection bias, implementation bias, detection bias, loss bias, reporting bias, and other biases. The tool divides quality risk into three categories: low, high, and unclear.
RevMan5.4.1 statistical software is used for data analysis. Standardized mean difference (SMD) of clinical scale change scores and their 95% confidence intervals were used for calculation. This study explored the long-term effects of hydrotherapy on PD patients, so we used the values of change in clinical scores from baseline to follow-up. 95% confidence intervals (CIs) were calculated using the z-test. Heterogeneity between groups was tested using the p-value test and I2 test. Heterogeneity between studies was indicated if p < 0.05 and I2 > 50%. Sensitivity analysis was performed to ensure the reliability of the results.
This study initially searched five databases and screened 149 potential studies, 43 of which were duplicates. We then screened the remaining 104 studies for titles and abstracts, 95 studies were excluded, and 9 studies were assessed in full text. Ultimately, only five studies were included in the analysis. The specific screening process is shown in Figure 1.
As shown in Table 1, a total of 5 studies including 135 PD patients were included. All five studies (Vivas et al., 2011; Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017; Da Silva and Israel, 2019) were RCTs by design, but four of them were parallel randomized controlled trials (Vivas et al., 2011; Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017), and one (Da Silva and Israel, 2019) was a non-equivalent randomized controlled trial. The smallest sample size included in the study was only 12 patients and the largest was 34 patients (Vivas et al., 2011; Palamara et al., 2017). Interventions for the control group included in the study included land-based therapy (Vivas et al., 2011), non-aquatic physical therapy (Volpe et al., 2017), intensive multidisciplinary rehabilitation (Palamara et al., 2017), dryland therapy (Pérez de la Cruz, 2017), and regular exercise (Da Silva and Israel, 2019). All five studies (Vivas et al., 2011; Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017; Da Silva and Israel, 2019) used BBS to assess balance function in patients with PD. Four studies (Vivas et al., 2011; Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017) used the Unified Parkinson’s Disease Rating Scale III (UPDRS III) to assess motor function in patients with PD. Four studies (Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017; Da Silva and Israel, 2019) used the Timed Up and Go Test (TUG) to assess mobility in patients with PD. Three studies (Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017) assessed the quality of life of patients with PD, two of which used the Unified Parkinson’s Disease Rating Scale II (UPDRS II)(Palamara et al., 2017; Pérez de la Cruz, 2017) and one of which used the Parkinson’s disease quality of life questionnaire-39 items (PDQ-39, 24].
All five studies (Vivas et al., 2011; Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017; Da Silva and Israel, 2019) were evaluated at follow-up, which ranged from a minimum of 17 days to a maximum of 6 months (Vivas et al., 2011; Palamara et al., 2017).
The results of the bias risk plot included in the study are shown in Figures 2A,B. The included articles showed low-risk random sequence generation (100%), allocation concealment (100%), Blind outcome assessment (40%), incomplete outcome data (100%), selective reporting (100%), and other biases (100%). Due to the particularity of intervention measures, the low risk of blinding of participants and personal ratio (40%), uncertain risk (60%). The percentage of other bias uncertainty is 100%.
All five included studies (Vivas et al., 2011; Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017; Da Silva and Israel, 2019) used BBS to follow balance function in patients with PD. The effect of the hydrotherapy group on balance function improvement could be maintained for a longer period compared to the control group (SMD = 0.69; 95% CI = 0.21, 1.17; p = 0.005; I2 = 44%; Figure 3).
Four studies (Vivas et al., 2011; Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017) of balance function in PD patients were followed. There was no significant difference in the maintenance of motor function in PD patients in the hydrotherapy group compared to the control group (SMD = 0.06; 95% CI = -0.33, 0.44; p = 0.77; I2 = 0%; Figure 4).
Four of the included studies (Vivas et al., 2011; Palamara et al., 2017; Pérez de la Cruz, 2017; Da Silva and Israel, 2019) assessed the mobility of PD patients by TUG at follow-up. The results showed no difference in the maintenance of mobility in PD patients in the hydrotherapy group compared to the control group (SMD = −0.53; 95% CI = −1.13, 0.08; p = 0.09; I2 = 63%; Figure 5A). The results yielded greater than 50% heterogeneity between studies, so we performed a case-by-case sensitivity analysis. The results showed that excluding the Volpe et al. study (Volpe et al., 2017) not only significantly reduced heterogeneity but also yielded inconsistent results (SMD = -0.80; 95% CI = −1.23, −0.37; p < 0.001; I2 = 0%; Figure 5B).
