- 1Department of Neurology, School of Medicine, Tokyo Women's Medical University, Tokyo, Japan
- 2Department of Neurology, Juntendo University Shizuoka Hospital, Shizuoka, Japan
- 3Department of Neurology, Dokkyo Medical University, Tochigi, Japan
- 4Department of Neurology, Saiseikai Shonan Hiratsuka Hospital, Kanagawa, Japan
- 5Department of Neurology, Tokai University Hachioji Hospital, Tokyo, Japan
- 6Department of Neurology, National Center of Neurology and Psychiatry, Tokyo, Japan
Objective: Parkinson’s disease (PD) is characterized by various non-motor symptoms (NMS), such as constipation, olfactory disturbance, sleep disturbance, mental disorders, and motor symptoms. This study aimed to investigate factors associated with NMS in patients with PD.
Methods: Symptoms of PD were evaluated using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), Parts I–IV. NMS was assessed using the MDS-UPDRS Part I (self-assessment of NMS) and rapid eye movement sleep behavior disorder (RBD) questionnaires. Patients were categorized by age into <70 years and ≥ 70 years (older adults) groups, according to disease duration into early-stage and advanced-stage groups with a cut-off value of 5 years for motor symptoms, and by sex into male and female groups.
Results: A total of 431 patients with PD (202 males and 229 females) with a mean age of 67.7 years, a mean disease duration of 6.4 years, and a mean Part I total score of 9.9 participated in this study. The Part I total score was significantly positively correlated (p < 0.01) with disease duration and Part II, III, and IV scores. For Part I sub-item scores, the older group had significantly higher scores for cognitive impairment, hallucinations, sleep problems, urinary problems, and constipation than the <70 years group, whereas the advanced-stage group had significantly higher scores for hallucinations, sleep problems, daytime sleepiness, pain, urinary problems, and constipation (p < 0.05) than the early-stage group. Anxiety was higher in female patients than in male patients, whereas daytime sleepiness, urinary problems, and RBD were higher in male patients than in female patients (p < 0.05). Factors affecting Part I included disease duration, Part II total scores, Part IV total scores, and RBD.
Conclusion: According to the self-questionnaire assessment, NMS was highly severe in older adult patients, those with longer illness duration, subjective and objective motor function impairments, and RBD. Sex-based differences were also observed.
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disease that causes various non-motor symptoms (NMS), including constipation, olfactory disturbances, sleep disturbances, psychiatric disorders, and motor symptoms (Marinus et al., 2018). Autonomic neuropathy symptoms, including constipation and frequent urination, along with NMS, such as rapid eye movement sleep behavior disorder (RBD), olfactory dysfunction, and depression, are frequently observed before the onset of motor symptoms. Insomnia, neurogenic bladder, and cognitive impairment develop after the onset of motor symptoms, with the progression of the PD (Savica et al., 2010; Kalia and Lang, 2015). Olfactory disturbances and RBD in NMS are considered clinical markers for the early diagnosis of PD because they are highly disease-specific and appear earlier than motor symptoms (Jellinger, 2011; Lang, 2011; Martinez-Martin et al., 2011).
NMS and motor symptoms are associated with health-related quality of life (HRQOL) (Zhao et al., 2021). In contrast, NMS have been reported to affect HRQOL more than motor symptoms, and its progression contributes to reduction in HRQOL (Martinez-Martin et al., 2011). In Japan, an association between the NMS and HRQOL has been reported in patients with advanced-stage PD (Maeda et al., 2017). However, 31.8–65.2% of patients PD experiencing NMS do not report these disturbances to their physicians; therefore, they may remain untreated (Chaudhuri et al., 2010). Capturing the characteristics of patients’ NMS and practicing personalized medicine to improve HRQOL are important for clinicians.
Additionally, there are approximately 2.9 million patients with PD in Japan, among them, 85% are aged 65 years or older, and this number is expected to increase with an aging population (Mori and Abe, 2017). Regarding the association between older patients with PD and quality of life (QOL), QOL in patients with middle-aged-onset PD (50–69 years) was associated with the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III and NMS, whereas QOL in patients with old-onset PD (≥70 years) was associated with NMS (Park et al., 2018). To improve the HRQOL of patients with PD, evaluating NMS and examining its associated factors may be crucial. The MDS-UPDRS Part I is a comprehensive framework for identifying NMS and was revised in 2007 based on a growing understanding of the impact of NMS on patients’ lives (Goetz et al., 2007). This study aimed to assess the characteristics of NMS in PD and the differences in NMS based on age, sex, and disease duration, and to examine the factors involved in NMS using the MDS-UPDRS Part I.
2. Methods
2.1. Participants
This study included ambulatory patients with PD who had achieved a Mini-Mental State Examination (MMSE) score of 24 or higher, who were registered between February 1, 2015 and July 31, 2022. The participating institutions were the Tokyo Women’s Medical University Hospital, Juntendo University Shizuoka Hospital, Dokkyo Medical University Hospital, Tokai University Hospital, Tokai University Hachioji Hospital, and Keio University Hospital. The clinical diagnosis of PD was based on UK Brain Bank Criteria (Hughes et al., 1992). The exclusion criteria included individuals with dementia or those who scored < 24 on the MMSE.
2.2. Evaluation of clinical symptoms
Age, sex, and disease duration were also recorded. PD severity was assessed using the Hoehn and Yahr (HY) scale, and clinical symptoms were assessed using the Japanese version of the MDS-UPDRS, Parts I–IV (Kashihara et al., 2014). HY scale was evaluated in the “on” patient status. Part I of the MDS-UPDRS is a self-assessment of NMS in daily life, in which the patient or caregiver is asked to complete a 13-item questionnaire (cognitive impairment, hallucinations, depressed mood, anxious mood, apathy, dopamine dysregulation syndrome (DDS), sleep problems, daytime sleepiness, pain, urinary problems, constipation, lightheadedness in standing, and fatigue); Part II is a self-assessment of motor symptoms in daily life, in which the patient or caregiver must complete a 13-item questionnaire; Part III is an investigation of the presence of 18 possible motor symptoms as assessed by a medical professional; and Part IV involves the assessment of motor complications and symptom variability by a medical professional on a scale in which scores are assigned as follows: 0, normal; 1, very mild; 2, mild; 3, moderate; and 4, severe. Olfactory function was assessed using the Odor Stick Identification Test for the Japanese (OSIT-J; Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan) or a card-based olfactory identification test (Open Essence; Wako Junyaku) to identify 12 odors (Martinez-Martin et al., 2011). RBD was assessed using the Japanese version of the RBD Screening Questionnaire (RBDSQ-J). The RBDSQ-J is a self-administered questionnaire on the behavioral characteristics of RBD consisting of 13 items, with a cut-off score of six points for the group with possible RBD (pRBD), based on previous studies (Miyamoto et al., 2009; Nomura et al., 2011). Each drug was evaluated using the levodopa equivalent daily dose (LEDD). All patients underwent magnetic resonance imaging to exclude other diseases. This study was approved by the Institutional Review Board of Tokyo Women’s Medical University (approval No. 3316) and the Institutional Review Board of each study site and was conducted in accordance with the “Ethical Guidelines for Clinical Research in Japan” and the “Declaration of Helsinki”. Written informed consent was obtained from all patients before the study began.
2.3. Statistical analysis
Results are expressed as mean ± SD. JMP Pro statistical software (version 16; SAS Institute, Tokyo, Japan) was used for statistical analyses. The relationship between the total score and sub-item scores of the MDS-UPDRS Part I and age, disease duration, and MDS-UPDRS Parts II, III, and IV was analyzed using regression analysis. Statistical significance was set at p < 0.05 and r > 0.20. The patients were divided into two groups: <70 years old (<70 years group), ≥70 years old (≥70 years group), <5 years after the onset of motor symptoms (early group), and > 5 years old (advanced group). The total and sub-item scores of the MDS-UPDRS Part I were compared between the <70 years and ≥ 70 years groups, the early-stage and advanced-stage groups, and males and females using Wilcoxon’s test. Factors (age, sex, disease duration, MDS-UPDRS Part II–IV, RBDSQ-J, and olfactory disorder) contributing to the MDS-UPDRS Part I total score were examined using logistic analysis.
