
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 21 November 2023
Sec. Neuroinflammation and Neuropathy
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1259668
This article is part of the Research Topic Effects of Vascular Function and Aging on Brain Circulation and Neurodegeneration View all 6 articles
Background: The hemoglobin to red cell distribution width ratio (HRR) has been experimentally associated with the prognosis of acute ischemic stroke (AIS). However, its relationship with mechanical thrombectomy (MT) for AIS remains unclear. Therefore, this study aimed to investigate the relationship between HRR at admission, follow-up HRR, and clinical outcomes in patients undergoing MT.
Methods: Acute ischemic stroke patients undergoing MT were consecutively enrolled from January 2017 to December 2022. Demographic, clinical, and laboratory information were collected. HRR was measured by dividing hemoglobin (Hb) by red cell distribution width (RDW) at admission and after 24 h of MT. Clinical outcomes after 3 months were evaluated using the modified Rankin Scale (mRS). The primary outcome was poor prognosis (mRS > 2) at 3 months, while the secondary outcome was death within 3 months.
Results: A total of 310 patients were analyzed, of whom 216 patients (69.7%) had poor prognosis, and 92 patients (29.6%) died. Patients with a poor prognosis and death had significantly lower HRR levels at admission and after 24 h. HRR at admission was not associated with clinical outcomes according to multivariable logistic regression analysis. However, HRR after 24 h was significantly associated with poor prognosis (adjusted odds ratio [OR]: 0.646, 95% confidence interval [CI]: 0.520–0.803, p < 0.001) and death (adjusted OR: 0.615, 95% CI: 0.508–0.744, p < 0.001). Receiver-operating characteristic curve analysis demonstrated the predictive ability of HRR after 24 h, with areas under the curves of 0.790 for poor prognosis and 0.771 for death.
Conclusion: Rapidly measurable HRR levels are an independent marker of outcome after MT in AIS patients. This may provide a reliable auxiliary outcome measure for clinical routine and interventional therapy.
The Global Burden of Disease Report identifies stroke as the second leading cause of worldwide mortality and disability (Krishnamurthi et al., 2020). Mechanical thrombectomy (MT) has emerged as one of the most efficacious therapies for acute ischemic stroke (AIS; Powers et al., 2018, 2019). Timely and effective thrombectomy significantly improves the prognosis for AIS patients. However, complications such as cerebral hemorrhage, vascular re-occlusion, and cerebral edema may occur after MT, and the limited treatment time window presents challenges to treatment efficacy (Kimberly et al., 2018; Luby et al., 2023; Sarraj et al., 2023). Hence, it is crucial to investigate simple, convenient, and effective clinical indicators that can predict the prognosis of AIS, guide clinical decision-making, and enhance treatment outcomes.
Hemoglobin (Hb) and red cell distribution width (RDW) are conventional blood test parameters. Hb levels determine the oxygen-carrying capacity, while RDW quantifies the variation in red blood cell size (Lippi and Plebani, 2014; Salvagno et al., 2015). These parameters not only reflect the balance between hematopoietic function and red blood cell survival but also play a critical role in inflammation, oxidative stress, and the vascular innate immune system (Emans et al., 2011; Patel et al., 2013; Kawabata, 2020). For instance, anemia has been shown to induce the release of interleukin-6 and tumor necrosis factor-α (Feret et al., 2022), and RDW has been linked to inflammatory markers and oxidative stress (Förhécz et al., 2009). Inflammation and oxidative stress can worsen cerebral edema and hemorrhage, delay cerebral ischemia, and contribute to poor prognosis (Zhao et al., 2017; Esposito et al., 2022; Westendorp et al., 2022). These findings suggest that both Hb and RDW, as essential markers of underlying inflammatory processes, may be correlated with the clinical outcome of ischemic stroke. Hb levels have been identified as significant predictors of AIS and coronary heart disease (Kwok et al., 2016; Chang et al., 2020; Zhang et al., 2021). Research has also demonstrated that RDW can predict adverse outcomes in patients with acute myocardial infarction receiving percutaneous coronary intervention (PCI) and in patients with AIS (Feng et al., 2017; Parizadeh et al., 2019; Xiao et al., 2022). Furthermore, RDW, regardless of anemia status, has been associated with stroke severity and adverse outcomes in AIS, thereby improving stroke prediction accuracy (Saliba et al., 2015; Xue et al., 2022).
Recently, the hemoglobin-to-red cell distribution width ratio (HRR) has emerged as a novel biomarker for cardiovascular diseases. It is calculated from Hb and RDW without additional costs (Rahamim et al., 2022; Xiu et al., 2022). HRR has been studied in relation to myocardial infarction stenting and ischemic stroke associated with atrial fibrillation (Qin et al., 2022; Sun et al., 2022). However, further investigation is needed to determine whether HRR can effectively predict the prognosis of AIS patients undergoing thrombectomy. Additionally, various components of the immune system undergo dynamic changes after AIS, which may have varying effects depending on the stage of stroke development. Therefore, our study aims to investigate the association between HRR and the prognosis of AIS patients undergoing thrombectomy, as well as explore the optimal time point at which HRR functions as a prognostic marker.
From January 2017 to December 2022, this retrospective study included consecutive patients who underwent MT at Chengdu Second People’s Hospital, based on a prospective database. The MT selection criteria and time window strictly adhered to the current guidelines of the American Heart Association/American Stroke Association for the Early Management of Acute Ischemic Stroke Patients (Powers et al., 2015, 2018; Greenberg et al., 2022). The choice of materials and thrombectomy approach, whether stent-retriever, aspiration, or a combined technique, was determined by the neuro-interventionalist. Follow-up computed tomography (CT) scans were performed approximately 24 h after MT to assess any intracranial hemorrhage.
The exclusion criteria for this study were as follows: (1) acute or chronic infection; (2) severe systemic illnesses such as malignancy, hematological disorders, severe heart failure, liver, or renal dysfunction; (3) incomplete clinical data; (4) modified Rankin Scale (mRS) score > 2 prior to the onset of stroke; and (5) patients who were lost to follow-up. 378 AIS patients who underwent MT were initially screened, and 37 patients were excluded based on the inclusion and exclusion criteria. Additionally, 21 patients were lost during the follow-up period. After excluding 10 patients who lacked baseline Hb and RDW values, the final sample size for the analysis was 310 patients (Figure 1). The retrospective study obtained approval from the ethics committee of Chengdu Second People’s Hospital, and patients or their families provided written informed consent.