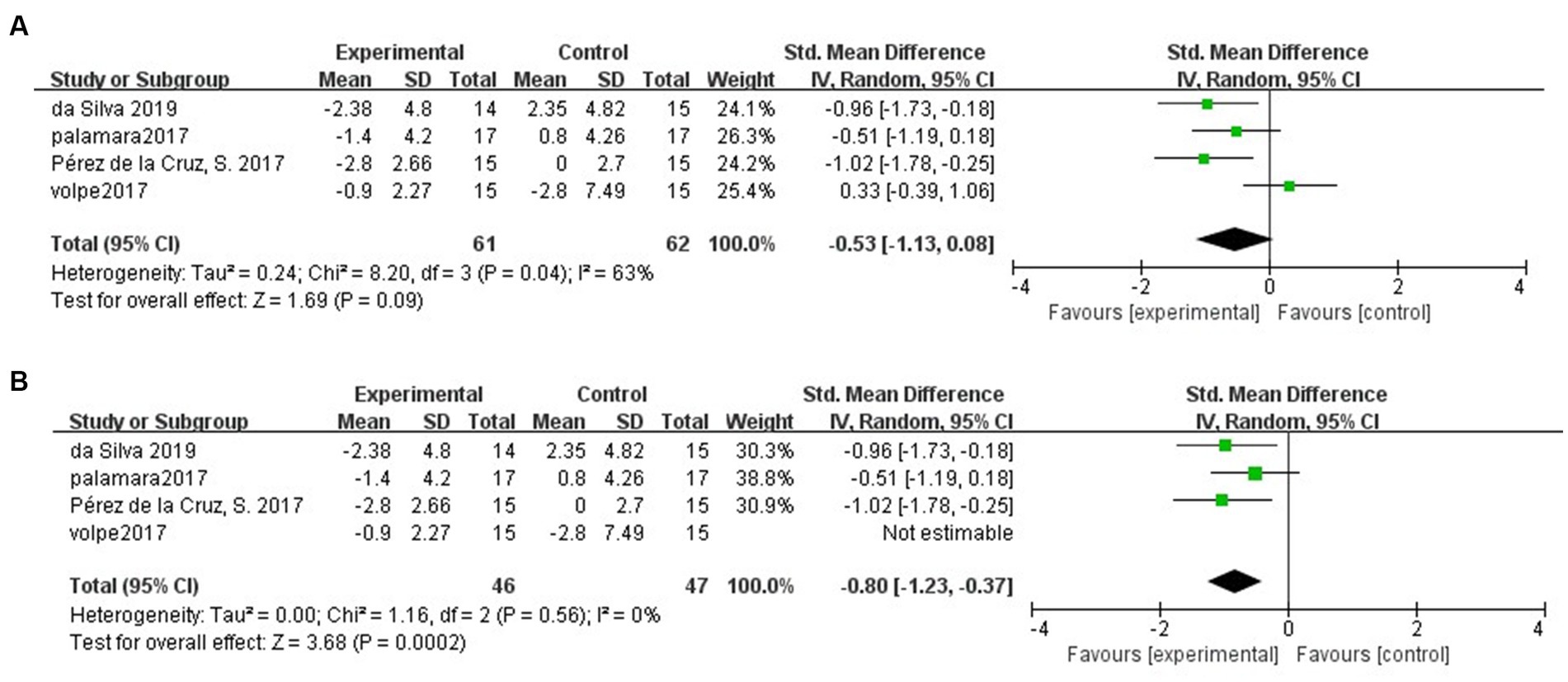
Figure 5. (A) Long-term effects of hydrotherapy in mobility. (B) Long-term effects of hydrotherapy in mobility (Sensitivity analysis).