3. Results
3.1. Patients’ background
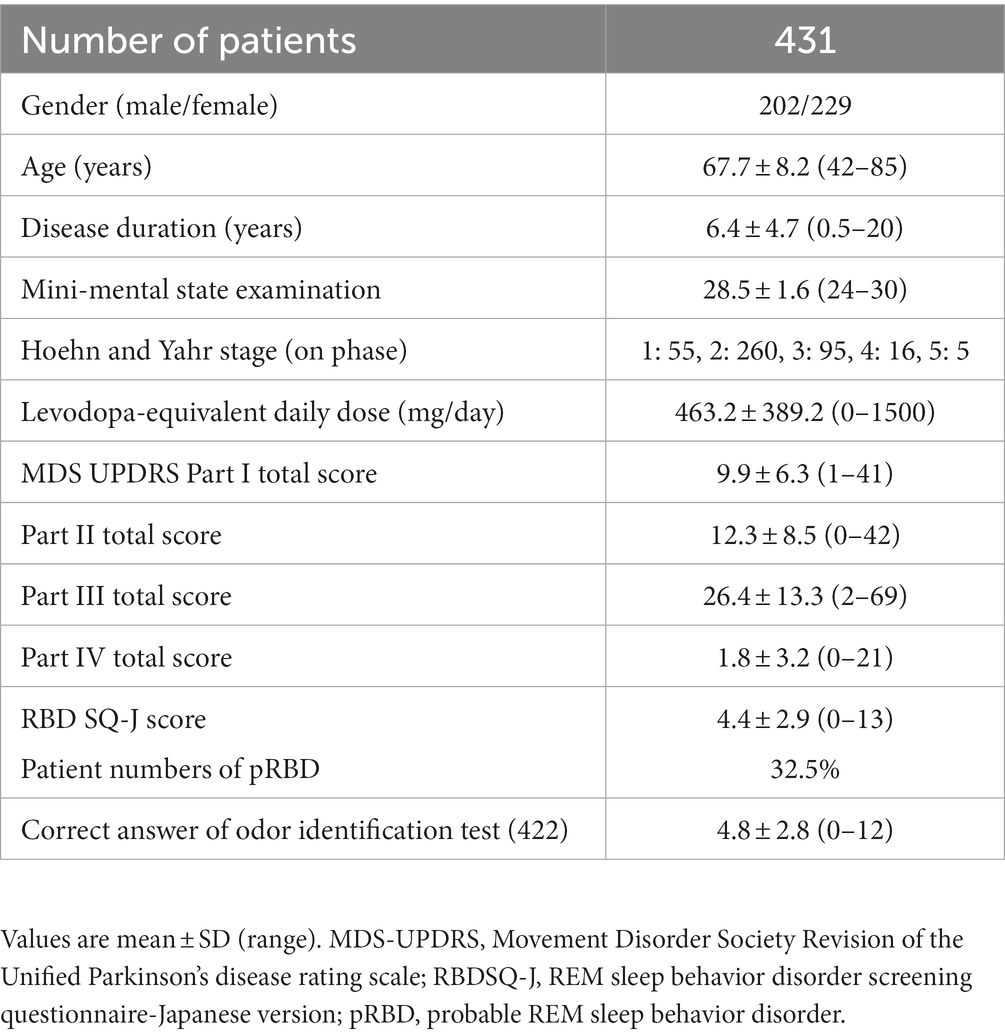
This study included 431 patients with PD without dementia (202 males and 229 females), aged 67.7 ± 8.2 years (age, 42–85 years) with a mean duration of disease of 6.4 ± 4.7 years (duration, 0.5–20 years), who visited one of the neurology departments of six hospitals in Japan. The mean MMSE was 28.5 points, the mean LEDD was 463.2 mg/day, and the HY scale (on status) was I for 55 patients, II for 260, III for 95, IV for 16, and V for 5. The mean total scores for MDS-UPDRS Parts I–IV were 9.9 ± 6.3, 12.3 ± 8.5, 26.4 ± 13.3, and 1.8 ± 3.2, respectively. pRBD was found in 32.5% of the patients, and the average number of correct answers on the olfactory identification test was 4.8 (Table 1).
3.2. Association of MDS-UPDRS Part I with age and disease duration
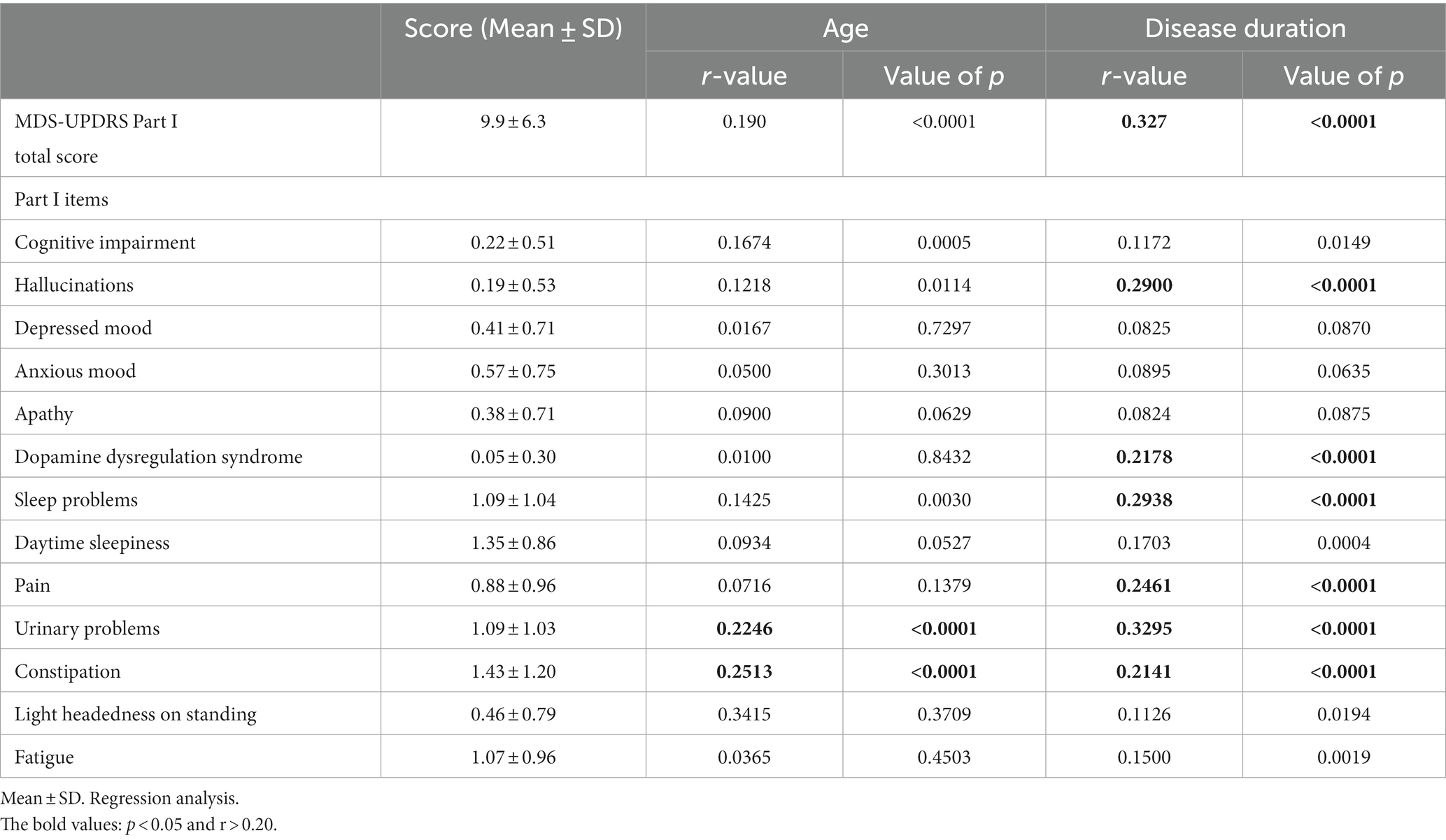
The correlation coefficient between the MDS-UPDRS Part I total score and age was low (r = 0.190, p < 0.0001), evidencing only a correlational trend. More specifically, the MDS-UPDRS Part I sub-items of urinary problems and constipation were found to be weakly correlated with age (Table 2). When the patients were divided into age groups of <70 and ≥70 years, significant differences were found in cognitive impairment, hallucinations, sleep and urinary problems, and constipation (p < 0.05; Figure 1).