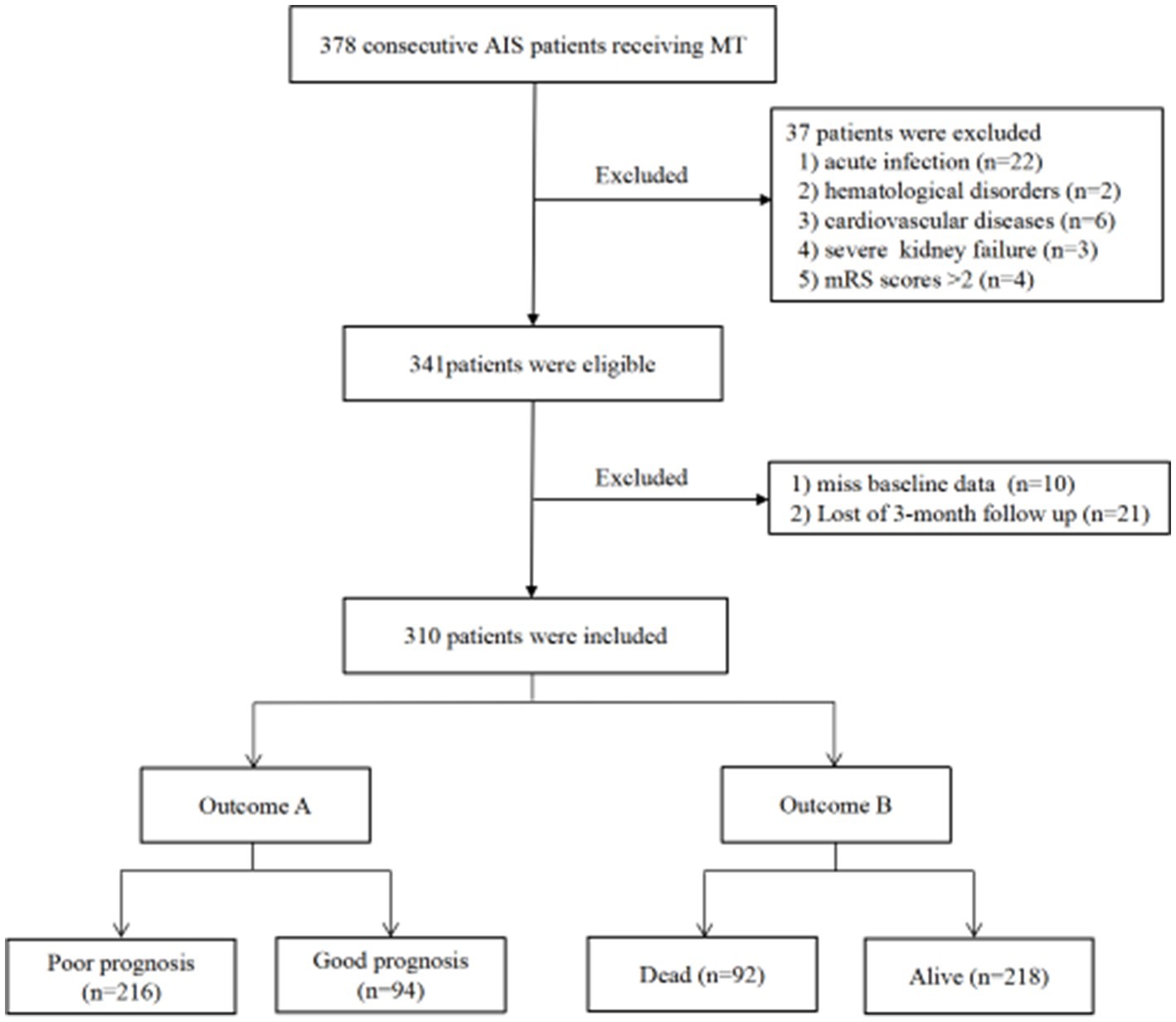
Figure 1. Study flow chart. AIS, Acute ischemic stroke; MT, Mechanical thrombectomy; mRS, Modified Rankin scale.
Demographic information, past medical history, vascular risk factors, National Institutes of Health Stroke Scale (NIHSS) scores at admission, pre-admission modified Rankin Scale (mRS) scores, results of computed tomography angiography or digital subtraction angiography, pre-treatment magnetic resonance imaging, and information regarding whether intravenous thrombolysis was administered before MT and post-MT recanalization rates were obtained from medical institution databases for analysis. Stroke etiology was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria (Adams et al., 1993). Blood samples were collected at admission and 24 h after MT to measure Hb and RDW levels. The HRR was calculated as the ratio of Hb to RDW. CT scans were conducted 24 h after MT to evaluate symptomatic intracranial hemorrhage (sICH). Follow-up data on current mRS scores were obtained through standardized telephone interviews from symptom onset to 3 months later.
Successful reperfusion was defined as the modified Thrombolysis in Cerebral Infarction (mTICI) ≥ 2b (Mokin et al., 2014; Kaesmacher et al., 2018). sICH was defined as intracranial hemorrhage with an increase of at least four points on the NIHSS scale (Hacke et al., 1998). The modified Rankin Scale (mRS) was used to assess the primary outcome at 3 months; good prognosis was defined as mRS ≤ 2, while poor prognosis was defined as mRS > 2 (Hill et al., 2020; Rebchuk et al., 2020). The secondary outcome was death within 3 months of MT. Death was defined as an all-cause passing resulting from a stroke.
