
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci., 08 January 2024
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1298166
This article is part of the Research TopicAdvances in Neuromodulation Treatment of Parkinson’s Disease and Aging-Related Movement DisordersView all 13 articles
Parkinson’s disease (PD) is featured by movement impairments, including tremors, bradykinesia, muscle stiffness, and imbalance. PD is also associated with many non-motor symptoms, such as cognitive impairments, dementia, and mental disorders. Previous studies identify the associations between PD progression and factors such as α-synuclein aggregation, mitochondrial dysfunction, inflammation, and cell death. The cannabinoid type-2 receptor (CB2 receptor) is a transmembrane G-protein-coupled receptor and has been extensively studied as part of the endocannabinoid system. CB2 receptor is recently emerged as a promising target for anti-inflammatory treatment for neurodegenerative diseases. It is reported to modulate mitochondrial function, oxidative stress, iron transport, and neuroinflammation that contribute to neuronal cell death. Additionally, CB2 receptor possesses the potential to provide feedback on electrophysiological processes, offering new possibilities for PD treatment. This review summarized the mechanisms underlying PD pathogenesis. We also discussed the potential regulatory role played by CB2 receptor in PD.
Parkinson’s disease (PD) is one of the most prevalent neurodegenerative diseases (de Lau and Breteler, 2006; Subramaniam and Chesselet, 2013). Patients with PD are commonly suffering from movement disorders, such as tremors, involuntary movements, rigidities, and imbalance. Many patients also demonstrate non-movement disorders, including cognitive impairments, sleep disorder, chronic pain, olfactory dysfunction, anxiety, and depressive disorder (Garcia-Ruiz et al., 2014; Tolosa et al., 2021). Many patients diagnosed with PD eventually develop dementia during the advanced stage (Szeto et al., 2020). The main pathological feature of PD includes gradual loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) located at the midbrain, and the accumulation of Lewy bodies (LBs) containing mainly α-synuclein (α-syn) intracellular inclusions all over the brain (Warren et al., 2017). Multiple mechanisms, including α-syn aggregation (Roy, 2017), mitochondrial dysfunction (Subramaniam and Chesselet, 2013), oxidative stress, abnormal iron accumulation (Hare and Double, 2016), and neuroinflammation (Gelders et al., 2018), have been implicated in the neurodegenerative process of PD (Figure 1). However, the exact cause of PD is still not clear. Consequently, clinical therapies for PD treatment, including medicines and surgeries, are mostly symptomatic. No treatment can stop or reverse the development of PD. The endocannabinoid system (ECS) comprises a network of endocannabinoids (eCBs) and their receptors that are widespread throughout the central nervous system (CNS) and immune system. This tightly regulated system modulates the transmission of chemical signals via an immediate feedback mechanism. Dysregulation of eCB signalling has been suggested in the development of neuropsychiatric disorders and neurodegenerative disease (Yin et al., 2019; Cooray et al., 2020). eCBs are recognized by cannabinoid receptors (CB receptors): the cannabinoid type-1 receptor (CB2 receptor) and the cannabinoid type-2 receptor (CB2 receptor). Among them, the CB2 receptor is mainly located in immune cells. Its activation is reported to exert protective effects in neurological disorders and thus receives extensive attention as a new treatment target. Here, we summarized the current research progress of how the CB2 receptor is involved in the pathogenesis and progression of PD and discussed the potential of targeting the CB2 receptor for the treatment of this disease.
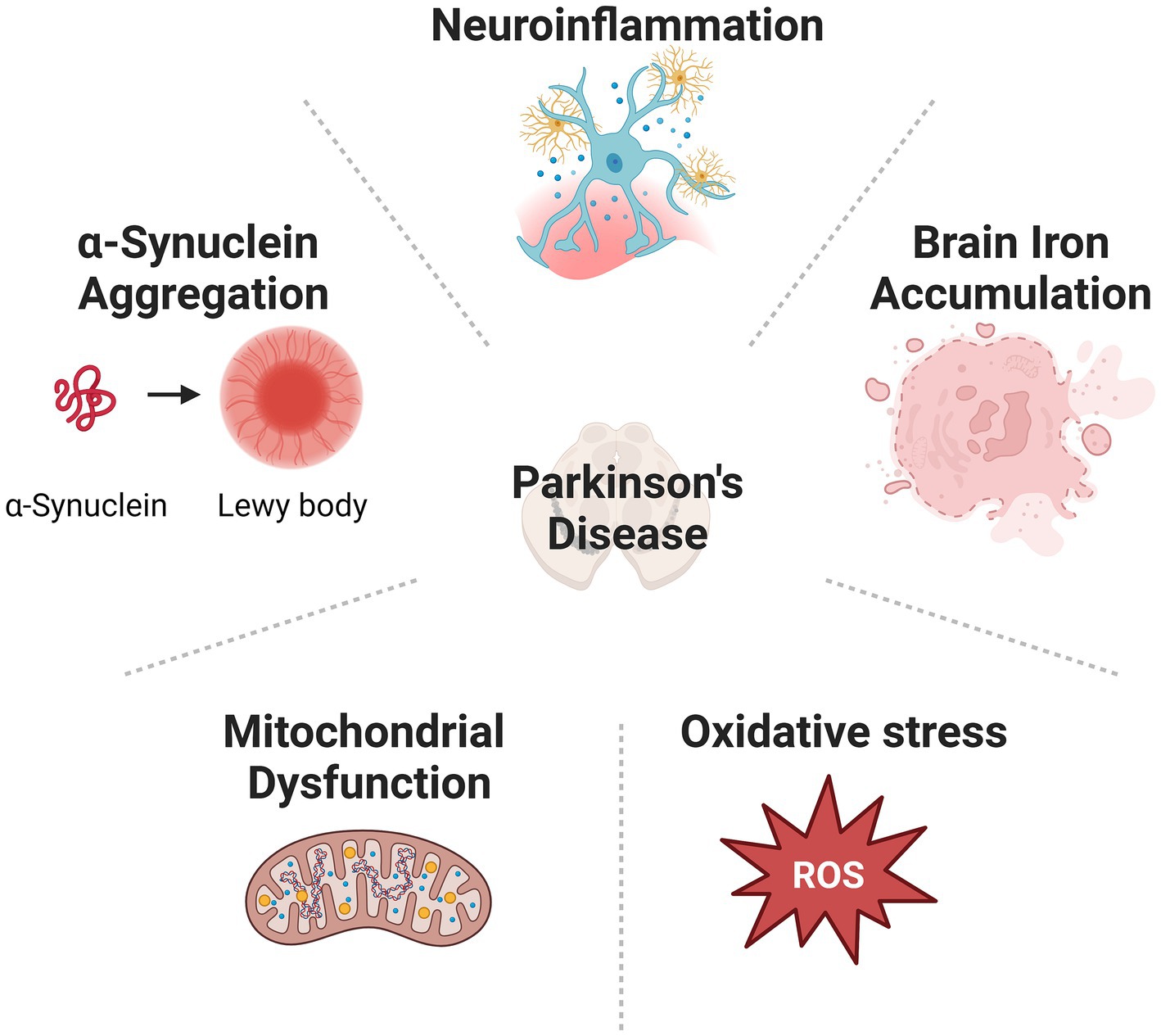
Figure 1. Cause of PD is various, including α-synuclein aggregation, mitochondrial dysfunction, oxidative stress, abnormal iron accumulation, and neuroinflammation.
Cannabinoids, as an emerging therapeutic agent, have attracted wide attention for their great potential in the treatment of various diseases. They are best understood for their inhibitory effects on the release of γ-aminobutyric acid (GABA) and glutamate through CB1 and CB2 receptors (Urits et al., 2020). The ECS consists of two major branches: the CB1 receptor is highly enriched in the brain and its surrounding nerves (Herkenham et al., 1991), meanwhile, the CB2 receptor is mainly found in the immune system (Facci et al., 1995). Cannabinoids are generally classified into three types based on their source: phytocannabinoids (found in cannabis plants, for example, Δ9-tetrahydrocannabinol, THC), synthetic cannabinoids (chemically synthesized), and endocannabinoids (eCBs, i.e., naturally occurring in the human body). Cannabinoids bind to CB receptors located on the cell membrane, exerting corresponding psychotropic effects (Howlett et al., 1990). The eCBs, CB receptors, and enzymes catalyze the synthesis and degradation collectively form the ECS. The activation of the ECS is related to decreased dopaminergic activity and can regulate various neural functions related to emotions, cognitions, motor controls, feeding behaviors, and pain (Castillo et al., 2012; Pacher and Kunos, 2013).
N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), which share highly similar structures with Δ9-THC, are the two major and most well-understood eCBs. Generally, they are released from the postsynaptic terminal after neuronal activation, modulate presynaptic neurotransmissions, and produce physiological feedback mechanisms dedicated in preventing excessive excitation of neurons (Lovinger, 2008; Zou and Kumar, 2018). This retrograde feedback initiates depolarization-induced suppression of inhibition (DSI) at GABAergic synapses and depolarization-induced suppression of excitation (DSE) at glutamatergic synapses (Makara et al., 2005). AEA and 2-AG, unlike other neurotransmitters and neuropeptides that are stored in the intracellular compartments, are produced on demand from the cleavage of their precursors, N-arachidonyl-phosphatidyl ethanolamine (NAPE) and diacylglycerol (DAG), respectively (Maccarrone and Finazzi-Agro, 2003).