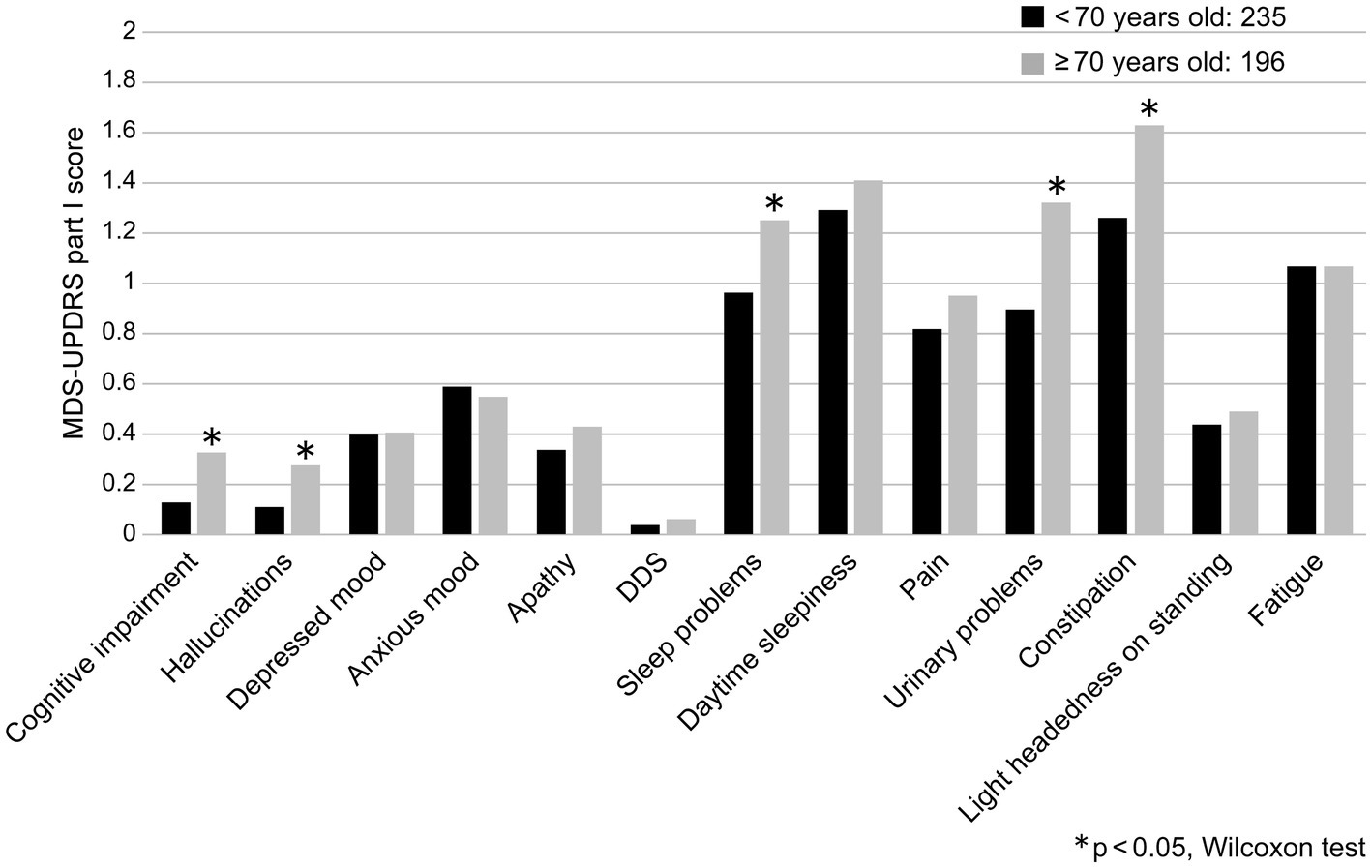
Figure 1. Comparison of MDS-UPDRS Part I sub-item scores and age groups (<70 years and ≥ 70 years). DDS, Dopamine dysregulation syndrome.
The MDS-UPDRS Part I total score and disease duration were positively correlated (r = 0.327, p < 0.0001), as were the sub-scores of Part I: hallucinations, DDS, sleep and urinary problems, pain, and constipation (Table 2). Comparing the total score of the MDS-UPDRS Part I total score and the early-stage and advanced-stage groups (early stage = < 5 years and advanced stage = ≥ 5 years), no correlation existed with the early-stage PD group (r = 0.144, p = 0.052); however, a positive correlation was observed with the advanced-stage PD group (r = 0.299, p < 0.0001). Hallucinations, sleep problems, daytime sleepiness, pain, urinary problems, and constipation were more common among patients with advanced-stage PD (Figure 2).
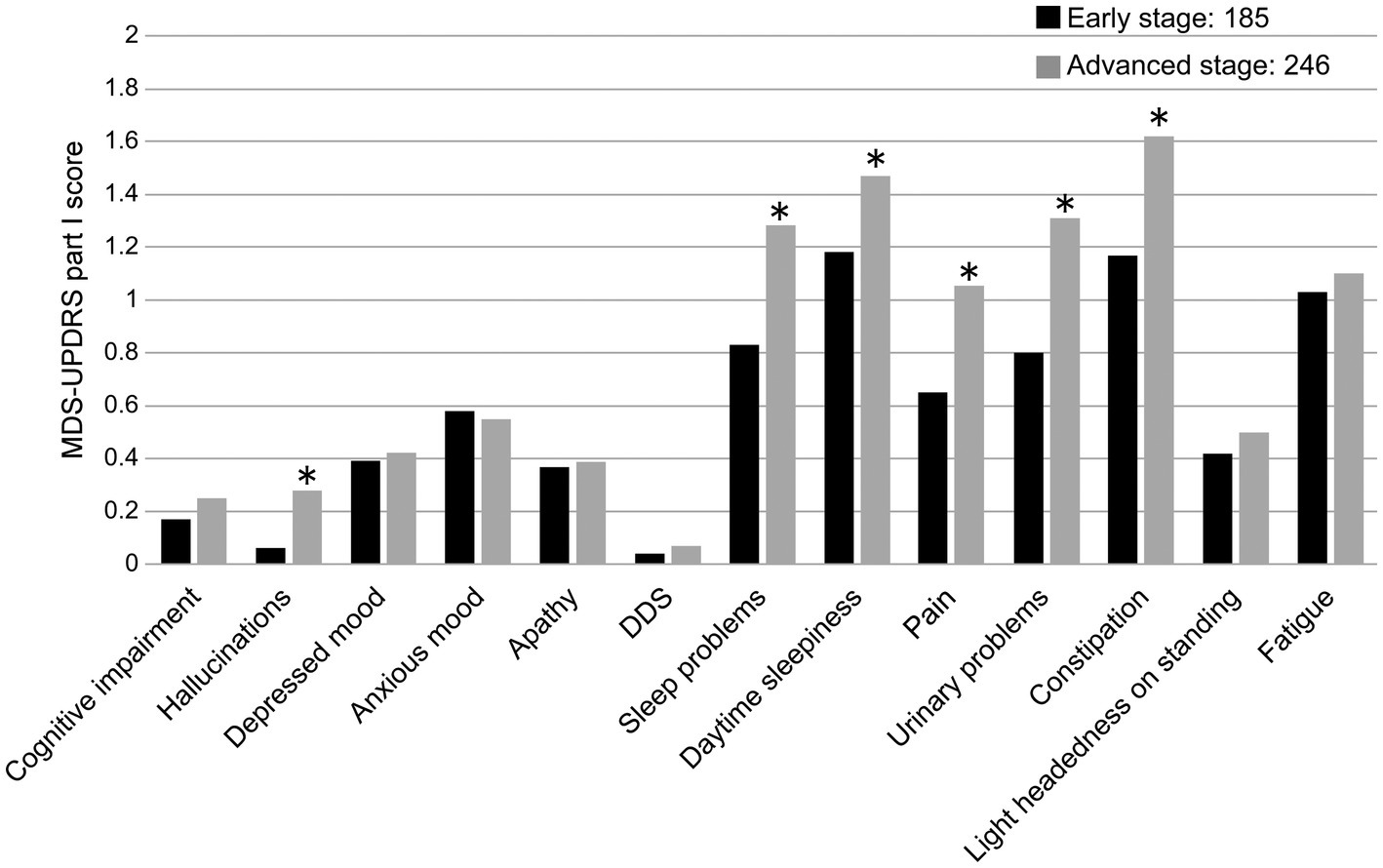
Figure 2. Comparison of MDS-UPDRS Part I sub-item scores and disease duration (early stage, advanced-stage cut-off: 5 years). DDS, Dopamine dysregulation syndrome. *p < 0.05.