All data were analyzed using SPSS version 22.0 (SPSS Inc., Chicago, IL, United States), GraphPad Prism version 8.0 (GraphPad Software, San Diego, California, United States), and MedCalc version 22.0 (MedCalc Software, Ostend, Belgium). The Kolmogorov–Smirnov test assessed the distributional normality. Categorical variables were presented as counts and percentages, and compared using the chi-squared test, while continuous variables were presented as mean ± standard deviation or median with interquartile range (IQR). Multivariate logistic regression was used to examine the impact of HRR on outcomes, adjusted for variables selected through the forward selection method, calculating odds ratios (ORs), and 95% confidence intervals (CIs). The Pearson correlation test was utilized to analyze the correlation between baseline laboratory data and data collected after 24 h. The discrimination of Hb, RDW, and HRR for outcomes was analyzed using the area under the receiver operating characteristic curve (AUC-ROC). Cut-off values for each biomarker were determined by Youden index. The generalized linear model was obtained by binary logistic regression analysis combining different parameters. Prediction probability was calculated from the regression equation as an additional parameter, which was further evaluated by ROC analysis. p < 0.05 was regarded as statistically significant.
The study included a total of 310 patients who underwent MT (Figure 1). The detailed characteristics of the patients are shown in Table 1. The median age of the patients was 72 (60–79) years, with 139 (44.8%) being female. The median NIHSS score at baseline was 15 (12–20). Prior to MT, 86 patients (27.7%) received intravenous thrombolysis. Successful reperfusion (mTICI ≥ 2b) was achieved in 82.3% of cases following MT. After MT, sICH was observed in 39 patients (12.6%). At 3 months, 216 patients (69.7%) had a poor prognosis, and 92 patients (29.6%) died. In addition, significant statistical differences were found between patients with good and poor prognosis concerning age, sex, atrial fibrillation, baseline NIHSS score at admission, NIHSS score after 24 h, sICH, and successful reperfusion (p < 0.05). Those who died within 3 months were more likely to be female, had older age, a higher NIHSS score at admission and after 24 h, a higher incidence of sICH, and a lower success rate of reperfusion (p < 0.05).

Table 1. Basic characteristics of study population according to 3-month prognosis and occurrence of death.
At admission, no differences in Hb and RDW levels were observed between patients with good prognosis and those without. However, patients with poor prognosis had lower HRR levels compared to those with a good prognosis [10.22 (8.97–12.05) vs. 11.18 (10.09–12.06); p = 0.016; Table 2; Figure 2A]. Nevertheless, multivariate analysis indicated that HRR at admission showed no correlation with the prognosis after 3 months in AIS patients undergoing MT (OR = 1.045; 95% CI: 0.886–1.232; p = 0.605; Table 3).
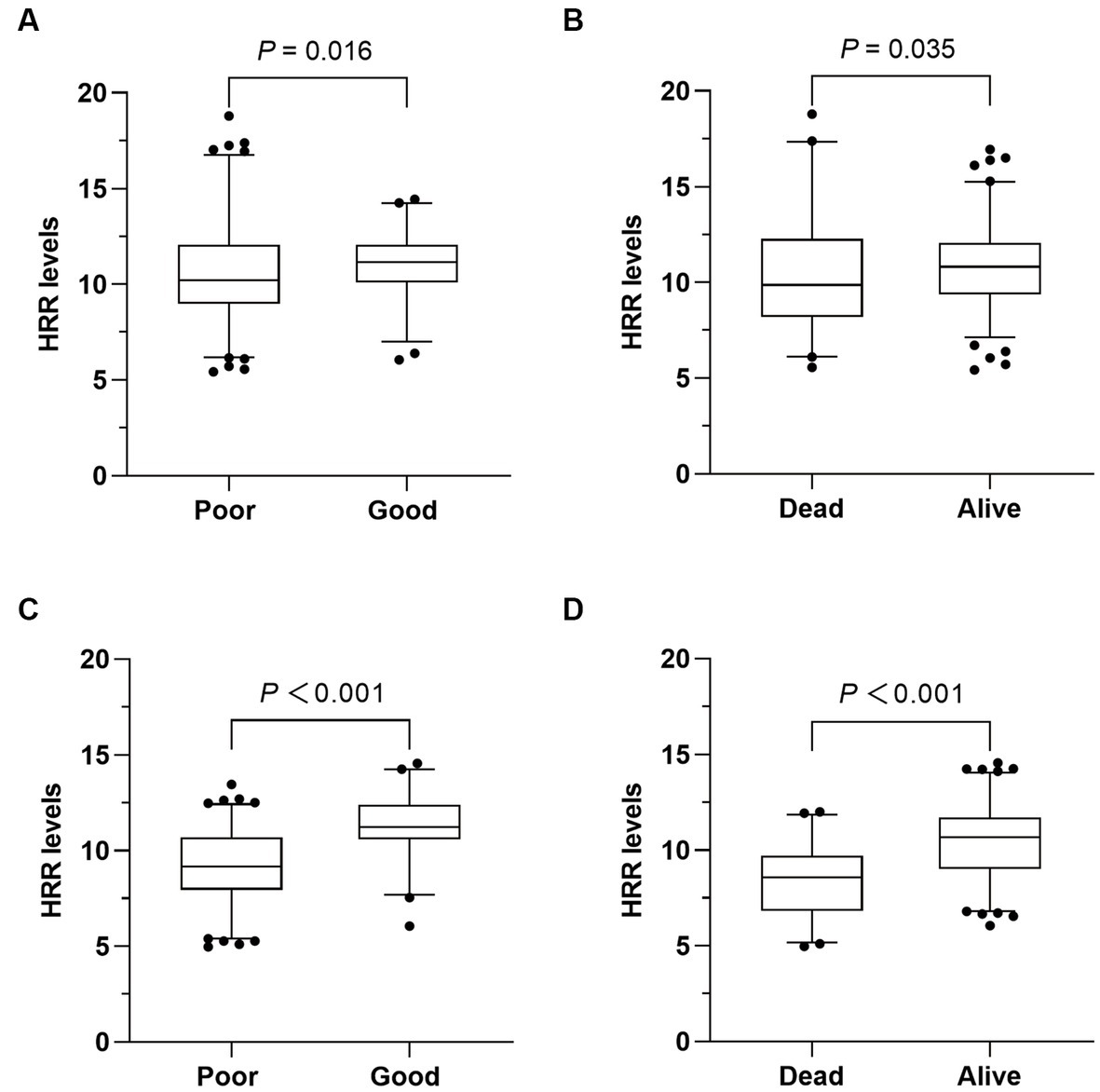
Figure 2. Comparison of HRR levels between different outcomes. (A,B) Comparison of hemoglobin to red cell distribution width ratio (HRR) at admission according to different outcomes. (C,D) Comparison of HRR after 24 h according to different outcomes.