In the CNS, the eCBs are synthesized by both neuronal cells and glial cells such as microglia (Kelly et al., 2020). In vitro study reveals the production of both AEA and 2-AG by microglia (Walter et al., 2003; Carrier et al., 2004). Adenosine triphosphate (ATP) stimulation of microglia increases the production of 2-AG through the activation of P2X purinoceptor 7 (P2X7) ionotropic receptor (Witting et al., 2004). Microglia is suggested as the one of the main source of eCBs under neuroinflammation (Stella, 2009). Upregulated eCB levels are implicated in anti-inflammatory effects, and therefore are believed to exert neuroprotective effects in various diseases. 2-AG is reported to limit acute neuroinflammation induced by the Theiler’s murine encephalomyelitis virus (TMEV) by modulating microglial activation and promoting the activation of brain-derived suppressor cells, indicating a potent regulatory function of 2-AG on peripheral and central immunity (Mecha et al., 2018). AEA treatment is found to attenuate the lipopolysaccharide (LPS)-induce microglia activation via the CB2 receptor (Malek et al., 2015). Clinical study recently reveals that deficiency of diacylglycerol lipase β (DAGLB), the synthase of 2-AG, is associated with early onset of PD. Knockdown of Daglb impairs locomotor skill learning in mice (Liu et al., 2022). In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse model, increased level of 2-AG is reported in the ventral midbrain after MPTP treatment (Mounsey et al., 2015). Exogenous addition of 2-AG or monoacylglycerol lipase (MAGL, enzyme for 2-AG hydrolysis) inhibitors demonstrate potent protective effect against MPTP-induced cell death (Mounsey et al., 2015). Collectively, those studies suggest the potential neuroprotective effects of eCBs in PD via regulation of microglia and neuroinflammation.
Biological effects of the eCBs and other synthetic cannabinoids (such as WIN55,212–2 and HU210) are mainly mediated by the G-protein-coupled CB receptors: the CB1 and CB2 receptors (Munro et al., 1993). The activation of CB1 receptors involves the coupling of pertussis toxin (PTX)-sensitive G proteins (Gαi/o), leading to the inhibition of adenylate cyclase (AC) and cyclic adenosine monophosphate (cAMP) formation. Activation of CB1 receptor also activates the mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathways, which both participate in the regulation of cell proliferation, cell cycle, and cell death (Pertwee, 2006; Howlett et al., 2010; Turu and Hunyady, 2010; Blázquez et al., 2015). CB1 receptors can also exert their effects through G protein-dependent or other ligand-dependent mechanisms (Demuth and Molleman, 2006). In addition to the typical G protein-dependent signaling, CB1 receptors also transmit signals through interaction with other molecules (such as β-arrestin) in a G protein-independent manner (Howlett et al., 2010). Moreover, CB1 receptors also regulate several types of ion channels (Turu and Hunyady, 2010). Upon activation of CB1 receptors, the inhibition of Gαi/o-mediated cAMP reduction regulates inwardly rectifying potassium channels (GIRKs) and inhibits N-type and P/Q-type voltage-gated calcium channels (Howlett et al., 2002; Fisyunov et al., 2006), thereby suppressing presynaptic neurotransmitter release. Research has shown that CB2 receptors regulate the activity of N-type Ca2+ channels located at the presynaptic membrane, thereby modulating calcium influx to inhibit GABA release in mouse hippocampal slices (Szabo et al., 2014).
Like the CB1 receptor, the CB2 receptor is coupled to Gαi/o proteins. However, unlike the CB1 receptor, the CB2 receptor does not appear to be coupled to potassium channels (McAllister et al., 1999). The CB2 receptor is initially thought to be predominantly expressed in the peripheral immune system. However, recent studies have found CB2 receptor expression in the CNS (Mackie, 2008). The CB2 receptor is expressed by microglia, astrocytes and certain subpopulations of neurons (Fernández-Ruiz et al., 2008). Upregulation of CB2 receptor has been implicated in neurodegenerative diseases. Activation of this receptor in animal models demonstrate disease-modifying effects against the process of neurodegeneration, suggesting CB2 receptor is a promising therapeutic target for the treatments of such disease. Also, compared to CB1 receptor, the activation of CB2 receptor has been shown to have fewer psychoactive and other side effects (Pacher et al., 2006; Liu et al., 2021), making selective CB2 receptor targeting a better option for this approach. In the following parts, we summarized the current understanding of how CB2 receptor participates in the progression of PD, and its potential as a treatment target in the treatment of this disease.
Both clinical and animal studies reveal the alternation of CB2 receptor in PD. Postmortem studies reveal the increased level of CB2 receptor in microglial cells at substantia nigra (SN) of PD patients, indicating the recruitment and activation of microglia at the site of lesion (Gómez-Gálvez et al., 2016). This finding is supported by the observation in animal models of PD. CB2 receptor level is significantly increased in both LPS- and 6-hydroxydopamine (6-OHDA)-induced PD model, and this elevation is associated with the activation of microglia (Concannon et al., 2015). Those findings suggest the upregulation of CB2 receptor in microglia. However, downregulation of CB2 receptor is also reported in neurons and other brain regions. Reduced level of CB2 receptor is reported in the tyrosine hydroxylase (TH)-containing in the SN of PD patients, indicating increased DA neuronal cell death (García et al., 2015). Reduced transcription of CB2 receptor is observed in the cerebellum and hippocampus of PD patients, as compared to healthy controls (Grünblatt et al., 2007). Similarly, in the MPTP-induced PD mouse model, a downregulation of CB2 receptor is observed 3 weeks after MPTP injection (Shi et al., 2017; Xin et al., 2020). Further research demonstrates neuroprotective potentials of CB2 receptor in PD. Specifically, CB2 receptor-deficient mice demonstrate more severe loss of tyrosine TH-containing neurons in the SN, indicating the protective role of CB2 receptor in PD (Gómez-Gálvez et al., 2016). In an in vitro PD model established by MPP+ treatment, JWH133 (a potent CB2 receptor agonist) is shown to promote cell survival (Aymerich et al., 2016). In vivo study also demonstrates that the administration of nonselective CB receptor agonist WIN55,212–2 and selective CB2 receptor agonist JWH015 alleviate the MPTP-induced neuron death and microglial activation in SN (Price et al., 2009). GW842166x (a selective CB2 receptor agonist) exerts protective effects against the 6-OHDA-induced loss of dopamine neurons (Yu et al., 2021). Another selective CB2 receptor agonist AM1241 is reported to alleviate the MPTP-induced PD-like symptoms and promote the regeneration of DA neurons in mice (Shi et al., 2017). Moreover, administration of β-caryophyllene (BCP, a CB2 receptor agonist) is reported to exert neuroprotective effects in both rotenone (ROT)-induced and MPTP-induced PD animal models (Javed et al., 2016; Viveros-Paredes et al., 2017). Those research findings collectively suggest the potential protective effects of CB2 receptor agonist in PD, rising the discussion of targeting CB2 receptor as a potential treatment approach for PD. Therefore, we further discuss the potential roles of CB2 receptor in PD from different perspectives and possible mechanisms in the following sections.
α-syn is one of the major components involved in the formation of LBs. α-syn oligomers exert strong cytotoxic effects to neuron (Ghosh et al., 2017; Calabresi et al., 2023). The formation of α-syn oligomers is influenced by multiple factors. Clinical studies have shown significantly elevated level of α-syn oligomers in the plasma, serum, and red blood cells of PD patients, as compared to healthy controls (Zhao et al., 2022). Interestingly, it has been reported that the peripheral autonomic nervous system may be a key pathway for the spread of α-syn pathology from the periphery to the CNS (Chen et al., 2020). Numerous research and clinical findings reemphasize the central role of α-syn, and α-syn-induced neurotoxicity and neuroinflammation in PD (Fayyad et al., 2019; Wang et al., 2019). However, the interaction between CB2 receptor and α-syn has been largely over-looked. Recently, Feng et al. demonstrate that the fibrillar α-syn treatment causes significantly promoted neuroinflammation and phagocytosis, as revealed by higher level of cluster of differentiation 68 (CD68) and interleukin-1β (IL-1β), reduced level of brain-derived neurotrophic factor (BDNF) in mice with CB2 receptor knockout, as compared to wild-type (WT) mice (Feng et al., 2023). Indeed, they also find that CB2 receptor knockout promotes the activation of microglia and pruning of cholinergic synapses induced by α-syn treatment (Feng et al., 2023), suggesting the important role played by CB2 receptor in α-syn pathology.