3.3. Sex differences of clinical features in PD patients
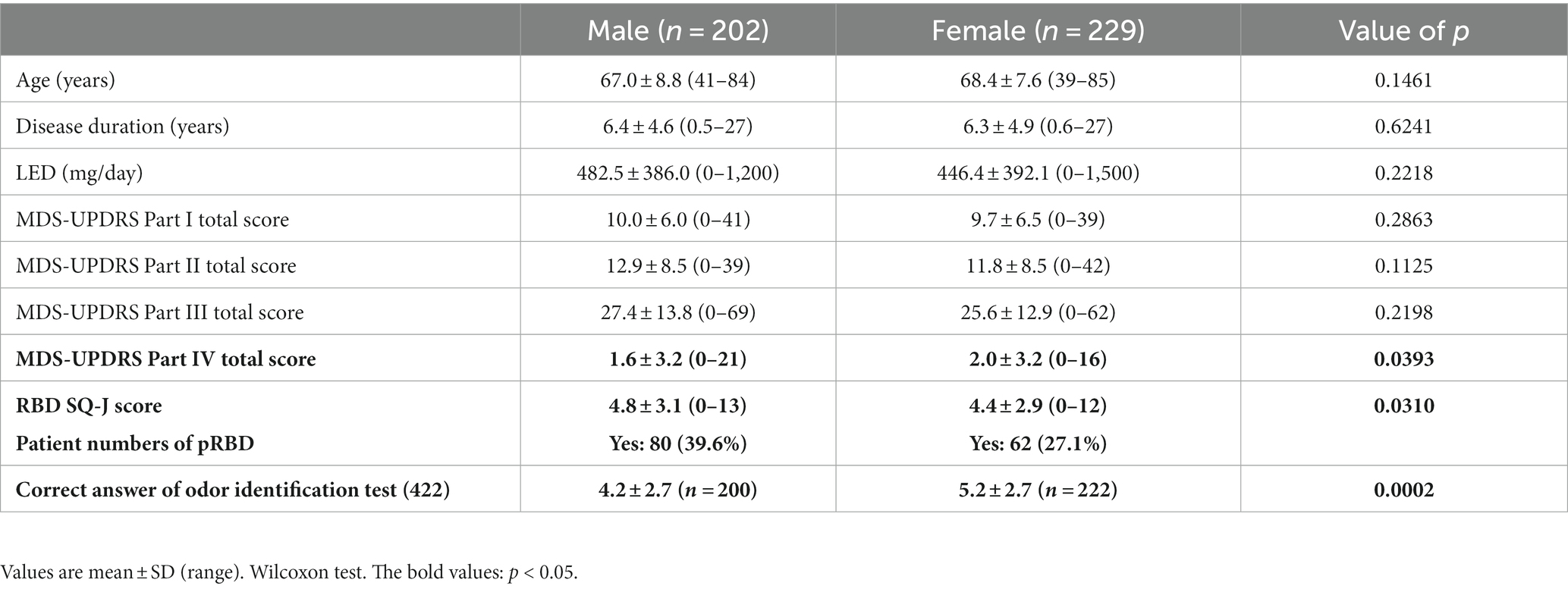
The results are summarized in Table 3. A total of 202 male patients and 229 female patients with no significant differences in age, disease duration, or LEDD were included in the two groups. The total scores of the MDS-UPDRS Parts I–IV were more severe in female patients in Part IV only (p < 0.05). A comparison of the MDS-UPDRS Part I sub-items revealed significantly higher scores for anxious mood in female patients than male patients (p < 0.05) and significantly higher scores for sleep and urinary problems in male patients than female patients (p < 0.05; Figure 3). pRBD was more common in male patients, and olfactory identification function was more severe in male patients (p < 0.05).
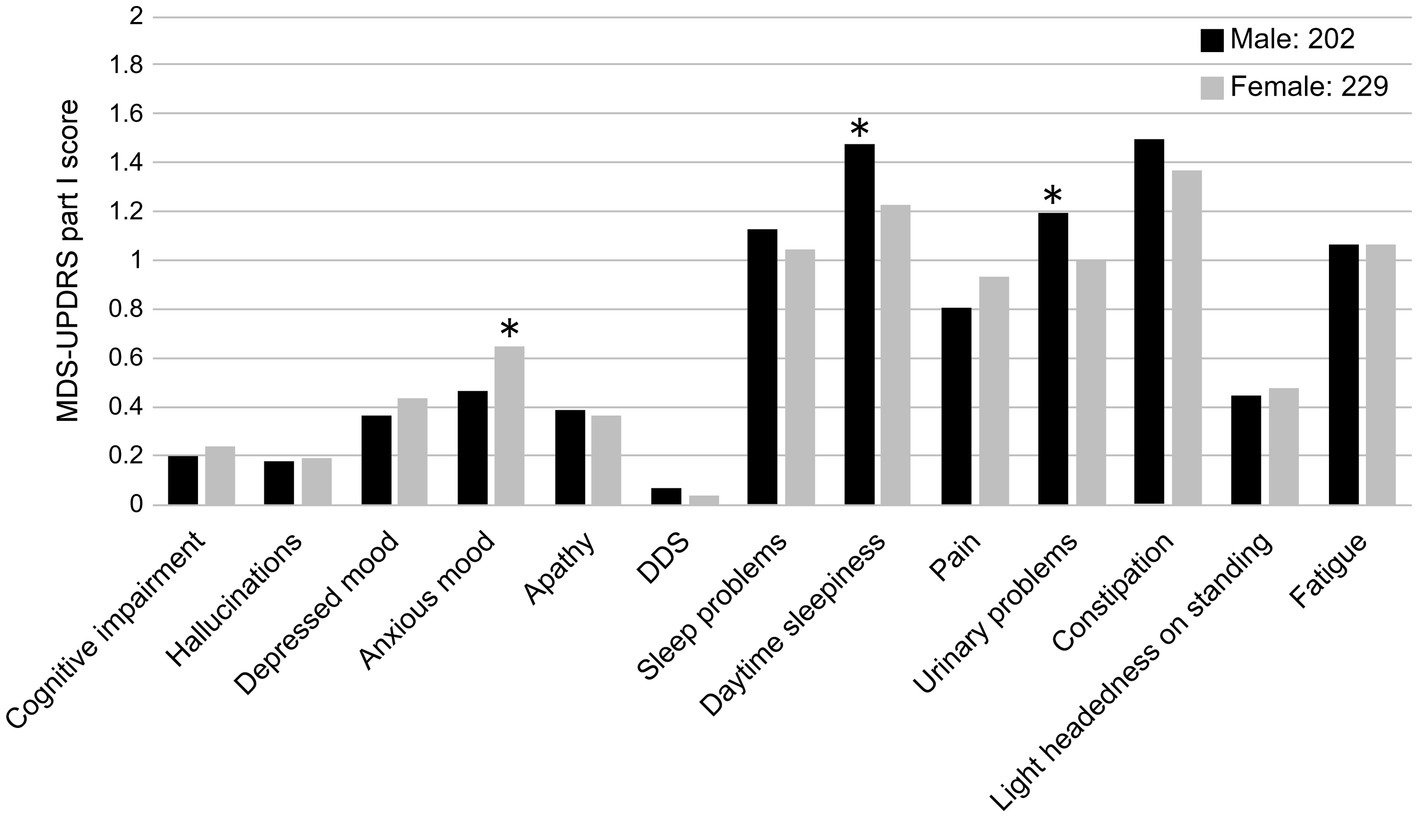
Figure 3. Comparison of MDS-UPDRS Part I sub-item scores and sex (male and female). DDS, Dopamine dysregulation syndrome. *p < 0.05.