After 24 h of MT, a significant difference in RDW was observed [13 (10.9–14.9) vs. 10.8 (9.0–12.9); p < 0.001; Table 2], while there was no difference in Hb levels between patients with good and poor prognosis. And HRR levels were significantly lower in patients with poor prognosis [9.18 (7.95–10.70) vs. 11.24 (10.60–12.40); p < 0.001; Table 2; Figure 2C]. In multivariate logistic regression, lower HRR was independently associated with a higher risk of poor prognosis (OR = 0.646; 95% CI: 0.520–0.803; p < 0.001; Table 3). Furthermore, the multivariate analysis revealed that older age, higher NIHSS score after 24 h, and lower successful reperfusion rate were risk factors for poor prognosis (Supplementary Table 1).
In addition, we observed strong and statistically significant positive correlations between Hb at admission and after 24 h (r = 0.983, p < 0.001), between RDW at admission and after 24 h (r = 0.703, p < 0.001), as well as a moderate and positive correlation between HRR at admission and after 24 h (r = 0.507, p < 0.001).
Similarly, the HRR levels exhibited a significant difference between dead and alive patients at admission [9.87 (8.21–12.27) vs. 10.82 (9.37–12.04); p = 0.035; Table 2; Figure 2B], as well as after 24 h [8.57 (6.83–9.70) vs. 10.67 (9.01–11.71); p < 0.001; Table 2; Figure 2D]. After adjusting for potential confounding variables, HRR after 24 h was found to be an independent predictor of death (OR 0.615; 95% CI 0.508–0.744; p < 0.001; Table 3). However, HRR at admission was not associated with death (OR = 1.022; 95% CI: 0.905–1.154; p = 0.728; Table 3). Other risk factors for death were showed in Supplementary Table 2.
Receiver operating characteristic analysis showed that HRR after 24 h was more discriminative of clinical outcome (poor prognosis and death) than HRR at admission (Supplementary Figure 1). Given that the results showed significant differences in HRR at 24 h among patients with poor prognosis, as well as the association between Hb, RDW, and HRR, we used ROC curves to evaluate their roles as prognostic markers (Figure 3A). Among all patients, RDW had good diagnostic accuracy in predicting poor prognosis, with an AUC of 0.697 (95% CI: 0.642–0.747, p < 0.001), sensitivity of 66.67%, and specificity of 63.83%. Notably, as a composite predictor calculated from Hb and RDW, HRR demonstrated even stronger discriminative ability, with an AUC of 0.790 (95% CI: 0.741–0.834, p < 0.001), sensitivity of 73.15%, and specificity of 76.60%. Furthermore, when combined with HRR, the AUC value of current NIHSS score for predicting poor prognosis increased from 0.859 (95% CI: 0.815–0.896) to 0.885 (95% CI: 0.845–0.919) (p = 0.025), with a sensitivity of 86.57% and specificity of 77.66%.
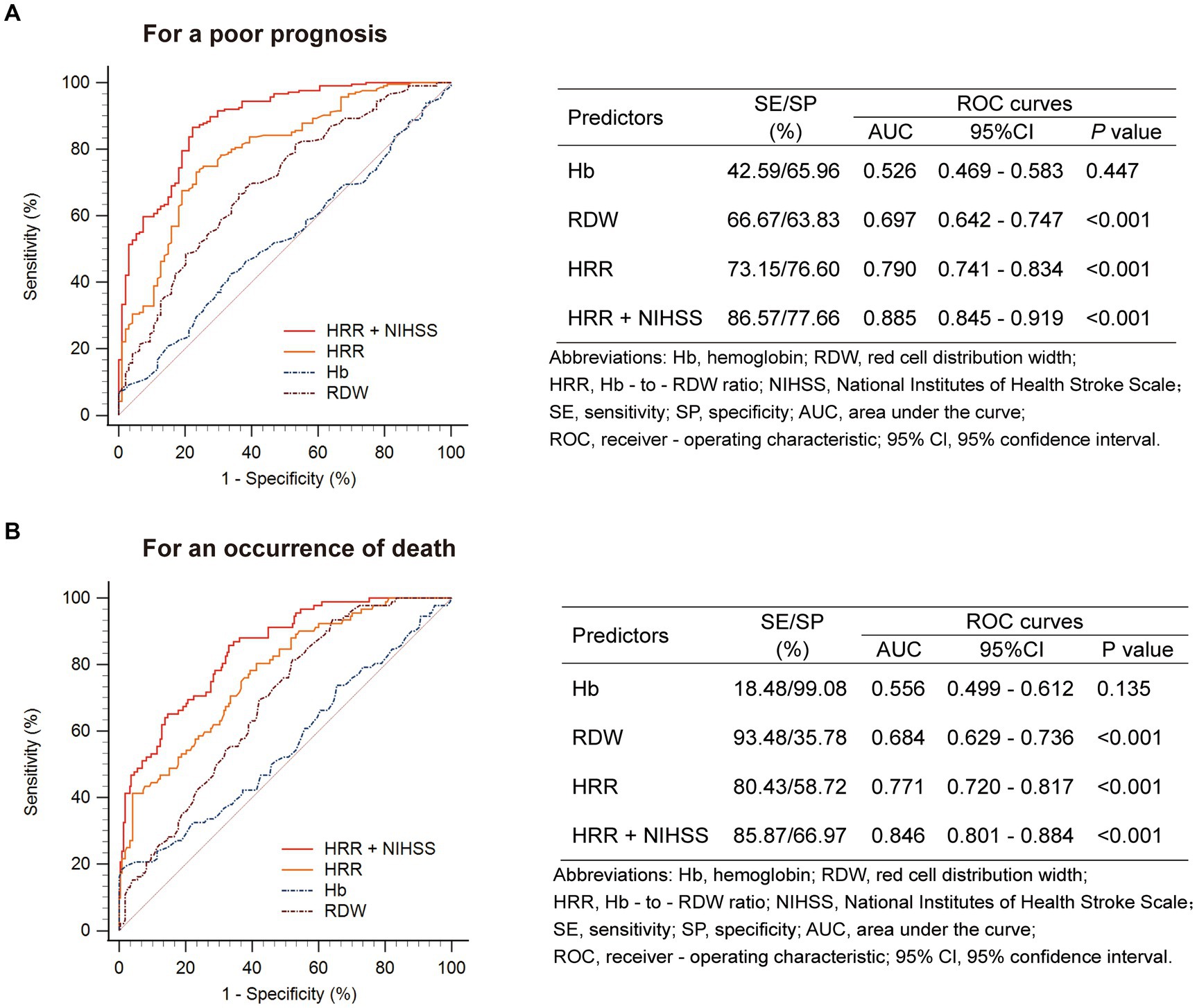
Figure 3. Prognostic accuracies of HRR as a predictor of (A) a poor prognosis or (B) an occurrence of death at 3 months after stroke.