Three studies (Palamara et al., 2017; Pérez de la Cruz, 2017; Volpe et al., 2017) have evaluated the quality of life of PD patients at follow-up. The meta-analysis showed: The results of the meta-analysis showed that there was no significant difference in the maintenance of the quality of life in PD patients in the hydrotherapy group compared to the control group (SMD = -0.21; 95% CI = -0.98, 0.57; p = 0.6; I2 = 71%; Figure 6). Results after performing sensitivity analyzes showed no significant change in heterogeneity or outcome.
The studies included in this study all evaluated PD patients at follow-up. The results of the meta-analysis showed that hydrotherapy had a more positive maintenance effect on balance function compared to land-based treatment, but no positives were seen in motor function, mobility, or quality of life.
The results of the analysis showed that hydrotherapy had a positive long-term effect on balance function compared with the control group. The impaired balance function in PD patients is altered processing of proprioceptive information, possibly due to the link between dysfunction of basal ganglia circuits and altered integration of peripheral inputs (Carpenter and Bloem, 2011; Conte et al., 2013). It has also been hypothesized that postural deformities in PD may also be associated with somatosensory integration dysfunction (Doherty et al., 2011).
It has been shown that the water environment can act on peripheral sensory receptors to stimulate the proprioceptive system in the balance control system (Schoneburg et al., 2013). It has been shown that the aquatic environment can increase proprioceptive input to the immersed body, resulting in better body alignment (Massion et al., 1995). In addition to this, the specific nature of the water environment plays an important role in improving and maintaining the equilibrium process (Volpe et al., 2017).
First, the hydrostatic pressure of the water supports the submerged body, providing a gravity-reducing environment that reduces the risk of falling (Caromano, 2002; Carregaro and AMd, 2008). Secondly, the buoyancy of water reduces the burden on the joints, thus improving their dynamic flexibility (Caromano, 2002). When hydrotherapy is combined with temperature, it can reduce pain and stiffness (Hinman et al., 2007; Hall et al., 2008). Thirdly, the viscosity of the water then provides a natural resistance to body movement, facilitating different exercise training as well as activating reactive postural adjustments in PD patients (Schoneburg et al., 2013; Cugusi et al., 2019). These characteristics of the aquatic environment allow some people with postural instability, high risk of falls, leg weakness, and gait disorders to exercise successfully in situations that would not be feasible or safe on land (Batterham et al., 2011). And finally, some findings suggest that balance-related training has the longest continuation effect compared to other training (Mak et al., 2017). The characteristics, as well as the benefits of hydrotherapy, seem to explain the positive long-term effects of hydrotherapy on homeostatic functioning in PD patients.
Previous studies have demonstrated that hydrotherapy improves motor function, mobility, and quality of life in PD patients (Carroll et al., 2017; Di Marco et al., 2022). However, no studies have examined the long-term effects of hydrotherapy. The results of this study showed no positive long-term effects of hydrotherapy on motor function, mobility, and quality of life compared to the control group. PD is a neurodegenerative disease with persistent impairment of motor function (Pringsheim et al., 2014), such as muscle weakness as well as decreased aerobic capacity, and gait disturbance (Allen et al., 2009; Nocera et al., 2010). However, some studies have established that exercise and physical therapy can change motor function in PD, but only if long-term training is achieved (Mak et al., 2017). In addition, maintaining a high level of regular physical activity and exercise habits is strongly associated with a favorable clinical course in PD (Tsukita et al., 2022). The maximum duration of the research interventions included in this study was no more than 10 weeks (Pérez de la Cruz, 2017) and the minimum was 2 weeks (Vivas et al., 2011). Therefore, this may explain the lack of positive results in terms of motor function.
In terms of mobility, a sensitivity analysis was performed due to the high heterogeneity between the included studies. The results showed a positive long-term effect of hydrotherapy on mobility compared to the control group. The included literature was assessed for mobility using the TUG. TUG was used to assess balance and mobility in PD patients (Zampieri et al., 2010). It has been suggested that an improvement in balance also represents to some extent an improvement in mobility (Zhu et al., 2023). Therefore, this is one of the reasons why the sensitivity analysis yielded a positive result in terms of mobility.
