- 1School of Nursing, Lanzhou University, Lanzhou, China
- 2School of Nursing, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Health cognitive promotion and protection is a critical topic. With the world’s aging population and rising life expectancy, there will be many people living with highly age-related dementia illnesses. Cardiovascular disease (CVD) and dementia share the same risk factors, such as unhealthy lifestyles and metabolic factors. These recognized risks associated with CVD and dementia frequently co-occur. CVD risk models may have a close association with dementia and cognitive decline. So, this systematic review aimed to determine whether CVD risk models were connected with dementia or cognitive decline and compare the predictive ability of various models.
Methods: PubMed, Web of Science, PsychINFO, Embase, Cochrane Library, CNKI, Sinomed, and WanFang were searched from 1 January 2014 until 16 February 2023. Only CVD risk models were included. We used the Newcastle-Ottawa scale (NOS) for the quality assessment of included cohort studies and the Agency for Healthcare Research and Quality (AHRQ) for cross-sectional studies. The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement’s guidelines were followed in this systematic study.
Results: In all, 9,718 references were screened, of which 22 articles were included. A total of 15 CVD risk models were summarized. Except for the Cardiovascular Health in Ambulatory Care Research Team (CANHEART) health index, the other 14 CVD risk models were associated with dementia and cognitive decline. In comparison, different CVD risk models and domain-specific cognitive function correlation variation depended on cohort characteristics, risk models, cognitive function tests, and study designs. Moreover, it needed to be clarified when comparing the predicting performance of different CVD risk models.
Conclusion: It is significant for public health to improve disease risk prediction and prevention and mitigate the potential adverse effects of the heart on the brain. More cohort studies are warranted to prove the correlation between CVD risk models and cognitive function. Moreover, further studies are encouraged to compare the efficacy of CVD risk models in predicting cognitive disorders.
1. Introduction
With the aging population and increasing life expectancy, people now have significantly higher chances of developing highly age-related dementia disorders (Livingston et al., 2020). Age-related dementia is caused by a complex combination of hereditary and environmental variables (Fayosse et al., 2020). In low- and middle-income nations, the number of people with dementia is increasing faster than in high-income countries, owing to a greater risk factor burden (Livingston et al., 2020). With less knowledge of the underlying pathology and no targeted treatments, modifiable factors have drawn particular attention for dementia prevention. According to the World Health Organization, health cognitive promotion and protection is a public health priority to achieve the goal of universal cognitive health coverage (WHO, 2020).
Increasing evidence demonstrates that heart and brain health are inextricably linked (Zhao et al., 2023). Patients diagnosed with cardiovascular disease (CVD) usually had poorer cognition than those without CVD (Covello et al., 2021). According to the most recent descriptions of novel pathological reasons, mixed dementia (Alzheimer’s disease and cerebrovascular injuries) is more common among the oldest seniors (those over 90 years old; Livingston et al., 2020). More and more population-based cohort studies have discovered that dementia and CVD may share the same risk factors. Modifiable health and lifestyle factors such as blood pressure (BP), total cholesterol (TC), blood glucose, physical activity, dietary habits, and smoking could improve vascular health and potentially reduce the risk of cognitive decline and dementia (Rasmussen et al., 2020). Cardiovascular risk factors may hasten cognitive decline by generating cerebral hypoperfusion, hypoxia, embolism, or infarcts, which result in vascular and degenerative brain lesions (Bancks et al., 2019; Lane et al., 2020). According to neuropathological research, dementia and CVD share pathogenic mechanisms linked to cardiovascular risk factors, and CVD may lower the threshold for cognitive impairment (Song et al., 2020).
According to the Lancet Commission on Dementia Prevention, 12 modifiable risk factors, namely, less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, low social contact, excessive alcohol consumption, traumatic brain injury, and air pollution, account for nearly 40% of all dementia cases globally, and this estimate highlights how relatively important potentially modifiable risk factors are in dementia (Livingston et al., 2020). Given that multiple risk factors overlap and may have an additive or synergistic effect (Lee et al., 2019), some risk models that treated incident dementia as the outcome were built, such as Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) risk algorithms (Kivipelto et al., 2006). These models, however, were mainly developed based on predictors that are challenging to obtain in primary prevention, such as apolipoprotein E ℇ4 (ApoE ℇ4), mean corpuscular volume, and peak expiratory flow (Exalto et al., 2014; You et al., 2022). In contrast, CVD risk models have been extensively constructed and applied. The Framingham risk models, which contain health parameters simple to measure, are the most frequently utilized in research and clinical settings (Peloso et al., 2020).
Surprisingly, relevant studies found that CVD risk models are associated with dementia and cognitive decline (Harrison et al., 2014). Using CVD risk models to predict dementia not only impedes the incidence of CVD but also favors putting off the progression of dementia. Harrison conducted a systematic review in 2014 to conclude the association between various CVD risk models and dementia or cognitive impairment (Harrison et al., 2014). In recent years, with the deepening of research, new CVD risk models have been constructed, such as the prediction for atherosclerotic cardiovascular disease (ASCVD) risk in China (China-PAR) (Yang et al., 2016) and Life’s Essential 8 (LE8) (Lloyd-Jones et al., 2022). Furthermore, some scholars have paid more attention to whether CVD risk models can predict dementia and which models have better forecast performance. So, it is necessary to find more evidence and update the results. This review has two objectives: (1) to comprehensively review the association between various CVD risk models and dementia or cognitive decline and (2) to compare the better risk models for predicting dementia and cognitive decline.
2. Methods
2.1. Search strategy
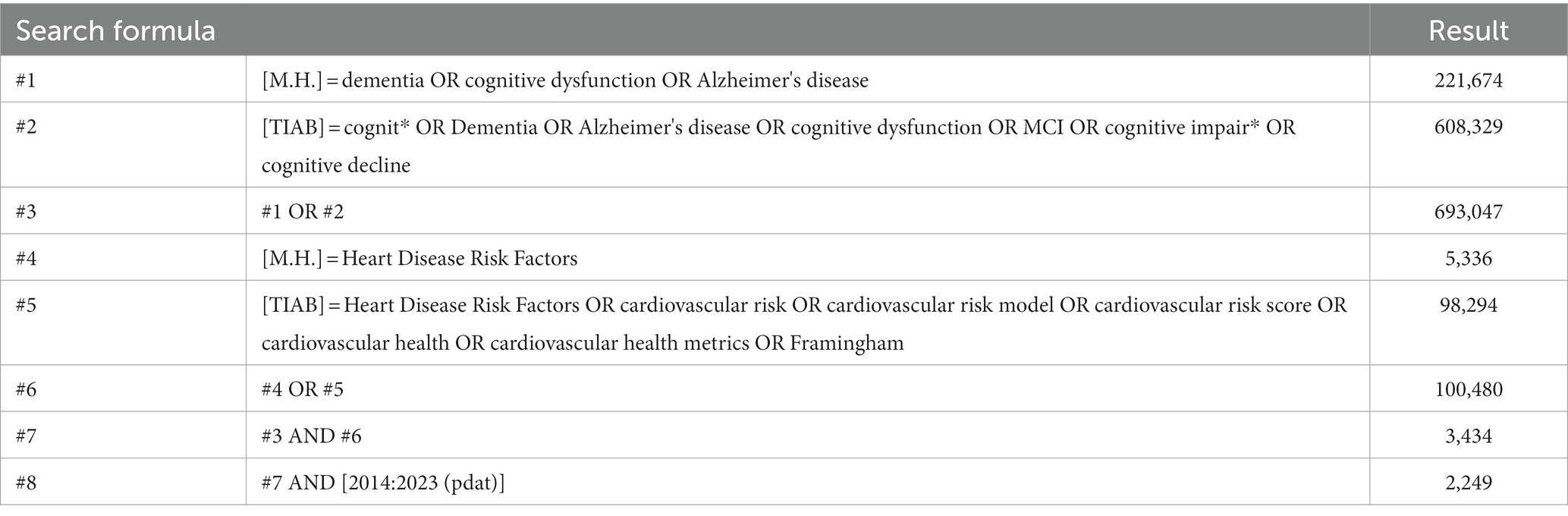
This systematic review followed the procedures of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (Page et al., 2021). The present study was registered in PROSPERO. Available at: https://www.crd.york.ac.uk/prospero/display_record.php? ID=CRD42023316549. Since Harrison reported related research in 2014 (Harrison et al., 2014), we have systematically reviewed articles from 1 January 2014 until 16 February 2023. PubMed, Web Of Science, PsychINFO, Embase, Cochrane Library, CNKI, Sinomed, and WanFang were searched. Combinations of the following terms were explored: “cognit*,” “dementia,” “Alzheimer’s disease,” “cognitive dysfunction,” “cognitive impair*,” “cognitive decline,” “heart disease risk factors,” “cardiovascular risk,” “cardiovascular risk model,” “cardiovascular risk score,” “cardiovascular health,” “cardiovascular health metrics,” and “Framingham”. The search strategy of PubMed is presented in Table 1.
2.2. Inclusion and exclusion criteria
The inclusion criteria for studies were the following: (1) assessed CVD risk models; (2) had access to specific information on cognitive test results (or how they changed); and (3) used a cross-sectional, longitudinal cohort study design. Articles were disqualified if they only examined a single risk factor or included dementia or cognitive impairment data in their risk models. Studies written in languages other than English or Chinese, those with incomplete abstracts, and those lacking full texts were also disqualified.
