
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 03 November 2023
Sec. Neurocognitive Aging and Behavior
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1264124
 Chu Chen1†
Chu Chen1† Yanfang Xie2†
Yanfang Xie2† Mingjun Pu1
Mingjun Pu1 Lan Deng1
Lan Deng1 Zuoqiao Li1
Zuoqiao Li1 Tiannan Yang1
Tiannan Yang1 Hao Yin1
Hao Yin1 Zhehao Zhang1
Zhehao Zhang1 Xinni Lv1
Xinni Lv1 Xueyun Liu2
Xueyun Liu2 Jing Cheng3
Jing Cheng3 Qi Li1*
Qi Li1*Background and purpose: Intracerebral hemorrhage (ICH) is a severe form of stroke that remains understudied in the young adults. We aimed to investigate the clinical presentation, and risk factors associated with ICH in this age group and compare them to older patients.
Methods: Our study included ICH patients admitted between March 2016 and December 2021 in the First Affiliated Hospital of Chongqing Medical University from our ongoing prospective cohort database. Demographic characteristics, etiology, risk factors, and clinical outcomes were compared between elderly and young patients. Furthermore, logistic regression analysis was employed to explore risk factors associated with the functional outcome at 3-months.
Results: We selected 1,003 patients (mean age, 59.9 ±13.8 years old), 746 (74.4%) patients were aged >50 years. The logistic regression analysis showed young patients have a higher proportion of secondary ICH, higher white blood cell count and higher body mass index (BMI), but less diabetes mellitus. Of all patients, predictors of 3-month functional independence was first-ever ICH and age ≤50 years. The history of nephropathy and stroke, higher baseline NIHSS score, larger hematoma volume, and the presence of hydrocephalus were associated with poor outcomes. And the white blood cell count could significantly influence the prognosis among young ICH patients. Three-month functional outcome based on modified Rankin scale score was better in young patients than the elderly (OR, 1.232; 95% CI, 1.095–1.388; p < 0.001).
Conclusions: The highest incidence of ICH occurs in the age groups of 50–59 and 60–69. ICH in young adults had higher white blood cell and BMI compared to the elderly, and differs in etiological distribution. The young patients also had similar short-term mortality but more favorable functional outcomes than the elderly. Furthermore, NIHSS score and larger hematoma volumes were associated with poor outcome in all patients.
Spontaneous intracerebral hemorrhage (ICH) is a devastating form of stroke that could results in focal neurological deficits and high mortality rates, which accounts for 10–20% of all strokes (van Asch et al., 2010). Although ICH traditionally perceived as a disease of older age, the prevalence in younger individuals is increasingly emerging as a public health issue (Krishnamurthi et al., 2014), often leading to permanent disability and imposing a heavy burden on society and economy.
The incidence of ICH in Asian populations was observed approximately double that in Western countries (Jacobs et al., 2002; van Asch et al., 2010), indicating a significant ethnic disparity in the occurrence. Considering the relatively longer life span of young patients, it is critical to clarify the risk factors affecting their functional outcome. However, few studies have investigated the risk factor profile and underlying etiologies for ICH among Chinese young patients (Qureshi et al., 2001, 2009; Keep et al., 2012). Therefore, there is a critical need for studies that targeting young adults to improve our understanding of the impact and develop appropriate prevention and treatment strategies.
In this study, we aimed to investigate the differences in risk factors and clinical characteristics in young and old patients, and to analyze the factors that influence early mortality and functional outcomes between different age groups.
We included ICH patients treated at the First Affiliated Hospital of Chongqing Medical University between March 2016 and December 2021, using data from our ongoing prospective cohort database. The study was conducted in accordance with local ethical framework, and informed consent was obtained from all participants or their legal surrogates.
The inclusion criteria were as follows: (1) age ≥18 years old, (2) diagnosed with non-traumatic ICH, and (3) undergone a CT scan within 24 h after the onset of symptoms. Patients with ICH due to primary intraventricular hemorrhage, tumor bleeding, hemorrhagic transformation of a cerebral infarction, or missing medical records were excluded.
This study assessed risk factors and symptoms in the study patients, including Glasgow Coma Scale (GCS) and National Institutes of Health Stroke Scale (NIHSS) scores, which were recorded by trained neurologist upon arrival. The location of hematoma was identified on baseline CT scans and classified into different regions, including basal ganglia, thalamus, lobar, brainstem, and cerebellum. We calculated hematoma volumes using the ABC/2 method (where A is the greatest hemorrhage diameter by CT, B is the diameter 90° to A, and C is the approximate number of CT slices with hemorrhage multiplied by the slice thickness) (Kothari et al., 1996). Moreover, we recorded the medical history of neurovascular risk factors for ICH, such as hypertension, diabetes mellitus, dyslipidemia, and body mass index (BMI).