This systematic review has several limitations. First, too few studies were included in this meta-analysis. Second, only the long-term effects of hydrotherapy on motor symptoms in PD patients were explored; non-motor symptoms were not analyzed. Finally, because of the limitations of the literature that met the inclusion criteria, there was no subgroup analysis of the different hydrotherapy intervention regimens to determine the optimal hydrotherapy regimen for PD treatment.
The results of the meta-analysis showed that hydrotherapy had a positive maintenance effect on balance function in PD patients, but the long-term effects on motor function, mobility, and life therapy were not significant. Thus, hydrotherapy may be a highly effective and long-lasting form of physical therapy for PD patients who need to improve and maintain balance function.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
ZL: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. MH: Funding acquisition, Resources, Visualization, Writing – original draft. YaL: Methodology, Project administration, Supervision, Validation, Writing – original draft. XX: Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft. PZ: Writing – original draft, Writing – review & editing, Supervision, Validation. YanL: Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CT: Conceptualization, Formal analysis, Project administration, Validation, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Special Funds for Postgraduate Innovation of Gannan Medical College (YC2022-X015).
The authors would like to thank all the professors and coworkers for their tireless assistance in this project. The authors would also thank the individuals who participated in this study as well as all of the administrative support staff for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Allen, N. E., Canning, C. G., Sherrington, C., and Fung, V. S. (2009). Bradykinesia, muscle weakness and reduced muscle power in Parkinson's disease. Mov. Disord. 24, 1344–1351. doi: 10.1002/mds.22609
Alves Da Rocha, P., McClelland, J., and Morris, M. E. (2015). Complementary physical therapies for movement disorders in Parkinson's disease: a systematic review. Eur. J. Phys. Rehabil. Med. 51, 693–704.
Batterham, S. I., Heywood, S., and Keating, J. L. (2011). Systematic review and meta-analysis comparing land and aquatic exercise for people with hip or knee arthritis on function, mobility and other health outcomes. BMC Musculoskelet. Disord. 12:123. doi: 10.1186/1471-2474-12-123
Caromano, F. A. (2002). Princípios físicos que fundamentam a hidroterapia. Fisioterapia Brasil 3, 394–402. doi: 10.33233/fb.v3i6.2991
Carpenter, M. G., and Bloem, B. R. (2011). Postural control in Parkinson patients: a proprioceptive problem? Exp. Neurol. 227, 26–30. doi: 10.1016/j.expneurol.2010.11.007
Carregaro, R. L., and AMd, T. (2008). Efeitos fisiológicos e evidências científicas da eficácia da fisioterapia aquática. Rev. Mov. 1:148.
Carroll, L. M., Morris, M. E., O'Connor, W. T., and Clifford, A. M. (2020). Is aquatic therapy optimally prescribed for Parkinson's disease? A systematic review and Meta-analysis. J. Parkinsons Dis. 10, 59–76. doi: 10.3233/JPD-191784
Carroll, L. M., Morris, M. E., O'Connor, W. T., Volpe, D., Salsberg, J., and Clifford, A. M. (2022). Evidence-based aquatic therapy guidelines for Parkinson's disease: an international consensus study. J. Parkinsons Dis. 12, 621–637. doi: 10.3233/JPD-212881
Carroll, L. M., Volpe, D., Morris, M. E., Saunders, J., and Clifford, A. M. (2017). Aquatic exercise therapy for people with Parkinson disease: a randomized controlled trial. Arch. Phys. Med. Rehabil. 98, 631–638. doi: 10.1016/j.apmr.2016.12.006
Conte, A., Khan, N., Defazio, G., Rothwell, J. C., and Berardelli, A. (2013). Pathophysiology of somatosensory abnormalities in Parkinson disease. Nat. Rev. Neurol. 9, 687–697. doi: 10.1038/nrneurol.2013.224