2.3. Data extraction and assessment for study quality
From each eligible study, we extracted data on (1) article information: authors, publication year, and country; (2) cohort information: name of the study, number of participants, percentage of women, mean age, and race/ethnicity; (3) study design: cross-sectional study, longitudinal cohort study (follow-up time); (4) CVD risk models: details of the risk model used and any modifications made; (5) primary outcome (dementia diagnoses, cognitive decline, or cognitive function); and (6) analytical strategy: details on models’ prognostic performance including odds ratio (OR), 95% confidence interval (95% CI), hazard ratio (HR), β coefficients, the area under the receiver operating characteristic curve (AUC), or C-statistic.
2.4. Risk of bias assessment
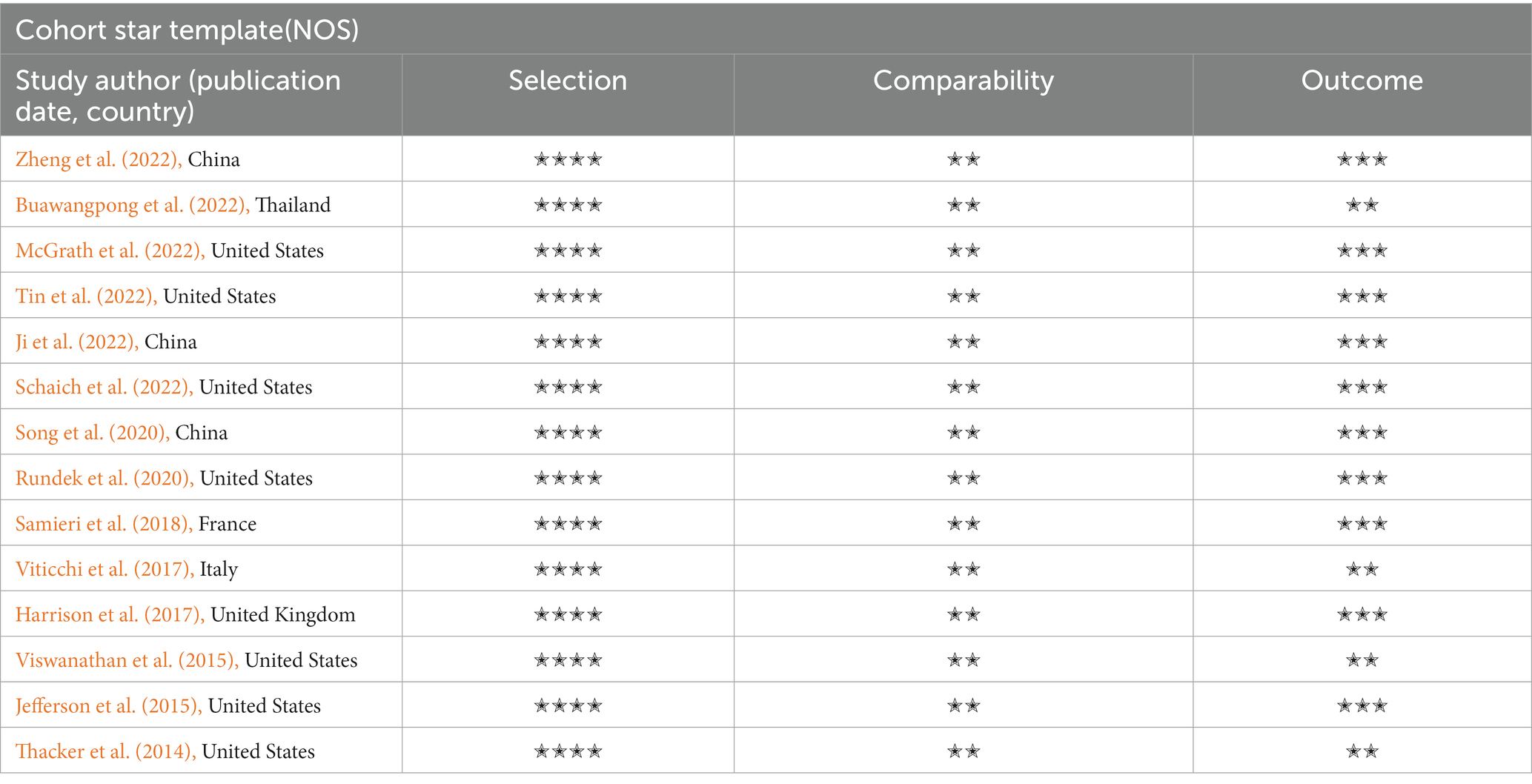
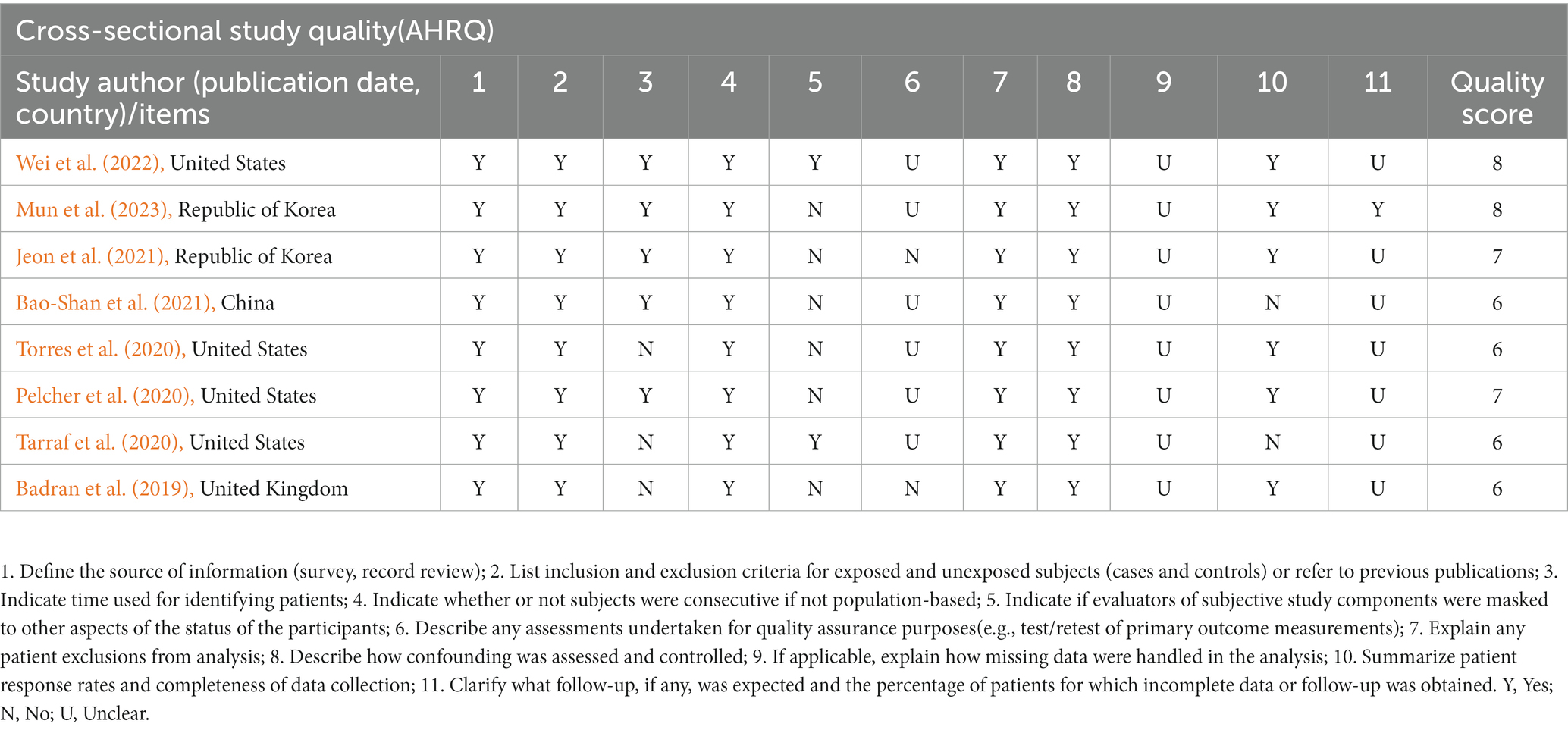
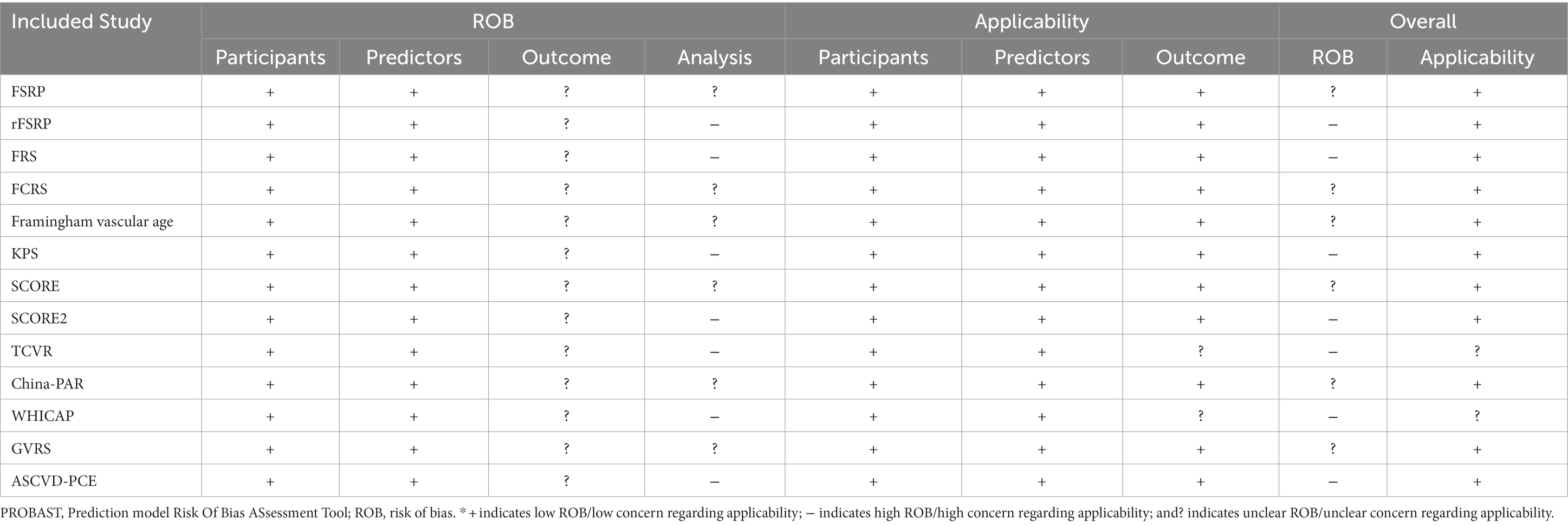
Cohort studies were evaluated for quality using the Newcastle-Ottawa scale (NOS) (Lo et al., 2014). In more detail, the scores of the related items in three domains–four items for the chosen domain, one item for the comparability domain, and three items for the resulting part–were added up to create a total score of the risk of bias. High-quality studies were those with a total score of six or higher. The Agency for Healthcare Research and Quality (AHRQ) provided an 11-item checklist for evaluating the methodological quality of the cross-sectional studies (Newman, 2000). If a question was answered “NO” or “UNCLEAR,” the item received a score of “0” and if the reply was “YES,” the item received a score of “1”. Low-quality articles were given a score of 0–3, moderate quality ones of 4–7, and high-quality ones of 8–11. The prediction model Risk Of Bias ASsessment Tool (PROBAST) includes 20 signaling questions across four domains: participants, predictors, outcome, and analysis (Moons et al., 2019). It is used to assess a prediction model’s risk of bias and applicability concerns. It can be downloaded from www.probast.org.