Extensive post-mortem examinations, brain imaging studies, epidemiological data, and animal studies have demonstrated the contribution of innate and adaptive immunities in neurodegeneration (McGeer et al., 1988; Gerhard et al., 2006; Theodore et al., 2008). It is widely believed that the degeneration and death of neurons in neurodegenerative diseases are primarily influenced by the release of inflammatory factors and neurotoxic mediators, such as IL-1β, tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-33 (IL-33), chemokine ligand 2 (CCL2), chemokine ligand 5 (CCL5), prostaglandin E2 (PGE2), cyclooxygenase-2 (COX-2), and increased ROS (Skaper et al., 2014; Kempuraj et al., 2015). These mediators bind to corresponding receptors on neurons or glia cells, directly or indirectly induce neurodegeneration and affect neuronal survival through interactions with neuroglial cells. Meanwhile, the activation of glial cells, including microglial cells and astrocytes, promote the expression of pro-inflammatory mediators in neurodegenerative diseases (Kim and Lee, 2014), causing aggravated neurodegeneration, which further exacerbates the progression of the disease course. In PD, the contribution of neuroinflammation has been intensively studied and suggested as a promising target for effective treatment (Tansey et al., 2022).
CB2 receptor has been identified as a potential anti-inflammatory component in various inflammation-related diseases. Its activation disrupts the self-sustained neuroinflammation status that contributes to the disease progression of neurodegeneration. Activation of CB2 receptor reduces the release of pro-inflammatory cytokine and thereby prevents neuronal cell death in neurodegeneration diseases. LPS injection in mice leads to an increase in TNF-α levels and oxidative stress in the brain, resulting in disease-like behavior. Acute injection of the CB2 receptor agonist 1-phenylisatin (PI) significantly rescues the behavioral changes induced by LPS administration in mice (Sahu et al., 2019). Moreover, PI inhibits the transcription of TNF-α and oxidative stress in the brain, demonstrating that both acute and long-term activation of CB2 receptor may exert protective effect against the development of various disease related to neuroinflammation and oxidative stress (Sahu et al., 2019). Activation of CB2 receptor is reported to inhibit the activation of NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome, a potent contributor of neuroinflammation and neurodegenerative diseases (Ke et al., 2016; Yu et al., 2019). In human microglial cells derived from the temporal lobe, JWH015 exerts neuroprotective effects by reducing the release of TNF-α and IL-1β (Klegeris et al., 2003).
Non-selective CB2 receptor agonist WIN55,212–2 and selective CB2 receptor agonist JWH015 have been shown to reduce MPTP-induced microglial infiltration. This effect can be reversed by the CB2 receptor antagonist JTE907, confirming the CB2 receptor-mediated inhibitory effect via the modulation of microglia (Price et al., 2009). CB2 receptor activation by JWH133 is reported to reduce the level of pro-inflammatory cytokines and promote the M2 polarization of microglia via the activation of the PI3K/Akt signalling pathway (Wang et al., 2023). In the MPTP-induced PD mouse model, CB2 receptor knockout exhibits aggrieved microglial activation, along with neuropathology and functional deficits (Komorowska-Muller and Schmole, 2020). In an environmental and viral inflammation-induced PD model established by unilateral intrastriatal injection of ROT or polyinosinic:polycytidylic acid (Poly I:C) in male rats, a significant increase of CB2 receptor expression is observed, which is strongly correlated with activated microglia in the model (Concannon et al., 2016). Similarly, in ROT-induced and MPTP-induced PD animal models, CB2 receptor agonizing using BCP demonstrates disease-alleviating effect via the suppression of neuroinflammation (Javed et al., 2016). ROT injection leads to microglial activation and subsequent inflammation. Further research has showed that the activation of CB2 receptor by BCP can inhibit ROT-induced microglial activation, improve the release and expression of inflammatory mediators in CNS, and attenuate the expression of inflammatory factors such as NF-κB, COX-2, and Inducible nitric oxide synthase (iNOS) (Javed et al., 2016).
Excessive inflammation not only involves the activation of microglial cells but also the activation and proliferation of astrocytes, which play a crucial regulatory role in the inflammatory response. Currently, there is limited research on the effects of CB2 receptor on astrocytes. It has been demonstrated that rat astrocytes express both CB1 receptor and CB2 receptor (Stella, 2004; Sheng et al., 2005). Recent studies also report the colocalization of CB2 receptor with astrocytes by immunohistochemical localization. Increased immunoreactivity of CB2 receptor in astrocytes is reported in PD patients (Navarrete et al., 2018). This suggests that the expression changes of CB2 receptor in astrocytes have potential regulatory roles in PD, and warrant further investigation. In primary cultured astrocytes, the nonspecific CB receptor agonist WIN 55,212–2 has been shown to regulate cell viability, inflammatory mediators, and oxidative stress. Specifically, the amyloid-β (Aβ) 1–42, the aberrant protein aggregation contributes to the pathogenesis of Alzheimer’s disease (AD), reduces astrocyte viability while increasing the expression of TNF-α, IL-1β, COX-2, and iNOS. Meanwhile, pre-treatment with WIN 55,212–2 significantly rescues the inflammatory and astrocyte vulnerability to Aβ1-42 treatment (Aguirre-Rueda et al., 2015). Furthermore, JWH133 is reported to exert neuroprotective effects by inhibiting blood–brain barrier (BBB) damage, astrocytic targeting myeloperoxidase (MPO) expression, peripheral immune cell infiltration, and the production of inflammatory and chemotactic factors by activated microglial cells (Chung et al., 2016). Collectively, those results indicate CB2 receptor as a promising disease-modifying treatment target for PD via its regulation of neuroinflammation.
The motor dysfunction in PD is caused by the loss of DA neurons in the SNpc. Increasing evidence suggests that oxidative stress is a key driving factor in the complex degenerative cascade of dopaminergic neurodegeneration in all forms of PD (Dias et al., 2013; Blesa et al., 2015). Markers of oxidative stress in the CNS increase with aging and the occurrence of neurodegenerative diseases (Boveris and Navarro, 2008). Oxidative stress arises from a disruption in cellular redox homeostasis, where the production of reactive oxygen species (ROS) exceeds the clearance rate by endogenous antioxidant enzymes and molecular chaperones. Uncontrolled oxidative reactions within cells cause destructive damage to normal cellular structures, leading to cellular degeneration and death (Wiseman and Halliwell, 1996; Rego and Oliveira, 2003). Accumulation of ROS induces oxidative damage to lipids, proteins, DNA, and RNA, impairing neuronal function and structural integrity (Schieber and Chandel, 2014). Due to the increased chances of spontaneous mutations resulting from oxidative stress, it may trigger mutations that make cells more susceptible to functional impairments, and the vulnerability of the SN to oxidative stress contributes to selective neuronal degeneration (Floor and Wetzel, 1998). The damaging effects of oxidative stress are well recognized, and research focusing on inhibiting neuronal oxidative stress has become a mainstream direction in PD treatment.
Previous research demonstrates that activation of CB2 receptor can protect DA neurons against degeneration in a ROT-induced PD model (Javed et al., 2016). ROT injection causes extensive loss of DA neurons in the SNpc and striatal fibers, leading to oxidative damage characterized by reduction of anti-oxidant enzymes and upregulated nitrite level (Thakur and Nehru, 2013). Treatment with the CB2 receptor agonist BCP prevents glutathione depletion, enhances antioxidant enzyme activity in the midbrain, and inhibits the elevation of nitrite levels. It has been found that the GW405833 (a CB2 receptor-specific agonist) administration inhibits inflammatory response by suppressing the levels of cytokine and oxidative stress (Parlar et al., 2018). Other research results report that the CB2 receptor agonist HU308 reduces the production of ROS-generating enzymes NOX4, NOX2, and NOX1, as well as subsequent renal oxidative stress in mice (Zhang et al., 2009). An in vitro study demonstrates that CB2 receptor is involved in the antioxidant stress process in RAW264.7 macrophages, blocking cell death (Giacoppo et al., 2017). These results indicate that activation of CB2 receptor can inhibit oxidative stress and protect neuronal cells.
Excessive accumulation of iron in the brain is a major characteristic of brain degeneration in patients with PD, known as brain iron accumulation. Non-physiological accumulation of iron in specific brain regions is associated with various diseases. This phenomenon is referred to as neurodegeneration with brain iron accumulation (NBIA) (Schneider et al., 2012). It has been reported that iron levels in the SN of PD patients increase significantly. This change is accompanied by upregulation of divalent metal transporter 1 (DMT1), a protein involved in iron transport (Jia et al., 2015). Iron accumulation may exert its pathogenic activity by increasing ROS and causing widespread damage to intracellular proteins. However, there is also evidence suggesting that it leads to neuronal death through interactions with pathological protein aggregates found in these diseases by promoting the process of cellular apoptosis (Ward et al., 2014).