Similar results were found when evaluating the predictive value of HRR for death (Figure 3B).
The purpose of this study was to investigate the association between the novel biomarker HRR and the outcomes of AIS patients who underwent MT. Our findings suggested that HRR at admission and after 24 h may be significantly lower in patients with poor prognosis and death. However, after adjusting for cerebrovascular risk factors, HRR after 24 h remained an independent risk factor for both poor prognosis and death, regardless of whether intravenous thrombolysis was performed prior to MT.
Anemia is a prevalent condition among AIS patients, and its pathogenesis involves various mechanisms such as reduced oxygen-carrying capacity, inflammation, energy imbalance, and hypercoagulation (Chang et al., 2020). Decreased levels of Hb imply a compromised oxygen-carrying capacity, leading to limited oxygen supply and an energy imbalance in the ischemic penumbra (Kimberly et al., 2013; Desai et al., 2023). Moreover, anemia can trigger the release of inflammatory factors like tumor necrosis factor-α (Feret et al., 2022). Recently, the role of inflammatory reactions in the progression of AIS and its association with poor prognosis has been widely recognized (Kehrel and Fender, 2016; Zhao et al., 2017; Shi et al., 2019; Esposito et al., 2022). Studies have indicated that overall poor nutritional status and weakened immune response may contribute to a worse prognosis (Bullock et al., 2020). Furthermore, anemic patients may experience hypercoagulability, further increasing the risk of ischemic events. Evidence suggests that severe anemia substantially raises the likelihood of stent thrombosis (Pilgrim et al., 2012), and lower Hb level was a strong predictor of post-PCI in-stent restenosis (Hu et al., 2021). Several studies have highlighted baseline Hb as a significant predictor of mortality and ischemic events in PCI (Reinecke, 2003; Dutsch et al., 2022). Additionally, a U-shaped association has been found between Hb levels and poor prognosis, all-cause mortality, and stroke severity in ischemic stroke (Chang et al., 2020; Zhang et al., 2021). Another study reported that lower Hb levels and variability were linked to mortality at 3 months in AIS patients undergoing MT (Nisar et al., 2021). In our investigation, AIS patients undergoing MT with poor prognosis and death had lower Hb levels compared to those with good prognosis and survival, although the difference was not statistically significant.
The value of RDW in cardiovascular disease reflects various mechanisms in the pathophysiological process. Firstly, an increased RDW indicates an underlying inflammatory state and impaired maturation of red blood cells (Ridker et al., 2001). RDW has been associated with inflammatory markers such as C-reactive protein with high sensitivity, interleukin-6, and fibrinogen levels (Förhécz et al., 2009). These inflammatory factors can disrupt red blood cell maturation by affecting the homeostasis of red blood cells, impairing iron metabolism, and inhibiting erythropoietin production (Rechavi and Rivella, 2008; Salvagno et al., 2015). Secondly, oxidative stress and microcirculatory damage play a significant role. Red blood cells possess substantial antioxidant capacity, and oxidative damage can lead to decreased cell survival rates (Emans et al., 2011; Lippi and Plebani, 2014). Changes in the morphology of red blood cells are associated with oxidative stress (Friedman et al., 2004). In addition, reduced red blood cell deformability can impede blood flow through the microcirculation, exacerbating ischemic conditions (Patel et al., 2013). Several studies have independently linked higher baseline RDW levels to mortality and adverse cardiovascular events in myocardial infarction patients undergoing PCI (Fatemi et al., 2013; Bujak et al., 2015; Zhao et al., 2015; Xiao et al., 2022). Research has also demonstrated that RDW may serve as a significant prognostic factor in AIS (Feng et al., 2017; Mohindra et al., 2020). Baseline RDW has been proposed as a prospective marker of mortality in AIS patients undergoing intravenous thrombolysis (Pinho et al., 2018; Ye et al., 2020). Similarly, in our study, AIS patients with poor prognosis and mortality tended to have elevated RDW levels 24 h after MT.
Although Hb and RDW have shown prognostic value in AIS patients, these two parameters can be influenced by various factors as mentioned above. Since HRR is calculated from Hb and RDW, it may provide a more effective and stable assessment compared to individual Hb or RDW measurements, objectively reflecting inflammatory and microcirculatory status, thus potentially serving as a superior biomarker. HRR has recently emerged as a crucial indicator for predicting cardiovascular disease mortality and prognosis (Rahamim et al., 2022). Lower HRR is associated with a higher risk of frailty and adverse outcomes in hospitalized older patients with coronary heart disease (Qu et al., 2021). Moreover, HRR may serve as a reliable indicator of mortality risk in patients with coronary artery disease after PCI (Sun et al., 2022; Xiu et al., 2022). Lower HRR levels have been found to increase the risk of death from all causes in AIS patients with atrial fibrillation (Qin et al., 2022). However, no studies have investigated the predictive significance of HRR for clinical outcomes in AIS patients undergoing MT. By excluding the influence of cardiovascular diseases, malignancies, infectious diseases, serious liver and kidney dysfunction, and adjusting for multivariate confounders, our study suggests that lower HRR significantly increases the risk of poor prognosis and death in AIS patients undergoing MT. Additionally, HRR demonstrates superior discriminative accuracy compared to the single parameter RDW. Globally, the NIHSS score is recognized as a valuable prognostic indicator for AIS, and incorporating HRR into the NIHSS enhances its predictive value.