2.5. Data synthesis
Due to the variation among studies, including variations in the variables included in the CVD risk models, the small number of studies using the same models, and even variations in adjusted variables across studies using the same models, as well as differences in cognitive outcomes and variations in the reporting of effect sizes, synthesis with meta-analysis is not possible.
3. Results
3.1. Description of included studies
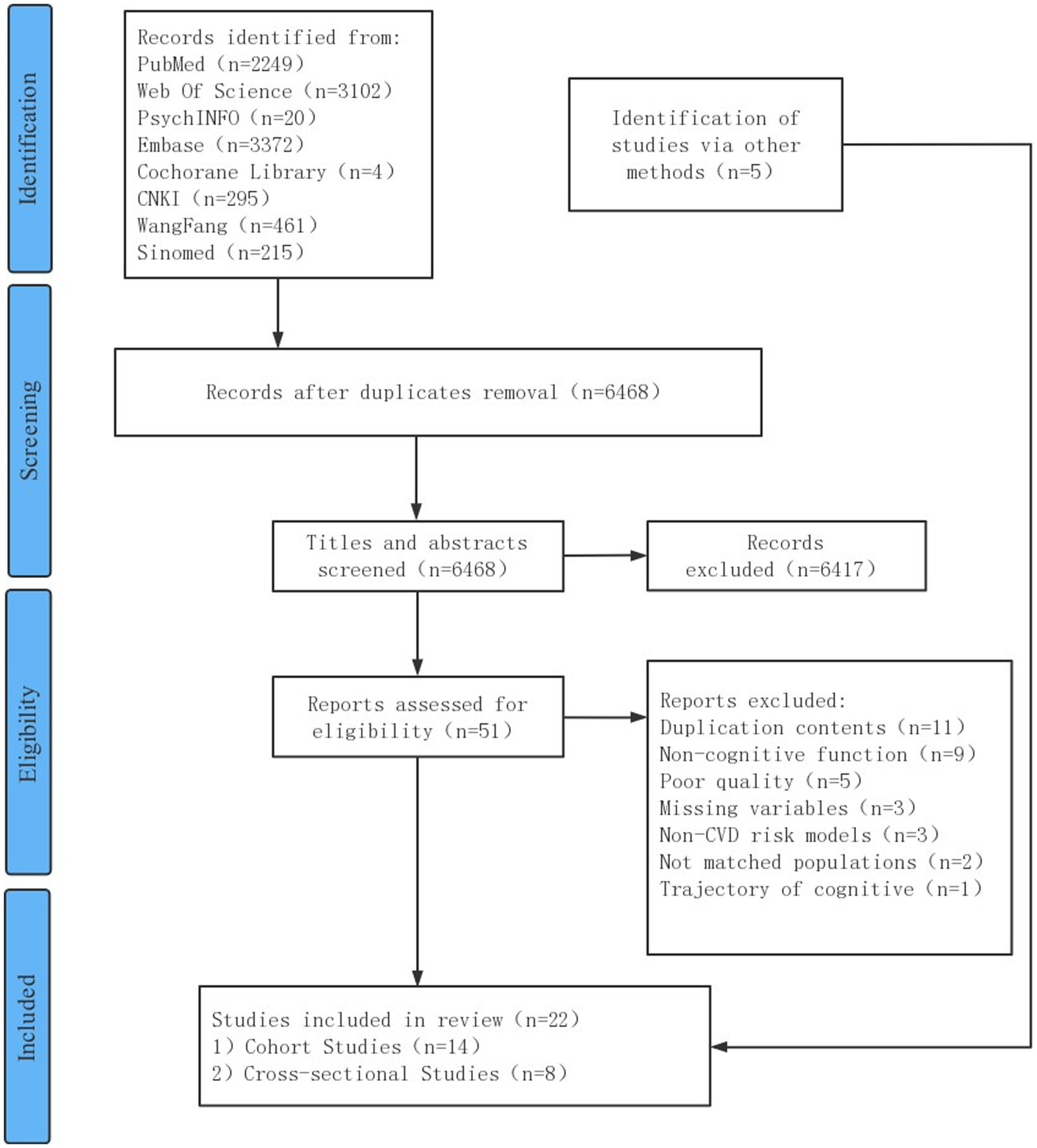
The search yielded a total of 9,718 articles. Of these, 3,250 were duplicates, and 6,417 were irrelevant after title and abstract reviews. A total of 51 articles underwent full-text screening and 34 were excluded due to unmet criteria. Other 5 extra studies were obtained from the references of the included articles and the citation retrieval of related systematic reviews, and 22 full-text articles, including 14 cohort studies and 8 cross-sectional studies, were included. Figure 1 summarizes the study selection process.
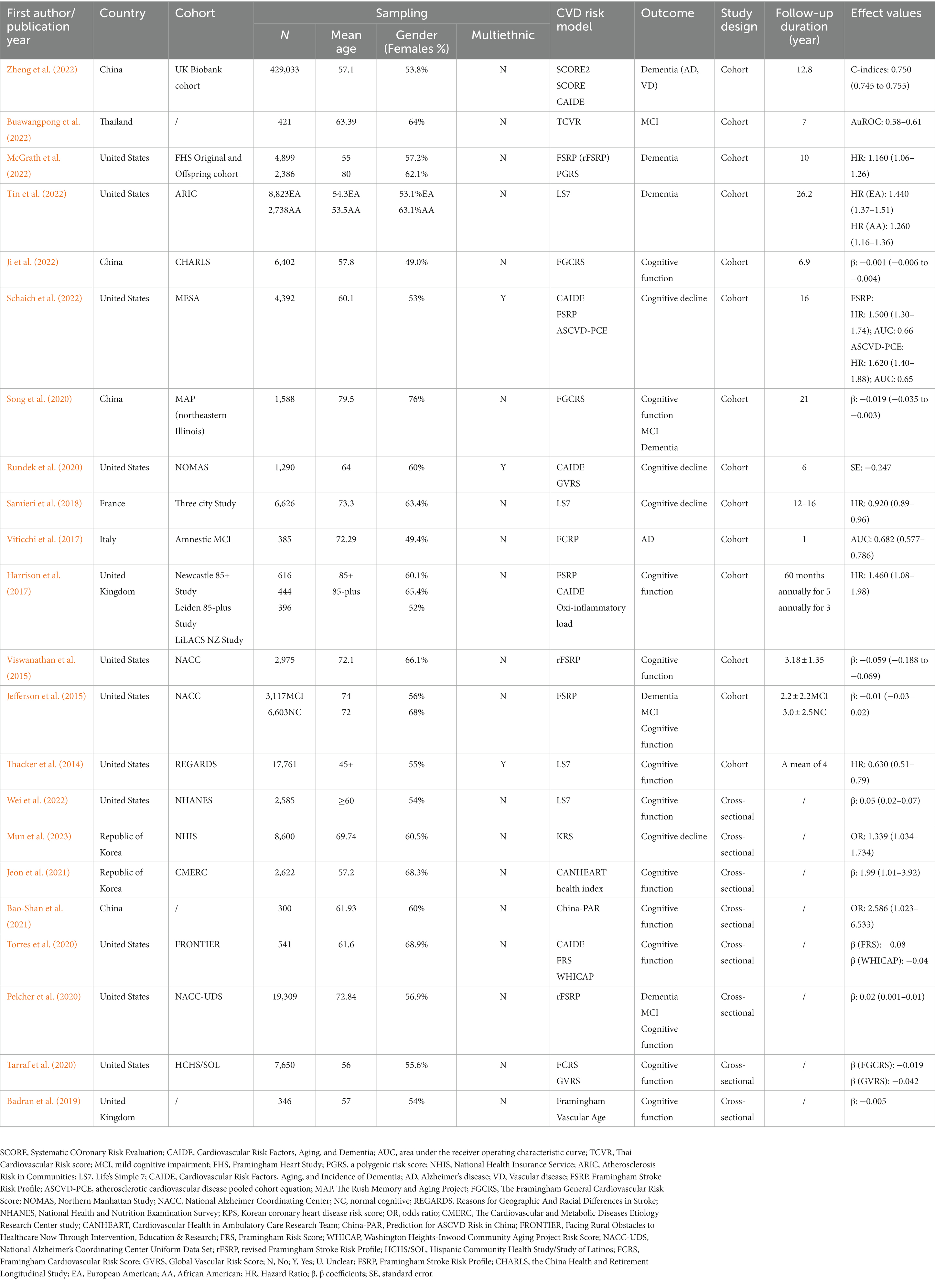
The included research was conducted in 10 different countries. The most significant articles came from the United States, China, Italy, France, Thailand, the Republic of Korea, and the United Kingdom. A total of 19 studies were derived from datasets based on data from a population-based cohort. Most studies used the National Alzheimer Coordinating Center (NACC) database (Jefferson et al., 2015; Viswanathan et al., 2015; Pelcher et al., 2020). Sample sizes ranged from 300 to 429,033 and were racially diverse as they included White people, Black people, Hispanic/Latino (H/L) people, and non-H/L people. The mean age at baseline ranged from 45 to 79.5 years. In cohort studies, the follow-up duration ranged from 1 to 26.2 years. Detailed information on the study cohort, sample traits, CVD risk models, cognitive function or dementia assessments, and model details are presented in Table 2.