Cugusi, L., Cadeddu, C., Nocco, S., Orrù, F., Bandino, S., Deidda, M., et al. (2015). Effects of an aquatic-based exercise program to improve cardiometabolic profile, quality of life, and physical activity levels in men with type 2 diabetes mellitus. PM R 7, 141–148; quiz 148. doi: 10.1016/j.pmrj.2014.09.004
Cugusi, L., Manca, A., Bergamin, M., Di Blasio, A., Monticone, M., Deriu, F., et al. (2019). Aquatic exercise improves motor impairments in people with Parkinson's disease, with similar or greater benefits than land-based exercise: a systematic review. J. Physiother. 65, 65–74. doi: 10.1016/j.jphys.2019.02.003
Da Silva, A. Z., and Israel, V. L. (2019). Effects of dual-task aquatic exercises on functional mobility, balance and gait of individuals with Parkinson's disease: a randomized clinical trial with a 3-month follow-up. Complement. Ther. Med. 42, 119–124. doi: 10.1016/j.ctim.2018.10.023
Denning, W. M., Bressel, E., Dolny, D., Bressel, M., and Seeley, M. K. (2012). A review of biophysical differences between aquatic and land-based exercise. Int. J. Aquat. Res. Educ. 6:7. doi: 10.25035/ijare.06.01.07
Di Marco, R., Pistonesi, F., Cianci, V., Biundo, R., Weis, L., Tognolo, L., et al. (2022). Effect of intensive rehabilitation program in thermal water on a Group of People with Parkinson's disease: A retrospective longitudinal study. Healthcare (Basel) 10:368. doi: 10.3390/healthcare10020368
Doherty, K. M., van de Warrenburg, B. P., Peralta, M. C., Silveira-Moriyama, L., Azulay, J. P., Gershanik, O. S., et al. (2011). Postural deformities in Parkinson's disease. Lancet Neurol. 10, 538–549. doi: 10.1016/S1474-4422(11)70067-9
Gage, H., and Storey, L. (2004). Rehabilitation for Parkinson's disease: a systematic review of available evidence. Clin. Rehabil. 18, 463–482. doi: 10.1191/0269215504cr764oa
Hall, J., Swinkels, A., Briddon, J., and McCabe, C. S. (2008). Does aquatic exercise relieve pain in adults with neurologic or musculoskeletal disease? A systematic review and meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 89, 873–883. doi: 10.1016/j.apmr.2007.09.054
Hariz, G. M., and Forsgren, L. (2011). Activities of daily living and quality of life in persons with newly diagnosed Parkinson's disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol. Scand. 123, 20–27. doi: 10.1111/j.1600-0404.2010.01344.x
Hayes, M. T. (2019). Parkinson's disease and parkinsonism. Am. J. Med. 132, 802–807. doi: 10.1016/j.amjmed.2019.03.001
Hinman, R. S., Heywood, S. E., and Day, A. R. (2007). Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys. Ther. 87, 32–43. doi: 10.2522/ptj.20060006
Mak, M. K., Wong-Yu, I. S., Shen, X., and Chung, C. L. (2017). Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat. Rev. Neurol. 13, 689–703. doi: 10.1038/nrneurol.2017.128
Massion, J., Fabre, J. C., Mouchnino, L., and Obadia, A. (1995). Body orientation and regulation of the center of gravity during movement under water. J. Vestib. Res. 5, 211–221. doi: 10.3233/VES-1995-5305
Nocera, J. R., Buckley, T., Waddell, D., Okun, M. S., and Hass, C. J. (2010). Knee extensor strength, dynamic stability, and functional ambulation: are they related in Parkinson's disease? Arch. Phys. Med. Rehabil. 91, 589–595. doi: 10.1016/j.apmr.2009.11.026
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Palamara, G., Gotti, F., Maestri, R., Bera, R., Gargantini, R., Bossio, F., et al. (2017). Land plus aquatic therapy versus land-based rehabilitation alone for the treatment of balance dysfunction in Parkinson disease: a randomized controlled study with 6-month follow-up. Arch. Phys. Med. Rehabil. 98, 1077–1085. doi: 10.1016/j.apmr.2017.01.025
Pérez de la Cruz, S. (2017). Effectiveness of aquatic therapy for the control of pain and increased functionality in people with Parkinson's disease: a randomized clinical trial. Eur. J. Phys. Rehabil. Med. 53, 825–832. doi: 10.23736/S1973-9087.17.04647-0