While our study investigated the association between HRR and outcomes in AIS patients undergoing MT, we acknowledge certain limitations. Firstly, we only collected HRR data at admission and after 24 h of MT, precluding analysis of HRR changes throughout the entire stroke process. Secondly, there was a loss to follow-up of 21 patients, potentially introducing bias into our results. Thirdly, this was a retrospective and single-center design study, raising the possibility of selection bias and limiting the generalizability of our findings. It is important to consider these limitations when interpreting the results, and future research should address these issues to provide more robust evidence on the relationship between HRR and outcomes in AIS patients undergoing MT.
Our study indicates that HRR levels after 24 h of MT, as a simple, novel, cost-effective, and valuable biomarker, are an independent predictor of poor prognosis and death for AIS patients undergoing MT. However, further research is necessary to elucidate the underlying biological mechanisms and confirm the clinical utility of HRR.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethics committee of Chengdu Second People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XF: Methodology, Data curation, Investigation, Writing – original draft, Funding acquisition. YZ: Investigation, Formal Analysis, Writing – review & editing. QL: Formal Analysis, Writing – review & editing, Software, Validation. BW: Writing – review & editing, Validation, Formal Analysis, Software. JS: Validation, Writing – review & editing, Project administration, Conceptualization, Methodology, Resources.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the comprehensive demonstration study on Chronic Disease Prevention and Control Technology in Southwest China (grant no. 2018YFC1311400).
The patients and their families are all sincerely appreciated for their cooperation, and we would like to thank the nurses for their contributions and assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1259668/full#supplementary-material
Adams, H. P., Bendixen, B. H., Kappelle, L. J., Biller, J., Love, B. B., Gordon, D. L., et al. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 24, 35–41. doi: 10.1161/01.STR.24.1.35
Bujak, K., Wasilewski, J., Osadnik, T., Jonczyk, S., Kołodziejska, A., Gierlotka, M., et al. (2015). The prognostic role of red blood cell distribution width in coronary artery disease: a review of the pathophysiology. Dis. Markers 2015, 1–12. doi: 10.1155/2015/824624
Bullock, A. F., Greenley, S. L., McKenzie, G. A. G., Paton, L. W., and Johnson, M. J. (2020). Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: systematic review, narrative synthesis and meta-analysis. Eur. J. Clin. Nutr. 74, 1519–1535. doi: 10.1038/s41430-020-0629-0
Chang, J. Y., Lee, J. S., Kim, B. J., Kim, J.-T., Lee, J., Cha, J. K., et al. (2020). Influence of hemoglobin concentration on stroke recurrence and composite vascular events. Stroke 51, 1309–1312. doi: 10.1161/STROKEAHA.119.028058
Desai, A., Oh, D., Rao, E. M., Sahoo, S., Mahajan, U. V., Labak, C. M., et al. (2023). Impact of anemia on acute ischemic stroke outcomes: a systematic review of the literature. PLoS One 18:e0280025. doi: 10.1371/journal.pone.0280025
Dutsch, A., Graesser, C., Voll, F., Novacek, S., Eggerstedt, R., Armbruster, N. L., et al. (2022). Association of in-Hospital Hemoglobin Drop with Decreased Myocardial Salvage and Increased Long-Term Mortality in patients with acute ST-segment-elevation myocardial infarction. J. Am. Heart Assoc. 11:e024857. doi: 10.1161/JAHA.121.024857
Emans, M. E., van der Putten, K., van Rooijen, K. L., Kraaijenhagen, R. J., Swinkels, D., van Solinge, W. W., et al. (2011). Determinants of red cell distribution width (RDW) in Cardiorenal patients: RDW is not related to erythropoietin resistance. J. Card. Fail. 17, 626–633. doi: 10.1016/j.cardfail.2011.04.009
Esposito, E., Zhang, F., Park, J.-H., Mandeville, E. T., Li, W., Cuartero, M. I., et al. (2022). Diurnal differences in immune response in brain, blood and spleen after focal cerebral ischemia in mice. Stroke 53, e507–e511. doi: 10.1161/STROKEAHA.122.040547
Fatemi, O., Paranilam, J., Rainow, A., Kennedy, K., Choi, J., Cutlip, D., et al. (2013). Red cell distribution width is a predictor of mortality in patients undergoing percutaneous coronary intervention. J. Thromb. Thrombolysis 35, 57–64. doi: 10.1007/s11239-012-0767-x
Feng, G.-H., Li, H.-P., Li, Q.-L., Fu, Y., and Huang, R.-B. (2017). Red blood cell distribution width and ischaemic stroke. Stroke Vasc. Neurol. 2, 172–175. doi: 10.1136/svn-2017-000071
Feret, W., Safranow, K., Kwiatkowska, E., Daniel, A., and Ciechanowski, K. (2022). Malnutrition and erythropoietin resistance among patients with end-stage kidney disease: where is the perpetrator of disaster? Nutrients 14:5318. doi: 10.3390/nu14245318
Förhécz, Z., Gombos, T., Borgulya, G., Pozsonyi, Z., Prohászka, Z., and Jánoskuti, L. (2009). Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am. Heart J. 158, 659–666. doi: 10.1016/j.ahj.2009.07.024
Friedman, J. S., Lopez, M. F., Fleming, M. D., Rivera, A., Martin, F. M., Welsh, M. L., et al. (2004). SOD2-deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood 104, 2565–2573. doi: 10.1182/blood-2003-11-3858