Furthermore, we classified the etiology of ICH based on patients' medical history and further brain imaging findings (computer tomographic angiography, magnetic resonance angiography) of each patient. Primary ICH is defined as caused by spontaneous rupture of small blood vessels in the brain, which is often associated with hypertension or cerebral amyloid angiopathy. Secondary ICH is mainly caused by underlying conditions such as intracranial cavernous hemangioma, arteriovenous malformations, arterial aneurysm, anticoagulant associated ICH, moyamoya vasculopathy (Cordonnier et al., 2018). Based on previous research, a young adult patient with ICH is typically defined as an individual between the age 18 and 50 (Tatlisumak et al., 2018).
Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg with repeated examination, or if they were or had been on antihypertensive medication at any time before onset (Unger et al., 2020). Diabetes mellitus was defined as self-reported history, or baseline fasting serum glucose ≥ 7.0 mmol/L (≥ 126 mg/dl), non-fasting serum glucose ≥ 11.1 mmol/L (≥ 200 mg/dl), glycated hemoglobin (HbA1C) ≥ 48 mmol/mol (≥ 6.5% by the Diabetes Control and Complications Trial) or the use of glucose-lowering drugs before onset (American Diabetes Association, 2010). Dyslipidemia was diagnosed if the fasting blood lipid test fulfilling at least one of the following criteria: total cholesterol (TC) ≥6.22 mmol/L, triglyceride (TG) ≥2.26 mmol/L, low-density lipoprotein-cholesterol (LDL-c) ≥4.14 mmol/L, high-density lipoprotein-cholesterol (HDL-c) <1.04 mmol/L, self-reported history, or long-term use of lipid-lowering agents (Stone et al., 2014). Chronic kidney disease (CKD) was ascertained from in-person interviews or baseline estimated glomerular filtration rate (eGFR). Patients were diagnosed as having CKD if they had an eGFR < 60 ml/min/1.73 m2 for ≥ 3 months (Levey et al., 2005). Patients with liver disease were defined as patients who had been diagnosed with chronic hepatitis or liver cirrhosis, or who showed abnormal laboratory data for aspartate aminotransferase (> 50 IU/l), alanine aminotransferase (> 50 IU/l), or gamma-glutamyl transferase (> 60 IU/l). Body mass index (BMI) was calculated based on measured body height and weight in the medical record.
The clinical outcome was measured using the modified Rankin Scale (mRS) score at 3-month, performed by phone interviews or outpatient clinic visits. Favorable outcomes were defined as mRS of 0–2. Case fatality was defined as death occurring within 30 days after the index stroke.
For baseline clinical characteristics, categorical variables were presented as percentages (%) and continuous variables were as mean ± standard deviation (SD) or median and interquartile range (IQR). Chi-square test, Student's t-test, or Mann–Whitney U-test were used to compare variables between the young and old groups as appropriate. Binary logistic regression analysis with the backward stepwise method was performed to determine the differences between the groups. Variables with a p < 0.1 were selected and entered into the logistic regression analysis, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A two-tailed p < 0.05 was considered significant. Statistical analysis was conducted using SPSS software version 25.0 (IBM Corp, Armonk, NY).
During the study period, a total of 1,861 patients with ICH were admitted to our medical center. The patient selection flowchart was illustrated in Figure 1, and 1,003 patients who met the inclusion criteria were included in the final analysis. The mean age of the patients was 59.9 ± 13.8 years, and 68.4% of them (n = 686) were male (Table 1). The most frequent location of hematoma was in the basal ganglia (47.7%, n = 478), with a median volume of 12.1 (IQR, 4.9–28.7) ml. The majority of cases (51.7%) were between 50–59 and 60–69 years old (Figure 2).
Of the 1003 patients, 746 (74.4%) patients were aged >50 years. Baseline characteristics stratified by age group (≤50 years vs. >50 years) were illustrated in Table 1. The younger ICH patients were more likely to be male (74.7 vs. 66.2%, p = 0.012), had larger baseline hematoma volume (median, 15.1 vs. 11.3, p < 0.001). Primary ICH was considerably more frequent among the elderly (94.5 vs. 87.3%, p < 0.01), while secondary ICH occurred more frequently among younger patients (16.3 vs. 5.5%), especially the arteriovenous malformations (9.3 vs. 1.2%).