3.4. Relationship between MDS-UPDRS Part I and Parts II–IV
The total scores of MDS-UPDRS Parts I and II, III, and IV were all significantly positively correlated (Figure 4). Part I of the MDS-UPDRS was divided into Parts IA (physician evaluation) and IB (self-evaluation), and their correlations with Parts II and III were compared. A stronger correlation was observed for Part II than for Part III in the physicians’ evaluation of Part IA. Even in Part IB, which was a self-evaluation, a stronger positive correlation was observed with Part II (Figure 5).

Figure 4. Associations between the MDS-UPDRS Part I total score and Parts II, III, and IV total scores. r: r-value, p: value of p.
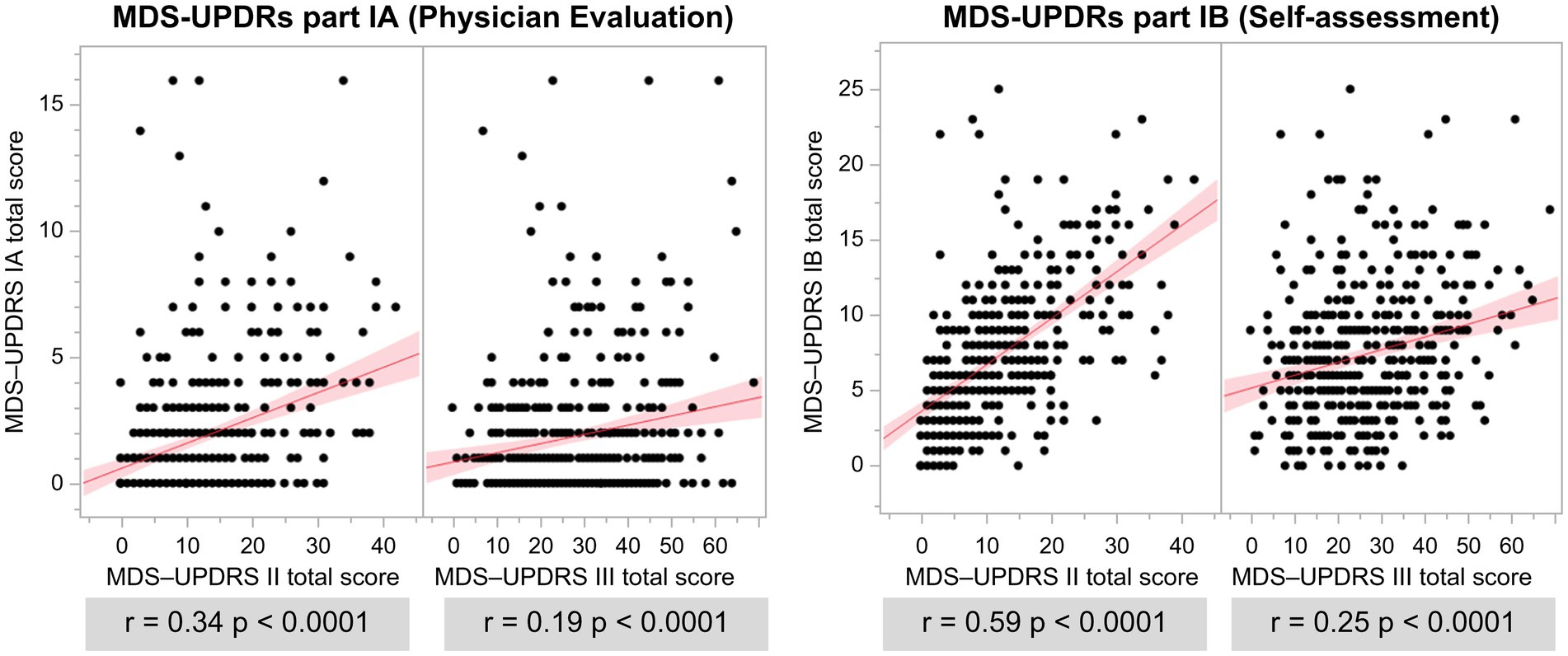
Figure 5. Association of MDS-UPDRS IA and IB with MDS-UPDRS II and III total scores. r: r-value, p: value of p.
3.5. Factors associated with the total score of MDS-UPDRS Part I
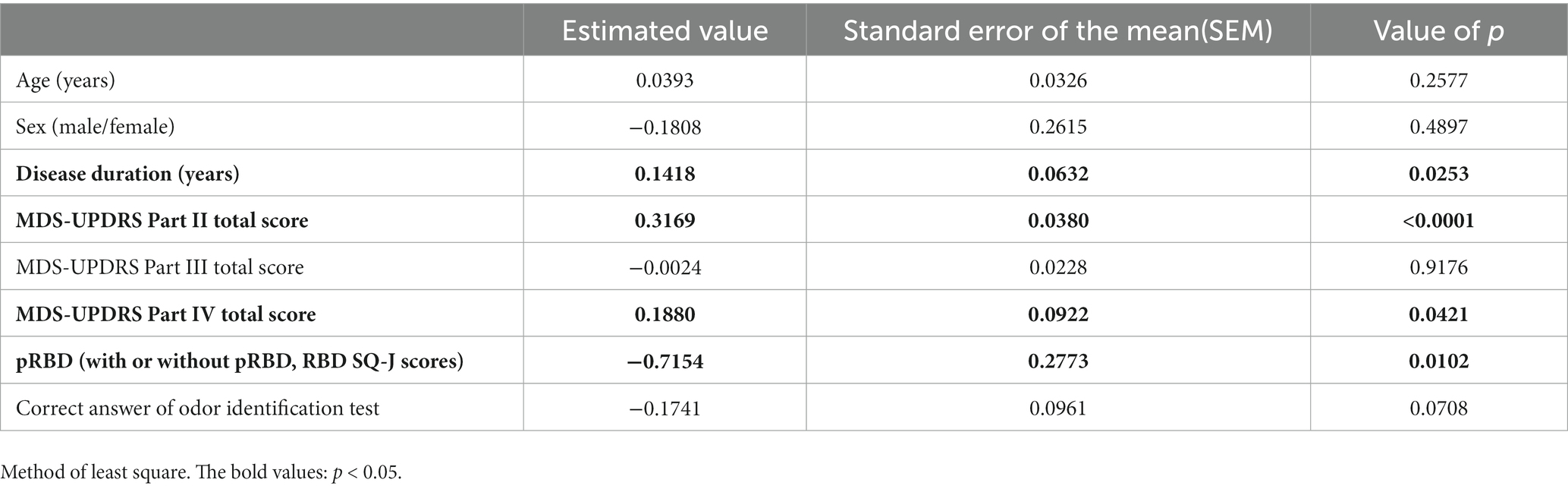
An investigation of the MDS-UPDRS Part I total score and patient background (age, sex, and disease duration), MDS-UPDRS Part II–IV total score, RBD (present and absent groups), and the correct number of olfactory identifications showed that the MDS-UPDRS Part I total score was associated with disease duration, Part II total scores, Part IV total scores, and pRBD (Table 4).
4. Discussion
4.1. Relationship between NMS, age, and disease duration
An FY2020 patient survey by the Ministry of Health, Labor, and Welfare showed that the total number of patients with PD in Japan was 289,000, of whom 234,000 were at least 70 years old, accounting for 81% (FY2020 patient survey: total number of patients, sex/age group × minor disease classification, Ministry of Health, Labor, and Welfare). The definition of elderly PD (i.e., elderly cut-off value) in this study was set at ≥70 years to better reflect clinical practice. Significant differences were observed between the <70-year group and ≥ 70-year group in terms of cognitive impairment, hallucinations, sleep problems, urinary problems, and constipation. This cross-sectional study included older adult patients with late-onset PD and those who grew older with continued treatment.
The pathological features of PD include the loss of midbrain substantia nigra neurons and the presence of eosinophilic inclusions called Lewy bodies, which are formed by the aggregation and deposition of α-synuclein in damaged neurons (Braak et al., 2003). Pathological progression is thought to begin in the dorsal vagus nucleus and olfactory bulb, which then progresses to the locus coeruleus pontine and midbrain substantia nigra, ascends the brain stem, and spreads to the cerebral cortex, with most cases exhibiting Lewy pathology from the peripheral to the central system (Braak et al., 2003). The diversity of NMS in patients with PD may be due to the extensive deposition of synucleins in the central, peripheral, and autonomic nervous systems (Braak et al., 2003; Jellinger, 2011; Schapira et al., 2017).