Greenberg, S. M., Ziai, W. C., Cordonnier, C., Dowlatshahi, D., Francis, B., Goldstein, J. N., et al. (2022). 2022 guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke 53, e282–e361. doi: 10.1161/STR.0000000000000407
Hacke, W., Kaste, M., Fieschi, C., von Kummer, R., Davalos, A., Meier, D., et al. (1998). Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 352, 1245–1251. doi: 10.1016/S0140-6736(98)08020-9
Hill, M. D., Goyal, M., Menon, B. K., Nogueira, R. G., McTaggart, R. A., Demchuk, A. M., et al. (2020). Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet 395, 878–887. doi: 10.1016/S0140-6736(20)30258-0
Hu, H., Wang, S., Tang, G., Zhai, C., and Shen, L. (2021). Impact of anemia on in-stent restenosis after percutaneous coronary intervention. BMC Cardiovasc. Disord. 21:548. doi: 10.1186/s12872-021-02355-1
Kaesmacher, J., Dobrocky, T., Heldner, M. R., Bellwald, S., Mosimann, P. J., Mordasini, P., et al. (2018). Systematic review and meta-analysis on outcome differences among patients with TICI2b versus TICI3 reperfusions: success revisited. J. Neurol. Neurosurg. Psychiatry 89, 910–917. doi: 10.1136/jnnp-2017-317602
Kawabata, H. (2020). The pathogenesis of anemia in inflammation. Rinsho Ketsueki 61, 1105–1111. doi: 10.11406/rinketsu.61.1105
Kehrel, B. E., and Fender, A. C. (2016). Resolving Thromboinflammation in the brain after ischemic stroke? Circulation 133, 2128–2131. doi: 10.1161/CIRCULATIONAHA.116.022858
Kimberly, W. T., Dutra, B. G., Boers, A. M. M., Alves, H. C. B. R., Berkhemer, O. A., van den Berg, L., et al. (2018). Association of Reperfusion with Brain Edema in patients with acute ischemic stroke: a secondary analysis of the MR CLEAN trial. JAMA Neurol. 75, 453–461. doi: 10.1001/jamaneurol.2017.5162
Kimberly, W. T., Lima, F. O., O’Connor, S., and Furie, K. L. (2013). Sex differences and hemoglobin levels in relation to stroke outcomes. Neurology 80, 719–724. doi: 10.1212/WNL.0b013e31828250ff
Krishnamurthi, R. V., Ikeda, T., and Feigin, V. L. (2020). Global, regional and country-specific burden of Ischaemic stroke, intracerebral Haemorrhage and subarachnoid Haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology 54, 171–179. doi: 10.1159/000506396
Kwok, C. S., Tiong, D., Pradhan, A., Andreou, A. Y., Nolan, J., Bertrand, O. F., et al. (2016). Meta-analysis of the prognostic impact of Anemia in patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 118, 610–620. doi: 10.1016/j.amjcard.2016.05.059
Lippi, G., and Plebani, M. (2014). Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin. Chem. Lab. Med. 52, 1247–1249. doi: 10.1515/cclm-2014-0585
Luby, M., Hsia, A. W., Lomahan, C. A., Davis, R., Burton, S., Kim, Y., et al. (2023). Post-ischemic hyperemia following endovascular therapy for acute stroke is associated with lesion growth. J. Cereb. Blood Flow Metab. 43, 856–868. doi: 10.1177/0271678X231155222
Mohindra, R., Mishra, U., Mathew, R., and Negi, N. S. (2020). Red cell distribution width (RDW) index as a predictor of severity of acute ischemic stroke: a correlation study. Adv. J. Emerg. Med. 4:e24. doi: 10.22114/ajem.v0i0.257
Mokin, M., Khalessi, A. A., Mocco, J., Lanzino, G., Dumont, T. M., Hanel, R. A., et al. (2014). Endovascular treatment of acute ischemic stroke: the end or just the beginning? Neurosurg. Focus. 36:E5. doi: 10.3171/2013.10.FOCUS13374
Nisar, T., Shapouran, S., Abu-Hadid, O., Shaulov, S., Tofade, T., Patel, J., et al. (2021). Association of anemia with functional outcomes in patients with mechanical thrombectomy. Clin. Neurol. Neurosurg. 211:107028. doi: 10.1016/j.clineuro.2021.107028
Parizadeh, S. M., Jafarzadeh-Esfehani, R., Bahreyni, A., Ghandehari, M., Shafiee, M., Rahmani, F., et al. (2019). The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors 45, 507–516. doi: 10.1002/biof.1518
Patel, K. V., Mohanty, J. G., Kanapuru, B., Hesdorffer, C., Ershler, W. B., and Rifkind, J. M. (2013). Association of the red cell distribution width with red blood cell deformability. Adv. Exp. Med. Biol. 765, 211–216. doi: 10.1007/978-1-4614-4989-8_29
Pilgrim, T., Vetterli, F., Kalesan, B., Stefanini, G. G., Räber, L., Stortecky, S., et al. (2012). The impact of Anemia on long-term clinical outcome in patients undergoing revascularization with the unrestricted use of drug-eluting stents. Circ. Cardiovasc. Interv. 5, 202–210. doi: 10.1161/CIRCINTERVENTIONS.111.965749
Pinho, J., Marques, S. A., Freitas, E., Araújo, J., Taveira, M., Alves, J. N., et al. (2018). Red cell distribution width as a predictor of 1-year survival in ischemic stroke patients treated with intravenous thrombolysis. Thromb. Res. 164, 4–8. doi: 10.1016/j.thromres.2018.02.002
Powers, W. J., Derdeyn, C. P., Biller, J., Coffey, C. S., Hoh, B. L., Jauch, E. C., et al. (2015). 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment. Stroke 46, 3020–3035. doi: 10.1161/STR.0000000000000074
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2018). 2018 guidelines for the early Management of Patients with Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49, e46–e110. doi: 10.1161/STR.0000000000000158
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American stroke. Stroke 50, e344–e418. doi: 10.1161/STR.0000000000000211
Qin, Z., Liao, N., Lu, X., Duan, X., Zhou, Q., and Ge, L. (2022). Relationship between the hemoglobin-to-red cell distribution width ratio and all-cause mortality in ischemic stroke patients with atrial fibrillation: an analysis from the MIMIC-IV database. Neuropsychiatr. Dis. Treat. 18, 341–354. doi: 10.2147/NDT.S350588