Older patients had a higher prevalence of hypertension (73.5 vs. 62.7%, p = 0.001), diabetes mellitus (19.8 vs. 13.1%, p = 0.018), and previous stroke (18.9 vs. 10.6%, p = 0.002). However, the admission SBP was significantly higher in the younger population. Furthermore, ICH patients in the younger population had higher white blood cell count (median, 11.1 vs. 8.9, p < 0.001) and platelet count (median, 197.0 vs. 175.0, p < 0.001). The young group also had a higher BMI (24.5 vs. 24, p = 0.017) and were more frequently treated with endotracheal intubation (46.8 vs. 33.8%, p < 0.001) and admission in the ICU (89.6 vs. 82.1%, p = 0.005) compared to the elderly.
After adjustment for confounding factors in the logistic regression (Table 2), diabetes mellitus history (OR, 2.076; 95% CI, 1.130–3.184; p = 0.019), secondary ICH (OR, 0.487; 95% CI, 0.238–0.994; p = 0.048), WBC (OR, 0.819; 95% CI, 0.772–0.869; p < 0.001), BMI (OR, 0.946; 95% CI, 0.899–0.996; p = 0.034) and hematoma location (p < 0.001) remained statistically different between the two groups. Young patients also had significant better 90-day outcomes than the elderly patients (OR, 1.232; 95% CI, 1.095–1.388; p < 0.001).
Of the 1,003 patients, 527 (52.5%) achieved functional independence (mRS 0–2) at 90-day follow-up (Table 3). Figure 3 showed the mRS scores distributionat the 3-month follow-up for each age group. Logistic regression analysis revealed that age ≤50 years (OR, 0.495; 95% CI, 0.323–0.757; p < 0.001) and first-ever ICH (OR, 0.424; 95% CI, 0.181–0.990; p = 0.047) decreased the likelihood of poor outcomes. However, the history of chronic kidney disease (OR, 0.4238; 95% CI, 1.045–4.988; p = 0.038), previous stroke (OR, 0.519; 95% CI, 0.286–0.940; p < 0.030), baseline NIHSS score (OR, 0.906; 95% CI, 0.876–0.937; p < 0.001), hematoma volume (OR, 0.965; 95% CI, 0.954–0.976; p < 0.001), and hydrocephalus (OR, 0.520; 95% CI, 0.319–0.874; p = 0.009) were associated with an increased risk of poor outcomes.