Regarding the pathological progression of PD, cognitive dysfunction is present in advanced PD (Savica et al., 2010; Kalia and Lang, 2015), but cognitive dysfunction in late-onset PD is already severe at the time of diagnosis (Pagano et al., 2016), and 21 autopsy cases of PD with an onset of ≥80 years developed dementia earlier than that reported in young-onset cases (Rajput and Rajput, 2017). A Japanese study indicated that the prevalence of mild cognitive dysfunction was 17.0% and often in those aged ≥65 years (Ninomiya et al., 2020). Moreover, cognitive dysfunction in PD occurs in approximately 83% of cases and may occur late in the pathological progression of PD or as a result of aging (Hely et al., 2008). Dysuria in PD patients is characterized by an overactive bladder, with nocturia being the most common at ≥60% and urinary incontinence at 26% for males and 28.5% for females (Sakakibara et al., 2012). Age-related vascular endothelial dysfunction, autonomic neuropathy, and inflammation are also important causes of an overactive bladder, with the number of patients and frequency increasing with age, which are major factors in the impairment of QOL among older adults (Homma et al., 2006; Irwin et al., 2006). Constipation in PD is often observed prior to PD diagnosis since α-synuclein aggregates have been confirmed in the enteric plexus (Wakabayashi et al., 1989). Constipation in the older adults is caused by a longer colon transit time and a higher rectal sensory threshold than in young people, and the 2019 Comprehensive Survey of Living Conditions by the Ministry of Health, Labor, and Welfare showed that 72.3% of females aged ≥65 years felt constipated, as did 64.1% of males aged ≥65 years. Therefore, cognitive dysfunction, dysuria, and constipation are important not only for the pathological progression of PD but also for older adult individuals.
Hallucinations often occur in the advanced stages of PD, particularly in the last 5 years, owing to pathological changes associated with the degeneration of the central nervous system, particularly the acetylcholine system, and the appearance of Lewy bodies (Kempster et al., 2010). The risk factors for hallucinations include old age, severe motor symptoms of PD, cognitive dysfunction, visual impairment, and sleep disturbances (Diederich et al., 2009). Regarding the pharmacokinetics of L-dopa in patients with elderly onset PD, higher doses of L-dopa are required in older adult patients than other patients with PD because of lower peak blood levels and increased time to reach peak blood levels (Nagayama et al., 2011), which may also be associated with an increased risk of hallucinations (Kalia and Lang, 2015). These findings suggest that hallucinations are often observed in older adult patients with PD undergoing long-term treatment. A 2017 report found that 96.5% of patients with PD exhibited sleep disorders such as RBD, sleep apnea, and excessive daytime drowsiness (Arnulf, 2005; Sobreira-Neto et al., 2017). PD-derived disorders of the sleep–wake center, nocturnal motor symptoms, cognitive dysfunction, and neurological symptoms, such as hallucinations, drugs, and nocturia, are complications of sleep disturbances. The risk factors for daytime drowsiness include disease duration, severity of motor symptoms, and total PD medication dose (Arnulf, 2005; Kang et al., 2017). Therefore, hallucinations, sleep problems, and daytime sleepiness may be related to disease duration and drug use, in addition to an older adult background.
There was a positive correlation between the MDS-UPDRS Part I total score and disease duration, whereas the advanced-period group showed a correlation with the Part I total score, although the early-stage group did not. The response to dopamine replacement therapy is good in the 3–5 year period after PD diagnosis, after which motor complications such as the wearing-off phenomenon and dyskinesia appear as side effects of L-dopa and the treatment response becomes poor (Aquino and Fox, 2015). A report on patients with early-onset PD (within 5 years) indicated that NMS progresses in parallel with motor symptoms (Santos-Garcia et al., 2020). In this study, the fact that no correlation was observed between NMS and the early-stage group may be because the neurodegenerative area was not extensive and patients with early-stage PD responded well to dopamine replacement therapy and had mild progression of NMS.
Comparisons between the early- and advanced-stage groups showed significant differences in hallucinations, sleep problems, daytime sleepiness, pain, urinary problems, and constipation compared with the MDS-UPDRS Part I sub-items. In PD cases, α-synuclein aggregates appear in the enteric plexus, and constipation is often seen prior to PD diagnosis (Wakabayashi et al., 1989). With pathological progression, hallucinations are associated with the occipital lobe; sleep disturbance is associated with the hypothalamus and reticular formation; pain is associated with the basal ganglia, coeruleus, raphe nucleus, amygdala, and thalamus; and dysuria, particularly urination function, is associated with the hypothalamus, cerebrum, frontal cortex, and basal ganglia, making the disease highly severe (Schapira et al., 2017). Drowsiness can be exacerbated by dopamine receptor agonists (Lim and Lang, 2010), and a multivariate analysis of patients with advanced PD showed that LEDD ≥400 mg/day was associated with an increased MDS-UPDRS Part I total score (Maeda et al., 2017).
4.2. Relationship between NMS and sex
Male patients had a higher severity of pRBD, daytime sleepiness, urinary problems, and olfactory disturbances than female patients. RBD is more common in male patients (Cerri et al., 2019; Iijima et al., 2021), and olfactory disturbances are more common in male patients (Iijima et al., 2011, 2021); these results are in agreement with those of previous studies. Excessive daytime drowsiness was found in 20–50% of patients with PD, and risk factors included male sex, disease duration, severity of motor symptoms, and drugs (Kang et al., 2017). Drowsiness is presumed to be caused by PD-derived disorders of the sleep–wake system, RBD, and drugs. The basal ganglia have an inhibitory effect on urination; therefore, PD patients with basal ganglia lesions have an overactive bladder (Sakakibara et al., 2008). When considering the age at the onset of PD, male patients are more likely to develop obstructive dysuria, which coincides with the age of prostatic hyperplasia (Homma et al., 2006), and middle-aged and older adult females are prone to stress urinary incontinence (Sakakibara et al., 2008). The frequency of dysuria, including urinary incontinence, in PD cases has been reported to be 53% in males and 63% in females (Sakakibara et al., 2001). The patients in the present study were older than those in previous reports; therefore, it is possible that dysuria was significantly more severe in males than in females.
Female patients had higher MDS-UPDRS Part IV total scores and anxious moods than male patients. In the West, PD onset is more common in male patients than in female patients, and the incidence of PD in males and postmenopausal female patients is roughly equivalent. Therefore, it is presumed that female hormones may prevent the risk of PD onset and have neuroprotective effects (Cerri et al., 2019). There are also sex differences in the bioavailability of L-dopa, suggesting the involvement of estrogen (Kumagai et al., 2014). Anxiety and dyskinesia are common among women in Western countries (Cerri et al., 2019). In Japan, the prevalence rate is 0.56 in females and the incidence rate is 0.61 in females, with both being higher in females (Yamawaki et al., 2009) than in males. The present study also showed significant differences in anxiety and higher rates in females than in males, which is consistent with previous reports. A report on the pharmacokinetics of L-dopa in older adult patients involved a body-weight-adjusted comparison between males and females, and the results showed that the L-dopa area under the blood concentration-time curve (AUC) was significantly higher in females (Kumagai et al., 2014) than in males. In a study involving 220 patients with PD in Japan, age and female sex were associated with an AUC of 4 h (Nishikawa et al., 2020). Female patients had a significantly greater bioavailability of L-dopa than male patients, and it was thought that female patients had more motor complications during the drug treatment of PD than that in male patients. The present study did not distinguish between MDS-UPDRS Part IV total score dyskinesia and the wearing-off phenomenon, and further examination is necessary. These results indicate that the clinical symptoms of NMS in PD differ between males and females and that it is necessary for clinicians to pay attention when treating patients with PD.