3.2. Quality of the included studies
After the studies’ quality assessment, the 14 cohort studies were rated as good quality, and the 8 cross-sectional studies were rated moderate to high. The deduction items are concentrated in 6 and 9–11. The key concern is that several cardiovascular parameters (physical activity and diet) were dependent on self-reports. In addition, some studies substituted similar variables because existing cohorts did not contain the desired variables. For example, Washington Heights-Inwood Community Aging Project Risk Score (WHICAP) studies used Body Mass Index (BMI) instead of the waist-to-hip ratio (Torres et al., 2020). The quality assessment of the reviewed studies is shown in Tables 3, 4.
3.3. Quality of the included CVD risk models
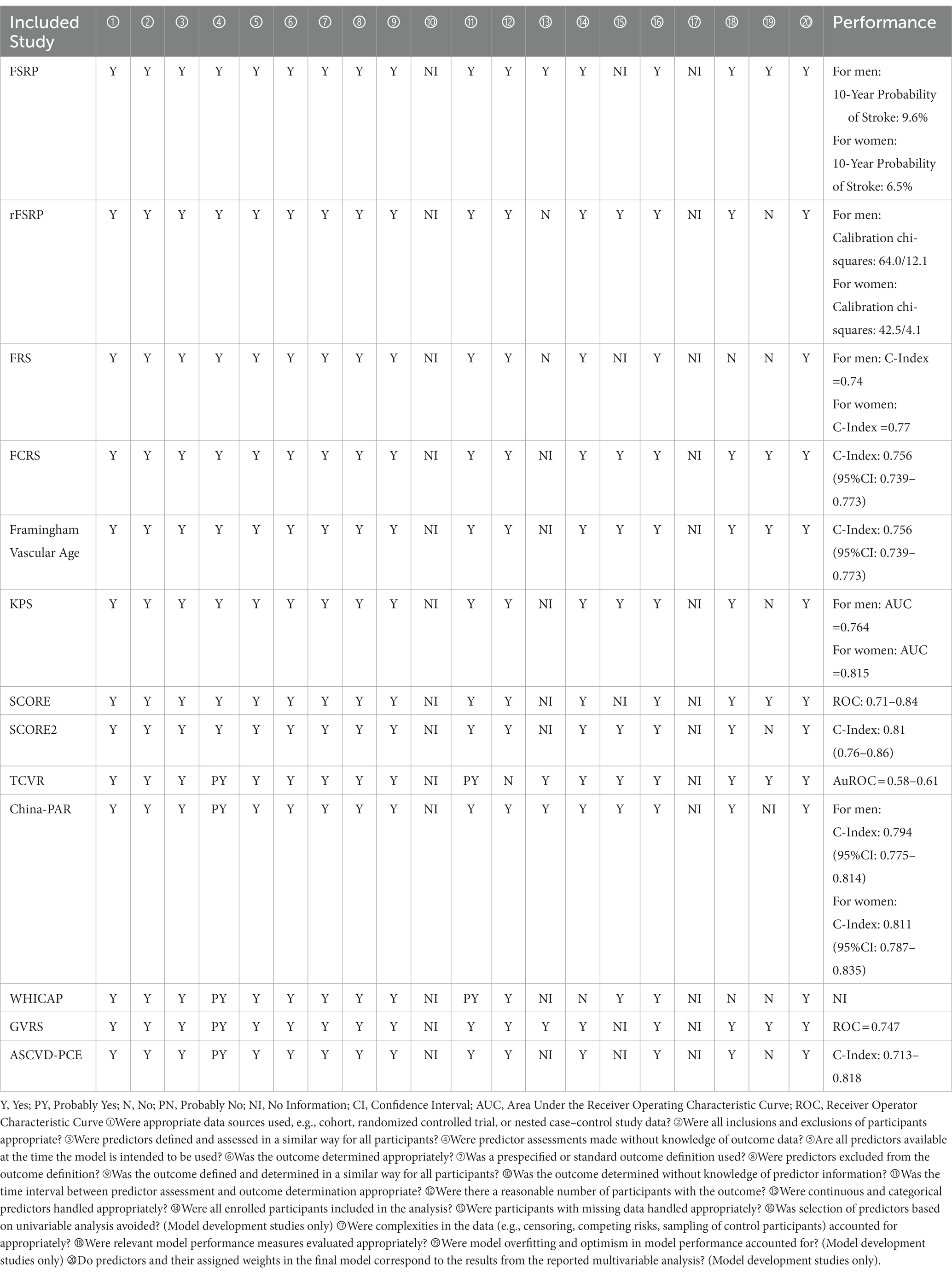
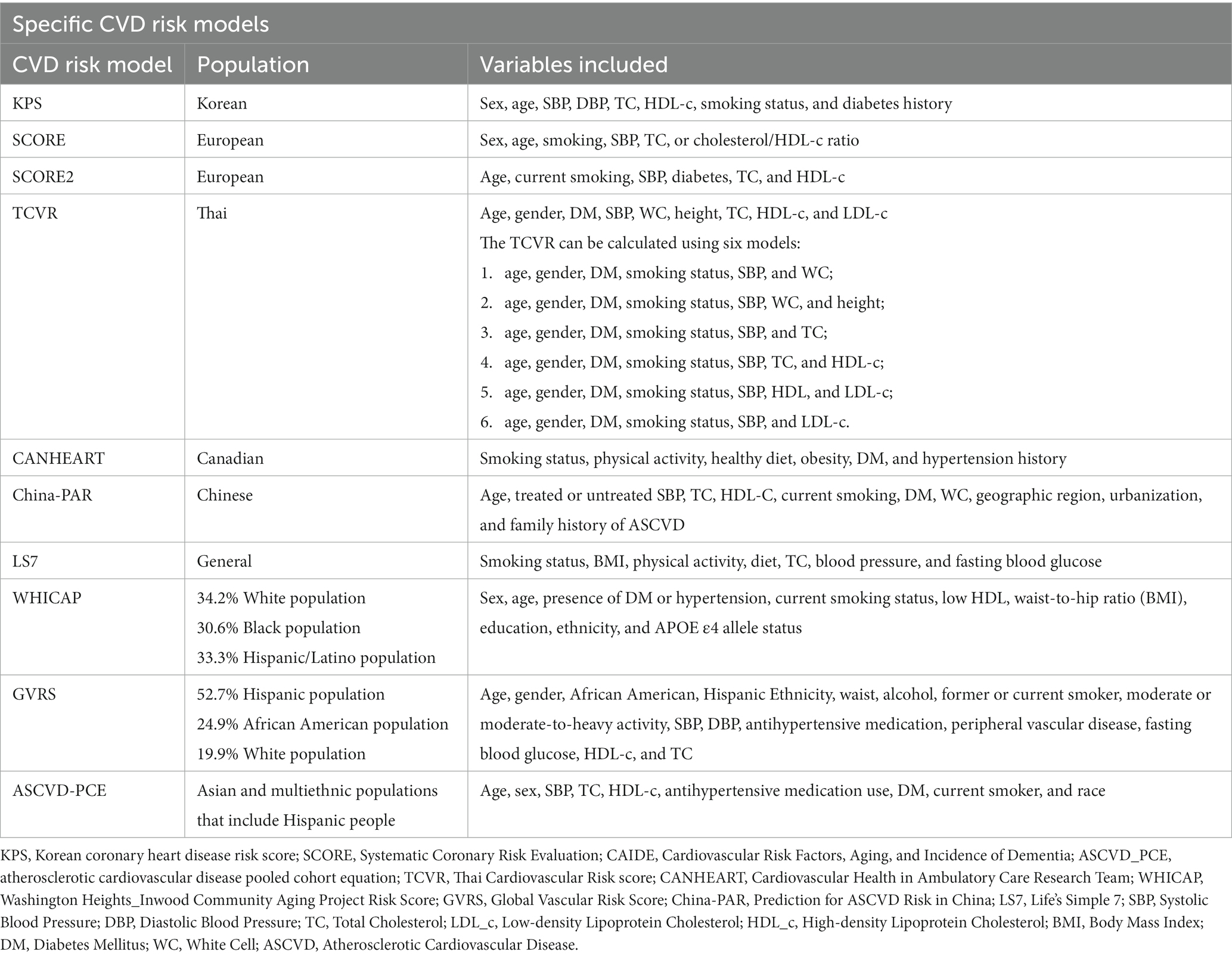
A total of 22 studies described 15 CVD prediction models (Kannel et al., 1976; D’Agostino et al., 1994, 2008; Conroy et al., 2003; Sacco et al., 2009; Lloyd-Jones et al., 2010; Reitz et al., 2010; Goff et al., 2014; Jee et al., 2014; Maclagan et al., 2014; Tsao and Vasan, 2015; Yang et al., 2016; Dufouil et al., 2017; Hageman et al., 2021; Buawangpong et al., 2022). Among them were two models without evaluation (Lloyd-Jones et al., 2010; Maclagan et al., 2014). Life’s Simple 7 (LS7) was defined as the concept and determining of the metrics done by the Goals and Metrics Committee of the Strategic Planning Task Force of the American Heart Association (AHA) (Lloyd-Jones et al., 2010). Furthermore, the Cardiovascular Health in Ambulatory Care Research Team (CANHEART) health index was a modified version of the AHA’s definition of ideal cardiovascular health (CVH) for Canadians (Maclagan et al., 2014). Besides, WHICAP claimed good prediction performance, with no specific values discovered (Reitz et al., 2010). Others found AUC or C-statistics ranging from 0.51 to 0.86. Except for the Thai Cardiovascular Risk score (TCVR) (Buawangpong et al., 2022), most of the models demonstrated good to acceptable discrimination (Receiver Operator Characteristic Curve>0.7). No studies exhibited a low risk of bias owing to insufficient analytical domain reporting (Tables 5, 6). The explanation could be that the CVD risk models were built and verified before the PROBAST report was proposed, and there needed to be a uniform and standard reporting checklist, resulting in a low assessment score. In contrast, its predictive performance was quite good, particularly for the Framingham series models.