Maintaining iron homeostasis in the brain has long been considered a potential target for drug treatment related to aging-related diseases. Iron is involved in various cellular functions, such as the synthesis of myelin phospholipid, mitochondrial respiration, and the biosynthesis and metabolism of neurotransmitters. Therefore, the regulation of iron transport through DMT1 plays a significant role in maintaining normal brain physiological function. It has been reported that Δ9-THC, CP 55940, WIN 55,212–2, and AEA inhibit the uptake of 55Fe and 54Mn in HEK293T cells expressing DMT1 by stabilizing the expression of the transporter protein and inhibiting DMT1 expression. Small-molecule tests have shown that Δ9-THC inhibits DMT1 activity (Wetli et al., 2006). Furthermore, gene knockout of the CB2 receptor eliminates its regulatory effects, indicating that the inhibitory effect of Δ9-THC is mediated by the CB2 receptor. Moreover, activation of CB2 receptor negatively regulates signaling cascades related to serine/threonine kinases. Immunoprecipitation experiments have shown that phosphorylation of serine 43 of DMT1 promotes its transport activity, thereby facilitating iron absorption. Δ9-THC blocks serine phosphorylation of DMT1, and CB2 receptor knockout abolishes the blockade of iron transport by Δ9-THC (Seo et al., 2016).
Mitochondria play a pivotal role in the vitality of eukaryotic cells as they are involved in bioenergetics, metabolism, and signaling, and are associated with many diseases (Pfanner et al., 2021). The involvement of mitochondrial dysfunction in the pathogenesis of PD is discovered when individuals who consumed illegally contaminated drugs containing MPTP developed PD-like symptoms (Langston et al., 1983). It has been demonstrated that mitochondrial dysfunction can induce degeneration and death of DA neurons (More and Choi, 2015), promoting the occurrence of neurodegenerative in PD (Bose and Beal, 2016).
Previous research has shown that cannabinoids such as Δ9-THC and synthetic cannabinoid HU210 impair mitochondrial respiratory function via the suppression of oxygen consumption and mitochondrial membrane potential (ΔΨm) (Athanasiou et al., 2007). ΔΨm manifests the functional status of mitochondria. Additionally, both AEA and 2-AG suppress the transcription of genes associated with mitochondrial biogenesis, and decrease mitochondrial DNA content and oxygen consumption in white adipocytes of mouse (Tedesco et al., 2010). Further studies have found that activation of CB2 receptor using JWH133 conveys an anti-apoptotic effect in animal model of myocardial ischemia (Li et al., 2013), which aligns with the protective outcome of JWH133 against ischemia-induced ΔΨm loss and cytochrome c release from mitochondria to the cytoplasm. Moreover, CB2 receptor is involved in AEA-stimulated mitochondrial cation transport (Zoratti et al., 2003). Collectively, CB2 receptor is believed to play a regulatory role in modulating mitochondrial respiratory activity. How this regulatory effect of CB2 receptor related to PD is therefore worth further investigation.
Autophagy is a lysosome-dependent self-degradation and recycling process. It is an essential metabolic process that targets protein and dysfunctional cellular components (Kim and Lee, 2014; Saha et al., 2018). Autophagy is a conserved cellular process that maintains cellular homeostasis. Autophagy impairments are closely related to the pathogenesis of PD (Cheng et al., 2020; Lu et al., 2020). Further studies reveal the association between autophagy and CB2 receptor. It has been demonstrated that autophagy is related to the protective functions of CB2 receptor in several diseases (Shao et al., 2014; Denaës et al., 2016). Ke et al. (2016) found that activation of CB2 receptor alleviates the effects of NLRP3 inflammasome activation by inducing autophagy in rat macrophages, thereby reducing inflammation in a mouse model of inflammatory bowel disease (IBD). Additionally, there is a similar association between CB2 receptor and autophagy in a mouse model of multiple sclerosis. It has been shown in mice that activation of CB2 receptor can induce autophagy to prevent diabetic cardiomyopathy (Wu et al., 2018). These studies suggest that inducing autophagy through the activation of CB2 receptor has potential therapeutic value in the progression of PD.
There is electrophysiological evidence suggesting that activation of CB2 receptors can regulate neuronal activity and excitability. CB2 receptors have been found to be expressed in ventral tegmental area (VTA) DA neurons (Foster et al., 2016), and systemic and local administration of JWH133 has been shown to enhance M-type potassium currents, leading to neuronal inhibition and hyperpolarization, significantly reducing the firing frequency of VTA DA neurons both in vivo and in vitro (Zhang et al., 2014, 2017). Specifically, in whole-cell perforated and cell-attached membrane patch clamp recordings from individual neurons or brain slices in wild-type mice, JWH133 dose-dependently suppressed the firing of VTA DA neurons, and this effect is blocked by AM630 and observed in CB2 receptor knockout mice. Similar effects have also been observed in rats, indicating that activation of CB2 receptor in the brain can regulate the firing of VTA DA neurons, exerting electrophysiological regulatory effects and providing new avenues for the treatment of PD.
Given that the main characteristic of PD is the loss of DA neurons in SN and a significant reduction in striatal dopamine, the current mainstay of PD clinical treatment involves the use of levodopa (L-DOPA). However, long-term use of L-DOPA often leads to fluctuations and motor complications that offset its beneficial effects (Utsumi et al., 2013). Therefore, many studies are focused on developing novel non-dopaminergic drugs that can prevent or even reverse the degeneration of DA neurons. CB2 receptors are detected in central nervous system regions including the striatum, hippocampus, basal ganglia, frontal cortex, amygdala as well as the VTA (Morris et al., 2021), and their activation is involved in various diseases associated with DA neuron injuries. Mice overexpressing CB2 receptor show significantly reduced damage to DA neurons induced by 6-OHDA, reduced motor impairment, and decreased activation of glial cells in the affected area (Ternianov et al., 2012). Activation of CB2 receptor using the CB2 receptor agonist AM1241 can protect against MPTP-induced PD mouse models, leading to an increase in the number of TH-positive cells in the SN, indicating the regeneration of DA neurons in PD mice and suggesting AM1241 as a potential candidate for PD treatment (Shi et al., 2017). Research data obtained from DA neuron-specific CB2 receptor knockout mice indicates that the absence of CB2 receptor in DA neurons modulate psychomotor and reward behavior (Liu et al., 2017). This further confirms the protective functions of CB2 receptor on DA neurons and establishes a new target for PD treatment.
Motor dysfunction is a prominent feature in the progression of PD and poses significant inconvenience and harm to patients (Bologna et al., 2020). In PD models established by unilateral lesion of DA neurons, induced by 6-OHDA or LPS injection in male Sprague Dawley rats, behavioral tests for motor dysfunction and CB2 receptor detection are conducted on days 7, 14, and 28. The animal exhibits motor dysfunction, and the expression of CB2 receptor is significantly upregulated in the PD models (Concannon et al., 2015). Previous studies have found that activation of CB2 receptor using agonists improve certain aspects of motor dysfunction, providing a solution to alleviate the motor deficits caused by PD. In C57BL mice, treatment with the CB2 receptor agonist JWH015 alleviates anxiety-like behavior during chronic mild stress, while AM630 enhances anxiety-like behavior (Ishiguro et al., 2018). An increase in CB1 and CB2 receptor expression in the striatum has been reported in chronic L-DOPA treatment for motor dysfunction, and a correlation between motor dysfunction, striatal activation, and microglial cell activation in the PD model after L-DOPA treatment (Navarro et al., 2018). In a mouse model of PD induced by MPTP treatment, treatment with AM1241 can mitigate weight loss, attenuate MPTP-induced motor impairment, and reduce climbing time in mice (Shi et al., 2017). This indicates the critical role of CB2 receptor in preventing MPTP toxicity and highlights the significant therapeutic value of the CB2 receptor agonist AM1241 in PD, including the potential regeneration of dopaminergic neurons following neurotoxicity induced by MPTP.
Currently, no selective CB2 receptor drug has been approved for the treatment of PD. However, several studies have proposed the use of cannabinoids in the treatment of PD (Stampanoni Bassi et al., 2017; Buhmann et al., 2019). In preclinical studies, different phytocannabinoids has demonstrated potent neuroprotective effect in animal models of PD and other neurodegenerative diseases. Phytocannabinoid Δ9-tetrahydrocannabivarin (Δ9-THCV), a potent agonist of CB2 receptor and antagonist of CB1 receptor, is reported to attenuate the loss of TH-containing neurons in the SN caused by 6-OHDA administration (García et al., 2011). Similar effect of Δ9-THCV is also reported in the PD animal model induce by L-DOPA (Espadas et al., 2020). However, its low BBB-permeability largely limits its application in clinic (Deiana et al., 2012). BCP, a phytocannabinoid and CB2 receptors agonist, is demonstrated to attenuates oxidative stress, neuroinflammation and apoptosis, and produces neuroprotective effects in PD animal models (Javed et al., 2016; al-Taee et al., 2019). Moreover, Δ9-THC has been shown to reduce agitation in the late stages of AD (Walther et al., 2006). In 2003, the FDA granted a patent for cannabinoids as antioxidants and neuroprotectants, but their clinical application in PD is yet to be determined (Krishnan et al., 2009).
Studies using synthetic cannabinoids recently have brought new exciting news in this research area. Nabilone, a synthetic form of Δ9-THC, mimicking the structure and pharmacological activities of Δ9-THC via both CB1 and CB2 receptors. This drug is approved by the U.S. Food and Drug Administration (FDA) for treatment of nausea and vomiting caused by chemotherapy. Recently, 2 clinical trials using Nabilone for the treatment of the non-motor symptoms of PD patients have completed (NCT03769896; NCT03773796). The obtained results indicate that Nabilone is able to produce beneficial effects on sleep disorders associated with PD (Peball et al., 2019, 2020, 2022).