4.3. Relationship between NMS and each part of MDS-UPDRS
A positive correlation was observed between MDS-UPDRS Part I and Part II/III total scores, suggesting that NMS in PD progresses in parallel with motor symptoms. Part I of the MDS-UPDRS was divided into Parts IA (physician evaluation) and IB (self-evaluation), which were compared with Parts II (self-evaluation of motor symptoms) and III (physician evaluation of motor symptoms). A 2022 report that examined patients with PD with an HY scale of 1 or 2 showed a positive correlation between MDS-UPDRS Parts IA/IB and II, no correlation between Parts IA and III, and a correlation between parts IB and III (Zolfaghari et al., 2022). The findings of this study including patients with PD with an HY scale of 1–5 were consistent with those of previous reports. The evaluation of side effects over time by physicians and patients showed that physician evaluations had lower values than patient evaluations at all time points (Basch, 2010). A previous study indicated that healthcare professionals underestimated adverse events in patients (Sonn et al., 2013). There was also a discrepancy between physician and patient evaluations in the present study, and NMS was more correlated with the Part II total score, which is a subjective symptom questionnaire, suggesting that subjectivity is correlated.
The MDS-UPDRS Part I and Part IV total scores were positively correlated. Patients in the advanced stages of PD may experience the wearing-off phenomenon, dyskinesia, and PD patients may face many symptoms associated with NMS (Sato et al., 2006).
4.4. Factors that contribute most to NMS
The factors that contributed to the MDS-UPDRS Part I total score were disease duration, MDS-UPDRS Part II total score, Part IV total score, and pRBD, with the Part II total score being the largest contributing factor. Neurodegenerative areas become extensive due to pathological progression; therefore, NMS is thought to become more severe during the disease duration (Braak et al., 2003; Jellinger, 2011; Schapira et al., 2017). The MDS-UPDRS Part IV total score, which evaluates motor complications, also evaluates dyskinesia and the wearing-off phenomenon that is observed in the advanced stages of PD, and NMS is thought to become severe because of the high score during the advanced stages of PD (Sato et al., 2006; Aquino and Fox, 2015). It has been reported that pRBD contributes to the MDS-UPDRS Part I total score (Iijima et al., 2021), and this is similar to the results of the present study. Moreover, NMS by self-question evaluation contributed the most to decreased motor dysfunction. Medical staff should avoid relying solely on their own evaluation and value the subjective symptoms of patients.
4.5. Limitations
This study has some limitations. First, cognitive impairment may have been underestimated because patients with obvious cognitive decline were excluded. Second, the motor score (MDS-UPDRS Part III) was evaluated in the “on” state, and this cannot be compared with the “off” state, and further study is needed in the future. Furthermore, this study was a cross-sectional study within a single ethnic group and could have been influenced by potential biases introduced through the use of different assessment tools with respect to olfactory function. We believe that future prospective studies involving patients from various regions and cultures must be conducted.
5. Conclusion
NMS, assessed using a self-administered questionnaire, was highly severe in older patients, those with a longer disease duration and subjective and objective motor function decline. There were also differences between the sexes. Additionally, the background and NMS of each patient must be evaluated to provide individualized medical care that satisfies different needs and improves the QOL of patients with PD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Tokyo Women’s Medical University (approval No. 3316) and the Institutional Review Board of each study site and was conducted in accordance with the “Ethical Guidelines for Clinical Research in Japan” and the “Declaration of Helsinki.” The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RM, MI, YO, KS, FY, SN, TO, and KK: conceptualization. RM, MI, YO, KS, FY, SN, and TO: data curation. RM and MI: formal analysis and methodology. MI, YO, KS, FY, SN, and TO: investigation. KK: project administration and supervision. RM: writing – original draft. YO, KS, FY, SN, TO, and KK: writing – review and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
This study was presented at the 64th Annual Meeting of the Japanese Society of Neurology and the International Congress of Parkinson’s Disease and Movement Disorders in 2023.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aquino, C. C., and Fox, S. H. (2015). Clinical spectrum of levodopa-induced complications. Mov. Disord. 30, 80–89. doi: 10.1002/mds.26125
Arnulf, I. (2005). Excessive daytime sleepiness in parkinsonism. Sleep Med. Rev. 9, 185–200. doi: 10.1016/j.smrv.2005.01.001
Basch, E. (2010). The missing voice of patients in drug-safety reporting. N. Engl. J. Med. 362, 865–869. doi: 10.1056/NEJMp0911494
Braak, H., Tredici, K. D., Rüb, U., de Vos, R. A. I., Jansen Steur, E. N. H., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/S0197-4580(02)00065-9
Cerri, S., Mus, L., and Blandini, F. (2019). Parkinson's disease in women and men: What's the difference? J. Parkinsons Dis. 9, 501–515. doi: 10.3233/JPD-191683
Chaudhuri, K. R., Prieto-Jurcynska, C., Naidu, Y., Mitra, T., Frades-Payo, B., Tluk, S., et al. (2010). The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov. Disord. 25, 704–709. doi: 10.1002/mds.22868
Diederich, N. J., Fénelon, G., Stebbins, G., and Goetz, C. G. (2009). Hallucinations in Parkinson disease. Nat. Rev. Neurol. 5, 331–342. doi: 10.1038/nrneurol.2009.62
Goetz, C. G., Fahn, S., Martinez-Martin, P., Poewe, W., Sampaio, C., Stebbins, G. T., et al. (2007). Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov. Disord. 22, 41–47. doi: 10.1002/mds.21198
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M., and Morris, J. G. (2008). The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov. Disord. 23, 837–844. doi: 10.1002/mds.21956
Homma, Y., Yamaguchi, O., and Hayashi, K., Neurogenic Bladder Society Committee (2006). Epidemiologic survey of lower urinary tract symptoms in Japan. Urology 68, 560–564. doi: 10.1016/j.urology.2006.03.035
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Iijima, M., Kobayakawa, T., Saito, S., Osawa, M., Tsutsumi, Y., Hashimoto, S., et al. (2011). Differences in odor identification among clinical subtypes of Parkinson's disease. Eur. J. Neurol. 18, 425–429. doi: 10.1111/j.1468-1331.2010.03167.x
Iijima, M., Okuma, Y., Suzuki, K., Yoshii, F., Nogawa, S., Osada, T., et al. (2021). Associations between probable REM sleep behavior disorder, olfactory disturbance, and clinical symptoms in Parkinson's disease: a multicenter cross-sectional study. PLoS One 16:e0247443. doi: 10.1371/journal.pone.0247443
Irwin, D. E., Milsom, I., Hunskaar, S., Reilly, K., Kopp, Z., Herschorn, S., et al. (2006). Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur. Urol. 50, 1306–1315. doi: 10.1016/j.eururo.2006.09.019
Jellinger, K. A. (2011). Synuclein deposition and non-motor symptoms in Parkinson disease. J. Neurol. Sci. 310, 107–111. doi: 10.1016/j.jns.2011.04.012
Kalia, L. V., and Lang, A. E. (2015). Parkinson's disease. Lancet 386, 896–912. doi: 10.1016/S0140-6736(14)61393-3
Kang, S. Y., Ryu, H. S., Sunwoo, M. K., Kim, S. J., Baik, J. S., Park, M. Y., et al. (2017). Sleepiness and depression in Parkinson's disease patients treated with ropinirole and levodopa. J. Mov. Disord. 10, 123–129. doi: 10.14802/jmd.17048
Kashihara, K., Kondo, T., Mizuno, Y., Kikuchi, S., Kuno, S., Hasegawa, K., et al. (2014). Official Japanese version of the Movement Disorder Society-unified Parkinson’s disease rating scale: validation against the original English version. Mov. Disord. Clin. Pract. 1, 200–212. doi: 10.1002/mdc3.12058
Kempster, P. A., O'sullivan, S. S., Holton, J. L., Revesz, T., and Lees, A. J. (2010). Relationships between age and late progression of Parkinson's disease: a clinico-pathological study. Brain 133, 1755–1762. doi: 10.1093/brain/awq059
Kumagai, T., Nagayama, H., Ota, T., Nishiyama, Y., Mishina, M., and Ueda, M. (2014). Sex differences in the pharmacokinetics of levodopa in elderly patients with Parkinson disease. Clin. Neuropharmacol. 37, 173–176. doi: 10.1097/WNF.0000000000000051
Lang, A. E. (2011). A critical appraisal of the premotor symptoms of Parkinson's disease: potential usefulness in early diagnosis and design of neuroprotective trials. Mov. Disord. 26, 775–783. doi: 10.1002/mds.23609
Lim, S. Y., and Lang, A. E. (2010). The nonmotor symptoms of Parkinson's disease-an overview. Mov. Disord. 25, S123–S130. doi: 10.1002/mds.22786
Maeda, T., Shimo, Y., Chiu, S. W., Yamaguchi, T., Kashihara, K., Tsuboi, Y., et al. (2017). Clinical manifestations of nonmotor symptoms in 1021 Japanese Parkinson's disease patients from 35 medical centers. Parkinsonism Relat. Disord. 38, 54–60. doi: 10.1016/j.parkreldis.2017.02.024
Marinus, J., Zhu, K., Marras, C., Aarsland, D., and Van Hilten, J. J. (2018). Risk factors for non-motor symptoms in Parkinson's disease. Lancet Neurol. 17, 559–568. doi: 10.1016/S1474-4422(18)30127-3
Martinez-Martin, P., Rodriguez-Blazquez, C., Kurtis, M. M., and Chaudhuri, K. R., Group, N.V (2011). The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov. Disord. 26, 399–406. doi: 10.1002/mds.23462
Miyamoto, T., Miyamoto, M., Iwanami, M., Kobayashi, M., Nakamura, M., Inoue, Y., et al. (2009). The REM sleep behavior disorder screening questionnaire: validation study of a Japanese version. Sleep Med. 10, 1151–1154. doi: 10.1016/j.sleep.2009.05.007
Mori, K., and Abe, K. (2017). Patient surveys overview. Ministry of health, Labour and Welfare Government Statistics 1–33. (in Japanese).