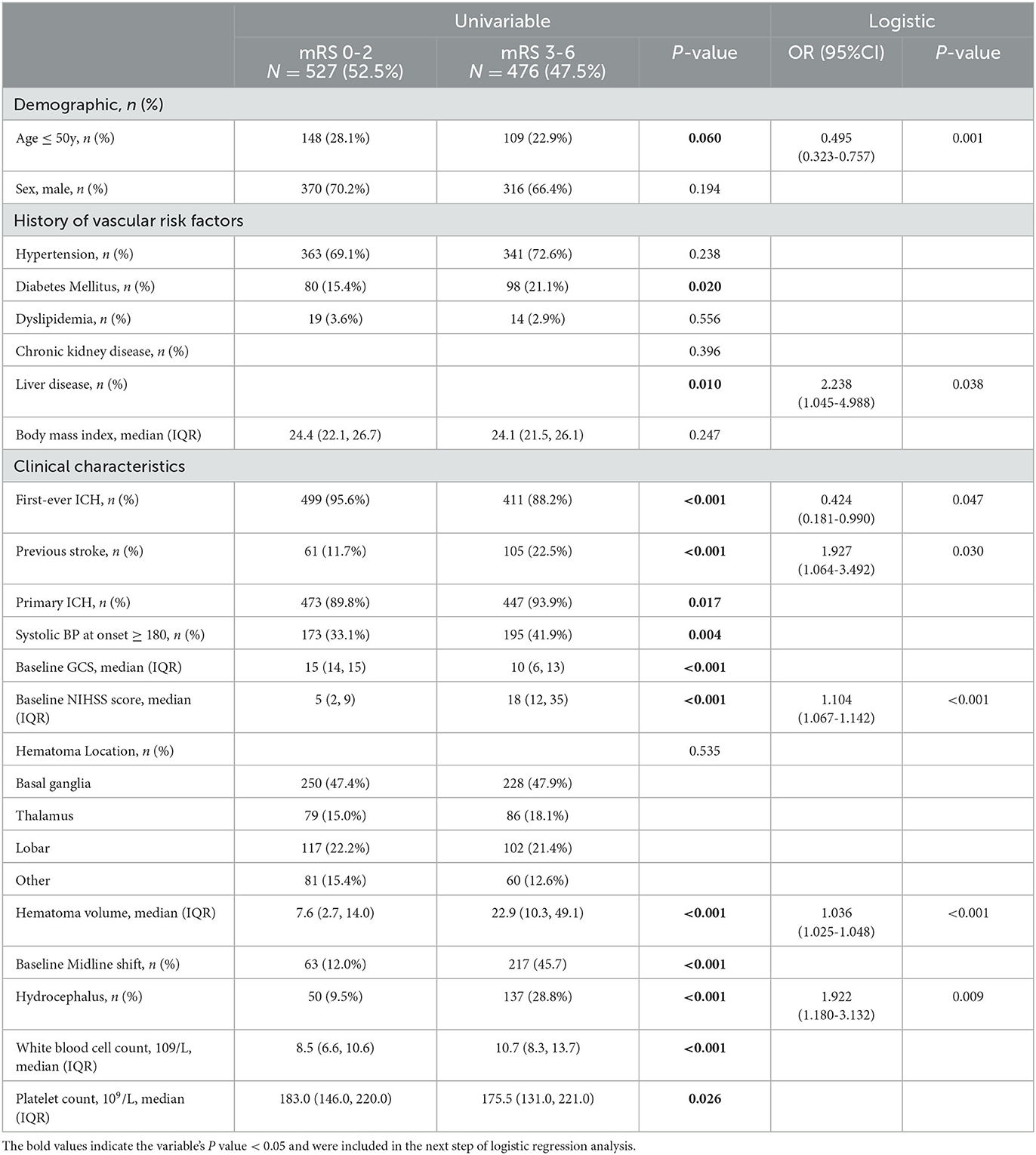
Table 3. Univariable and logistic regression comparison on factors associated with 3-month functional independence after ICH of the study cohort.
We further subdivided the young population (n = 257) into two groups based on outcome at the 90-day follow-up (Table 4). After adjusting for confounding factors in the logistic regression analysis, we found that first-ever ICH (OR, 0.063; 95% CI, 0.011–0.361; p = 0.002), baseline NIHSS score (OR, 1.085; 95% CI, 1.048–1.124; p < 0.001), hematoma volume (OR, 1.050; 95% CI, 1.028–1.072; p < 0.001), and white blood cell count (OR, 1.123; 95% CI, 1.022–1.234; p = 0.016) were associated with poor functional outcome in young ICH patients.
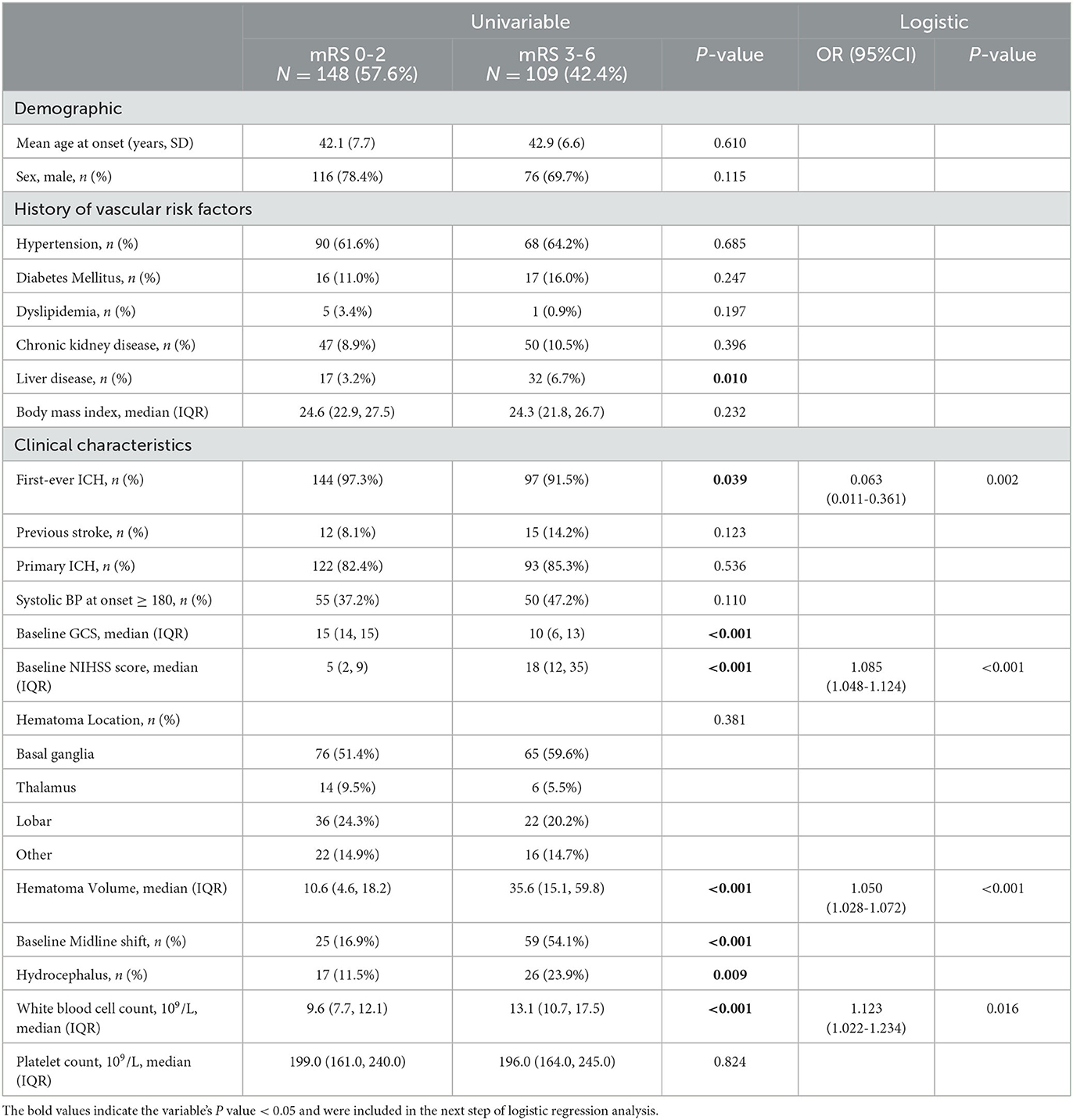
Table 4. Univariable and logistic regression comparison on factors associated with 3-month functional independence in young ICH patients.
Our results provided the clinical characteristics and prognostic factors of non-traumatic ICH in young patients, and compared the results to those of the elderly. The incidence of ICH is considerably affected by factors such as age, gender, and ethnic disparity (van Asch et al., 2010). Over the past few decades, there has been a global decline in the incidence of ischemic strokes. However, the incidence of hemorrhagic strokes has not followed the same trend (Feigin et al., 2009). Furthermore, non-traumatic ICH in young individuals differs in several key aspects from that in older individuals, and there is a lack of attention and comprehensive guidance for physicians regarding the diagnosis and treatment strategies for ICH in young patients.