Poewe, W., and Seppi, K. (2001). Treatment options for depression and psychosis in Parkinson's disease. J. Neurol. 248 Suppl 3:Iii12-21. doi: 10.1007/PL00007821
Pringsheim, T., Jette, N., Frolkis, A., and Steeves, T. D. (2014). The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 29, 1583–1590. doi: 10.1002/mds.25945
Reinoso, G., Allen, J. C. Jr., Au, W. L., Seah, S. H., Tay, K. Y., and Tan, L. C. (2015). Clinical evolution of Parkinson's disease and prognostic factors affecting motor progression: 9-year follow-up study. Eur. J. Neurol. 22, 457–463. doi: 10.1111/ene.12476
Samoudi, G., Jivegård, M., Mulavara, A. P., and Bergquist, F. (2015). Effects of stochastic vestibular galvanic stimulation and LDOPA on balance and motor symptoms in patients with Parkinson's disease. Brain Stimul. 8, 474–480. doi: 10.1016/j.brs.2014.11.019
Schoneburg, B., Mancini, M., Horak, F., and Nutt, J. G. (2013). Framework for understanding balance dysfunction in Parkinson's disease. Mov. Disord. 28, 1474–1482. doi: 10.1002/mds.25613
Simon, D. K., Tanner, C. M., and Brundin, P. (2020). Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin. Geriatr. Med. 36, 1–12. doi: 10.1016/j.cger.2019.08.002
Simpkins, C., and Yang, F. (2023). Do dance style and intervention duration matter in improving balance among people with Parkinson's disease? A systematic review with meta-analysis. Parkinsonism Relat. Disord. 106:105231. doi: 10.1016/j.parkreldis.2022.105231
Sveinbjornsdottir, S. (2016). The clinical symptoms of Parkinson’s disease. J. Neurochem. 139, 318–324. doi: 10.1111/jnc.13691
Titova, N., and Chaudhuri, K. R. (2018). Non-motor Parkinson disease: new concepts and personalised management. Med. J. Aust. 208, 404–409. doi: 10.5694/mja17.00993
Tomlinson, C. L., Patel, S., Meek, C., Herd, C. P., Clarke, C. E., Stowe, R., et al. (2012). Physiotherapy intervention in Parkinson's disease: systematic review and meta-analysis. BMJ 345:e5004. doi: 10.1136/bmj.e5004
Tsukita, K., Sakamaki-Tsukita, H., and Takahashi, R. (2022). Long-term effect of regular physical activity and exercise habits in patients with early Parkinson disease. Neurology 98, e859–e871. doi: 10.1212/WNL.0000000000013218
Vivas, J., Arias, P., and Cudeiro, J. (2011). Aquatic therapy versus conventional land-based therapy for Parkinson's disease: an open-label pilot study. Arch. Phys. Med. Rehabil. 92, 1202–1210. doi: 10.1016/j.apmr.2011.03.017
Volpe, D., Giantin, M. G., Manuela, P., Filippetto, C., Pelosin, E., Abbruzzese, G., et al. (2017). Water-based vs. non-water-based physiotherapy for rehabilitation of postural deformities in Parkinson's disease: a randomized controlled pilot study. Clin. Rehabil. 31, 1107–1115. doi: 10.1177/0269215516664122
Zampieri, C., Salarian, A., Carlson-Kuhta, P., Aminian, K., Nutt, J. G., and Horak, F. B. (2010). The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 81, 171–176. doi: 10.1136/jnnp.2009.173740
Keywords: hydrotherapy, Parkinson’s disease, balance, meta-analysis, PD
Citation: Liu Z, Huang M, Liao Y, Xie X, Zhu P, Liu Y and Tan C (2023) Long-term efficacy of hydrotherapy on balance function in patients with Parkinson’s disease: a systematic review and meta-analysis. Front. Aging Neurosci. 15:1320240. doi: 10.3389/fnagi.2023.1320240
Received: 12 October 2023; Accepted: 17 November 2023;
Published: 13 December 2023.
Edited by:
Danúbia Da Cunha De Sá Caputo, Rio de Janeiro State University, BrazilReviewed by:
Vicki A. Nejtek, University of North Texas Health Science Center, United StatesCopyright © 2023 Liu, Huang, Liao, Xie, Zhu, Liu and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zicai Liu, MTQ1NDI2MjA2NUBxcS5jb20=; Yangyou Liu, MjYzODcyODkzOEBxcS5jb20=; Cheng Tan, U2d0YW5jaGVuZ0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.