As a progressive neurodegenerative disorder, the prevalence of PD significantly increases in the past decades. Meanwhile, the incidence of PD is also demonstrating a trend of early onset at younger ages. Intensive studies unravel multiple theories that contributes to the pathogenesis of PD. However, the fundamental mechanisms are not fully understood. Consequently, the current treatment for PD is most symptomatic. For this reason, identifying effective therapeutic targets for PD is critically important. The discovery of CB2 receptor by Munro in 1993 (Munro et al., 1993) and subsequent evidence of CB2 receptor expression in the brain and neurons of rodents and primates (Zhang et al., 2014; Stempel et al., 2016), as well as alterations in CB2 receptor expression in PD, have led to investigations in this area. CB2 receptor, as an important component of ECS, plays a protective role in various neurodegenerative diseases (Jordan and Xi, 2019). Selective activation of CB2 receptor regulates mitochondrial function, inhibits oxidative stress, suppresses the release of inflammatory factors, and involves in various regulations such as iron transport, electrophysiology, and autophagy. CB2 receptor agonists have emerged as promising neuroprotective drugs with considerable therapeutic potential (Spinelli et al., 2017). However, many questions about CB2 receptor and its function in PD still remain open, which potentially limits the development and application of the CB2 receptor-targeting therapy. First, most of the current research on the neuroprotective effects of CB2 receptor has focused on its anti-inflammatory properties in microglia and astrocytes. As neurons also express CB2 receptor, its function in neuron and association with neurodegeneration warrant further studies. Second, clinical researches intensively focus on the use of phytocannabinoids such as Δ9-THC in the treatment of neurodegenerative disease such as AD. As those compounds are potent agonist of both CB1 and CB2 receptors, further in-depth clinical research of selective CB2 receptor agonists is necessary to fully understand the therapeutic potential of CB2 receptor in PD. Finally, the function of CB2 receptor in PD is generally believed as neuroprotective and anti-inflammatory, and results little or no adverse CNS effects. However, giving its abundance in the immune system, further investigation of the potential adverse effects of CB2 receptor agonizing is critically important for the clinical application of selective CB2 receptor agonists.
XY: Writing – original draft. YJ: Writing – original draft. YD: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (82003729) and the Natural Science Foundation of Shandong Province (ZR2022QH144, ZR2020QH357).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aguirre-Rueda, D., Guerra-Ojeda, S., Aldasoro, M., Iradi, A., Obrador, E., Mauricio, M. D., et al. (2015). WIN 55,212-2, agonist of cannabinoid receptors, prevents amyloid beta1-42 effects on astrocytes in primary culture. PLoS One 10:e0122843. doi: 10.1371/journal.pone.0122843
al-Taee, H., Azimullah, S., Meeran, M. F. N., Alaraj Almheiri, M. K., al Jasmi, R. A., Tariq, S., et al. (2019). beta-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: an in vitro and in vivo study. Eur. J. Pharmacol. 858:172467. doi: 10.1016/j.ejphar.2019.172467
Athanasiou, A., Clarke, A. B., Turner, A. E., Kumaran, N. M., Vakilpour, S., Smith, P. A., et al. (2007). Cannabinoid receptor agonists are mitochondrial inhibitors: a unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem. Biophys. Res. Commun. 364, 131–137. doi: 10.1016/j.bbrc.2007.09.107
Aymerich, M. S., Rojo-Bustamante, E., Molina, C., Celorrio, M., Sánchez-Arias, J. A., and Franco, R. (2016). Neuroprotective effect of JZL184 in MPP(+)-treated SH-SY5Y cells through CB2 receptors. Mol. Neurobiol. 53, 2312–2319. doi: 10.1007/s12035-015-9213-3
Blázquez, C., Chiarlone, A., Bellocchio, L., Resel, E., Pruunsild, P., García-Rincón, D., et al. (2015). The CB(1) cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 22, 1618–1629. doi: 10.1038/cdd.2015.11
Blesa, J., Trigo-Damas, I., Quiroga-Varela, A., and Jackson-Lewis, V. R. (2015). Oxidative stress and Parkinson's disease. Front. Neuroanat. 9:91. doi: 10.3389/fnana.2015.00091
Bologna, M., Paparella, G., Fasano, A., Hallett, M., and Berardelli, A. (2020). Evolving concepts on bradykinesia. Brain 143, 727–750. doi: 10.1093/brain/awz344
Bose, A., and Beal, M. F. (2016). Mitochondrial dysfunction in Parkinson's disease. J. Neurochem. 139, 216–231. doi: 10.1111/jnc.13731
Boveris, A., and Navarro, A. (2008). Brain mitochondrial dysfunction in aging. IUBMB Life 60, 308–314. doi: 10.1002/iub.46
Buhmann, C., Mainka, T., Ebersbach, G., and Gandor, F. (2019). Evidence for the use of cannabinoids in Parkinson's disease. J. Neural Transm. (Vienna) 126, 913–924. doi: 10.1007/s00702-019-02018-8
Calabresi, P., Mechelli, A., Natale, G., Volpicelli-Daley, L., di Lazzaro, G., and Ghiglieri, V. (2023). Alpha-synuclein in Parkinson's disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 14:176. doi: 10.1038/s41419-023-05672-9
Carrier, E. J., Kearn, C. S., Barkmeier, A. J., Breese, N. M., Yang, W., Nithipatikom, K., et al. (2004). Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol. Pharmacol. 65, 999–1007. doi: 10.1124/mol.65.4.999
Castillo, P. E., Younts, T. J., Chávez, A. E., and Hashimotodani, Y. (2012). Endocannabinoid signaling and synaptic function. Neuron 76, 70–81. doi: 10.1016/j.neuron.2012.09.020
Chen, Z., Li, G., and Liu, J. (2020). Autonomic dysfunction in Parkinson's disease: implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 134:104700. doi: 10.1016/j.nbd.2019.104700
Cheng, J., Liao, Y., Dong, Y., Hu, H., Yang, N., Kong, X., et al. (2020). Microglial autophagy defect causes parkinson disease-like symptoms by accelerating inflammasome activation in mice. Autophagy 16, 2193–2205. doi: 10.1080/15548627.2020.1719723
Chung, Y. C., Shin, W. H., Baek, J. Y., Cho, E. J., Baik, H. H., Kim, S. R., et al. (2016). CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson's disease. Exp. Mol. Med. 48:e205. doi: 10.1038/emm.2015.100
Concannon, R. M., Okine, B. N., Finn, D. P., and Dowd, E. (2015). Differential upregulation of the cannabinoid CB(2) receptor in neurotoxic and inflammation-driven rat models of Parkinson's disease. Exp. Neurol. 269, 133–141. doi: 10.1016/j.expneurol.2015.04.007
Concannon, R. M., Okine, B. N., Finn, D. P., and Dowd, E. (2016). Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson's disease. Exp. Neurol. 283, 204–212. doi: 10.1016/j.expneurol.2016.06.014
Cooray, R., Gupta, V., and Suphioglu, C. (2020). Current aspects of the endocannabinoid system and targeted THC and CBD Phytocannabinoids as potential therapeutics for Parkinson's and Alzheimer's diseases: a review. Mol. Neurobiol. 57, 4878–4890. doi: 10.1007/s12035-020-02054-6
de Lau, L. M., and Breteler, M. M. (2006). Epidemiology of Parkinson's disease. Lancet Neurol. 5, 525–535. doi: 10.1016/S1474-4422(06)70471-9
Deiana, S., Watanabe, A., Yamasaki, Y., Amada, N., Arthur, M., Fleming, S., et al. (2012). Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Delta(9)-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 219, 859–873. doi: 10.1007/s00213-011-2415-0
Demuth, D. G., and Molleman, A. (2006). Cannabinoid signalling. Life Sci. 78, 549–563. doi: 10.1016/j.lfs.2005.05.055
Denaës, T., Lodder, J., Chobert, M. N., Ruiz, I., Pawlotsky, J. M., Lotersztajn, S., et al. (2016). The cannabinoid receptor 2 protects against alcoholic liver disease via a macrophage autophagy-dependent pathway. Sci. Rep. 6:28806. doi: 10.1038/srep28806
Dias, V., Junn, E., and Mouradian, M. M. (2013). The role of oxidative stress in Parkinson's disease. J. Parkinsons Dis. 3, 461–491. doi: 10.3233/JPD-130230
Espadas, I., Keifman, E., Palomo-Garo, C., Burgaz, S., García, C., Fernández-Ruiz, J., et al. (2020). Beneficial effects of the phytocannabinoid Delta(9)-THCV in L-DOPA-induced dyskinesia in Parkinson's disease. Neurobiol. Dis. 141:104892. doi: 10.1016/j.nbd.2020.104892
Facci, L., Dal Toso, R., Romanello, S., Buriani, A., Skaper, S. D., and Leon, A. (1995). Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. U. S. A. 92, 3376–3380. doi: 10.1073/pnas.92.8.3376
Fayyad, M., Salim, S., Majbour, N., Erskine, D., Stoops, E., Mollenhauer, B., et al. (2019). Parkinson's disease biomarkers based on alpha-synuclein. J. Neurochem. 150, 626–636. doi: 10.1111/jnc.14809
Feng, L., Lo, H., You, H., Wu, W., Cheng, X., Xin, J., et al. (2023). Loss of cannabinoid receptor 2 promotes alpha-Synuclein-induced microglial synaptic pruning in nucleus accumbens by modulating the pCREB-c-Fos signaling pathway and complement system. Exp. Neurol. 359:114230. doi: 10.1016/j.expneurol.2022.114230
Fernández-Ruiz, J., Pazos, M. R., García-Arencibia, M., Sagredo, O., and Ramos, J. A. (2008). Role of CB2 receptors in neuroprotective effects of cannabinoids. Mol. Cell. Endocrinol. 286, S91–S96. doi: 10.1016/j.mce.2008.01.001
Fisyunov, A., Tsintsadze, V., Min, R., Burnashev, N., and Lozovaya, N. (2006). Cannabinoids modulate the P-type high-voltage-activated calcium currents in purkinje neurons. J. Neurophysiol. 96, 1267–1277. doi: 10.1152/jn.01227.2005
Floor, E., and Wetzel, M. G. (1998). Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 70, 268–275. doi: 10.1046/j.1471-4159.1998.70010268.x
Foster, D. J., Wilson, J. M., Remke, D. H., Mahmood, M. S., Uddin, M. J., Wess, J., et al. (2016). Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron 91, 1244–1252. doi: 10.1016/j.neuron.2016.08.017
García, M. C., Cinquina, V., Palomo-Garo, C., Rábano, A., and Fernández-Ruiz, J. (2015). Identification of CB(2) receptors in human nigral neurons that degenerate in Parkinson's disease. Neurosci. Lett. 587, 1–4. doi: 10.1016/j.neulet.2014.12.003
García, C., Palomo-Garo, C., García-Arencibia, M., Ramos, J. A., Pertwee, R. G., and Fernández-Ruiz, J. (2011). Symptom-relieving and neuroprotective effects of the phytocannabinoid Delta(9)-THCV in animal models of Parkinson's disease. Br. J. Pharmacol. 163, 1495–1506. doi: 10.1111/j.1476-5381.2011.01278.x
Garcia-Ruiz, P. J., Chaudhuri, K. R., and Martinez-Martin, P. (2014). Non-motor symptoms of Parkinson's disease a review…From the past. J. Neurol. Sci. 338, 30–33. doi: 10.1016/j.jns.2014.01.002
Gelders, G., Baekelandt, V., and Van der Perren, A. (2018). Linking Neuroinflammation and neurodegeneration in Parkinson's disease. J Immunol Res 2018:4784268.