Analysis of the 2010 Global Burden of Disease data revealed the incidence of cerebral hemorrhage doubled in younger age groups and was concentrated in low- and middle-income countries (Krishnamurthi et al., 2014). A previous report in 2010 highlighted that the incidence of ICH was 1.9 cases per 100, 000 individuals worldwide among those under 45 years of age, with an escalation to tenfold in the 45–54 age group, and a nearly twentyfold rise in the 55–64 age group (van Asch et al., 2010). Our finding was consistent with this, showing that the highest frequency of ICH in our center occurs in the age groups of 50–59 and 60–69.
Among the participants in our analysis, 68.4% were male, and men appeared to have a higher likelihood of experiencing ICH than women across various age groups. This finding is consistent with a 2015 study in Finland (Koivunen et al., 2015a), where the incidence of non-traumatic ICH was notably higher in men (6.2 cases per 100, 000 individuals; 95% CI 5.0–7.3) than in women (4.0 cases per 100, 000 individuals; 95% CI 3.4–4.7). The male predominance in stroke events can potentially be attributed to the higher prevalence but lower control rate of risk factors (Nzwalo et al., 2018; On et al., 2022).
It has been reported that the etiology of ICH in young patients more often involves structural abnormalities and uncommon underlying factors (Koivunen et al., 2015a). The majority of new evidence in the field of ICH in young individuals comes from genetic research, revealing numerous previously unknown genetic loci associated with brain hemorrhage. These studies have the capacity to offer new insights into the mechanisms of ICH in young patients. In this study, 91.7% cases were caused by primary ICH. We noted that younger population was more frequently associated with secondary ICH, such as arteriovenous malformations, arterial aneurysms, or moyamoya vasculopathy, markedly differing from older patients.