Qu, J., Zhou, T., Xue, M., Sun, H., Shen, Y., Chen, Y., et al. (2021). Correlation analysis of hemoglobin-to-red blood cell distribution width ratio and frailty in elderly patients with coronary heart disease. Front. Cardiovasc. Med. 8:728800. doi: 10.3389/fcvm.2021.728800
Rahamim, E., Zwas, D. R., Keren, A., Elbaz-Greener, G., Ibrahimli, M., Amir, O., et al. (2022). The ratio of hemoglobin to red cell distribution width: a strong predictor of clinical outcome in patients with heart failure. J. Clin. Med. 11:886. doi: 10.3390/jcm11030886
Rebchuk, A. D., O’Neill, Z. R., Szefer, E. K., Hill, M. D., and Field, T. S. (2020). Health utility weighting of the modified Rankin scale: a systematic review and Meta-analysis. JAMA Netw. Open 3:e203767. doi: 10.1001/jamanetworkopen.2020.3767
Rechavi, G., and Rivella, S. (2008). Regulation of Iron absorption in Hemoglobinopathies. Curr. Mol. Med. 8, 646–662. doi: 10.2174/156652408786241401
Reinecke, H. (2003). Haemoglobin-related mortality in patients undergoing percutaneous coronary interventions. Eur. Heart J. 24, 2142–2150. doi: 10.1016/j.ehj.2003.09.008
Ridker, P. M., Rifai, N., Clearfield, M., Downs, J. R., Weis, S. E., Miles, J. S., et al. (2001). Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 344, 1959–1965. doi: 10.1056/NEJM200106283442601
Saliba, W., Barnett-Griness, O., Elias, M., and Rennert, G. (2015). The association between red cell distribution width and stroke in patients with atrial fibrillation. Am. J. Med. 128, 192.e11–192.e18. doi: 10.1016/j.amjmed.2014.09.020
Salvagno, G. L., Sanchis-Gomar, F., Picanza, A., and Lippi, G. (2015). Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 52, 86–105. doi: 10.3109/10408363.2014.992064
Sarraj, A., Pujara, D. K., Churilov, L., Sitton, C. W., Ng, F., Hassan, A. E., et al. (2023). Mediation of successful reperfusion effect through infarct growth and cerebral edema: a pooled, patient-level analysis of EXTEND-IA trials and SELECT prospective cohort. Ann. Neurol. 93, 793–804. doi: 10.1002/ana.26587
Shi, K., Tian, D.-C., Li, Z.-G., Ducruet, A. F., Lawton, M. T., and Shi, F.-D. (2019). Global brain inflammation in stroke. Lancet Neurol. 18, 1058–1066. doi: 10.1016/S1474-4422(19)30078-X
Sun, X., Zhang, R., Fan, Z., Liu, Z., and Hua, Q. (2022). Predictive value of hemoglobin-to-red blood cell distribution width ratio for contrast-induced nephropathy after emergency percutaneous coronary intervention. Perfusion 2676591221119422:026765912211194. doi: 10.1177/02676591221119422
Westendorp, W. F., Dames, C., Nederkoorn, P. J., and Meisel, A. (2022). Immunodepression, infections, and functional outcome in ischemic stroke. Stroke 53, 1438–1448. doi: 10.1161/STROKEAHA.122.038867
Xiao, Q., Yan, D., Qin, J., Chen, W., Jiang, K., Zhao, J., et al. (2022). Dynamic changes in red cell distribution width can predict major adverse cardiovascular events after PCI in patients with unstable angina pectoris: a retrospective cohort study. Dis. Markers 2022:2735717. doi: 10.1155/2022/2735717
Xiu, W.-J., Zheng, Y.-Y., Wu, T.-T., Hou, X.-G., Yang, Y., Ma, Y.-T., et al. (2022). Hemoglobin-to-red-cell distribution width ratio is a novel predictor of long-term patient outcomes after percutaneous coronary intervention: a retrospective cohort study. Front. Cardiovasc. Med. 9:726025. doi: 10.3389/fcvm.2022.726025
Xue, J., Zhang, D., Zhang, X.-G., Zhu, X.-Q., Xu, X.-S., and Yue, Y.-H. (2022). Red cell distribution width is associated with stroke severity and unfavorable functional outcomes in ischemic stroke. Front. Neurol. 13:938515. doi: 10.3389/fneur.2022.938515
Ye, W.-Y., Li, J., Li, X., Yang, X.-Z., Weng, Y.-Y., Xiang, W.-W., et al. (2020). Predicting the one-year prognosis and mortality of patients with acute ischemic stroke using red blood cell distribution width before intravenous thrombolysis. Clin. Interv. Aging 15, 255–263. doi: 10.2147/CIA.S233701
Zhang, R., Xu, Q., Wang, A., Jiang, Y., Meng, X., Zhou, M., et al. (2021). Hemoglobin concentration and clinical outcomes after acute ischemic stroke or transient ischemic attack. J. Am. Heart Assoc. 10:e022547. doi: 10.1161/JAHA.121.022547
Zhao, N., Mi, L., Liu, X., Pan, S., Xu, J., Xia, D., et al. (2015). Combined value of red blood cell distribution width and global registry of acute coronary events risk score for predicting cardiovascular events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. PLoS One 10:e0140532. doi: 10.1371/journal.pone.0140532
Keywords: acute ischemic stroke, mechanical thrombectomy, hemoglobin to red cell distribution width ratio, poor prognosis, death, marker
Citation: Feng X, Zhang Y, Li Q, Wang B and Shen J (2023) Hemoglobin to red cell distribution width ratio as a prognostic marker for ischemic stroke after mechanical thrombectomy. Front. Aging Neurosci. 15:1259668. doi: 10.3389/fnagi.2023.1259668
Received: 31 July 2023; Accepted: 16 October 2023;
Published: 21 November 2023.
Edited by:
Stefano Tarantini, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Andreia Morais, Harvard Medical School, United StatesCopyright © 2023 Feng, Zhang, Li, Wang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Shen, MzcwMzc1NjA2QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.