Gerhard, A., Pavese, N., Hotton, G., Turkheimer, F., Es, M., Hammers, A., et al. (2006). In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol. Dis. 21, 404–412. doi: 10.1016/j.nbd.2005.08.002
Ghosh, D., Mehra, S., Sahay, S., Singh, P. K., and Maji, S. K. (2017). Alpha-synuclein aggregation and its modulation. Int. J. Biol. Macromol. 100, 37–54. doi: 10.1016/j.ijbiomac.2016.10.021
Giacoppo, S., Gugliandolo, A., Trubiani, O., Pollastro, F., Grassi, G., Bramanti, P., et al. (2017). Cannabinoid CB2 receptors are involved in the protection of RAW264.7 macrophages against the oxidative stress: an in vitro study. Eur. J. Histochem. 61:2749. doi: 10.4081/ejh.2017.2749
Gómez-Gálvez, Y., Palomo-Garo, C., Fernández-Ruiz, J., and García, C. (2016). Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinson's disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 64, 200–208. doi: 10.1016/j.pnpbp.2015.03.017
Grünblatt, E., Zander, N., Bartl, J., Jie, L., Monoranu, C. M., Arzberger, T., et al. (2007). Comparison analysis of gene expression patterns between sporadic Alzheimer's and Parkinson's disease. J. Alzheimers Dis. 12, 291–311. doi: 10.3233/JAD-2007-12402
Hare, D. J., and Double, K. L. (2016). Iron and dopamine: a toxic couple. Brain 139, 1026–1035. doi: 10.1093/brain/aww022
Herkenham, M., Lynn, A. B., Johnson, M. R., Melvin, L. S., de Costa, B. R., and Rice, K. C. (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 11, 563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991
Howlett, A. C., Barth, F., Bonner, T. I., Cabral, G., Casellas, P., Devane, W. A., et al. (2002). International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 54, 161–202. doi: 10.1124/pr.54.2.161
Howlett, A. C., Bidaut-Russell, M., Devane, W. A., Melvin, L. S., Johnson, M. R., and Herkenham, M. (1990). The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 13, 420–423. doi: 10.1016/0166-2236(90)90124-S
Howlett, A. C., Blume, L. C., and Dalton, G. D. (2010). CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 17, 1382–1393. doi: 10.2174/092986710790980023
Ishiguro, H., Horiuchi, Y., Tabata, K., Liu, Q. R., Arinami, T., and Onaivi, E. (2018). Cannabinoid CB2 receptor gene and Environmental interaction in the development of psychiatric disorders. Molecules 23:1836. doi: 10.3390/molecules23081836
Javed, H., Azimullah, S., Haque, M. E., and Ojha, S. K. (2016). Cannabinoid type 2 (CB2) receptors activation protects against oxidative stress and Neuroinflammation associated dopaminergic neurodegeneration in rotenone model of Parkinson's disease. Front. Neurosci. 10:321. doi: 10.3389/fnins.2016.00321
Jia, W., Xu, H., du, X., Jiang, H., and Xie, J. (2015). Ndfip1 attenuated 6-OHDA-induced iron accumulation via regulating the degradation of DMT1. Neurobiol. Aging 36, 1183–1193. doi: 10.1016/j.neurobiolaging.2014.10.021
Jordan, C. J., and Xi, Z. X. (2019). Progress in brain cannabinoid CB(2) receptor research: from genes to behavior. Neurosci. Biobehav. Rev. 98, 208–220. doi: 10.1016/j.neubiorev.2018.12.026
Ke, P., Shao, B. Z., Xu, Z. Q., Wei, W., Han, B. Z., Chen, X. W., et al. (2016). Activation of cannabinoid receptor 2 ameliorates DSS-induced colitis through inhibiting NLRP3 Inflammasome in macrophages. PLoS One 11:e0155076. doi: 10.1371/journal.pone.0155076
Kelly, R., Joers, V., Tansey, M. G., McKernan, D. P., and Dowd, E. (2020). Microglial phenotypes and their relationship to the cannabinoid system: therapeutic implications for Parkinson's disease. Molecules 25:453. doi: 10.3390/molecules25030453
Kempuraj, D., Thangavel, R., Yang, E., Pattani, S., Zaheer, S., Santillan, D. A., et al. (2015). Dopaminergic toxin 1-Methyl-4-Phenylpyridinium, proteins alpha-Synuclein and glia maturation factor activate mast cells and release inflammatory mediators. PLoS One 10:e0135776. doi: 10.1371/journal.pone.0135776
Kim, K. H., and Lee, M. S. (2014). Autophagy—a key player in cellular and body metabolism. Nat. Rev. Endocrinol. 10, 322–337. doi: 10.1038/nrendo.2014.35
Klegeris, A., Bissonnette, C. J., and McGeer, P. L. (2003). Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br. J. Pharmacol. 139, 775–786. doi: 10.1038/sj.bjp.0705304
Komorowska-Muller, J. A., and Schmole, A. C. (2020). CB2 receptor in microglia: the Guardian of self-control. Int. J. Mol. Sci. 22:19. doi: 10.3390/ijms22010019
Krishnan, S., Cairns, R., and Howard, R. (2009). Cannabinoids for the treatment of dementia. Cochrane Database Syst. Rev. 2009:CD007204. doi: 10.1002/14651858.CD007204.pub2
Langston, J. W., Ballard, P., Tetrud, J. W., and Irwin, I. (1983). Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980. doi: 10.1126/science.6823561
Li, Q., Wang, F., Zhang, Y. M., Zhou, J. J., and Zhang, Y. (2013). Activation of cannabinoid type 2 receptor by JWH133 protects heart against ischemia/reperfusion-induced apoptosis. Cell. Physiol. Biochem. 31, 693–702. doi: 10.1159/000350088
Liu, Q. R., Canseco-Alba, A., Zhang, H. Y., Tagliaferro, P., Chung, M., Dennis, E., et al. (2017). Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci. Rep. 7:17410. doi: 10.1038/s41598-017-17796-y
Liu, Z., Yang, N., Dong, J., Tian, W., Chang, L., Ma, J., et al. (2022). Deficiency in endocannabinoid synthase DAGLB contributes to early onset parkinsonism and murine nigral dopaminergic neuron dysfunction. Nat. Commun. 13:3490. doi: 10.1038/s41467-022-31168-9
Liu, Q. R., Aseer, K. R., Yao, Q., Zhong, X., Ghosh, P., O’Connell, J. F., et al. (2021). Anti-inflammatory and pro-autophagy effects of the cannabinoid receptor CB2R: possibility of modulation in type 1 diabetes. Front. Pharmacol. 12:809965. doi: 10.3389/fphar.2021.809965
Lovinger, D. M. (2008). Presynaptic modulation by endocannabinoids. Handb. Exp. Pharmacol. 184, 435–477. doi: 10.1007/978-3-540-74805-2_14
Lu, J., Wu, M., and Yue, Z. (2020). Autophagy and Parkinson's disease. Adv. Exp. Med. Biol. 1207, 21–51. doi: 10.1007/978-981-15-4272-5_2
Maccarrone, M., and Finazzi-Agro, A. (2003). The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 10, 946–955. doi: 10.1038/sj.cdd.4401284
Mackie, K. (2008). Cannabinoid receptors: where they are and what they do. J. Neuroendocrinol. 20, 10–14. doi: 10.1111/j.1365-2826.2008.01671.x
Makara, J. K., Mor, M., Fegley, D., Szabó, S. I., Kathuria, S., Astarita, G., et al. (2005). Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat. Neurosci. 8, 1139–1141. doi: 10.1038/nn1521
Malek, N., Popiolek-Barczyk, K., Mika, J., Przewlocka, B., and Starowicz, K. (2015). Anandamide, acting via CB2 receptors, alleviates LPS-induced Neuroinflammation in rat primary microglial cultures. Neural Plast. 2015:130639. doi: 10.1155/2015/130639
McAllister, S. D., Griffin, G., Satin, L. S., and Abood, M. E. (1999). Cannabinoid receptors can activate and inhibit G protein-coupled inwardly rectifying potassium channels in a xenopus oocyte expression system. J. Pharmacol. Exp. Ther. 291, 618–626.