Nagayama, H., Ueda, M., Kumagai, T., Tsukamoto, K., Nishiyama, Y., Nishimura, S., et al. (2011). Influence of ageing on the pharmacokinetics of levodopa in elderly patients with Parkinson's disease. Parkinsonism Relat. Disord. 17, 150–152. doi: 10.1016/j.parkreldis.2010.11.002
Ninomiya, T., Nakaji, S., Maeda, T., Yamada, M., Mimura, M., Nakashima, K., et al. (2020). Study design and baseline characteristics of a population-based prospective cohort study of dementia in Japan: the Japan prospective studies collaboration for aging and dementia (JPSC-AD). Environ. Health Prev. Med. 25:64. doi: 10.1186/s12199-020-00903-3
Nishikawa, N., Iwaki, H., Shiraishi, T., Mukai, Y., Takahashi, Y., and Murata, M. (2020). Female, aging, difference formulations of DCI, or lower body weight increases AUC(4hr) of levodopa in patients with Parkinson's disease. Parkinsonism Relat. Disord. 76, 16–20. doi: 10.1016/j.parkreldis.2020.05.020
Nomura, T., Inoue, Y., Kagimura, T., Uemura, Y., and Nakashima, K. (2011). Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson's disease patients. Sleep Med. 12, 711–713. doi: 10.1016/j.sleep.2011.01.015
Pagano, G., Ferrara, N., Brooks, J. D., and Pavese, N. (2016). Age at onset and Parkinson disease phenotype. Neurology 86, 1400–1407. doi: 10.1212/WNL.0000000000002461
Park, H. R., Youn, J., Cho, J. W., Oh, E. S., Kim, J. S., Park, S., et al. (2018). Characteristic motor and nonmotor symptoms related to quality of life in drug-naive patients with late-onset Parkinson disease. Neurodegener Dis 18, 19–25. doi: 10.1159/000484249
Rajput, A. H., and Rajput, E. F. (2017). Octogenarian parkinsonism - Clinicopathological observations. Parkinsonism Relat. Disord. 37, 50–57. doi: 10.1016/j.parkreldis.2017.01.009
Sakakibara, R., Shinotoh, H., Uchiyama, T., Sakuma, M., Kashiwado, M., Yoshiyama, M., et al. (2001). Questionnaire-based assessment of pelvic organ dysfunction in Parkinson’s disease. Auton. Neurosci. 92, 76–85. doi: 10.1016/S1566-0702(01)00295-8
Sakakibara, R., Tateno, F., Kishi, M., Tsuyuzaki, Y., Uchiyama, T., and Yamamoto, T. (2012). Pathophysiology of bladder dysfunction in Parkinson's disease. Neurobiol. Dis. 46, 565–571. doi: 10.1016/j.nbd.2011.10.002
Sakakibara, R., Uchiyama, T., Yamanishi, T., Shirai, K., and Hattori, T. (2008). Bladder and bowel dysfunction in Parkinson’s disease. J. Neural Transm. (Vienna) 115, 443–460. doi: 10.1007/s00702-007-0855-9
Santos-Garcia, D., De Deus Fonticoba, T., Suarez Castro, E., Aneiros Diaz, A., Mcafee, D., Catalan, M. J., et al. (2020). Non-motor symptom burden is strongly correlated to motor complications in patients with Parkinson’s disease. Eur. J. Neurol. 27, 1210–1223. doi: 10.1111/ene.14221
Sato, K., Hatano, T., Yamashiro, K., Kagohashi, M., Nishioka, K., Izawa, N., et al. (2006). Prognosis of Parkinson's disease: time to stage III, IV, V, and to motor fluctuations. Mov. Disord. 21, 1384–1395. doi: 10.1002/mds.20993
Savica, R., Rocca, W. A., and Ahlskog, J. E. (2010). When does Parkinson disease start? Arch. Neurol. 67, 798–801. doi: 10.1001/archneurol.2010.135
Schapira, A. H. V., Chaudhuri, K. R., and Jenner, P. (2017). Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450. doi: 10.1038/nrn.2017.62
Sobreira-Neto, M. A., Pena-Pereira, M. A., Sobreira, E. S. T., Chagas, M. H. N., Fernandes, R. M. F., Tumas, V., et al. (2017). High frequency of sleep disorders in Parkinson’s disease and its relationship with quality of life. Eur. Neurol. 78, 330–337. doi: 10.1159/000481939
Sonn, G. A., Sadetsky, N., Presti, J. C., and Litwin, M. S. (2013). Differing perceptions of quality of life in patients with prostate cancer and their doctors. J. Urol. 189, S59–S65. doi: 10.1016/j.juro.2012.11.032
Wakabayashi, K., Takahashi, H., Takeda, S., Ohama, E., and Ikuta, F. (1989). Lewy bodies in the enteric nervous system in Parkinson's disease. Arch. Histol. Cytol. 52, 191–194. doi: 10.1679/aohc.52.Suppl_191
Yamawaki, M., Kusumi, M., Kowa, H., and Nakashima, K. (2009). Changes in prevalence and incidence of Parkinson's disease in Japan during a quarter of a century. Neuroepidemiology 32, 263–269. doi: 10.1159/000201565
Zhao, N., Yang, Y., Zhang, L., Zhang, Q., Balbuena, L., Ungvari, G. S., et al. (2021). Quality of life in Parkinson’s disease: a systematic review and meta-analysis of comparative studies. CNS Neurosci. Ther. 27, 270–279. doi: 10.1111/cns.13549
Keywords: Parkinson’s disease, non-motor symptoms, older adults, self-questionnaire, Japan
Citation: Morimoto R, Iijima M, Okuma Y, Suzuki K, Yoshii F, Nogawa S, Osada T and Kitagawa K (2023) Associations between non-motor symptoms and patient characteristics in Parkinson’s disease: a multicenter cross-sectional study. Front. Aging Neurosci. 15:1252596. doi: 10.3389/fnagi.2023.1252596
Edited by:
Atsushi Takeda, National Hospital Organization Sendai Nishitaga Hospital, JapanReviewed by:
Shinsuke Fujioka, Fukuoka University, JapanTadashi Ichikawa, Saitama Prefectural Rehabilitation Center, Japan
Copyright © 2023 Morimoto, Iijima, Okuma, Suzuki, Yoshii, Nogawa, Osada and Kitagawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mutsumi Iijima, aWlqaW1hLm11dHN1bWlAdHdtdS5hYy5qcA==
 Remi Morimoto
Remi Morimoto Mutsumi Iijima
Mutsumi Iijima Yasuyuki Okuma
Yasuyuki Okuma Keisuke Suzuki
Keisuke Suzuki Fumihito Yoshii
Fumihito Yoshii Shigeru Nogawa
Shigeru Nogawa Takashi Osada
Takashi Osada Kazuo Kitagawa
Kazuo Kitagawa