3.4. The different CVD risk models and dementia or cognitive decline
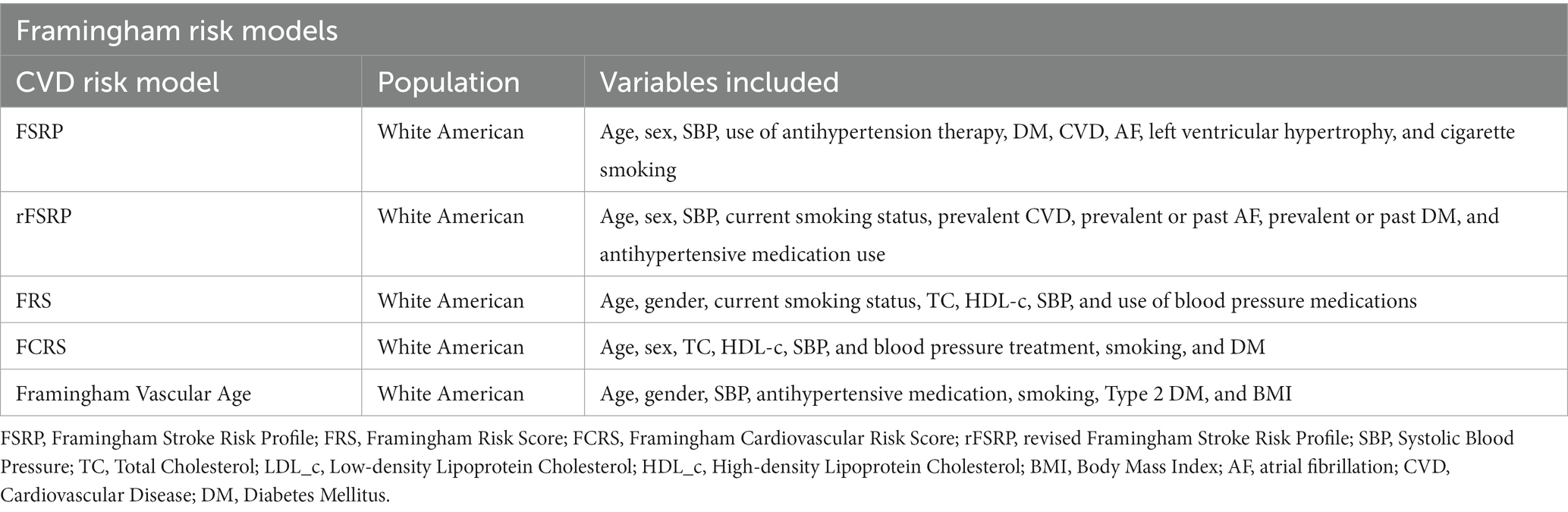
Framingham risk models and CAIDE, and other nine CVD risk models were used to predict dementia or cognitive decline, namely, the Korean Coronary Heart Disease Risk score (KPS), Systematic COronary Risk Evaluation (SCORE), SCORE2, atherosclerotic cardiovascular disease pooled cohort equation (ASCVD-PCE), TCVR, CANHEART, Global Vascular Risk score (GVRS), China-PAR, and LS7. Generally, the included CVD risk models had 5 to 15 factors. GVRS had the most number of factors, whereas SCORE had the least (Conroy et al., 2003; Sacco et al., 2009). Except for CANHEART and LS7, other models all included sex, age (age-stratified), systolic blood pressure (SBP), and smoking habits. Notably, race was taken into consideration by ASCVD-PCE, GVRS, and WHICAP (Rundek et al., 2020; Tarraf et al., 2020; Torres et al., 2020; Schaich et al., 2022). WHICAP is a simple summary risk score for predicting Alzheimer’s disease (AD) in older adults (Reitz et al., 2010). Given that its construction was based on cardiovascular risk factors, we decided that WHICAP could be included. All of the risk factors can be divided into traditional factors, behavioral factors, and anthropometric factors, including demographic data (age and sex), laboratory data (BP, blood glucose, and APOE ℇ4), questionnaire data (physical activity and dietary habits) and self-report data (physical activity, dietary habits, and smoking habits). Please see Tables 7, 8 for more details.
Compared with previous research, more studies focused on cognitive function than dementia (Harrison et al., 2014). Dementia was further diagnosed in different subcategories, including AD and vascular disease (VD) (Zheng et al., 2022). There were two ways for included studies to assess outcomes: one was cognitive diagnoses, including International Classification of Diseases 9 or 10 (ICD-9, ICD-10), an expert committee, clinical examination, or Clinical Dementia Rating (CDR), and the other way was neuropsychological assessments including Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MOCA), or domain-specific tests. Furthermore, global cognitive function could be acquired by calculating Z scores.
Except for one study (Jeon et al., 2021), the various risk models were found to be significantly connected with a higher chance of dementia in the future. In this study, CVH levels did not correlate with early cognitive decline in the Korean study population. The validity of the CANHEART model used to measure CVH was not established for the Korean population because it was initially developed by Canadian academics for the Canadian population. In addition, the CANHEART model used in Canadian samples for predicting cognitive decline was unclear.
The results were ambiguous. AUC was the prognostic performance metric in only four studies (Viticchi et al., 2017; Buawangpong et al., 2022; Schaich et al., 2022; Zheng et al., 2022). More studies just analyzed β efficiency. According to the individual risk factor profiles in midlife, the CAIDE risk score was explicitly created as an easy method to forecast the likelihood of dementia in the future (Kivipelto et al., 2006). With an initial C-index of 0.78 and a repeated C-index of 0.75 following external validation, the score accurately predicted dementia (Exalto et al., 2014).
3.5. The Framingham CVD risk models and dementia or cognitive decline
A total of 12 studies used the Framingham risk model: five studies used the Framingham General Cardiovascular Risk Score (FGCRS) (Viticchi et al., 2017; Song et al., 2020; Tarraf et al., 2020; Torres et al., 2020; Ji et al., 2022), three studies used the Framingham Stroke Risk Profile (FSRP) (Jefferson et al., 2015; Harrison et al., 2017; Schaich et al., 2022), while three studies used the revised Framingham Stroke Risk Profile (rFSRP) (Viswanathan et al., 2015; Pelcher et al., 2020; McGrath et al., 2022), which eliminates left ventricular hypertrophy (LVEF), and one study used Framingham Vascular Age (Badran et al., 2019). All studies found higher Framingham risk scores associated with an increased risk of subsequent cognitive decline or impairment. Which cognitive abilities were linked to a higher risk of CVD or stroke varied among research due to various measures of cognitive function, sample sizes, or statistical performance.
3.6. Specific CVD risk models and dementia or cognitive decline
Except for Framingham models, we concluded that nine specific CVD risk models may be associated with dementia and cognitive decline. Five CVD risk models were designed for a single country (Bao-Shan et al., 2021; Jeon et al., 2021; Buawangpong et al., 2022; Zheng et al., 2022; Mun et al., 2023). KPS, TCVR, the China-PAR, SCORE2, and the CANHEART health index were developed to measure cardiovascular risk among Korean, Thai, Chinese, European, and Canadian populations.
Four risk models were for the multiethnic cohort. A longitudinal cohort of non-Hispanic White people and African Americans served as the basis for the ASCVD-PCE (Schaich et al., 2022) and has since been validated for predicting atherosclerotic CVD risk in Asian and multiethnic populations, including Hispanics. A diverse group with almost equal representations of White people (34.2%), Black people (30.6%), and H/L (33.3%) participants created the WHICAP summary risk score to predict AD in older adults based on their vascular profiles associated with late-onset AD (Torres et al., 2020). GVRS is a model for assessing global vascular risk developed in the Northern Manhattan Study (NOMAS) that incorporates traditional, behavioral, and anthropometric risk factors and uses continuous variables (Rundek et al., 2020; Tarraf et al., 2020). A simple tool, LS7 only has seven modified factors, including four behavioral and three biological factors, similar to CANHEART (Lloyd-Jones et al., 2010). The AHA first suggested this metric for keeping CVH and has since been advised for brain health (Gorelick et al., 2017).