McGeer, P. L., Itagaki, S., Boyes, B. E., and McGeer, E. G. (1988). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–1291. doi: 10.1212/WNL.38.8.1285
Mecha, M., Feliú, A., Machín, I., Cordero, C., Carrillo-Salinas, F., Mestre, L., et al. (2018). 2-AG limits Theiler's virus induced acute neuroinflammation by modulating microglia and promoting MDSCs. Glia 66, 1447–1463. doi: 10.1002/glia.23317
More, S. V., and Choi, D. K. (2015). Promising cannabinoid-based therapies for Parkinson's disease: motor symptoms to neuroprotection. Mol. Neurodegener. 10:17. doi: 10.1186/s13024-015-0012-0
Morris, G., Walder, K., Kloiber, S., Amminger, P., Berk, M., Bortolasci, C. C., et al. (2021). The endocannabinoidome in neuropsychiatry: opportunities and potential risks. Pharmacol. Res. 170:105729. doi: 10.1016/j.phrs.2021.105729
Mounsey, R. B., Mustafa, S., Robinson, L., Ross, R. A., Riedel, G., Pertwee, R. G., et al. (2015). Increasing levels of the endocannabinoid 2-AG is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Exp. Neurol. 273, 36–44. doi: 10.1016/j.expneurol.2015.07.024
Munro, S., Thomas, K. L., and Abu-Shaar, M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65. doi: 10.1038/365061a0
Navarrete, F., García-Gutiérrez, M. S., Aracil-Fernández, A., Lanciego, J. L., and Manzanares, J. (2018). Cannabinoid CB1 and CB2 receptors, and Monoacylglycerol lipase gene expression alterations in the basal ganglia of patients with Parkinson's disease. Neurotherapeutics 15, 459–469. doi: 10.1007/s13311-018-0603-x
Navarro, G., Borroto-Escuela, D., Angelats, E., Etayo, Í., Reyes-Resina, I., Pulido-Salgado, M., et al. (2018). Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB(1) and CB(2) receptors and relevance for Alzheimer's disease and levodopa-induced dyskinesia. Brain Behav. Immun. 67, 139–151. doi: 10.1016/j.bbi.2017.08.015
Pacher, P., Batkai, S., and Kunos, G. (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 58, 389–462. doi: 10.1124/pr.58.3.2
Pacher, P., and Kunos, G. (2013). Modulating the endocannabinoid system in human health and disease – successes and failures. FEBS J. 280, 1918–1943. doi: 10.1111/febs.12260
Parlar, A., Arslan, S. O., Doğan, M. F., Çam, S. A., Yalçin, A., Elibol, E., et al. (2018). The exogenous administration of CB2 specific agonist, GW405833, inhibits inflammation by reducing cytokine production and oxidative stress. Exp. Ther. Med. 16, 4900–4908. doi: 10.3892/etm.2018.6753
Peball, M., Krismer, F., Knaus, H. G., Djamshidian, A., Werkmann, M., Carbone, F., et al. (2020). Non-motor symptoms in Parkinson's disease are reduced by Nabilone. Ann. Neurol. 88, 712–722. doi: 10.1002/ana.25864
Peball, M., Seppi, K., Krismer, F., Knaus, H. G., Spielberger, S., Heim, B., et al. (2022). Effects of Nabilone on sleep outcomes in patients with Parkinson's disease: a post-hoc analysis of NMS-nab study. Mov Disord Clin Pract 9, 751–758. doi: 10.1002/mdc3.13471
Peball, M., Werkmann, M., Ellmerer, P., Stolz, R., Valent, D., Knaus, H. G., et al. (2019). Nabilone for non-motor symptoms of Parkinson's disease: a randomized placebo-controlled, double-blind, parallel-group, enriched enrolment randomized withdrawal study (the NMS-nab study). J. Neural Transm. (Vienna) 126, 1061–1072. doi: 10.1007/s00702-019-02021-z
Pertwee, R. G. (2006). The pharmacology of cannabinoid receptors and their ligands: an overview. Int. J. Obes. 30, S13–S18. doi: 10.1038/sj.ijo.0803272
Pfanner, N., Warscheid, B., and Wiedemann, N. (2021). Author correction: mitochondrial proteins: from biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 22:367. doi: 10.1038/s41580-021-00361-x
Price, D. A., Martinez, A. A., Seillier, A., Koek, W., Acosta, Y., Fernandez, E., et al. (2009). WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Eur. J. Neurosci. 29, 2177–2186. doi: 10.1111/j.1460-9568.2009.06764.x
Rego, A. C., and Oliveira, C. R. (2003). Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 28, 1563–1574. doi: 10.1023/A:1025682611389
Roy, S. (2017). Synuclein and dopamine: the Bonnie and Clyde of Parkinson's disease. Nat. Neurosci. 20, 1514–1515. doi: 10.1038/nn.4660
Saha, S., Panigrahi, D. P., Patil, S., and Bhutia, S. K. (2018). Autophagy in health and disease: a comprehensive review. Biomed. Pharmacother. 104, 485–495. doi: 10.1016/j.biopha.2018.05.007
Sahu, P., Mudgal, J., Arora, D., Kinra, M., Mallik, S. B., Rao, C. M., et al. (2019). Cannabinoid receptor 2 activation mitigates lipopolysaccharide-induced neuroinflammation and sickness behavior in mice. Psychopharmacology 236, 1829–1838. doi: 10.1007/s00213-019-5166-y
Schieber, M., and Chandel, N. S. (2014). ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462. doi: 10.1016/j.cub.2014.03.034
Schneider, S. A., Hardy, J., and Bhatia, K. P. (2012). Syndromes of neurodegeneration with brain iron accumulation (NBIA): an update on clinical presentations, histological and genetic underpinnings, and treatment considerations. Mov. Disord. 27, 42–53. doi: 10.1002/mds.23971
Seo, Y. A., Kumara, R., Wetli, H., and Wessling-Resnick, M. (2016). Regulation of divalent metal transporter-1 by serine phosphorylation. Biochem. J. 473, 4243–4254. doi: 10.1042/BCJ20160674
Shao, B. Z., Wei, W., Ke, P., Xu, Z. Q., Zhou, J. X., and Liu, C. (2014). Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis via activation of autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci. Ther. 20, 1021–1028. doi: 10.1111/cns.12349
Sheng, W. S., Hu, S., Min, X., Cabral, G. A., Lokensgard, J. R., and Peterson, P. K. (2005). Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia 49, 211–219. doi: 10.1002/glia.20108
Shi, J., Cai, Q., Zhang, J., He, X., Liu, Y., Zhu, R., et al. (2017). AM1241 alleviates MPTP-induced Parkinson's disease and promotes the regeneration of DA neurons in PD mice. Oncotarget 8, 67837–67850. doi: 10.18632/oncotarget.18871
Skaper, S. D., Facci, L., and Giusti, P. (2014). Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS Neurol. Disord. Drug Targets 13, 1654–1666. doi: 10.2174/1871527313666141130224206
Spinelli, F., Capparelli, E., Abate, C., Colabufo, N. A., and Contino, M. (2017). Perspectives of cannabinoid type 2 receptor (CB2R) ligands in neurodegenerative disorders: structure-affinity relationship (SAfiR) and structure-activity relationship (SAR) studies. J. Med. Chem. 60, 9913–9931. doi: 10.1021/acs.jmedchem.7b00155
Stampanoni Bassi, M., Sancesario, A., Morace, R., Centonze, D., and Iezzi, E. (2017). Cannabinoids in Parkinson's disease. Cannabis Cannabinoid Res. 2, 21–29. doi: 10.1089/can.2017.0002
Stella, N. (2009). Endocannabinoid signaling in microglial cells. Neuropharmacology 56, 244–253. doi: 10.1016/j.neuropharm.2008.07.037
Stempel, A. V., Stumpf, A., Zhang, H. Y., Özdoğan, T., Pannasch, U., Theis, A. K., et al. (2016). Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the Hippocampus. Neuron 90, 795–809. doi: 10.1016/j.neuron.2016.03.034
Subramaniam, S. R., and Chesselet, M. F. (2013). Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Prog. Neurobiol. 106-107, 17–32. doi: 10.1016/j.pneurobio.2013.04.004