We also showed that the risk factors differ between age groups. Nevertheless, hypertension emerged as the predominant risk factor within both age categories. Younger patients with ICH manifested less frequent diabetes rates but higher body mass index (BMI) compared to older individuals. Prior investigations have established a correlation between elevated BMI, increased high-density lipoprotein (HDL) cholesterol, and the incidence of ICH (Chen et al., 2017; Yang et al., 2020). Therefore, it is necessary to control the aforementioned modifiable risk factors, such as hypertension, diabetes and BMI, to reduce the hemorrhagic stroke occurrence.
Furthermore, our results indicated the white blood cell count was independently associated with poor outcomes in young patients. On the progress of intracerebral hemorrhage, an inflammatory response occurs as a consequence of cerebral vascular bleeding and acute stress reaction, which is accompanied by an elevation in inflammatory markers such as WBC (Di Napoli et al., 2011). In addition, severely ICH patients may experience impaired consciousness and swallowing dysfunction, increasing the risk of developing aspiration pneumonia (Wang et al., 2021). These acute infections can also lead to an elevation in WBC count. Prior research suggested a significant association between elevated inflammatory markers and hematoma growth (Di Napoli et al., 2014; Morotti et al., 2016), as well as a higher incidence of unfavorable clinical outcomes (He et al., 2023).
Prior studies reported that increasing age, baseline NIHSS, baseline GCS, hematoma volume, the presence of hydrocephalus, herniation, renal or heart disease, and multiple hemorrhages were associated with the case-fatality in ICH patients (Hemphill et al., 2001; van Asch et al., 2010; Koivunen et al., 2014, 2015b; Rutten-Jacobs et al., 2014; Yang et al., 2020). Our study were also consistent with these studies, we found the factor associated with patients' 3-month functional independence were the previous medical history of nephropathy and stroke events, age > 50 years, higher NIHSS scores, larger hematoma volumes and the presence of hydrocephalus.
The severity of neurological impairment upon admission demonstrated no significant age-related differences. Similarly, there was no statistical difference between the two groups in 1-month mortality rate (17.5 and 18.8% for young and elderly patients, respectively). However, a marked improvement in outcomes at 90 days was significantly evident among younger ICH patients, suggesting that the rehabilitation rate is more favorable among younger survivors. As evidenced by our study, potential explanations for the observed improved outcomes in younger patients could be attributed to their enhanced neuroplasticity and a lower likelihood of experiencing complications (Koivunen et al., 2014; Yang et al., 2020). Furthermore, the prevalence of vascular risk factors increased with age, younger patients typically exhibit fewer individual risk factors, which contribute to their more favorable outcomes. Emerging evidence supported an aggressive therapeutic approach for spontaneous ICH in young patients, including aggressive blood pressure reduction (Anderson et al., 2013; Qureshi et al., 2016), selective surgical hematoma evacuation (Lai et al., 2005; Koivunen et al., 2014), and intensive rehabilitation.
We recognized several limitations in this study. First, this is a single-center study, our results need to be confirmed by multi-center study. Second, there are additional important young-adult-specific risk factors, including drug abuse, pregnancy, and the postpartum period, however, our current research was lacking in data pertaining to these factors. Third, different levels of nursing care quality may also contribute to the prognosis of ICH patients. It's important to acknowledge that there are variations in the nursing care quality among the patients in this study.
In summary, our study demonstrates that spontaneous ICH in young adults had higher white blood cell and BMI compared to the elderly, and differs in etiological distribution. The young patients also had similar short-term mortality but more favorable functional outcomes. Furthermore, NIHSS score and larger hematoma volumes were associated with poor outcome in all age groups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent was obtained from all participants or their legal surrogates.