Other than CANHEART, specific CVD risk models were associated with a lower risk for dementia or cognitive decline (Covello et al., 2021; Jeon et al., 2021; Speh et al., 2021). CVD risk models from multiethnic cohorts were proven to predict dementia or cognitive decline in diverse race populations, such as White, H/L, Black, or Asian. Specifically, an article reported the association between QRISK and cognitive function, but we did not report it due to its poor quality (Hsu et al., 2015).
Only SCORE2, FSRP, ASCVD-PCE, and FCRP reported the models’ performance for dementia (Viticchi et al., 2017; Schaich et al., 2022; Zheng et al., 2022). The AUC or C-statistics of these models ranged from 0.65 to 0.75. Additionally, the C-indices of SCORE2 risk could distinguish between incident all-cause dementia, AD, VD, and all-cause death (Zheng et al., 2022).
3.7. Comparing the association between different CVD risk models and dementia or cognitive decline
Seven studies compared the predictive performance of two or three risk models (Harrison et al., 2017; Rundek et al., 2020; Tarraf et al., 2020; McGrath et al., 2022; Schaich et al., 2022; Zheng et al., 2022). CAIDE, as a reference substance, was used for five articles. Some studies reported that CAIDE is better for predicting dementia. For instance, one study that evaluated FSRP, ASCVD-PCE, and CAIDE concluded that CAIDE had more robust relationships with cognitive performance than FSRP and ASCVD-PCE but that ASCVD-PCE’s associations with the Digit Symbol Coding (DSC) and Digit Span (DS) were comparable to CAIDE’s (β = −0.57 and − 0.21, respectively) (Schaich et al., 2022).
Other studies showed that CAIDE had an inferior association with dementia or cognitive function compared with other risk models, such as SCOPE2, WHICAP, or GVRS. One study that compared SCORE2 with SCORE and CAIDE showed that the C-index of SCORE2 risk for discriminating against all-cause dementia was 0.750, which was significantly higher than the C-indices of SCORE and CAIDE risk (SCORE2 vs. SCORE: 0.014; SCORE2 vs. CAIDE: 0.093). The study further found that SCORE2 risk significantly improved the ability to distinguish between AD and VD (Zheng et al., 2022). Two studies showed that higher GVRS presented stronger associations with lower cognitive function than the FCRS or CAIDE (Rundek et al., 2020; Tarraf et al., 2020).
4. Discussion
This systematic review synthesizes the current trends regarding the association between different CVD risk models and incident dementia or cognitive decline. We updated Harrison’s systematic review and searched the articles from 2014 to 2023 (Harrison et al., 2014). As a result, 22 studies were included, and 15 CVD risk models were included; besides the Framingham series of models (FSRP, rFSRP, FRS, FGCRS, and Framingham vascular age), there were also specific CVD risk models, namely, KPS, TCVR, China-PAR, SCORE1, SCORE2, CANHEART, ASCVD-PCE, WHICAP, GVRS, and LS7. Our results show that higher CVD risk scores were associated with an increased risk of subsequent dementia or cognitive impairment. Furthermore, compared to the traditional dementia predicting model, multiethnic CVD risk models had a higher connection with cognitive tests.
Regarding the target outcome, only CAIDE was built to predict incident dementia, while other risk models treated fatal or non-fatal CVD events as outcomes. However, more studies found that CVD risk models were associated with dementia and cognitive impairment, and even some articles that compared CAIDE with CVD risk models proved that some CVD risk models’ dementia-predicting performance may be similar to or better than CAIDE. Kaffashian et al. (2013) discovered that the Framingham risk scores may be more accurate at predicting future cognitive decline than the CAIDE score by contrasting the FGCRS and FSPR with the CAIDE risk score (Kaffashian et al., 2013). One study compared CAIDE, FSRP, and ASCVD-PCE with exceptional cognitive performance in 4,392 multiethnic studies of participants with atherosclerosis and found that CAIDE had stronger associations with cognitive abilities performance than the FSRP and ASCVD-PCE. However, associations of ASCVD-PCE with processing speed and working memory were similar to CAIDE (Schaich et al., 2022). It is clear that Framingham risk models, consisting of cardiovascular factors, are more appropriate for predicting cognitive impairment. Similar to how SCORE2, WHICAP, and GVRS were created to address a variety of incident CVD risk variables, the findings of this study showed that those models could also predict incident dementia better than the CAIDE risk algorithm (Rundek et al., 2020; Tarraf et al., 2020; Torres et al., 2020; Zheng et al., 2022).
Higher scores from the CVD risk models have been linked to an increased risk of cognitive deterioration or dementia in many studies. Still, when specific cognitive domains have been examined, the results have been mixed regarding which cognitive domains have higher cardiovascular risk (Harrison et al., 2014; Wu et al., 2023). Notably, the identified studies had different primary cognitive outcome measures, which impede inferences regarding which vascular risk scores are the most responsive to specific cognitive domains. Many cognitive tests were used to identify incident dementia or cognitive decline, including verbal fluency, short-term and long-term memory, computation, processing speed, and exclusion function. Some studies reported that FGCRS and GVRS were associated with episodic memory, while others proved that no association was observed for episodic memory when the rFSRP was used (Viswanathan et al., 2015). However, in the NACC cohort, after controlling for age, FSRP independently contributed to a decline in processing speed (Jefferson et al., 2015). The variation of cohort characteristics (e.g., ethnicity and education level), methodological variances, follow-up time, and cognitive assessment instruments may all contribute to the differences in the results.
Traditional risk factors for CVD, such as hypertension, diabetes, dyslipidemia, obesity, and smoking, have been linked to a rapid loss of cognitive function. Moreover, most studies that showed an association between CVD risk models and worse cognitive function were focused on a single population, such as White people, whereas there were fewer studies conducted within non-White populations, such as H/L. It has been shown that race/ethnicity, as a critical element, modified associations (Tarraf et al., 2020; Torres et al., 2020; Schaich et al., 2022). Schaich et al. (2022) found that associations between CAIDE and dementia were more significant in African Americans and Hispanics than in White individuals (difference in β = 0.69 and 1.67, respectively). CVD risk models, such as the multiethnic GVRS, that are tailored to specific risks based on racial/ethnic background and that can offer significant insight into cognitive risk are practical to use in primary care settings as opposed to FCRP, which ignores race (Tarraf et al., 2020).
Better than GVRS, which contains too many factors, LS7 and CANHEART emphasize modifiable risk factors more than other risk models and may have more immediate implications for health promotion and disease prevention. As Canadian researchers originally created the CANHEART for the Canadian population, it does not have the TC constraint and its validity has not been demonstrated for the Korean population. Unlike CANHEART, LS7 was proposed by the AHA and includes seven modifiable cardiovascular risk factors. The relationships between the seven individual components and cognitive outcomes have been extensively researched. Nonetheless, the results were mixed since the connections between vascular risk factors and dementia are frequently complex, non-linear, and age-dependent. It is generally accepted that midlife CVH tends to have a linear association with late-life dementia risk.
In contrast, a J-shaped association was seen between the late-life CVH score and dementia, according to a systematic review and meta-analysis (Wu et al., 2023). Furthermore, in 2022, the AHA updated LS7 to LE8, optimizing the scoring algorithm and adding sleep information (Lloyd-Jones et al., 2022). Following the AHA’s CVH recommendations and keeping CVH at its best will significantly lower the risk of developing dementia in old age.
A limitation of this review is that it did not involve quantifying the association between CVD risk models and cognitive function through meta-analysis. Another limitation is that we included cross-sectional studies. Furthermore, only cross-sectional studies were used to explore the correlation between CVD risk models and dementia because the latest models have not yet been used for longitudinal cohorts. We aimed to maximize the search for suitable studies on the correlation between CVD risk models and dementia, therefore, cross-sectional and longitudinal cohorts were included. It is expected that more cohorts can be conducted in the future. The aim of this analysis was not to come to any firm conclusions about risk models’ powers to predict performance on cognitive decline, but to provide an overarching viewpoint on this trend that can inform future efforts to regulate CVD and dementia and promote health.
5. Implications for clinical practice and policy
Growing evidence points to a tight link between heart and brain health, with CVD possibly leading to brain illnesses such as stroke, dementia, and cognitive impairment. The overall picture of the anatomical and functional connections between the heart and the brain is still unknown at this time. Early detection and prompt management of modifiable risk factors are essential for CVD or dementia, therefore, there is a need for medical professionals to focus more on health promotion.
By understanding human health from a multi-organ perspective, medical professionals can improve disease risk prediction and prevention and mitigate the adverse effects of disease in one organ on other organs at risk. Using mature CVD risk models to predict cognitive decline or dementia incidence has several implications for optimizing clinical practice in escalating care among professional staff. First, two outcomes, CVD and dementia incidence, may be acquired at a one-time assessment, simplifying the preventive services. Second, compared with CAIDE, CVD risk models have few items and few experimental indicators, which are easier to collect, and patients also have the opportunity to self-monitor and self-manage. Finally, it is critical to increase public awareness of the link between CVD and dementia and to reaffirm the significance of preventing and controlling CVD risk factors. Keeping the heart healthy can help prevent cognitive decline in non-CVD or CVD patients.