Szabo, G. G., Lenkey, N., Holderith, N., Andrasi, T., Nusser, Z., and Hajos, N. (2014). Presynaptic calcium channel inhibition underlies CB(1) cannabinoid receptor-mediated suppression of GABA release. J. Neurosci. 34, 7958–7963. doi: 10.1523/JNEUROSCI.0247-14.2014
Szeto, J. Y. Y., Walton, C. C., Rizos, A., Martinez-Martin, P., Halliday, G. M., Naismith, S. L., et al. (2020). Dementia in long-term Parkinson's disease patients: a multicentre retrospective study. NPJ Parkinsons Dis. 6:2. doi: 10.1038/s41531-019-0106-4
Tansey, M. G., Wallings, R. L., Houser, M. C., Herrick, M. K., Keating, C. E., and Joers, V. (2022). Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 22, 657–673. doi: 10.1038/s41577-022-00684-6
Tedesco, L., Valerio, A., Dossena, M., Cardile, A., Ragni, M., Pagano, C., et al. (2010). Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes 59, 2826–2836. doi: 10.2337/db09-1881
Ternianov, A., Pérez-Ortiz, J. M., Solesio, M. E., García-Gutiérrez, M. S., Ortega-Álvaro, A., Navarrete, F., et al. (2012). Overexpression of CB2 cannabinoid receptors results in neuroprotection against behavioral and neurochemical alterations induced by intracaudate administration of 6-hydroxydopamine. Neurobiol. Aging 33:421.e1. doi: 10.1016/j.neurobiolaging.2010.09.012
Thakur, P., and Nehru, B. (2013). Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by sodium salicylate in a chronic rotenone model of Parkinson's disease. Neuroscience 231, 420–431. doi: 10.1016/j.neuroscience.2012.11.006
Theodore, S., Cao, S., McLean, P. J., and Standaert, D. G. (2008). Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol. 67, 1149–1158. doi: 10.1097/NEN.0b013e31818e5e99
Tolosa, E., Garrido, A., Scholz, S. W., and Poewe, W. (2021). Challenges in the diagnosis of Parkinson's disease. Lancet Neurol. 20, 385–397. doi: 10.1016/S1474-4422(21)00030-2
Turu, G., and Hunyady, L. (2010). Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 44, 75–85. doi: 10.1677/JME-08-0190
Urits, I., Gress, K., Charipova, K., Li, N., Berger, A. A., Cornett, E. M., et al. (2020). Cannabis use and its association with psychological disorders. Psychopharmacol. Bull. 50, 56–67.
Utsumi, H., Okuma, Y., Kano, O., Suzuki, Y., Iijima, M., Tomimitsu, H., et al. (2013). Evaluation of the efficacy of pramipexole for treating levodopa-induced dyskinesia in patients with Parkinson's disease. Intern. Med. 52, 325–332. doi: 10.2169/internalmedicine.52.8333
Viveros-Paredes, J. M., González-Castañeda, R., Gertsch, J., Chaparro-Huerta, V., López-Roa, R., Vázquez-Valls, E., et al. (2017). Neuroprotective effects of beta-Caryophyllene against dopaminergic neuron injury in a murine model of Parkinson's disease induced by MPTP. Pharmaceuticals (Basel) 10:60. doi: 10.3390/ph10030060
Walter, L., Franklin, A., Witting, A., Wade, C., Xie, Y., Kunos, G., et al. (2003). Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. 23, 1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003
Walther, S., Mahlberg, R., Eichmann, U., and Kunz, D. (2006). Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology 185, 524–528. doi: 10.1007/s00213-006-0343-1
Wang, Z., Gao, G., Duan, C., and Yang, H. (2019). Progress of immunotherapy of anti-alpha-synuclein in Parkinson's disease. Biomed. Pharmacother. 115:108843. doi: 10.1016/j.biopha.2019.108843
Wang, M. Y., Liu, M., and Ma, Z. G. (2023). Cannabinoid type 2 receptor activation inhibits MPP+-induced M1 differentiation of microglia through activating PI3K/Akt/Nrf2 signal pathway. Mol. Biol. Rep. 50, 4423–4433. doi: 10.1007/s11033-023-08395-4
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R., and Zecca, L. (2014). The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 13, 1045–1060. doi: 10.1016/S1474-4422(14)70117-6
Warren, N., O’Gorman, C., Lehn, A., and Siskind, D. (2017). Dopamine dysregulation syndrome in Parkinson's disease: a systematic review of published cases. J. Neurol. Neurosurg. Psychiatry 88, 1060–1064. doi: 10.1136/jnnp-2017-315985
Wetli, H. A., Buckett, P. D., and Wessling-Resnick, M. (2006). Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chem. Biol. 13, 965–972. doi: 10.1016/j.chembiol.2006.08.005
Wiseman, H., and Halliwell, B. (1996). Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 313, 17–29. doi: 10.1042/bj3130017
Witting, A., Walter, L., Wacker, J., Möller, T., and Stella, N. (2004). P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc. Natl. Acad. Sci. U. S. A. 101, 3214–3219. doi: 10.1073/pnas.0306707101
Wu, A., Hu, P., Lin, J., Xia, W., and Zhang, R. (2018). Activating cannabinoid receptor 2 protects against diabetic cardiomyopathy through autophagy induction. Front. Pharmacol. 9:1292. doi: 10.3389/fphar.2018.01292
Xin, Q., Xu, F., Taylor, D. H., Zhao, J. F., and Wu, J. (2020). The impact of cannabinoid type 2 receptors (CB2Rs) in neuroprotection against neurological disorders. Acta Pharmacol. Sin. 41, 1507–1518. doi: 10.1038/s41401-020-00530-2
Yin, A. Q., Wang, F., and Zhang, X. (2019). Integrating endocannabinoid signaling in the regulation of anxiety and depression. Acta Pharmacol. Sin. 40, 336–341. doi: 10.1038/s41401-018-0051-5
Yu, W., Jin, G., Zhang, J., and Wei, W. (2019). Selective activation of cannabinoid receptor 2 attenuates myocardial infarction via suppressing NLRP3 Inflammasome. Inflammation 42, 904–914. doi: 10.1007/s10753-018-0945-x
Yu, H., Liu, X., Chen, B., Vickstrom, C. R., Friedman, V., Kelly, T. J., et al. (2021). The neuroprotective effects of the CB2 agonist GW842166x in the 6-OHDA mouse model of Parkinson's disease. Cells 10:3548. doi: 10.3390/cells10123548
Zhang, H. Y., Gao, M., Liu, Q. R., Bi, G. H., Li, X., Yang, H. J., et al. (2014). Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl. Acad. Sci. U. S. A. 111, E5007–E5015. doi: 10.1073/pnas.1413210111
Zhang, H. Y., Gao, M., Shen, H., Bi, G. H., Yang, H. J., Liu, Q. R., et al. (2017). Expression of functional cannabinoid CB(2) receptor in VTA dopamine neurons in rats. Addict. Biol. 22, 752–765. doi: 10.1111/adb.12367
Zhang, D. W., Shao, J., Lin, J., Zhang, N., Lu, B. J., Lin, S. C., et al. (2009). RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336. doi: 10.1126/science.1172308
Zhao, X., He, H., Xiong, X., Ye, Q., Feng, F., Zhou, S., et al. (2022). Lewy body-associated proteins A-Synuclein (a-syn) as a plasma-based biomarker for Parkinson's disease. Front. Aging Neurosci. 14:869797. doi: 10.3389/fnagi.2022.869797
Zoratti, C., Kipmen-Korgun, D., Osibow, K., Malli, R., and Graier, W. F. (2003). Anandamide initiates ca(2+) signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br. J. Pharmacol. 140, 1351–1362. doi: 10.1038/sj.bjp.0705529
Keywords: Parkinson’s disease, CB2 receptor, mitochondrial function, neuroinflammation, iron transport
Citation: Yu X, Jia Y and Dong Y (2024) Research progress on the cannabinoid type-2 receptor and Parkinson’s disease. Front. Aging Neurosci. 15:1298166. doi: 10.3389/fnagi.2023.1298166
Received: 21 September 2023; Accepted: 18 December 2023;
Published: 08 January 2024.
Edited by:
Fangang Meng, Capital Medical University, ChinaReviewed by:
Rengasamy Balakrishnan, Konkuk University, Republic of KoreaCopyright © 2024 Yu, Jia and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Dong, anVsaWFkb25nODI5QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.