CC: Methodology, Writing—original draft. YX: Methodology, Resources, Writing—original draft. MP: Resources, Writing—review & editing. LD: Resources, Writing—review & editing. ZL: Resources, Writing—review & editing. TY: Resources, Writing—review & editing. HY: Resources, Writing—review & editing. ZZ: Resources, Writing—review & editing. XLv: Resources, Writing—review & editing. XLi: Resources, Writing—review & editing. JC: Resources, Writing—review & editing. QL: Conceptualization, Data curation, Funding acquisition, Supervision, Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant no. 82071337), the Chongqing Innovation Support Program for Returned Overseas Chinese Scholars (grant no. cx2020002), the Chongqing Science Fund for Distinguished Young Scholars (grant no. cstc2021jcyj-jqX0029), and Research Fund of Anhui Institute of Translational Medicine (grant no. 2022zhyx-C38).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
American Diabetes Association (2010). Standards of medical care in diabetes−2010. Diab. Care. 33, S11–61. doi: 10.2337/dc10-S011
Anderson, C. S., Heeley, E., Huang, Y., Wang, J., Stapf, C., Delcourt, C., et al. (2013). INTERACT2 investigators rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N. Engl. J. Med. 368, 2355–2365. doi: 10.1056/NEJMoa1214609
Chen, C. J., Brown, W. M., Moomaw, C. J., Langefeld, C. D., and Osborne, J., Worrall, B. B., et al. (2017). Alcohol use and risk of intracerebral hemorrhage. Neurology 88, 2043–2051. doi: 10.1212/WNL.0000000000003952
Cordonnier, C., Demchuk, A., Ziai, W., and Anderson, C. S. (2018). Intracerebral haemorrhage: current approaches to acute management. The Lancet 392, 1257–1268. doi: 10.1016/S0140-6736(18)31878-6
Di Napoli, D., Godoy, M., Campi, D. A., del Valle, M., Piñero, G., Mirofsky, M., et al. (2011). C-reactive protein level measurement improves mortality prediction when added to the spontaneous intracerebral hemorrhage score. Stroke 42, 1230–1236. doi: 10.1161/STROKEAHA.110.604983
Di Napoli, D., Parry-Jones, M., Smith, A. R., Hopkins, C. J., Slevin, S. J., Masotti, M., et al. (2014).C-reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke 45, 59–65. doi: 10.1161/STROKEAHA.113.001721
Feigin, V. L., Lawes, C. M. M., Bennett, D. A., Barker-Collo, S. L., and Parag, V. (2009). Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 8, 355–369. doi: 10.1016/S1474-4422(09)70025-0
He, J., Zhang, Y., Cheng, X., Li, T., Xiao, Y., Peng, L., et al. (2023). White blood cell count predicts mortality in patients with spontaneous intracerebral hemorrhage. Neurocrit. Care 10, 1–14. doi: 10.1007/s12028-023-01716-2
Hemphill, J. C., Bonovich, D. C., Besmertis, L., Manley, G. T., and Johnston, S. C. (2001). The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32, 891–897. doi: 10.1161/01.STR.32.4.891
Jacobs, B. S., Boden-Albala, B., Lin, I. F., and Sacco, R. L. (2002). Stroke in the young in the northern manhattan stroke study. Stroke 33, 2789–2793. doi: 10.1161/01.STR.0000038988.64376.3A
Keep, R. F., Hua, Y., and Xi, G. (2012). Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 11, 720–731. doi: 10.1016/S1474-4422(12)70104-7
Koivunen, R. J., Satopää, J., Haapaniemi, E., Strbian, D., Meretoja, A., Mustanoja, S., et al. (2014). Predictors of early mortality in young adults after intracerebral hemorrhage. Stroke 45, 2454–2456. doi: 10.1161/STROKEAHA.114.006020
Koivunen, R. J., Satopää, J., Meretoja, A., Strbian, D., and Haapaniemi, E., Niemelä, M., et al. (2015a). Incidence, risk factors, etiology, severity and short-term outcome of non-traumatic intracerebral hemorrhage in young adults. Eur. J. Neurol. 22, 123–132. doi: 10.1111/ene.12543
Koivunen, R. J., Tatlisumak, T., Satopää, J., Niemelä, M., and Putaala, J. (2015b). Intracerebral hemorrhage at young age: long-term prognosis. Eur. J. Neurol. 22, 1029–1037. doi: 10.1111/ene.12704
Kothari, R. U., Brott, T., Broderick, J. P., Barsan, W. G., Sauerbeck, L. R., Zuccarello, M., et al. (1996). The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27, 1304–1305. doi: 10.1161/01.STR.27.8.1304