6. Conclusion
The current systematic review shows the rapidly spreading use of present CVD risk models to predict dementia or cognitive decline. With the rapid development of CVD risk scores, we updated Harrison’s study published in 2014 (Harrison et al., 2014). This review presents findings from a large variety of cross-sectional studies and cohorts published between 2014 and 2023, showing significant progress in this field. Our findings prove that a positive association was observed between nationally or multiethnic-based CVD risk scores and subsequent dementia or cognitive impairment. Although meta-analysis was not conducted for different models’ risk, this study supports findings that indicate that the included models may be associated with CVD, cognitive function, and dementia.
Given that these factors are easily accessible in clinical and research settings and may be used to identify the members of a population who are most at risk for future cognitive decline and dementia, future efforts should be concentrated on developing vascular factor-based dementia or cognitive decline risk models. More cohorts could clarify the link between CVD risk models and dementia or cognitive decline by unifying the outcome assessment. Additionally, constructing models applicable to low-income and middle-income countries or multiethnic populations is becoming increasingly significant.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RRJ: Data curation, Methodology, Writing – original draft. QW: Methodology, Supervision, Writing – review & editing. HYH: Data curation, Methodology, Writing – review & editing. TL: Supervision, Writing – review & editing. CFY: Supervision, Writing – review & editing. YLY: Data curation, Methodology, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the following sources: Science and Technology Project of Gansu Province, China (20JR10RA637); Fundamental Research Funds for the Central Universities, Lanzhou University, China (lzujbky2022-30, lzujbky-2019-58); and ‘Double-First Class’ Lanzhou University Project Grant (561119204).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Badran, A., Hollocks, M. J., Brookes, R. L., Morris, R. G., and Markus, H. S. (2019). Framingham vascular age is associated with worse cognitive performance in the middle-aged and elderly. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 26, 531–540. doi: 10.1080/13825585.2018.1499866
Bancks, M., Alonso, N., Allen, N., Yaffe, K., and Carnethon, M. (2019). Temporal trends in cognitive function of older US adults associated with population changes in demographic and cardiovascular profiles. J. Epidemiol. Comm. Health 73, 612–618. doi: 10.1136/jech-2018-211985
Bao-Shan, H., Wen-Fa, W. U., and Meng, C. A. O. (2021). A study on the relationship of different cardiovascular risk levels with cognitive function and sleep quality in middle-aged and elderly patients based on the China-PAR model. Chin. J. Pract. Intern. Med. 41:11.
Buawangpong, N., Aramrat, C., Pinyopornpanish, K., Phrommintikul, A., Soontornpun, A., Jiraporncharoen, W., et al. (2022). Risk prediction performance of the Thai cardiovascular risk score for mild cognitive impairment in adults with metabolic risk factors in Thailand. Healthcare (Basel) 10:1959. doi: 10.3390/healthcare10101959
Conroy, R. M., Pyorala, K., Fitzgerald, A. P., Sans, S., Menotti, A., De Backer, G., et al. (2003). Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur. Heart J. 24, 987–1003. doi: 10.1016/S0195-668X(03)00114-3
Covello, A. L., Horwitz, L. I., Singhal, S., Blaum, C. S., Li, Y., and Dodson, J. A. (2021). Cardiovascular disease and cumulative incidence of cognitive impairment in the health and retirement study. BMC Geriatr. 21:274. doi: 10.1186/s12877-021-02191-0
D’Agostino, R. B., Wolf, P. A., Belanger, A. J., and Kannel, W. B. (1994). Stroke risk profile: adjustment for antihypertensive medication. The Framingham study. Stroke 25, 40–43. doi: 10.1161/01.STR.25.1.40
D’Agostino, R. B., Vasan, R. S., Pencina, M. J., Wolf, P. A., Cobain, M., Massaro, J. M., et al. (2008). General cardiovascular risk profile for use in primary care: the Framingham Heart study. Circulation 117, 743–753. doi: 10.1161/CIRCULATIONAHA.107.699579
Dufouil, C., Beiser, A., McLure, L. A., Wolf, P. A., Tzourio, C., Howard, V. J., et al. (2017). Revised Framingham stroke risk profile to reflect temporal trends. Circulation 135, 1145–1159. doi: 10.1161/CIRCULATIONAHA.115.021275
Exalto, L. G., Quesenberry, C. P., Barnes, D., Kivipelto, M., Biessels, G. J., and Whitmer, R. A. (2014). Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 10, 562–570. doi: 10.1016/j.jalz.2013.05.1772
Fayosse, A., Nguyen, D. P., Dugravot, A., Dumurgier, J., Tabak, A. C., Kivimaki, M., et al. (2020). Risk prediction models for dementia: role of age and cardiometabolic risk factors. BMC Med. 18:107. doi: 10.1186/s12916-020-01578-x
Goff, D. C., Lloyd-Jones, D. M., Bennett, G., Coady, S., D'Agostino, R. B., Gibbons, R., et al. (2014). 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation 129:98. doi: 10.1161/01.cir.0000437741.48606.98
Gorelick, P. B., Furie, K. L., Iadecola, C., Smith, E. E., Waddy, S. P., Lloyd-Jones, D. M., et al. (2017). Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 48, e284–e303. doi: 10.1161/STR.0000000000000148
Hageman, S., Pennells, L., Ojeda, F., Kaptoge, S., Kuulasmaa, K., de Vries, T., et al. (2021). SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 42, 2439–2454. doi: 10.1093/eurheartj/ehab309
Harrison, S. L., de Craen, A. J., Kerse, N., Teh, R., Granic, A., Davies, K., et al. (2017). Predicting risk of cognitive decline in very old adults using three models: the Framingham stroke risk profile; the cardiovascular risk factors, aging, and dementia model; and Oxi-inflammatory biomarkers. J. Am. Geriatr. Soc. 65, 381–389. doi: 10.1111/jgs.14532
Harrison, S. L., Ding, J., Tang, E. Y. H., Siervo, M., Robinson, L., Jagger, C., et al. (2014). Cardiovascular disease risk models and longitudinal changes in cognition: a systematic review. PLoS One 9:e114431. doi: 10.1371/journal.pone.0114431
Hsu, D. C., Munro, C. E., Rao, V., Chute, M., Dagley, A., Schultz, A., et al. (2015). Hippocampal volume and cardiovascular risk using the QRISK2 prediction tool in cognitively normal elderly. Am. J. Geriatr. Psychiatr. 23, S96–S97. doi: 10.1016/j.jagp.2014.12.098
Jee, S. H., Jang, Y., Oh, D. J., Oh, B.-H., Lee, S. H., Park, S.-W., et al. (2014). A coronary heart disease prediction model: the Korean Heart study. BMJ Open 4:e005025. doi: 10.1136/bmjopen-2014-005025
Jefferson, A. L., Hohman, T. J., Liu, D. D., Haj-Hassan, S., Gifford, K. A., Benson, E. M., et al. (2015). Adverse vascular risk is related to cognitive decline in older adults. J. Alzheimers Dis. 44, 1361–1373. doi: 10.3233/JAD-141812
Jeon, Y. J., Lee, J. H., Kim, H. C., and Jung, S. J. (2021). Exploring the associations between cardiovascular health measured with the CANHEART model and early cognitive impairment in a middle-aged population in Korea. Epidemiol. Health 43:e2021044. doi: 10.4178/epih.e2021044
Ji, X., Gao, H., Sun, D., Zhao, W., Zhuang, J., Wang, K., et al. (2022). Association of baseline level of cardiovascular risk burden and its temporal changes with cognitive decline. Front. Aging Neurosci. 14:895188. doi: 10.3389/fnagi.2022.895188
Kaffashian, S., Dugravot, A., Elbaz, A., Shipley, M. J., Sabia, S., Kivimäki, M., et al. (2013). Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology 80, 1300–1306. doi: 10.1212/WNL.0b013e31828ab370
Kannel, W. B., Mcgee, D., and Gordon, T. (1976). A general cardiovascular risk profile: the Framingham study. Am. J. Cardiol. 38, 856–862. doi: 10.1016/0002-9149(76)90061-8
Kivipelto, M., Ngandu, T., Laatikainen, T., Winblad, B., Soininen, H., and Tuomilehto, J. (2006). Risk score for the prediction of dementia risk in 20 years among middle-aged people: a longitudinal, population-based study. Lancet Neurol. 5, 735–741. doi: 10.1016/S1474-4422(06)70537-3
Lane, C. A., Barnes, J., Nicholas, J. M., Sudre, C. H., Cash, D. M., Malone, I. B., et al. (2020). Associations between vascular risk across adulthood and brain pathology in late life: evidence from a British birth cohort. JAMA Neurol. 77, 175–183. doi: 10.1001/jamaneurol.2019.3774
Lee, W. J., Liao, Y. C., Wang, Y. F., Lin, Y. S., Wang, S. J., and Fuh, J. L. (2019). Summative effects of vascular risk factors on the progression of Alzheimer's disease. J. Am. Geriatr. Soc. 68, 129–136. doi: 10.1111/jgs.16181
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Lloyd-Jones, D. M., Hong, Y., Labarthe, D., Mozaffarian, D., Appel, L. J., Van Horn, L., et al. (2010). Defining and setting National Goals for cardiovascular health promotion and disease reduction. Circulation 121, 586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