Krishnamurthi, R. V., Moran, A. E., Forouzanfar, M. H., Bennett, D. A., and Mensah, G. A. (2014). The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob. Heart 9, 101–106. doi: 10.1016/j.gheart.2014.01.003
Lai, S. L., Chen, S. T., Lee, T. H., Ro, L. S., and Hsu, S. P. (2005). Spontaneous intracerebral hemorrhage in young adults. Eur. J. Neurol. 12, 310–316. doi: 10.1111/j.1468-1331.2004.00957.x
Levey, A. S., Eckardt, K. U., Tsukamoto, Y., Levin, A., Coresh, J., Rossert, J., et al. (2005). Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 67, 2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x
Morotti, A., Phuah, C. L., Anderson, C. D., Jessel, M. J., Schwab, K., Ayres, A. M., et al. (2016). Leukocyte count and intracerebral hemorrhage expansion. Stroke 47, 1473–1478. doi: 10.1161/STROKEAHA.116.013176
Nzwalo, H., Nogueira, J., Félix, A. C., Guilherme, P., Abreu, P., Figueiredo, T., et al. (2018). Short-term outcome of spontaneous intracerebral hemorrhage in algarve, portugal: retrospective hospital-based study. J. Stroke Cerebrovasc. Dis. 27, 346–351. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.006
On, S., Poh, R., Salor, R. S., Philip, R. G., Chekkattu, R. H., Lim, M. A., et al. (2022). The burden and risks factors for intracerebral hemorrhage in a Southeast Asian population. Clin. Neurol. Neurosurg. 214, 107145. doi: 10.1016/j.clineuro.2022.107145
Qureshi, A. I., Mendelow, A. D., and Hanley, D. F. (2009). Intracerebral haemorrhage. Lancet 373, 1632–1644. doi: 10.1016/S0140-6736(09)60371-8
Qureshi, A. I., Palesch, Y. Y., Barsan, W. G., Hanley, D. F., Hsu, C. Y., Martin, R. L., et al. (2016). ATACH-2 trial investigators and the neurological emergency treatment trials network. intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N. Engl. J. Med. 375, 1033–1043. doi: 10.1056/NEJMoa1603460
Qureshi, A. I., Tuhrim, S., Broderick, J. P., Batjer, H. H., Hondo, H., Hanley, D. F., et al. (2001). Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 344, 1450–1460. doi: 10.1056/NEJM200105103441907
Rutten-Jacobs, L. C., Maaijwee, N. A., Arntz, R. M., Schoonderwaldt, H. C., Dorresteijn, L. D., Van Dijk, E. J., et al. (2014). Outcome of intracerebral hemorrhage in young adults. J. Neurol. 261, 2143–2149. doi: 10.1007/s00415-014-7469-6
Stone, N. J., Robinson, J. G., Lichtenstein, A. H., Bairey Merz, C. N., Blum, C. B., Eckel, R. H., et al. (2014). 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S1–45. doi: 10.1161/01.cir.0000437738.63853.7a
Tatlisumak, T., Cucchiara, B., Kuroda, S., Kasner, S. E., and Putaala, J. (2018). Nontraumatic intracerebral haemorrhage in young adults. Nat. Rev. Neurol. 14, 237–250. doi: 10.1038/nrneurol.2018.17
Unger, T., Borghi, C., Charchar, F., Khan, N. A., Poulter, N. R., Prabhakaran, D., et al. (2020). International society of hypertension global hypertension practice guidelines. J. Hypertens. 38, 982–1004. doi: 10.1097/HJH.0000000000002453
van Asch, C. J., Luitse, M. J., Rinkel, G. J., van der Tweel, I., Algra, A., Klijn, C. J., et al. (2010). Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 9, 167–176. doi: 10.1016/S1474-4422(09)70340-0
Wang, Q., Liu, Y., Han, L., He, F., Cai, N., Zhang, Q., et al. (2021). Risk factors for acute stroke-associated pneumonia and prediction of neutrophil-to-lymphocyte ratios. Am. J. Emerg. Med. 41, 55–59. doi: 10.1016/j.ajem.2020.12.036
Keywords: intracerebral hemorrhage, outcome, predictor, risk factor, young patients
Citation: Chen C, Xie Y, Pu M, Deng L, Li Z, Yang T, Yin H, Zhang Z, Lv X, Liu X, Cheng J and Li Q (2023) Age-related differences in risk factors, clinical characteristics, and outcomes for intracerebral hemorrhage. Front. Aging Neurosci. 15:1264124. doi: 10.3389/fnagi.2023.1264124
Received: 20 July 2023; Accepted: 16 October 2023;
Published: 03 November 2023.
Edited by:
Allison B. Reiss, New York University, United StatesReviewed by:
Sangeetha Sukumari Ramesh, Augusta University, United StatesCopyright © 2023 Chen, Xie, Pu, Deng, Li, Yang, Yin, Zhang, Lv, Liu, Cheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Li, cWlsaV9tZEAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.