Lloyd-Jones, D. M., Allen, N. B., Anderson, C. A. M., Black, T., Brewer, L. C., Foraker, R. E., et al. (2022). Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation 146, e18–e43. doi: 10.1161/CIR.0000000000001078
Lo, C. K.-L., Mertz, D., and Loeb, M. (2014). Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 14, 1471–2288. doi: 10.1186/1471-2288-14-45
Maclagan, L. C., Park, J., Sanmartin, C., and Mathur, K. R. (2014). The CANHEART health index: a tool for monitoring the cardiovascular health of the Canadian population. CMAJ 186, 180–187. doi: 10.1503/cmaj.131358
McGrath, E. R., Beiser, A. S., O'Donnell, A., Himali, J. J., Pase, M. P., Satizabal, C. L., et al. (2022). Determining vascular risk factors for dementia and dementia risk prediction across mid- to later-life: the Framingham Heart study. Neurology 99, e142–e153. doi: 10.1212/WNL.0000000000200521
Moons, K. G. M., Wolff, R. F., Riley, R. D., Whiting, P. F., Westwood, M., Collins, G. S., et al. (2019). PROBAST: a tool to assess risk of Bias and applicability of prediction model studies: explanation and elaboration. Ann. Intern. Med. 170, W1–W33. doi: 10.7326/M18-1377
Mun, H., Shim, J. Y., Kimm, H., and Kang, H. C. (2023). Associations between Korean coronary Heart disease risk score and cognitive function in dementia-free Korean older adults. J. Korean Med. Sci. 38:e11. doi: 10.3346/jkms.2023.38.e11
Newman, L. (2000). AHRQ's evidence-based practice centers prove viable. Agency for Healthcare Research and Quality. Lancet 356:1990. doi: 10.1016/S0140-6736(05)72963-9
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Pelcher, I., Puzo, C., Tripodis, Y., Aparicio, H. J., Steinberg, E. G., Phelps, A., et al. (2020). Revised Framingham stroke risk profile: association with cognitive status and MRI-derived volumetric measures. J. Alzheimers Dis. 78, 1393–1408. doi: 10.3233/JAD-200803
Peloso, G. M., Beiser, A. S., Satizabal, C. L., Xanthakis, V., Vasan, R. S., Pase, M. P., et al. (2020). Cardiovascular health, genetic risk, and risk of dementia in the Framingham Heart study. Neurology 95, e1341–e1350. doi: 10.1212/WNL.0000000000010306
Rasmussen, I. J., Rasmussen, K. L., Nordestgaard, B. G., Tybjaerg-Hansen, A., and Frikke-Schmidt, R. (2020). Impact of cardiovascular risk factors and genetics on 10-year absolute risk of dementia: risk charts for targeted prevention. Eur. Heart J. 41, 4024–4033. doi: 10.1093/eurheartj/ehaa695
Reitz, C., Tang, M. X., Schupf, N., Manly, J. J., Mayeux, R., and Luchsinger, J. A. (2010). A summary risk score for the prediction of Alzheimer's disease in elderly persons. Arch. Neurol. 67, 835–841. doi: 10.1001/archneurol.2010.136
Rundek, T., Gardener, H., Dias Saporta, A. S., Loewenstein, D. A., Duara, R., Wright, C. B., et al. (2020). Global vascular risk score and CAIDE dementia risk score predict cognitive function in the northern Manhattan study. J. Alzheimers Dis. 73, 1221–1231. doi: 10.3233/JAD-190925
Sacco, R. L., Khatri, M., Rundek, T., Xu, Q., Gardener, H., Boden-Albala, B., et al. (2009). Improving global vascular risk prediction with behavioral and anthropometric factors. J. Am. Coll. Cardiol. 54, 2303–2311. doi: 10.1016/j.jacc.2009.07.047
Samieri, C., Perier, M. C., Gaye, B., Proust-Lima, C., Helmer, C., Dartigues, J. F., et al. (2018). Association of Cardiovascular Health Level in older age with cognitive decline and incident dementia. JAMA 320, 657–664. doi: 10.1001/jama.2018.11499
Schaich, C. L., Yeboah, J., Espeland, M. A., Baker, L. D., Ding, J., Hayden, K. M., et al. (2022). Association of Vascular Risk Scores and Cognitive Performance in a diverse cohort: the multi-ethnic study of atherosclerosis. J. Gerontol. A Biol. Sci. Med. Sci. 77, 1208–1215. doi: 10.1093/gerona/glab189
Song, R., Xu, H., Dintica, C. S., Pan, K. Y., Qi, X., Buchman, A. S., et al. (2020). Associations between cardiovascular risk, structural brain changes, and cognitive decline. J. Am. Coll. Cardiol. 75, 2525–2534. doi: 10.1016/j.jacc.2020.03.053
Speh, A., Wang, R., Winblad, B., Kramberger, M. G., Bäckman, L., Qiu, C., et al. (2021). The relationship between cardiovascular health and rate of cognitive decline in young-old and old-old adults: a population-based study. J. Alzheimers Dis. 84, 1523–1537. doi: 10.3233/JAD-210280
Tarraf, W., Kaplan, R., Daviglus, M., Gallo, L. C., Schneiderman, N., Penedo, F. J., et al. (2020). Cardiovascular risk and cognitive function in middle-aged and older Hispanics/Latinos: results from the Hispanic community health study/study of Latinos (HCHS/SOL). J. Alzheimers Dis. 73, 103–116. doi: 10.3233/JAD-190830
Thacker, E. L., Gillett, S. R., Wadley, V. G., Unverzagt, F. W., Judd, S. E., McClure, L. A., et al. (2014). The American Heart Association Life's simple 7 and incident cognitive impairment: the reasons for geographic and racial differences in stroke (REGARDS) study. J. Am. Heart Assoc. 3:e000635. doi: 10.1161/JAHA.113.000635
Tin, A., Bressler, J., Simino, J., Sullivan, K. J., Mei, H., Windham, B. G., et al. (2022). Genetic risk, midlife Life's simple 7, and incident dementia in the atherosclerosis risk in communities study. Neurology 99, E154–E163. doi: 10.1212/WNL.0000000000200520
Torres, S., Alexander, A., O'Bryant, S., and Medina, L. D. (2020). Cognition and the predictive utility of three risk scores in an ethnically diverse sample. J. Alzheimers Dis. 75, 1049–1059. doi: 10.3233/JAD-191284
Tsao, C. W., and Vasan, R. S. (2015). Cohort profile: the Framingham Heart study (FHS): overview of milestones in cardiovascular epidemiology. Int. J. Epidemiol. 44, 1800–1813. doi: 10.1093/ije/dyv337
Viswanathan, A., Macklin, E. A., Betensky, R., Hyman, R., Smith, E. E., and Blacker, D. (2015). The influence of vascular risk factors and stroke on cognition in late life: analysis of the NACC cohort. Alzheimer Dis. Assoc. Disord. 29, 287–293. doi: 10.1097/WAD.0000000000000080
Viticchi, G., Falsetti, L., Buratti, L., Sajeva, G., Luzzi, S., Bartolini, M., et al. (2017). Framingham risk score and the risk of progression from mild cognitive impairment to dementia. J. Alzheimers Dis. 59, 67–75. doi: 10.3233/JAD-170160
Wei, J., Wang, L., Kulshreshtha, A., and Xu, H. (2022). Adherence to Life's simple 7 and cognitive function among older adults: the National Health and nutrition examination survey 2011 to 2014. J. Am. Heart Assoc. 11:e022959. doi: 10.1161/JAHA.121.022959
WHO. The world health report: Health systems financing: The path to universal coverage. Geneva: World Health Organization. (2020).
Wu, J., Xiong, Y., Xia, X., Orsini, N., Qiu, C., Kivipelto, M., et al. (2023). Can dementia risk be reduced by following the American Heart Association's Life's simple 7? A systematic review and dose-response meta-analysis. Ageing Res. Rev. 83:101788. doi: 10.1016/j.arr.2022.101788
Yang, X., Li, J., Hu, D., and Chen, J. (2016). Predicting the ten-year risks of atherosclerotic cardiovascular disease in Chinese population: the China-PAR project. Circulation 134, 1430–1440. doi: 10.1161/CIRCULATIONAHA.116.022367
You, J., Zhang, Y. R., Wang, H. F., Yang, M., Feng, J. F., Yu, J. T., et al. (2022). Development of a novel dementia risk prediction model in the general population: a large, longitudinal, population-based machine-learning study. EClinicalMedicine 53:101665. doi: 10.1016/j.eclinm.2022.101665
Zhao, B., Li, T., Fan, Z., Yang, Y., Shu, J., Yang, X., et al. (2023). Heart-brain connections: phenotypic and genetic insights from magnetic resonance images. Science 380:abn6598. doi: 10.1126/science.abn6598
Zheng, F., Xie, W., Li, C., Gao, D., and Liang, J. (2022). Prediction abilities of SCORE2 risk algorithms for incident dementia and all-cause mortality: results from the UK biobank cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 78, 704–710. doi: 10.1093/gerona/glac251
Glossary
Keywords: cardiovascular disease risk models, cardiovascular health, dementia, cognitive decline, health promotion
Citation: Jia R, Wang Q, Huang H, Yang Y, Chung YF and Liang T (2023) Cardiovascular disease risk models and dementia or cognitive decline: a systematic review. Front. Aging Neurosci. 15:1257367. doi: 10.3389/fnagi.2023.1257367
Edited by:
Ying Wang, Fudan University, ChinaReviewed by:
Lídia Vaqué-Alcázar, University of Barcelona, SpainNing Li, Southeast University, China
Copyright © 2023 Jia, Wang, Huang, Yang, Chung and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Liang, bGlhbmd0YW8xQGhvdG1haWwuY29t
†These authors share first authorship
 Ruirui Jia
Ruirui Jia Qing Wang
Qing Wang Hengyi Huang
Hengyi Huang Yanli Yang1
Yanli Yang1